Abstract
Background
Electrical cardioversion (ECV) is an effective method for restoring sinus rhythm after atrial fibrillation (AF). However, early recurrence of AF occurs in a significant number of patients after ECV. This study aimed to identify electrocardiographic (ECG) predictors of early AF recurrence after ECV.
Methods
A total of 272 patients with persistent AF undergoing successful ECV were consecutively enrolled in this study. We investigated clinical, echocardiographic, and ECG data. The 12‐lead ECG parameters were measured during sinus rhythm right after ECV using a digital caliper. The early AF recurrence was defined as recurrence within 2 months.
Results
Of the 272 patients, 165 patients (60.7%) experienced an early AF recurrence. Maximum P‐wave duration (PWD) in limb leads (OR: 1.086; 95% CI: 1.019–1.157; p = .012) and P‐terminal force (PTF) in V1 (OR: 1.019; 95% CI: 1.004–1.033; p = .011) were independent predictors of early AF recurrence after ECV. The optimal cutoff value of the maximum PWD in limb leads for predicting early AF recurrence was 134 ms, characterized by 90.3% sensitivity and 72.0% specificity. Likewise, the optimal cutoff value of PTF in V1 was 50 ms × mm, characterized by 80.0% sensitivity and 64.5% specificity.
Conclusion
A longer PWD (>134 ms) and a larger PTF (>50 ms × mm) were useful predictors of early recurrence of AF after successful ECV in clinical practice. A more effective rhythm control therapy such as catheter ablation or rate control strategy rather than a repeat ECV should be considered.
Keywords: atrial fibrillation, cardioversion, P‐terminal force, P‐wave duration, recurrence
1. INTRODUCTION
Atrial fibrillation (AF) is the most prevalent sustained arrhythmia seen in clinical practice and is associated with high healthcare costs (Delaney et al., 2018; Go et al., 2001). There are two strategies for addressing persistent AF. One approach is to control the ventricular rate using an atrioventricular node blocking agent, and the other is to restore the sinus rhythm (SR) using antiarrhythmic drugs (AADs), electrical cardioversion (ECV), and catheter ablation. Rhythm control is performed to improve the quality of life by reducing symptoms such as palpitation, chest pain, and dyspnea, although there is no clear evidence of survival benefit (Wyse et al., 2002). Previous studies reported that the immediate overall success rate for ECV converting AF to SR is up to 90% (Berry et al., 2001; Pisters et al., 2012; Toso et al., 2012). However, maintenance of SR is often challenging because almost half of the patients return to AF within 1 year post‐cardioversion (Toso et al., 2012). Moreover, most recurrence happens within 1 month after ECV (Bertaglia et al., 2002; Tieleman et al., 1998). Therefore, careful early selection of the therapeutic strategy is crucial to reduce unnecessary medical costs and prevent possible ECV complications.
Various clinical, demographic, and echocardiographic risk factors have been proposed as predictors for AF recurrence after successful ECV. A prolonged AF duration, especially longer than 6 months, was associated with a significant increase of AF recurrence (Toso et al., 2012). Concerning demographics, older patients have a higher risk of recurrence of AF (Pisters et al., 2012). Besides, multiple comorbidities including hypertension, diabetes, and chronic obstructive pulmonary disease (COPD) have been suggested as independent risk factors that reduce the likelihood of SR maintenance (Berry et al., 2001; Pisters et al., 2012; Soran et al., 2008). Also, several studies have shown that left ventricular (LV) systolic dysfunction and left atrial (LA) enlargement are beneficial for predicting AF recurrence (Marchese et al., 2011; Raitt et al., 2006). Regarding ECG parameters, P‐wave duration and dispersion were suggested as ECG predictors of AF recurrence after ECV (Fujimoto et al., 2018; Gonna et al., 2014). However, evidence for these parameters in predicting AF recurrence is still lacking, so these predictors are not commonly used in clinical practice. In addition, these ECG parameters have not been thoroughly evaluated for early recurrence of AF after ECV. We hypothesized that simple ECG predictors could be useful for patients who experience early AF recurrence to determine future treatment strategy including catheter ablation or rate control therapy. This prospective cohort study aimed to identify predictors of early AF recurrence after successful ECV, including clinical, echocardiographic, and especially ECG parameters.
2. METHODS
2.1. Study population
All consecutive adult patients (≥18 years of age) with symptomatic persistent AF who underwent ECV at the Samsung Medical Center in Korea from November 2017 to October 2019 were enrolled prospectively in this study. This study complied with the Declaration of Helsinki, and the research protocol was approved by the ethics committee of the Samsung Medical Center. We reviewed the medical records, and the clinical, echocardiographic, and electrocardiographic data were investigated. Persistent AF was defined as AF duration over 3 months that did not convert to SR even with at least two kinds of class Ic or class III AADs. Exclusion criteria were as follows: a previous ECV within 1 year, previous AF ablation, moderate‐to‐severe valvular heart disease, hypertrophic cardiomyopathy, cardiac implantable electrical devices, congenital heart disease, previous cardiac surgery, and thyroid disease.
2.2. Electrical cardioversion
All patients undergoing ECV were anticoagulated for at least 1 month before ECV. Transesophageal echocardiography was routinely performed right before the procedure to identify the intracardiac thrombus. ECV was performed under sedation in an electrophysiology laboratory equipped with cardiopulmonary resuscitation devices and under supervision by a cardiologist while the patient had continuous blood pressure, heart rate, and oxygen saturation monitoring. According to our hospital protocol, the first biphasic synchronous shock through self‐adhesive electrodes was positioned anterolaterally and delivered at 150J and then increased to 200J if AF persisted. Successful ECV was defined as a case when SR was maintained at least 2 min after ECV without immediate AF recurrence (Fuster et al., 2006).
2.3. ECG parameter measurements
Standard 12‐lead ECGs were digitally recorded using the CardioLab™ electrophysiology recording system (GE Healthcare) (Figure 1). The P‐wave duration (PWD) and amplitude were measured on all 12 leads, and other conventional ECG parameters, including the PR interval, QRS duration, and QT interval, were measured in lead II. The maximum PWD was defined as the longest value for each limb and precordial leads. The P‐wave terminal force (PTF) is a product of the amplitude (millimeter) and the duration (millisecond) of the terminal phase of P‐wave in lead V1. All ECG parameters were measured with digital calipers with magnification (200%–800%) by two cardiology fellows (JHC, HJK), unaware of the AF recurrence status.
FIGURE 1.

Schematic presentation of electrocardiographic parameter measurements used for the study cases using digital caliper with magnification at a speed of 100 mm/s (Top: lead II, bottom: lead V1). (a). A case with early AF recurrence, (b). A case without early AF recurrence, AF, atrial fibrillation; PTF, P‐terminal force
2.4. Echocardiographic examination
The transthoracic echocardiography was always performed together with transesophageal echocardiography before ECV. The LA diameter was measured in the M mode parasternal long‐axis view with the probe located in the third or fourth intercostal space (Lang et al., 2015). The LA volume index (LAVI) was measured by the biplane area length method using the apical four‐chamber and apical two‐chamber view at the ventricular end‐systole and indexed to the calculated body surface area using the Du Bois formula (Lang et al., 2015). The LV ejection fraction (LVEF) was accessed by the biplane Simpson method at ventricular end‐diastole (Lang et al., 2015).
2.5. Follow‐up after discharge
Clinical follow‐up was scheduled monthly until 3 months after ECV. At each visit, recurrence of AF‐related symptoms and 12‐lead ECG were obtained. Routine 24‐h Holter monitoring was applied 2 months after ECV if no AF‐related symptoms were obtained each outpatient visit. Patients who had recurrent AF‐related symptoms were evaluated immediately using Holter or event monitoring regardless of follow‐up schedule and educated to receive a 12‐lead ECG at a nearby hospital as soon as symptoms occur. After 2 months, follow‐up visits were planned with ECG and Holter monitoring at 4, 8, and 12 months for patients whose sinus rhythm was maintained. Diagnosis of early AF recurrence was made when AF was confirmed on 12‐lead ECG, or AF lasting at least 30 s was documented on Holter monitoring within 2 months after ECV. Time to AF recurrence was calculated from successful ECV to the first AF rhythm documentation on ECG or Holter monitoring after successful ECV.
2.6. Statistical analysis
SPSS statistical software (version 25; SPSS Inc.) was utilized to perform data analysis. Continuous variables are reported as mean values ± SD, and categorical variables as the number of patients (percentage). The chi‐square test, Fisher's exact test, and independent sample t tests were performed to compare the two groups. Univariate and multivariate logistic regression were performed to identify predictors for AF recurrence, and an odds ratio (OR) presented with a 95% confidence interval (CI). For the AF‐free survival analysis, a Kaplan–Meier survival curve was performed. Receiver operating characteristic (ROC) analysis was performed to evaluate the optimal cutoff value of the ECG parameters for early recurrence of AF after successful ECV. ROC analysis for the model combining the two parameters was carried out using predicted probability from a logistic regression analysis with two parameters. All statistical tests were two‐tailed, and a p‐value of <.05 was considered significant.
3. RESULTS
3.1. Baseline characteristics
The study flow chart with the number of patients is presented in Figure 2. A total of 380 patients underwent ECV, and 325 patients were successfully converted to SR. The overall ECV success rate was 85.5%. Excluding 48 patients based on exclusion criteria, 277 patients were finally included in this study. Of these 277 patients, 5 patients were lost to follow‐up. We divided 272 patients into two groups (Group A and Group B) based on the early AF recurrence. At 2 months after ECV, 165 patients (Group A, 60.7%) experienced early AF recurrence, and 107 patients (Group B, 39.3%) maintained the SR. In Group B, only 42 patients (39.3%) experienced AF recurrence during the follow‐up period (10.1 ± 6.0 months). The mean time to AF recurrence was significantly different between the two groups (Group A vs. Group B; 30 ± 8 days vs. 260 ± 132 days; p < .0001). The baseline demographic, clinical, echocardiographic, and electrocardiographic characteristics of each study group are summarized in Table 1. The mean age was similar in Group A vs. Group B (60 ± 9 years vs. 60 ± 11; p = .960). Male sex was dominant in both groups (p = .073). Patients with chronic kidney disease were more common in Group B (5.6%) than Group A (0%) (p = .003). Most patients in both study groups were taking AADs before (Group A vs. Group B, 82.4% vs. 85.0%, p = .570) and after ECV (Group A vs. Group B, 93.9 vs. 97.2%, p = .219). 10 (6.1%) patients in Group A and 3 (2.8%) patients in Group B were not taking AADs, due to symptomatic bradycardia. The patients with early AF recurrence were found to be taking more propafenone (Group A vs. Group B, 37.0 vs. 21.5%; p = .007) and less amiodarone (Group A vs. Group B, 33.9 vs. 46.7%; p = .035) compared with the patients without early AF recurrence.
FIGURE 2.
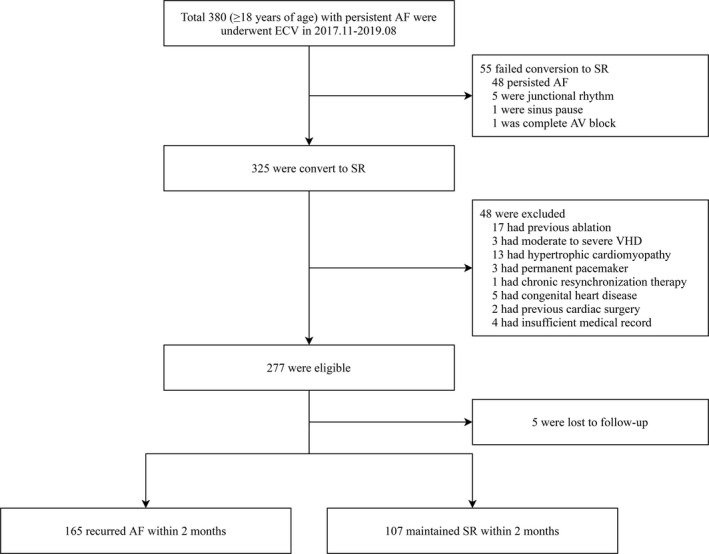
Study flow chart with a number of patients. AF, atrial fibrillation; AV, atrioventricular; ECV, electrical cardioversion; SR, sinus rhythm; VHD, valvular heart disease
TABLE 1.
Baseline clinical, echocardiographic, and electrocardiographic characteristics
| Variables |
Group A (n = 165) |
Group B (n = 107) |
p‐value |
|---|---|---|---|
| Demographic variables | |||
| Age, years | 60 ± 9 | 60 ± 11 | .960 |
| Age >65 years, n (%) | 43 (26.1) | 34 (31.8) | .307 |
| Male, n (%) | 138 (83.6) | 80 (74.8) | .073 |
| BMI, kg/m2 | 25.9 ± 2.7 | 26.0 ± 3.3 | .785 |
| CHA2DS2‐VASc score | 0.98 ± 0.87 | 1.04 ± 0.76 | .589 |
| CHA2DS2‐VASc score ≥2, n (%) | 37 (22.4) | 29 (27.1) | .379 |
| Previous ECV, n (%) | 8 (4.8) | 7 (5.9) | .550 |
| AF duration, months | 23 ± 33 | 21 ± 32 | .499 |
| AF duration >6 months, n (%) | 84 (50.9) | 54 (50.5) | .943 |
| AF duration >12 months, n (%) | 71 (43.0) | 38 (35.5) | .217 |
| AF duration >24 months, n (%) | 52 (31.5) | 29 (27.1) | .437 |
| Medical history | |||
| Hypertension, n (%) | 89 (53.9) | 64 (59.8) | .340 |
| Diabetes, n (%) | 34 (20.6) | 15 (14.0) | .167 |
| Chronic kidney disease, n (%) | 0 | 6 (5.6) | .003 |
| Coronary artery disease, n (%) | 1 (0.6) | 1 (0.9) | 1.000 |
| Stroke/transient ischemic attack, n (%) | 11 (6.7) | 3 (2.8) | .159 |
| Antiarrhythmic drugs before cardioversion, n (%) | 136 (82.4) | 91 (85.0) | .570 |
| Flecainide, n (%) | 18 (10.9) | 15 (14.0) | .443 |
| Propafenone, n (%) | 58 (35.2) | 23 (21.5) | .016 |
| Amiodarone, n (%) | 50 (30.3) | 47 (43.9) | .022 |
| Dronedarone, n (%) | 10 (6.1) | 4 (3.7) | .397 |
| Sotalol, n (%) | 0 | 2 (1.9) | .154 |
| Pilsicainide, n (%) | 0 | 0 | |
| Antiarrhythmic drugs after cardioversion, n (%) | 155 (93.9) | 104 (97.2) | .219 |
| Flecainide, n (%) | 24 (14.5) | 20 (18.7) | .364 |
| Propafenone, n (%) | 61 (37.0) | 23 (21.5) | .007 |
| Amiodarone, n (%) | 56 (33.9) | 50 (46.7) | .035 |
| Dronedarone, n (%) | 14 (8.5) | 8 (7.5) | .766 |
| Sotalol, n (%) | 0 | 2 (1.9) | .154 |
| Pilsicainide, n (%) | 0 | 1 (0.9) | .393 |
| Echocardiographic parameters | |||
| LVEF, % | 57.9 ± 8.6 | 56.5 ± 10.4 | .251 |
| LA diameter, mm | 45.9 ± 7.3 | 46.6 ± 5.8 | .425 |
| LA diameter >40 mm, n (%) | 144 (87.3) | 90 (84.1) | .463 |
| LAVI, ml/m2 | 52.1 ± 15.1 | 51.5 ± 16.8 | .772 |
| Electrocardiographic parameters | |||
| Maximum PWD in limb leads, ms | 148 ± 14 | 136 ± 14 | <.001 |
| Maximum PWD in precordial leads, ms | 146 ± 15 | 134 ± 15 | <.001 |
| Duration of P‐terminal force, ms | 80 ± 16 | 67 ± 16 | <.001 |
| P‐terminal force, ms × mm | 74.9 ± 33.2 | 49.4 ± 26.3 | <.001 |
| Maximum P‐wave amplitude in limb leads, mV | 0.15 ± 0.05 | 0.16 ± 0.06 | .396 |
| PR interval, ms | 199 ± 35 | 194 ± 33 | .277 |
| QRS duration, ms | 93 ± 15 | 93 ± 16 | .882 |
| QT interval, ms | 425 ± 37 | 435 ± 50 | .093 |
| Corrected QT interval, ms | 419 ± 35 | 423 ± 42 | .419 |
Variables are expressed as n (%) or mean ± SD.
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CHA2DS2‐VASc score, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age 65–74 years, sex category; ECV, electrical cardioversion; LA, left atrium; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; PWD, P‐wave duration.
The mean CHA2DS2‐VASc score was 1 in both groups, and the distribution of this score was not different between the two groups. There was no significant difference between the two groups in other variables such as comorbidities, LV systolic function, and LA size. More than half of the patients had AF duration longer than 6 months, and over 80% of patients had LA diameter larger than 40 mm in both groups. The maximum PWD in limb leads and precordial lead, duration of PTF, and PTF were strongly associated with early AF recurrence after ECV. Other conventional ECG parameters such as P‐wave amplitude, PR interval, QRS duration, and QT interval were not significantly different between groups.
3.2. PWD and PTF results as predictors of early AF recurrence
The results of the logistic regression analysis are summarized in Table 2. In univariate analysis, four electrocardiographic parameters were strongly associated with early recurrence of AF: the maximum PWD measured in the limb and precordial leads, duration of PTF, and P‐terminal force. After multivariate analysis using variables with p < .2 in univariate analysis, only maximum PWD in limb leads (OR: 1.086; 95% CI: 1.019 –1.157; p = .012) and PTF (OR: 1.019; 95% CI: 1.004–1.033; p = .011) were independent predictors of early AF recurrence after adjusting for age, sex, diabetes, stroke (or transient ischemic attack), propafenone, amiodarone, and QT interval. Each millisecond increase in the maximum PWD in limb leads and each millisecond × millimeter increased in the PTF were associated with an 8.6% and 1.9% increase in the risk of early AF recurrence, respectively.
TABLE 2.
Univariate and multivariate predictors of early AF recurrence
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p‐value | OR | 95% CI | p‐value | |
| Demographic variables | ||||||
| Age, per year | 0.999 | 0.975–1.024 | .959 | 0.983 | 0.951–1.015 | .296 |
| Male sex | 1.725 | 0.946–3.144 | .075 | 1.343 | 0.620–2.910 | .455 |
| BMI, per kg/m2 | 0.989 | 0.915–1.069 | .779 | |||
| CHA2DS2‐VASc score, per 1 point | 0.922 | 0.687–1.237 | .587 | |||
| CHA2DS2‐VASc score ≥2, | 0.777 | 0.443–1.363 | .380 | |||
| Previous ECV | 0.728 | 0.256–2.070 | .551 | |||
| AF duration, per month | 1.003 | 0.995–1.010 | .499 | |||
| >6 months | 1.018 | 0.626–1.656 | .943 | |||
| >12 months | 1.372 | 0.830–2.265 | .217 | |||
| >24 months | 1.238 | 0.723–2.120 | .437 | |||
| Medical history | ||||||
| Hypertension | 0.787 | 0.481–1.288 | .340 | |||
| Diabetes | 1.592 | 0.820–3.091 | .170 | 1.558 | 0.718–3.380 | .262 |
| Coronary artery disease | 0.646 | 0.040–10.445 | .759 | |||
| Stroke/transient ischemic attack | 2.476 | 0.674–9.091 | .172 | 3.343 | 0.834–13.393 | .088 |
| Antiarrhythmic drugs after ECV | ||||||
| Flecainide | 0.740 | 0.386–1.419 | .365 | |||
| Propafenone | 2.142 | 1.225–3.747 | .008 | 1.472 | 0.679–3.193 | .327 |
| Amiodarone | 0.586 | 0.356–0.964 | .035 | 1.047 | 0.524–2.091 | .897 |
| Dronedarone | 1.147 | 0.464–2.836 | .766 | |||
| Echocardiographic parameters | ||||||
| LVEF, per percent | 1.015 | 0.989–1.042 | .251 | |||
| LA diameter, per mm | 0.985 | 0.949–1.022 | .425 | |||
| LA diameter >40 mm | 1.295 | 0.649–2.586 | .463 | |||
| LAVI, per ml/m2 | 1.002 | 0.987–1.018 | .771 | |||
| Electrocardiographic parameters | ||||||
|
Maximum PWD in limb leads , per ms |
1.079 | 1.053–1.106 | <.001 | 1.086 | 1.019–1.157 | .012 |
|
Maximum PWD in precordial leads, per ms |
1.061 | 1.039–1.083 | <.001 | 0.979 | 0.929–1.032 | .436 |
| Duration of PTF, per ms | 1.060 | 1.039–1.081 | <.001 | 1.003 | 0.970–1.037 | .855 |
| PTF, per ms × mm | 1.032 | 1.021–1.043 | <.001 | 1.019 | 1.004–1.033 | .011 |
|
Maximum P‐wave amplitude in limb leads, per mV |
0.137 | 0.001–13.464 | .396 | |||
| PR interval, per ms | 1.004 | 0.997–1.011 | .277 | |||
| QRS duration, per ms | 0.999 | 0.983–1.015 | .882 | |||
| QT interval, per ms | 0.995 | 0.989–1.001 | .075 | 0.995 | 0.987–1.003 | .234 |
| Corrected QT interval, per ms | 0.997 | 0.991–1.004 | .418 | |||
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CHA2DS2‐VASc score, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age 65–74 years, sex category; ECV, electrical cardioversion; LA, left atrium; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; PTF, P‐terminal force; PWD, P‐wave duration.
In Figure 3, the ROC curve for the maximum PWD in limb leads (Figure 3a) and PTF (Figure 3b) are presented, in addition to a model combining the two predictors (Figure 3c). The area under the ROC curve of maximum PWD in limb leads for early AF recurrence was 0.794 (95% CI: 0.732–0.855; p < .001) and that for PTF was 0.745 (95% CI: 0.685–0.806; p < .001); the model combining the two predictors was 0.814 (95% CI: 0.756–0.871; p < .001). Table 3 shows independent predictors with optimal cutoff value and measures of diagnostic accuracy. The optimal cutoff value using the Youden index of the maximum PWD in limb leads to predict early AF recurrence was 134 ms, characterized by 90.3% sensitivity and 72.0% specificity. Likewise, the optimal cutoff value of PTF was 50 ms × mm, characterized by 80.0% sensitivity and 64.5% specificity. Maximum PWD in limb leads showed better diagnostic performance than PTF, but there was no significant difference between the two predictors when analyzed using Delong's test (p = .132). The specificity of the model combining the two predictors was 75.7%, which was better than that of the two predictors. A detailed explanation of the model combining the two predictors is in supplementary material.
FIGURE 3.
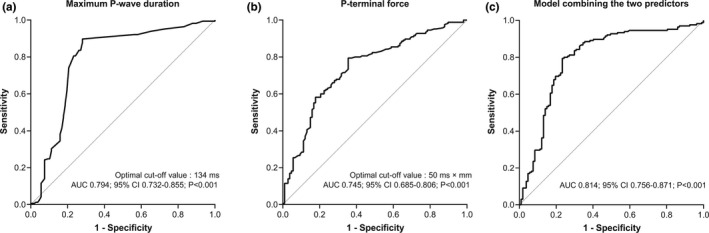
Receiver operating characteristic curves for independent predictors of early AF recurrence. (a). Maximum P‐wave duration in limb leads, (b). P‐terminal force, (c). Model combining the two predictors, AF, atrial fibrillation; AUC, area under the curve
TABLE 3.
Optimal cutoff value and measures of diagnostic accuracy
| Parameters | AUC | Cut‐off value | Sen (%) | Spe (%) | PPV (%) | NPV (%) | ACC (%) |
|---|---|---|---|---|---|---|---|
| Maximum PWD in limb leads | 0.794 | 134 ms | 90.3 | 72.0 | 83.2 | 82.8 | 83.1 |
| P‐terminal force | 0.745 | 50 ms × mm | 80.0 | 64.5 | 77.7 | 67.7 | 73.9 |
| Model combining the two predictors | 0.814 | 81.8 | 75.7 | 83.9 | 73.0 | 79.4 |
Abbreviations: ACC, accuracy; AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value; PWD, P‐wave duration; Sen, sensitivity; Spe, specificity.
3.3. Intra‐ and inter‐rater reliability of measurements
The intra‐ and inter‐rater correlation coefficients for maximum PWD in limb leads were 0.941 and 0.932, respectively. The intra‐ and inter‐rater correlation coefficients for the P‐terminal force were 0.957 and 0.935, respectively. There was no disagreement on the presence of early recurrence between raters.
4. DISCUSSION
In our study, we aimed to identify ECG predictors for early AF recurrence after successful ECV. The results of this study suggest that two ECG parameters, maximum PWD and PTF, could be useful for predicting early AF recurrence after successful ECV. In particular, the maximum PWD in limb leads with a cutoff value of 134 ms showed the highest sensitivity, and the model combining the two predictors showed the highest specificity for predicting early AF recurrence.
The overall recurrence rate of AF within 1 month after ECV in this study was approximately 55%, which was similar to previous studies (Bertaglia et al., 2002; Tieleman et al., 1998). The current study included a large number of patients at higher risk of AF recurrence. AF duration was longer than 6 months in more than half of these patients, and the LA dimension was greater than 40 mm in over 80% of patients. Prediction of early recurrence of AF after ECV would be necessary, especially in patients with a high risk of AF recurrence, to guide future treatment strategies. 12‐lead ECG is a simple, inexpensive, and reproducible examination method routinely performed in AF patients. Therefore, prediction of early recurrence using ECG parameters would be beneficial in real‐world clinical practices. In the AFFIRM study, the authors suggested that a history of coronary artery disease (OR: 0.52; 95% CI: 0.35–0.76; p = .001) and a PWD longer than 135 ms (OR: 1.60; 95% CI: 1.11–2.31; p = .001) were predictors for AF recurrence within 2 months after ECV (Raitt et al., 2006). However, the PWD was only measured in lead II in this study. In a prospective study that involved 77 patients, Gonna et al. also showed that the maximum PWD in 12 leads could predict AF recurrence after ECV (OR: 3.17; 95% CI: 1.16–8.67; p = .02). A maximum PWD longer than 142 ms was suggested as an optimal cutoff point (sensitivity 58.6%, specificity 62.1%) (Gonna et al., 2014). In our study, we showed that maximum PWD is a good predictor for AF recurrence after ECV, and these results are consistent with those of a previous study. Besides, we propose PTF as a new predictor of early AF recurrence after successful ECV. PTF showed comparable diagnostic accuracy compared with maximum PWD in limb leads. Along with these results, the model combining these two predictors showed better specificity to predict early recurrence of AF after ECV.
The PWD, which was a significant predictor in this study, represents the atrial conduction time. Since right atrial depolarization precedes the LA depolarization, the total depolarization time of the atrium largely depends on the LA conduction time. Thus, the maximum PWD reflects the total conduction time of LA. Two large prospective cohort studies showed an association between prolonged PWD and the risk of AF (Magnani et al., 2015; Smith et al., 2017). Moreover, prolonged PWD has been consistently suggested as a strong predictor for recurrence after AF ablation as well as incident AF (Wang et al., 2017). LA remodeling is a key process in AF generation and is a consequence of structural and functional maladaptation against external stress. LA remodeling, in turn, promotes electrical disturbance that can increase incident AF (Casaclang‐Verzosa et al., 2008; Nattel & Harada, 2014). This electrical disturbance generates disorganized anisotropic propagation of electrical activity across the atria, manifested as PWD prolongation in the surface ECG (Hari et al., 2018). The second negative deflection in the lead V1, called PTF in our study, represents LA depolarization because depolarization of the LA is directed posteriorly. The P‐terminal force in V1 has also been associated with AF incidence (Baturova et al., 2016; Magnani et al., 2015). In our study, maximum PWD in limb leads and PTF were significantly associated with early AF recurrence, and our findings reflect this theoretical background well.
ECV is a widely conducted procedure that restores the AF to SR in a patient with persistent AF, despite AADs in clinical practice. The clinical significance of this study is that a simple ECG predictor may be helpful for predicting early AF recurrence after successful ECV, even with various AADs. This prospective study evaluated predictors for early AF recurrence after ECV in patients with persistent AF despite taking AADs who have longer AF duration and larger LA size. This study differs from other studies in that (1) the enrolled patients tended to show longer AF duration and larger LA size, (2) it is the first study to show that PTF, as well as PWD, is valuable predictors for early AF recurrence after ECV, and (3) the model combining PWD and PTF showed better specificity to predict early recurrence of AF. This approach could guide clinicians to determine treatment plans from a simple rate control strategy to a more effective rhythm control therapy such as catheter ablation for patients with persistent AF refractory to AADs and ECV.
This study had several limitations. First, this study is based on data from a single center. A subsequent definitive multicenter study or external validation of our results must be conducted to confirm our results. Second, by using magnification to measure ECG parameters, it is possible that a small measurement error created a statistically significant change. To minimize measurement error, we performed tests for intra‐ and inter‐observer reliability. Third, AF recurrence might be underestimated because continuous ECG monitoring was not performed during the follow‐up period. Fourth, it may be difficult to apply the cutoff value of ECG parameters to patients with normal LA diameter or shorter AF duration because our findings came from patients with long AF duration and large LV size.
5. CONCLUSIONS
Maximum PWD in limb leads and PTF in V1 could be useful for predicting early recurrence of AF after successful ECV in clinical practice. An AF patient with a longer PWD and larger PTF on SR ECG should be observed closely after successful ECV even with various AADs. A more effective rhythm control therapy such as catheter ablation or rate control strategy rather than a repeat ECV should be considered in these patients.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
J.H.Choi., S.J.Park., and K.M.Park. involved in conceptualization; J.H.Choi., H.J.Kwon., and H.R.Kim. investigated the study; J.H.Choi. and K.M.Park. formally analyzed the study; J.H.Choi. involved in original draft preparation; J.S.Kim., Y.K.On., S.J.Park., and K.M.Park. reviewed and edited the study; J.H.Choi. visualized the study; K.M.Park involved in supervision.
ETHICAL APPROVAL
This study complied with the Declaration of Helsinki, and the research protocol was approved by the local institutional ethics board (Samsung Medical Center Institutional Review Board).
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
None.
Choi, J.‐H. , Kwon, H.‐J. , Kim, H. R. , Park, S.‐J. , Kim, J. S. , On, Y. K. , & Park, K.‐M. (2021). Electrocardiographic predictors of early recurrence of atrial fibrillation. Annals of Noninvasive Electrocardiology, 26, e12884. 10.1111/anec.12884
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Baturova, M. A. , Sheldon, S. H. , Carlson, J. , Brady, P. A. , Lin, G. , Rabinstein, A. A. , Friedman, P. A. , & Platonov, P. G. (2016). Electrocardiographic and Echocardiographic predictors of paroxysmal atrial fibrillation detected after ischemic stroke. BMC Cardiovascular Disorders, 16(1), 209. 10.1186/s12872-016-0384-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, C. , Stewart, S. , Payne, E. M. , McArthur, J. D. , & McMurray, J. J. (2001). Electrical cardioversion for atrial fibrillation: outcomes in "real‐life" clinical practice. International Journal of Cardiology, 81(1), 29–35. 10.1016/s0167-5273(01)00522-8 [DOI] [PubMed] [Google Scholar]
- Bertaglia, E. , D'Este, D. , Zerbo, F. , Zoppo, F. , Delise, P. , & Pascotto, P. (2002). Success of serial external electrical cardioversion of persistent atrial fibrillation in maintaining sinus rhythm; a randomized study. European Heart Journal, 23(19), 1522–1528. 10.1053/euhj.2002.3167 [DOI] [PubMed] [Google Scholar]
- Casaclang‐Verzosa, G. , Gersh, B. J. , & Tsang, T. S. (2008). Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. Journal of the American College of Cardiology, 51(1), 1–11. 10.1016/j.jacc.2007.09.026 [DOI] [PubMed] [Google Scholar]
- Delaney, J. A. C. , Yin, X. , Fontes, J. D. , Wallace, E. R. , Skinner, A. , Wang, N. A. , Hammill, B. G. , Benjamin, E. J. , Curtis, L. H. , & Heckbert, S. R. (2018). Hospital and clinical care costs associated with atrial fibrillation for Medicare beneficiaries in the Cardiovascular Health Study and the Framingham Heart Study. SAGE Open Med, 6, 2050312118759444. 10.1177/2050312118759444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto, Y. , Yodogawa, K. , Maru, Y.‐J. , Oka, E. , Hayashi, H. , Yamamoto, T. , Iwasaki, Y.‐K. , Hayashi, M. , & Shimizu, W. (2018). Advanced interatrial block is an electrocardiographic marker for recurrence of atrial fibrillation after electrical cardioversion. International Journal of Cardiology, 272, 113–117. 10.1016/j.ijcard.2018.07.135 [DOI] [PubMed] [Google Scholar]
- Fuster, V. , Rydén, L. E. , Cannom, D. S. , Crijns, H. J. , Curtis, A. B. , Ellenbogen, K. A. , Halperin, J. L. , Le Heuzey, J.‐Y. , Kay, G. N. , Lowe, J. E. , Olsson, S. B. , Prystowsky, E. N. , Tamargo, J. L. , Wann, S. , Smith, S. C. , Jacobs, A. K. , Adams, C. D. , Anderson, J. L. , Antman, E. M. , … Zamorano, J. L. (2006). ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation, 114(7), e257–e354. 10.1161/CIRCULATIONAHA.106.177292 [DOI] [PubMed] [Google Scholar]
- Go, A. S. , Hylek, E. M. , Phillips, K. A. , Chang, Y. , Henault, L. E. , Selby, J. V. , & Singer, D. E. (2001). Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA, 285(18), 2370–2375. 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- Gonna, H. , Gallagher, M. M. , Guo, X. H. , Yap, Y. G. , Hnatkova, K. , & Camm, A. J. (2014). P‐wave abnormality predicts recurrence of atrial fibrillation after electrical cardioversion: a prospective study. Annals of Noninvasive Electrocardiology, 19(1), 57–62. 10.1111/anec.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari, K. J. , Nguyen, T. P. , & Soliman, E. Z. (2018). Relationship between P‐wave duration and the risk of atrial fibrillation. Expert Review of Cardiovascular Therapy, 16(11), 837–843. 10.1080/14779072.2018.1533814 [DOI] [PubMed] [Google Scholar]
- Lang, R. M. , Badano, L. P. , Mor‐Avi, V. , Afilalo, J. , Armstrong, A. , Ernande, L. , Flachskampf, F. A. , Foster, E. , Goldstein, S. A. , Kuznetsova, T. , Lancellotti, P. , Muraru, D. , Picard, M. H. , Rietzschel, E. R. , Rudski, L. , Spencer, K. T. , Tsang, W. , & Voigt, J.‐U. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography, 28(1), 1–39 e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Magnani, J. W. , Zhu, L. , Lopez, F. , Pencina, M. J. , Agarwal, S. K. , Soliman, E. Z. , Benjamin, E. J. , & Alonso, A. (2015). P‐wave indices and atrial fibrillation: cross‐cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. American Heart Journal, 169(1), 53–61 e51. 10.1016/j.ahj.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese, P. , Bursi, F. , Delle Donne, G. , Malavasi, V. , Casali, E. , Barbieri, A. , Melandri, F. , & Modena, M. G. (2011). Indexed left atrial volume predicts the recurrence of non‐valvular atrial fibrillation after successful cardioversion. European Journal of Echocardiography, 12(3), 214–221. 10.1093/ejechocard/jeq176 [DOI] [PubMed] [Google Scholar]
- Nattel, S. , & Harada, M. (2014). Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. Journal of the American College of Cardiology, 63(22), 2335–2345. 10.1016/j.jacc.2014.02.555 [DOI] [PubMed] [Google Scholar]
- Pisters, R. , Nieuwlaat, R. , Prins, M. H. , Le Heuzey, J.‐Y. , Maggioni, A. P. , Camm, A. J. , & Crijns, H. J. G. M. (2012). Clinical correlates of immediate success and outcome at 1‐year follow‐up of real‐world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace, 14(5), 666–674. 10.1093/europace/eur406 [DOI] [PubMed] [Google Scholar]
- Raitt, M. H. , Volgman, A. S. , Zoble, R. G. , Charbonneau, L. , Padder, F. A. , O'Hara, G. E. , & Kerr, D. (2006). Prediction of the recurrence of atrial fibrillation after cardioversion in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study. American Heart Journal, 151(2), 390–396. 10.1016/j.ahj.2005.03.019 [DOI] [PubMed] [Google Scholar]
- Smith, J. W. , O'Neal, W. T. , Shoemaker, M. B. , Chen, L. Y. , Alonso, A. , Whalen, S. P. , & Soliman, E. Z. (2017). PR‐interval components and atrial fibrillation risk (from the Atherosclerosis Risk in Communities Study). American Journal of Cardiology, 119(3), 466–472. 10.1016/j.amjcard.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soran, H. , Younis, N. , Currie, P. , Silas, J. , Jones, I. R. , & Gill, G. (2008). Influence of diabetes on the maintenance of sinus rhythm after a successful direct current cardioversion in patients with atrial fibrillation. QJM, 101(3), 181–187. 10.1093/qjmed/hcm123 [DOI] [PubMed] [Google Scholar]
- Tieleman, R. G. , Van Gelder, I. C. , Crijns, H. J. G. M. , De Kam, P. J. , Van Den Berg, M. P. , Haaksma, J. , Van Der Woude, H. J. , & Allessie, M. A. (1998). Early recurrences of atrial fibrillation after electrical cardioversion: A result of fibrillation‐induced electrical remodeling of the atria? Journal of the American College of Cardiology, 31(1), 167–173. 10.1016/s0735-1097(97)00455-5 [DOI] [PubMed] [Google Scholar]
- Toso, E. , Blandino, A. , Sardi, D. , Battaglia, A. , Garberoglio, L. , Miceli, S. , Azzaro, G. , Capello, A. L. , & Gaita, F. (2012). Electrical cardioversion of persistent atrial fibrillation: Acute and long‐term results stratified according to arrhythmia duration. Pacing and Clinical Electrophysiology, 35(9), 1126–1134. 10.1111/j.1540-8159.2012.03453.x [DOI] [PubMed] [Google Scholar]
- Wang, Y. S. , Chen, G. Y. , Li, X. H. , Zhou, X. , & Li, Y. G. (2017). Prolonged P‐wave duration is associated with atrial fibrillation recurrence after radiofrequency catheter ablation: A systematic review and meta‐analysis. International Journal of Cardiology, 227, 355–359. 10.1016/j.ijcard.2016.11.058 [DOI] [PubMed] [Google Scholar]
- Wyse, D. G. , Waldo, A. L. , DiMarco, J. P. , Domanski, M. J. , Rosenberg, Y. , Schron, E. B. , Kellen, J. C. , Greene, H. L. , Mickel, M. C. , Dalquist, J. E. , Corley, S. D. , Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) Investigators (2002). A comparison of rate control and rhythm control in patients with atrial fibrillation. New England Journal of Medicine, 347(23), 1825–1833. 10.1056/NEJMoa021328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


