Abstract
Objective:
The purpose of this study was to estimate the associations of genetically determined maternal blood glucose levels with obesity-related outcomes among children from pregnancies with and without gestational diabetes mellitus (GDM).
Methods:
A total of 1,114 mothers with (N = 560) and without (N = 554) GDM and their children were included in the present study. A maternal genetic risk score (GRS) for blood glucose was constructed on the basis of 17 single-nucleotide polymorphisms identified from a recent genome-wide association study.
Results:
It was found that maternal GRS for blood glucose showed different associations with offspring risk of overweight and obesity, as well as adiposity measures (all P for interaction < 0.05). Among mothers without GDM, genetically determined maternal blood glucose levels were associated with an 89% higher risk of overweight in their children (95% CI: 42%−152% per SD increase in GRS, P = 1.40 × 10−5) and a 120% higher risk of obesity (44%−235%, P = 2.61 × 10−4) after adjustment for covariates. In addition, higher maternal GRS for blood glucose was associated with children’s increased obesity-related traits (all P < 0.05). However, no significant associations were observed among children of mothers with GDM.
Conclusions:
This study indicates that GDM status may modify the relation between genetically determined glucose levels and obesity risk among children.
Introduction
The prevalence of childhood obesity has increased markedly in the past decades (1,2), from 0.9% in 1975 to 7.8% in 2016 in boys and from 0.7% in 1975 to 5.6% in 2016 in girls worldwide (1). Compelling evidence has shown that maternal hyperglycemia and diabetes may affect offspring risk of obesity beginning in childhood (3–7). In particular, maternal glucose levels during pregnancy, ranging from normal to gestational diabetes mellitus (GDM), have been consistently related to childhood obesity (8–11).
In a 2016 study, it was found that genetically determined higher maternal fasting glucose concentrations were associated with higher birth weight, which is a risk factor for childhood obesity (12). Glucose-associated genes of mothers may influence the long-term growth and development of children by modulating maternal glycemia during pregnancy. However, to our knowledge, no studies hitherto have explored the associations between genetically determined maternal blood glucose levels and obesity-related outcomes among children. Moreover, whether maternal GDM status may modify such relations remains unknown.
Therefore, the present study aimed to estimate the associations of maternal genetically determined fasting glucose levels, characterized by a combined genetic risk score (GRS) for blood glucose, with obesity-related outcomes among children from pregnancies with and without GDM. We particularly tested the interaction between maternal GRS and GDM status on childhood obesity-related outcomes.
Methods
Participants
This study was approved by the Human Subjects Committee of the Tianjin Women’s and Children’s Health Center, and written informed consent was obtained from all participants.
Our study was conducted based on Tianjin GDM Screening, an urban universal screening of GDM using the 1999 World Health Organization (WHO) criteria in all six central districts of Tianjin, China, launched by the Tianjin Women’s and Children’s Health Center in 1999 (13). All pregnant women participated in a 1-hour, 50-g glucose screening test at 26 to 30 weeks’ gestation, and those with a glucose level ≥7.8 mmol/L were invited to take a 75-g, 2-hour oral glucose tolerance test (OGTT) at Tianjin Women’s and Children’s Health Center. According to criteria from WHO, GDM is defined by confirming either of the following 75-g OGTT results: (1) diabetes (fasting glucose ≥7 mmol/L or 2-hour glucose ≥11.1 mmol/L); or (2) impaired glucose tolerance (2-hour glucose ≥7.8 and <11.1 mmol/L) (14).
From 2005 to 2009, a total of 76,325 pregnant women were screened, with a screening rate >91%, among whom 4,644 pregnant women were diagnosed with GDM (15,16). We invited all 4,644 women to join the Tianjin Gestational Diabetes Mellitus Prevention Program (TGDMPP), a 4-year randomized clinical trial among women with GDM (15,17). Ultimately, a total of 1,263 women with GDM completed the baseline survey, among whom 1,180 attended the TGDMPP and were randomly assigned to either a lifestyle intervention or a control group including four follow-up visits (15,17–20). In brief, mothers with GDM would receive intensive and individually designed diet and exercise programs, which included six face-to-face sessions with dietitians and two telephone calls in the first year and two additional sessions and four telephone calls in each subsequent year. The control group received usual care, including the provision of general information on the awareness of diabetes, dietary modification, and increased physical activity at subsequent annual visits, but no specific individualized programs were offered (15,17).
Subsequently, we randomly selected 578 mother-child pairs who finished the baseline and follow-up surveys of the TGDMPP, and we enrolled 578 mother-child pairs from 71,681 women without GDM who finished the GDM screening at the same period, with age and sex frequency matched to the 578 children of mothers with GDM (16,21). Among them, 1,114 mothers had available genome-wide association study (GWAS) data (560 GDM and 554 non-GDM mother-child pairs), which formed the present transgeneration cross-sectional study (see flowchart in Supporting Information Figure S1).
Measurements and questionnaires
All mother-child pairs underwent a physical examination, according to a standard protocol as previously described (16,21). Moreover, the children’s physical examination also included waist circumference, hip circumference, skinfolds (triceps, subscapular, suprailiac), and body fat percentage. More details are given in Supporting Information Appendix S1. All the measurements were conducted twice by a trained medical examiner, and the averages of both measurements were used. BMI was calculated by dividing weight in kilograms by height in meters squared. Weight-for-age z score and BMI-for-age z score were calculated based on the standards for the WHO Child Growth Standards (22).
Mothers’ general information was collected by a self-administered questionnaire, including sociodemographic characteristics (age, marital status, education [<13, 13–16, and ≥16 years], family monthly income [<¥5,000, ¥5,000-¥8,000 and ≥¥8,000], and family history of diabetes), basic information during pregnancy (prepregnancy weight, gestational age at delivery, gestational weight gain, self-reported hypertensive disorders of pregnancy, treatment of GDM [no, insulin, lifestyle control]), and lifestyle questions (smoking status [no, past, current], drinking status [no, yes]) (16,21). Of note, the proportion of mothers with GDM who received insulin treatment was limited, mainly because the traditional Chinese view is that insulin treatment would affect babies, and most mothers with GDM maintained their glucose levels well after lifestyle intervention.
Children’s general information, including sex, age, birth weight, birth length, feeding patterns within the first 6 months (exclusive breast feeding, mixed breast and formula feeding, or exclusive formula feeding), dietary habits assessed using a validated food frequency questionnaire (23), routine activities (indoor and outdoor activities, screening-watching time, and sleep duration), and history of diseases and medication, was collected by another questionnaire completed by their mothers (16,21).
Genotyping and GRS calculation
DNA was extracted from the buffy coat fraction of centrifuged blood using a QIAamp DNA Blood Maxi Kit (Qiagen, Chatsworth, California). Seventeen single-nucleotide polymorphisms (SNPs; P < 5 × 10−8, Supporting Information Table S1) significantly associated with blood glucose and identified from a large-scale GWAS (24) conducted by BioBank Japan Project among 93,146 Japanese individuals were selected and genotyped using the Illumina HumanOmniExpress (San Diego, California) covering around 750,000 SNPs. The genotyping success rate was more than 98%. For quality control, 10% of replicated samples were genotyped, and the concordance rate was more than 99%. The allele frequencies of all SNPs in total participants or in women without GDM were in Hardy-Weinberg equilibrium (all P > 0.05, Supporting Information Table S1).
A weighted GRS for blood glucose was calculated based on the 17 selected SNPs. The genotypes of each SNP were coded as 0, 1, and 2 according to the number of glucose-increasing alleles. Each SNP was weighted by its relative effect size (β coefficient) obtained from the original study (24) by using the following equation: weighted GRS = (β1 × SNP1 + β2 × SNP2 + … + βn × SNPn) × (total number of SNPs/sum of the β coefficients) (25). A higher GRS indicated a higher genetic predisposition to higher levels of blood glucose.
Exposure and outcomes
In the present study, exposure was genetically determined maternal blood glucose levels, represented by a combined GRS of 17 glucose-related SNPs. The primary outcome was children’s overweight and obesity status, defined based on WHO Child Growth Standards. For children under 5 years of age, overweight and obesity are weight-for-height >2 and 3 SD above the WHO Child Growth Standards median, respectively; for children aged between 5 and 19 years, overweight and obesity are BMI-for-age >1 and 2 SD above the WHO Growth Reference median (26). The secondary outcomes were obesity-related quantitative traits, including weight, weight-for-age z score, BMI, BMI-for-age z score, waist circumference, hip circumference, sum of skinfolds, and body fat percentage.
Statistical analysis
Data were expressed using mean and SD for continuous variables or number and percentage for categorical variables. An χ2 test for categorical variables and general linear models for continuous variables were applied to compare proportions or means of characteristics across quartiles of maternal GRS for blood glucose.
Multivariable logistic regression models were used to examine the association of maternal GRS for blood glucose with overweight and obesity status in children, and general linear models were used to examine the association of GRS with children’s obesity-related quantitative traits. We performed stratified analyses by GDM status to explore the modification effect of GDM status on such associations. To test for interaction effects, we examined GRS, GDM status, and their interaction term as independent predictors of children’s obesity-related outcomes, adjusted for potential confounders.
Covariates were included in the multivariate models as follows: Model 1: adjusted for children’s age and sex; Model 2: Model 1 plus children’s birth weight, maternal age at pregnancy, prepregnancy BMI, gestational weight gain, and gestational age at delivery; Model 3: Model 2 plus maternal lifestyle and socioeconomic and other related factors: smoking status, drinking status, marital status, education, family monthly income, hypertensive disorders of pregnancy, treatment of GDM, and any family history of diabetes; Model 4: Model 3 plus children’s variables: feeding patterns, outdoor physical activity time, screen-watching time, sleeping time, vegetable intake frequency, fruit intake frequency, and illness within the last 3 months. Especially for overweight, obesity, weight-for-age z score, and BMI-for-age z score, which were defined based on sex- and age-specific standards, children’s age and sex were not adjusted in all models. The missing rates for covariates in the present study were low, ranging from 0.1% to 1.6%. Therefore, our analyses were conducted using the complete data.
In addition, because some glucose SNPs or their proxy SNPs, including KCNQ1 rs60808706 (27), GCKR rs1260326 (in high linkage disequilibrium [LD] with rs780094 (28)), CDKAL1 rs9358356 (in high LD with rs2206734 (29)), and SLC30A8 rs13266634 (in high LD with rs3802177 (27)), were also known to be associated with BMI/obesity in East Asians, we performed a sensitivity analysis excluding these SNPs and constructed a new GRS for blood glucose.
A two-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Maternal and child characteristics according to GRS quartiles
Descriptive characteristics of mothers and their children across quartiles of maternal GRS for blood glucose were presented in Table 1. Overall, maternal characteristics among the four GRS groups (in quartiles) were similar (all P > 0.05), except GDM status (P = 0.008). There were also no significant differences in children’s characteristics across the GRS quartiles (all P > 0.05), except sleeping time (P = 0.006) and BMI (P = 0.040). However, we observed a tendency toward higher height (P = 0.096), weight (P = 0.070), hip circumference (P = 0.057), and body fat percentage (P = 0.050) in children in higher quartiles of maternal GRS for blood glucose compared with those in the lowest quartile. We also provided the children’s characteristics according to GDM status in Supporting Information Table S2. Children born to mothers with GDM were more likely to have higher obesity measures (all P < 0.05).
TABLE 1.
Characteristics of mothers and their children, stratified by maternal glucose GRS quartiles
| Maternal glucose GRS quartiles | |||||
|---|---|---|---|---|---|
| Q1 (n = 279) | Q2 (n = 279) | Q3 (n = 275) | Q4 (n = 281) | P value | |
| GRS | 10.37~16.49 | 16.50~18.31 | 18.32~20.05 | 20.06~26.81 | — |
| Maternal characteristics | |||||
| Age, y | 30.3 ± 3.2 | 30.3 ± 3.4 | 30.0 ± 3.3 | 30.0 ± 3.3 | 0.466 |
| Gestational age, wk | 39.2 ± 1.5 | 39.0 ± 1.7 | 39.1 ± 1.3 | 39.1 ± 1.3 | 0.445 |
| Prepregnancy BMI, kg/m2 | 22.4 ± 3.5 | 21.9 ± 2.8 | 22.2 ± 2.9 | 22.1 ± 3.1 | 0.233 |
| Gestational weight gain, kg | 17.4 ± 6.4 | 17.7 ± 6.1 | 17.3 ± 6.2 | 17.1 ± 6.7 | 0.788 |
| Education, n (%) | |||||
| <13 y | 54 (19.4) | 33 (11.8) | 43 (15.6) | 41 (14.6) | 0.156 |
| 13–16 y | 198 (71.0) | 208 (74.6) | 207 (75.3) | 213 (75.8) | |
| ≥16 y | 27 (9.7) | 38 (13.6) | 25 (9.1) | 27 (9.6) | |
| Income, ¥, n (%) | |||||
| <5,000 | 46 (16.9) | 33 (12.0) | 44 (16.1) | 45 (16.2) | 0.396 |
| 5,000~8,000 | 66 (24.2) | 72 (26.3) | 63 (23.1) | 81 (29.1) | |
| ≥8,000 | 161 (59.0) | 169 (61.7) | 166 (60.8) | 152 (54.7) | |
| Current smokers, n (%) | 3 (1.1) | 9 (3.2) | 11 (4.0) | 8 (2.9) | 0.344 |
| Alcohol drinkers, n (%) | 75 (26.9) | 79 (28.3) | 66 (24.0) | 75 (26.7) | 0.710 |
| Family history of diabetes, n (%) | 79 (28.3) | 81 (29.0) | 91 (33.1) | 102 (36.3) | 0.146 |
| GDM, n (%) | 138 (49.5) | 119 (42.7) | 143 (52.0) | 160 (56.9) | 0.008 |
| Treatment of GDM, n (%) | |||||
| No | 20 (14.5) | 20 (16.8) | 17 (11.9) | 16 (10.0) | 0.506 |
| Insulin | 5 (3.6) | 1 (0.8) | 4 (2.8) | 4 (2.5) | |
| Lifestyle control | 113 (81.9) | 98 (82.4) | 122 (85.3) | 140 (87.5) | |
| Hypertensive disorders of pregnancy, n (%) | 11 (3.9) | 11 (3.9) | 11 (4.0) | 13 (4.6) | 0.972 |
| Children’s characteristics | |||||
| Age, y | 5.8 ± 1.2 | 6.0 ± 1.3 | 5.8 ± 1.2 | 6.0 ± 1.3 | 0.119 |
| Sex, boys, n (%) | 144 (51.6) | 158 (56.6) | 145 (52.7) | 137 (48.8) | 0.311 |
| Feeding pattern, n (%) | |||||
| Exclusive breast feeding | 120 (43.0) | 120 (43.2) | 119 (43.3) | 122 (43.4) | 0.865 |
| Mixed breast and formula feeding | 124 (44.4) | 120 (43.2) | 124 (45.1) | 115 (40.9) | |
| Exclusive formula feeding | 35 (12.5) | 38 (13.7) | 32 (11.6) | 44 (15.7) | |
| Vegetable intake frequency, n (%) | |||||
| ≤1 time/d | 23 (8.2) | 25 (9.0) | 21 (7.6) | 21 (7.5) | 0.801 |
| 2 times/d | 247 (88.5) | 238 (85.3) | 239 (86.9) | 248 (88.3) | |
| ≥3 times/d | 9 (3.2) | 16 (5.7) | 15 (5.5) | 12 (4.3) | |
| Fruit intake frequency, n (%) | |||||
| <1 time/d | 5 (1.8) | 12 (4.3) | 9 (3.3) | 12 (4.3) | 0.448 |
| 1 times/d | 93 (33.3) | 101 (36.2) | 95 (34.6) | 107 (38.1) | |
| >3 times/d | 181 (64.9) | 166 (59.5) | 171 (62.2) | 162 (57.7) | |
| Sleeping time, n (%) | |||||
| ≤8 h/d | 29 (10.4) | 45 (16.1) | 37 (13.5) | 35 (12.5) | 0.006 |
| 9–10 h/d | 193 (69.2) | 181 (64.9) | 180 (65.7) | 216 (76.9) | |
| ≥11 h/d | 57 (20.4) | 53 (19.0) | 57 (20.8) | 30 (10.7) | |
| Screen-watching time, h/d | 1.0 ± 0.8 | 1.1 ± 0.8 | 1.1 ± 0.8 | 1.0 ± 0.7 | 0.562 |
| Outdoor activity, h/d | 2.2 ± 0.9 | 2.2 ± 0.9 | 2.1 ± 0.8 | 2.1 ± 0.9 | 0.423 |
| Birth length, cm | 50.7 ± 1.7 | 50.5 ± 2.0 | 50.8 ± 2.0 | 50.8 ± 2.2 | 0.206 |
| Birth weight, g | 3,487.5 ± 483.2 | 3,425.9 ± 495.1 | 3,500.4 ± 490.4 | 3,471.8 ± 475.8 | 0.293 |
| Height, cm | 117.1 ± 9.4 | 118.3 ± 9.5 | 118.6 ± 9.9 | 119.0 ± 9.5 | 0.096 |
| Weight, kg | 22.2 ± 6.5 | 22.1 ± 5.5 | 22.9 ± 7.2 | 23.4 ± 6.8 | 0.070 |
| BMI, kg/m2 | 15.9 ± 2.6 | 15.6 ± 1.9 | 16.0 ± 2.7 | 16.2 ± 2.6 | 0.040 |
| Waist circumference, cm | 55.4 ± 7.0 | 55.0 ± 5.2 | 55.7 ± 7 | 56.1 ± 6.7 | 0.199 |
| Hip circumference, cm | 64.0 ± 7.4 | 63.8 ± 6.5 | 64.7 ± 7.6 | 65.3 ± 7.7 | 0.057 |
| Sum of skinfolds, mm | 30.8 ± 15.4 | 29.7 ± 13.5 | 31.6 ± 16.4 | 32.5 ± 16.3 | 0.189 |
| Body fat percentage, % | 19.6 ± 7.8 | 19.1 ± 6.8 | 20.4 ± 8.1 | 20.8 ± 8.3 | 0.050 |
Data are shown as mean ± SD or n (%). Significant differences in maternal and children’s characteristics among the four groups are highlighted in bold.
GDM, gestational diabetes mellitus; GRS, genetic risk score.
Association of GRS with maternal glucose/GDM
In the present study, maternal glucose levels were available only among women with GDM. the association of GRS with maternal fasting glucose among women with GDM is shown in Supporting Information Figure S2. We found that maternal GRS for blood glucose was significantly associated with maternal fasting glucose levels during pregnancy (β [SE] = 0.05 (0.02), P = 0.015). In addition, it was also significantly associated with GDM status (odds ratio [OR] = 1.13, 95% CI: 1.02–1.25, P = 0.024).
Associations with childhood obesity outcomes
As presented in Table 2, among children of mothers without GDM, per SD increase in maternal GRS for blood glucose was positively associated with a 63% higher risk of childhood overweight and an 86% higher risk of childhood obesity (model 1), and the associations became stronger after adjusting for children’s birth weight, maternal age at pregnancy, prepregnancy BMI, gestational weight gain, gestational age at delivery, and other maternal lifestyle, socioeconomic factors (models 2 and 3). In model 4, after additional adjustment for children’s variables, the associations became more statistically significant (overweight: OR = 1.89, 95% CI: 1.42–2.52, P = 1.40 × 10−5; obesity: OR = 2.20, 95% CI: 1.44–3.35, P = 2.61 × 10−4). No significant associations were observed among children of mothers with GDM.
TABLE 2.
Associations of genetically determined maternal blood glucose levels with overweight and obesity status among children with and without maternal GDM
| Non-GDM (N = 554) | GDM (N = 560) | |||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | P for interaction | ||
| Model 1 a | ||||||
| Overweight | 1.63 (1.27–2.09) | 1.42 × 10−4 | 1.00 (0.83–1.21) | 0.976 | 0.002 | |
| Obesity | 1.86 (1.29–2.67) | 8.20 × 10−4 | 1.07 (0.82–1.38) | 0.626 | 0.015 | |
| Model 2 b | ||||||
| Overweight | 1.75 (1.34–2.28) | 3.17 × 10−5 | 1.01 (0.83–1.23) | 0.924 | 0.001 | |
| Obesity | 2.02 (1.38–2.94) | 2.68 × 10−4 | 1.07 (0.81–1.40) | 0.646 | 0.008 | |
| Model 3 c | ||||||
| Overweight | 1.86 (1.40–2.45) | 1.38 × 10−5 | 0.98 (0.80–1.20) | 0.851 | 6.91 × 10−4 | |
| Obesity | 2.18 (1.46–3.27) | 1.40 × 10−4 | 1.08 (0.81–1.42) | 0.605 | 0.007 | |
| Model 4 d | ||||||
| Overweight | 1.89 (1.42–2.52) | 1.40 × 10−5 | 0.94 (0.76–1.16) | 0.573 | 5.66 × 10−4 | |
| Obesity | 2.20 (1.44–3.35) | 2.61 × 10−4 | 1.04 (0.78–1.39) | 0.777 | 0.008 | |
Significant associations are highlighted in bold.
Model 1: unadjusted for any covariates because the definitions of overweight and obesity were based on sex- and age-specific standards.
Model 2: Model 1 + children’s birth weight, maternal age at pregnancy, prepregnancy BMI, gestational weight gain, gestational age at delivery.
Model 3: Model 2 + maternal lifestyle, socioeconomic and other related factors: smoking status, drinking status, marital status, education, family monthly income, hypertensive disorders of pregnancy, treatment of GDM, and any family history of diabetes.
Model 4: Model 3 + children’s variables: feeding patterns, outdoor physical activity time, screen-watching time, sleeping time, vegetable intake frequency, fruit intake frequency, illness within the last 3 months.
GDM, gestational diabetes mellitus; OR, odds ratio.
The interactions between maternal GRS for blood glucose and GDM status were significant on childhood overweight (P for interaction = 5.66 × 10−4 ~ 0.002) and obesity (P for interaction = 0.007 ~ 0.015) in all models.
Associations with obesity-related quantitative traits in children
As shown in Table 3 and Figure 1, genetically determined maternal blood glucose levels were positively associated with obesity-related quantitative traits among women without GDM (all P < 0.05). The results were highly consistent in all models. In model 4 (fully adjusted model), per SD increase in maternal GRS for blood glucose was significantly associated with 0.79 kg higher weight (P = 1.05 × 10−4), 0.19 higher weight-for-age z score (P = 2.45 × 10−4), 0.39 kg/m2 higher BMI (P = 5.53 × 10−5), 0.20 higher BMI-for-age z score (P = 2.60 × 10−4), 0.72 cm higher waist circumference (P = 0.003), 0.76 cm higher hip circumference (P = 0.002), 2.34 mm higher sum of skinfolds (P = 1.38 × 10−4), 1.35% higher body fat percentage (P = 3.49 × 10−5). No such associations were observed among children of mothers with GDM.
TABLE 3.
Associations of genetically determined maternal blood glucose levels with obesity-related quantitative traits among children with and without maternal GDM
| Model 1a | Model 2b | Model 3c | Model 4d | |||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | |
| Non-GDM (N = 554) | ||||||||
| Weight, kg | 0.66 (0.21) | 0.002 | 0.74 (0.19) | 1.61 × 10−4 | 0.79 (0.20) | 9.61 × 10−5 | 0.79 (0.20) | 1.05 × 10−4 |
| Weight-for-age z score | 0.16 (0.05) | 0.002 | 0.19 (0.05) | 1.55 × 10−4 | 0.19 (0.05) | 1.35 × 10−4 | 0.19 (0.05) | 2.45 × 10−4 |
| BMI, kg/m2 | 0.32 (0.10) | 0.001 | 0.36 (0.09) | 1.29 × 10−4 | 0.39 (0.10) | 7.27 × 10−5 | 0.39 (0.10) | 5.53 × 10−5 |
| BMI-for-age z score | 0.17 (0.06) | 0.003 | 0.19 (0.05) | 2.88 × 10−4 | 0.20 (0.05) | 2.15 × 10−4 | 0.20 (0.05) | 2.60 × 10−4 |
| Waist circumference, cm | 0.56 (0.24) | 0.022 | 0.66 (0.23) | 0.004 | 0.69 (0.24) | 0.004 | 0.72 (0.24) | 0.003 |
| Hip circumference, cm | 0.61 (0.25) | 0.015 | 0.70 (0.23) | 0.003 | 0.77 (0.24) | 0.001 | 0.76 (0.24) | 0.002 |
| Sum of skinfolds, mm | 1.93 (0.61) | 0.002 | 2.13 (0.59) | 3.23 × 10−4 | 2.32 (0.61) | 1.59 × 10−4 | 2.34 (0.61) | 1.38 × 10−4 |
| Body fat percentage, % | 1.17 (0.33) | 3.86 × 10−4 | 1.30 (0.31) | 4.09 × 10−5 | 1.36 (0.32) | 3.19 × 10−5 | 1.35 (0.32) | 3.49 × 10−5 |
| GDM (N = 560) | ||||||||
| Weight, kg | 0.08 (0.21) | 0.720 | 0.12 (0.20) | 0.572 | 0.06 (0.20) | 0.767 | 0.08 (0.21) | 0.717 |
| Weight-for-age z score | 0.01 (0.05) | 0.853 | 0.01 (0.05) | 0.825 | −0.002 (0.05) | 0.965 | −0.01 (0.05) | 0.844 |
| BMI, kg/m2 | 0.0004 (0.1) | 0.997 | 0.03 (0.10) | 0.763 | 0.01 (0.10) | 0.941 | 0.01 (0.10) | 0.948 |
| BMI-for-age z score | −0.01 (0.06) | 0.876 | 0.001 (0.05) | 0.990 | −0.01 (0.05) | 0.844 | −0.02 (0.05) | 0.743 |
| Waist circumference, cm | −0.08 (0.24) | 0.740 | −0.04 (0.24) | 0.876 | −0.09 (0.24) | 0.718 | −0.07 (0.24) | 0.759 |
| Hip circumference, cm | 0.12 (0.24) | 0.620 | 0.17 (0.23) | 0.478 | 0.12 (0.23) | 0.607 | 0.10 (0.24) | 0.683 |
| Sum of skinfolds, mm | −0.27 (0.61) | 0.661 | −0.14 (0.59) | 0.818 | −0.28 (0.59) | 0.630 | −0.31 (0.60) | 0.600 |
| Body fat percentage, % | −0.03 (0.32) | 0.918 | −0.01 (0.31) | 0.964 | −0.10 (0.31) | 0.736 | −0.16 (0.32) | 0.606 |
Significant associations are highlighted in bold.
Model 1: adjusted for children’s age and sex. For weight-for-age z score and BMI-for-age z score, which were calculated based on sex- and age-specific standards, children’s age and sex were excluded in the adjustment.
Model 2: Model 1 + children’s birth weight, maternal age at pregnancy, prepregnancy BMI, gestational weight gain, gestational age at delivery.
Model 3: Model 2 + maternal lifestyle, socioeconomic and other related factors: smoking status, drinking status, marital status, education, family monthly income, hypertensive disorders of pregnancy, treatment of GDM, any family history of diabetes.
Model 4: Model 3 + children’s variables: feeding patterns, outdoor physical activity time, screen-watching time, sleeping time, vegetable intake frequency, fruit intake frequency, illness within the last 3 months.
GDM, gestational diabetes mellitus.
Figure 1.
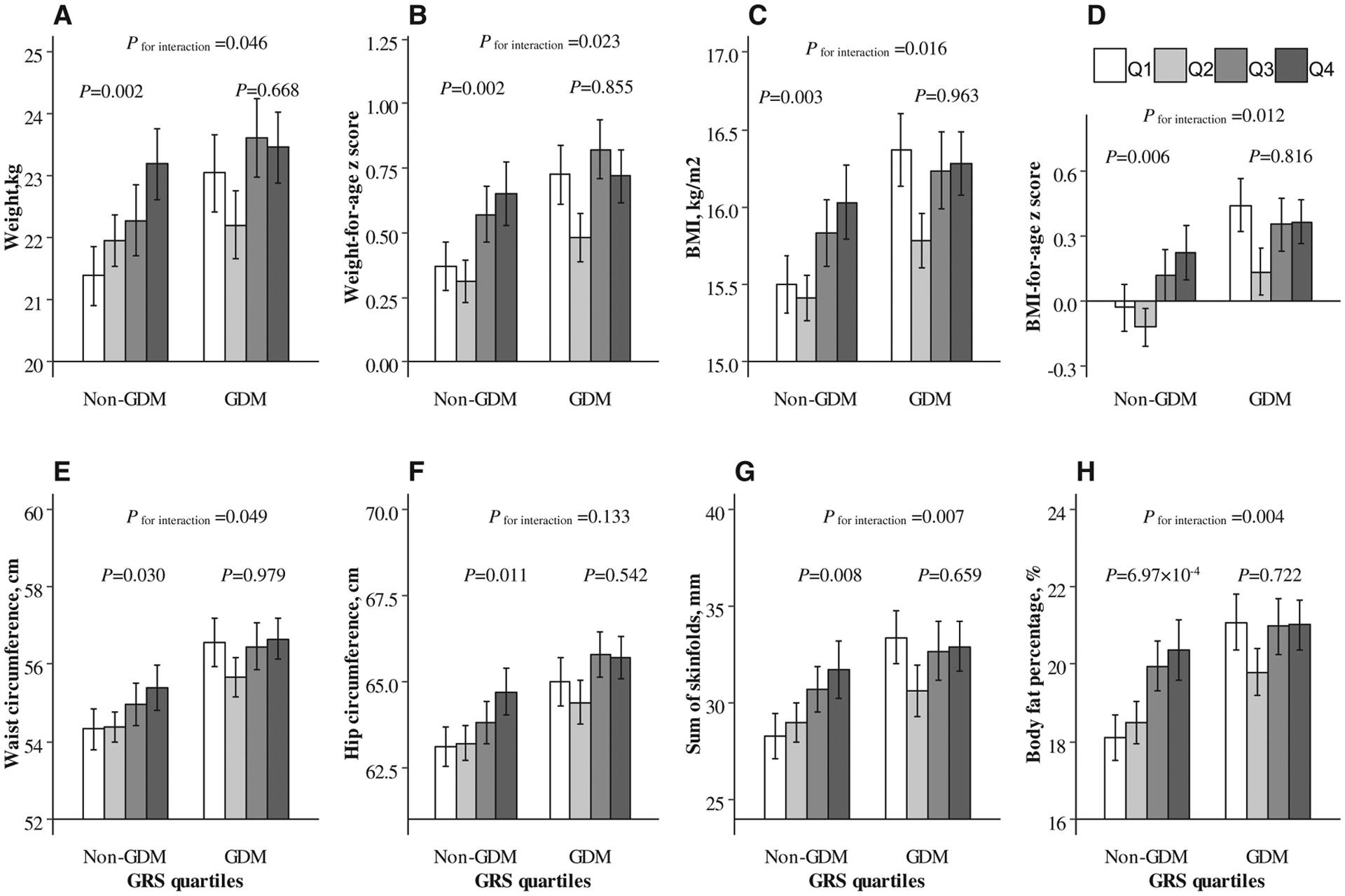
Interaction between genetically determined maternal blood glucose levels and GDM status on children’s obesity-related quantitative traits. General linear models were performed to explore the associations of genetically determined maternal blood glucose levels (in quartiles: Q1~Q4) with (A) weight, (B) weight-for-age z score, (C) BMI, (D) BMI-for-age z score, (E) waist circumference, (F) hip circumference, (G) sum of skinfolds, and (H) body fat percentage, respectively. Interactions were also tested, adjusted for children’s age, sex, birth weight, feeding patterns, outdoor physical activity time, screen-watching time, sleeping time, vegetable intake frequency, fruit intake frequency, illness within the last 3 months, and maternal age at pregnancy, gestational weight gain, gestational age at delivery, smoking status, drinking status, marital status, education, family monthly income, hypertensive disorders of pregnancy, treatment of GDM, any family history of diabetes, and maternal prepregnancy BMI. For weight-for-age z score and BMI-for-age z score, which were calculated based on sex- and age-specific standards, children’s age and sex were excluded in the adjustment. GDM, gestational diabetes mellitus; GRS, genetic risk score.
Figure 1 shows the interaction between genetically determined maternal blood glucose levels and GDM status on children’s obesity-related quantitative traits in the fully adjusted model. The interactions between maternal GRS for blood glucose and GDM status were significant on weight (P for interaction = 0.046), weight-for-age z score (P for interaction = 0.023), BMI (P for interaction = 0.016), BMI-for-age z score (P for interaction = 0.012), waist circumference (P for interaction = 0.049), sum of skinfolds (P for interaction = 0.007), and body fat percentage (P for interaction = 0.004).
Sensitivity analyses
When we excluded SNPs associated with BMI/obesity in East Asians, the interaction between the new glucose GRS and GDM status on overweight, obesity, and most measures of childhood obesity remained significant (P for interaction < 0.05, Supporting Information Table S3). Genetically determined maternal blood glucose levels were positively associated with all the obesity-related traits among women without GDM (all P < 0.05, Supporting Information Table S3).
Discussion
In this study, for the first time, we found significantly different associations of genetically determined maternal blood glucose levels with offspring overweight and obesity status according to maternal GDM status. Maternal genetically determined blood glucose levels were significantly associated with childhood overweight and obesity risk among children of mothers without GDM but not among children of mothers with GDM. We also found similarly significant interactions between genetically determined maternal blood glucose levels and GDM status on children’s other obesity-related outcomes, including weight, weight-for-age z score, BMI, BMI-for-age z score, waist circumference, sum of skinfolds, and body fat percentage.
A group of previous studies has examined the relation of in utero exposure to higher glucose levels during pregnancy with obesity in offspring, especially during childhood (3–9,30–32). However, in these observational studies, the associations might be influenced by confounding factors. In the present study, we found positive associations of genetically determined maternal blood glucose levels, which were characterized by a weighted GRS that was calculated based on 17 SNPs genome-wide significantly associated with blood glucose, with obesity and other obesity-related outcomes among children of mothers without GDM. Because genotypes are randomly determined at conception, such associations between genetically determined maternal blood glucose levels and children’s obesity outcomes are less likely to be influenced by potential confounding (12).
In a 2016 study, genetically elevated maternal blood glucose levels were found to be associated with increased birth weight, which is a risk factor for childhood obesity (12). Women with a higher GRS for blood glucose had relatively higher glucose levels during pregnancy (33,34). Maternal glucose, but not insulin, could cross the placenta, leading to increased fetal insulin secretion (12,35). Because insulin is a key intrauterine growth factor, altered fetal insulin secretion would consequently influence the development of the fetus in utero, leading to outcomes such as higher birth weight, and even long-term health, such as childhood obesity (35,36). In addition, it has been suggested that other metabolites related to maternal glucose, such as lipids, were important contributors to excess fetal growth and fat accretion (8,37). Jacob et al. (38) found that maternal glucose levels were associated with maternal lipids levels, including triacylglycerol, nonesterified fatty acids, β-hydroxybutyrate, and several amino acids. Therefore, the transplacental transfer of mixed nutrients from mothers to fetus might also contribute to the link between maternal glucose and offspring obesity, which was consistent with the hypothesis proposed by Freinkel and colleagues (39).
One of our predominant findings was the modification effect of GDM status on the association between genetically determined glucose levels and offspring obesity during childhood. Significant associations of maternal GRS for blood glucose with obesity-related outcomes were observed only among children whose mothers had GDM and not among children whose mothers did not have GDM. One possible explanation for such observations might be that children of mothers with GDM have been found to generally be at an increased risk for the development of overweight and obesity, having a higher weight, BMI, and other adiposity-related measures (3–7); in this case, the variance in obesity outcomes among children of mothers with GDM was smaller than children without maternal GDM, and, therefore, the genetic associations could not be detected, as demonstrated in Figure 1. Another explanation might be partly related to treatment effects of mothers with GDM, which would modify the relationship between the genetically determined glucose levels in mothers and offspring adiposity. However, more studies are needed to elucidate the underlying mechanism of the interaction between genetically determined maternal blood glucose levels and GDM status in the future.
Our findings have great public health implications. During the past decades, the prevalence of overweight and obesity among children and adolescents has risen dramatically (1,2). It is well acknowledged that obesity during childhood is associated with higher risks of subsequent obesity and unfavorable cardiometabolic outcomes in adolescence and adulthood (40–42). Thus, in order to develop early intervention strategies for primordial obesity prevention, identifying risk factors in early prenatal and postnatal life that are related to later obesity is of great significance. Our study indicated that genetically determined maternal blood glucose levels were significantly associated with obesity-related outcomes among children without maternal GDM. In other words, among women without GDM and with normal glucose levels, their children were still at higher risk to develop obesity if the mothers were genetically predisposed to higher glycemia, highlighting the potentially causal role of maternal glucose in development of childhood obesity, which was also demonstrated by another larger study using Mendelian randomization (12). Therefore, it is necessary to optimize maternal glucose values in pregnancy for long-term benefit by reducing childhood obesity. Furthermore, we should focus more on preconception health and reduction of obesity and metabolic risk before pregnancy.
To the best of our knowledge, our study is the first to evaluate the association of genetically determined maternal blood glucose levels with obesity among children from pregnancies with and without GDM and the first to test the interaction between maternal GRS of blood glucose and GDM status. In addition, GDM was diagnosed according to the standard 1999 WHO criteria in our study (14). Moreover, a variety of potential confounding factors have been measured and controlled in our analyses. Particularly, in the present study, we adjusted for maternal prepregnancy BMI, which is usually considered as a major confounding factor in the association of maternal hyperglycemia with childhood obesity, as maternal hyperglycemia is generally accompanied by higher maternal BMI (5–7). The results were robust in differently adjusted models.
However, there were several potential limitations. First, our study participants were restricted to being of Chinese descent, and it was unknown whether our results could be generalized to other ethnic groups. Second, some covariates, such as maternal prepregnancy weight and gestational weight gain, were self-reported, which may introduce recall bias. However, several validation studies in the United States have found that maternal and infant health indicators reported by mothers were in good concordance with data abstracted from hospital records (43–45). Third, glucose values were not available for women without GDM, and thus we could not validate the association between glucose GRS and glucose values among women without GDM.
Conclusion
The present study indicates for the first time that maternal GDM status may modify the relation between genetically determined maternal glucose levels and obesity risk among children. Genetically determined maternal blood glucose levels were significantly associated with childhood obesity among children of mothers without GDM. Measures to optimize maternal nutrition and health to ensure normoglycemia during pregnancy are needed for the long-term benefits of reducing childhood obesity.
Supplementary Material
Study Importance.
What is already known?
Compelling evidence has shown that maternal glucose levels during pregnancy and gestational diabetes mellitus (GDM) may affect offspring risk of obesity.
However, no studies hitherto have explored the associations between genetically determined maternal blood glucose levels and obesity-related outcomes among children.
What does this study add?
In the present study involving 1,114 mother-child pairs, we found that GDM status modified the associations between genetically determined glucose levels and offspring obesity during childhood.
Maternal genetic risk score for blood glucose was associated with childhood obesity–related outcomes only among children of mothers without GDM.
How might these results change the focus of clinical practice?
Our findings highlight the importance of maintaining healthy gestational glucose levels, even among women without GDM, in prevention of offspring obesity.
Acknowledgments
We thank all participants of the study for their dedication and contribution to the research.
Funding agencies:
This work was supported by the European Foundation for the Study of Diabetes/Chinese Diabetes Society/Lilly programme for Collaborative Research between China and Europe. LQ was partly supported by NIH grants from the National Heart, Lung, and Blood Institute (HL034594, HL071981, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383), and Fogarty International Center (TW010790). GH was partly supported by NIH grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100790) and the National Institute of General Medical Sciences (U54GM104940). QS is a recipient of a scholarship under the China Scholarship Council to pursue her study in the United States (201806010383).
Footnotes
Disclosure: The authors declared no conflict of interest.
Supporting information: Additional Supporting Information may be found in the online version of this article.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel S, Fraser A, Davey Smith G, et al. Associations of gestational diabetes, existing diabetes, and glycosuria with offspring obesity and cardiometabolic outcomes. Diabetes Care 2012;35:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao P, Liu E, Qiao Y, et al. Maternal gestational diabetes and childhood obesity at age 9–11: results of a multinational study. Diabetologia 2016;59:2339–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SY, England JL, Sharma JA, Njoroge T. Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: a systematic review. Exp Diabetes Res 2011;2011:541308. doi: 10.1155/2011/541308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philipps LH, Santhakumaran S, Gale C, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia 2011;54:1957–1966. [DOI] [PubMed] [Google Scholar]
- 7.Lowe WLJ, Scholtens DM, Lowe LP, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 2018;320:1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowe WLJ, Lowe LP, Kuang A, et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up study. Diabetologia 2019;62:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubo A, Ferrara A, Windham GC, et al. Maternal hyperglycemia during pregnancy predicts adiposity of the offspring. Diabetes Care 2014;37:2996–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles M-A, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 2007;30:2287–2292. [DOI] [PubMed] [Google Scholar]
- 11.Hillier TA, Pedula KL, Vesco KK, Oshiro CES, Ogasawara KK. Impact of maternal glucose and gestational weight gain on child obesity over the first decade of life in normal birth weight infants. Matern Child Health J 2016;20:1559–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyrrell J, Richmond RC, Palmer TM, et al. Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA 2016;315:1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Dong L, Zhang CP, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med 2011;28:652–657. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; 1999. [Google Scholar]
- 15.Hu G, Tian H, Zhang F, et al. Tianjin Gestational Diabetes Mellitus Prevention Program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pract 2012;98:508–517. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Zhang S, Li W, et al. Maternal gestational diabetes is associated with offspring’s hypertension. Am J Hypertens 2019;32:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Wang L, Zhang S, et al. One-year weight losses in the Tianjin Gestational Diabetes Mellitus Prevention Programme: a randomized clinical trial. Diabetes Obes Metab 2018;20:1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Liu H, Wang L, et al. Zinc-associated variant in SLC30A8 gene interacts with gestational weight gain on postpartum glycemic changes: a longitudinal study in women with prior gestational diabetes mellitus. Diabetes 2016;65:3786–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Zhang S, Liu H, et al. Different associations of diabetes with β-cell dysfunction and insulin resistance among obese and nonobese Chinese women with prior gestational diabetes mellitus. Diabetes Care 2014;37:2533–2539. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Liu H, Zhang C, et al. Maternal glucose during pregnancy and after delivery in women with gestational diabetes mellitus on overweight status of their children. Biomed Res Int 2015;2015:543038. doi: 10.1155/2015/543038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Wang L, Liu H, et al. Maternal gestational diabetes and different indicators of childhood obesity - a large study. Endocr Connect 2018;7:1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Child Growth Standards. Geneva, Switzerland; 2006. http://www.who.int/childgrowth/standards/en [Google Scholar]
- 23.Li Y, He Y, Zhai F, et al. Comparison of assessment of food intakes by using 3 dietary survey methods [in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi 2006;40:273–280. [PubMed] [Google Scholar]
- 24.Kanai M, Akiyama M, Takahashi A, et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet 2018;50:390–400. [DOI] [PubMed] [Google Scholar]
- 25.Huang T, Qi Q, Zheng Y, et al. Genetic predisposition to central obesity and risk of type 2 diabetes: two independent cohort studies. Diabetes Care 2015;38:1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Obesity and overweight. Updated. April 1, 2020. Accessed October 16, 2020. https://www.who.int/en/news-room/fact-sheets/detail/obesity-andoverweight
- 27.Akiyama M, Okada Y, Kanai M, et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat Genet 2017;49: 1458–1467. [DOI] [PubMed] [Google Scholar]
- 28.Qi Q, Wu Y, Li H, et al. Association of GCKR rs780094, alone or in combination with GCK rs1799884, with type 2 diabetes and related traits in a Han Chinese population. Diabetologia 2009;52:834–843. [DOI] [PubMed] [Google Scholar]
- 29.Okada Y, Kubo M, Ohmiya H, et al. Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nat Genet 2012;44: 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam WH, Ma RCW, Yang X, et al. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics 2008;122:1229–1234. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor DA, Fraser A, Lindsay RS, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 2010;53:89–97. [DOI] [PubMed] [Google Scholar]
- 32.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens 2009;22:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freathy RM, Hayes MG, Urbanek M, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: common genetic variants in GCK and TCF7L2 are associated with fasting and postchallenge glucose levels in pregnancy and with the new consensus definition of gestational diabetes mellitus from the I. Diabetes 2010;59: 2682–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes MG, Urbanek M, Hivert M-F, et al. Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes 2013;62:3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser A, Lawlor DA. Long-term health outcomes in offspring born to women with diabetes in pregnancy. Curr Diab Rep 2014;14:489. doi: 10.1007/s11892-014-0489-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freathy RM, Weedon MN, Bennett A, et al. Type 2 diabetes TCF7L2 risk genotypes alter birth weight: a study of 24,053 individuals. Am J Hum Genet 2007;80:1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbour LA, Hernandez TL. Maternal non-glycemic contributors to fetal growth in obesity and gestational diabetes: spotlight on lipids. Curr Diab Rep 2018;18:37. doi: 10.1007/s11892-018-1008-2 [DOI] [PubMed] [Google Scholar]
- 38.Jacob S, Nodzenski M, Reisetter AC, et al. Targeted metabolomics demonstrates distinct and overlapping maternal metabolites associated with BMI, glucose, and insulin sensitivity during pregnancy across four ancestry groups. Diabetes Care 2017;40:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freinkel N Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980;29:1023–1035. [DOI] [PubMed] [Google Scholar]
- 40.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med 2017;377:2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Y, Hou D, Zhao X, et al. Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine 2015;50:87–92. [DOI] [PubMed] [Google Scholar]
- 42.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011;365:1876–1885. [DOI] [PubMed] [Google Scholar]
- 43.Tomeo CA, Rich-Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology 1999;10:774–777. [PubMed] [Google Scholar]
- 44.Dietz P, Bombard J, Mulready-Ward C, et al. Validation of self-reported maternal and infant health indicators in the Pregnancy Risk Assessment Monitoring System. Matern Child Health J 2014;18:2489–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han E, Abrams B, Sridhar S, Xu F, Hedderson M. Validity of self-reported pre-pregnancy weight and body mass index classification in an integrated health care delivery system. Paediatr Perinat Epidemiol 2016;30:314–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


