Abstract
Background
Health-promoting interventions are important for preventing frailty and sarcopenia in older adults. However, there is limited evidence that nutritional interventions yield additional effects when combined with resistance training. This systematic review and meta-analysis aimed to compare the effectiveness of nutritional interventions with resistance training and that of resistance training alone.
Methods
Randomized controlled trials published in peer-reviewed journals prior to July 2020 were retrieved from databases and other sources. The articles were screened according to the inclusion and exclusion criteria. The methodological quality of the included studies was assessed using Cochrane’s risk of bias tool 2. A meta-analysis was performed using the RevMan 5.4 program and STATA 16 program.
Results
A total of 22 studies were included in the meta-analysis. The results of the meta-analysis showed no significant differences between groups in muscle mass, muscle strength, or physical functional performance. In the subgroup analysis regarding the types of nutritional interventions, creatine showed significant effects on lean body mass (n = 4, MD 2.61, 95% CI 0.51 to 4.72). Regarding the other subgroup analyses, there were no significant differences in appendicular skeletal muscle mass (p = .43), hand grip strength (p = .73), knee extension strength (p = .09), chair stand test results (p = .31), or timed up-and-go test results (p = .31). In the meta-regression, moderators such as the mean age of subjects and duration of interventions were not associated with outcome variables.
Conclusions
This meta-analysis showed that nutritional interventions with resistance training have no additional effect on body composition, muscle strength, or physical function. Only creatine showed synergistic effects with resistance training on muscle mass.
Trial registration
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-021-02491-5.
Keywords: Exercise, Frailty, Nutrition, Older adults, Resistance training, Sarcopenia
Background
Age-related conditions and chronic diseases increase the risk of disability and dependence, which are considered nearly irreversible conditions. Increasingly more older adults are becoming interested in ‘active aging’, which refers to the process of optimizing opportunities for health, participation, and security later in life [1]. A growing research topic is the identification of factors that increase the risk of negative events and the development of preventive interventions against disability. In this context, frailty and sarcopenia have increasingly emerged as research interests.
Although there is still no consensus on the definition and measurement of frailty for diagnosis, frailty is defined as a geriatric condition characterized by a cumulative decline in functioning and accompanied by increased vulnerability to stressors and dependency [2]. In 2001, Fried et al. [3] suggested the following criteria of frailty as a physical phenotype, focusing on physiological components: unintentional weight loss, exhaustion, decreased physical activity, a slow walking speed, and muscle weakness. Rockwood and Mitnitski [4] introduced a frailty index based on the accumulation of age-related deficits. A recent consensus more broadly suggested that frailty is a multidimensional syndrome including sensory limitations, cognitive decline, mood-related conditions, changes in the social environment, comorbidities and disability in addition to physical impairment [5]. The specific pathological pathway of frailty remains unclear, but frailty has a biological component resulting from inflammation and cumulative cellular damage over one’s lifetime. Although it occurs independently of chronological age, frailty is more prevalent in people of an older age; females; those who are living alone; those with low educational and socioeconomic statuses, multimorbidity, malnourishment, depression, polypharmacy, cognitive impairment, and a low physical activity level; and those who smoke and drink alcohol regularly [6–8].
Sarcopenia is considered a muscle disorder associated with poor muscle function; low muscle mass is considered a principal determinant. Although sarcopenia occurs in people who are not elderly, muscle mass decreases with age [9]. There are several operational definitions of sarcopenia; for example, the European working group on sarcopenia in older people defines sarcopenia as a combination of low muscle mass and strength and/or poor physical function [10]. Inconsistency in the definition leads to a wide range of prevalence rates, ranging from 9.9 to 40.4% [11]. Although the concepts of both frailty and sarcopenia are still being developed, the physical phenotypes of frailty, including low grip strength and slow gait speed described by Fried et al. [3], overlap substantially with those of sarcopenia [12]. In addition, as the etiology of frailty, such as inflammation, cellular damage, and protein degradation, is also related to that of sarcopenia, sarcopenia is an essential component of physical frailty. Frailty with sarcopenia can result in falls and fractures, a loss of independence, disability, morbidities, social isolation, institutionalization, and hospitalization [6, 13, 14], which lead to increases in healthcare costs and social burden [15]. Physical frailty and sarcopenia are transitional processes that increase individuals’ vulnerability to reduced functional capacity and adverse health outcomes. Issues related to healthcare and support for frail and sarcopenic older adults are expected to increase with population aging [16].
Health-promoting behaviors are important to prevent disability and dependence and to reduce the need for care [17]. Physical inactivity and malnutrition are common conditions in older adults and are major modifiable risk factors for frailty and sarcopenia [18, 19]. An increasing amount of research has suggested that physical inactivity can lead to the loss of muscle mass, decreases in muscle strength and poor physical performance. Several evidence-based systematic reviews and meta-analyses of RCTs have shown that exercise affects muscle mass, strength, and physical performance [17, 20]. For optimal effects, multimodal exercise combined with moderate- to high-intensity progressive resistance training and functional balance and mobility training at least twice a week for 30–45 min per session is recommended [19, 21].
Several nutrients, such as protein and vitamins D and E, have been known to affect anabolic stimuli, lead to the synthesis of muscle proteins, and protect against oxidative damage and the loss of muscle mass [22]. Although nutrition plays a key role in the pathogenesis of physical frailty and sarcopenia, the effects of nutritional interventions on muscle mass, strength and physical function are unclear. A systematic review showed that dietary supplementation, when combined with exercise training, has been shown to yield additional effects on muscle mass, strength and physical performance in some studies, but the existing evidence was inconsistent [23]. A more recent systematic review and meta-analysis by Hita-Contreras et al. showed that nutritional interventions do not provide additional or synergistic benefits when combined with resistance exercise in terms of muscle strength and mobility improvements among older adults with sarcopenic obesity [20].
There is evidence suggesting that there is an interaction effect between exercise and various nutritional factors, particularly protein and some multinutrient supplements, that can slow age-related decline and preserve muscle function in older adults. However, whether this has a meaningful preventive effect on frailty and sarcopenia remains unclear. Some previous reviews did not provide a quantitative synthesis, combined community-dwelling and institutionalized populations, or included and analyzed diverse types of interventions together [17, 23, 24], making it difficult to interpret the results. Thus, we focused on the primary prevention and synergistic effects of nutritional interventions, that is, the changes in muscle function after resistance training and nutritional interventions, in healthy community-dwelling older adults. The aim of this systematic review and meta-analysis was to compare the combination of resistance training and nutritional interventions with resistance training alone. A preferred reporting items for systematic reviews and meta-Analyses (PRISMA) checklist is presented in Additional file 1.
Methods
Search strategy
Electronic databases and the reference lists of related studies were searched by two investigators. First, for the electronic search, MEDLINE (PubMed), Cochrane CENTRAL, and Embase were searched for articles published prior to July 2020 by entering the following combinations of keywords: (“nutrition” OR “food” OR “diet”) OR (“exercise” OR “resistance training”) AND “aged” AND (“muscle mass” or “skeletal muscle” OR “muscle strength” OR “physical performance” OR “physical functional performance” OR “walking speed” OR “gait speed”). Second, the reference lists of related studies were searched to identify additional articles. The searches were limited to articles published in the English language, studies involving humans, and RCTs. Only peer-reviewed articles were included, and gray literature such as dissertations, proceedings, and government reports was excluded.
Study selection
The inclusion criteria for this systematic review were as follows: (a) studies including community-dwelling healthy older adults aged 60 years or above; (b) those including experimental groups that underwent resistance training and nutritional interventions; (c) those including comparison groups that underwent resistance training alone with or without a nutritional placebo supplement; (d) studies that reported the outcome measures of muscle mass, muscle strength, and physical functional performance; and (e) randomized controlled parallel-group trials with at least one arm. We set the age of 60 years or above, the age at which the activity level can decrease after retirement [25] and the loss of muscle strength accelerates [26]. We included only studies in healthy subjects to reduce the level of heterogeneity between studies. We accepted the various authors’ own definitions of ‘healthy’. The experimental interventions included any form of resistance training and nutritional (dietary) interventions that involved repeated practice during standardized programs for the purpose of enhancing muscle mass, muscle strength, and physical function. Nutritional interventions were defined as those that provided at least one nutrient through nutritional supplementation or whole food to obtain biologically beneficial effects. There was no minimum duration of follow-up. However, all included trials had to report outcomes at a minimum of one time point after the completion of the intervention.
Articles were excluded if (a) the participants had malignant tumors, severe chronic diseases, or levels of frailty and sarcopenia that limited their physical activity, diet, and level of independence in daily life; (b) the study was conducted in an animal model; (c) the experimental intervention was combined with any other form of interventions, such as medication and hormone therapy; (d) the nutritional intervention was designed for calorie intake reduction and weight loss; (e) the study evaluated the effectiveness of experimental interventions by only examining inflammatory factors or biological markers related to muscle synthesis; or (f) the study had a non-RCT design, such as case reports or cohort studies, without a comparison group.
Studies were selected based on the inclusion and exclusion criteria by two independent researchers; these researchers screened the studies according to the titles and abstracts of all studies and then reviewed the full texts of the remaining studies. Disagreements between researchers were resolved by discussion.
Data extraction
Two independent researchers extracted key data from the included articles in a standardized Excel sheet, and the results were cross checked. For each article, data about (a) the article, including the authors, year of publication, and country; (b) characteristics of the study population, including the number of participants, mean age, sex, health status, and attrition rate; (c) characteristics of the experimental intervention, including the contents of resistance training, contents of nutritional intervention, delivery mode, amount, frequency and duration of intervention, and treatment for comparison group; and (d) outcome evaluation, including the follow-up period and the method of measurement. As the aim of the study was to compare the effects of the combination of resistance training and nutritional interventions with those of resistance training alone on muscle mass, strength, and physical performance, when more than two groups were present, only the data we intended to compare were recorded.
Assessment of risk of Bias
Methodological quality was assessed using Cochrane’s risk of bias 2 (RoB2) tool by two independent researchers. The RoB2 tool consists of five domains: the randomization process, deviation from intended intervention, missing outcome data, measurement of outcome, and selection of the reported result. The risk of bias for each domain is evaluated as “low risk”, “some concerns”, or “high risk” by an algorithm with several signaling questions. Overall, “low risk of bias” was recorded when the study was judged to have a low risk of bias for all domains, “some concerns” was recorded when the study was judged to have some concerns in at least one domain, and “high risk of bias” was recorded when the study was judged to have a high risk of bias in at least one domain. This process was carried out by two independent researchers, and inconsistencies were resolved through discussion.
Data synthesis and statistical analysis
The effect sizes of the combination of resistance training and a nutritional intervention were calculated using the mean difference (MD) or standardized MD (SMD) for continuous outcome data for muscle mass, muscle strength, and physical functional performance. When a study provided data on more than one outcome for the same construct (ex: timed up-and-go and 4-m walk tests for physical functional performance), valid, reliable and commonly used measures for frailty and sarcopenia were selected by reviewing the associated literature and considering the frequencies of their use in the included studies. As a result, lean body mass and appendicular skeletal muscle mass were selected for muscle mass, hand grip strength and knee extension strength for muscle strength, and the chair stand tests and timed up-and-go tests for physical functional performance. Fat-free mass was included in the analysis when lean body mass was not available.
In addition, if a study used different lengths of intervention and follow-up periods, we used the outcome values at the postintervention endpoint. When only the mean change scores and standard deviation (SD) of each group were available, they were used instead of the postintervention endpoint mean and SD for the mean difference. SMDs were used for studies using different units (scale) of the same measure (ex: kg and Nm for strength). If there were more than two groups that could be considered experimental groups in the study, the groups were combined to create a single pairwise comparison in the meta-analysis to avoid unit-of-analysis error from multiple comparisons as recommended [27]. Studies for which we could not identify the outcome data necessary for quantitative synthesis after contacting the authors were excluded from this meta-analysis.
Meta-analysis was conducted using Review Manager (RevMan) 5.4. Individual MDs and SMDs were pooled using random effects models and the inverse variance method. The statistical significance of each effect size and overall effect size were checked using 95% confidence intervals. The chi-squared test and Higgin’s I2 test were used to examine between-trial heterogeneity. When the p value for the chi-squared test was less than 0.1 and I2 was greater than 50%, substantial heterogeneity was considered to be present. Subgroup analysis was conducted by nutritional intervention type. All subgroup differences were tested regarding the significance of the effect sizes and heterogeneity. Meta-regression was conducted to identify the potential effect moderator using the STATA 16 program. p values from random effect meta-regression were calculated using restricted maximum likelihood for continuous moderators.
Certainty of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool approach was used to assess the quality of evidence. It compared the resistance training combined diet compared to resistance training only for muscle function. The GRADE tool comprised of risk of bias, inconsistency, indirectness, imprecision, publication bias. The grading was estimated as high, moderate, low and very low certainty of quality.
Results
Search results
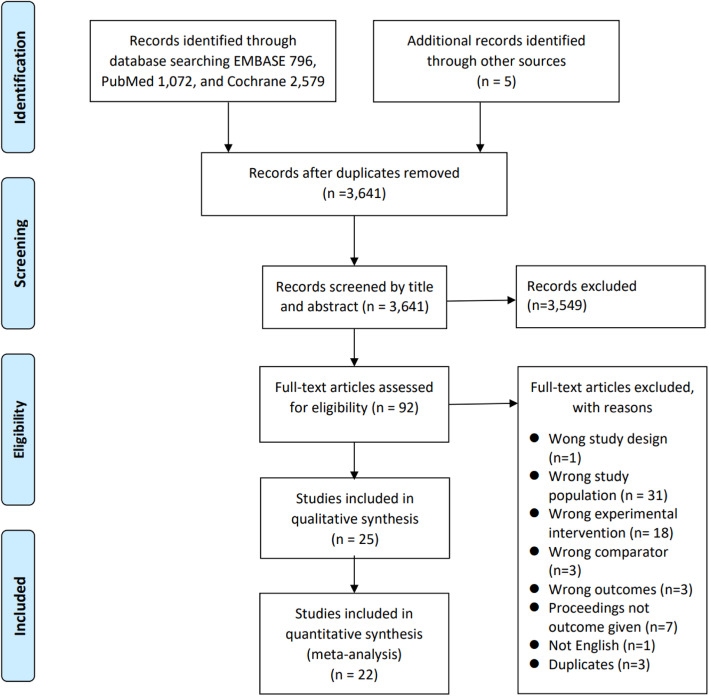
Figure 1 demonstrates the study selection process. After duplicates were removed, 3641 articles remained. After 3549 articles were excluded through title and abstract review, the full texts of 92 articles were reviewed. Sixty-seven articles were additionally excluded, and consequently, 25 articles were included in this systematic review. In some papers, the necessary values for meta-analysis could not be identified, so 22 articles were included in the quantitative synthesis.
Fig. 1.
Flow diagram of the study selection process
Description of included studies
Table 1 shows the characteristics of the included studies. Six RCTs were conducted in Canada, six in Japan, four in Brazil, two in the Netherlands, two in the USA and one each in France, Iceland, Norway, Sweden, and the UK. The studies were published between 1998 and 2020. The sample sizes ranged from 14 to 161. Six studies were conducted in males only, five studies were conducted in females only, and 14 studies were conducted in both males and females. There were 12 studies with a mean age of participants of less than 70 years and 13 studies with a mean age of more than 70 years.
Table 1.
Summary of included study characteristics
| First author (year), study location | Sample characteristics: n, mean age ± SD, sex (female ratio) | Intervention group | Control groupa | Follow-up period (weeks)b | Body composition assessment method | Muscle strength assessment method | Physical performance assessment method | |
|---|---|---|---|---|---|---|---|---|
| Exercise | Nutrition | |||||||
| Aguiar (2013) [28], Brazil |
Ic: 9, 64 ± 4, female Cd: 9, 65 ± 6, female |
Resistance training using machines, 60 min sessions, 3 times a week, for 24 weeks | 5 g Creatine monohydrate, once a day, for 12 weeks | Placebo: maltodextrin | 24 |
Body mass Fat free mass Muscle mass |
Bench press Biceps curl Knee extension |
Chair stand |
| Aoki (2018) [29], Japan |
I: 43, 68.8 ± 5.3, mixed (74.4%) C: 45, 71.2 ± 6.8, mixed (75.6%) |
Lower body resistance training, daily | 25mcg vitamin D3 (1000 IUe), divided into 3 times, daily | Usual diet | 24 |
BMIf Lower limb muscle mass |
Hip flexion Knee extension |
Chair stand Single leg stance Two step test Functional reach test |
| Arnarson (2013) [30], Iceland |
I: 83, 73.3 ± 6, mixed (unknown) C: 78, 74.6 ± 5.8, mixed (unknown) |
Resistance training using machines, 3 times a week | 20 g of whey protein 3 times a week | Placebo: 250 ml isocaloric carbohydrate drink | 12 |
ASMMg Lean body mass |
Grip strength Knee extension |
TUGh 6-min walk |
| Bermon (1998) [31], France |
I: 8, 71.0 ± 5.4, mixed (50%) C: 8, 69.3 ± 1.1, mixed (50%) |
Resistance training using machines, 3 times a week, for 7 weeks |
First 5 days: 5 g creatine monohydrate and 2 g glucose, 4 times a day Following 47 days: 3 g creatine monohydrate and 2 g glucose once a day |
Placebo: First 5 days:7 g glucose 4 times a day Following 47 days: 5 g glucose once a day |
8 |
Body mass BMI Lower limb muscle volume |
Leg press Chest press Knee extension |
– |
| Bjørnsen (2016) [32], Norway |
I: 17, 69 ± 7, male C: 17, 67 ± 5, male |
Free weight exercises, 3 times a week | 500 mg vitamin C and 117.5 mg vitamin E, twice a day | Placebo: cellulose and dicalsium phosphate | 12 |
Lean body mass Muscle thickness |
Biceps curl Knee extension Leg press |
– |
| Bobeuf (2011) [33], Canada |
I: 14, 64.3 ± 3.8, mixed (50%) C: 17, 67 ± 3.7, mixed (52.9%) |
Resistance training, 60 min sessions, 3 times a week | 1000 mg vitamin C ascorbate and vitamin E, once a day | Placebo: 100 mg lactose | 24 |
ASMM Muscle mass |
– | – |
| Brose (2003) [34], Canada |
I: 14, 69.6 ± 5.4, mixed (42.8%) C: 14, 69.1 ± 4.8, mixed (50%) |
Resistance training using machines, 3 times a week | 5 g creatine and 2 g dextrose, once a day |
Placebo: 7 g dextrose |
14 | Fat free mass |
Grip strength Knee extension Leg press |
– |
| Chrusch (2001) [35], Canada |
I:16, 70.4 ± 6.4, male C:14, 71.1 ± 6.7, male |
Resistance training using machines, 3 times a week | Creatine supplement: 0.3 g/kg/day for the first 5 days (loading phase) and 0.07 g/kg/day thereafter, once a day, for 11 weeks | Placebo: sucrose-flour mixture | 12 | Lean body mass |
Bench press Knee extension Leg press |
– |
| Cornish (2018) [36], Canada |
I:11, 71.4 ± 6.2, male C:12, 70.9 ± 5, male |
Resistance training, 60 min sessions, 3 times a week |
3.0 g omega-3 fatty acid combined 1.98 g EPAi and 0.99 g DHAj, once a day |
Placebo: 3.0 g omega 3–6-9 blend |
12 |
Body mass Lean body mass |
Chest press Leg press |
TUG 6-min walk |
| Da Boit (2017) [37], UK |
I: 27, 70.1 ± 4, mixed (48.1%) C: 23, 70.9 ± 4.2, mixed (43.4%) |
Lower body resistance training, twice a week | 3.0 g omega–3 fatty acids containing 2.1 g EPA and 0.6 g DHA, once a day |
Placebo: 3.0 g safflower oil |
18 | Muscle anatomic cross-sectional area | Knee extension |
Chair stand SPPBk 4 m walk |
| Edholom (2017) [38], Sweden |
I: 20, 67.2 ± 1.3, female C: 17, 67.9 ± 2.1, female |
Resistance training, 60 min sessions, twice a week |
Healthy diet: following a dietary consultation and a diet plan with the current dietary guidelines in Europe and US |
Usual diet | 24 | Lean body mass |
Knee extension Leg press |
Chair stand Single leg stance Squat jump TUG |
| Holwerda (2018) [39], Netherlands |
I: 21, 69 ± 4.6, male C: 20, 71 ± 4.5, male |
Resistance exercise training, 3 times a week |
21 g leucine-enriched whey protein (3 g total leucine), once a day | Placebo | 12 |
ASMM BMI Body mass Lean body mass |
Knee extension Leg press |
Chair stand SPPB 4 m walk |
| Kawada (2013) [40], Japan |
Ia: 10, 65 ± 1, mixed (50%) Ib: 11, 67 ± 3, mixed (72.3%) C: 8, 70 ± 1, mixed (50%) |
Low-intensity resistance training, twice a week |
Ia: 3.0 g essential amino acid supplements with milk, twice a day Ib: 6.0 g essential amino acid supplements with milk, twice a day |
Placebo: 3 g dextrin-contained powder with milk, once a day |
24 | Cross sectional area of Psoas major muscle | – |
Gait speed Obstacle course walk 6-min walk |
| Leenders (2013) [41], Netherlands |
I: 27,70.9 ± 5.4, mixed (44.4%) C: 26, 69.5 ± 3.6, mixed (46.2%) |
Resistance training, 3 times a week | 15 g milk protein, once a day |
Placebo: 7.13 g lactose and 0.42 g calcium only |
24 |
BMI Lean body mass Lower limb lean mass |
Grip strength Leg press |
Chair stand |
| Mori (2018) [42], Japan |
I: 25, 70.6 ± 4.2, female C: 25, 70.6 ± 4.2, female |
Resistance training, twice a week | 25 g leucine enriched whey protein, once a day | Usual diet | 24 |
BMI Lower limb lean mass SMIl Upper limb lean mass |
Grip strength Knee extension |
Gait speed |
| Nabuco (2018) [43], Brazil |
Ia: 23, 66.2 ± 9.4, female Ib: 24, 67.5 ± 5.2, female C: 23, 66.5 ± 7.2, female |
Resistance training, 3 times a week |
Ia: 27.1 g whey protein after resistance training, 3 times a week Ib: 27.1 g whey protein before resistance training, 3 times a week |
Placebo: maltodextrin drink |
12 |
Lower limb lean mass Skeletal muscle mass Upper limb lean mass |
Biceps curl Chest press Knee extension |
Chair stand Gait speed |
| Nagai (2019) [44], Japan |
I: 17, 72.7 ± 1.4, mixed (64.7%) C: 19, 73.5 ± 2.3, mixed (68.4%) |
Latex band training, squat, and tai chi, 90 min sessions, once a week | 60 mg maslinic acid, once a day |
Placebo: jelly without maslinic acid |
12 |
BMI Body mass Fat free mass Skeletal muscle mass Segmental muscle mass |
Grip strength |
Chair stand Gait speed |
| Nakayama (2020) [45], Japan |
I: 61, 71.4 ± 6.2, mixed (74%) C: 61, 70.4 ± 5.5, mixed (77%) |
Body weight exercises and 5 medicine ball exercises, daily |
Low-dose milk protein: 10.1 g protein, once a day |
Placebo: Isocaloric carbohydrate |
24 |
Body mass Lean body mass |
Grip strength Knee extension Knee flexion Push up |
Chair stand Gait speed TUG |
| Nilsson (2020) [46], Canada |
I: 16, 77.4 ± 11.2, male C: 16, 74.4 ± 5.2, male |
Home based resistance training with elastic bands, 3 times a week | Multi nutrients: 24 g whey protein, 16 g micellar casein contained 416 mg calcium, 3 g creatine, vitamin D 1000 IU, and omega-3 fish-oil containing 1.51 g EPA and 0.95 g DHA, once a day | Placebo: collagen and sunflower oil | 12 |
ASMM Body mass BMI Lean body mass |
Grip strength Knee extension Leg press |
Chair stand SPPB Stair climb TUG 4 m walk |
| Pinto (2016) [47], Brazil |
I: 13, 67.4 ± 4.7, mixed (unknown) C: 14, 67.1 ± 6.3, mixed (unknown) |
Resistance training, 3 times a week | 5 g Creatine monohydrate, once a day | Placebo: 5 g maltodextrin | 12 |
Body mass Lean body mass SMI |
Bench press Leg press |
– |
| Seino (2018) [48], Japan |
I: 40, 73.4 ± 4.3, mixed (85%) C: 40, 73.7 ± 4.3, mixed (82.5%) |
Weight-bearing exercise and exercises using a resistance band and Pilates ball, 60 min sessions, twice a week | Fortified milk containing 10.5 g total milk protein, 3.9 g fat, 9.3 g carbohydrate, and 337 mg calcium at lunch and micronutrient beverage at breakfast, daily | Usual diet | 12 |
Body mass Lean body mass Lower limb lean mass SMI |
Grip strength Knee extension |
Chair stand Gait speed One leg standing with eyes open TUG |
| Stout (2013) [49], USA |
I: 24, 73 ± 4.9, mixed (54.2%) C: 24, 73 ± 4.9, mixed (54.2%) |
Resistance exercise, 3 times a week for 21 weeks | 1.5 g calcium and 4 g carbohydrate, twice a day | Placebo: 200 mg calcium and 4 g carbohydrate | 24 |
Lean body mass Lower limb lean mass Upper limb lean mass |
Grip strength | Chair stand |
| Sugihara Junior (2018) [50], Brazil |
I: 15, 67.4 ± 4.1, female C: 16, 67.8 ± 4.1, female |
Resistance training using a combination of free weights and machines, 45 ~ 50 min sessions, 3 times a week | 35 g whey protein, immediately after each resistance training |
Placebo: 35 g maltodextrin |
12 |
Body mass BMI Lower limb lean mass SMI Upper limb lean mass |
Biceps curl Chest press Knee extension |
– |
| Tarnopolsky (2007) [51], Canada |
I: 21,70.7 ± 4.5, mixed (47.6%) C: 18, 71.1 ± 5.5, mixed (55.6%) |
Resistance exercise using machines, twice a week | 5 g creatine monohydrate and 6 g conjugated linoleic acid, once a day | Placebo: dextrose and safflower oil | 24 |
Body mass BMI Fat free mass |
Biceps curl Chest press Knee extension Leg press |
Chair stand Gait speed Stair climb Standing balance |
| Villanueva (2014) [52], USA |
I: 7, 68.7 ± 6.8, male C: 7, 68.7 ± 6.6, male |
Progressive overload, total body periodized resistance program, 3 times a week |
First 5 days: 0.1 g/kg body weight of creatine 3 times a day and 35 g whey protein once a day Following days: 0.07 g/kg body weight of creatine and 35 g whey protein, once a day |
Usual diet | 12 | Lean body mass |
Bench press Leg press |
Stair climb 400 m walk |
aThe resistance exercise of the control group applied in the same manner as in the experimental group
b The duration of intervention in most studies were the same as the follow-up period
c I, intervention group d C, control group e IU, international unit f BMI, body mass index g ASMM, appendicular skeletal muscle mass, h TUG, timed up and go i EPA, eicosapentaenoic acid j DHA, docosahexaenoic acid k SPPB, short physical performance battery l SMI, skeletal muscle mass index
All of the studies administered supervised exercise programs except one study [46] which included home-based resistance training with consistent encouragement. In almost all the studies, the exercise programs were performed twice (6 studies) or three times (16 studies) a week on nonconsecutive days; the exercise programs were performed daily in two studies and once a week in one study.
The RCTs provided protein (eight studies), creatine (five studies), long chain n-3 polyunsaturated fatty acids (PUFA omega-3) (two studies), calcium (one study), maslinic acid (one study), vitamins C and E (two studies), creatine and linoleic acid (one study), creatine and protein (one study), vitamin D (one study), and multinutrients containing more than three nutrients (three studies). Most studies provided nutritional supplements in pill, capsule, powder or drink forms, and a study provided a personalized and nutritionally balanced diet [38]. Most studies provided control groups with an isocaloric placebo. Two studies provided the control groups with pills or capsules containing some nutrients, such as calcium or omega-3 [36, 49]. The intervention period ranged between 8 and 24 weeks: eight weeks in one study, 12 weeks in 13 studies, 14 weeks in one study, 18 weeks in one study, and 24 weeks in nine studies.
Risk of Bias
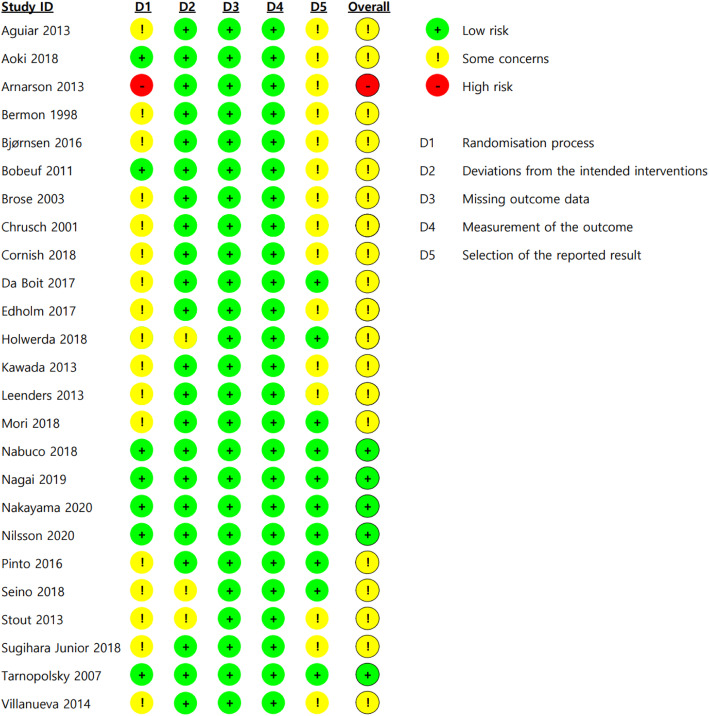
The risk of bias results for the 25 RCTs are demonstrated in Fig. 2. Regarding the randomization process, seven studies had a low risk of bias, 17 had some concerns, and one study had a high risk of bias because of a failure to conceal group allocation. Regarding deviation from the intended intervention, three had some concerns, and the others had a low risk of bias. As there were no studies in which missing values were judged to have an impact on the study results, all studies had a low risk of bias in the domain of missing outcome data. All studies had a low risk of bias in the domain of measurement of outcome, either because the outcome assessor was blinded or the outcome assessor’s awareness of the group assignments was judged to not affect the measurement of muscle mass, strength, or physical function. In the fifth domain, the selection of the reported results, 10 studies had a low risk of bias, while the other 15 studies had some concerns because of the absence of a prespecified trial protocol. Overall, five RCTs had a low risk of bias, 19 RCTs had some concerns, and one study had a high risk of bias.
Fig. 2.
Risk of bias of the included studies
Effects of resistance training and nutritional interventions compared with those of resistance training only on muscle mass, muscle strength, and physical functional performance
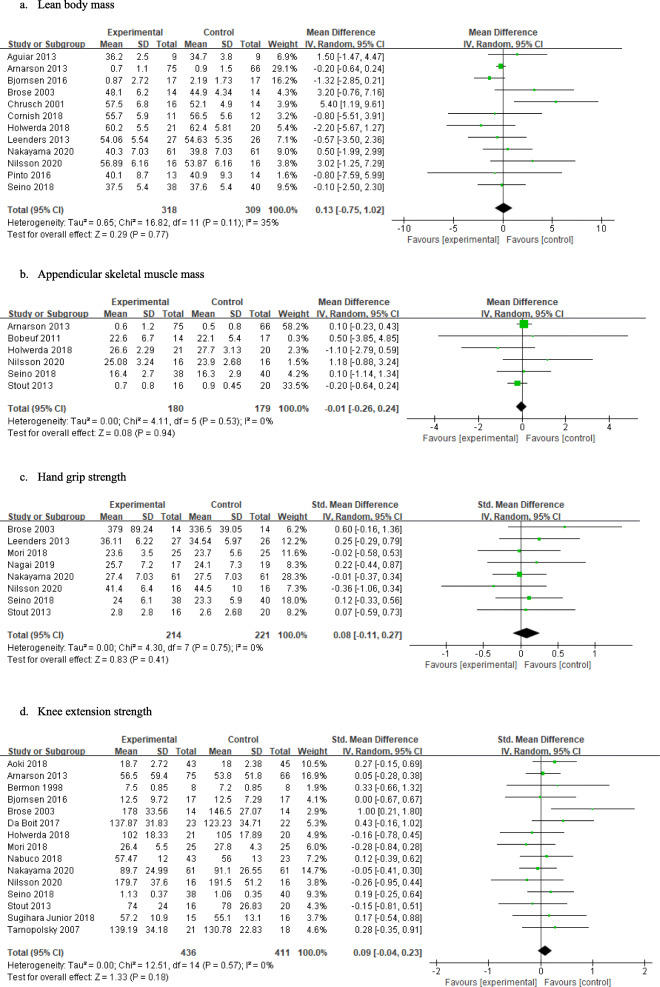
The effect sizes and 95% confidence intervals (95% CIs) for individual studies and all studies are shown in Fig. 3. The results of the meta-analysis showed no significant effects on lean body mass (n = 12, MD 0.13, 95% CI − 0.75 to 1.02), appendicular skeletal muscle mass (n = 6, MD -0.01, 95% CI − 0.26 to 0.24), hand grip strength (n = 8, SMD 0.08, 95% CI − 0.11 to 0.27), knee extension strength (n = 15, SMD 0.09, 95% CI − 0.04 to 0.23), the chair stand test results (n = 7, MD -0.13, 95% CI − 0.43 to 0.17), or the timed up-and-go test results (n = 6, MD 0.02, 95% CI − 0.16 to 0.20). The I2 values for all outcomes except lean body mass were zero, indicating that heterogeneity might not be important for these outcomes and that lean body mass might represent moderate heterogeneity (I2 = 35%).
Fig. 3.
Effects of resistance training and nutritional interventions compared with those of resistance training only on muscle mass, muscle strength, and physical functional performance
Subgroup analysis according to type of nutritional interventions
The results of the subgroup analyses according to the type of nutritional interventions are shown in Table 2. The subgroup analyses for lean body mass showed significant differences between the types of nutritional interventions (Chi2 = 7.28, p = .03). Among the nutritional interventions, only those with creatine showed significant effects on lean body mass (n = 4, MD 2.61, 95% CI 0.51 to 4.72). Regarding the other subgroup analyses, there were no significant differences in appendicular skeletal muscle mass (χ2 = 0.62, p = .43), hand grip strength (χ2 = 0.12, p = .73), knee extension strength (χ2 = 4.89, p = .09), chair stand test results (χ2 = 1.05, p = .43), or timed up-and-go test results (χ2 = 1.02, p = .31). Although creatine showed significant effects on knee extension strength (n = 2, SMD 0.74, 95% CI 0.09 to 1.38), there were no significant subgroup differences.
Table 2.
Subgroup analysis: effects by type of nutritional interventions on muscle mass, muscle Strength, and physical functional performance
| Subgroups | Lean body mass | Appendicular skeletal muscle mass | ||||||||
| n | MD | 95% CI | I2 | Subgroup differences | n | MD | 95% CI | I2 | Subgroup differences | |
| Creatine | 4 | 2.61 | 0.51 ~ 4.72 | 9% | χ2 = 7.28 (p = .03) | – | – | – | – | χ2 = 0.62 (p = .43) |
| Multi-nutrients | 2 | 0.94 | −1.94 ~ 3.82 | 36% | 2 | 0.39 | −0.68 ~ 1.45 | 0% | ||
| Protein | 4 | −0.21 | − 0.57 ~ 0.14 | 0% | 2 | −0.20 | −1.23 ~ 0.82 | 47% | ||
| Subgroups | Hand grip strength | Knee extension strength | ||||||||
| n | SMD | 95% CI | I2 | Subgroup differences | n | SMD | 95% CI | I2 | Subgroup differences | |
| Creatine | – | – | – | – | χ2 = 0.12 (p = .73) | 2 | 0.74 | 0.09 ~ 1.38 | 7% | χ2 = 4.89 (p = .09) |
| Multi-nutrients | 2 | −0.04 | −0.48 ~ 0.40 | 21% | 2 | 0.05 | −0.36 ~ 0.46 | 12% | ||
| Protein | 3 | 0.05 | −0.21 ~ 0.31 | 0% | 6 | −0.02 | −0.20 ~ 0.17 | 0% | ||
| Subgroups | Chair stand test | Timed up and go test | ||||||||
| n | MD | 95% CI | I2 | Subgroup differences | n | MD | 95% CI | I2 | Subgroup differences | |
| Multi-nutrients | 2 | 0.15 | −0.41 ~ 0.72 | 0% | χ2 = 1.05 (p = .31) | 2 | 0.26 | −0.31 ~ 0.83 | 32% | χ2 = 1.02 (p = .31) |
| Protein | 3 | −0.21 | −0.60 ~ 0.19 | 0% | 2 | −0.06 | −0.28 ~ 0.17 | 0% | ||
Moderator analysis with Meta-regression
As recommended [27], meta-regression was conducted when there were more than ten studies in a meta-analysis; therefore, meta-regression was performed on lean body mass (n = 12) and knee extension strength (n = 15). The mean age of the subjects and duration of interventions were included as explanatory variables in a univariate regression model. In the meta-regression, neither the mean age of the subjects (β = 0.01, SE = 0.04, t = 0.18, p = .858) nor the duration of interventions (β = − 0.001, SE = 0.02, t = − 0.04, p = .965) were associated with lean body mass. These moderators were not contributing variables to knee extension strength (β = − 0.03, SE = 0.03, t = − 0.90, p = .383; β = − 0.01, SE = 0.01, t = − 0.45, p = .660, respectively).
Sensitivity analysis
Sensitivity analysis was conducted to compare the effect sizes, 95% CIs, and I2 values by excluding two studies that provided some nutrients to control groups. There were no significant differences in lean body mass (n = 11, MD 0.20, 95% CI − 0.74 to 1.14), appendicular skeletal muscle mass (n = 5, MD 0.09, 95% CI − 0.23 to 0.40), hand grip strength (n = 7, MD 0.08, 95% CI − 0.12 to 0.28), knee extension strength (n = 14, MD 0.10, 95% CI − 0.04 to 0.24), chair stand test results (n = 7, MD -0.13, 95% CI − 0.43 to 0.17), or timed up-and-go test results (n = 4, MD 0.01, 95% CI − 0.18 to 0.20). The I2 values for all outcomes except for lean body mass were zero, indicating that heterogeneity might not be important for these outcomes and that lean body mass might have moderate heterogeneity (I2 = 40%).
Certainty of evidence
There were six outcomes to compare the quality of evidence for resistance training combined a nutritional intervention compared to resistance training alone for muscle function. The hand grip strength and chair stand test were of moderate certainty. The other outcomes were low. The GRADE summary of the findings is shown in Additional file 2.
Discussion
Nutrient-dense foods that ensure sufficient intake of energy, protein and micronutrients are important to prevent frailty and sarcopenia and promote physical activity. However, to date, the optimal type of nutritional intervention or supplementation for the prevention of frailty and sarcopenia is unclear. This study was conducted to compare the synergistic effect of nutritional interventions combined with resistance training with that of resistance training alone. This study can provide insight into resource optimization and strategies to prevent frailty and sarcopenia.
This systematic review and meta-analysis showed that there were no additional effects of nutritional interventions when combined with resistance training on muscle mass, strength, or physical function. Of note, in two studies, the control conditions included some nutrients that have biological benefits [36, 49], which likely reduced the calculated effect size when the data for the control conditions were pooled. However, the findings of the sensitivity analysis showed little possibility of blunted effects. One of the possible reasons for this lack of significant results is that the analysis included studies of healthy older adults who might not have nutrient deficiencies with the usual diets [23]. Healthy diets provide a broad range of micronutrients and bioactive nonnutrients as well as macronutrients that might not be included in the experimental supplements in trials. In addition, since diets are patterned, isolating the effects of individual experimental supplements might not be possible without controlling for the usual diet. Thus, the effects of nutritional interventions might be blunted among older adults who habitually consume sufficient nutrients. However, in previous studies that provided vitamin D-deficient and mobility-limited older adults with a protein mixture containing 20 g protein, 800 IU vitamin D, 350 mg calcium, and other minerals once a day for 6 months with an exercise program, there were no differences in muscle function parameters such as leg strength, gait speed, and short physical performance battery between this group and the exercise-only control group except in muscle density [53, 54]. In another study that also provided sarcopenic older adults who had low protein intake with multinutrient supplements containing 21 g protein, 800 IU vitamin D and other nutrients once a day for 3 months with an exercise program, there were no differences between the two groups, although both groups exhibited improved muscle function [55]. It is necessary to additionally consider the dose of the nutrient and duration of intervention and monitor dietary energy intake. Despite the lack of evidence, greater benefits of resistance training along with nutritional supplementation are expected in older adults who already have poor muscle function or habitually have low nutrient intake.
In the subgroup analysis of the types of nutrients, only creatine showed significant effects on lean body mass. Among the five studies included in this meta-analysis, four administered 5 g creatine daily combined with resistance training 3 times a week for 12 weeks [28, 34, 47] or twice a week for 24 weeks [51], and one administered 5 g creatine four times a day for the first 5 days of the loading phase and 3 g creatine daily for the maintenance phase combined with resistance training 3 times a week for 7 weeks [31]. Recent systematic reviews similarly identified the additive effect of creatine during resistance training on body composition, muscle strength, and physical function [56, 57]. As skeletal muscle has no capacity for creatine biosynthesis, the consumption of creatine-containing food or supplementation of creatine increases creatine and phosphocreatine levels in skeletal muscle and elevates phosphate resynthesis (energy buffer) during high-energy demanded exercise, such as repetitive resistance training training [58, 59, 60]. Creatine helps to increase muscle mass and strength by indirectly increasing work capacity, and the combination of creatine supplementation and resistance training promotes muscle protein synthesis. Alternatively, creatine supplementation may enhance muscle protein synthesis stimulating signaling pathways (myogenic regulatory factors), which facilitate myosatellite cell proliferation and differentiation [61]. Controversy exists as to whether creatine stores and metabolism are affected by aging, but creatine supplements can account for dietary changes and reductions in physical activity with aging [57]. The effect sizes for variables other than lean body mass were not significant in this study. Additional meta-analyses including more experimental studies are needed to verify the effects of creatine on muscle mass and function in older adults.
As proteins provide amino acids that are essential for muscle protein synthesis and act as anabolic stimuli, protein consumption increases muscle mass, and protein consumption following resistance training enhances net protein utilization, attenuating exercise-induced muscle protein breakdown [60, 62]. The combination of protein supplements and exercise was expected to have a synergistic effect on muscle function, but the findings of this study did not support this hypothesis. On the other hand, in a previous meta-analysis, protein supplements for sarcopenic older adults along with exercise showed a larger effect size than exercise alone and no intervention [24]. The previous meta-analysis was conducted in frail, sarcopenic, or mobility-limited older adults and included not only community-dwelling older adults but also institutionalized older adults. Individuals with existing nutritional deficiencies or poor muscle function might have been shown to respond better to accompanying nutritional supplements than to exercise alone. Additional studies are needed to determine whether the inconsistency in findings resulted from the characteristics of the subjects.
Muscle protein synthesis through protein intake in older adults should be maximized with consideration of the frequency, distribution, and other nutritional components, such as creatine, vitamins, and fatty acids [22, 60]. It is recommended that older adults consume ≥0.4 g/kg per meal and 1.2–1.6 g/kg per day to induce muscle protein synthesis saturation to thus support muscle function [60]. Among the included studies, most studies provided 10.1 g–25 g protein once a day [39, 41, 42, 45] or 20 g ~ 35 g protein 3 times a week on the days exercise was performed [30, 43, 50], which could not take into account frequency and distribution. A previous review showed that multi-ingredient protein supplements have the potential to increase the benefits of resistance training, but there were no differences in the effects on muscle mass and strength between multi-ingredient protein and single protein [63]. The impact of multiple nutrients is unclear, but there are complex interactions between food components inducing potential synergistic effects. Thus, nutritional interventions involving dietary modifications with various and balanced nutrients or whole food approaches rather than a single specific nutrient can be effective in improving muscle mass and function [64]. Among the 25 RCTs, three provided multinutrients that were arbitrarily defined as containing three or more nutrients. Of the three studies, only one used a whole-diet approach. The number of studies was too small to verify the effect of the whole-food or whole-diet approach.
Nutritional effects may not manifest following dietary interventions of short durations. Although the meta-regression results did not show that the intervention period was associated with effect sizes, a 6-year longitudinal study showed a positive relationship between daily protein intake and muscle strength [65]; nutritional contributions can be expected to be observed in the long term. Thus, despite the nonsignificant results, nutritional interventions may still be beneficial for older adults who do not lack nutrients. With aging, muscle loss (breakdown) occurs more rapidly than muscle synthesis, so additional supplements may be required. In addition, older adults experience declines in food intake because of changes in appetite and a lack of hunger, which is referred to as ‘anorexia of aging’ [66]. As consumed food is metabolized to synthesize energy for organ function, poor nourishment leads to body fat and muscle being catabolized to provide energy. Not only a lack of specific nutrients but also the consumption of an insufficient amount of food contributes to weight loss and declines in muscle mass, strength and physical function, which can lead to physical frailty and sarcopenia. Thus, the consumption of an adequate amount of food containing nutrients essential for muscle function is important to maintain muscle mass, strength, and physical function [22, 58]. Considering that changes occur in various physiological functions as well as muscle function, interventions with a balanced diet are important in older people. As nutritional interventions have the advantages of low costs and high availability and accessibility, additional studies are necessary to determine whether they can be effective in preventing frailty and sarcopenia.
This study has several limitations. First, this meta-analysis included only retrievable RCTs that were published in English, which may have contributed to language bias. Second, this study in healthy older adults might not have demonstrated significant effects on muscle mass, muscle strength, and physical function due to the ceiling effect. Additional systematic reviews and meta-analyses are needed to identify the additional effects of nutritional interventions when combined with resistance training among dynapenic, sarcopenic, or frail older adults. Third, the range of nutritional interventions included in this study were vast because each nutrient has a different mechanism that affects muscle synthesis, function, or prevention of muscle damage. Future studies need to be more focused on a specific nutritional intervention. Finally, as mentioned above, the amount, frequency, and distribution of nutrients administered are important to consider to fully assess the effects of nutritional interventions; however, these factors were not assessed in the meta-analysis.
As the levels of variability in muscle mass and functional measurements are quite high in older adults, it is hard to obtain adequate statistical power to verify differences between groups in many studies on nutritional interventions. This meta-analysis showed that nutritional interventions have no additional effect on body composition, muscle strength, or physical function when combined with resistance training. Only creatine showed synergistic effects with resistance training on muscle mass. The enhanced effect of nutritional interventions for unhealthy older adults, such as frail, sarcopenic, or nutritionally deficient older adults, needs to be investigated in future studies. The long-term effects of nutrition on muscle function also need to be studied. In addition, additional studies should be conducted to identify the dietary parameters that maximize nutritional effects on muscle protein synthesis, including dose, frequency, distribution, and recipes that take into account interactions with other nutrients. Health-promoting interventions such as exercise and diet are important for at-risk older adults to prevent clinically evident disability. This systematic review and meta-analysis provides a comprehensive synthesis of the experimental results available to date for health practitioners and researchers to establish intervention strategies or public health policies.
Supplementary Information
Additional file 2. Summary of findings and analysis of the quality of evidence.
Acknowledgments
Not applicable.
Abbreviations
- CI
Confidence interval
- GRADE
Grading of recommendations, assessment, development and evaluation
- IU
International unit
- MD
Mean difference
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- PUFA
Polyunsaturated fatty acid
- RCT
Randomized controlled trial
- RoB
Risk of bias
- SD
Standard deviation
- SMD
Standardized mean difference.
- UK
United Kingdom
- USA
United States of America
Authors’ contributions
MK designed this study, collected and selected articles, extracted data from the included studies, evaluated the risk of bias, performed meta-analyses, and drafted the manuscript. HY performed data collection, selection of studies according to criteria, and data extraction. JY evaluated the risk of bias of the included studies, checked the results of meta-analyses, and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1G1A1003901).
Availability of data and materials
The authors can confirm that all relevant data are included in the article.
Declarations
Ethics approval and consent to participate
This study performed in accordance with the Declaration of Helsinki. This study was approved for a review exemption from the institutional review board of a university, Chuncheon, Korea (KWNUIRB-2020–07–006).
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Active ageing: a policy framework. Geneva: World Health Organization; 2002. [PubMed]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, Scuteri A, Sinclair A, Pelaez M, van der Cammen T, Beland F, Bickenbach J, Delamarche P, Ferrucci L, Fried LP, Gutiérrez-Robledo LM, Rockwood K, Rodríguez Artalejo F, Serviddio G, Vega E, FOD-CC group (Appendix 1) Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 7.Shmuel S, Lund JL, Alvarez C, Hsu CD, Palta P, Kucharska-Newton A, Jordan JM, Nelson AE, Golightly YM. Polypharmacy and incident frailty in a longitudinal community-based cohort study. J Am Geriatr Soc. 2019;67(12):2482–2489. doi: 10.1111/jgs.16212. [DOI] [PubMed] [Google Scholar]
- 8.Yadav UN, Tamang MK, Thapa TB, Hosseinzadeh H, Harris MF, Yadav KK. Prevalence and determinants of frailty in the absence of disability among older population: a cross sectional study from rural communities in Nepal. BMC Geriatr. 2019;19(1):283. doi: 10.1186/s12877-019-1290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Classification of Disease. 2021 ICD-10-CM diagnosis code M62.84. http://www.icd10data.com/ICD10CM/Codes/M00-M99/M60-M63/M62-/M62.84 (2021). Accessed 15 Feb 2021.
- 10.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Bautmans I, Baeyens JP, Cesari M, Cherubini A, Kanis J, Maggio M, Martin F, Michel JP, Pitkala K, Reginster JY, Rizzoli R, Sánchez-Rodríguez D, Schols J. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, De Souza RJ, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56. doi: 10.1093/ageing/afy106. [DOI] [PubMed] [Google Scholar]
- 12.Bernabei R, Martone AM, Vetrano DL, Calvani R, Landi F, Marzetti E. Frailty, physical frailty, sarcopenia: a new conceptual model. Stud Health Technol Inform. 2014;203:78–84. [PubMed] [Google Scholar]
- 13.Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Sarcopenia and physical independence in older adults: the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle. 2017;8(2):245–250. doi: 10.1002/jcsm.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaap LA, Van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: the longitudinal aging study Amsterdam. J Gerontol A Biol Sci Med Sci. 2018;73(9):1199–1204. doi: 10.1093/gerona/glx245. [DOI] [PubMed] [Google Scholar]
- 15.Steffl M, Sima J, Shiells K, Holmerova I. The increase in health care costs associated with muscle weakness in older people without long-term illnesses in the Czech Republic: results from the survey of health, ageing and retirement in Europe (SHARE) Clin Interv Aging. 2017;12:2003–2007. doi: 10.2147/CIA.S150826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Howard K, Hayes A, Monaghan N, Langron C, Aggar C, Cameron ID. Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. J Am Med Dir Assoc. 2015;16(1):41–48. doi: 10.1016/j.jamda.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Frost R, Belk C, Jovicic A, Ricciardi F, Kharicha K, Gardner B, Iliffe S, Goodman C, Manthorpe J, Drennan VM, Walters K. Health promotion interventions for community-dwelling older people with mild or pre-frailty: a systematic review and meta-analysis. BMC Geriatr. 2017;17(1):157. doi: 10.1186/s12877-017-0547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28(6):1817–1833. doi: 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly RM. Exercise and nutritional approaches to prevent frail bones, falls and fractures: an update. Climacteric. 2017;20(2):119–124. doi: 10.1080/13697137.2017.1286890. [DOI] [PubMed] [Google Scholar]
- 20.Hita-Contreras F, Bueno-Notivol J, Martínez-Amat A, Cruz-Díaz D, Hernandez AV, Pérez-López FR. Effect of exercise alone or combined with dietary supplements on anthropometric and physical performance measures in community-dwelling elderly people with sarcopenic obesity: a meta-analysis of randomized controlled trials. Maturitas. 2018;116:24–35. doi: 10.1016/j.maturitas.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Dulac MC, Aubertin-Leheudre M. Exercise: an important key to prevent physical and cognitive frailty. J Frailty Aging. 2016;5(1):3–5. doi: 10.14283/jfa.2015.72. [DOI] [PubMed] [Google Scholar]
- 22.Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, Bischoff-Ferrari H, Bruyère O, Cesari M, Dawson-Hughes B, Fielding RA, Kaufman JM, Landi F, Malafarina V, Rolland Y, van Loon LJ, Vellas B, Visser M, Cooper C, al-Daghri N, Allepaerts S, Bauer J, Brandi ML, Cederholm T, Cherubini A, Cruz Jentoft A, Laviano A, Maggi S, McCloskey EV, Petermans J, Roubenoff R, Rueda R. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. 2018;37(4):1121–1132. doi: 10.1016/j.clnu.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denison HJ, Cooper C, Sayer AA, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging. 2015;10:859–869. doi: 10.2147/CIA.S55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao CD, Chen HC, Huang SW, Liou TH. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: a systematic review and meta-regression analysis of randomized trials. Nutrients. 2019;11(8):1713. doi: 10.3390/nu11081713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenholm S, Pulakka A, Kawachi I, Oksanen T, Halonen JI, Aalto V, Kivimäki M, Vahtera J. Changes in physical activity during transition to retirement: a cohort study. Int J Behav Nutr Phys Act. 2016;13(1):51. doi: 10.1186/s12966-016-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baum K, Hildebrandt U, Edel K, Bertram R, Hahmann H, Bremer FJ, Böhmen S, Kammerlander C, Serafin M, Rüther T, Miche E. Comparison of skeletal muscle strength between cardiac patients and age-matched healthy controls. Int J Med Sci. 2009;6(4):184–191. doi: 10.7150/ijms.6.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane. 2021. www.training.cochrane.org/handbook. Accessed 24 Aug 2021.
- 28.Aguiar AF, Januário RS, Junior RP, Gerage AM, Pina FL, Do Nascimento MA, et al. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur J Appl Physiol. 2013;113:987–96. 10.3390/nu10050563. [DOI] [PubMed]
- 29.Aoki K, Sakuma M, Endo N. The impact of exercise and vitamin D supplementation on physical function in community-dwelling elderly individuals: A randomized trial. J Orthop Sci. 2018;23(4):682–7. 10.1016/j.jos.2018.03.011. [DOI] [PubMed]
- 30.Arnarson A, Gudny Geirsdottir O, Ramel A, Briem K, Jonsson PV, Thorsdottir I. Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: double blind, randomised controlled trial. Eur J Clin Nutr. 2013;67(8):821–6. 10.1038/ejcn.2013.40. [DOI] [PubMed]
- 31.Bermon S, Venembre P, Sachet C, Valour S, Dolisi C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol Scand. 1998;164(2):147–55. 10.1046/j.1365-201X.1998.00427.x. [DOI] [PubMed]
- 32.Bjørnsen T, Salvesen S, Berntsen S, Hetlelid KJ, Stea TH, Lohne-Seiler H, et al. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand J Med Sci Sports. 2016;26(7):755–63. 10.1111/sms.12506. [DOI] [PubMed]
- 33.Bobeuf F, Labonte M, Dionne IJ, Khalil A. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J Nutr Health Aging. 2011;15(10):883–9. 10.1007/s12603-011-0097-2. [DOI] [PubMed]
- 34.Brose A, Parise G, Tarnopolsky MA. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J Gerontol A Biol Sci Med Sci. 2003;58(1):11–9. 10.1093/gerona/58.1.b11. [DOI] [PubMed]
- 35.Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc. 2001;33(12):2111–7. 10.1097/00005768-200112000-00021. [DOI] [PubMed]
- 36.Cornish SM, Myrie SB, Bugera EM, Chase JE, Turczyn D, Pinder M. Omega-3 supplementation with resistance training does not improve body composition or lower biomarkers of inflammation more so than resistance training alone in older men. Nutr Res. 2018;60:87–95. 10.1016/j.nutres.2018.09.005. [DOI] [PubMed]
- 37.Da Boit M, Sibson R, Sivasubramaniam S, Meakin JR, Greig CA, Aspden RM, et al. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am J Clin Nutr. 2017;105(1):151–8. 10.3945/ajcn.116.140780. [DOI] [PMC free article] [PubMed]
- 38.Edholm P, Strandberg E, Kadi F. Lower limb explosive strength capacity in elderly women: effects of resistance training and healthy diet. J Appl Physiol (2017). 123(1):190–6. 10.1152/japplphysiol.00924.2016. [DOI] [PubMed]
- 39.Holwerda AM, Overkamp M, Paulussen KJM, Smeets JSJ, Van Kranenburg J, Backx EMP, et al. Protein supplementation after exercise and before sleep does not further augment muscle mass and strength gains during resistance exercise training in active older men. J Nutr. 2018;148(11):1723–32. 10.1093/jn/nxy169. [DOI] [PubMed]
- 40.Kawada S, Okamoto Y, Ogasahara K, Yanagisawa S, Ohtani M, Kobayashi K. Resistance exercise combined with essential amino acid supplementation improved walking ability in elderly people. Acta Physiol Hung. 2013;100(3):329–39. 10.1556/APhysiol.100.2013.008. [DOI] [PubMed]
- 41.Leenders M, Verdijk LB, Van der Hoeven L, Van Kranenburg J, Nilwik R, Wodzig WK, et al. Protein supplementation during resistance-type exercise training in the elderly. Med Sci Sports Exerc. 2013;45(3):542–52. 10.1249/MSS.0b013e318272fcdb. [DOI] [PubMed]
- 42.Mori H, Tokuda Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: a randomized controlled trial. Geriatr Gerontol Int. 2018;18(9):1398–404. 10.1111/ggi.13499. [DOI] [PubMed]
- 43.Nabuco HCG, Tomeleri CM, Sugihara Junior P, Fernandes RR, Cavalcante EF, Antunes M, et al. Effects of whey protein supplementation pre- or post-resistance training on muscle mass, muscular strength, and functional capacity in pre-conditioned older women: a randomized clinical trial. Nutrients. 2018;10(5):563. 10.3390/nu10050563. [DOI] [PMC free article] [PubMed]
- 44.Nagai N, Yagyu S, Hata A, Nirengi S, Kotani K, Moritani T, et al. Maslinic acid derived from olive fruit in combination with resistance training improves muscle mass and mobility functions in the elderly. J Clin Biochem Nutr. 2019;64(3):224–30. 10.3164/jcbn.18-104. [DOI] [PMC free article] [PubMed]
- 45.Aguiar AF, Januário RS, Junior RP, Gerage AM, Pina FL, Do Nascimento MA, et al. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur J Appl Physiol. 2012;113(4):987–96. 10.1007/s00421-012-2514-6. [DOI] [PubMed]
- 46.Nilsson MI, Mikhail A, Lan L, Di Carlo A, Hamilton B, Barnard K, et al. A five-ingredient nutritional supplement and home-based resistance exercise improve lean mass and strength in free-living elderly. Nutrients. 2020;12(8):2391. 10.3390/nu12082391. [DOI] [PMC free article] [PubMed]
- 47.Pinto CL, Botelho PB, Carneiro JA, Mota JF. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J Cachexia Sarcopenia Muscle. 2016;7(4):413–21. 10.1002/jcsm.12094. [DOI] [PMC free article] [PubMed]
- 48.Seino S, Sumi K, Narita M, Yokoyama Y, Ashida K, Kitamura A, et al. Effects of Low-Dose Dairy Protein Plus Micronutrient Supplementation during Resistance Exercise on Muscle Mass and Physical Performance in Older Adults: A Randomized, Controlled Trial. J Nutr Health Aging. 2018;22(1):59–67. 10.1007/s12603-017-0904-5. [DOI] [PubMed]
- 49.Stout JR, Smith-Ryan AE, Fukuda DH, Kendall KL, Moon JR, Hoffman JR, et al. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: a randomized, double-blind pilot trial. Exp Gerontol. 2013;48(11):1303–10. 10.1016/j.exger.2013.08.007. [DOI] [PubMed]
- 50.Sugihara Junior P, Ribeiro AS, Nabuco HCG, Fernandes RR, Tomeleri CM, Cunha PM, et al. Effects of whey protein supplementation associated with resistance training on muscular strength, hypertrophy, and muscle quality in preconditioned older women. Int J Sport Nutr Exerc Metab. 2018;28(5):528–35. 10.1123/ijsnem.2017-0253. [DOI] [PubMed]
- 51.Tarnopolsky M, Zimmer A, Paikin J, Safdar A, Aboud A, Pearce E, et al. Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS One. 2007;2(10):e991. 10.1371/journal.pone.0000991. [DOI] [PMC free article] [PubMed]
- 52.Villanueva MG, He J, Schroeder ET. Periodized resistance training with and without supplementation improve body composition and performance in older men. Eur J Appl Physiol. 2014;114(5):891–905. 10.1007/s00421-014-2821-1. [DOI] [PubMed]
- 53.Englund DA, Kirn DR, Koochek A, Zhu H, Travison TG, Reid KF, et al. Nutritional supplementation with physical activity improves muscle composition in mobility-limited older adults, the VIVE2 study: a randomized, double-blind, placebo-controlled trial. J Gerontol A Biol Sci Med Sci. 2018;73(1):95–101. 10.1093/gerona/glx141. [DOI] [PMC free article] [PubMed]
- 54.Fielding RA, Travison TG, Kirn DR, Koochek A, Reid KF, Von Berens Å, et al. Effect of structured physical activity and nutritional supplementation on physical function in mobility-limited older adults: results from the VIVE2 randomized trial. J Nutr Health Aging. 2017;21(9):936–42. 10.1007/s12603-017-0936-x. [DOI] [PMC free article] [PubMed]
- 55.de Carvalho BA, Nobre LN, de Souza MB, Rosa IF, Ferreira GB, Santos DDL, et al. Independent and combined effect of home-based progressive resistance training and nutritional supplementation on muscle strength, muscle mass and physical function in dynapenic older adults with low protein intake: a randomized controlled trial. Arch Gerontol Geriatr. 2020;89:104098. 10.1016/j.archger.2020.104098. Epub 2020 May 13. PMID: 32446170. [DOI] [PubMed]
- 56.Chilibeck PD, Kaviani M, Candow DG, Zello GA. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med. 2017;8:213–26. 10.2147/OAJSM.S123529. [DOI] [PMC free article] [PubMed]
- 57.Stares A, Bains M. The additive effects of creatine supplementation and exercise training in an aging population: a systematic review of randomized controlled trials. J Geriatr Phys Ther. 2020;43(2):99–112. 10.1519/JPT.0000000000000222. [DOI] [PubMed]
- 58.Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017;29(1):43–8. 10.1007/s40520-016-0709-0. [DOI] [PubMed]
- 59.Devries MC, Phillips SM. Creatine supplementationduring resistance training in older adults-a meta-analysis. Med Sci SportsExerc. 2014;46(6):1194–203. 10.1249/MSS.0000000000000220. [DOI] [PubMed]
- 60.McKendry J, Currier BS, Lim C, McLeod JC, Thomas ACQ, Phillips SM. Nutritional supplements to support resistance exercise in countering the sarcopenia of aging. Nutrients. 2020;12(7):2057. 10.3390/nu12072057. [DOI] [PMC free article] [PubMed]
- 61.Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci. 2013;70(21):4117–30. 10.1007/s00018-013-1330-4. [DOI] [PMC free article] [PubMed]
- 62.Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–84. 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed]
- 63.O'Bryan KR, Doering TM, Morton RW, Coffey VG,Phillips SM, Cox GR. Do multi-ingredient protein supplements augment resistancetraining-induced gains in skeletal muscle mass and strength? A systematicreview and meta-analysis of 35 trials. Br J Sports Med. 2020;54(10):573–81.10.1136/bjsports-2018-099889. [DOI] [PubMed]
- 64.Yannakoulia M, Ntanasi E, Anastasiou CA, Scarmeas N. Frailty and nutrition: from epidemiological and clinical evidence to potential mechanisms. Metabolism. 2016;68:64–76. 10.1016/j.metabol.2016.12.005. [DOI] [PubMed]
- 65.McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham offspring cohort. J Gerontol A Biol Sci Med Sci. 2016;71(3):356–61. 10.1093/gerona/glv184. [DOI] [PMC free article] [PubMed]
- 66.Morley JE, Vellas B, Van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Summary of findings and analysis of the quality of evidence.
Data Availability Statement
The authors can confirm that all relevant data are included in the article.