Abstract
Background and Objectives
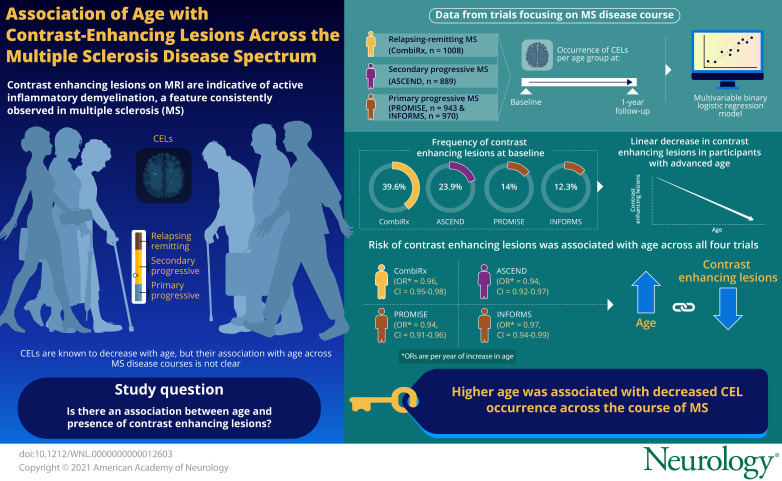
To investigate the association of age and the presence of contrast-enhancing lesions (CELs) on cranial MRI scans in different disease courses of multiple sclerosis (MS), we describe the frequency of CELs as a function of age in 4 large randomized controlled trial (RCT) datasets.
Methods
Using original trial data from CombiRx (Combination Therapy in Patients With Relapsing-Remitting Multiple Sclerosis; clinicaltrials.gov identifier NCT00211887), a trial in relapsing-remitting MS; ASCEND (A Clinical Study of the Efficacy of Natalizumab on Reducing Disability Progression in Participants With Secondary Progressive Multiple Sclerosis; clinicaltrials.gov identifier NCT01416181), a trial in secondary progressive MS; and the 2 primary progressive MS trials PROMISE and INFORMS; clinicaltrials.gov identifier NCT00731692), we describe the occurrence of CELs per age group at baseline for the entire trial cohort and at 1 year follow-up in the treatment arms.
Results
CombiRx included 1,008, ASCEND 889, PROMISE 943, and INFORMS 970 participants. At baseline, CEL frequency differed between datasets according to disease course: 39.6% of CombiRx, 23.9% of ASCEND, 14.0% of PROMISE, and 12.3% of INFORMS participants had CELs. This distribution by disease course was largely preserved within each age group. In all datasets, there was an almost linear decrease of the percentage of participants with CELs with advancing age. After 1 year of experimental treatment, CEL occurrence was reduced in all trial datasets, and almost absent in ASCEND. The decrease of CEL occurrence with advancing age was preserved in CombiRx, PROMISE, and INFORMS after 1 year of treatment. We investigated the association of the baseline factors age, disease duration, sex, and EDSS with having CELs at baseline with multivariable binary logistic regression models. Age was the only characteristic associated with the risk of CELs at baseline in all datasets, with higher age associated with a lower risk of CELs (odds ratios for having CELs at baseline per year increase in age: CombiRx: 0.96, 95% confidence interval [CI] 0.95–0.98; ASCEND: 0.94, 95% CI 0.92–0.97; PROMISE: 0.94, 95% CI 0.91–0.96; INFORMS: 0.97, 95% CI 0.94–0.99).
Discussion
Our analysis of 4 large, well-characterized RCT datasets shows that the association of age and CEL occurrence is a general phenomenon across the spectrum of MS disease courses. Our findings should be replicated in real-world MS datasets.
The disease process of multiple sclerosis (MS) is characterized by inflammation as well as neurodegeneration.1 Common to all forms of MS are foci of inflammation in the brain and spinal cord, which can be visualized with MRI. Active inflammatory demyelination within such lesions leads to damage to the blood–brain barrier, so that actively demyelinating lesions can be identified using a gadolinium-based contrast agent. Contrast-enhancing lesions (CELs) on MRI scans are indicative of active inflammatory demyelination at the time of the scan, and are therefore seen as a reliable radiologic biomarker of current inflammatory disease activity. The presence and number of CELs are an important secondary outcome in relapsing-remitting MS (RRMS) trials, but CELs occur in all disease courses of MS.
Several studies showed that CEL frequency decreases with age,2-5 a finding that is in keeping with the observation that relapses decrease with age and disease duration,6 and with the general idea that inflammatory disease activity in MS is more pronounced in younger and less prevalent in older people. However, these studies were dominated by patients with RRMS, and it is less clear whether the observed association of age and CEL occurrence is present across all MS disease courses.
In this study, we used the datasets from 4 large randomized controlled trials (RCTs) in RRMS, secondary progressive MS (SPMS), and primary progressive MS (PPMS) to investigate the relation of age on the presence of CELs at baseline and after 1 year of experimental treatment.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The dataset of CombiRx (Combination Therapy in Patients With Relapsing-Remitting Multiple Sclerosis) was obtained from the Coordinating Center at the University of Alabama at Birmingham. The ASCEND (A Clinical Study of the Efficacy of Natalizumab on Reducing Disability Progression in Participants With Secondary Progressive Multiple Sclerosis) and PROMISE and INFORMS RCT datasets were obtained from Biogen, TEVA, and Novartis, the pharmaceutical companies that conducted and oversaw these trials. Ethical approval for the original trial is described in the original publications.7-10 Ethical approval for this analysis was sought and granted by the University of Calgary Conjoint Health Research Ethics Board.
Trial Datasets
CombiRx
CombiRx7 was a multicenter phase 3 RCT of interferon-β plus placebo (25%) or glatiramer acetate plus placebo (25%) or combination of the 2 active agents in RRMS (50%), so that all included participants received a form of immunomodulatory treatment. The inclusion criteria were a diagnosis of RRMS by Poser11 or 2001 McDonald12 diagnostic criteria, age 18–60 years inclusive, and Expanded Disability Status Scale (EDSS) score13 of 0–5.5 inclusive, with at least 2 exacerbations in the 3 years before inclusion (where one exacerbation could be an MRI change meeting the 2001 McDonald MRI criteria for dissemination in time). CombiRx participants were naive to immunomodulatory treatment at the time of enrollment.7
ASCEND
ASCEND8 was a multicenter phase 3 RCT investigating the efficacy of natalizumab treatment in SPMS. Entry criteria included age 18–58 years inclusive, SPMS for 2 or more years, disability progression over the previous year (as assessed using a standardized form), and a screening EDSS of 3.0–6.5 inclusive.
PROMISE
PROMISE9 was a multicenter phase 3 RCT investigating the efficacy of glatiramer acetate treatment in PPMS. Key inclusion criteria included a participant age between 30 and 65 years with an entry EDSS score of 3.0–6.5 inclusive and a diagnosis of PPMS confirmed by the principal investigator at each study site. Participants with a history of relapses were excluded.9
INFORMS
INFORMS10 was a multicenter phase 3 RCT investigating the efficacy of fingolimod treatment in PPMS. Key inclusion criteria were age 25–65 years, a clinical diagnosis of PPMS, disease progression for 1 year or more, a disease duration of 2–10 years, and objective evidence of disability worsening in the 2 years before inclusion.10
Outcome Measures
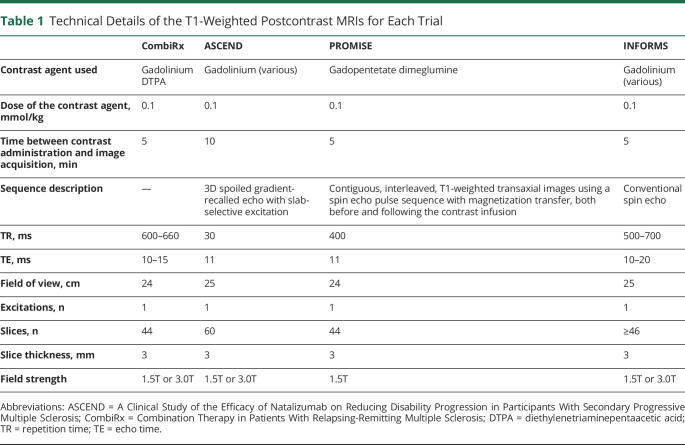
All clinical trials included gadolinium-enhanced cranial MRI scans at the beginning and throughout the trial. Technical details of the postcontrast scans are shown in Table 1. We selected the first scan before treatment initiation (performed at the screening visit in ASCEND and at baseline in the other trials) for the entire trial cohorts and the 1-year follow-up scan (performed at 48 weeks in ASCEND) of the treatment arm for our analyses. We determined the number of patients with CELs on these scans.
Table 1.
Technical Details of the T1-Weighted Postcontrast MRIs for Each Trial
To investigate whether patients with CELs at baseline also had CELs at 1 year follow-up, we constructed 2 × 2 tables and performed χ2 tests.
We investigated the association of the baseline factors age, disease duration, sex, and EDSS at baseline with having CELs at baseline with multivariable binary logistic regression models. We also investigated the possible interaction of age and disease duration with an interaction term in these models.
Given the heterogeneity of the datasets, we decided not to perform further analyses. All statistical analyses were performed with the R statistical software package for Windows, version 4.0.2.14 Statistical significance was taken to be at the 2-tailed 0.05 level.
Data Availability
CombiRx
Access to the CombiRx dataset can be requested from the Coordinating Center or MS Center at the University of Alabama at Birmingham by completing a data use agreement that is reviewed by a committee overseeing the use of the data. Qualified researchers have or will obtain appropriate institutional review board approval for the study request. Depending on the complexity of the request, researchers may need to cover the cost of producing the deidentified data.
ASCEND
The ASCEND dataset is available upon request from Biogen. Individual participant data collected during the trial will be shared after anonymization and on approval of a research proposal and data sharing agreement. Research proposals can be submitted online (biogenclinicaldatarequest.com).
PROMISE
The PROMISE dataset was acquired through TEVA Pharmaceutical's Data Sharing and Transparency office after agreeing on a research proposal and data sharing agreement. It is available to other qualified researchers upon request from TEVA Pharmaceuticals.
INFORMS
The INFORMS dataset is available upon request from Novartis. Individual participant data collected during the trial will be shared after anonymization and on approval of a research proposal and data sharing agreement. Research proposals can be submitted online (clinicalstudydatarequest.com).
Results
Baseline Characteristics
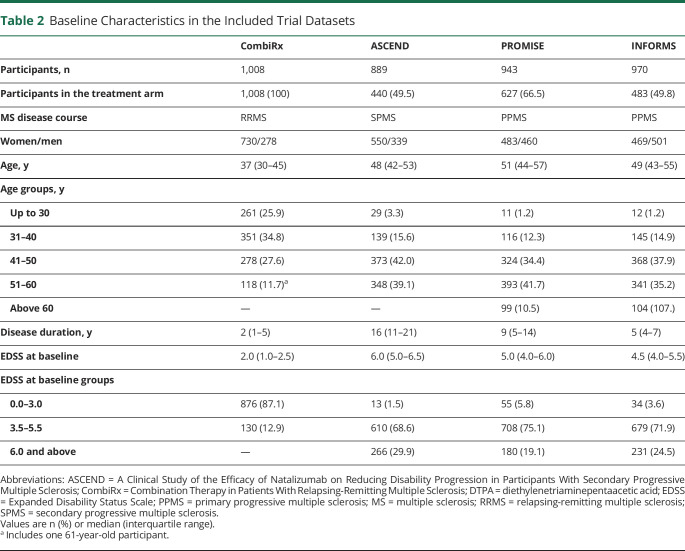
The baseline characteristics of the trial datasets are shown in Table 2. The datasets reflect the disease course included in each trial, with CombiRx including younger patients than the 3 progressive trials. As expected, and as a consequence of the inclusion criteria and disease definitions, disability at baseline was much higher in the progressive trials. We divided age at baseline into 5 categories: up to 30 years, 31–40 years, 41–50 years, 51–60 years, and above 60 years. One participant in CombiRx was 61 years of age at baseline; we decided to include this participant’s data in the 51–60 years group.
Table 2.
Baseline Characteristics in the Included Trial Datasets
CELs at Baseline by Age
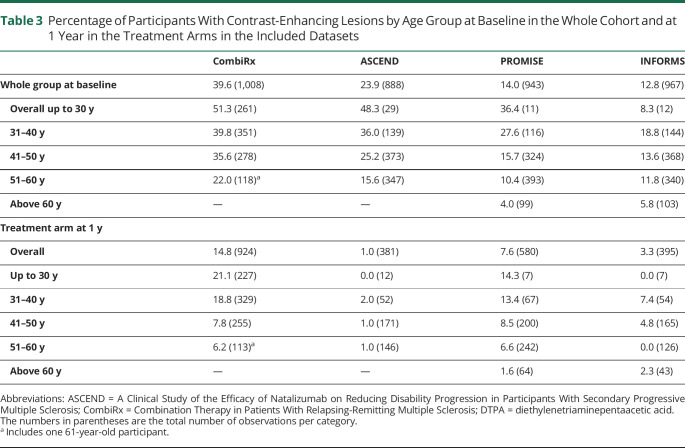
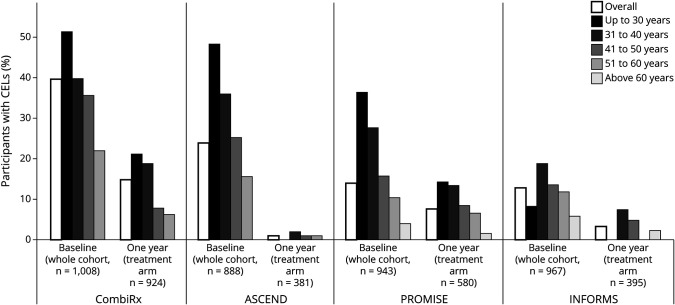
CEL occurrence differed between datasets according to disease course, with 39.6% of CombiRx participants having CELs, followed by ASCEND participants with 23.9%, and PROMISE and INFORMS participants with 14.0% and 12.8%. This distribution by disease course was largely preserved within each age group (Table 3 and Figure). In all datasets, there was an almost linear decrease of the percentage of participants with CELs with advancing age (Table 3 and Figure).
Table 3.
Percentage of Participants With Contrast-Enhancing Lesions by Age Group at Baseline in the Whole Cohort and at 1 Year in the Treatment Arms in the Included Datasets
Figure. Percentage of Trial Participants With Contrast-Enhancing Lesions (CELs) by Age Group.
Percentage of trial participants with CELs by age group at baseline in the 4 included trial datasets. The percentage of individuals with CELs decreases as a function of age and disease course both at baseline and after 1 year of treatment.
CELs in the Treatment Arms at 1 Year
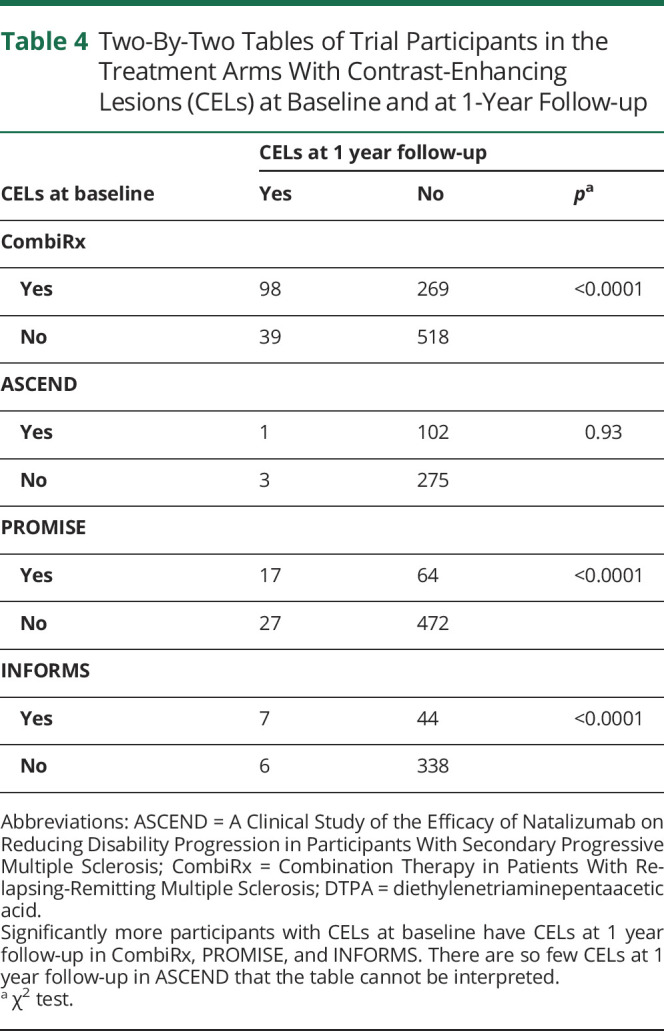
After 1 year of experimental treatment, CEL occurrence was meaningfully reduced in all trial datasets. As expected, this reduction was less pronounced in CombiRx and PROMISE, which used the platform treatments interferon-β and glatiramer acetate, and in which the percentage of CEL occurrence was reduced to about half of the baseline percentage after 1 year of treatment (CombiRx: from baseline overall 39.6% to 14.8% at 1 year in the treatment arm; PROMISE: from 14.0% to 7.6%) (Table 3 and Figure). After 1 year of fingolimod treatment in INFORMS, CELs were reduced to about a quarter of their baseline frequency (from 12.8% to 3.3%), and 1 year of natalizumab treatment almost abolished CELs in ASCEND (from 23.9% to 1%) (Table 3 and Figure). The almost linear decrease of CEL occurrence with advancing age, however, was preserved in CombiRx, PROMISE, and INFORMS after 1 year of treatment. Table 4 shows the resulting 2 × 2 tables for CELs at baseline and 1 year follow-up for the treatment arms of the trials. Significantly more trial participants with CELs at baseline remained CEL positive at 1 year follow-up compared to those without CELs at baseline in CombiRx (with 27% of participants with CELs at baseline also having CELs at follow-up, compared to only 7% of participants without CELs at baseline being CEL positive at follow up, χ2 p < 0.0001), PROMISE (21% vs 5%, p < 0.0001), and INFORMS (14% vs 2%, p < 0.0001). In ASCEND, there were so few CELs at 1 year follow-up that the 2 × 2 table could not be interpreted (Table 4).
Table 4.
Two-By-Two Tables of Trial Participants in the Treatment Arms With Contrast-Enhancing Lesions (CELs) at Baseline and at 1-Year Follow-up
CELs and Baseline Characteristics
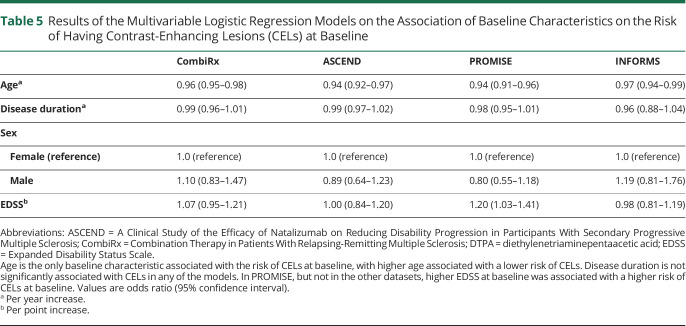
Table 5 shows the results of the multivariable logistic regression models on the association of baseline characteristics and CEL occurrence. Age was the only baseline characteristic associated with the risk of CELs at baseline in all datasets, with higher age associated with a lower risk of CELs. The strength of the association of age and CEL occurrence was similar between trial datasets, with odds ratios of between 0.94 and 0.96 per year increasing age and comparable 95% confidence intervals between trial datasets (Table 5). Disease duration was not significantly associated with CELs and there was no significant interaction between age and disease duration in any of the datasets. In PROMISE, but not in the other datasets, higher EDSS at baseline was associated with a higher risk of CELs at baseline (odds ratio per EDSS point increase 1.20, 95% confidence interval 1.03 to 1.41).
Table 5.
Results of the Multivariable Logistic Regression Models on the Association of Baseline Characteristics on the Risk of Having Contrast-Enhancing Lesions (CELs) at Baseline
Discussion
Our investigation into 4 large phase 3 RCT datasets shows that the age dependence of CELs is a general phenomenon across the spectrum of disease courses in MS. The observation that CELs occurrence decreases with age was previously described in several observational and interventional studies, including an observational study of 200 untreated people with RRMS and SPMS,4 a cohort including 1,543 individuals with all forms of MS and clinically isolated syndrome,5 and in the linomide in RRMS and SPMS trial.2 Two studies using triple-dose gadolinium contrast in 45 people showed that CELs decrease with time in PPMS, from 32% at baseline15 to 26% 5 years later.16 Our investigation confirms the observation that CELs are also age-dependent in PPMS, and adds that the effect of age on CEL occurrence is preserved in treated patients.
In the ASCEND dataset, we see a very powerful treatment effect of natalizumab in SPMS, which almost abolishes CELs 1 year after treatment. It should be kept in mind, however, that ASCEND was a negative trial and that natalizumab treatment, despite its impressive effect on CEL occurrence, could not prevent the steady functional decline the participants experienced in this trial over the observation period.8 This lacking effect on functional outcomes despite a powerful effect on CELs supports the idea that disease progression in SPMS is driven by a pathophysiology that is separate from focal inflammatory demyelination. The hypotheses about the nature of this pathophysiology range from a different form of inflammation17 to the idea that MS may be primarily a neurodegenerative disease in which inflammation is an early reaction to neurodegenerative processes.18
In the CombiRx dataset we see the natural decrease of inflammatory disease activity in RRMS as a function of age, even though the inclusion criteria of this trial selected for patients with comparatively active RRMS, with at least 2 demyelinating events (2 relapses or 1 relapse and new MRI activity in the 3 years before inclusion). Furthermore, it recruited previously treatment naive participants so that the CEL positivity in the higher age groups may also indicate a more benign preexisting disease course in the older participants, since they were recruited in their 40s or 50s without prior exposure to immunomodulatory drugs. Nevertheless, the almost linear relation between age and CEL occurrence is preserved after 1 year of treatment with the platform therapies interferon-β, glatiramer acetate, or their combination. The effect of aging on disease activity can also be seen in relapse frequency measured in clinical practice. A study in 2,477 people with RRMS in the British Columbia Natural History Cohort showed that annualized relapse rates decline with age.6
In our study, disease duration was not significantly associated with CELs at baseline in any of the trial datasets, nor did we find a significant interaction between age and disease duration for the risk of having CELs. These findings contrast the investigation of factors associated with gadolinium enhancement in the linomide in RRMS and SPMS trial, where disease duration as well as age were associated with having CELs.2 The previously mentioned study on relapse frequency in the British Columbia Natural History Cohort also found that disease duration was associated with decreasing relapse frequency in addition to age, and across age groups.6
Taken together, these investigations suggest that there is a natural “mellowing” of focal inflammatory disease activity with age across the MS disease spectrum. There is some support for this idea from an autopsy study investigating the characteristics of demyelinating white matter lesions in 120 individuals with different MS disease courses.19 This study showed that “active” white matter lesions dominate in younger individuals and make up around 80% of demyelinating lesions at age 20, but decline almost linearly to around 20% at age 60. In older individuals, “inactive” and “smoldering” lesions form the majority of white matter lesions.19 It has been speculated that this reduction in active focal inflammatory demyelination is due to a shift from adaptive T- and B-cell mediated immunity underlying active focal inflammation to innate immunity resulting in chronic inflammation and microglial activation.20 For RRMS, this suggests that younger patients may benefit from a more aggressive treatment approach. On the other hand, it also suggests that older patients may naturally age out of the risk of highly active disease, and that immunomodulatory treatment focused on focal inflammation may be less beneficial with advancing age. Weideman et al.21 examined the effect of immunomodulatory drug treatment on disability progression in a meta-analysis of 38 clinical trials in MS and found that the efficacy of such treatment decreases with age, to the point where it may not reduce disability progression in participants above age 53. Based on these findings, several RCTs are underway to investigate whether immunomodulatory drugs can be safely discontinued in older patients or in patients without disease activity for a prolonged period. In the DISCOMS trial (Discontinuation of Disease Modifying Therapies in Multiple Sclerosis; clinicaltrials.gov identifier NCT03073603), participants are randomized to either remain on their current treatment or to discontinue it, and are then followed clinically and with cranial MRIs every 6 months to determine differences in clinical and radiologic disease activity. Other trials following a similar design are the STOP-I-SEP trial (Disease Modifying Therapies Withdrawal in Inactive Secondary Progressive Multiple Sclerosis Patients Older Than 50 Years; clinicaltrials.gov identifier NCT03653273) and the DOT-MS trial (Discontinuing Disease-modifying Therapies in Stable Relapsing-Onset Multiple Sclerosis; clinicaltrials.gov identifier NCT04260711). These trials will contribute to a better understanding of age as a determinant of inflammatory disease activity and treatment response in different forms of MS. Similarly, the effect of age demonstrated in our study could also be used to enrich a trial cohort, for example by including younger patients for a phase 2 study of a treatment for active inflammatory demyelination, or by deliberately including older participants for trials targeting smoldering lesions or chronic microglial activation.
Our study has several limitations. While our investigation shows that the effect of aging on focal inflammatory demyelination is present in all MS disease courses, we caution against comparing between these datasets, and against generalizing the findings from these datasets to the general clinical population of people with MS. Clinical trials always include a selected subgroup of the real-world population of people with MS, and are often enriched for activity to increase or enhance the occurrence of outcome events. Our findings should be replicated in real-world datasets, ideally in population-based cohorts including people with all subtypes of MS.
Author Statement
The principal author takes full responsibility for the data, the analyses and interpretation, and the conduct of the research; the principal author had full access to all of the data and has the right to publish any and all data separate and apart from any sponsor.
Acknowledgment
The CombiRx trial (clinicaltrials.gov identifier NCT00211887), one of the data sources used in this study, was funded by the NIH National Institute of Neurological Disorders and Stroke phase III study grant UO1NS045719.
Glossary
- ASCEND
A Clinical Study of the Efficacy of Natalizumab on Reducing Disability Progression in Participants With Secondary Progressive Multiple Sclerosis
- CEL
contrast-enhancing lesion
- CombiRx
Combination Therapy in Patients With Relapsing-Remitting Multiple Sclerosis
- EDSS
Expanded Disability Status Scale
- MS
multiple sclerosis
- PPMS
primary progressive multiple sclerosis
- RCT
randomized controlled trial
- RRMS
relapsing-remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
Appendix. Authors
Footnotes
Infographic: http://links.lww.com/WNL/B508
Study Funding
No targeted funding reported.
Disclosure
M.W. Koch received consulting fees and travel support from Biogen, Novartis, Roche, Sanofi Genzyme, and EMD Serono. J. Mostert and Y. Zhang report no disclosures. J.S. Wolinsky received compensation for consulting, scientific advisory boards, or other activities with Acorda Therapeutics, Actelion, Alkermes, Avotres, Brainstorm Cell Therapeutics, EMD Serono, GeNeuro, GW Pharma, MedDay Pharmaceuticals, NervGen Pharma Corp, Novartis, Roche/Genentech, Sanofi Genzyme, University of Alabama, and University of Miami; royalties are received through UTHealth for outlicensed monoclonal antibodies to Millipore (Chemicon International) Corporation. F.D. Lublin received honoraria from consulting agreements and from serving on scientific advisory boards and data safety and monitoring boards and speaker's bureau of Biogen, EMD Serono, Novartis, Teva, Actelion/Janssen, Sanofi/Genzyme, Acorda, Roche/Genentech, MedImmune/Viela Bio, Receptos/Celgene/BMS, TG Therapeutics, MedDay, Atara Biotherapeutics, Mapi Pharma, Innate Immunotherapeutics, Apitope, Orion Biotechnology, Brainstorm Cell Therapeutics, Jazz Pharmaceuticals, GW Pharma, Mylan, Immunic, Population Council, Avotres, and Neurogene; received research funding from Novartis, Actelion, Biogen, Sanofi, NMSS, NIH, and Brainstorm Cell Therapeutics; and holds stock options of Avotres. E.M.M. Strijbis reports no disclosures. G. Cutter served on data and safety monitoring boards for Astra-Zeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Mapi Pharmaceuticals LTD, Merck, Merck/Pfizer, Opko Biologics, OncoImmune, Neurim, Novartis, Ophazyme, Sanofi-Aventis, Reata Pharmaceuticals, Teva Pharmaceuticals, VielaBio Inc, Vivus, NHLBI (Protocol Review Committee), and NICHD (OPRU oversight committee); consulting or advisory boards for Biodelivery Sciences International, Biogen, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Immunic, Klein-Buendel Incorporated, Medimmune, MedDay, Neurogenesis LTD, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion/Cerexis Pharmaceuticals, Roche, and TG Therapeutics; is employed by the University of Alabama at Birmingham and is President of Pythagoras, Inc., a private consulting company located in Birmingham. Go to Neurology.org/N for full disclosures.
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolinsky JS, Narayana PA, Noseworthy JH, et al. Linomide in relapsing and secondary progressive MS: part II: MRI results: MRI analysis center of the University of Texas-Houston, Health science Center, and the North American linomide investigators. Neurology. 2000;54(9):1734-1741. [DOI] [PubMed] [Google Scholar]
- 3.Barkhof F, Held U, Simon JH, et al. Predicting gadolinium enhancement status in MS patients eligible for randomized clinical trials. Neurology. 2005;65(9):1447-1454. [DOI] [PubMed] [Google Scholar]
- 4.Tortorella C, Bellacosa A, Paolicelli D, et al. Age-related gadolinium-enhancement of MRI brain lesions in multiple sclerosis. J Neurol Sci. 2005;239(1):95-99. [DOI] [PubMed] [Google Scholar]
- 5.Koch MW, Mostert J, Greenfield J, Liu W-Q, Metz L. Gadolinium enhancement on cranial MRI in multiple sclerosis is age dependent. J Neurol. 2020;267(9):2619-2624. [DOI] [PubMed] [Google Scholar]
- 6.Tremlett H, Zhao Y, Joseph J, Devonshire V; UBCMS Clinic Neurologists. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79(12):1368-1374. [DOI] [PubMed] [Google Scholar]
- 7.Lublin FD, Cofield SS, Cutter GR, et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol. 2013;73(3):327-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor R, Ho P-R, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415. [DOI] [PubMed] [Google Scholar]
- 9.Wolinsky JS, Narayana PA, O'Connor P, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61(1):14-24. [DOI] [PubMed] [Google Scholar]
- 10.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075-1084. [DOI] [PubMed] [Google Scholar]
- 11.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231. [DOI] [PubMed] [Google Scholar]
- 12.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50(1):121-127. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. R: A Language and Environment for Statistical Computing [online]. R Foundation for Statistical Computing; 2021. R-project.org/. [Google Scholar]
- 15.Ingle GT, Sastre-Garriga J, Miller DH, Thompson AJ. Is inflammation important in early PPMS? A longitudinal MRI study. J Neurol Neurosurg Psychiatry. 2005;76(9):1255-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khaleeli Z, Ciccarelli O, Mizskiel K, Altmann D, Miller DH, Thompson AJ. Lesion enhancement diminishes with time in primary progressive multiple sclerosis. Mult Scler. 2010;16(3):317-324. [DOI] [PubMed] [Google Scholar]
- 17.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647-656. [DOI] [PubMed] [Google Scholar]
- 18.Stys PK, Zamponi GW, van Minnen J, Geurts JJG. Will the real multiple sclerosis please stand up? Nat Rev Neurosci. 2012;13(7):507-514. [DOI] [PubMed] [Google Scholar]
- 19.Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78(5):710-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiner HL. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J Neurol. 2008;255(suppl 1):3-11. [DOI] [PubMed] [Google Scholar]
- 21.Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
CombiRx
Access to the CombiRx dataset can be requested from the Coordinating Center or MS Center at the University of Alabama at Birmingham by completing a data use agreement that is reviewed by a committee overseeing the use of the data. Qualified researchers have or will obtain appropriate institutional review board approval for the study request. Depending on the complexity of the request, researchers may need to cover the cost of producing the deidentified data.
ASCEND
The ASCEND dataset is available upon request from Biogen. Individual participant data collected during the trial will be shared after anonymization and on approval of a research proposal and data sharing agreement. Research proposals can be submitted online (biogenclinicaldatarequest.com).
PROMISE
The PROMISE dataset was acquired through TEVA Pharmaceutical's Data Sharing and Transparency office after agreeing on a research proposal and data sharing agreement. It is available to other qualified researchers upon request from TEVA Pharmaceuticals.
INFORMS
The INFORMS dataset is available upon request from Novartis. Individual participant data collected during the trial will be shared after anonymization and on approval of a research proposal and data sharing agreement. Research proposals can be submitted online (clinicalstudydatarequest.com).