Abstract
Background:
Tourette syndrome (TS) is a neurodevelopmental disorder involving chronic motor and phonic tics. Most individuals with Tourette syndrome can suppress their tics for at least a short period of time. Yet, the brain correlates of tic suppression are still poorly understood.
Methods:
In the current study, high-density electroencephalography (EEG) was recorded during a resting-state and a tic suppression session in 72 children with TS. Functional connectivity between cortical regions was assessed in the alpha band (8-13 Hz) using an EEG source connectivity method. Graph theory and network-based statistics were used to assess the global network topology and to identify brain regions showing increased connectivity during tic suppression.
Results:
Graph theoretical analyses revealed distinctive global network topology during tic suppression, relative to rest. Using network-based statistics, we found a subnetwork of increased connectivity during tic suppression (p < .001). That subnetwork encompassed many cortical areas, including the right superior frontal gyrus and the left precuneus, which are involved in the default mode network. We also found a condition by age interaction, suggesting age-mediated increases in connectivity during tic suppression.
Conclusions:
These results suggest that children with TS suppress their tics through a brain circuit involving distributed cortical regions, many of which are part of the default mode network. Brain connectivity during tic suppression also increases as youths with TS mature. These results highlight a mechanism by which children with TS may control their tics, which could be relevant for future treatment studies.
Introduction
Tourette syndrome (TS) is a neurodevelopmental disorder characterized by chronic motor and phonic tics (1). While tics are presumed to be automatic, they can be suppressed for short periods, even in children (2–4). Tics follow a somatotopic organization: body parts with large representations in sensorimotor areas, such as the face or the hands, are more affected by tics (5). Voluntary tic suppression follows an inverse pattern: tics are more easily suppressed in body parts that are less affected by tics, suggesting that tic suppression has no direct impact on a global tic generation mechanism, but attenuates the output of this mechanism by suppressing tics in less affected body areas (5).
Functional magnetic resonance imaging (fMRI) studies reported several regions within the cortico-striato-thalamo-cortical circuitry that were preferentially activated or deactivated during tic suppression. The first fMRI study of tic suppression found decreased activations in the thalamus, putamen, and globus pallidus, and increased activation in the temporal gyrus, right anterior cingulate cortex (ACC) and right frontal cortex (6). Following studies reported tic suppression-related activation increases in the left inferior frontal gyrus (IFG; 7), as well as in the bilateral middle and right lateral temporal cortices, right anterior and dorsolateral prefrontal (dlPFC) cortices, bilateral superior frontal gyrus (SFG)/dorsomedial PFC (dmPFC), right inferior occipital gyrus and right inferior parietal cortex (IPC; 8). Additionally, compared to healthy controls suppressing eyeblinks, the right anterior PFC, right dlPFC, right ACC, and left superior frontal and premotor cortices were more activated in adults with TS during tic suppression (8). However, these studies had small samples, only included adults, and have not assessed brain connectivity during tic suppression.
Regarding presumed brain mechanisms of tic suppression, parallels have been drawn between tic suppression and response inhibition (9), implicating a response inhibition network consisting of the right IFG, pre-supplementary motor area, and subthalamic nucleus (10). These regions partly overlap with some regions identified by tic suppression studies, particularly in the prefrontal cortex. Other brain regions activated during tic suppression, such as the dmPFC and IPC, are also part of the default mode network (DMN; 11, 12). This network is usually activated while individuals are at rest or performing an internally focused task, and deactivated when focusing on the external world (13–15). Tic suppression could constitute a good example of an internally focused task. Along these lines, involvement of the DMN in tic suppression has been suggested previously (16) but remains to be formally investigated.
In this study, we use electroencephalography (EEG), which can provide valuable information regarding brain processes involved in movement control and inhibition. Thus, in healthy children, eyeblink inhibition has been found to be associated with increased activity in the left dlPFC (17). Furthermore, an EEG study of cued eyeblinks in children with TS reported increased connectivity from occipital to frontal and motor areas (18). The first study of the connectivity dynamics of tic suppression reported increased alpha coherence between prefrontal and sensorimotor areas in 9 adults with TS (9). Another tic suppression study conducted in 9 children with TS reported increased EEG coherence from frontal electrodes to electrodes spanning the contralateral motor areas (19). These two studies suggest increased cortical connectivity during tic suppression but are limited by small samples and poor spatial resolution due to small number of electrodes (10 & 18). Sensor-level EEG analyses also limit the interpretation of the brain regions involved (20, 21), a shortcoming that can be overcome by dense-array EEG data collection and source connectivity analysis (22).
Regarding data-analytic tools, connectivity studies may benefit from graph theory, allowing for characterization of a network’s global topology (i.e. network organization) and local connectivity patterns (23). Graph theory conceptualizes brain networks as multiple nodes (i.e. brain regions) connected together by one or multiple edges (24) and allows for assessment of the segregation and integration properties of a network. Segregation, within a network, refers to the ability of brain regions to form densely connected clusters, whereas integration refers to the ability of brain regions to easily communicate with one another (23, 25). To date, only two studies applied graph theory in children with TS. In one study, compared to controls, children with TS were found to have decreased connectivity degree, local and global efficiency, and clustering coefficient, as well as increased characteristic path length (26). Similarly, in a second study, decreased local efficiency and clustering coefficients were reported for the DMN, and were negatively associated with tic severity (27).
The current study aimed at understanding the brain circuits involved in tic suppression, using EEG source connectivity. Following Serrien et al. (9) and given that alpha oscillations show high coherence over large distances (28), analyses were focused on the alpha band. First, we aimed to investigate the global topology of whole brain connectivity during tic suppression using graph theory analyses. Since tic severity was shown to be negatively associated with local efficiency and clustering coefficient (27), we hypothesized that tic suppression would lead to altered topological network organization in comparison to rest, reflecting increased integration and segregation. Second, we aimed to use network-based statistics to identify a subnetwork of brain regions showing increased connectivity during tic suppression. According to prior EEG studies of tic suppression, we hypothesized that tic suppression would lead to increased connectivity. If tic suppression recruits the response inhibition network, we could expect the right IFG and motor areas to be part of that subnetwork. If, however, tic suppression recruits the DMN, we could expect increased connectivity between the precuneus, the dmPFC, the posterior cingulate cortex (PCC), and parietal areas. Third, we aimed to assess whether tic severity impacted brain connectivity during tic suppression. Since tic frequency is positively associated with tic suppressibility (4), we hypothesized that children with more severe tics would show increased connectivity during tic suppression. Given the detrimental impact of ADHD on inhibitory functions in TS (29), we also investigated the association between ADHD symptomatology and brain circuits during tic suppression.
Methods and Materials
Participants
Seventy-seven children with TS aged 8–16 years old participated in this study. Participants were recruited through the Yale TS/OCD specialty clinic and the Tourette Association of America’s Connecticut chapter. Criteria for inclusion/exclusion are presented in the Supplement. Since five children had less than 20 non-artifacted EEG epochs in either the tic suppression or the rest condition (see Supplement), the final sample comprised 72 children. Demographic and clinical characteristics are reported in Table 1.
Table 1.
Demographic and clinical characteristics of study participants
| Age in years, mean (SD) | 11.3 (1.9) |
| Sex, number (%) | |
| Boys | 64 (88.9%) |
| Girls | 8 (11.1%) |
| Handedness, number (%) | |
| Right-handed | 60 (83.3%) |
| Left-handed | 9 (12.5%) |
| Ambidextrous | 3 (4.2%) |
| Race, numbera (%) | |
| White | 62 (87.3%) |
| Black | 5 (7.0%) |
| Asian | 4 (5.6%) |
| Ethnicity, numberb (%) | |
| Hispanic | 3 (4.3%) |
| Non-Hispanic | 67 (95.7%) |
| Full Scale IQ, mean (SD) | 112.9 (15.5) |
| Clinical scores, mean (SD) | |
| YGTSS total tic score | 24.0 (7.8) |
| Motor tic subscale | 14.2 (3.4) |
| Phonic tic subscale | 9.8 (5.9) |
| SNAP-IV | 15.2 (11.2) |
| DBRS | 6.0 (5.1) |
| SCARED | 12.6 (11.3) |
| CBCL Anxious/Depressed scale T-score | 56.8 (7.5) |
| CBCL Withdrawn/Depressed scale T-score | 54.9 (7.0) |
| Comorbid diagnoses, number (%) | |
| ADHD | 26 (36.1%) |
| OCD | 10 (13.9%) |
| ODD | 10 (13.9%) |
| Anxiety disorder | 12 (16.7%) |
| Depressive disorder | 6 (8.3%) |
| Psychotherapy/counseling, numberc (%) | |
| Concomitant psychotherapy/counseling | 11 (15.9%) |
| Past psychotherapy/counseling | 23 (33.3%) |
| Medication status, numberd (%) | |
| Not taking medication | 40 (56.3%) |
| On psychotropic medication | 31 (43.7%) |
| Stimulantse | 2 (2.9%) |
| α-Agonistsf | 19 (26.8%) |
| Atomoxetine | 3 (4.3%) |
| Antipsychoticsg | 11 (15.9%) |
| SSRIsh | 8 (11.6%) |
| Otheri | 2 (2.9%) |
Note: ADHD: attention deficit hyperactivity disorder, CBCL: Child Behavior Checklist, DBRS: Disruptive Behavior Rating Scale, OCD: obsessive-compulsive disorder, ODD: oppositional defiant disorder, SCARED: Screen for Child Anxiety Related Disorders, SD: standard deviation, SNAP-IV: Swanson, Nolan and Pelham Questionnaire for ADHD, SSRI: selective serotonin reuptake inhibitors, YGTSS: Yale Global Tic Severity Scale.
One participant with missing data.
Two participants with missing data.
Three participants with missing data.
Three participants with missing data.
Stimulant medications included methylphenidate (n = 2).
α-Agonists included guanfacine (n = 15) and Clonidine (n = 4)
Antipsychotics included risperidone (n = 8), haloperidol (n = 2), aripiprazole (n = 1), and quetiapine (n = 1).
SSRIs included Citalopram (n = 2), fluvoxamine (n = 2), sertraline (n = 2), escitalopram (n = 1), and fluoxetine (n = 1).
Other medications included benztropine (n = 1) and gabapentin (n = 1).
This study was approved by the local institutional review board and conducted in accordance with the Declaration of Helsinki. Informed consent and assent were respectively obtained from parents and children.
Procedures
Clinical assessment
An expert clinician conducted a semi-structured interview (K-SADS; 30) to assess psychiatric diagnoses. Tic severity, ADHD symptoms, anxiety, and disruptive behaviors were respectively assessed with the Yale Global Tic Severity Scale (YGTSS; 31), the Swanson, Nolan and Pelham Questionnaire (SNAP-IV; 32), the Screen for Child Anxiety Related Disorders (SCARED; 33), and the Disruptive Behavior Rating Scale (DBRS; 34). Best estimate DSM-IV-TR diagnoses of TS and concomitant disorders were assigned using structured interviews and clinical ratings (35). See the Supplement for complete details.
Tic suppression and rest sessions
Following the earlier EEG study of tic suppression (9), EEG was recorded during three 2-minute tic suppression sessions during which children were asked to suppress all tics. These sessions were contrasted with a 7-minute resting-state session during which children were asked not to try to suppress their tics. In each session, they were asked to keep their eyes open while looking at the computer screen. Further details are provided in the Supplement.
EEG recordings and signal processing
EEG recordings
EEG was continuously recorded during both conditions with a 128-channel HydroCel Geodesic Sensor Net, a Net Amps 200 amplifier, and Net Station Acquisition software version 4.2.1 (EGI, Inc.). The net was soaked in a potassium chloride solution and electrode impedance was assessed at or under 40 kΩ prior to data collection. Data were online filtered with a 0.01 Hz high-pass filter and a 100 Hz low-pass filter at 250 Hz sampling rate. EEG signal was referenced to the vertex electrode (Cz) during recordings (36, 37).
EEG pre-processing
The Maryland Analysis of Developmental EEG pipeline (38), running on Matlab R2020a, was used for preprocessing. This pipeline uses EEGLAB’s (39) functions and data structure and was designed specifically to preprocess EEG signals in youth. Preprocessing steps involved filtering, artifact removal through independent component analysis (ICA) and threshold-based rejection, removal and interpolation of bad channels, segmentation of continuous EEG in 2-second epochs, and re-referencing. Complete details are provided in the Supplement.
Source-based connectivity pipeline
Sources were reconstructed in Brainstorm (40) using weighted minimum norm imaging (wMNE) and were projected onto the Desikan-Killiany atlas, consisting of 34 regions of interests (ROI) per hemisphere (41). The phase-locking value (PLV) was used as a measure of connectivity and was computed in Brainstorm. PLV values range from 0 to 1 and reflect the instantaneous phase difference of two signals (42). Larger PLV values indicate stronger coupling between the phases of two signals (43). The combination of wMNE and PLV was shown to outperform other methods for EEG source-reconstructed connectivity (22, 44). The PLV was extracted using a Hilbert transform in the alpha band (8–13 Hz). Supplementary analyses were performed in neighboring frequency bands (theta (4–8 Hz) and beta (13–30 Hz)). The PLV was computed for each epoch and averaged by condition, yielding two 68X68 connectivity matrices per individual. Each row/column of these matrices is considered as a node, and the association between two nodes is designed as an edge. Further details are provided in the supplement.
Graph theoretical analyses
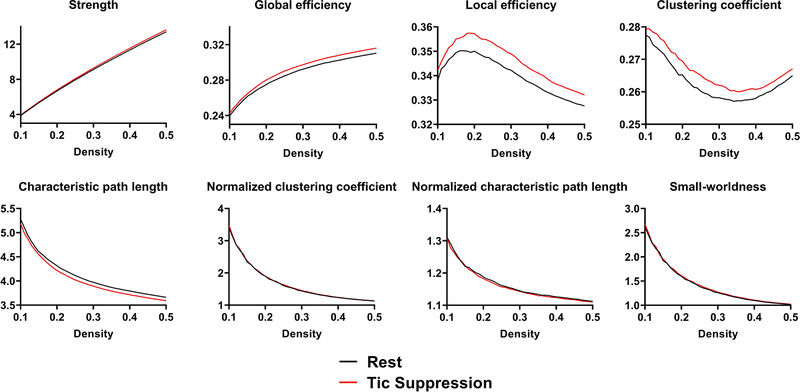
Graph theory analyses were performed in two steps, using the Brain Connectivity Toolbox (23). In a first step, eight metrics related to global network topology were computed from thresholded weighted matrices, over 41 sparsity thresholds (45, 46). These metrics were network strength, clustering coefficient, characteristic path length, global efficiency, local efficiency, normalized clustering coefficient, normalized characteristic path length, and small-worldness. Following previous work (47), we also computed the following graph theory metrics pertaining to individual nodes: node strength, nodal clustering coefficient, and nodal efficiency. Resulting graph theory metrics can be plotted as a function of graph density (see Figure 1). We computed the area under the curve for each metric, yielding a single measure per condition/child for global network analyses, and one measure per node/condition/child for nodal analyses. In a second step, graph theory analyses were repeated on the NBS subnetwork. Further details are provided in the Supplement.
Figure 1: Global network topology.
Each graph theory metrics was computed over a range of graph density (between 10% and 50%, in increments of 1%). For our analyses, we computed the area under the curve of the tic suppression and rest condition. We then used the area under the curve to compare both conditions. Significant differences between conditions were found for characteristic path length (p = .0003) and global efficiency (p = .004).
Data analyses
Network topology
To assess differences between rest and tic suppression, we conducted paired t-tests on the area under the curve of the graph theory metrics pertaining to global network topology. A Bonferroni-corrected threshold of α = .05 / 8 = .00625 was used. Effect sizes were assessed with Cohen’s d.
Nodal properties
Paired t-tests comparing both conditions were also conducted on the area under the curve of the graph theory metrics indexing nodal properties. Here, our significance threshold was adjusted for the number of nodes (α = .05 / 68 = .0007).
Network-based statistics
The NBS toolbox (48) was used to identify a subnetwork involved in tic suppression. A detailed description of NBS is provided in the Supplement. Network visualization was performed with BrainNet Viewer (49), Cytoscape (50), and aMatReader (51). To ascertain our findings, analyses were also performed in adjacent theta and beta bands (see Supplementary results). Connectivity degree (number of connections per node) was assessed to find hubs in subnetworks identified with NBS. Hubs are nodes that show high connectivity with multiple nodes and play a central role in a network (52). Nodes were considered as hubs if their degree z-score was > 1 (53).
Association with demographic and clinical measures
We performed several exploratory analyses in NBS to test whether continuous variables (age, ADHD symptoms (SNAP-IV total score), tic severity (YGTSS total tic score, motor and phonic subscales)) or categorical variables (ADHD (yes/no), OCD (yes/no), tic severity (high/low median split) and medication (yes/no)) interacted with the condition factor (tic suppression/rest). For these analyses, we used a more liberal threshold (t = 3.21, (corresponding to α = .001)). Given the possible mediation of response to Comprehensive Behavioral Intervention for Tics (CBIT) relative to Psychoeducation and Supportive Therapy (54), we additionally tested whether alpha-agonists interacted with the condition factor.
Results
Global network topology
Graph theory analyses conducted on thresholded connectivity matrices revealed smaller characteristic path length [t(71) = 3.82, p = .0003, d = .20] and larger global efficiency [t(71) = −3.00, p = .004, d = .14] during tic suppression, relative to rest. The condition difference for global network strength [t(71) = −2.69, p = .009, d = .13] and local efficiency [t(71) = −2.16, p = .034, d = .12] did not reach the Bonferroni-corrected threshold. No significant condition effect was found for clustering coefficient, normalized clustering coefficient, normalized characteristic path length, and small-worldness [all t-values < |1.3|, all p-values > .21] (see Figure 1).
Nodal properties
Analyses of nodal properties did not reveal condition effects reaching the Bonferroni-corrected threshold of .0007 [all t-values < |3.3|, all p-values > .001].
Network-based statistics
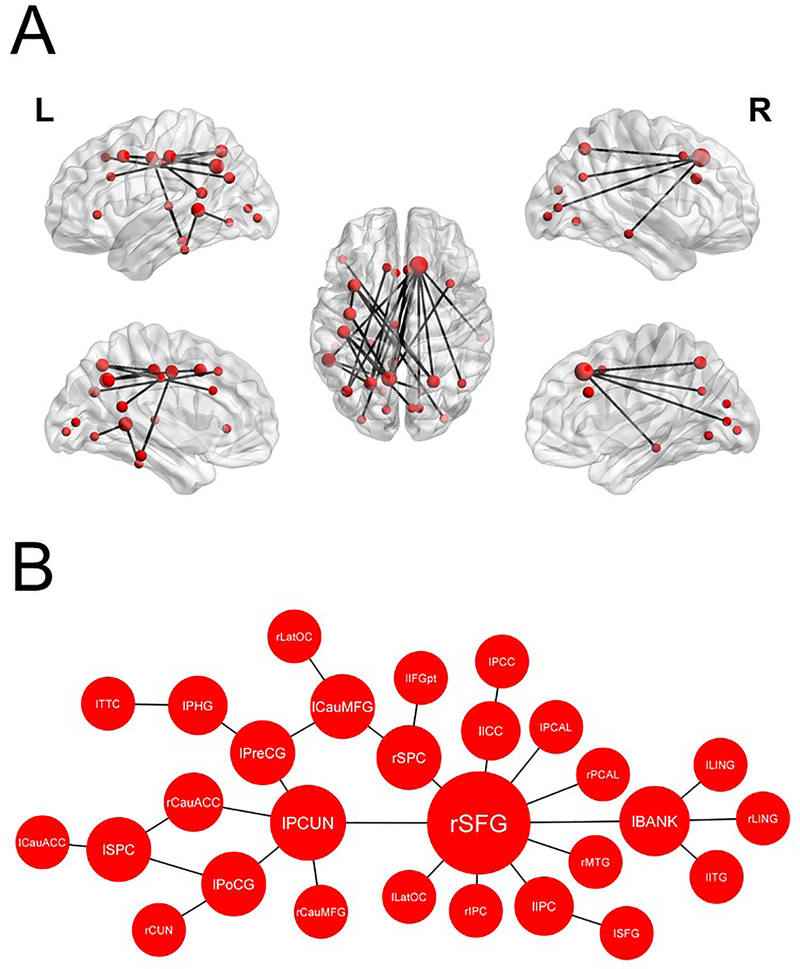
NBS revealed a subnetwork of increased connectivity in the alpha frequency band during tic suppression relative to rest [p < .001, FWER-corrected], which included 28 nodes and 29 edges (see Table S1 for list of edges). Of note, the right SFG (10 connections, degree z-score: 4.2), the left precuneus (5 connections, degree z-score: 1.5), and the left banks of superior temporal sulcus (4 connections, degree z-score: 1.01) were considered as hubs in that network (see Figure 2). Another smaller subnetwork involving 4 nodes and 3 edges also reached the significance threshold [p = .037, FWER-corrected] (see Figure S1). Subnetworks showing increased connectivity during tic suppression were also found in theta (Figure S2) and beta (Figure S3) bands.
Figure 2: Subnetwork of functional connectivity in the alpha band during tic suppression.
Nodes depicted in this figure showed increased connectivity during tic suppression in comparison to rest. Node size corresponds to connectivity degree (number of connections per node). (A) Hubs in this network were the right superior frontal gyrus (10 connections, degree z-score: 4.2), the left precuneus (5 connections, degree z-score: 1.5), and the left banks of superior temporal sulcus (4 connections, degree z-score: 1.01). (B) Graphical representation of the alpha-band subnetwork. Note: “l” & “r” denotes the lateralization of the nodes; CauACC: caudal anterior cingulate cortex, CauMFG: caudal middle frontal gyrus, CUN: cuneus, ICC: isthmus of the cingulate cortex, IFGpt: pars triangularis of the inferior frontal gyrus, IPC: inferior parietal cortex, ITG: inferior temporal gyrus, LatOC: lateral occipital cortex, LING: lingual gyrus, MTG: middle temporal gyrus, PCAL: pericalcarine cortex, PCC: posterior cingulate cortex, PCUN: precuneus, PHG: parahippocampal gyrus, PoCG: postcentral gyrus, PreCG: precentral gyrus, SFG: superior frontal gyrus, SPC: superior parietal cortex, TTC: transverse temporal cortex.
Topology of the NBS subnetwork
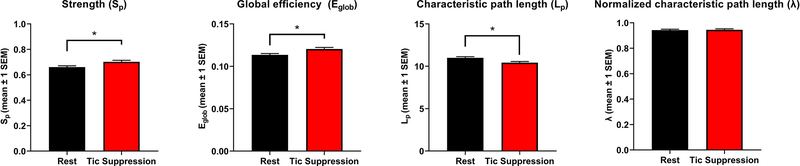
Graph theory analyses were also performed on the NBS subnetwork. Since triplets of nodes were not found within this subnetwork, the clustering coefficient, local efficiency, normalized clustering coefficient, and small-worldness could not be assessed. The subnetwork showed increased strength [t(71) = −.8.02, p < .001, d = .44], smaller characteristic path length [t(71) = 7.26, p < .001, d = .47], and larger global efficiency [t(71) = −7.39, p < .001, d = .45] during tic suppression. There was however no condition effect regarding the normalized characteristic path length (Figure 3).
Figure 3: Topology of the NBS subnetwork.
Graph theory analyses were repeated on the NBS subnetwork. Significant differences between conditions were observed for network strength, global efficiency, and characteristic path length. The asterisk shows significant differences between conditions. Error bars represent the standard error of the mean.
Nodal properties of the NBS subnetwork
Graph theoretical analyses of nodal properties were also conducted in the subnetwork identified by NBS. As mentioned above, it was only possible to conduct these analyses on node strength. Since 25 nodes were identified in the NBS subnetwork, we used a Bonferroni-corrected threshold of α = .05 / 25 = .002. In that subnetwork, all nodes showed larger strength during tic suppression compared to rest [all t-values > 3.4, all p-values < .001] (Figure S4).
Association with demographic and clinical measures
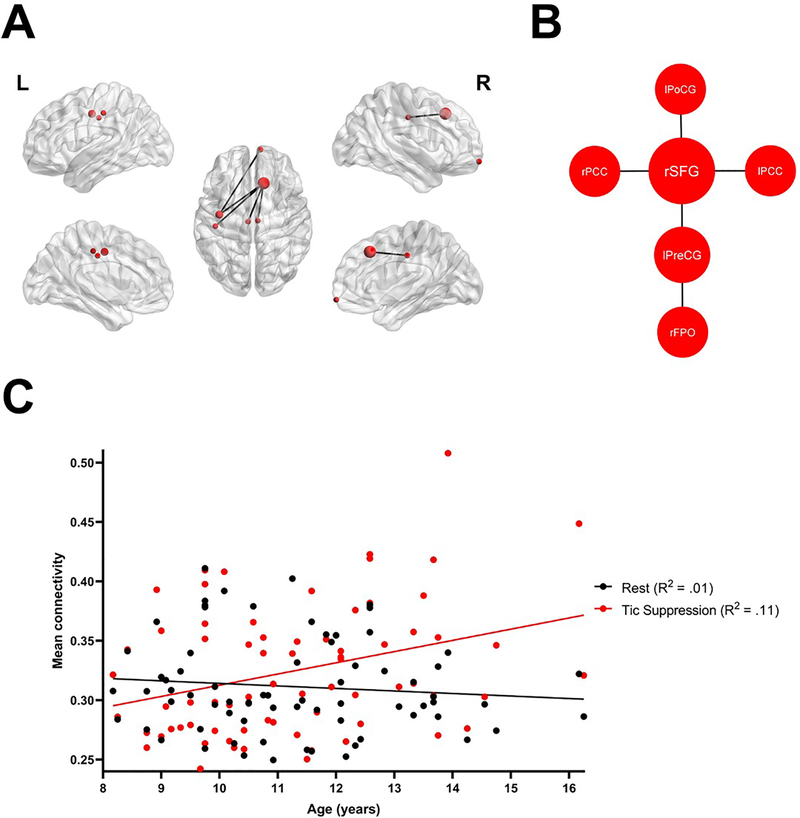
Exploratory analyses revealed a significant condition by age interaction (p = .040, FWER-corrected), suggesting that connectivity within a specific subnetwork increased more with age during tic suppression than rest (Figure 4). There was no significant interaction between condition and medication status, ADHD or OCD comorbidity, or severity of ADHD and tic symptoms (all p-values > .09). Scatterplots of mean connectivity in the alpha subnetwork and YGTSS (Figure S5) and SNAP-IV (Figure S6) scores are presented in the Supplement.
Figure 4: Condition by age interaction.
(A) Exploratory analyses revealed a condition by age interaction in a subnetwork involving 6 nodes and 5 edges. The right superior frontal gyrus had a degree z-score of 1.9 and was considered as a hub in this subnetwork. (B) Graphical representation of the subnetwork interacting with age. (C) Within this subnetwork, connectivity during tic suppression increased linearly with age, while it remained stable in the rest condition.
Discussion
Our study focused on identifying brain circuits involved in tic suppression. Graph theoretical analyses revealed statistically significant yet subtle differences in global network topology during tic suppression, such as more direct and efficient brain-wide communication. Modulation of brain connectivity was however more focused within a specific subnetwork, as revealed with NBS. That subnetwork encompassed many cortical areas, including the right SFG and the left precuneus, which are involved in the DMN. Increased connectivity during tic suppression was confirmed by NBS analyses on neighboring frequency bands and by graph theoretical analyses performed on the alpha-band subnetwork. Partly consistent with prior findings (9, 19), our analyses allowed for a refined identification of involved brain regions.
Two hubs identified in the alpha-subnetwork of the present study – the SFG and the precuneus – are frontal and parietal midline structures. Recently, Uddin et al. (55) proposed an anatomical taxonomy of large-scale brain networks, where the DMN is called the medial frontoparietal network, since its main nodes are frontal and parietal midline brain regions. Thus, these two hubs map well onto this medial frontoparietal network. In adults with TS, increased resting-state connectivity between the left dmPFC and other DMN regions has been reported (16). Since participants were instructed to lie still during the scan, the authors argued that increased DMN connectivity could reflect an effect of tic suppression. Importantly, the dmPFC cluster showing increased connectivity in that latter study is encompassed in the SFG region used in our study (41).
The precuneus – a hub in the alpha-band subnetwork – is one of the most connected structures in the brain (24, 56, 57) and a key node in the DMN (58). It is involved in self-awareness (57) – an important process in tic suppression (59). For instance, greater awareness of premonitory urges is linked to better self-rated tic suppression capacity (60). The precuneus also plays a role in agency (i.e. the sensation of being in control of our actions) (61), which could be impaired in TS (62, 63). Here, it seems likely that the sense of agency increased while children with TS were concentrating on tic suppression. Along these lines, an earlier awareness of one’s own intention, suggesting a better sense of agency, has been associated with increased observer-rated tic suppression capacity (64). Decreased amplitude of low-frequency fluctuation (ALFF) and fractional ALFF in the PCC/precuneus during resting-state fMRI has been reported in children with TS (65). This reduced activity suggests a functional disturbance in TS, thus leading to impaired tic regulation. Therefore, it is plausible that the active regulation of tics would lead to an increase in precuneus connectivity. Likewise, recent graph theoretical investigations of the DMN in children with TS found decreased connectivity relative to healthy controls (26, 27). Yet, another study reported paradoxically increased DMN connectivity in children with TS, TS+OCD, and OCD, compared to healthy controls (66). Similar to TS, decreased DMN connectivity has been reported in children and adolescents with ADHD (67, 68).
Increased DMN connectivity during tic suppression runs counter to the hypothesis that tic suppression relies on a response inhibition network. However, a recent fMRI study reported that tic suppression recruits different brain regions than eyeblink inhibition (8). In healthy controls, eyeblink inhibition recruited brain regions such as the right ventrolateral PFC, the core hub of the classical “stop” network (69). In individuals with TS, tic suppression recruited mostly the medial PFC, the anterior hub of the medial frontoparietal network or DMN (55). These findings suggest that brain mechanisms of tic suppression differ from those of eyeblink inhibition and are not analogous to classical response inhibition mechanisms. Furthermore, performance on an attention-demanding task usually decreases during tic suppression (70).
Given the anti-correlation between the DMN and the lateral frontoparietal network (71, 72), such diminished behavioral performance during tic suppression is expected.
An additional explanation for the involvement of the DMN in tic suppression comes from its interaction with the sensorimotor network (SMN). The DMN stands in a reciprocal relationship with the SMN, and balance or imbalance of these networks can lead to wide range of psychomotor symptoms (73). In TS, enhanced activity within the SMN has been associated with tic expression (74, 75). The SMN also has important connections with subcortical structures. For instance, enhanced structural connectivity in tracts connecting the SMN to thalamus was found in TS and correlated positively with tic severity (76). Thus, increased DMN connectivity during tic suppression could allow balancing enhanced SMN activity associated with tic expression.
We also found that connectivity in a specific subnetwork, in which the right SFG was a hub, followed a developmental trajectory: connectivity during tic suppression, but not rest, increased with age. Likewise, an fMRI study of eyeblink inhibition in children and adults with TS reported that brain activation in the right dlPFC, right inferolateral PFC, caudate, and ACC increased with age in individuals with TS, while remaining stable or declining in healthy controls (77). A recent investigation of resting-state connectivity reported differences in brain networks among children with TS which effectively distinguished them from control children (78). These alterations could reflect the development of compensatory tic suppression mechanisms. The DMN also follows a developmental trajectory (79–81). Disruptions in DMN functional connectivity that were found among children with TS (26, 27) could reflect abnormal or delayed development of that network. Normal or increased DMN connectivity has been reported in adults with TS (16, 75, 82), potentially reflecting a distinct developmental trajectory of the DMN in TS. Tics generally follow a developmental curve characterized by a peak severity around 10 years of age and a gradual waning thereafter (83). Accordingly, tic suppression capacities typically increase with age (4). As shown in Figure 4, connectivity differences between tic suppression and rest began to diverge around the age of 10. At this age, most children are becoming aware of premonitory urges (84, 85). It is thus possible that developmental connectivity patterns presented here represent increasing awareness of urges that in turn facilitates tic suppression (60).
Connectivity between DMN hubs and basal ganglia has been associated with self-referential processing, notably in relation to premonitory urges (82). During voluntary tic suppression, such attention to tics and premonitory urges is probably increased. Awareness training, one of the components of CBIT, could involve such self-referential thinking by promoting self-monitoring of tics and internal triggers like premonitory urges (86). Mindfulness-based interventions also aim to increase awareness of tic-related processes and have been shown as promising treatment options for TS (87, 88). Thus, future clinical trials should test whether DMN connectivity and other carefully selected biomarkers (89) can be considered as mechanisms of behavioral therapies and mindfulness-based interventions.
It would also be important to investigate how the SFG and the precuneus communicate during tic suppression. Prior work on the DMN suggest that the PCC/precuneus directs attention towards external and internal information and relays it to anterior structures involved in continuous monitoring of internal states (11, 90). However, this remains speculative at the current stage and requires further investigation.
Our results must be interpreted in the light of some limitations. Our sample was skewed toward boys, thus limiting the generalizability of our findings. Additionally, most children were non-Hispanic and white. Members of these majority ethnic and racial groups are more likely to be diagnosed with TS than Hispanic white and non-Hispanic Black individuals (91), a difference that likely reflects inequalities in access to healthcare. More effort should be made to include girls and non-white and Hispanic individuals in TS research. While the use of EEG over fMRI may have some advantages such as better temporal resolution, direct measurement of neural oscillations, and increased feasibility in youth with TS, a well-known limitation of EEG is its reduced spatial precision and its inability to measure subcortical sources. Also, some children had comorbid conditions and/or were taking psychotropic medication. However, our results were not moderated by comorbidity or medication status. Finally, while the instructions for the tic suppression and rest conditions only differed in regard to suppressing tics vs. ticcing freely, we cannot exclude the possibility that other cognitive processes were involved during the rest or the tic suppression conditions. Using a third condition where children would perform a cognitively demanding task while suppressing their tics, such as in previous behavioral studies (70), could increase our understanding of networks involved in tic suppression.
Despite these limitations, our study has many strengths, such as the largest sample of individuals with TS among all studies of tic suppression, the consideration of age maturation effects and comorbidity, and the assessment of connectivity at the source level. Importantly, our findings open interesting avenues for future research, especially regarding the involvement of this subnetwork in symptom improvement following behavioral therapy.
Supplementary Material
Acknowledgements
This project was supported by NIMH grants K01MH079130 and R03MH094583 to DGS. SMB was supported by a postdoctoral fellowship award from the Canadian Institutes of Health Research (#415541).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.American Psychiatric Association (2013): Diagnostic and statistic manual of mental disorders. 5th ed. Arlington, VA: Author. [Google Scholar]
- 2.Sambrani T, Jakubovski E, Müller-Vahl KR (2016): New Insights into Clinical Characteristics of Gilles de la Tourette Syndrome: Findings in 1032 Patients from a Single German Center. Frontiers in neuroscience. 10:415–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Specht MW, Woods DW, Nicotra CM, Kelly LM, Ricketts EJ, Conelea CA, et al. (2013): Effects of tic suppression: ability to suppress, rebound, negative reinforcement, and habituation to the premonitory urge. Behav Res Ther. 51:24–30. [DOI] [PubMed] [Google Scholar]
- 4.Conelea CA, Wellen B, Woods DW, Greene DJ, Black KJ, Specht M, et al. (2018): Patterns and Predictors of Tic Suppressibility in Youth With Tic Disorders. Frontiers in psychiatry. 9:188-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganos C, Bongert J, Asmuss L, Martino D, Haggard P, Münchau A (2015): The somatotopy of tic inhibition: Where and how much? Movement Disorders. 30:1184–1189. [DOI] [PubMed] [Google Scholar]
- 6.Peterson BS, Skudlarski P, Anderson AW, et al. (1998): A functional magnetic resonance imaging study of tic suppression in tourette syndrome. Archives of general psychiatry. 55:326–333. [DOI] [PubMed] [Google Scholar]
- 7.Ganos C, Kahl U, Brandt V, Schunke O, Baumer T, Thomalla G, et al. (2014): The neural correlates of tic inhibition in Gilles de la Tourette syndrome. Neuropsychologia. [DOI] [PubMed] [Google Scholar]
- 8.van der Salm SMA, van der Meer JN, Cath DC, Groot PFC, van der Werf YD, Brouwers E, et al. (2018): Distinctive tics suppression network in Gilles de la Tourette syndrome distinguished from suppression of natural urges using multimodal imaging. NeuroImage: Clinical. 20:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrien DJ, Orth M, Evans AH, Lees AJ, Brown P (2005): Motor inhibition in patients with Gilles de la Tourette syndrome: functional activation patterns as revealed by EEG coherence. Brain. 128:116–125. [DOI] [PubMed] [Google Scholar]
- 10.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007): Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of neuroscience: the official journal of the Society for Neuroscience. 27:3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews-Hanna JR, Smallwood J, Spreng RN (2014): The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences. 98:4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashwal S (2017): Disorders of Consciousness in Children. In: Swaiman KF, Ashwal S, Ferriero DM, Schor NF, Finkel RS, Gropman AL, et al. , editors. Swaiman's Pediatric Neurology (Sixth Edition): Elsevier, pp 767–780. [Google Scholar]
- 14.Mulders PC, van Eijndhoven PF, Beckmann CF (2016): Identifying Large-Scale Neural Networks Using fMRI. In: Frodl T, editor. Systems Neuroscience in Depression. San Diego: Academic Press, pp 209–237. [Google Scholar]
- 15.Buckner RL, Andrews-Hanna JR, Schacter DL (2008): The Brain's Default Network. Annals of the New York Academy of Sciences. 1124:1–38. [DOI] [PubMed] [Google Scholar]
- 16.Fan S, van den Heuvel OA, Cath DC, de Wit SJ, Vriend C, Veltman DJ, et al. (2018): Altered Functional Connectivity in Resting State Networks in Tourette's Disorder. Frontiers in human neuroscience. 12:363-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyakoshi M, Jurgiel J, Dillon A, Chang S, Piacentini J, Makeig S, et al. (2020): Modulation of Frontal Oscillatory Power during Blink Suppression in Children: Effects of Premonitory Urge and Reward. Cerebral Cortex Communications. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loo SK, Miyakoshi M, Tung K, Lloyd E, Salgari G, Dillon A, et al. (2019): Neural activation and connectivity during cued eye blinks in Chronic Tic Disorders. NeuroImage: Clinical. 24:101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong HJ, Sohn H, Cha M, Kim S, Oh J, Chu MK, et al. (2013): Increased Frontomotor Oscillations During Tic Suppression in Children With Tourette Syndrome. Journal of Child Neurology. 28:615–624. [DOI] [PubMed] [Google Scholar]
- 20.Brunner C, Billinger M, Seeber M, Mullen TR, Makeig S (2016): Volume Conduction Influences Scalp-Based Connectivity Estimates. Frontiers in computational neuroscience. 10:121–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van de Steen F, Faes L, Karahan E, Songsiri J, Valdes-Sosa PA, Marinazzo D (2019): Critical Comments on EEG Sensor Space Dynamical Connectivity Analysis. Brain topography. 32:643–654. [DOI] [PubMed] [Google Scholar]
- 22.Hassan M, Dufor O, Merlet I, Berrou C, Wendling F (2014): EEG Source Connectivity Analysis: From Dense Array Recordings to Brain Networks. PLOS ONE. 9:e105041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- 24.Bullmore E, Sporns O (2009): Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 10:186–198. [DOI] [PubMed] [Google Scholar]
- 25.Rangaprakash D, Dretsch MN, Katz JS, Denney TS Jr, Deshpande G (2019): Dynamics of Segregation and Integration in Directional Brain Networks: Illustration in Soldiers With PTSD and Neurotrauma. Frontiers in neuroscience. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen H, Liu Y, Rekik I, Wang S, Chen Z, Zhang J, et al. (2018): Combining Disrupted and Discriminative Topological Properties of Functional Connectivity Networks as Neuroimaging Biomarkers for Accurate Diagnosis of Early Tourette Syndrome Children. Molecular neurobiology. 55:3251–3269. [DOI] [PubMed] [Google Scholar]
- 27.Openneer TJC, Marsman J-BC, van der Meer D, Forde NJ, Akkermans SEA, Naaijen J, et al. (2020): A graph theory study of resting-state functional connectivity in children with Tourette syndrome. Cortex. 126:63–72. [DOI] [PubMed] [Google Scholar]
- 28.Nunez PL, Wingeier BM, Silberstein RB (2001): Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Human brain mapping. 13:125–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morand-Beaulieu S, Grot S, Lavoie J, Leclerc JB, Luck D, Lavoie ME (2017): The puzzling question of inhibitory control in Tourette syndrome: A meta-analysis. Neurosci Biobehav Rev. 80:240–262. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- 31.Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. (1989): The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 28:566–573. [DOI] [PubMed] [Google Scholar]
- 32.Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. (2001): Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 40:168–179. [DOI] [PubMed] [Google Scholar]
- 33.Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M (1999): Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 38:1230–1236. [DOI] [PubMed] [Google Scholar]
- 34.Barkley RA (1997): Defiant children: A clinician's manual for assessment and parent training, 2nd ed. New York, NY, US: Guilford Press. [Google Scholar]
- 35.Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM (1982): Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 39:879–883. [DOI] [PubMed] [Google Scholar]
- 36.Morie KP, Wu J, Landi N, Potenza MN, Mayes LC, Crowley MJ (2019): Oscillatory Dynamics of Feedback Processing in Adolescents with Prenatal Cocaine Exposure. Developmental neuropsychology. 44:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley MJ, van Noordt SJR, Wu J, Hommer RE, South M, Fearon RMP, et al. (2014): Reward feedback processing in children and adolescents: Medial frontal theta oscillations. Brain and Cognition. 89:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debnath R, Buzzell GA, Morales S, Bowers ME, Leach SC, Fox NA (2020): The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology. 57:e13580. [DOI] [PubMed] [Google Scholar]
- 39.Delorme A, Makeig S (2004): EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 134:9–21. [DOI] [PubMed] [Google Scholar]
- 40.Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM (2011): Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Computational intelligence and neuroscience. 2011:879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- 42.Lachaux J-P, Rodriguez E, Martinerie J, Varela FJ (1999): Measuring phase synchrony in brain signals. Human Brain Mapping. 8:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aydore S, Pantazis D, Leahy RM (2013): A note on the phase locking value and its properties. NeuroImage. 74:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassan M, Merlet I, Mheich A, Kabbara A, Biraben A, Nica A, et al. (2017): Identification of Interictal Epileptic Networks from Dense-EEG. Brain topography. 30:60–76. [DOI] [PubMed] [Google Scholar]
- 45.Brauchli C, Leipold S, Jäncke L (2020): Diminished large-scale functional brain networks in absolute pitch during the perception of naturalistic music and audiobooks. NeuroImage. 216:116513. [DOI] [PubMed] [Google Scholar]
- 46.Xu T, Cullen KR, Mueller B, Schreiner MW, Lim KO, Schulz SC, et al. (2016): Network analysis of functional brain connectivity in borderline personality disorder using resting-state fMRI. NeuroImage: Clinical. 11:302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue K, Luo C, Zhang D, Yang T, Li J, Gong D, et al. (2014): Diffusion tensor tractography reveals disrupted structural connectivity in childhood absence epilepsy. Epilepsy research. 108:125–138. [DOI] [PubMed] [Google Scholar]
- 48.Zalesky A, Fornito A, Bullmore ET (2010): Network-based statistic: Identifying differences in brain networks. NeuroImage. 53:1197–1207. [DOI] [PubMed] [Google Scholar]
- 49.Xia M, Wang J, He Y (2013): BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLOS ONE. 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. (2003): Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research. 13:2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Settle B, Otasek D, Morris JH, Demchak B (2018): aMatReader: Importing adjacency matrices via Cytoscape Automation. F1000Research. 7:ISCB Comm J-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Heuvel MP, Sporns O (2013): Network hubs in the human brain. Trends in cognitive sciences. 17:683–696. [DOI] [PubMed] [Google Scholar]
- 53.Worbe Y, Malherbe C, Hartmann A, Pélégrini-Issac M, Messé A, Vidailhet M, et al. (2012): Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain. 135:1937–1946. [DOI] [PubMed] [Google Scholar]
- 54.Sukhodolsky DG, Woods DW, Piacentini J, Wilhelm S, Peterson AL, Katsovich L, et al. (2017): Moderators and predictors of response to behavior therapy for tics in Tourette syndrome. Neurology. 88:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uddin LQ, Yeo BTT, Spreng RN (2019): Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain topography. 32:926–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomasi D, Volkow ND (2011): Functional connectivity hubs in the human brain. NeuroImage. 57:908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavanna AE, Trimble MR (2006): The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 129:564–583. [DOI] [PubMed] [Google Scholar]
- 58.Utevsky AV, Smith DV, Huettel SA (2014): Precuneus is a functional core of the default-mode network. The Journal of neuroscience: the official journal of the Society for Neuroscience. 34:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cavanna AE, Nani A (2013): Tourette syndrome and consciousness of action. Tremor and other hyperkinetic movements (New York, NY). 3:tre-03-181-4368-4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuda N, Nonaka M, Kono T, Fujio M, Nobuyoshi M, Kano Y (2020): Premonitory Awareness Facilitates Tic Suppression: Subscales of the Premonitory Urge for Tics Scale and a New Self-Report Questionnaire for Tic-Associated Sensations. Frontiers in psychiatry. 11:592–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, et al. (2001): Mind Reading: Neural Mechanisms of Theory of Mind and Self-Perspective. NeuroImage. 14:170–181. [DOI] [PubMed] [Google Scholar]
- 62.Delorme C, Salvador A, Voon V, Roze E, Vidailhet M, Hartmann A, et al. (2016): Illusion of agency in patients with Gilles de la Tourette Syndrome. Cortex. 77:132–140. [DOI] [PubMed] [Google Scholar]
- 63.Sigurdsson HP, Jackson SR, Jolley L, Mitchell E, Jackson GM (2020): Alterations in cerebellar grey matter structure and covariance networks in young people with Tourette syndrome. Cortex. 126:1–15. [DOI] [PubMed] [Google Scholar]
- 64.Ganos C, Asmuss L, Bongert J, Brandt V, Münchau A, Haggard P (2015): Volitional action as perceptual detection: Predictors of conscious intention in adolescents with tic disorders. Cortex. 64:47–54. [DOI] [PubMed] [Google Scholar]
- 65.Cui Y, Jin Z, Chen X, He Y, Liang X, Zheng Y (2014): Abnormal baseline brain activity in drug-naïve patients with Tourette syndrome: a resting-state fMRI study. Frontiers in human neuroscience. 7:913–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tikoo S, Cardona F, Tommasin S, Giannì C, Conte G, Upadhyay N, et al. (2020): Resting-state functional connectivity in drug-naive pediatric patients with Tourette syndrome and obsessive-compulsive disorder. Journal of psychiatric research. 129:129–140. [DOI] [PubMed] [Google Scholar]
- 67.Castellanos FX, Aoki Y (2016): Intrinsic Functional Connectivity in Attention-Deficit/Hyperactivity Disorder: A Science in Development. Biological psychiatry Cognitive neuroscience and neuroimaging. 1:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao Y, Shuai D, Bu X, Hu X, Tang S, Zhang L, et al. (2019): Impairments of large-scale functional networks in attention-deficit/hyperactivity disorder: a meta-analysis of resting-state functional connectivity. Psychological Medicine. 49:2475–2485. [DOI] [PubMed] [Google Scholar]
- 69.Aron AR, Robbins TW, Poldrack RA (2014): Inhibition and the right inferior frontal cortex: one decade on. Trends in cognitive sciences. 18:177–185. [DOI] [PubMed] [Google Scholar]
- 70.Conelea CA, Woods DW (2008): Examining the impact of distraction on tic suppression in children and adolescents with Tourette syndrome. Behaviour Research and Therapy. 46:1193–1200. [DOI] [PubMed] [Google Scholar]
- 71.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences. 15:483–506. [DOI] [PubMed] [Google Scholar]
- 72.Sridharan D, Levitin DJ, Menon V (2008): A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Northoff G, Hirjak D, Wolf RC, Magioncalda P, Martino M (2021): All roads lead to the motor cortex: psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Molecular psychiatry. 26:92–102. [DOI] [PubMed] [Google Scholar]
- 74.Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. (2006): Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 129:2029–2037. [DOI] [PubMed] [Google Scholar]
- 75.Neuner I, Werner CJ, Arrubla J, Stocker T, Ehlen C, Wegener HP, et al. (2014): Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Frontiers in human neuroscience. 8:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Worbe Y, Marrakchi-Kacem L, Lecomte S, Valabregue R, Poupon F, Guevara P, et al. (2015): Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain. 138:472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazzone L, Yu S, Blair C, Gunter BC, Wang Z, Marsh R, et al. (2010): An FMRI study of frontostriatal circuits during the inhibition of eye blinking in persons with Tourette syndrome. The American journal of psychiatry. 167:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nielsen AN, Gratton C, Church JA, Dosenbach NUF, Black KJ, Petersen SE, et al. (2020): Atypical Functional Connectivity in Tourette Syndrome Differs Between Children and Adults. Biological Psychiatry. 87:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, et al. (2008): The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences. 105:4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rebello K, Moura LM, Pinaya WHL, Rohde LA, Sato JR (2018): Default Mode Network Maturation and Environmental Adversities During Childhood. Chronic Stress. 2:2470547018808295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M (2014): Development of the default mode and central executive networks across early adolescence: a longitudinal study. Developmental cognitive neuroscience. 10:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramkiran S, Heidemeyer L, Gaebler A, Shah NJ, Neuner I (2019): Alterations in basal ganglia-cerebello-thalamo-cortical connectivity and whole brain functional network topology in Tourette's syndrome. NeuroImage: Clinical. 24:101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, et al. (1998): Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 102:14–19. [DOI] [PubMed] [Google Scholar]
- 84.Woods DW, Piacentini J, Himle MB, Chang S (2005): Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. Journal of developmental and behavioral pediatrics: JDBP. 26:397–403. [DOI] [PubMed] [Google Scholar]
- 85.Gulisano M, Calì P, Palermo F, Robertson M, Rizzo R (2015): Premonitory Urges in Patients with Gilles de la Tourette Syndrome: An Italian Translation and a 7-Year Follow-up. Journal of Child and Adolescent Psychopharmacology. 25:810–816. [DOI] [PubMed] [Google Scholar]
- 86.Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. (2010): Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA: the journal of the American Medical Association. 303:1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reese HE, Brown WA, Summers BJ, Shin J, Wheeler G, Wilhelm S (2021): Feasibility and acceptability of an online mindfulness-based group intervention for adults with tic disorders. Pilot and Feasibility Studies. 7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reese HE, Vallejo Z, Rasmussen J, Crowe K, Rosenfield E, Wilhelm S (2015): Mindfulness-based stress reduction for Tourette syndrome and chronic tic disorder: A pilot study. Journal of Psychosomatic Research. 78:293–298. [DOI] [PubMed] [Google Scholar]
- 89.Essoe JK-Y, Ramsey KA, Singer HS, Grados M, McGuire JF (2021): Mechanisms Underlying Behavior Therapy for Tourette’s Disorder. Current developmental disorders reports. [Google Scholar]
- 90.Zhang R, Volkow ND (2019): Brain default-mode network dysfunction in addiction. NeuroImage. 200:313–331. [DOI] [PubMed] [Google Scholar]
- 91.Bitsko RH, Holbrook JR, Visser SN, Mink JW, Zinner SH, Ghandour RM, et al. (2014): A National Profile of Tourette Syndrome, 2011–2012. Journal of Developmental & Behavioral Pediatrics. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.