Abstract
Introduction
Noninfectious keratitis is a painful corneal inflammation treated with topical cyclosporine and other immunosuppressants. Additional treatment options are needed for keratitis that does not improve with standard therapies. Repository corticotropin injection (RCI; Acthar® Gel) is approved to treat severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa, including keratitis. This phase 4, multicenter, open-label study assessed the efficacy and safety of RCI for refractory severe noninfectious keratitis.
Methods
Patients were ≥ 18 years old with persistent severe keratitis despite treatment with topical immunosuppressants. Patients received 80 U of RCI subcutaneously twice weekly for 12 weeks followed by a 4-week taper. Assessments included all domains of the Impact of Dry Eye on Everyday Life (IDEEL) Questionnaire, Ocular Discomfort and 4-Symptom Questionnaire, and Visual Analog Scale (VAS). Corneal fluorescein and conjunctival lissamine green staining, Conjunctival Redness Scale, tear production (Schirmer’s test), visual acuity, slit lamp examination, and intraocular pressure were also assessed. Safety was evaluated via treatment-emergent adverse events. Analyses were performed using the modified intent-to-treat (mITT) population (patients who received ≥ 1 dose of RCI and contributed any post-baseline efficacy data).
Results
In the mITT population (N = 35), 50.0% (95% confidence interval, 33.2% to 66.8%) of patients experienced clinically important improvements in the symptom bother domain of the IDEEL Questionnaire at week 12 of RCI therapy. All domains of the IDEEL and the Ocular Discomfort and 4-Symptom Questionnaire showed improvements at week 12 of RCI treatment. The most pronounced improvements in the VAS at week 12 were for eye dryness and eye discomfort. Corneal staining, conjunctival staining, conjunctival redness, and tear production showed early improvements that were sustained through week 12. No new safety signals for RCI were identified.
Conclusions
RCI is safe and effective for refractory severe noninfectious keratitis that has not improved with other approved therapies.
Trial registration number
ClinicalTrials.gov NCT04169061.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-021-00400-y.
Keywords: Acthar Gel, Corneal inflammation, Keratitis, Repository corticotropin injection
Key Summary Points
| Why carry out this study? |
| Noninfectious keratitis is a painful inflammation of the cornea that is associated with considerable morbidity and severe complications, including loss of vision. |
| Keratitis can be treated with topical lubricants, cyclosporine, lifitegrast, corticosteroids, and immunosuppressants; however, alternative treatments are needed for patients in whom these treatments are not effective. |
| We conducted a phase 4, multicenter, open-label study to assess the efficacy and safety of repository corticotropin injection (RCI; Acthar® Gel) in patients with refractory severe noninfectious keratitis that had not improved after treatment with standard therapies. |
| What was learned from this study? |
| After 12 weeks of treatment with RCI, 50.0% of patients experienced clinically important improvements in the symptom bother domain of the Impact of Dry Eye on Everyday Life (IDEEL) Questionnaire; improvements were also observed in the following assessments: IDEEL Questionnaire, Ocular Discomfort and 4-Symptom Questionnaire, Visual Analog Scale, corneal fluorescein staining, conjunctival lissamine green staining, Conjunctival Redness Scale, and tear production (Schirmer’s test). Adverse events (AEs) were mild, with blurry vision and double vision being the only ocular AEs reported. |
| Results of this study showed RCI to be a safe and effective treatment for refractory severe noninfectious keratitis. |
Introduction
Keratitis is an inflammation of the cornea that can have infectious or noninfectious causes [1–3]. Noninfectious keratitis can result from dry eye or local injury, such as prolonged use of contact lenses, or it can be an ocular manifestation of systemic autoimmune disorders including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) [1–3]. Fluctuating vision, foreign body sensation, irritation, pain, photophobia, and redness in the affected eye(s) are common symptoms of noninfectious keratitis [1, 4]. Repeated episodes and long-lasting, persistently active keratitis can lead to serious complications such as permanent corneal damage and loss of vision [4]. Additionally, the following complications may develop in patients with severe keratitis and concomitant systemic inflammatory conditions: corneal neovascularization, scarring, or thinning; keratinization of the ocular surface; sterile or microbial ulceration of the cornea with possible perforation; and severe loss of vision [5].
Treatment of noninfectious keratitis is typically focused on symptomatic relief and prevention of corneal damage. Standards of care for noninfectious keratitis include topical lubricants and immunosuppressants, such as cyclosporine, as well as lifitegrast and topical or systemic corticosteroids. Systemic immunosuppressants may be required in more severe or refractory cases or in patients with an underlying autoimmune condition. However, some patients have noninfectious keratitis that does not improve after treatment with conventional therapies. With prolonged use, corticosteroids and immunosuppressants have unfavorable safety profiles [6]. Further, there are few treatment options for patients with persistent keratitis that is difficult to treat. Therefore, alternative treatments may be appropriate for these patients.
Repository corticotropin injection (RCI; Acthar® Gel) is a naturally sourced complex mixture of adrenocorticotropic hormone (ACTH) analogs and other pituitary peptides [7]. The RCI manufacturing process converts the initial porcine pituitary extract with low ACTH content into a mixture with modified porcine ACTH and other related peptide analogs solubilized in gelatin [7]. A major component in the formulated complex mixture is N-25 deamidated porcine ACTH(1–39) [7]. RCI engages all five melanocortin receptors (MCRs) and has demonstrated direct immunomodulatory and indirect anti-inflammatory effects [8–13]. MCRs are expressed on immune cells and other tissues in the central nervous system and the eye [14–17].
RCI is approved by the US Food and Drug Administration for the treatment of severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa, including keratitis [7]. RCI has also been found effective for the treatment of persistently active systemic inflammatory disorders that have not responded to corticosteroids and other standard-of-care therapies [18–21].
A recent consensus panel recommended RCI for the treatment of keratitis in patients with underlying inflammatory disorders but acknowledged that additional research is needed to assess the effectiveness of RCI for this purpose [22]. The objective of this study was to evaluate the efficacy and safety of RCI for the treatment of refractory severe noninfectious keratitis that had not adequately responded to treatment with standard-of-care therapies.
Methods
Ethics and Compliance
The study protocol was reviewed and approved by the Alpha Institutional Review Board (San Clemente, CA, USA). The study was conducted in agreement with the Declaration of Helsinki and with requirements for registered clinical trials (ClinicalTrials.gov identifier NCT04169061). Patients provided written informed consent prior to participation.
Study Design
This phase 4, multicenter, open-label study was conducted across 8 study sites in the United States (supplemental Table S1). Investigators enrolled adults who had previously been diagnosed with keratitis sicca that was determined not to have an infectious cause such as bacteria or viruses. Patients had severe noninfectious keratitis that did not improve after treatment with topical cyclosporine or lifitegrast or could not tolerate such therapies.
Patients were eligible for participation if they met all enrollment criteria (Table 1) at the screening and baseline visits. The following treatments were prohibited during the study period: topical, inhaled, intra-articular, intra-ocular, or systemic corticosteroids; systemic immunosuppressants; systemic immunomodulators; and systemic biologic agents for a concomitant condition. Lid hygiene treatments, warm compresses, and artificial tears were permitted provided the patient had been on a stable dose for ≥ 4 weeks prior to screening. Patients were instructed not to use ophthalmic preparations within 2 h prior to each study visit.
Table 1.
Enrollment criteria
| Key inclusion criteria |
|
1. Be age ≥ 18 years at the screening visit 2. Have normal lid anatomy 3. Have a reported history of severe keratitis in one or both eyes and a history of previous treatment for keratitis within the previous 6 months 4. Did not have symptomatic improvement or did not tolerate previous treatment with topical cyclosporine or lifitegrast 5. Have all the following in at least one eye (the same eye) at screening and baseline: (a) Inferior corneal fluorescein staining score ≥ 2 in any field (b) Corneal sum fluorescein staining score ≥ 4 (c) Conjunctival sum lissamine green staining score ≥ 2 (d) Conjunctival redness score ≥ 1 (e) Schirmer score ≥ 1 mm/5 min and ≤ 10 mm/5 min (f) Ocular discomfort score (ODS) ≥ 2 |
| Key exclusion criteria |
|
1. Have any ocular condition that, in the opinion of the investigator, could affect study parameters 2. Have a history of laser-assisted in situ keratomileusis (LASIK) and/or any other ocular surgical procedure within 12 months prior to the baseline visit; ocular trauma, penetrating intraocular surgery, refractive surgery, corneal transplantation, or eyelid surgery within 12 weeks prior to the screening visit; or any scheduled ocular surgical procedure during the study phase 3. Have active or any history of ocular herpes or other ocular infection within 30 days prior to the baseline visit 4. Have current punctal plugs, punctal occlusion, or a history of nasolacrimal duct obstruction 5. Be unwilling to avoid wearing contact lenses for 7 days prior to the screening visit and for the duration of the study phase 6. Have a best-corrected visual acuity < 0.7 logarithm of the minimum angle of resolution (logMAR) in each eye at the screening and baseline visits 7. Be under treatment with any corticosteroids, immunosuppressants, immunomodulators, or biologic agents for a concomitant condition 8. Use prohibited medications or devices and be unable to discontinue their use for the required period before entry into the study, as follows: (a) Ophthalmic cyclosporine or lifitegrast within 12 weeks prior to the screening visit (b) Topical and systemic corticosteroids, antibiotics, mast cell stabilizers, and vasoconstrictors and ocular autologous serum within 30 days prior to the screening visit (c) Systemic tetracyclines, unless the dose has been stable for ≥ 30 days prior to the screening visit and will remain stable during the study phase (d) Any topical or systemic medication known to cause ocular drying, unless the dose has been stable for ≥ 30 days prior to the screening visit and will remain stable during the study phase (e) Topical and systemic antihistamines within 7 days prior to the screening visit (f) Topical or nasal vasoconstrictors within 14 days prior to the screening visit (g) Unable to refrain from using artificial tears within 2 h prior to each study visit (h) Has had glucocorticoid implants within 3 years prior to the baseline visit or has had complications related to the device and/or has had the device removed within 90 days prior to the baseline visit (i) Any US Food and Drug Administration (FDA)-approved medical devices other than punctal plugs for dry eye within 12 weeks prior to the screening visit |
FDA US Food and Drug Administration, LASIK laser-assisted in situ keratomileusis, logMAR Logarithm of the Minimum Angle of Resolution, ODS ocular discomfort score
Study Procedures and Data Collection
Patients or their caregivers administered 80 U of RCI subcutaneously twice weekly for 12 weeks followed by a tapering period of 4 weeks during which RCI was reduced to 40 U twice weekly for 2 weeks, then to 40 U once weekly for 2 weeks. Study drug records were used to monitor treatment compliance, and records and empty drug vials were assessed at each visit.
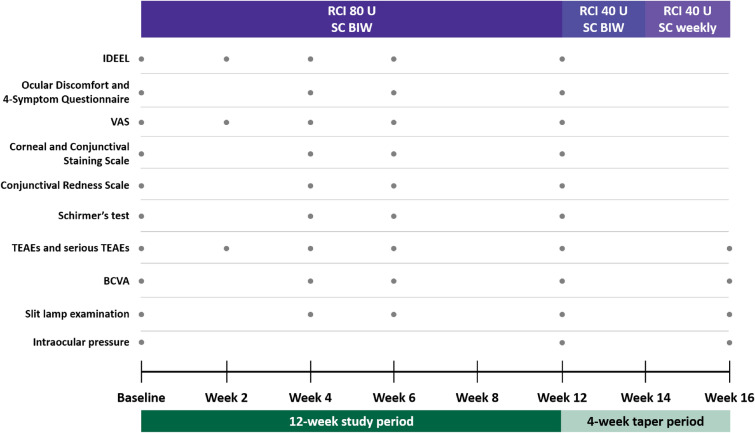
The following efficacy assessments were conducted via clinician evaluation or patient self-reports (Fig. 1). The Impact of Dry Eye on Everyday Life (IDEEL) Questionnaire [23] was assessed at baseline and weeks 2, 4, 6, and 12. The IDEEL Questionnaire consists of 6 domains: impact on daily activities, emotional impact, impact on work, satisfaction with treatment effectiveness, treatment-related bother, and symptom bother. Each domain scored patients’ self-reported experiences in the 2 weeks prior on a scale from 0 to 100. Higher scores indicated better quality of life or treatment satisfaction in each respective domain except symptom bother, in which higher scores indicated more bothersome symptoms. The Ora Calibra™ Ocular Discomfort and 4-Symptom Questionnaire was assessed at baseline and weeks 4, 6, and 12. The questionnaire allowed patients to rate 5 common symptoms of keratoconjunctivitis sicca (ocular discomfort, dryness, grittiness, burning, and stinging) on a scale from 0 (none) to 5 (severe). The Visual Analog Scale (VAS) was conducted at baseline and weeks 2, 4, 6, and 12. The VAS is a 7-item symptom index in which patients rated each symptom on a scale from 0 (no discomfort) to 100 (maximal discomfort); the symptoms assessed were eye dryness, burning/stinging, itching, foreign body sensation, eye discomfort, photophobia, and pain. The Ora Calibra™ Corneal and Conjunctival Staining Scale was conducted at baseline and weeks 4, 6, and 12. Fluorescein staining was used for assessment of the inferior, superior, and central regions of the cornea individually and as the sum. Lissamine green staining was used to assess the nasal and temporal regions of the conjunctiva. Corneal and conjunctival regions were scored from 0 (none) to 4 (severe). The Ora Calibra™ Conjunctival Redness Scale measured conjunctival redness on a scale from 0 (none) to 4 (severe) and was assessed at baseline and weeks 4, 6, and 12. Tear production was assessed at baseline and weeks 4, 6, and 12 by an unanesthetized Schirmer’s test [24] using a paper strip to determine the amount (mm) of tears produced over 5 min. Other ophthalmic assessments included best-corrected visual acuity (logarithm of the minimum angle of resolution [logMAR]) at baseline and weeks 4, 6, 12, and 16, with lower logMAR scores indicating better visual acuity; slit lamp examination at baseline and weeks 4, 6, 12, and 16; and intraocular pressure (mm Hg) at baseline and weeks 12 and 16. Treatment-emergent adverse events (TEAEs) and serious TEAEs at weeks 2, 4, 6, 12, and 16 were also recorded.
Fig. 1.
Study design and data collection. BCVA best-corrected visual acuity, BIW twice weekly, IDEEL Impact of Dry Eye on Everyday Life, RCI repository corticotropin injection, TEAE treatment-emergent adverse event, SC subcutaneous, VAS Visual Analog Scale
Paper versions of the IDEEL and VAS were converted to an electronic platform and were validated by cognitive interviews with patients to assess equivalence with the paper versions. Responses were collected on electronic devices during a predetermined recall period at which time patients were permitted to respond to a given assessment. Patients were not permitted to enter data retroactively after the recall period closed.
Outcomes
The primary endpoints were the proportion of patients with ≥ 12-point improvement in the symptom bother domain of the IDEEL Questionnaire at week 12 and the proportion of patients with at least 20%, 30%, and 50% improvement in the symptom bother domain of the IDEEL Questionnaire at week 12. The 12-point improvement was chosen on the basis of the minimal clinically important difference threshold proposed by Fairchild et al. [25]. Other endpoints included the mean change from baseline to week 12 in the following assessments: each domain of the IDEEL Questionnaire; each item of the Ocular Discomfort and 4-Symptom Questionnaire; each item of the VAS; corneal and conjunctival sums of the Corneal and Conjunctival Staining Scale; Conjunctival Redness Scale; Schirmer’s test; visual acuity; and slit lamp examination. We also assessed the proportion of patients with complete resolution of symptoms at week 12 as measured by the Ocular Discomfort and 4-Symptom Questionnaire. Safety endpoints included the incidence and severity of ocular TEAEs, new or worsening cataracts, and mean change from baseline to week 12 in intraocular pressure.
Statistical Analyses
Efficacy endpoints were analyzed in the modified intent-to-treat (mITT) population, defined as all patients who received at least one dose of RCI and who contributed any post-baseline efficacy data to the study. For patients with both eyes affected, change from baseline was determined by using the eye with the worst severity at baseline; if both eyes had the same severity at baseline, the right eye was used. Safety endpoints were analyzed in the safety population, defined as all patients who received at least one dose of RCI. Safety results were summarized descriptively. No formal sample size calculations were performed; 95% confidence intervals (CIs) were calculated based on normal approximation. A target sample size of 36 patients was determined empirically.
Results
Patient Disposition, Demographics, and Baseline Characteristics
The trial was initiated on September 30, 2019, and was completed on December 7, 2020. Thirty-six patients enrolled in the study and were included in the safety population. Thirty-one patients completed the study; five patients did not complete the study owing to TEAEs (n = 2), study withdrawal (n = 1), protocol violation (n = 1), and death (n = 1). Thirty-five patients were included in the mITT population.
Most patients were female, White, and not of Hispanic or Latino ethnicity (Table 2). All patients had keratitis in both eyes. The mean (SD) and median duration of keratitis were 4.4 (5.4) and 2.6 years, respectively. The ophthalmic medical and surgical history of participating patients is provided in supplemental Table S2.
Table 2.
Demographics and baseline characteristics
| n | Modified intent-to-treat (mITT) populationa (N = 35) | |
|---|---|---|
| Age, years, mean (SD) | 35 | 63.3 (10.2) |
| Sex, no. (%) | 35 | |
| Male | 10 (28.6) | |
| Female | 25 (71.4) | |
| Race, no. (%) | 35 | |
| White | 28 (80.0) | |
| Black or African American | 7 (20.0) | |
| Ethnicity, no. (%) | 35 | |
| Hispanic or Latino | 2 (5.7) | |
| Not Hispanic or Latino | 33 (94.3) | |
| Impact of Dry Eye on Everyday Life (IDEEL) Questionnaire, mean (SD) [scale 0–100] | ||
| Impact on daily activities | 34 | 64.5 (20.1) |
| Emotional impact | 34 | 65.6 (21.8) |
| Impact on work | 17 | 60.3 (19.4) |
| Satisfaction with treatment effectiveness | 16 | 22.7 (21.0) |
| Treatment-related bother | 21 | 59.1 (32.6) |
| Symptom bother | 34 | 65.4 (15.5) |
| Ocular Discomfort and 4-Symptom Questionnaire, mean (SD) [scale 0–5] | ||
| Ocular discomfort score (ODS) | 35 | 3.2 (0.9) |
| Burning | 35 | 1.7 (1.3) |
| Dryness | 35 | 3.5 (1.0) |
| Grittiness | 35 | 2.5 (1.3) |
| Stinging | 35 | 2.1 (1.4) |
| Visual Analog Scale (VAS), mean (SD) [scale 0–100] | ||
| Eye dryness | 29 | 77.6 (18.2) |
| Burning/stinging | 29 | 45.3 (29.1) |
| Itching | 29 | 44.1 (29.5) |
| Foreign body sensation | 29 | 50.9 (27.8) |
| Eye discomfort | 29 | 71.3 (20.3) |
| Photophobia | 29 | 57.0 (25.7) |
| Pain | 29 | 34.5 (23.3) |
| Corneal Staining Scale, mean (SD) | ||
| Inferior [scale 0–4] | 35 | 2.2 (0.3) |
| Superior [scale 0–4] | 35 | 1.8 (0.6) |
| Central [scale 0–4] | 35 | 1.4 (0.5) |
| Corneal sum for fluorescein | 35 | 5.3 (0.9) |
| Conjunctival Staining Scale, mean (SD) | ||
| Temporal [scale 0–4] | 35 | 1.8 (0.7) |
| Nasal [scale 0–4] | 35 | 1.9 (0.7) |
| Conjunctival sum for lissamine green | 35 | 3.5 (1.3) |
| Conjunctival Redness Scale, mean (SD) [scale 0–4] | 35 | 1.6 (0.6) |
| Schirmer’s test, mm, mean (SD) | 35 | 3.2 (1.8) |
| Visual acuity, Logarithm of the Minimum Angle of Resolution (logMAR), mean (SD) | 35 | 0.1 (0.1) |
| Intraocular pressure, mm Hg | 35 | 15.9 (3.0) |
aAll patients who received at least one dose of RCI and who contributed any post-baseline efficacy data to the study
As mentioned previously, patient responses to the IDEEL Questionnaire and VAS were collected on electronic devices during a predetermined recall period and were not permitted to be collected retroactively after the recall period closed. Consequently, some patient responses were not collected throughout the study for certain domains of the IDEEL Questionnaire and VAS (Table 2).
IDEEL Questionnaire
At week 12, 17 patients (50.0%) experienced a ≥ 12-point improvement from baseline in the symptom bother domain of the IDEEL Questionnaire (Table 3). The proportions of patients with ≥ 12-point improvement in the symptom bother domain at each time point assessed are presented in Table 3, while the proportions with ≥ 20%, ≥ 30%, or ≥ 50% improvement in the symptom bother domain score at week 12 are presented in Table 4.
Table 3.
Proportion of patients with at least 12-point improvement in the symptom bother domain score of the IDEEL Questionnaire
| No. (%) | 95% CI | |
|---|---|---|
| Week 2 | 18 (52.9) | 36.2–69.7% |
| Week 4 | 18 (52.9) | 36.2–69.7% |
| Week 6 | 19 (55.9) | 39.2–72.6% |
| Week 12 | 17 (50.0) | 33.2–66.8% |
Table 4.
Proportion of patients with at least 20%, 30%, or 50% improvement in the symptom bother domain score of the IDEEL Questionnaire at week 12
| No. (%) | 95% CI | |
|---|---|---|
| ≥ 20% | 17 (50.0) | 33.2–66.8% |
| ≥ 30% | 15 (44.1) | 27.4–60.8% |
| ≥ 50% | 5 (14.7) | 2.8–26.5% |
Improvements from baseline were observed as early as week 2 and were sustained through week 12 for the following dimensions of the IDEEL Questionnaire: impact on daily activities, emotional impact, treatment-related bother, and symptom bother (Fig. 2). Improvements from baseline for the impact on work domain were also observed at week 2, although a slight decrease in this domain score was observed from week 2 to week 4 before increasing again at week 6 (Fig. 2).
Fig. 2.
Change from baseline for each domain of the IDEEL Questionnaire. Data are mean (95% confidence interval)
Ocular Discomfort and 4-Symptom Questionnaire
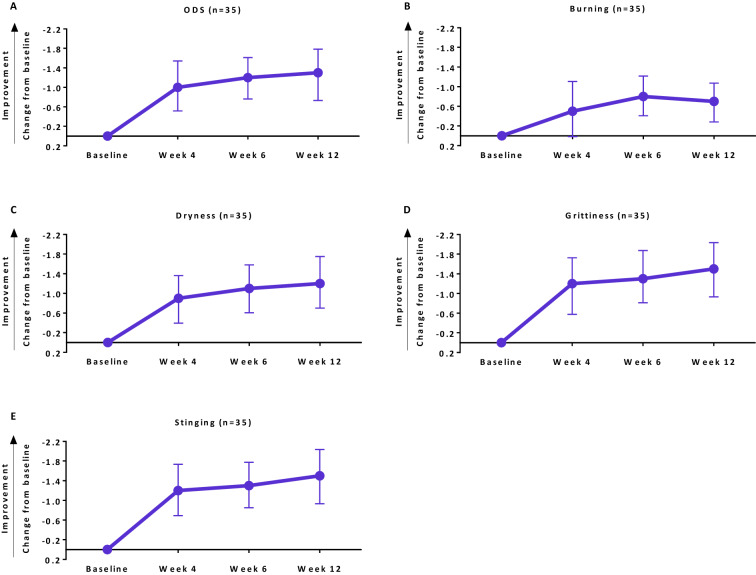
Improvements from baseline at week 12 and multiple time points prior were observed for each item of the Ocular Discomfort and 4-Symptom Questionnaire after initiation of RCI treatment (Fig. 3). The proportions of patients who had complete resolution of each symptom by week 12 are presented in Table 5.
Fig. 3.
Changes from baseline for each item of the Ocular Discomfort and 4-Symptom Questionnaire. Data are mean (95% confidence interval)
Table 5.
Proportion of patients with complete resolution of each symptom of the Ocular Discomfort and 4-Symptom Questionnaire at week 12
| No. (%) | 95% CI | |
|---|---|---|
| Ocular discomfort | 7 (20.0) | 6.7–33.3% |
| Dryness | 3 (8.6) | 0–17.8% |
| Grittiness | 16 (45.7) | 29.2–62.2% |
| Burning | 15 (42.9) | 26.5–59.3% |
| Stinging | 22 (62.9) | 46.8–78.9% |
VAS
At week 12, improvements from baseline were observed for each item of the VAS (Table 6). The most pronounced improvements were observed for eye dryness at week 12 and eye discomfort at weeks 2 and 12.
Table 6.
Change from baseline for each item of the VAS
| Week 2 (n = 24) | Week 12 (n = 26) | |||
|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | |
| Eye dryness | −19.3 (29.9) | −31.9 to −6.7 | −22.2 (25.6) | −32.6 to −11.8 |
| Burning/stinging | −16.6 (28.6) | −28.7 to −4.5 | −13.5 (24.3) | −23.3 to −3.7 |
| Itching | −10.4 (25.6) | −21.2 to 0.4 | −10.1 (27.3) | −21.1 to 0.9 |
| Foreign body sensation | −19.4 (21.6) | −28.5 to −10.3 | −17.7 (22.5) | −26.7 to −8.6 |
| Eye discomfort | −23.1 (27.5) | −34.7 to −11.5 | −23.9 (25.4) | −34.2 to −13.7 |
| Photophobia | −21.2 (27.4) | −32.7 to −9.6 | −19.5 (26.5) | −30.2 to −8.8 |
| Pain | −12.0 (15.1) | −18.4 to −5.6 | −15.0 (20.2) | −23.1 to −6.9 |
Ophthalmic Assessments
Improvements in the corneal sum by fluorescein staining, conjunctival sum by lissamine green staining, and Schirmer’s test were observed as early as week 4 after initiation of RCI treatment and were sustained through week 12 (Table 7). An improvement in conjunctival redness was observed as early as week 6 after initiation of RCI treatment and was sustained through week 12 (Table 7). No clinically meaningful changes in visual acuity (supplemental Table S3) or slit lamp examination (supplemental Table S4) were observed.
Table 7.
Change from baseline for each ophthalmic assessment
| Week 4 (n = 33) | Week 6 (n = 32) | Week 12 (n = 31) | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | |
| Corneal sum—fluorescein | −1.0 (1.5) | −1.5 to −0.4 | −1.3 (1.4) | −1.8 to −0.8 | −1.1 (1.4) | −1.6 to −0.6 |
| Conjunctival sum—lissamine green | −0.6 (0.9) | −0.9 to −0.2 | −0.8 (1.3) | −1.2 to −0.3 | −0.7 (1.4) | −1.2 to −0.2 |
| Conjunctival Redness Scale | −0.2 (0.7) | −0.4 to 0.1 | −0.3 (0.6) | −0.5 to −0.1 | −0.4 (0.8) | −0.6 to −0.1 |
| Schirmer’s test, mm | 2.1 (4.9) | 0.4 to 3.9 | 1.6 (4.4) | 0.01 to 3.2 | 1.3 (3.9) | −0.1 to 2.7 |
Safety
One-third of patients experienced at least one TEAE after initiation of RCI treatment (Table 8). Almost all TEAEs were single incidences. Blurred vision and double vision were the only ocular TEAEs reported. One serious TEAE of intentional overdose was reported and resulted in death; although the specific drug implicated in the overdose is unknown, investigators identified this event as unrelated to RCI treatment. No new or worsening cataracts (supplemental Table S4) or clinically meaningful changes in intraocular pressure (supplemental Table S5) were observed.
Table 8.
Safety results
| Safety populationa (N = 36) | |
|---|---|
| Patients who experienced any treatment-emergent adverse event (TEAE), no. (%) | 12 (33.3) |
| TEAEs, no. (%) | |
| Hypertension | 2 (5.6) |
| Abdominal pain | 1 (2.8) |
| Ankle fracture | 1 (2.8) |
| Blurred vision | 1 (2.8) |
| Double vision | 1 (2.8) |
| Fever | 1 (2.8) |
| Increased viscosity of upper respiratory secretions | 1 (2.8) |
| Intentional overdose | 1 (2.8) |
| Irritability | 1 (2.8) |
| Polymyalgia rheumatica | 1 (2.8) |
| Weight gain | 1 (2.8) |
| Wrist fracture | 1 (2.8) |
| Upper respiratory tract infection | 1 (2.8) |
| Serious TEAE, no. (%) | 1 (2.8) |
| Intentional overdose | 1 (2.8) |
| TEAE with fatal outcome, no. (%) | 1 (2.8) |
| Intentional overdose | 1 (2.8) |
aAll patients who received at least one dose of RCI
Discussion
The results from this phase 4, multicenter, open-label study showed improvements across multiple symptoms of keratitis after initiation of RCI treatment. By week 12, half of the patients in this study had experienced a ≥ 12-point improvement or a ≥ 20% improvement in the IDEEL symptom bother domain score. These findings were supported by improvements in other domains of the IDEEL as well as conjunctival redness and symptoms of burning, discomfort, dryness, foreign body sensation, grittiness, pain, photophobia, and stinging. Furthermore, improvements in corneal fluorescein staining, conjunctival lissamine staining, and tear production (Schirmer’s test) were also observed. Many of these improvements were observed as early as week 2 or week 4 after RCI initiation, which is especially meaningful given that the trial had enrolled patients with severe and long-lasting, persistently active keratitis that had not improved with prior treatments.
Punctate keratitis is a characteristic sign of corneal damage and of dry eye [2, 26–28]. If untreated, its sequelae can lead to partial or total vision loss, particularly when the central region of the cornea is affected [29–32]. Fluorescein and lissamine staining are key markers for evaluating the health of the corneal and conjunctival epithelium, respectively [27–29]. Importantly, the improvements in fluorescein staining and lissamine staining observed after RCI treatment in our study suggest that RCI may help clear ocular surface changes caused by keratitis. Although these results were not reinforced by improvements from baseline in visual acuity, a lack of improvement in visual acuity could be attributed to the small number of patients in this study.
Additionally, corneal damage and dry eye trigger a self-perpetuating immune response that can further intensify ocular cell and tissue damage [27, 30]. Components of both the innate and adaptive immune response have been implicated in this process [27, 30]. Homeostasis of the ocular surface is regulated by lymphocytes; in response to epithelial injury, T cells activate an inflammatory cascade that results in apoptosis of corneal epithelial cells, further altering ocular homeostasis [30, 33]. This process stimulates the production of proinflammatory cytokines such as interferon gamma, tumor necrosis factor alpha, and interleukin (IL)-1, IL-6, IL-8, and IL-17 on the ocular surface and in tear film [26, 27, 33]. Although inflammatory cells and proinflammatory cytokine profiles were not directly assessed in this study, the effectiveness of RCI for improving corneal epithelium staining and symptoms of keratitis may be attributed to a reduction in the inflammatory changes of the ocular surface. It is thought that RCI reduces the activity of helper T cells and increases the activity and number of regulatory T cells via activation of MCRs [15, 34, 35]. Additionally, previous studies have suggested that RCI has an immunomodulatory effect on B cell activation, differentiation, and development and inhibits the inflammatory cytokine response [9–13]. The direct immunomodulatory effects of RCI in patients with refractory noninfectious keratitis should be evaluated in a future study.
The results of this study also showed that RCI was safe and well tolerated. No new safety signals for RCI were identified. Notably, the only ocular TEAEs reported were blurred vision (n = 1) and double vision (n = 1), and there were no serious TEAEs related to RCI treatment. These findings are consistent with the known safety profile of RCI when used to treat refractory RA [19] and refractory SLE [36]. Additionally, a previous systematic review found that RCI has a comparable safety profile to that of low-dose corticosteroids, and these findings were further supported by pharmacovigilance data for RCI [37]. However, to date, no head-to-head studies have directly compared the safety profile of RCI with that of corticosteroids or other standard-of-care therapies for keratitis.
Patients in this study had refractory severe noninfectious keratitis that did not improve after treatment with various standard-of-care therapies or were previously unable to tolerate such therapies, including topical ophthalmic preparations. The corneal anatomy can limit topical drug absorption, and many topical drugs are cleared rapidly from the ocular surface after administration because of tear turnover [38]. The systemic effects of the RCI gel preparation may provide a sustained therapeutic response that contrasts with the rapid clearance and low bioavailability of topical ophthalmic treatments. Because of the previously mentioned limitations of topical treatments, these preparations often require frequent daily administration that contributes to a low treatment compliance rate [38, 39]. Therefore, the twice-weekly administration of RCI may be preferred by some patients.
Keratitis is also associated with various autoimmune diseases, such as RA and SLE, and is sometimes the presenting sign of disease [40, 41]. In addition to ocular inflammatory disorders, RCI is approved for the treatment of systemic inflammatory, collagen, and rheumatic disorders [7]; thus, the efficacy of RCI for treating keratitis is a potential additional benefit of RCI when used to treat these other conditions. However, this was not directly evaluated in this study and warrants further investigation.
This study adds to the body of literature for a condition that is still being explored extensively. Limitations of this study include a relatively small sample size, a short treatment period of 12 weeks, and a lack of a placebo comparator. However, because the patients were not permitted to receive standard therapies such as cyclosporine, lifitegrast, and corticosteroids during the study period, the observed improvements in the symptoms of keratitis are likely the result of RCI treatment. Although the open-label study design was appropriate for this target population of patients with severe refractory keratitis, the efficacy and safety results warrant further investigation in a randomized, placebo-controlled trial.
Conclusions
Keratitis is associated with substantial morbidity and reduced quality of life [26, 28]. Consequently, patients who have keratitis that has not improved with conventional therapies have a need for efficacious alternatives. Treatment with 80 U of RCI subcutaneously twice weekly for 12 weeks was associated with rapid and sustained improvements in clinical and symptomatic assessments of keratitis. These results support the utility of RCI as a safe and effective treatment option for refractory severe noninfectious keratitis that has not improved after treatment with other medications approved to treat this condition.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of this study.
Funding
Sponsorship for this study and the journal’s Rapid Service Fee were funded by Mallinckrodt Pharmaceuticals.
Medical Writing and Editorial Assistance
Professional writing and editorial support were provided by Amber Watson, PharmD, of MedLogix Communications, LLC, Itasca, Illinois, under the direction of the authors and were funded by Mallinckrodt Pharmaceuticals.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
David Wirta, Eugene McLaurin, George Ousler, Jingyu Liu, R. Oktay Kacmaz, and Joseph Grieco were involved in the conception or design of the study, data analysis or interpretation, and critical revision of the manuscript. All named authors approved the final version of the manuscript.
Prior Presentation
This manuscript is based on work previously presented in part at the virtual Association for Research in Vision and Ophthalmology Annual Meeting (May 1–7, 2021) and the virtual International Society for Pharmacoeconomics and Outcomes Research Annual Meeting (May 17–20, 2021).
Disclosures
David Wirta has received research grant support from Mallinckrodt Pharmaceuticals. Eugene McLaurin has financial relationships with Aldeyra Therapeutics; Allergan; Aurinia Pharmaceuticals; Hanall Biopharma; Mitotech; Ocular Therapeutix; ReGenTree, LLC; Santen Pharmaceutical Co., Ltd.; Shire; Sun Pharma; and TopiVert Ltd. George Ousler has a financial relationship with Mallinckrodt Pharmaceuticals. Jingyu Liu, R. Oktay Kacmaz, and Joseph Grieco are employees of Mallinckrodt Pharmaceuticals.
Compliance with Ethics Guidelines
The study protocol was reviewed and approved by Alpha Institutional Review Board (San Clemente, CA). The study was conducted in agreement with the Declaration of Helsinki and with requirements of registered clinical trials (ClinicalTrials.gov identifier NCT04169061). Patients provided written informed consent prior to participation.
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available. Individual patient data may be requested if allowed per informed consent and appropriately anonymized. Requests should be sent to Mallinckrodt Pharmaceuticals’ department for Clinical Trial Disclosure and Transparency at clinicaltrials@mnk.com.
References
- 1.Singh P, Gupta A, Tripathy K. Keratitis. StatPearls [Internet] Treasure Island: StatPearls Publishing; 2020. [Google Scholar]
- 2.Srigyan D, Gupta M, Behera H. Keratitis: an inflammation of cornea. EC Ophthalmol. 2017:171–7.
- 3.Srinivasan M, Mascarenhas J, Prashanth CN. Distinguishing infective versus noninfective keratitis. Indian J Ophthalmol. 2008;56(3):203–207. doi: 10.4103/0301-4738.40358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dargin JM, Lowenstein RA. The painful eye. Emerg Med Clin North Am. 2008;26(1):199–216. doi: 10.1016/j.emc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Lin A, Rhee MK, et al. Dry eye syndrome preferred practice pattern. Ophthalmology. 2019;126(1):P286–P334. doi: 10.1016/j.ophtha.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Durrani K, Zakka FR, Ahmed M, Memon M, Siddique SS, Foster CS. Systemic therapy with conventional and novel immunomodulatory agents for ocular inflammatory disease. Surv Ophthalmol. 2011;56(6):474–510. doi: 10.1016/j.survophthal.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Acthar Gel [package insert]. Bedminster: Mallinckrodt Pharmaceuticals; 2021.
- 8.Huang YJ, Galen K, Zweifel B, Brooks LR, Wright AD. Distinct binding and signaling activity of Acthar Gel compared to other melanocortin receptor agonists. J Recept Signal Transduct Res. 2020:1–9. [DOI] [PubMed]
- 9.Decker DA, Grant C, Oh L, Becker PM, Young D, Jordan S. Immunomodulatory effects of H.P. Acthar Gel on B cell development in the NZB/W F1 mouse model of systemic lupus erythematosus. Lupus. 2014;23(8):802–812. doi: 10.1177/0961203314531840. [DOI] [PubMed] [Google Scholar]
- 10.Olsen NJ, Decker DA, Higgins P, Becker PM, McAloose CA, Benko AL, et al. Direct effects of HP Acthar Gel on human B lymphocyte activation in vitro. Arthritis Res Ther. 2015;17:300. doi: 10.1186/s13075-015-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins P, Decker D, Becker P. Immunomodulatory effects of repository corticotropin injection (H.P. Acthar® gel) on the MRL/lpr model of lupus. J Immunol. 2016;196(suppl 1):210–211. [Google Scholar]
- 12.Healy LM, Jang JH, Lin YH, Rao V, Antel JP, Wright D. Melanocortin receptor mediated anti-inflammatory effect of repository corticotropin injection on human monocyte-derived macrophages [ECTRIMS-ACTRIMS abstract EP1481] Mult Scler J. 2017;23(suppl 3):777. [Google Scholar]
- 13.Benko AL, McAloose CA, Becker PM, Wright D, Sunyer T, Kawasawa YI, et al. Repository corticotrophin injection exerts direct acute effects on human B cell gene expression distinct from the actions of glucocorticoids. Clin Exp Immunol. 2018;192(1):68–81. doi: 10.1111/cei.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56(1):1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. Sci World J. 2010;10:1840–1853. doi: 10.1100/tsw.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor AW, Lee D. Applications of the role of alpha-MSH in ocular immune privilege. Adv Exp Med Biol. 2010;681:143–149. doi: 10.1007/978-1-4419-6354-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor AW. Immunosuppressive and anti-inflammatory molecules that maintain immune privilege of the eye. Reference module in neuroscience and biobehavioral psychology. Elsevier; 2017. [Google Scholar]
- 18.Furie R, Mitrane M, Zhao E, Das M, Li D, Becker PM. Efficacy and tolerability of repository corticotropin injection in patients with persistently active SLE: results of a phase 4, randomised, controlled pilot study. Lupus Sci Med. 2016;3(1):e000180. doi: 10.1136/lupus-2016-000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleischmann R, Furst DE, Connolly-Strong E, Liu J, Zhu J, Brasington R. Repository corticotropin injection for active rheumatoid arthritis despite aggressive treatment: a randomized controlled withdrawal trial. Rheumatol Ther. 2020;7(2):327–344. doi: 10.1007/s40744-020-00199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Askanase AD, Zhao E, Zhu J, Bilyk R, Furie RA. Repository corticotropin injection for persistently active systemic lupus erythematosus: results from a phase 4, multicenter, randomized, double-blind, placebo-controlled trial. Rheumatol Ther. 2020;7(4):893–908. doi: 10.1007/s40744-020-00236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan J, Miller T, Baker M, Due B, Zhao E. A prospective observational registry of repository corticotropin injection (Acthar® Gel) for the treatment of multiple sclerosis relapse. Front Neurol. 2020;11(1688):598496. doi: 10.3389/fneur.2020.598496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez VL, Ayres B, Toyos M, Toyos R. Noninfectious keratitis: an expert panel recommendation. Evolve Medical Education LLC; 2019. [Google Scholar]
- 23.Abetz L, Rajagopalan K, Mertzanis P, Begley C, Barnes R, Chalmers R, et al. Development and validation of the Impact of Dry Eye on Everyday Life (IDEEL) Questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health Qual Life Outcomes. 2011;9:111. doi: 10.1186/1477-7525-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schirmer O. Studies on the physiology and pathology of the secretion and drainage of tears. Graefe Arch Ophthal. 1903;56:197. doi: 10.1007/BF01946264. [DOI] [Google Scholar]
- 25.Fairchild CJ, Chalmers RL, Begley CG. Clinically important difference in dry eye: change in IDEEL-symptom bother. Optom Vis Sci. 2008;85(8):699–707. doi: 10.1097/OPX.0b013e3181824e0d. [DOI] [PubMed] [Google Scholar]
- 26.Mantopoulos D, Cruzat A, Hamrah P. In vivo imaging of corneal inflammation: new tools for clinical practice and research. Semin Ophthalmol. 2010;25(5–6):178–185. doi: 10.3109/08820538.2010.518542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonardi A, Flamion B, Baudouin C. Keratitis in dry eye disease and topical ciclosporin A. Ocul Immunol Inflamm. 2017;25(4):577–586. doi: 10.1080/09273948.2016.1276933. [DOI] [PubMed] [Google Scholar]
- 28.Pflugfelder SC, Stern ME. The cornea in keratoconjunctivitis sicca. Exp Eye Res. 2020;201:108295. doi: 10.1016/j.exer.2020.108295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Constad WH, Bhagat N. Keratitis sicca and dry eye syndrome. Immunol Allergy Clin North Am. 1997;17(1):89–102. doi: 10.1016/S0889-8561(05)70292-7. [DOI] [Google Scholar]
- 30.Baudouin C, Irkec M, Messmer EM, Benitez-Del-Castillo JM, Bonini S, Figueiredo FC, et al. Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018;96(2):111–119. doi: 10.1111/aos.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efron N, Morgan PB. Rethinking contact lens associated keratitis. Clin Exp Optom. 2006;89(5):280–298. doi: 10.1111/j.1444-0938.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 32.Verhelst D, Koppen C, Van Looveren J, Meheus A, Tassignon MJ, Belgian Keratitis Study G Clinical, epidemiological and cost aspects of contact lens related infectious keratitis in Belgium: results of a seven-year retrospective study. Bull Soc Belge Ophtalmol. 2005;297:7–15. [PubMed] [Google Scholar]
- 33.Yamaguchi T. Inflammatory response in dry eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES192–DES199. doi: 10.1167/iovs.17-23651. [DOI] [PubMed] [Google Scholar]
- 34.Taylor AW, Lee DJ. The alpha-melanocyte stimulating hormone induces conversion of effector T cells into treg cells. J Transplant. 2011;2011:246856. doi: 10.1155/2011/246856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright D, Zweifel B, Prabha S, Galen K, Fitch R. Reduced steriodogenic activity of repository corticotropin injection (RCI) induces a distinct cytokine response following T cell activation [EULAR abstract AB0082] Ann Rheum Dis. 2019;78(suppl 2):1504. [Google Scholar]
- 36.Askanase AD, Zhao E, Zhu J, Bilyk R, Furie RA. Repository corticotropin injection for persistently active systemic lupus erythematosus: results from a phase 4, multicenter, randomized, double-blind, placebo-controlled trial. Rheumatol Ther. 2020;7(4):839–908. doi: 10.1007/s40744-020-00236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleischmann R, Furst DE. Safety of repository corticotropin injection as an adjunctive therapy for the treatment of rheumatoid arthritis. Expert Opin Drug Saf. 2020;19(8):935–944. doi: 10.1080/14740338.2020.1779219. [DOI] [PubMed] [Google Scholar]
- 38.Patel PB, Shastri DH, Shelat PK, Shukla AK. Ophthalmic drug delivery system: challenges and approaches. Sys Rev Pharm. 2010;1(2):113–120. doi: 10.4103/0975-8453.75042. [DOI] [Google Scholar]
- 39.Vandenbroeck S, De Geest S, Dobbels F, Fieuws S, Stalmans I, Zeyen T. Prevalence and correlates of self-reported nonadherence with eye drop treatment: the Belgian Compliance Study in Ophthalmology (BCSO) J Glaucoma. 2011;20(7):414–421. doi: 10.1097/IJG.0b013e3181f7b10e. [DOI] [PubMed] [Google Scholar]
- 40.Zlatanovic G, Veselinovic D, Cekic S, Zivkovic M, Dordevic-Jocic J, Zlatanovic M. Ocular manifestation of rheumatoid arthritis-different forms and frequency. Bosn J Basic Med Sci. 2010;10(4):323–327. doi: 10.17305/bjbms.2010.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoughy SS, Tabbara KF. Ocular findings in systemic lupus erythematosus. Saudi J Ophthalmol. 2016;30(2):117–121. doi: 10.1016/j.sjopt.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available. Individual patient data may be requested if allowed per informed consent and appropriately anonymized. Requests should be sent to Mallinckrodt Pharmaceuticals’ department for Clinical Trial Disclosure and Transparency at clinicaltrials@mnk.com.