Abstract
Independent of its antiapoptotic function, Bcl-2 can, through an undetermined mechanism, retard entry into the cell cycle. Cell cycle progression requires the phosphorylation by cyclin-dependent kinases (Cdks) of retinoblastoma protein (pRB) family members to free E2F transcription factors. We have explored whether retarded cycle entry is mediated by the Cdk inhibitor p27 or the pRB family. In quiescent fibroblasts, enforced Bcl-2 expression elevated levels of both p27 and the pRB relative p130. Bcl-2 still slowed G1 progression in cells deficient in pRB but not in those lacking p27 or p130. Hence, pRB is not required, but both p27 and p130 are essential mediators. The ability of p130 to form repressive complexes with E2F4 is implicated, because the retardation by Bcl-2 was accentuated by coexpressed E2F4. A plausible relevant target of p130/E2F4 is the E2F1 gene, because Bcl-2 expression delayed E2F1 accumulation during G1 progression and overexpression of E2F1 overrode the Bcl-2 inhibition. Hence, Bcl-2 appears to retard cell cycle entry by increasing p27 and p130 levels and maintaining repressive complexes of p130 with E2F4, perhaps to delay E2F1 expression.
In addition to its well-established function in controlling cell survival, the Bcl-2 family (1) has been found to influence the cell cycle. Although Bcl-2 and its prosurvival relatives do not affect the growth rate in proliferating cultures, they can both accelerate withdrawal from the cycle (46) and retard reentry (4, 29, 30, 35). Conversely, a shortened G1 is found in lymphocytes that lack Bcl-2 (29) or express the Bcl-2 antagonist Bax (4). Thus, Bcl-2 appears to have a physiological role in influencing the transition between the quiescent and cycling states. That this ability is separate from its role in cell survival (46) is most clearly shown by mutations of Bcl-2 that eliminate its cell cycle activity but spare its antiapoptotic function (17, 45).
The cell cycle control function of Bcl-2 has ramifications for cellular homeostasis. Cycling cells are often more vulnerable to apoptosis, perhaps because, under conditions unfavorable for proliferation, certain cell cycle effectors promote apoptosis (11). Hence, promoting quiescence under conditions of stress may provide Bcl-2 with an additional, albeit indirect, means to enhance cell survival (30, 46). Interference with Bcl-2's cell cycle effect may also augment its oncogenic role (see Discussion).
Progression through the cell cycle requires the action of cyclin-dependent kinases (Cdks) (38, 39). As cells enter the cycle, newly synthesized D-type cyclins associate with and activate their Cdk-4/6 catalytic partners in mid- to late G1 phase, while cyclin E appears later in G1 and activates its Cdk-2 kinase subunit near the G1/S boundary. Opposing their activity are Cdk inhibitors (Cki) of two classes: INK4 proteins, such as p16, specifically inhibit D-cyclin kinases, whereas Cip/Kip proteins, such as p21 and p27, also inhibit Cdk-2 (38, 39). Other key negative regulators include the best-known Cdk substrates, the retinoblastoma (RB) family of nuclear “pocket” proteins: pRB itself, p130, and p107 (10, 34). They are thought to act by forming repressive complexes with E2F transcription factors, which control the expression of genes essential for cell cycle progression. Phosphorylation of the pocket protein by Cdks frees the E2F and thereby derepresses or activates E2F target genes.
The G1-S-phase transition is thought to be controlled by pRB, which binds E2Fs 1 to 3. Hence, p107 or p130, both of which bind E2F4 or E2F5 and probably regulate a distinct subset of E2F target genes (18), might govern the poorly understood exit from G0. p107 is unlikely, because its expression is restricted to cycling cells and it associates with E2F4 only late in G1, but p130 is prominent during quiescence, and p130-E2F4 complexes appear almost exclusively during G0 (20, 32, 47). Hence, regulation of E2F4 activity by p130 has been implicated in control of the G0/G1 transition, but no antiproliferative pathway is yet known to rely on p130.
How Bcl-2 favors the quiescent state is largely unknown. Neither p53 nor p16 can be essential, because Bcl-2 promoted quiescence in cells lacking those genes (35, 46). In bcl-2 transgenic cells, delayed cycle entry correlated with increased expression of p27 (4, 29), hypophosphorylated pRB (30), and increased p130 (28), but those alterations might simply be an indirect consequence of the increased proportion of noncycling cells.
To establish the negative regulators through which Bcl-2 acts, we have used cells derived from mice deficient in pRB, p130, and p27. We report that pRB is dispensable for the inhibitory effect but that both p27 and p130 are essential, and we present evidence that p130 may act through E2F4 to control the level of E2F1. These findings thus establish the framework through which Bcl-2 impacts the cell cycle. They also identify the first antiproliferative pathway requiring p130 and provide the first functional evidence that p130 plays an essential role in a pathway controlling G0 exit.
MATERIALS AND METHODS
Cell lines, expression vectors, transfection, and DNA analysis.
The RB−/− fibroblast cell lines, immortalized by the 3T3 protocol, have been described (50), and fibroblast lines were generated similarly (T. M. Upton and M. E. Ewen, unpublished) from embryonic fibroblasts of p130−/− and wild-type mice (18). The NIH 3T3 lines stably transfected with bcl-2 (cl.AH2) or its Y28A mutant (cl.FF3) were characterized previously (17).
The expression vectors pCDNA1-HAp130 (47) and pEFhBcl-2pGKpuro and the parent vector pEFpGKpuro (35) have been described previously. pGFP-E2F1 and pGFP-E2F4 were constructed by inserting the human E2F1 and E2F4 cDNAs (15), respectively, into pEGFP-C1 (Clontech).
Exponentially growing cultures of the fibroblast lines (106 cells) were electroporated (500 V and 25 μF capacitance) with 15 μg of linearized expression vector using a Gene Pulser (Bio-Rad). The cells were then resuspended in ∼44 ml of Dulbecco's modified Eagle's medium (DME) with 10% fetal bovine serum (FBS) and dispensed into 10-cm dishes, and puromycin (3 μg/ml; Sigma) was added 2 days later. Puromycin-resistant clones, picked from separate dishes to ensure independent derivation, that had closely matched levels of hBcl-2, determined by immunoblot analysis or by flow cytometric analysis using a monoclonal antibody specific for human Bcl-2 (35, 46), were chosen for study. For transient transfection, exponential cultures of control or Bcl-2-expressing fibroblasts in 10-cm dishes were lipofected with 1 μg of pEGFP-C1 together with 8 μg of pCDNA1-HAp130 or empty pCDNA1 vector or with 8 μg of pGFP-E2F4, pGFP-E2F1, or parent GFP vector. DNA premixed with 20 μl (2 mg/ml) of lipofectAMINE (Gibco-BRL) was added to the cells in antibiotic-free DME for ∼6 h according to the manufacturer's instructions. The medium was then replaced with DME–10% FBS. After incubation overnight, the cells were harvested by trypsinization and resuspended in 0.5 ml of DME–2% FBS containing 0.5 μg of propidium iodide per ml for sorting. Viable (propidium iodide excluding) cells that displayed similar levels of GFP-E2F were isolated using a FACStar Plus cell sorter (Becton Dickinson). Recovered cells were resuspended in DME–10% FBS and seeded into six-well plates (Falcon) at 5 × 104 cells/well. After 2 days they were washed and resuspended in DME–0.1% FBS and cultured for a further 5 days with medium changes on days 3 and 4 to render them quiescent. The cells were stimulated to reenter the cell cycle by replacing the medium with DME–10% FBS, and the entire contents of the well were collected after ∼20 h. Concurrent cell cycle and apoptosis analyses were performed as described previously (46).
Immunoblots and antibodies.
Immunoblotting was performed essentially as described (47) except that fibroblasts were harvested and lysed at 107 cells/ml in radioimmunoprecipitation (RIPA) buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 50 mM Tris [pH 7.5], 1 mM dithiothreitol, 50 mM NaF) containing protease inhibitors. Samples containing equivalent amounts of protein were subjected to SDS-polyacrylamide gel electrophoresis and transferred to Immobolin-P membranes (Millipore). Bands were visualized by enhanced chemiluminescence (Amersham). Where indicated, membranes were stripped by incubation in 2% SDS–100 mM β-mercaptoethanol–62.5 mM Tris-HCl (pH 6.8) at 50°C for 30 to 60 min, washed, and reprobed.
Rabbit polyclonal antibodies against p130 (C-20), p107 (C-18), p27 (C-19), p27 (N-20), Cdk-2 (M-2), cyclin E (M-20), E2F1 (C-18), and E2F4 (C-18) came from Santa Cruz Biotechnology. Mouse monoclonal antibody to HSP70 (SPA820) was from StressGen Technologies.
Transgenic mice and lymphocytes.
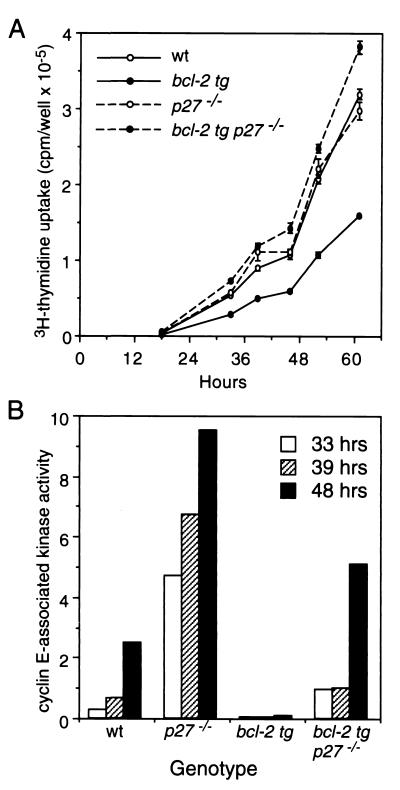
Mice carrying mutant p27 alleles (22) were crossed with Eμ-bcl-2 transgenic mice (strain bcl-2-36) (42). We used strains backcrossed onto a C57BL/6 genetic background. Mice were genotyped for p27 status and the bcl-2 transgene by PCR. The bcl-2 transgene did not alter the ratio of CD4 to CD8 single-positive lymphocytes in either the wild-type (wt) or p27−/− background (data not shown). Single-cell suspensions from spleens of 8- to 12-week-old mice were cleared of red blood cells. Incubation over nylon wool and passage through a murine T-cell isolation column (Pierce) yielded >90% pure CD4 and CD8 single-positive T cells. For cell proliferation assays, the purified T cells were seeded into 96-well plates at 2 × 106 cells/ml (0.2 ml/well) and treated with soluble anti-CD3e antibody (1.5 μg/ml) (Pharmingen, LE 01080 D) and recombinant human interleukin-2 (IL-2; 100 U/ml; Stem Cell Technologies). The cells were pulsed with [3H]thymidine (1 μCi/well) for the last 6 h prior to harvest, and thymidine incorporation was determined by liquid scintillation counting. For cyclin E-associated kinase assays, lysates were prepared from the activated lymphocytes, protein content was determined, and equivalent amounts of protein (30 to 50 μg) were immunoprecipitated as described previously (22), using 2 μg of anti-cyclin E or a control antibody.
RESULTS
Cell cycle retardation by Bcl-2 is independent of pRB.
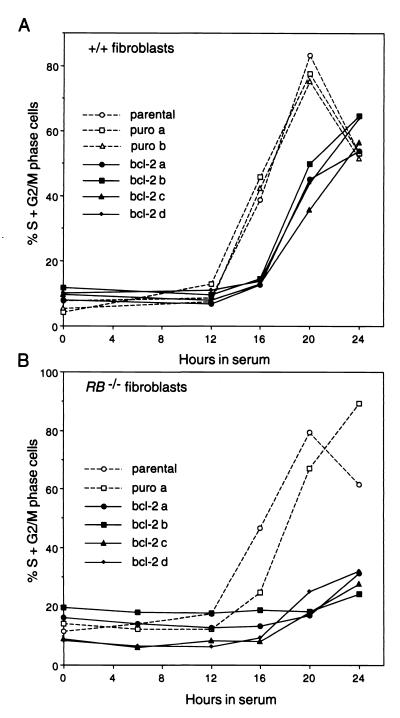
To explore whether pRB is required for the Bcl-2 cell cycle effect, 3T3 fibroblast cell lines derived from wt or pRB-null mice (50) were transfected with either an empty vector or one directing Bcl-2 expression, and independent clonal lines were isolated. After their synchronization in G0 by serum deprivation, the cells were stimulated with serum to reenter the cycle. As reported (17, 35), Bcl-2-overexpressing wt fibroblasts entered S phase more slowly than the control cells (Fig. 1A), although their rates of proliferation once in cycle were equivalent (data not shown; see also Fig. 2). In the RB−/− fibroblasts, Bcl-2 retained its ability to retard cell cycle entry (Fig. 1B). Thus, Bcl-2 can retard G0-to-S-phase progression independently of pRB. Although the experiment in Fig. 1B suggests a more pronounced effect of Bcl-2 in the RB-null cells than the wt cells, in other experiments Bcl-2 had equivalent effects in the two cell types.
FIG. 1.
Retardation of cell cycle reentry by Bcl-2 is independent of pRB. Quiescent (A) wt or (B) RB−/− fibroblasts were stimulated to reenter the cell cycle with 10% FBS, and their cell cycle distribution was analyzed at the indicated times by DNA content. The data represent the percentage of cells in S and G2/M phases relative to all cells with ≥2N DNA content for the untransfected parental cell line and independent clonal derivatives stably transfected with the empty vector (puro) or the bcl-2 expression vector. The results are representative of three similar experiments performed for each genotype.
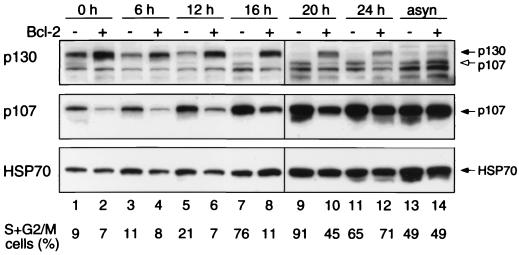
FIG. 2.
Reciprocal regulation of p130 and p107 protein levels by Bcl-2. Wt fibroblast lines, stably transfected with the control vector (odd-numbered lanes) or a vector expressing Bcl-2 (even-numbered lanes), were stimulated for the indicated times with 10% FBS to reenter the cell cycle or growing asynchronously (asyn). Immunoblot analysis was performed on lysates (50 μg of protein per lane) using an 8% polyacrylamide gel. The membrane was probed with p130 antibody, then stripped and reprobed for p107 and finally for HSP70 as a control for loading and protein integrity. (Note that HSP70 levels reproducibly increased as quiescent cells entered the cycle.) The proportion of cells in the S and G2/M phases, determined from replicate cultures, is also shown. Lanes 1 to 8 and lanes 9 to 14 were from separate gels run simultaneously in the same tank. The p130 C-terminal peptide antibody used cross-reacts with murine p107 (open arrow).
Bcl-2 augments p130 levels but decreases p107.
To address whether Bcl-2 might instead act through p130, we first assessed whether it affected the level of the protein (Fig. 2, top panel). As expected (10, 34), in the control cells the p130 level was highest in G0 and gradually fell as cells went into cycle, becoming almost undetectable by 16 h (early to mid-S phase) (lane 7). In the presence of Bcl-2, however, p130 was elevated during quiescence (Fig. 2, top panel, c.f. lanes 1 and 2) and persisted for at least 24 h (lane 12). The elevation in p130 levels is unlikely to be an indirect consequence of retarded entry into cycle because it was observed even in the quiescent cells and the p130 level remained higher in the Bcl-2 line even when its proportion of cycling cells was similar to the control (e.g., compare 24-h Bcl-2 cells in lane 12 with 16-h control cells in lane 7). Thus, p130 is likely to lie on the pathway regulated by Bcl-2.
The p107 levels were also influenced by Bcl-2, but in the opposite manner (Fig. 2, middle panel). As expected (10, 34), p107 levels in the control cells were lowest in quiescence and gradually rose. The resting cells expressing Bcl-2 displayed less p107 than the controls (compare lanes 1 and 2), and the increase was delayed (lanes 7 and 8), although their p107 level approached that of the controls by S phase (lanes 11 and 12) and remained comparable in asynchronous cultures (lanes 13 and 14). Reprobing for the housekeeping protein HSP70 (lower panel) confirmed that the differences did not reflect variations in loading or transfer. The concomitant rise in p130 and fall in p107 is consistent with the ability of p130 to regulate p107 transcription (see Discussion).
p130 is essential for the retardation of cycle reentry by Bcl-2.
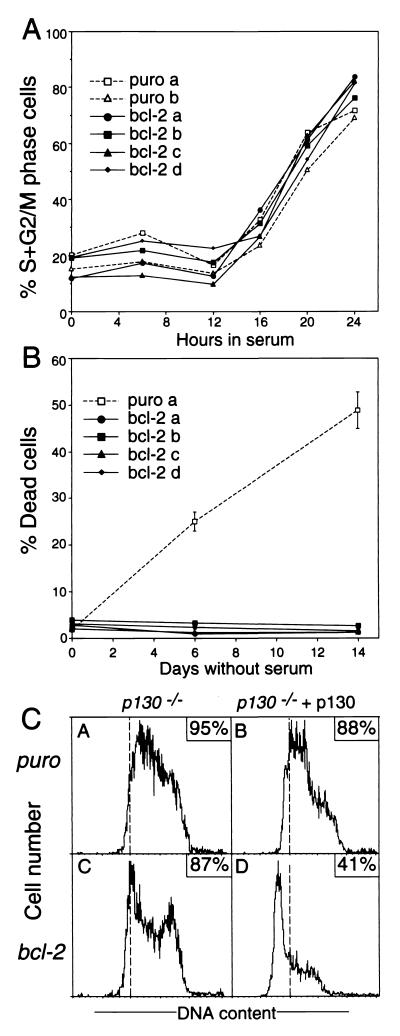
To test whether p130 was an essential mediator of the retardation by Bcl-2, we investigated whether Bcl-2 retarded cycle entry in fibroblast lines derived from mice lacking p130 (8). Notably, Bcl-2 did not significantly affect their cycle entry (Fig. 3A). This result did not reflect an inadequate Bcl-2 level, because the levels in these lines were comparable to those in the transfectants in Fig. 1A (data not shown), and the transgene prevented apoptosis in serum-deprived cultures of the p130−/− fibroblasts (Fig. 3B). Furthermore, the inability of Bcl-2 to retard cell cycle entry of the p130-deficient 3T3 fibroblasts was specifically due to loss of the p130 gene and not some other mutation arising during immortalization, because reintroduction of p130 restored this effect of Bcl-2 (Fig. 3C, compare subpanels C and D). The ectopic p130 had little effect on the cycling of the control p130−/− cells (compare subpanels A and B), even though its level was similar in the two lines (data not shown). We conclude that p130 is essential for the retardation of cell cycle entry by Bcl-2 and hence must have a function in G0-S-phase control that cannot be met by p107 or pRB.
FIG. 3.
Bcl-2 fails to retard cell cycle reentry of p130−/− fibroblasts. (A) Kinetics of cell cycle reentry of p130−/− fibroblast lines. Quiescent p130−/− fibroblasts were stimulated by addition of 10% FBS, and their cell cycle distribution was followed. The results, shown for two independent control cell lines (puro) and four independent Bcl-2-expressing lines, are representative of four similar experiments. (B) Bcl-2 enhances survival of p130−/− fibroblasts deprived of serum. The p130−/− fibroblasts were incubated in medium without any FBS, and cell death was monitored (see Materials and Methods). The results, shown for one of the control cell lines and the four Bcl-2 lines used in A, depict the averages ± SEM of triplicate cultures and are representative of two separate experiments. (C) Reintroduction of p130 restores the cell cycle-inhibitory activity of Bcl-2 in p130−/− fibroblasts. The p130−/− fibroblasts stably expressing control puro vector (subpanels A and B) or the Bcl-2 vector (subpanels C and D) were transiently cotransfected with GFP and the p130 or empty vector (control) as indicated. Viable GFP-expressing cells were isolated, cultured for another 2 days, and rendered quiescent by serum deprivation (see Materials and Methods). DNA content was determined 20 h after serum addition. Boxed numbers are the percentages of the live cells that were in the S and G2/M phases.
Bcl-2 retards E2F1 accumulation.
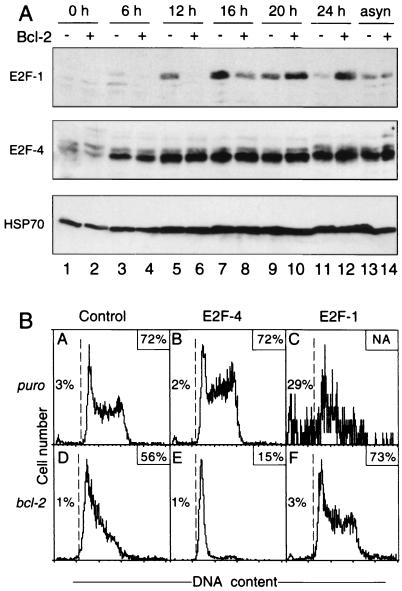
The ability of p130 to restrain cell cycle progression probably involves its complexes with E2F4 (47), which can repress the gene for E2F1, a critical inducer of cell cycle entry (see Discussion). Since the rise in E2F1 protein during G0-S-phase progression largely reflects increased transcription (10, 34), we reasoned that if Bcl-2 acted through p130-E2F4 complexes, it might retard the accumulation of E2F1. Indeed, Figure 4A shows that to be the case. As expected (15), E2F1 levels in the control fibroblasts peaked at 16 h (lane 7) and then declined rapidly. In the Bcl-2-expressing cells, E2F1 appeared later (compare 12-h data in lanes 5 and 6) and reached a plateau at 20 h (lane 10). Bcl-2 did not affect the level of E2F1 in asynchronously growing fibroblasts (lanes 13 and 14) or retard the serum-induced appearance of E2F1 in p130−/− fibroblasts (data not shown). Nor did Bcl-2 affect the E2F4 level (Fig. 4A), which is low in quiescent cells and increases with entry into cycle (15, 47). Thus, the retarded accumulation of E2F1 provoked by Bcl-2 paralleled the retarded cell cycle entry.
FIG. 4.
E2F4 accentuates but E2F1 overrides the retardation by Bcl-2. (A) Bcl-2 retards expression of E2F1 but not E2F4 during G0-S-phase progression. The wt fibroblast lines stably transfected with the control vector (odd-numbered lanes) or that expressing Bcl-2 (even-numbered lanes) were stimulated for the indicated times with 10% FBS or growing asynchronously (asyn). Immunoblot analysis was then performed on lysates containing 50 μg of protein using a 7.5% polyacrylamide gel. The membrane was probed with E2F1 antibody, stripped and reprobed for E2F4, and finally for HSP70. (B) Cell cycle inhibition by Bcl-2 is enhanced by E2F4 but overcome by E2F1. A wt fibroblast line bearing the control puro vector (subpanels A to C) or one that expresses Bcl-2 (subpanels D to F) was transiently transfected with the GFP vector GFP-E2F-4 or GFP-E2F-1, as indicated. Viable GFP-expressing cells were isolated, cultured for another 2 days, and rendered quiescent by serum deprivation (see Materials and Methods). DNA content was determined 20 h after serum addition. The boxed number in each subpanel is the percentage of cells in S and G2/M phases relative to all cells with ≥2N DNA content (NA, not applicable); the other number in each panel is the percentage of dead (i.e., subdiploid) cells to the left of the dashed line that arose during the serum treatment. The data were reproduced on four separate occasions.
Bcl-2 effect was augmented by E2F4 but overridden by E2F1.
Our evidence that Bcl-2 retards the elevation of E2F1 but not E2F4 is consistent with a model in which Bcl-2, by promoting formation of p130-E2F4 complexes, represses E2F1 gene expression (see Discussion). To explore the functional consequences of this model, we tested the effects of coexpressing E2F1 or E2F4 with Bcl-2 in transiently transfected fibroblasts. To facilitate isolation of transfectants and to ensure that transfectant pools contained comparable E2F levels, some experiments used E2Fs having a green fluorescent protein (GFP) tag, but non-GFP-tagged E2Fs gave similar results. Figure 4B shows the cell cycle analysis of the transfectants rendered quiescent by serum starvation for 5 days and restimulated with serum for 20 h.
E2F4 alone had little effect on the proportion of cells residing in the S and G2/M phases (∼70%) (Fig. 4B, compare subpanels A and B), but its coexpression with Bcl-2 profoundly inhibited cycling: only ∼15% of cells expressing both were in those phases (subpanel E), versus ∼55% of those expressing Bcl-2 alone (subpanel D). This result reflects retention in G0/G1 rather than accelerated entry into the next G1 phase, because cells prevented from exiting mitosis by nocodazole behaved similarly (data not shown). Thus, E2F4 enhanced the retardation by Bcl-2. In striking contrast, coexpression of E2F1 with Bcl-2 increased the cycling population to the levels in control cells without Bcl-2 (compare subpanels F and A). This result may implicate the E2F1 gene as a target for inhibition by Bcl-2 (see Discussion).
Ectopic E2F1 but not E2F4 induces apoptosis (9, 43). Accordingly, almost no cells expressing E2F4 alone were subdiploid, i.e., apoptotic (Fig. 4B, subpanel B), whereas few E2F1 transfectants lacking the bcl-2 transgene remained at harvest, and a substantial fraction of those (29%) were apoptotic (subpanel C)—the scatter reflects the low cell numbers remaining for analysis. The decreased cell number and accumulation of apoptotic cells by E2F1 was completely blocked by coexpression of Bcl-2 (subpanel F).
To account for the synergy between E2F4 and Bcl-2 in retarding entry into cycle, we suggest that the ectopic E2F4 together with the increased p130 levels produced by Bcl-2 (see Fig. 2) generate higher levels of repressive p130-E2F4 complexes (see Discussion). Accordingly, the amount of endogenous E2F4 that coprecipitated with p130 was ∼40% higher in quiescent Bcl-2-expressing fibroblasts than in quiescent control cells (data not shown), even though the total E2F4 level was the same (Fig. 4A). That the introduced E2F4 cooperates with Bcl-2 rather than overcoming its cell cycle effect, as might have been expected, is probably due to the relatively modest levels of ectopic E2Fs achieved under the serum-deprived conditions employed (data not shown). Although the marked difference between the effects of E2F4 and E2F1 is consistent with evidence that E2F1 is a much more potent promoter of cycle progression (9), to our knowledge this is the first evidence that ectopic E2F4 can under certain conditions act to restrain G0-S-phase transit.
Enhanced p27 accumulation requires a critical Bcl-2 N-terminal residue.
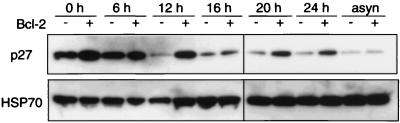
Since Bcl-2-overexpressing lymphocytes have elevated p27 levels (4, 29), we examined whether Bcl-2 also affected p27 levels in fibroblasts entering the cell cycle. As expected (39), the p27 level in the control fibroblasts was maximal in quiescence and almost indiscernible by 12 h (Fig. 5A), as they began to enter S phase (see cell cycle data in Fig. 2). Although Bcl-2 did not affect the p27 level during asynchronous growth, its level was augmented in quiescent cells (compare Fig. 5A, lanes 1 and 2) and remained elevated for 16 to 20 h. Like the elevation in p130, the increase in p27 in the quiescent cells is compatible with a direct effect of Bcl-2 rather than an indirect consequence of slower cell cycle progression. As in other cell types (4, 29), Bcl-2 did not alter the level of the closely related Cki p21 (data not shown). As expected from the ability of p27 to inhibit Cdk-2 activity, Bcl-2-expressing cells emerging from quiescence displayed slower Cdk-2 activation than control fibroblasts (data not shown, but Fig. 7B provides data for lymphocytes).
FIG. 5.
Accumulation of p27 induced by Bcl-2 requires a critical N-terminal residue. (A) Bcl-2 enhances p27 levels in wt fibroblasts. Wt fibroblast lines stably transfected with the control vector (odd-numbered lanes) or that expressing Bcl-2 (even-numbered lanes) were stimulated for the indicated times with 10% FBS to reenter the cycle or growing asynchronously (asyn). The cells were collected at the indicated times, lysates were prepared, and p27 and HSP70 levels were determined. The immunoblots used a 12% polyacrylamide gel with 50 μg of protein per lane. (B) Tyrosine 28 of Bcl-2 is required for increased p27 levels. Quiescent NIH 3T3 fibroblasts which overexpressed either wt Bcl-2 or the Y28A mutant were either left untreated or stimulated with 10% FBS for 12 h prior to collection for immunoblot analysis.
FIG. 7.
Inhibition by Bcl-2 of S-phase entry and cyclin E-associated kinase activity in T lymphocytes requires p27. (A) To assess entry into cycle, splenic T cells isolated from littermates of the four genotypes were stimulated with IL-2 and CD3 antibody. Six hours before harvest, the cells were pulsed with [3H]thymidine, and incorporation of label was determined. The data represent the averages ± SEM of at least five replicate wells; at least four mice of each genotype yielded similar results. (B) To assay cyclin E-associated kinase activity, equivalent amounts of protein from extracts of T cells stimulated as above were immunoprecipitated with cyclin E antibody, and the associated histone H1 kinase activity was measured (arbitrary units).
The cell cycle effects of Bcl-2 depend upon a region near its N terminus: a Bcl-2 mutant with alanine replacing tyrosine 28 (Y28A) cannot retard cycle entry of fibroblasts but retains full survival activity (17). To determine whether tyrosine 28 was required for the p27 accumulation, we compared p27 levels in NIH 3T3 cell lines that stably overexpress comparable amounts of either wt Bcl-2 or the Y28A mutant (17), both during quiescence and 12 h after readdition of serum. The p27 levels fell considerably more in the cells expressing the Y28A mutant than in those expressing wt Bcl-2 (Fig. 5B). Hence, maintenance of elevated p27 levels requires a residue essential for the cell cycle effects of Bcl-2.
Bcl-2 elevates p27 independently of cell cycle retardation.
Could the elevated p27 levels be a consequence rather than a cause of the retarded cell cycle entry? The failure of Bcl-2 to affect cycle entry for cells deficient in p130 (Fig. 3) prompted us to use those cells to determine whether p27 levels also rose when cell cycle entry was unperturbed (Fig. 6). As in wt fibroblasts (see Fig. 5A), p27 levels were augmented by Bcl-2 in the quiescent cells and declined more slowly. Hence, the rise in p27 does not merely reflect the slower cell cycle kinetics normally elicited by Bcl-2. This finding also establishes that the inhibitory signals still emanate from Bcl-2 in the p130−/− cell line. The increased p27 is likely to be functional, because Cdk-2 kinase activity in the p130−/− cells was reduced by Bcl-2 (data not shown).
FIG. 6.
Bcl-2 directly enhances p27 levels, independently of p130-mediated cell cycle effects. The p130−/− fibroblast-transfected lines were either growing asynchronously (asyn) or had been stimulated for the indicated times with 10% FBS to reenter the cell cycle. The immunoblots (50 μg of protein per lane) involved a 4 to 20% polyacrylamide gradient minigel (Novex). The line separating the 16-h and 20-h lanes indicates that the data came from different gels run simultaneously in the same tank. The less pronounced effect of Bcl-2 at the 16-h time point compared to later times was not seen in other experiments and reflects experimental variation.
p27 is essential for cell cycle retardation by Bcl-2 in lymphocytes.
We next wished to determine whether p27 was required for the Bcl-2 effect. As immortal p27−/− fibroblast lines were unavailable and the limited proliferative potential of primary p27−/− fibroblasts (7) renders them unsuitable for stable transfection, we instead examined the entry of nullizygous T lymphocytes into cycle. Splenic T cells from either wt or p27−/− mice that either did or did not express a bcl-2 transgene were stimulated with IL-2 and an agonistic antibody to the T-cell antigen receptor (anti-CD3). Figure 7A compares the cell cycle entry, measured by [3H]thymidine incorporation, for the four genotypes. As expected, the bcl-2 transgene slowed entry of the p27+/+ cells into cycle. Consistent with other reports (7, 22), the rate of cycle entry was similar for the wt and p27−/− lymphocytes. Importantly, the inhibition by Bcl-2 was completely reversed in lymphocytes lacking p27. The elevated p27 levels must therefore be essential for the cell cycle retardation by Bcl-2, and other Cki such as p21 cannot functionally compensate.
Because p27 is a major regulator of cyclin E and Cdk-2 activity in lymphocytes (7), we also followed cyclin E-associated kinase activity as cells progressed into S phase (Fig. 7B). As previously found, loss of p27 significantly elevated the kinase activity. In the p27+/+ cells, Bcl-2 expression markedly suppressed kinase activity, consistent with its ability to enhance p27 levels (see above). However, Bcl-2 also significantly reduced kinase activity in cells that lacked p27, even though the activity remained higher than in the nontransgenic p27+/+ cells and was therefore unlikely to be limiting for cell cycle progression, as was observed. This unexpected result indicates that Bcl-2 can also inhibit cyclin E and Cdk-2 kinase activity via a p27-independent mechanism. Although normal mouse lymphocytes usually have a relatively low level of p130, the ability of Bcl-2 to enhance p130 levels might nevertheless be involved, because p130, under certain conditions, can functionally substitute for loss of p27 (7).
DISCUSSION
Bcl-2 is functionally linked to negative regulators of the cell cycle.
By exploiting cells lacking important cell cycle inhibitors, we have established the framework by which Bcl-2 interacts with the cell cycle machinery (Fig. 8). Consistent with earlier evidence that Bcl-2 acts primarily during entry into G1 (see Introduction), pRB, which controls G1/S progression, proved dispensable for the Bcl-2 effect. However, Bcl-2 increased the levels of its relative p130 and of the Cdk inhibitor p27, both previously implicated in control of G0 exit. The increases in both inhibitors elicited by Bcl-2 in quiescent cells argue for a direct effect rather than an indirect effect of the cell cycle perturbation. Importantly, the results with cells lacking each inhibitor showed that both p130 and p27 were essential for the Bcl-2 cell cycle effect. Moreover, because the p27 increase did not require p130, it cannot be merely a consequence of slower cycle entry (Fig. 6). Hence, as would be expected (40), p27 appears to act upstream of p130 or in parallel with it, probably by inhibiting Cdk-2 activity. It is likely that p130 functions mainly via its ability to complex with E2F4. Thus, the simplest interpretation of our results is that, by enhancing the levels of p27 and p130, Bcl-2 produces repressive p130-E2F4 complexes that inhibit cell cycle entry (Fig. 8).
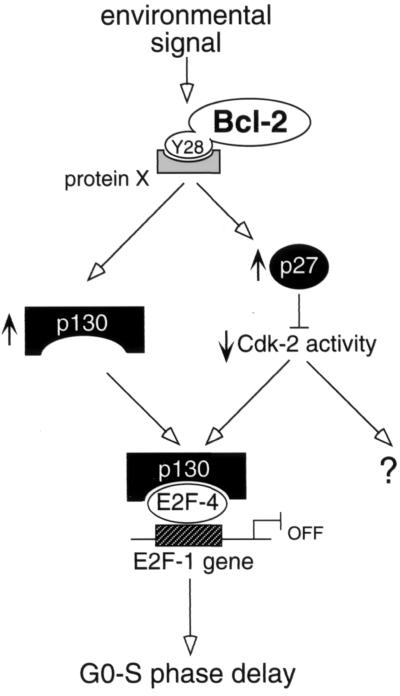
FIG. 8.
Model for the cell cycle-inhibitory effect of Bcl-2, based on the findings presented here and current understanding of cell cycle control (see text). In normal quiescent cells having only a low Bcl-2 level, mitogenic stimulation inhibits p27 synthesis and provokes destruction of both p130 and p27, facilitating cell cycle entry. The enhanced Bcl-2 activity induced by certain environmental cues, however, increases the levels of both p27 and p130. Bcl-2 presumably acts through the binding of its N-terminal domain near tyrosine 28 to an unknown protein (X here). The increased p27 levels lower the kinase activity of Cdk-2, and one consequence is the maintenance of repressive pocket protein-E2F4 complexes; for example, the phosphorylation of p130 and p27 by Cdk-2 can target them for destruction. The resulting increase in p130 also promotes a higher level of p130-E2F4 complexes, and they repress transcription of a gene (such as E2F1) encoding a product that allows cell cycle entry. The p130-E2F4 complexes thus have an essential role in regulating G0 exit. Key features of the model therefore are that Bcl-2 acts via p27 and p130 to maintain p130-E2F4 complexes, which then repress a gene, such as that for E2F1, which is required for entry from quiescence into cycle.
Role of pocket proteins and E2Fs.
Previously, p130 was implicated in control of G0 exit by its expression pattern, timing of association with E2Fs, and the arrest elicited by its overexpression (10, 34). Its inhibitory activity is ascribed to its ability to bind to and negatively regulate E2F4, thereby repressing E2F4 target genes required for cell cycle entry (47). The finding that E2F4 but not E2F1 (see below) potently cooperated with Bcl-2 to retard entry (Fig. 4B) supports our suggestion that Bcl-2 acts by increasing the repressive complexes of E2F4 with pocket proteins. Since E2F4 binds predominantly to p130 during quiescence (20, 32, 47), p130-E2F4 may govern the G0/G1 transition, much as pRB-E2F1 (or E2F3) does the G1/S transition (47). The evidence that these partnerships regulate distinct subsets of genes is growing (9, 18, 49). Our findings support such models, because the Bcl-2 cell cycle effects are confined to the G0-S transition (17, 35, 46). Thus, p130 has for the first time been shown to have an essential role in a pathway controlling G0 exit. It seems relevant that p130-E2F4 complexes have been shown recently to accumulate in cells treated with the growth inhibitors transforming growth factor beta (19) and alpha interferon (14).
As indicated in Fig. 8, the E2F1 gene is a plausible candidate for a relevant E2F4 target. Repression of E2F1 during G0/early G1 requires the E2F sites within its promoter (10, 34) and involves p130 (21). E2F4 is now thought to control induction of E2F1 expression during cycle entry (14). Importantly, E2F1 has the remarkable ability to push quiescent cells into cycle (10). Moreover, E2F1 loss delays G0-S phase entry but is dispensable in actively dividing cells (48), features that mirror the cell cycle effects of Bcl-2 in diverse cells (see Introduction). We found that Bcl-2 retarded the serum-mediated induction of E2F1 by several hours (Fig. 4A), in good agreement with the G0-S delay caused by Bcl-2. Furthermore, overexpression of E2F1 overcame cell cycle inhibition by Bcl-2 (Fig. 4B), as expected if Bcl-2 acted by negatively regulating its level. Collectively, these considerations implicate E2F1 as a critical regulator affected by Bcl-2 during exit from quiescence. Although potential involvement of E2Fs 2 and 3, which are also subject to E2F-mediated repression (34), cannot be excluded, neither is likely, because E2F2 protein and DNA-binding activity are essentially undetectable in quiescent cells entering the cell cycle (26, 32) and E2F3 appears to be needed mainly after cells have begun cycling (26). Other potential targets for p130-E2F4 repression include the genes for cyclin E (24) and Cdc25A (19), which are subject to E2F control in early to mid-G1, but unlike E2F1, their cell cycle roles are not restricted to quiescent cells entering the cell cycle.
The activity of E2F1 is controlled not only by its level but also by its inhibitory interaction with pRB. However, loss of pRB, which might be expected to free E2F1 to induce its target genes, did not overcome the effect of Bcl-2 (Fig. 1B). This is consistent with earlier findings indicating that loss of pRB is not necessarily functionally equivalent to deregulated E2F1 expression. For instance, although ectopic E2F1 is sufficient to drive cells into S phase (10, 34), this process remains mitogen dependent in RB-null cells (see reference 18 for an example). This seeming paradox might be due to the known ability of other pocket proteins such as p107 to functionally compensate for the absence of pRB (25, 37).
Interestingly, the activities of p130 and p107 appear to be coupled. Their reciprocal expression has been noted previously, with p130 prevalent in quiescent cells and p107 prevalent during proliferation (10, 34). Bcl-2 expression during cell cycle reentry dramatically increased p130 and concomitantly reduced p107 levels. The reciprocal effects may well reflect the ability of p130 to repress transcription of the p107 gene in an E2F-dependent manner (34, 41, 51). Hence, the drop in p107 expression is likely to be secondary to enhanced p130 function. Our hypothesis that Bcl-2 acts through inhibitory E2F4 complexes is supported by the demonstration that Bcl-2 reduces expression of both p107 and E2F1, genes normally repressed during G0/early G1, in an E2F-dependent manner.
Role of p27.
The function of p27 is strongly linked to control of G0-S progression. Its overexpression induces G1 arrest, while ablation of its synthesis delays withdrawal from the cell cycle. In normal cells, p27 is elevated during quiescence and declines as cells enter the cycle, whereas the related p21 is low during quiescence and increases in cycling cells (38, 39). As reported for lymphocytes (4, 29), Bcl-2 markedly enhanced p27 levels in quiescent fibroblasts, whereas no effects on the p21 level have been observed by us or others. The elevation in p27 required tyrosine 28 of Bcl-2, which is required specifically for its cell cycle effects (17). The rise in p27 cannot be merely an indirect consequence of delayed cell cycle entry, because p27 also increased in fibroblasts lacking p130 (Fig. 6), in which Bcl-2 did not influence the cell cycle. Hence, Bcl-2 influences p27 levels in a direct fashion, independent of p130.
It might seem puzzling that the increase in p27 caused by Bcl-2 was insufficient to retard cell cycle entry in cells that lack p130. G1 progression is thought to be determined, however, by a balance between the p27 level and Cdk-2 activity, the so-called threshold model (38, 39). Cells lacking p130 have elevated Cdk-2 activity (5; M. Lahda, G. Vairo, and M. E. Ewen, unpublished), probably because p130 can bind to and inhibit cyclin E/A-Cdk-2 kinases via a domain similar to those in p21 and p27 (see reference 5 and references therein). Accordingly, the threshold model predicts that elevated levels of p27 would be required to overcome the increased Cdk-2 activity in p130−/− cells. Thus, in addition to repressing E2F4 target genes, enhanced p130 function apparently can also raise the threshold needed for Cdk-2 activity.
Consistent with this notion, in fibroblasts lacking p27 or p21, p130 can assume the role of negatively regulating Cdk-2 activity (7). In lymphocytes, however, our data suggest that p130 cannot compensate for loss of p27, probably because lymphocytes have a much lower basal level of p130. Thus, the relative contribution of p130 and p27 to the cell cycle-inhibitory effect of Bcl-2 may well vary in different cell types, particularly in those where one of the two regulators is present in limiting amounts. Nevertheless, in cells such as fibroblasts where we found that Bcl-2 increased expression of both p130 and p27, it seems very likely that the increased p27, via Cdk-2 inhibition, enhances the cell cycle-inhibitory activity of p130. That hypothesis is consistent with evidence that the ability of p130, like pRB, to bind E2Fs and inhibit cell cycle progression is regulated by phosphorylation (10, 34). Cdk-2 is a likely kinase, because the phosphorylation of p130 coincides with Cdk-2 activation and is prevented by ectopic p21 (41), while cyclins E and A can overcome the inhibitory effects of p130 (6). In further support of a functional role for p27 upstream of p130 and E2F, its conditional expression provoked accumulation of p130-E2F complexes and inhibited E2F-dependent transcription (40). Moreover, E2F1 can override the arrest caused by inhibition of Cdk-2 activity and must therefore act downstream of it (10). Hence, we propose that p27 mediates the cell cycle-inhibitory effects of Bcl-2 at least in part by inhibiting the phosphorylation of p130 by Cdk-2, thereby increasing the abundance of repressive p130-E2F4 complexes.
Our evidence that p130 but not pRB is required for the Bcl-2 effect implicates p130 as an important mediator of cell cycle control by Cip/Kip proteins and is consistent with their ability to inhibit cell cycle passage independently of pRB (38, 39). However, the regulation of other proteins by Cdk-2 phosphorylation presumably would also be modulated by p27, and those unknown Cdk-2 substrates might also contribute to the cell cycle-inhibitory effects of Bcl-2.
How might Bcl-2 influence p27 and p130 levels?
Bcl-2 affected p130 and p27 similarly: both were elevated during G0 and persisted longer during cell cycle reentry. Their levels are governed predominantly by posttranscriptional mechanisms that include regulated destruction by the proteasome pathway (10, 34, 38, 39) and, at least for p27, translational control (see below). A report that both p27 and p21 are caspase targets (27) suggested that Bcl-2 might enhance p27 levels through inhibition of caspase activation, but several considerations make that unlikely. First, despite the similar putative caspase recognition site in p21, its levels increase as cells reenter the cycle. Second, the proline in the putative caspase site of human p27 (27) would make it a poor substrate for the known caspases (44). Finally, immunoblots using an antibody to the N terminus of p27 revealed no C-terminal processing of p27 during entry of fibroblasts into cycle, even though p27 levels declined notably (G. Vairo, unpublished results).
Interference with proteasome-mediated destruction cannot readily account for the p27 and p130 increases induced by Bcl-2 in quiescent cells, because that mechanism occurs only during S-phase progression after they have been targeted through phosphorylation by cyclin E and Cdk-2 (34, 38, 39, 41). However, in G0 cells, there is growing evidence for translational control of at least p27 (2, 16, 31). Mitogenic and antimitogenic signals determine the frequency with which p27 mRNA is translated by controlling factors that bind to its untranslated regions (Millard et al., unpublished results). Hence, we favor the notion that Bcl-2 increases the p27 level in quiescent cells by enhancing translation of p27 mRNA. The elevated p27 levels in quiescent cells would prolong the period during which p27 remained above the threshold required to inhibit Cdk-2 activity and accordingly prolong the G0-S-phase interval. The p130 increase might also involve a translational mechanism, but because less is established about regulation of its levels, other mechanisms such as protein stability might be relevant.
Whatever the mechanism, the N-terminal region of Bcl-2 near tyrosine 28 is strongly implicated as the critical binding site for a potential regulator of its cell cycle effects (17). Importantly, that residue was shown here to be required for the rise in p27 (Fig. 5). The relevant Bcl-2-binding protein (X in Fig. 8) remains unknown, but Raf-1 and calcineurin have been reported to bind to that portion of Bcl-2 (see reference 1 for a review).
Significance of Bcl-2 cell cycle activity for oncogenesis.
The antiproliferative activity of Bcl-2 may have evolved to modulate the oncogenic potential of excessive cell survival (1, 11, 35, 46). In accord with that notion, a recent study demonstrated a tumor suppressor activity of Bcl-2 that correlated with its ability to restrain proliferation (33). Conversely, in T-antigen-dependent mammary tumor development, the ability of Bcl-2 to accelerate tumor progression correlated with a selective loss of its antiproliferative activity (13). Our data may provide a molecular explanation for that result, because T-antigen transformation requires its ability to inactivate the pocket proteins, including p130 (10, 34), which we have shown to be essential for the proliferation-restraining effect of Bcl-2.
Evidence that the cell cycle-inhibitory activity of Bcl-2 may impact on human cancers comes from findings that the translocated bcl-2 gene in follicular lymphoma is often mutated in regions associated with its cell cycle activity (1). Moreover, elevated Bcl-2 levels are associated with decreased proliferation and sometimes also a favorable prognosis in diverse malignancies, including breast and colorectal cancer (3, 23) and multiple myeloma (36). Our evidence that p27 is a key downstream target of Bcl-2 seems relevant, because in many tumor types, elevated p27 is strongly associated with a favorable outcome (39). Our results suggest that, as well as specific mutations in Bcl-2, loss of p130 or p27 function (or gain of E2F1 function) would diminish the Bcl-2 cycle-inhibitory activity and potentially enhance its oncogenic impact. Although no mutations of p130 in human tumors have yet been reported (10, 34), a disrupted p27 allele can contribute to tumorigenesis (12). Thus, lesions in any of the steps in the cell cycle pathway extending from Bcl-2 (Fig. 8) could contribute to the development of several types of tumors.
ACKNOWLEDGMENTS
We thank G. Lindeman, B. Warner, and E. Harlow for discussions; T. Diem and L.-C. Zhang for expert technical assistance; M. Ladha for performing some of the Cdk-2 assays; and J. Birtles and G. Filby for secretarial assistance. We also thank N. Saunders, D. C. S. Huang, L. A. O'Reilly, A. Strasser, J. Nikolic-Zuglic, D. Ginsberg, W. Krek, D. Livingston, N. Dyson, T. Jacks, and E. Harlow for their kind gifts of plasmids, antibodies, or cells and are grateful to the WEHI flow cytometry facility for assistance with cell sorting.
This work was supported by grants from the National Health and Medical Research Council of Australia (Reg. Key 973002) and the U.S. National Institutes of Health (CA80188 and CA43540). G.V. was supported by a Queen Elizabeth II Fellowship from the Australian Research Council and by an AMRAD Corporation Postdoctoral Award.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal D, Hauser P, McPherson F, Dong F, Garcia A, Pledger W J. Repression of p27kip1 synthesis by platelet-derived growth factor in BALB/c 3T3 cells. Mol Cell Biol. 1996;16:4327–4336. doi: 10.1128/mcb.16.8.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biden K G, Simms L A, Cummings M, Buttenshaw R, Schoch E, Searle J, Gobe G, Jass J R, Meltzer S J, Leggett B A, Young J. Expression of Bcl-2 protein is decreased in colorectal adenocarcinomas with microsatellite instability. Oncogene. 1999;18:1245–1249. doi: 10.1038/sj.onc.1202413. [DOI] [PubMed] [Google Scholar]
- 4.Brady H J M, Gil-Gómez G, Kirberg J, Berns A J M. Baxα perturbs T cell development and affects cell cycle entry of T cells. EMBO J. 1996;15:6991–7001. [PMC free article] [PubMed] [Google Scholar]
- 5.Castaño E, Kleyner Y, Dynlacht B D. Dual cyclin-binding domains are required for p107 to function as a kinase inhibitor. Mol Cell Biol. 1998;18:5380–5391. doi: 10.1128/mcb.18.9.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claudio P P, De Luca A, Howard C M, Baldi A, Firpo E J, Koff A, Paggi M G, Giordano A. Functional analysis of pRb2/p130 interaction with cyclins. Cancer Res. 1996;56:2003–2008. [PubMed] [Google Scholar]
- 7.Coats S, Whyte P, Fero M L, Lacy S, Chung G, Randel E, Firpo E, Roberts J M. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27Kip1-deficient cells. Curr Biol. 1999;9:163–173. doi: 10.1016/s0960-9822(99)80086-4. [DOI] [PubMed] [Google Scholar]
- 8.Cobrinik D, Lee M-H, Hannon G, Mulligan G, Bronson R T, Dyson N, Harlow E, Beach D, Weinberg R A, Jacks T. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- 9.DeGregori J, Leone G, Miron A, Jakoi L, Nevins J R. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci USA. 1997;94:7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 11.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1321. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 12.Fero M L, Randel E, Gurley K E, Roberts J M, Kemp C J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furth P A, Bar-Peled U, Li M, Lewis A, Laucirica R, Jäger R, Weiher H, Russell R G. Loss of anti-mitotic effects of Bcl-2 with retention of anti-apoptotic activity during tumor progression in a mouse model. Oncogene. 1999;18:6589–6596. doi: 10.1038/sj.onc.1203073. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa Y, Iwase S, Kikuchi J, Nakamura M, Yamada H, Matsuda M. Transcriptional repression of the E2F-1 gene by interferon-α is mediated through induction of E2F-4/pRB and E2F-4/p130 complexes. Oncogene. 1999;18:2003–2014. doi: 10.1038/sj.onc.1202500. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg D, Vairo G, Chittenden T, Xiao Z X, Xu G, Wydner K L, DeCaprio J A, Lawrence J B, Livingston D M. E2F-4, a new member of the E2F transcription factor family, interacts with p107. Genes Dev. 1994;8:2665–2679. doi: 10.1101/gad.8.22.2665. [DOI] [PubMed] [Google Scholar]
- 16.Hengst L, Reed S I. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 17.Huang D C S, O'Reilly L A, Strasser A, Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurford R K, Jr, Cobrinik D, Lee M-H, Dyson N. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 1997;11:1447–1463. doi: 10.1101/gad.11.11.1447. [DOI] [PubMed] [Google Scholar]
- 19.Iavarone A, Massague J. E2F and histone deacetylase mediate transforming growth factor beta repression of cdc25A during keratinocyte cell cycle arrest. Mol Cell Biol. 1999;19:916–922. doi: 10.1128/mcb.19.1.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda M, Jakoi L, Nevins J R. A unique role for the Rb protein in controlling E2F accumulation during cell growth and differentiation. Proc Natl Acad Sci USA. 1996;93:3215–3220. doi: 10.1073/pnas.93.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson D G. Regulation of E2F-1 gene expression by p130 (Rb2) and D-type cyclin kinase activity. Oncogene. 1995;11:1685–1692. [PubMed] [Google Scholar]
- 22.Kiyokawa H, Kineman R D, Manova-Todorova K A, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 23.Knowlton K, Mancini M, Creason S, Morales C, Hockenbery D, Anderson B O. Bcl-2 slows in vitro breast cancer growth despite its antiapoptotic effect. J Surg Res. 1998;76:22–26. doi: 10.1006/jsre.1998.5277. [DOI] [PubMed] [Google Scholar]
- 24.Le Cam L, Polanowska J, Fabbrizio E, Olivier M, Philips A, Ng Eaton E, Classon M, Geng Y, Sardet C. Timing of cyclin E gene expression depends on the regulated association of a bipartite repressor element with a novel E2F complex. EMBO J. 1999;18:1878–1890. doi: 10.1093/emboj/18.7.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M-H, Williams B O, Mulligan G, Mukai S, Bronson R T, Dyson N, Harlow E, Jacks T. Targeted disruption of p107: functional overlap between p107 and Rb. Genes Dev. 1996;10:1621–1632. doi: 10.1101/gad.10.13.1621. [DOI] [PubMed] [Google Scholar]
- 26.Leone G, DeGregori J, Yan Z, Jakoi L, Ishida S, Williams R S, Nevins J R. E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev. 1998;12:2120–2130. doi: 10.1101/gad.12.14.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levkau B, Koyama H, Raines E W, Clurman B E, Herren B, Orth K, Roberts J M, Ross R. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol Cell. 1998;1:553–563. doi: 10.1016/s1097-2765(00)80055-6. [DOI] [PubMed] [Google Scholar]
- 28.Lind E F, Wayne J, Wang Q-Z, Staeva T, Stolzer A, Petrie H T. Bcl-2-induced changes in E2F regulatory complexes reveal the potential for integrated cell cycle and cell death functions. J Immunol. 1999;162:5374–5379. [PubMed] [Google Scholar]
- 29.Linette G P, Li Y, Roth K, Korsmeyer S J. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proc Natl Acad Sci USA. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazel S, Burtrum D, Petrie H T. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J Exp Med. 1996;183:2219–2226. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millard S S, Yan J S, Nguyen H, Pagano M, Kiyokawa H, Koff A. Enhanced ribosomal association of p27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem. 1997;272:7093–7098. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- 32.Moberg K, Starz M A, Lees J A. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy K L, Kittrell F S, Gay J P, Jäger R, Medina D, Rosen J M. Bcl-2 expression delays mammary tumor development in dimethylbenz(a)anthracene-treated transgenic mice. Oncogene. 1999;18:6597–6604. doi: 10.1038/sj.onc.1203099. [DOI] [PubMed] [Google Scholar]
- 34.Nevins J R. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 35.O'Reilly L A, Huang D C S, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 36.Puthier D, Pellat-Deceunynck C, Barille S, Robillard N, Rapp M J, Juge-Morineau N, Harousseau J L, Bataille R, Amiot M. Differential expression of Bcl-2 in human plasma cell disorders according to proliferation status and malignancy. Leukemia. 1999;13:289–294. doi: 10.1038/sj.leu.2401302. [DOI] [PubMed] [Google Scholar]
- 37.Robanus-Maandag E, Dekker M, van der Valk M, Carrozza M L, Jeanny J C, Dannenberg J H, Berns A, te Riele H. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 1998;12:1599–1609. doi: 10.1101/gad.12.11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 39.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 40.Shiyanov P, Hayes S, Chen N, Pestov D G, Lau L F, Raychaudhuri P. p27Kip1 induces an accumulation of the repressor complexes of E2F and inhibits expression of the E2F-regulated genes. Mol Biol Cell. 1997;8:1815–1827. doi: 10.1091/mbc.8.9.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith E J, Leone G, Nevins J R. Distinct mechanisms control the accumulation of the Rb-related p107 and p130 proteins during cell growth. Cell Growth Differ. 1998;9:297–303. [PubMed] [Google Scholar]
- 42.Strasser A, Harris A W, Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 43.Strom D K, Cleveland J L, Chellappan S, Nip J, Hiebert S W. E2f-1 and E2f-3 are functionally distinct in their ability to promote myeloid cell cycle progression and block granulocyte differentiation. Cell Growth Differ. 1998;9:59–69. [PubMed] [Google Scholar]
- 44.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 45.Uhlmann E J, D'Sa-Eipper C, Subramanian T, Wagner A J, Hay N, Chinnadurai G. Deletion of a nonconserved region of Bcl-2 confers a novel gain of function: suppression of apoptosis with concomitant cell proliferation. Cancer Res. 1996;56:2506–2509. [PubMed] [Google Scholar]
- 46.Vairo G, Innes K M, Adams J M. Bcl-2 has a cell cycle inhibitory function separable from its enhancement of cell survival. Oncogene. 1996;13:1511–1519. [PubMed] [Google Scholar]
- 47.Vairo G, Livingston D M, Ginsberg D. Functional interaction between E2F-4 and p130: evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995;9:869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z M, Yang H, Livingston D M. Endogenous E2F-1 promotes timely G0 exit of resting mouse embryo fibroblasts. Proc Natl Acad Sci USA. 1998;95:15583–15586. doi: 10.1073/pnas.95.26.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe G, Albanese C, Lee R J, Reutens A, Vairo G, Henglein B, Pestell R G. Inhibition of cyclin D1 kinase activity is associated with E2F-mediated inhibition of cyclin D1 promoter activity through E2F and Sp1. Mol Cell Biol. 1998;18:3212–3222. doi: 10.1128/mcb.18.6.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zalvide J, DeCaprio J A. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol Cell Biol. 1995;15:5800–5810. doi: 10.1128/mcb.15.10.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu L, Zhu L, Xie E, Chang L S. Differential roles of two tandem E2F sites in repression of the human p107 promoter by retinoblastoma and p107 proteins. Mol Cell Biol. 1995;15:3552–3562. doi: 10.1128/mcb.15.7.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]