Abstract
Alcohol drinking and tobacco smoking are hazardous behaviors associated with a wide range of adverse health outcomes. In this study, we explored the association of polygenic risk scores (PRS) related to drinks per week, age of smoking initiation, smoking initiation, cigarettes per day, and smoking cessation with 433 psychiatric and behavioral traits in 4498 children and young adults (aged 8–21) of European ancestry from the Philadelphia neurodevelopmental cohort. After applying a false discovery rate multiple testing correction accounting for the number of PRS and traits tested, we identified 36 associations related to psychotic symptoms, emotion and age recognition social competencies, verbal reasoning, anxiety-related traits, parents’ education, and substance use. These associations were independent of the genetic correlations among the alcohol-drinking and tobacco-smoking traits and those with cognitive performance, educational attainment, risk-taking behaviors, and psychopathology. The removal of participants endorsing substance use did not affect the associations of each PRS with psychiatric and behavioral traits identified as significant in the discovery analyses. Gene-ontology enrichment analyses identified several neurobiological processes underlying mechanisms of the PRS associations we report. In conclusion, we provide novel insights into the genetic overlap of smoking and drinking behaviors in children and young adults, highlighting their independence from psychopathology and substance use.
Subject terms: Genomics, Pathogenesis
Introduction
Alcohol drinking and tobacco smoking may result in direct or indirect health concerns. Psychoactive compounds such as ethanol and nicotine act primarily on mental processes and therefore can affect mood, feelings, and behavior [1], but they are also related to many negative health outcomes [2–4]. Drinking and smoking represent two of the three leading preventable causes of death in the US [5]. Understanding the molecular and behavioral processes underlying the predisposition to alcohol drinking and tobacco smoking could lead to better strategies aiming to prevent the cascade of psychiatric and behavioral impairments associated with problematic drinking and smoking. Large-scale genome-wide association studies (GWAS) of traits related to alcohol drinking and tobacco smoking demonstrated that the predisposition to these complex behavioral traits is highly polygenic (i.e., thousands of variants with small effects) [6–12]. To date, GSCAN (GWAS & Sequencing Consortium of Alcohol and Nicotine) has completed the largest genome-wide meta-analysis across multiple drinking and smoking behaviors short of dependence on either of these substances, analyzing up to 1.2 million individuals [13]. GSCAN investigated one alcohol-drinking phenotype (drinks per week, DPW) and four tobacco-smoking phenotypes. These included cigarettes per day (CPD, average number of cigarettes smoked per day), smoking initiation (SI, smoker versus non-smoker), smoking cessation (SC, current versus former smoker), and age of smoking initiation (ASI, age at which an individual started smoking regularly). While ASI is negatively genetically correlated with all the other traits (from rg = −0.10 with respect to DPW to rg = −0.71 for SI), DPW and the other smoking phenotypes share positive genetic correlations ranging from rg = 0.07 (CPD vs. DPW) to rg = 0.42 (SC vs. CPD). These traits also showed a broad spectrum of genetic correlations including behavioral traits (e.g., risk tolerance and neuroticism), psychiatric disorder (e.g., major depressive disorder and schizophrenia), and physical health outcomes (e.g., obesity and coronary artery disease) [13]. Due to the large effects of tobacco and alcohol on human health, it is challenging and important to distinguish whether the genetic correlations observed are due to the consequences of alcohol drinking and tobacco smoking or to the genetic etiology shared between these traits and other complex phenotypes. To dissect the pleiotropic mechanisms related to alcohol drinking and tobacco smoking, we investigated their genetic liability through the polygenetic risk scores (PRS) derived from GSCAN genome-wide association data with respect to psychiatric and behavioral traits assessed in the Philadelphia Neurodevelopmental Cohort (PNC). Similar to other studies [14–20], we used a high-resolution phenome-wide screening approach investigating hundreds of traits related to different neurodevelopmental domains. Due to the limited alcohol and tobacco use in the PNC participants, testing the association of genetic liability to alcohol drinking and tobacco smoking with a wide range of elements of psychiatric and behavioral assessment can permit us to generate novel hypotheses regarding the mechanisms leading to smoking and drinking behaviors, independently of the effects of psychoactive compounds. Additionally, we also verified that the associations observed are not due to the genetic overlap across the GSCAN phenotypes or to other genetically correlated psychiatric and behavioral traits including psychopathology, risk tolerance, educational attainment, and socioeconomic status.
Materials and methods
Philadelphia Neurodevelopmental Cohort
Phenotype and genotype data for PNC participants were obtained, after authorized access, from the National Center for Biotechnology Information database of Genotypes and Phenotypes (dbGaP; available at http://www.ncbi.nlm.nih.gov/gap) through dbGaP accession number phs000607.v3.p2 (Neurodevelopmental Genomics: Trajectories of Complex Phenotypes). The PNC is a population-based sample including more than 9500 individuals aged 8–21 years not enriched for any epidemiologically ascertained specific disorder, behavior, or trait [21]. The PNC participants were selected after stratification by sex, age, and ethnicity from a pool of approximately 50,000 subjects previously recruited from patients undergoing bloodwork in the Children’s Hospital of Philadelphia care network [22, 23]. Each participant was assessed for psychiatric and behavioral traits with a structured interview and completed a CNB following the Kiddie-SADS Family Study Interview [24, 25]. The structured interview included a panel of questions related to demographics, the timeline of life events, education, medical history, psychopathological assessment, and a global assessment of cognitive and executive functioning. The screening for symptoms related to psychiatric diagnoses was based on items defined by the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [26]. The CNB consisted of 14 tests assessing executive control, episodic memory, complex cognition, social cognition, and sensorimotor speed. A complete list of neurodevelopmental domains assessed and the specific features of each domain tested in the neurocognitive battery is available at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000607.v3.p2. In our analysis, we tested phenotypes that were assessed in at least 500 participants. Supplementary Table 1 reports the sample size for each phenotype investigated. These included a total of 433 traits (Supplementary Table S1) that were grouped in 18 domains: attention deficit disorder, depression, generalized anxiety disorder, neuropsychiatric assessment, mania/hypomania, medical concerns, obsessive-compulsive disorder, oppositional defiant disorder, panic disorder, specific phobia, psychosis, post-traumatic stress, general probes, separation anxiety, structured interview for prodromal symptoms (SIPS) to assess psychotic risk, social anxiety, substance use, and other (i.e., phenotypes not classifiable in previous domains).
To account for the overall health, we included medical rating assessed at the administration of the tests among the covariates of the regression models. Additionally, we included the type of interview as a further covariate in our model to account for possible differences among participants in the assessment. Indeed, as for participants 8–10 years of age, the assessment was not direct, but caregivers or legal guardians were asked for information regarding the subject tested, and for probands aged 11–17, both the participants and their caregivers/legal guardians were interviewed. For the latter group, we investigated only the self-reported information to avoid a duplicated assessment. We decided to not explore differences in the PRS association between data derived from participants’ interviews and from caregivers’ interviews because of the limited sample size of the probands aged 11–17. The inclusion of “medical rating” and “type of interview" variables as covariates is in line with the design of previous PNC studies [27–29].
Our analysis was restricted to PNC participants of genetically confirmed European descent due to the lack of availability of large-scale GWAS data for other populations and known biases of cross-ancestry PRS analysis [30]. Considering these inclusion criteria, we investigated 433 psychiatric and behavioral traits (Supplementary Table S1) in 4498 children and young adults of European descent. Data quality control was performed as detailed in Wendt et al. [31]. Briefly, preimputation quality control was performed using PGC analysis pipeline specifically designed to handle datasets consisting of multiple genotyping platforms (see https://sites.google.com/a/broadinstitute.org/ricopili/preimputation-qc). Individuals of European descent were verified with genetic information via principal component analysis and the 1000 Genomes Project reference panel for populations with European ancestry (N = 503). For sample pairs with relatedness PI-HAT > 0.2, the sample with more informative phenotypes was retained. Imputation was performed for unrelated individuals of European ancestry using SHAPEIT for pre-phasing, IMPUTE2 for imputation, and the human 1000 Genomes Project Phase 3 as a reference panel [32–34].
Genome-wide association data
Genome-wide association data for drinking and smoking traits were derived from GSCAN [13] and accessed via the Data Repository of the University of Minnesota (available at https://conservancy.umn.edu/handle/11299/201564). GSCAN GWAS included only individuals of European descent. GWAS data were generated in each study included in the GSCAN GWAS using RVTEST [35], accounting for the family-based studies and unrelated samples [33]. GSCAN investigated five traits, one related to alcohol drinking and four related to tobacco smoking. DPW (N = 941,280) was defined based on the average number of alcoholic drinks a participant reported consuming in a week. SI (N = 1,232,091) is a binary trait considering regular smokers as cases and non-smokers as controls, while ASI (N = 341,427) is a quantitative trait related to the age when an individual started to smoke tobacco-based cigarettes. SC (N = 547,219) was defined considering current smokers as cases and former smokers as controls. CPD (N = 337,334) was calculated in both current and former smokers by binning the quantitative measure of CPD (bin1 = 1–5; bin2 = 6–15; bin3 = 16–25; bin4 = 26–35; bin5 = 36+). Considering the risk variants identified in the GSCAN GWAS and available in the PNC cohort, we observed several associations (p < 0.05) with substance use phenotypes (Supplementary Table S2) although only 21% of the participants are informative for these phenotypes. The limited sample size and the characteristics of the PNC cohorts do not permit us to investigate single-variant effects.
SNP-based heritability and genetic correlation
SNP-based heritability and genetic correlation for the smoking and drinking traits, and the additional phenotypes were estimated using the Linkage Disequilibrium Score Regression (LDSC) method [36, 37]. As provided by the LDSC developers (details available at https://github.com/bulik/ldsc), the analysis was conducted considering the HapMap 3 reference panel and pre-computed LD scores based on the 1000 Genomes Project reference data for individuals of European ancestry.
PRS analysis
PRS based on drinking and smoking GWAS data were investigated with respect to psychiatric and behavioral traits assessed in the PNC (Supplementary Table S1). PRSice v. 2.3.1.c [38] was used to compute PRS using the clumping-thresholding method where the clumping step is used to obtain independent effect estimates from base datasets (smoking and drinking GWAS in the present study) and the thresholding step is used to maximize the predictive ability of the derived polygenic scores [39]. SNPs were clumped based on 250 kb windows, based on clump-r2 threshold = 0.1 and clump-p threshold = 1, respectively. The step size of the threshold was set to 5e−05, and the range of p value thresholds was from 5e−08 to 1 using an additive model for regression at each threshold. Although epistatic models could provide additional information regarding the genetics of drinking and smoking behaviors [40], a recent study showed that additive variance should account for the vast majority of the SNP-based heritability of complex traits [41]. All PRSs were covaried for age, sex, the first ten within-ancestry principal components (PCs), medical rating (i.e., the PNC code indicating the severity of a patient’s medical condition), and type of interview. False discovery rate (FDR) at 5% was applied to correct the results obtained for multiple testing, accounting for the number of phenotypes and PRS tested. The Manhattan plot related to the phenome-wide association study using the phenotypic binning was generated in R using the ggplot2 package [42]. To assess the independence of the phenotypes identified as associated with drinking and smoking PRS, we also calculated Spearman’s rank-order correlations via R using the rcorr function of the Hmisc library [43]. Correlation p values were adjusted for the number of tests performed using FDR q < 0.05. Finally, we verified whether the significant PRS associations with psychiatric and behavioral traits were independent of the genetic correlation among alcohol and tobacco phenotypes and between them and other psychiatric and behavioral traits. Results that survived multiple testing correction in the initial analysis were subsequently tested in two additional models. In model 2, we included as covariates the other PRS related to alcohol and tobacco use. Accordingly, if two among PRS tested are associated with the same trait and the association remained significant after covarying for each other effect, we can assume that the effects observed are independent of each other.
To verify whether the PRS associations were due to the genetic overlap of alcohol drinking and tobacco smoking with other complex traits, significant PRS associations were also covaried using PRS related to other psychiatric and behavioral traits in addition to the covariates defined in the model 1. Specifically, we used large-scale GWAS datasets, including Psychiatric Genomics Consortium cross-disorder (PGC-CD; N = 438,997 [44]), Social Science Genetic Association Consortium (SSGAC) cognitive performance (N = 257,828 [45]), SSGAC educational attainment (N = 766,345 [45]), SSGAC general-risk-tolerance (N = 466,571 [46]), and household income (N = 286,301 [47]). The PGC-CD study is a cross-disorder analysis including anorexia nervosa, attention-deficit/hyperactivity disorder, autism spectrum disorder, bipolar disorder, major depression, obsessive-compulsive disorder, schizophrenia, and Tourette syndrome [44]. We used PGC-CD GWAS data to account for the genetic overlap of alcohol and tobacco use with psychopathology and psychiatric comorbidities. SSGAC educational attainment (EA) was defined by mapping the major educational qualification of each participant to relevant categories from the International Standard Classification of Education (ISCED), then imputing the years-of-education equivalent for each ISCED category, facilitating comparison across different systems [45]. The SSGAC cognitive performance (CP) data were generated by meta-analyzing data from the COGENT (Cognitive Genomics Consortium) study and the UK Biobank (UKB). The COGENT study analyzed a phenotype defined as the first principal component derived from three or more neuropsychological tests [46]. In UKB, cognitive performance was defined based on the respondent’s score on a test of verbal-numerical reasoning. The SSGAC GWAS of general-risk tolerance (GR) meta-analyzed cohorts with different assessments capturing an individual’s tendency, preparedness, or willingness to take risks in general [47]. Annual household income (HI) GWAS was conducted in UKB using self-reported pre-tax household income binned to create a five-point scale (bin1 < £18,000, bin2 = £18,000–£29,999, bin3 = £30,000–£51,999, bin4 = £52,000–£100,000, bin5 > £100,000) [48]. HI data were analyzed as a proxy of socioeconomic status.
Enrichment analysis
The SNPs used to generate each significant PRS association were analyzed for pathway enrichment using PRSet implemented in PRSice v. 2.3.1.c [38, 49]. Briefly, the PRSset method stratifies the PRS by gene sets. In our study, we used the Molecular Signatures Database (MSigDB) to derive gene sets related to gene ontologies and defined gene boundaries using the human gene annotation (GTF file). REVIGO [50] was employed to summarize GO terms by removing redundant items based on Jiang and Conrath semantic distance [51] and a similarity degree of 0.5.
Results
SNP-based heritability and genetic correlation
The SNP-based heritability of the alcohol-drinking and tobacco-smoking traits ranged from 0.032 ± 0.002 for SC to 0.072 ± 0.007 for CPD (Supplementary Table S3). LDSC-based correlations were examined among the substance-use phenotypes. Their genetic correlations are highly significant, but their absolute value ranges from 0.083 to 0.684. DPW showed a positive genetic correlation with each tobacco-smoking trait (SI rg = 0.407, P = 1.40E−92; CPD rg = 0.083, P = 3.93E−03; SC rg = 0.108, P = 1.02E−03; Fig. 1 and Supplementary Table S4) with the exception of ASI (rg = −0.160, P = 2.73E−07). Similarly, ASI is negatively genetically correlated with the other tobacco-smoking phenotypes (ASI vs. SI, rg = −0.684, P = 3.09E−199; ASI vs. CPD, rg = −0.369, P = 1.07E−23; ASI vs. SC, rg = −0.291, P = 1.71E−12). Considering other psychiatric, behavioral, and social traits (Supplementary Table S5), PGC-CD showed a positive genetic correlation with CPD (rg = 0.21, P = 2.13E−18), SI (rg = 0.215, P = 2.33E−26), DPW (rg = 0.107, P = 6.01E−06), and SC (rg = 0.102, P = 1.54E−03), while a negative correlation was present with respect to ASI (rg = −0.147; P = 9.22E−09). CP was positively correlated with ASI (rg = 0.314, P = 6.31E−32), while the other smoking traits showed only a weak negative rg (CP vs. SI rg = −0.172, P = 2.34E−22; CP vs. CPD rg = −0.103, P = 2.35E−05, and CP vs. SC rg = −0.298, P = 2.80E−28). EA was the most correlated additional behavioral trait respect to ASI (rg = 0.599, P = 7.08E−167), SC (rg = −0.502, P = 4.15E−95), SI (rg = −0.362, P = 2.75E−128), and CPD (rg = −0.285, P = 8.32E−29). HI followed the same correlation pattern of EA, while GR is the only adjunctive trait negatively related to ASI (rg = −0.228, P = 8.46E−13), showing a positive association with the other smoking phenotypes (GR vs. SI rg = 0.327, P = 1.54E−46; R vs. CPD rg = 0.175, P = 7.87E−07; GR vs. SC rg = 0.180, P = 4.22E−07). Finally, GR was also the only additional trait with a moderate positive genetic correlation with DPW (rg = 0.286, P = 4.26E−30). Supplementary Table S4 provides details of the genetic correlations calculated among the GWAS datasets investigated.
Fig. 1. Genetic correlation matrix among smoking and drinking traits assessed by the GWAS & Sequencing Consortium of Alcohol and Nicotine.
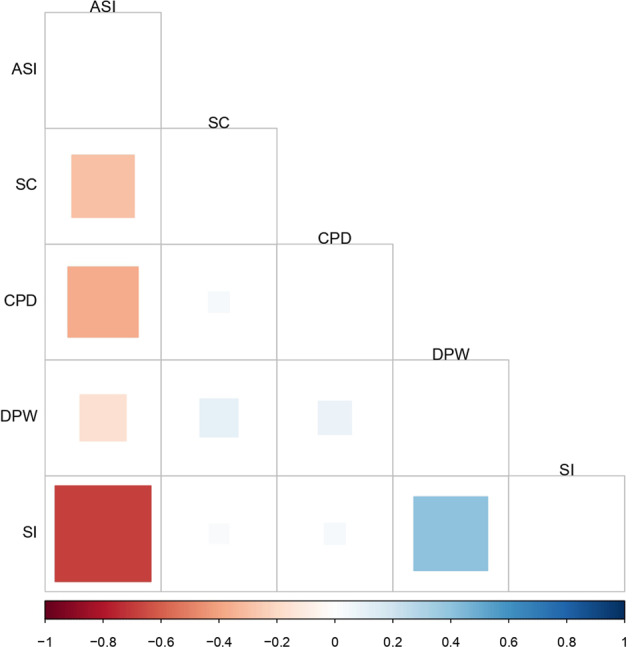
The square size is proportional to the magnitude of the correlation. Blank squares relate to not significant correlations (P > 0.01). ASI age of smoking initiation, CPD cigarettes per day, DPW drinks per week, SC smoking cessation, SI smoking initiation.
Phenotypic correlations among psychiatric and behavioral traits associated with smoking and drinking polygenic risk
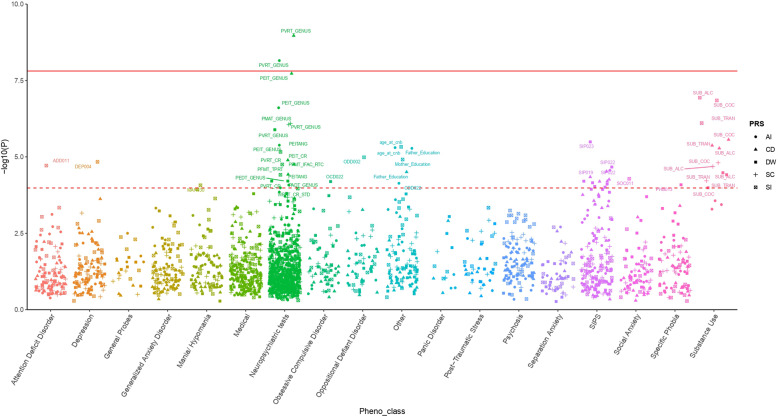
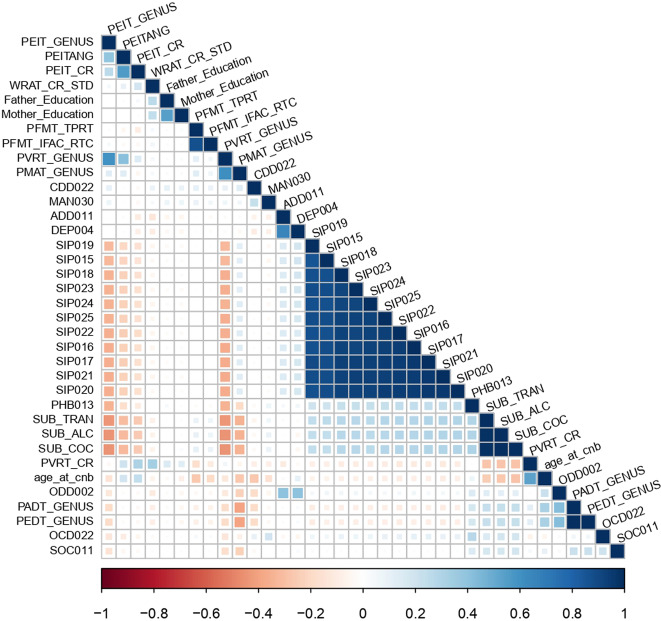
The polygenic risk drinking and smoking behaviors were tested with respect to 433 psychiatric and behavioral traits assessed in PNC children and young adults and a total of 36 phenotypes were significantly associated after accounting for the number of PRS and phenotypes tested (FDR < 5%; Table 1, Fig. 2 and Supplementary Table S6). To assess the independence of the phenotypes identified as associated with drinking and smoking PRS, we conducted a Spearman’s correlation analysis among these psychiatric and behavioral traits, observing several significant correlations (Fig. 3 and Supplementary Table S7). We observed a high correlation among the psychosis-related SIPS outcomes [52] (Spearman’s ρ > 0.87, P < 0.001). Penn computerized individual tests outcomes were also positively correlated to each other: Median Response Time for Correct Responses to Target Faces (PFMT_TPRT) and Median Response Time for Total Correct Test Trial Responses (PFMT_IFAC_RTC) (Spearman’s ρ = 0.87, P < 0.0001) for Penn Face Memory Test; Penn Verbal Reasoning Test Genus (PVRT_GENUS) and Penn Matrix Reasoning Test Genus (PMAT_GENUS) (Spearman’s ρ = 0.61, P < 0.0001) for verbal and reasoning tests; Penn Age Differentiation Test (PADT_GENUS) and Penn Emotion Differentiation Test (PEDT_GENUS) (Spearman’s ρ = 1, P < 0.0001) for age and emotion recognition. Alcohol, cocaine, and tranquilizer use were also highly correlated (Spearman’s ρ > 0.98, P < 0.0001).
Table 1.
Association of smoking and drinking PRS with psychiatric and behavioral traits surviving the FDR 5% threshold.
| PRS | Phenotype | Threshold | SNP N | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Z- score | P | R2 | Z- score | P | R2 | Z- score | P | ||||
| ASI | PEITANG | 0.408 | 59,052 | 0.49% | 4.61 | 4.14E−06 | 0.44% | 4.36 | 1.31E−05 | 0.21% | 3.06 | 0.002 |
| Father_Education | 0.0016 | 897 | 0.47% | 4.57 | 5.00E−06 | 0.41% | 4.27 | 2.03E−05 | 0.28% | 3.62 | 3.04E−04 | |
| SIP019 | 0.1 | 21,081 | 0.41% | −4.2 | 2.73E−05 | 0.34% | −3.84 | 1.24E−04 | 0.15% | −2.52 | 0.012 | |
| CDD022 | 0.093 | 19,832 | 0.34% | 3.97 | 7.34E−05 | 0.28% | 3.62 | 3.03E−04 | 0.25% | 3.4 | 6.76E−04 | |
| WRAT_CR_STD | 1 | 99,097 | 0.35% | 3.89 | 1.03E−04 | 0.28% | 3.46 | 5.36E−04 | 0.05% | 1.47 | 0.142 | |
| CPD | PVRT_GENUS | 0.0013 | 1039 | 0.82% | −6.11 | 1.08E−09 | 0.72% | −5.8 | 7.20E−09 | 0.41% | −4.44 | 9.35E−06 |
| PEIT_GENUS | 4.00E−04 | 520 | 0.69% | −5.63 | 1.87E−08 | 0.49% | −4.78 | 1.83E−06 | 0.33% | −3.99 | 6.76E−05 | |
| PEIT_CR | 2.50E−04 | 413 | 0.42% | −4.37 | 1.28E−05 | 0.35% | −3.96 | 7.51E−05 | 0.28% | −3.57 | 3.66E−04 | |
| PVRT_CR | 0.0015 | 1159 | 0.31% | −4.3 | 1.77E−05 | 0.31% | −4.26 | 2.07E−05 | 0.15% | −3.09 | 0.002 | |
| SIP017 | 0.0013 | 1039 | 0.39% | 4.08 | 4.54E−05 | 0.33% | 3.82 | 1.37E−04 | 0.20% | 2.95 | 0.003 | |
| SIP018 | 0.0013 | 1039 | 0.38% | 4.04 | 5.48E−05 | 0.27% | 3.42 | 6.24E−04 | 0.16% | 2.64 | 0.008 | |
| SIP020 | 0.0013 | 1039 | 0.37% | 4 | 6.37E−05 | 0.35% | 3.92 | 8.82E−05 | 0.20% | 2.99 | 0.003 | |
| DPW | SIP023 | 0.0024 | 2025 | 0.50% | −4.66 | 3.23E−06 | 0.44% | −4.37 | 1.28E−05 | 0.36% | −4 | 6.37E−05 |
| SIP022 | 0.0059 | 3468 | 0.42% | −4.25 | 2.15E−05 | 0.35% | −3.89 | 1.03E−04 | 0.27% | −3.42 | 6.24E−04 | |
| PADT_GENUS | 5.00E−08 | 48 | 0.31% | 4.01 | 6.18E−05 | 0.28% | 3.81 | 1.43E−04 | 0.28% | 3.82 | 1.33E−04 | |
| PEDT_GENUS | 5.00E−08 | 48 | 0.31% | 4.01 | 6.18E−05 | 0.28% | 3.81 | 1.43E−04 | 0.28% | 3.82 | 1.33E−04 | |
| SIP021 | 0.0059 | 3468 | 0.37% | −4.01 | 6.24E−05 | 0.30% | −3.59 | 3.30E−04 | 0.22% | −3.12 | 0.002 | |
| OCD022 | 5.01E−05 | 299 | 0.38% | 4 | 6.42E−05 | 0.38% | 4.01 | 6.18E−05 | 0.34% | 3.8 | 1.44E−04 | |
| SIP015 | 0.0071 | 3877 | 0.37% | −3.98 | 7.03E−05 | 0.30% | −3.58 | 3.42E−04 | 0.24% | −3.22 | 0.001 | |
| SIP016 | 0.0071 | 3893 | 0.36% | −3.96 | 7.76E−05 | 0.38% | −4.06 | 4.92E−05 | 0.28% | −3.48 | 4.99E−04 | |
| PHB013 | 5.01E−05 | 299 | 0.36% | 3.94 | 8.19E−05 | 0.39% | 4.1 | 4.26E−05 | 0.32% | 3.72 | 2.02E−04 | |
| SIP024 | 0.0071 | 3893 | 0.36% | −3.91 | 9.27E−05 | 0.39% | −4.12 | 3.87E−05 | 0.30% | −3.62 | 2.99E−04 | |
| SIP025 | 0.0024 | 2025 | 0.35% | −3.9 | 9.79E−05 | 0.32% | −3.73 | 1.92E−04 | 0.27% | −3.47 | 5.27E−04 | |
| SC | PMAT_GENUS | 5.01E−05 | 73 | 0.51% | 4.94 | 8.06E−07 | 0.50% | 4.91 | 9.23E−07 | 0.46% | 4.72 | 2.47E−06 |
| SI | SUB_ALC | 0.077 | 20,981 | 0.58% | −5.31 | 1.16E−07 | 0.68% | −5.78 | 7.83E−09 | 0.90% | −6.72 | 2.04E−11 |
| SUB_COC | 0.072 | 20,123 | 0.57% | −5.27 | 1.42E−07 | 0.66% | −5.72 | 1.17E−08 | 0.89% | −6.69 | 2.58E−11 | |
| SUB_TRAN | 0.077 | 20,981 | 0.51% | −4.95 | 7.85E−07 | 0.61% | −5.44 | 5.76E−08 | 0.83% | −6.4 | 1.67E−10 | |
| age_at_cnb | 0.072 | 20,138 | 0.01% | 4.58 | 4.70E−06 | 0.02% | 5.16 | 2.56E−07 | 0.02% | 5.6 | 2.35E−08 | |
| ODD002 | 0.149 | 32,093 | 0.69% | 4.41 | 1.03E−05 | 0.74% | 4.58 | 4.65E−06 | 0.40% | 3.37 | 7.64E−04 | |
| Mother_Education | 0.081 | 21,799 | 0.44% | −4.38 | 1.22E−05 | 0.36% | −3.95 | 7.88E−05 | 0.25% | −3.35 | 8.28E−04 | |
| DEP004 | 0.01 | 6639 | 0.63% | 4.34 | 1.45E−05 | 0.57% | 4.11 | 4.04E−05 | 0.43% | 3.58 | 3.42E−04 | |
| PFMT_IFAC_RTC | 0.012 | 7229 | 0.42% | −4.3 | 1.77E−05 | 0.36% | −4 | 6.42E−05 | 0.25% | −3.33 | 8.62E−04 | |
| ADD011 | 5.01E-05 | 677 | 0.59% | 4.27 | 1.93E−05 | 0.45% | 3.74 | 1.87E−04 | 0.31% | 3.13 | 0.002 | |
| PFMT_TPRT | 0.011 | 6963 | 0.37% | −4.12 | 3.86E−05 | 0.39% | −4.21 | 2.56E−05 | 0.25% | −3.42 | 6.35E−04 | |
| SOC011 | 0.0084 | 5875 | 0.38% | −4.05 | 5.21E−05 | 0.36% | −3.94 | 8.45E−05 | 0.27% | −3.41 | 6.60E−04 | |
| MAN030 | 0.0078 | 5636 | 0.36% | −3.93 | 8.48E−05 | 0.34% | −3.83 | 1.30E−04 | 0.23% | −3.15 | 0.002 | |
The definition of the phenotype abbreviations is available in Supplementary Table S1. The results were assessed with respect to three regression models, including different sets of covariates. Model 1 covariates: 10 principal components, Sex, Age, Type of Interview, Medical Rating; Model 2 covariates: Model 1 + PRSs for smoking and alcohol traits; Model 3 covariates: Model 1 + PRSs for PGC cross-disorder, cognitive performance, educational attainment, general-risk tolerance, and household income.
ASI age of smoking initiation, CPD cigarettes per day, DPW drinks per week, SC smoking cessation, SI smoking initiation.
Fig. 2. Association of drinking and smoking polygenic risk scores for 433 psychiatric and behavioral traits.
The dashed line represents the FDR 5% threshold, while the solid line refers to Bonferroni 5% correction. The definition of the phenotype abbreviations is available in Supplementary Table S1. ASI age of smoking initiation, CPD cigarettes per day, DPW drinks per week, SC smoking cessation, SI smoking initiation.
Fig. 3. Spearman’s rank-order correlation matrix across 36 neurobehavioral traits significantly associated with the PRS analyzed.
The square size is proportional to the magnitude of the correlation. Blank squares relate to not significant correlations (P > 0.01). The definition of the phenotype abbreviations is available in Supplementary Table S1.
Drinks per week
The PRS for DPW was negatively associated with seven outcomes derived from the psychosis-related SIPS (SIP015: “I think I have felt that there are odd or unusual things going on that I can’t explain” Z-score = −3.98, R2 = 0.4%, P = 7.03E−05; SIP016: “I think that I might be able to predict the future” Z-score = −3.96, R2 = 0.4%, P = 7.76E−05; SIP021: “I wonder if people may be planning to hurt me or even may be about to hurt me” Z-score = −4.01, R2 = 0.4%, P = 6.24E−05; SIP022: “I believe that I have special natural or supernatural gifts beyond my talents and natural strengths” Z-score = −4.25, R2 = 0.4%, P = 2.15E−05; SIP023: “I think I might feel like my mind is “playing tricks” on me” Z-score = −4.66, R2 = 0.5%, P = 3.23E−06; SIP024: “I have had the experience of hearing faint or clear sounds of people or a person mumbling or talking when there is no one near me” Z-score = −3.91, R2 = 0.4%, P = 9.27E−05; SIP025: “I think that I may hear my own thoughts being said out loud” Z-score = −3.90, R2 = 0.4%, P = 9.79E−05). DPW polygenic risk was positively associated with the specific phobia-related item (PHB013: “Thinking about all of the time that you were afraid of (insert worst fear), whether or not you actually faced it, how long did this fear last? (Weeks)” Z-score = 3.94, R2 = 0.4%, P = 8.19E−05), one of the obsessive-compulsive disorder traits (OCD022 Z-score = 4.00, R2 = 0.4%, P = 6.42E−05), and PADT_GENUS and PEDT_GENUS (Penn Age Differentiation and Emotion Differentiation Test, both featuring a Z-score = 4.01, R2 = 0.3%, P = 6.18E−05).
Age of smoking initiation
The PRS for ASI was positively associated with the ability to recognize the angry facial emotions of others (PEITANG: Number of Correct Responses to Anger Trials, Z-score = 4.61, R2 = 0.5%, P = 4.14E−06), the standardized score from the Wide Range Achievement Test [53] (WRAT_CR_STD: age-adjusted WRAT test determining participants’ ability to complete the battery and to provide an estimate of IQ, Z-score = 3.89, R2 = 0.3%, P = 1.03E–04); an item related to conduct disorder (CDD022: “How much did these behaviors change your relationships with your friends?” Z-score = 3.97, R2 = 0.3%, P = 7.34E–05), and years of father’s education (Z-score = 4.57, R2 = 0.5%, P = 5.00E-06). The only negative association was found with respect to item SIP019 (“I think that I may get confused at times whether something I experience or perceive may be real or may be just part of my imagination or dreams” Z-score = −4.20, R2 = 0.4%, P = 2.73E−05).
Smoking initiation
The polygenic risk of SI positively associated with the age at completion of the CNB (Z-score = 4.58, R2 = 0.01%, P = 4.70E−06), one of the oppositional defiant disorder traits (ODD002: “Was there a time when you often got into trouble with adults for refusing to do what they told you to do or for breaking rules at home/school” Z-score = 4.41, R2 = 0.7%, P = 1.03E−05), a depression-related item (DEP004: “Has there ever been a time when you felt grouchy, irritable or in a bad mood most of the time; even little things would make you mad?” Z-score = 4.34, R2 = 0.6%, P = 1.45E−05), and attention deficit disorder (ADD011: “Did you often have trouble paying attention or keeping your mind on your school, work, chores, or other activities that you were doing?” Z-score = 4.27, R2 = 0.6%, P = 1.93E−05). A negative association was observed for the PRS of SI and the years of mother’s education (Z-score = −4.38, R2 = 0.4%, P = 1.22E−05), social anxiety (SOC011: “Thinking about all of the time that you were afraid of (insert worst fear), whether or not you actually faced it, how long did your fear of this situation last? (Months)” Z-score = −4.05, R2 = 0.4%, P = 5.21E−05), and a mania-related item (MAN030: “How much did your feeling (too happy/excited/grouchy/energetic) upset or bother you?” Z-score = −3.93, R2 = 0.4%, P = 8.48E−05). The two highly correlated phenotypes accounting for the Penn Face Memory Test were also negatively associated with the PRS of SI (PFMT_TPRT: Median Response Time for Correct Responses to Target Faces Z-score = −4.12, R2 = 0.4%, P = 3.86E−05, and PFMT_IFAC_RTC: Median Response Time for Total Correct Test Trial Responses Z-score = −4.30, R2 = 0.4%, P = 1.77E−05, respectively). SI polygenic risk was also negatively associated with the alcohol (Z-score = −5.31, R2 = 0.6%, P = 1.16E−07), cocaine (Z-score = −5.27, R2 = 0.6%, P = 1.42E−07), and tranquilizer (Z-score = −4.95, R2 = 0.5%, P = 7.85E−07) endorsement phenotypes.
Cigarettes per day
The PRS for CPD was positively associated with three SIPS-derived psychosis outcomes (SIP017: “I may have felt that there could possibly be something interrupting or controlling my thoughts, feelings, or actions” Z-score = 4.08, R2 = 0.4%, P = 4.54E−05, SIP018: “I have had the experience of doing something differently because of my superstitions” Z-score = 4.04, R2 = 0.4%, P = 5.48E−05, and SIP020: “I have thought that it might be possible that other people can read my mind, or that I can read others’ minds” Z-score = 4.00, R2 = 0.4%, P = 6.37E−05. Furthermore, the Penn Emotion Identification Test-related traits and those included in the CNB for the Penn Verbal Reasoning Test were negatively associated to PRS for CPD (PEIT_GENUS Z-score = −5.64, R2 = 0.7%, P = 1.87E−08, and PEIT_CR Z-score = −4.37, R2 = 0.4%, P = 1.28E−05, PVRT_GENUS Z-score = −6.11, R2 = 0.8%, P = 1.08E−09, and PVRT_CR Z-score = −4.30, R2 = 0.3%, P = 1.77E−05).
Smoking cessation
After multiple testing correction, a single association was found for SC polygenic risk with respect to the ability to perform the Penn Matrix Reasoning Test, which assesses reasoning by geometric analogy and contrast principle (PMAT_GENUS Z-score = 4.94, R2 = 0.5%, P = 8.06E−07).
Independence of PRS associations with respect to the genetic overlap among alcohol drinking, tobacco smoking, psychopathology, and other behavioral traits
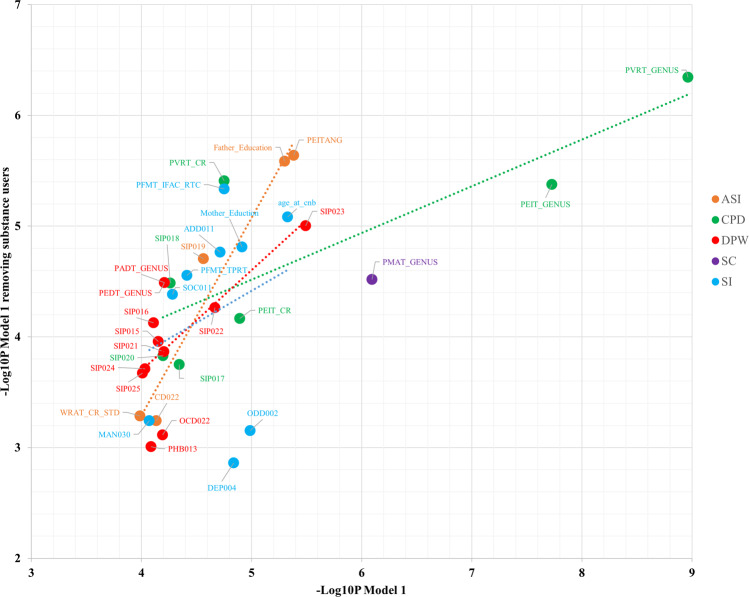
To test whether the associations of drinking and smoking polygenic risk with psychiatric and behavioral traits were due to the genetic overlap with psychopathology, EA, CP, GR, and HI, we added additional covariates to the regression model used in the initial analysis (model 1). Building upon the sets of covariates included in model 1 (i.e., sex, age, the first ten within-ancestry PCs, medical rating, and type of interview), we added the PRS of the other alcohol-drinking and tobacco-smoking traits as covariates (model 2). In model 3, the PRS for psychopathology (i.e., PGC-CD), EA, CP, GR, and HI were added to the set of covariates included in model 1. The significant associations observed in model 1 remained significant in the model 2 and model 3 analysis with the exception of the association of PRS for ASI with WRAT_CR_STD (“age-adjusted WRAT test determining participants ability to complete the battery and to provide an estimate of IQ”) when model 3 covariates were applied (model 1: Z-score = 3.89, R2 = 0.35%, P = 1.03E−04; model 3: Z-score = 1.47, R2 = 0.05%, P = 0.142). To further test the independence of the PRS associations from the effects of psychoactive substance use, we removed the participants endorsing the use of alcohol or drugs (N = 964; 21%). Applying model 1 covariates, we observed consistent PRS associations between the full PNC cohort and the sample excluding substance users (Fig. 4 and Supplementary Table S8).
Fig. 4. Relationship between the statistical significance (−log10Pvalue) of PRS association including and excluding participants endorsing substance use (x axis and y axis, respectively).
The analyses were conducted on the covariates of model 1 (i.e., ten principal components, Sex, Age, Type of Interview, Medical Rating). The definition of the phenotype abbreviations is available in Supplementary Table S1. The dashed lines represent the linear relationship between the results obtained from these analyses with respect to the different drinking and smoking polygenic risk scores tested. PRS polygenic risk score, DPW drinks per week, ASI age of smoking initiation, SI smoking initiation, CPD cigarettes per day, SC smoking cessation, PGC-CD Psychiatric Genomics Consortium Cross Disorder, CP cognitive performance, EA educational attainment, GR general-risk-taking behavior, HI household income, PNC Philadelphia Neurodevelopmental Cohort.
Enrichment analysis
We interrogated the biological processes to characterize the molecular mechanisms underlying the alcohol-drinking and tobacco-smoking PRS associations, identifying several GO enrichments surviving Bonferroni multiple testing correction (Supplementary Table S9). The associations identified for CPD polygenic risk involved several biological domains, including neuromuscular junction development (GO:0007528, P = 3.50E−15) and amyloid precursor protein metabolic process (GO:0042982, P = 1.68E−13), among the most significant. Strong enrichments related to immune systems functions were also observed, including leukocyte activation involved in inflammatory response (GO:0002269, P = 1.62E−13), positive regulation of interleukin-6 biosynthetic process (GO:0045410, P = 2E−13), and erythrocyte maturation (GO:0043249, P = 2.61E−13). The genes underpinning the associations related to the ASI polygenic risk were enriched for several biological pathways, whose primary roles seemed to be linked to the muscle tissue and brain development, including myotube cell development (GO:0014904, P = 4.00E−8), striated muscle cell differentiation (GO:0051146, P = 5.53E−8), and response to manganese ion (GO:0010042, P = 7.17E−8). The associations of the PRS for DPW were enriched for negative regulation of cell projection organization (GO:0031345, P = 5.60E−8) and negative regulation of cell development (GO:0031345, P = 6.54E−8). With respect to the associations identified for the PRS for SI, we observed enrichment for DNA geometric change (GO:0032392, P = 2.34E−13), cellular response to carbohydrate stimulus (GO:0071322, P = 5.13E−12), axodendritic protein transport (GO:0099640, P = 1.75E−10), positive regulation of Phospholipase A2 activity (GO:0032430, P = 2.67E−10), phosphatidylethanolamine acyl-chain remodeling (GO:0036152, P = 1.05E−10), and intracellular receptor signaling pathway (GO:0030522, P = 1.13E−10). Finally, no enrichment related to the SC polygenic risk associations survived multiple testing correction.
Discussion
Leveraging well-powered genome-wide information generated by the GSCAN study of alcoholic drinks-per-week and four traits related to tobacco smoking [13], we investigated the genetic liability for these five traits with respect to psychiatric and behavioral traits in children and young adults. The present study expands the findings provided by recent investigations of drinking and smoking PRS in predicting alcohol use disorder remission in adults and in dissecting the association of prenatal alcohol exposure and offspring alcohol use in mother-child pairs [54, 55]. Our findings increase the understanding of the possible psychiatric and behavioral consequences of smoking and alcohol polygenic risk in childhood and early adulthood. In particular, we observed a wide range of PRS associations and the majority of them were not affected by the genetic overlap with the psychopathology spectrum (i.e., PGC-CD), GR, EA, and HI, or to substance use among PNC participants. The strength of the PRS associations identified is in line with other cross-phenotype PRS analyses done with respect to psychiatric and behavioral traits [56–58]. Since the associations observed appear to be largely mutually independent, we discussed the results for each smoking/drinking PRS separately.
Drinks per week
This was the only alcohol-related trait analyzed. The PRS was negatively associated with the outcome of several psychosis-related SIPS items capturing the time-length of the specific symptoms as part of an assessment of psychosis presence and severity [59]. Differently from the previously hypothesized “self-medication” and diathesis-stress model [60], our results indicated that children and young adults with low genetic liability to alcohol drinking reported longer periods of prodromal psychotic symptoms. This is the opposite of what is expected for problematic alcohol use and alcohol dependence. Accordingly, we hypothesize that the association in our study is due to the “moderate alcohol consumption” component present in DPW and not to the component of this trait associated with alcohol misuse, reflecting the genetic differences between DPW and alcohol use disorder [61].
The main biological processes underlying the PRS associations correspond to the negative regulation of critical cell activities such as the development and the projection organization, ultimately impacting brain activity. Aberrations in these processes have previously been implicated in neurodevelopmental changes underlying psychosis [62, 63] and in altered synapses in the limbic brain areas that drive drinking behavior [64, 65]. DPW polygenic risk was also associated with the number of test trials administered to evaluate the social cognition and behavioral function in the psychometric tests related to emotion differentiation and age differentiation [66–68]. Finally, PRS for DPW was also positively associated with anxiety-related obsessive-compulsive disorder. The mesolimbic dopaminergic pathway originating in the ventral tegmental area (VTA) is critical for the onset of reward processing and emotional responses related to anxiety-related disorders [69–71].
Age of smoking initiation
Individuals with high genetic liability to early smoking initiation had a longer duration of psychosis symptoms. Despite the well-known association between schizophrenia, and cigarette smoking, a previous study suggested that individuals with psychosis started smoking at a similar age as non-psychotic comparison subjects [72]. Our findings are consistent with the hypothesis that psychosis and the predisposition to early smoking share some genetic liability. PRS for ASI is, unsurprisingly, positively associated with the years of paternal education. Adolescents whose parents had less or no college education are much more likely to smoke and to smoke earlier than those whose parents have a higher education [73]. Our results showed that the association between ASI polygenic risk and father’s education held even when the polygenic components of educational attainment, cognitive performance, and socioeconomic status were added as covariates in the model. This suggests that other mechanisms (e.g., dynastic effects and assortative mating) may be responsible for the genetic overlap between tobacco-smoking behaviors and parents’ education.
ASI genetic liability was positively associated with higher scores for correct recognition of angry faces (PEITANG) during the Penn Emotional Identification Test. This relationship suggests that genetic predisposition for smoking initiation may share some liability toward preferential processing of negative social information or perhaps a heightened experience of interpersonal stressors [74, 75]. This association was enriched for biological processes mainly related to cellular response to manganese ions (Mn2+), which is a key element in brain activation induced by chronic psychosocial stress [76, 77].
The genetic predisposition to later smoking initiation was also positively associated with the ratings of the effects of conduct disorder impacting social relationships (i.e., referencing a pattern of disruptive and violent behaviors following rule-breaking encounters; CDD022: “How much did these behaviors change your relationships with your friends?”) suggesting a more consciousness of their disruptive and antisocial behavior in people starting smoking later. A shared genetic etiology among substance abuse and conduct disorder has been previously described [78], and our data extend this relationship to the age of smoking initiation.
The WRAT total standardized score (WRAT_CR_STD) [79] was positively associated with ASI polygenic risk. An impact of smoking on cognitive decline has been observed in adulthood [80, 81] and childhood [82]. Nonetheless, our study suggests that this relationship might be also due to a shared genetic predisposition rather than the sole effect of tobacco smoking. Indeed, this association is not significant when covaried for the other PRS investigated.
While early tobacco use was already associated with specific non-affective psychosis [83], the association of PRS for ASI with SIP019 phenotype (i.e., “I think that I may get confused at times whether something I experience or perceive may be real or maybe just part of my imagination or dreams”) supports a partial contribution from horizontal pleiotropy (i.e., shared genetic basis) between these traits.
Cigarettes per day
PRS for CPD was positively associated with several psychosis-related SIPS items indicating the duration of prodromal psychotic symptoms. This finding is consistent with the shared genetic liability observed in discordant twin and sibling studies of schizophrenia [84]. With respect to this PRS association, we observed multiple biological processes related to cellular signaling homeostasis, including terms related to Synaptic Vesicles Membrane (GO:0030672) and Signal Release (GO:0023061). The disruption of synaptic plasticity is known to be associated with psychotic behaviors [85, 86].
The genetic liability to smoking quantity was negatively associated with emotional identification and verbal reasoning independently from psychopathology, substance use, and other behavioral traits. Accordingly, we can hypothesize that certain molecular mechanisms involved in the predisposition to smoking quantity are shared with these psychiatric and behavioral traits.
We observed multiple neuroinflammatory pathways among the biological processes enriched for CPD PRS associations. Neuroinflammation appears to correlate with neurocognitive changes in the context of aging [87–90]. Experimental manipulations of IL-6 signaling appear capable of producing related effects in animal models [91, 92]. Interaction between smoking history and genetic variation in the IL-6 promoter was predictive of circulating IL-6 and CRP levels [93]. Thus, pleiotropic effects between smoking quantity and cognitive development may involve the IL-6 pathway. Moreover, CPD genetic liability was previously shown to be enriched with biological processes related to amyloid metabolism and neuroinflammation [94].
Smoking initiation and cessation
Genetic predisposition to smoking initiation was positively associated with items related to oppositional defiant disorder in children and young adults. The positive phenotypic correlation of ODD002 (Oppositional Defiant Disorder: Was there a time when you often got into trouble with adults for refusing to do what they told you to do or for breaking rules at home/school?) with ADD011 (Attention Deficit Disorder: Did you often have trouble paying attention or keeping your mind on your school, work, chores, or other activities that you were doing?) and DEP004 (Depression: Has there ever been a time when you felt grouchy, irritable or in a bad mood most of the time; even little things would make you mad?) was already described [95, 96] and might account for the associations’ consistency with SI genetic liability.
The dysregulations of dopaminergic and nicotinic pathways are shared mechanisms between smoking habits and the onset of attention deficit disorders [97]. Conversely, the relationship between tobacco smoking and depressive status and anxiety is inconsistent in terms of the direction of association [98]. Our results supported the sharing of genetic determinants for smoking initiation and these survey item reports, even when the genetic predisposition for psychopathology was included as a covariate in the model. Nevertheless, environmental factors, such as the family context and parental behaviors, play a crucial role in the onset of these behaviors [99–101]. Indeed, higher levels of maternal education were associated with a lower likelihood of being a smoker [4]. This association held on even after we included PRSs for psychosocial and psychopathological traits as covariates, supporting the interplay between familiar factors and SI genetic predisposition in early adolescence [102].
Two more traits, SOC011 (Social Anxiety: Thinking about all of the time that you were afraid of your worst fear, whether or not you actually faced it, how long did your fear of this situation last in months?) and the age when the computerized neurocognitive battery was completed, were associated with the SI genetic predisposition, and, as expected, it is also positively related to the substance use. A child with a higher genetic vulnerability for smoking initiation may have more behavioral issues in general [103], which might prompt parents to seek evaluation at an earlier age. Indeed, even though the PNC cohort is not enriched for any disorder, the participants were recruited through a pediatric healthcare network. Accordingly, PNC participants include individuals reporting psychiatric traits and outcomes. We hypothesize that the association of PRS for SI with age at CNB administration may reflect a participation bias in the PNC cohort.
The positive relationship between the PRS for SI and age at neurocognitive testing was enriched with genetic variants involved in brain-relevant biological pathways, including positive regulation of the phospholipase A2 (PLA2) (GO: 0032430), which is involved in a pro-inflammatory status [104]. It may also contribute to nervous system degeneration [105], and appears to impact iron accumulation in the globus pallidus, the substantia nigra, and the dentate nucleus [106]. Our data are consistent with the previously reported association between PLA2 genetic variation and the development of smoking habits in people affected by psychiatric disorders [107]. Other genetic variants underpinning this association are also involved in the axodendritic protein transport (GO: 0099640), suggesting their role in the proper synapse development [108].
Our data confirm that tobacco smoking and alcohol drinking share a genetic liability involving multiple biological processes [109], and the most significant functional enriched process in substance use association was the DNA geometric change (GO:0032392).
Genetic variants underlying substance use associations with the genetic liability for tobacco smoking recognized cellular response to carbohydrate stimuli, that are increasingly considered to alter brain circuitry, leveraging the induction of dopamine reward and craving that are comparable in magnitude to those induced by addictive drugs or alcohol use [110, 111].
The substance associations with the genetic liability to SI were also driven by genetic variants involved in the metabolism of phosphatidylethanolamine, a phospholipid critical for white matter establishment [112, 113]. Finally, the genetic liability to SC partially overlaps with the outcome of the Penn Matrix Reasoning Test, suggesting that people who opted for fewer practice trials before the test are less genetically predisposed to quit smoking.
In conclusion, our study provided evidence that the polygenic risk for tobacco-smoking and alcohol-use phenotypes overlap with several neurobehavioral traits assessed in a population-based cohort of children and adolescence, and that these relationships appeared independent of actual psychoactive substance use. The associations were also independent of the genetic effects exerted by genetically correlated phenotypes, including other substance use phenotypes, psychopathology, and psychosocial factors. Our findings highlight plausible pleiotropic mechanisms linking genetic liability to smoking and drinking behaviors to aspects of cognitive and behavioral development.
Supplementary information
Acknowledgements
We would like to acknowledge support from the National Institutes of Health [grants R21 DA047527, R33 DA047527, R21 DC018098, F32 MH122058].
Author contributions
FDA and RP designed the study. FDA conducted the data analysis. FDA, FRW, GAP, DST, AG, JG, and RP participated in the interpretation of data and critical revision of the manuscript for important intellectual content. FRW and RP obtained the funding. RP supervised the study. All the authors revised and approved the final version of this manuscript.
Competing interests
Drs. Polimanti and Gelernter are paid for their editorial work on the journal Complex Psychiatry. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01713-z.
References
- 1.World Health Organization. Guidance on the WHO review of psychoactive substances for international control. 2008. https://www.who.int/medicines/areas/quality_safety/GLS_WHORev_PsychoactSubst_IntC_2010.pdf. Accessed 4 December 2020.
- 2.World Health Organization. Global report mortality attributable to tobacco. 2012. https://apps.who.int/iris/bitstream/handle/10665/44815/9789241564434_eng.pdf;jsessionid=F6FB9406A950C0B25897ADD22F542507?sequence=1. Accessed 4 December 2020.
- 3.World Health Organization. Global status report on alcohol and health 2018. 2018. https://www.who.int/substance_abuse/publications/global_alcohol_report/en/. Accessed 4 December 2020.
- 4.Kõks G, Fischer K, Kõks S. Smoking-related general and cause-specific mortality in Estonia. BMC Public Health. 2017;18:34. Erratum in: BMC Public Health. 2017;17:736. [DOI] [PMC free article] [PubMed]
- 5.NSDUH. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. 2019. https://www.samhsa.gov/data/ Accessed 4 December 2020.
- 6.Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N = 112 117). Mol Psychiatry. 2017. 10.1038/mp.2017. [DOI] [PMC free article] [PubMed]
- 7.Jorgenson E, Thai KK, Hoffmann TJ, Sakoda LC, Kvale MN, Banda Y, et al. Genetic contributors to variation in alcohol consumption vary by race/ethnicity in a large multi-ethnic genome-wide association study. Mol Psychiatry. 2017. 10.1038/mp.2017.101. [DOI] [PMC free article] [PubMed]
- 8.Gelernter J, Zhou H, Nuñez YZ, Mutirangura A, Malison RT, Kalayasiri R. Genome-wide association study of alcohol dependence and related traits in a Thai population. Alcohol Clin Exp Res. 2018. 10.1111/acer.13614. [DOI] [PMC free article] [PubMed]
- 9.Kranzler HR, Zhou H, Kember RL, Vickers Smith R, Justice AC, Damrauer S, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019. 10.1038/s41467-019-09480-8. [DOI] [PMC free article] [PubMed]
- 10.Erzurumluoglu AM, Liu M, Jackson VE, Barnes DR, Datta G, Melbourne CA, et al. Meta-analysis of up to 622,409 individuals identifies 40 novel smoking behaviour associated genetic loci. Mol Psychiatry. 2019. 10.1038/s41380-018-0313-0. [DOI] [PMC free article] [PubMed]
- 11.Matoba N, Akiyama M, Ishigaki K, Kanai M, Takahashi A, Momozawa Y, et al. GWAS of smoking behaviour in 165,436 Japanese people reveals seven new loci and shared genetic architecture. Nat Hum Behav. 2019. 10.1038/s41562-019-0557-y. [DOI] [PubMed]
- 12.Evans LM, Jang S, Hancock DB, Ehringer MA, Otto JM, Vrieze SI, Keller MC. Genetic architecture of four smokingbehaviors using partitioned SNP heritability. Addiction. 2021. 10.1111/add.15450. [DOI] [PMC free article] [PubMed]
- 13.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019. 10.1038/s41588-018-0307-5. [DOI] [PMC free article] [PubMed]
- 14.Fritsche LG, Gruber SB, Wu Z, Schmidt EM, Zawistowski M, Moser SE, et al. Association of polygenic risk scores for multiple cancers in a phenome-wide study: results from The Michigan Genomics Initiative. Am J Hum Genet. 2018. 10.1016/j.ajhg.2018.04.001. [DOI] [PMC free article] [PubMed]
- 15.Shen X, Howard DM, Adams MJ, Hill WD, Clarke TK; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, et al. A phenome-wide association and Mendelian Randomisation study of polygenic risk for depression in UK Biobank. Nat Commun. 2020. 10.1038/s41467-020-16022-0. [DOI] [PMC free article] [PubMed]
- 16.Meng X, Li X, Timofeeva MN, He Y, Spiliopoulou A, Wei WQ, et al. Phenome-wide Mendelian-randomization study of genetically determined vitamin D on multiple health outcomes using the UK Biobank study. Int J Epidemiol. 2019. 10.1093/ije/dyz182. [DOI] [PMC free article] [PubMed]
- 17.Richardson TG, Harrison S, Hemani G, Davey Smith G. An atlas of polygenic risk score associations to highlight putative causal relationships across the human phenome. Elife. 2019. 10.7554/eLife.43657. [DOI] [PMC free article] [PubMed]
- 18.Leppert B, Millard LAC, Riglin L, Davey Smith G, Thapar A, Tilling K, et al. A cross-disorder PRS-pheWAS of 5 major psychiatric disorders in UK Biobank. PLoS Genet. 2020. 10.1371/journal.pgen.1008185. [DOI] [PMC free article] [PubMed]
- 19.Kember RL, Merikangas AK, Verma SS, Verma A, Judy R; Regeneron Genetics Center, et al. Polygenic risk of psychiatric disorders exhibits cross-trait associations in electronic health record data from European Ancestry Individuals. Biol Psychiatry. 2021. 10.1016/j.biopsych.2020.06.026. [DOI] [PMC free article] [PubMed]
- 20.Zheutlin AB, Dennis J, Karlsson Linnér R, Moscati A, Restrepo N, Straub P, et al. Penetrance and pleiotropy of polygenic risk scores for schizophrenia in 106,160 patients across four health care systems. Am J Psychiatry. 2019. 10.1176/appi.ajp.2019.18091085. [DOI] [PMC free article] [PubMed]
- 21.Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, et al. The Philadelphia Neurodevelopmental Cohort: a publicly available resource for the study of normal and abnormal brain development in youth. NeuroImage. 2016. 10.1016/j.neuroimage.2015.03.056. [DOI] [PMC free article] [PubMed]
- 22.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014. 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed]
- 23.Robinson EB, Kirby A, Ruparel K, Yang J, McGrath L, Anttila V, et al. The genetic architecture of pediatric cognitive abilities in the Philadelphia Neurodevelopmental Cohort. Mol Psychiatry. 2015. 10.1038/mp.2014.65. [DOI] [PMC free article] [PubMed]
- 24.Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014. 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed]
- 25.Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn computerized neurocognitive battery. Neuropsychology. 2015. 10.1037/neu0000093. [DOI] [PMC free article] [PubMed]
- 26.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text rev.). 10.1176/appi.books.9780890423349. 2000.
- 27.Moore TM, Martin IK, Gur OM, Jackson CT, Scott JC, Calkins ME, et al. Characterizing social environment’s association with neurocognition using census and crime data linked to the Philadelphia Neurodevelopmental Cohort. Psychol Med. 2016. 10.1017/S0033291715002111. [DOI] [PMC free article] [PubMed]
- 28.Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015. 10.1111/jcpp.12416. [DOI] [PMC free article] [PubMed]
- 29.Robinson EB, Kirby A, Ruparel K, Yang J, McGrath L, Anttila V, et al. The genetic architecture of pediatric cognitive abilities in the Philadelphia Neurodevelopmental Cohort. Mol Psychiatry. 2015. 10.1038/mp.2014.65. [DOI] [PMC free article] [PubMed]
- 30.Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, et al. Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet. 2017. 10.1016/j.ajhg.2017.03.004. [DOI] [PMC free article] [PubMed]
- 31.Wendt F, Muniz Carvalho C, Pathak G, Gelernter J, Polimanti R. Polygenic risk for autism spectrum disorder associates with anger recognition in a neurodevelopment-focused phenome-wide scan of unaffected youths from a population-based cohort. PLoS Genet. 2020. 10.1371/journal.pgen.1009036. [DOI] [PMC free article] [PubMed]
- 32.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015. 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed]
- 33.O’Connell J, Gurdasani D, Delaneau O, Pirastu N, Ulivi S, Cocca M, et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet. 2014. 10.1371/journal.pgen.1004234. [DOI] [PMC free article] [PubMed]
- 34.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009. 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed]
- 35.Zhan X, Hu Y, Li B, Abecasis GR, Liu DJ. RVTESTS: an efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics. 2016. 10.1093/bioinformatics/btw079. [DOI] [PMC free article] [PubMed]
- 36.Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015. 10.1038/ng.3406. [DOI] [PMC free article] [PubMed]
- 37.Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh PR, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet. 2015. 10.1038/ng.3404. [DOI] [PMC free article] [PubMed]
- 38.Choi SW, O’Reilly PF. PRSice-2: Polygenic Risk Score software for biobank-scale data. GigaScience. 2019. 10.1093/gigascience/giz082. [DOI] [PMC free article] [PubMed]
- 39.Choi SW, Mak TS, O’Reilly PF. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc. 2020. 10.1038/s41596-020-0353-1. [DOI] [PMC free article] [PubMed]
- 40.Kõks G, Prans E, Ho XD, Duy BH, Tran HD, Ngo NB, et al. Genetic interaction between two VNTRs in the MAOA gene is associated with the nicotine dependence. Exp Biol Med. 2020. 10.1177/1535370220916888. [DOI] [PMC free article] [PubMed]
- 41.Hivert V, Sidorenko J, Rohart F, Goddard ME, Yang J, Wray NR, et al. Estimation of non-additive genetic variance in human complex traits from a large sample of unrelated individuals. Am J Hum Genet. 2021. 10.1016/j.ajhg.2021.02.014. [DOI] [PMC free article] [PubMed]
- 42.Wickham H. ggplot2: elegant graphics for data analysis. New York, NY: Springer-Verlag; 2009.
- 43.Alzola C, Harrell F. An introduction to S and the Hmisc and design libraries. 2006. https://cran.r-project.org/doc/contrib/Alzola+Harrell-Hmisc-Design-Intro.pdf. Accessed 4 December 2020.
- 44.Cross-Disorder Group of the Psychiatric Genomics Consortium, et al. Genome wide meta-analysis identifies genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Preprint at 10.1101/528117 (2019). [DOI] [PMC free article] [PubMed]
- 45.Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher, M et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018. 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed]
- 46.Trampush JW, Yang ML, Yu J, Knowles E, Davies G, Liewald DC, et al. GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: a report from the COGENT consortium. Mol Psychiatry. 2017. 10.1038/mp.2016.244. [DOI] [PMC free article] [PubMed]
- 47.Karlsson Linnér R, Biroli P, Kong E, Meddens SFW, Wedow R, Fontana MA, et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet. 2019. 10.1038/s41588-018-0309-3. [DOI] [PMC free article] [PubMed]
- 48.Hill WD, Davies NM, Ritchie SJ, Skene NG, Bryois J, Bell S, et al. Genome-wide analysis identifies molecular systems and 149 genetic loci associated with income. Nat Commun. 2019. 10.1038/s41467-019-13585-5. [DOI] [PMC free article] [PubMed]
- 49.Yates AD, Achuthan P, Akanni W, Allen J, Allen J, Alvarez-Jarreta J. et al. Ensembl 2020. Nucleic Acids Res. 2020. 10.1093/nar/gkz966. [DOI] [PMC free article] [PubMed]
- 50.Supek F, Bošnjak M, Škunca N, Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011. 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed]
- 51.Couto FM, Silva MJ, Coutinho PM. Measuring semantic similarity between Gene Ontology terms. Data Knowl Eng. 2007. 10.1016/j.datak.2006.05.003.
- 52.Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999 doi: 10.1023/A:1022034115078. [DOI] [PubMed] [Google Scholar]
- 53.Wilkinson G, Robertson G. Psychological Assessment Resources I. WRAT 4: wide range achievement test; professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2006.
- 54.Kinreich S, McCutcheon VV, Aliev F, Meyers JL, Kamarajan C, Pandey AK, et al. Predicting alcohol use disorder remission: a longitudinal multimodal multi-featured machine learning approach. Transl Psychiatry. 2021. 10.1038/s41398-021-01281-2. [DOI] [PMC free article] [PubMed]
- 55.Easey KE, Wootton RE, Sallis HM, Haan E, Schellhas L, Munafò MR, et al. Characterization of alcohol polygenic risk scores in the context of mental health outcomes: within-individual and intergenerational analyses in the Avon Longitudinal Study of Parents and Children. Drug Alcohol Depend. 2021. 10.1016/j.drugalcdep.2021.108654. [DOI] [PMC free article] [PubMed]
- 56.Barr PB, Ksinan A, Su J, Johnson EC, Meyers JL, Wetherill L, et al. Using polygenic scores for identifying individuals at increased risk of substance use disorders in clinical and population samples. Transl Psychiatry. 2020. 10.1038/s41398-020-00865-8. [DOI] [PMC free article] [PubMed]
- 57.Khouja JN, Wootton RE, Taylor AE, Davey Smith G, Munafò MR. Association of genetic liability to smoking initiation with e-cigarette use in young adults: a cohort study. PLoS Med. 2021. 10.1371/journal.pmed.1003555. [DOI] [PMC free article] [PubMed]
- 58.Bray MJ, Chen LS, Fox L, Ma Y, Grucza RA, Hartz SM, et al. Studying the utility of using genetics to predict smoking-related outcomes in a population-based study and a selected cohort. Nicotine Tob Res. 2021. 10.1093/ntr/ntab100. [DOI] [PMC free article] [PubMed]
- 59.Griffa A, Baumann PS, Klauser P, Mullier E, Cleusix M, Jenni R. Brain connectivity alterations in early psychosis: from clinical to neuroimaging staging. Transl Psychiatry. 2019. 10.1038/s41398-019-0392-y. [DOI] [PMC free article] [PubMed]
- 60.Polimanti R, Agrawal A, Gelernter J. Schizophrenia and substance use comorbidity: a genome-wide perspective. Genome Med. 2017. 10.1186/s13073-017-0423-3. [DOI] [PMC free article] [PubMed]
- 61.Zhou H, Sealock JM, Sanchez-Roige S, Clarke TK, Levey DF, Cheng Z, et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci. 2020. 10.1038/s41593-020-0643-5. [DOI] [PMC free article] [PubMed]
- 62.Leong AT, Chan RW, Gao PP, Chan YS, Tsia KK, Yung WH, et al. Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proc Natl Acad Sci USA. 2016. 10.1073/pnas.1616361113. [DOI] [PMC free article] [PubMed]
- 63.Haarsma J, Fletcher PC, Griffin JD, Taverne HJ, Ziauddeen H, Spencer TJ, et al. Precision weighting of cortical unsigned prediction error signals benefits learning, is mediated by dopamine, and is impaired in psychosis. Mol Psychiatry. 2020. 10.1038/s41380-020-0803-8. [DOI] [PMC free article] [PubMed]
- 64.Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, et al. Role of ventral subiculum in context-induced relapse to alcohol seeking after punishment-imposed abstinence. J Neurosci. 2016. 10.1523/JNEUROSCI.4299-15.2016. [DOI] [PMC free article] [PubMed]
- 65.Sayette MA. The effects of alcohol on emotion in social drinkers. Behav Res Ther. 2017. 10.1016/j.brat.2016.06.005. [DOI] [PMC free article] [PubMed]
- 66.Swagerman SC, de Geus EJC, Kan KJ, van Bergen E, Nieuwboer HA, Koenis MMG, et al. The computerized neurocognitive battery: validation, aging effects, and heritability across cognitive domains. Neuropsychology. 2016. 10.1037/neu0000248. [DOI] [PubMed]
- 67.Gur RE, Moore TM, Calkins ME, Ruparel K, Gur RC. Face processing measures of social cognition: a dimensional approach to developmental psychopathology. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2017. 10.1016/j.bpsc.2017.03.010. [DOI] [PubMed]
- 68.Oruc I, Balas B, Landy MS. Face perception: a brief journey through recent discoveries and current directions. Vis Res. 2019. 10.1016/j.visres.2019.06.005. [DOI] [PMC free article] [PubMed]
- 69.Wang Y, Yao L, Zhao X. Amygdala network in response to facial expression following neurofeedback training of emotion. Brain Imaging Behav. 2020. 10.1007/s11682-019-00052-4. [DOI] [PubMed]
- 70.Sun F, Lei Y, You J, Li C, Sun L, Garza J, et al. Adiponectin modulates ventral tegmental area dopamine neuron activity and anxiety-related behavior through AdipoR1. Mol Psychiatry. 2019. 10.1038/s41380-018-0102-9. [DOI] [PMC free article] [PubMed]
- 71.Juarez B, Morel C, Ku SM, Liu Y, Zhang H, Montgomery S, et al. Midbrain circuit regulation of individual alcohol drinking behaviors in mice. Nat Commun. 2017. 10.1038/s41467-017-02365-8. [DOI] [PMC free article] [PubMed]
- 72.Gurillo P, Jauhar S, Murray RM, MacCabe JH. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry. 2015. 10.1016/S2215-0366(15)00152-2. [DOI] [PMC free article] [PubMed]
- 73.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975−2015: overview of key findings on adolescent drug use. Institute for Social Research. Available at http://monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf (Ann Arbor, MI: The University of Michigan; 2016).
- 74.Brondolo E, Rieppi R, Erickson SA, Bagiella E, Shapiro PA, McKinley P. Hostility, interpersonal interactions, and ambulatory blood pressure. Psychosom Med. 2003. 10.1097/01.PSY.0000097329.53585.A1. [DOI] [PubMed]
- 75.Kahler CW, McHugh RK, Leventhal AM, Colby SM, Gwaltney CJ, Monti PM. High hostility Among Smokers Predicts Slower Recognition of Positive Facial Emotion. Pers Individual Differ. 2012. 10.1016/j.paid.2011.11.009. [DOI] [PMC free article] [PubMed]
- 76.Bouabid S, Tinakoua A, Lakhdar‐Ghazal N, Benazzouz A. Manganese neurotoxicity: behavioral disorders associated with dysfunctions in the basal ganglia and neurochemical transmission. J Neurochem. 2016. 10.1111/jnc.13442. [DOI] [PubMed]
- 77.Laine MA, Sokolowska E, Dudek M, Callan SA, Hyytiä P, Hovatta I. Brain activation induced by chronic psychosocial stress in mice. Sci Rep. 2017. 10.1038/s41598-017-15422-5. [DOI] [PMC free article] [PubMed]
- 78.Grant JD, Lynskey MT, Madden PA, Nelson EC, Few LR, Bucholz KK, et al. The role of conduct disorder in the relationship between alcohol, nicotine and cannabis use disorders. Psychol. Med. 2015. 10.1017/S0033291715001518. [DOI] [PMC free article] [PubMed]
- 79.Harvey PD, Friedman JI, Bowie C, Reichenberg A, McGurk SR, Parrella M, et al. Validity and stability of performance-based estimates of premorbid educational functioning in older patients with schizophrenia. J Clin Exp Neuropsychol. 2006. 10.1080/13803390500360349. [DOI] [PubMed]
- 80.Sabia S, Elbaz A, Dugravot A, Head J, Shipley M, Hagger-Johnson G, et al. Impact of smoking on cognitive decline in early old age: The Whitehall II cohort study. Arch Gen Psychiatry. 2012. 10.1001/archgenpsychiatry.2011.2016. [DOI] [PMC free article] [PubMed]
- 81.Sabia S, Marmot M, Dufouil C, Singh-Manoux A. Smoking history and cognitive function in middle age from the Whitehall II study. Arch Intern Med. 2008. 10.1001/archinte.168.11.1165. [DOI] [PMC free article] [PubMed]
- 82.Julvez J, Ribas-Fitó N, Torrent M, Forns M, Garcia-Esteban R, Sunyer J. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int J Epidemiol. 2007. 10.1093/ije/dym107. [DOI] [PubMed]
- 83.McGrath JJ, Alati R, Clavarino A, Williams GM, Bor W, Najman JM, et al. Age at first tobacco use and risk of subsequent psychosis-related outcomes: a birth cohort study. Aust NZ J Psychiatry. 2016. 10.1177/0004867415587341. [DOI] [PubMed]
- 84.Kendler KS, Lönn SL, Sundquist J, Sundquist K. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015. 10.1176/appi.ajp.2015.15010126. [DOI] [PMC free article] [PubMed]
- 85.Crabtree GW, Gogos JA. Synaptic plasticity, neural circuits, and the emerging role of altered short-term information processing in schizophrenia. Front Synaptic Neurosci. 2014. 10.3389/fnsyn.2014.00028. [DOI] [PMC free article] [PubMed]
- 86.Mizuno T, Matsumoto H, Mita K, Kogauchi S, Kiyono Y, Kosaka H, et al. Psychosis is an extension of mood swings from the perspective of neuronal plasticity impairments. Med Hypotheses. 2019. 10.3389/fnsyn.2014.00028. [DOI] [PubMed]
- 87.Bradburn S, Sarginson J, Murgatroyd CA. Association of peripheral interleukin-6 with global cognitive decline in non-demented adults: a meta-analysis of prospective studies. Front Aging Neurosci. 2018. 10.3389/fnagi.2017.00438. [DOI] [PMC free article] [PubMed]
- 88.Fominykh V, Vorobyeva A, Onufriev MV, Brylev L, Zakharova MN, Gulyaeva NV. Interleukin-6, S-Nitrosothiols, and neurodegeneration in different central nervous system demyelinating disorders: is there a relationship? J Clin Neurol. 2018. 10.3988/jcn.2018.14.3.327. [DOI] [PMC free article] [PubMed]
- 89.Bobbo VCD, Jara CP, Mendes NF, Morari J, Velloso LA, Araújo EP. Interleukin-6 expression by hypothalamic microglia in multiple inflammatory contexts: a systematic review. BioMed Res Int. 2019. 10.1155/2019/1365210. [DOI] [PMC free article] [PubMed]
- 90.Jung YH, Shin NY, Jang JH, Lee WJ, Lee D, Choi Y, et al. Relationships among stress, emotional intelligence, cognitive intelligence, and cytokines. Medicine. 2019. 10.1097/MD.0000000000015345. [DOI] [PMC free article] [PubMed]
- 91.Donegan JJ, Girotti M, Weinberg MS, Morilak DA. A novel role for brain interleukin-6: facilitation of cognitive flexibility in rat orbitofrontal cortex. J Neurosci. 2014. 10.1523/JNEUROSCI.3968-13.2014. [DOI] [PMC free article] [PubMed]
- 92.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA. 2014. 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed]
- 93.Sunyer J, Forastiere F, Pekkanen J, Plana E, Kolz M, Pistelli R, et al. Interaction between smoking and the interleukin-6 gene affects systemic levels of inflammatory biomarkers. Nicotine Tob Res. 2009. 10.1093/ntr/ntp144. [DOI] [PubMed]
- 94.Winer JR, Maass A, Pressman P, Stiver J, Schonhaut DR, Baker SL, et al. Associations between tau, β-amyloid, and cognition in Parkinson disease. JAMA Neurol. 2018. 10.1001/jamaneurol.2017.3713. [DOI] [PMC free article] [PubMed]
- 95.Noordermeer SDS, Luman M, Weeda WD, Buitelaar JK, Richards JS, Hartman CA, et al. Risk factors for comorbid oppositional defiant disorder in attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2017. 10.1007/s00787-017-0972-4. [DOI] [PMC free article] [PubMed]
- 96.Ding W, Meza J, Lin X, He T, Chen H, Wanget Y, et al. Oppositional defiant disorder symptoms and children’s feelings of happiness and depression: mediating roles of interpersonal relationships. Child Indic Res. 2020. 10.1007/s12187-019-09685-9.
- 97.van Amsterdam J, van der Velde B, Schulte M, van den Brink W. Causal factors of increased smoking in ADHD: a systematic review. Subst Use Misuse. 2018. 10.1080/10826084.2017.1334066. [DOI] [PubMed]
- 98.Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 2017. 10.1093/ntr/ntw140. [DOI] [PMC free article] [PubMed]
- 99.Deault LC. A systematic review of parenting in relation to the development of comorbidities and functional impairments in children with attention-deficit/hyperactivity disorder (ADHD). Child Psychiatry Hum Dev. 2010. 10.1007/s10578-009-0159-4. [DOI] [PubMed]
- 100.Banerjee J, Bhojani S, Emcy N. Co-existence of ADHD, autoimmune hypothyroidism and pituitary macroadenoma presenting in a behaviour clinic: a case report and brief review of the literature. J Pediatr Endocrinol Metab. 2011. 10.1515/jpem.2011.122. [DOI] [PubMed]
- 101.Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013. 10.1111/j.1469-7610.2012.02611.x. [DOI] [PMC free article] [PubMed]
- 102.Maes HH, Prom-Wormley E, Eaves LJ, Rhee SH, Hewitt JK, Young S, et al. A genetic epidemiological mega analysis of smoking initiation in adolescents. Nicotine Tob Res. 2017. 10.1093/ntr/ntw294. [DOI] [PMC free article] [PubMed]
- 103.Minichino A, Bersani FS, Calò WK, Spagnoli F, Francesconi M, Vicinanza R, et al. Smoking behaviour and mental health disorders—mutual influences and implications for therapy. Int J Environ Res Public Health. 2013. 10.3390/ijerph10104790. [DOI] [PMC free article] [PubMed]
- 104.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004. 10.1194/jlr.R300016-JLR200. [DOI] [PubMed]
- 105.Collins MA, Tajuddin N, Moon KH, Kim HY, Nixon K, Neafsey EJ. Alcohol, phospholipase A2-associated neuroinflammation, and ω3 docosahexaenoic acid protection. Mol Neurobiol. 2014. 10.1007/s12035-014-8690-0. [DOI] [PMC free article] [PubMed]
- 106.Lehéricy S, Roze E, Goizet C, Mochel F. MRI of neurodegeneration with brain iron accumulation. Curr Opin Neurol. 2020. 10.1097/WCO.0000000000000844. [DOI] [PubMed]
- 107.Nadalin S, Rebić J, Šendula Jengić V, Peitl V, Karlović D, Buretić-Tomljanović A. Association between PLA2G6 gene polymorphism for calcium-independent phospholipase A2 and nicotine dependence among males with schizophrenia. Prostaglandins, Leukot Essent Fatty Acids. 2019. 10.1016/j.plefa.2019.07.005. [DOI] [PubMed]
- 108.Law MH, Cotton RGH, Berger GE. The role of phospholipases A2 in schizophrenia. Mol Psychiatry. 2006. 10.1038/sj.mp.4001819. [DOI] [PubMed]
- 109.Prom-Wormley EC, Ebejer J, Dick DM, Bowers MS. The genetic epidemiology of substance use disorder: a review. Drug Alcohol Depend. 2017. 10.1016/j.drugalcdep.2017.06.040. [DOI] [PMC free article] [PubMed]
- 110.Gordon EL, Ariel-Donges AH, Bauman V, Merlo LJ. What is the evidence for “food addiction?” A systematic review. Nutrients. 2018. 10.3390/nu10040477. [DOI] [PMC free article] [PubMed]
- 111.Winterdahl M, Noer O, Orlowski D, Schacht AC, Jakobsen S, Alstrup AKO, et al. Sucrose intake lowers μ-opioid and dopamine D2/3 receptor availability in porcine brain. Sci Rep. 2019. 10.1038/s41598-019-53430-9. [DOI] [PMC free article] [PubMed]
- 112.Fledrich R, Abdelaal T, Rasch L, Bansal V, Schütza V, Brügger B, et al. Targeting myelin lipid metabolism as a potential therapeutic strategy in a model of CMT1A neuropathy. Nat Commun. 2018. 10.1038/s41467-018-05420-0. [DOI] [PMC free article] [PubMed]
- 113.Unterrainer HF, Hiebler-Ragger M, Koschutnig K, Fuchshuber J, Ragger K, Perchtold CM, et al. Brain structure alterations in poly-drug use: reduced cortical thickness and white matter impairments in regions associated with affective, cognitive, and motor functions. Front Psychiatry. 2019. 10.3389/fpsyt.2019.00667. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.