Abstract
For over 70 years experimental autoimmune encephalomyelitis (EAE) has been induced with myelin autoantigens emulsified in complete Freund’s adjuvant (CFA) which has significant side effects such as pain, inflammation, and tissue necrosis at the injection site. β-1,3-D-glucan particles (GPs) are hollow microcapsules prepared from Saccharomyces cerevisiae cell walls that induce potent Th17 cell responses without causing strong injection site tissue reactions. We evaluated the potential of GPs complexed with neuroantigens to induce EAE while avoiding undesirable side effects. GPs loaded with myelin oligodendrocyte glycoprotein 35–55 (MOG35–55) or proteolipid protein 139–151 (PLP139–151) peptides effectively induced EAE in C57BL/6 mice and SJL mice. Disease severity, CNS pathology and immune responses were comparable between GP- and CFA-immunized mice. Importantly, injection with GPs resulted in significantly decreased inflammation compared with CFA. We posit that use of GPs provides an alternative means for inducing EAE that results in comparable disease, but less discomfort to animals.
Keywords: Multiple sclerosis, EAE, Glucan particles
1. Introduction
Multiple sclerosis (MS) is a debilitating neuroinflammatory disease of the central nervous system (CNS). A hallmark of MS pathogenesis is the occurrence of lesions in the CNS, characterized by infiltration of CD4+ and CD8+ T cells, B cells/plasma cells, macrophages, and dendritic cells (DC) that generate an inflammatory milieu of cytokines, chemokines, and antibodies [1, 2]. Much of our understanding of the pathology of MS as well as advances in treatment have come from preclinical animal models of neuroinflammation and demyelination, the most common being experimental autoimmune encephalomyelitis (EAE).
EAE recapitulates the clinical and histopathological hallmarks of MS with the infiltration of mononuclear cells in the CNS resulting in demyelination and ultimately paralysis [3]. EAE can be induced actively by injection of myelin antigens in adjuvant, passively by the transfer of autoreactive T cells, or disease can develop spontaneously in transgenic animals [4]. Active and passive induction of autoimmune pathology in most models rely on the use of complete Freund’s adjuvant (CFA), a method that is problematic due to pain, inflammation and resulting granuloma formation at the injection site as well as distal tissues [5]. Though its exact mechanism for inducing autoimmune disease is not fully delineated, CFA contains Mycobacterium tuberculosis (Mtb) that provides pathogen associated molecular patterns (PAMPs) recognized by the innate system ultimately leading to antigen-specific adaptive immune responses [6]. The oil-in-water emulsion also serves as an antigen depot for release into the body to facilitate immune responses [7]. CFA has been an invaluable tool for induction of disease in many autoimmune animal disease animals since its description over seven decades ago and has remained the standard due to of lack of suitable alternatives [8].
Here we show the use of glucan particles (GPs) as a novel method of inducing active EAE, as well as for inducing autoreactive T cells for induction of passive EAE. GPs are hollow vesicles composed primarily of β-1,3-D-glucans obtained from purified cell walls of Saccharomyces cerevisiae. They can be loaded with a variety of particles, including peptides and proteins. The β-1,3-D-glucans serve as PAMPs that are recognized by Dectin-1 receptors and CR3 found predominantly on professional antigen-presenting cells (APCs) [9]. Their utility as potential vaccine adjuvants has been shown recently for several infectious pathogens, including Cryptococcus spp., Histoplasma capsulatum, Francisella tularensis, and Coccidioides posadasii [10–14]. Of particular relevance is the observation that GPs skew the immune response towards Th17 immunity, which has been implicated as driver of autoimmune pathology in EAE and MS [15]. Based on these findings we hypothesized that EAE could be induced in mice by the administration of GPs loaded with myelin autoantigens.
Our results show that GPs loaded with the autoantigen peptides myelin oligodendrocyte glycoprotein (MOG) 35–55 or proteolipid protein (PLP) 139–151 induced active EAE in B6 or SJL mice, respectively, and that the resulting disease incidence and severity was comparable to that when these peptides were injected in CFA. Autoantigen-containing GPs as adjuvants were also comparable to CFA in that they produced similar immune cell infiltration in the CNS and generated comparable peripheral immune responses. Additionally, we showed that GP:MOG35–55 immunization generated autoreactive T cells that when transferred to C57BL/6 mice induced passive EAE. Importantly, injection with GPs resulted in significantly less inflammation than injection with CFA. Thus, our results suggest that GPs may be an effective alternative for CFA for the induction of experimental autoimmune diseases while causing less distress to the animals.
2. Materials and Methods
2.1. GP Packaging
GPs were prepared as previously described [11, 16–18]. The MOG35–55 (Peptide 2.0, 98.2% purity) or PLP139–151 peptides (Peptide 2.0, 98.6% purity) were dissolved at 100 mg/ml in sterile, pyrogen-free water and 100 µl of peptide solution was mixed with 20 mg GPs, allowed to swell at 4°C for 30 minutes and then frozen. Following lyophilization the peptides were trapped inside the GPs by gradually mixing in 150 µl of 20 mg/ml dextran sulfate, sodium salt (Millipore Sigma RES2029D-A707X). Following washing with sterile saline the GP:MOG35–55 or GP:PLP139–151 peptide vaccines were suspended in a volume of sterile saline to deliver 400 µg GPs and 200 µg peptide in a 100 µl injection. To confirm that mice receive the expected amount of peptide delivered in GPs, efficiency of packaging was validated by SDS-PAGE comparing 10 µg of packaged GP:Peptide to 10 µg of free peptide. To further validate the efficiency of packaging, the supernatant containing the unbound fraction of the packaging reaction was also run to confirm the absence of unbound peptide.
2.2. EAE Induction
All experiments were conducted in accordance with animal protocols approved by the University of Texas at San Antonio Institutional Animal Care and Use Committee. Active EAE was induced in female C57BL/6 mice (C57BL/6J, The Jackson Laboratory, Bar Harbor, ME) by subcutaneous (s.c.) injection of 200 μg MOG35–55 peptide loaded into either GPs or emulsified in 50 μl CFA containing 5 mg/ml heat inactivated M. tuberculosis H37 RA (DIFCO Laboratories, Detroit, MI). Mice also received intraperitoneal (i.p.) injections of 200 ng pertussis toxin (List Biological Laboratories, Campbell, CA) on day 0 and day 2, with the GP group receiving a GP: MOG35–55 boost on day 4 post immunization. Active disease was induced similarly in female SJL mice (SJL/J, The Jackson Laboratory) using 100 μg PLP139–151 either packaged in GPs or emulsified in CFA.
For induction of passive EAE by adoptive transfer, female C57BL/6 donor mice were immunized s.c. with 200 µg MOG35–55 either loaded in GPs or emulsified in CFA [19]. The GP donor group was boosted with GP:MOG day 7 post immunization. Splenocytes and draining lymph nodes were collected from donor mice on Day 10 and restimulated with 20 μg/ml MOG35–55 for 3 days at 37°C in RPMI containing 10% fetal bovine serum and L-glutamine, in the presence of Th17 culture conditions: 20 ng/ml recombinant mouse IL-23 (BioLegend, San Diego, CA), 10 μg/ml anti-mouse IFN-γ monoclonal antibody (R4–6A2, Bio X Cell West Lebanon, NH). Recipient mice were injected with 15x106 cells i.p.
2.3. EAE Monitoring
Mice were monitored and scored daily for clinical EAE for up to 30 days using the following standard EAE scoring system [20]: 0, no abnormality; 1, limp tail; 2, moderate hind limb weakness; 3, complete hind limb paralysis; 4, quadriplegia or premoribund state; 5, death.
2.4. Cytokine ELISPOT Assay
Cytokine ELISPOT was performed as previously described [19, 21]. ELISPOT plates (MilliporeSigma, Burlington, MA) were coated with functional-grade purified anti-mouse IFN-γ mAB (AN-18, eBioscience, San Diego, CA), anti-mouse IL-17A mAB (17F3, Bio X Cell), or anti-mouse IL-5 mAB (TRFK5, Bio X Cell) at 4°C overnight. Splenocytes (0.5 x106 cells per well for IFN-γ and 1x106 cells per well for IL-17 and IL-5) were restimulated with MOG35–55 peptide in HL-1 medium (Lonza, Walkersville, MD) at 37°C for 24 hours for IFN-γ and IL-17 and 48 hours IL-5. Biotinylated anti-mouse IFN-γ mAB (R4–6A2, eBioscience), IL-17 mAB (TC11–8H4, BioLegend), and IL-5 (TRFK4, eBioscience) were added to corresponding plates and incubated overnight at 4°C, followed by incubation with streptavidin-alkaline phosphatase conjugate (Invitrogen, Eugene, OR) for 2 hours at from temperature and developing with BCIP/NBT Phosphatase Substrate (Seracare Life Sciences, Milford, MA). After plate developing, image analysis of spots was performed on an S6 Universal-V Analyzer (Cellular Technology Limited, Cleveland, OH).
2.5. Bio-Plex Cytokine Assay
Supernatants from cytokine ELISPOT were removed and cytokine and chemokine levels were analyzed by Bio-Plex Pro Mouse Cytokine 23-Plex Assay (BioRad, Hercules, CA) and read on the Bio-Plex 200 System following the manufacturer’s instructions.
2.6. H&E
Tissues (brain, spinal cord, injection site tissue) were harvested from the respective mice and fixed by immersion in 10% buffered formalin overnight. Tissues were then rinsed in PBS, dehydrated through increasing concentrations of ethanol, cleared, and embedded in paraffin. Each tissue block was cut into three 5 μm-thick sections, with each section 20 µm apart and mounted on slides. The sections were stained with hematoxylin and eosin (H&E) followed by microscopic examination for cellular infiltrates (Olympus microscope with DP72 camera, CellSens Standard 1.5 software).
2.7. Immunohistochemistry (IHC)
Murine brains and spinal cords were obtained at various time points, placed in OCT freezing compound (Fisher HealthCare, Houston, TX) and frozen at −80°C. Tissues were cut in 10 μm-thick sections and staggered on glass slides. Sections were fixed, permeabilized and blocked with bovine serum albumin. Labelled primary antibody FITC-conjugated anti-mouse CD45 antibody (30-F11, eBioscience) and DAPI or Fluoromyelin Green Fluorescent Myelin Stain (Invitrogen) and DAPI were incubated for one hour at room temperature in a closed, humid chamber. Slides were rinsed with permeabilization buffer and coverslips were mounted with Fluoromount-G (SouthernBiotech, Birmingham, AL) and allowed to dry and cure overnight at room temperature in the dark. Staining was followed by microscopic examination for cellular infiltrates and demyelination (Olympus microscope with DP72 camera, CellSens Standard 1.5 software). Contrast was adjusted minimally for each image and performed uniformly between corresponding images.
2.8. Flow cytometry
EAE brains were collected and depleted of myelin using myelin removal beads (Miltenyi Biotec, Bergisch Gladbach, Germany). For cell surface staining, cell suspensions were stained with fluorochrome-conjugated anti-mouse-CD45 (30-F11, eBioscience), anti-mouse-CD4 (RM4–5, BD Biosciences, San Jose, CA), anti-mouse-CD8b (H35–17.2, eBioscience), anti-mouse-Ly6G (1A8, BD Biosciences), anti-mouse-CD11b (M1/70, eBioscience), and anti-mouse-CD11c (N418, eBioscience). All samples were run on BD LSR II and analyzed with FlowJo software.
3. Results
3.1. Glucan particles packaged with myelin autoantigens induce active EAE comparable to CFA-induced disease
To begin to investigate the potential of GPs as an adjuvant for the induction of EAE, we generated GPs loaded with the autoantigen peptide MOG35–55 as described in Methods and outlined in Supplementary Fig. S1A. After synthesis, loading efficiency and quantification of MOG35–55 packaging into particles was validated by SDS-PAGE (Supplementary Fig. S1B). A 100 µl immunization dose containing approximately 400 µg of particles loaded with 200 μg MOG35–55 peptide was administered subcutaneously (s.c.) in the shoulder of C57BL/6 mice with an equivalent boost in the flank on day 4. Pertussis toxin (PTx) was administered on day 0 and day 2 post immunization. For comparison, a control group of mice was immunized with the equal amount of MOG35–55 peptide emulsified in 50 µl of the conventionally used CFA with 200 ng PTx on day 0 and 2.
As shown in Fig. 1A, C57BL/6 mice injected with CFA:MOG35–55 developed EAE around day 10–11 after immunization and EAE peaked by day 17 and remitted by day 19 (closed symbols). Importantly, mice injected with GP:MOG35–55 developed EAE with a similar monophasic disease course as compared to CFA:MOG35–55-immunized mice (Fig. 1A, open symbols). Immunization with each of the adjuvants resulted in similar disease incidence (Table 1). The mean onset of C57BL/6 GP:MOG35–55-induced EAE was comparable to that of CFA:MOG35–55-immunized mice, with GP-immunized mean onset at 11.6 days post immunization as compared with 12.3 days post immunization for CFA immunized mice (Table 1). Additionally, no significant differences were noted in peak disease scores between the experimental groups (Table 1).
Figure 1. GPs loaded with myelin autoantigens induce clinical EAE disease comparable to CFA-induced EAE.
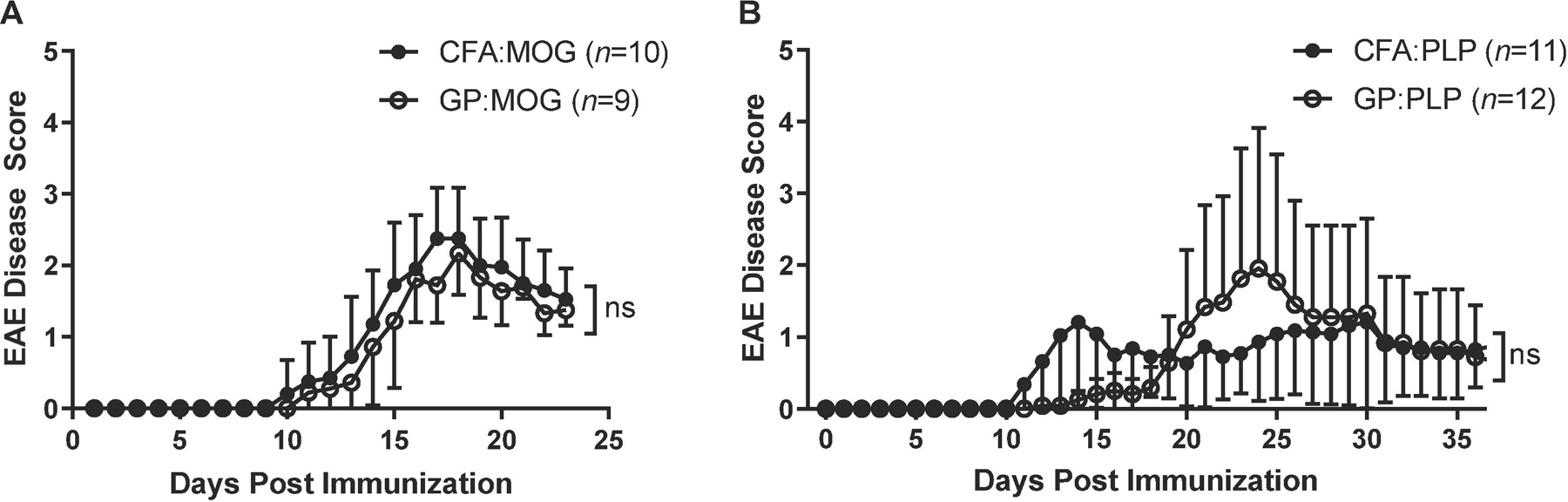
EAE was induced by s.c. injection of myelin autoantigens either packaged into GPs or emulsified in CFA. Mice were scored daily for clinical EAE. Shown are the clinical disease scores for (A) C57BL/6 active disease with 200 μg MOG35–55 administered once for CFA and twice for GP and (B) SJL active disease with 100 μg PLP139–151 administered once for CFA and twice for GP. Data shown are EAE disease score ± SD of one experiment representative of two independent C57BL/6 experiments and three independent SJL experiments (n=10–15 per group, unpaired t-test performed for each day).
Table 1.
Disease phenotype of C57BL/6 and SJL active immunization EAE
| C57BL/6 |
SJL |
|||||
|---|---|---|---|---|---|---|
| CFA: MOG35-55 | GP: MOG35-55 | t-testp value | CFA: PLP139–151 | GP: PLP139–151 | t-testp value | |
| Incidence | 19/25 (76%) | 24/30 (80%) | 34/45 (76%) | 30/40 (75%) | ||
| Survival | 100% | 100% | 97% | 90% | ||
| OnsetDay ± SD | 12.3 ± 1.7 | 11.6 ± 2.9 | 0.2688 | 13.0 ± 4.4 | 15.1 ± 2.6 | 0.7110 |
| PeakEAE Score ± SD | 2.4 ± 0.8 | 2.0 ± 0.7 | 0.1456 | 2.3 ± 0.8 | 2.2 ± 0.8 | 0.3299 |
Similar results were obtained in SJL mice immunized with GP:PLP139–151 on day 0 and day 4 and injected with PTx on day 0 and 2. Accordingly, CFA:PLP139–151 was administered in another group of mice for comparison (Fig. 1B). Onset of disease in SJL GP:PLP139–151 mice was slightly delayed (Table 1) compared with CFA:PLP139–151; however, peak disease scores were comparable (Table 1). The incidence of disease was also comparable in SJL EAE induced with either CFA or GP adjuvant (Table 1). Relapses occurred in both groups, occurring at a frequency of less than 50% for both (data not shown).
Taken together, we showed in two independent EAE models using two different strains of mice that myelin autoantigens packaged in GPs induced EAE similar to CFA induced EAE as evidenced by disease curve, disease incidence, mean onset, and mean peak of disease.
3.2. Subcutaneous immunization with peptide-loaded GPs results in less inflammation at the injection sites as compared with CFA
Previous work showed that immunization with antigens in GP resulted in transient minimal swelling and erythema at the injection sites [22]. Therefore, we investigated the effects of immunization with autoantigens in GP on swelling, histopathology, and inflammation at the injection site as compared with CFA.
To better characterize the differences in local inflammatory reaction at the injection site, we injected mice s.c. with either CFA:MOG35–55 or GP:MOG35–55 adjuvant and compared the percent increase in paw width before and after injection. Shown in Fig. 2A and 2B, paw thickness increased in CFA- and GP-injected animals within 24 hours after injection. Paw thickness peaked in GP mice by 48 hours and then significantly decreased over the following 48 hours, whereas swelling continued to increase in CFA mice until it reached a peak by day 11 (Fig. 2B). By day 30, the increase in paw width of mice injected with GP adjuvant was below 50% and paws were approaching their original width. In strong contrast, the paws of CFA-injected mice swelled to greater than 150% of their original size and remained that way through day 35 (Fig. 2B). Daily measurements were discontinued at day 35 when the statistically significant difference between the two groups was clearly established (p < 0.001 for 15 days), but paws were monitored up to day 50 to ensure consistency in foot inflammation even at a more extended timepoint. At day 50 post injection the paws of mice injected with GP:MOG35–55 had returned to their normal appearance (Fig 2A, bottom right) whereas the paws of mice injected with CFA:MOG35–55 remained swollen (Figure 2A, bottom left).
Figure 2. Subcutaneous immunization with peptide-loaded GPs results in less inflammation at the injection sites as compared with CFA.
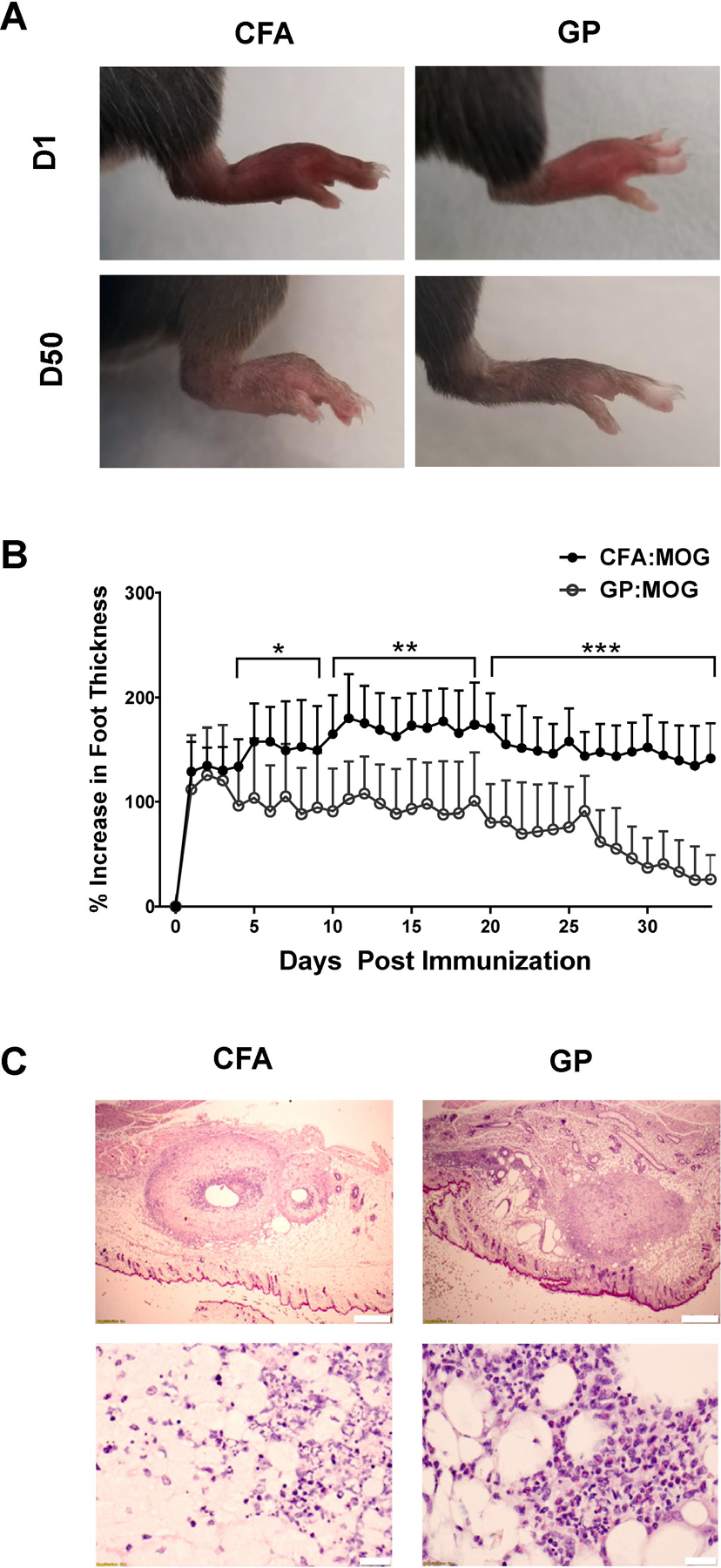
(A and B) C57BL/6 mice were immunized s.c. in the dorsum of the paw with either 50 μl of CFA:MOG35–55 or 100 μl of GP:MOG35–55 and paw width at the widest point was measured daily with a digital caliper to determine percent increase in thickness. (A) Images indicate representative swelling one day post injection (top) and 50 days post injection (bottom) for CFA-immunized mice (left) and GP-immunized mice (right). (B) Percent increase in foot thickness, n=10 per group, bars represent SD (* p < 0.05, **p < 0.01, and ***p < 0.001, unpaired t-test performed for each day). (c) C57BL/6 mice were injected s.c. on the flank with 50 μl CFA:MOG35–55 on day 0 or 100 μl GP:MOG35–55 in the shoulder on day 0 and on the flank day 4. The CFA injection site was removed at day 18 post injection and H&E stained (left). The day 4 boost GP flank injection site was removed at day 18 post initial immunization and H&E stained (right). Top scale bars represent 500 μm and bottom represent 20 μm.
Next, we investigated the effect of GP versus CFA injection on resulting histopathology and inflammatory reaction at the injection site of mice induced for EAE with MOG35–55 peptide. Because GP mice received booster injections on day 4, which is generally not permissible for CFA injections, we compared tissue from the primary CFA injection site with tissue from the site of secondary GP injection to better contrast the histopathological effects of the two immunization protocols. Skin and underlying subcutaneous tissue from mice immunized for EAE was removed from flank injection sites 18 days post immunization for CFA:MOG35–55 and for GP:MOG35–55 tissue from the secondary injection site was removed on day 14 after secondary injection (i.e., day 18 after primary injection).
These tissues were stained by H&E for histopathological evaluation and analyzed by a pathologist blinded to the groups. Shown in Fig. 2C, injection of CFA and GP both resulted in inflammatory infiltrates present in the subcutaneous tissue at the injection site (top). However, the CFA-injected tissue showed notably increased acute inflammation, characterized by pronounced necrosis, surface ulceration and epidermal thickening as compared with GP injection sites (bottom).
Taken together, the results show that injection of GP resulted in decreased inflammatory pathology at the injection site and significantly decreased swelling that was reduced close to normal by 30 days after immunization.
3.3. CNS histopathology and inflammatory infiltrates are comparable between EAE induced with CFA:MOG35–55 or GP:MOG35–55
We showed that GPs induce similar clinical EAE disease while causing significantly less acute and chronic inflammation at the injection site. To corroborate that GPs induced similar histopathological changes in the CNS during EAE as compared to CFA, brains and spinal cords of C57BL/6 mice were removed following the acute phase of disease and stained by H&E. Shown in Fig. 3, H&E staining revealed a similar extent of inflammatory infiltrates in the brain and spinal cord between CFA:MOG35–55- and GP:MOG35–55-injected groups (Figs. 3A and 3B top).
Figure 3. CNS histopathology is comparable between EAE disease induced with CFA:MOG and GP:MOG.
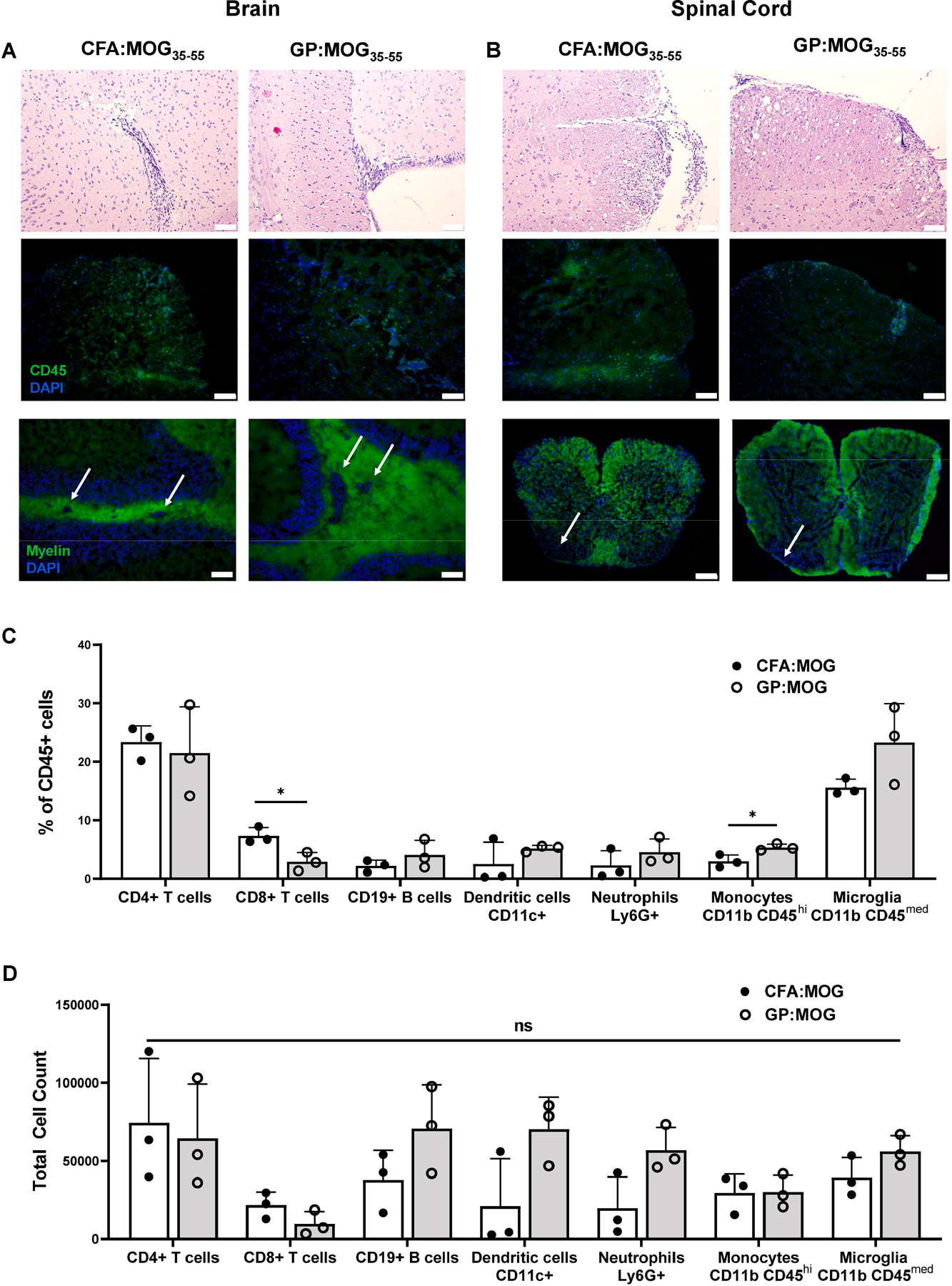
Inflammatory infiltrates, lesions, and demyelination in the CNS were characterized by H&E staining (top), IF staining for CD45 (middle), and myelin staining (bottom) in both the (A) brain and (B) spinal cord tissues collected day 20 post immunization. Top, middle, and bottom left scale bars represent 100 μm and bottom right scale bars represent 200 μm. (C) Composition of singlet CD45+ cells as measured by flow cytometry of brain tissues day 30 post immunization are compared between the CFA:MOG35–55- and GP:MOG35–55-immunized mice. (D) Total cell numbers of immune population in the brain day 30 post immunization compared between CFA:MOG35–55- and GP:MOG35–55-immunized mice. Bars represent mean ± SD and data are from one study representative of three independent flow cytometry experiments, n=3 per group for each experiment. (unpaired t-test performed for each cell type, * p <0.05)
To further characterize the extent and nature of immune infiltration and resulting demyelination in the CNS, we performed CD45 staining for analysis by immunofluorescence microscopy (IF). Analysis of brain and spinal cord sections by IF revealed CD45+ inflammatory infiltrates of similar extent in GP:MOG35–55 as compared to CFA:MOG35–55-injected animals (Figs. 3A and 3B, middle). Brain inflammatory infiltrates were located predominantly periventricularly and in the cerebellum (periventricular lesions shown in Fig. 3A, middle). Shown in Fig. 3b, middle, are similar inflammatory CD45+ infiltrates in the spinal cords.
To compare the extent of the demyelination between disease induced by the two adjuvants, brain and spinal cord tissue sections were stained for myelin. Of note, CNS tissues of mice immunized with GP:MOG35–55 showed regions of demyelination around lesions in both the brain and spinal cord that was comparable to demyelination observed after induction of EAE with CFA:MOG35–55 (Figs. 3A and 3B, bottom).
Lastly, flow cytometry analysis of whole brains for inflammatory cell types associated with lesions showed that the composition of inflammatory infiltrates and microglia was comparable between CFA:MOG35–55- and GP:MOG35–55-injected groups of mice, with the exception of infiltrating CD8+ T cells that were slightly decreased in GP:MOG35–55-immunized mice and monocytes which were slightly increased (Fig. 3C). Additionally, total cell counts of immune populations in the brain were all comparable (Fig. 3D). Taken together, CNS pathology and inflammatory infiltrates of mice induced for EAE with GP:MOG35–55 was comparable to mice immunized with CFA:MOG35–55.
3.4. Autoantigen-specific immune responses are comparable between mice immunized with CFA or GP adjuvants
Administering microbial antigens packaged in GPs induces robust antigen-specific Th1 and Th17 immune responses [23]. However, the effect of GPs on induction of immunity against autoantigens remains unresolved. To address this question, Th1 and Th17 cytokine responses were analyzed on day 24 after immunization by cytokine ELISPOT assay. Splenocytes were procured from C57BL/6 or SJL mice induced for EAE with MOG35–55 or PLP139–151 peptide, respectively. The splenocytes were restimulated in culture with MOG35–55 or PLP139–151. Shown in Fig. 4, C57BL/6 and SJL mice immunized with MOG35–55 or PLP139–151 peptides, respectively, in either CFA or GP showed similar frequencies of IFN-γ secretion (Fig. 4A and 4B). Induction of IL-17 responses was also similar for C75BL/6 mice induced with CFA:MOG35–55 or GP:MOG35–55 peptide (Fig. 4C). However, the frequencies of IL-17 producing T cells was somewhat lower in SJL mice, but the difference did not achieve statistical significance (p=0.054). In contrast to the strong induction of Th1 and Th17 responses by myelin peptides in GP or CFA, induction of Th2 responses using IL-5 as a surrogate was minimal in C57BL/6 or SJL mice immunized with MOG35–55 or PLP139–151 peptide in CFA or GP (Supplementary Fig. S2) and no significant difference was noted in C57BL/6 mice, and only a minor increase in IL-5 responses was observed in SJL mice immunized with GP.
Figure 4. Antigen-specific Th1 and Th17 immune responses are comparable in mice immunized with CFA and GP adjuvants.
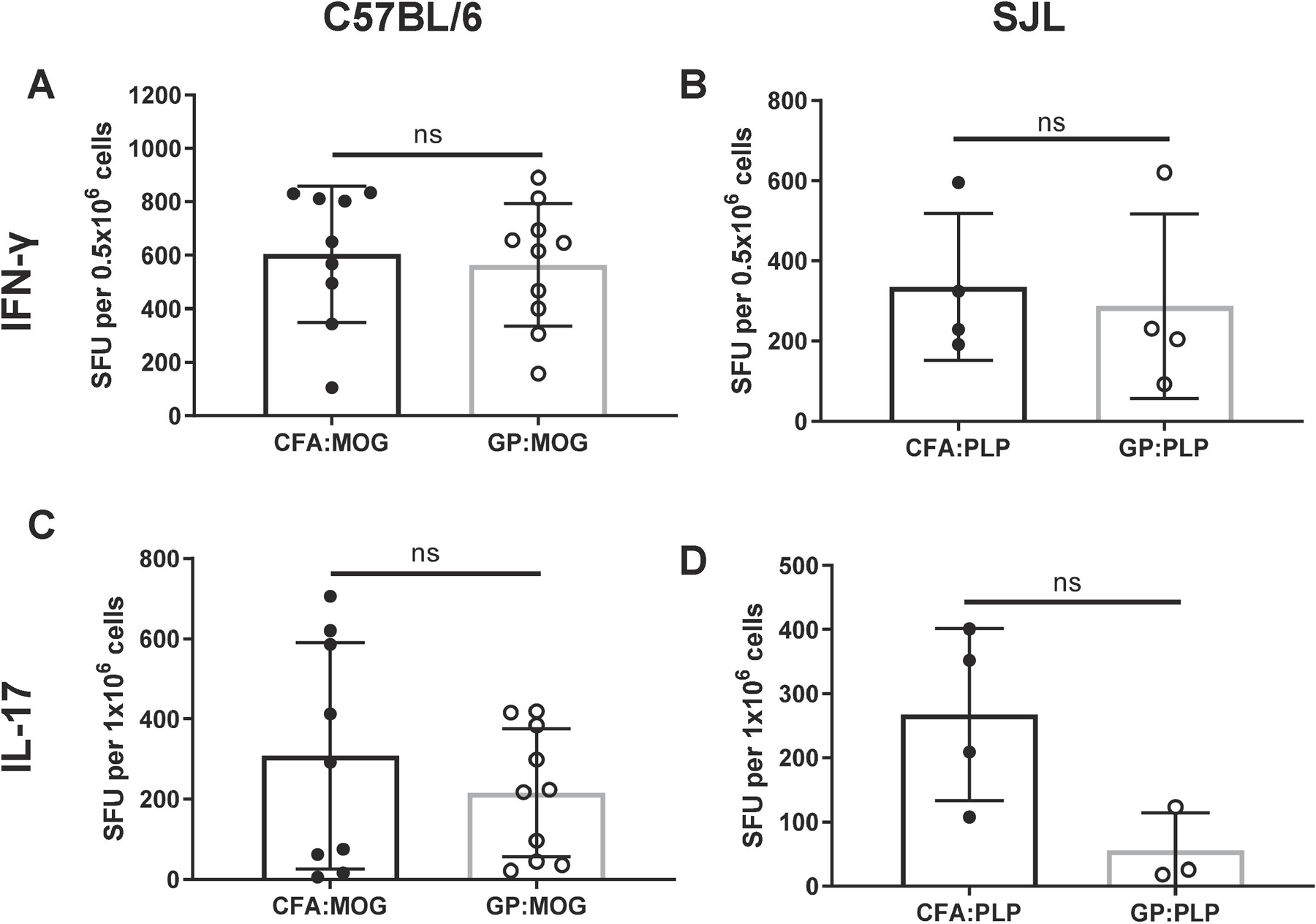
Immunization of myelin autoantigens with either CFA or GP adjuvants elicited similar antigen-specific IFN-γ and IL-17 responses on day 24 post immunization as measured by spleen ELISPOT for both (A and C) C57BL/6 immunized with MOG35–55 or (B and D) SJL mice immunized with PLP139–151. Experiments shown are two representative studies of four independent C57BL/6 experiments and one representative of three independent SJL experiments with similar results, n=3–5 mice per group for each experiment analyzed by unpaired t-test.
To gain broader insight into the cytokine profiles induced by GP:MOG35–55 immunization as compared with CFA:MOG35–55 we performed a 23-plex assay of cytokines and chemokines of the supernatants from MOG35–55-restimulated splenocyte cultures procured from immunized mice just after the acute phase of disease, day 24 post immunization. Shown in Fig. 5, the profile and level of pro-inflammatory cytokines, anti-inflammatory cytokines and chemokines secreted by cultured splenocytes was comparable between mice immunized with GP:MOG35–55 and CFA:MOG35–55 and revealed no significant differences in the observed cytokine profiles (Fig. 5A–5C).
Figure 5. Splenocytes from GP:MOG35–55- and CFA:MOG35–55 -immunized mice show similar cytokine profiles and levels of immune mediators.
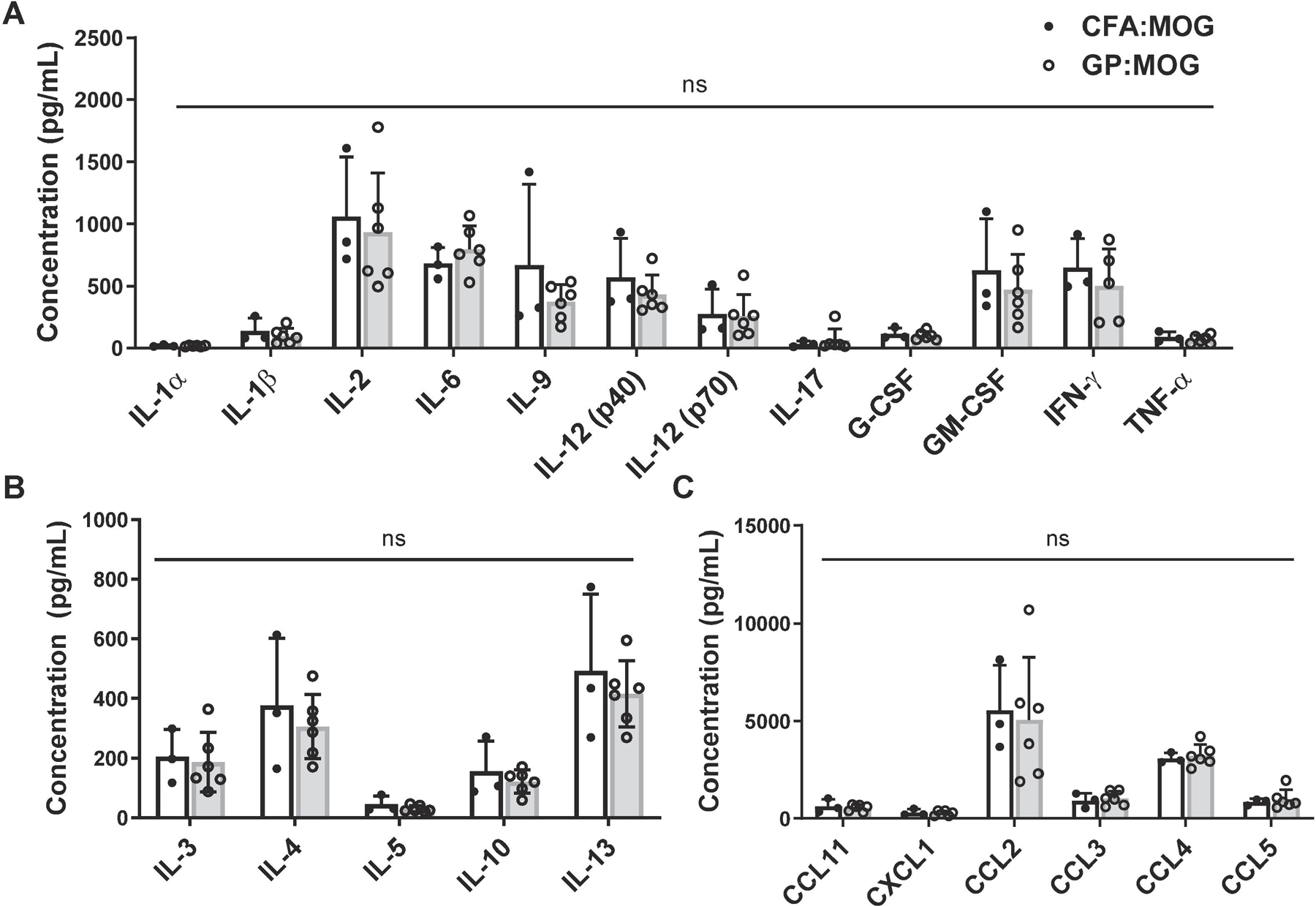
Supernatants of GP:MOG35–55- and CFA:MOG35–55-immunized mouse splenocyte culture were assayed using a mouse cytokine and chemokine 23-plex immunoassay. Shown are the following inflammatory mediators: (A) proinflammatory cytokines, (B) anti-inflammatory cytokines and (C) chemokines. Data show mean expression level ± SD, unpaired t-test performed for each molecule, n=3–6 per group.
Taken together, the results showed that induction of autoreactive Th1 and Th17 cells was comparable between GP and CFA immunized mice and no significant differences in cytokine profiles or magnitudes of the induced autoreactive T cells were detected. The results further support the potential of GPs as useful replacement for CFA for induction of EAE.
3.5. Adoptive transfer of encephalitogenic T cells derived from GP:MOG35–55-immunized mice efficiently induces EAE
Induction of passive EAE by adoptive transfer (AT) of encephalitogenic T cells is a standard protocol for induction of disease. Therefore, we investigated the ability of autoreactive T cells generated with GP:MOG35–55 immunization to adoptively transfer EAE.
C57BL/6 mice were immunized with CFA:MOG35–55 or GP:MOG35–55. Draining lymph nodes and spleen were recovered on D10 after immunization and cells cultured for 3 days in vitro with MOG35–55 peptide and Th17-skewing conditions as described in Methods. CFA:MOG35–55- or GP:MOG35–55-induced autoreactive cells were adoptively transferred to naïve recipient mice and the animals observed for up to 30 days. Shown in Fig. 6 is the disease course for both groups in a representative experiment. T cells generated with either immunization protocol induced onset of EAE by day 8 after transfer (Fig. 6A). EAE reached peak disease in both groups by day 13 after transfer. Mice from both groups showed a monophasic disease course and animals from either group started to remit by day 15. No major difference was observed in the incidence, onset, and peak of disease (Table 2). Thus, the results showed that GP-primed autoreactive T cells potently induced EAE after adoptive transfer.
Figure 6. Transfer of encephalitogenic T cells generated by immunization with GP:MOG35–55 induces EAE in recipient mice.
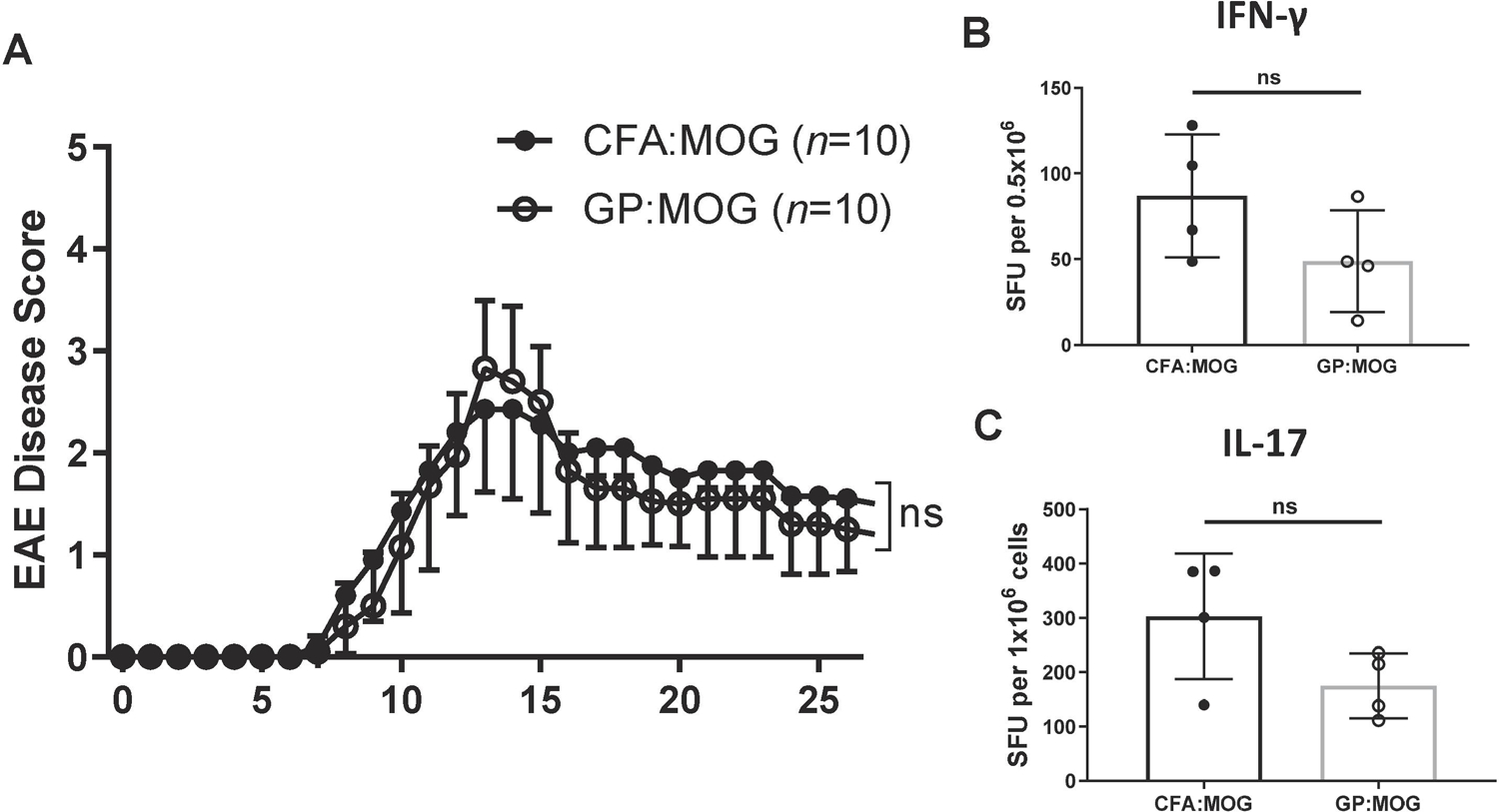
C57BL/6 donor mice were immunized with 200 μg MOG35–55 administered in either CFA or GPs, with GP mice receiving an equivalent boost on Day 7. On day 10 post immunization, splenocytes and draining lymph node cells were cultured in Th17-inducing conditions. 15x106 total cells were transferred to C57BL/6 recipient mice. (A) Shown are the mean clinical disease scores ± SD representative of three independent adoptive transfer studies, n=5–10 per group for each study. (unpaired t-tests were performed for each day) (B and C) Spleen ELISPOTS were performed 26 days post adoptive transfer and (B) IFN-γ and (C) IL-17 antigen-specific immune responses were compared (unpaired t-test). ELISPOT data are representative of two independent ELISPOT experiments.
Table 2.
Disease phenotype of C57BL/6 adoptive transfer EAE.
| C57BL/6 |
|||
|---|---|---|---|
| CFA:MOG35–55 | GP:MOG35–55 | t-testp value | |
| Incidence | 18/18 (100%) | 14/15 (93%) | |
| Survival | 100% | 100% | |
| OnsetDay ± SD | 9.6 ± 2.1 | 9.9 ± 2.6 | 0.0267 |
| PeakEAE Score ± SD | 2.5 ± 0.8 | 2.8 ± 0.7 | 0.4972 |
To determine the cytokine profile of the adoptively transferred T cells, we recovered splenic T cells on day 26 after AT and performed cytokine ELISPOT for the cytokines IFN-γ and IL-17. The results showed that T cells from both groups mounted comparable T cell responses as evidenced by the antigen-specific secretion of IFN-γ and IL-17 (Fig. 6B and 6C). The results show that autoreactive T cells generated using GPs as an adjuvant induced passive EAE with similar disease characteristics and cytokine profiles as EAE induced by CFA primed cells. Thus, the data support that GP can be a suitable alternative to CFA for the generation of autoreactive cells for adoptive transfer EAE as well as for the induction of active autoimmune disease.
4. Discussion
Despite strong reservations against the use of CFA in animals, it continues to be an essential component of most protocols for the induction of EAE as well as other autoimmune animal models. We have herein demonstrated that GP adjuvant loaded with myelin autoantigens induce EAE comparable to CFA emulsified with the same antigen in all important measures of disease including disease phenotype, CNS pathology, and induction of immune responses. Importantly, glucan particles induced much less inflammation and tissue necrosis and ulceration at the injection sites as CFA. Thus, we posit that glucan particles are a viable option for replacement of CFA for the induction of disease in animal models of EAE.
CFA is discouraged by animal use committees because of the inflammation and pain elicited. This is evidenced by the fact that CFA injection is a widely used pain and inflammation model [24–26]. Human case-studies of accidental needle sticks with CFA highlight the pain and inflammation caused by the injection of CFA, with locations of needle stick injury often requiring surgical debridement [27, 28]. We showed that GP adjuvants did not induce the same amount of inflammation as CFA therefore facilitating the humane treatment of experimental animals and protection of researchers.
The immunogenicity of CFA can be attributed largely to the PAMPs provided by Mtb, primarily, but not limited to the dominant glycolipids trehalose-6,6-dimycolate, lipoarabinomannans and lipomannan as well as their modified forms [29–31]. Myeloid cells recognize Mtb PAMPs through Toll-like receptors (TLRs), C-type lectin receptors and Nod-like receptors [32]. It is well-documented that Mtb infection promotes Th1 and Th17 cell responses in line with CFA’s capacity to induce autoimmunity [33–36].
In contrast to the severe, broad inflammation induced by the milieu of Mtb PAMPs, GPs are composed primarily of β-1–3-D-glucans, which signal preferentially via the C-type lectin receptor Dectin-1 and CR3 and therefore trigger a more restricted pattern of pattern recognition receptors (PRR). Recent work showed that PRRs triggered by GPs are well-suited to elicit Th17 immunity [14, 37]. GPs are recognized by Dectin-1 receptors and CR3 on professional APCs, leading to phagocytosis and induction of TNF-α, IL-6 and reactive oxygen species [15, 37, 38]. Here, we showed that autoantigens presented in the context of GPs activated and primed autoreactive T cells and promoted the development of autoimmunity with minimal inflammation at the injection sites.
Adjuvants act through several mechanisms including: 1) enhancement of antigen uptake/presentation and targeting to APCs in the draining lymph nodes, 2) immune modulation through cytokines and chemokine secretion and 3) depot formation [39–41]. As mentioned above, GPs effectively facilitate antigen presentation through activation of Dectin-1 on APCs and ultimately lead to the generation of Th17 immune responses.
One limitation of the use of GPs administered as an aqueous suspension is that they may not provide an antigen depot similar to CFA and therefore may require a booster injection. It is possible that administration of GP vaccines in an oil or emulsion will overcome this limitation. In contrast, however, the transient immune stimulation provided by GPs compared to the sustained antigen dosage provided by CFA may more closely parallel the etiology of the human condition, such as viral infections known to precede the onset of MS [42]. Moreover, while the protocol for generation of CFA adjuvants involves a simple emulsification step with a 1:1 solution of soluble antigens, GPs requires loading the antigen of choice into the particles.
Although this protocol may need adjustments for the biophysical properties of the antigens of choice, once the conditions for loading antigens such as proteins or peptides into GPs is established, production of immunogenic particles can be easily accomplished, and resulting preparations can be stored as a ready-to-use frozen particle suspension or as a lyophilized powder. It is important to note that we confirmed that the GP boost does not result in significant inflammation in the way of a secondary CFA injection. The ability to deliver booster injections with GPs is noteworthy since secondary injections of CFA are contraindicated and in most cases requires the use of incomplete Freund’s adjuvant lacking Mtb [24].
Additionally, the composition of the GPs can be modified to enhance delivery to DCs promoting enhanced autoimmunity and potentially reducing the amount of peptide and GPs needed. Indeed, previous studies using GPs modified with the addition of chitin and chitosan resulted in the enhancement of anti-viral and anti-fungal T cell responses [14, 43]. Along these lines, it is feasible to load GPs with autoantigen and include a tolerogenic payload, such as immunoregulatory cytokines, for the induction of immune tolerance. This is an area worthy of further exploration.
Our studies showed the effectiveness of inducing EAE through stimulation of Th17 mechanisms and thereby point to their utility for other Th17-dependent autoimmune disease models such as experimental autoimmune myocarditis, collagen-induced arthritis, experimental autoimmune uveitis, and other autoimmune models [44–46]. GPs can be loaded with specific autoantigens required to induce autoreactive T cells against a wide range of tissues, and therefore we posit that this approach should be feasible for disease induction in other autoimmune disease models.
In summary, previously the use of CFA for induction of experimental autoimmune disease in animal models was indispensable. However, our studies may provide an alternative approach for induction of experimental autoimmune diseases through the use of GPs loaded with autoantigens.
Supplementary Material
Highlights.
β-1,3-D-glucan particles (GPs) induce potent Th17 immune responses
GPs loaded with myelin autoantigens induce EAE and CNS pathology
Subcutaneous injection with GPs results in minimal inflammation at the injection site compared with CFA
GPs may have utility for other autoimmune disease models
Acknowledgments:
We thank Dr. Itay Raphael (University of Pittsburgh) for reviewing this manuscript and for critical suggestions. This work was supported by grants NS084201 and AI144731 from the National Institutes of Health and grants RG5501 and RG1602 from the National Multiple Sclerosis Society (T.G.F.). C.C.H. was supported by RISE R25GM060655.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All of the authors are in full agreement with the contents of the manuscript. The contents of manuscript have not been published previously and are not under consideration for publication elsewhere. None of the authors have any financial conflicts or financial interests to report regarding the publication of the contents of the manuscript.
References
- [1].Bruck W, The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage, J Neurol, 252 Suppl 5 (2005) v3–9. [DOI] [PubMed] [Google Scholar]
- [2].Olsson T, Zhi WW, Hojeberg B, Kostulas V, Jiang YP, Anderson G, Ekre HP, Link H, Autoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon-gamma, J Clin Invest, 86 (1990) 981–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Robinson AP, Harp CT, Noronha A, Miller SD, The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment, Handb Clin Neurol, 122 (2014) 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miller SD, Karpus WJ, Experimental autoimmune encephalomyelitis in the mouse, Curr Protoc Immunol, Chapter 15 (2007) Unit 15 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Broderson JR, A retrospective review of lesions associated with the use of Freund’s adjuvant, Lab Anim Sci, 39 (1989) 400–405. [PubMed] [Google Scholar]
- [6].Billiau A, Matthys P, Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases, J Leukoc Biol, 70 (2001) 849–860. [PubMed] [Google Scholar]
- [7].Herbert WJ, The mode of action of mineral-oil emulsion adjuvants on antibody production in mice, Immunology, 14 (1968) 301–318. [PMC free article] [PubMed] [Google Scholar]
- [8].Freund J, Casals J, Hosmer EP, Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil, P Soc Exp Biol Med, 37 (1937) 509–513. [Google Scholar]
- [9].Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SYC, The β-Glucan Receptor, Dectin-1, Is Predominantly Expressed on the Surface of Cells of the Monocyte/Macrophage and Neutrophil Lineages, The Journal of Immunology, 169 (2002) 3876–3882. [DOI] [PubMed] [Google Scholar]
- [10].Hester MM, Lee CK, Abraham A, Khoshkenar P, Ostroff GR, Levitz SM, Specht CA, Protection of mice against experimental cryptococcosis using glucan particle-based vaccines containing novel recombinant antigens, Vaccine, 38 (2020) 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Specht CA, Lee CK, Huang H, Hester MM, Liu J, Luckie BA, Torres Santana MA, Mirza Z, Khoshkenar P, Abraham A, Shen ZT, Lodge JK, Akalin A, Homan J, Ostroff GR, Levitz SM, Vaccination with Recombinant Cryptococcus Proteins in Glucan Particles Protects Mice against Cryptococcosis in a Manner Dependent upon Mouse Strain and Cryptococcal Species, MBio, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deepe GS Jr., Buesing WR, Ostroff GR, Abraham A, Specht CA, Huang H, Levitz SM, Vaccination with an alkaline extract of Histoplasma capsulatum packaged in glucan particles confers protective immunity in mice, Vaccine, 36 (2018) 3359–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Whelan AO, Flick-Smith HC, Homan J, Shen ZT, Carpenter Z, Khoshkenar P, Abraham A, Walker NJ, Levitz SM, Ostroff GR, Oyston PCF, Protection induced by a Francisella tularensis subunit vaccine delivered by glucan particles, PLoS One, 13 (2018) e0200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hung CY, Zhang H, Castro-Lopez N, Ostroff GR, Khoshlenar P, Abraham A, Cole GT, Negron A, Forsthuber T, Peng T, Galgiani JN, Ampel NM, Yu JJ, Glucan-chitin particles enhance Th17 response and improve protective efficacy of a multivalent antigen (rCpa1) against pulmonary Coccidioides posadasii infection, Infect Immun, (2018). [DOI] [PMC free article] [PubMed]
- [15].Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM, Characterization and optimization of the glucan particle-based vaccine platform, Clin Vaccine Immunol, 20 (2013) 1585–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Specht CA, Lee CK, Huang H, Tipper DJ, Shen ZT, Lodge JK, Leszyk J, Ostroff GR, Levitz SM, Protection against Experimental Cryptococcosis following Vaccination with Glucan Particles Containing Cryptococcus Alkaline Extracts, MBio, 6 (2015) e01905–01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Soto ER, Caras AC, Kut LC, Castle MK, Ostroff GR, Glucan particles for macrophage targeted delivery of nanoparticles, J Drug Deliv, 2012 (2012) 143524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mirza Z, Soto ER, Dikengil F, Levitz SM, Ostroff GR, Beta-Glucan Particles as Vaccine Adjuvant Carriers, Methods Mol Biol, 1625 (2017) 143–157. [DOI] [PubMed] [Google Scholar]
- [19].Raphael I, Gomez-Rivera F, Raphael RA, Robinson RR, Nalawade S, Forsthuber TG, TNFR2 limits proinflammatory astrocyte functions during EAE induced by pathogenic DR2b-restricted T cells, JCI Insight, 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ji N, Somanaboeina A, Dixit A, Kawamura K, Hayward NJ, Self C, Olson GL, Forsthuber TG, Small molecule inhibitor of antigen binding and presentation by HLA-DR2b as a therapeutic strategy for the treatment of multiple sclerosis, J Immunol, 191 (2013) 5074–5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ji N, Forsthuber TG, ELISPOT Techniques, Methods Mol Biol, 1304 (2016) 63–71. [DOI] [PubMed] [Google Scholar]
- [22].Hung C-Y, Zhang H, Castro-Lopez N, Ostroff GR, Khoshlenar P, Abraham A, Cole GT, Negron A, Forsthuber T, Peng T, Galgiani JN, Ampel NM, Yu J-J, Glucan-Chitin Particles Enhance Th17 Response and Improve Protective Efficacy of a Multivalent Antigen (rCpa1) against Pulmonary Coccidioides posadasii Infection, Infection and immunity, 86 (2018) e00070–00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM, Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles, MBio, 1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].NIH Animal Research Advisory Committee, Guidelines for the use of adjuvants in research-special emphasis on Freund’s adjuvant, (2016).
- [25].Fehrenbacher JC, Vasko MR, Duarte DB, Models of inflammation: Carrageenan- or complete Freund’s Adjuvant (CFA)-induced edema and hypersensitivity in the rat, Curr Protoc Pharmacol, Chapter 5 (2012) Unit5 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kopach O, Viatchenko-Karpinski V, Belan P, Voitenko N, Development of inflammation-induced hyperalgesia and allodynia is associated with the upregulation of extrasynaptic AMPA receptors in tonically firing lamina II dorsal horn neurons, Front Physiol, 3 (2012) 391–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chapel HM, August PJ, Report of nine cases of accidental injury to man with Freund’s complete adjuvant, Clin Exp Immunol, 24 (1976) 538–541. [PMC free article] [PubMed] [Google Scholar]
- [28].Gaspar MP, Landes G, Safavi F, Osterman AL, Accidental Injection of Freund Complete Adjuvant With Mycobacterium Tuberculosis, The Journal of Hand Surgery, 43 (2018) 873.e871–873.e874. [DOI] [PubMed] [Google Scholar]
- [29].Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S, Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle, Journal of Experimental Medicine, 206 (2009) 2879–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ, The CD14 Ligands Lipoarabinomannan and Lipopolysaccharide Differ in Their Requirement for Toll-Like Receptors, The Journal of Immunology, 163 (1999) 6748. [PubMed] [Google Scholar]
- [31].Quesniaux VJ, Nicolle DM, Torres D, Kremer L, Guérardel Y, Nigou J, Puzo G, Erard F, Ryffel B, Toll-Like Receptor 2 (TLR2)-Dependent-Positive and TLR2-Independent-Negative Regulation of Proinflammatory Cytokines by Mycobacterial Lipomannans, The Journal of Immunology, 172 (2004) 4425. [DOI] [PubMed] [Google Scholar]
- [32].Reiling N, Hölscher C, Fehrenbach A, Kröger S, Kirschning CJ, Goyert S, Ehlers S, Cutting Edge: Toll-Like Receptor (TLR)2- and TLR4-Mediated Pathogen Recognition in Resistance to Airborne Infection with <em>Mycobacterium tuberculosis</em>, The Journal of Immunology, 169 (2002) 3480. [DOI] [PubMed] [Google Scholar]
- [33].Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J, Li BQ, Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis, Cell Mol Immunol, 5 (2008) 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tigno-Aranjuez JT, Jaini R, Tuohy VK, Lehmann PV, Tary-Lehmann M, Encephalitogenicity of complete Freund’s adjuvant relative to CpG is linked to induction of Th17 cells, J Immunol, 183 (2009) 5654–5661. [DOI] [PubMed] [Google Scholar]
- [35].Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR, An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection, The Journal of experimental medicine, 178 (1993) 2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kuchroo VK, Martin CA, Greer JM, Ju ST, Sobel RA, Dorf ME, Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis, The Journal of Immunology, 151 (1993) 4371–4382. [PubMed] [Google Scholar]
- [37].Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, Weiss A, Vasilakos JP, Underhill DM, Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’, Nature, 472 (2011) 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Camilli G, Tabouret G, Quintin J, The Complexity of Fungal β-Glucan in Health and Disease: Effects on the Mononuclear Phagocyte System, Front Immunol, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stils HF Jr., Adjuvants and Antibody Production: Dispelling the Myths Associated with Freund’s Complete and Other Adjuvants, ILAR Journal, 46 (2005) 280–293. [DOI] [PubMed] [Google Scholar]
- [40].Cox JC, Coulter AR, Adjuvants--a classification and review of their modes of action, Vaccine, 15 (1997) 248–256. [DOI] [PubMed] [Google Scholar]
- [41].Awate S, Babiuk LA, Mutwiri G, Mechanisms of action of adjuvants, Front Immunol, 4 (2013) 114–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sospedra M, Martin R, Molecular mimicry in multiple sclerosis, Autoimmunity, 39 (2006) 3–8. [DOI] [PubMed] [Google Scholar]
- [43].Soares E, Groothuismink ZMA, Boonstra A, Borges O, Glucan Particles Are a Powerful Adjuvant for the HBsAg, Favoring Antiviral Immunity, Molecular Pharmaceutics, 16 (2019) 1971–1981. [DOI] [PubMed] [Google Scholar]
- [44].Inglis JJ, Criado G, Medghalchi M, Andrews M, Sandison A, Feldmann M, Williams RO, Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen, Arthritis Res Ther, 9 (2007) R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gutowski MB, Wilson L, Van Gelder RN, Pepple KL, In Vivo Bioluminescence Imaging for Longitudinal Monitoring of Inflammation in Animal Models of Uveitis, Investigative Ophthalmology & Visual Science, 58 (2017) 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW, Cardiac myosin induces myocarditis in genetically predisposed mice, The Journal of Immunology, 139 (1987) 3630–3636. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


