Abstract
How do brains shape social networks, and how do social ties shape the brain? Social networks are complex webs by which ideas spread among people. Brains consist of webs by which information is processed and transmitted among neural units. While brain activity and structure offer biological mechanisms for human behaviors, social networks offer external inducers or modulators of those behaviors. Together, these two axes represent fundamental contributors to human experience. Integrating foundational knowledge from social and developmental psychology and sociology on how individuals function within dyads, groups, and societies with recent advances in network neuroscience can offer new insights in both domains. We use the example of how ideas and behaviors spread to illustrate the potential of multi-layer network models.
Keywords: networks, neuroscience, social networks, neuroimaging, multiscale, communication
The structure of our social world is incredibly complex and involves multiple interacting units [1]. Questions that hinge on understanding when, how, and why these units interact require theories and methods that address this heterogeneous pattern of interpersonal connectivity. Network science offers theories and methods that can capture the richness of interconnection patterns [2], pinpoint local network nodes that influence global function [3], and offer tools to intervene in a way that drives social network change [4,5]. Integrating these theories and methods with empirical studies offers a powerful means to uncover a set of principles that describe behavior in terms of network structure and function across domains. For example, there are intriguing similarities between the patterns of interconnectivity among individuals in a close-knit family [6], members in a team [7], activists in political uprisings [8], companies in the corporate world [9], and countries engaging in efforts supporting global diplomacy [10]. Indeed, the term “social network” is perhaps a misnomer – a general term for what is in fact a set of interacting networks at multiple levels of analysis that form the fabric of our social and cultural lives.
Although units in social networks can be countries, companies, or compatriots, typically the smallest unit studied in social network research is that of a single person: the atom of the social network universe. Yet, as the atom is composed of first protons and neutrons, then quarks – a person is in fact composed of smaller units that interact in networks of their own [11]. This is the fodder of the emerging field of network neuroscience [12], which pursues new ways to map, record, analyze, and model the elements and interactions of neurobiological systems. In humans, this enterprise seeks to understand the pattern of connections in an individual’s brain that code for their personality [13,14], behavior [15,16], and risk for disease [17–19], as well as their potential to adapt to their surroundings [20], engage in meaningful relationships with others [21], and participate in a larger team [22]. Such predictive patterns of connections exist across diverse spatiotemporal scales [23] and can be argued to form a separate intra-human layer in the multiscale social network hierarchy. These recent advances in network neuroscience complement substantial advances in social and cognitive neuroscience that have mapped patterns of activity and the structure of the human brain within and across brain regions.
Here, we review recent studies that have brought together questions of brain structure and function with insights from social network analysis. It is beyond the scope of this piece to provide a substantial review of the foundational literatures in social and developmental psychology and sociology, on how individuals function within dyads, groups, and societies, and within neuroscience on how brain structure and function relate to the psychology of the individual. Instead, we argue that understanding brains and social networks in the context of one another is not only important but indeed critically necessary, because the two are interacting systems: brain dynamics shape learning and behavior [24], including social interaction [25]; likewise, social contexts alter brain structure and function [26,27]. Integration of theories and methods linking brain activity and structure to activity and structure within social networks holds great promise to improve our knowledge of the single human by improving our ability to predict behavior, derive core psychological and neurocognitive principles, and distinguish brain health and disease [28]. In addition, integration of social network and brain dynamics holds great promise to improve our knowledge of the collective by improving our ability to predict group behavior [29,30], gain deeper insight into underlying mechanisms, and potentially intervene more efficiently. For example, questions of communication between individuals and across groups, as well as broader classes of learning and decision making, can be conceptualized both in terms of the neural and psychological mechanisms supporting the decisions and behaviors of each actor (e.g., a decision to share a piece of information, an unconscious facial response to learning a piece of information), as well as how individuals mutually influence one another. In these ways, linking brain and social networks opens new avenues for discovering principles that fundamentally underlie individual decision making, person to person interactions, and the broader organization of society.
Although several pieces of this puzzle remain to be discovered, we focus on one example – the neurobiology of how ideas and behaviors spread within social networks – to illustrate ways in which brain network dynamics and social network dynamics might be studied in parallel. Specifically, ideas and behaviors are transmitted and adopted over a period of time, either through verbal or non-verbal communication. This process involves biological coupling (e.g., of language patterns [31–33], non-verbal signals [34–36], and brain activity between communicators and receivers [37–39]). Because the behavioral and cognitive processes supporting idea and behavior spread can change over time, tools to reveal their neurophysiological underpinnings must be tuned to characterize and quantify temporally expansive phenomena. Complementing time-varying univariate approaches including parametric modulation analysis [40], here we focus on dynamic network approaches as a natural set of tools that meet these requirements, and that can specifically be used to understand interactions between brain systems implicated in idea and behavior spread, including the brain’s value system and the default mode network [41]. By characterizing patterns of functional connectivity and their changes over fine scale temporal windows, these approaches can be used to map communication patterns between functionally specialized brain areas that either directly relate to interpersonal communication patterns occurring during the experiment, or that predict features of interpersonal communication occurring outside of the experiment over longer time windows. More generally, markers of brain connectivity (short time scale) can be linked in a correlative or causative manner to communication patterns between people in social networks (longer time scale), thereby offering an explicit mathematical framework for integration across this multiscale network.
To unpack how multiscale network perspectives can inform both neural and social scientific questions related to the spread of ideas and behaviors, we begin with a discussion of selected brain networks that are broadly relevant to the spread of ideas and behaviors, and we review how a network perspective has aided our understanding of these processes. We then move to a discussion of recent studies that have examined brain dynamics in dyads and groups, which comprise intermediate building blocks of social networks, and we consider the ways in which broader social network structure might influence these processes. In doing so, we review work that has conceptualized social network structure as a type of individual difference that might affect and be affected by a broader array of psychological functions and individual behaviors [42,43]. The studies we review and the concepts we grapple with are illustrative of the broader potential for integrating brain network and social network perspectives, and motivate a set of open questions at the intersection of these domains. Tackling these questions calls for new methodological approaches and conceptual theories addressing the multiscale nature of social networks, with the smallest scales being composed of individual humans whose brains are teaming with interconnected units performing diverse computations and communicating complex information, and scaling up to explain collective phenomena.
How do information and behaviors spread from person to person in networks?
As a central example to illustrate the idea that brain and social networks mutually influence one another, and that understanding such interactions has value, we will consider the fact that ideas [44,45], emotions [46–49], and behaviors [46–48,50,51] can spread from person to person in both online and offline social networks [52]. What are biological mechanisms for these phenomena? Social scientists have asserted that belonging and coordination are critical for human survival [53,54]; being part of a group confers many advantages including the potential to guard or defend from predators and the elements, and to learn optimal behaviors from others, thereby ensuring maintenance and availability of resources and safety. In parallel, neuroscientists have characterized a wide range of brain systems relevant to communication and decision making. However, the question of how ideas and behaviors spread between brains, through social networks, has only begun to be addressed [55]. In the following sections, we review studies in individuals, as well as research branching into dyads and networks, that speak to the cognitive mechanisms of, neurophysiological manifestations accompanying, and network influences on idea and behavior spread. Collectively, this growing body of literature provides an example for thinking about a broader framework for traversing levels of analysis from brains to social networks. Bringing these ideas together, we then argue that there are important open questions about how ideas and behaviors spread that could be solved by modeling brains and social networks within a multiscale network framework.
Brain networks within individuals
Cognitive processes supporting the spread of ideas and behaviors
Although many cognitive processes may support the spread of ideas and behaviors from person to person, reward-driven learning in ventral striatum (VS) and ventromedial prefrontal cortex (vMPFC) is central to the successful spread of ideas and behaviors, such that people are more likely to share ideas when they believe the outcome of sharing will be positive [55]. In this context, communicators’ intentions to share information [56,57], and their success in doing so [57–59], are associated with activity within the communicator’s value system, which can weigh the potential value of sharing one piece of information over another, or over not sharing it at all. In this case, sharers may find value in sharing content that they themselves find valuable, but self-disclosure and social bonding may also be inherently valuable ends that can motivate sharing ([56,60–62], for reviews and information on inputs to the value calculation, see [44,55]). In parallel, neural activation in the analogous brain systems of receivers who are exposed to information about others’ beliefs, preferences, and actions also contributes to the likelihood that the receivers updates their own beliefs, preferences, and actions to align with those of the communicators [55,63–67].
Brain network dynamics supporting the spread of ideas and behaviors
Brain areas implicated in the communication or reception of ideas and behaviors, however, do not operate in isolation. Instead, recent advances in network neuroscience [12] suggest that these brain areas form hotspots within a wider, more dynamic web. In this view, individual brain regions interact with each other (e.g., via synchronization, correlation, or other measures of functional connectivity) on the backbone of crisscrossing white matter tracts comprised of large bundles of myelinated neuronal axons [68]. Recent advances in neuroscience and computational science have opened unprecedented opportunities to consider the dynamics within and between brain networks as they evolve over time in accordance with people’s changing mental states and behaviors [69,70]. Importantly, reconfiguration of regions working together provides a complementary view of brain activity to the more modular view that a given region tends to achieve a specific function in a relatively fixed manner. The dynamic network perspective can thus augment prediction of different behavioral outcomes in humans [24] and provide tools that are particularly appropriate for the study of idea and behavior spread.
Differences in the architectures and functional dynamics of key brain regions and networks across people can alter the tendency for a person to either spread or receive ideas or behaviors via a host of mechanisms. In other words, some people might tend to exhibit brain dynamics that promote a greater tendency to share ideas, or a greater receptivity to assimilating ideas. For example, preliminary evidence demonstrates that individual differences in connectivity within the value system (i.e., between VMPFC and VS) of sedentary adults [71] and smokers [72] predict their receptivity to persuasive appeals aimed at changing those behaviors, and explain variance in real-world health outcomes in physical activity and smoking cessation above and beyond univariate activity in either region alone. More broadly, different brain network dynamics have also been associated with variation in factors that might indirectly alter the tendency for a person to either spread or receive ideas or behaviors such as social anxiety [73], emotional state [74], and perceived social support [75].
Recent efforts also demonstrate that the manner in which brain networks reconfigure over time can predict our future decisions [76], and can track the processing of linguistic stimuli from words to sentences to stories [77–79], suggesting a role in the processing and uptake of information that is naturally provided in social contexts. Importantly, these patterns of brain network reconfiguration differ across different people and, thus, can be used to predict individual differences in learning [80,81], working memory [82], and cognitive flexibility [82,83], all cognitive processes that are fundamental to idea and behavior spread. Finally, the intrinsic dynamics of these brain networks encoding patterns of time-varying functional connectivity can change within a single person over short time scales as a function of mood, fatigue, arousal, and attention [74,84], highlighting the value of using tools capable of capturing network configuration over time and at different temporal scales (e.g., within and between experimental sessions).
As a whole, network neuroscience thus offers a perspective on human cognition [70,85] and provides insights into specific processes supporting the spread of ideas and behaviors. More broadly, the emerging consideration of network organization in the brain has the potential to improve our understanding of other cognitive processes that cannot be explained by the workings of individual regions [86]. The approach capitalizes on tools from network science, graph theory, and systems engineering [87], which together have offered fundamental insights into the architecture of multiple brain systems relevant to the spread of ideas and behaviors such as the default mode system [88], the valuation system [89], and the mentalizing system [14].
From individuals to dyads and multiscale networks
Work on the brain network dynamics characteristic of single individuals has recently taken a more ‘social turn’, and begun to uncover the neural basis of how pairs of individuals or groups of individuals interact [90]. For example, although measures of functional connectivity have traditionally focused on intra-brain connectivity within brain regions in single subjects, they can just as straightforwardly be computed between two brain regions in different subjects. These sorts of calculations are referred to as inter-subject functional connectivity [77]. The resulting brain-to-brain networks provide an analogue to social networks and other social scientific approaches to studying dyads and groups, but fractionate the ‘atomic’ definition of the person into brain regions. These within-brain networks can then be integrated in a comprehensive framework of dyads, groups, or even larger communities or cultures. Taking this step is more than a mere thought exercise, but instead can offer new insight about the underpinnings of thoughts and emotions and how they spread in our inherently social world.
In the case of ideas spreading through social networks, the strength of coupling between brain activity in communicators and receivers [38,39,91], as well as between receivers exposed to shared content [92], is associated with the success of the communication process, i.e., the successful transmission of signals into the brain networks of the receiver [37,93]. For example, greater synchrony in several parts of the value system and default mode network more broadly (medial prefrontal cortex, MPFC; striatum; posterior cingulate; and temporal parietal junction, TPJ) has been associated with more successful communication between a communicator telling a story and a listener who was later asked to recall details of that story [38]. Furthermore, activity within a subset of the synchronized brain regions (MPFC; striatum; and dorsolateral prefrontal cortex, dLPFC) in listeners actually precede the corresponding activity in the speaker’s brain, a process termed ‘anticipatory coupling’, emphasizing the bidirectional nature of communication. Moving from communicating pairs to even larger networks, analyses of inter-subject brain connectivity across networks of individuals demonstrate that brain-to-brain networks become increasingly efficiently organized as the level of interaction between subjects increases [94]. Complementary work demonstrates that people who are closer to one another in their social networks show more similar brain responses to stimuli such as movie clips, even controlling for demographic similarity [95]. These data suggest that social network variables may influence how people process information, or that people who process information more similarly may more easily communicate and become friends.
Biological synchrony is thought to allow communicating pairs of individuals to co-regulate their actions according to shared goals [96]. The growing body of research directly examining the brains of people communicating [90] lays the foundation on which to consider how ideas and behaviors flow in social networks by pointing towards dyads and chains of person-to-person communication as the key building blocks of social networks. Yet, both brain and social systems are known to operate as interconnected networks with emergent collective behaviors [97,98]. Thus, work examining neural processes in dyads, chains and more complex configurations such as triangles, might help illuminate how and why ideas spread in small groups and larger populations [99]. What are the network factors – both in brains and social systems – that influence how ideas are propagated and how behaviors are transmitted across groups? Likewise, similar questions can be asked regarding how social network structure might influence a much wider array of social, cognitive and emotional processes in individuals and groups.
Drawing on methods from social sciences that study dyads and groups, neuroscientists have begun to consider how brain activity across individuals synchronizes and what effects this might have on interpersonal communication and decision-making more broadly. Although preliminary evidence illustrates that brain activity in one actor can be correlated with activity in other actors, many questions remain unanswered, including those about the brain networks involved, their interrelationships across actors, and the influence of broader social network structures on brain activity within individuals and pairs. Understanding when, why, and how ideas and behaviors spread can benefit from linking these levels of analysis.
Multiscale networks spanning brain and social networks
To address questions of mechanism across levels of analysis, it is important to note that because network architectures are present and influential across levels of the social hierarchy – from individuals, to pairs, to groups – techniques from network science offer ways in which to link mechanisms of cross-scale phenomena [23]. Beyond the fact that network analysis is applicable to both brain and social networks independently, we envision that multiscale network analysis could provide some unique insights. Specifically, mathematical modeling of such a multiscale network could offer a new and parsimonious way of integrating findings from individual brains with findings relevant to social groups (e.g., about how ideas and behaviors spread). This notion builds on (i) recent advances in the mathematics of multilayer networks [100], including tools for representation [101], characterization [102], and inference regarding function [103], and (ii) the application of these tools to real-world multilayer systems with structures that are analogous to those observed in brain-human-group networks [104–106]. These studies offer concrete intuitions for how information can be created, manipulated, and processed in regions and networks in one brain and, based on those computations, the information could be transmitted from that brain to another brain in the social network, via processes of active transmission, learning, diffusion, or contagion to name a few (See Box 2, Multilayer Brain-Social Networks, and Box 3, A multilayer network model of how ideas and behaviors spread).
Box 2. Multilayer Brain-Social Networks.
Intuitively, the brain-social multiscale network has two layers: the brain network layer in which regions are nodes, and structural or functional connections are edges, and the social network layer in which people are nodes, and inter-personal relationships are edges. An important question is how these distinct layers get connected up with one another to form a multilayer structure. Arguably the simplest way in which to link the two layers it to note that all brain areas of person 1 are linked by interlayer links to the node in the social network that represents person 1. Similarly, all brain areas of person 2 are linked by interlayer links to the node in the social network that represents person 2. In this multilayer network, information in one region in person 1 can be transmitted to another region in person 1 via the brain network; that transmission of information can lead to a change in the idea of person 1, which can then be transmitted from person 1 to person 2 via the social network.
To summarize, this architecture is composed of region-to-region links in the brain network layer only, person-to-person links in the social network layer only, and region-to-person links that bridge across layers in the multilayer network. While a useful starting point, recent data suggests that this multilayer network also contains a fourth kind of link: a region-to-region link across persons where a brain area in one person can be linked by temporal synchronization of activity to the brain area of another person. Such brain-to-brain connectivity could reflect shared neural representations that arise from assumptions based on cultural knowledge or other variables, which may be, but are not required to be directly reflected in behavior.
The key advantage of the multilayer framework is that it enables one to formally develop and integrate (i) models of information transmission across links in the brain network, (ii) models of idea transmission across links in the social network, (iii) models of brain-to-brain synchrony, and (iv) models of computation, cognition, emotion, and perception that translate information into ideas across region-to-person links. Such models can then be used to probe and predict how perturbations at one node in one network layer (e.g., brain) can impact on another node in another network layer (e.g., social).
Box 3. A multi-layer network model of how ideas and behaviors spread.
Ideas and behaviors spread from brain to brain through social networks. We offer one example of the type of multi-scale network model we envision linking neuro- and social scientific models to explain when, why and how ideas and behaviors spread. From the neuroscience side, decisions, including whether to share information as a communicator, or act on that information as a receiver, can be modeled in terms of reinforcement learning [159,160]. The reinforcement learning perspective argues that personal subjective value maximization guides behavior [161–164]. Under this framework, when making choices, the brain is thought to compute a predicted reward for each potential course of action. Choices that result in more reward than expected are reinforced, and choices that result in less reward (or more punishment) than expected are devalued [165]. In parallel, social psychologists have highlighted that learning can take place not only with respect to an actor’s own experience, but also through observational and social learning from the experiences of others [93,166,167]. This learning occurs in conjunction with brain systems supporting simulation [167] and understanding of other’s experiences and perspectives (i.e., mentalizing) [93,168,169]. Likewise, the spread of ideas and other group-based problems are also critically influenced by the structure and composition of the social network (e.g., [2,8,170,171]).
Bringing these ideas together with a multi-scale network perspective, the flow of ideas and behaviors through social networks could be mathematically modeled by integrating concepts from reinforcement learning and social learning with network science. Here, an actor’s choices could be modeled using terms that capture information about the structure and function of brain networks within and between individuals. For example, at the individual level, such a model would characterize activity within and interactions between brain systems within the individual that support valuation, simulation and mentalizing as the actor learns from her own choices and the choices she observes in others [172]. Consistent with foundational research in social and developmental psychology and sociology on how individuals function within dyads, groups, and societies, such a model would also explicitly model the actions of social referents surrounding the actor. Finally, to capture the multi-scale nature of networks of brains communicating with one another, the model could account for the strength of coupling between key brain systems of actors within the network, as well as the structure of the individuals in the network (e.g., Figure 3).
For example, the probability that an idea spreads from a sharer (s) to receiver (r), could be modeled in terms of activation (A) and connectivity (C) matrices capturing (i) activity within the potential sharer’s brain (e.g., with nodes in the value and mentalizing systems) when describing the idea, (ii) activity and connectivity within parallel regions within the receiver’s brain during exposure to the ideas, and (iii) coupling between the two. Further, one could include a matrix capturing the number and position of other social referents (relative to the sender and receiver) who hold the same view within the social network (N). An example model could be written as follows:
Although each of these pieces has been modeled in isolation in prior work, such an integrated social reinforcement learning model mathematically links models of brain dynamics with social network structure and dynamics, and allows a more integrated theory of how ideas and behaviors spread in a social world [24].
Given that people, and hence their brains, do not function in isolation, it also makes sense to consider how the structure of interconnections between people who influence one another might change the way a given individual operates. Thus, in the next section, we turn our attention to the ways in which the structural properties of a social network might moderate the brains of individual actors and vice versa.
In what ways do different social network structures and dynamics shape brain and behavior? In what ways do different brains shape the network positions we occupy?
The idea that the social fabric that surrounds humans contributes to psychological and biological functioning is not new. For example, decades of research have demonstrated links between social support and biological function in animals and humans [107–110] and cultural psychologists have argued that individuals both influence and are influenced by their social environments [111]. Extending this logic, the brain influences the social networks people are in and how individuals interact within them, but the social dynamics and social network structure also influence how people’s brains work. By formalizing these relationships, mathematical models of social network structure and resources with models of brain function may provide new insight in both domains (see Box 4). In line with this goal, a growing body of literature has begun to explore the ways in which social network properties (including specific structural features) relate to brain structure and function. Several of these findings suggest that the structure and function of the brain’s mentalizing system, as well as regions involved in affective processing, display particularly strong covariation with social network features.
Box 4. Multiscale network models: description, prediction, and perturbation.
In principle, the multilayer network framework can be used to model how the change in time-varying activation of a single region (node) in the brain network layer can alter the state (e.g., mental or behavioral) of a person (node) in the social network layer. Key types of open questions in the field can be divided into questions of description, prediction, and perturbation.
First efforts to describe the architecture of the multilayer brain-social network could focus on quantifying the relative strengths of the different sorts of links present. Quite generally, one set of studies has focused on quantifying the strength and topology of inter-regional links present in brain networks during tasks related to idea transmission and receptivity; a second set of studies has focused on quantifying the strength and topology of brain-to-brain synchrony during perception; and a third set of studies has focused on quantifying the strength and topology of interpersonal links present in social networks that support idea spread and idea contagion. However, at present no single study has attempted to quantify the strength of region-to-region links within brain networks, brain-to-brain links across individuals, person-to-person links within social networks, and region-to-person links bridging brain networks to social networks during a single task and with the same individuals. The challenge in doing so will be to address the fact that each of these links may need to be estimated on different time scales – with region-to-region links being estimable by fMRI over short time windows, and person-to-person transmission of ideas potentially occurring over longer time windows in social networks.
After quantifying the strength of different sorts of links, one might wish to predict how far an idea might be expected to spread through a given sort of social network when the pattern of activity and connectivity in a single brain changes. Answering this question will require the construction of causal models for information transmission across links in a brain network, idea transmission across links in a social network as potentially modulated by the likelihood of brain-to-brain synchrony, and the translation of information within a brain region to an idea that is transmittable across persons via intra-personal processes related to computation, cognition, emotion, and perception. The construction and integration of such causal models will provide specific predictions about how perturbations at one node in one network layer can impact on another node in another network layer.
The development of such predictions then motivates perturbative empirical studies that can validate or disprove the predictions. One particularly interesting way in which to test causal predictions in brain-social multilayer networks is to expand behavioral network science experiments [171,173,174] by integrating neuroimaging and neurocognitive phenotyping. Specifically, one could recruit a large group of individuals (e.g., 30<N<50), place each individual on a network of communication according to their neural markers of receptivity or influence, and quantify idea or behavior transmission in the group. Such an experimental setup would allow one to test causal predictions such as that a person whose brain network has been statistically linked to high capacity for social influence would have a bigger impact on population-level behaviors if placed in a point of high betweenness in the communication network than if placed in a point of low betweenness and low degree.
Mentalizing, operationalized as accurately reporting on someone else’s mental state [112], and empathizing with others [113] are both processes that covary with social network size. In monkeys, experimentally manipulating social group size increases the structure and function of brain regions associated with the processing of social cues [114]. In humans, social network size also covaries with grey matter volume within regions associated with mentalizing [115], as well as broader emotion processing systems composed of the amygdala, orbitofrontal cortex, and connectivity between amygdala and cortical regions [116–118]. Also in humans, social network diversity, but not size, is associated with global white matter integrity at the borders of dmPFC, a key component of the mentalizing system [119], and in the corpus callosum.
Differing social network properties are also associated with functional differences in processing within systems necessary for navigating the social world. For example, during resting state scans, those with larger social networks show greater connectivity between the amygdala and parts of the value system implicated in social affiliative behavior, as well as brain regions implicated in social perception [118]. It is likely that social network structures both shape the types of social interactions that people have and are also shaped by individual differences in the tendency to use the brain in particular ways. To this end, teens who occupy social network positions with greater potential for information brokerage (i.e., connect more friends who did not otherwise know one another) also use brain regions implicated in mentalizing more when making recommendations to others [120]. It is possible that those who have more opportunities to translate information between others would need to practice mentalizing more, or that people who tend to do so more would naturally gravitate to network positions that make use of that tendency.
Mentalizing tendencies may also be associated with other advantages that are reflected in terms of brain function in perceivers and social targets. First, more popular people are perceived by others differently than less popular people are; when network members viewed the faces of more popular people in their social networks, popular faces elicited increased activity in functionally localized brain regions associated with mentalizing and valuation, with the effects of mentalizing mediated by value-related activity [121]. Interestingly, these effects were strongest in the most popular individuals, who were also the most accurate in knowing how others viewed them [121]. These data are consistent with the idea that brain and social network variables may mutually influence each other, with activity in value and mentalizing systems helping perceivers identify important social referents.
The brain’s mentalizing system in lateral temporal cortex and temporal parietal junction may also encode information about the social network position of people more broadly; in one study, these regions automatically distinguished the social network position of people within a social network whose faces participants observed in an fMRI scanner [122]. Separate regions that have been associated with processing social status, including mPFC, temporal poles, and fusiform gyrus, also encoded information about eigenvector centrality, a measure of prestige that captures the degree to which a participant is connected to well-connected others [122]. The authors note that these features of the social network were encoded automatically upon perception of the social referents (the task involved viewing of brief videos of network members and indicating whether the video was the same as the previous video played, and did not call any specific attention to social judgment). This highlights one value of integrating brain data with social network data, since the social perception processes in question occur quickly and often outside of conscious awareness. Thus, determining the mechanisms would likely be difficult with self-reports alone.
Moving forward, a more comprehensive and systematic investigation of how different social network properties (e.g., size, closure) relate to brain structure and function across a range of tasks, groups, and stages of development will help fill in this picture. Recent findings have begun to address different types of social ties (e.g., friends versus kin; varying levels of closeness in a social network) may be processed differently in the brain [123], highlighting the need for more nuanced hypotheses and analyses that account for multiscale structure in both social networks and brain networks. In addition, although the growing body of studies reviewed above suggest promise in integrating social network analysis with measures of brain structure and function, almost none of this work has explicitly modeled networks both within the brain and between individuals (c.f. [124]), which we argue is an important intersection to consider.
Current Frontiers and Open Questions
Frontiers for network neuroscience
Adopting a multiscale perspective suggests several possible extensions to extant network neuroscience findings. For example, it remains an exciting open question to determine the extent to which the brain network dynamics described in early network neuroscience investigations might vary across social contexts (e.g., between people who inhabit different social network structures), cultures, and stages of development. Likewise, variation in brain network dynamics might contribute to variation in social network structures, and it would be interesting to examine these dynamics in different cultural contexts.
Frontiers at the intersection of brain and social network science
As noted in our introduction, the term ‘social network’ encompasses multiple forms of interacting, multiscale networks of friends, family, co-workers, and communities. Work at the intersection of brain science and social network science has only begun to scratch the surface of how a wide range of social network types (e.g., core networks versus full networks [125–130]; strong versus weak ties [131–133]; online versus offline networks [134,135]) might influence and be influenced by brain function across the lifespan. In addition, tools for describing the social networks that individuals inhabit range from objective logs of specific ego-centric networks (e.g., Facebook [120,136]) to subjective assessments of the support available from a range of others (e.g., [136]) to interactions with supportive others [137]. Finally, within a given network, a number of features– ranging from measures of size to measures of topological complexity– might be differentially associated with brain structure and function for different types of tasks.
Many open questions remain at the intersection between brain networks and social networks. Arguably some of the most fundamental questions relate to notions of causality. To what extent do social network dynamics cause changes in brain dynamics? And to what extent do brain dynamics cause changes in social network dynamics? Most existing research at the intersection of brain and social network science has been correlative; in addition to building a deeper and broader picture of how a wide range of cognitive, affective, and social processes interact, experiments that determine causality will also substantially advance science in both domains. Longitudinal studies that span key transitions (e.g., from high school to college) will also provide insight into the direction of causality because both brain networks and social networks can change appreciably over this time scale. Indeed, tracking associations between changes in brain network and social network functioning over time provides a standard of evidence between cross-sectional and experimental designs [138].
A second set of questions relates to how other external processes might change the workings of brain and social networks. What environmental factors moderate the interactions between social and brain network dynamics? In what ways do cultural variables influence social and brain network dynamics? In what ways might interactions between brain networks and social networks vary across development (e.g., in children versus adolescents versus adults)? In each case, we hold that the time is ripe for hypotheses bridging brain and social networks, and building on both recent preliminary data and recently developed computational tools and methods.
Potential applications
Efforts aimed at understanding and integrating the study of social and brain network dynamics will advance understanding of basic psychological principles and aid in deriving fundamental principles about the organization of society. But even beyond fundamental knowledge, work at this intersection has the potential to improve real-world practice in clinical treatments for mental and physical disorders, predicting behavior change in response to persuasive messages, and improving educational outcomes including learning and creativity. For example, if people whose brain and/or social networks show differential response to treatments, logged information (e.g., from social media) could aid in providing tailored interventions. Similarly, educational environments could be constructed in which groups of students work with one another in tailored social networks to maximize individual learning potential. Indeed, improved knowledge in these domains also has the potential to aid in constructing optimal teams for group learning and for task performance in education, corporate, medical, defense, or other contexts. These possibilities motivate collaborative alliances between social scientists, neuroscientists, and network scientists in building and fine-tuning laboratory experiments, real-world studies, computational infrastructure, and fundamental mathematical theory that bridges the divide between individual brains and social groups by depicting the two as fundamentally interconnected levels in a multiscale network.
Figure 1. Building hypotheses bridging brain networks and social networks.
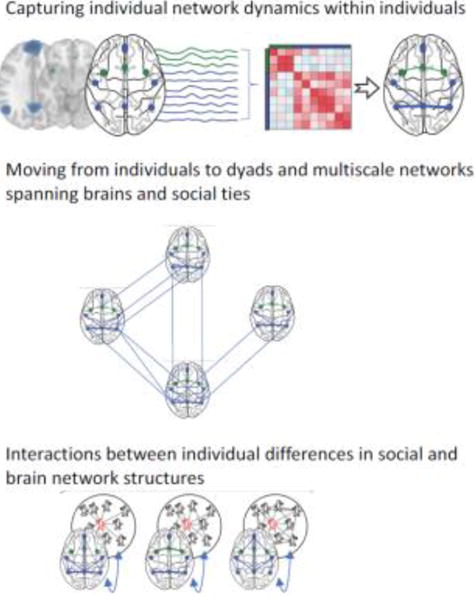
How can we move from typical data collected in a neuroimaging experiment to a multiscale network? Neural regions of interest (e.g., blue and green dots in the top panel) can be treated as nodes in a network, connected to one another by estimates of white matter structure or by estimates of functional connectivity such as a Pearson correlation between pairs of regional mean BOLD time series (e.g., in the top panel, the blue and green lines represent the timeseries from each region of interest over the course of a task in an MRI scanner; see also Box 1). By encoding these relationships in a connectivity matrix (depicted in shades of red in the top panel), one can first determine the strength of connectivity between brain regions in a single individual during different task conditions (in the top panel, the weight of the edges connecting the regions of interest in the right-most brain image represents the strength of the correlation between the timeseries of those nodes). Creating such a matrix can also address hypotheses not only about individuals acting in isolation, but also about the interplay between brain networks supporting mutual influence between individuals in social networks (e.g., processes facilitating the spread of ideas or behaviors), as depicted by lines connecting brain regions across different people’s brains in the middle panel. In addition, it is possible to model how an individual’s social network resources or placement within their social network relate to their brain or behavior as depicted in the bottom panel. Adapted from Schmaezle et al. (2017).
Figure 2. Multiscale network nature of idea and behavior spread.
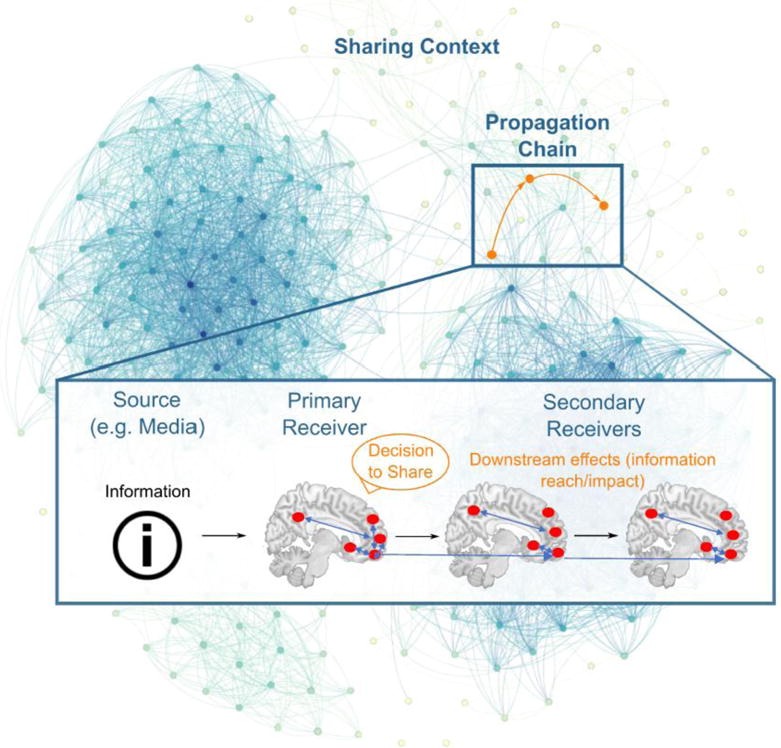
Ideas and behaviors spread from mass media and from person to person through brain networks within social networks. Here we illustrate the complex and heterogeneous organization of social networks where people (nodes) are connected by relationships of various strengths (edges), which can be defined in different ways depending on the study being conducted and the hypothesis being tested. A propagation chain between three individuals within this social network begins with the person who is the source of the information, who is connected to the primary receiver, who may in turn decide to share to a secondary receiver. Adapted from Scholz & Falk, in press.
Figure 3. Mathematical models of the multiscale mechanisms of idea and behavior spread.
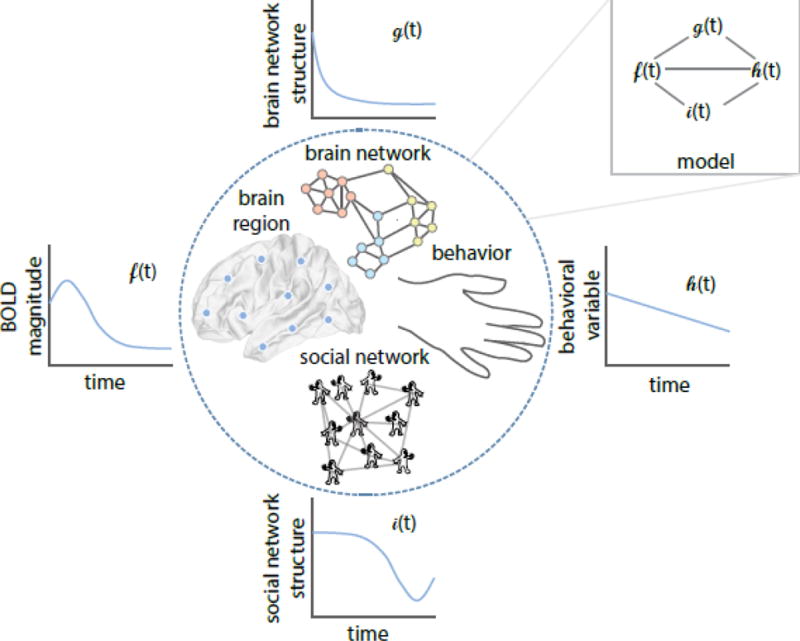
Building an integrated theory of how ideas and behaviors spread from person-to-person could benefit from models that explicitly bridge network models of brain structure and function, mathematical models of human behavior, and quantitative statistics summarizing social network structure and function, as well as interactions between each of these levels of analysis. In this conceptual schematic, we illustrate the idea that time-varying changes in regional activation, brain network architecture, behavioral measures, and social network resources can be linked mathematically in a formal modeling framework. As detailed in Box 3, it is possible to bridge reinforcement learning with network science such that an actor’s choices are modeled in terms of their own behavior and neurophysiology, but also with terms that account for the actions of social referents surrounding the actor, as well as the brain and social network structures of the individuals in the network. Adapted with permission from Bassett & Mattar, TICS (2017).
Figure 4. Example findings linking brain dynamics and social network position.
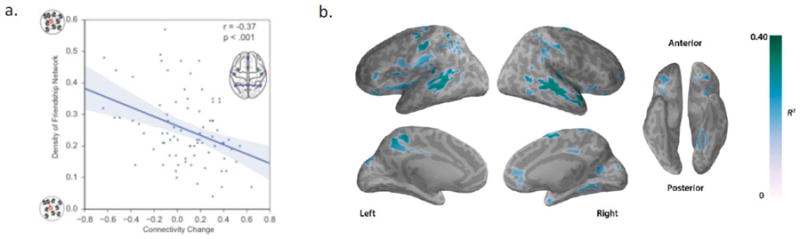
a) Greater changes in connectivity within the mentalizing network during social exclusion versus inclusion are associated with less dense friendship networks among adolescent males. Reproduced with permission from Schmaelzle et al. (2017). b) Multi-voxel patterns within the mentalizing system and other key brain regions are systematically associated the social network position of classmates seen in an fMRI experiment. Reproduced with permission from Parkinson et al. (2017).
Trends Box.
Network neuroscience provides new ways of understanding the complex patterns of structural connections and functional coupling in the human brain.
Social network analysis offers systematic ways to quantify social environments and interactions among persons and people groups.
Recent trends bring these two types of analysis together to understand how brain networks and social networks interact to influence the behaviors of individuals, groups and populations.
The confluence of these fields is beginning to shed light on how ideas and behaviors spread from person-to-person, and has the potential to inform health, education and community intervention.
Outstanding questions box.
To what extent do social network dynamics cause changes in brain dynamics?
To what extent to do brain dynamics cause changes in social network dynamics?
What factors moderate the interactions between social and brain network dynamics?
In what ways do other cultural and environmental variables influence social and brain dynamics and the relationship between them?
In what ways does development across the lifespan influence social and brain network dynamics and the relationship between them?
To what extent can understanding and integrating the study of social and brain network dynamics support practical advances such as tailoring clinical treatments for mental and physical disorders, predicting behavior change in response to persuasive messages, and improving educational outcomes, including learning and creativity.
To what extent can understanding and integrating the study of social and brain network dynamics advance understanding of basic psychological principles and aid in deriving fundamental principles about the organization of society?
Could improved knowledge in these domains aid in constructing optimal teams, for group learning and for task performance in education, corporate, medical, defense, or other contexts?
Box 1. Measurement of integrated social and brain networks.
Scaling up beyond individuals to pairs or groups of subjects requires either simultaneous measurement in the form of hyperscanning [139], or posthoc analyses that take data from individuals who engaged in the same experience and link that data together after acquisition [140]. One way in which the latter can be accomplished is by exposing participants to the same time locked stimuli and then examining the degree to which different participants show intersubject correlation in response to those stimuli [37,92,141]. An extension of this approach involves collecting brain activity in communicators as they communicate and receivers as they receive – this method similarly allows time locked analysis of a common time series [37,91,92,141]. Scaling this approach from dyads to networks, one study constructed multi-brain networks of 2 speakers and 10 listeners, connected in a single network, to measure synchronous communication between network members [142]. These approaches pave the way to understand the manners in which brain network dynamics in one person might influence or reflect the brain network dynamics in another.
In addition, it is important to note that both brains and social systems have both structural and functional network organization, and both aspects of the systems may be important in understanding system-system interactions. In the human brain, while functional networks are defined based on similar time-varying patterns of regional activity, structural networks are defined based on estimates of white matter tracts connecting region pairs [143,144]. Structural network organization in the brain varies appreciably across individuals [145], over developmental time scales [146], and over healthy aging [147]. This organization has been linked to individual differences in cognitive function [148], and to differences in the patterns of functional connectivity that support it [149–151].
Just as brain networks can be described in terms of structure and function, social networks can likewise be described in terms of the structure of social ties that surround an individual (i.e., who knows or is connected to whom [1,42,131,134,152]) and infrastructure that connects them [153] as well as functional interactions in which people engage (e.g., communication networks [154–156]) and the quality of the relationships between people (e.g., liking [121]). As with brain networks, the structure of social networks varies appreciably across individuals [42,134], and within individuals over development [157] and as their context changes [158], though there is some consistency in an individual’s “signature” network characteristics [154].
Acknowledgments
The authors thank Richard F. Betzel, Bruce Dore, David Lydon-Staley, Christin Scholz, Ralf Schmaelzle, Joe Bayer, Ally Paul, and Steve Tompson for helpful comments on earlier versions of this manuscript, and Matt O’Donnell for helpful discussions related to links between social networks and brain activity. DSB and EBF would also like to acknowledge support from the Army Research Laboratory through contract number W911NF-10-2-0022; further support for DSB comes from the John D. and Catherine T. MacArthur foundation, the Alfred P. Sloan Foundation, and an NSF CAREER grant PHY-1554488 and for EBF from an NIH New Innovator Award NIH-1 DP2 DA035156-01, a DARPA Young Faculty Award YFA-D14AP00048, and Hope Lab.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moreno JL, Jennings HH. Statistics of Social Configurations. Sociometry. 1938;1:342–374. [Google Scholar]
- 2.Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 3.Radicchi F, Castellano C. Fundamental difference between superblockers and superspreaders in networks. Phys Rev E. 2017;95:012318. doi: 10.1103/PhysRevE.95.012318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long JC, et al. Structuring successful collaboration: a longitudinal social network analysis of a translational research network. Implement Sci. 2016;11:19. doi: 10.1186/s13012-016-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson K, et al. Can mental health interventions change social networks? A systematic review. BMC Psychiatry. 2015;15:297. doi: 10.1186/s12888-015-0684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widmer ED, et al. How central and connected am I in my family?: Family-based social capital of individuals with intellectual disability. Res Dev Disabil. 2008 Mar;29:176–187. doi: 10.1016/j.ridd.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Bae SH, et al. Nursing teams: behind the charts. J Nurs Manag. 2017 doi: 10.1111/jonm.12473. [DOI] [PubMed] [Google Scholar]
- 8.González-Bailón S, et al. The dynamics of protest recruitment through an online network. Sci Rep. 2011;1:197. doi: 10.1038/srep00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bond M. Social influences on corporate political donations in Britain. Br J Sociol. 2004;55:55–77. doi: 10.1111/j.1468-4446.2004.00006.x. [DOI] [PubMed] [Google Scholar]
- 10.Ward MD, et al. Identifying international networks: Latent spaces and imputation. 2003 na. [Google Scholar]
- 11.Butts CT. Revisiting the foundations of network analysis. Science. 2009;325:414–416. doi: 10.1126/science.1171022. [DOI] [PubMed] [Google Scholar]
- 12.Bassett DS, Sporns O. Network Neuroscience. Nat Neurosci. 2016 doi: 10.1038/nn.4502. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Servaas MN, et al. Associations between genetic risk, functional brain network organization and neuroticism. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaty RE, et al. Personality and complex brain networks: The role of openness to experience in default network efficiency. Hum Brain Mapp. 2016;37:773–779. doi: 10.1002/hbm.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marek S, et al. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biol. 2015;13:e1002328. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spielberg JM, et al. Flexible brain network reconfiguration supporting inhibitory control. Proc Natl Acad Sci U S A. 2015;112:10020–10025. doi: 10.1073/pnas.1500048112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peeters S, et al. Semi-metric analysis of the functional brain network: Relationship with familial risk for psychotic disorder. Neuroimage Clin. 2015;9:607–616. doi: 10.1016/j.nicl.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang P, et al. The Effects of an APOE Promoter Polymorphism on Human White Matter Connectivity during Non-Demented Aging. J Alzheimers Dis. 2017;55:77–87. doi: 10.3233/JAD-160447. [DOI] [PubMed] [Google Scholar]
- 19.Braun U, et al. Dynamic reconfiguration of brain networks: a potential schizophrenia genetic risk mechanism modulated by NMDA receptor function. Submitted. 2016 doi: 10.1073/pnas.1608819113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karuza EA, et al. Local Patterns to Global Architectures: Influences of Network Topology on Human Learning. Trends Cogn Sci. 2016:S1364–6613. 30071–30077. doi: 10.1016/j.tics.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borchardt V, et al. Dynamic disconnection of the supplementary motor area after processing of dismissive biographic narratives. Brain Behav. 2015;5:e00377. doi: 10.1002/brb3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vico Fallani F, et al. Defecting or not defecting: how to “read” human behavior during cooperative games by EEG measurements. PLoS One. 2010;5:e14187. doi: 10.1371/journal.pone.0014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betzel RF, Bassett DS. Multi-scale brain networks. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.11.006. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassett DS, Mattar MG. A Network Neuroscience of Human Learning: Potential to Inform Quantitative Theories of Brain and Behavior. Trends Cogn Sci. 2017;21:250–264. doi: 10.1016/j.tics.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmaelzle R, et al. Brain connectivity dynamics during social interaction reflect social network structure. bioRxiv. 2017 Jan 02;:096420. doi: 10.1073/pnas.1616130114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackman DA, et al. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donnell MB, Falk EB. Big Data under the Microscope and Brains in Social Context. Ann Am Acad Pol Soc Sci. 2015;659:274–289. [Google Scholar]
- 29.O’Donnell MB, et al. Big data in the new media environment. Behav Brain Sci. 2014;37:94–95. doi: 10.1017/S0140525X13001672. [DOI] [PubMed] [Google Scholar]
- 30.Meshi D, et al. The Emerging Neuroscience of Social Media. Trends Cogn Sci. 2015;19:771–782. doi: 10.1016/j.tics.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Branigan HP, et al. Syntactic co-ordination in dialogue. Cognition. 2000;75:B13–25. doi: 10.1016/s0010-0277(99)00081-5. [DOI] [PubMed] [Google Scholar]
- 32.Gonzales AL, et al. Language Style Matching as a Predictor of Social Dynamics in Small Groups. Communic Res. 2009 doi: 10.1177/0093650209351468. [DOI] [Google Scholar]
- 33.Niederhoffer KG, Pennebaker JW. Linguistic style matching in social interaction. J Lang Soc Psychol. 2002;21:337–360. [Google Scholar]
- 34.Cappella JN. Why biological explanations? J Commun. 1996;46:4–7. [Google Scholar]
- 35.Richardson DC, Dale R. Looking to understand: the coupling between speakers’ and listeners’ eye movements and its relationship to discourse comprehension. Cogn Sci. 2005;29:1045–1060. doi: 10.1207/s15516709cog0000_29. [DOI] [PubMed] [Google Scholar]
- 36.Lakin JL, Chartrand TL. Using Nonconscious Behavioral Mimicry to Create Affiliation and Rapport. Psychol Sci. 2003;14:334–339. doi: 10.1111/1467-9280.14481. [DOI] [PubMed] [Google Scholar]
- 37.Hasson U, et al. Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends Cogn Sci. 2012;16:114–121. doi: 10.1016/j.tics.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephens GJ, et al. Speaker-listener neural coupling underlies successful communication. Proc Natl Acad Sci U S A. 2010;107:14425–14430. doi: 10.1073/pnas.1008662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silbert LJ, et al. Coupled neural systems underlie the production and comprehension of naturalistic narrative speech. Proc Natl Acad Sci U S A. 2014;111:E4687–96. doi: 10.1073/pnas.1323812111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Büchel C, et al. Characterizing Stimulus–Response Functions Using Nonlinear Regressors in Parametric fMRI Experiments. Neuroimage. 1998 Aug;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- 41.Ankit Khambhati Ann E, Sizemore Richard F, Betzel Danielle S, Bassett Modeling and Interpreting Network Dynamics. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burt RS, et al. Social network analysis: foundations and frontiers on advantage. Annu Rev Psychol. 2013;64:527–547. doi: 10.1146/annurev-psych-113011-143828. [DOI] [PubMed] [Google Scholar]
- 43.Landis B. Personality and social networks in organizations: A review and future directions. J Organ Behav. 2015;37:S107–S121. [Google Scholar]
- 44.Scholz C, Falk EB. The neuroscience of viral ideas. In: González-Bailón S, Foucault Welles B, editors. Handbook of Communication in the Networked Age. Oxford University Press; 2017. [Google Scholar]
- 45.Goel S, et al. The Structural Virality of Online Diffusion. Manage Sci 2015 [Google Scholar]
- 46.Mednick SC, et al. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010;5:e9775. doi: 10.1371/journal.pone.0009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenquist JN, et al. The spread of alcohol consumption behavior in a large social network. Ann Intern Med. 2010;152:426–33, W141. doi: 10.1059/0003-4819-152-7-201004060-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christakis NA, Fowler JH. The Spread of Obesity in a Large Social Network over 32 Years. N Engl J Med. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 49.Fowler JH, Christakis NA. Dynamic spread of happiness in a large social network: longitudinal analysis over 20 years in the Framingham Heart Study. BMJ. 2008;337:a2338–a2338. doi: 10.1136/bmj.a2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DA, et al. Social network targeting to maximise population behaviour change: a cluster randomised controlled trial. Lancet. 2015;386:145–153. doi: 10.1016/S0140-6736(15)60095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strully KW, et al. Aspirin use and cardiovascular events in social networks. Soc Sci Med. 2012;74:1125–1129. doi: 10.1016/j.socscimed.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gleeson JP, et al. Effects of Network Structure, Competition and Memory Time on Social Spreading Phenomena. Phys Rev X. 2016;6:021019. [Google Scholar]
- 53.Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. 1995;117:497–529. [PubMed] [Google Scholar]
- 54.Lieberman MD, Eisenberger NI. Neuroscience. Pains and pleasures of social life. Science. 2009;323:890–891. doi: 10.1126/science.1170008. [DOI] [PubMed] [Google Scholar]
- 55.Falk EB, Scholz C. Persuasion, Influence and Value: Perspectives from communication and social neuroscience. Annu Rev Psychol. 2018 doi: 10.1146/annurev-psych-122216-011821. [DOI] [PubMed] [Google Scholar]
- 56.Baek EC, et al. Neural correlates of selecting and sharing information. Psychol Sci [Google Scholar]
- 57.Falk EB, et al. Creating buzz: the neural correlates of effective message propagation. Psychol Sci. 2013;24:1234–1242. doi: 10.1177/0956797612474670. [DOI] [PubMed] [Google Scholar]
- 58.Falk EB, et al. Getting the word out: neural correlates of enthusiastic message propagation. Front Hum Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dietvorst RC, et al. A sales force–specific theory-of-mind scale: Tests of its validity by classical methods and functional magnetic resonance imaging. J Mark Res. 2009;46:653–668. [Google Scholar]
- 60.Scholz C, et al. A neural model of information virality. Proceedings of the National Academy of Science. 2017 doi: 10.1073/pnas.1615259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamir DI, et al. Informing others is associated with behavioral and neural signatures of value. J Exp Psychol Gen. 2015;144:1114–1123. doi: 10.1037/xge0000122. [DOI] [PubMed] [Google Scholar]
- 62.Tamir DI, Mitchell JP. Disclosing information about the self is intrinsically rewarding. Proc Natl Acad Sci U S A. 2012;109:8038–8043. doi: 10.1073/pnas.1202129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaki J, et al. Social Influence Modulates the Neural Computation of Value. Psychol Sci. 2011;22:894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]
- 64.Nook EC, Zaki J. Social norms shift behavioral and neural responses to foods. J Cogn Neurosci. 2015;27:1412–1426. doi: 10.1162/jocn_a_00795. [DOI] [PubMed] [Google Scholar]
- 65.Klucharev V, et al. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 66.Cascio CN, et al. Social influence and the brain: persuasion, susceptibility to influence and retransmission. Current Opinion in Behavioral Sciences. 2015;3:51–57. [Google Scholar]
- 67.Cascio CN, et al. Neural Correlates of Susceptibility to Group Opinions in Online Word-of-Mouth Recommendations. J Mark Res. 2015;52:559–575. [Google Scholar]
- 68.Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- 69.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 70.Medaglia JD, et al. Cognitive network neuroscience. J Cogn Neurosci. 2015;27:1471–1491. doi: 10.1162/jocn_a_00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooper N, et al. Coherent activity between brain regions that code for value is linked to the malleability of human behavior. Sci Rep. 2017;7:43250. doi: 10.1038/srep43250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cooper N, Tompson S, O’Donnell MB, Vettel JV, Bassett DS, Falk EB. Coherent neural activity in the brain’s value system during antismoking messages predicts reductions in smoking. International Communication Association. 2017 doi: 10.1037/hea0000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu H, et al. Altered Topological Properties of Brain Networks in Social Anxiety Disorder: A Resting-state Functional MRI Study. Sci Rep. 2017;7:43089. doi: 10.1038/srep43089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Betzel RF, et al. Positive affect, surprise, and fatigue are correlates of network flexibility. Sci Rep. 2017;7:520. doi: 10.1038/s41598-017-00425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Che X, et al. Synchronous activation within the default mode network correlates with perceived social support. Neuropsychologia. 2014;63:26–33. doi: 10.1016/j.neuropsychologia.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 76.Ekman M, et al. Predicting errors from reconfiguration patterns in human brain networks. Proc Natl Acad Sci U S A. 2012;109:16714–16719. doi: 10.1073/pnas.1207523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simony E, et al. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat Commun. 2016;7:12141. doi: 10.1038/ncomms12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chai LR, et al. Functional Network Dynamics of the Language System. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doron KW, et al. Dynamic network structure of interhemispheric coordination. Proc Natl Acad Sci U S A. 2012;109:18661–18668. doi: 10.1073/pnas.1216402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bassett DS, et al. Learning-induced autonomy of sensorimotor systems. Nat Neurosci. 2015;18:744–751. doi: 10.1038/nn.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bassett DS, et al. Cross-linked structure of network evolution. Chaos. 2014;24:013112. doi: 10.1063/1.4858457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Braun U, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A. 2015;112:11678–11683. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alavash M, et al. Persistency and flexibility of complex brain networks underlie dual-task interference. Hum Brain Mapp. 2015;36:3542–3562. doi: 10.1002/hbm.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shine JM, et al. Temporal metastates are associated with differential patterns of time-resolved connectivity, network topology, and attention. Proc Natl Acad Sci U S A. 2016;113:9888–9891. doi: 10.1073/pnas.1604898113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petersen SE, Sporns O. Brain Networks and Cognitive Architectures. Neuron. 2015;88:207–219. doi: 10.1016/j.neuron.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 87.Bassett DS, et al. Emerging Frontiers of Neuroengineering: A Network Science of Brain Connectivity. arXiv [q-bio.NC] 2016 Dec 23; doi: 10.1146/annurev-bioeng-071516-044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Achard S, et al. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mattar MG, et al. The network architecture of value learning. arXiv [q-bio.NC] 2016 Jul 14; [Google Scholar]
- 90.Schilbach L, et al. Toward a second-person neuroscience. Behav Brain Sci. 2013;36:393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- 91.Scholz C, et al. A neural propagation system: Neurocognitive and preference synchrony in information sharers and their receivers [Google Scholar]
- 92.Schmälzle R, et al. Engaged listeners: shared neural processing of powerful political speeches. Soc Cogn Affect Neurosci. 2015 doi: 10.1093/scan/nsu168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hasson U, Frith CD. Mirroring and beyond: coupled dynamics as a generalized framework for modelling social interactions. Philos Trans R Soc Lond B Biol Sci. 2016;371 doi: 10.1098/rstb.2015.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toppi J, et al. Graph theory in brain-to-brain connectivity: A simulation study and an application to an EEG hyperscanning experiment. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:2211–2214. doi: 10.1109/EMBC.2015.7318830. [DOI] [PubMed] [Google Scholar]
- 95.Parkinson C, et al. Neural Homophily: Similar Neural Responses Predict Friendship. bioRxiv. 2016 Dec 07;:092130. [Google Scholar]
- 96.Semin GR, Cacioppo JT. Grounding Social Cognition: Synchronization, Entrainment, and Coordination. In: Semin GR, Smith ER, editors. Embodied grounding: Social, cognitive, affective, and neuroscientific approaches. Cambridge University Press; 2008. pp. 119–147. [Google Scholar]
- 97.Bassett DS, Gazzaniga MS. Understanding complexity in the human brain. Trends Cogn Sci. 2011;15:200–209. doi: 10.1016/j.tics.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Templeton A, et al. From Mindless Masses to Small Groups: Conceptualizing Collective Behavior in Crowd Modeling. Rev Gen Psychol. 2015;19:215–229. doi: 10.1037/gpr0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Falk EB, et al. What is a representative brain? Neuroscience meets population science. Proc Natl Acad Sci U S A. 2013;110:17615–17622. doi: 10.1073/pnas.1310134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kivelä M, et al. Multilayer networks. complex networks. 2014 at http://comnet.oxfordjournals.org/content/2/3/203.short.
- 101.De Domenico M, et al. MuxViz: a tool for multilayer analysis and visualization of networks. J Complex Netw. 2015;3:159–176. [Google Scholar]
- 102.De Domenico M, et al. The physics of spreading processes in multilayer networks. Nat Phys. 2016;12:901–906. [Google Scholar]
- 103.Solé-Ribalta A, et al. Congestion Induced by the Structure of Multiplex Networks. Phys Rev Lett. 2016;116:108701. doi: 10.1103/PhysRevLett.116.108701. [DOI] [PubMed] [Google Scholar]
- 104.Yağan O, Gligor V. Analysis of complex contagions in random multiplex networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2012;86:036103. doi: 10.1103/PhysRevE.86.036103. [DOI] [PubMed] [Google Scholar]
- 105.Battiston F, et al. Efficient exploration of multiplex networks. New J Phys. 2016;18:043035. [Google Scholar]
- 106.Nicosia V, et al. Collective Phenomena Emerging from the Interactions between Dynamical Processes in Multiplex Networks. Phys Rev Lett. 2017;118:138302. doi: 10.1103/PhysRevLett.118.138302. [DOI] [PubMed] [Google Scholar]
- 107.Taylor SE, et al. Toward a biology of social support. Oxford University Press; 2002. [Google Scholar]
- 108.Uchino BN, et al. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- 109.Kiecolt-Glaser JK, et al. Psychoneuroimmunology: psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- 110.Cohen S, Herbert TB. Health psychology: psychological factors and physical disease from the perspective of human psychoneuroimmunology. Annu Rev Psychol. 1996;47:113–142. doi: 10.1146/annurev.psych.47.1.113. [DOI] [PubMed] [Google Scholar]
- 111.Markus HR, Kitayama S. Cultures and Selves: A Cycle of Mutual Constitution. Perspect Psychol Sci. 2010;5:420–430. doi: 10.1177/1745691610375557. [DOI] [PubMed] [Google Scholar]
- 112.Stiller J, Dunbar RIM. Perspective-taking and memory capacity predict social network size. Soc Networks. 2007;29:93–104. [Google Scholar]
- 113.Kardos P, et al. Empathic people have more friends: Empathic abilities predict social network size and position in social network predicts empathic efforts. Soc Networks. 2017;50:1–5. [Google Scholar]
- 114.Sallet J, et al. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- 115.Kanai R, et al. Online social network size is reflected in human brain structure. Proc Biol Sci. 2012;279:1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Von Der Heide R, et al. The social network-network: size is predicted by brain structure and function in the amygdala and paralimbic regions. Soc Cogn Affect Neurosci. 2014;9:1962–1972. doi: 10.1093/scan/nsu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Powell J, et al. Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proc Biol Sci. 2012;279:2157–2162. doi: 10.1098/rspb.2011.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bickart KC, et al. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci. 2012;32:14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Molesworth T, et al. Social network diversity and white matter microstructural integrity in humans. Soc Cogn Affect Neurosci. 2015;10:1169–1176. doi: 10.1093/scan/nsv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Donnell MB, et al. Neural bases of recommendations differ according to social network structure. Soc Cogn Affect Neurosci. 2017 doi: 10.1093/scan/nsw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zerubavel N, et al. Neural mechanisms tracking popularity in real-world social networks. Proc Natl Acad Sci U S A. 2015;112:15072–15077. doi: 10.1073/pnas.1511477112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parkinson C, et al. Spontaneous Neural Encoding of Social Network Position. 2017 [Google Scholar]
- 123.Wlodarski R, Dunbar RIM. When BOLD is thicker than water: processing social information about kin and friends at different levels of the social network. Soc Cogn Affect Neurosci. 2016;11:1952–1960. doi: 10.1093/scan/nsw101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schmälzle R, et al. Brain connectivity dynamics during social interaction reflect social network structure. Proc Natl Acad Sci U S A. 2017;114:5153–5158. doi: 10.1073/pnas.1616130114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dunbar RIM. The Social Brain. Curr Dir Psychol Sci. 2014;23:109–114. [Google Scholar]
- 126.Hampton KN, et al. CORE NETWORKS, SOCIAL ISOLATION, AND NEW MEDIA. Inf Commun Soc. 2011;14:130–155. [Google Scholar]
- 127.Ling R, et al. Small Circles: Mobile Telephony and the Cultivation of the Private Sphere. The Information Society. 2014;30:282–291. [Google Scholar]
- 128.Sutcliffe A, et al. Relationships and the social brain: integrating psychological and evolutionary perspectives. Br J Psychol. 2012;103:149–168. doi: 10.1111/j.2044-8295.2011.02061.x. [DOI] [PubMed] [Google Scholar]
- 129.Roberts SGB, et al. Individual differences and personal social network size and structure. Pers Individ Dif. 2008;44:954–964. [Google Scholar]
- 130.Roberts SGB, Dunbar RIM. Communication in social networks: Effects of kinship, network size, and emotional closeness. Pers Relatsh. 2010;18:439–452. [Google Scholar]
- 131.Granovetter MS. The Strength of Weak Ties. Am J Sociol. 1973;78:1360–1380. [Google Scholar]
- 132.Vanhalst J, Leary MR. Sociotropic differentiation: Differential anticipatory reactions to rejection by close versus distal others predict well-being. Pers Individ Dif. 2014;68:176–182. [Google Scholar]
- 133.Wright KB, Miller CH. A Measure of Weak-Tie/Strong-Tie Support Network Preference. Commun Monogr. 2010;77:500–517. [Google Scholar]
- 134.Burt RS. Network-Related Personality and the Agency Question: Multirole Evidence from a Virtual World. Am J Sociol. 2012;118:543–591. [Google Scholar]
- 135.Dunbar RIM, et al. The structure of online social networks mirrors those in the offline world. Soc Networks. 2015;43:39–47. [Google Scholar]
- 136.Cohen S, et al. Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- 137.Eisenberger NI, et al. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feld SL, et al. Describing Changes in Personal Networks over Time. Field methods. 2007;19:218–236. [Google Scholar]
- 139.Montague PR, et al. Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage. 2002;16:1159–1164. doi: 10.1006/nimg.2002.1150. [DOI] [PubMed] [Google Scholar]
- 140.Babiloni F, Astolfi L. Social neuroscience and hyperscanning techniques: past, present and future. Neurosci Biobehav Rev. 2014;44:76–93. doi: 10.1016/j.neubiorev.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hasson U, et al. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- 142.Tadić B, et al. Algebraic Topology of Multi-Brain Connectivity Networks Reveals Dissimilarity in Functional Patterns during Spoken Communications. PLoS One. 2016;11:e0166787. doi: 10.1371/journal.pone.0166787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sporns O, et al. The human connectome: A structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hagmann P, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bassett DS, et al. Conserved and variable architecture of human white matter connectivity. Neuroimage. 2011;54:1262–1279. doi: 10.1016/j.neuroimage.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 146.Baum GL, et al. Modular Segregation of Structural Brain Networks Supports the Development of Executive Function in Youth. Curr Biol. doi: 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Betzel RF, et al. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 2014;102(Pt 2):345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 148.Hermundstad AM, et al. Structurally-constrained relationships between cognitive states in the human brain. PLoS Comput Biol. 2014;10:e1003591. doi: 10.1371/journal.pcbi.1003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hermundstad AM, et al. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proc Natl Acad Sci U S A. 2013;110:6169–6174. doi: 10.1073/pnas.1219562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Goñi J, et al. Resting-brain functional connectivity predicted by analytic measures of network communication. Proc Natl Acad Sci U S A. 2014;111:833–838. doi: 10.1073/pnas.1315529111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Honey CJ, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arnaboldi V, et al. Online Social Networks: Human Cognitive Constraints in Facebook and Twitter Personal Graphs. Elsevier; 2015. [Google Scholar]
- 153.Wellman B. Computer Networks As Social Networks. Science. 2001;293:2031–2034. doi: 10.1126/science.1065547. [DOI] [PubMed] [Google Scholar]
- 154.Saramäki J, et al. Persistence of social signatures in human communication. Proc Natl Acad Sci U S A. 2014;111:942–947. doi: 10.1073/pnas.1308540110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eckmann JP, et al. Entropy of dialogues creates coherent structures in e-mail traffic. Proc Natl Acad Sci U S A. 2004;101:14333–14337. doi: 10.1073/pnas.0405728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Onnela JP, et al. Structure and tie strengths in mobile communication networks. Proc Natl Acad Sci U S A. 2007;104:7332–7336. doi: 10.1073/pnas.0610245104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wrzus C, et al. Social network changes and life events across the life span: a meta-analysis. Psychol Bull. 2013;139:53–80. doi: 10.1037/a0028601. [DOI] [PubMed] [Google Scholar]
- 158.Small ML, et al. How stable is the core discussion network? Soc Networks. 2015;40:90–102. [Google Scholar]
- 159.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 160.Dayan P. Chapter 2 - Models of Value and Choice. In: Dolan R, Sharot T, editors. Neuroscience of Preference and Choice. Academic Press; 2012. pp. 33–52. [Google Scholar]
- 161.Von Neumann J, Morgenstern O. Theory of games and economic behavior. Princeton university press; 1944. [Google Scholar]
- 162.Savage Leonard J. The foundations of statistics. NY: John Wiley; 1954. [Google Scholar]
- 163.Samuelson PA. A Note on Measurement of Utility. Rev Econ Stud. 1937;4:155. [Google Scholar]
- 164.Camerer C, et al. Neuroeconomics: How Neuroscience Can Inform Economics. J Econ Lit. 2005;43:9–64. [Google Scholar]
- 165.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 166.Bandura A. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- 167.Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 168.Dufour N, et al. Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS One. 2013;8:e75468. doi: 10.1371/journal.pone.0075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Frith CD, Frith U. The Neural Basis of Mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 170.Centola D. The spread of behavior in an online social network experiment. Science. 2010;329:1194–1197. doi: 10.1126/science.1185231. [DOI] [PubMed] [Google Scholar]
- 171.Judd S, et al. Behavioral dynamics and influence in networked coloring and consensus. Proc Natl Acad Sci U S A. 2010;107:14978–14982. doi: 10.1073/pnas.1001280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zaki J, et al. Social Cognition as Reinforcement Learning: Feedback Modulates Emotion Inference. J Cogn Neurosci. 2016;28:1270–1282. doi: 10.1162/jocn_a_00978. [DOI] [PubMed] [Google Scholar]
- 173.Kearns M, et al. An experimental study of the coloring problem on human subject networks. Science. 2006;313:824–827. doi: 10.1126/science.1127207. [DOI] [PubMed] [Google Scholar]
- 174.Carlson JM, et al. Measuring and modeling behavioral decision dynamics in collective evacuation. PLoS One. 2014;9:e87380. doi: 10.1371/journal.pone.0087380. [DOI] [PMC free article] [PubMed] [Google Scholar]


