Abstract
Background: Understanding the impact of comorbidities and competing risks of death when caring for older adults with thyroid cancer is key for personalized management. The objective of this study was to determine whether older adults with thyroid cancer are more likely to die from thyroid cancer or other etiologies, and determine patient factors associated with each.
Methods: The Surveillance, Epidemiology, and End Results (SEER)-Medicare database was used to identify patients aged ≥66 years diagnosed with thyroid cancer (papillary, follicular, Hürthle cell, medullary, anaplastic, and other) between 2000 and 2015 (median follow-up, 50 months). We analyzed time to event (i.e., death from other causes or death from thyroid cancer) using cumulative incidence functions. Competing risk hazards regression was used to determine the association between patient (e.g., age at diagnosis and specific comorbidities) and tumor characteristics (e.g., SEER stage) with two competing mortality outcomes: death from other causes and death from thyroid cancer.
Results: Of 21,509 patients with a median age of 72 years (range 66–106), 4168 (19.4%) died of other causes and 2644 (12.3%) died of thyroid cancer during the study period. For differentiated thyroid cancer patients, likelihood of dying from other causes exceeds likelihood of dying from thyroid cancer, whereas the opposite is true for anaplastic thyroid cancer. For medullary thyroid cancer, after 6.25 years patients are more likely to die from other etiologies than thyroid cancer. Using competing risks hazards regression, male sex (hazards ratio [HR] 1.47; 95% confidence interval [CI 1.37–1.57]), black race (HR 1.30; CI [1.16–1.46]), and comorbidities (e.g., heart disease, HR 1.34; CI [1.25–1.44]; chronic lower respiratory disease, HR 1.25; CI [1.17–1.34]) were associated with death from other causes. Tumor characteristics such as histology, tumor size, and stage correlated with death from thyroid cancer (e.g., distant SEER stage compared with localized, HR 12.65; CI [10.91–14.66]).
Conclusions: The clinical context, including patients' specific comorbidities, should be considered when diagnosing and managing thyroid cancer. Our findings can be used to develop decision models that account for competing causes of death, as an aid for clinical decision making.
Keywords: death, older adults, thyroid cancer
Introduction
The median age of patients dying from thyroid cancer is 73 years with >70% of all thyroid cancer deaths occurring in patients aged 65 years and older (1). In addition, this same age group is at higher risk of dying from other causes, such as heart disease and chronic lower respiratory disease (2). Considering this age group is at higher risk of dying from both thyroid cancer and other causes, potential risks and costs related to thyroid cancer diagnosis and treatments in older adults must be balanced against benefits (3,4). Importantly, older adults are the most vulnerable to morbidity related to surgical complications from thyroid surgery, as well as cardiac and skeletal complications secondary to suppressive doses of thyroid hormone therapy (4–11).
In view of the health and economic impact of thyroid cancer on older adults, advancing patient age, the burden of comorbidities, and life expectancy are important considerations in the clinical decision-making process regarding thyroid cancer diagnosis and management. Understanding competing causes of death could lead to more personalized patient care, with consideration of comorbidities and life expectancy in addition to severity of thyroid cancer. Although prior studies in other cancers have evaluated the relationship between presence of comorbidities and death from other causes, this topic is largely unexplored and perhaps even more relevant for thyroid cancer, a cancer with a low disease-specific mortality rate (12–14). Therefore, in the context of a rapidly growing aging and multimorbid population and low overall thyroid cancer-specific mortality, accounting for competing causes of death in these patients is key to personalized management.
Using data from Surveillance, Epidemiology, and End Results (SEER)-Medicare, we investigated whether older adults with thyroid cancer are more likely to die from thyroid cancer or other etiologies and factors associated with each. We hypothesized that a subgroup of older adults is more likely to die of other causes than their thyroid cancer and, therefore, individual patient context should be considered when diagnosing and managing thyroid cancer in older adults.
Methods
Data sources
We conducted a retrospective cohort study by using linked SEER-Medicare data files. The SEER database captures cancer incidence and survival data from population-based cancer registries representing ∼35% of the U.S. population (15). More than 97% of older adults (aged 65 years and older) are eligible to enroll in Medicare, which covers claims for health services (16,17). SEER data linked with Medicare administrative claims provide billing information and diagnoses from inpatient, outpatient, and noninstitutional settings (18).
Study population
Using SEER-Medicare we identified all patients diagnosed with any type of thyroid cancer (papillary, follicular, Hürthle cell, medullary, anaplastic, and other) at age 66 years or older between 2000 and 2015. We subsequently excluded those who had another cancer first (i.e., a cancer other than thyroid cancer) since SEER only records cause of death from the primary cancer (19). Since our study focuses on older adults who are most at risk for death, and the vast majority of Americans join Medicare at age 65 years, we restricted our SEER-Medicare cohort to patients aged ≥66 years to capture at least 1 year of Medicare claims before thyroid cancer diagnosis to extract reliable information on comorbidities.
The study did not require Institutional Review Board approval because it involves research using publicly available deidentified data.
Outcome measures
Cause of death and survival time were obtained from the SEER records. Cause of death was used to determine whether each patient died as a result of their thyroid cancer or another cause, or whether they were alive at the end of the follow-up period.
Covariates
Patient characteristics
Patient demographic variables were obtained from the SEER records, including age at the time of thyroid cancer diagnosis, sex, race, and ethnicity. Comorbidities were selected to represent the top leading noncancer causes of death in older adults aged 65 years and older in the United States and were identified from Medicare data using ICD-9-CM codes before thyroid cancer diagnosis (2). These comorbidities included heart disease (coronary artery disease, myocardial infarction, and heart failure), chronic lower respiratory disease (chronic obstructive pulmonary disease, asthma), cerebrovascular disease (stroke, ischemic cerebrovascular disease, and bilateral carotid artery occlusion), Alzheimer's disease, and diabetes mellitus.
Tumor characteristics
SEER data were used to obtain information on tumor characteristics, including histology (papillary, follicular, Hürthle, medullary, anaplastic, and other), tumor size (categorized as ≤1 cm, >1 and ≤2 cm, >2 and ≤4 cm, >4 cm, and unknown) and SEER stage (categorized as localized, regional, distant, and unknown).
Statistical analysis
We analyzed the time to event (i.e., death from other causes or death from thyroid cancer) by performing regression analysis of competing risks using cumulative incidence functions. The competing risks considered were death from other causes and death from thyroid cancer. Patients who were alive at the end of the follow-up period were considered to have censored survival times. We used a semiparametric proportional hazards model for the subdistribution hazards proposed by Fine and Gray to determine the association between patient and tumor characteristics and time to event. This approach takes into account the competing causes of death in thyroid cancer patients and allows estimation of the impact of covariates on the outcome of interest (20–23).
All statistical analyses were performed using SAS 9.4 software. A two-tailed p < 0.05 was considered statistically significant.
Results
A total of 21,509 thyroid cancer patients were included in the study with a median follow-up of 50 months (range 0–191 months). Median follow-up differed by thyroid cancer type with more aggressive types, such as anaplastic and medullary thyroid cancer, having shorter median follow-up times (2 and 44 months, respectively), and less aggressive types, such as papillary thyroid cancer, having longer median follow-up times (55 months). Table 1 shows the characteristics of the study population, including demographics and tumor characteristics. Patients had a median age of 72 years (range 66–106). The majority of patients were female (69.5%) and white (79.3%), with approximately one-third having heart disease (35.4%), chronic lower respiratory disease (31.9%), or diabetes mellitus (34.9%). Three-fourths of the patients had papillary thyroid cancer. Overall, 4168 (19.4%) patients died of other causes and 2644 (12.3%) patients died of thyroid cancer during the study period.
Table 1.
Characteristics of Patients with Thyroid Cancer (N = 21,509)
| Patient characteristics | N (%) |
|---|---|
| Age at diagnosis (years) | |
| 66–69 | 6991 (32.5) |
| 70–74 | 6209 (28.9) |
| 75–79 | 4240 (19.7) |
| 80–84 | 2461 (11.4) |
| ≥85 | 1608 (7.5) |
| Sex | |
| Female | 14,957 (69.5) |
| Male | 6552 (30.5) |
| Race | |
| White | 17,057 (79.3) |
| Black | 1517 (7.1) |
| Other | 2935 (13.6) |
| Ethnicity | |
| Non-Hispanic | 19,394 (90.2) |
| Hispanic | 2115 (9.8) |
| Comorbidities | |
| Heart disease | 7609 (35.4) |
| Chronic lower respiratory disease | 6856 (31.9) |
| Cerebrovascular disease | 3259 (15.2) |
| Alzheimer's disease | 435 (2.0) |
| Diabetes mellitus | 7510 (34.9) |
| Tumor characteristics | |
| Histology | |
| Papillary | 16,323 (75.9) |
| Follicular | 1690 (7.9) |
| Hürthle cell | 1033 (4.8) |
| Medullary | 589 (2.7) |
| Anaplastic | 695 (3.2) |
| Other | 1179 (5.5) |
| Tumor size (cm) | |
| ≤1 | 6697 (31.1) |
| >1 and ≤2 | 4195 (19.5) |
| >2 and ≤4 | 4379 (20.4) |
| >4 | 3813 (17.7) |
| Unknown | 2425 (11.3) |
| SEER stage | |
| Localized | 13,488 (62.7) |
| Regional | 4522 (21.0) |
| Distant | 2653 (12.4) |
| Unknown | 846 (3.9) |
SEER, Surveillance, Epidemiology, and End Results.
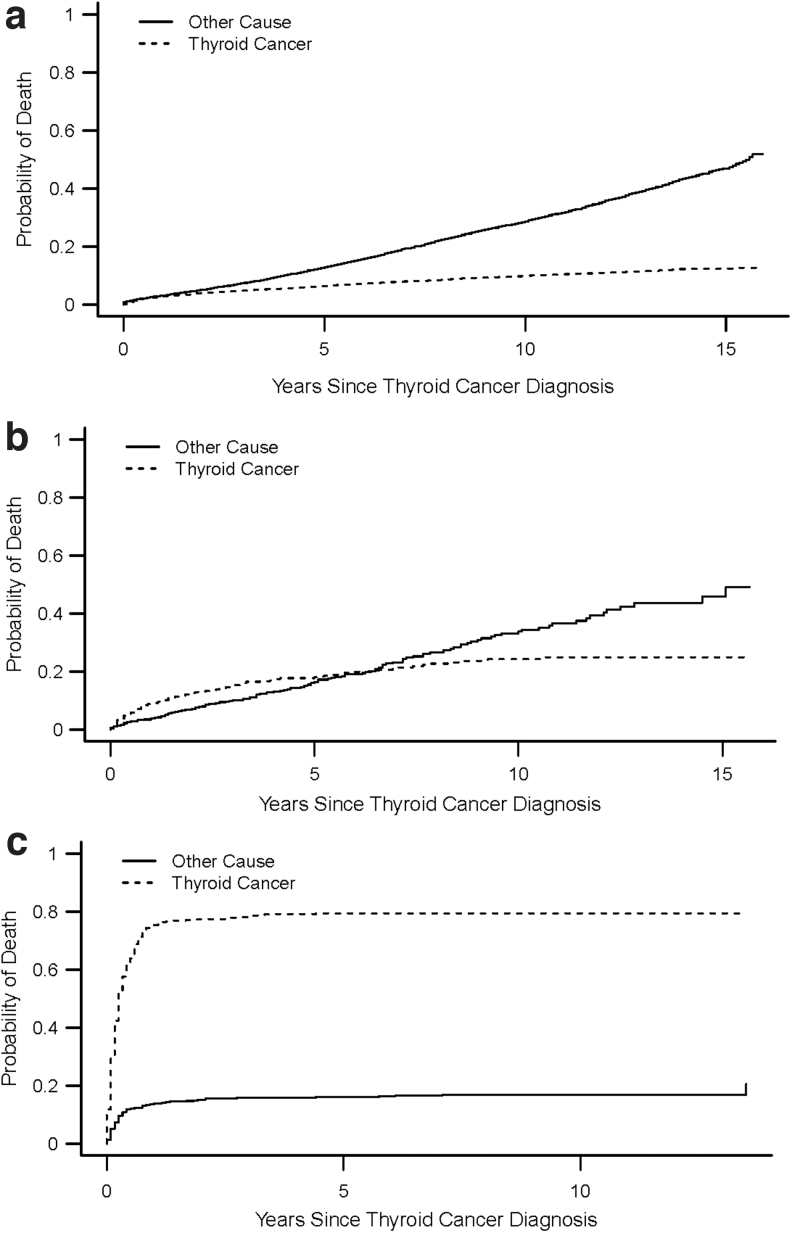
Figure 1a–c shows the crude cumulative incidence curves demonstrating cause-specific death in differentiated thyroid cancer, medullary thyroid cancer, and anaplastic thyroid cancer patients over time. Figure 1a demonstrates that a higher proportion of patients with differentiated thyroid cancer die of other causes over time. For patients with medullary thyroid cancer, a higher proportion die from thyroid cancer initially, but after 6.25 years the proportion of patients dying from other causes is higher (Fig. 1b). In contrast, a higher proportion of anaplastic thyroid cancer patients die from thyroid cancer than from other causes over time, with rate of death being highest in the first year after diagnosis (Fig. 1c).
FIG. 1.
(a–c) Cumulative incidence curves demonstrating cause-specific death in differentiated thyroid cancer patients (a), medullary thyroid cancer patients (b), and anaplastic thyroid cancer patients (c).
Results from the competing risk hazards regression analysis are shown in Table 2. Older age was associated with both a greater probability of death from other causes (e.g., age ≥85 compared with age 66–69 years, hazards ratio [HR] 4.01; 95% confidence interval [CI 3.55–4.54]) and death from thyroid cancer (e.g., age ≥85 compared with age 66–69 years, HR 1.96; CI [1.69–2.26]). Male sex (HR 1.47; CI [1.37–1.57], compared with female sex), black race (HR 1.30; CI [1.16–1.46], compared with white race) and presence of comorbidities (e.g., heart disease, HR 1.34; CI [1.25–1.44]; chronic lower respiratory disease, HR 1.25; CI [1.17–1.34]) were associated with death from other causes. Tumor characteristics such as histology (e.g., anaplastic compared with papillary, HR 5.51; CI [4.82–6.31]), tumor size (e.g., >4 cm compared with ≤1 cm, HR 3.35; CI [2.71–4.15]) and stage (e.g., distant SEER stage compared with localized, HR 12.65; CI [10.91–14.66]) correlated with death from thyroid cancer.
Table 2.
Competing Risk Analyses for Death in Thyroid Cancer Patients
| Death from other causes, HR [95% CI] | Death from other causes, p-value | Death from thyroid cancer, HR [95% CI] | Death from thyroid cancer, p-value | |
|---|---|---|---|---|
| Age at diagnosis (years) | ||||
| 66–69 | Ref. | Ref. | ||
| 70–74 | 1.47 [1.33 to 1.61] | <0.001 | 1.21 [1.07 to 1.36] | 0.003 |
| 75–79 | 2.23 [2.03 to 2.46] | <0.001 | 1.32 [1.16 to 1.50] | <0.001 |
| 80–84 | 3.32 [3.00 to 3.68] | <0.001 | 1.61 [1.40 to 1.85] | <0.001 |
| ≥85 | 4.01 [3.55 to 4.54] | <0.001 | 1.96 [1.69 to 2.26] | <0.001 |
| Sex | ||||
| Female | Ref. | Ref. | ||
| Male | 1.47 [1.37 to 1.57] | <0.001 | 1.00 [0.91 to 1.09] | 0.922 |
| Race | ||||
| White | Ref. | Ref. | ||
| Black | 1.30 [1.16 to 1.46] | <0.001 | 0.91 [0.77 to 1.08] | 0.298 |
| Other | 0.77 [0.70 to 0.86] | <0.001 | 1.00 [0.89 to 1.13] | 0.950 |
| Ethnicity | ||||
| Non-Hispanic | Ref. | Ref. | ||
| Hispanic | 1.03 [0.92 to 1.16] | 0.595 | 1.03 [0.90 to 1.17] | 0.697 |
| Comorbidities | ||||
| Heart disease | 1.34 [1.25 to 1.44] | <0.001 | 0.95 [0.86 to 1.05] | 0.308 |
| Chronic lower respiratory disease | 1.25 [1.17 to 1.34] | <0.001 | 0.99 [0.90 to 1.09] | 0.782 |
| Cerebrovascular disease | 1.09 [1.00 to 1.18] | 0.053 | 0.88 [0.78 to 1.01] | 0.065 |
| Alzheimer's disease | 1.72 [1.44 to 2.05] | <0.001 | 1.14 [0.87 to 1.49] | 0.343 |
| Diabetes mellitus | 1.14 [1.06 to 1.21] | <0.001 | 0.97 [0.88 to 1.07] | 0.571 |
| Histology | ||||
| Papillary | Ref. | Ref. | ||
| Follicular | 1.04 [0.94 to 1.16] | 0.442 | 1.29 [1.12 to 1.48] | <0.001 |
| Hürthle cell | 0.92 [0.81 to 1.05] | 0.199 | 1.49 [1.25 to 1.78] | <0.001 |
| Medullary | 1.05 [0.88 to 1.26] | 0.599 | 1.96 [1.61 to 2.38] | <0.001 |
| Anaplastic | 0.59 [0.47 to 0.74] | <0.001 | 5.51 [4.82 to 6.31] | <0.001 |
| Other | 0.85 [0.72 to 0.99] | 0.040 | 3.01 [2.65 to 3.43] | <0.001 |
| Tumor size (cm) | ||||
| ≤1 | Ref. | Ref. | ||
| >1 and ≤2 | 1.00 [0.91 to 1.10] | 0.983 | 1.26 [0.99 to 1.60] | 0.059 |
| >2 and ≤4 | 1.03 [0.94 to 1.13] | 0.519 | 2.35 [1.91 to 2.90] | <0.001 |
| >4 | 1.14 [1.04 to 1.27] | 0.009 | 3.35 [2.71 to 4.15] | <0.001 |
| Unknown | 1.22 [1.09 to 1.38] | 0.001 | 4.10 [3.29 to 5.11] | <0.001 |
| SEER stage | ||||
| Localized | Ref. | Ref. | ||
| Regional | 0.94 [0.86 to 1.01] | 0.101 | 4.59 [3.98 to 5.31] | <0.001 |
| Distant | 0.81 [0.72 to 0.91] | <0.001 | 12.65 [10.91 to 14.66] | <0.001 |
| Unknown | 0.93 [0.78 to 1.11] | 0.440 | 5.04 [4.11 to 6.17] | <0.001 |
CI, 95% confidence interval; HR, hazards ratio.
Discussion
The results of this population-based study of a large cohort of older adults with thyroid cancer demonstrate that likelihood of death from other causes versus thyroid cancer differs by specific thyroid cancer type. Importantly, likelihood of dying of other causes correlated with patient characteristics, including specific comorbidities, while likelihood of dying from thyroid cancer correlated with tumor characteristics, such as histology, tumor size, and stage. These findings are relevant to providing personalized care for thyroid cancer patients as they highlight the importance of considering patient context when balancing benefits and harms of thyroid cancer diagnosis and management in older adults.
Prior studies evaluating other cancer types have assessed competing causes of death and the effect of comorbidities; however, this topic is understudied and perhaps even more relevant for thyroid cancer, a cancer with low disease-specific mortality (24–30). Although some studies on thyroid cancer have attempted to assess death from other etiologies, many of these prior studies primarily used single institution data (30,31), did not assess the effect of comorbidities (32,33), or only focused on number of comorbidities and not specific types of comorbidities (30,34,35). In a population-based study of 29,225 patients diagnosed with thyroid cancer tumors ≤2 cm between 1988 and 2003 using data from SEER, Yang et al. developed a nomogram based on a competing risks model to predict the probability of death for patients with thyroid cancer (32). They found a nearly twofold higher risk of dying from causes other than thyroid cancer (32). However, Yang et al. did not consider specific comorbidities. In contrast to this prior study (32), we used a more contemporary cohort, focused on a high-risk population (i.e., older adults), included all tumor sizes, and most importantly, evaluated the effect of specific comorbidities such as heart disease and chronic lower respiratory disease, which are more relevant to risk of death in the clinical setting.
Our finding that likelihood of death from other causes versus thyroid cancer differs by cancer type and specific comorbidities highlights the importance of considering patient context, including pre-existing comorbidities, in addition to risk of death from thyroid cancer when deciding management in older adults. This finding provides clinical context for future patients and appeals to physicians to continuously reassess competing risks over time in older adults with thyroid cancer. This is important as for older patients who are more likely to survive several years from their thyroid cancer, death from other causes may become more relevant shifting focus of patient management. Therefore, we propose a personalized approach, instead of a “one size fits all” treatment, when caring for older adults with thyroid cancer. This approach should take into consideration that physicians must balance the significance of prompt recognition of aggressive thyroid cancers more likely to be associated with increased disease-specific mortality against the indolent course of the majority of older patients with thyroid cancer.
Controversy remains as to whether age thresholds should guide medical decision making in cancer care, as older adults of similar age can have complex and heterogeneous health trajectories and functional status (36–40). However, much of this heterogeneity in outcome is secondary to some older adults having specific comorbidities that will lead to death and other older adults having very few comorbidities or comorbidities that have low risk of death (36,39–42). By assessing risk of death from thyroid cancer versus other causes in a population-based cohort of older adults, we elucidate the importance of understanding the entire clinical context and personalizing care to the patient. For example, older adults with high risk of death from heart disease or chronic lower respiratory disease may not benefit from additional work-up and evaluation of thyroid nodules, the precursor to thyroid cancer (31,43). In addition, if life expectancy from another pre-existing comorbidity is low, then the harms associated with thyroid cancer treatments such as external beam radiation should be balanced with the likelihood of improved overall survival. Overall, our findings suggest that understanding the influence of specific comorbid conditions on mortality can enable a better and more patient-centered assessment of prognosis for older thyroid cancer patients.
Strengths of our study include the population-based design with inclusion of a large cohort representative of older adults with thyroid cancer in the United States, a 98% registry completeness for incident cancers in SEER (18), the focus on specific comorbidities known to be common etiologies of death in older patients instead of simply using a crude count of number of comorbidities (2), and the use of an analytic approach that accounted for the presence of competing risks. As actual prognosis measures have been traditionally unavailable for older patients and those with comorbidities, mainly because cancer registries do not routinely report comorbid conditions on cancer patients, we utilized two complementary databases (SEER and Medicare) to address this limitation and longitudinally track patients until death. Study limitations include those inherent to studies using claims data, such as risk for coding errors and reporting bias. However, even though SEER acquires cause of death from death certificates that may be prone to inaccuracies, it has been shown that validity of cause of death in SEER is high (44). In addition, we excluded patients who had another cancer diagnosis first (i.e., a cancer other than thyroid cancer) since SEER only records cause of death from the primary cancer. Although this is a limitation, it likely leads to an underestimation of death due to other causes, further emphasizing the importance of understanding clinical context. Moreover, Medicare lacks information on the severity and duration of comorbidities. Since this study lacks detail on severity and duration of comorbidities, it is possible that life expectancy of individuals with specific comorbidities varies, and could have impacted physician decision making. Finally, we were not able to assess the effects of treating specific comorbidities on death.
Our study underscores the need for careful consideration of competing causes of death and presence of comorbidities when caring for older adults with thyroid cancer, in view of the excellent prognosis and relatively indolent course of most thyroid cancer tumors. Older adults with thyroid cancer represent a heterogeneous cohort of patients and incorporating competing risks of death and comorbidities in the decision-making process will help physicians better understand the patients' overall health status to pursue a balanced clinical individualized approach. Given the strong relevance of quality of life when considering any intervention in the geriatric population, our findings emphasize the need to reappraise which older patients are expected to benefit from thyroid cancer diagnosis and treatment, especially in the context of comorbidities that could substantially increase the risk of nonthyroid cancer death. Ultimately, our results can be used to develop decision models that account for competing causes of death, as a step toward personalized care and an aid for clinical decision making in older adults with thyroid cancer.
Acknowledgment
The authors thank Ms. Brittany Gay who assisted with article formatting and review.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study is supported by the Agency for Healthcare Research and Quality (AHRQ) Grant No. R01 HS024512 to principal investigator M.R.H. M.R.H. also receives funding from R01 CA201198 from the National Cancer Institute (NCI). M.P. receives funding from K08 AG049684 (NIA).
References
- 1. NIH National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Thyroid Cancer. Available at https://seer.cancer.gov/statfacts/html/thyro.html (accessed October 13, 2020).
- 2. CDC: 10 Leading Causes of Death by Age Group, United States. Available at https://www.cdc.gov/injury/wisqars/pdf/leading_causes_of_death_by_age_group_2018-508.pdf (accessed June 15, 2020).
- 3. Boltz MM, Hollenbeak CS, Schaefer E, Goldenberg D, Saunders BD. 2013. Attributable costs of differentiated thyroid cancer in the elderly Medicare population. Surgery 154:1363–1370. [DOI] [PubMed] [Google Scholar]
- 4. Tuggle CT, Park LS, Roman S, Udelsman R, Sosa JA. 2010. Rehospitalization among elderly patients with thyroid cancer after thyroidectomy are prevalent and costly. Ann Surg Oncol 17:2816–2823. [DOI] [PubMed] [Google Scholar]
- 5. Papaleontiou M, Hughes DT, Guo C, Banerjee M, Haymart MR. 2017. Population-based assessment of complications following surgery for thyroid cancer. J Clin Endocrinol Metab 102:2543–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vini L, Hyer SL, Marshall J, A'Hern R, Harmer C. 2003. Long-term results in elderly patients with differentiated thyroid carcinoma. Cancer 97:2736–2742. [DOI] [PubMed] [Google Scholar]
- 7. Haymart MR, Esfandiari NH, Stang MT, Sosa JA. 2017. Controversies in the management of low-risk differentiated thyroid cancer. Endocr Rev 38:351–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sosa JA, Mehta PJ, Wang TS, Boudourakis L, Roman SA. 2008. A population-based study of outcomes from thyroidectomy in aging Americans: at what cost? J Am Coll Surg 206:1097–1105. [DOI] [PubMed] [Google Scholar]
- 9. Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D'Agostino RB. 1994. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 331:1249–1252. [DOI] [PubMed] [Google Scholar]
- 10. Bauer DC, Ettinger B, Nevitt MC, Stone KL; Study of Osteoporotic Fractures Research Group. 2001. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med 134:561–568. [DOI] [PubMed] [Google Scholar]
- 11. Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA. 2004. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34:195–202. [DOI] [PubMed] [Google Scholar]
- 12. Satariano WA, Ragland DR. 1994. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med 120:104–110. [DOI] [PubMed] [Google Scholar]
- 13. Strasser-Weippl K, Goss PE 2013 Competing risks in low-risk breast cancer. Am Soc Clin Oncol Educ Book 32-9. DOI: 10.14694/EdBook_AM.2013.33.32. [DOI] [PubMed] [Google Scholar]
- 14. Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. 2014. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 120:1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Cancer Institute, Surveillance, Epidemiology, and End Results Program: Overview of the SEER Program. Available at https://seer.cancer.gov/about/overview.html (accessed October 13, 2020).
- 16. Moon M 1996. What Medicare has meant to older Americans. Health Care Financ Rev 18:49–59. [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Centers for Medicare & Medicaid Services. The Official U.S. Government Site for Medicare. Available at https://www.medicare.gov (accessed October 1, 2020).
- 18. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. 2002. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 40:IV-3-18. [DOI] [PubMed] [Google Scholar]
- 19. National Cancer Institute Surveillance, Epidemiology, and End Results Program (SEER): Cause-specific Death Classification. Available at https://seer.cancer.gov/causespecific/index.html (accessed September 4, 2020).
- 20. Putter H, Fiocco M, Geskus RB. 2007. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26:2389–2430. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC, Fine JP. 2017. Accounting for competing risks in randomized controlled trials: a review and recommendations for improvement. Stat Med 36:1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gray RJ 1988. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154. [Google Scholar]
- 23. Fine JP, Gray RJ. 1999. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509. [Google Scholar]
- 24. Howlader N, Mariotto AB, Woloshin S, Schwartz LM. 2014. Providing clinicians and patients with actual prognosis: cancer in the context of competing causes of death. J Natl Cancer Inst Monogr 2014:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. 2011. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol 29:1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daskivich TJ, Fan KH, Koyama T, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Stanford JL, Stroup AM, Litwin MS, Penson DF. 2013. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med 158:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Newschaffer CJ, Otani K, McDonald MK, Penberthy LT. 2000. Causes of death in elderly prostate cancer patients and in a comparison nonprostate cancer cohort. J Natl Cancer Inst 92:613–621. [DOI] [PubMed] [Google Scholar]
- 28. Tan KS, Eguchi T, Adusumilli PS. 2018. Competing risks and cancer-specific mortality: why it matters. Oncotarget 9:7272–7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. 2011. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst 103:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee YK, Hong N, Park SH, Shin DY, Lee CR, Kang SW, Lee J, Jeong JJ, Nam KH, Chung WY, Lee EJ. 2019. The relationship of comorbidities to mortality and cause of death in patients with differentiated thyroid carcinoma. Sci Rep 9:11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Z, Vyas CM, Van Benschoten O, Nehs MA, Moore FD Jr., Marqusee E, Krane JF, Kim MI, Heller HT, Gawande AA, Frates MC, Doubilet PM, Doherty GM, Cho NL, Cibas ES, Benson CB, Barletta JA, Zavacki AM, Larsen PR, Alexander EK, Angell TE. 2018. Quantitative analysis of the benefits and risk of thyroid nodule evaluation in patients >/ = 70 years old. Thyroid 28:465–471. [DOI] [PubMed] [Google Scholar]
- 32. Yang L, Shen W, Sakamoto N. 2013. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol 31:468–474. [DOI] [PubMed] [Google Scholar]
- 33. Wang K, Xu J, Li S, Liu S, Zhang L. 2019. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer among patients with papillary thyroid microcarcinoma. Cancer Med 8:6977–6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, Meyer JE. 2017. Causes of death among cancer patients. Ann Oncol 28:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karadaghy OA, Kallogjeri D, Piccirillo JF. 2017. Development of a new clinical severity staging system for patients with nonmetastatic papillary thyroid carcinoma. JAMA Otolaryngol Head Neck Surg 143:1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walter LC, Covinsky KE. 2001. Cancer screening in elderly patients: a framework for individualized decision making. JAMA 285:2750–2756. [DOI] [PubMed] [Google Scholar]
- 37. Lansdorp-Vogelaar I, Gulati R, Mariotto AB, Schechter CB, de Carvalho TM, Knudsen AB, van Ravesteyn NT, Heijnsdijk EA, Pabiniak C, van Ballegooijen M, Rutter CM, Kuntz KM, Feuer EJ, Etzioni R, de Koning HJ, Zauber AG, Mandelblatt JS. 2014. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med 161:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Force USPST, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., Garcia FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. 2016. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 315:2564–2575. [DOI] [PubMed] [Google Scholar]
- 39. Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. 2014. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci 69:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Force USPST, Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, Doubeni CA, Ebell M, Epling JW Jr., Kemper AR, Krist AH, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Siu AL, Tseng CW. 2018. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 319:1901–1913. [DOI] [PubMed] [Google Scholar]
- 41. Stenholm S, Westerlund H, Head J, Hyde M, Kawachi I, Pentti J, Kivimaki M, Vahtera J. 2015. Comorbidity and functional trajectories from midlife to old age: the Health and Retirement Study. J Gerontol A Biol Sci Med Sci 70:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schoenborn NL, Massare J, Park R, Pollack CE, Choi Y, Boyd CM. 2020. Clinician perspectives on overscreening for cancer in older adults with limited life expectancy. J Am Geriatr Soc 68:1462–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papaleontiou M, Haymart MR. 2012. Approach to and treatment of thyroid disorders in the elderly. Med Clin North Am 96:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu C, Xing Y, Cormier JN, Chang GJ. 2009. The validity of cause of death coding within the Surveillance, Epidemiology, and End Results (SEER) Registry. Journal of Clinical Oncology 27(15_suppl):6544–6544. [Google Scholar]