Abstract
Background
Primary membranous nephropathy (PMN) is a common cause of nephrotic syndrome in adults. Without treatment, approximately 30% of patients will experience spontaneous remission and one third will have persistent proteinuria. Approximately one‐third of patients progress toward end‐stage kidney disease (ESKD) within 10 years. Immunosuppressive treatment aims to protect kidney function and is recommended for patients who do not show improvement of proteinuria by supportive therapy, and for patients with severe nephrotic syndrome at presentation due to the high risk of developing ESKD. The efficacy and safety of different immunosuppressive regimens are unclear. This is an update of a Cochrane review, first published in 2004 and updated in 2013.
Objectives
The aim was to evaluate the safety and efficacy of different immunosuppressive treatments for adult patients with PMN and nephrotic syndrome.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 1 April 2021 with support from the Cochrane Kidney and Transplant Information Specialist using search terms relevant to this review. Studies in the Register were identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials (RCTs) investigating effects of immunosuppression in adults with PMN and nephrotic syndrome were included.
Data collection and analysis
Study selection, data extraction, quality assessment, and data synthesis were performed using Cochrane‐recommended methods. Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) and 95% CI for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
Sixty‐five studies (3807 patients) were included. Most studies exhibited a high risk of bias for the domains, blinding of study personnel, participants and outcome assessors, and most studies were judged unclear for randomisation sequence generation and allocation concealment.
Immunosuppressive treatment versus placebo/no treatment/non‐immunosuppressive treatment
In moderate certainty evidence, immunosuppressive treatment probably makes little or no difference to death, probably reduces the overall risk of ESKD (16 studies, 944 participants: RR 0.59, 95% CI 0.35 to 0.99; I² = 22%), probably increases total remission (complete and partial) (6 studies, 879 participants: RR 1.44, 95% CI 1.05 to 1.97; I² = 73%) and complete remission (16 studies, 879 participants: RR 1.70, 95% CI 1.05 to 2.75; I² = 43%), and probably decreases the number with doubling of serum creatinine (SCr) (9 studies, 447 participants: RR 0.46, 95% CI 0.26 to 0.80; I² = 21%). However, immunosuppressive treatment may increase the number of patients relapsing after complete or partial remission (3 studies, 148 participants): RR 1.73, 95% CI 1.05 to 2.86; I² = 0%) and may lead to a greater number experiencing temporary or permanent discontinuation/hospitalisation due to adverse events (18 studies, 927 participants: RR 5.33, 95% CI 2.19 to 12.98; I² = 0%). Immunosuppressive treatment has uncertain effects on infection and malignancy.
Oral alkylating agents with or without steroids versus placebo/no treatment/steroids
Oral alkylating agents with or without steroids had uncertain effects on death but may reduce the overall risk of ESKD (9 studies, 537 participants: RR 0.42, 95% CI 0.24 to 0.74; I² = 0%; low certainty evidence). Total (9 studies, 468 participants: RR 1.37, 95% CI 1.04 to 1.82; I² = 70%) and complete remission (8 studies, 432 participants: RR 2.12, 95% CI 1.33 to 3.38; I² = 37%) may increase, but had uncertain effects on the number of patients relapsing, and decreasing the number with doubling of SCr. Alkylating agents may be associated with a higher rate of adverse events leading to discontinuation or hospitalisation (8 studies 439 participants: RR 6.82, 95% CI 2.24 to 20.71; I² = 0%). Oral alkylating agents with or without steroids had uncertain effects on infection and malignancy.
Calcineurin inhibitors (CNI) with or without steroids versus placebo/no treatment/supportive therapy/steroids
We are uncertain whether CNI with or without steroids increased or decreased the risk of death or ESKD, increased or decreased total or complete remission, or reduced relapse after complete or partial remission (low to very low certainty evidence). CNI also had uncertain effects on decreasing the number with a doubling of SCr, temporary or permanent discontinuation or hospitalisation due to adverse events, infection, or malignancy.
Calcineurin inhibitors (CNI) with or without steroids versus alkylating agents with or without steroids
We are uncertain whether CNI with or without steroids increases or decreases the risk of death or ESKD. CNI with or without steroids may make little or no difference to total remission (10 studies, 538 participants: RR 1.01, 95% CI 0.89 to 1.15; I² = 53%; moderate certainty evidence) or complete remission (10 studies, 538 participants: RR 1.15, 95% CI 0.84 to 1.56; I² = 56%; low certainty evidence). CNI with or without steroids may increase relapse after complete or partial remission. CNI with or without steroids had uncertain effects on SCr increase, adverse events, infection, and malignancy.
Other immunosuppressive treatments
Other interventions included azathioprine, mizoribine, adrenocorticotropic hormone, traditional Chinese medicines, and monoclonal antibodies such as rituximab. There were insufficient data to draw conclusions on these treatments.
Authors' conclusions
This updated review strengthened the evidence that immunosuppressive therapy is probably superior to non‐immunosuppressive therapy in inducing remission and reducing the number of patients that progress to ESKD. However, these benefits need to be balanced against the side effects of immunosuppressive drugs. The number of included studies with high‐quality design was relatively small and most studies did not have adequate follow‐up. Clinicians should inform their patients of the lack of high‐quality evidence.
An alkylating agent (cyclophosphamide or chlorambucil) combined with a corticosteroid regimen had short‐ and long‐term benefits, but this was associated with a higher rate of adverse events.
CNI (tacrolimus and cyclosporin) showed equivalency with alkylating agents however, the certainty of this evidence remains low.
Novel immunosuppressive treatments with the biologic rituximab or use of adrenocorticotropic hormone require further investigation and validation in large and high‐quality RCTs.
Plain language summary
Immunosuppressive treatment for adults with idiopathic membranous nephropathy
What is the issue?
Primary membranous nephropathy (PMN) is an autoimmune disease, where the body's immune system attacks the kidneys. The term "primary" is used to describe membranous nephropathy that is not caused by another disease in the body. PMN is a leading cause of nephrotic syndrome in adults. Nephrotic syndrome is a condition, where the membrane of the kidney is damaged and becomes permeable for proteins. Primary membranous nephropathy is diagnosed through findings in a kidney biopsy and the presence of nephrotic syndrome.
PMN is not harmful in about one‐third of patients, who will have a spontaneous "complete remission", which means that the disease will resolve by itself. However, about another one third will experience spontaneous remission but will have some protein in the urine that continues with normal kidney function. These patients usually only require supportive treatments that do not interact with the immune system. Without treatment, about 15% to 50% of patients progress to end‐stage kidney disease (ESKD) within 10 years.
In some patients, PMN can be severe or continues to get worse even after using 6 months of supportive treatments. In these patients, extra treatment that dampens the activity of the immune system may be used to reduce damage to the kidney. It is not clear which of these treatment(s) is the most helpful and what side effects can occur. Therefore, the duration and intensity of immunosuppressive treatment need to be balanced against possible side effects. There are different classes of drugs used in immunosuppressive therapy. These drugs may or may not be combined with corticosteroids (drugs based on the body's stress response hormone cortisol).
What did we do?
We searched the Cochrane Kidney and Transplant specialised register up to 1 April 2021. We have combined studies to compare different treatment regimens with immunosuppressive therapy to assess which treatments help to treat patients with PMN and nephrotic syndrome with the least side effects.
What did we find?
This review identified sixty‐five studies with 3807 patients. Different types of immunosuppressive treatment include alkylating agents (cyclophosphamide and chlorambucil), calcineurin inhibitors (tacrolimus and cyclosporine), antimetabolites (mycophenolate mofetil, azathioprine), biologicals (e.g. rituximab) and adrenocorticotropic hormone. These drugs may or may not be combined with corticosteroids (e.g. prednisone), which also suppresses the immune system. After combining the results of available studies together, we found that compared with no treatment, supportive treatment or steroids alone, the use of immunosuppressive treatment probably reduced the number of patients who progressed to ESKD by about 40% and increased the number of patients that achieved complete remission. However, immunosuppressive treatment may lead to more adverse events, which can cause treatment to be stopped or lead to the patients needing to go to hospital.
The different drugs that can be used in the immunosuppressive treatment were also examined in our review. We found that alkylating agents probably increases complete remission but may lead to more adverse events. We are uncertain whether alkylating agents increase infection or cancer. Based on the currently available evidence, the effectiveness of using calcineurin inhibitors is still unclear, but there is low certainty of the evidence, that CNI may lead to similar remission rates compared to alkylating agents.
Furthermore, other treatment options such as mycophenolate mofetil, adrenocorticotropic hormone, rituximab and others have only been examined in a few studies. There is not enough data to draw final conclusions on the use of these treatments in adults with PMN and nephrotic syndrome.
Conclusions
The treatment of patients with PMN and nephrotic syndrome with immunosuppressive therapy compared to no treatment or supportive therapy alone probably protects the kidney but may increase side effects. A combination of immunosuppressive therapy with steroids may decrease disease activity and the use of alkylating agent combined with steroids probably has the short‐term and long‐term benefits of limiting damage to the kidney. Other therapies such as calcineurin inhibitors, mycophenolate mofetil, rituximab and adrenocorticotropic hormone have less certainty regarding their safety and effectiveness from these studies.
Summary of findings
Background
Description of the condition
Membranous nephropathy is the most common cause of primary nephrotic syndrome in adults, and particularly affects elderly patients (Cameron 1996; Hofstra 2012; Vendemia 2001). Approximately 75% of membranous nephropathy cases are considered primary/idiopathic (Abe 1986) with the other 25% due to secondary causes, such as infections, autoimmune diseases, certain medications, or malignant diseases. Primary membranous nephropathy (PMN) shows a benign or indolent course in about one‐third of patients, with a high rate of spontaneous remission in about 30% of patients (Polanco 2010). Approximately one third develops nephrotic syndrome but maintain normal kidney function. Despite this, 15% to 50% of patients who do not receive immunosuppressive treatment progress to end‐stage kidney disease (ESKD) within 10 years (Deegens 2005; Ponticelli 2010; Waldman 2009). Recent findings of anti‐phospholipase‐A2‐receptor‐antibodies (anti‐PLA2R) (Beck 2009) and anti‐thrombospondin type‐1 domain‐containing protein 7A‐antibodies (anti‐THSD7A) (Tomas 2014) have improved understanding of the autoimmune pathophysiology of PMN. PMN is caused by the subepithelial formation of immune complex deposits in the kidney's glomerular basement membrane (GBM) (Lai 2015). The exact mechanisms behind this remain unclear, however, there are a number of presumptive hypotheses. Firstly, systemically pre‐formed immune‐complexes may deposit in the GBM, suggesting a similar pathophysiological mechanism as in lupus‐associated nephritis (Lai 2015). Secondly, circulating antigens (such as during infection) might be targeted by antibodies, thus forming immune complexes that deposit in this site. this has especially been observed in infection‐related (i.e. secondary) forms of membranous nephropathy, such as during infection with hepatitis B virus (Bhimma 2004; Lai 2000; Lai 2015). Thirdly, based on Heymann's model of nephritis (Heymann 1959), podocyte‐antigens (such as megalin) may lead to binding of autoantibodies to the GBM's podocytes which cause the subepithelial deposits that are present in PMN (Tramontano 2006). However, thus far, this connection has not been clearly established through the extraction of anti‐megalin‐antibodies in PMN. Finally, the complement system and genetic factors might contribute to the autoimmune aetiology of PMN. So far, two associated genomic loci have been identified: chromosome 2q24 encodes for the anti‐PLA2R‐receptor auto‐antibody and chromosome 6p21 encodes for HLADQA1, which might play pivotal roles in the pathogenesis of PMN (Bullich 2014; Stanescu 2011).
In a kidney biopsy, diagnosis of membranous nephropathy can be established by the presence of subepithelial immune deposits. In light‐microscopy, a thickened, prominent GBM with "spikes" (local thickening of the membrane due to matrix reactions to the deposits) may indicate PMN, however electron microscopy and immunofluorescence are superior techniques in establishing the diagnosis of PMN. Immunofluorescence may show staining for PLA2R, complement (C3) and immunoglobulin (Fogo 2015; Lai 2015), whereas electron microscopy allows pathological staging of PMN into four stages according to the classification first suggested by Churg and Ehrenreich (Ehrenreich 1976). Electron microscopy may show "extensive foot process effacement and subepithelial deposits with increasing matrix spike reaction with advancing disease. As the disease progresses, an increase in matrix production can envelop these deposits and lead to a "laddering appearance" (Fogo 2015). The diagnosis of PMN is one of exclusion and secondary causes of membranous nephropathy must be ruled out.
Description of the intervention
Several immunosuppressive treatments have been used to treat patients with PMN and nephrotic syndrome, including corticosteroids, alkylating agents (chlorambucil and cyclophosphamide (CPA)), azathioprine (AZA), and mizoribine. More recently, other treatments such as calcineurin inhibitors (CNI) (cyclosporine (CSA) and tacrolimus (TAC)), mycophenolate mofetil (MMF), adrenocorticotropic hormone (ACTH), Tripterygium wilfordii (a traditional Chinese immunosuppressive medicine), and therapeutic approaches such as biologics (rituximab and eculizumab) and high dose gamma‐globulin have also been considered for PMN. However, due to the uncertain risk‐benefit profile of immunosuppressive treatment and the lack of definite evidence on altering the long‐term course of the disease, the most appropriate therapy remains unclear.
Currently, "Kidney Disease: Improving Global Outcomes" (KDIGO) guidelines suggest supportive therapy for all patients with PMN and immunosuppressive therapy should be considered only in patients with urinary protein exceeding 3.5 g/24 hours and eGFR ≤ 60 mL/min/1.73 m², or in patients with one risk for disease progression is present. Initial suggested therapy consists of a six‐month course of alternating monthly cycles of oral and intravenous (IV) corticosteroids and CPA or TAC or rituximab as alternatives (KDIGO 2020).
How the intervention might work
Given the autoimmune aetiology of PMN, immunosuppressive treatment is used to decrease the overall activity of the immune system, leading to reduced damage to the kidneys. Most immunosuppressive drugs suppress the immune system more broadly, whereas some therapies such as rituximab aim to target specific parts of the immune system.
Why it is important to do this review
In the 2004 Cochrane review (Schieppati 2004), 19 studies with 1025 participants were included. This review found that immunosuppressive treatments could increase complete or partial remission. However, the long‐term effects of immunosuppressive treatments on definite endpoints such as death (any cause) or the prevention of ESKD could not be demonstrated. Immunosuppressive treatments were found to lead to a significantly higher risk of severe adverse events.
In the 2014 update of the Cochrane review (Chen 2014), 39 studies with 1825 participants overall were included, which further strengthened the certainty of the evidence. New treatments have more recently been investigated in randomised controlled trials (RCTs) for the treatment of PMN, and studies have reported on the use of new therapies such as monoclonal antibodies in patients with PMN and traditional Chinese medicine (Shenqi particles) (Chen 2013e). Most notably, rituximab (GEMRITUX 2017; MENTOR 2015) have been tested in studies for PMN.
Objectives
Our objective was to assess the evidence and evaluate the safety and efficacy of immunosuppressive treatments for adult patients with PMN and nephrotic syndrome. The following questions relating to the management of PMN and nephrotic syndrome were addressed:
Is immunosuppressive therapy superior to non‐immunosuppressive therapy?
If so, which immunosuppressive agent/s is the most effective and safe in treating patients with IMN and nephrotic syndrome?
What routes of administration and duration of therapy should be used?
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs that assessed the effects of immunosuppressive treatments in adult patients with IMN and nephrotic syndrome.
Types of participants
Inclusion criteria
Adults (at least 18 years of age)
Diagnosis of PMN, established by kidney biopsy (and possibly be further proven by detection of anti‐PLA2R‐ or anti‐THSD7‐antibodies). Prior to 2009, membranous nephropathy was determined by kidney biopsy. Other underlying causes of membranous nephropathy were ruled out clinically to establish the diagnosis of primary membranous nephropathy
Diagnosis of nephrotic syndrome as defined by the authors in each study. In studies that included > 50% non‐nephrotic patients, analyses were restricted to nephrotic patients only. In the absence of an explicit definition of nephrotic syndrome, the cut‐off value of proteinuria above 3.5 g/24 hours was used.
Exclusion criteria
Secondary forms of membranous nephropathy were excluded. We also excluded studies where it was impossible to identify how many adult PMN patients had nephrotic syndrome.
Types of interventions
We considered the following immunosuppressive treatments: corticosteroids, alkylating agents (chlorambucil and CPA), CNI (CSA and TAC), sirolimus, MMF, and synthetic ACTH. Other less commonly studied immunosuppressive regiments such as Tripterygium wilfordii (a traditional Chinese immunosuppressive medicine); Shenqi particles (a traditional Chinese immunosuppressive medicine), leflunomide, AZA, mizoribine, methotrexate, and levamisole were also investigated. Furthermore, high dose gamma‐globulin and biologics (rituximab and eculizumab) were included in this review.
Non‐immunosuppressive treatments were excluded: drugs aimed to reduce proteinuria through inhibition of the renin‐angiotensin system (e.g. angiotensin‐converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) or aliskiren); drugs aimed to correct dyslipidaemia (e.g. statins); anti‐aldosterone drugs (e.g. spironolactone); nonsteroidal anti‐inflammatory drugs (e.g. indomethacin).
Types of outcome measures
Primary outcomes
Death (any cause)
ESKD (requiring kidney replacement therapy) at the last follow‐up
Complete or partial (total) remission, complete remission alone, and partial remission alone at different time points and at the last follow‐up.
Complete and partial remission of nephrotic syndrome was assessed according to the definition provided in each study. In the absence of an explicit definition, complete remission was defined as proteinuria < 0.3 g/24 hours and with a normal or stable serum creatinine (SCr) (within 50% of baseline value). In the absence of an explicit definition, partial remission was defined as a reduction in proteinuria by at least 50% and remaining between 0.3 to 3.5 g/24 hours with a normal or stable SCr (within 50% of baseline value).
Secondary outcomes
Relapse (recurrence of disease) after initial remission
100% increase (doubling) in SCr from baseline at different time points and at the last follow‐up
Quality of Life (as measured by study investigators).
The following side effects (toxicity) of treatments were considered.
-
Adverse events (as defined by the study investigators)
Temporary or permanent discontinuation or hospitalisation due to adverse events
Infection
Malignancy.
The following continuous kidney function outcomes were analysed at the end of follow‐up.
SCr (μmol/L)
Serum albumin (g/L)
Glomerular filtration rate (GFR) (mL/min/1.73 m²)
Proteinuria (g/24 hours)
50% increase in SCr from baseline at different time points and at the last follow‐up.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 1 April 2021 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals, and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Handsearching proceedings of major rheumatology conferences.
Contacting relevant individuals/organisations seeking information about unpublished or incomplete studies.
Data collection and analysis
Selection of studies
A search was performed to identify relevant studies. In this update, study selection was done by two authors (GW, TvG). The titles and abstracts of retrieved citations, and where necessary the full‐text articles, were independently evaluated by two authors (GW, TvG). Disagreements were resolved by consulting a third author (DT). Where duplicated reports of the same study were confirmed, the initial first complete publication was selected (the index publication) and was the primary data source, but any other additional prior or subsequent reports were also included. These additional prior or subsequent reports containing supplementary outcome data (such as longer‐term follow‐up, or different outcomes) also contributed to the review and meta‐analysis.
Data extraction and management
Data extraction was carried out independently by two authors (GW, TvG) using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. In case of duplicates, reports were grouped together and the publication with the most complete data was included. When relevant outcomes were only published in earlier versions, these data were used. Any differences between published versions were highlighted. A third author (DT) resolved these discrepancies. If needed, further details were requested by written correspondence to principal investigators and any relevant information obtained in this manner was included in this review. We also contacted principal investigators for missing data whenever necessary.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors (GW, TvG) using the risk of bias assessment tool (Higgins 2011) (see Appendix 2). Publication bias was especially investigated for the comparison of immunosuppressive treatments versus no immunosuppression.
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
Dichotomous data
For dichotomous outcomes (death, ESKD, total remission, complete remission, partial remission, relapse, doubling of SCr, 50% increase in SCr, adverse events, infection, malignancy) results were expressed as risk ratios (RR) with 95% confidence intervals (CI). RR was the selected effect measure because it describes the multiplication of risk and is relatively easy to understand, is a bounded measure of effect that provides a consistent estimate of effect.
Continuous data
When a continuous scale of measurement was used (eGFR, SCr, 24‐hour proteinuria, quality of life), the mean difference (MD) with 95% CI was chosen or the standardised mean difference (SMD) was considered if a different scale was adopted or SMDs were reported in a publication.
Unit of analysis issues
In studies with multiple intervention arms we considered the following:
If different classes (for example, CPA, or MMF versus steroids), we included each treatment group in a separate meta‐analysis, ensuring that we did not include outcome data for the control group participants more than once in a single meta‐analysis
If interventions were the same therapy (for example Mizoribine 150 mg once/day versus Mizoribine 50 mg three times/day), we compared the two intervention arms with each other as in the study.
Dealing with missing data
Missing data were assessed for each included study. For missing participants due to drop‐out, intention‐to‐treat analyses (ITT) were performed if the data were reported elsewhere or were provided by principal investigators in response to our requests for additional information. For missing statistics such as standard deviations, these studies were not considered in the meta‐analysis unless the missing data could be appropriately imputed using methods recommended by the Cochrane Collaboration. We included missing participants in the analyses. Issues of missing data and imputation methods (for example last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
In one study that reported median and interquartile ranges (GEMRITUX 2017), we calculated mean and standard deviations, using the formula suggested by Hozo 2005 for larger sample sizes, given the sample sizes of both groups in the study exceeded 25. We used the Vassarstats calculator (http://vassarstats.net/median_range.html), which is based on the Hozo formula.
We also contacted principal investigators to request missing data where possible.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot, by examining the direction of the effect estimates and the overlap of confidence intervals. Heterogeneity was then further assessed by using the Chi² test, with a p‐value less than 0.1 used to denote statistical significance, and with the I² statistic calculated to measure the proportion of total variation in the estimates of treatment effect that was due to heterogeneity rather than chance (Higgins 2011). A guide to the interpretation of I² values (Higgins 2003) is as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test, or a confidence interval for I²).
Assessment of reporting biases
We planned to assess for publication bias for the primary outcomes. We made every attempt to minimise publication bias by including unpublished studies (for example, including abstract‐only publications and searching online trial registries). To assess publication bias we used funnel plots of the log odds ratio (OR) (effect versus standard error of the effect size) when a sufficient number of studies were available (10 studies or more) (Harbord 2009; Higgins 2011). For the analysis and interpretation of the funnel plots, other reasons for asymmetry besides publication bias were considered (differences in methodological quality and true heterogeneity in intervention effects). However, the limited amount of study data did not enable meaningful interpretation.
Data synthesis
Data were abstracted from individual studies and then pooled for summary estimates using a random‐effects model. The random‐effects model was chosen because it provides a more conservative estimate of effect in the presence of known or unknown potential heterogeneity (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses are hypothesis‐generating rather than hypothesis testing and should be treated with caution. Subgroup analysis was used to explore possible sources of heterogeneity (e.g. participants and interventions). Heterogeneity among participants could be related to age and disease severity. Heterogeneity in treatments could be related to the route, dose, and duration of therapies in the studies. Subgroup analysis was also performed to explore the following covariates: the language of publication, source of funding and sample size calculation as well as anti‐PLA2R‐levels. However, there was limited data reported to undertake these subgroup analyses, in particular the reporting of anti‐PLA2R‐levels.
Sensitivity analysis
We considered the following sensitivity analyses in order to explore the influence of the following factors.
Repeating the analysis excluding unpublished studies or low‐quality studies based on the assessment of the risk of bias
-
Repeating the analysis excluding studies that were of insufficient follow‐up for the primary outcome
Death: 10‐year follow‐up
ESKD:10‐year follow‐up
Complete remission:2‐year follow‐up
Repeating the analysis excluding any very long or very large study to determine the extent to which they unduly influenced the results.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also includes an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as to the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of the within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, the precision of effect estimates and risk of publication bias (Schunemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Death
ESKD
Total remission (complete or partial)
Complete remission
Recurrence (relapse) of disease
Doubling of SCr from baseline
-
Adverse events
Temporary or permanent discontinuation or hospitalisation due to adverse events
Infection
Malignancy
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
We searched the Cochrane Kidney and Transplant Register of Studies 1 April 2021 and identified 82 new reports. After full‐text assessment, 57 new studies were identified; 24 new included studies (40 reports), 13 new studies (14 reports) were excluded, and 16 new ongoing studies were identified. Four new studies are awaiting assessment (recently completed but no data available). We also identified eight new reports of existing included and excluded studies.
In this update, we also reassessed the existing studies.
Included studies: 1 study moved to excluded (not RCT)
Excluded studies: 1 study moved to included; 10 studies deleted (not RCT, wrong population)
Studies awaiting classification: 5 studies moved to included; 1 study deleted
Ongoing studies: 8 studies moved to included studies; 3 studies move to awaiting classification.
A total of 65 studies (127 reports, 3807 randomised participants; Figure 1) were included, 25 excluded, 5 are awaiting assessment, and there are 20 ongoing studies.
1.
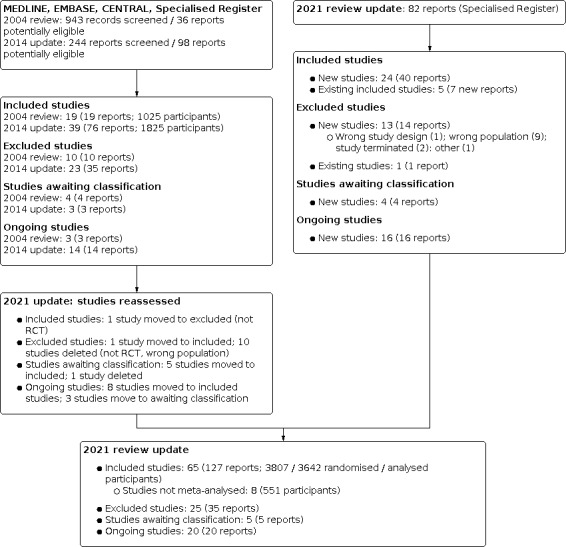
2021 review update: study selection flow diagram.
Included studies
A total of 65 studies (3807 randomised participants) investigating immunosuppressive therapy in adults with primary membranous nephropathy and nephrotic syndrome were included in this updated review (Figure 1). The median sample size was 57 (range 9 to 190) patients. The median follow‐up time was 26 months (range 6 months to 12 years). Unpublished data were provided by the authors of two studies (Braun 1995; CYCLOMEN 1994). Eight studies (Appel 2002; Austin 1996a; Dyadyk 2001a; Hladunewich 2014; Sahay 2002; Stegeman 1994; Sun 2014; Zhang 2015d) could not be included in the meta‐analyses as we were unable to extract the necessary data. One study was prematurely terminated due to a low accrual rate (Stegeman 1994).
Four studies only investigated patients with deteriorating kidney function (Cattran 1995; CYCLOMEN 1994; Falk 1992; Reichert 1994). Some studies did not report whether or not they included patients with deteriorating kidney function.
Five studies involved patients who were resistant to corticosteroids monotherapy (Koshikawa 1993; Saito 2014; Shibasaki 2004) or corticosteroids plus alkylating agents (Cattran 2001; Naumovic 2011). Eleven studies included patients who had previously received immunosuppressive treatment before inclusion in the study or who had previously received immunosuppressive treatments if a defined wash‐out period of not receiving any immunosuppressive treatment was completed (Cattran 1989; Chan 2007; Chen 2010a; Donadio 1974; Jha 2007; Liu 2009b; Murphy 1992; Praga 2007; Reichert 1994; Shibasaki 2004; Tiller 1981).
Studies were arranged into the following comparison groups.
Corticosteroids versus placebo/no treatment
Immunosuppressive treatments ± steroids versus placebo/no treatment/non‐immunosuppressive treatments
Immunosuppressive treatments ± steroids versus steroids monotherapy
CPA + leflunomide + steroid versus CPA + steroid
Oral alkylating agents ± steroids versus placebo/no treatment/supportive treatment/steroids
CPA + steroids versus chlorambucil + steroids
Early (immediate) CPA + steroids versus late (when SCr increased > 25%) CPA + steroids
CPA + leflunomide + steroids versus leflunomide + steroids
MMF + CNI versus CNI
CNI ± steroids versus placebo/no treatment/supportive treatment/steroids
CNI ± steroids versus alkylating agents ± steroids
Short‐course tacrolimus + steroids short‐course versus long‐course tacrolimus + steroids
Cyclosporine + steroids versus steroids alone
Cyclosporine + steroids (3.0 mg/kg, once/day) versus cyclosporine + steroids (1.5 mg/kg, twice/day)
Cyclosporine + steroids versus tacrolimus + steroids
Cyclosporin versus AZA
AZA ± steroids versus no treatment
MMF versus no treatment/supportive therapy
MMF ± steroids versus alkylating agents ± steroids
MMF ± steroids versus CNI ± steroids
ACTH versus no treatment
ACTH versus alkylating agents + steroids
Mizoribine ± steroids versus placebo/no treatment/corticosteroids
Mizoribine: 150 mg (once/day) versus 50 mg (3 times/day)
Rituximab + supportive therapy versus supportive therapy alone
Rituximab versus cyclosporine
Traditional Chinese medicine versus immunosuppressive therapy (Shenqi particles; Tripterygium wilfordii)
The following comparisons were planned however no data were available.
Two non‐steroid immunosuppressive agents versus one non‐steroid immunosuppressive agent
CPA + leflunomide + steroid versus CPA + steroids
ACTH 40 IU versus ACTH 80 IU
Studies awaiting classification
Five studies are awaiting assessment (NCT00302523; NCT00518219; NCT01093157; NCT01386554; NCT01845688) and will be assessed in a future update when the methods and results become available.
Ongoing studies
We identified 20 ongoing studies which will be assessed in a future update (Chen 2020; ChiCTR‐INR‐15007440; ChiCTR‐INR‐17011400; ChiCTR‐INR‐17012070; ChiCTR‐INR‐17012212; ChiCTR‐IPR‐16008344; ChiCTR‐IPR‐16008527; ChiCTR‐IPR‐17011386; ChiCTR‐IPR‐17011702; ChiCTR‐TRC‐11001144; CTRI/2017/05/008648; EudraCT2007‐005410‐39; HIGHNESS 2011; ISRCTN17977921; ISRCTN70791258; MMF‐STOP‐IMN 2017; NCT02173106; RI‐CYCLO 2020; STARMEN 2015; UMIN000001099).
Excluded studies
Twenty‐five studies (35 records) were excluded. Reasons for exclusion were: wrong study design or conduct (Branten 1998; Michail 2004; Sharma 2009; Sun 2008); wrong or mixed population (Ambalavanan 1996; Badri 2013; Black 1970; ChiCTR‐IPR‐14005366; Edefonti 1988; Heimann 1987; Krasnova 1998; Lagrue 1975; Li 2012e; Liu 2016c; Majima 1990; MRCWP 1971; Nand 1997; Plavljanic 1998; Ponticelli 1993a; Sharpstone 1969; Xu 2011; Yang 2016a); study was terminated (EudraCT2011‐000242‐38; NCT01762852); and the status of one study is unknown 10 years after initial registration (ChiCTR‐TRC‐09000539).
Risk of bias in included studies
See Figure 2.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Twenty‐seven studies (48%) specified appropriate methods for random sequence generation and were considered to be at low risk of bias. Appropriate methods of randomisation were not reported in 39 studies (51%). These studies were thus considered to have an unclear risk of bias. One study (1%) was considered to have a high risk of bias for random sequence generation.
Allocation concealment
Twenty studies (31%) reported appropriate allocation concealment methods and were considered to be at low risk of bias, while the remaining45 studies (69%) did not provide details about allocation concealment and were considered to have an unclear risk of bias.
Blinding
Performance bias
Appropriate procedure relating to the blinding of participants was reported in five studies (8%) and were considered to be at low risk of bias. Five studies (5%) were considered to have an unclear risk of bias, and the remaining 55 studies (84%) did not perform adequate blinding of participants and were considered to be at high risk of bias.
Detection bias
Adequate blinding of personnel and outcome assessors was reported in four studies (6%) and were considered to be at low risk of bias. Fifty‐five studies (85%) were considered to have an unclear risk of bias, and the remaining six studies (9%) did not perform adequate blinding of personnel and outcome assessors and were considered to be at high risk of bias.
Incomplete outcome data
Forty‐four studies (68%) were considered to be at low risk of bias; 11 studies (17%) were considered to have an unclear risk of bias, and 10 studies (15%) were considered to be at high risk of bias.
Selective reporting
Forty‐seven studies (72%) were considered to be at low risk of bias and, three studies (5%) were considered to have an unclear risk of bias. Fifteen studies (23%) were considered to be at high risk of bias.
Publication bias
It has been recommended that tests for publication bias should be used only when at least 10 studies are included in the meta‐analysis (Harbord 2009). Given the wide variety of different treatments tested in studies, comparisons did not include more than 10 studies, so that publication bias could not be assessed properly (Figure 3).
3.
Publication bias of comparison: 1 Immunosuppressive treatment versus placebo/no treatment/non‐immunosuppressive treatments, outcome: 1.1 death or risk of ESKD (Harbord test) (A); 1.6 complete or partial remission (Harbord test) (B); 1.1 death or risk of ESKD (funnel plot) (C); and 1.6 complete or partial remission (funnel plots) (D).
Other potential sources of bias
Twenty‐nine studies (45%) were considered to be at low risk of bias; twenty‐five studies (38%) were considered to have an unclear risk of bias. The remaining 11 studies (17%) were assessed as having a high risk of bias using GRADE in this section as there were concerns about potential financial interest or other significant conflicts of interest. Four studies were primarily funded and executed by private companies. These studies were evaluated to be at high risk of bias. Five studies received substantial financial and/or technical support or donated medicines from private companies. These studies were rated as low risk of bias if no employees of private companies were directly involved in the execution of the trial, data analysis and/or publication. Funding from foundations, not‐for‐profit and philanthropic organisations were not considered to increase the risk of bias. The underlying rationale has been detailed in the risk of bias tables in the Characteristics of included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Immunosuppressive treatment versus placebo/no treatment/non‐immunosuppressive supportive treatment.
| Immunosuppressive treatment versus placebo/no treatment/non‐immunosuppressive supportive treatment for primary membranous nephropathy in adults with nephrotic syndrome | |||||
| Patient or population: primary membranous nephropathy in adults with nephrotic syndrome Setting: primary care Intervention: immunosuppressive treatment Comparison: control (placebo/no treatment/non‐immunosuppressive supportive treatment) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with immunosuppressive treatment | ||||
| Death at final follow‐up (range: 9 months to 12 years) |
40 per 1000 | 30 per 1000 (14 to 64) | RR 0.73 (0.34 to 1.59) | 944 (16) | ⊕⊕⊕⊖ Moderate1 |
| End‐stage kidney disease at final follow‐up (range: 9 months to 12 years) |
124 per 1000 | 73 per 1000 (43 to 123) | RR 0.59 (0.35 to 0.99) | 944 (16) | ⊕⊕⊕⊖ Moderate1 |
| Total remission (complete or partial) at final follow‐up (range: 6 months to 12 years) |
337 per 1000 | 485 per 1000 (355 to 663) | RR 1.44 (1.05 to 1.97) | 879 (16) | ⊕⊕⊕⊖ Moderate1 |
| Complete remission at final follow‐up (range: 6 months to 12 years) | 127 per 1000 | 216 per 1000 (133 to 349) | RR 1.70 (1.05 to 2.75) | 879 (16) | ⊕⊕⊕⊖ Moderate1 |
| Recurrence of disease (relapse) at final follow‐up (range: 21 months to 12 years) |
114 per 1000 | 181 per 1000 (102 to 316) |
RR 1.73 (1.05 to 2.86) |
310 (3) | ⊕⊕⊖⊖ Low1,2 |
| 100% increase in serum creatinine at final follow‐up (range: 12 months to 12 years) |
299 per 1000 | 138 per 1000 (78 to 240) |
RR 0.46 (0.26 to 0.80) |
447 (8) | ⊕⊕⊕⊖ Moderate1 |
| Adverse events: temporary/permanent discontinuation or hospitalisation at final follow‐up (range: 6 months to 12 years) |
2 per 1000 | 13 per 1000 (5 to 31) | RR 5.33 (2.19 to 12.98) | 927 (16) | ⊕⊕⊕⊖ Moderate1 |
| Adverse events: infection at 3 years | 54 per 1000 | 159 per 1000 (37 to 682) |
RR 2.95 (0.69 to 12.61) |
106 (1) | ⊕⊖⊖⊖ Very low1,3 |
| Adverse events: malignancy at final follow‐up (range: 17 months to 3 years) |
13 per 1000 | 14 per 1000 (2 to 120) |
RR 1.03 (0.12 to 9.14) |
182 (2) | ⊕⊖⊖⊖ Very Low1,3 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level: studies generally unclear or high risk of bias for many domains
2Downgraded one level: serious imprecision ‐ due few events and participants in the included studies
3 Downgraded two levels: very serious imprecision ‐ only one study and very wide confidence intervals indicating appreciable benefit and harm
4Downgraded one level: serious imprecision ‐ very wide confidence intervals indicating appreciable benefit and harm
Summary of findings 2. Oral alkylating agents ± steroids versus placebo/no treatment/steroids.
| Oral alkylating agents ±steroids versus placebo/no treatment/steroids for primary membranous nephropathy in adults with nephrotic syndrome | |||||
|
Patient or population: primary membranous nephropathy in adults with nephrotic syndrome
Setting: primary care Intervention: oral alkylating agents ± steroids Comparison: control (placebo/no treatment/steroids) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with alkylating agents ± steroids | ||||
| Death at final follow‐up (range: 9 months to 12 years |
37 per 1000 | 28 per 1000 (9 to 84) | RR 0.76 (0.25 to 2.30) | 440 (7) | ⊕⊕⊖⊖ Low1,2 |
| End‐stage kidney disease at final follow‐up (range: 9 months to 12 years) |
146 per 1000 | 61 per 1000 (35 to 108) | RR 0.42 (0.24 to 0.74) | 537 (9) | ⊕⊕⊕⊖ Moderate1 |
| Total remission (complete or partial) at final follow‐up (range: 6 months to 12 years) |
411 per 1000 | 604 per 1000 (459 to 803) | RR 1.37 (1.04 to 1.82) | 468 (9) | ⊕⊕⊕⊖ Moderate1 |
| Complete remission at final follow‐up (range: 9 months to 12 years) |
171 per 1000 | 362 per 1000 (227 to 577) | RR 2.12 (1.33 to 3.38) | 432 (8) | ⊕⊕⊕⊖ Moderate1 |
| Recurrence of disease (relapse) at final follow‐up (range: 21 months to 12 years) |
190 per 1000 | 152 per 1000 (76 to 307) |
RR 0.80 (0.40 to 1.61) |
161 (3) | ⊕⊖⊖⊖ Very low1,3 |
| 100% increase in serum creatinine at final follow‐up (range: 12 months to 12 years) |
329 per 1000 | 194 per 1000 (99 to 382) |
RR 0.59 (0.30 to 1.16) |
332 (7) | ⊕⊕⊕⊖ Moderate1 |
| Adverse events ‐ temporary/permanent discontinuation or hospitalisation at final follow‐up (range: 9 months to 12 years) |
5 per 1000 | 33 per 1000 (11 to 101) | RR 1.44 (0.96 to 2.15 |
184 (3) | ⊕⊕⊖⊖ Low1,4 |
| Adverse events ‐ infection at 3 years | 54 per 1000 | 91 per 1000 (16 to 511) |
RR 1.68 (0.30 to 9.45) |
70 (1) | ⊕⊖⊖⊖ 1,3 Very low |
| Adverse events ‐ malignancy at final follow‐up (range: 3 to 4 years) |
12 per 1000 | 19 per 1000 (2 to 146) |
RR 1.63 (0.21 to 12.37) |
199 (2) | ⊕⊖⊖⊖ Very low1,3 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Study limitations: studies generally unclear or high risk of bias for many domains
2 Imprecision: estimate of effect includes negligible difference and considerable benefit and harm
3Downgraded two levels: very serious imprecision ‐ only one study and very wide confidence intervals indicating appreciable benefit and harm
4 Serious imprecision (few participants and few events)
Summary of findings 3. Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids.
| Calcineurin inhibitors ± steroids versus to placebo/no treatment/supportive treatment/steroids for primary membranous nephropathy in adults with nephrotic syndrome | |||||
|
Patient or population: primary membranous nephropathy in adults with nephrotic syndrome
Setting: primary care Intervention: calcineurin inhibitors ± steroids Comparison: control (placebo/no treatment/supportive treatment/steroids) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with CNI | ||||
| Death at final follow‐up (range: 9 to 60 months) |
15 per 1000 | 25 per 1000 (7 to 92) | RR 1.69 (0.46 to 6.14) | 296 (7) | ⊕⊖⊖⊖ Verylow1,2,3 |
| End‐stage kidney disease at final follow‐up (range: 9 to 60 months) |
82 per 1000 | 97 per 1000 (44 to 263) | RR 1.18 (0.54 to 2.60) | 296 (7) | ⊕⊖⊖⊖ Verylow1,3,4 |
| Total remission at final follow‐up (range: 9 to 60 months) |
416 per 1000 | 503 per 1000 (258 to 989) | RR 1.21 (0.62 to 2.38) | 206 (5) | ⊕⊕⊖⊖ Low1,5 |
| Complete remission at final follow‐up (range: 9 to 60 months) |
146 per 1000 | 156 per 1000 (74 to 327) | RR 1.07 (0.51 to 2.24) | 206 (5) | ⊕⊕⊖⊖ Low1,5 |
| Recurrence of disease (relapse) at final follow‐up (range: 18 to 60 months) |
259 per 1000 | 404 per 1000 (205 to 801) |
RR 1.56 (0.79 to 3.09) |
92 (2) | ⊕⊖⊖⊖ VeryLow1,4 |
| 100% increase in SCr at final follow‐up (range: 18 to 60 months) |
178 per 1000 | 149 per 1000 (66 to 331) |
RR 0.84 (0.37 to 1.86) |
117 (2) | ⊕⊖⊖⊖ VeryLow1,4 |
| Adverse events ‐ temporary or permanent discontinuation/hospitalisation at final follow‐up (range: 9 to 60 months) |
0/63 | 2/98** | RR 5.45 (0.29 to 101.55) |
156 (5) | ⊕⊖⊖⊖ VeryLow1,4 |
| Adverse events ‐ infection at 36 months | 54 per 1000 | 222 per 1000 (51 to 976) |
RR 4.11 (0.94 to 18.06) |
73 (1) | ⊕⊖⊖⊖ VeryLow1,4 |
| Adverse events ‐ malignancy at 36 months | 0/38 | 2/69** | RR 2.79 (0.14 to 56.57) |
107 (1) | ⊕⊖⊖⊖ VeryLow1,2 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Event rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the control group CI: Confidence interval; CNI: calcineurin inhibitors; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Study limitations: studies generally unclear or high risk of bias for many domains
2 Very serious imprecision (2 grades): few events, and estimate of effect includes negligible difference and considerable benefit and harm
3 Serious Indirectness: insufficient follow‐up for the outcome to occur ≤ 10 years
4 Very serious imprecision: few events and estimate of effect includes negligible difference and considerable benefit and harm
5 Serious imprecision: estimate of effect includes negligible difference and considerable benefit and harm
6 Serious imprecision: only one study
Summary of findings 4. Calcineurin inhibitors ± steroids versus alkylating agents ± steroids.
| Calcineurin inhibitors ± steroids versus alkylating agents ± steroids for primary membranous nephropathy in adults with nephrotic syndrome | |||||
|
Patient or population: primary membranous nephropathy in adults with nephrotic syndrome
Setting: primary care Intervention: calcineurin inhibitors ± steroids Comparison: alkylating agents ± steroids | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with alkylating agents ± steroids | Risk with CNI ± steroids | ||||
| Death at final follow‐up (range: 9 to 60 months) |
38 per 1000 | 34 per 1000 (13 to 89) | RR 0.90 (0.35 to 2.34) | 394 (7) | ⊕⊖⊖⊖ Verylow 1,2,3 |
| End‐stage kidney disease at final follow‐up (range: 9 to 60 months) |
15 per 1000 | 36 per 1000 (10 to 134) | RR 2.40 (0.64 to 9.01) | 293 (5) | ⊕⊖⊖⊖ Very low 1,2,3 |
| Total remission at final follow‐up (range: 9 to 60 months) |
784 per 1000 | 791 per 1000 (697 to 901) | RR 1.01 (0.89 to 1.15) | 529 (10) | ⊕⊕⊕⊖ Moderate 1 |
| Complete remission at final follow‐up (range: 9 to 60 months) |
429 per 1000 | 493 per 1000 (360 to 669) | RR 1.15 (0.84 to 1.56) | 533 (10) | ⊕⊕⊖⊖ Low 4,5 |
| Recurrence of disease (relapse) at final follow‐up (range: 9 to 18 months) |
61 per 1000 | 130 per 1000 (43 to 390) |
RR 2.13 (0.71 to 6.37) |
295 (6) | ⊕⊕⊖⊖ Low 1,2 |
| 100% increase in SCr at final follow‐up (range: 9 to 60 months) |
136 per 1000 | 95 per 1000 (41 to 226) |
RR 0.70 (0.30 to 1.67) |
132 (2) | ⊕⊖⊖⊖ Verylow 1,2,3 |
| Adverse events ‐ temporary or permanent discontinuation/hospitalisation at final follow‐up (range: 9 to 12 months) |
42 per 1000 | 60 per 1000 (13 to 278) |
RR 1.43 (0.31 to 6.67) | 151 (3) | ⊕⊖⊖⊖ VeryLow 1,6 |
| Adverse events ‐ infection (range: 9 to 30 months) |
223 per 1000 | 191 per 1000 (96 to 381) |
RR 0.86 (0.43 to 1.71) |
552 (9) | ⊕⊕⊖⊖ Low 1,2 |
| Adverse events ‐ malignancy (range 30 to 36 months |
33 per 1000 | 6 per 1000 (0 to 121) |
RR 0.18 (0.01 to 3.69) |
127 (2) | ⊕⊖⊖⊖ VeryLow1,6 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CNI: calcineurin inhibitors; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Study limitations (studies generally at unclear or high risk of bias for many domains)
2 Serious imprecision: estimate of effect includes negligible difference and considerable benefit and harm
3 Serious indirectness: Follow‐up less than 10 years
4 Serious study limitations: Unclear randomisation sequence generation and allocation concealment
5 Serious inconsistency: point estimates vary widely, and the magnitude of statistical heterogeneity was high, with I2 =53%
6 Very serious imprecision (2 grades): few events, and estimate of effect includes negligible difference and considerable benefit and harm
See Summary of findings tables for the main comparisons:
Table 1: Immunosuppressive treatments versus placebo/no treatment/non‐immunosuppressive treatments
Table 2: Oral alkylating agent with or without steroids versus placebo/no treatment/steroids
Table 3: CNI versus placebo/no treatment/supportive therapy/steroids
Table 4: CNI with or without steroids versus alkylating agents with or without steroids.
1) Corticosteroids versus placebo or no treatment
Four studies (Cameron 1990; Cattran 1989; Coggins 1979; Donadio 1974) investigated monotherapy with corticosteroids versus placebo or no treatment.
Compared to placebo or no treatment, corticosteroids may make little or no difference to death (Analysis 1.1 (3 studies. 33 participants): RR 0.59, 95% CI 0.11 to 3.23, I² = 32%), ESKD (Analysis 1.2 (3 studies 333 participants): RR 0.83, 95% CI 0.35 to 1.98; I² = 17%), total (complete or partial) remission (Analysis 1.3 (3 studies, 295 participants): RR 1.15, 95% CI 0.58 to 2.27; I² = 69%), complete remission (Analysis 1.4 (2 studies, 192 participants): RR 0.64, 95% CI 0.29 to 1.42; I² = 0%), or partial remission (Analysis 1.5 (2 studies, 192 participants): RR 1.34, 95% CI 0.34 to 5.21; I² = 75%)
1.1. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 1: Death
1.2. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 2: ESKD (dialysis/transplantation)
1.3. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 3: Complete or partial remission
1.4. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 4: Complete remission
1.5. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 5: Partial remission
Compared to placebo or no treatment, corticosteroids may make little or no difference to the number with doubling of SCr (Analysis 1.6.1 (3 studies, 120 participants): RR 0.41, 95% CI 0.11 to 1.53; I² = 19%) or adverse events (Analysis 1.7 (2 studies, 175 participants): RR 1.04, 95% CI 0.11 to 9.82; I² = 0%).
1.6. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 6: Increase in serum creatinine
1.7. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 7: Adverse events
It is unclear whether corticosteroids compared to placebo or no treatment improve kidney function (Analysis 1.8; Analysis 1.9; Analysis 1.10). The number relapsing after complete or partial remission was not reported.
1.8. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 8: Final serum creatinine
1.9. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 9: Final CrCl
1.10. Analysis.

Comparison 1: Corticosteroids versus placebo/no treatment, Outcome 10: Final proteinuria
2) Immunosuppressive treatment versus placebo, no treatment or non‐immunosuppressive treatment
Eighteen studies investigated immunosuppressive treatment versus placebo/no treatment/non‐immunosuppressive treatments (Arnadottir 2006; Badri 2013; Braun 1995; Cattran 1989; Coggins 1979; CYCLOMEN 1994; Donadio 1974; Dussol 2008; GEMRITUX 2017; Imbasciati 1980; Jha 2007; Koshikawa 1993; Kosmadakis 2010; Murphy 1992; Praga 2007; Sharma 2009; Shibasaki 2004; Silverberg 1976).
Compared to placebo/no treatment/non‐immunosuppressive treatment, immunosuppressive treatment probably makes little or no difference to death (Analysis 2.1 (16 studies, 944 participants): RR 0.73, 95% CI 0.34 to 1.59; I² = 0%; moderate certainty evidence) but may reduce the overall risk of ESKD by 40% (Analysis 2.2 (16 studies, 944 participants): RR 0.59, 95% CI 0.35 to 0.99; I² = 22%; moderate certainty evidence) at final follow‐up (9 months to 12 years), and in studies with follow‐up of ≥ 10 years immunosuppressive treatment probably decreases ESKD by 71% (Analysis 2.2.2 (2 studies, 185 participants): RR 0.29, 95% CI 0.13 to 0.63; I² = 0%).
2.1. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 1: Death
2.2. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 2: ESKD (dialysis/transplantation)
Compared to placebo/no treatment/non‐immunosuppressive treatment, immunosuppressive treatment probably increases the number who achieve total remission (Analysis 2.3 (16 studies, 879 participants): RR 1.44, 95% CI 1.05 to 1.97; I² = 73%; moderate certainty evidence) and complete remission (Analysis 2.4 (16 studies, 879 participants): RR 1.70, 95% CI 1.05 to 2.75; I² = 43%; moderate certainty evidence), and may increase the number achieving partial remission (Analysis 2.5 (16 studies, 879 participants): RR 1.36, 95% CI 0.93 to 1.98; I² = 60%). The number relapsing after complete or partial remission may increase with immunosuppressive treatment (Analysis 2.6 (3 studies, 148 participants): RR 1.73, 95% CI 1.05 to 2.86; I² = 0%; low certainty evidence).
2.3. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 3: Complete or partial remission
2.4. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 4: Complete remission
2.5. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 5: Partial remission
2.6. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 6: Relapse after complete or partial remission
Immunosuppressive treatment probably decreases the number with doubling of SCr (Analysis 2.7 (9 studies, 447 participants): RR 0.46, 95% CI 0.26 to 0.80; I² = 21%; moderate certainty of the evidence), but may increase the number experiencing temporary or permanent discontinuation/hospitalisation due to adverse events (Analysis 2.9 (18 studies, 927 participants): RR 5.33, 95% CI 2.19 to 12.98; I² = 0%; low certainty evidence). Immunosuppressive treatment has uncertain effects on infection and malignancy.
2.7. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 7: 100% increase in serum creatinine
2.9. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 9: Temporary or permanent discontinuation/hospitalisation due to adverse events
Immunosuppressive treatment may improve GFR (Analysis 2.14), proteinuria (Analysis 2.15), but not SCr (Analysis 2.13).
2.14. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 14: Final GFR [mL/min/1.73 m²]
2.15. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 15: Final proteinuria
2.13. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 13: Final serum creatinine
3) Immunosuppressive treatments with or without steroids versus steroids alone
Five studies (Ahmed 1994; Falk 1992; Hasegawa 2017; Pahari 1993; Ponticelli 1992) compared immunosuppressive treatment with steroids alone.
Immunosuppressive treatment may make little or no difference to death (Analysis 3.1) or ESKD (Analysis 3.2), but may increase the number achieving total remission (Analysis 3.3 (5 studies, 241 participants): (RR 1.47, 95% CI 1.19 to 1.82; I² = 0%) and complete remission (Analysis 3.4 (4 studies, 205 participants): RR 1.89, 95% CI 1.34 to 2.65; I² = 0%). There were no differences between studies that had a follow‐up of less than 2 years and studies with 2 years or more of follow‐up.
3.1. Analysis.

Comparison 3: Immunosuppressive treatment ± steroids versus steroids, Outcome 1: Death
3.2. Analysis.

Comparison 3: Immunosuppressive treatment ± steroids versus steroids, Outcome 2: ESKD (dialysis/transplantation)
3.3. Analysis.

Comparison 3: Immunosuppressive treatment ± steroids versus steroids, Outcome 3: Complete or partial remission
3.4. Analysis.

Comparison 3: Immunosuppressive treatment ± steroids versus steroids, Outcome 4: Complete remission
Immunosuppressive treatment had uncertain effects on doubling of SCr (Analysis 3.7 (3 studies, 97 participants): RR 1.19, 95% CI 0.52 to 2.71; I² = 0%), adverse events (Analysis 3.9; Analysis 3.8), and relapse after complete or partial remission (Analysis 3.6).
3.7. Analysis.

Comparison 3: Immunosuppressive treatment ± steroids versus steroids, Outcome 7: Increase in serum creatinine
3.9. Analysis.

Comparison 3: Immunosuppressive treatment ± steroids versus steroids, Outcome 9: Adverse events
3.8. Analysis.

Comparison 3: Immunosuppressive treatment ± steroids versus steroids, Outcome 8: Temporary or permanent discontinuation/hospitalisation due to adverse events
3.6. Analysis.

Comparison 3: Immunosuppressive treatment ± steroids versus steroids, Outcome 6: Relapse after complete or partial remission
4) Cyclophosphamide plus leflunomide plus steroids versus cyclophosphamide plus steroids
Liu 2015e reported CPA plus leflunomide plus steroids may increase complete remission compared to leflunomide plus steroids (Analysis 4.1 (1 study. 48 participants): RR 1.50, 95% CI 1.04 to 2.17). No other outcomes were reported.
4.1. Analysis.

Comparison 4: Cyclophosphamide + leflunomide + steroid versus cyclophosphamide + steroid, Outcome 1: Complete remission
5) Oral alkylating agents with or without steroids versus placebo/no treatment/supportive treatment/steroids
Nine studies (Ahmed 1994; Braun 1995; Donadio 1974; Hasegawa 2017; Imbasciati 1980; Jha 2007; Kosmadakis 2010; Pahari 1993) investigated oral alkylating agents with or without steroids versus placebo/no treatment/supportive treatments/steroids only.
Oral alkylating agents may have little or no effects on death (Analysis 5.1 (7 studies, 440 participants): RR 0.76, 95% CI 0.25 to 2.30; I² = 0%; low certainty evidence) compared with no treatment/placebo/steroids alone but probably decreases ESKD at final follow‐up (Analysis 5.2 (9 studies, 537 participants): RR 0.42, 95% CI 0.24 to 0.74; I² = 0%; moderate certainty evidence). In moderate certainty evidence, total and complete remission may increase using oral alkylating agents with or without steroids (Analysis 5.3 (9 studies, 468 participants): RR 1.37, 95% CI 1.04 to 1.82; I² = 70%; Analysis 5.4 (8 studies, 432 participants): RR 2.12, 95% CI 1.33 to 3.38; I² = 37%), but uncertain effects on partial remission (Analysis 5.5 (8 studies, 432 participants): RR 0.94, 95% CI 0.57 to 1.55; I² = 57%) and the number relapsing after complete or partial remission (Analysis 5.7). There was no evidence of difference for studies with < 10 years follow‐up and the study with ≥ 10 years follow‐up.
5.1. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 1: Death
5.2. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 2: ESKD (dialysis/transplantation)
5.3. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 3: Complete or partial remission
5.4. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 4: Complete remission
5.5. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 5: Partial remission
5.7. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 7: Relapse after complete or partial remission
It is uncertain whether oral alkylating agents decrease the doubling of SCr (Analysis 5.6.1 (7 studies, 332 participants): RR 0.59, 95% CI 0.30 to 1.16; I² = 42%; low certainty evidence). Oral alkylating agents compared with placebo/no treatment/steroids may increase temporary or permanent discontinuation or hospitalisation due to adverse events (Analysis 5.8 (8 studies 439 participants): RR 6.82, 95% CI 2.24 to 20.71; I² = 0%; low certainty evidence). Oral alkylating agents with or without steroids had uncertain effects on infection (Analysis 5.9.2), malignancy (Analysis 5.9.3) and final GFR (Analysis 5.10).
5.6. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 6: Increase in serum creatinine
5.8. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 8: Temporary or permanent discontinuation/hospitalisation due to adverse events
5.9. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 9: Adverse events
5.10. Analysis.

Comparison 5: Oral alkylating agents ± steroids versus placebo/no treatment/steroids, Outcome 10: Final GFR [mL/min/1.73 m²]
6) Cyclophosphamide plus steroids versus chlorambucil plus steroids
Two studies (Ponticelli 1998; Reichert 1994) investigated CPA plus steroids versus chlorambucil plus steroids.
There was only one death reported in the CPA group in Reichert 1994. We are uncertain whether CPA plus steroids increases the risk of ESKD (Analysis 6.2 (2 studies, 115 participants): RR 3.01, 95% CI 0.61 to 14.81; I² = 0%).
6.2. Analysis.

Comparison 6: Cyclophosphamide + steroids versus chlorambucil + steroids, Outcome 2: ESKD (dialysis/transplantation)
CPA plus steroids compared with chlorambucil plus steroids may increase total remission (Analysis 6.3 (2 studies, 115 participants): RR 1.23, 95% CI 1.01 to 1.50; I² = 0%; low certainty evidence), however, it had uncertain effects on complete (Analysis 6.4) and partial remission (Analysis 6.5) (low certainty evidence). Relapse after complete or partial remission was not reported.
6.3. Analysis.

Comparison 6: Cyclophosphamide + steroids versus chlorambucil + steroids, Outcome 3: Complete or partial remission
6.4. Analysis.

Comparison 6: Cyclophosphamide + steroids versus chlorambucil + steroids, Outcome 4: Complete remission
6.5. Analysis.

Comparison 6: Cyclophosphamide + steroids versus chlorambucil + steroids, Outcome 5: Partial remission
It is uncertain whether CPA plus steroids decreases the number with doubling of SCr (Analysis 6.6), decreases temporary or permanent discontinuation or hospitalisation due to adverse events (Analysis 6.7), improves kidney function (Analysis 6.8), or decreases proteinuria (Analysis 6.9).
6.6. Analysis.

Comparison 6: Cyclophosphamide + steroids versus chlorambucil + steroids, Outcome 6: Increase in serum creatinine
6.7. Analysis.

Comparison 6: Cyclophosphamide + steroids versus chlorambucil + steroids, Outcome 7: Temporary or permanent discontinuation/hospitalisation due to adverse events
6.8. Analysis.

Comparison 6: Cyclophosphamide + steroids versus chlorambucil + steroids, Outcome 8: Final serum creatinine
6.9. Analysis.

Comparison 6: Cyclophosphamide + steroids versus chlorambucil + steroids, Outcome 9: Final proteinuria
7) Early (immediate) cyclophosphamide versus late (serum creatinine increase > 25%) cyclophosphamide plus steroids
Hofstra 2010 investigated early (immediate) initiation of therapy with CPA versus late (SCr increase by > 25%) initiation of therapy with CPA and steroids. Participants were followed up for a mean period of 72 ± 22 months.
Hofstra 2010 reported one death in the initiation group (Analysis 7.1), and one patient reached ESKD in the early initiation group (Analysis 7.2).
7.1. Analysis.

Comparison 7: Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids, Outcome 1: Death
7.2. Analysis.

Comparison 7: Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids, Outcome 2: ESKD (dialysis/transplantation)
We are uncertain whether early initiation of CPA improved total (Analysis 7.3), complete (Analysis 7.4) or partial remission (Analysis 7.5) due to very low certainty evidence. We are also uncertain whether early initiation of CPA improves temporary or permanent discontinuation or hospitalisation due to adverse events (Analysis 7.6), SCr (Analysis 7.7), eGFR (Analysis 7.8), or proteinuria (Analysis 7.9), because the certainty of the evidence is very low.
7.3. Analysis.

Comparison 7: Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids, Outcome 3: Complete or partial remission
7.4. Analysis.

Comparison 7: Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids, Outcome 4: Complete remission
7.5. Analysis.

Comparison 7: Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids, Outcome 5: Partial remission
7.6. Analysis.

Comparison 7: Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids, Outcome 6: Temporary or permanent discontinuation/hospitalisation due to adverse events
7.7. Analysis.

Comparison 7: Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids, Outcome 7: Final serum creatinine
7.8. Analysis.

Comparison 7: Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids, Outcome 8: Final GFR [mL/min/1.73 m²]
7.9. Analysis.

Comparison 7: Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids, Outcome 9: Final proteinuria
Relapse and other adverse events were not reported.
8) Cyclophosphamide plus leflunomide plus steroids versus leflunomide plus steroids
Liu 2015e reported CPA plus leflunomide plus steroids versus leflunomide plus steroids may make little or no difference to complete remission (Analysis 8.1 (1 study, 48 participants): RR 1.40, 95% CI 0.99 to 1.98) or malignancy (Analysis 8.2). No other outcomes were reported.
8.1. Analysis.

Comparison 8: Cyclophosphamide + leflunomide + steroid versus leflunomide + steroid, Outcome 1: Complete remission
8.2. Analysis.

Comparison 8: Cyclophosphamide + leflunomide + steroid versus leflunomide + steroid, Outcome 2: Malignancy
9) Mycophenolate mofetil plus calcineurin inhibitors versus calcineurin inhibitors alone
Jurubita 2012 investigated CSA plus MMF versus CSA alone and Nikolopoulou 2019 investigated TAC plus MMF versus TAC alone.
Nikolopoulou 2019 reported one patient in each group reached ESKD (Analysis 9.1). CNI plus MMF may increase both total remission (Analysis 9.2 (2 studies, 58 participants): RR 1.21, 95% CI 0.99 to 1.48; I² = 0%; low certainty of the evidence) and complete remission (Analysis 9.3 (2 studies, 58 participants): RR 1.18, 95% CI 0.93 to 1.51; I² = 0%), but not partial remission (Analysis 9.4). Nikolopoulou 2019 reported no difference in the number of relapses after complete or partial remission (Analysis 9.5) but more adverse events with MMF plus TAC (Analysis 9.6).
9.1. Analysis.

Comparison 9: Mycophenolate mofetil + calcineurin inhibitors versus calcineurin inhibitors, Outcome 1: ESKD (dialysis/transplantation)
9.2. Analysis.

Comparison 9: Mycophenolate mofetil + calcineurin inhibitors versus calcineurin inhibitors, Outcome 2: Complete or partial remission
9.3. Analysis.

Comparison 9: Mycophenolate mofetil + calcineurin inhibitors versus calcineurin inhibitors, Outcome 3: Complete remission
9.4. Analysis.

Comparison 9: Mycophenolate mofetil + calcineurin inhibitors versus calcineurin inhibitors, Outcome 4: Partial remission
9.5. Analysis.

Comparison 9: Mycophenolate mofetil + calcineurin inhibitors versus calcineurin inhibitors, Outcome 5: Relapse after complete or partial remission
9.6. Analysis.

Comparison 9: Mycophenolate mofetil + calcineurin inhibitors versus calcineurin inhibitors, Outcome 6: Severe adverse events
No other outcomes were reported.
10) Calcineurin inhibitors versus placebo/no treatment/supportive treatment/steroids
Seven studies compared CNI with placebo/no treatment/supportive treatments/steroids (Braun 1995; Cattran 1995; Cattran 2001; CYCLOMEN 1994; Howman 2013; Kosmadakis 2010; Praga 2007)
We are uncertain whether CNI increased or decreased the risk of death or ESKD because of very low certainty evidence. The certainty was downgraded because of few events reported in studies which resulted in wide CIs (Analysis 10.1; Analysis 10.2).
10.1. Analysis.

Comparison 10: Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids, Outcome 1: Death
10.2. Analysis.

Comparison 10: Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids, Outcome 2: ESKD (dialysis/transplantation)
We are uncertain whether CNI increases or decreases total remission (Analysis 10.3 (5 studies, 206 participants): RR 1.21, 95% CI 0.62 to 2.38; I² = 77%), complete remission (Analysis 10.4 (5 studies, 206 participants): RR 1.07, 95% CI 0.51 to 2.24; I² = 15%), partial remission (Analysis 10.5 (5 studies. 206 participants): RR 1.08, 95% CI 0.53 to 2.22; I² = 65%), or relapse after complete or partial remission (Analysis 10.6).
10.3. Analysis.

Comparison 10: Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids, Outcome 3: Complete or partial remission
10.4. Analysis.

Comparison 10: Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids, Outcome 4: Complete remission
10.5. Analysis.

Comparison 10: Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids, Outcome 5: Partial remission
10.6. Analysis.

Comparison 10: Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids, Outcome 6: Relapse after complete or partial remission
CNI had uncertain effects on SCr increase (Analysis 10.7), temporary or permanent discontinuation or hospitalisation due to adverse events (Analysis 10.8), serious adverse events (Analysis 10.9.1), infection (Analysis 10.9.2), or malignancy (Analysis 10.9.3).
10.7. Analysis.

Comparison 10: Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids, Outcome 7: Increase in serum creatinine
10.8. Analysis.

Comparison 10: Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids, Outcome 8: Temporary or permanent discontinuation/hospitalisation due to adverse events
10.9. Analysis.

Comparison 10: Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids, Outcome 9: Adverse events
11) Calcineurin inhibitors with or without steroids versus alkylating agents with or without steroids
Eleven studies (Agarwal 2012a; Braun 1995; Chen 2010a; He 2013; Howman 2013; Kosmadakis 2010; Liang 2017; Peng 2016; Ramachandran 2016; Xu 2010; Xu 2013a) investigated CNI with or without steroids versus alkylating agents with or without steroids.
We are uncertain whether CNI with or without steroids increases or decreases the risk of death (Analysis 11.1) or ESKD (Analysis 11.2) because the certainty of the evidence is very low (due to serious risk of bias, imprecision, indirectness and insufficient follow‐up).
11.1. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 1: Death
11.2. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 2: ESKD (dialysis/transplantation)
CNI with or without steroids may make little or no difference to total remission (Analysis 11.3.1 (10 studies, 538 participants): RR 1.01, 95% CI 0.89 to 1.15; I² = 53%; moderate certainty evidence), complete remission (Analysis 11.4.1 (10 studies, 538 participants): RR 1.15, 95% CI 0.84 to 1.56; I² = 56%; low certainty evidence), or partial remission (Analysis 11.5.1 (10 studies, 528 participants): RR 0.82, 95% CI 0.58 to 1.18; I² = 48%) compared to alkylating agents at final follow‐up (9 to 60 months). For studies with a final follow‐up of ≥ 2 years, there was little or no difference to total, complete or partial remission. CNI with or without steroids may increase relapse at final follow‐up < 2 years (Analysis 11.6 (6 studies, 295 participants): RR 2.13, 95% CI 0.71 to 6.37; I² = 29%; low certainty of the evidence) and at ≥ 2 years (to 60 months) (Analysis 11.5.2 (3 studies, 169 participants): RR 0.34, 95% CI 0.09 to 1.32; I² = 67%).
11.3. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 3: Complete or partial remission
11.4. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 4: Complete remission
11.5. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 5: Partial remission
11.6. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 6: Relapse after complete or partial remission
CNI with or without steroids had uncertain effects on SCr increase (Analysis 11.7), adverse events (Analysis 11.8; Analysis 11.9), and kidney function (Analysis 11.10; Analysis 11.11; Analysis 11.12; Analysis 11.13; Analysis 11.14).
11.7. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 7: Increase in serum creatinine
11.8. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 8: Temporary or permanent discontinuation/hospitalisation due to adverse events
11.9. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 9: Adverse events
11.10. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 10: Final serum creatinine
11.11. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 11: Final serum albumin
11.12. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 12: Final GFR [mL/min/1.73 m²]
11.13. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 13: Loss of GFR > 20%
11.14. Analysis.

Comparison 11: Calcineurin inhibitors ± steroids versus alkylating agents ± steroids, Outcome 14: Final proteinuria
12) Short‐course tacrolimus plus steroids versus long‐course tacrolimus plus steroids
Two studies compared short‐ versus long‐course TAC (Di 2018; Yuan 2013). Di 2018 compared 6 months of TAC (short course) plus steroids versus 12 months of TAC (long course) plus steroids, and Yuan 2013 compared 6 months TAC (short course) plus steroids with 24 months TAC (long course) plus steroids. Both studies had a follow‐up period of 24 months.
Yuan 2013 reported no deaths in either group; neither study reported ESKD.
Short‐course TAC plus steroids had uncertain effects on total remission (Analysis 12.2 (2 studies, 106 participant): RR 0.68, 95% CI 0.42 to 1.10; I² = 72%), complete remission (Analysis 12.3 (2 studies, 106 participants): RR 0.52, 95% CI 0.28 to 0.97; I² = 0%), partial remission (Analysis 12.4 (2 studies, 106 participants): RR 0.77, 95% CI 0.30 to 1.99; I² = 78%), and relapse after complete or partial remission (Analysis 12.5 (2 studies, 82 participants): RR 7.25, 95% CI 0.41 to 129.75; I² = 75%).
12.2. Analysis.

Comparison 12: Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids, Outcome 2: Complete or partial remission
12.3. Analysis.

Comparison 12: Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids, Outcome 3: Complete remission
12.4. Analysis.

Comparison 12: Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids, Outcome 4: Partial remission
12.5. Analysis.

Comparison 12: Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids, Outcome 5: Relapse after complete or partial remission
Short‐course TAC plus steroids may make little or no difference to adverse events (Analysis 12.6.1) and infection (Analysis.12.6.2), SCr (Analysis 12.7), but may decrease final serum albumin (Analysis 12.8) and raise final proteinuria (Analysis 12.9).
12.6. Analysis.

Comparison 12: Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids, Outcome 6: Adverse events
12.7. Analysis.

Comparison 12: Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids, Outcome 7: Final serum creatinine
12.8. Analysis.

Comparison 12: Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids, Outcome 8: Final serum albumin
12.9. Analysis.

Comparison 12: Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids, Outcome 9: Final proteinuria
13) Cyclosporine plus steroids versus cyclosporine alone
Two studies (CYPMEN 2006; Li 2015) compared CSA plus steroids versus CSA alone.
Li 2015 reported no deaths in either group; neither study reported ESKD.
CSA plus steroids had uncertain effects on total remission (Analysis 13.2) and partial remission (Analysis 13.4), but may increase complete remission (Analysis 13.3 (2 studies, 55 participants): RR 2.20, 95% CI 1.07 to 4.49; I² = 0%; low certainty evidence) compared to CSA alone.
13.2. Analysis.

Comparison 13: Cyclosporine + steroids versus cyclosporine alone, Outcome 2: Complete or partial remission
13.4. Analysis.

Comparison 13: Cyclosporine + steroids versus cyclosporine alone, Outcome 4: Partial remission
13.3. Analysis.

Comparison 13: Cyclosporine + steroids versus cyclosporine alone, Outcome 3: Complete remission
CSA plus steroids had uncertain effects on SCr increase (Analysis 13.5) and infection (Analysis 13.6.2), but may reduce adverse events (Analysis 13.6.1 (1 study 27 participants): RR 2.37, 95% CI 1.13 to 4.97) compared to CSA alone.
13.5. Analysis.

Comparison 13: Cyclosporine + steroids versus cyclosporine alone, Outcome 5: 50% increase in serum creatinine
13.6. Analysis.

Comparison 13: Cyclosporine + steroids versus cyclosporine alone, Outcome 6: Adverse events
14) Cyclosporine (3.0 mg/kg, once/day) plus steroids versus cyclosporine (1.5 mg/kg, twice/day) plus steroids
Saito 2014 compared CSA given twice/day at a dose of 1.5 mg/kg with CSA given once/day at a dose of 3.0 mg/kg. Both groups received additional therapy with steroids.
Once/day CSA plus steroids had uncertain effects on total remission (Analysis 14.1) complete remission (Analysis 14.2) or partial remission (Analysis 14.3). Relapse was not reported.
14.1. Analysis.

Comparison 14: Cyclosporine (3.0 mg/kg, once/day) + steroids versus cyclosporine (1.5 mg/kg, twice/day) + steroids, Outcome 1: Complete or partial remission
14.2. Analysis.

Comparison 14: Cyclosporine (3.0 mg/kg, once/day) + steroids versus cyclosporine (1.5 mg/kg, twice/day) + steroids, Outcome 2: Complete remission
14.3. Analysis.

Comparison 14: Cyclosporine (3.0 mg/kg, once/day) + steroids versus cyclosporine (1.5 mg/kg, twice/day) + steroids, Outcome 3: Doubling of serum creatinine
It is uncertain whether once/day CSA reduces the number of patients with doubling of SCr (Analysis 14.3), infection (Analysis 14.4.2), or malignancy (Analysis 14.4.3) compared to twice/day CSA.
14.4. Analysis.

Comparison 14: Cyclosporine (3.0 mg/kg, once/day) + steroids versus cyclosporine (1.5 mg/kg, twice/day) + steroids, Outcome 4: Adverse events
15) Cyclosporine plus steroids versus tacrolimus plus steroids
Li 2017c and Omrani 2017 compared CSA plus steroids with TAC plus steroids however, Omrani 2017 only provided data for adverse events.
Li 2017c reported no difference between the groups for total (Analysis 15.1), complete (Analysis 15.2) and partial remission (Analysis 15.3). Omrani 2017 reported no difference between the two groups for serious adverse events (Analysis 15.4).
15.1. Analysis.

Comparison 15: Cyclosporine + steroids versus tacrolimus + steroids, Outcome 1: Complete or partial remission
15.2. Analysis.

Comparison 15: Cyclosporine + steroids versus tacrolimus + steroids, Outcome 2: Complete remission
15.3. Analysis.

Comparison 15: Cyclosporine + steroids versus tacrolimus + steroids, Outcome 3: Partial remission
15.4. Analysis.

Comparison 15: Cyclosporine + steroids versus tacrolimus + steroids, Outcome 4: Serious adverse events
16) Cyclosporine versus azathioprine
Naumovic 2011 compared CSA with AZA in 23 participants.
No deaths occurred during the study period (Analysis 16.1). Naumovic 2011 reported no differences between the groups for ESKD (Analysis 16.2), total remission (Analysis 16.3), complete remission (Analysis 16.4), partial remission (Analysis 16.5, increase in SCr (Analysis 16.6), temporary or permanent discontinuation or hospitalisation due to adverse events (Analysis 16.7), final SCr (Analysis 16.8), final GFR (Analysis 16.9), and final proteinuria (Analysis 16.10).
16.1. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 1: Death
16.2. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 2: ESKD (dialysis/transplantation)
16.3. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 3: Complete or partial remission
16.4. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 4: Complete remission
16.5. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 5: Partial remission
16.6. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 6: Increase in serum creatinine
16.7. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 7: Temporary or permanent discontinuation/hospitalisation due to adverse events
16.8. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 8: Final serum creatinine
16.9. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 9: Final GFR [mL/min/1.73 m²]
16.10. Analysis.

Comparison 16: Cyclosporine versus azathioprine, Outcome 10: Final proteinuria
17) Azathioprine with or without steroids versus no treatment/supportive treatment
Silverberg 1976 compared AZA with no treatment/supportive treatment.
Silverberg 1976 reported no differences between the groups for total remission (Analysis 17.3), complete remission (Analysis 17.4), increase in SCr (Analysis 17.6), final SCr (Analysis 17.8), final GFR (Analysis 17.9), or final proteinuria (Analysis 17.10).
17.3. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 3: Complete or partial remission
17.4. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 4: Complete remission
17.6. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 6: Increase in serum creatinine
17.8. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 8: Final serum creatinine
17.9. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 9: Final GFR [mL/min/1.73 m²]
17.10. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 10: Final proteinuria
There were no reported deaths, progression to ESKD, partial remissions, or temporary or permanent discontinuation of treatment or hospitalisation due to adverse events during the study period.
18) Mycophenolate mofetil versus no treatment/supportive therapy
Dussol 2008 compared MMF with no treatment.
There were no reported deaths, progression to ESKD, or increase in SCr during the study period.
Dussol 2008 reported no differences between the groups for total remission (Analysis 18.3), complete remission (Analysis 18.4), partial remission (Analysis 18.5), or final GFR (Analysis 18.8).
18.3. Analysis.

Comparison 18: Mycophenolate mofetil versus no treatment/supportive therapy, Outcome 3: Complete or partial remission
18.4. Analysis.

Comparison 18: Mycophenolate mofetil versus no treatment/supportive therapy, Outcome 4: Complete remission
18.5. Analysis.

Comparison 18: Mycophenolate mofetil versus no treatment/supportive therapy, Outcome 5: Partial remission
18.8. Analysis.

Comparison 18: Mycophenolate mofetil versus no treatment/supportive therapy, Outcome 8: Final GFR [mL/min/1.73 m²]
19) Mycophenolate mofetil with or without steroids versus alkylating agents with or without steroids
Four studies (Chan 2007; Fu 2012a; Peng 2016; Senthil Nayagam 2008) compared MMF with or without steroids versus alkylating agents with or without steroids. Fu 2012a followed‐up patients over a period of 36 months.
There was only one death reported by one of the four studies (Peng 2016) in the MMF group, and there was no progression to ESKD reported by three studies (Chan 2007; Peng 2016; Senthil Nayagam 2008). Peng 2016 reported no increase in SCr.
MMF with or without steroids may make little or no difference to total remission (Analysis 19.3.1 (4 studies, 124 participants): RR 0.90, 95% CI 0.71 to 1.13; I² = 0%); complete remission (Analysis 19.4.1 (4 studies, 124 participants): RR 1.01, 95% CI 0.58 to 1.73; I² = 0%), partial remission (Analysis 19.5.1 (4 studies, 124 participants): RR 0.89, 95% CI 0.58 to 1.37; I² = 0%) (low certainty of the evidence). This is consistent with findings from Fu 2012a which reported total remission (Analysis 19.3.2: RR 0.90 95% CI 0.71 to 1.13), complete remission (Analysis 19.4.2: RR 1.00, 95% CI 0.44 to 2.29) and partial remission (Analysis 19.5.2: RR 1.33, 95% CI 0.37 to 4.82) at 36 months in 24 participants.
19.3. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 3: Complete or partial remission
19.4. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 4: Complete remission
19.5. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 5: Partial remission
It is uncertain whether MMF with or without steroids increases or decreases, temporary or permanent discontinuation or hospitalisation due to adverse events (Analysis 19.8), adverse events (Analysis 19.9), infection (Analysis 19.9.3) or kidney function measures (Analysis 19.10; Analysis 19.11; Analysis 19.12; Analysis 19.13).
19.8. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 8: Temporary or permanent discontinuation/hospitalisation due to adverse events
19.9. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 9: Adverse events
19.10. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 10: Final serum creatinine
19.11. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 11: Final serum albumin
19.12. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 12: Final GFR [mL/min/1.73 m²]
19.13. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 13: Final proteinuria
20) Mycophenolate mofetil with or without steroids versus calcineurin inhibitors with or without steroids
Choi 2018 and Peng 2016 compared MMF with or without steroids versus CNI with or without steroids.
Peng 2016 reported one death in each group (Analysis 20.1), no progression to ESKD (Analysis 20.2), no increase in SCr (Analysis 20.7), and no temporary or permanent discontinuation or hospitalisation due to adverse events (Analysis 20.8).
20.1. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 1: Death
20.2. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 2: ESKD (dialysis/transplantation)
20.7. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 7: Increase in serum creatinine
20.8. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 8: Temporary or permanent discontinuation/hospitalisation due to adverse events
MMF plus steroids may make little or no difference to total remission (Analysis 20.3 (2 studies, 97 participants): RR 0.94, 95% CI 0.70 to 1.27; I² = 37%); complete remission (Analysis 20.4 (2 studies, 97 participants): RR 0.57, 95% CI 0.20 to 1.63; I² = 48%), or partial remission (Analysis 20.5 (2 studies, 97 participants): RR 1.36, 95% CI 0.88 to 2.10; I² = 0%) (low certainty of the evidence). Peng 2016 reported no difference in relapse between the two groups (Analysis 20.6).
20.3. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 3: Complete or partial remission
20.4. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 4: Complete remission
20.5. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 5: Partial remission
20.6. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 6: Relapse after complete remission
MMF with or without steroids compared to CNI with or without steroids may make little or no difference to adverse events (Analysis 20.9), infection (Analysis 20.9.2), malignancy (Analysis 20.9.3), final serum albumin (Analysis 20.11), and final proteinuria (Analysis 20.13).
20.9. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 9: Adverse events
20.11. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 11: Final serum albumin
20.13. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 13: Final proteinuria
Choi 2018 reported no differences in final SCr (Analysis 20.11) and final GFR (Analysis 20.12) between the two groups.
20.12. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 12: Final GFR [mL/min/1.73 m²]
21) Adrenocorticotropic hormone versus no treatment
Arnadottir 2006 compared ACTH with no treatment.
Arnadottir 2006 reported ACTH increased total remission (Analysis 21.1 (30 participants): RR 7.00, 95% CI 1.91 to 25.62), complete remission (Analysis 21.2 (30 participants): RR 11.00, 95% CI 1.62 to 74.88), but not partial remission (Analysis 21.3 (30 participants): RR 3.00, 95% CI 0.35 to 25.68).
21.1. Analysis.

Comparison 21: Adrenocorticotropic hormone versus no treatment, Outcome 1: Complete or partial remission
21.2. Analysis.

Comparison 21: Adrenocorticotropic hormone versus no treatment, Outcome 2: Complete remission
21.3. Analysis.

Comparison 21: Adrenocorticotropic hormone versus no treatment, Outcome 3: Partial remission
No other outcomes were reported.
22) Adrenocorticotropic hormone versus alkylating agents plus steroids
Ponticelli 2006 compared ACTH with alkylating agents plus steroids.
Ponticelli 2006 reported no deaths, and one patient progressed to ESKD by the end of the study in the ACTH group.
There were no reported differences between the two groups for total remission (Analysis 22.3 (32 participants): RR 0.93, 95% CI 0.75 to 1.17); more patients achieved complete remission in the ACTH group (Analysis 22.4 (32 participants): RR 2.00, 95% CI 0.88 to 4.54); while more achieved partial remission in the alkylating agents plus steroids group (Analysis 22.5 (32 participants): RR 0.40, 95% CI 0.16 to 1.01).
22.3. Analysis.

Comparison 22: Adrenocorticotropic hormone versus alkylating agents + steroids, Outcome 3: Complete or partial remission
22.4. Analysis.

Comparison 22: Adrenocorticotropic hormone versus alkylating agents + steroids, Outcome 4: Complete remission
22.5. Analysis.

Comparison 22: Adrenocorticotropic hormone versus alkylating agents + steroids, Outcome 5: Partial remission
There were no reported differences between the groups for increases in SCr (Analysis 22.6), temporary or permanent discontinuation or hospitalisation due to adverse events (Analysis 22.7), or final SCr (Analysis 22.8). Final proteinuria was reported to be lower in the ACTH group (Analysis 22.9).
22.6. Analysis.

Comparison 22: Adrenocorticotropic hormone versus alkylating agents + steroids, Outcome 6: Increase in serum creatinine
22.7. Analysis.

Comparison 22: Adrenocorticotropic hormone versus alkylating agents + steroids, Outcome 7: Temporary or permanent discontinuation/hospitalisation due to adverse events
22.8. Analysis.

Comparison 22: Adrenocorticotropic hormone versus alkylating agents + steroids, Outcome 8: Final serum creatinine
22.9. Analysis.

Comparison 22: Adrenocorticotropic hormone versus alkylating agents + steroids, Outcome 9: Final proteinuria
23) Mizoribine with or without steroids versus placebo/no treatment/steroids
Three studies (Hasegawa 2017; Koshikawa 1993; Shibasaki 2004) compared mizoribine with or without steroids with placebo/no treatment/steroids only. Data from Hasegawa 2017 could not be extracted.
We are uncertain whether mizoribine with or without steroids increases or decreases total remission (Analysis 23.1), complete remission (Analysis 23.2), or partial remission (Analysis 23.3) because the certainty of the evidence is very low.
23.1. Analysis.

Comparison 23: Mizoribine ± steroids versus placebo/no treatment/corticosteroids, Outcome 1: Complete or partial remission
23.2. Analysis.

Comparison 23: Mizoribine ± steroids versus placebo/no treatment/corticosteroids, Outcome 2: Complete remission
23.3. Analysis.

Comparison 23: Mizoribine ± steroids versus placebo/no treatment/corticosteroids, Outcome 3: Partial remission
Koshikawa 1993 reported two patients discontinued treatment due to serious adverse events (Analysis 23.4).
23.4. Analysis.

Comparison 23: Mizoribine ± steroids versus placebo/no treatment/corticosteroids, Outcome 4: Temporary or permanent discontinuation/hospitalisation due to adverse events
No other outcomes were reported.
24) Mizoribine (150 mg) once a day versus mizoribine (50 mg) 3 times a day
Saito 2017 compared mizoribine (150 mg) once/day versus mizoribine (50 mg) 3 times/day.
Saito 2017 reported no differences between the groups for total remission (Analysis 24.1) complete remission (Analysis 24.2), or relapse after complete or partial remission (Analysis 24.4). More patients achieved partial remission with 50 mg 3 times/day (Analysis 24.3)
24.1. Analysis.

Comparison 24: Mizoribine: 150 mg (once/day) versus 50 mg (3 times/day), Outcome 1: Complete or partial remission
24.2. Analysis.

Comparison 24: Mizoribine: 150 mg (once/day) versus 50 mg (3 times/day), Outcome 2: Complete remission
24.4. Analysis.

Comparison 24: Mizoribine: 150 mg (once/day) versus 50 mg (3 times/day), Outcome 4: Relapse after complete or partial remission
24.3. Analysis.

Comparison 24: Mizoribine: 150 mg (once/day) versus 50 mg (3 times/day), Outcome 3: Partial remission
No adverse events or infections were reported in either group. Malignancy was reported in two patients in the once/day group (Analysis 24.5.3).
24.5. Analysis.

Comparison 24: Mizoribine: 150 mg (once/day) versus 50 mg (3 times/day), Outcome 5: Adverse events
No other outcomes were reported.
25) Rituximab plus supportive therapy versus supportive therapy alone
GEMRITUX 2017 compared the biologic agent rituximab with supportive therapy (ACEi/ARB) versus supportive therapy alone (ACEi/ARB).
GEMRITUX 2017 reported rituximab plus supportive therapy may improve total remission at 6 months (Analysis 25.1.1 (75 participants): RR 2.21, 95% CI 1.37 to 3.57) and final follow‐up (median 17 months) Analysis 25.1.2 (75 participants): RR 1.90, 95% CI 1.15 to 3.13) (low certainty of the evidence). More patients achieved complete remission (Analysis 25.2) and partial remission (Analysis 25.3) with rituximab.
25.1. Analysis.

Comparison 25: Rituximab + supportive therapy versus supportive therapy alone, Outcome 1: Complete or partial remission
There were no reported differences in adverse events between the two groups (Analysis 25.4.1), and malignancy was reported in one patient in the control group.
At the end of follow‐up, GEMRITUX 2017 reported rituximab plus supportive therapy may improve serum albumin (Analysis 25.6 (75 participants): MD 5.70 g/L, 95% CI 4.59 to 6.81), protein‐to‐creatinine ratio (Analysis 25.8 (75 participants): MD ‐1348.50 mg/g, 95% CI ‐1993.39 to ‐703.61), and PLA2R antibody titre (Analysis 25.9 (75 participants): MD ‐81.80 RU/mL, 95% CI ‐105.38 to ‐58.22) compared to supportive therapy. However, rituximab with supportive therapy was reported to make little or no difference to SCr (Analysis 25.5 (75 participants): MD ‐0.40 µmol/L, 95% CI ‐5.44 to 4.64) or eGFR (Analysis 25.7 (75 participants): MD ‐4.00 mL/min/1.7 m², 95% CI ‐8.91 to 0.91) compared to supportive therapy (low certainty of the evidence).
No other outcomes were reported.
26) Rituximab versus cyclosporine
MENTOR 2015 compared rituximab plus supportive therapy with CSA plus supportive therapy.
MENTOR 2015 reported no deaths, and one patient progressed to ESKD in the CSA group by the end of the 24‐month study period.
MENTOR 2015 reported rituximab may increase total remission (Analysis 26.3 (130 participants): RR 3.00, 95% CI 1.77 to 5.07) and complete remission (Analysis 26.4 (130 participants): RR 47.00, 95% CI 2.91 to 757.81) at 24 months but not partial remission (Analysis 26.5 (130 participants): RR 1.23, 95% CI 0.65 to 2.35) (low certainty of the evidence). The number relapsing after complete or partial remission was higher in the CSA group (Analysis 26.6 (73 participants): RR 0.10, 95% CI 0.02 to 0.39).
26.3. Analysis.

Comparison 26: Rituximab versus cyclosporine, Outcome 3: Complete or partial remission
26.4. Analysis.

Comparison 26: Rituximab versus cyclosporine, Outcome 4: Complete remission
26.5. Analysis.

Comparison 26: Rituximab versus cyclosporine, Outcome 5: Partial remission
26.6. Analysis.

Comparison 26: Rituximab versus cyclosporine, Outcome 6: Relapse after complete or partial remission
In patients with any form of remission (complete or partial), quality of life as measured by SF‐12 scores (score range: 0‐100 points) for physical health (Analysis 26.7.1) and mental health (Analysis 26.7.2) may be slightly lower in patients who receive rituximab compared with CSA. There were more reported serious adverse events in the CSA group (Analysis 26.8.1); the number of infections was similar (Analysis 26.8.2).
26.7. Analysis.

Comparison 26: Rituximab versus cyclosporine, Outcome 7: Quality of Life in patients with any remission
26.8. Analysis.

Comparison 26: Rituximab versus cyclosporine, Outcome 8: Adverse events
No other outcomes were reported.
27) Traditional Chinese medicine versus immunosuppressive therapy
Chen 2013e and Liu 2009b investigated the efficacy and safety of traditional Chinese medicine versus immunosuppressive therapy. Chen 2013e compared Shenqi particles with CPA plus steroids and Liu 2009b compared Tripterygium wilfordii plus steroids with Tripterygium wilfordii alone.
Chen 2013e reported three deaths with immunosuppressive therapy and none with Shenqi particles. Liu 2009b reported no deaths in either group, and no patients progressed to ESKD (Analysis 27.1; Analysis 27.2).
27.1. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 1: Death
27.2. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 2: ESKD (dialysis/transplantation)
Chen 2013e reported no difference in total, complete and partial remission between Shenqi particles and immunosuppressive therapy. Liu 2009b reported an increase in the number achieving total and complete remission with Tripterygium wilfordii plus steroids compared to Tripterygium wilfordii alone, but no difference in partial remission (Analysis 27.3; Analysis 27.4; Analysis 27.5).
27.3. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 3: Complete or partial remission
27.4. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 4: Complete remission
27.5. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 5: Partial remission
Chen 2013e reported one case of doubling of SCr in the immunosuppressive therapy group and none in the Shenqi particle group; Liu 2009b reported no cases in either group.
Chen 2013e reported more severe adverse events in the immunosuppressive therapy (Analysis 27.7). The number of severe adverse events was similar in Liu 2009b.
27.7. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 7: Severe adverse events
Chen 2013e reported no differences between the groups for final serum albumin (Analysis 27.8) and proteinuria (Analysis 27.10); while final GFR was higher in the Shenqi particle group (Analysis 27.9).
27.8. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 8: Final serum albumin
27.10. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 10: Final proteinuria
27.9. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 9: Final GFR [mL/min/1.73 m²]
Eculizumab 8 mg/kg every 2 weeks versus eculizumab 8 mg/kg every 4 weeks
Appel 2002 investigated IV eculizumab 8 mg/kg every two weeks versus IV eculizumab IV 8 mg/kg every four weeks. However, the only reports identified were a conference abstract and its associated press release; these reports did not contain any data that could be meta‐analysed. The study enrolled 117 patients and reported no major hypersensitivity reactions and treatment with eculizumab was generally well tolerated. We could not identify published outcome data from this study.
Adrenocorticotropic hormone 40 IU versus adrenocorticotropic hormone 80 IU
Hladunewich 2014 investigated 40 IU ACTH versus 80 IU ACTH, however, we were not able to extract data because many patients switched treatment arms and results were not reported according to the two intervention groups as defined at the start of the study. We have provided a brief narrative summary of the main findings of this study.
The study was a phase Ib/II trial using ACTH in the form of H.P. Acthar® Gel (Questcor Pharmaceuticals, Inc.) in 20 adult patients with IMN with nephrotic syndrome. ACTH was generally well‐tolerated and did not lead to any significant adverse events or discontinuation of treatment. By 12 months of follow‐up, there was a significant improvement in proteinuria in the entire cohort, decreasing from baseline proteinuria of 9.07 ± 3.38 g/day to 3.87 ± 4.24 g/day (P < 0.001). Proteinuria decreased by more than 50% in 65% of the patients. A likely dose‐response relationship was established during the trial period with better efficacy of the treatment in patients treated at higher doses than 40 IU.
Discussion
Treatment of patients with PMN and nephrotic syndrome is complex and difficult to navigate because of multiple interventions and studies, which have compared numerous different treatment regimens. As a result, the efficacy and safety of different immunosuppressive regimens remain unclear.
This original review (Schieppati 2004) included 19 RCTs with 1025 participants and found that immunosuppressive treatments, could increase the rates of complete or partial remission. However, the long‐term effects of immunosuppressive treatments on definite endpoints such as death (any cause) or kidney survival rate could not be demonstrated. Immunosuppressive treatments also had a significantly higher risk of severe adverse events. The first update of this review (Chen 2014) included 39 studies with 1825 participants, which further strengthened the certainty of the evidence.
There was limited evidence available on other treatments such as MMF, AZA or traditional Chinese medicine and these studies did not show promising results in terms of superiority of these treatments over standard therapy.
The role of other therapies remains an ongoing topic of investigation and discussion.
Summary of main results
This review update included 65 studies that randomised 3807 participants and answered two aims of this systematic review.
Is immunosuppressive therapy superior to non‐immunosuppressive therapy in treating patients with PMN and nephrotic syndrome?
If so, which immunosuppressive agent/s is most effective and safe in treating patients with PMN and nephrotic syndrome?
Immunosuppressive treatments compared with no treatment or non‐immunosuppressive treatment probably provides a clinical benefit for the outcomes of reducing ESKD, doubling of SCr, and an increase in the rate of total remission and complete remission. However, the use of immunosuppressive treatments compared with no treatment/non‐immunosuppressive treatments probably increased temporary or permanent discontinuation of treatment or hospitalisation due to adverse events of therapy.
This review firstly showed that immunosuppressive therapy with non‐steroid immunosuppressive drugs with or without concomitant steroids may be superior in the induction of remission compared to immunosuppression with corticosteroids only.
Secondly, immunosuppressive therapy with oral alkylating agents with or without steroids compared to no treatment or supportive therapy or steroids alone probably increases remission rates but may lead to a decrease in rates of ESKD by up to 70%. However, there may be a three‐fold increase in rates of serious adverse events. There was little difference in efficacy or safety when comparing alkylating agents CPA with steroids versus chlorambucil with steroids, except that CPA might increase rates of total remission. These findings may justify the use of CPA combined or alternated with steroids as first‐line therapy for adults with PMN and nephrotic syndrome, who do not achieve remission within six months of supportive therapy, as recommended by KDIGO guidelines (KDIGO 2020).
Comparing CNI (CSA and TAC) with alkylating agents showed little or no difference in remission rates or improvement of other secondary outcomes, including adverse events. Due to the very low certainty of the evidence, no conclusion can be made with regards to death or progression to ESKD.
The effectiveness and safety of many other interventions remain unclear, and the clinical use of these therapies, therefore, warrants caution. MMF showed similar effectiveness in inducing remission as alkylating agents or CNI, however, the certainty of the evidence is low, due to the small number of studies with a low number of events and insufficient length of follow‐up to determine long‐term efficacy and safety of this therapy in patents with PMN.
The combination of two non‐steroidal immunosuppressive treatments (e.g. TAC with MMF) may improve rates of complete remission compared with one non‐steroidal immunosuppressive treatment alone. Treatment regimens with two non‐steroidal immunosuppressive drugs may be considered in patients with contraindications or severe side effects from treatment with steroids. However, this was only investigated in a small number of studies, therefore requiring further investigation. In our meta‐analysis of this comparison, we included two studies that combined CSA with MMF and one study that combined CPA and leflunomide. It is noteworthy, that there was an unexpected lack of statistical heterogeneity.
Additionally, other studies examining mizoribine monotherapy, ACTH and rituximab have demonstrated some potential efficacy benefits, but the long‐term efficacy and safety of these treatments are unknown and should be further examined in future RCTs.
Overall completeness and applicability of evidence
Our review was based on a standardised and highly sensitive electronic search of the Cochrane Kidney and Transplant Specialised Register, which includes a review of journal alerts and handsearching of all relevant conference proceedings. Many recent studies are registered with clinical trial registries such as clinicaltrials.gov, which leads to transparency and accountability and a smaller possibility of selective reporting. Furthermore, it is noteworthy that most recent studies report on remission rates as primary outcomes, which improves the consistency of reporting and comparability of results among different studies.
One major limitation was the relatively small numbers of included studies in some comparisons of immunosuppressive regimens, especially for the newer immunosuppressive treatments such as ACTH and rituximab. This issue is common in systematic reviews carried out in the field of glomerulonephritis (e.g. in lupus nephritis Tunnicliffe 2018 or IgA nephropathy Natale 2020). Another major concern is the relatively short follow‐up period in most of the included studies (median follow‐up of 24 months). It has been recognised that for long‐term endpoints such as ESKD or death a follow‐up period of at least seven to 10 years should be considered. For surrogate outcomes such as complete or partial remission, an adequate follow‐up period should be of at least two to three years (du Buf‐Vereijken 2005). This is especially important to monitor rates of relapse from remission as this is a frequent complication of membranous nephropathy, even under continued immunosuppressive treatment. Furthermore, most studies did not perform blinding of participants, personnel, and outcome assessors, leading to risk of bias. Finally, some of the investigated treatments, especially CNI, may have additional non‐immunosuppressive actions that may positively or negatively influence their efficiency in the treatment of PMN however lack of data has made it difficult to investigate this further.
Patient‐reported outcomes, such as quality of life, are increasingly recognised as critical to healthcare decision making but these outcomes are often not measured nor reported in RCTs. A core outcome set that includes critically important outcomes from the perspective of patients, caregivers, researchers, and physicians alike, is vital to ensure that the evidence from RCTs is used to inform clinical decision making that is appropriate and valuable to all stakeholders. In addition, standardised measures of important efficacy outcomes such as remission would allow for ease of comparison across studies and help build the evidence for the treatment of patients with IMN and nephrotic syndrome. Such a set of core outcomes is currently under development by the SONG initiative (Standardised Outcomes in Nephrology), including a working group for glomerular disease.
Many recent studies have been conducted in Asian countries and it is unclear whether differences in response to treatment exist among patients of different ethnicity. Furthermore, other differences among patients with PMN require further investigation to assess whether certain patients may benefit from different therapeutic approaches such as whether the presence or level of certain antibodies influences treatment response.
Finally, current RCTs may not reflect the entire range of therapies that are used in clinical practice, such as biologic therapies which have been used increasingly and have been reported on in observational studies. However, given the greater potential for bias in observational studies, these treatments should be further investigated in RCTs.
Quality of the evidence
Certainty of the evidence was graded using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (GRADE 2011). In general, most studies did not perform blinding and had several study limitations (Begg 1996; Clarke 2000). Therefore, the risk of bias was high or moderate in most studies. The internal validity of the design, conduct and analysis of the included RCTs was difficult to assess in some studies because of the omission of important methodological details and not all trials had published trial protocols or registered their study with a clinical trial registry.
The generalisability of the evidence is limited by the small number of studies for many treatment options and the limited number of studies that examine differences between patient subgroups. For example, many studies did not report the number of patients with positive anti‐PLA2R‐antibodies or the histopathological stage of the kidney damage.
We were not able to assess for the presence of language bias through subgroup analysis, as only three studies were published in a language other than English. No studies were excluded on the basis of language. Sensitivity analysis could not be performed to explore the effect of dominating studies with very long follow‐up or very large same size. Additionally, publication bias (the effect of small or unpublished studies on treatment effects) could often not be assessed given the small number of trials available. To reduce publication bias, new reports and existing reports from the hand‐searching of conference proceedings from the Cochrane Kidney and Transplants registry were included in this systematic review.
Potential biases in the review process
This systematic review update is reported using Cochrane methods and includes a comprehensive search of literature by the Cochrane Kidney and Transplant Information Specialist. As with any systematic review and meta‐analysis, this review is limited to the outcomes reported in the included studies. For example, there was a lack of reporting of patient‐reported outcomes in most RCTs. Many of the included studies were of insufficient follow‐up to detect important clinical outcomes, such as death, ESKD, and complete remission. Subgroup analyses have been undertaken according to the duration of follow‐up of studies to minimise indirectness. However, there were only a small number of studies, each with small numbers of participants, that were of sufficient follow‐up and imprecision may be present in the overall effect estimate. Additionally, the small number of studies might have limited the power of statistical testing to detect important differences between studies. Heterogeneity was found to be substantial in certain comparisons. Study authors had no affiliation to any trial investigators. The review did not receive private industry funding.
Agreements and disagreements with other studies or reviews
Three systematic reviews were published before 1995 (Couchoud 1994; Hogan 1995; Imperiale 1995). Imperiale 1995 included five prospective studies, four RCTs and one non‐RCT, in which alkylating agents were compared with corticosteroids, placebo or symptomatic treatments. They found a beneficial effect of alkylating agents on complete or partial remission in 228 patients. However, there was not enough evidence related to the effects of alkylating agents on the long‐term endpoints. Hogan 1995 performed a pooled analysis of 35 retrospective and prospective studies in 1815 patients. Complete remission was more frequent with the use of alkylating agents compared with no treatment or corticosteroids. However, there was again insufficient evidence that corticosteroids or alkylating therapy could improve long‐term kidney survival in patients with PMN and nephrotic syndrome.
Systematic reviews which included observational studies of rituximab treatment in PMN (Bomback 2009; Zou 2018) showed potential efficacy of rituximab in inducing remission in PMN with a generally good safety profile with mostly mild adverse reactions. The limited evidence available from RCTs that were included in our review showed a treatment effect in the same direction.
Authors' conclusions
Implications for practice.
In this review update, we found that immunosuppressive therapy compared to non‐immunosuppressive therapy is probably beneficial for inducing remission and improving kidney survival in adult patients with PMN and nephrotic syndrome. The combination of an alkylating agent and corticosteroid regimen had short‐ and long‐term benefits, including greater induction of remission and lower rates of ESKD. It should be emphasised that the number of included studies with high‐quality design and appropriate blinding was relatively small and most of the included studies did not have adequate follow‐up or enough power to assess the prespecified definite endpoints, such as death and ESKD. Clinicians and patients should be aware of the low certainty of the evidence for these benefits as well as the well‐recognised adverse events of therapy. Whether this combined therapy should be indicated in all adult patients at high risk of progression to ESKD or only restricted to those with deteriorating kidney function remains unclear.
An alkylating agent (CPA or chlorambucil) combined with a corticosteroid regimen may be beneficial for adult patients with PMN and nephrotic syndrome, however, this was associated with a higher rate of adverse events.
Therapy with a CNI such as TAC was recommended by the 2020 KDIGO Clinical Practice Guideline as a treatment regimen for adults with PMN and nephrotic syndrome (KDIGO 2020); however, it remains uncertain whether CNI could alter clinical outcomes such as death or ESKD. We found that treatment regimens of alkylating agents were equivalent to CNI with or without steroids on complete or total remission rates. Given the low certainty of the evidence, we cannot conclude that there is superiority over alkylating agents with the currently available evidence. Compared with no treatment or non‐immunosuppressive supportive treatment, CNI showed little or no effect on complete and total remission rates; however, the certainty of this evidence is low because of study limitations and only a few RCTs with a small number of patients have been conducted.
There is low certainty of the evidence for the use of MMF in PMN. The number of corresponding studies for rituximab, ACTH, are still too sparse to draw firm conclusions for clinical practice. Observational trials may support the limited body of evidence from RCTs on both the use of rituximab (Fiorentino 2016; Ruggenenti 2006; Ruggenenti 2016) and ACTH (Berg 1999; Bomback 2011; Kittanamongkolchai 2016; Ponticelli 2006) until more high‐quality RCTs become available.
Finally, the presence and the level of circulating antibodies, such as anti‐PLA2R‐, anti‐THSD7A‐ or NELL1‐antibodies may provide guidance in assessing immunological disease activity and response to treatment. This has been acknowledged in the scientific literature and recent updates to international treatment guidelines (KDIGO 2020). This review did not assess immunological disease activity based on antibody titres as only a few of the included studies have provided this data.
Implications for research.
There is a need for more methodologically sound studies with an emphasis on adequate sample size and follow‐up. This may require international multi‐centre collaboration and the use of registry‐based RCTs to clarify the risks and eventual benefits and harms of therapy, with the use of registry databases capturing important longer‐term clinical outcomes. When possible, blinding of participants, clinicians and outcome assessors should be performed. Studies should also report the histopathological subclasses of PMN that are present at the initial biopsy. Furthermore, priority should be given to the use of definite rather than surrogate endpoints in studies. Moving forward, immunosuppressive treatments should be directly compared with alkylating agents and corticosteroids after the superiority of this treatment over no treatment, non‐immunosuppressive treatment and corticosteroid‐monotherapy has now been established in patients with persistent nephrotic syndrome, deteriorating kidney function and those at high risk of developing ESKD.
The optimal dose/s, route/s of administration, and duration of therapies that are most beneficial and least harmful to patients of different ethnicity, ages, and clinical and pathological severity still need to be clarified. It is noteworthy that many of the recently published trials were conducted in China, and the generalisability of these findings to patients of other ethnicities is unclear. Therefore, a greater geographical and ethnic diversity of study participants may be beneficial in future studies. Standardised outcomes (as currently developed by the Standardised Outcomes in Nephrology (SONG-Glomerular Disease group) should be considered in the design of new studies to ensure better comparability of results between different trials and to ensure that both clinical outcomes and patient‐reported outcomes are assessed and reported in studies.
Certainty of the evidence for CNI and MMF remains low and with an unclear profile of side effects. Therefore, further research into the efficacy and side effects of MMF and CNI treatment regimens with long‐term follow‐up is needed to better inform this evidence. As for tacrolimus, a shorter treatment period of six months compared to longer treatment periods demonstrated encouraging results in Yuan 2013 and Di 2018. Further studies of this treatment regimen would be helpful to further strengthen the evidence for this practice, which may be beneficial to patients.
A combination of two non‐steroidal immunosuppressive treatments compared with one non‐steroidal immunosuppressive treatment combined with steroids should be investigated further to evaluate whether steroid‐free treatment regimens may be appropriate for the treatment of PMN with nephrotic syndrome. Future studies in this area should investigate and report adverse events so that the safety of dual treatment can be assessed.
Following up on the promising early results in observational or dose‐finding studies, new therapies such as rituximab (Remuzzi 2002; Zou 2018) and ACTH (Hladunewich 2014) require further investigation with RCTs with more participants and longer follow‐up to inform clinical practice.
Finally, there is growing insight into the role of anti‐PLA2R antibodies (Beck 2009) and anti‐THSD71 antibodies (Tomas 2014) both in research and the clinical management of PMN. Therefore, it would be helpful for future studies to include serial measurement of anti‐PLA2R antibodies and anti‐THSD7A antibodies to help guide immunosuppressive therapy in PMN and to improve the understanding of treatment effects.
Future studies should provide adequate follow‐up of patients in order to better understand complications (such as adverse events, infections, development of malignancies) and the rates of relapse in patients that initially achieved remission.
What's new
| Date | Event | Description |
|---|---|---|
| 8 November 2021 | New citation required and conclusions have changed | New comparisons in this review for included studies that investigated novel treatments (rituximab, ACTH, traditional Chinese medicine, mizoribine) |
| 8 November 2021 | New search has been performed | Search strategy update; recently published studies included in this review for already existing comparisons |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 19 November 2014 | Amended | Minor edit to study names and number of reports of studies excluded and awaiting classification |
| 30 June 2014 | New citation required and conclusions have changed | The conclusion has been changed in this update |
| 30 June 2014 | New search has been performed | New search undertaken, new studies identified and included |
| 9 October 2008 | Amended | Converted to new review format. |
| 30 April 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank Ms Gail Higgins, Cochrane Kidney and Transplant's Senior Information Specialist, who provided us with the Cochrane Library search strategy and relevant information, and Dr Fiona Russell, the Cochrane Kidney and Transplant Managing Editor, for their help and support. We are grateful to Professor G Remuzzi, Dr A Chianca, Dr LA Tjosvold, Dr L Tammuzzo who were involved in the original design of the review, and we thank Dr A Schieppati, Dr X Chen, Dr G Cai, Dr J Zamora, Dr GA Giuliano, Professor N Braun, and Dr A Perna for their contribution to the Cochrane 2013 review update.
We are indebted to the principal investigators of the completed and ongoing studies considered in the review, who provided additional information or clarification for the original review (Professor Daniel C Cattran, Professor Peter Mathieson, Dr Roberto Pisoni, Professor Sanjay Kumar Agarwal and Professor Teut Risler, Professor Wetzels). We are grateful for Dr. John Carlisle and Dr. Bernardo Sousa Pinto for their statistical advice regarding the simdistir‐method for assessing baseline data. Finally, we are grateful to Przemyslaw Holko, Institute of Public Health, Jagiellonian University for the translation of articles from Polish to English.
Appendices
Appendix 1. Electronic search strategies
| Databases | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
NOTE: Search strategies used in the original review can be found in Schieppati 2004
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Corticosteroids versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.11, 3.23] |
| 1.2 ESKD (dialysis/transplantation) | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.35, 1.98] |
| 1.3 Complete or partial remission | 3 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.58, 2.27] |
| 1.4 Complete remission | 2 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.46, 1.28] |
| 1.5 Partial remission | 2 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.34, 5.21] |
| 1.6 Increase in serum creatinine | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 100% increase in serum creatinine | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.11, 1.53] |
| 1.6.2 50% increase in serum creatinine | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.34, 0.94] |
| 1.7 Adverse events | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.11, 9.82] |
| 1.8 Final serum creatinine | 1 | 87 | Mean Difference (IV, Random, 95% CI) | 48.00 [‐21.30, 117.30] |
| 1.9 Final CrCl | 1 | 86 | Mean Difference (IV, Random, 95% CI) | 8.00 [‐9.88, 25.88] |
| 1.10 Final proteinuria | 1 | 86 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐1.99, 1.99] |
Comparison 2. Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Death | 16 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.34, 1.59] |
| 2.1.1 Final follow‐up < 10 years | 15 | 840 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.36, 1.85] |
| 2.1.2 Final follow‐up ≥ 10 years | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.22] |
| 2.2 ESKD (dialysis/transplantation) | 16 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 0.99] |
| 2.2.1 Final follow‐up < 10 years | 14 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.49, 1.44] |
| 2.2.2 Final follow‐up ≥ 10 years | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.13, 0.63] |
| 2.3 Complete or partial remission | 16 | 879 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.05, 1.97] |
| 2.3.1 Final follow‐up < 2 years | 11 | 524 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.91, 2.14] |
| 2.3.2 Final follow‐up ≥ 2 years | 5 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.90, 2.65] |
| 2.4 Complete remission | 16 | 879 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [1.05, 2.75] |
| 2.4.1 Final follow‐up < 2 years | 12 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.84, 2.95] |
| 2.4.2 Final follow‐up ≥ 2 years | 4 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.87, 4.54] |
| 2.5 Partial remission | 16 | 879 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.93, 1.98] |
| 2.5.1 Final follow‐up < 2 years | 11 | 524 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.87, 2.53] |
| 2.5.2 Final follow‐up ≥ 2 years | 5 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.65, 2.16] |
| 2.6 Relapse after complete or partial remission | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.05, 2.86] |
| 2.6.1 Final follow‐up (≥ 2 years) | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.05, 2.86] |
| 2.7 100% increase in serum creatinine | 8 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.26, 0.80] |
| 2.7.1 Steroids versus placebo/no treatment at 24 months | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.05, 0.85] |
| 2.7.2 Alkylating agents ± steroids versus placebo/no treatment/supportive therapy at final follow‐up (≤ 2 years) | 3 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.05, 2.94] |
| 2.7.3 Alkylating agents ± steroids versus placebo/no treatment/supportive therapy at final follow‐up (> 2 years) | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.24, 1.20] |
| 2.7.4 Calcineurin inhibitors + steroids versus placebo/no treatment (60 months) | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.11] |
| 2.7.5 MMF versus placebo/no treatment at final follow‐up (12 months) | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.7.6 Azathioprine versus placebo/no treatment at final follow‐up (12 months) | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.07, 9.18] |
| 2.8 50% increase in serum creatinine | 8 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.33, 0.81] |
| 2.8.1 Steroids versus placebo/no treatment at 36 months | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.34, 0.94] |
| 2.8.2 Alkylating agents versus placebo/no treatment at final follow‐up (≤ 2 years) | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.20, 4.91] |
| 2.8.3 Alkylating agents ± steroids versus placebo/no treatment at final follow‐up (> 2 years) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.15, 0.68] |
| 2.8.4 Calcineurin inhibitors versus placebo/no treatment (30 months) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.18] |
| 2.8.5 MMF versus placebo/no treatment (12 months) | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.8.6 Azathioprine versus placebo/no treatment (12 months) | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [0.25, 68.16] |
| 2.8.7 Mizoribine versus placebo/no treatment (6 months) | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.23, 2.16] |
| 2.9 Temporary or permanent discontinuation/hospitalisation due to adverse events | 16 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 5.33 [2.19, 12.98] |
| 2.9.1 Steroids versus placebo/no treatment | 3 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [0.37, 12.96] |
| 2.9.2 Alkylating agents ± steroids versus placebo/no treatment/supportive therapy | 7 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 8.14 [2.22, 29.82] |
| 2.9.3 Calcineurin inhibitors versus placebo/no treatment/supportive therapy | 5 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 5.45 [0.29, 101.55] |
| 2.9.4 MMF versus placebo/no treatment | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 8.10 [0.47, 140.24] |
| 2.9.5 Azathioprine versus placebo/no treatment | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.9.6 Mizoribine versus placebo/no treatment | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 4.29 [0.21, 86.80] |
| 2.10 Adverse events | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.85, 1.89] |
| 2.10.1 Alkylating agents + steroids versus supportive therapy | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.83, 1.95] |
| 2.10.2 Rituximab versus supportive therapy | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.41, 3.69] |
| 2.11 Infection | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.69, 12.61] |
| 2.12 Malignancy | 2 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.12, 9.14] |
| 2.13 Final serum creatinine | 5 | 198 | Mean Difference (IV, Random, 95% CI) | 25.43 [10.09, 40.78] |
| 2.13.1 Steroids versus placebo/no treatment at final follow‐up (36 months) | 1 | 87 | Mean Difference (IV, Random, 95% CI) | 48.00 [‐42.71, 138.71] |
| 2.13.2 Alkylating agents ± steroids versus placebo/no treatment at final follow‐up (24 to 120 months) | 2 | 81 | Mean Difference (IV, Random, 95% CI) | 26.41 [10.24, 42.58] |
| 2.13.3 Calcineurin inhibitors versus placebo/no treatment at final follow‐up (12 months) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 11.50 [‐50.19, 73.19] |
| 2.13.4 Azathioprine versus placebo/no treatment at final follow‐up (12 months) | 1 | 9 | Mean Difference (IV, Random, 95% CI) | ‐53.10 [‐219.98, 113.78] |
| 2.14 Final GFR [mL/min/1.73 m²] | 8 | 296 | Mean Difference (IV, Random, 95% CI) | 9.59 [3.84, 15.33] |
| 2.14.1 Steroids versus placebo/no treatment at final follow‐up (36 months) | 1 | 86 | Mean Difference (IV, Random, 95% CI) | 8.00 [‐11.49, 27.49] |
| 2.14.2 Alkylating agents ± steroids versus placebo/no treatment/supportive therapy at final follow‐up (9 to 120 months) | 3 | 125 | Mean Difference (IV, Random, 95% CI) | 6.06 [‐6.74, 18.87] |
| 2.14.3 Calcineurin inhibitors versus placebo/no treatment/supportive therapy at final follow‐up (9 to 24 months) | 3 | 44 | Mean Difference (IV, Random, 95% CI) | 4.20 [‐10.65, 19.05] |
| 2.14.4 MMF versus placebo/no treatment at final follow‐up (12 months) | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 12.37 [‐4.93, 29.67] |
| 2.14.5 Azathioprine versus placebo/no treatment at final follow‐up (12 months) | 1 | 9 | Mean Difference (IV, Random, 95% CI) | 33.00 [‐19.01, 85.01] |
| 2.15 Final proteinuria | 9 | 402 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.75, ‐0.08] |
| 2.15.1 Steroids versus placebo/no treatment (36 months) | 1 | 86 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐1.99, 1.99] |
| 2.15.2 Alkylating agents ± steroids versus placebo/no treatment/supportive therapy (12 months) | 2 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.85, ‐0.07] |
| 2.15.3 Alkylating agents ± steroids versus placebo/no treatment/supportive therapy at final follow‐up (24 to 120 months) | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐3.69, ‐0.44] |
| 2.15.4 Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive therapy (24 months) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐4.53, 7.13] |
| 2.15.5 Calcineurin inhibitors + steroids versus supportive therapy at final follow‐up (9 to 21 months) | 2 | 32 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐6.62, 3.22] |
| 2.15.6 Azathioprine versus placebo/no treatment (12 months) | 1 | 9 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐2.79, 4.99] |
2.8. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 8: 50% increase in serum creatinine
2.10. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 10: Adverse events
2.11. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 11: Infection
2.12. Analysis.

Comparison 2: Immunosuppressive treatment ± steroids versus placebo/no treatment/non‐immunosuppressive supportive treatment, Outcome 12: Malignancy
Comparison 3. Immunosuppressive treatment ± steroids versus steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Follow‐up < 10 years | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.19, 12.01] |
| 3.2 ESKD (dialysis/transplantation) | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.36, 2.58] |
| 3.3 Complete or partial remission | 5 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.19, 1.82] |
| 3.3.1 Complete or partial remission (< 2 years) | 3 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.93, 2.37] |
| 3.3.2 Complete or partial remission at final follow‐up (≥ 2 years) | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.15, 1.86] |
| 3.4 Complete remission | 4 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.34, 2.65] |
| 3.4.1 Complete remission at final follow‐up (< 2 years) | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.60, 4.60] |
| 3.4.2 Complete remission at final follow‐up (≥ 2 years) | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.93, 3.22] |
| 3.5 Partial remission | 4 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.42, 3.97] |
| 3.5.1 Partial remission at final follow‐up (< 2 years) | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.50, 6.98] |
| 3.5.2 Partial remission at final follow‐up (≥ 2 years) | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.01, 18.32] |
| 3.6 Relapse after complete or partial remission | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.33, 2.28] |
| 3.7 Increase in serum creatinine | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.7.1 100% increase in serum creatinine | 3 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.52, 2.71] |
| 3.7.2 50% increase in serum creatinine | 3 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.34, 1.59] |
| 3.8 Temporary or permanent discontinuation/hospitalisation due to adverse events | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 4.18 [0.49, 35.97] |
| 3.9 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.9.1 Adverse events | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.55, 3.30] |
| 3.9.2 Malignancy | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.07, 16.20] |
3.5. Analysis.

Comparison 3: Immunosuppressive treatment ± steroids versus steroids, Outcome 5: Partial remission
Comparison 4. Cyclophosphamide + leflunomide + steroid versus cyclophosphamide + steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Complete remission | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.04, 2.17] |
Comparison 5. Oral alkylating agents ± steroids versus placebo/no treatment/steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Death | 7 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.25, 2.30] |
| 5.1.1 Follow‐up < 10 years | 5 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.37, 8.22] |
| 5.1.2 Follow‐up ≥ 10 years | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.58] |
| 5.2 ESKD (dialysis/transplantation) | 9 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.24, 0.74] |
| 5.2.1 Final follow‐up < 10 years | 7 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.29, 1.44] |
| 5.2.2 Final follow‐up ≥ 10 years | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.13, 0.63] |
| 5.3 Complete or partial remission | 9 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.04, 1.82] |
| 5.3.1 Complete or partial remission at final follow‐up (< 2 years) | 4 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.76, 2.09] |
| 5.3.2 Complete or partial remission at final follow‐up (≥ 2 years) | 5 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.04, 2.04] |
| 5.4 Complete remission | 8 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [1.33, 3.38] |
| 5.4.1 Complete remission at final follow‐up (< 2 years) | 3 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.46, 18.52] |
| 5.4.2 Complete remission at final follow‐up (≥ 2 years) | 5 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.22, 3.60] |
| 5.5 Partial remission | 8 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.57, 1.55] |
| 5.5.1 Partial remission at final follow‐up (< 2 years) | 3 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.37, 1.87] |
| 5.5.2 Partial remission at final follow‐up (≥ 2 years) | 5 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.48, 1.91] |
| 5.6 Increase in serum creatinine | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.6.1 100% increase in serum creatinine | 7 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.30, 1.16] |
| 5.6.2 50% increase in serum creatinine | 6 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.33, 1.08] |
| 5.7 Relapse after complete or partial remission | 3 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.40, 1.61] |
| 5.8 Temporary or permanent discontinuation/hospitalisation due to adverse events | 8 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 6.82 [2.24, 20.71] |
| 5.9 Adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.9.1 Adverse events | 3 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.96, 2.15] |
| 5.9.2 Infection | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.30, 9.45] |
| 5.9.3 Malignancy | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.21, 12.37] |
| 5.10 Final GFR [mL/min/1.73 m²] | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐5.33 [‐26.46, 15.80] |
Comparison 6. Cyclophosphamide + steroids versus chlorambucil + steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Death | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.14, 65.90] |
| 6.2 ESKD (dialysis/transplantation) | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 3.01 [0.61, 14.81] |
| 6.3 Complete or partial remission | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.01, 1.50] |
| 6.4 Complete remission | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.84, 2.90] |
| 6.5 Partial remission | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.41, 2.15] |
| 6.6 Increase in serum creatinine | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.6.1 100% increase in serum creatinine (15 months) | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 6.00 [0.87, 41.21] |
| 6.6.2 50% increase in serum creatinine (15 to 39 months) | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.93, 4.39] |
| 6.7 Temporary or permanent discontinuation/hospitalisation due to adverse events | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.13, 1.82] |
| 6.8 Final serum creatinine | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 28.25 [‐73.04, 129.54] |
| 6.9 Final proteinuria | 1 | 87 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.53, 0.69] |
6.1. Analysis.

Comparison 6: Cyclophosphamide + steroids versus chlorambucil + steroids, Outcome 1: Death
Comparison 7. Early (immediate) cyclophosphamide + steroids versus late (when SCr increase > 25%) cyclophosphamide + steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Death | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.50] |
| 7.2 ESKD (dialysis/transplantation) | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [0.12, 58.48] |
| 7.3 Complete or partial remission | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.77, 1.69] |
| 7.4 Complete remission | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.45] |
| 7.5 Partial remission | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 4.29 [0.58, 31.79] |
| 7.6 Temporary or permanent discontinuation/hospitalisation due to adverse events | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.07, 1.16] |
| 7.7 Final serum creatinine | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐12.00 [‐73.26, 49.26] |
| 7.8 Final GFR [mL/min/1.73 m²] | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 8.00 [‐8.59, 24.59] |
| 7.9 Final proteinuria | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐0.64, 1.82] |
Comparison 8. Cyclophosphamide + leflunomide + steroid versus leflunomide + steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Complete remission | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.99, 1.98] |
| 8.2 Malignancy | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 5.59 [0.28, 112.34] |
Comparison 9. Mycophenolate mofetil + calcineurin inhibitors versus calcineurin inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 ESKD (dialysis/transplantation) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1.1 MMF + TAC versus TAC alone | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.07, 14.90] |
| 9.2 Complete or partial remission | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.99, 1.48] |
| 9.3 Complete remission | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.93, 1.51] |
| 9.3.1 MMF + CSA versus CSA alone | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.86, 1.86] |
| 9.3.2 MMF + TAC versus TAC alone | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.83, 1.55] |
| 9.4 Partial remission | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.56, 3.18] |
| 9.5 Relapse after complete or partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.5.1 MMF + TAC versus TAC alone | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.41, 1.73] |
| 9.6 Severe adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.6.1 MMF + TAC versus TAC alone | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.70, 7.76] |
Comparison 10. Calcineurin inhibitors ± steroids versus placebo/no treatment/supportive treatment/steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Death | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1.1 Death (any cause) | 7 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.46, 6.14] |
| 10.1.2 Death due to deteriorating kidney function | 3 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 2.27 [0.35, 14.75] |
| 10.2 ESKD (dialysis/transplantation) | 7 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.54, 2.60] |
| 10.3 Complete or partial remission | 5 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.62, 2.38] |
| 10.3.1 Complete or partial remission (< 2 years) | 3 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.30, 3.22] |
| 10.3.2 Complete or partial remission (≥ 2 years) | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.39, 7.28] |
| 10.4 Complete remission | 5 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.51, 2.24] |
| 10.4.1 Patients with normal kidney function | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.51, 2.92] |
| 10.4.2 Patients with deteriorating kidney function | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.03] |
| 10.5 Partial remission | 5 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.53, 2.22] |
| 10.5.1 Patients with normal kidney function | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.51, 2.78] |
| 10.5.2 Patients with deteriorating kidney function | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.15, 3.53] |
| 10.6 Relapse after complete or partial remission | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.79, 3.09] |
| 10.7 Increase in serum creatinine | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.7.1 100% increase in serum creatinine | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.86] |
| 10.7.2 50% increase in serum creatinine | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.05, 5.75] |
| 10.8 Temporary or permanent discontinuation/hospitalisation due to adverse events | 5 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 5.45 [0.29, 101.55] |
| 10.9 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.9.1 Serious adverse events | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.70, 1.90] |
| 10.9.2 Infection | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 4.11 [0.94, 18.06] |
| 10.9.3 Malignancy | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.14, 56.57] |
Comparison 11. Calcineurin inhibitors ± steroids versus alkylating agents ± steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Death | 7 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.35, 2.34] |
| 11.2 ESKD (dialysis/transplantation) | 5 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [0.64, 9.01] |
| 11.3 Complete or partial remission | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.3.1 Complete or partial remission at final follow‐up | 10 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.15] |
| 11.3.2 Complete or partial remission at final follow‐up (≥ 2 years) | 3 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.35] |
| 11.4 Complete remission | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.4.1 Complete remission at final follow‐up | 10 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.84, 1.56] |
| 11.4.2 Complete remission at final follow‐up (≥ 2 years) | 3 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.47, 2.18] |
| 11.5 Partial remission | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.5.1 Partial remission at final follow‐up | 10 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.18] |
| 11.5.2 Partial remission at final follow‐up (≥ 2 years) | 3 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.32] |
| 11.6 Relapse after complete or partial remission | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.6.1 Relapse after complete or partial remission (< 2 years) | 6 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [0.71, 6.37] |
| 11.6.2 Relapse after complete or partial remission (≥ 2 years) | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 3.78 [1.01, 14.18] |
| 11.7 Increase in serum creatinine | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.7.1 100% increase in serum creatinine | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.30, 1.67] |
| 11.7.2 50% increase in serum creatinine | 4 | 286 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.8 Temporary or permanent discontinuation/hospitalisation due to adverse events | 3 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.31, 6.67] |
| 11.9 Adverse events | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.9.1 Serious adverse events | 10 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.20] |
| 11.9.2 Infection | 9 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.43, 1.71] |
| 11.9.3 Malignancy | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.69] |
| 11.10 Final serum creatinine | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.04, 0.16] |
| 11.11 Final serum albumin | 5 | 227 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐1.82, 4.49] |
| 11.12 Final GFR [mL/min/1.73 m²] | 4 | 206 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐6.94, 5.90] |
| 11.13 Loss of GFR > 20% | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.00, 1.95] |
| 11.14 Final proteinuria | 8 | 443 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.66, 0.26] |
Comparison 12. Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.2 Complete or partial remission | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.42, 1.10] |
| 12.2.1 6 months versus 12 months TAC | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.33, 0.81] |
| 12.2.2 12 months versus 24 months TAC | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.65, 1.07] |
| 12.3 Complete remission | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.97] |
| 12.3.1 6 months versus 24 months TAC | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.14] |
| 12.3.2 12 months versus 24 months TAC | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.25, 1.01] |
| 12.4 Partial remission | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.30, 1.99] |
| 12.4.1 6 months versus 24 months TAC | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.23, 0.94] |
| 12.4.2 12 months versus 24 months TAC | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.71, 2.06] |
| 12.5 Relapse after complete or partial remission | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 7.25 [0.41, 129.75] |
| 12.6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.6.1 Adverse events | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.35, 2.87] |
| 12.6.2 Infection | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.20, 2.88] |
| 12.7 Final serum creatinine | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐10.98, 7.69] |
| 12.7.1 6 months versus 24 months TAC | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐18.10, 13.50] |
| 12.7.2 12 months versus 24 months TAC | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐12.87, 10.27] |
| 12.8 Final serum albumin | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐6.40 [‐8.75, ‐4.05] |
| 12.9 Final proteinuria | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 1.70 [1.34, 2.06] |
12.1. Analysis.

Comparison 12: Short‐course tacrolimus + steroids versus long‐course tacrolimus + steroids, Outcome 1: Death
Comparison 13. Cyclosporine + steroids versus cyclosporine alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.2 Complete or partial remission | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.77, 1.33] |
| 13.3 Complete remission | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [1.07, 4.49] |
| 13.4 Partial remission | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.06, 3.17] |
| 13.5 50% increase in serum creatinine | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.39, 5.23] |
| 13.6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.6.1 Adverse events | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [1.13, 4.97] |
| 13.6.2 Infection | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.22, 21.03] |
13.1. Analysis.

Comparison 13: Cyclosporine + steroids versus cyclosporine alone, Outcome 1: Death
Comparison 14. Cyclosporine (3.0 mg/kg, once/day) + steroids versus cyclosporine (1.5 mg/kg, twice/day) + steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Complete or partial remission | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.18] |
| 14.2 Complete remission | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.73, 2.27] |
| 14.3 Doubling of serum creatinine | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.17, 7.10] |
| 14.4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.4.1 Adverse events | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 14.4.2 Infection | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [0.14, 76.01] |
| 14.4.3 Malignancy | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.45] |
Comparison 15. Cyclosporine + steroids versus tacrolimus + steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Complete or partial remission | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 15.2 Complete remission | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.31, 1.89] |
| 15.3 Partial remission | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.40, 2.10] |
| 15.4 Serious adverse events | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.49, 1.19] |
Comparison 16. Cyclosporine versus azathioprine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 16.2 ESKD (dialysis/transplantation) | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.02, 9.43] |
| 16.3 Complete or partial remission | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.68, 2.48] |
| 16.4 Complete remission | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.40, 9.54] |
| 16.5 Partial remission | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.37, 2.90] |
| 16.6 Increase in serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.6.1 50% increase in serum creatinine | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.87] |
| 16.7 Temporary or permanent discontinuation/hospitalisation due to adverse events | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.01, 4.78] |
| 16.8 Final serum creatinine | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐102.50 [‐280.28, 75.28] |
| 16.9 Final GFR [mL/min/1.73 m²] | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 23.20 [‐1.98, 48.38] |
| 16.10 Final proteinuria | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐2.02, 4.02] |
Comparison 17. Azathioprine ± steroids versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.2 ESKD (dialysis/transplantation) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.3 Complete or partial remission | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 5.43] |
| 17.4 Complete remission | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 5.43] |
| 17.5 Partial remission | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 17.6 Increase in serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.6.1 100% increase in serum creatinine | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.07, 9.18] |
| 17.6.2 50% increase in serum creatinine | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [0.25, 68.16] |
| 17.7 Temporary or permanent discontinuation/hospitalisation due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.8 Final serum creatinine | 1 | 9 | Mean Difference (IV, Random, 95% CI) | ‐53.10 [‐219.98, 113.78] |
| 17.9 Final GFR [mL/min/1.73 m²] | 1 | 9 | Mean Difference (IV, Random, 95% CI) | 33.00 [‐19.01, 85.01] |
| 17.10 Final proteinuria | 1 | 9 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐2.79, 4.99] |
17.1. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 1: Death
17.2. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 2: ESKD (dialysis/transplantation)
17.5. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 5: Partial remission
17.7. Analysis.

Comparison 17: Azathioprine ± steroids versus no treatment, Outcome 7: Temporary or permanent discontinuation/hospitalisation due to adverse events
Comparison 18. Mycophenolate mofetil versus no treatment/supportive therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.2 ESKD (dialysis/transplantation) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.3 Complete or partial remission | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.52, 2.48] |
| 18.4 Complete remission | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.06, 5.64] |
| 18.5 Partial remission | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.52, 3.56] |
| 18.6 Temporary or permanent discontinuation/hospitalisation due to adverse events | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 8.10 [0.47, 140.24] |
| 18.7 Increase in serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.7.1 100% increase in serum creatinine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 18.7.2 50% increase in serum creatinine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 18.8 Final GFR [mL/min/1.73 m²] | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 12.37 [‐4.93, 29.67] |
18.1. Analysis.

Comparison 18: Mycophenolate mofetil versus no treatment/supportive therapy, Outcome 1: Death
18.2. Analysis.

Comparison 18: Mycophenolate mofetil versus no treatment/supportive therapy, Outcome 2: ESKD (dialysis/transplantation)
18.6. Analysis.

Comparison 18: Mycophenolate mofetil versus no treatment/supportive therapy, Outcome 6: Temporary or permanent discontinuation/hospitalisation due to adverse events
18.7. Analysis.

Comparison 18: Mycophenolate mofetil versus no treatment/supportive therapy, Outcome 7: Increase in serum creatinine
Comparison 19. Mycophenolate mofetil ± steroids versus alkylating agents ± steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Death | 4 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.83] |
| 19.2 ESKD (dialysis/transplantation) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 19.3 Complete or partial remission | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.3.1 Complete or partial remission at final follow‐up) | 4 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.71, 1.13] |
| 19.3.2 Complete or partial remission at follow‐up (≥ 2 years) | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.56, 1.44] |
| 19.4 Complete remission | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.4.1 Complete remission at final follow‐up | 4 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.58, 1.73] |
| 19.4.2 Complete remission at follow‐up (≥ 2 years) | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.44, 2.29] |
| 19.5 Partial remission | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.5.1 Partial remission at final follow‐up | 4 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.58, 1.37] |
| 19.5.2 Partial remission at follow‐up (≥ 2 years) | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.37, 4.82] |
| 19.6 Relapse after complete or partial remission | 3 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.33, 5.43] |
| 19.7 Doubling of serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 19.8 Temporary or permanent discontinuation/hospitalisation due to adverse events | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 19.9 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.9.1 Severe adverse events | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 19.9.2 Infection | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.49, 2.60] |
| 19.10 Final serum creatinine | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐18.14, 14.94] |
| 19.11 Final serum albumin | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐1.63, 3.43] |
| 19.12 Final GFR [mL/min/1.73 m²] | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 3.75 [‐6.12, 13.62] |
| 19.13 Final proteinuria | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.89, 1.09] |
19.1. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 1: Death
19.2. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 2: ESKD (dialysis/transplantation)
19.6. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 6: Relapse after complete or partial remission
19.7. Analysis.

Comparison 19: Mycophenolate mofetil ± steroids versus alkylating agents ± steroids, Outcome 7: Doubling of serum creatinine
Comparison 20. Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Death | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.07, 15.26] |
| 20.2 ESKD (dialysis/transplantation) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 20.3 Complete or partial remission | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.70, 1.27] |
| 20.4 Complete remission | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.20, 1.63] |
| 20.5 Partial remission | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.88, 2.10] |
| 20.6 Relapse after complete remission | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.18, 7.74] |
| 20.7 Increase in serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 20.7.1 50% increase in serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 20.8 Temporary or permanent discontinuation/hospitalisation due to adverse events | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 20.9 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.9.1 Adverse events | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.63, 2.07] |
| 20.9.2 Infection | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.80, 3.12] |
| 20.9.3 Malignancy | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.06, 12.75] |
| 20.10 Final serum creatinine | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.12, 0.32] |
| 20.11 Final serum albumin | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.28, 0.10] |
| 20.12 Final GFR [mL/min/1.73 m²] | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐13.90 [‐31.05, 3.25] |
| 20.13 Final proteinuria | 2 | 97 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.45, 1.07] |
20.10. Analysis.

Comparison 20: Mycophenolate mofetil ± steroids versus calcineurin inhibitors ± steroids, Outcome 10: Final serum creatinine
Comparison 21. Adrenocorticotropic hormone versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Complete or partial remission | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [1.91, 25.62] |
| 21.2 Complete remission | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 11.00 [1.62, 74.88] |
| 21.3 Partial remission | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.35, 25.68] |
Comparison 22. Adrenocorticotropic hormone versus alkylating agents + steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 22.1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 22.2 ESKD (dialysis/transplantation) | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.57] |
| 22.3 Complete or partial remission | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.17] |
| 22.4 Complete remission | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.88, 4.54] |
| 22.5 Partial remission | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.16, 1.01] |
| 22.6 Increase in serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.6.1 100% increase in serum creatinine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.57] |
| 22.6.2 50% increase in serum creatinine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.57] |
| 22.7 Temporary or permanent discontinuation/hospitalisation due to adverse events | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.16, 6.25] |
| 22.8 Final serum creatinine | 1 | 31 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐19.07, 17.07] |
| 22.9 Final proteinuria | 1 | 31 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.19, ‐0.41] |
22.1. Analysis.

Comparison 22: Adrenocorticotropic hormone versus alkylating agents + steroids, Outcome 1: Death
22.2. Analysis.

Comparison 22: Adrenocorticotropic hormone versus alkylating agents + steroids, Outcome 2: ESKD (dialysis/transplantation)
Comparison 23. Mizoribine ± steroids versus placebo/no treatment/corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 23.1 Complete or partial remission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.1.1 Complete or partial remission at final follow‐up | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 2.24 [1.14, 4.38] |
| 23.1.2 Complete or partial remission at final follow‐up (≥ 2 years) | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 4.71 [0.66, 33.61] |
| 23.2 Complete remission | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.2.1 Complete remission at final follow‐up | 3 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.69, 3.84] |
| 23.2.2 Complete remission at final follow‐up (> 2 years) | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 5.60 [0.32, 98.21] |
| 23.3 Partial remission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.3.1 Partial remission at final follow‐up | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.90, 3.97] |
| 23.3.2 Partial remission at final follow‐up (≥ 2 years) | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [0.28, 19.66] |
| 23.4 Temporary or permanent discontinuation/hospitalisation due to adverse events | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 4.29 [0.21, 86.80] |
Comparison 24. Mizoribine: 150 mg (once/day) versus 50 mg (3 times/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 24.1 Complete or partial remission | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.51, 1.13] |
| 24.2 Complete remission | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.66, 2.78] |
| 24.3 Partial remission | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.97] |
| 24.4 Relapse after complete or partial remission | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.05, 3.51] |
| 24.5 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.5.1 Adverse events | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 24.5.2 Infection | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 24.5.3 Malignancy | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [0.24, 92.65] |
Comparison 25. Rituximab + supportive therapy versus supportive therapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 25.1 Complete or partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 25.1.1 Complete or partial remission (6 months) | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.37, 3.57] |
| 25.1.2 Complete or partial remission (median 17 months) | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.15, 3.13] |
| 25.2 Complete remission | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.78, 3.55] |
| 25.3 Partial remission | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [1.25, 7.62] |
| 25.4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 25.4.1 Serious adverse events | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.41, 3.69] |
| 25.4.2 Malignancy | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.14] |
| 25.5 Final serum creatinine | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐5.44, 4.64] |
| 25.6 Final serum albumin | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 5.70 [4.59, 6.81] |
| 25.7 Final GFR [mL/min/1.73 m²] | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐8.91, 0.91] |
| 25.8 Final protein:creatinine ratio | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.99, ‐0.70] |
| 25.9 Final PLA2R‐Ab titre | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐81.80 [‐105.38, ‐58.22] |
25.2. Analysis.

Comparison 25: Rituximab + supportive therapy versus supportive therapy alone, Outcome 2: Complete remission
25.3. Analysis.

Comparison 25: Rituximab + supportive therapy versus supportive therapy alone, Outcome 3: Partial remission
25.4. Analysis.

Comparison 25: Rituximab + supportive therapy versus supportive therapy alone, Outcome 4: Adverse events
25.5. Analysis.

Comparison 25: Rituximab + supportive therapy versus supportive therapy alone, Outcome 5: Final serum creatinine
25.6. Analysis.

Comparison 25: Rituximab + supportive therapy versus supportive therapy alone, Outcome 6: Final serum albumin
25.7. Analysis.

Comparison 25: Rituximab + supportive therapy versus supportive therapy alone, Outcome 7: Final GFR [mL/min/1.73 m²]
25.8. Analysis.

Comparison 25: Rituximab + supportive therapy versus supportive therapy alone, Outcome 8: Final protein:creatinine ratio
25.9. Analysis.

Comparison 25: Rituximab + supportive therapy versus supportive therapy alone, Outcome 9: Final PLA2R‐Ab titre
Comparison 26. Rituximab versus cyclosporine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 26.1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 26.2 ESKD (dialysis/transplantation) | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.03] |
| 26.3 Complete or partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.3.1 Complete or partial remission at end of therapy (12 months) | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.85, 1.56] |
| 26.3.2 Complete or partial remission at final follow‐up (2 years) | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [1.77, 5.07] |
| 26.4 Complete remission | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 47.00 [2.91, 757.81] |
| 26.5 Partial remission | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.65, 2.35] |
| 26.6 Relapse after complete or partial remission | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.02, 0.39] |
| 26.7 Quality of Life in patients with any remission | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 26.7.1 SF‐12 Score Physical Health | 1 | 130 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐5.03, 0.83] |
| 26.7.2 SF‐12 Score Mental Health | 1 | 130 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.56, 0.36] |
| 26.8 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.8.1 Serious adverse events | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.29, 1.05] |
| 26.8.2 Infection | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.55, 1.63] |
26.1. Analysis.

Comparison 26: Rituximab versus cyclosporine, Outcome 1: Death
26.2. Analysis.

Comparison 26: Rituximab versus cyclosporine, Outcome 2: ESKD (dialysis/transplantation)
Comparison 27. Traditional Chinese medicine versus immunosuppressive therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 27.1 Death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1.1 Shenqi particle versus CPA+steroids | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.73] |
| 27.1.2 Tripterygium wilfordii versus Tripterygium wilfordii+steroids | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 27.2 ESKD (dialysis/transplantation) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 27.2.1 Tripterygium wilfordii versus Tripterygium wilfordii+steroids | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 27.3 Complete or partial remission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.3.1 Shenqi particle versus CPA+steroids | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.77, 1.13] |
| 27.3.2 Tripterygium wilfordii versus Tripterygium wilfordii+steroids | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.32, 0.76] |
| 27.4 Complete remission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.4.1 Shenqi particle versus CPA+steroids | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.31, 1.16] |
| 27.4.2 Tripterygium wilfordii versus Tripterygium wilfordii+steroids | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.03, 0.54] |
| 27.5 Partial remission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.5.1 Shenqi particle versus CPA+steroids | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.81, 1.56] |
| 27.5.2 Tripterygium wilfordii versus Tripterygium wilfordii+steroids | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.47, 1.54] |
| 27.6 Doubling of serum creatinine | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.6.1 Shenqi particle versus CPA+steroids | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.79] |
| 27.6.2 Tripterygium wilfordii versus Tripterygium wilfordii+steroids | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 27.7 Severe adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.7.1 Shenqi particle versus CPA+steroids | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.77] |
| 27.7.2 Tripterygium wilfordii versus Tripterygium wilfordii+steroids | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.33, 5.87] |
| 27.8 Final serum albumin | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 27.8.1 Shenqi particle versus CPA+steroids | 1 | 132 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐3.40, 2.46] |
| 27.9 Final GFR [mL/min/1.73 m²] | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 27.9.1 Shenqi particle versus CPA+steroids | 1 | 132 | Mean Difference (IV, Random, 95% CI) | 19.00 [7.85, 30.15] |
| 27.10 Final proteinuria | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 27.10.1 Shenqi particle versus CPA+steroids | 1 | 132 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.69, 1.01] |
27.6. Analysis.

Comparison 27: Traditional Chinese medicine versus immunosuppressive therapy, Outcome 6: Doubling of serum creatinine
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2012a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Primary and secondary endpoints comprehensively reported; trial registered at clinical trial registry |
| Other bias | Unclear risk | Incomplete reporting. No financial disclosures provided |
Ahmed 1994.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Not sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Appel 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | No publication found 19 years after study ended |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Arnadottir 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Only remission data could be extracted from the abstract |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Austin 1996a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Data could not be extracted |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Braun 1995.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The patients were randomised into one of the two treatment groups (1986 to 1990) using sealed envelopes that contained the treatment protocol and that were numbered according to a table of randomisation. The study group decided to change the randomisation protocol in 1990 by adding a control group to the two treatment arms. Patients were then randomised into one of the two treatment groups or the control group (1991 to 1996) using a computer based‐randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method described could usually not allow investigators/participants to know or influence intervention group before eligible participant entered in the study. But the authors failed to clarify the randomisation was centrally performed and it was possible for investigators to open the sealed envelopes in advance |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 97/124 (78%) randomised patients were entered to the final analysis. Furthermore, of these 97 patients 18 were lost to follow‐up and 11 did not complete the five‐year follow‐up. Eventually only 68/124 (55%) completed the five‐year follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | High risk | Only abstract was available and unpublished data were included |
Cameron 1990.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomization was performed centrally, and coded tablets given locally from bottles supplied from the co‐ordinator" |
| Allocation concealment (selection bias) | Low risk | Randomisation method described could not allow investigators/participants to know or influence intervention group before eligible participant entered in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical tablets were used, that contained either 5 mg of prednisolone or placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 patients (8%) in the treatment group were lost at 4, 6, 21, and 24 months and 3 (6%) in the placebo group at 9, 18, and 21 months. Their data to the point of loss have been included in the analysis on an intention‐to‐treat basis. No patient lost was in remission or had a plasma Cr of over 400 μmol/L when lost. Thus, missing outcome data balanced in numbers across intervention groups and have been imputed using appropriate methods |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Cattran 1989.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned by the study coordinator in Toronto Glomerulonephritis Registry according to a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Central Randomisation method described could not allow investigators/participants to know or influence intervention group before eligible participant entered in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27/158 (17%) patients were lost during follow‐up of 48 months: 10/81 (12%) in the prednisolone group and 17/77 (22%) in the control group |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | High risk | 158 patients were properly randomised, only 120 of them were diagnosed with nephrotic syndrome. The randomisation was not stratified according to nephrotic syndrome or non‐nephrotic syndrome |
Cattran 1995.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomly assigned to either CSA or placebo in blocks stratified by centre |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The patients were masked in regard to their assignment, but for safety reasons the physician in charge was not |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Cattran 2001.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the clinical coordinating centre from a table of random numbers and was stratified by centre in blocks of two to ensure a balance between groups |
| Allocation concealment (selection bias) | Low risk | Central randomisation method described could not allow investigators/participants to know or influence intervention group before eligible participant entered in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The patients were masked in regard to CSA versus placebo assignment. Novartis Canada Ltd. (Whitby, Ontario, Canada) provided CSA in a drink solution (100 mg/mL) and an identical placebo made from the same carrier. The physicians were not masked in regard to CSA versus placebo assignment for safety reasons |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The end points were objective and measured centrally by a lab blinded to patient designation. No further information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients except 2 patients completed the study. The reasons were relocation outside of North America and noncompliance |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Chan 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Patients who satisfied the selection criteria were randomised by drawing envelope into either one of two treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Chen 2010a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a clinical coordinating centre using a table of random numbers and was stratified by centres |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was performed by enclosing assignments in sequentially numbered, opaque‐closed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 13/73 patients (18%) did not finish the 12‐month follow‐up. 6/39 patients (15%) withdrew in the TAC group (infection (3); severe gastrointestinal complaint (1); elevated aminotransferase (1); patient's intention (1)). In the CPA group 7/34 patients (21%) did not finish the follow‐up: 3 patients withdrew (severe gastrointestinal complaint (1); elevated aminotransferase (1); patient's intention (1)) and 4 patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Chen 2013e.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of treatment
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation as per random sequence. SAS program PROC PLAN |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High drop‐out rate (58/190); main reasons were a) took other medication, b) missed follow‐up visit |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes reported |
| Other bias | Low risk | Study appears free of other biases |
Choi 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group:
Duration
Co‐medications
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation technique, using SAS randomisation program, managed by statisticians in external department |
| Allocation concealment (selection bias) | Low risk | Sealed sequential numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of any blinding, except that both drugs were provided as prepacked drugs in identical bottles |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43 screened, 39 included, high drop‐out (5/18 and 9/21) however all randomised patients were included in analysis (intention‐to‐treat) |
| Selective reporting (reporting bias) | Low risk | Complete and partial remission are appropriate outcomes. However, improvement in hypoalbuminaemia and hypercholesterolaemia were not reported as secondary outcomes on clinicaltrials.gov but were reported in the trial |
| Other bias | Low risk | Drugs were provided free of charge by pharmaceutical company that however was not involved in the study in any other way |
Coggins 1979.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Immediately after admission to the study, patients were randomly allocated to prednisone or placebo. Randomization was stratified according to initial histologic diagnosis with the light microscope (before review by the Central Pathology Board) in the participating hospital |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were assigned without the knowledge of either the patient or physician to prednisone therapy or identical placebo control tablets (supplied by the Upjohn Company) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
CYCLOMEN 1994.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation method described could not allow investigators/participants to know or influence intervention group before eligible participant entered in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/22 (95%) randomised completed the treatment and were finally analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
CYPMEN 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Di 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment details
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete data. Comprehensive reporting. Low‐drop‐out rate (all due to severe adverse effects, which are reported) |
| Selective reporting (reporting bias) | High risk | No protocol reported, not all kidney outcomes reported |
| Other bias | Low risk | Conflict of Interest of authors not declared. Sources of Funding declared (public funding). No evidence of other bias |
Donadio 1974.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Only after a patient was deemed eligible was the treatment ascertained by referral to a list created from a table of random numbers (by WFT). The table was maintained by the renal pathologist (KEH) and was not seen by the clinicians (JVD and CFA) |
| Allocation concealment (selection bias) | Low risk | Neither patient nor clinician knew what treatment was going to be given before the patient agreed to enter study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/11 patients (18%) in the CPA group and 1/11 patients (9%) in the no‐drug group did not complete the 12‐month follow‐up. In 2 patients in the CPA group, the drug was stopped after 8 months, on the advice of the clinicians, when data analysis to that point revealed no treatment benefit either to these patients or to the 19 patients who had completed the study. 1 patient in the no‐drug group was dropped from the study because the patient was not considered to have purely IMN |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Dussol 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomization was performed by each centre through a centralized Internet on‐line application provided by the sponsor (minimization method). Randomization was stratified according to sex and centre" |
| Allocation concealment (selection bias) | Low risk | Central randomisation method described could not allow investigators/participants to know or influence intervention group before eligible participant entered in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Dyadyk 2001a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Data could not be extracted for meta‐analysis |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Falk 1992.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All patients were randomised under the same computer‐generated randomisation table through the central Glomerular Disease Collaborative Network office. Patients were stratified on the basis of whether they had deterioration in kidney function or persistent proteinuria with morbid complications |
| Allocation concealment (selection bias) | Low risk | Central randomisation method described could not allow investigators/participants to know or influence intervention group before eligible participant entered in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two (one in each group) patients had less than 18 months of follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Fu 2012a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
Follow‐up at 3, 12, 24 and 36 months |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. Groups are very similar in baseline characteristics, unlikely by chance. indicating some form of matching |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. all patients completed course of the study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. comprehensive reporting on all outcomes |
| Other bias | Low risk | No evidence of other bias. authors declare no conflict of interest |
GEMRITUX 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Does say analysis was performed blind but not specifically outcome determination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/77 (2.6%) excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Most appropriate outcome, remission was reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Hasegawa 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" and no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Very limited information provided, much data not reported, including primary outcome on 3/4 of measurement‐points |
| Other bias | Unclear risk | Insufficient information to permit judgement |
He 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pre‐printed randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not sure how many were screened or whether more were randomised however outcomes are comprehensively and appropriately chosen and reported |
| Selective reporting (reporting bias) | Low risk | Outcomes reasonably complete and appropriate |
| Other bias | Low risk | No evidence of other bias; no evidence for conflict of interest |
Hladunewich 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred in 1:1 ratio using a block randomisation technique |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome‐assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Poor reporting of outcomes within the randomised groups. no intention‐to‐treat analysis. many patients switched treatment arms. |
| Selective reporting (reporting bias) | High risk | Outcomes not properly reported for randomised groups separately |
| Other bias | High risk | High number of patients that changed the treatment‐arm during the study. SCr not reported in outcome measures. Industry‐funded trial, however the pharmaceutical company had no role in the design and/or evaluation of the study, nor the writing of the manuscript |
Hofstra 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐medication
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | During the first year of the study, 3 patients were excluded because of the following reasons: discovery of a malignancy and withdrawal from the study within 3 months; protocol violation (start of prednisone by a physician in another hospital) and loss to follow‐up due to emigration 7 months after randomisation. Thus, the final analysis included 26 patients |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Howman 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐medications: not reported |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table had been prepared to allocate patients to one of three groups. Patients were randomly assigned by a member of staff in the clinical trials office at the Glasgow Royal Infirmary, Glasgow, UK, who was not otherwise involved in the trial |
| Allocation concealment (selection bias) | Low risk | Allocation was not influenced by patient characteristics, random allocation by a non‐otherwise involved person at the clinical trials office at the Glasgow Royal Infirmary |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. "Treatment allocation was communicated by fax to the clinician entering the patient into the trial. We did not attempt to mask patients or investigators." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/108 (1.9%) excluded post randomisation; no evidence for missing data |
| Selective reporting (reporting bias) | Low risk | Outcomes appropriate and reasonably extensive; registered trial including outcomes |
| Other bias | Low risk | Methods, details and results well reported; no evidence of other risk of bias; no evidence for Conflict of Interest |
Imbasciati 1980.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | For all patients, the indications for therapy were contained in sealed, completely opaque envelopes numbered in sequence according to a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation method described could not allow investigators/participants to know or influence intervention group before eligible participant entered in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four patients in the treatment group did not complete the 6‐month therapy, these patients were continued to be followed up because of side effects. They were considered to be treated patients in the data analysis, according to the intention‐to‐treat principle. In the case of patients who died, data obtained before the time of death were included. 3/81 patients (3%) were lost to 5‐year follow‐up: two controls and one treated patient were lost to follow‐up 22, 28, and 24 months after randomisation, respectively. At the second analysis, 9/42 (21%) treated patients and 10/39 (26%) controls were lost to follow‐up from 12 to 96 months after randomisation. These 3 patients were also considered in the analyses |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Jha 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/104 (11%) patients were lost to follow‐up, 4/51 (8%) in treatment group and 7/53 (13%) in control group, between 18 to 48 month of randomisation and excluded from analysis |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Study appears free of other biases |
Jurubita 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient was lost to follow‐up, and an intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | High risk | Only remission data were provided in the abstract |
| Other bias | Unclear risk | Only abstract was available and unpublished data were not used |
Koshikawa 1993.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; no information on blinding of outcome‐assessors provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2/48 patients in the treatment group did not complete 24‐week follow‐up |
| Selective reporting (reporting bias) | High risk | The primary outcome such as death and ESKD were not reported |
| Other bias | High risk | The data were abstracted from a RCT aiming to investigate the effect of mizoribine on steroid‐resistant primary nephrotic syndrome. This study included all different pathologic variants of nephrotic syndrome. The randomisation was not stratified according to the pathologic diagnosis |
Kosmadakis 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group (supportive therapy only)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | High risk | There was a significant difference in the baseline GFR (P = 0.021). The sample size was also small for a 3‐arm study |
Li 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasonable and comprehensive outcome reporting. Unable to determine if more were randomised than reported |
| Selective reporting (reporting bias) | Low risk | Reports on remissions, most appropriate outcome. All outcomes were reported |
| Other bias | Low risk | No evidence for other bias. no evidence for conflict of interest, however, no trial protocol published |
Li 2017c.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to a randomisation list generated from the table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be reported |
| Other bias | Low risk | Study appears free of other biases |
Liang 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐medications
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported how random allocation was performed. Patients were able to switch their randomised intervention group after randomisation based on personal preferences, which some patients did |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not reported whether more were screened or allocated than were reported in the analysis however outcomes reported comprehensively |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest reported |
| Other bias | Low risk | No evidence for other bias; no evidence for financial conflict of interest |
Liu 2009b.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3/84 patients (all in treatment group 2) lost to 12‐month follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Published in Chinese |
Liu 2015e.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly divided into three groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Many outcomes not reported; abstract‐only publication |
| Other bias | Unclear risk | No information on potential conflict of interests and funding sources |
MENTOR 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐intervention (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization schedule was computer‐generated, stratified according to site, blocked with randomly varied block sizes of two and four, and concealed with the use of a Web‐based, locked central randomization system" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization schedule was computer‐generated, stratified according to site, blocked with randomly varied block sizes of two and four, and concealed with the use of a Web‐based, locked central randomization system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label study; many outcomes are based on laboratory results, however not stated how these results are interpreted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients have been accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes of relevant to this review have been reported |
| Other bias | High risk | Industry‐funded. Genentech provides its own drug free of charge for evaluation in this trial. Study PIs have a conflict of interest as they received funding from Genentech. |
Murphy 1992.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Insufficient information about the sequence generation process to permit judgement. However, it could be done |
| Allocation concealment (selection bias) | Low risk | After consent was obtained from patient, randomisation was performed by opening sealed envelops |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All except 1 patient completed the 2 years of follow‐up. One treatment group patient died 8 months after study entry, 2 months after cessation of CPA. As this patient had a severe nephrotic syndrome and was the only patient with progressive deterioration in kidney function, his death and consequent removal from the remainder of the study could have biased data at time points after 6 months in favour of a benefit of therapy. Accordingly, it was decided to enter dummy values for SCr and proteinuria. These dummy values were chosen to be higher (900 μmol/L for SCr and 30g/24 h for proteinuria) than all the other patients at that time point, in order to ensure that any bias introduced due to the death of this patient would be against an effect of treatment |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | High risk | 40 patients were properly randomised, only 26 were diagnosed with nephrotic syndrome, 13 in each group. The randomisation was not stratified according to nephrotic syndrome or non‐nephrotic syndrome |
Naumovic 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | High risk | This study was not fully randomised |
Nikolopoulou 2019.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up (2 in each group); major deviations from protocol (2 in MMF/TAC group); ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported and could be meta‐analysed |
| Other bias | Low risk | Study appears free of other potential biases |
Omrani 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to ascertain, only provides final numbers in analysed group |
| Selective reporting (reporting bias) | High risk | Primary outcome was not reported; SDs not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Pahari 1993.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 90 patients were randomised, only 71/90 (79%) were finally analysed. The missing outcome data were not balanced in numbers across intervention groups: 6/42 (14%) in CPA group and 13/48 (27%) in prednisolone group |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | High risk | The inclusion criteria of proteinuria was 2 g/24 hours rather than 3.5 g/24 hours |
Peng 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Duration of treatment
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 lost to follow‐up, 2 died, 1 ceased due to leucopenia |
| Selective reporting (reporting bias) | Low risk | Data on primary outcome comprehensive, all outcomes reported data. intention‐to‐treat analysis was performed |
| Other bias | Low risk | No evidence for other sources of bias. no evidence for potential conflict of interest however, no study protocol was published beforehand |
Ponticelli 1992.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The coordinating centre assigned the patients consecutively to one of the two treatment regimens in random order |
| Allocation concealment (selection bias) | Low risk | Central randomisation method described could not allow investigators/participants to know or influence intervention group before eligible participant entered in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients who could not complete treatment were included in the analysis according to the intention‐to‐treat principle. For the two patients who died and the one who was lost to follow‐up, data obtained at the last observation were considered |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Ponticelli 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At the coordinating centre, patients were assigned consecutively to one of the two treatment regimens, according to a centre‐stratified random order |
| Allocation concealment (selection bias) | Low risk | Central randomisation method described above could not allow investigators/participants to know or influence intervention group before eligible participant entered in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 8/95 (8%) patients did not complete the 6‐month regimen and then excluded in some final analyses: treatment group 1 (6/50), treatment group 2 (2/45). Two patients did not present at the follow‐up visit and a 51‐yr‐old woman died because of a deep‐vein thrombosis with acute kidney failure and cardiac shock 3 months after the diagnosis of membranous nephropathy, before treatment was started. Four patients in treatment group 1 and one in treatment group 2, who completed the treatment, did not present at the follow‐up visit and were considered lost to follow‐up after the sixth month |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ponticelli 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The coordinating centre assigned patients consecutively by telephone to 1 of the 2 treatment regimens in a centralized randomised order, with assignation produced by a table from a statistical textbook |
| Allocation concealment (selection bias) | Low risk | The sequence was concealed until intervention was assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Praga 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the clinical coordinating centre using a table of random numbers and was stratified by centres |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was performed by enclosing assignments in sequentially numbered, opaque‐closed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 8/48 (17%) randomised patients did not complete the 18‐month regimen. Two patients of the treated group (personal decision because lack of response after 6 months of treatment and a partial seizure in a patient with history of epilepsy) and one of the control group (severe oedema six months after randomisation and deafness attributed to high‐dose diuretics) withdrew from the study. Five patients (three in the control group and two in the treatment group) were lost to follow‐up between 3 and 18 months after randomisation. But they were all included in the final analyses according to the intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Ramachandran 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐medications
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer based random numbers. Random sequence generation was performed by an author, who was not otherwise involved in the enrolment and allocation of treatment of the participants |
| Allocation concealment (selection bias) | Low risk | Labelled sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70 eligible and randomised, all are included in outcome analyses |
| Selective reporting (reporting bias) | Low risk | Remission most relevant and is reported. Generally comprehensive reporting of outcome data |
| Other bias | Low risk | No evidence of other sources of bias. No evidence for conflict of interest or financial interests |
Reichert 1994.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18/20 patients completed the study. 2 (1 from each treatment group) immediately withdrew after assignment: one had to receive regular dialysis before treatment with methylprednisolone and CPA had begun, and the other became psychotic 2 weeks after starting prednisone treatment. Because these 2 patients received neither chlorambucil nor CPA, their data are not used for analysis |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Sahay 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reports 12/20 in the Ponticelli regime completed the study and were analysed; no other data provided |
| Selective reporting (reporting bias) | High risk | Data could not be meta‐analysed (percentages reported and unsure of numbers per group) |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Saito 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐medications
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comprehensive reporting of primary outcomes |
| Other bias | Low risk | Industry co‐funded trial. Otherwise, no evidence for other sources of bias |
Saito 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 51 randomised, 37 reported in outcomes data |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Senthil Nayagam 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation was based on minimization, using the following parameters: (MN or FSGS), sex and GFR. Minimization is a valid alternative to randomisation, and ensures uniformity between the two groups with respect to the characteristics used in the allocation process |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/11 patients in MMF group was lost to follow‐up after 1.5 months and was included in the non‐responder category |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Shibasaki 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information to permit judgement, likely no blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Approximate 32% (8/25) of patients were lost in the two‐year follow‐up: 21% (3/14) in the mizoribine group and 45% (5/11) in the control group. The proportion of loses in the follow‐up could have a substantial influence on the results. The reason for missing data were not specified and the missing data were not imputed using appropriate methods |
| Selective reporting (reporting bias) | High risk | Only complete or partial remission were reported. The primary outcome such as death and ESKD were not stated; side effects leading to patient withdrawal were not recorded |
| Other bias | High risk | The data were abstracted from a RCT aiming to investigate the effect of mizoribine on steroid‐resistant nephrotic syndrome. This study included all different pathologic variants of nephrotic syndrome. The randomisation was not stratified according to the pathologic diagnosis |
Silverberg 1976.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Low risk | Closed‐envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Only the pharmacist knew which tablets were AZA and which were placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Stegeman 1994.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients stratified centrally according to the clinical characteristics during the pre‐treatment phase |
| Allocation concealment (selection bias) | Low risk | Central trial coordinator will randomly allocate eligible patients after stratification |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study terminated due to poor accrual rate |
| Selective reporting (reporting bias) | High risk | Study terminated due to poor accrual rate |
| Other bias | High risk | Study terminated due to poor accrual rate |
Sun 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported, says only patients were divided randomly equally into the groups |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients reported as included are in the primary outcome analysis |
| Selective reporting (reporting bias) | Unclear risk | Secondary outcomes not clearly defined. No pre‐published trial protocol available |
| Other bias | Low risk | No evidence of other sources of bias. No evidence of conflicts of interest |
Tiller 1981.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29/54 patients (54%) completed the 36‐month follow‐up: 14/27 (52%) in the treatment group and 15/27 (56%) in the control group. The missing numbers of patients were balanced and the missing reason was specified in each patient. The rate of loss to follow‐up was high (54%), intention‐to‐treat principle was used to deal with these data to avoid potential bias |
| Selective reporting (reporting bias) | Low risk | The primary outcomes and key adverse effects were detailed in the publication, although other outcomes were not available to be included in this meta‐analysis |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Xu 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail about concealment of the random allocation sequence before or during enrolment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was claimed that double‐blind was performed, however no further details were provided because it was only published in the conference abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 of 24 randomised patients completed the study. Only 2 patients in FK506 group dropped out at 2 years |
| Selective reporting (reporting bias) | Unclear risk | No pre‐published protocol was available. Outcomes are randomly described at different time points and not all measured time points are reported. Reason for drop‐out of patients in intervention‐group not reported. |
| Other bias | Unclear risk | Only abstract was available. Financial disclosure was not provided. |
Xu 2013a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Duration of treatments and follow up details
Co‐medications
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no other details reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Uncertain if total number included |
| Selective reporting (reporting bias) | Low risk | No evidence for missing data; outcomes comprehensively reported |
| Other bias | Low risk | No evidence of other sources of bias; no evidence for Conflict of Interest |
Yuan 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
TAC dose
Prednisone dose
Co‐medications
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pre‐printed randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No suggestion of missing data. However, not certain of total number analysed |
| Selective reporting (reporting bias) | Low risk | Comprehensive reporting of all outcomes. no evidence of selective reporting |
| Other bias | Unclear risk | Poorly reported methods. Conflict of interest of authors not declared. Sources of funding declared and no suggestion for bias |
Zhang 2015d.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Very limited reporting. no pre‐published protocol available. primary/secondary outcomes not clearly defined. selective outcome reporting. outcomes not reported in absolute numbers |
| Other bias | Unclear risk | Insufficient information to permit judgement |
ACTH ‐ adrenocorticotropic hormone; AZA ‐ azathioprine, BP ‐ blood pressure; BP ‐ blood pressure; BUN ‐ blood urea nitrogen; ACEi ‐ angiotensin converting enzyme inhibitors; ARB ‐ angiotensin receptor blockers; Cr ‐ creatinine; CrCl ‐ creatinine clearance; CPA ‐ cyclophosphamide; CSA ‐ cyclosporine; DBP ‐ diastolic blood pressure; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; (e)GFR ‐ (estimated) glomerular filtration rate; HIV ‐ human immunodeficiency virus; IM ‐ intramuscular; IMN ‐ idiopathic membranous nephropathy; IQR ‐ interquartile range; ITT ‐ intention‐to‐treat; IU ‐ international units; IV ‐ intravenous; KRT ‐ kidney replacement therapy; MDRD ‐ modified Diet in Renal Disease; MGN ‐ membranous glomerular nephritis; MMF ‐ mycophenolate mofetil; NIAT ‐ non‐immunosuppressive antiproteinuric treatment; NSAID ‐ nonsteroidal anti‐inflammatory drugs; PLA2R ‐ anti‐phospholipase A2 receptor; RAS ‐ renin angiotensin system; RCT ‐ randomised controlled trial; RTX ‐ rituximab; SBP ‐ systolic blood pressure; SC ‐ subcutaneous; SCr ‐ serum creatinine; SLE ‐ systemic lupus erythematosus; TAC ‐ tacrolimus; TCM ‐ traditional Chinese medicine; UACR ‐ urinary albumin:creatinine ratio; UPCR ‐ urinary protein:creatinine ratio; WCC ‐ white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ambalavanan 1996 | Mixed population / wrong intervention duration: cross‐over design compared the efficacy of CSA versus ACEi in the treatment of adult PMN and secondary MN. We could not determine the number of patients with IMN in each intervention group. The first period of the cross‐over was only 3 months (< 6 months) |
| Badri 2013 | Wrong population: not PMN |
| Black 1970 | Mixed population: RCT compared prednisone and supportive treatment in patients with nephrotic syndrome; we could not determine the number of patients diagnosed with PMN and nephrotic syndrome in each intervention group |
| Branten 1998 | Wrong study design: study details a combination of RCT and observational data after the RCT were stopped |
| ChiCTR‐IPR‐14005366 | Wrong population: atypical MN |
| ChiCTR‐TRC‐09000539 | Unknown review status: RCT over 10 years old and no published data |
| Edefonti 1988 | Wrong population: 35/66 patients received renal biopsy and all patients were diagnosed with MCN and FSGS; no PMN were included |
| EudraCT2011‐000242‐38 | Study terminated: ended prematurely without results being reported |
| Heimann 1987 | Wrong population: not PMN |
| Krasnova 1998 | Mixed population: MN (12), MSGN (16), MSGN (3) We could not determine the number of patients with PMN and nephrotic syndrome in each intervention group |
| Lagrue 1975 | Mixed population: we could not determine the number of patients diagnosed with PMN and nephrotic syndrome in each intervention group |
| Li 2012e | Wrong population: secondary MN |
| Liu 2016c | Wrong population: refractory nephrotic syndrome, not PMN |
| Majima 1990 | Mixed population: we could not determine whether all included patients had the diagnosis of nephrotic syndrome. The age of included patients was not available for us to make sure they were all adults |
| Michail 2004 | Wrong study design: unclear whether randomisation was used |
| MRCWP 1971 | Mixed population: we could not determine the number of patients with PMN and nephrotic syndrome in each intervention group |
| Nand 1997 | Mixed population: we could not determine the number of patients with PMN and nephrotic syndrome in each intervention group |
| NCT01762852 | Study terminated: study was withdrawn due to poor recruitment |
| Plavljanic 1998 | Wrong population: patients with MN; it was uncertain that MN were primary or secondary; the clinical diagnosis of nephrotic syndrome was unclear |
| Ponticelli 1993a | Wrong population: all patients were diagnosed with MCN and FSGS. No PMN were included |
| Sharma 2009 | Wrong study design: not RCT; patients divided into 2 groups ‐ control group included only those cases of GN who dropped out of the study or refused their inclusion |
| Sharpstone 1969 | Wrong population: proliferative glomerulonephritis |
| Sun 2008 | Study design/conduct: RCT compared 24‐month TAC plus steroids with 6‐month TAC plus steroids in 20 adults diagnosed as PMN and nephrotic syndrome. The recruiting of patients was from March 2004 to August 2007; the publication of this study was submitted to that journal on February 2008. Thus, we concluded that some of randomised patients did not complete the 24‐month treatment of TAC plus steroids |
| Xu 2011 | Wrong population: Hepatitis B virus MN (secondary MN) |
| Yang 2016a | Wrong population: Hepatitis B virus MN (secondary MN) |
ACEi ‐ angiotensin‐converting enzyme inhibitors; AZA ‐ azathioprine; CPA ‐ cyclophosphamide; CKD ‐ chronic kidney disease; CSA ‐ cyclosporine; FSGS ‐ focal segmental glomerulosclerosis; I/PMN ‐ idiopathic/primary membranous nephropathy; MCGN ‐ mesangiocapillary glomerulonephropathy; MCN ‐ minimal change nephropathy; MN ‐ membranous nephropathy; MSGN ‐ mesangial proliferative glomerulonephropathy; RCT ‐ randomised controlled trial; TAC ‐ tacrolimus
Characteristics of studies awaiting classification [ordered by study ID]
NCT00302523.
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Notes |
|
NCT00518219.
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Notes |
|
NCT01093157.
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
Both therapies will be administered subcutaneously and given in a dose escalating frequency beginning at once every 2 weeks escalating to a maximum of twice/week over a total of 3 months exposed |
| Outcomes |
|
| Notes |
|
NCT01386554.
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
Group 3
|
| Outcomes |
|
| Notes |
|
NCT01845688.
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Notes |
|
ACEi ‐ angiotensin converting enzyme inhibitors; ACTH ‐ adrenocorticotropic hormone; ARB ‐ angiotensin receptor blockers; AZA ‐ azathioprine; BP ‐ blood pressure; CPA ‐ cyclophosphamide; CrCl ‐ creatinine clearance; CSA ‐ cyclosporine; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; I/PMN ‐ idiopathic/primary membranous nephropathy; MMF ‐ mycophenolate mofetil; NYHA ‐ New York Heart Association; MN ‐ membranous nephropathy; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SLE ‐ systemic lupus erythematosus; TAC ‐ tacrolimus; UPCR ‐ urinary protein:creatinine ratio
Characteristics of ongoing studies [ordered by study ID]
Chen 2020.
| Study name | Comparison of the efficacy and safety of tacrolimus monotherapy and cyclophosphamide combined with glucocorticoid in the treatment of adult primary membranous nephropathy: protocol of a multicenter, randomised, controlled, open study |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date |
|
| Contact information | Daqing Hong: Renal Division and Institute of Nephrology, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, Medical School of University of Electronic Science and Technology of China, Chengdu, 610072, China |
| Notes |
|
ChiCTR‐INR‐15007440.
| Study name | Multitarget therapy for treatment of refractory idiopathic membranous nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
Group 3
|
| Outcomes |
|
| Starting date |
|
| Contact information |
|
| Notes |
|
ChiCTR‐INR‐17011400.
| Study name | The effect of the treatment of idiopathic membranous nephropathy was observed in the treatment of idiopathic membranous nephropathy, and the effect of the treatment on Th17 / Treg |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date |
|
| Contact information |
|
| Notes |
|
ChiCTR‐INR‐17012070.
| Study name | Yongquan acupoint Shenque moxibustion curative effect of traditional Chinese medicine in the treatment of membranous nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | Registration site updated; 20 July 2017 |
| Contact information | Shi Wei 593224713@qq.com |
| Notes | States not yet recruiting (20/7/2017) |
ChiCTR‐INR‐17012212.
| Study name | Use of sirolimus in patients with primary idiopathic membranous nephropathy: a prospective randomised control trial |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | 28 July 2017 |
| Contact information | Fang Wang, wangfang@bjmu.edu.cn |
| Notes | Sponsor: Huabei Pharmaceutical Company Status: recruiting (refreshed 1 Aug 2017) |
ChiCTR‐IPR‐16008344.
| Study name | A study for comparing alternating glucocorticoid and cyclophosphamide versus glucocorticoid plus tacrolimus in idiopathic membranous nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | |
| Contact information | |
| Notes |
|
ChiCTR‐IPR‐16008527.
| Study name | Rituximab in the treatment of refractory membranous nephropathy: a multicenter, randomised, controlled clinical study |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | |
| Contact information | |
| Notes |
|
ChiCTR‐IPR‐17011386.
| Study name | Study on the effect and mechanism of interleukin‐2 in the treatment of idiopathic membranous nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | 1 June 2017 |
| Contact information | Yinghui Jiang, 176305893@qq.com |
| Notes | http://www.chictr.org.cn/showprojen.aspx?proj=19215 |
ChiCTR‐IPR‐17011702.
| Study name | Compare of the treatment of membranous nephropathy with mizoribine and steroid or cyclophosphamide and steroid |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | 1 July 2017 |
| Contact information | Wang Xichao ctxichao@outlook.com and Tu Yangke tuyangke@aliyun.com |
| Notes | States pending recruitment |
ChiCTR‐TRC‐11001144.
| Study name | A prospective randomised study on the efficacy of steroid combined with CTX or tacrolimus in IMN patients with NS |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | 2008/01/01 |
| Contact information | Chen Nan, Zhang Wen, Tel: +86 021 64370045, Fax: +86 021 64456419, chen‐nan@medmail.com.cn, zhangwen255@163.com, nephrology department, Shanghai Jiaotong university affiliated Ruijin hospital, No.197, Ruijin NO.2 Road, Luwan District, Shanghai, 200025, China |
| Notes | Recruiting in December 2011 |
CTRI/2017/05/008648.
| Study name | Randomised controlled trial of aPLA2R‐targeted therapy versus standard treatment in PLA2R related membranous nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | 1 June 2017 |
| Contact information | Raja Ramachandran drraja_1980@yahoo.co.in |
| Notes |
EudraCT2007‐005410‐39.
| Study name | Estudio piloto aleatorizado comparativo de tacrolimus vs ciclofosfamida‐prednisona en la nefropatía membranosa idiopática ‐ MEMTAC |
| Methods |
|
| Participants |
|
| Interventions | Group1
Group 2
|
| Outcomes |
|
| Starting date | 11/06/2008 |
| Contact information | Spain |
| Notes | None |
HIGHNESS 2011.
| Study name | High‐dose gamma‐globulin therapy for nephrotic membranous nephropathy patients |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | 2012/02/01 |
| Contact information | Hitoshi Yokoyama, Kanazawa Medical University Hospital Nephrology, 1‐1 Daigaku, Uchinada, Ishikawa, Japan, Telephone: 076‐286‐2211(3401), Email: h‐yoko@kanazawa‐med.ac.jp |
| Notes |
|
ISRCTN17977921.
| Study name | A randomised controlled study of tacrolimus for the treatment of idiopathic membranous nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | |
| Contact information | |
| Notes | Funding source: The First Affiliated Hospital of Zhengzhou University (China) Data from trial registration site only (June 2018). Contact; Zhanzheng Zhao 13938525666@139.com. Emailed 11 July 2018 |
ISRCTN70791258.
| Study name | Treatment with adrenocorticotropic hormone in idiopathic membranous nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Control group
|
| Outcomes |
|
| Starting date | |
| Contact information | |
| Notes | Funding source: Department of Nephrology, University Hospital in Lund (Sweden) Information from trial registration site only (June 2018), emailed Ann‐lena.berg@njur.lu.se and sponsor kerstin.wihlborg@med.lu.se on 13 Jun 2018 |
MMF‐STOP‐IMN 2017.
| Study name | Mycophenolate mofetil plus steroid in the treatment of patients with progressive idiopathic membranous nephropathy (MMF‐STOP‐IMN) |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | 1 June 2018 |
| Contact information | Contact: Xinling Liang, MD, PhD 86‐13808819770 xinlingliang_ggh@163.com |
| Notes | Details obtained from trial registration site 11 July 2018 |
NCT02173106.
| Study name | A controlled study of steroids plus cyclosporin therapy for patients of idiopathic membranous nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | |
| Contact information | |
| Notes | Data from trial registry site only Status; Unknown, no update since Jul 2014 Investigator Yanhong Deng, at Sun Yat‐sen University. Contact; jx.home@medmail.com.cn. Emailed 11 Jul 2018 |
RI‐CYCLO 2020.
| Study name | Rituximab versus steroids and cyclophosphamide in the treatment of idiopathic membranous nephropathy (RI‐CYCLO) |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | January 2012 Status: recruiting |
| Contact information | Contacts: pravani@ucalgary.ca and ceccoscolari@gmail.com |
| Notes | Details from trial registration site |
STARMEN 2015.
| Study name | Sequential therapy with tacrolimus and rituximab in primary membranous nephropathy (STARMEN) |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | January 2014 |
| Contact information | MANUEL PRAGA, mpragat@senefro.org and Jorge Rojas jerori2003@yahoo.com |
| Notes |
|
UMIN000001099.
| Study name | Optimal use of cyclosporine in idiopathic membranous nephropathy associated with nephrotic syndrome |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | July 2007 |
| Contact information | Masaaki Izumi, Hyogo College of Medicine, Division of Kidney and Dialysis, Department of Internal Medicine, 1‐1, Mukogawa, NIshinomiya, Hyogo, Japan, TEL +81‐798‐45‐6521, Email izumi@hyo‐med.ac.jp |
| Notes | Last follow‐up date: 2010/07 |
ACEi ‐ angiotensin converting enzyme inhibitors; ACTH ‐ adrenocorticotropic hormone; ARB ‐ angiotensin receptor blockers; CKD ‐ chronic kidney disease; CPA ‐ cyclophosphamide; CrCl ‐ creatinine clearance; CSA ‐ cyclosporine; ECG ‐ electrocardiogram, eGFR ‐ estimated glomerular filtration rate; GFR ‐ glomerular filtration rate; IMN/PMN ‐ idiopathic/primary membranous nephropathy; MN ‐ membranous nephropathy; RCT ‐ randomised controlled trial; RTX‐ rituximab; SCr ‐ serum creatinine; TCM ‐ traditional Chinese medicine; UPCR ‐ urinary protein/creatinine ratio
Differences between protocol and review
This review update includes several differences from the previous Cochrane review update (Chen 2013)
The updated Cochrane risk of bias tool has replaced the previous Risk of bias tool
Further sensitivity analysis of follow‐up (death and ESKD ≥ 10 years; remission ≥ 2 years) has been included in this review update
We referred to the disease as "primary" membranous nephropathy as opposed to "idiopathic" membranous nephropathy because this terminology is now more commonly used and easier to understand
Performing subgroup‐analysis for levels of anti‐PLA2R was not possible due to only few studies reporting this outcome.
Contributions of authors
Study selection: TvG, GW, DJT
Quality assessment: TvG, GW, DJT
Data extraction and data entry: TvG, GW, DJT
Resolution of disagreements: DJT
Manuscript draft: TvG, DJT
Manuscript review: GW, EA, AM, YC, EH
Sources of support
Internal sources
Division of Nephrology, State Key Discipline and State Key Laboratory of Kidney Diseases (2011DAV00088), Chinese People's Liberation Army (PLA) General Hospital (301 Hospital), Chinese PLA Medical Academy, Fuxing Road 28, Haidian District, Beijing 100853, China
External sources
No sources of support provided
Declarations of interest
Thilo C von Groote has declared that they have no conflict of interest
Gabrielle Williams has declared that they have no conflict of interest
Eric H Au has declared that they have no conflict of interest
Yizhi Chen has declared that they have no conflict of interest
Anna T Mathew has declared that they have no conflict of interest
Elisabeth M Hodson has declared that they have no conflict of interest
David J Tunnicliffe has declared that they have no conflict of interest
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Agarwal 2012a {published data only}
- Agarwal SK, Dogra PM, Gupta S, Bhowmik DM. Prospective study to compare efficacy of tacrolimus versus cyclophosphamide in inducing remission in idiopathic membranous nephropathy [abstract no: SA-PO357]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):719A. [Google Scholar]
Ahmed 1994 {published data only}
- Ahmed S, Rahman M, Alam MR, Islam S, Chowdhury MN, Chowdhury SM, et al. Methyl prednisolone plus chlorambucil as compared with prednisolone alone for the treatment of idiopathic membranous nephropathy - a preliminary study. Bangladesh Renal Journal 1994;13(2):51-4. [EMBASE: 25084107] [Google Scholar]
- Rahman M, Ahmed S, Alam MR, Selim SI, Chowdhury MN, Chowdhury SM, et al. Methyl prednisolone plus chlorambucil as compared with prednisolone alone for the treatment of idiopathic membranous nephropathy - a preliminary study [abstract]. In: 10th Asian Colloquium in Nephrology; 1994, Dec 2-6; Karachi, Pakistan. 1994:66.
Appel 2002 {published data only}
- Appel G, Nachman P, Hogan S, Radhakrishnan J, Old C, Hebert L, et al. Eculizumab (C5 complement inhibitor) in the treatment of idiopathic membranous nephropathy (IMN): preliminary baseline and pharmacokinetic (PK)/pharmacodynamic (PD) data [abstract no: SU-PO970]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):668A. [Google Scholar]
- Radhakrishnan J, Diamond B, Kumor K, Appel G. Lipoprotein-(a)[Lp(a)] levels in nephrotic syndrome [NS] [abstract no: A0642]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):122A. [CENTRAL: CN-00447320] [Google Scholar]
Arnadottir 2006 {published data only}
- Arnadottir M, Stefansson B, Berg A. A randomized study on treatment with adrenocorticotropic hormone in idiopathic membranous nephropathy [abstract no: F-PO1112]. Journal of the American Society of Nephrology 2006;17(Abstracts):571A. [CENTRAL: CN-00782355] [Google Scholar]
Austin 1996a {published data only}
- Austin HA, Vaughan EM, Boumpas DT, Balow JE. Randomized trial of pulse cyclophosphamide and prednisone vs. prednisone alone in idiopathic membranous nephropathy [abstract no: A0410]. Journal of the American Society of Nephrology 1996;7(9):1327. [CENTRAL: CN-00583858] [Google Scholar]
Braun 1995 {published and unpublished data}
- Braun N, Dietz K, Vonthein R, Erley C, Risler T. Remission and relapses in idiopathic membranous nephropathy. Description by a longitudinal multi-state Markov-model [abstract no: SP054]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:34-5. [CENTRAL: CN-00509101]
- Braun N, Erley CM, Benda N, Zauner I, Kanis R, Grupp C, et al. Therapy of membranous glomerulonephritis with nephrotic syndrome. 5 years follow-up of a prospective, randomized multi-center study [abstract no: 726]. Journal of the American Society of Nephrology 1995;6(3):413. [CENTRAL: CN-00261096] [Google Scholar]
- Braun N, Erley CM, Benda N, Zauner I, Kanis R, Grupp C, et al. Therapy of membranous glomerulonephritis with nephrotic syndrome. 5 years follow-up of a prospective, randomized multi-centre study [abstract]. Nephrology Dialysis Transplantation 1995;10(6):967. [CENTRAL: CN-00261096] [Google Scholar]
Cameron 1990 {published data only}
- Cameron JS, Healy MJ, Adu D. The Medical Research Council trial of short-term high-dose alternate day prednisolone in idiopathic membranous nephropathy with nephrotic syndrome in adults. The MRC Glomerulonephritis Working Party. Quarterly Journal of Medicine 1990;74(274):133-56. [MEDLINE: ] [PubMed] [Google Scholar]
- Cameron JS, Working party of the UK MRC Glomerulonephritis Registry. The MRC trial of prednisolone in membranous nephropathy. The Working Party of the UK MRC Glomerulonephritis Registry [abstract]. Nephrology Dialysis Transplantation 1988;3(4):507-8. [CENTRAL: CN-00260389] [Google Scholar]
- Cameron JS, Working Party of the MRC Glomerulonephritis Registry. The MRC trial of prednisolone in membranous nephropathy. The Working Party of the MRC Glomerulonephritis Registry [abstract]. Nephrology Dialysis Transplantation 1988;3(6):844. [CENTRAL: CN-00260412] [Google Scholar]
Cattran 1989 {published data only}
- Cattran D, Cardella C, Charron R, Roscoe J, Cole E, Bear R, et al. Results of a controlled trial of alternate day prednisone (ADS) in idiopathic membranous glomerulonephritis (IMGN) [abstract]. Kidney International 1985;27(3):600. [CENTRAL: CN-00550495] [Google Scholar]
- Cattran D, Cardella C, Charron R, Roscoe J, Rance P, Cusimano M, et al. Preliminary results of controlled trial of alternate-day prednisone in idiopathic membranous glomerulonephritis [abstract]. Kidney International 1981;19(2):388-9. [CENTRAL: CN-00644365] [Google Scholar]
- Cattran DC, Cole E, Cardella C, Charron R, Roscoe J, Delmore T, et al. Results of a controlled trial of alternate day prednisone (P) in membranous nephropathy (IMGN) [abstract]. Kidney International 1989;35(1):208. [CENTRAL: CN-00644367] [Google Scholar]
- Cattran DC, Delmore T, Roscoe J, Cole E, Cardella C, Charron R, et al. A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. New England Journal of Medicine 1989;320(4):210-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cattran 1995 {published data only}
- Cattran D, Greenwood CM, Ritchie S, Bernstein K, Churchill DN, Clarke WF, et al. A controlled trial of cyclosporine in patients with progressive membranous nephropathy [abstract no: 118P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):271. [CENTRAL: CN-00483446] [Google Scholar]
- Cattran DC, Greenwood C, Ritchie S, Bernstein K, Churchill DN, Clark WF, et al. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney International 1995;47(4):1130-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cattran DC. Cyclosporine in the treatment of membranous nephropathy (MGN) [abstract]. In: 12th International Congress of Nephrology; 1993, Jun 13-18; Jerusalem, Israel. 1993:535. [CENTRAL: CN-00602121]
Cattran 2001 {published data only}
- Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, et al. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney International 2001;59(4):1484-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cattran DC, Pohl M, Maxwell D, Appel G, Hunsicker GP, Kunis C, et al. Randomized controlled trial of cyclosporine (D) vs placebo (P) in steroid resistant, nephrotic patients (NS) with membranous nephropathy (SR-MGN) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):85A. [CENTRAL: CN-00444711] [Google Scholar]
- Cattran DC, Wald R, Brenchley PE, Coupes B, North American Nephrotic Syndrome Group and the Genes Gender and Glomerulonephritis Group. Clinical correlates of serial urinary membrane attack complex estimates in patients with idiopathic membranous nephropathy. Clinical Nephrology 2003;60(1):7-12. [MEDLINE: ] [PubMed] [Google Scholar]
Chan 2007 {published data only}
- Chan TM, Lin AW, Tang SC, Qian JQ, Lam MF, Ho YW, et al. Prospective controlled study on mycophenolate mofetil and prednisolone in the treatment of membranous nephropathy with nephrotic syndrome. Nephrology 2007;12(6):576-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2010a {published data only}
- Chen M, Chen J, Li X, Lu F, Xu FF, Qian JQ, et al. A randomized controlled study comparing prednisone plus tacrolimus versus prednisone plus cyclophosphamide in patients with membranous nephropathy and nephrotic syndrome [abstract no: SA-PO1086]. Journal of the American Society of Nephrology 2006;17(Abstracts):802A. [CENTRAL: CN-00782317] [Google Scholar]
- Chen M, Li H, Li XY, Lu FM, Ni ZH, Xu FF, et al. Tacrolimus combined with corticosteroids in treatment of nephrotic idiopathic membranous nephropathy: a multicenter randomized controlled trial. American Journal of the Medical Sciences 2010;339(3):233-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2013e {published data only}
- Chen Y, Deng Y, Ni Z, Chen N, Chen X, Shi W, et al. Efficacy and safety of traditional Chinese medicine (Shenqi particle) for patients with idiopathic membranous nephropathy: a multicenter randomized controlled clinical trial. American Journal of Kidney Diseases 2013;62(6):1068-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Choi 2018 {published data only}
- Choi JY, Kim DK, Kim YW, Yoo TH, Lee JP, Chung HC, et al. The effect of mycophenolate mofetil versus cyclosporine as a combination therapy to low dose corticosteroid in high risk patients with idiopathic membranous nephropathy: a multicenter randomized trial [abstract no: FR-OR113]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):61A. [CENTRAL: CN-01657834] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Kim DK, Kim YW, Yoo TH, Lee JP, Chung HC, et al. The effect of mycophenolate mofetil versus cyclosporine as combination therapy with low dose corticosteroids in high-risk patients with idiopathic membranous nephropathy: a multicenter randomized trial. Journal of Korean Medical Science 2018;33(9):e74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coggins 1979 {published data only}
- Abrass CK, Hall CL, Border WA, Brown CA, Glassock RJ, Coggins CH. Circulating immune complexes in adults with idiopathic nephrotic syndrome. Collaborative Study of the Adult Idiopathic Nephrotic Syndrome. Kidney International 1980;17(4):545-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- A controlled study of short-term prednisone treatment in adults with membranous nephropathy. Collaborative Study of the Adult Idiopathic Nephrotic Syndrome. New England Journal of Medicine 1979;301(24):1301-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coggins CH, Bostom MA, Collaborative Study of the Adult Nephrotic Syndrome. The effect of short-term prednisone treatment in adult nephrotics with minimal change (MC) and membranous (M) histology [abstract]. Kidney International 1977;12(6):463. [CENTRAL: CN-00784389] [Google Scholar]
CYCLOMEN 1994 {published and unpublished data}
- Laurens W, Ruggenenti P, Perna A, Vanrenterghem Y, Remuzzi G. A randomised and controlled study to assess the effect of cyclosporin in nephrotic patients with membranous nephropathy and reduced renal function (CYCLOMEN). Journal of Nephrology 1994;7(4):237-47. [EMBASE: 24283126] [Google Scholar]
- Pisoni R, Grinyo JM, Salvadori M, Laurens W, Garini G, Oliver YA, et al. Cyclosporine versus conservative therapy in patients with idiopathic membranous nephropathy (IMN) and deteriorating renal function: results of the CYCLOMEN trial [abstract no: A0514]. Journal of the American Society of Nephrology 2000;11(Sept):95A. [CENTRAL: CN-00583866] [Google Scholar]
CYPMEN 2006 {published data only}
- Hirayama K, Koyama A. Randomized prospective trial of cyclosporine with/without low-dose prednisolone in idiopathic membranous nephropathy in adults with nephrotic syndrome. www.anzctr.org.au/ACTRN12609000996268.aspx (first received 31 March 2006).
Di 2018 {published data only}
- Di J, Qian Q, Yang M, Jiang Y, Zhou H, Li M, et al. Efficacy and safety of long-course tacrolimus treatment for idiopathic membranous nephropathy. Experimental & Therapeutic Medicine 2018;16(2):979-84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donadio 1974 {published data only}
- Donadio JV, Holley KE, Anderson CF, Taylor WF. Controlled trial of cyclophosphamide in idiopathic membranous nephropathy. Kidney International 1974;6(6):431-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dussol 2008 {published data only}
- Dussol B, Morange S, Burtey S, Indreies M, Cassuto E, Mourad G, et al. Mycophenolate mofetil monotherapy in membranous nephropathy: a 1-year randomized controlled trial. American Journal of Kidney Diseases 2008;52(4):699-705. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dussol B, Sichez H, Burtey S, Cassuto E, Kaaraslan H, Villar E, et al. Mycophenolate mofetil (MMF) in patients with idiopathic membranous nephropathy with nephrotic syndrome: a multicenter randomized trial [abstract no: TH-F-DS1092]. Journal of the American Society of Nephrology 2006;17(Abstracts):566A. [CENTRAL: CN-00653744] [Google Scholar]
Dyadyk 2001a {published data only}
- Dyadyk AI, Bagriy AE, Yarovaya NF, Dyadyk IA. Results of long-term randomised study of immunosuppressive treatment of patients with idiopathic membranous glomerulonephritis [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A64. [CENTRAL: CN-00487685] [Google Scholar]
Falk 1992 {published data only}
- Falk RJ, Hogan SL, Muller KE, Jennette JC. Treatment of progressive membranous glomerulopathy. A randomized trial comparing cyclophosphamide and corticosteroids with corticosteroids alone. The Glomerular Disease Collaborative Network. Annals of Internal Medicine 1992;116(6):438-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fu 2012a {published data only}
- Fu P, Yuan A, Yu J, Wang J, Wu H, Cui R. Mycophenolate mofetil combined with prednisone for treatment of idiopathic membranous nephropathy with nephrotic syndrome: a 36-month prospective controlled study. Academic Journal of Second Military Medical University 2012;33(3):270-3. [EMBASE: 373957733] [Google Scholar]
GEMRITUX 2017 {published data only}
- Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. Journal of the American Society of Nephrology 2017;28(1):348-58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco PM, Dahan K, Debiec H, Plaisier EM, Cachanado M, Rousseau A, et al. A randomized controlled trial of rituximab for severe idiopathic membranous nephropathy (IMN) [abstract no: SA-OR011]. Journal of the American Society of Nephrology 2015;26(Abstracts):62A. [CENTRAL: CN-01657835] [Google Scholar]
- Rosenzwajg M, Languille E, Debiec H, Hygino J, Dahan K, Simon T, et al. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney International 2017;92(1):227-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Seitz-Polski B, Debiec H, Rousseau A, Dahan K, Zaghrini C, Payre C, et al. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. Journal of the American Society of Nephrology 2018;29(2):401-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasegawa 2017 {published data only}
- Hasegawa H, Mitarai T, Tomino Y, Yokoyama H, Yamagata K, Iwano M, et al. Clinical advantage of concomitant use of low dose mizoribine and prednisolone on primary membranous nephropathy in the elderly [abstract no: TH-PO130]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):136. [EMBASE: 633698258] [Google Scholar]
- Hasegawa H, Mitarai T, Tomino Y, Yokoyama H, Yamagata K, Iwano M, et al. Clinical advantage of concomitant use of mizoribine and prednisolone on primary membranous nephropathy in the elderly [abstract no: MP226]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii509. [EMBASE: 617291046] [Google Scholar]
He 2013 {published data only}
- He L, Peng Y, Liu H, Liu Y, Yuan S, Liu F, et al. Treatment of idiopathic membranous nephropathy with combination of low-dose tacrolimus and corticosteroids. Journal of Nephrology 2013;26(3):564-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hladunewich 2014 {published data only}
- Hladunewich MA, Cattran D, Beck LH, Odutayo A, Sethi S, Ayalon R, et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar R Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrology Dialysis Transplantation 2014;29(8):1570-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladunewich MA, Fervenza FC, Beck LH, Reich HN, Sethi S, Irazabal MV, et al. A pilot study to determine dose, effectiveness and depletion of anti-PLA2R antibodies of adrencorticotrophic hormone (ACTH acthar gel) in subjects with nephrotic syndrome and idiopathic membranous nephropathy (iMN) [abstract no: FR-OR125]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):58A. [Google Scholar]
Hofstra 2010 {published data only}
- Branten AJ, du Buf-Vereijken PW, Wetzels JF. Defining the optimal time of treatment start in patients with membranous nephropathy: a randomized study [abstract no: SU-PO969]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):668A. [CENTRAL: CN-00444526] [Google Scholar]
- Branten AJ, Du Buf-Vereijken PW, Wetzels JF. Early versus late start of immunosuppressive therapy in patients with membranous nephropathy; a randomized study [abstract no: SA-PO188]. Journal of the American Society of Nephrology 2004;15(Oct):341A. [CENTRAL: CN-00583772] [Google Scholar]
- Hofstra JM, Branten AJ, Wetzels FM. No benefit of early start of immunosuppressive therapy in idiopathic membranous nephropathy [abstract no: F-FC264]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):60A. [CENTRAL: CN-00747283] [Google Scholar]
- Hofstra JM, Branten AJ, Wirtz JJ, Noordzij TC, du Buf-Vereijken PW, Wetzels JF. Early versus late start of immunosuppressive therapy in idiopathic membranous nephropathy: a randomized controlled trial. Nephrology Dialysis Transplantation 2010;25(1):129-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Howman 2013 {published data only}99959692
- Howman A, Chapman TL, Langdon MM, Ferguson C, Adu D, Feehally J, et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet 2013;381(9868):744-51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson PW. The UK randomized controlled trial of immunosuppression for progressive membranous nephropathy [abstract no: THOR048]. Journal of the American Society of Nephrology 2011;22(Abstracts):11A. [Google Scholar]
Imbasciati 1980 {published data only}
- Imbasciati E, Cagnoli L, Case N, Pasquali S, Di Filippo G, Limido D, et al. Controlled study of treatment of steroids and chlorambucil, in alternate months, for membranous nephropathy and focal glomerulosclerosis. Preliminary evaluation of the results [Studio controllato di un trattamento a mesi alterni di steroidi e clorambucil nella nefropatia membranosa e nella glomerulosclerosi focale. Valutazione preliminare dei risultati]. Minerva Nefrologica 1980;27(4):571-5. [MEDLINE: ] [PubMed] [Google Scholar]
- Ponticelli C, Zucchelli P, Imbasciati E, Cagnoli L, Pozzi C, Grassi C, et al. Controlled trial of monthly alternated courses of steroid and chlorambucil for idiopathic membranous nephropathy. Proceedings of the European Dialysis & Transplant Association 1983;19:717-23. [MEDLINE: ] [PubMed] [Google Scholar]
- Ponticelli C, Zucchelli P, Imbasciati E, Cagnoli L, Pozzi C, Passerini P, et al. Controlled trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. New England Journal of Medicine 1984;310(15):946-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Zucchelli P, Locatelli F, Grassi G, Limido D, Volpini T, et al. Controlled trial of monthly alternated courses of steroid and chlorambucil in idiopathic membranous nephropathy [abstract]. Kidney International 1983;23(3):554. [CENTRAL: CN-00644373] [PubMed] [Google Scholar]
- Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C, et al. A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. New England Journal of Medicine 1989;320(1):8-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney International 1995;48(5):1600-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C. Treatment of membranous nephropathy (MN) [abstract]. In: 9th Asian Colloquium in Nephrology; 1992, May 17-21; Seoul, Korea. 1992:22-4. [CENTRAL: CN-00461530]
- Ponticelli C. Treatment of membranous nephropathy [abstract]. In: 12th International Congress of Nephrology; 1993, Jun 13-18; Jerusalem, Israel. 1993:565.
- Zucchelli P, Cagnoli L, Pasquali S, Casanova S, Donini U. Clinical and morphologic evolution of idiopathic membranous nephropathy. Clinical Nephrology 1986;25(6):282-8. [MEDLINE: ] [PubMed] [Google Scholar]
- Zucchelli P, Ponticelli C, Aroldi A, Cagnoli I. Predictive indices of good prognosis in idiopathic membranous nephropathy [abstract]. Kidney International 1985;28(2):268. [Google Scholar]
- Zucchelli P, Ponticelli C, Cagnoli L, Aroldi A, Beltrandi E. Prognostic value of T lymphocyte subset ratio in idiopathic membranous nephropathy. American Journal of Nephrology 1988;8(1):15-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jha 2007 {published data only}
- Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. Journal of the American Society of Nephrology 2007;18(6):1899-904. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jha V, Kohli HS, Sud K, Gupta KL, Sakhuja V. A randomized controlled trial of steroids and cyclophosphamide in adults with idiopathic membranous nephropathy [abstract no: FC3-02]. Nephrology 2003;8(Suppl):A16-7. [CENTRAL: CN-01658628] [DOI] [PubMed] [Google Scholar]
- Jha V, Kohli HS, Sud K, Krishan L, Joshi K, Sakhuja V. Randomized controlled trial of steroids and cyclophosphamide in adults with idiopathic membranous nephropathy [abstract no: A1100]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):213A. [CENTRAL: CN-00445921] [DOI] [PubMed] [Google Scholar]
- Jha V, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, et al. A randomized controlled trial of steroids and cyclophosphamide in adults with idiopathic membranous nephropathy [abstract]. In: 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998, Jun 6-9; Rimini, Italy. 1998:114.
Jurubita 2012 {published data only}
- Jurubita R, Ismail G, Bobeica G, Rusu E, Zilisteanu D, Andronesi A, et al. Efficacy and safety of triple therapy with MMF, cyclosporine and prednisolone versus cyclosporine and prednisolone in adult patients with idiopathic membranous nephropathy and persistent heavy proteinuria [abstract no: FP347]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii186. [EMBASE: 70765843] [Google Scholar]
Koshikawa 1993 {published data only}
- Koshikawa S. Clinical evaluation of an immunosuppressive drug, mizoribine (HE-69) on steroid-resistant nephrotic syndrome. A multicenter double-blind comparison study with placebo. Jin ToTohseki [Kidney and Dialysis] 1993;34(4):631-50. [CENTRAL: CN-00602134] [Google Scholar]
Kosmadakis 2010 {published data only}
- Kosmadakis G, Filiopoulos V, Smirloglou D, Skarlas P, Georgoulias C, Michail S. Comparison of immunosuppressive therapeutic regimens in patients with nephrotic syndrome due to idiopathic membranous nephropathy. Renal Failure 2010;32(5):566-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2015 {published data only}
- Li MX, Yu YW, Zhang ZY, Zhao HD, Xiao FL. Administration of low-dose cyclosporine alone for the treatment of elderly patients with membranous nephropathy. Genetics & Molecular Research 2015;14(1):2665-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2017c {published data only}
- Li QH, Yang ZJ, Li L, Gou R, Guo YY, Yin LL, et al. Comparison of efficacy and safety between tacrolimus and cyclosporine combined with corticosteroids in patients with idiopathic membranous nephropathy: a randomized controlled trial. International Journal of Clinical & Experimental Medicine 2017;10(6):9764-70. [EMBASE: 616970461] [Google Scholar]
Liang 2017 {published data only}
- Liang Q, Li H, Xie X, Qu F, Li X, Chen J. The efficacy and safety of tacrolimus monotherapy in adult-onset nephrotic syndrome caused by idiopathic membranous nephropathy. Renal Failure 2017;39(1):512-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2009b {published data only}
- Liu ZH, Li SJ, Wu Y, Zuo K, Wang B, Zeng CH, et al. Treatment of membranous nephropathy with Tripterygium wilfordii and steroid: a prospective randomized control trial. Shenzangbing Yu Touxi Shenyizhi Zazhi [Chinese Journal of Nephrology Dialysis & Transplantation] 2009;18(4):303-9. [Google Scholar]
Liu 2015e {published data only}
- Liu S. Clinical trial of treatment for idiopathic membranous nephropathy with leflunomide combined with cyclophosphamide and glucocorticoid [abstract]. Hong Kong Journal of Nephrology 2015;17(2 Suppl 1):S64. [EMBASE: 72227084] [Google Scholar]
MENTOR 2015 {published data only}
- Beanlands H, Cattran DC, Fervenza FC. Membranous nephropathy (MN): baseline health related quality of life (HRQOL) in patients with severe nephrotic syndrome (SNS) in the Membranous Nephropathy Trial of Rituximab (MENTOR) RCT [abstract no: SA-PO946]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):851A. [CENTRAL: CN-01657773] [Google Scholar]
- Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. New England Journal of Medicine 2019;381(1):36-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fervenza FC, Canetta PA, Barbour S, Lafayette RA, Rovin BH, Aslam N, et al. A multi-center randomized controlled trial of rituximab versus cyclosporine in the treatment of idiopathic membranous nephropathy (MENTOR) [abstract no: SA-OR127]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):B2. [EMBASE: 633704501] [Google Scholar]
- Fervenza FC, Canetta PA, Barbour SJ, Lafayette RA, Rovin BH, Aslam N, et al. A multicenter randomized controlled trial of rituximab versus cyclosporine in the treatment of idiopathic membranous nephropathy (MENTOR). Nephron 2015;130(3):159-68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murphy 1992 {published data only}
- Murphy BF, McDonald I, Fairley KF, Kincaid-Smith PS. Randomized controlled trial of cyclophosphamide, warfarin and dipyridamole in idiopathic membranous glomerulonephritis. Clinical Nephrology 1992;37(5):229-34. [MEDLINE: ] [PubMed] [Google Scholar]
Naumovic 2011 {published data only}
- Naumovic R, Jovanovic D, Pavlovic S, Stosovic M, Marinkovic J, Basta-Jovanovic G. Cyclosporine versus azathioprine therapy in high-risk idiopathic membranous nephropathy patients: a 3-year prospective study. Biomedicine & Pharmacotherapy 2011;65(2):105-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nikolopoulou 2019 {published data only}
- Nikolopoulou A, Condon M, Turner-Stokes T, Cook HT, Duncan N, Galliford JW, et al. Mycophenolate mofetil and tacrolimus versus tacrolimus alone for the treatment of idiopathic membranous glomerulonephritis: a randomised controlled trial. BMC Nephrology 2019;20(1):352. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulou AK, Condon MB, Cook HT, Duncan ND, Galliford JW, Levy JB, et al. Mycophenolate mofetil and tacrolimus versus tacrolimus alone for the treatment of idiopathic membranous glomerulonephritis: a randomised controlled trial [abstract no: TH-PO1021]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):385. [EMBASE: 633734270] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omrani 2017 {published data only}
- Omrani H, Golmohamadi S, Hichi F, Sadeghi M. Comparison of the efficacy of tacrolimus versus cyclosporine in the treatment of idiopathic membranous nephropathy. Nephro-Urology Monthly 2017;9(1):e42473. [EMBASE: 614165929] [Google Scholar]
Pahari 1993 {published data only}
- Pahari DK, Das S, Dutta BN, Banerjee D. Prognosis and management of membraneous nephropathy. Journal of the Association of Physicians of India 1993;41(6):350-1. [MEDLINE: ] [PubMed] [Google Scholar]
Peng 2016 {published data only}
- Peng L, Wei SY, Li LT, He YX, Li B. Comparison of different therapies in high-risk patients with idiopathic membranous nephropathy. Journal of the Formosan Medical Association 2016;115(1):11-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ponticelli 1992 {published data only}
- Ponticelli C, Zucchelli P, Passerini P, Cesana B. Methylprednisolone plus chlorambucil as compared with methylprednisolone alone for the treatment of idiopathic membranous nephropathy. The Italian Idiopathic Membranous Nephropathy Treatment Study Group. New England Journal of Medicine 1992;327(9):599-603. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C. Treatment of idiopathic membranous nephropathy (IMN) [abstract]. In: 10th Asian Colloquium in Nephrology; 1994, Dec 2-6; Karachi, Pakistan. 1994:38. [CENTRAL: CN-00461529]
Ponticelli 1998 {published data only}
- Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. Journal of the American Society of Nephrology 1998;9(3):444-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ponticelli 2006 {published data only}
- Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, et al. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. American Journal of Kidney Diseases 2006;47(2):233-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Praga 2007 {published data only}
- Praga M, Barrio V, Fernandez Juarez G, Luno J. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial [abstract no: S-FC-010]. In: 4th World Congress of Nephrology.19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21-25; Rio de Janeiro, Brazil. 2007:42. [CENTRAL: CN-00587433] [DOI] [PubMed]
- Praga M, Barrio V, Juárez GF, Luño J, Grupo Español de Estudio de la Nefropatía Membranosa. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney International 2007;71(9):924-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramachandran 2016 {published data only}
- Gupta KL, Ramachandran R, Kohli HS, Jha V. Two year outcomes of patients with idiopathic membranous nephropathy, previously randomized to either modified Ponticelli regimen or to a combination of tacrolimus and steroids [abstract no: SA-OR012]. Journal of the American Society of Nephrology 2015;26(Abstracts):63A. [Google Scholar]
- Kohli HS, Ramachandran R, Nada R, Gupta KL. Tacrolimus with oral prednisolone versus cyclical cyclophosphamide and steroids in primary membranous nephropathy: 5-year outcome of the randomised trial [abstract no: SUN-027]. Kidney International Reports 2019;4(7 Suppl):S163. [EMBASE: 2002179361] [Google Scholar]
- Ramachandran R, Hn HK, Kumar V, Nada R, Yadav AK, Goyal A, et al. Tacrolimus combined with corticosteroids versus modified Ponticelli regimen in treatment of idiopathic membranous nephropathy: randomized control trial. Nephrology 2016;21(2):139-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Hn HK, Sharma V, Kumar A, Yadav AK, Nada R, et al. Tacrolimus combined with corticosteroids versus modified Ponticelli regimen in treatment of idiopathic membranous nephropathy: randomized control trial [abstract no: FR-OR066]. Journal of the American Society of Nephrology 2014;25(Abstracts):62A. [CENTRAL: CN-01657837] [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Kumar V, Nada R, Kohli HS, Jha V, Gupta KL. Tacrolimus with corticosteroids is inferior to modified Ponticelli regimen in the management of primary membranous nephropathy: results at 2 years of randomization [abstract no: TO038]. Nephrology Dialysis Transplantation 2016;31(Suppl 1):i77. [EMBASE: 72326088] [Google Scholar]
- Ramachandran R, Yadav AK, Kumar V, Siva Tez Pinnamaneni V, Nada R, Ghosh R, et al. Two-year follow-up study of membranous nephropathy treated with tacrolimus and corticosteroids versus cyclical corticosteroids and cyclophosphamide. Kidney International Reports 2017;2(4):610-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reichert 1994 {published data only}
- Reichert LJ, Huysmans FT, Assmann K, Koene RA, Wetzels JF. Preserving renal function in patients with membranous nephropathy: daily oral chlorambucil compared with intermittent monthly pulses of cyclophosphamide. Annals of Internal Medicine 1994;121(5):328-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Reichert LJ, Koene RA, Wetzels JF. Chlorambucil but not pulse cyclophosphamide reverses the deterioration of renal function in patients with membranous glomerulonephropathy [abstract no: 98P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):285. [Google Scholar]
Sahay 2002 {published data only}
- Gopal KA, Sahay M, Raman A, Narayen G. Ponticelli regime for membranous nephropathy - do Indians respond differently? [abstract no: W216]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):615. [CENTRAL: CN-00445523] [Google Scholar]
- Sahay M, Anuradha, Narayen G. Ponticelli regimen for membranous nephropathy - do Indians respond differently? [abstract]. Indian Journal of Nephrology 2002;12(4):180. [CENTRAL: CN-00461642] [Google Scholar]
Saito 2014 {published data only}
- Saito T, Iwano M, Matsumoto K, Mitarai T, Yokoyama H, Yorioka N, et al. Significance of combined cyclosporine-prednisolone therapy and cyclosporine blood concentration monitoring for idiopathic membranous nephropathy with steroid-resistant nephrotic syndrome: a randomized controlled multicenter trial. Clinical & Experimental Nephrology 2014;18(5):784-94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Mitarai T, Yokoyama H, Yorioka N, Iwano M, Nishi S, et al. Effect of preprandial once-a-day administration of cyclosporine (CyA) for steroid resistant nephrotic syndrome (SRNS) [abstract no: SA-PO997]. Journal of the American Society of Nephrology 2007;18(Abstracts):562A. [CENTRAL: CN-01657682] [Google Scholar]
- Saito T, Watanabe M, Ogahara S, Shuto H, Kataoka H. Benefits of once-a-day preprandial administration and C2 monitoring for the cyclosporine (CyA) treatment of idiopathic membranous nephropathy (IMN) with steroid resistant nephrotic syndrome (SRNS) [abstract no: SA-PO2293]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):634A. [Google Scholar]
- Saito T, Watanabe M, Ogahara S, Shuto H, Kataoka Y. Significance of blood concentration of cyclosporine at 2 hours after administration for the treatment of idiopathic membranous nephropathy with steroid resistant nephrotic syndrome [abstract no: SA285]. In: World Congress of Nephrology; 2009 May, 22-26; Milan, Italy. 2009. [CENTRAL: CN-01657683]
Saito 2017 {published data only}
- Saito T, Iwano M, Matsumoto K, Mitarai T, Yokoyama H, Yorioka N, et al. Mizoribine therapy combined with steroids and mizoribine blood concentration monitoring for idiopathic membranous nephropathy with steroid-resistant nephrotic syndrome. Clinical & Experimental Nephrology 2017;21(6):961-70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Watanabe M, Ogahara S, Shuto H, Kataoka Y. Effects of mizoribin combined with prednisolone for the treatment of idiopathic membranous nephropathy with steroid resistant nephrotic syndrome [abstract]. NDT Plus 2010;3(Suppl 3):iii430-1. [EMBASE: 70484583] [Google Scholar]
Senthil Nayagam 2008 {published data only}
- Senthil Nayagam L, Ganguli A, Rathi M, Kohli HS, Gupta KL, Joshi K, et al. Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: a pilot study. Nephrology Dialysis Transplantation 2008;23(6):1926-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shibasaki 2004 {published data only}
- Shibasaki T, Koyama A, Hishida A, Muso E, Osawa G, Yamabe H, et al. A randomized open-label comparative study of conventional therapy versus mizoribine onlay therapy in patients with steroid-resistant nephrotic syndrome (postmarketing survey). Clinical & Experimental Nephrology 2004;8(2):117-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silverberg 1976 {published data only}
- Western Canadian Glomerulonephritis Study Group. Controlled trial of azathioprine in the nephrotic syndrome secondary to idiopathic membranous glomerulonephritis. Canadian Medical Association Journal 1976;115(12):1209-10. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Stegeman 1994 {published data only}
- De Jong PE. ACE inhibition versus corticosteroids in membranous nephropathy (ACIMEN). In: European Union Biomedical and Health Research: the BIOMED 1 programme. Vol. 1. Amsterdam: IOS Press, 1995:606-607. [Google Scholar]
- Stegeman CA, De Zeeuw D, De Jong PE, Vanrenterghem YF, Rostoker G, Mann JF, et al. ACE inhibition versus corticosteroids in membranous nephropathy (ACIMEN). Journal of Nephrology 1994;7(5):294-300. [EMBASE: 24342908] [Google Scholar]
Sun 2014 {published data only}
- Sun Z, Ren M, Wu Q, Du X. Co-administration of Wuzhi capsules and tacrolimus in patients with idiopathic membranous nephropathy: clinical efficacy and pharmacoeconomics. International Urology & Nephrology 2014;46(10):1977-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tiller 1981 {published data only}
- Mathew TH. The treatment of idiopathic glomerulonephritis. In: Proceedings of the Second Asian Pacific Congress of Nephrology. 1983:164-72.
- Tiller D. Australian glomerulonephritis trial: membranous glomerulonephritis, mesangiocapillary glomerulonephritis [abstract]. Australian & New Zealand Journal of Medicine 1981;11(4):603. [CENTRAL: CN-00583749] [Google Scholar]
- Tiller DJ, Clarkson AR, Mathew T, Thompson N, Row G, Lauer C, et al. A prospective randomised trial of cyclophosphamide, dipyridamole and warfarin in membranous and mesangiocapillary glomerulonephritis. In: Zurukzoglu W, Papadimitriou M, Pyrpasopoulos M, Sion M, Zamboulis C, editors(s). Advances in Basic and Clinical Nephrology. 8th International Congress of Nephrology; 1981 June 7-12; Athens, Greece. Basel: Karger, 1981:345-51. [Google Scholar]
Xu 2010 {published data only}
- Xu J, Zhang W, Xu Y, Chen N. A double-blinded prospective randomised study on the efficacy of corticosteroid plus cyclophosphamide or FK506 in idiopathic membranous nephropathy patients with nephrotic syndrome [abstract no: FP05-05]. Nephrology 2010;15(Suppl 3):43. [EMBASE: 70467393] [Google Scholar]
- Xu J, Zhang W, Xu YW, Chen N. A double-blinded prospective randomised study on the efficacy of corticosteroid plus cyclophosphamide or FK506 in idiopathic membranous nephropathy patients with nephrotic syndrome [abstract no: SU364]. NDT Plus 2010;3(Suppl 3):iii431. [EMBASE: 70484584] [Google Scholar]
Xu 2013a {published data only}
- Xu J, Zhang W, Xu Y, Shen P, Ren H, Wang W, et al. Tacrolimus combined with corticosteroids in idiopathic membranous nephropathy: a randomized, prospective, controlled trial. Contributions to Nephrology 2013;181:152-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yuan 2013 {published data only}
- Yuan H, Liu N, Sun GD, Jia Y, Luo P, Miao LN. Effect of prolonged tacrolimus treatment in idiopathic membranous nephropathy with nephrotic syndrome. Pharmacology 2013;91(5-6):259-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2015d {published data only}
- Zhang D, Zheng H, Shang LI. Long-term efficacy and safety study of tacrolimus combined with corticosteroids in the treatment of idiopathic membranous nephropathy [abstract]. Hong Kong Journal of Nephrology 2015;17(2 Suppl 1):S65. [EMBASE: 72227086] [Google Scholar]
References to studies excluded from this review
Ambalavanan 1996 {published data only}
- Ambalavanan S, Fauvel J-P, Sibley RK, Myers BD. Mechanism of the antiproteinuric effect of cyclosporine in membranous nephropathy. Journal of the American Society of Nephrology 1996;7(2):290-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Badri 2013 {published data only}
- Badri S, Dashti-Khavidaki S, Ahmadi F, Mahdavi-Mazdeh M, Abbasi MR, Khalili H. Effect of add-on pentoxifylline on proteinuria in membranous glomerulonephritis: a 6-month placebo-controlled trial. Clinical Drug Investigation 2013;33(3):215-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Black 1970 {published data only}
- Black DA, Rose G, Brewer DB. Controlled trial of prednisone in adult patients with the nephrotic syndrome. British Medical Journal 1970;3(5270):412-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Branten 1998 {published data only}
- Branten AJ, Reichert LJ, Koene RA, Wetzels JF. Cyclophosphamide is superior to chlorambucil in the treatment of patients with membranous nephropathy and renal insufficiency [abstract no: A0395]. Journal of the American Society of Nephrology 1997;8(Programs & Abstracts):82A. [CENTRAL: CN-00444527] [Google Scholar]
- Branten AJ, Reichert LJ, Koene RA, Wetzels JF. Oral cyclophosphamide versus chlorambucil in the treatment of patients with membranous nephropathy and renal insufficiency. Qjm 1998;91(5):359-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ChiCTR‐IPR‐14005366 {published data only}
- Li W. A prospective study of safety and efficacy in atypical membranous nephropathy by low-dose hormone combined immunosuppressive therapy. www.chictr.org.cn/showproj.aspx?proj=9713 (first received 10 January 2014).
ChiCTR‐TRC‐09000539 {published data only}
- Miao L. Clinical studies of tacrolimus in idiopathic membranous nephropathy with nephrotic syndrome therapy. www.chictr.org.cn/showproj.aspx?proj=8996 (first received 30 September 2009).
Edefonti 1988 {published data only}
- Edefonti A, Ghio L, Bettinelli A, Paterlini G, Giani M, Nebbia G, et al. Unconjugated hyperbilirubinemia due to ciclosporin administration in children with nephrotic syndrome. Contributions to Nephrology 1988;67:121-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Edefonti A, Ghio L, Bettinelli A, Paterlini G. Unconjugated hyperbilirubinemia due to cyclosporin a (CyA) administration in children with nephrotic syndrome (NS) [abstract]. Pediatric Nephrology 1987;1(4):C8. [CENTRAL: CN-00465774] [DOI] [PubMed] [Google Scholar]
- Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, et al. Cyclosporine (CSA) vs cyclophosphamide (CYC) for children with frequently relapsing/steroid dependant nephrotic syndrome (FR/SDNS): long term study [abstract]. In: 9th Congress. International Pediatric Nephrology Association; 1992 Aug 30 - Sept 4; Jerusalem, Israel. 1992:C70. [CENTRAL: CN-00483820]
- Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, et al. Cyclosporine (CSA) vs cyclophosphamide (CYC) for children with frequently relapsing/steroid dependent nephrotic syndrome (FR/SDNS): long term study [abstract]. Journal of the American Society of Nephrology 1992;3(3):310. [CENTRAL: CN-00520331] [Google Scholar]
- Ponticelli C, Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, et al. Cyclosporin versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrology Dialysis Transplantation 1993;8(12):1326-32. [MEDLINE: ] [PubMed] [Google Scholar]
- Ponticelli C, Rivolta E. Ciclosporin in minimal-change glomerulopathy and in focal segmental glomerular sclerosis. American Journal of Nephrology 1990;10(Suppl 1):105-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C. A multicenter controlled prospective trial with cyclosporine vs cyclophosphamide in frequent relapsers and steroid dependent patients with idiopathic nephrotic syndrome. Journal of Nephrology 1989;2(2):147-51. [EMBASE: 21014828] [Google Scholar]
- Ponticelli C. Ciclosporin in the treatment of idiopathic nephrotic syndrome [abstract]. In: 10th Asian Colloquium in Nephrology; 1994, Dec 2-6; Karachi, Pakistan. 1994:116. [CENTRAL: CN-00461528]
- Tirelli AS, Paterlini G, Ghio L, Edefonti A, Assael BM, Bettinelli A, et al. Renal effects of cyclosporin A in children treated for idiopathic nephrotic syndrome. Acta Paediatrica 1993;82(5):463-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EudraCT2011‐000242‐38 {published data only}
- EudraCT2011-000242-38. A 2 year study to investigate belimumab in membranous nephropathy. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2011-000242-38 (first received 3 October 2012).
Heimann 1987 {published data only}
- Heimann JC, Pestalozzi MS, Praxedes JN, Penna DO, Saldanha LB, Marcondes M. Treatment of nephrotic syndrome: a prospective, double-blind study (prednisone vs placebo) [abstract]. In: 10th International Congress of Nephrology; 1987 Jul 26-31; London, UK. 1987:66. [CENTRAL: CN-01658289]
Krasnova 1998 {published data only}
- Krasnova TN, Shilov EM, Tareeva IE, Gordovskaia NB, Lavrova ON, Miroshnichenko NG, et al. Comparison of two cyclophosphamide treatment regimens in nephrotic patients with chronic glomerulonephritis [Sravnenie dvukh metodov lecheniia tsiklofosfanom bol'nykh khronicheskim glomerulonefritom s nefroticheskim sindromom]. Terapevticheskii Arkhiv 1998;70(6):14-7. [MEDLINE: ] [PubMed] [Google Scholar]
- Shilov EM, Krasnova TN, Ivanov AA, Ivanova LV, Shnyakina OV, Tareyeva IE. Oral cyclophosphamide versus cyclophosphamide (CPA) pulses in membranous and mesangial GN [abstract]. In: 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998, Jun 6-9; Rimini, Italy. 1998:116. [CENTRAL: CN-00485850]
Lagrue 1975 {published data only}
- Lagrue G, Bernard D, Bariety J, Druet P, Guenel J. Treatment with chlorambucil and azathioprine in primary glomerulonephritis. Results of a "controlled" study [Traitement par le chlorambucil et l'azathioprine dans les glomérulonéphrites primitives: Résultats d'une étude "controlée"]. Journal d Urologie et de Nephrologie 1975;81(9):655-72. [MEDLINE: ] [PubMed] [Google Scholar]
Li 2012e {published data only}
- Li K, He Y. Tacrolimus in the treatment of HBV associated with membranous nephropathy: a prospective study [abstract no: SA-PO354]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):718A. [Google Scholar]
Liu 2016c {published data only}
- Liu Y, Qu X, Chen W, Zhang Y, Liu L. Efficacy of leflunomide combined with prednisone in the treatment of refractory nephrotic syndrome. Renal Failure 2016;38(10):1616-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Majima 1990 {published data only}
- Majima K, Hori Y, Inoue T, Ishizaki K, Kinoshita E, Nomura G. The effects of treatment with prednisolone in patients with idiopathic membranous nephropathy [abstract]. In: 11th International Congress of Nephrology; 1990, Jul 15-20; Tokyo, Japan. 1990:117A. [CENTRAL: CN-00784437]
Michail 2004 {published data only}
- Michail S, Filiopoulos V, Kosmadakis G, Tentolouris N, Georgoulias C, Gobou A, et al. Comparison of three regimens in patients with idiopathic membranous nephropathy (IMN) associated with nephrotic syndrome (NS) [abstract no: SP053]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004, May 15-18; Lisbon, Portugal. 2004:34. [CENTRAL: CN-00509354]
MRCWP 1971 {published data only}
- Controlled trial of azathioprine and prednisone in chronic renal disease. Report by Medical Research Council Working Party. British Medical Journal 1971;2(5756):239-41. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Nand 1997 {published data only}
- Nand N, Das R, Jaswal TS. Evaluation of efficacy of methylprednisolone, prednisolone and chlorambucil in idiopathic glomerulonephritis. Journal of the Indian Medical Association 1997;95(6):163-5, 174. [MEDLINE: ] [PubMed] [Google Scholar]
NCT01762852 {published data only}
- NCT01762852. Efficacy and safety study of intravenous belimumab versus placebo in subjects with idiopathic membranous nephropathy. clinicaltrials.gov/ct2/show/NCT01762852 (first received 8 January 2008).
Plavljanic 1998 {published data only}
- Plavljanic D, Spoljar-Scukanec M, Milutinovic S. Tubular damage in patients with membranous nephropathy. The response to treatment [abstract]. In: 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998, Jun 6-9; Rimini, Italy. 1998:193. [CENTRAL: CN-00485443]
Ponticelli 1993a {published data only}
- Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney International 1993;43(6):1377-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sharma 2009 {published data only}
- Sharma M, Nand N, Sangwan V, Binani S. Evaluation of immunosuppressive therapy in cases of steroid resistant idiopathic glomerulonephritis. Journal International Medical Sciences Academy 2009;22(2):87-8. [EMBASE: 355293077] [Google Scholar]
Sharpstone 1969 {published data only}
- Sharpstone P, Ogg CS, Cameron JS. Nephrotic syndrome due to primary renal disease in adults: II. A controlled trial of prednisolone and azathioprine. British Medical Journal 1969;2(656):535-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2008 {published data only}
- Sun GD, Xu ZG, Luo P, Miao LN. The effect of tacrolimus combined with small dose of hormone on idiopathic membranous nephropathy. Zhongguo Shiyong Neike Zazhi [Chinese Journal of Practical Internal Medicine] 2008;28(7):542-545. [Google Scholar]
Xu 2011 {published data only}
- Xu G, Duang Z, Wu X, Zou H, Fang X, Tu W. Treatment of hepatitis B virus-associated membranous nephritis patients in Chinese: an open parallel controlled trial. Clinical Chemistry & Laboratory Medicine 2011;49(6):1077-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2016a {published data only}
- Yang Y, Ma L, Wang C, Kong D, Wang Y, Mei C. Effectiveness of sulodexide might be associated with inhibition of complement system in hepatitis B virus-associated membranous nephropathy: an inspiration from a pilot trial. European Journal of Internal Medicine 2016;32:96-104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00302523 {published data only}
- Liu Z. Tacrolimus treatment of patients with idiopathic membranous nephropathy. clinicaltrials.gov/ct2/show/NCT00302523 (first received 14 March 2006).
NCT00518219 {published data only}
- Liu Z. To compare the efficacy and safety of Tripterygium wilfordii (TW) versus valsartan in the membranous nephropathy (MN). clinicaltrials.gov/ct2/show/NCT00518219 (first received 20 August 2007).
NCT01093157 {published data only}
- Cattran D. A dose escalation study of long-acting ACTH gel in membranous nephropathy. clinicaltrials.gov/ct2/show/NCT01093157 (first received 25 March 2010).
NCT01386554 {published data only}
- NCT01386554. Acthar for treatment of proteinuria in membranous nephropathy patients (CHART). www.clinicaltrials.gov/ct2/show/NCT01386554 (first received 1 July 2011).
NCT01845688 {published data only}
- Wang L. Clinical study of QingReMoShen granule to treat idiopathic membranous nephropathy. clinicaltrials.gov/ct2/show/NCT01845688 (first received 3 May 2013).
References to ongoing studies
Chen 2020 {published data only}
- Chen S, Ren S, Wang AY, Tran H, Li Z, Cheng X, et al. Comparison of the efficacy and safety of tacrolimus monotherapy and cyclophosphamide combined with glucocorticoid in the treatment of adult primary membranous nephropathy: protocol of a multicenter, randomized, controlled, open study. Trials [Electronic Resource] 2020;21(1):219. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR‐INR‐15007440 {published data only}
- Liu D. Multitarget therapy for treatment of refractory idiopathic membranous nephropathy. www.chictr.org.cn/showproj.aspx?proj=12311 (first received 30 October 2015).
ChiCTR‐INR‐17011400 {published data only}
- Liu Y. The effect of the treatment of idiopathic membranous nephropathy was observed in the treatment of idiopathic membranous nephropathy, and the effect of the treatment on Th17 / Treg. www.chictr.org.cn/showproj.aspx?proj=19368 (first received 14 May 2017).
ChiCTR‐INR‐17012070 {published data only}
- Shi W. Yongquan acupoint Shenque moxibustion curative effect of traditional Chinese medicine in the treatment of membranous nephropathy. www.chictr.org.cn/showproj.aspx?proj=20524 (first received 20 July 2017).
ChiCTR‐INR‐17012212 {published data only}
- Zhao M. Use of sirolimus in patients with primary idiopathic membranous nephropathy: a prospective randomized control trial. www.chictr.org.cn/showproj.aspx?proj=20792 (first received 1 August 2017).
ChiCTR‐IPR‐16008344 {published data only}
- Su W. A study for comparing alternating glucocorticoid and cyclophosphamide versus glucocorticoid plus tacrolimus in idiopathic membranous nephropathy. www.chictr.org.cn/showproj.aspx?proj=14061 (first received 22 April 2016).
ChiCTR‐IPR‐16008527 {published data only}
- Chen N. Rituximab in the treatment of refractory membranous nephropathy: a multicenter, randomized, controlled clinical study. www.chictr.org.cn/showproj.aspx?proj=14456 (first received 24 May 2016).
ChiCTR‐IPR‐17011386 {published data only}
- Jiang Y. Study on the effect and mechanism of interleukin -2 in the treatment of idiopathic membranous nephropathy. www.chictr.org.cn/showproj.aspx?proj=19215 (first received 12 May 2017).
ChiCTR‐IPR‐17011702 {published data only}
- Tu Y. Compare of the treatment of membranous nephropathy with mizoribine and steroid or cyclophosphamide and steroid. www.chictr.org.cn/showproj.aspx?proj=18548 (first received 19 June 2017).
ChiCTR‐TRC‐11001144 {published data only}
- Zhang W. A prospective randomized study on the efficacy of steroid combined with CTX or tacrolimus in IMN patients with NS. www.chictr.org/en/proj/show.aspx?proj=261 (first received 2 January 2011).
CTRI/2017/05/008648 {published data only}
- Ramachandran R. Antibody targeted therapy in membranous nephropathy. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=18965 (first received 25 May 2017).
EudraCT2007‐005410‐39 {published data only}
- EudraCT2007-005410-39-ES. Randomized pilot study comparing tacrolimus vs. cyclophosphamide-prednisone in idiopathic membranous nephropathy - MEMTAC [Estudio piloto aleatorizado comparativo de tacrolimus vs ciclofosfamida-prednisona en la nefropatía membranosa idiopática - MEMTAC]. www.clinicaltrialsregister.eu/ctr-search/trial/2007-005410-39/ES (first received 4 August 2008).
HIGHNESS 2011 {published data only}
- Matsuo S. High-dose gamma-globulin therapy for nephrotic membranous nephropathy patients. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000007975 (first received 24 December 2011).
ISRCTN17977921 {published data only}
- Zhao Z. A randomized controlled study of tacrolimus for the treatment of idiopathic membranous nephropathy. isrctn.com/ISRCTN17977921 (first received 11 May 2016).
ISRCTN70791258 {published data only}70791258
- Berg AL. Treatment with adrenocorticotropic hormone in idiopathic membranous nephropathy. www.isrctn.com/ISRCTN70791258 (first received 26 January 2007).
MMF‐STOP‐IMN 2017 {published data only}
- Liang X. Mycophenolate mofetil plus steroid in the treatment of patients with progressive idiopathic membranous nephropathy (MMF-STOP-IMN). clinicaltrials.gov/ct2/show/NCT03170323 (first received 31 May 2017).
NCT02173106 {published data only}
- Deng Y. A controlled study of steroids plus cyclosporin therapy for patients of idiopathic membranous nephropathy. clinicaltrials.gov/ct2/show/NCT02173106 (first received 24 June 2014).
RI‐CYCLO 2020 {published data only}
- Delbarba E, Santoro D, Gesualdo L, Pani A, Alberici F, Ghiggeri GM, et al. Rituximab vs cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO trial [abstract]. Journal of the American Society of Nephrology 2020;31(Abstract Suppl):B9. [EMBASE: 633696884] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari F, Dallera N, Gesualdo L, Santoro D, Pani A, Santostefano M, et al. Rituximab versus steroids and cyclophosphamide for the treatment of primary membranous nephropathy: protocol of a pilot randomised controlled trial. BMJ Open 2019;9(12):e029232. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari F, Delbarba E, Santoro D, Gesualdo L, Pani A, Dallera N, et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. Journal of the American Society of Nephrology 2021;32(4):972-82. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
STARMEN 2015 {published data only}
- Rojas-Rivera J, Fernandez-Juarez G, Ortiz A, Hofstra J, Gesualdo L, Tesar V, et al. A European multicentre and open-label controlled randomized trial to evaluate the efficacy of Sequential treatment with TAcrolimus-Rituximab versus steroids plus cyclophosphamide in patients with primary MEmbranous Nephropathy: the STARMEN study. Clinical Kidney Journal 2015;8(5):503-10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
UMIN000001099 {published data only}
- Nakanishi T. Optimal use of ciclosporin in membranous nephropathy (OCIM-NS study). upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000001321 (first received 31 Mar 2009).
Additional references
Abe 1986
- Abe S, Amagasaki Y, Konishi K, Kato E, Iyori S, Sakaguchi H. Idiopathic membranous glomerulonephritis: aspects of geographical differences. Journal of Clinical Pathology 1986;39(11):1193-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 2009
- Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. New England Journal of Medicine 2009;361(1):11-21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA 1996;276(8):637-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berg 1999
- Berg AL, Nilsson-Ehle P, Arnadottir M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney International 1999;56(4):1534-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bhimma 2004
- Bhimma R, Coovadia HM. Hepatitis B virus-associated nephropathy. American Journal of Kidney Diseases 2004;24(2):198-211. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bomback 2009
- Bomback AS, Derebail VK, McGregor JG, Kshirsagar AV, Falk RJ, Nachman PH. Rituximab therapy for membranous nephropathy: asystematic review. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(4):734-44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bomback 2011
- Bomback AS, Tumlin JA, Baranski J, Bourdeau JE, Besarab A, Appel A, et al. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Design, Development & Therapy 2011;5:147-53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bullich 2014
- Bullich G, Ballarin J, Oliver A, Ayasreh N, Silva I, Santin S, et al. HLA-DQA1 and PLA2R1 polymorphisms and risk of idiopathic membranous nephropathy. Clinical Journal of The American Society of Nephrology: CJASN 2014;9(2):335-43. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Cameron 1996
- Cameron JS. Nephrotic syndrome in the elderly. Seminars in Nephrology 1996;16(4):319-29. [MEDLINE: ] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M. The QUORUM statement. Lancet 2000;355(9205):756-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Couchoud 1994
- Couchoud C, Laville M, Boissel JP. Treatment of membranous nephropathy: a meta-analysis. Nephrology Dialysis Transplantation 1994;9(5):469-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deegens 2005
- Deegens JK, Wetzels JF. Diagnosis and treatment of primary glomerular diseases. Membranous nephropathy, focal segmental glomerulosclerosis and IgA nephropathy. Minerva Urologica e Nefrologica 2005;57(3):211-36. [MEDLINE: ] [PubMed] [Google Scholar]
du Buf‐Vereijken 2005
- du Buf-Vereijken PW, Branten AJ, Wetzels JF. Idiopathic membranous nephropathy: outline and rationale of a treatment strategy. American Journal of Kidney Diseases 2005;46(6):1012-29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ehrenreich 1976
- Ehrenreich T, Porush JG, Churg J, Garfinkel L, Glabman S, Goldstein MH, et al. Treatment of idiopathic membranous nephropathy. New England Journal of Medicine 1976;295(14):741-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fiorentino 2016
- Fiorentino M, Tondolo F, Bruno F, Infante B, Grandaliano G, Gesualdo L, et al. Treatment with rituximab in idiopathic membranous nephropathy. Clinical Kidney Journal 2016;9(6):788-93. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fogo 2015
- Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD atlas of renal pathology: membranous nephropathy. American Journal of Kidney Diseases 2015;66(3):e15-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harbord 2009
- Harbord RM, Harris RJ, Sterne JA. Updated tests for small-study effects in meta-analyses. Stata Journal 2009;9(2):197-210. [DOI: 10.1177/1536867X0900900202] [DOI] [Google Scholar]
Heymann 1959
- Heymann W, Hackel DB, Harwood S, Wilson SG, Hunter JL. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proceedings of the Society for Experimental Biology & Medicine 1959;100(4):660-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hofstra 2012
- Hofstra JM, Wetzels JF. Management of patients with membranous nephropathy. Nephrology Dialysis Transplantation 2012;27(1):6-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hogan 1995
- Hogan SL, Muller KE, Jennette JC, Falk RJ. A review of therapeutic studies of idiopathic membranous glomerulopathy. American Journal of Kidney Diseases 1995;25(6):862-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imperiale 1995
- Imperiale TF, Goldfarb S, Berns JS. Are cytotoxic agents beneficial in idiopathic membranous nephropathy? A meta-analysis of the controlled trials. Journal of the American Society of Nephrology 1995;5(8):1553-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2020
- Kidney Disease: Improving Global Outcomes Glomerular Diseases Guideline Work Group. 2020 Clinical Practice Guidelines on Glomerular Diseases. www.kdigo.org/guidelines/gn/ 2020;Public review.
Kittanamongkolchai 2016
- Kittanamongkolchai W, Cheungpasitporn W, Zand L. Efficacy and safety of adrenocorticotropic hormone treatment in glomerular diseases: a systematic review and meta-analysis. Clinical Kidney Journal 2016;9(3):387-96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2000
Lai 2015
- Lai WL, Yeh TH, Chen PM, Chan CK, Chiang WC, Chen YM, et al. Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. Journal of the Formosan Medical Association 2015;114(2):102-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Natale 2020
- Natale P, Palmer SC, Ruospo M, Saglimbene VM, Craig JC, Vecchio M, et al. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD003965. [DOI: 10.1002/14651858.CD003965.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Polanco 2010
- Polanco N, Gutierrez E, Covarsi A, Ariza F, Carreno A, Vigil A, Baltar J, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. Journal of the American Society of Nephrology 2010;21(4):697-704. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Ponticelli 2010
- Ponticelli C, Passerini P. Management of idiopathic membranous nephropathy. Expert Opinion on Pharmacotherapy 2010;11(13):2163-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Remuzzi 2002
- Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P. Rituximab for idiopathic membranous nephropathy. Lancet 2002;360(9337):923-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ruggenenti 2006
- Ruggenenti P, Chiurchiu C, Abbate M, Perna A, Cravedi P, Bontempelli M, et al. Rituximab for idiopathic membranous nephropathy: who can benefit? Clinical Journal of The American Society of Nephrology: CJASN 2006;1(4):738-48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ruggenenti 2016
- Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasà M, Remuzzi G. Rituximab in Idiopathic Membranous Nephropathy. Journal of the AMerican Society of Nephrology: JASN 2012; Aug 23;(23):1416-1425. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Stanescu 2011
- Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. New England Journal of Medicine 2011;364(7):616-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tomas 2014
- Tomas NM, Beck, LH, Meyer-Schwesinger C, Seitz-Polski B, Hong M, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G. Thrombospondin- type-1-domain-containing 7A in idiopathic membranous nephropathy. New England Journal of Medicine: NEJM 2014; Dec 11;371(24):2277-2287. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Tramontano 2006
- Tramontano A, Knight T, Vizzuso D, Makker SP. Nested N-terminal megalin fragments induce high-titer autoantibody and attenuated Heymann nephritis. Journal of the American Society of Nephrology 2006;17(7):1979-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tunnicliffe 2018
- Tunnicliffe DJ, Palmer SC. Immunosuppressive treatment for proliferative lupus nephritis: summary of a Cochrane review. American Journal of Kidney Diseases 2018;72(5):756-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vendemia 2001
- Vendemia F, Gesualdo L, Schena FP, D'Amico G, Renal Immunopathology Study Group of the Italian Society of Nephrology. Epidemiology of primary glomerulonephritis in the elderly. Report from the Italian Registry of Renal Biopsy. Journal of Nephrology 2001;14(5):340-52. [MEDLINE: ] [PubMed] [Google Scholar]
Waldman 2009
- Waldman M, Austin HA 3rd. Controversies in the treatment of idiopathic membranous nephropathy. Nature Reviews Nephrology 2009;5(8):469-79. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zou 2018
- Zou PM, Li H, Cai JF, Chen ZJ, Li C, Li XW. Therapy of rituximab in idiopathic membranous nephropathy with nephrotic syndrome: asystematic review and meta-analysis. Chinese Medical Sciences Journal 2018;33(1):9-19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chen 2013
- Chen Y, Schieppati A, Cai G, Chen X, Zamora J, Giuliano GA, et al. Immunosuppression for membranous nephropathy: a systematic review and meta-analysis of 36 clinical trials. Clinical Journal of The American Society of Nephrology: CJASN 2013;8(5):787-96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2014
- Chen Y, Schieppati A, Chen X, Cai G, Zamora J, Giuliano GA, et al. Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD004293. [DOI: 10.1002/14651858.CD004293.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perna 2004
- Perna A, Schieppati A, Zamora J, Giuliano GA, Braun N, Remuzzi G. Immunosuppressive treatment for idiopathic membranous nephropathy: a systematic review. American Journal of Kidney Diseases 2004;44(3):385-401. [MEDLINE: ] [PubMed] [Google Scholar]
Schieppati 2004
- Schieppati A, Perna A, Zamora J, Giuliano GA, Braun N, Remuzzi G. Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD004293. [DOI: 10.1002/14651858.CD004293.pub2] [DOI] [PubMed] [Google Scholar]


