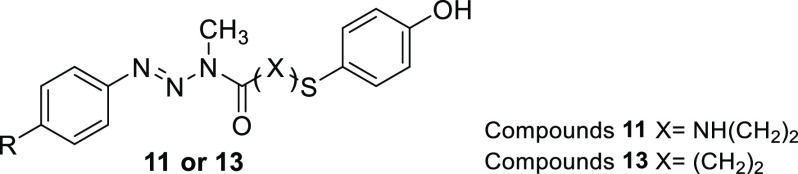
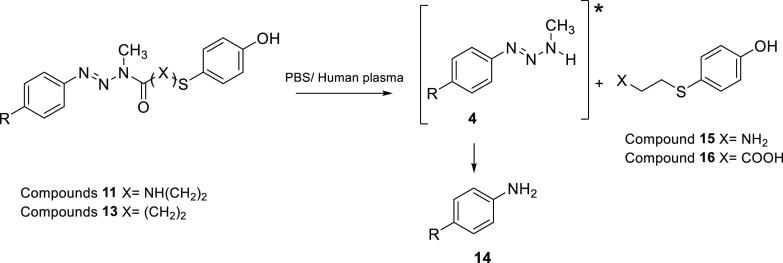
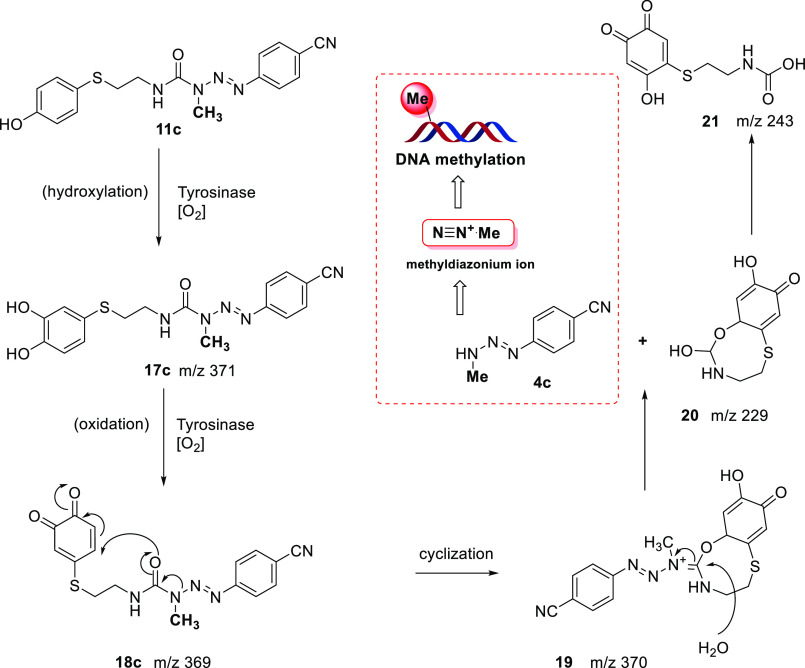
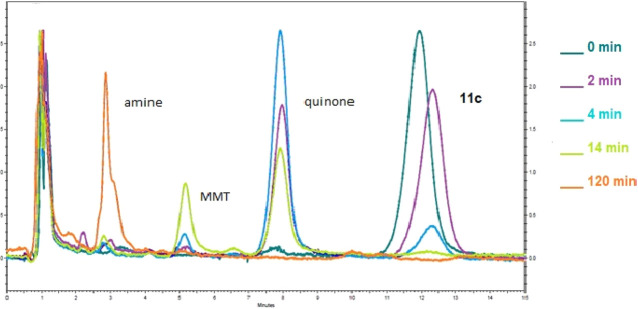
Abstract
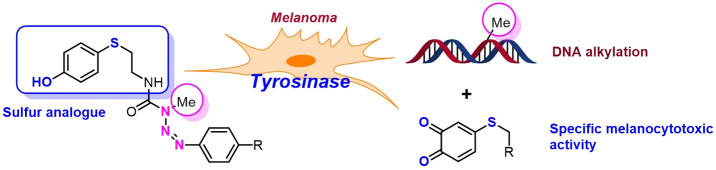
Malignant melanoma is the major cause of death from skin cancer. Treatment of metastatic melanoma remains an enormous challenge. In this study we developed hybrid compounds and studied their potential use in malignant melanoma chemotherapy. They were designed to act by a double mechanism of action, being composed of two pharmacophores: the tyrosine sulfur analogue 4-S-cysteaminylphenol (4-S-CAP, 10), with immunomodulatory properties and specific melanocytotoxic activity, and triazene 4, with DNA alkylating properties. The design of these compounds aims to achieve selective activation by the enzyme tyrosinase overexpressed in melanoma cells. Compounds 11a–e, 13a, and 13b were found to be excellent tyrosinase substrates (0.5 min ≤ t1/2 ≤ 3.7 min). Furthermore, derivatives 11 and 13 were evaluated for their molecular properties, hepatotoxicity, in vivo toxicity profile, and assessment of cytotoxic activity in melanoma and non-melanoma cell lines. The results were compared with those obtained for temozolomide, a triazene used in melanoma therapy. It was discovered that the hybrids are selective and effective drugs, representing a valuable model for the development of new multitarget melanoma therapy. In particular, compound 10 may be an important component for these strategies that use a metabolic pathway of melanin synthesis. Molecular hybridization of 10 with triazenes 4 renders the hybrids (11 and 13) unexpectedly devoid of hepatotoxicity while maintaining cytotoxic activity in malignant cells.
Keywords: Triazenes, melanoma, tyrosinase, hybrid compounds, target therapy
Although malignant melanoma represents less than 5% of all malignant skin diseases, it is the major cause of death from skin cancer. Compared with other cancers, the incidence and mortality of melanoma have risen so rapidly in the last 50 years that melanoma has become a heavy health and economic burden.1,2 In the ranking of the primary tumors that most easily metastasize in the brain, melanoma ranks third (after lung and breast cancers), greatly worsening the prognosis of patients with melanoma.3
Treatment of metastatic melanoma depends on the tumor stage at the time of diagnosis. Surgery remains the mainstay for local primary melanoma. Radiotherapy is a valid and effective therapeutic option especially in situations of medical inoperability or for most melanomas with few systemic options.4 With regard to chemotherapy, dacarbazine (DTIC) is the only drug specifically approved by the U.S. Food and Drug Administration for the treatment of malignant melanoma. It is a bioprecursor of the DNA alkylating agent, 5-(3-methyltriazeno)imidazole-4-carboxamide (MTIC).5 Unfortunately, DTIC is only able to promote medium survivals ranging from 6 to 11 months. The modest activity of DTIC in the treatment of metastatic melanoma patients has been attributed in part to the lower activity of cytochrome P450 (CYP450) in humans compared with rodents.6 Another triazene, temozolomide (TMZ), approved for the treatment of anaplastic astrocytoma, has been used to treat brain metastases of melanoma since after oral administration it is able to cross the blood–brain barrier. Albeit TMZ is also a prodrug, in the brain it is transformed into MTIC by chemical hydrolysis.7
Although not so widely used as chemotherapy and radiotherapy, immunotherapy is a therapeutic strategy that seeks to help the immune system fight cancer. The most commonly used immunostimulators for treatment of melanoma are interleukin (IL)-2, interferon (IFN)-α, thymosin α1, and monoclonal antibodies such as ipilimumab, prembolizumab, and nivolumab. Immunotherapy has contributed to improve the overall survival of patients at advanced melanoma stages.2
Targeted therapies namely, small-molecule drugs, are able to specifically act on particular mechanisms of melanoma occurrence and progression. These therapies include BRAF inhibitors, namely sorafenib (a multitargeted tyrosine kinase inhibitor), vemurafenib and dabrafenib, and/or MEK inhibitors such as binimetinib.2,4
Regrettably, current therapeutic strategies display limited clinical success, mainly because of the severe side effects, lack of specificity, and acquired resistance, which determine not only the high aggressiveness of the melanoma but also the high rate of tumor relapses.2 To overcome some of these drawbacks, combination therapy is recommended in clinical practice.2,4
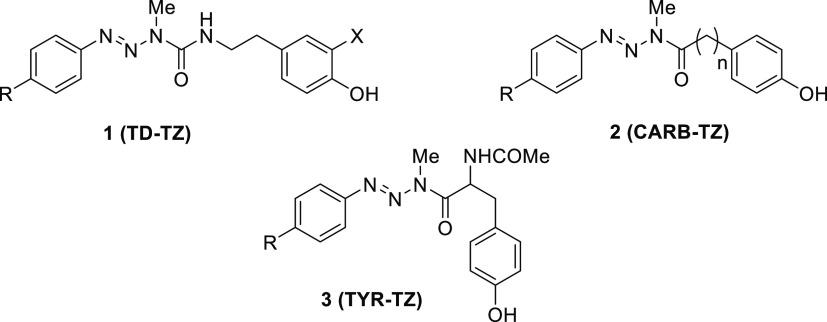
The design and synthesis of multitarget hybrid molecules has become a promising new strategy in anticancer therapy. Hybrid molecules, designed and synthesized with two or more different pharmacophore moieties that act simultaneously on the same or different targets at tumor cells, may be more potent and efficient than combination therapies.8−12 In recent years, our team has designed hybrid molecules that combine an antitumor triazene and a substrate for the enzyme tyrosinase, which is overexpressed in melanoma cells (Figure 1).13−15
Figure 1.
Previously synthesized hybrid drugs containing different phenols as tyrosinase substrates: TD-TZ, 1 (tyramine and dopamine); CARB-TZ, 2 (hydroxyphenyl carboxylic acids); TYR-TZ, 3 (N-acetyltyrosine).13−15
Tyrosinase (monophenol, 3,4-β-dihydroxy-l-phenylalanine oxygen oxidoreductase, EC 1.14.18.1) is involved in the biosynthetic pathways of melanin pigment. It catalyzes the two-step oxidation of phenolic compounds into the corresponding catechols (phenolase activity) and o-quinones (catecholase activity).16 The tyrosinase overexpression and the high turnover rate of its catalytic activity render this enzyme an attractive molecular target for the development of melanoma-specific therapies.8,9,11,12 In the present work, taking advantage of the same biosynthetic pathway that leads to the production of melanin, we assessed different phenols for tyrosinase-mediated selective release of cytotoxic triazenes. Oxidation of the phenol carrier leads to the formation of a corresponding o-quinone, and a subsequent cyclization-based mechanism finally allows the release of the cytotoxic triazene.13−16
Supported by our previous results, we envisioned a new strategy involving the design of hybrid compounds conjugating two pharmacophores with antimelanoma activity: aryltriazenes 4 and a sulfur analogue of tyrosine, 4-S-cysteaminylphenol (4-S-CAP, 10), a substrate of melanoma tyrosinase that exhibits a significant in vivo depigmenting effect.17
4-S-CAP 10 was identified as a much better substrate for mushroom tyrosinase than l-tyrosine and also a good substrate for mammalian tyrosinase. Moreover, compound 10 is known to inhibit the growth of malignant melanoma and cause depigmentation of dark skin. One possible explanation for such activity is that in melanocytes this phenol is oxidized by tyrosinase to the corresponding o-quinone, which conjugates with sulfhydryl enzymes through cysteine residues, thus exerting cytotoxic effects.17,18
Herein we report the synthesis, stability studies in physiological conditions, toxicity evaluation, and in vitro antimelanoma activity of these hybrid molecules. For the design of these new hybrid molecules, two pharmacophore moieties, triazene 4 and 4-S-CAP 10, were conjugated by insertion of urea or amide linkages. These two linkages were chosen because in general they are chemically stable, thus allowing the hybrids to reach tumor cells intact. Compounds 11 and 13 were aimed to be used in a targeted therapeutic strategy based on overexpression of the enzyme tyrosinase in malignant melanocytes. It was expected that the compounds would manifest cytotoxic activity only after tyrosinase oxidation, thus increasing the selectivity while minimizing side effects.
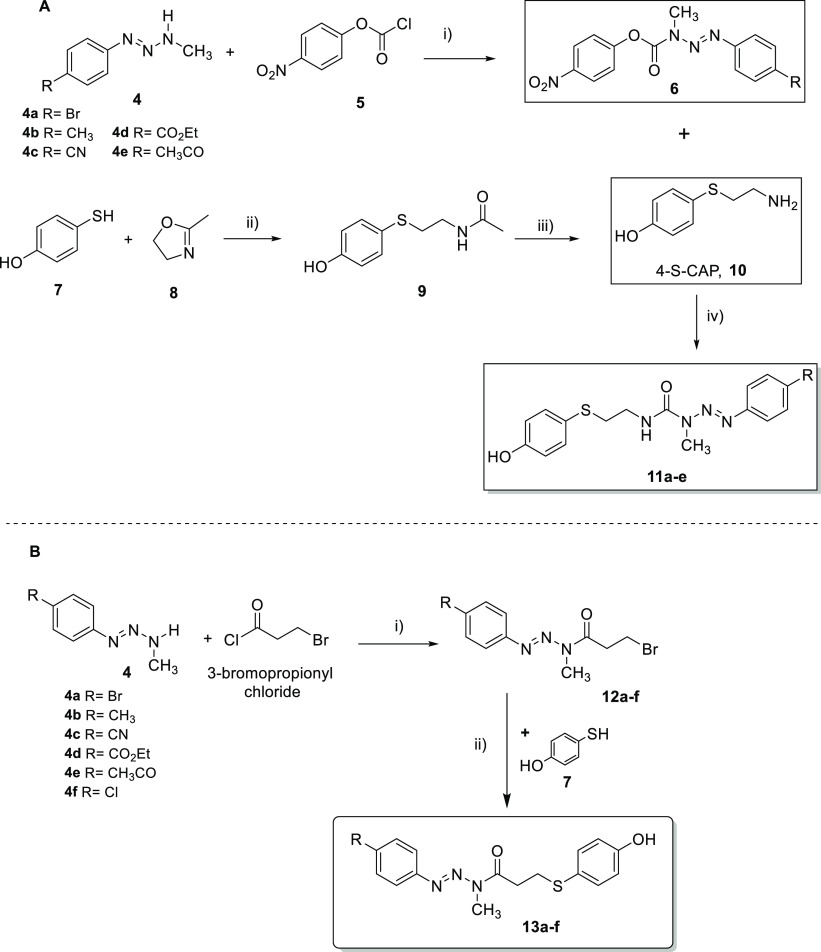
The synthetic approach used for the preparation of triazene hybrid compounds 11a–e is shown in Scheme 1A. Key intermediates 6a–e were synthesized by reaction of monomethyltriazenes (MMTs) 4a–e with p-nitrophenyl chloroformate (5), allowing the insertion of urea functionality in moderate to good yields (43–91%).19 The final hybrid compounds 11a–e were obtained by coupling intermediates 6 with 4-S-CAP under basic conditions. These reactions also occurred in moderate to good yields (53–80%), and the compounds were fully identified and characterized by spectroscopic methods (Supporting Information, S3).
Scheme 1. Synthetic Pathways for (A) Triazene Derivatives 11 and 4-S-CAP (10)20 and (B) Triazene Derivatives 13a–f.
Reagents and conditions: (A) (i) CH2Cl2, pyridine; (ii) N2 atmosphere, reflux (130 °C); (iii) conc. HCl, reflux (170 °C); (iv) THF, Et3N, rt. (B) (i) CH2Cl2, pyridine; (ii) K2CO3, THF.
Compound 10 was synthesized by the reaction between 4-mercaptophenol (7) and 2-methyl-2-oxazoline (8) followed by acid-catalyzed hydrolysis of the amide bond, a method also called the Wehrmeister reaction (Scheme 1A). Compound 10 was obtained in 70% overall yield, which is in accordance with previous published results.20
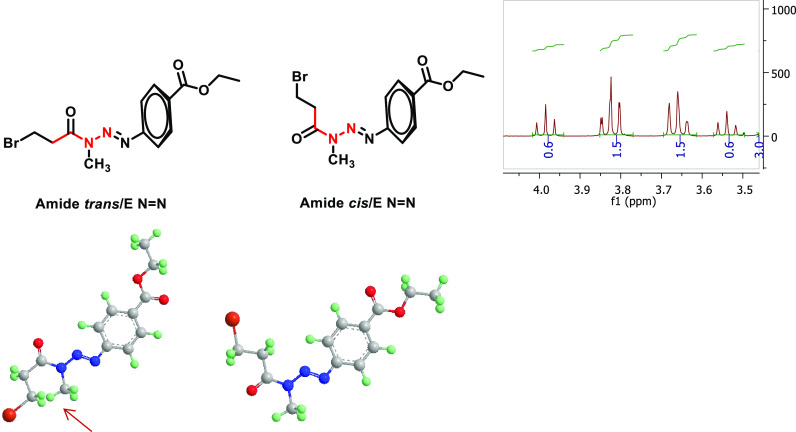
Amide-linked hybrids 13a–f were synthesized by the reactions outlined in Scheme 1B. Intermediate amides 12a–f were synthesized by the Schotten–Baumann reaction between MMTs 4 and 3-bromopropionyl chloride, followed by the reaction of these intermediates 12a–f with mercaptophenol 7 in basic medium.21 The intermediate amides 12 were generally obtained in low yields (15–35%), and the final hybrid compounds were obtained in moderate or good yields depending on the R group (41–75%). Compounds 13a–f were also fully identified and characterized by spectroscopic methods (Supporting Information S7). Interestingly, in the 1H NMR spectra, intermediate amides 12a–f showed the presence of a mixture of E and Z diastereomers in the deuterated solvent solution (as shown for compound 12d in Figure 2). In contrast to intermediate amides 12a–f, E/Z diastereomers were not observed for hybrids 13a–f in the same deuterated solvent solutions (Supporting Information, S6).
Figure 2.
General structures of the cis- and trans-amide isomers for compound 12d.
The existence of E and Z diastereomers is attributed to the partial double-bond character of the amide bond (O)C–N, which prevents the free rotation of atoms or groups of atoms around the axis of the bond. For asymmetric tertiary amides, the limited rotation implies the existence of nonisolable E and Z diastereomers. In the case of compound 12d, the predominant diastereomer is possibly the cis one. In the 3D representations of the two isomers (Figure 2) a spatial repulsion between the close −NCH3 and −CH2Br groups is observed (signaled in the figure with a red arrow), which probably shifts the balance to the cis isomer (1.5/0.5), as noticed in the spectrum integration values.
The stabilities of triazene derivatives 11a–e and 13a–f were studied both in phosphate buffered saline (PBS) and in 80% human plasma at 37 °C. These reactions were followed by HPLC by monitoring both the loss of compounds 11a–e and 13a–f and the formation of hydrolysis products. Half-lives for the hydrolysis of triazene derivatives in PBS and in human plasma are presented in Tables 1, S2, and S3.
Table 1. Half-Lives (t1/2) for Triazene Derivatives 11a–e and 13a–f in PBS or 80% Human Plasma at 37 °C, log P and clogP Values, and Ability to Act as Tyrosinase Substrates (100 units/mL).
|
t1/2 |
||||||
|---|---|---|---|---|---|---|
| compound | R | log P | clogPa | PBS pH 7.4 (days) | 80% human plasma (h) | tyrosinase (min) |
| 11a | Br | 3.95 ± 0.04 | 4.31 | >10 | 104.6 ± 3.2 | 0.5 ± 0.2 |
| 11b | Me | 4.21 ± 0.06 | 3.88 | >10 | 88.5 ± 0.5 | 1.9 ± 0.1 |
| 11c | CN | 3.49 ± 0.05 | 3.54 | >10 | 74.0 ± 5.6 | 0.5 ± 0.1 |
| 11d | EtOCO | 4.08 ± 0.10 | 3.88 | >10 | 30.5 ± 2.9 | 3.7 ± 0.1 |
| 11e | MeCO | 3.44 ± 0.05 | 3.51 | >10 | 61.6 ± 11.9 | 1.0 ± 0.2 |
| 13a | Br | ndb | 5.06 | 4.4 ± 0.4 | 8.3 ± 0.7 | 2.1 ± 0.1 |
| 13b | Me | nd | 4.43 | 4.2 ± 1.1 | 8.7 ± 0.4 | 2.3 ± 0.2 |
| 13c | CN | nd | 4.15 | 3.6 ± 0.2 | 2.99 ± 0.17 | nd |
| 13d | EtOCO | nd | 4.68 | 5.4 ± 0.8 | 6.57 ± 0.41 | nd |
| 13e | MeCO | nd | 3.99 | 5.8 ± 0.6 | 4.90 ± 0.33 | nd |
| 13f | Cl | nd | 4.71 | nd | nd | nd |
Calculated using Virtual Computational Chemistry Laboratory (VCCLAB) (http://www.vcclab.org, 2005).
nd: not determined.
These reactions followed pseudo-first-order kinetics and were monitored during at least 3 half-lives. Pseudo-first-order rate constants (kobs) were calculated from the slopes of plots of ln(concentration) vs time (eq 1), and half-lives (t1/2) were calculated using eq 2:
| 1 |
| 2 |
Analysis of the obtained results showed that hybrids 11a–e, with the urea linker, are extremely stable in PBS, remaining undecomposed for more than 10 days. These results are in accordance with the previous ones obtained by our group for triazene derivatives of tyramine 1 (X = H), also conjugated through an urea linker, which were stable in PBS for more than 15 days.13 However, the presence of a urea linkage is not a guarantee of stability since in the same study dopamine derivatives 1 (X = OH) proved to be much more unstable, with half-lives of around 15 to 20 h.13
Compounds 13a–f with an amide linkage are also quite stable in PBS, with half-lives between 4 and 6 days at 37 °C. Hydrolysis of hybrids 13a–f in this medium yielded the respective anilines (Scheme 2) in quantitative amounts. Again, these results are in line with the previous ones obtained by our group for compounds 2 (n = 2) with half-lives between 2.5 and 4 days. Amides 2 with longer alkyl chains separating the triazene and the phenol (n = 4) are more stable and remain undecomposed for more than 10 days in PBS.15 Overall, comparing the reactivities of hybrids 11a–e and 13a–f confirmed that urea derivatives are more stable than amides in PBS at 37 °C.
Scheme 2. Decomposition Pathways in PBS and Human Plasma to Afford Hydrolysis Products of Hybrid Compounds 11 and 13.
Not detected in PBS.
Human plasma is rich in hydrolytic enzymes that can recognize ureas and amides and decompose hybrid compounds 11 and 13. In fact, both types of compounds decompose in human plasma, but with a remarkable difference in reactivity. Indeed, ureas 11a–e showed half-lives between 30 and 105 h, while amides 13a–f presented half-lives between 3 to 9 h. Compound 11a is 35 times more stable than compound 13c, the most reactive. The products of reaction are MMTs 4 and the corresponding anilines 14 (Scheme 2).
The results for ureas 11a–e are in line with our previous ones obtained for compounds 1 (X = H), highlighting the great stability of the urea bond in plasma. On the contrary, the amide bond is much more unstable, reflecting the higher concentration of amidases in plasma that, acting on the compounds, break down the linkage, which results in shorter plasma half-lives. It can be stated that urea derivatives 11a–e are very stable in human plasma, and the potential hydrolysis promoted by plasma enzymes is not significant at 37 °C. Because they are poor substrates for the plasmatic enzymes, their premature hydrolysis is prevented, and the compounds can circulate freely through the body and reach tumor sites at high concentration. With regard to our original statement that hybrid compounds must target affected areas intact, the urea linker seems more adequate to accomplish this objective than the amide linker.
Next, derivatives 11a–e and 13a–f were evaluated as tyrosinase substrates. Looking at the half-lives in the presence of mushroom tyrosinase (Table 1), we can state that these hybrid compounds (11 and 13) are easily recognized and oxidized by the enzyme with half-lives between 0.5 and 4 min. In these assays, the first metabolite detected by HPLC is quinone 18 (Scheme 3). After the initial formation of these metabolites, we propose that they were decomposed into MMT 4 and cyclic compound 19, although they were not detected by HPLC. Nevertheless, by analysis of the composition of the reaction mixtures from the tyrosinase assays by mass spectrometry, it was possible to observe the presence of peaks with m/z corresponding to compounds 17, 18, 19, 20, and 21. The formation and complete disappearance of compound 18 after 2 h of reaction was also confirmed by HPLC. Moreover, at this time, the concomitant formation of a 100% yield of amine 14, which results from decomposition of monomethyltriazene 4, was detected. Figure 3 depicts the overlap of the chromatograms obtained from samples taken at different reaction time points. As an example, the disappearance of hybrid compound 11c over time is shown, along with the appearance of intermediate quinone 18, which later degrades into MMT 4c and the corresponding amine 14c (Figure S1).
Scheme 3. Proposed Mechanism for MMT Release from Derivative 11c after Tyrosinase Activation.
Figure 3.
Overlapping chromatograms showing the disappearance of hybrid compound 11c over time following tyrosinase oxidation, which results in formation of an intermediate quinone, that later degrades into MMT 4c and the corresponding amine 14c.
For amides 13, a parallel study involving mass spectrometry analysis of samples from the tyrosinase assays corroborated the presence of the corresponding dihydroxylated compound. For example, compound 13a displayed two peaks at m/z 408 and 410 for the dihydroxylated compound (M – H+), while compound 13b presented one peak at m/z 344 (M – H+) (Scheme S1 and Figure S2).
Evaluation of the kinetic parameters for hybrid compounds 11 showed that these compounds are very good substrates for mushroom tyrosinase with Km values ranging from 0.12 to 0.064 mM. In fact, these new compounds exhibited a 2- to 4-fold higher affinity in comparison with the natural substrate l-tyrosine. This effect was not observed in our previous work on triazene derivatives13−15 (Table S4).
The values of log P presented in Table 1 for compounds 11 (3.51 < log P < 4.31) and 13 (3.99 < log P < 5.06) demonstrate their lipophilicity. The presence of the linker amide (compounds 13) increases the lipophilic character of the hybrids. With regard to compounds 11, the experimental and calculated log P values are close. Comparison of compounds 13 with derivatives 2 shows that the introduction of sulfur in the ethyl chain increases the log P value by one unit.15 Other molecular properties that must be contextualized with log P values are included in Table S5.
The cytotoxic activities of hybrid compounds 11 and 13 (Table 2) were also investigated. Compounds were screened in terms of antiproliferative properties following their incubation in two human melanoma cell lines (MNT-1 and A375), one murine cell line (B16F10), and a healthy human keratinocyte cell line (HaCaT). In general, the compounds were less cytotoxic to HaCaT cells, with a selectivity index ranging from 2 to 3. The hybrid compounds showed similar antiproliferative properties toward human and murine melanoma cell lines. Hybrid compound 11d displayed the highest antiproliferative properties, with IC50 values ranging from 49 to 51 μM for melanotic cell lines (MNT-1 and B16F10) and 43.8 μM for the amelanotic cell line (A375).
Table 2. Inhibitory Effects of the Tested Compounds 11 and 13 after Incubation for 72 h with MNT-1 (Human Melanoma), B16F10 (Murine Melanoma), HaCaT (Human Keratinocyte), and A375 (Human Melanoma) Cells.
| IC50 (μM) |
||||
|---|---|---|---|---|
| compound | MNT-1 | B16F10 | A375 | HaCaT |
| 11a | >75 | >75 | nda | >150 |
| 11b | 74.1 ± 2.9 | 78.3 ± 2.7 | nd | >130 |
| 11c | >75 | >75 | nd | >150 |
| 11d | 49.4 ± 1.6 | 51.0 ± 1.5 | 43.8 ± 3.3 | >100 |
| 11e | >75 | >75 | nd | >150 |
| 13a | >75 | >75 | 55.0 ± 3 | nd |
| 13b | Nd | >75 | 47.0 ± 1.6 | 45.8 ± 1.2 |
| 13c | >75 | >75 | >75 | nd |
| 13d | >75 | >75 | 61.0 ± 4 | nd |
| 13e | >75 | >75 | >75 | nd |
| 13f | nd | >75 | >75 | >75 |
| 4a | >60 | >60 | nd | >60 |
| 4c | >60 | >60 | nd | >60 |
| 4d | >60 | >60 | nd | >60 |
| 4e | >75 | >75 | nd | >130 |
| 10 + 4e | 37.1 ± 1.9 | >75 | nd | 130 ± 8.7 |
| 10 | 42.8 ± 1.4 | >75 | nd | >125 |
| TMZ | >75 | >75 | >75 | >75 |
nd: not determined.
Surprisingly, compounds 13a, 13b, and 13d exhibited higher antiproliferative properties for the amelanotic A375 cell line. This cytotoxic activity probably does not depend exclusively on the presence of tyrosinase, in contrast to what was previously observed for compounds 1, 2, and 3 (without sulfur in their structure), in which the cytotoxic activity was slightly superior in melanotic cell lines.14
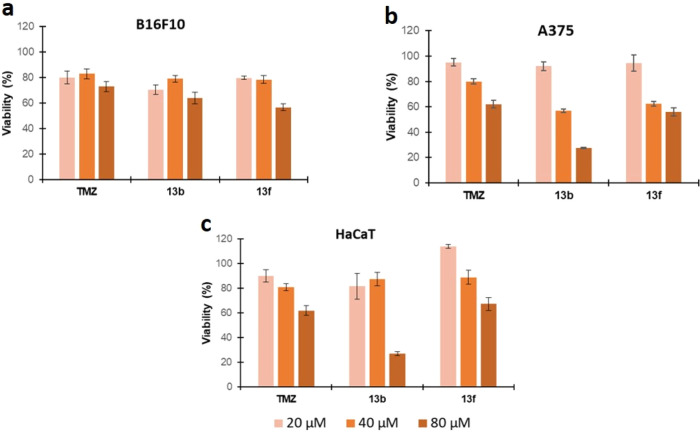
The individual cytotoxicities of the two pharmacophores in hybrids 11 were also determined. For that, 4-S-CAP 10 and MMTs 4a–e, either separately or in a physical mixture at the same concentration as the one tested for hybrid compounds 11, were evaluated in the same cell lines. MMTs 4a–e alone did not show cytotoxicity in any of the cell lines under study. On the other hand, 10 exhibited greater cytotoxicity toward MNT-1 cells, with an IC50 value of 42.8 μM and a selectivity index of ≥3. The incubation of both compounds (10 + 4e) displayed cytotoxicity toward the MNT-1 cell line with a selectivity index of ≥3. The results of this study showed that the conjugation of the two pharmacophores in a hybrid molecule resulted in antitumor activity that depends mostly on the triazene structure. Furthermore, the type of linker between the two pharmacophores also influences the antitumor activity, since compounds with the amide linker showed cytotoxic activity only against the A375 cell line. Another important observation results from the data obtained for TMZ, the triazene compound used in the assay as a positive control (Table 2). Compound 11d displayed superior antiproliferative activity compared with TMZ. Also, Figure 4 shows the results of cellular viability (%) for compounds 13b and 13f compared with the positive control. Again, compound 13b exhibited higher cytotoxic properties than TMZ toward the A375 cell line. However, the selectivity index was <1.
Figure 4.
Cellular viability of the (a) murine melanoma cell line (B16F10), (b) human melanoma cell line (A375), and (c) human epidermal keratinocyte cell line (HaCaT) in the presence of two hybrid compounds, 13b and 13f, with potential antiproliferative properties. TMZ was used as a positive control. The compounds were incubated for 48 h, and the cell viability was determined by the MTS assay. The cell concentration was 1.5 × 103 cells/mL. The tested concentrations of all compounds were 20, 40, and 80 μM.
Next, the hepatotoxicities of derivatives 11 were evaluated. Quinones have two chemical properties that must be addressed with regard to their potential overall toxicity: electrophilicity and oxidative stress promotion. As electrophiles, they can bind to sulfhydryl proteins, causing protein denaturation or enzyme inhibition. As oxidants, they can promote oxidative stress by generation of reactive oxygen radicals and the depletion of antioxidants from cells. Consequently, quinones are associated with the in vivo occurrence of acute cytotoxicity, immunotoxicity, or carcinogenesis.22 In studies on the antimelanoma effect of 4-S-CAP 10 in in vivo models, the existing information on the metabolic fate of this compound is very scarce, namely, the in vivo formation of quinones as a result of oxidation by hepatic microsomes. Jimbow et al.23 disclosed that in a female C57BL/6J murine melanoma metastases model possessing both B16F10 subcutaneous melanoma nodules and melanoma lung colonies, after a single intraperitoneal injection of radiolabeled phenolic thioether N-acetyl-4-S-cysteaminylphenol (NA-CAP), the radioactive material was cleared from the body and was not detectable in any normal organs except the lumen of the large intestine. On their interpretation, the significant detoxication of NA-CAP occurred in the liver and was followed by the excretion of NA-CAP metabolites into the bile. However, the metabolites that would have resulted from hepatic metabolism were not described or quantified. The authors suggested that reactive intermediates of NA-CAP (quinones or semiquinones) may result from the interaction of the compound with enzymes such as CYP450. Our previous studies regarding the potential hepatotoxicity of compounds 2 (derived from 4-hydroxypropanoic acid) revealed high concentrations of quinones and increased glutathione (GSH) depletion (35% to 64% in 180 min) in the incubation assays with rat microsomes, a common feature of 3-hydroxy- or 4-hydroxyphenyl compounds.15,24 (Figure S3 and Table S6). Since the presence of a phenol group in hybrid compounds 11 raises a potential hepatotoxicity risk, we evaluated this risk by measuring the GSH depletion promoted by the compounds. In this study with rat microsomes we did not observe the depletion of GSH (Figure S4b). The study was complemented with HPLC analysis to search for o-quinones as CYP450 metabolites. These studies confirmed the absence of quinones (Figure S4a). The results allow us to claim that compounds 11 do not generate o-quinones in the presence of rat microsomes, giving them high therapeutic potential.
To provide further insights into the safety of the hybrid compounds, in a very preliminary in vivo study, the most promising compound, 11d, was intravenously administered to BALB/c mice at a dose of 12 mg/kg of body weight 3 times per week for 1 week. The animals’ behavior was monitored every day. Two days after the last injection, the mice were sacrificed, and blood and organs were collected and analyzed. The control group was constituted by mice that received only the vehicle for compound 11d solubilization, a mixture of PBS and Cremophor (5% p/v). The tissue index was calculated according to eq 3:
| 3 |
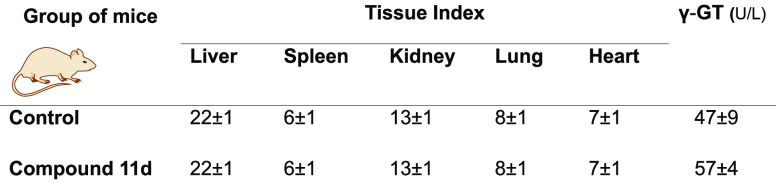
The tissue index and hepatic biomarkers were assessed, as depicted in Table 3. Determination of the tissue index allows the evaluation of changes in the weights of specific organs that may occur relative to the whole body weight. Changes in organ weight have long been accepted as a sensitive indicator of chemically induced toxicity.25 In particular, tissue indexes of the liver, kidneys, and spleen are considered the most valuable in different areas such as pharmaceutical, veterinary, chemical, and food/nutritional/consumer.25 While an increased tissue index indicates organ hypertrophy, congestion, or edema, a decreased ratio indicates organ atrophy and degenerative changes.26
Table 3. Preliminary In Vivo Safety Assays of 11d: Tissue Indexes and γ-GT Values.
In terms of tissue index, no changes for the analyzed organs were observed among the two groups of mice (Table 3). The γ-glutamyl transferase (γ-GT) blood levels were also determined, since these levels are used as an index of liver dysfunction and the enzyme plays an essential role in glutathione metabolism. No statistically significant differences between the control group and the animals that received compound 11d were observed.
To complement the evaluation of the in vivo safety of compound 11d, a hemolytic assay using human red blood cells was performed. The results showed that compound 11d at concentrations ranging from 0.2 to 400 μM did not promote hemolysis (less than 1%) after 1 h of incubation at 37 °C.27 Taken together, these results highlight the safety profile of compound 11d.
In conclusion, the studies presented herein led to the synthesis and identification of novel hybrid compounds designed to be activated by tyrosinase, the enzyme overexpressed in melanoma tumor cells. Under physiological conditions, compounds 11 and 13 proved to be very stable, being slowly hydrolyzed by plasma enzymes. The parameters of enzymatic kinetics characterize these compounds as excellent substrates of tyrosinase, as confirmed by mass spectroscopy studies regarding the identification of the generated metabolites. Some of these compounds reveal an increased and selective potency toward melanoma cell lines. Cytotoxicity assays highlighted 11d as having the highest antiproliferative properties (IC50 values of 49.4 and 51.0 μM for MNT-1 and B16F10, respectively). The hepatotoxicity and hemolysis studies for the most promising compound, 11d, revealed a very good safety profile since the formation of oxidative quinones occurred only in melanoma tumor cells (promoted by tyrosinase) and not by hepatic microsomal oxidation (CYP450). In addition, preliminary safety in vivo assays with BALB/c mice confirmed the absence of chemically induced toxicity over vital organs and no toxic hepatic effects. On the other hand, the cytotoxic effect demonstrated by compounds 13 on the A375 amelanotic cell line opens new perspectives about other potential pathways involved in tumor cell death promoted by sulfur analogues. Currently, encapsulation studies in lipid-based delivery systems of the most promising compound, 11d, are underway, as well as in vivo evaluation.
Acknowledgments
This research was funded by Fundação para a Ciência e Tecnologia (FCT) Projects PTDC/MED-QUI/31721/2017, SAICTPAC/0019/2015, UIDB/04138/2020 and UIDP/04138/2020. The authors also acknowledge financial support from the National Program for Scientific Re-equipment for the acquisition of the LC–MS/MS equipment that is part of the Portuguese National Mass Spectrometry Network (Contract RNEM; Lisboa-01-0145-FEDER-402-022125).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00252.
Experimental procedures for the synthesis and full NMR characterization of compounds 11–13; description of stability studies under physiological conditions and in the presence of mushroom tyrosinase; general procedures for determination of apparent partition coefficients, cytotoxicity screening assays, and hepatotoxicity studies; and assay protocols for the in vivo toxicity profile in BALB/c mice for compound 11d (PDF)
Author Contributions
§ M.G. and E.M. contributed equally.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Medicinal Chemistry Letters virtual special issue “Medicinal Chemistry in Portugal and Spain: A Strong Iberian Alliance”.
Supplementary Material
References
- Melanoma of Skin. The Global Cancer Observatory, International Agency for Reserch on Cancer , December 2020. https://gco.iarc.fr/today/data/factsheets/cancers/16-Melanoma-of-skin-fact-sheet.pdf
- Cutaneous Melanoma: Etiology and Therapy; Ward W. H., Farma J. M., Eds.; Codon Publications, 2017. [PubMed] [Google Scholar]
- Nayak L.; Lee E. Q.; Wen P. Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- Mishra H.; Mishra P. K.; Ekielski A.; Jaggi M.; Iqbal Z.; Talegaonkar S. Melanoma treatment: from conventional to nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 2283–2302. 10.1007/s00432-018-2726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibba J. L.; Beal D. D.; Ramirez G.; Bryan G. T. N-Demethylation of the Antineoplastic Agent 4(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide by Rats and Man. Cancer Res. 1970, 4, 147–150. [PubMed] [Google Scholar]
- Reid J. M.; Kuffel M. J.; Miller J. K.; Rios R.; Ames M. M. Metabolic activation of dacarbazine by human cytochromes P450: The role of CYP1A1, CYP1A2, and CYP2E1. Clin. Cancer Res. 1999, 5, 2192–2197. [PubMed] [Google Scholar]
- Stevens M. F. G.Temozolomide: From cytotoxic to molecularly-targeted agent. In Cancer Drug Design and Discovery; Elsevier, 2008; Chapter 7, pp 157–172. 10.1016/B978-012369448-5.50010-0. [DOI] [Google Scholar]
- Braga C.; Vaz A. R.; Oliveira M. C.; Matilde Marques M.; Moreira R.; Brites D.; Perry M. J. Targeting gliomas with triazene-based hybrids: Structure-activity relationship, mechanistic study and stability. Eur. J. Med. Chem. 2019, 172, 16–25. 10.1016/j.ejmech.2019.03.048. [DOI] [PubMed] [Google Scholar]
- Fortin S.; Bérubé G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discovery 2013, 8, 1029–1047. 10.1517/17460441.2013.798296. [DOI] [PubMed] [Google Scholar]
- Kucuksayan E.; Ozben T. Hybrid Compounds as Multitarget Directed Anticancer Agents. Curr. Top. Med. Chem. 2017, 17, 907–918. 10.2174/1568026616666160927155515. [DOI] [PubMed] [Google Scholar]
- Pinheiro R.; Braga C.; Santos G.; Bronze M. R.; Perry M. J.; Moreira R.; Brites D.; Falcão A. S. Targeting Gliomas: Can a New Alkylating Hybrid Compound Make a Difference?. ACS Chem. Neurosci. 2017, 8, 50–59. 10.1021/acschemneuro.6b00169. [DOI] [PubMed] [Google Scholar]
- Francisco A. P.; Mendes E.; Santos A. R.; Perry M. J. Anticancer Triazenes: from Bioprecursors to Hybrid Molecules. Curr. Pharm. Des. 2019, 25, 1623–1642. 10.2174/1381612825666190617155749. [DOI] [PubMed] [Google Scholar]
- Perry M. J.; Mendes E.; Simplício A. L.; Coelho A.; Soares R. V.; Iley J.; Moreira R.; Francisco A. P. Dopamine- and tyramine-based derivatives of triazenes: Activation by tyrosinase and implications for prodrug design, Eur. Eur. J. Med. Chem. 2009, 44, 3228–3234. 10.1016/j.ejmech.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Monteiro A. S.; Almeida J.; Cabral G.; Severino P.; Videira P. A.; Sousa A.; Nunes R.; Pereira J. D.; Francisco A. P.; Perry M. J.; Mendes E. Synthesis and evaluation of N-acylamino acids derivatives of triazenes. Activation by tyrosinase in human melanoma cell lines, Eur. Eur. J. Med. Chem. 2013, 70, 1–9. 10.1016/j.ejmech.2013.09.040. [DOI] [PubMed] [Google Scholar]
- Sousa A.; Santos F.; Gaspar M. M.; Calado S.; Pereira J. D.; Mendes E.; Francisco A. P.; Perry M. J. The selective cytotoxicity of new triazene compounds to human melanoma cells, Bioorganic. Bioorg. Med. Chem. 2017, 25, 3900–3910. 10.1016/j.bmc.2017.04.049. [DOI] [PubMed] [Google Scholar]
- Buitrago E.; Hardré R.; Haudecoeur R.; Jamet H.; Belle C.; Boumendjel A.; Bubacco L.; Réglier M. Are Human Tyrosinase and Related Proteins Suitable Targets for Melanoma Therapy?. Curr. Top. Med. Chem. 2016, 16, 3033–3047. 10.2174/1568026616666160216160112. [DOI] [PubMed] [Google Scholar]
- Jimbow K.; Iwashina T.; Alena F.; Yamada K.; Pankovich J.; Umemura T. Exploitation of pigment biosynthesis pathway as a selective chemotherapeutic approach for malignant melanoma. J. Invest. Dermatol. 1993, 100, S231–S238. 10.1038/jid.1993.82. [DOI] [PubMed] [Google Scholar]
- Shosuke I.; Toshiaki K.; Kiichi I.; Tsutomu K.; Kowichi J. Mechanism of selective toxicity of 4-S-cysteinylphenol and 4-S-cysteaminylphenol to melanocytes. Biochem. Pharmacol. 1987, 36, 2007–2011. 10.1016/0006-2952(87)90501-6. [DOI] [PubMed] [Google Scholar]
- Capucha V.; Mendes E.; Francisco A. P.; Perry M. J. Development of triazene prodrugs for ADEPT strategy: New insights into drug delivery system based on carboxypeptidase G2 activation. Bioorg. Med. Chem. Lett. 2012, 22, 6903–6908. 10.1016/j.bmcl.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Padgette S. R.; Herman H. H.; Han J. H.; Pollock S. H.; May S. W. Antihyperten sive activities of phenyl aminoethyl sulfides, a class of synthetic substrates for dopamine.beta.-hydroxylase. J. Med. Chem. 1984, 27, 1354–1357. 10.1021/jm00376a024. [DOI] [PubMed] [Google Scholar]
- Smith M. B.; March J.. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th ed.; Wiley, 2006. 10.1002/0470084960. [DOI] [Google Scholar]
- Bolton J. L.; Dunlap T. Formation and biological targets of quinones: Cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 2017, 30, 13–37. 10.1021/acs.chemrestox.6b00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alena F.; Iwashina T.; Gili A.; Jimbow K. Selective in Vivo Accumulation of N-Acetyl-4-S-cysteaminylphenol in B16F10 Murine Melanoma and Enhancement of Its In Vitro and In Vivo Antimelanoma Effect by Combination of Buthionine Sulfoximine. Cancer Res. 1994, 54, 2661–2666. [PubMed] [Google Scholar]
- Moridani M. Y.; Moore M.; Bartsch R. A.; Yang Y.; Heibati-Sadati S. Structural toxicity relationship of 4-alkoxyphenols’ cytotoxicity towards murine B16-F0 melanoma cell line. J. Pharm. Pharm. Sci. 2005, 8, 348–360. [PubMed] [Google Scholar]
- Michael B.; Yano B.; Sellers R. S.; Perry R.; Morton D.; Roome N.; Johnson J. K.; Schafer K. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007, 35, 742–750. 10.1080/01926230701595292. [DOI] [PubMed] [Google Scholar]
- Sellers R. S.; Morton D.; Michael B.; Roome N.; Johnson J. K.; Yano B. L.; Perry R.; Schafer K. Society of toxicologic pathology position paper: Organ weight recommendations for toxicology studies. Toxicol. Pathol. 2007, 35, 751–755. 10.1080/01926230701595300. [DOI] [PubMed] [Google Scholar]
- Nave M.; Castro R. E.; Rodrigues C. M. P.; Casini A.; Soveral G.; Gaspar M. M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine 2016, 11, 1817–1830. 10.2217/nnm-2016-0086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.