ABSTRACT.
Antimicrobial resistance (AMR), largely driven by irrational use of antimicrobials, is a global, multifaceted problem calling for a complete understanding of all contributory factors for effective containment. In conflict settings, war-wounds and malnutrition can combine with existing social determinants to increase demand for antibiotics, compounding irrational use. In this study, we focus on Yemen, a low-income country with active conflict for the last 5 years, and analyze the current status of awareness and stewardship efforts regarding AMR. We performed a survey of prescribers/physicians and pharmacists to describe perceptions of AMR prevalence, antibiotic use practices, and stewardship in Yemen, supported by a nonsystematic scoping literature review and a key informant interview. Participants (96%, N = 54) reported a perceived high AMR prevalence rate. Prescribers (74%, 20/27) reported pressure to prescribe broad-spectrum antibiotics. In the majority of cases (81%, 22/27), antimicrobial sensitivity tests (AST) were not performed to inform antibiotic choice. The main barrier to AST was cost. Most pharmacists (67%, 18/27) sold antibiotics without prescriptions. Amoxicillin (including amoxicillin-clavulanate) was the most-commonly prescribed (63%, 17/27) or dispensed (81%, 22/27) antibiotic. AST was rated the least important solution to AMR in Yemen. While there was awareness of a high AMR rate, stewardship is poor in Yemen. We note that barriers to the use of AST could be addressed through the deployment of reliable, affordable, quality rapid diagnostics, and AST kits. Compulsory continuing education emphasizing the use of AST to guide prescribing and patients’ awareness programs could help avoid irrational use.
INTRODUCTION
Antimicrobial resistance (AMR)—the ability of disease-causing microorganisms such as bacteria to withstand therapeutic doses of an antimicrobial agent, leading to treatment failure/death—is a multidimensional biosocial problem affecting countries worldwide and requires urgent measures aimed at containment.1,2 Low- and middle-income countries (LMICs) are particularly projected to bear the greater impact of AMR.1 This may be even more so in humanitarian and armed conflict contexts.3–5
While a natural adaptive strategy, AMR has been accelerated by anthropogenic factors promoting inappropriate use, including nonevidence-based prescribing, or empirical prescribing, self-medication, nonadherence to prescribed dosages, use of substandard and falsified (SF) medicines, and nontherapeutic uses in livestock.6–10 Social determinants, including poor infection prevention and control practices, poor water, sanitation, and hygiene (WASH) infrastructure, and lack of access to quality health services further exacerbate AMR, resulting in situations whereby antibiotics are used as substitutes for disease-preventive activities in some settings.9,11,12 Overall, more than 50% of all antibiotic prescriptions are inappropriate, calling for better antimicrobial stewardship (AMS) to preserve efficacy.13
Specifically, in active conflicts, increased injuries associated with war wounds predisposing to AMR infections contribute additional contextual challenges.4,14–18 Some studies report or suggest a high carriage of AMR and transmission among refugees and the forcibly displaced fleeing conflicts in five World Health Organization (WHO) Eastern Mediterranean Region countries.16,19 Thus, conflicts aggregate multiple social determinants potentially promoting inappropriate antibiotic use and AMR.
Yemen, following upon upheavals arising from the Arab Spring in 2011, slipped into conflict in March 2015 and is now described as the world’s largest humanitarian crisis.20 In a population of 29.1 million in 2019, 24 million are in need of aid with about 3.3 million internally displaced persons (IDPs). Access to healthcare, as well as to other services essential to life, remains limited. Since 2016, Yemen has been struggling with a cholera outbreak, leading to over 1.2 million cases as of January 2020, mostly in children under five.21 Malnutrition in young children predisposing to infections is high.22,23 Several studies already suggest a high AMR burden.24–30 In 2018, for example, among patients admitted to the Médecins Sans Frontiers (MSF) hospital in Aden, more than 60% had a drug-resistant infection.31 These conditions coincide with the current COVID-19 pandemic to further exacerbate the problem.32
Understanding and accounting for contextual factors impacting AMR, including individual-, social-, and national-level dimensions, is necessary for the design and implementation of successful containment programs. Globally, the WHO’s Global Action Plan on AMR Containment, launched in 2015, articulates a strategic framework of actions. The follow-up 2017 Access, Watch, Reserve (AWaRe) classification of antibiotics aims to promote rational use at the community level. Both lay emphasis on antimicrobial sensitivity tests (AST) to guide antimicrobial prescribing and prescription-only-sale of antibiotics as two complementary AMS approaches. In general, surveillance data on AMR prevalence and antimicrobial consumption inform country-level measures to contain AMR.
There are surveillance gaps for Yemen that lead to a poor understanding of AMR drivers and containment efforts. There are no records on national AMR prevalence on several databases including the WHO’s GLASS, the PFIZER database, and CDDEP resistance bank.33 There is also no national action plan on AMR. Conflict and a weak National Health Information System make it difficult to access reliable, up-to-date data.
The aim of this exploratory mixed-method study was to assess current awareness and perceptions of AMR and AMS status in Yemen. The objectives were to: (1) assess perceptions on AMR prevalence, (2) assess antibiotic prescribing and dispensing practices, and (3) evaluate AMS activities, with a view to both understanding current challenges with AMR and providing evidence for policy interventions.
METHODS
Study design: The study used a mixed-method study design. The mixed-method approach was judged appropriate to provide robust results, recognizing both the challenge of conducting research during conflicts in the middle of a pandemic and the need for accurate data. In designing, implementing, and reporting this study, we adopted the mixed-method guidelines of Leech and Onwuegbuzie.34 It used a combination of a literature review, questionnaire survey, and a focused key stakeholder interview.
Literature review.
To better understand the context and to evaluate our findings, we performed a targeted nonsystematic literature review of grey, peer-reviewed, and unpublished documents, reports, and articles obtained from various sources. Documents selected a priori for evaluation included the National Essential Medicines List (EML). Peer-reviewed literature retrieved from a search on PubMed and Web of Science and reports identified from researchers’ experience in Yemen from 1999 were selected and evaluated, with a focus on the time period from 2015 to 2020. Articles related to AMR prevalence, prescribing and dispensing practices, antibiotic use, and use of AST in Yemen, as well as other relevant policies including prescribing guidelines were selected, and data were subsequently extracted. Thus, the literature review was iterative—performed before the survey to guide questionnaire development and after the survey to triangulate survey findings as well as support the key stakeholder’s interview to summarize and synthesize the current evidence.34
Survey.
Study participants: Study participants were physicians in private and public facilities that prescribe medicines and pharmacists in private pharmacies. Participants were purposively selected via two separate existing professional WhatsApp groups in Yemen.
Sample size: We targeted a sample size of 40–60, comprising at least 30 physicians and 10 pharmacists, considered appropriate for a pilot or exploratory study.35,36
Study location: Facilities across Yemen, encompassing the “regional” north (Alamana, Sana’a, Dhamar, Marib) and south (mainly Aden) to account for the current administrative structures, comprising all five tiers of health units, health centers, and hospitals (district, regional, and central specialized). This dispersion allowed for a maximum variation sampling scheme to collect a wide range of perspectives.
Study instrument: Two different semi-structured questionnaires were used to collect data with tailored questions for healthcare workers/physicians and pharmacists (Supplemental Appendix 1). Questions were based on a rational construct to scope perceptions, practices, and AMS activities in the relevant facility type. The construct included selected aspects of AMS with a focus on rational prescribing—the use of guides, antibiotic choice, prescribing (or consumption) patterns, the presence of Drug and Therapeutic Committees (DTC), and AST derived from WHO guidelines.37,38 The questionnaires contained four sections: (i) demographic information on both the clients of the facilities and participants; (ii) perceptions of the AMR burden and approaches aimed as solution; (iii) AMS; and (iv) practices related to antibiotic prescribing and dispensing, including the major antibiotics prescribed or dispensed. Questionnaires were made available in English and Arabic versions. Translations were provided by a proficient bilingual speaker (SB) and checked by a researcher (NA) who is also a proficient bilingual professional for accuracy, cultural suitability, and content validity.
Survey administration and data collection: The questionnaires were distributed online via links on WhatsApp for self-completion in August–September 2020.
Key informant interview.
The interview was conducted with a public official in Yemen Republic to triangulate information from the survey and literature review, both to understand public policies and to provide contextual information. Questions were formulated based on the survey results. The pertinent questions asked the key informant were: What factors would improve the uptake of AST, the average cost of one AST, and policies around prescription guides and AMR. To improve data quality, we purposively identified and interviewed a high-ranking official. Using this politically important case sampling strategy, we hoped to obtain accurate information on policies. The interview was conducted over the telephone using specific questions prepared in advance from survey findings.
Analysis: The results from the survey were analyzed by both descriptive and inferential statistics. Descriptive statistics are presented as percentages. To test if there was a significant difference in selected binary responses between the two groups of participants, we used the χ2 statistic, with significance at P < 0.05. Survey responses with incompletely or incorrectly filled variables were screened out before data analysis. Interview findings are reported qualitatively as received.
RESULTS
The survey response rate was 41% (57/140). There was a higher response rate among pharmacists (68%, 27/40) than among physicians (30%, 30/100). Following screening, three questionnaires were excluded from the physicians’ responses. Total valid responses were, therefore, 54, comprising an equal number (27) of physicians and pharmacists.
Supplemental Table 1 presents the results for the survey. (Detailed demographic information is attached in Supplemental Appendix 2.)
AMR: prevalence and solutions.
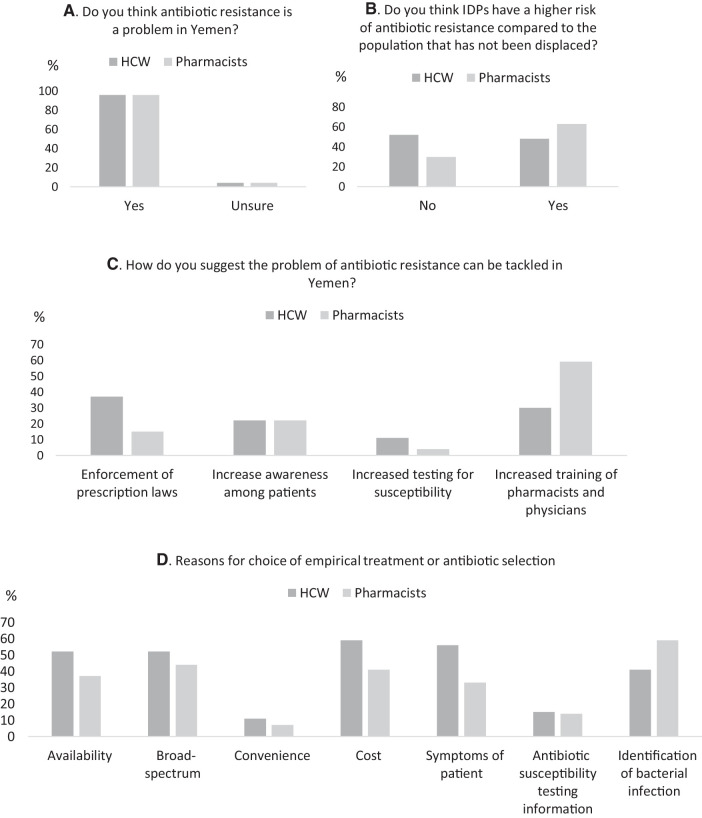
Participants were aware of a high AMR prevalence rate, if understudied/underreported. Almost all (96%, 52/54) reported AMR to be a problem in Yemen. The majority (56%, 15/27 of physicians and 70%, 19/27 of pharmacists) perceived AMR to be an understudied or underreported problem; with no significant differences between physicians and pharmacists, P = 0.73.
More pharmacists perceived a higher prevalence of AMR in IDPs relative to the general population (63%, 17/27) compared with physicians (48%, 13/27). However, there was no statistically significant difference either within or between participant groups (P = 0.150).
Physicians and pharmacists differed in their ranked perception of solutions to the high burden of AMR in Yemen. Physicians were more (37%, 10/27) in support of the enforcement of prescription laws—sale of antimicrobials without prescription—and increased training (30%, 8/27) than in increasing awareness among clients (22%, 6/27). On the other hand, pharmacists were more in support of increased training (59%, 16/27) and increased awareness among clients (22%, 6/27), than in enforcement of prescription laws (15%, 4/27).
Interestingly, both ranked the use of AST the lowest—only 11% (3/27) of physicians and 4% (1/27) of pharmacists reported increased testing for susceptibility as an approach to tackling AMR.
Antibiotic utilization—prescribing/dispensing practices and major antibiotics.
Most physicians (74%, 20/27) reported that they felt under pressure to prescribe broad-spectrum versus narrow-spectrum antibiotics.
There were multiple reasons for empirical treatment with antibiotics with the major ones being cost (59%, 16/27) and symptoms of patient (56%, 15/27) and for antibiotic choice, with availability, and broad-spectrum nature (52%, 14/27), respectively, being the main reasons. Other reasons for empirical treatment and antibiotic choice were identification (from signs and symptoms) of bacterial infection (41%, 11/27) and convenience (11%, 3/27).
The practice of selling antibiotic without a prescription was reported by most pharmacists (67%, 18/27) as with counter-prescribing (63%, 17/27). The conditions for which antibiotics were demanded or counter-prescribed were: inflammation affecting the throat/mouth or ear (37%, 10/27), followed by infections—urinary tract infections (UTIs) and respiratory tract infections (RTIs) (33%, 9/27, each) and fever with other symptoms (11%, 3/27).
The most important considerations for dispensing of antibiotics by pharmacists were disease (59%, 16/27); price patient can afford (22%, 6/27) and cost (15%, 4/27).
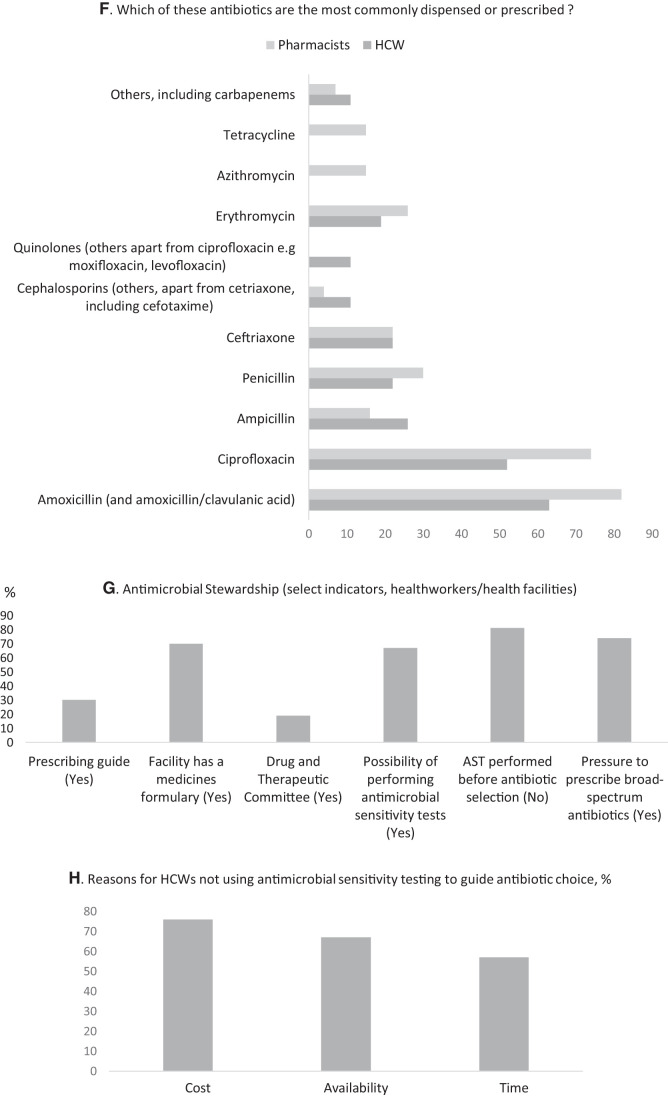
Penicillins were the most commonly prescribed and dispensed antibiotic group, followed by quinolones, macrolides, and tetracyclines. Of the penicillins, amoxicillin (and amoxicillin/clavulanic acid) was the most commonly prescribed (63%, 17/27) by physicians or dispensed (81%, 22/27) by pharmacists. Ciprofloxacin was the most common quinolone antibiotic prescribed (52%, 14/27) or dispensed (74%, 20/27). There was more community dispensing of macrolides, with pharmacies dispensing more of erythromycin (26%, 7/27) than the healthcare facilities (19%, 5/27), and with azithromycin dispensed only by pharmacists (15%, 4/27). Similarly, the dispensing of tetracyclines was reported only in pharmacies (15%, 4/27).
By comparison, cephalosporins were not as commonly prescribed or dispensed. Physicians reported 22% (6/27) of prescribed ceftriaxone—similar to pharmacists—and 11% (3/27) for other cephalosporins compared with 4% (1/27) of pharmacists.
Antimicrobial stewardship.
In terms of capacity to perform AST, two-thirds, 67% (18/27), reported the possibility of performing AST at their facilities: most (56%, 10/18) could do so on site. In the majority of cases (81%, 22/27), however, AST was not performed to inform choice of antibiotics.
The major barriers to the routine use of AST were cost, availability, and waiting time for AST results. The average cost of one AST in Yemen is estimated at between 3,500 and 5,000 Yemeni Rial (Key informant), equivalent to USD14–20.39 Even allowing for a lower 6-month average exchange rate of 600 Rial to USD1, the cost is USD6–9. At a daily wage of USD3.39 in 2017, amid delayed salary payments and inflation, the cost of one AST is prohibitive for three-thirds of the population who live below the poverty line (USD1.9) in 2020.40–42 This is in addition to poor access to laboratory facilities and equipment (Key informant, 2020).
Prescribing guides were available only in 30% (8/27) of health workers’ practices, though most participants, 70% (19/27), reported that their facility had a Medicines Formulary. Less than one-fifth, 19% (5/27), reported that their facilities had a DTC. There is no antibiotic policy for Yemen (Key informant, 2020).
The key findings from the survey are summarized in Figure 1.
Figure 1.
Summary of select results from a survey assessing antimicrobial resistance, use, and stewardship in healthcare workers (HCW) and pharmacists in Yemen. (A, B, and C) Antimicrobial resistance (AMR): The majority of respondents (96%) thought AMR to be a problem in Yemen (A) but were more split in terms of if IDPs had the greater risk (B). Among solution options to tackling AMR, both respondents ranked the use of increased antimicrobial sensitivity testing the lowest and ranked increased training of pharmacists and physicians (HCW) the highest. (D, E, and F) Antimicrobial use: There were several overlapping reasons for choice of empirical treatment or empirical antibiotic selection both in prescribing physicians and dispensing pharmacists with cost (price patient can afford), signs and symptoms (including “identification of bacterial infection”) of patient and availability with a preference for broad-spectrum antibiotics. In terms of antibacterial agents, amoxicillin (including amoxicillin/clavulanic acid) was the most commonly prescribed or dispensed followed by ciprofloxacin. (G and H) Antimicrobial stewardship: Among select criteria, physicians reported to be under pressure to prescribe broad-spectrum antibiotics, and even where there was the possibility to perform antimicrobial sensitivity tests, these were not used to guide prescribing in the majority of cases. The reason for this was largely cost (H).
DISCUSSION
AMR is correlated with an increase in the consumption of antimicrobials.43–46 The containment of AMR requires a complete understanding of all factors promoting misuse. This mixed-methods study provides current knowledge of individual-level (awareness and practices) as well as contextual factors related to AMR and stewardship in Yemen—a low-income country in conflict since 2015. There are three salient findings:
-
•
Awareness of a high AMR prevalence rate
-
•
Inappropriate use of antibiotics
-
•
Overall sub-optimal AMS activities, including low prioritization and cost of AST.
AMR prevalence: perception and solutions.
The high level of AMR in Yemen perceived by participants indicates awareness among physicians and pharmacists, considered frontline health workers, in this survey and is corroborated by the earlier mentioned studies reporting laboratory investigations.24–30
In Yemen, IDPs may not necessarily be distinguished from the nondisplaced. Since 2020, displacement has been more marked among 4 out of a total of 22 governates (Marib, Al Hudaydah, Al Dhale'e, Taizz, and Al Jawf)—mostly in the north—with the greater proportion moving in with relatives or other nonfamily hosts, rather than into any settlement camps.47 IDPs, as they experience the same conditions as their hosts, would, thus, be hypothesized, or expected, to have the same risks, or prevalence, of AMR as the general population, as the results also seem to suggest.
The difference in the ranking by physicians and pharmacists of solutions to the perceived high burden of AMR in Yemen may reflect differential clinical training and practice environments as well as highlight training needs or gaps.48,49 In this, our findings are in line with a previous 2016 study among healthcare workers (physicians, pharmacists, and nurses) assessing awareness of AMR, possible contributory factors, as well as solutions.49
Interestingly, despite the level of awareness, both professionals surveyed in our study ranked the use of AST as the least important solution to the problem of AMR in Yemen.
Antimicrobial utilization.
Prescription and dispensing practices.
Antibiotic prescribing is a complex activity heavily influenced by contextual individual, social, and policy factors, creating ethical dilemmas.50 Physicians may feel obligated to prescribe antibiotics both in response to direct patients’ demand and (on the need) to induce rapid relief of symptoms. Both of these, as shown in a systematic review by Rodrigues et al (2013) on physician prescribing behavior, can create a state of tension or fear—one of several factors leading to irrational prescribing.51 While the extent to which pressure to prescribe broad-spectrum antibiotics for symptom relief may be related to actual need, for instance infected war-wounds, was not investigated, patients’ demand for antibiotics when not clinically indicated is well documented in the literature.
Failure to prescribe antibiotics when demanded could lead the patient to other physicians willing to do so, or to pharmacists. Hence, this culture is perpetuated. This is common in many geographical regions. As one approach to reduce patient demand, the United Kingdom mounted a patient awareness campaign to create awareness among the lay population that not all colds require an antibiotic, as well as institute stricter prescribing guidelines such that general practitioners would not prescribe an antibiotic for acute upper RTIs, including cough, common cold, acute pharyngitis, tonsillitis, and otitis media, except for the systematically very unwell or with high risk of complications.52,53 Generally, educational interventions aimed at creating awareness among clinicians and the public alike have been shown to reduce irrational prescribing, especially for RTIs.54
In resource-limited settings, affordability—the price a patient can pay for their medicines—also affects prescribing pattern. This is the case in this study, where physicians reported cost as a major factor both in the choice of antibiotics and in empirical treatment, creating another layer of complexity.
There was pharmacy sale and counter-prescribing of antimicrobials, in common with many others countries especially in the global south, and as previously documented for Yemen.55,56 In these contexts, pharmacies are usually the first port-of-call for clients with minor ailments, and, thus, serve an important public health function. In Yemen with only 50% of fully functional health facilities and with 5 physicians to 10,000 population, this is particularly so.57,58 These reduce patient access to physicians, along with other geographical, economic, and social factors. Our results conform with a 2014 study by Belkina et al in which 78.2% (N = 400) teachers indicated having obtained a nonprescribed antibiotic.8 The WHO GAP, as one step to containing AMR, recommends the prescription-only sale of antimicrobials in pharmacies and medicine outlets. However, in the global north, the role of the pharmacist is evolving to include the prescription of medicines, with some evidence of their impact in decreasing irrational prescribing in hospitals.59 Enhancing their capacity by equipping private pharmacies in LMICs with rapid diagnostic devices, including the use of mobile-based decision-making algorithms, could leverage this professional capacity in the containment of AMR.60 Using these tools, pharmacists can, for example, screen bacterial from viral infections, and advise clients with viral infections including the common cold more appropriately on the choice of medicines, and whether antibiotics are required. These tools should also be available at healthcare facilities.25
Consumption pattern and AMR.
Amoxicillin (including amoxicillin/clavulanic acid), a penicillin, was the most commonly prescribed or dispensed antibiotic among the participants. This corresponds with results from several studies assessing antimicrobial utilization in the community in Yemen.61–63 In addition, it mirrors the high usage of this antibiotic in the greater Middle Eastern region.1 In Yemen, ciprofloxacin is the first-choice antibiotic treatment of several infections including UTIs and gastrointestinal infections including typhoid.24 Both of these antibiotics were for oral use, with amoxicillin a preferred choice in susceptible RTIs, especially in children, as contained in the national EML and guidelines for child health programs in Yemen, which is widely supported by international organizations.
In a recent study of AMR in Aden, Yemen, by Badulla et al (2020), the resistance to amoxicillin/clavulanic acid among seven bacterial species isolated from various clinical specimens—urine, pus, and wound—was an average of 65.2%.24 This study raises questions about the continued efficacy of amoxicillin and amoxicillin/clavulanic acid in Yemen.
Resistance against ceftriaxone, the most commonly prescribed/dispensed cephalosporin in this survey, was also fairly high at about 62% of microorganisms in their study. In contrast, resistance to ciprofloxacin was relatively low at about 26%.
This study by Badulla et al., while limited to Aden and, therefore, potentially not generalizable, might account in part for the treatment failure incidences reported by participants in this survey. However, a number of other studies conducted in other parts of Yemen report high resistance against antibiotics noted as commonly dispensed by the survey conducted.25,26,28–30 For instance, a study from Sana’a found that clinical isolates of Escherichia coli collected in 2017 were 96% resistant to amoxicillin-clavulanic acid29 and another study from Al-Mukalla found UTI E. coli isolates from 2003 to 2006 were 78.8% resistant to penicillin and 73.1% resistant to cephalosporins.26
Other possible reasons for treatment failure may include the use of SF medicines, noncompliance with recommended dosages, and the empirical use of broad-spectrum antibiotics. The prevalence of poor-quality medicines is thought to be high64 and has been estimated by various sources to be between 10% and 60% in Yemen.65 As part of its pharmacovigilance, Yemen collects information on suspected therapeutic failure due to poor-quality medicines. Current pharmacovigilance reports on poor-quality antimicrobials put this at < 0.012% (Key informant, 2020).
The potential to induce AMR differs according to antibiotic, with the broad-spectrum macrolides and fluoroquinolones being among the most potent inducers (fluoroquinolones can induce AMR even with a single use). The AWaRe classification aims to reduce the use of potent inducers—Watch/Reserve antibiotics—or last choice antibiotics—Reserve—to only serious cases of infection resistant to the Access group of antibiotics. One target of this classification is to increase the use of Access antibiotics, such as penicillins, to 60% by 2050, as one approach to tackling AMR. In this regard, the results of this survey, in almost equal proportions, are both encouraging and concerning.
Antimicrobial stewardship.
Overall, the use of AST to identify resistance and to better guide the prescription process was suboptimal. While there was capacity to perform AST in the majority of the facilities where the surveyed physicians practice, routine use to guide the choice of antimicrobial agent was limited and, in some cases, nonexistent. Physicians rather prescribed antibiotics based on clinical and financial considerations, with preference for a broad-spectrum antibiotic. This preference for initial (empirical) treatment with a broad-spectrum antibiotic is possibly informed by the perception of an assumed (pre)existing antibiotic resistance to first-line antibiotics in patients. The irrational prescribing of antibiotics potentially leading to AMR has been described for public hospitals in Yemen, including up to several antibiotics at one time, as also found from this study.66
In recognition of the high levels of AMR among certain impacted populations in its health facilities in conflict-affected countries in this region, the MSF routinely use AST including rapid diagnostics and has developed a propriety field device to facilitate AST.5,67 However, the challenges in both the development and deployment of these devices, and of AST in certain contexts, must be acknowledged.68
Facilitators to increase the uptake of AST might include increased access to quality-assured AST kits or equipment and consumables including media for both aerobic and anaerobic bacteria. and capacity increase and training for laboratory personnel, as well as logistical support. Development partners could play a role here in increasing access to AST including rapid diagnostics, through subsidies.
Other structures to ensure AMS were also suboptimal. Many facilities lacked prescribing guides. The majority had no DTC—an institutional mechanism for ensuring the rational use of medicines, including antibiotics, in a healthcare facility—though most had a Medicines Formulary. These results are in line with a previous survey on AMS in health facilities in Yemen.69 Educational interventions to increase the use of AST among professionals, including of AMS in community pharmacies, may be needed to address the observed AMS gaps.70 Actively promoting AST use in pharmacies as collaborative efforts aimed at AMR containment may also be needed.71,72
Limitation: The small sample size of this study limits its generalizability across the country, as earlier indicated. However, this study advances our understanding of some current challenges in the country with respect to AMR. There is need for more robust studies.
CONCLUSION
Active conflicts aggregate multiple social determinants promoting irrational use and AMR. Challenges to AMS in these settings are multifactorial, demanding complete understanding for containment. This study found that while there is awareness of a high AMR prevalence in Yemen among health workers surveyed, there were suboptimal antimicrobial use practices and AMS. The main barrier to AMS was cost.
There is the need for educational/awareness interventions targeting both providers and consumers of antibiotics to reduce irrational use. Policy changes to the use of amoxicillin as a preferred antibiotic may be required. There is also a strong need to build AMS structures as a priority. These activities could be built using the WHO antimicrobial stewardship toolkit as a guide.73 Barriers to the use of AST could be addressed through the deployment of quality-assured, reliable, low-cost, or affordable AST devices including rapid diagnostics. In all aspects, strong international support would be required.
Supplemental Material
ACKNOWLEDGMENTS
This work was made possible by support from the Boston University Social Innovation on Drug Resistance (SIDR) Postdoctoral Program to ESFO.
Note: Supplemental table and appendices appear at www.ajtmh.org
REFERENCES
- 1. World Bank , 2017. Drug-Resistant Infections: A Threat to Our Economic Future. Washington, DC: The World Bank. [Google Scholar]
- 2. Kirchhelle C. et al. , 2020. Setting the standard: multidisciplinary hallmarks for structural, equitable and tracked antibiotic policy. BMJ Glob Health 5: e003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haraoui DL-P, 2019. Armed conflicts and antimicrobial resistance: A deadly convergence. AMR Control 2019: 63–73. [Google Scholar]
- 4. Abbara A. et al. , 2018. A summary and appraisal of existing evidence of antimicrobial resistance in the Syrian conflict. Int J Infect Dis 75: 26–33. [DOI] [PubMed] [Google Scholar]
- 5. Kanapathipillai R. et al. , 2019. Antibiotic resistance in conflict settings: lessons learned in the Middle East. JAC-Antimicrob Res 1: dlz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collignon P, McEwen S, 2019. One health—its importance in helping to better control antimicrobial resistance. TropicalMed 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, Goossens H, Laxminarayan R, 2018. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA 115: E3463–E3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belkina T, Al Warafi A, Hussein Eltom E, Tadjieva N, Kubena A, Vlcek J, 2014. Antibiotic use and knowledge in the community of Yemen, Saudi Arabia, and Uzbekistan. J Infect Dev Ctries 8: 424–429. [DOI] [PubMed] [Google Scholar]
- 9. Nwokike J, Clark A, Nguyen PP, 2018. Medicines quality assurance to fight antimicrobial resistance. Bull World Health Organ 96: 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinstein ZB, Zaman MH, 2019. Evolution of rifampin resistance in Escherichia coli and Mycobacterium smegmatis due to substandard drugs. Antimicrob Agents Chemother 63: e01243–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramay BM. et al. , 2020. Antibiotic use and hygiene interact to influence the distribution of antimicrobial-resistant bacteria in low-income communities in Guatemala. Sci Rep 10: 13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO , 2020. Antimicrobial Resistance. Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed October 21, 2020.
- 13. WHO , 2019. AWaRe Policy Brief. Available at: https://adoptaware.org/assets/pdf/aware_policy_brief.pdf. Accessed October 23, 2020.
- 14. Dau AA, Tloba S, Daw MA, 2013. Characterization of wound infections among patients injured during the 2011 Libyan conflict. East Mediterr Health J 19: 356–361. [PubMed] [Google Scholar]
- 15. Bazzi W. et al. , 2020. Heavy metal toxicity in armed conflicts potentiates AMR in A. baumannii by selecting for antibiotic and heavy metal co-resistance mechanisms. Front Microbiol 11: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kassem DF, Hoffmann Y, Shahar N, Ocampo S, Salomon L, Zonis Z, Glikman D, 2017. Multidrug-resistant pathogens in hospitalized Syrian children. Emerg Infect Dis 23: 166–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen K, Riddle MS, Danko JR, Blazes DL, Hayden R, Tasker SA, Dunne JR, 2007. Trauma-related infections in battlefield casualties from Iraq. Ann Surg 245: 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Älgå A, Wong S, Shoaib M, Lundgren K, Giske CG, von Schreeb J, Malmstedt J, 2018. Infection with high proportion of multidrug-resistant bacteria in conflict-related injuries is associated with poor outcomes and excess resource consumption: a cohort study of Syrian patients treated in Jordan. BMC Infect Dis 18: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maltezou HC, Theodoridou M, Daikos GL, 2017. Antimicrobial resistance and the current refugee crisis. J Glob Antimicrob Resist 10: 75–79. [DOI] [PubMed] [Google Scholar]
- 20. United Nations , 2019. Humanitarian Crisis in Yemen Remains the Worst in the World, Warns UN. Available at: https://news.un.org/en/story/2019/02/1032811. Accessed February 14, 2019.
- 21. WHO EMRO , 2020. Outbreak Update – Cholera in Yemen, 12 January 2020, Cholera | Epidemic and Pandemic Diseases. Available at: http://www.emro.who.int/pandemic-epidemic-diseases/cholera/outbreak-update-cholera-in-yemen-12-january-2020.html. Accessed October 21, 2020.
- 22. United Nations , 2020. Yemeni Children Suffer Record Rates of Acute Malnutrition, Putting ‘Entire Generation’ at Risk. Available at: https://news.un.org/en/story/2020/10/1076272. Accessed October 27, 2020.
- 23. Ibrahim MK, Zambruni M, Melby CL, Melby PC, 2017. Impact of childhood malnutrition on host defense and infection. Clin Microbiol Rev 30: 919–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Badulla WFS, Alshakka M, Mohamed Ibrahim MI, 2020. Antimicrobial resistance profiles for different isolates in Aden, Yemen: a cross-sectional study in a resource-poor setting. BioMed Res Int 2020: 1810290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. AL-Magrami RTF, Al-Shamahy HA , 2019. Pseudomonas aeruginosa skin-nasopharyngeal colonization in the in-patients: Prevalence, risk factors and antibiotic resistance in tertiary hospitals in Sana’a City - Yemen . Univ J Pharm Res. doi: 10.22270/ujpr.v3i6.219. [DOI] [Google Scholar]
- 26. Al-Haddad AM, Ghouth A, El-Hosseiny M, 2010. Microbial resistance in patients with urinary tract infections in Al-Mukalla, Yemen. Sudan J Med Sci 5: 145–149. [Google Scholar]
- 27. Al-Haroni MH, Skaug N, Al-Hebshi NN, 2006. Prevalence of subgingival bacteria resistant to aminopenicillins and metronidazole in dental patients from Yemen and Norway. Int J Antimicrob Agents 27: 217–223. [DOI] [PubMed] [Google Scholar]
- 28. Al-Moyed KA, Harmal NS, Al-Harasy AH, Al-Shamahy HA, 2006. Increasing single and multi-antibiotic resistance in Shigella species isolated from shigellosis patients in Sana’a, Yemen. Saudi Med J 27: 1157–1160. [PubMed] [Google Scholar]
- 29. Al-Hammadi MA, Al-Shamahy HA, Ali AQ, Abdulghani MAM, Pyar H, AL-Suboal I, 2020. Class 1 integrons in clinical multi drug resistance E. coli, Sana’a Hospitals, Yemen. Pak J Biol Sci 23: 231–239. [DOI] [PubMed] [Google Scholar]
- 30. Banajeh SM, 2001. Bacterial aetiology and anti-microbial resistance of childhood diarrhoea in Yemen. J Trop Pediatr 47: 301–302. [DOI] [PubMed] [Google Scholar]
- 31. Anon , 2020. Yemen: Going Behind the Front Lines of a Hidden War. Doctors Without Borders – USA. Available at: https://www.doctorswithoutborders.org/what-we-do/news-stories/story/yemen-going-behind-front-lines-hidden-war. Accessed October 20, 2020.
- 32. Nasser A, 2020. COVID-19 in Yemen – A Perfect Storm. Human Rights Watch. Available at: https://www.hrw.org/news/2020/04/14/covid-19-yemen-perfect-storm. Accessed April 14, 2020.
- 33. WHO , 2020. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 34. Leech NL, Onwuegbuzie AJ, 2010. Guidelines for conducting and reporting mixed research in the field of counseling and beyond. J Couns Dev 88: 61–69. [Google Scholar]
- 35. Hertzog MA, 2008. Considerations in determining sample size for pilot studies. Res Nurs Health 31: 180–191. [DOI] [PubMed] [Google Scholar]
- 36. Viechtbauer W, Smits L, Kotz D, Budé L, Spigt M, Serroyen J, Crutzen R, 2015. A simple formula for the calculation of sample size in pilot studies. J Clin Epidemiol 68: 1375–1379. [DOI] [PubMed] [Google Scholar]
- 37. WHO , 2015. Global Action Plan on Antimicrobial Resistance. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 38. World Health Organization , 2019. The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 39. XE , 2020. 3500 YER to USD, Convert Yemeni Rials to US Dollars. Available at: https://www.xe.com/currencyconverter/convert/?Amount=3%2C500&From=YER&To=USD. Accessed October 22, 2020.
- 40. World Bank , 2020. Yemen Overview. Available at: https://www.worldbank.org/en/country/yemen/overview. Accessed October 1, 2020.
- 41. World Bank , 2020. Yemen Monthly Economic Update April 2020. Available at: https://www.worldbank.org/en/country/yemen/publication/yemen-monthly-economic-update-april-2020. Accessed October 20, 2020.
- 42. Save the Children , 2018. YEMEN: Cost of Food Nearly Doubles, Putting Thousands of Lives at Risk. Available at: https://www.savethechildren.org/us/about-us/media-and-news/2018-press-releases/yeme-cost-of-food-nearly-doubles. Accessed October 20, 2020).
- 43. Davies J, Davies D, 2010. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74: 417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olesen SW, Barnett ML, MacFadden DR, Brownstein JS, Hernández-Díaz S, Lipsitch M, Grad YH, 2018. The distribution of antibiotic use and its association with antibiotic resistance. eLife 7: e39435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Boeckel TP, Pires J, Silvester R, Zhao C, Song J, Criscuolo NG, Gilbert M, Bonhoeffer S, Laxminarayan R, 2019. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 365: eaaw1944. [DOI] [PubMed] [Google Scholar]
- 46. Kimera ZI, Mshana SE, Rweyemamu MM, Mboera LEG, Matee MIN, 2020. Antimicrobial use and resistance in food-producing animals and the environment: an African perspective. Antimicrob Resist Infect Control 9: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. International Displacement Monitoring Centre , 2020. Yemen. Available at: https://www.internal-displacement.org/countries/yemen. Accessed October 20, 2020.
- 48. Ali Al-Worafi YM, Fathelrahman AI, Ibrahim MIM, Wertheimer AI. Pharmacy Practice in Developing Countries. Boston, MA: Academic Press, 267–287. [Google Scholar]
- 49. Halboup A, Alzoubi K, Othman G, 2018. Awareness of bacterial resistance to antibiotics among healthcare providers in Sana’a City, Yemen. YJMS 12: 12–21. [Google Scholar]
- 50. Basu S, Garg S, 2018. Antibiotic prescribing behavior among physicians: ethical challenges in resource-poor settings. J Med Ethics Hist Med 11: 5. [PMC free article] [PubMed] [Google Scholar]
- 51. Teixeira Rodrigues A, Roque F, Falcão A, Figueiras A, Herdeiro MT, 2013. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents 41: 203–212. [DOI] [PubMed] [Google Scholar]
- 52. Centre for Clinical Practice at NICE (United Kingdom) , 2008. Respiratory Tract Infections - Antibiotic Prescribing: Prescribing of Antibiotics for Self-Limiting Respiratory Tract Infections in Adults and Children in Primary Care. London, United Kingdom: National Institute for Health and Clinical Excellence. [PubMed] [Google Scholar]
- 53. NICE , 2020. Summary of Antimicrobial Prescribing Guidance – Managing Common Infections. Public Health England. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/906668/Common_Infect_PHE_context_references_and_rationale_August_2020.pdf. Accessed October 20, 2020.
- 54. Cross ELA, Tolfree R, Kipping R, 2017. Systematic review of public-targeted communication interventions to improve antibiotic use. J Antimicrob Chemother 72: 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Halboup A, Abdi A, Ahmed M, Al-Qadasi F, Othman GQ, 2020. Access to antibiotics without prescription in community pharmacies in Yemen during the political conflict. Public Health 183: 30–35. [DOI] [PubMed] [Google Scholar]
- 56. Negarandeh R, Shayan SJ, Nazari R, Kiwanuka F, Rad SA, 2020. Self-Medication with Antibiotics in WHO Eastern Mediterranean Region: A Systematic Review and Meta-Analysis [Review]. doi: 10.21203/rs.3.rs-39213/v1. [DOI]
- 57. WHO , 2019. Yemen: Health Resources and Services Availability Mapping System 2018 (HeRAMS). Available at: https://www.who.int/initiatives/herams. Accessed December 12, 2020.
- 58. WHO , 2020. Global Health Observatory, By Category, Medical Doctors: Yemen. Available at: https://apps.who.int/gho/data/node.main.HWFGRP_0020?lang=en. Accessed 2020.
- 59. Sakeena MHF, Bennett AA, McLachlan AJ, 2018. Enhancing pharmacists’ role in developing countries to overcome the challenge of antimicrobial resistance: a narrative review. Antimicrob Resist Infect Control 7: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gubbins PO, Klepser ME, Adams AJ, Jacobs DM, Percival KM, Tallman GB, 2017. Potential for pharmacy-public health collaborations using pharmacy-based point-of-care testing services for infectious diseases. J Public Health Manag Pract 23: 593–600. [DOI] [PubMed] [Google Scholar]
- 61. Alshakka M, Said K, Babakri M, Ansari M, Aldhubhani A, Azmi Hassali M, Mohamed Ibrahim MI, 2016. A study on antibiotics prescribing pattern at outpatient department in four hospitals in Aden-Yemen. JPPCM 2: 88–93. [Google Scholar]
- 62. Alfadly S, Ballaswad MR, Amra AS, Alghadeer SM, Wajid S, Al-Arifi MN, Babelghaith SD, 2017. Self-medication with antibiotic amongst adults attending community pharmacies in Mukalla district, Yemen. Lat Am J Pharm 36: 224–228. [Google Scholar]
- 63. Mohanna M, 2010. Self-medication with antibiotic in children in Sana’a City, Yemen. Oman Med J 25: 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diack A, Seiter A, Hawkins L, Dweik IS, 2010. Assessment of Governance and Corruption in the Pharmaceutical Sector. Washington, DC: The International Bank for Reconstruction and Development/The World Bank.
- 65. Alshakka M, Mohamed Ibrahim MI, Bahattab A, Badulla WFS, Shankar PR, 2020. An insight into the pharmaceutical sector in Yemen during conflict: challenges and recommendations. Med Confl Surviv 36: 232–248. [DOI] [PubMed] [Google Scholar]
- 66. Mohamed Ibrahim MI, Alshami AK, Abdorabbo A, 2011. The quality of prescriptions with antibiotics in Yemen. J Clin Diagn Res 5: 808–812. [Google Scholar]
- 67. MSF , 2020. MSF Mini-Lab Project. Médecins Sans Frontières Access Campaign. Available at: https://msfaccess.org/msf-mini-lab-project. Accessed October 20, 2020.
- 68. Hays JP. et al. , 2019. The successful uptake and sustainability of rapid infectious disease and antimicrobial resistance point-of-care testing requires a complex “mix-and-match” implementation package. Eur J Clin Microbiol Infect Dis 38: 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alshakka M, Badulla WF, Alawi S, Al-abd N, Mahmoud MA, Alahmadi Y, Wajid S, Attef O, 2020. Status of antimicrobial stewardship in hospitals in Aden, Yemen. Lat Am J Pharm 39: 936–943. [Google Scholar]
- 70. Alshakka M, Badulla WF, Bahattab A, Al-Abd N, Mahmoud MA, Alahmadi Y, Wajid S, Attef O, 2019. Perception and practices of antimicrobial stewardship by community pharmacists in Aden-Yemen. Biomed Res 30: 819–825. [Google Scholar]
- 71. Dickerson LM, Mainous AG, Carek PJ, 2000. The pharmacist’s role in promoting optimal antimicrobial use. Pharmacotherapy 20: 711–723. [DOI] [PubMed] [Google Scholar]
- 72. Weber NC, Klepser ME, Akers JM, Klepser DG, Adams AJ, 2016. Use of CLIA-waived point-of-care tests for infectious diseases in community pharmacies in the United States. Expert Rev Mol Diagn 16: 253–264. [DOI] [PubMed] [Google Scholar]
- 73. WHO , 2019. Antimicrobial Stewardship Programmes in Health-care Facilities in Low- and Middle-income Countries: A WHO Practical Toolkit. Geneva, Switzerland: World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.