Abstract
Objective:
To analyze serial biomarkers of the persistent inflammation, immunosuppression, and catabolism syndrome (PICS) to gain insight into the pathobiology of chronic critical illness (CCI) after surgical sepsis.
Background:
Although early deaths after surgical intensive care unit sepsis have decreased and most survivors rapidly recover (RAP), one third develop the adverse clinical trajectory of CCI. However, the underlying pathobiology of its dismal long-term outcomes remains unclear.
Methods:
PICS biomarkers over 14 days from 124 CCI and 225 RAP sepsis survivors were analyzed to determine associations and prediction models for (1) CCI (≥ 14 intensive care unit days with organ dysfunction) and (2) dismal 1-year outcomes (Zubrod 4/5 performance scores). Clinical prediction models were created using PIRO variables (predisposition, insult, response, and organ dysfunction). Biomarkers were then added to determine if they strengthened predictions.
Results:
CCI (vs RAP) and Zubrod 4/5 (vs Zubrod 0–3) cohorts had greater elevations in biomarkers of inflammation (interleukin [IL]-6, IL-8, interferon gamma-induced protein [IP-10], monocyte chemoattractant protein 1), immunosuppression (IL-10, soluble programmed death ligand-1), stress metabolism (C-reactive protein, glucagon-like peptide 1), and angiogenesis (angiopoietin-2, vascular endothelial growth factor, vascular endothelial growth factor receptor-1, stromal cell-derived factor) at most time-points. Clinical models predicted CCI on day 4 (area under the receiver operating characteristics curve [AUC] = 0.89) and 1 year Zubrod 4/5 on day 7 (AUC = 0.80). IL-10 and IP-10 on day 4 minimally improved prediction of CCI (AUC = 0.90). However, IL-10, IL-6, IL-8, monocyte chemoattractant protein 1, IP- 10, angiopoietin-2, glucagon-like peptide 1, soluble programmed death ligand-1, and stromal cell-derived factor on day 7 considerably improved the prediction of Zubrod 4/5 status (AUC = 0.88).
Conclusions:
Persistent elevations of PICS biomarkers in the CCI and Zubrod 4/5 cohorts and their improved prediction of Zubrod 4/5 validate that PICS plays a role in CCI pathobiology.
Keywords: biomarkers, chronic critical illness, immunosuppression and catabolism syndrome, long-term outcomes, persistent inflammation, sepsis
Sepsis is defined by a dysregulated systemic immune response that causes life threatening organ dysfunctions.1,2 It is recognized to be a leading cause of death in hospitalized patients.3,4 However, many septic surgical intensive care unit (ICU) patients who previously died early of unremitting multiple organ failure (MOF) are now surviving as a result of the early implementation of the Surviving Sepsis Campaign (SSC) evidence-based guidelines (EBGs).3,5-7 However, a large number of these “sepsis survivors” develop a clinical trajectory of chronic critical illness (CCI) characterized by prolonged ICU stays and poor long-term outcomes.8-10
In a recently published prospective study of 301 surgical ICU patients treated for new onset of sepsis, we reported that only 4% died early (within 14 days), the majority (63%) experienced rapid recovery (RAP), whereas the remaining (33%) progressed into CCI.11 Despite having similar good baseline performance status, CCI survivors experienced severe functional disabilities at 3 months after sepsis (and did not recover), whereas RAP patients experienced mild disability at three months (and most recovered). Of note, 1-year mortality was much worse for CCI patients (41% vs 5%). Although baseline predisposition and the type of inciting infection certainly play a role in these poor long-term outcomes, we and others believe that a persistent dysregulated immune response drives the underlying pathobiology.12
In a 2012 review article, the term Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS) was coined to provide a mechanistic explanation for CCI and its dismal long-term outcomes (defined as death or full functional dependence at 1 year).13 We have collected serial blood biomarkers reflecting different aspects of PICS in high-risk patients to study its validity.13 In this manuscript, we report the results of these biomarker studies in CCI patients and those with dismal 1-year outcomes (Zubrod performance score of 4 or 5) as well as explore clinical prediction models using readily available clinical variables categorized by the PIRO (predisposition, insult, response, and organ dysfunction) classification system, with and without biomarkers included, to gain insight into causality.14 We hypothesize that CCI patients and those with 1-year Zubrod 4/5 scores will have persistent aberrations in PICS-related biomarkers and that these biomarkers will enhance clinical predictions models for CCI and 1 year Zubrod 4/5.
METHODS
Study Design and Population
This is a prospective, longitudinal study that enrolled surgical ICU patients over four years ending January 2019. This study was conducted in the trauma and surgical ICUs at the University of Florida (UF) Health Shands Hospital (Gainesville, Florida, USA). The study was approved by the UF Institutional Review Board and registered with clinicaltrials.gov (NCT02276417). The patient or legally authorized representative provided informed consent within 96 hours. If not obtained within 96 hours, all patient data and biological samples were destroyed.
Details of the study design with specific goals, inclusion/exclusion criteria, clinical/laboratory standard operating procedures (SOPs) plus interim results have been published.11,12,15 In brief, inclusion criteria included (1) age ≥18 years, (2) clinical diagnosis of sepsis as defined by 2001 consensus guidelines, and (3) entrance into electronic medical record clinical SOPs based on the SSC EBGs. Exclusion criteria eliminated patients whose baseline immunosuppression, end-stage comorbidities, or severe injuries would be a primary determinant of their long-term outcomes. The clinical course was documented using an established sepsis database. The diagnosis of sepsis, site of infection, and sepsis severity of each case was adjudicated weekly by a team of bedside clinicians. Infections were defined using CDC definitions and sepsis was classified as “present on admission” if diagnosed within 48 hours and “hospital-acquired” if diagnosed after 48 hours. Secondary infections were defined as occurring at least 48 hours after sepsis protocol onset during the index hospitalization. Infections within 48 hours were considered coexisting and excluded. Predictive mortality was assessed by the acute physiology age chronic health evaluation (APACHE) II. MOF was defined by the Denver MOF score and acute kidney injury was defined by Kidney Disease Improving Global Outcomes score. Longitudinal follow-up was performed for one year. After discharge, patients (or proxy) were contacted monthly by telephone concerning subsequent hospitalizations, and current disposition, including mortality which was cross-check validated via the United States Social Security Death Index. Among survivors, prospective follow-up assessments were conducted at 3, 6, and 12 months after sepsis onset. These were conducted at the UF Institute on Aging, the patient’s home, or via telephone (as feasible in that priority sequence).
Patients were categorized into 3 inpatient clinical trajectories: (1) early death, (2) CCI, and (3) RAP. Early death was defined as death within 14 days. CCI was defined as an ICU stay greater than or equal to 14 days with evidence of persistent organ dysfunction based upon components of the SOFA score. RAP was defined as those discharged from the ICU within 14 days with resolution of organ dysfunction, or those not meeting criteria for early death or CCI.
Performance status was assessed using the WHO/Zubrod scale with a scores of (0) for fully active, (1) symptomatic but ambulatory and capable of light work, (2) mild disability, in bed ≤50% of daytime, capable of self-care but no work, (3) moderate disability in bed for >50% of daytime, capable of limited self-care, (4) severe disability, completely bedridden with full functional dependence and (5) denoting death.16
Study Enrollment and Cohort Selection for This Analysis
In a recent manuscript, we described the current epidemiology of sepsis in this study population based on 301 subjects enrolled over 36-month period (ending January 2018).11 Subsequent to this report, the 4-year final enrollment of 363 subjects was completed. Updated previously published tables are included in the Supplementary Digital Content (SDC) Tables 1 to 4, http://links.lww.com/SLA/D258. Of the 363 subjects, 14 (4%) patients were classified as early deaths and were not included in the current manuscript looking at long-term outcomes. Of the remaining 349 included patients, 225 (62%) were classified as RAP and 124 (34%) were classified as CCI. In regards to long-term follow-up, 36 patients withdrew from the study and 23 patients were lost to follow-up. This left 304 patients who completed 1-year follow-up, of which 215 (71%) had a 1-year Zubrod score of 0 to 3 and 89 (29%) had a 1-year Zubrod score 4/5. We found no significant difference in clinical risk factors or outcome. SDC Table 5, http://links.lww.com/SLA/D258 and SCD Figure 1, http://links.lww.com/SLA/D257 depict clinical characteristics and biomarkers of the 59 dropouts versus the 304 patients who completed 1 year follow-up.
Healthy Controls
Consented age, sex, and race/ethnicity matched healthy controls (n = 37) had a single blood sample collected. Limited clinical data were collected, but individuals with known history of autoimmune disease, taking immunosuppressive medication, active cancer treatment, or active infection were excluded.
Blood Draws and Biomarker Analyses
Blood samples were collected at 1, 4, 7, and 14 days after sepsis onset for subjects remaining inpatient and analyzed for biomarkers reflecting underlying pathobiology of PICS including inflammation (interleukin [IL]-6, IL-8, interferon gamma-induced protein 10 [IP-10]), monocyte chemoattractant protein 1, granulocyte-macrophage colony-stimulating factor [GM-CSF]); stress metabolism (C-reactive protein [CRP], glucagon-like peptide 1 [GLP-1]); anabolism (insulin-like growth factor [IGF], IGF binding protein-3 [IGFBP3]); immunosuppression (soluble programmed death ligand-1 [sPDL-1] and IL-10), and angiogenesis (vascular endothelial growth factor [VEGF], soluble vascular endothelial growth factor receptor-1 [sFlt-1], angiopoietin-2 [Ang-2], stromal cell-derived factor-1 [SDF-1]). Plasma biomarker concentrations were determined by multiplex (MILLIPLEX multiplex assay, EMD Millipore Corp. Billerica, MA) or ELISA. Complete blood counts with differential were performed by the Clinical and Diagnostic Laboratories at the UF Health Shands Hospital. A summary of the selected PICS biomarkers is reported in SDC Table 6, http://links.lww.com/SLA/D258.
Statistical Analysis
Data are presented as frequency and percentage, mean and standard deviation, or median and 25th/75th percentiles. Fisher exact test and the Kruskal-Wallis test were used for comparison of categorical and continuous variables, respectively. Biomarkers were compared using the Kruskal-Wallis test at each time point for significant differences based on clinical trajectory (CCI vs RAP) and functional status at 1-year (Zubrod 0–3 vs Zubrod 4/5). The log-rank test was used to compare Kaplan-Meier product limit estimates of survival between groups.
Two clinical prediction models were performed on (1) day 4 for CCI and (2) day 7 for 1-year Zubrod 4/5 using variables within the PIRO categories of Predisposition, Insult characterization, Response, and Organ dysfunction.17 A list of these variables by category can be found in SDC Table 7, http://links.lww.com/SLA/D258. For the CCI model, univariate analyses on CCI versus RAP patients were performed on readily available variables on day 4 ± 24 hours within each of the PIRO categories (see results in SDC Table 8, http://links.lww.com/SLA/D258). To reduce the number of variables in the final model, multivariate stepwise logistic regression (MSLR) was performed within each PIRO category. Nine independent predictors were identified including (1) 3 predisposition (chronic lung disease, number of comorbidities, and reason for admission), (2) 2 insult (site of infection and sepsis severity), (3) 2 response (Acute Physiology Score [APS] from APACHE II and day 4 Neutrophil/Lymphocyte ratio), and (4) 2 organ dysfunction (day 4 PaO2/FiO2 and on ventilator on day 4) variables. These were included in the final MSLR clinical prediction model.
For the second clinical model, univariate analyses were performed on Zubrod 1 – 3 vs Zubrod 4/5 patients using readily available variables on day 7 ± 24 hours within each of the PIRO categories (see results in SDC Table 9, http://links.lww.com/SLA/D258). To reduce the number of variables, MSLR was performed within each PIRO category. Nine independent predictors were identified including (1) 4 predisposition (age, solid cancer, heart failure, and prior stroke), (2) 2 insult (site of infection and sepsis severity), (3) 1 response (lymphocyte count), and (4) 2 organ dysfunction (platelet count and on ventilator on day 7) variables. These were included in the final MSLR clinical prediction model.
In regards to biomarkers added to the clinical models, initial selection was based on whether they were significantly different on nonparametric testing between groups at pertinent time points (day 4 for CCI or day 7 for Zubrod 4/5). Selected biomarkers were then subjected to univariate logistic regression. Those found to be significant independent predictors (2 for CCI on day 4; 9 for Zubrod 4/5 on day 7) were then added to clinical models to establish improved prognostic value as determined by the area under the receiver operating characteristics curve (AUC) values. All significance tests were 2-sided, with P value ≤ 0.05 considered statistically significant. Statistical analyses were performed with SAS (v.9.4, Cary, NC).
RESULTS
Epidemiology of the Study Patient Cohorts
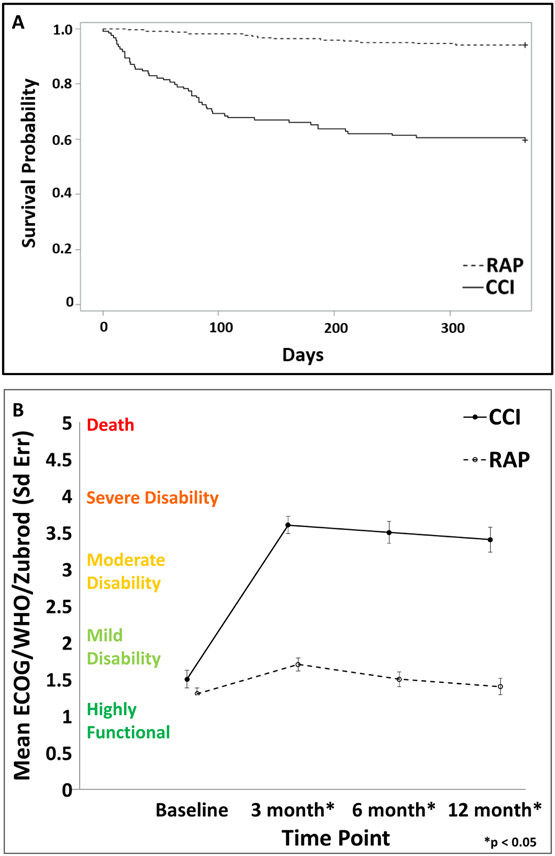
Table 1 depicts baseline predisposition, insult characteristics, and outcomes of RAP versus CCI and 1-year Zubrod 0–3 versus Zubrod 4/5 patient cohorts. Compared to the RAP, CCI patients were more likely to be older males with more comorbidities (including strokes, heart, and lung disease). They had more hospital-acquired infections (with more pneumonia) as the inciting cause of sepsis; and substantially more CCI patients presented in septic shock and had higher APACHE II scores. Notable more CCI patients required mechanical ventilation and developed MOF. Additionally, as can be seen in Figure 1, CCI patients had much worse 1-year survival, as well as higher Zubrod performance scores at 3 months that persisted to 1-year after sepsis onset.
TABLE 1.
Baseline Predisposition, Insult Characteristics and Outcomes of RAP Versus CCI and 1 year Zubrod 0-3 Versus Zubrod 4/5 Patient Cohorts
| RAP (n=225) |
CCI (n=124) |
p-value - CCI vs. RAP |
1 year Zubrod 0-3 (n=215) |
1year Zubrod 4/5 (n=89) |
p-value - Zubrod 4/5 vs. Zubrod 0-3 |
|
|---|---|---|---|---|---|---|
| Male, n (%) | 109 (48) | 79 (64) | < 0.01 | 111 (52) | 54 (61) | 0.17 |
| Age in years, median (25th, 75th) | 58 (45, 69) | 64 (56, 72) | < 0.01 | 59 (46, 68) | 69 (62, 74) | <0.01 |
| Race, n (%) | 0.48 | 0.23 | ||||
| Caucasian | 198 (88) | 113 (91) | 190 (88) | 82 (92) | ||
| African American | 25 (11) | 9 (7) | 23 (11) | 5 (6) | ||
| Other | 2 (1) | 2 (2) | 2 (1) | 2 (2) | ||
| Charlson comorbidity index, median (25th, 75th) | 2 (1, 4) | 4 (2, 6) | < 0.01 | 2 (1, 4) | 5 (3, 7) | <0.01 |
| Comorbidities | ||||||
| Solid cancer | 31 (14) | 23 (19) | 0.28 | 23 (11) | 29 (33) | <0.01 |
| Hematologic cancer | 0 (0) | 1 (1) | 0.36 | 0 (0) | 1 (1) | 0.29 |
| Heart failure | 21 (9) | 25 (20) | < 0.01 | 16 (7) | 23 (26) | <0.01 |
| Chronic lung disease | 30 (13) | 36 (29) | < 0.01 | 34 (16) | 25 (28) | 0.02 |
| Prior Stroke | 11 (5) | 15 (12) | 0.02 | 11 (5) | 12 (14) | 0.02 |
| Coronary disease | 44 (20) | 40 (32) | < 0.01 | 39 (18) | 37 (42) | <0.01 |
| Diabetes | 74 (33) | 47 (38) | 0.35 | 65 (30) | 36 (40) | 0.11 |
| Morbid obesity | 36 (16) | 25 (20) | 0.38 | 39 (18) | 16 (18) | 1 |
| Peripheral artery disease | 25 (11) | 17 (14) | 0.49 | 18 (8) | 21 (24) | < 0.01 |
| ESRD | 4 (2) | 4 (3) | 0.46 | 2 (1) | 4 (5) | 0.06 |
| Active cancer diagnosis | 27 (12) | 21 (17) | 0.26 | 21 (10) | 26 (29) | <0.01 |
| History of cancer | 52 (23) | 34 (27) | 0.44 | 42 (20) | 41 (46) | <0.01 |
| Reason for hospital admission, n (%) | 0.88 | 0.47 | ||||
| Active infection | 153 (68) | 64 (52) | 135 (63) | 53 (60) | ||
| Non-infectious complication | 13 (6) | 17 (14) | 16 (7) | 11 (12) | ||
| Planned surgery | 43 (19) | 24 (19) | 43 (20) | 19 (21) | ||
| Trauma | 16 (7) | 19 (15) | 21 (10) | 6 (7) | ||
| Hospital-acquired sepsis (>48 hrs), n (%) | 66 (29) | 67 (54) | < 0.01 | 75 (35) | 41 (46) | 0.07 |
| Site of infection, n (%) | < 0.01 | 0.02 | ||||
| Intra-abdominal | 95 (42) | 57 (46) | 92 (43) | 46 (52) | ||
| Pneumonia | 29 (13) | 36 (29) | 34 (16) | 18 (20) | ||
| Skin/Soft Tissue | 49 (22) | 17 (14) | 45 (21) | 11 (12) | ||
| Genitourinary | 40 (18) | 5 (4) | 33 (15) | 5 (6) | ||
| Vascular | 12 (5) | 9 (7) | 11 (5) | 9 (10) | ||
| Sepsis severity, n (%) | < 0.01 | < 0.01 | ||||
| Sepsis | 94 (42) | 15 (12) | 77 (36) | 17 (19) | ||
| Severe sepsis | 98 (44) | 55 (44) | 98 (46) | 35 (39) | ||
| Septic shock | 33 (15) | 54 (44) | 40 (19) | 37 (42) | ||
| APACHE II, median (25th, 75th) | 14 (10, 19) | 22 (16, 26) | < 0.01 | 15 (10, 21) | 21 (15, 26) | < 0.01 |
| Type of Sepsis Source Control Procedure, n (%) | 166 (74) | 79 (64) | 0.05 | 156 (73) | 60 (67) | 0.54 |
| Invasive procedures | 110 (49) | 59 (48) | 107 (50) | 44 (49) | ||
| Non-invasive procedures | 56 (25) | 20 (16) | 49 (23) | 16 (18) | ||
| Culture Positive, n (%) | 146 (65) | 80 (65) | 0.76 | 139 (65) | 56 (63) | 0.58 |
| Bacterial – gram positive | 32 (14) | 21 (17) | 30 (14) | 17 (19) | ||
| Bacterial – gram negative | 63 (28) | 30 (24) | 58 (27) | 20 (23) | ||
| Fungal | 5 (2) | 5 (4) | 6 (3) | 4 (5) | ||
| Polymicrobial | 46 (20) | 24 (19) | 45 (21) | 15 (17) | ||
| Need for mechanical ventilation, n (%) | 112 (50) | 116 (94) | < 0.01 | 129 (60) | 73 (82) | < 0.01 |
| MOF Denver Score, n (%) | 5 (2) | 43 (35) | < 0.01 | 17 (8) | 30 (36) | < 0.01 |
FIGURE 1.
Comparison of outcomes over 1-year after sepsis for CCI versus RAP sepsis cohorts. (A) One-year survival probability and (B) Twelve-month Zubrod score. Data presented as mean ± standard error with statistical significance set at P < 0.05.
Of the 304 patients who completed 1-year follow-up, 215 (71%) had a Zubrod score of 0–3 and 89 (29%) had a Zubrod score 4/5. Compared to the Zubrod 0–3 cohort, the Zubrod 4/5 patients were more likely to be older with more comorbidities (including cancer and heart disease). Interestingly, there were no differences in the characteristics of the septic insult (including community versus hospital-acquired and site of infection). However, Zubrod 4/5 patients had twice the incidence of septic shock and 4 times the incidence of MOF.
Biomarkers of Based on the Patient Cohorts
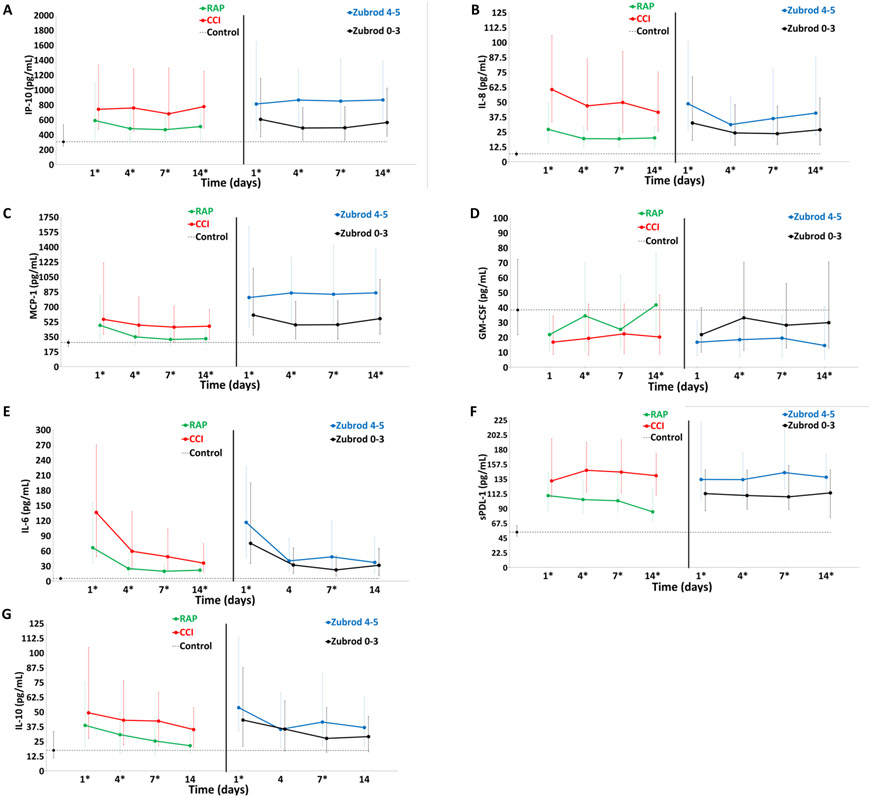
Figure 2 depicts comparisons of the inflammation (A–E) and immunosuppression (F and G) biomarkers over 14 days after sepsis for the CCI (vs RAP) and 1-year Zubrod 4/5 (vs Zubrod 0–3) patient cohorts. At all time points the CCI patients had higher levels of IP-10, IL-8, monocyte chemoattractant protein 1, and sPDL-1. IL-6 and IL-10 were significantly higher in CCI patients at every time point except day 14 (no difference). Interestingly, GM-CSF was significant lower at days 4 and 14. In the comparisons of the 1-year Zubrod 4/5 patients, they had higher levels of IL-8, IP-10, and sPDL-1 at all time points. IL-6 and IL-10 were higher only at days 1 and 7, whereas GM-CSF was lower at days 4, 7, and 14.
FIGURE 2.
Comparison of inflammation and immunosuppression biomarkers for CCI versus RAP and Zubrod 4/5 versus Zubrod 0–3.
Inflammation: (A) interferon gamma-induced protein 10 (IP-10), (B) interleukin-8 (IL-8), (C) monocyte chemoattractant protein 1 (MCP-1), (D) granulocyte-macrophage colony-stimulating factor (GM-CSF) and (E) interleukin-6 (IL-6). Immunosuppression: (F) soluble programmed death ligand 1 (sPDL-1) and (G) interleukin-10 (IL-10).
Data presented as median (25%, 75%) with * denoting statistical significance set at P < 0.05. Dotted black line represents median of biomarker levels in matched control subjects.
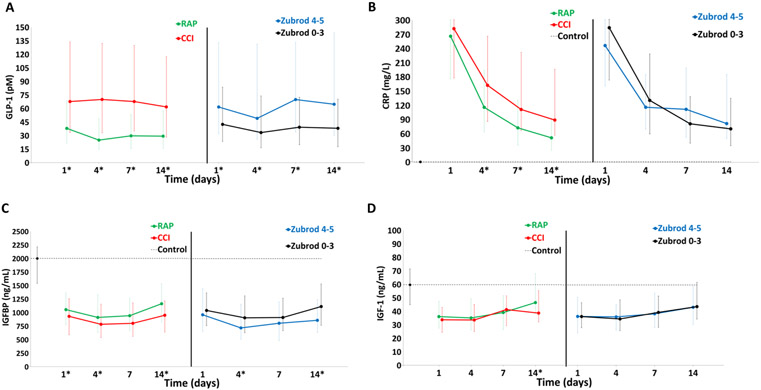
Figure 3 depicts comparisons of stress metabolism (A and B) and anabolic (C and D) biomarkers in the CCI (versus RAP) and Zubrod 4/5 (vs Zubrod 0–3) patient cohorts. For CCI, stress metabolism GLP-1 was higher at all time point and CRP was higher on days 4, 7, and 14. For CCI, anabolic IGFBP3 was lower at days 1, 4, and 14; whereas IGF was only lower on day 14. For Zubrod 4/5 patient comparisons, stress metabolism GLP-1 is higher at all time points, whereas CRP is not different at any time points. Anabolic IGFBP3 was significantly lower in Zubrod 4/5 patients at days 4 and 14, whereas IGF was not different at any time point.
FIGURE 3.
Comparison of stress metabolism and anabolism biomarkers for CCI versus RAP and Zubrod 4/5 versus Zubrod 0–3.
Stress metabolism: (A) glucagon-like peptide 1 (GLP-1) and C-reactive protein (CRP). Anabolism: (C) insulin-like growth factor binding protein (IGFBP3) and (D) insulin-like growth factor (IGF).
Dotted line represents biomarker levels in control subjects. Data presented as median (25%, 75%) with * denoting statistical significance set at P < 0.05. Dotted black line represents median biomarker levels in matched control subjects.
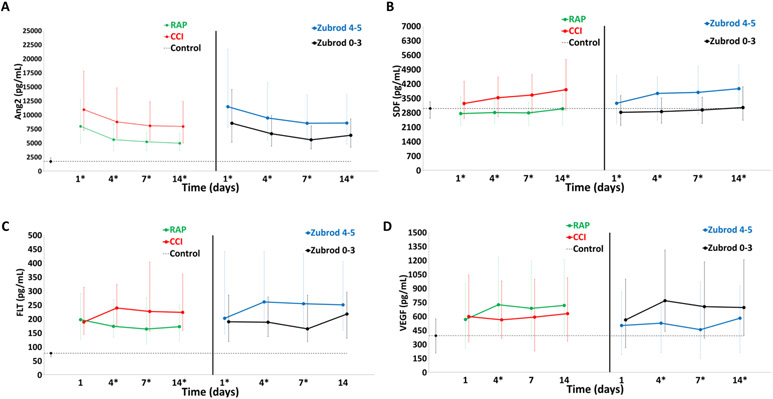
Figure 4 depicts comparisons of angiogenesis biomarkers in CCI (vs RAP) and Zubrod 4/5 (vs Zubrod 0–3) patient cohorts. For CCI (vs RAP), Ang-2 and SDF were higher at all time points. sFlt-1 was significantly higher at days 4, 7, and 14 in CCI patients. However, VEGF trended lower at all time points and was significantly lower on day 4 in CCI patients. Interestingly, patients with Zubrod 4/5 (vs Zubrod 0–3) had significantly lower levels of VEGF on days 4, 7, and 14. Whereas sFlt-1 was significantly higher in Zubrod 4/5 on days 1, 4, and 7.
FIGURE 4.
Angiogenesis biomarkers for CCI versus RAP and Zubrod 4/5 versus Zubrod 0–3.
Angiogenesis: (A) angiopoietin 2 (ANG-2) (B) stromal cell derived factor 1 (SDF-1), (C) soluble vascular endothelial growth factor receptor 1 (sFLT-1), and (D) vascular endothelial growth factor (VEGF). Data presented as median (25%, 75%) with * denoting statistical significance set at P < 0.05. Dotted black line represents median biomarker levels in matched control subjects.
Predicting CCI and 1-year Zubrod 4/5 based on PIRO Variables With and Without Biomarkers
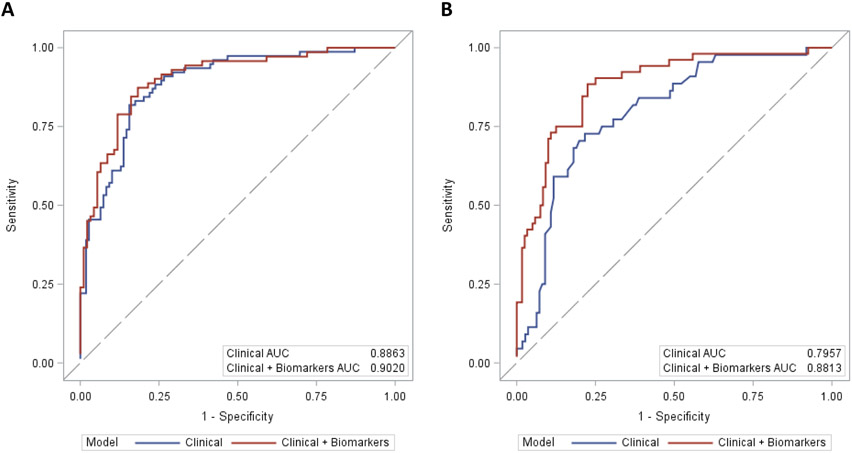
Table 2A depicts the multivariate prediction models for CCI with Day 4 variables with and without biomarkers. For the clinical model, prediction of CCI at day 4 based on 6 variables resulting in an AUC of 0.89 (0.84, 0.93). On univariate logistic regression analysis, IL-10 and IP-10 on day 4 were found to be independent predictors of CCI. However, their addition to the clinical model only minimally improved the prediction of CCI with an AUC = 0.90 (0.85, 0.95). Figure 5A depicted receiver operator curves for multivariate models predicting CCI at day 4 with and without biomarkers.
TABLE 2.
Multivariate prediction models with and without biomarkers for (A) CCI and (B) 1-year Zubrod 4/5.
| A. CCI with Day 4 variables | Clinical Model Multivariate (AUC=0.89) |
Clinical + Biomarkers Model Multivariate (AUC=0.90) |
||
|---|---|---|---|---|
| Parameter | adjusted OR |
P-value | adjusted OR |
P-value |
| Reason for Admission | 0.01 | 0.78 | ||
| Active infection vs. Trauma | 0.44 | 0.455 | ||
| Non-infectious/Chronic medical problems vs. Trauma | 10.157 | >999.999 | ||
| Planned surgery vs. Trauma | 0.349 | 0.391 | ||
| Site of Infection | <0.01 | 0.06 | ||
| Gastrointestinal vs. Pulmonary | 0.365 | 0.36 | ||
| Genitourinary vs. Pulmonary | 0.055 | 0.035 | ||
| Skin and Soft Tissue/Musculoskeletal vs. Pulmonary | 0.25 | 0.229 | ||
| Vascular vs. Pulmonary | 2.576 | 1.478 | ||
| Sepsis Severity | 0.02 | 0.23 | ||
| Severe Sepsis vs. Sepsis | 3.404 | 2.282 | ||
| Septic Shock vs. Sepsis | 6.121 | 3.552 | ||
| Acute Physiology Score from APACHE II | 1.095 | 0.02 | 1.095 | 0.05 |
| Neutrophil/Lymphocyte ratio, day 4 | 1.08 | <0.01 | 1.07 | 0.04 |
| On ventilator, day 4 | 5.796 | <0.01 | 5.842 | <0.01 |
| IL-10 day 4 | 1.005 | 0.4 | ||
| IP-10 day 4 | 1.001 | 0.06 | ||
| B. 1 year Zubrod 4/5 with day 7 variables | Clinical Model Multivariate (AUC=0.80) |
Clinical Model + Biomarkers Multivariate (AUC=0.88) |
||
| Parameter | adjusted OR |
P-value | adjusted OR |
P-value |
| Age | 1.039 | 0.02 | 1.046 | 0.02 |
| Solid Cancer | 3.888 | <0.01 | 3.503 | 0.02 |
| Heart Failure | 3.798 | <0.01 | 8.198 | <0.01 |
| On ventilator, day 7 | 2.977 | 0.01 | 3.435 | 0.02 |
| IL-10, day 7 | 1.006 | 0.12 | ||
| IL-6, day 7 | 1.007 | 0.05 | ||
| IL-8, day 7 | 0.998 | 0.80 | ||
| IP-10, day 7 | 1 | 0.16 | ||
| MCP-1, day 7 | 0.999 | 0.34 | ||
| Ang2, day 7 | 1 | 0.40 | ||
| GLP-1, day 7 | 0.999 | 0.84 | ||
| sPDL-1, day 7 | 1 | 0.89 | ||
| SDF, day 7 | 1 | 0.33 | ||
FIGURE 5.
Receiver operator curves (ROC) curves for multivariate models predicting CCI and Zubrod. (A) ROC for models predicting CCI at day 4 with and without biomarkers, and (B) ROC for models predicting Zubrod 4/5 at day 7 with and without biomarkers.
Table 2B depicts the multivariate prediction models for 1 year Zubrod 4/5 using Day 7 variables with and without biomarkers. For the clinical model, prediction of 1 year Zubrod 4/5 at day 7 based on four variables resulting in an AUC of 0.80 (0.72, 0.87). On univariate logistic regression analysis, IL-10, IL-6, IL-8, MCP-1, IP-10, Ang-2, GLP-1, sPDL-1, and SDF on day 7 emerged as independent predictors of 1 year Zubrod 4/5. Their addition to the clinical model considerably improved the prediction of 1 year Zubrod 4/5 to AUC = 0.88 (0.83, 0.94). Figure 5B depicted receiver operator curves for multivariate models predicting 1 year Zubrod 4/5 at day 7 with and without biomarkers.
DISCUSSION
In this prospective study of surgical ICU patients treated for sepsis, early mortality was surprisingly low. However, one third of survivors progressed into CCI. Unfortunately, most CCI patients were discharged to nonhome destinations with severe functional and cognitive disabilities from which they do not recovery, and 40% were dead at 1 year. The elderly patients (40% of our study patients) were especially vulnerable.8,11,18,19 These poor long-term outcomes after sepsis are consistent with other reports which additionally document a high rate of hospital readmission with mortality from sepsis recidivism and cardiovascular events. 5,20,21 In the present study, the CCI and 1-year Zubrod 4/5 cohorts were found to have persistent aberrations in the biomarkers reflecting different aspects of PICS. Both could be accurately predicted using readily available clinical parameters. Adding PICS-related biomarkers minimally improved the clinical prediction of CCI but notably strengthened the prediction of 1-year Zubrod 4/5. These data provide evidence that PICS plays a role in the multifactorial pathobiology of CCI after sepsis in the surgical ICU. These data are also consistent with research showing that trauma, burns, and sepsis induce a similar host response that when dysregulated causes an injurious systemic inflammatory response syndrome (SIRS, with multiple organ dysfunctions), an immunosuppressive compensatory anti-inflammatory response syndrome (with secondary infections), and catabolic stress metabolism (with loss of lean body mass).22-25 Recent studies show that these occur simultaneously and it is the failure to return to homeostasis that characterizes a complicated clinical course.9,18,26-29 Previous studies in burns and now reports from sepsis indicate that these dysregulated responses may persist for up to 1-year.30,31
The current study findings have important therapeutic implications. Previous sepsis trials tested a variety of immune modulators in the early phase of SIRS but consistently failed to decrease ICU mortality. There multiple reasons for these failures.17,32-34 The most notable is early heterogeneity with the inability to predict appropriate high-risk patients. As early mortality continues to decrease, treating persistent dysregulated immunity to improve CCI long-term outcomes has become more relevant. These interventions can likely be initiated later in the clinical course when CCI can be more accurately predicted. Although our day 4 CCI model was not developed to devise entry criteria into interventional trials, it does provide proof of concept. More robust data sets would be required to develop and validate prediction models for this purpose. As in trauma, we believe an early genomic metric to could enhance prediction and hopefully focus interventions on specific pathologic endotypes.35,36
The second major failure in previous trials is the concept that there is a “silver bullet” to treat sepsis. The current data indicate that multiple aspects of the PICS response remain deranged and that multiple interventions will be required to optimize CCI outcomes. Success in other chronic diseases with similar phenotypes (such as chronic cancer, burns, and sarcopenia) would likely provide potential beneficial interventions. For example, cancer immunotherapies are being tested in ongoing phase II sepsis trials (with IL-7 targeting lymphopenia and nivolumab targeting elevated PDL-1) to reduce sepsis recidivism.37,38 Additionally, despite aggressive ICU nutritional support, CCI patients lose substantial lean body mass resulting cachexia and anabolic resistance. This provides the rationale for anabolic nutrition with high protein, leucine, and arginine supplementation.39 High protein diets have been shown to overcome anabolic resistance in chronic cancer, pediatric burns, and sarcopenia. Leucine has been shown to overcome anabolic resistance in sarcopenia and is believed to increase muscle protein synthesis through the mamalian target of rapamycin pathway. Although arginine use in acute sepsis remains controversial, we have shown that the persistent expansion of myeloid-derived suppressor cells characterizes CCI and is predictive secondary infections and late mortality. Myeloid-derived suppressor cells upregulate arginase-1 causing an arginine deficiency which adversely affects lymphocyte proliferation and collagen synthesis.40 Perioperative arginine supplementation has also been shown to reduce postoperative complications after major cancer surgery.41,42 Long-term studies in pediatric burns have also shown improved protein synthesis when anabolic agents (eg, intensive insulin, oxandrolone, and propranolol) are combined with exercise. Likewise, resistance exercise interventions have been shown to be an effective adjunctive in aging sarcopenia.43 Preventing unnecessary muscle loss provides the rationale for early ICU-based physical therapy programs. These have been best studied in acute respiratory distress syndrome survivors and have been associated with a reduction in ventilator and ICU days with improved physical function after discharge.44,45 When combined with adequate nutritional support, exercise training substantially improves muscle synthesis and functional outcomes.46
Strengths and Limitations
The strengths include that this is a prospective study of new onset sepsis managed by clinical SOPs that ensure high compliance with the SSC EBGs. Sepsis diagnosis, site of infection, and sepsis severity were adjudicated weekly by a team of bedside clinicians and the clinical course was documented using an established sepsis database. The study also has several limitations. First, this observational study is using statistical association and prediction models to strengthen the argument for causation. These will require validation and intervention(s) to be definitive. Second, PICS biomarkers were measured during hospitalization. As a future goal, these biomarkers will be obtained after hospital discharge to study long-term biomarker aberrations and their resolution to better understand the pathobiology of dismal long-term outcomes. Third, this study was performed in the trauma and surgical ICUs at a single tertiary regional medical center which limits the generalizability of the observations. Trauma (type and severity) or type of planned surgery likely contributes to PICS-CCI but it is difficult to ascertain their role because of limited numbers of these patients in our cohort and variability of these pre-sepsis insults. Fourth, although RAP and CCI had similar baseline functional status by the Zubrod scale, comorbid disease plays an important role in the predisposition and outcomes after sepsis. We obtained comorbidity data by concurrent chart review, but a more in-depth interview with the patient/family plus specific biomarkers (such as HbA1C for diabetes) would have allowed quantitation of poor control or severity.
CONCLUSIONS
This prospective study of new onset sepsis in surgical ICU patients provides biomarker evidence that PICS plays a role in the underlying pathobiology of CCI after sepsis and presents potential targets for interventional studies aimed at improving long-term outcomes.
Supplementary Material
DISCUSSANT.
Dr. Jeffrey D. Kerby
Hello!
My name is Jeff Kerby from the University of Alabama at Birmingham. I would like to start with thanking the American Surgical Association for the honor of discussing this paper from Dr. Darden and Associates from the University of Florida at Gainesville.
This group has previously shown that although early mortality for those that survive a septic episode is surprisingly low, around 1/3 of patients develop CCI associated with persistent organ dysfunction with a high rate of poor functional performance and mortality at 1 year.
The question then that needs to be answered is to prevent this from occurring to provide better outcomes for our patients.
This study using the same population of patient as the original study with an additional year’s worth of enrollment, evaluates a panel of biomarker results at multiple time points in these patients, revealing persistent elevations for those that develop CCI and poor functional outcomes as defined by Zubrod scores. Importantly, the use of biomarker results enhanced predictive modeling for those who develop poor functional outcomes. I have a couple of questions:
You had 59 patients (~17%) who either withdrew or were lost to follow-up. Were there any major differences between these patients and those that remained in the study that could have affected your results?
What was the rationale for choosing Day 4 biomarkers for CCI and Day 7 biomarkers for Zubrod scores in your prediction models? It appears that statistical differences in biomarkers were apparent for other time points as well. Were other time points for this modeling also considered?
I noticed that you did not discriminate between RAP Patients and CCI patients when reporting the results of the Zubrod Scores. How many RAP Patients poor functional outcome and how many CCI patients had good functional outcomes. Is there anything we can learn from these subsets of patients that can help us clinically?
Finally, I want to give you an opportunity to tell us about next steps. This is very important work and can potentially inform future clinical trial design. Are you currently externally validating your prediction models and do you have plans to use this information in any upcoming clinical trials?
I would like to congratulate Dr. Darden on a well done and superbly presented study.
Response Dr. Dijoia Darden
First, I would like to thank the American Surgical Association for the opportunity to respond to Dr. Kerby’s insightful questions. Regarding the first question, since 59 patients were withdrawn or lost to follow up after hospital discharge, their loss would have affected the comparisons of the Zubrod 0–3 versus Zubrod 4/5 outcomes at 1 year but not the comparisons of the RAP versus CCI cohorts. We were able to compare the biomarkers that made it into the prediction models for Zubrod 4/5, and we found no significant differences in biomarker levels at every time point between the 2 groups, except for GLP at day 7. GLP was lower in the dropout group. We were also able to run additional analyses of clinical data to compare the patients that withdrew or were lost to follow up to those that completed the study. We did not find any significant differences in demographics, past medical history, sepsis severity, organ dysfunctions, CCI clinical trajectory nor poor discharge disposition. So no, we did not find any major differences between the 59 withdrawn or lost-to-follow-up patients and those that remained in the study that would have affected our study results.
In regards to the second question, it is true that there were significant differences in most of the biomarkers at days 1 and 14. Historically, innumerable sepsis trials dating back to the late 1980s tested a host of promising “silver bullet” immune modulators in the early phase of SIRS. Unfortunately, they have consistently failed to decrease ICU mortality. There are multiple reasons for these failures. The most notable is early heterogeneity with the inability to predict appropriate high-risk patients since early SIRS is characterized by a genomic and cytokine storm of dysregulated immunity. Additionally, resuscitation and operations confound the physiologic and phenotypic responses. As a result from a statistical perspective, the clinical trajectories do not start diverging until after 48 hours. As ICU mortality continues to decrease, treating persistent dysregulated immunity to improve CCI and dismal long-term outcomes have become more relevant. These interventions can likely be initiated after 48 hours when CCI and dismal long-term outcomes can be accurately predicted. Additionally, when thinking about the conversations you have had with your septic patient families. After 4 to 7 days of seeing a loved one on the ventilator, the psychology starts to change. At this time, families are more receptive to honest discussions concerning prognosis and the need for “life-altering” interventions such as tracheostomy, permanent feeding access, withdraw from care etc.
In regards to the third question, remembering that Zubrod 0–3 reflects good functional outcomes and Zubrod 4/5 reflects very poor functional outcomes at 1 year, within the CCI cohort there were 47 patients with Zubrod 0–3 and 55 patients with Zubrod 4/5. Within the RAP cohort there were 168 patients with Zubrod 0–3 and only 20 patients with Zubrod 4/5. So about half of the CCI patients continue to have poor functional outcomes at 1 year whereas only about 10% of RAP patients have poor functional outcomes. To inform your clinical decisions on post-discharge plans, this tells you that most RAP patients will return to baseline function while you have a 50/50 chance that your CCI patient will die or be completely disabled and incapable of any self-care at 1-year. Although we did not fully explore this in our paper, our group has also shown cognitive dysfunction and frailty in this subset of CCI patients at 3 months follow-up from which they do not recovery. In contrast, the RAP patients show modest impaired quality of life indicators and limitations in physical performance testing at 3 months; however, most have recovered by 1 year. We also found that the elderly (40% of our patients) are especially vulnerable. And within those who progress into CCI, over half are dead at 1 year whereas most of the survivors remain severely disabled.
Finally, Dr. Kerby asks about our next steps. To this, I would first like to clarify that these models were not developed to devise any entry criteria into interventional trials but rather to provide proof of concept validation of the PICS pathobiology in CCI and poor outcomes after sepsis. A more robust data set is required to develop and validate prediction models for this purpose. Thus, we have not started to externally validate these prediction models. However, our group has been interested in creating genomic and proteomic metrics to identify the dominant pathophysiologic endotypes driving the CCI phenotype in specific patients. Our data have indicated that multiple aspect of PICS remain deranged and that more targeted multimodality interventions will be needed to improve outcomes.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (NIGMS) grants: R01 GM-113945 (PE), P50 GM-111152 (GLG, BB, LLM, PAE, SCB and FAM), and T32 GM-008721 (DBD, BPF, LSK). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore LJ, McKinley BA, Turner KL, et al. The epidemiology of sepsis in general surgery patients. J Trauma. 2011;70:672–680. [DOI] [PubMed] [Google Scholar]
- 5.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croft CA, Moore FA, Efron PA, et al. Computer versus paper system for recognition and management of sepsis in surgical intensive care. J Trauma Acute Care Surg. 2014;76:311–317. discussion 318-319. [DOI] [PubMed] [Google Scholar]
- 7.McKinley BA, Moore LJ, Sucher JF, et al. Computer protocol facilitates evidence-based care of sepsis in the surgical intensive care unit. J Trauma. 2011;70:1153–1166. discussion 1166-1157. [DOI] [PubMed] [Google Scholar]
- 8.Gardner AK, Ghita GL, Wang Z, et al. The development of chronic critical illness determines physical function, quality of life, and long-term survival among early survivors of sepsis in surgical ICUs. Crit Care Med. 2019;47:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stortz JA, Mira JC, Raymond SL, et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg. 2018;84:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brakenridge SC, Efron PA, Cox MC, et al. Current epidemiology of surgical sepsis: discordance between inpatient mortality and 1-year outcomes. Ann Surg. 2019;270:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stortz JA, Cox MC, Hawkins RB, et al. Phenotypic heterogeneity by site of infection in surgical sepsis: a prospective longitudinal study. Crit Care. 2020;24:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno RP, Metnitz B, Adler L, et al. Sepsis mortality prediction based on predisposition, infection and response. Intensive Care Med. 2008;34:496–504. [DOI] [PubMed] [Google Scholar]
- 15.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7:e015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 17.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intens Care Med. 2003;29:530–538. [DOI] [PubMed] [Google Scholar]
- 18.Mankowski RT, Anton SD, Ghita GL, et al. Older adults demonstrate biomarker evidence of the persistent inflammation, immunosuppression and catabolism syndrome (PICS) after sepsis. J Gerontol A Biol Sci Med Sci. 2021;glab080. doi: 10.1093/gerona/glab080. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mankowski RT, Anton SD, Ghita GL, et al. Older sepsis survivors suffer persistent disability burden and poor long-term survival. J Am Geriatr Soc. 2020;68:1962–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yende S, Linde-Zwirble W, Mayr F, et al. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;189:1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winters BD, Eberlein M, Leung J, et al. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283. [DOI] [PubMed] [Google Scholar]
- 22.Horn DL, Bettcher LF, Navarro SL, et al. Persistent metabolomic alterations characterize chronic critical illness after severe trauma. J Trauma Acute Care Surg. 2021;90:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore FA, Moore EE, Billiar TR, et al. The role of NIGMS P50 sponsored team science in our understanding of multiple organ failure. J Trauma Acute Care Surg. 2017;83:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeschke MG, Gauglitz GG, Finnerty CC, et al. Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann Surg. 2014;259:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuenca AG, Maier RV, Cuschieri J, et al. The Glue Grant experience: characterizing the post injury genomic response. Eur J Trauma Emerg Surg. 2011;37:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenthal MD, Kamel AY, Rosenthal CM, et al. Chronic critical illness: application of what we know. Nutr Clin Pract. 2018;33:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stortz JA, Murphy TJ, Raymond SL, et al. Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock. 2018;49:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mira JC, Cuschieri J, Ozrazgat-Baslanti T, et al. The epidemiology of chronic critical illness after severe traumatic injury at two level-one trauma centers. Crit Care Med. 2017;45:1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathias B, Delmas AL, Ozrazgat-Baslanti T, et al. Human myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg. 2017;265:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yende S, Kellum JA, Talisa VB, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2:e198686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riche F, Chousterman BG, Valleur P, et al. Protracted immune disorders at one year after ICU discharge in patients with septic shock. Crit Care. 2018;22:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenner BP, Darden DB, Kelly LS, et al. Immunological endotyping of chronic critical illness after severe sepsis. Front Med (Lausanne). 2020;7:616694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horiguchi H, Loftus TJ, Hawkins RB, et al. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol. 2018;9:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20:195–203. [DOI] [PubMed] [Google Scholar]
- 35.Brakenridge SC, Wang Z, Cox M, et al. Distinct immunologic endotypes are associated with clinical trajectory after severe blunt trauma and hemorrhagic shock. J Trauma Acute Care Surg. 2021;90:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Council NAGMS. NAGMSC Working Group on Sepsis: Final Report. 2019. [Google Scholar]
- 37.Hotchkiss RS, Colston E, Yende S, et al. Immune checkpoint inhibition in sepsis: a phase 1b randomized, placebo-controlled, single ascending dose study of antiprogrammed cell death-ligand 1 antibody (BMS-936559). Crit Care Med. 2019;47:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francois B, Jeannet R, Daix T, et al. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore FA, Phillips SM, McClain CJ, et al. Nutrition support for persistent inflammation, immunosuppression, and catabolism syndrome. Nutr Clin Pract. 2017;32(1_suppl):121S–127S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darcy CJ, Minigo G, Piera KA, et al. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care. 2014;18:R163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drover JW, Dhaliwal R, Weitzel L, et al. Perioperative use of arginine-supplemented diets: a systematic review of the evidence. J Am Coll Surg. 2011;212:385–399. 399 e381. [DOI] [PubMed] [Google Scholar]
- 42.Braga M, Gianotti L, Vignali A, et al. Hospital resources consumed for surgical morbidity: effects of preoperative arginine and omega-3 fatty acid supplementation on costs. Nutrition. 2005;21:1078–1086. [DOI] [PubMed] [Google Scholar]
- 43.Rivas E, Sanchez K, Cambiaso-Daniel J, et al. Burn injury may have age-dependent effects on strength and aerobic exercise capacity in males. J Burn Care Res. 2018;39:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashem MD, Nelliot A, Needham DM. Early mobilization and rehabilitation in the ICU: moving back to the future. Respir Care. 2016;61:971–979. [DOI] [PubMed] [Google Scholar]
- 45.Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cermak NM, Res PT, de Groot LC, et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96:1454–1464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.