Abstract
Treatment with CD19 or CD22-targeted chimeric antigen receptor-engineered T (CD19/CD22 CAR-T) cells achieve complete responses in approximately 60 to 90% of adults and children with refractory or relapsed (R/R) acute lymphoblastic leukemia (ALL). This led to the approval of tisagenlecleucel (Kymriah) by the FDA and several European regulatory agencies in ALL patients up to 25 years of age. Although CAR T-cell therapy is likely to transform the ALL therapeutic landscape, its development and wide dissemination have been impacted by the occurrence of significant toxicities; namely, cytokine release syndrome (CRS) and Immune effector cell-Associated Neurotoxicity Syndrome (ICANS) have been reported at higher rates in ALL patients compared to other B cell malignancies, particularly in the adult population. Here, we review recent data suggesting a significant proportion of ALL patients are at risk of developing severe, sometimes life-threatening, CRS and ICANS after CD19 and CD22 CAR T-cell therapy. After describing the key clinical and laboratory features of severe CRS and ICANS, we explore the disease and treatment-related factors thay may predict the severity of these toxicities. Last, we review strategies under investigation in the prophylactic and therapeutic settings to improve the safety of CAR T-cells for ALL.
Keywords: CAR T cells, ALL, CRS, ICANS, toxicity
Introduction
CD19 and CD22-targeted chimeric antigen receptor (CAR)-engineered (CD19/CD22 CAR) T cells have demonstrated high efficacy against relapsed or refractory (R/R) B cell malignancies and particularly high response rates in patients with acute lymphoblastic leukemia (ALL). In children and adults with R/R ALL, complete remission (CR) rates have been reported in approximately 60 to 90% patients receiving CD19 CAR T cell therapy1-14. A CD19 CAR T-cell product, tisagenlecleucel, was approved in 2017 by the FDA for patients with B-ALL up to the age of 25 with refractory disease, or in second or later relapse. Of note, there is to date no approved CAR T-cell product for adult patients with R/R ALL. While CAR T-cell therapy has become an area of intense focus, cytokine release syndrome (CRS) and Immune effector cell-Associated Neurotoxicity Syndrome (ICANS) remain significant barriers to the development and the wide dissemination of these promising therapies15; this is especially true for adult ALL patients, in whom these toxicities might be more severe compared to patients with other B cell malignancies. Here, we review the incidence and severity of CRS and ICANS across clinical trials of CAR T-cell therapy for ALL. We examine the key disease and treatment-related factors associated with these toxicities, and discuss several practical approaches to improve the safety of CAR T cells while preserving their anti-tumor effects.
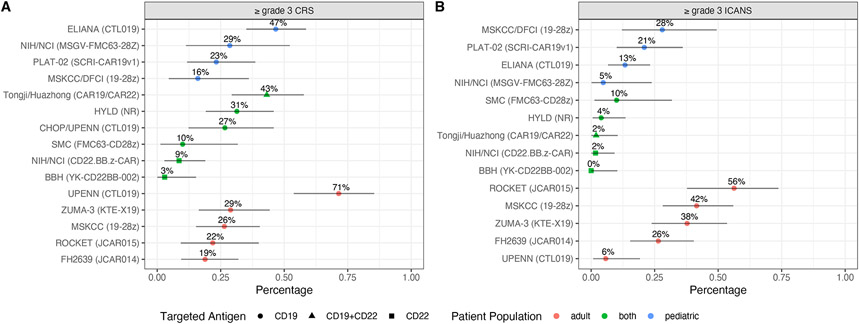
High incidence of severe cytokine release syndrome after CD19 CAR T-cell therapy for ALL (Figure 1A)
Figure 1. Grade ≥3 CRS (A) and ICANS (B) in ALL patients receiving CD19 CAR T-cell therapy.
Dots, observed proportions; grey horizontal lines, 95% confidence intervals using the Clopper-Pearson method. CRS grading systems differ across clinical trials. ICANS was graded using the Common Terminology Criteria for Adverse Events (CTCAE). Abbreviations: AE, adverse event; BBH, Beijin Boren Hospital; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; CHOP, Children’s Hospital of Pennsylvania; DFCI, Dana Farber Cancer Institute; FHCRC, Fred Hutchinson Cancer Research Center; Flu, fludarabine; HYLD, Hebei Yanda Lu Daopei Hospital; ICANS, immune effector cell-associated neurotoxicity syndrome; MSKCC, Memorial Sloan Kettering Cancer Center; MRD; minimal residual disease; NCI, National Cancer Institute; NIH, National Institute of Health; NR, not reported; SMC, Sheba Medical Center; UPENN, University of Pennsylvania.
Upon ligation of the CAR to its target on ALL blasts and normal B cells, CAR T-cells undergo marked in vivo activation and proliferation16,17 This is associated with the subsequent activation of other cell types, e.g., monocytes, endothelial and stromal cells, leading to high serum concentrations of pro-inflammatory cytokines18,19. As detailed later in this review, preclinical models suggest monocytes and macrophages may be paramount to the development of CRS and ICANS20,21. Clinically, fever remains the hallmark of CRS. Rigors, tachycardia, hypotension, tachypnea, hypoxemia, and other signs of systemic inflammation are also commonly observed8. CRS is mild in reversible in most patients, but severe cases of refractory shock with fatal multi-organ failure have been reported, often with clinical and cytokinic features mimicking hemophagocytic lymphohistocytosis (HLH) 19,22,23. Key patient and treatment characteristics of the main CD19 CAR T-cell clinical trials to date are detailed in Table 1 and Table 2 for adult and pediatric patients, respectively. High rates of CRS have been reported across clinical trials of CD19 CAR T-cell therapy for R/R ALL (any grade CRS, 56-100%)1-11. A significant fraction of these patients presented with grade ≥ 3 CRS (3-71%). In our experience using JCAR014, a 4-1BB costimulated CD19 CAR T-cell product of defined composition (1:1 ratio of CD8+ and CD4+ CD19 CAR T-cells) in adults with relapsed or refractory ALL, any grade CRS occurred in 75% of cases, and 10% of patients developped grade ≥3 CRS using the 2014 Lee consensus criteria24. Severe grade ≥3 CRS was characterized by early (<24 hours) and profound hemodynamic instability, capillary leak syndrome, and consumptive coagulopathy. Four of 47 ALL patients (8%) developed grade ≥4 CRS during the dose escalation stage of the study (2x106 CAR Tcells/kg, n=3; 2x107 CAR T cells/kg, n=1). We did not observe grade ≥4 CRS in ALL patients treated at the lowest dose level (2x105 CAR T cells/kg). In the phase I stage of the ZUMA-3 trial, evaluating KTE-X19 in adults with R/R ALL, grade ≥ 3 CRS was reported in 29% of patients10. There were two KTE-C19-related fatal events: 1 cerebral infarction at the 0.5 × 106 cells/kg dose and 1 previously reported multiorgan failure at the 2 × 106 cells/kg dose, both events occurring in the context of CRS. This led the investigators to select 1x106/kg as the maximum tolerated dose, and to revise CRS and ICANS management for this ongoing clinical trial. This revision required corticosteroids to be initiated for grade ≥2 ICANS, and tocilizumab not to be administered for ICANS unless in the context of CRS.
Table 1.
Summary of CD19 CAR-T cell therapy clinical trials for relapsed or refractory adult ALL.
AE adverse event, CAR chimeric antigen receptor, CR complete remission, CRS cytokine release syndrome, Cy cyclophosphamide, FC flow cytometry, FHCRC Fred Hutchinson Cancer Research Center, Flu fludarabine; Hebei Yanda Lu Daopei Hospital, ICANS immune effector cell-associated neurotoxicity syndrome, MFC multiparameter flow cytometry; MSKCC Memorial Sloan Kettering Cancer Center, MOF multiorgan failure, MRD minimal residual disease.
| Institution | Multicenter10 | UPENN11 | FHCRC3 | MSKCC7 | Multicenter24 | HYLDH4 |
|---|---|---|---|---|---|---|
| Trial identification | ZUMA-3 NCT02614066 |
NCT01029366
NCT02030847 |
FH2639 NCT01865617 |
NCT01044069 | ROCKET NCT02535364 |
ChiCTR-IIh-16008711 |
| Number of subjects infused | 45 | 35 | 53 | 53 | 32 | 42 including both children and adults |
| CAR T-cell product | KTE-X19 (brexucabtagene autoleucel) | CTL019 (tisagenlecleucel) | JCAR014 | 19-28z | JCAR015 | Not reported |
| Co-stimulatory domain | 19-28z | 4-1BB | 4-1BB | CD28 | CD28 | 4-1BB |
| scFv | FMC63 | FMC63 | FMC63 | SJ25C1 | SJ25C1 | Not reported |
| CAR T-cell dose | 0.5 x106/kg 1 x 106/kg 2 x 106/kg |
5x108 total dose
(single) 5x107 (single or fractionated*) 5x108 (fractionated*) |
2 x105/kg 2 x106/kg 6 2 x 107/kg |
1 x 106/kg 3 x 106/kg |
1 x106/kg 3 x106/kg |
0.05-1.6x106/kg |
| Lymphodepletion | Cy 900mg/m2x1 + Flu 25mg/m2x3 | Cy 300 mg/m2 every 12 hours x3,
71% Cy 500 mg/m2 x2 + Flu 30 mg/m2 x4, 29% |
Cy 60mg/kg x1 + Flu 25mg/m2x3,
45% Cy 300mg/m2 x1 + Flu 30mg/m2x3, 45%, 21% |
Cy 3g/m2, 71% Cy + Flu 19% |
Cy 1-3g/m2 x1 Cy 30-60mg/kg x1+Flu 25mg/m2x3 |
Cy 250mg/m2x3 + Flu 30mg/m2x3 |
| Median BM blasts prior to lymphodepletion | 59% | >5% in 94% | 28% | 63% | ≥5% in 100% | 32.6% |
| MRD negative CR by MFC | 75% | Overall 69% 90% in 5x108 fractionated cohort |
85% | 67% | 55% | 85% |
| Median CRS duration (days) | Not reported | Not reported | 3 | Not reported | Not reported | Not reported |
| Median ICANS duration (days) | Original AE management,
20.5 Revised AE management, 11 |
Not reported | 5 | Not reported | Not reported | Not reported |
| Seizures (n) | 0 | Not reported | 3 | Not reported | Not reported | 8 |
| Cerebral edema (n) | 0 | 0 | 1 | 0 | 5 | Not reported |
| Deaths attributed to CAR-T cell therapy (n) | 2 (4%) | 3 (8%) | 2 (4%) | 1 (2%) | 5 (16%) | 2 |
| Causes of death | Severe CRS with MOF Cerebral infarction |
Refractory hypotension in the context of
sepsis and CRS Intracranial hemorrhage |
Cerebral edema Severe CRS with MOF |
Severe CRS with MOF | Cerebral edema | Intracranial hemorrhage Heart failure |
Fractionated dosing: day 1, 10%; day 2, 30%; day 3, 60%.
Table 2.
Summary of CD19 CAR-T cell therapy clinical trials for relapsed or refractory pediatric (children and young adults) ALL. AE adverse event, BBH Beijin Boren Hospital, CAR chimeric antigen receptor, CR complete remission, CRS cytokine release syndrome, CHOP Children’s Hospital of Pennsylvania, Cy cyclophosphamide, DFCI Dana Farber Cancer Institute, FC flow cytometry, Flu fludarabine, ICANS immune effector cell-associated neurotoxicity syndrome, MFC multiparameter flow cytometry, MSKCC Memorial Sloan Kettering Cancer Center, MOF multiorgan failure, MRD minimal residual disease, NA not applicable, NIH National Institute of Health, NCI National Cancer Institute, TCS CD8+ and CD4+ T cell selection, UPENN University of Pennsylvania.
| Institution | CHOP/UPENN1 | Multicenter6 | NIH/NCI | Seattle Children’s5 | MSKCC/DFCI8 | BBH26 | |
|---|---|---|---|---|---|---|---|
| Target Antigen | CD19 | CD19 | CD22 | CD19 | CD22 | ||
| Trial identification |
NCT01626495
NCT01029366 |
ELIANA NCT02435849 |
NCT01593696 2 | NCT02315612 46 | PLAT-02 NCT02028455 |
NCT01860937 | hiCTR-OIC-17013523 |
| Number of subjects infused | 30 | 75 | 21 | 58 | 43 | 25 | 34 |
| CAR T-cell product | CTL019 (tisagenlecleucel) | MSGV-FMC63-28Z | CD22.BB.z-CAR | SCRI-CAR19v1 | 19-28z | YK-CD22BB-002 | |
| Co-stimulatory domain | 4-1BB | CD28 | 4-1BB | 4-1BB | CD28 | 4-1BB | |
| scFv | FMC63 | FMC63 | m971 | FMC63 | SJ25C1 | YK | |
| CAR T-cell dose | 1-17x106/kg | 0.2-5.4×106/kg | 1x106/kg 3 x 106/kg |
1x106/kg
bulk 1x106/kg TCS 3 x 105/kg TCS (de-escalated due to toxicity) |
0.5 x106/kg 1 x 106/kg 5 x106/kg 10 x 106/kg |
1 x 106/kg | No prior allo: median dose, 7.5 ×
105/kg (range, 0.3–34.7 ×
105/kg) Prior allo: median dose, 1 × 105/kg (range, 0.2 to 10 × 105/kg |
| Lymphodepletion | Cy-based, 83% Cy 500mg/m2 x1 + Flu 25mg/m2x3 in 43% |
Cy 500mg/m2x + Flu
30mg/m2x4 or Ara-c 500mg/m2x2 + etoposide 150mg/m2x3 |
Cy 900mg/m2x1 + Flu 25mg/m2x3 | Cy 2-4mg/m2x1 Cy 500mg/m2x2+30mg/m2x4 |
Cy 3 g/m2 +/− Flu
(n=3), 68% Cy ≤1.5 g/m2 +/− Flu (n=3), 32% |
Cy 250mg/m2x3 + Flu 30mg/m2x3 | |
| Median BM blasts prior to lymphodepletion | Detectable disease by morphology in 80% | 74% | 25.5% | >5% in 76% | >25% in 51% | 4% | 63% |
| MRD negative CR by MFC | 73% | 81% | 57% | 60% | 89% | 88% | 76% |
| Median CRS duration (days) | Not reported | 8 | 5 | 5 | Not reported | Not reported | 8 |
| Median ICANS duration (days) | 2-3 | 50% resolved within 10 days 75% within 18 days 4 patients had no resolution |
Not reported. Two weeks in one patient | Not reported | Not reported | Not reported | 7 |
| Seizures (n) | 1 | 1 | 0 | 0 | 5 | 5 | 1 |
| Cerebral edema (n) | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Deaths attributed to CAR-T cell therapy (n) | 0 | 0 | 0 | 2 (3%) | 0 | 1 (4%) | 4 (12%) |
| Causes of death | NA | 0 | NA | Sepsis with MOF Severe CRS with ARDS | NA | Sepsis with MOF after severe CRS | Grade 5 CRS (n=1) SOS (n=1) Seosis with MOF (n=2) |
Factors associated with CRS severity (Table 3)
Table 3.
Factors and biomarkers associated with CRS and ICANS severity in ALL patients treated with CD19 CAR T cells. All biomarkers are peak serum concentrations, unless specified.
allo-HCT allogeneic hematopoietic cell transplantation, ALL acute lymphoblastic leukemia, BM bone marrow, CAR chimeric antigen receptor, CNS central nervous system, CRS cytokine release syndrome, Cy cyclophosphamide, FC flow cytometry, FHCRC Fred Hutchinson Cancer Research Center, Flu fludarabine, ICANS immune effector cell-associated neurotoxicity syndrome, IT intrathecal, MSKCC Memorial Sloan Kettering Cancer Center, qPCR quantitative polymerase chain reaction.
| Publication | CART cell product |
N | Factors associated with CRS severity | Factors associated with ICANS severity | Method |
|---|---|---|---|---|---|
| Maude et al, NEJM. 20141 | CTL019 | 30 |
Pretreatment BM tumor
burden CRP, IL-6, Ferritin, IFN-γ, sIL-2Rα |
Not reported | Wilcoxon rank-sum test |
| Teachey, Cancer Discovery. 201617 | CTL019 | 51 | Ferritin LDH CRP Fibrinogen trough |
Wilcoxon rank-sum test | |
|
Model 1 (combined adult and
pediatric cohort): sgp130+IFN-γ+IL-1Rα (peak
within 3 days post infusion) Model 2 (pediatric cohort): IFN-γ+IL-15+ MIP-1α |
Multivariable logistic regression | ||||
|
Model 1 (combined adult and
pediatric cohort): sgp130 + MCP-1 +
eotaxin Model 2 (pediatric cohort): IL-10 + pretreatment BM tumor burden Model 3 (pediatric cohort): IFN-γ+MIP;-1α |
Classification tree models
(CRS) Validation in small independent cohort (n=12) |
||||
| Davila et al, STM. 201425 | 19-28z | 22 |
Pretreatment BM tumor
burden CRP, IL-6, IFN-γ, Flt-3-L, Fractalkine, IL-5, IL-10, GM-CSF |
Spearman rank-order correlation | |
| Lee et al, Lancet. 20152 | MSGV-FMC63-CD28Z | 21 |
Pretreatment BM tumor
burden Peak CAR T cell in vivo expansion (FC) CRP IL-6 fold change IFN-γ fold change |
Wilcoxon rank-sum test | |
| Hay et al, Blood. 201716 / Gust et al, Cancer Discovery 201723 | JCAR014 | 47 |
Pretreatment BM tumor
burden Prior allo-HCT CD8+ selection method CAR T-cell dose |
Severe CRS* Preexisting neurologic comorbidities Pretreatment BM tumor burden Cy-Flu lymphodepletion CAR T-cell dose |
Multivariable proportional odds model |
| Peak CAR T cell in vivo expansion (FC) | Peak CAR T cell blood count | Univariate logistic regression | |||
| CRP, IL-6, IFN-γ, IL-8, IL-10, IL-15, MCP-1, TNFRp55, MIP-1β | CRP, ferritin, IL-6, IFN-γ | Wilcoxon rank-sum test | |||
| Fever + MCP-1 peak within 36 hours post infusion | Not reported | Classification trees (CRS) | |||
| Gardner et al, Blood. 20175 | SCRI-CAR19v1 | 43 |
CAR T-cell dose Did not confirm association with pretreatment BM tumor burden, CD19 antigen load, Cy-Flu lymphodepletion |
Severe CRS Did not confirm association with disease burden, CD19 antigen load, Cy-Flu lymphodepletion |
Univariate proportional odds regression |
| Park et al, NEJM. 20187 | 19-28z | 53 | Pretreatment BM tumor burden | Pretreatment BM tumor burden | Fisher’s test |
| Peak CAR T cell in vivo expansion (gPCR; p=0.06) | Peak CAR T cell blood count*(gPCR; association between CAR T cell count and ICANS was preserved after adjusting for BM tumor burden in multivariable logistic regression | Wilcoxon rank-sum test | |||
| Curran KJ, Blood. 20198 | 19-28z | 25 | Did not confirm association between with pretreatment BM tumor burden, peak CAR T cell in vivo expansion (qPCR) | Severe CRS | Fisher’s test |
| CRP, IL-6 | Not reported | Wilcoxon rank-sum test | |||
| Gilbert et al, SITC. 201724 | JCAR015 | 31 | Not reported | IL-15 | Descriptive statistics |
| CMC attributes of the CAR T-cell
product: Annexin V- CD8+ CAR+ cell dose % of CD4+CAR+ producing IL-2, TNF-α* % of CD8+CAR+ producing TNFα* |
Univariate logistic
regression Approximately 140 variables screened No adjustment for multiplicity |
||||
| Cy-Flu lymphodepletion Did not confirm associations with prior CNS irradiation, prior IT chemotherapy, prior CNS disease, prior allo-HCT, higher ECOG performance status, prior blinatumomab |
Multivariable logistic regression
adjusting for: high-intensity bridging, age, prior lines of
therapy Approximately 500 variables screened No adjustment for multiplicity |
||||
| Non-Philadelphia gene expression signature (ROCKET) | Not reported | ||||
| JCAR015, JCAR014 | 154 | IL-15 (ROCKET + FHCRC phase 1 [B cell malignancies]) | Classification trees | ||
| JCAR015, JCAR014, 19-28z | 222 | Platelet count (ROCKET + FHCRC phase 1+ MSKCC phase I [B cell malignancies]) | Classification trees | ||
| JCAR014 | 109 | ALL (FHCRC phase 1) | Classification trees |
After 18-h in vitro stimulation of 0.25 × 106 cryopreserved CD3+CAR+ cells with a CD19 presenting cell line (effector:target ratio, 1:1).
To date, validated models to predict CRS severity are lacking. Several groups, including ours, have reported associations between clinical, biological, product-related characteristics and CRS and ICANS severity and applied various modeling strategies (Table 3). Some simple clinical variables, such as the CAR T-cell dose and the pretreatment BM tumor burden, are strongly associated with toxicity in most studies. In contrast, biomarker identification remains exploratory at this stage in the CAR T-cell field, mainly suggesting new avenues for future research. Many of the identified biomarkers have failed to replicate across studies (e.g., CRP, IL-15).
Davila et al from the MSKCC2 showed that higher serum concentrations of C-reactive protein (CRP; ≥20mg/dL) measured after CD19 CAR T-cell infusion were associated with severe CRS. The authors reported high specificity and sensitivity using a model including CRP, although the timepoint of measurement was not specified, and the metrics likely to be biased due to overfitting in this small dataset. In addition, they measured higher serum concentrations of 7 cytokines (INF-γ, IL-6, FLT3-ligand, Fractalkine, IL-5, IL-10, GM-CSF) and higher pre-treatment tumor burden in patients who developped occurrence of severe CRS. The predictive ability of the CRP using the 20mg/dL threshold was not confirmed by Maude et al using tisagenlecleucel1, illustrating the limitations of “optimal” thresholds who rarely replicate in independent cohorts.
Using data from patients treated at our center on phase I/II clinical trial with JCAR014, we used multivariable ordinal regression to a subgroup of patients with ALL patients (n=47)18. We identified prior allogeneic hematopoietic cell transplantation, the pre-lymphodepletion percentage of marrow blasts, a higher CAR T-cell dose, and CAR T-cell manufacturing from bulk CD8+ T cells were independent predictors of CRS severity. This suggests CAR T-cell therapy administered earlier in the course of ALL, and after tumor reduction – “debulking” or “bridging” – could reduce CRS severity. In addition, peak CD8+ CAR T-cell expansion in blood was strongly associated with both severe CRS and efficacy. Aside from multivariable ordinal regression, we applied classification tree modeling to our entire dataset (n=133) including ALL, NHL and CLL patients. This algorithm was successful at identifying patients with grade ≥4 CRS based on developing fever ≥38.9°C within 36 hours of CAR T-cell infusion, and a serum MCP-1 concentration ≥1343.5 pg/mL. Note that models including MCP-1 performed better – in terms of discrimination – than those with CRP, ferritin, or other cytokines (IFN-γ, IL-6, IL-8, IL-10, IL-15). Although this approach performed well on our dataset (sensitivity, 1.00; specificity, 0.95), our model has not been validated on an independent cohort and might suffer from overfitting. Additionally, JCAR014 is formulated using a fixed CD8+/CD4+ CAR T-cell ratio, thereby differing from commercial CAR T-cell products. As such, our models may not generalize to other CAR T cell products.
Teachey et al from the University of Pennsylvania, investigated 24 serum biomarkers to predict high grade (grade 3-4) CRS19. Using classification trees they developed 2-step and 3-step models, (with or without disease burden) including peak serum concentrations of cytokines (sgp30, INF-γ, IL-1Rα, IL-10, MIP-1α) measured within the first three days after CAR T-cell infusion. Each of these models are detailed in Table 3. Other biomarkers, potentially targetable, such as soluble IL-2Rα and IL-6R were also associated with CRS severity. The authors reported near-perfect discrimination (area under the Receiver Operator Curve, 0.93-0.98) to predict severe CRS with these models. While promising, these findings should be interpreted with caution due the low number of patients (n=48) and events (n=14), suggesting overfitting. In addition, the validation cohort only included 12 patients. Further studies are needed to validate these models in larger and independent cohorts.
Overall, the predictive value of the biomarkers of CRS in ALL patients treated with CD19 CAR T cells should be properly estimated and validated in larger studies. Interestingly, two studies did not confirm the association between the pretreatment BM tumor burden and CRS severity, potentially due to low statistical power. Low statistical power can also be a consequence of inappropriate modeling, for instance by dichotomizing CRS severity (e.g., not severe versus severe)25,26. In addition to the gain in statistical power, modeling CRS grade as an ordinal variable has many advantages: i) probabilities for any given CRS grade can be computed; ii) it respects the clinical relevance of each category on the scale; ii) odds ratios from the model can easily be compared across studies, which is not possible when investigators use different cutpoints across studies (e.g., grade ≥3 versus grade ≥4).
High incidence of severe ICANS after CD19 CAR T-cell therapy for ALL
The exact mechanisms responsible for severe ICANS after CD19 CAR T-cell therapy are not well understood. Increased blood-brain barrier (BBB) permeability may lead to high concentrations of pro-inflammatory cytokines (e.g., IL-6, IFN-γ and TNF-α) in the CSF. Autopsy tissues from the brains of two ALL patients treated with JCAR014 on a phase I/II clinical trial at our institution who developped fatal cerebral edema showed multifocal microhemorrhages, parenchymal necrosis, endothelial activation, endothelial damage, and CAR T-cell infiltration of the brain27. Gilbert et al, also performed neuropathologic examination of autopsy tissue from patients treated on the ROCKET trial and who developed fatal cerebral edema after CD19 CAR T cell therapy. They also observed endothelial damage and microglial activation; in contrast with our findings, there was complete absence of CAR T cells and immune cells28.
ICANS spans a variety of neurologic symptoms such as headache, tremor, speech impairment (e.g., expressive aphasia), delirium, confusion, impaired consciousness (stupor, lethargy, obtundation), and less commonly focal deficits. In our experience, delirum with preserved alertness was the most common presentation of ICANS, accounting for 66% of ICANS cases. In its most severe forms, ICANS can be associated with life-threatening features such as seizures and cerebral edema. While CRS classically precedes ICANS, temporal overlaps are common. Importantly, it is rare to observe severe ICANS in the absence of severe CRS27. In patients who developed severe ICANS, we found evidence of profound vascular dysfunction with capillary leak syndrome (weight gain, hypotension, hypoalbuminemia) in addition to consumptive coagulopathy.
In a phase II clinical trial of CD28-costimulated, defined-composition CD19 CAR T cells in adult patients with high tumor burden ALL (>5% blasts in the bone marrow; ROCKET), investigators observed significant neurologic toxicity, which led to the early termination of the study. Of 32 patients, they observed severe grade ≥3 ICANS in 18 patients (56%), and fatal cerebral edema in 5 patients (16%). Of note, in the MSKCC 09-114 phase 1 cohort (53 patients) that used a similar CAR construct but with a distinct in-house manufacturing, the rates of severe CRS and ICANS were 26% and 42%, respectively29. Cerebral edema has been reported in other clinical trials of CAR T cell therapy for ALL and NHL. In an updated analysis of the ZUMA 3 phase I data (45 adult ALL patients), ≥ grade 3 ICANS was observed in 38% of the patients across all dose levels (0.5-2x106/kg). Fatal cerebral edema occurred in one of 16 patients (6%) who received 0.5x106 CAR T cells/kg. As mentioned earlier in this manuscript, this led to the revision of the management of toxicities10. In our experience using JCAR014 on a phase I/II clinical trial, fatal cerebral edema occurred in 1 ALL patient27. Overall, the observed rates of ICANS vary greatly across clinical trials of CD19 CAR T cells for ALL (29-72%); Severe ≥ grade 3 ICANS may be more frequent in adult patients compared to pediatric patients (6-56% versus 0-28%, respectively, Figure 1B).
Factors associated with severe ICANS (Table 3)
In a cohort of 133 patients treated at our center with JCAR014 on a phase I/II clinical trial, we identified the following variables as independent predictors of ICANS severity using ordinal regression: bone marrow disease burden, cyclophosphamide and fludarabine lymphodepletion, and the presence of any pre-existing neurologic comorbidity27. CD8+ CAR T-cell dose and peak expansion were also strongly associated with ICANS severity. Retrospective analysis of the ROCKET trial data suggested cyclophosphamide and fludarabine lymphodepletion was also associated with severe ICANS. A pooled analysis of the FHCRC JCAR014 phase I and the ROCKET data (all disease types) found an association between serum concentrations of IL-15 – a key cytokine involved in T cell homeostasis – and the development of severe ICANS. Using univariate logistic regression, the authors screened approximately 140 CAR T-cell product and manufacturing-related variables. They found associations between the percentage of TNF-α-producing CD8+CAR T cells, TNF-α-producing CD4+CAR T cells, IL-2-producing CD8+CAR T cells and the risk of cerebral edema. This suggests severe ICANS may be associated with more functional T cells, in terms of cytokine production, after ex vivo stimulation through the CAR with a CD19-presenting cell line. Importantly, these analyses were unspecified and not corrected for multiplicity – hence with a high risk of false positives.
CRS and ICANS beyond the CD19 target
Among the many potential candidate targets, CAR T cell-based approaches targeting the CD22 antigen are being extensively evaluated in both the CD19 CAR-naïve and CD19 CAR-exposed setting. The goals are manifold: to improve response durability, to reduce the risk or rescue antigen-negative relapses. Investigators from Beijin Boren Hospital reported the use of CD22-targeted CAR T cells in 34 B-ALL pediatric and adult ALL patients with persistent disease after CD19 CAR T-cell therapy30. MRD-negative CR was achieved in 76% of patients. CRS was very common (any grade CRS, 91%) and mild in most cases; they did observe one case of grade 5 CRS. They also reported one case of grade 2 seizures, and grade 1 ICANS in 15% of patients. In a recent publication from the NIH/NCI, Shah et al analyzed data from 58 patients treated with CD22-targeted CAR T cells. CRS occurred in 50 (86%) and CRS was ≥ grade 3 in 5 (10%) patients. Symptoms of ICANS were minimal and transient in most patients (grade 3 only in one patient). In contrast with CD19 CAR T cell therapy, HLH-like manifestations were frequently seen in 19 of 58 (33%) subjects, prompting utilization of anakinra31. The same investigators recently identified the following factors to be associated with HLH after CD22 CAR T cell therapy using multivariable logistic regression: lower baseline blood and marrow percentage of NK cells and higher percentage of CD3+ cells, pre-manufacturing CD4+/CD8+ T cell selection, and higher IL-18 serum concentrations32. Last, Wang et al assessed the sequential administration of CD19 and CD22-targeted CAR T cells in a multicenter trial in patients with refractory B cell malignancies33. Eleven of 51 ALL patients (22%) developed grade ≥3 CRS, and 7 patients (14%) developed grade 1-2 ICANS (grade 4 ICANS, n=1).
CRS and ICANS prevention and treatment (Table 4)
Table 4.
Summary of current and investigational strategies for CRS and ICANS management after CAR T-cell therapy for ALL
| Reference | Study design | CRS | ICANS | |
|---|---|---|---|---|
| Current management according to the Kymriah REMS | ||||
| Prophylaxis | Maude SL et al. NEJM 2018 (ELIANA) | Phase 2 | Not recommended | Not recommended |
| Mild symptoms | Supportive care only | Supportive care only | ||
| Moderate to severe symptoms | Tocilizumab Corticosteroids in the absence of clinical improvement after the first dose of tocilizumab |
Corticosteroids1 | ||
| Investigational strategies | ||||
| Prophylaxis | ||||
| Tumor burden-based CAR T-cell dose reduction | Turtle CJ et al, JCI 2016 | Retrospective analysis of Phase I/II data after ad hoc amendments | Lower rates of ICU admission in high tumor burden patients | Lower rates of severe ICANS in high tumor burden patients |
| Split-dosing (day 1, 10%; day 2, 30%; day 3, 60%) | Frey NV et al JCO 2020 | Lower rates of severe CRS | Not specifically reported per cohort | |
| Early intervention | ||||
| Tocilizumab for mild2 CRS | Gardner RA et al. Blood 2019 | Retrospective analysis of Phase I/II data after ad hoc amendments | Comparable rates of any grade
CRS Lower rates of severe CRS |
Comparable rates of severe ICANS |
| Tocilizumab for fever if high tumor burden (≥40% marrow blasts) | Kadauke S et al. Cytotherapy 2019 | Prospective, non-randomized, two cohort (high and low tumor burden) | Low rates of severe CRS Met primary endpoint (grade ≥ 4 CRS <30%) |
Not reported |
| Steroid for grade ≥2 ICANS | Shah BD et al ASCO 2019 (ZUMA-3) | Retrospective analysis of
Phase I/II data after ad hoc amendments |
Comparable rates of CRS (any grade and severe) | Lower rates of severe ICANS Shorter duration of ICANS |
| HLH-type manifestations 3 | ||||
| Anakinra (5-8mg/kg/day subcutaneously) +/− corticosteroids | Shah NN, JCO 2020 | Retrospective analysis of
Phase I/II data after ad hoc amendments |
Resolution of HLH-like manifestations in all patients | Not specifically reported Overall mild ICANS severity |
| Refractory ICANS or HLH | ||||
| Anakinra (1mg/kg/day subcutaneously) +/− corticosteroids | Strati P et al, Blood Advances, 2020 | Retrospective analysis of case series (n=8) after axicatagene ciloleucel treatment for LBCL | Not specifically reported All patients had presented with grade ≥3 CRS prior to severe ICANS or HLH |
Response in 4 of 8 patients Recurrence of ICANS in 1 or 4 responders |
Per the Kymriah Risk Evaluation and Mitigation Strategy (REMS) only supportive care is recommended for ICANS but most institutions would recommend corticosteroids.
Mild CRS symptoms were defined as follows: persistent fever for 10 hours unresponsive to acetaminophen, recurrent hypotension unresponsive to fluid bolus, hypoxia requiring oxygen supplementation
Defined by peak ferritin >100,000μg/L and at least two of the following criteria: hepatic aminotransferases or bilirubin grade ≥3; creatinine increase grade ≥3; pulmonary edema grade ≥3; evidence of hemophagocytosis on bone marrow aspirate/biopsy. Abbreviations: ASCO, American Society of Clinical Oncology; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; HLH, hemophagocytic lymphohistocytosis; ICANS, immune effector cell-associated neurotoxicity syndrome; JCI, Journal of Clinical Investigation; JCO, Journal of Clinical Oncology; LBCL, large B cell lymphoma; NEJM, New England Journal of Medicine.
CAR T-cell dose modifications
Tumor burden-based CAR T-cell dose reduction
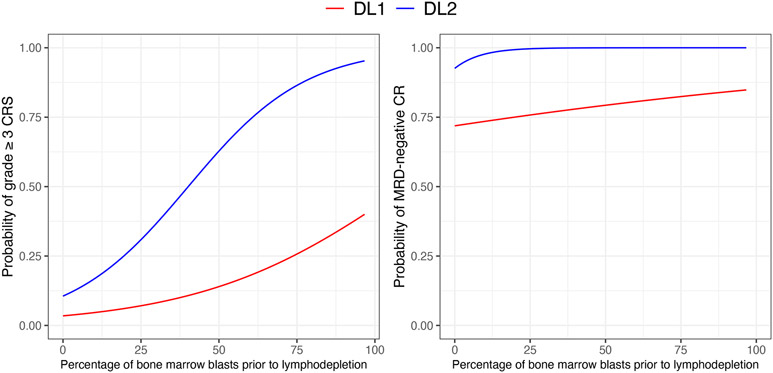
An in-depth understanding of the CAR T-cell dose-outcome relationship may be critical to prevent the occurrence of severe toxicities. We have demonstrated that the CAR T-cell dose and the bone marrow (BM) tumor burden (e.g., percentage of abnormal B cells in the bone marrow) prior to lymphodepletion were strongly associated with CRS severity after CD19 CAR T-cell therapy18. The relationship between CAR T-cell dose, BM tumor burden and outcomes can be inspected easily by plotting probabilities from logistic or ordinal regression models (Figure 2). We hypothesized a lower CAR T-cell dose might: i) reduce the risk of severe CRS at the same level of BM tumor burden; ii) “flatten” the relationship between the BM tumor burden and CRS severity. This can be easily modeled by adding an interaction term between CAR T-cell dose and BM tumor burden, allowing the slope of the relationship to vary across dose levels. At both dose levels, a higher BM tumor burden was associated with an increase in the risk of developping severe CRS. For very low BM tumor burden (< 5-10%), the risk of severe CRS was comparable across dose levels (≤10%). At the higher CAR T-cell dose (2x106/kg), the relationship between BM tumor burden and grade ≥3 CRS became very steep (e.g., 25% abnormal B cells in the BM was associated with a 30% risk of grade ≥3 CRS compared to 7% at the lower dose; 50% abnormal B cells in the BM was associated with a 60% risk of grade ≥3 CRS compared to 15% at the lower dose). The higher CAR T-cell dose was only associated with a modest increase in efficacy (90-100%) compared to the lower dose (70-80%). Based on these observations, we implemented a CAR T-cell dose reduction algorithm based on the percentage of abnormal B cells in the BM prior to lymphodepletion (>20%: 2x105 CAR T cells/kg; ≤20%: 2x106 CAR T cells/kg)3. Identifying an “optimal” cutpoint is challenging. If a threshold needs to be set, we would recommend to threshold the prediction itself (provided predictions are perfectly calibrated), based on the following: i) subject matter knowledge (e.g., “expert” opinion of what constitutes an “acceptable” risk in light of the predicted efficacy); ii) resource utilization (e.g., how many ICU beds are available?); iii) patient’s preference after being informed of the risks associated with each dose. While risk-adapted CAR T-cell dose adjustment might be beneficial in ALL, the CAR T-cell dose-outcome relationship may differ markedly across distinct B cell malignancies. We have shown that decreasing the CAR T-cell dose in NHL patients could be associated with a significant decrease in efficacy18. Approaches beyond CAR T-cell dose reduction are likely needed to prevent toxicity while preserving efficacy in patients other B cell malignancies. We would like to advocate for phase I/II designs allowing dose determination in larger groups of patients compared to conventional 3+3 designs34.
Figure 2. Prediction of dose-outcomes relationships after CD19 CAR T cell therapy (adapted from Hay et al, Blood 2017).
Left panel, probabilities of developing grade ≥3 CRS from a proportional odds model adjusting for Cy-Flu lymphodepletion and including two interaction terms (CAR T cell dose level [DL] and the pre-lymphodepletion bone marrow blast percentage; Cy-Flu lymphodepletion and the pre-lymphodepletion bone marrow blast percentage). Right panel, probabilities of achieving an MRD-negative CR from a logistic regression model.
Abbreviations: CAR, chimeric antigen receptor, CR, complete remission; DL, dose level, CRS, cytokine release syndrome, ICANS, immune effector cell-associated neurotoxicity syndrome; MRD, minimal residual disease.
CAR T-cell dose fractionation (“split dosing”)
Frey al recently reported the outcomes of 35 adult ALL patients treated with CD19 CAR T cell therapy (CTL019)11. Patients were treated in one of three cohorts regardless of their tumor burden: high-dose single-infusion (HDS; 5x108 CAR T cells; n=6), low-dose single or fractionated infusion (LD; 5x107 CAR T cells; n = 9), HD fractionated infusion (HDF; 5x108 CAR T cells; n=20). The CTL019 product was fractionated over 3 days (day 1, 10%; day 2, 30%; day 3, 60%), allowing the day 2 and day 3 doses to be held if the patient experienced any grade CRS, including isolated fever. In favor of CAR T cell dose fractionation, the investigators reported lower rates of severe grade ≥4 CRS in the HDF cohort (n=1; 5%) compared to the HDS (n=3; 50%) and the LD cohorts (n=2; 22%). This more favorable toxicity profile in the HDF cohort was associated with high anti-tumor efficacy (MRD-negative CR, n=18 [ 90%]) and prolonged overall survival (73% at 2 years). In the HDF cohort, most patients only received the first dose of CAR T cells (45%); 4 patients received 2 doses (10%), and 7 patients (35%) received all 3 doses. The very favorable response rates and long-term outcomes make CAR T-cell dose fractionation a very promising approach, with the benefit of performing “individualized titration” of CAR T cells. However, the authors did not specifically study the effects of dose fractionation on CAR T-cell in vivo expansion and more specifically on long-term persistence. Increased immunogenicity has also be observed after repeat infusion of T cells engineered with murine scFv-bearing CARs35.
Concurent administration of pathway inhibitors
Ibrutinib
We recently reported the feasibility and efficacy of the concurrent administration of ibrutinib with JCAR014 in 19 patients with refractory chronic lymphocytic leukemia36. Beyond the anti-tumor effects of ibrutinib, our goal was to harvest its immunomodulatory effects on T cells. Indeed, prior studies suggested ibrutinib was associated with improved T cell numbers and function in CLL patients37. In addition, ibrutinib was associated with better CAR T-cell proliferation and antitumor efficacy in mice treated with human CD19 CAR T cells38 and appeared to attenuate cytokine release syndrome (CRS)39. Patients enrolled on our pilot study were scheduled to receive ibrutinib for at least 2 weeks prior to leukapheresis and for at least 3 months after CAR T-cell infusion. This approach was well tolerated and 13 patients (68%) received ibrutinib as planned without dose reduction. While CD19 CAR T cell therapy with concurrent ibrutinib led to high response rates (83% by iwCLL 2018 criteria) and robust CAR T cell in vivo expansion, we observed lower CRS severity and lower serum concentration of CRS-associated cytokines compared to a cohort of patients treated with CD19 CAR T cells without ibrutinib. Our findings suggest ibrutinib might alter CAR T cell function and the cytokine milieu, reducing CRS severity while maintaining potent anti-tumor effects.
Prophylactic tocilizumab (IL-6 receptor-directed antibody)
To our knowledge, prophylactic tocilizumab has not been formally evaluated in ALL patients. Locke et al. reported at the ASH 2017 annual meeting comparisons between the Primary Analysis Cohort (n=101) and the Cohort 3 (Safety Management Cohort, n=34) of the ZUMA-1 clinical trial in patients with refractory large B cell lymphoma40. Patients on Cohort 3 received prophylactic tocilizumab on day +2 after CAR T cell infusion. Grade ≥3 CRS was reported in 1 patient (3%) in the SMS Cohort 3 compared to 13 (13%) in the Primary Analysis Cohort. Grade ≥3 ICANS was reported in 14 (48%) and 28 (28%) patients in the SMS Cohort 3 and the Primary Analysis cohort, respectively, suggesting tocilizumab may not prevent ICANS. This may be explained by its poor penetration into the CNS. Using a Rhesus macaque model, Nella et al measured concentrations of tocilizumab approximately 1,000 times lower in the CSF compared to the serum after IV administration of the antibody41. Whether tocilizumab can worsen ICANS severity remains to be demonstrated in a larger cohort.
Prophylactic anakinra (IL-1 receptor antagonist)
As preclinical models of CRS and ICANS are being developped, several groups have suggested an important role of the IL-1/IL-1R axis in the pathogenesis of these toxicities. Using immunodeficient beige mice treated with human CD19 CAR T cells, Giavridis et al managed to induce CRS after intraperitoneal injection of Raji cells, but only in mice who developped high tumor burden. Importantly, the authors failed to induce CRS under the same conditions using NSG mice, which are known to harbor development and maturation defects of monocytes and macrophages. In the beige mice, human CD19 CAR T cells activated peritoneal macrophages, which were the main source of IL-6. Treatment with a blocking antibody against the murine IL-6R in this model prevented CRS-related mortality. Furthermore, peritoneal macrophages produced high levels of iNOS, and CRS severity could be diminished using iNOS inhibitors. Since iNOS production was both IL-6 and IL-1-dependent, the authors administered anakinra intraperitoneally to antagonize the IL-1R, which successfully prevented CRS while preserving anti-tumor efficacy. Despite the limitations of a non-humanized mouse model (e.g., peritoneal disease, murine myeloid lineage, anakinra administered intraperitoneally instead of subcutaneously in humans), the data suggest anakinra may be efficacious at preventing or treating CRS.21 In the same issue of Nature, Norelli et al described a humanized mouse model more closely recapitulating human biology, by providing bystander human hematopoiesis and generating xenotolerant CAR T cells.20 To achieve this, the authors transplanted human cord blood hematopoietic stem and progenitor cells into sublethally irradiated triple transgenic NSG (SGM3) mice expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-3. CAR T cells were then engineered from human T cells that matured in the newborn humanized SGM3 mice (nHuSGM3). Infusion of CD19 or CD44v6-targeted nHuSGM3 CAR T cells did not cause CRS in non-humanized SGM3 mice. In contrast, when infused into humanized mice CAR T cells caused a significant systemic inflammatory syndrome resembling CRS, characterized by severe weight loss, increased systemic human IL-6, TNF-α, IL-10 levels and high fever. In this model, CRS occurred in the absence of tumor, due to the presence of normal human CD19+ B cells and CD44v6+ myeloid cells, but was worsened in the presence of tumor. Human monocytes were the predominant source of IL-1 and IL-6 during CRS. They observed distinct cytokine kinetics, IL-1 production preceding IL-6 production by 24 hours. Although both IL-1R and IL-6R blockage prevented CRS without impacting CAR T-cell expansion or antitumor effects, only anakinra prevented the development of fatal ICANS. These findings, if confirmed in humans, may challenge the current management of CRS and ICANS after CAR T cell therapy. Anakinra is a particularly promising agent to prevent both CRS and ICANS while potentially maintaining antitumor effects. Several clinical trials are currently evaluating the use of anakinra to prevent CRS and ICANS in the prophylactic (NCT04359784) and therapeutic settings (NCT04148430).
Early intervention for CRS or ICANS
The best timing of intervention for CRS and ICANS in ALL patients is unknown, although preliminary data suggested earlier interventions may be beneficial. An important concern for the field is the impact of high dose corticosteroids on CAR T cell function, expansion, and long-term persistence. Gardner et al from Seattle Children’s evaluated the use of an early-intervention (EI) strategy with tocilizumab and dexamethasone in 20 pediatric ALL patients who developped CRS after CD19 CAR T cell therapy (SCRI-CAR19v1)42. CRS management in the EI cohort consisted of the administration of tocilizumab for persistent mild CRS symptoms and prior to the development of dose-limiting or life-threatening toxicities. Mild CRS symptoms were defined as follows: persistent fever for 10 hours unresponsive to acetaminophen, recurrent hypotension unresponsive to fluid bolus, hypoxia requiring oxygen supplementation. Dexamethasone was administered in the absence of response to tocilizumab, or if need for vasopressors or mechanical ventilation. Subjects on the EI cohort were retrospectively compared to 23 patients treated previously on the same trial (DLT cohort). Patients on the DLT cohort received tocilizumab and/or corticosteroids only if they developed dose-limiting and/or life-threating toxicities associated with CRS or ICANS. Severe CRS was defined as the use of vasopressors at any dose, intubation for respiratory failure, or the use of inotropes. The investigators reported comparable rates of any grade CRS in the two cohorts (EI cohort, 95%; DLT cohort, 91%), respectively. The rate of severe CRS was lower in the EI cohort (15%; 95% CI, 3-38) compared to the DLT cohort (30%; 95% CI, 13-53). As expected since tocilizumab has poor CNS penetration, similar rates of severe neurotoxicity were observed in the DLT and EI cohorts (DLT cohort, 22%; EI, 25%). The authors measured comparable CAR T cell in vivo expansion and persistence, and similar duration of B cell aplasia. Response rates in both the EI and DLT cohorts were also comparable.
Kadauke et al reported at the 2019 ISCT annual meeting the results of a two-cohort pilot study (NCT02906371) investigating the efficacy of the early administration of tocilizumab to prevent severe CRS in children or young adults with ALL treated with CTL01943. The primary endpoint was the frequency of grade ≥4 CRS using the Penn scale44. Tumor burden was assessed after lymphodepletion, 1 to 5 days before CTL019 infusion. High and low tumor burden (HTB and LTB, respectively) were defined as a percentage of abnormal B cells in the BM ≥40% and <40%, respectively. LTB patients received standard CRS management. HTB patients received a single dose of tocilizumab (8-12 mg/kg) after developing a fever to >38.5 C measured twice in 24 hours and were subsequently treated using standard CRS management. The incidence of any grade CRS was 72% (HTB cohort, 100%; LTB cohort, 64%). The primary endpoint was just met, with a 27% incidence of grade 4 CRS in the HTB cohort (predefined as <30%); this was also numerically lower compared to prior studies of CTL019 therapy in pediatric ALL patients (42% in the CHP959 trial and 47% in ELIANA6). The CR/CRi rate at day 28 was 97% (HTB cohort, 87%; LTB cohort, 100%), suggesting the early administration of tocilizumab in HTB ALL patients did not impact the efficacy of CTL019. The investigators reported a re-analysis of their dataset at the 2020 ASTCT annual meeting (NCT01626495)45. Patients in the early intervention cohort (EI) were retrospectively compared to another cohort of HTB ALL patients treated previously at their institution and who received standard CRS management (SM). All pts developed grade ≥ 2 CRS; median time to fever was longer in the EI cohort [EI, 3 days; SM, 2 days]. Grade 4 CRS was observed in 4 of 15 patients (27%) in the EI cohort compared to 13 of 26 (50%) in the SM cohort (RR, 0.53; 95% CI, 0.21-1.34). They observed modest reductions in the rates of ICU admission, duration of mechanical ventilation, use of vasopressors, and duration of vasopressor treatment. The incidence and severity of ICANS events were not reported.
As mentioned earlier in this manuscript, investigators of the ZUMA-3 clinical trial (adult ALL) also evaluated a modified toxicity management algorithm after observing high rates of grade ≥3 ICANS10. Only 1 of 9 patients in the revised toxicity management cohort (11%) developed grade 3 ICANS compared to 8 of 14 (57%) in patients who received the original toxicity management. ICANS duration was also shorter with the revised toxicity management (median, 11 days; range, 3-19) compared to the original toxicity management (median 20.5 days; range, 3-72).
Taken together, these three studies suggest early interventions may ameliorate the toxicity profile of CD19 CAR T cell therapy for ALL. Additional studies should clarify – and perhaps, simplify –current treatment algorithms, and further investigate the prophylactic setting in patients with high marrow tumor burden.
Tocilizumab/corticosteroids-refractory or recurrent CRS and ICANS
There are to date very limited data to guide our management of patients with CRS or ICANS when tocilizumab and corticosteroids fail, or when symptoms recur after treatment cessation. When life-threatening toxicities occur, a “kitchen sink” approach is often embraced, and many anecdotal approaches have been reported: high-dose methylprednisolone18, ventriculostomy46, antithymocyte globulin46, siltuximab46, cyclophosphamide47, intrathecal administration4,46,48,49 (e.g., cytarabine, methotrexate, dexamethasone). Recent data by Shah et al showed anakinra can prevent the occurrence of severe CRS with hemophagocytic lymphohistiocytosis features in ALL patients treated with CD22-targeted CAR T cells31. A recent case series from the MD Anderson Cancer Center suggested responses can be seen in NHL patients developing refractory ICANS or HLH using low doses of anakinra50 after treatment with axicabtagene ciloleucel. The authors reported clinical improvement in 4 of 8 patients, and recurrence of ICANS in 1 of the 4 responding patients. These two publications signal anakinra as a key contender to improve CRS and ICANS management after CAR T cell therapy.
While severe CRS and ICANS can in some rare cases lead to organ damage and death, another major issue is the risk of infections due to the profound immunosuppression induced by cytokine-directed therapies and high-dose corticosteroids. Hill et al. from our institution reported a multivariable analysis showing that CRS severity after CD19 CAR T cell therapy was the only factor independently associated with an increased risk of infections51. In our recent experience, CAR T-cell-related mortality has not been related directly to CRS and ICANS, but primarily to sepsis in the context of prolonged glucocorticosteroids exposure. This highlights a critical need for steroid-sparing strategies to reduce the risk of infections during CRS and ICANS. 31
Conclusions
CD19 CAR T cells are showing great promise for patients with refractory ALL, yet broad dissemination will necessitate improvement in the toxicity profile of these new therapies. We have reviewed a range of strategies under investigation to prevent and treat severe CRS and ICANS; there is mounting evidence that earlier, and potentially more targeted, interventions can reduce these toxicities. While informative, prudence is warranted when comparing point-estimates across single-arm clinical trials, given the high risk of confounding and selection bias. In addition, CRS and ICANS management has significantly changed over the past 5 years, with a natural trend towards intervening earlier and for lower CRS grades. Efforts at harmonizing toxicity grading system24,52 and thresholds for intervention are underway. They will be particularly helpful to design and interpret data from future clinical trials of CAR T-cell therapy.
Future efforts should also aim at developping better prognostic models incorporating key biomarkers such as infusion product characteristics, CAR T cell counts and cytokine serum concentrations. Furthermore, the development of experimental models of CRS and ICANS (e.g., humanized mice, non-human primates) will identify targetable pathways and guide future clinical trials.
Although advances in gene editing and cell engineering technologies will help develop CAR T cells that may be intrinsically safer, the bench-to-bedside development of CAR T cell therapies remains a costly and lengthy process. Until then, our field have to “make do and mend” with the products and the data at hand. We are hopeful recent and future progress in CRS and ICANS management will facilitate the approval of CAR T cell products for adult ALL (e.g., KTE-X19).
Footnotes
No conflicts of interest to declare
References
- 1.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. The New England Journal of Medicine 2014; 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England) 2015; 385: 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. Journal of Clinical Investigation 2016; 126: 2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan J, Yang J, Deng B, Zhao X, Zhang X, Lin Y et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 2017; 31: 2587. [DOI] [PubMed] [Google Scholar]
- 5.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K et al. Intent to treat leukemia remission by CD19CAR T cells of defined formulation and dose in children and young adults. Blood 2017; 129: blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. The New England Journal of Medicine 2018; 378: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. The New England Journal of Medicine 2018; 378: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran KJ, Margossian S, Kernan NA, Silverman LB, Williams DA, Shukla N et al. Toxicity and Response following CD19-specific CAR T cells in pediatric/young adult relapsed/refractory B-ALL. Blood 2019. doi: 10.1182/blood.2019001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood 2019; 133: 1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah BD, Bishop M, Oluwole OO, Logan A, Baer MR, Donnellan W et al. End of phase I results of ZUMA-3, a phase 1/2 study of KTE-X19, anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in adult patients (pts) with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL). AsCo Abstract 2019; 37: 7006–7006. [Google Scholar]
- 11.Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J Clin Oncol 2020; 38: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauthier J, Yakoub-Agha I. Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: Clinical data to date, current limitations and perspectives. Current Research in Translational Medicine 2017; 65: 93–102. [DOI] [PubMed] [Google Scholar]
- 13.Jacoby E, Bielorai B, Avigdor A, Itzhaki O, Hutt D, Nussboim V et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am J Hematol 2018; 93: 1485–1492. [DOI] [PubMed] [Google Scholar]
- 14.Danylesko I, Chowers G, Shouval R, Besser MJ, Jacoby E, Shimoni A et al. Treatment with anti CD19 chimeric antigen receptor T cells after antibody-based immunotherapy in adults with acute lymphoblastic leukemia. Curr Res Transl Medicine 2019; 68: 17–22. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Current Research in Translational Medicine 2018. doi: 10.1016/j.retram.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCR$zeta/CD28 receptor. Nature biotechnology 2002; 20: 70–75. [DOI] [PubMed] [Google Scholar]
- 17.Sommermeyer D, Hudecek M, Kosasih P, Gogishvili T, Maloney D, Turtle C et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 2015; 30: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay KA, Hanafi L-A, Li D, Gust J, Liles CW, Wurfel MM et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy. Blood 2017; 130: 2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer discovery 2016; 6: 664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nature Medicine 2018; 24: 739–748. [DOI] [PubMed] [Google Scholar]
- 21.Giavridis T, van der Stegen SJ, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nature Medicine 2018; 24: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. The Lancet Oncology 2018. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandler R, Tattersall R, Schoemans H, Greco R, Badoglio M, Labopin M et al. Diagnosis and Management of Secondary HLH/MAS Following HSCT and CAR-T Cell Therapy in Adults; A Review of the Literature and a Survey of Practice Within EBMT Centres on Behalf of the Autoimmune Diseases Working Party (ADWP) and Transplant Complications Working Party (TCWP). Front Immunol 2020; 11: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell FE, Lee KL, Mark DB. Multivariable Prognostic Models: Issues In Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med 1996; 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Shepherd BE, Li C, Harrell FE. Modeling continuous response variables using ordinal regression. Statistics in Medicine 2017; 36: 4316–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gust J, Hay KA, Hanafi L-A, Li D, Myerson D, Gonzalez-Cuyar LF et al. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discovery 2017; 7: 1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert MJ. Severe neurotoxicity in the phase 2 trial of JCAR015 in adult B-ALL (ROCKET study): analysis of patient, protocol and product attributes. Proceedings from the Society for Immunotherapy of Cancer 2017; : 8–12. [Google Scholar]
- 29.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Science Translational Medicine 2014; 6: 224ra25–224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia 2019; : 1–13. [DOI] [PubMed] [Google Scholar]
- 31.Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 Car T-Cell Trial. J Clin Oncol 2020; : JCO.19.03279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtenstein DA, Steinberg SM, Highfill SL, Yates B, Jin P, Jin J et al. Abstract 4231: Factors predictive of CAR T cell associated hemophagocytic lymphohistiocytosis (HLH). 2020; : 4231–4231. [Google Scholar]
- 33.Wang N, Hu X, Cao W, Li C, Xiao Y, Cao Y et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood 2020; 135: 17–27. [DOI] [PubMed] [Google Scholar]
- 34.Gauthier J, Yuan Y, Thall P. Bayesian Phase 1/2 trial designs and cellular immunotherapies: a practical primer. Cell & Gene Therapy Insights 2019.https://insights.bio/cell-and-gene-therapy-insights/journal/articles/bayesian-phase-1-2-trial-designs-and-cellular-immunotherapies-a-practical-primer/. [Google Scholar]
- 35.Gauthier J, Bezerra E, Hirayama AV, Pender BS, Vakil A, Steinmetz RN et al. Repeat Infusions of CD19 CAR-T Cells: Factors Associated with Response, CAR-T Cell In Vivo Expansion, and Progression-Free Survival. Biol Blood Marrow Tr 2020; 26: S267–S268. [Google Scholar]
- 36.Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH et al. Feasibility and efficacy of CD19-targeted CAR-T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood 2020. doi: 10.1182/blood.2019002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM et al. Ibrutinib treatment improves T cell number and function in CLL patients. Journal of Clinical Investigation 2017; 127: 3052–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 2016; 127: 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruella M, Kenderian S, Shestova O, Klichinsky M, Melenhorst J, Wasik M et al. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms. Leukemia 2017; 31: 246. [DOI] [PubMed] [Google Scholar]
- 40.Locke FL, Neelapu SS, Bartlett NL, Lekakis LJ, Jacobson CA, Braunschweig I et al. Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (axicel; KTE-C19) Treatment for Patients with Refractory,Aggressive Non-Hodgkin Lymphoma (NHL). ASH Abstract 2017; 130: 1547–1547. [Google Scholar]
- 41.Nellan A, McCully CM, Garcia R, Jayaprakash N, Widemann BC, Lee DW et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood 2018; 132: 662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner RA, Ceppi F, Rivers J, Annesley C, Summers C, Taraseviciute A et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood 2019; 134: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadauke S, Maude S, Gladney W, Motley L, Shenoy V, Callahan C et al. Early administration of tocilizumab (Toci) for the prevention of grade 4 cytokine release syndrome (CRS) after CD19-directed CAR T-cell therapy (CTL019). Cytotherapy 2019; 21: e2–e3. [Google Scholar]
- 44.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. Journal of hematology & oncology 2018; 11: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers RM, Kadauke S, Li Y, Callahan CA, Gladney W, Fitzgerald JC et al. Risk-Adapted Preemptive Tocilizumab Decreases Severe Cytokine Release Syndrome (CRS) after CTL019 CD19-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for Pediatric B-Cell Acute Lymphoblastic Leukemia (B-ALL). ASTCT Abstract 2020; 26: S39. [Google Scholar]
- 46.Wang ML, Munoz Goy A, Locke FLL, Jacobson CA, Hill BT et al. 754 KTE-X19, an Anti-CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy, in Patients (Pts) With Relapsed/Refractory (R/R) Mantle Cell Lymphoma (MCL): Results of the Phase 2 ZUMA-2 Study. Blood (ASH Abstract) 2019. [Google Scholar]
- 47.Garfall AL, Lancaster E, Stadtmauer EA, Lacey SF, Dengel K, Ambrose DE et al. Posterior Reversible Encephalopathy Syndrome (PRES) after Infusion of Anti-Bcma CAR T Cells (CART-BCMA) for Multiple Myeloma: Successful Treatment with Cyclophosphamide. Blood 2016; 128: 5702–5702. [Google Scholar]
- 48.Shah NN, Johnson BD, Fenske TS, Raj RV, Hari P. Intrathecal chemotherapy for management of steroid-refractory CAR T-cell–associated neurotoxicity syndrome. Blood Adv 2020; 4: 2119–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yucebay F, Maakaron J, Grana A, Jaglowski S, Roddy J. Intrathecal Chemotherapy: An Alternative Treatment Strategy to Prolonged Corticosteroids for Severe CAR T Associated Neurotoxicity. ASTCT Abstract 2020; 26: S312. [Google Scholar]
- 50.Strati P, Ahmed S, Kebriaei P, Nastoupil LJ, Claussen CM, Watson G et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy–associated toxicity in large B-cell lymphoma. Blood Adv 2020; 4: 3123–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X et al. Infectious complications of CD19-targeted chimeric antigen receptor–modified T-cell immunotherapy. Blood 2018; 131: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biology of Blood and Marrow Transplantation 2019; 25: 625–638. [DOI] [PubMed] [Google Scholar]