Abstract
Context
Circulating microRNAs (miR) have revolutionized the field of molecular biology owing to their potential as a diagnostic as well as a prognostic biomarker of cardiovascular disease and dysfunctions. The present study aims to identify the circulating miR-126 and -122 as an independent risk predictors of coronary artery disease cases.
Methods and material
Blood samples were collected from coronary artery disease cases (n=100) and non-CAD cases (n=100). Serum RNA was isolated by Trizol method. MiR levels were measured by quantitative real-time polymerase chain reaction with the specific primer probe set.
Results
MiR-126 levels were significantly down-regulated in CAD cases compared to non-CAD cases (controls) (80.0% vs. 39.0%, χ2=14.95, p<0.001). The level of miR-122 was significantly up-regulated in CAD cases in comparison to its non-CAD variant (14.0% vs. 63.0%, χ2=21.23, p<0.001). Multivariate analysis found chest pain (OR=37.07, 95% CI=3.21-169.04, p=0.017) and miR-126 (OR=0.01, 95% CI=0.00-0.63, p=0.030) as independent risk predictors of CAD.
Conclusion
The results of our study show the potential of circulating miR-126 as a novel non-invasive biomarker in the risk prediction of CAD. Further unraveling of the role of miR-122 and miR-126 in the pathogenesis and progression of CAD will add to our understanding of the disease process leading to a new diagnostic approach.
Highlights
Mir-122 and -126 significantly differentiate non CAD cases from angiographically proven CAD cases
Chest pain and miR-126 might work as an independent risk predictor of coronary artery disease
Key words: circulating biomarker, coronary artery disease, chest pain, microRNA
INTRODUCTION
Globally, cardiac disease is the predominant cause of death in the past several years. Multitude factors are associated with the disease; the major risk factors are hypertension, high cholesterol levels, unhealthy diet, obesity, diabetes, and addiction in different forms like alcohol and tobacco chewing.[1] Atherosclerosis and thrombosis due to unhealthy diet, lack of physical exercise, and hypertension ultimately lead to the development of coronary artery disease (CAD).[2] Diagnosis of CAD is based on the invasive coronary angiogram (CAG) and radiologic techniques such as an echocardiogram (Echo), computed tomography (CT), electrocardiogram (ECG), and exercise treadmill test (ETT). Several blood-based markers have been studied in the diagnosis of CAD but without any significant clinical result.[3] Circulating microRNAs (miRNAs) have revolutionized the field of molecular biology owing to their potential as diagnostic as well as prognostic biomarkers of cardiovascular diseases and dysfunctions. MicroRNAs are short, single-stranded non-coding RNAs that regulate cellular functions by degradation and translational repression of mRNAs. They assist in cell proliferation, differentiation, metabolism, apoptosis, development, aging, and in the pathophysiology of many diseases namely, oncogenesis, cardiovascular, and neurological disorders. Several studies have reported dysregulation of miRNAs in heart diseases such as myocardial infarction (MI), cardiac hypertrophy, fibrosis, and developmental heart disease. [4-7] Studies reported the change in the number of miRNAs in such pathological processes and the abnormal expressions of miRNAs are associated with a different type of heart disease. [8-10]
In earlier studies reduced plasma concentration of miR-126 in patients with heart failure (HF) compared to healthy controls were found to have an inverse co-relationship with brain natriuretic peptide (BNP), thus proving as a classic marker of HF in past studies. Higher levels of miR-126 indicated the better clinical condition of patients. Xiao Sun et al in 2012 demonstrated the relationship between miR-126 and LDL cholesterol in patients with or without CAD, which may have significant implications for identifying the potential role of miR-126 in cholesterol metabolism. [11] Different miRNAs related to cardiac origin have been studied to investigate the diagnostic potential. Reduced expression of miR-122 has been found in patients with MI. Thus, the current study was designed to assess the levels of miR-126 and 122 in CAD cases for evaluating the risk predictors in coronary artery disease cases in India.
SUBJECTS AND METHODS
Study population
The study subjects were enrolled from January 2019 to December 2019. Coronary angiograms were evaluated by a clinician, who made a visual estimation of luminal narrowing in multiple segments based on the AHA/ACC classification of the coronary tree.[12] All the subjects fulfilling the inclusion criteria were enrolled for the study. All the cases were newly diagnosed. The blood sample was collected before the start of medication-related to the CAD and before the coronary stent implantation. Written informed consent was taken from the subjects. Approval to conduct the research was obtained from the Institutional Ethical Committee (approval number IEC95) before the start of the study and complies with the ethical principles for medical research involving human subjects, by the Declaration of Helsinki. Patients attending the OPD and who were not suffering from either detectable coronary stenosis or atherosclerotic vascular disease were considered as controls (CAD-ve). Patients were interviewed to collect information about their medical history and lifestyle habits. Risk factors were determined by a physician. Subjects were excluded from the study if they are affected by the hepatic failure, renal failure, abnormal liver function, hepatitis, cardiomyopathy, congenital heart disease, bleeding disorders, previous thoracic irradiation therapy, and malignant diseases.
Samples collection and serum isolation
Peripheral blood (3.5 ml) was collected from 200 cases in plain and EDTA vials (NOVAC, POLYMED, POLY MEDICURE LTD, India). Serum was separated by centrifugation of peripheral blood in plain vial at 1900g for 10 min, followed by a 10 min high-speed centrifugation at 16,000g and stored at -80°C until further processing.
Biochemical examination
Biochemical parameters including HbA1c (%) (D-10 Bio-Rad, USA), total cholesterol (TC), (mg/dl), triglyceride (TG), (mg/dl), high density lipoproteins (HDL-C) (mg/dl), low density lipoproteins (LDL-C) (mg/dl), very low density lipoproteins (VLDL-C) (mg/dl), folate II (nmol/L), Vitamin B12 (pg/ml), small dense low density lipoproteins (sdLDL) (mmol/l), and total homocysteine (HCY2) (umol/L) were recorded. All biochemical parameters were measured with a fully automated biochemical analyzer (ARCHITECT i2000SR, Abbott Diagnostic & Selectra ProXL, ELITech Group).
RNA isolation and cDNA synthesis
Total RNA was extracted using a Trizol-based miRNA isolation protocol (Invitrogen, Carlsbad, CA, USA) by the addition of 750μl of Trizol reagent to 250μl of plasma. The RNA concentrations were measured with a Nanodrop and cDNA was prepared using a commercially available MuLV reverse transcriptase kit (Cat. no. K1622, Thermo Fisher Scientific, USA). [13]
Quantitative real-time PCR
cDNA was amplified with specific primer sets miR-122 (hsa-miR-122-5p, Cat. no. 4427975), miR126 (hsa-miR-126-5p, Cat no. 4427975), and RNU6 (Cat no. 4427975). Quantitative realtime PCR (qRT PCR) was carried out using 7500 real-time PCR system (Applied Biosystems, USA) using TaqMan® Universal Master Mix II No UNG (Applied Biosystem, USA) according to the manufacturers’ instructions. Data were normalized for RNU6 (housekeeping gene) expression by the comparative threshold cycle method. Duplicate Ct values were averaged, the relative expression levels of miR were calculated using formula ΔCt = Ct[Target]-Ct[Housekeeping] and ΔΔCt = (ΔExp.)- (ΔControl) and got the -ΔΔCt log-fold-change and fold-changes were calculated for each miRNA. [13]
VEGF ELISA
Serum VEGF level (pg/ml) was determined by ELISA using RayBio Human VEGF ELISA kit and the reading were recorded by iMark™ microplate absorbance reader (BIOS) at 450 nm. The level of VEGF concentration (pg/ml) in cases was determined by comparing the OD of the samples with the standard curve.
Statistical analysis
Discrete (categorical) data are presented in number (n) and percentage (%). Categorical groups were compared by the chi-square (χ2) test. Independent predictor(s) of CAD were assessed using univariate (crude or unadjusted odds ratio) and multivariate (adjusted odds ratio) binary logistic regression (BLR) analysis. Receiver operating characteristics (ROC) curve analysis was done to assess diagnostic accuracy (sensitivity and specificity) of markers (miR-122 and miR-126) for CAD assessment. Based on the data, a cut-off point was chosen, where the higher sensitivity and specificity were obtained (Data not shown). All continuous data were categorized into two groups (non-CAD and CAD cases). A two-tailed (α=2) p<0.05 was considered statistically significant. Analyses were performed on SPSS software (windows version 17.0).
RESULTS
A total of 200 cases 100 CAD and 100 non-CAD patients of age between 20-80 yrs were enrolled. The outcome measures of the study were demographic and clinical characteristics (age, sex, height, weight, BMI, educational status, nature of work, diet, smoking, alcohol, exercise, hypertension, and chest pain), biochemical parameters (HbA1c, HDL, LDL, VLDL, TG, TC, sdLDL, folate II, Vitamin B12, Vitamin D, TSH, total HCY2), markers (miR-122, miR-126 fold expression (AACt) and VEGF (pg/ml).
Demographic and clinical characteristics of CAD and non-CAD cases
The demographic and clinical characteristics of the two groups (non-CAD cases and CAD cases) are summarized in table 1. The age of non-CAD and CAD cases ranged from 25-79 yrs and 28-76 yrs with a mean (± SE) of 50.90 ± 2.08 yrs and 52.15 ± 1.13 yrs, respectively, and a median age of 52 yrs. Further, in non-CAD cases, there were 26 (26.0%) females and 74 (74.0%) males whereas in CAD cases, this was 25 (25.0%) and 75 (75.0%), respectively. Comparing the demographic and clinical characteristics of the two groups, χ2 test showed significantly different and higher frequency (%) of illiterate (14.0% vs. 61.0%, χ2=19.14, p<0.001), smokers (16.0% vs. 37.0%, χ2=4.39, p=0.03), those suffering from hypertension (24.0% vs. 62.0%, χ2=10.55, p=0.001) and those from chest pain (17.0% vs. 71.0%, χ2=44.42, p<0.001) whereas, less frequency of exercise (53.0% vs. 30.0%, χ2=4.73, p=0.03) in CAD cases as compared to non-CAD suggests that these parameters may be associated with CAD. However, other demographic and clinical characteristics (age, sex, height, weight, BMI, occupation, nature of work, diet, and alcohol) were found to be similar (p>0.05) between the two groups indicating these parameters may not be associated with CAD development.
Table 1.
Demographic and clinical characteristics of two groups
| Variable | Controls (n=100) (%) |
Cases (n=100) (%) |
χ2 value |
p value |
|---|---|---|---|---|
| Age (yrs.): | ||||
| ≤52 | 56(56.0) | 52 (52.0) | 0.15 | 0.702 |
| >52 | 44 (44.0) | 48 (48.0) | ||
| Sex: | ||||
| Female | 26 (26.0) | 25 (25.0) | 0.01 | 0.913 |
| Male | 74 (74.0) | 75 (75.0) | ||
| Height (cm): | ||||
| ≤165 | 53 (53.0) | 69 (69.0) | 2.40 | 0.121 |
| >165 | 47 (47.0) | 31(31.0) | ||
| Weight (kg): | ||||
| ≤65 | 60 (60.0) | 56(56.0) | 0.11 | 0.735 |
| >65 | 40 (40.0) | 44 (44.0) | ||
| BMI (kg/m2): | ||||
| ≤26 | 67 (67.0) | 56(56.0) | 0.94 | 0.331 |
| >26 | 33 (33.0) | 44 (44.0) | ||
| Educational status: | ||||
| Literate | 86 (86.0) | 39 (39.0) | 19.14 | <0.001 |
| Illiterate | 14 (14.0) | 61 (61.0) | ||
| Occupation: | ||||
| No | 53 (53.0) | 34 (34.0) | 3.17 | 0.075 |
| Yes | 47 (47.0) | 66 (66.0) | ||
| Nature of work: | ||||
| Sedentary | 22 (22.0) | 27 (22.0) | 0.29 | 0.591 |
| Hard | 78 (78.0) | 73 (73.0) | ||
| Diet: | ||||
| Vegetarian | 43 (43.0) | 27 (27.0) | 0.43 | 0.513 |
| Non-vegetarian | 57 (57.0) | 73 (73.0) | ||
| Smoking: | ||||
| No | 84 (84.0) | 63 (63.0) | 4.39 | 0.036 |
| Yes | 16(16.0) | 37 (37.0) | ||
| Alcohol: | ||||
| No | 83 (83.0) | 90(90) | 0.18 | 0.670 |
| Yes | 17 (17.0) | 10 (10.0) | ||
| Exercise: | ||||
| No | 67 (67.0) | 70 (70.0) | 4.73 | 0.030 |
| Yes | 53 (53.0) | 30 (30.0) | ||
| Hypertension: | ||||
| No | 76 (76.0) | 38 (38.0) | 10.55 | 0.001 |
| Yes | 24 (24.0) | 62 (62.0) | ||
| Chest pain: | ||||
| No | 83 (83.0) | 29 (29.0) | 44.42 | <0.001 |
| Yes | 17 (17.0) | 71 (71.0) |
Demographic and clinical characteristics of two groups were summarised in number (n) and percentage (%) and compared by χ2 test.
Values of biochemical parameters values in CAD and non-CAD cases
The level of biochemical parameters in the level of the two groups is summarized in table 2. Comparing the biochemical parameter levels of two groups, χ2 test showed significantly different and higher level of (%) of HbA1c (10.0% vs. 41.0%, χ2=9.52, p=0.002), sdLDL (17.0% vs. 62.0%, χ2=17.46, p<0.001), folate II (20.0% vs. 60.0%, χ2=14.05, p<0.001), Vitamin B12 (27.0% vs. 59.0%, χ2=9.05, p=0.003) and VEGF (pg/ml) (20.0% vs. 62.0%, χ2=14.95, p<0.001) whereas, less frequency of HDL (63.0% vs. 43.0%, χ2=3.84, p=0.05) and LDL (70.0% vs. 40.0%, χ2=7.95, p=0.005) in CAD cases as compared to non CAD cases suggesting that these may be associated with CAD. However, biochemical parameters namely VLDL, TG, TC, and Vitamin D, TSH, and total HCY2 did not differ (p>0.05) between the two groups indicating that these may not be associated with CAD. We have also observed the correlation of miR-122 and -126 with demographic and clinical characteristics of the cases, however, the difference was not significant (data not shown).
Table 2.
Biochemical parameter levels of two groups
| Variable | Controls (n=100) (%) |
Cases (n=100) (%) |
χ2 value | P value |
|---|---|---|---|---|
| HbA1c (%): | ||||
| ≤6 | 90 (90.0) | 59 (59.0) | 9.52 | 0.002 |
| >6 | 10 (10.0) | 41 (41.0) | ||
| HDL(mg/dl): | ||||
| ≤43 | 37 (37.0) | 57 (57.0) | 3.84 | 0.05 |
| >43 | 63 (63.0) | 43 (43.0) | ||
| LDL(mg/dl): | ||||
| ≤56 | 30 (30.0) | 60 (60.0) | 7.95 | 0.005 |
| >56 | 70 (70.0) | 40 (40.0) | ||
| VLDL(mg/dl): | ||||
| ≤29 | 50 (50.0) | 53 (53.0) | 0.06 | 0.81 |
| >29 | 50 (50.0) | 47 (47.0) | ||
| TG (mg/dl): | ||||
| ≤133 | 57 (57.0) | 47 (47.0) | 0.74 | 0.39 |
| >133 | 43 (43.0) | 53 (53.0) | ||
| TC (mg/dl): | ||||
| ≤135 | 37 (37.0) | 56(56.0) | 3.38 | 0.06 |
| >135 | 63 (63.0) | 44 (44.0) | ||
| sdLDL(mmol/l): | ||||
| ≤15 | 83 (83.0) | 38 (38.0) | 17.46 | <0.001 |
| >15 | 17 (17.0) | 62 (62.0) | ||
| Folate II (mmol/l): | ||||
| ≤9 | 80 (80.0) | 40 (40.0) | 14.05 | <0.001 |
| >9 | 20 (20.0) | 60 (60.0) | ||
| Vitamin B12 (pg/ml): | ||||
| ≤238 | 73 (73.0) | 41 (41.0) | 9.05 | 0.003 |
| >238 | 27 (27.0) | 59 (59.0) | ||
| Vitamin D (ng/ml): | ||||
| ≤21 | 40 (40.0) | 60 (60.0) | 3.59 | 0.05 |
| >21 | 60 (60.0) | 40 (40.0) | ||
| TSH (μmol/l): | ||||
| ≤2 | 63 (63.0) | 64 (64.0) | 0.01 | 0.94 |
| >2 | 37 (37.0) | 36 (36.0) | ||
| Total HCY2 (μmol/l): | ||||
| ≤19 | 47 (47.0) | 52 (52.0) | 0.30 | 0.58 |
| >19 | 53 (53.0) | 48 (48.0) | ||
| VEGF(pg/ml): | ||||
| ≤120 | 80 (80.0) | 38 (38.0) | 14.95 | <0.001 |
| >120 | 20 (20.0) | 62 (62.0) |
Biochemical parameter levels of two groups were summarised in number (n) and percentage (%) and compared by χ2 test.
Circulating miR-122 and miR -126 expression
The marker expression levels in the two groups are summarized in Table 3. Comparing the marker expression levels of the two groups, χ2 test showed significantly different and higher frequency (%) of miR-122 (14.0% vs. 63.0%, χ2=21.23, p<0.0001) whereas the significantly different and lower frequency of miR-126 (80.0% vs. 39.0%, χ2=14.95, p<0.0001) in CAD cases as compared to non-CAD suggests that both markers may be associated with CAD as depicted in figure 1a and 1b.
Table 3.
Circulating miRNA 122 &126 expression levels of two groups
| Variable | Cut off | Controls (n=100) (%) |
Cases (n=100) (%) |
χ2 value |
p value |
|---|---|---|---|---|---|
| miR-122: | |||||
| ≤1.24 | 86 (86.0) | 37 (37.0) | 21.23 | <0.0001 | |
| >1.24 | 14 (14.0) | 63 (63.0) | |||
| miR-126: | |||||
| ≤0.90 | 20 (20.0) | 61 (61.0) | 14.95 | <0.0001 | |
| >0.90 | 80 (80.0) | 39 (39.0) |
Expression levels of two groups were summarised in number (n) and percentage (%) and compared by χ2 test.
Figure 1.
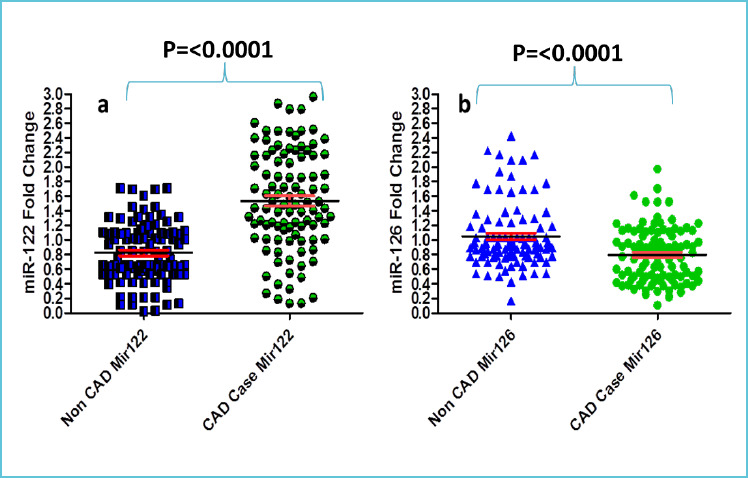
Scatter plot showing values of the a) miR-122 and b) miR-126 in CAD cases and non-CAD cases
Independent predictors of CAD
Unadjusted odds ratio
To evaluate the risk predictors of CAD, all variables (demographic and clinical, biochemical and marker expression) were first subjected to univariate (crude or unadjusted) binary logistic regression analysis (controls=0 and cases=l). These are summarized in Table 4. The univariate analysis found educational status (OR=9.86, 95% CI=3.13-31.01, p<0.001), smoking (OR=3.02, 95% CI=1.04-8.76, p=0.04), exercise (OR=0.39, 95% CI=0.16-0.92, p=0.03), hypertension (OR=4.40, 95% CI=1.74-11.14, p=0.002), chest pain (OR=27.50, 95% CI=8.79-86.01, p<0.001), HbA1c (OR=6.26, 95% CI=1.75-22.41, p=0.005), LDL (OR=0.28, 95% CI=0.12-0.70, p=0.006), sdLDL (OR=8.00, 95% CI=2.76-23.16, p<0.001), folate II (OR=6.07, 95% CI=2.22-16.53, p<0.001), Vitamin B12 (OR=3.95, 95% CI = 1.57-9.98, p=0.004), VEGF (OR=6.40, 95% CI=2.35-17.47, p<0.001), miR-122 (OR=10.98, 95% CI=3.48-34.63, p<0.001) and miR-126 (OR=0.16, 95% CI=0.06-0.43, p<0.001) as the significant predictors of CAD. Predictors that have shown significant p values in univariate (crude or unadjusted) binary logistic regression analysis were subjected to multivariable repression model to find the independent risk predictors of CAD.
Table 4.
Predictors of CAD using univariate binary logistic regression analysis
| Predictor | OR (95% CI) | p value |
|---|---|---|
| Educational status: | ||
| Literate | Ref. | |
| Illiterate | 9.86 (3.13-31.01) | <0.001 |
| Smoking: | ||
| No | Ref. | |
| Yes | 3.02 (1.04-8.76) | 0.04 |
| Exercise: | ||
| No | Ref. | |
| Yes | 0.39 (0.16-0.92) | 0.03 |
| Hypertension: | ||
| No | Ref. | |
| Yes | 4.40 (1.74-11.14) | 0.002 |
| Chest pain: | ||
| No | Ref. | |
| Yes | 27.50 (8.79-86.01) | <0.001 |
| HbA1c (%): | ||
| ≤6 | Ref. | |
| >6 | 6.26 (1.75-22.41) | 0.005 |
| LDL(mg/dl): | ||
| ≤56 | Ref. | |
| >56 | 0.28 (0.12-0.70) | 0.006 |
| sdLDL(mmol/l): | ||
| ≤15 | Ref. | |
| >15 | 8.00 (2.76-23.16) | <0.001 |
| Folate II (mmol/l): | ||
| ≤9 | Ref. | |
| >9 | 6.07 (2.22-16.53) | <0.001 |
| Vit. B12 (pg/ml): | ||
| ≤238 | Ref. | |
| >238 | 3.95 (1.57-9.98) | 0.004 |
| VEGF (pg/ml): | ||
| ≤120 | Ref. | |
| >120 | 6.40 (2.35-17.47) | <0.001 |
| miR-122: | ||
| ≤1.24 | Ref. | |
| >1.24 | 10.98 (3.48-34.63) | <0.001 |
| miR-126: | ||
| ≤0.90 | Ref. | |
| >0.90 | 0.16 (0.06-0.43) | <0.001 |
OR: odds ratio, CI: confidence interval, Ref: reference category. All odds ratio were evaluated against reference category.
Adjusted odds ratio
To find out the independent risk predictors of CAD, the significant variables (found in Table 4) were further subjected to multivariate (adjusted) binary logistic regression analysis and are summarized in Table 5. The multivariate analysis further found chest pain (OR=37.07, 95% CI=3.21-169.04, p=0.01) and miR-126 (OR=0.01, 95% CI=0.00-0.63, p=0.03) as significant. Thus, chest pain and miR-126 may serve as significant and independent predictors of CAD.
Table 5.
Independent predictors of CAD using multivariate binary logistic regression analysis
| Predictor | OR (95% CI) | p value |
|---|---|---|
| Educational status: | ||
| Literate | Ref. | |
| Illiterate | 4.94 (0.32-76.49) | 0.25 |
| Smoking: | ||
| No | Ref. | |
| Yes | 13.76 (0.54-353.17) | 0.11 |
| Exercise: | ||
| No | Ref. | |
| Yes | 0.12 (0.00-5.31) | 0.27 |
| Hypertension: | ||
| No | Ref. | |
| Yes | 1.15 (0.08-16.36) | 0.91 |
| Chest pain: | ||
| No | Ref. | |
| Yes | 6.60 (3.21-169.04) | 0.01 |
| HbA1c (%): | ||
| ≤6 | Ref. | |
| >6 | 3.32 (0.16-68.21) | 0.43 |
| LDL(mg/dl): | ||
| ≤56 | Ref. | |
| >56 | 0.15 (0.01-2.23) | 0.16 |
| sdLDL(mmol/l): | ||
| ≤15 | Ref. | |
| >15 | 18.20 (0.62-534.65) | 0.09 |
| Folate II (mmol/l): | ||
| ≤9 | Ref. | |
| >9 | 6.34 (0.44-90.60) | 0.17 |
| Vitamin B12 (pg/ml): | ||
| ≤238 | Ref. | |
| >238 | 26.62 (0.49-1454.89) | 0.10 |
| VEGF: (pg/ml) | ||
| ≤120 | Ref. | |
| >120 | 1.31(0.11-15.18) | 0.82 |
| miR-122: | ||
| ≤1.24 | Ref. | |
| >1.24 | 13.25 (0.68-259.03) | 0.08 |
| miR-126: | ||
| ≤0.90 | Ref. | |
| >0.90 | 0.01 (0.00-0.63) | 0.03 |
OR: odds ratio, CI: confidence interval, Ref: reference category. All odds ratio were evaluated against reference category
Diagnostic accuracy of circulatory miR-122 and miR-126
To see the diagnostic accuracy (sensitivity and specificity) of markers (miR-122 and miR-126) in predicting CAD, both the markers were subjected to ROC curve analysis. The ROC curve analysis showed a significant diagnostic accuracy of miR-122 area under the curve (AUC= 0.8057, Z=6.66, p<0.001) and a cut-off value of >1.24, it discriminated the subjects of the two groups (controls and cases) with 64.00% sensitivity (95% CI=53.79-73.36) and 84.00% specificity (95% CI=75.32-90.57), and 79.75% positive predictive value and 69.42% negative predictive value. Similarly, miR-126 also showed significant diagnostic accuracy (AUC=0.806, Z=3.52, p<0.001), and at a cutoff value of ≤0.90, it discriminated the subjects of the two groups with 61.54% sensitivity (95% CI=49.8-72.3) and 80.00% specificity (95% CI=61.4-92.2), and 88.9% positive predictive value and 44.4% negative predictive value and also depicted in figure 2.
Figure 2.
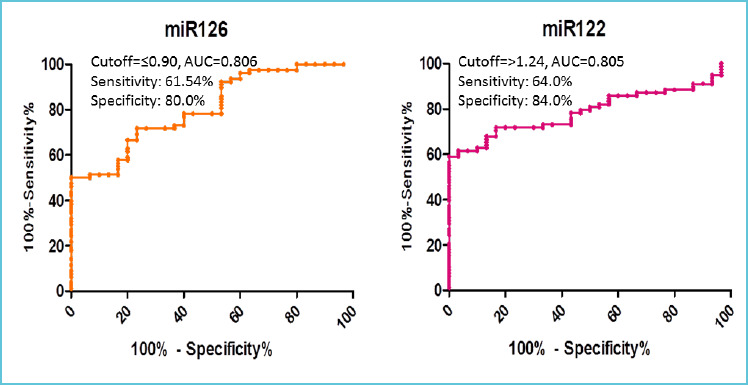
ROC curve values for miR-126 and miR-122 to differentiate between CAD cases and non-CAD cases
DISCUSSION
The role of circulating miRNAs as a new biomarker in diagnosing cardiovascular diseases has kindled a brighter outlook worldwide. Despite recent advances and a stupendous rise in interest in miRNA for CAD diagnosis and prognosis, we are still unraveling the complexity superficially. Circulating miRNAs are a sensitive, diagnostic, and prognostic biomarker for therapeutic interventions. MiRNAs show tissue-specific expression, are rapidly released into circulation, and are remarkably stable in serum/plasma. The emerging role of miRNA in CAD and cardiac arrhythmia has been demonstrated.[14] Analysis of circulating plasma/serum miRNA may be of importance in disease prediction and improvement of the diagnostic accuracy of cardiovascular diseases.[15, 16] Circulating levels of miR-1, miR-126, miR-133, miR-1291, miR-663b, and miR423-5p have been reported to be promising biomarkers in acute myocardial infarction (AMI) cases.[17, 18] Studies reported the up-regulation of miR-208a, miR-133a, miR-1, miR-133b, miR-337-5p, miR-122, and miR-433 in CAD cases, however, the circulating levels of miR-126, miR-17, miR-145, miR-155, miR-92a, and miR-199a were significantly down regulated in CAD cases, as compared to non-CAD cases (controls).[19] Hoeckstra et al. in 2010, for the first time, analyzed human circulating miR in CAD, and miR-135a and -147 were found to be significantly elevated in CAD cases compared to non-CAD healthy controls.[20] The findings highlight the role of circulating miRNAs as biomarkers in the detection of CAD. Different miRNAs related to cardiac origin have been studied to investigate the diagnostic potential. Reduced expression of miR-122 has been found in patients with MI. [21, 22] Our study evaluated the potential signature of circulating miRNAs 122 and 126 in differentiating CAD from non-CAD cases. We have found significant downregulation of miR-126 (p=<0.001) in CAD cases as compared to non-CAD cases. The level of circulating miR-122 was significantly upregulated in CAD cases compared to non-CAD cases (p=0.001). A study by Wang X et al. 2017, reported a significant down regulation of miR-126 in CAD cases, and it was also associated with increased placental growth factor (PGF) levels in CAD and AMI cases.[23] Our findings of miR-126 are consistent with those of Li HY et al. 2016, where plasma miR-126-5p was significantly downregulated in patients with severe CAD.[24] The levels of miR-126 were also lower in CAD patients with either intermediate or high SYNTAX scores, instead of low SYNTAX scores. In contrast to our finding, the study by Sridhar M et al. 2021 reported that miR-122 was significantly downregulated in CAD patients, however, the level of miR-126 did not show any change. This may be due to the frequent use of aspirin and β blockers medication of the study participants that may affect the miR-126 level.[13] These findings collectively indicate that anti-platelet therapies did not affect the miRNA levels.[25-28]
In our study, a univariate analysis of demographic, clinical characteristics and miR-126 & 122 has been carried out to find the risk predictor of CAD. We have found educational status (p<0.001), smoking (p=0.042), exercise (p=0.032), hypertension (p=0.002), chest pain (p<0.001), HbA1c (p=0.005), LDL (p=0.006), sdLDL (p<0.001), Folate II (p<0.001), Vitamin B12 (p=0.004), VEGF (p<0.001), miR-122 (p<0.001) and miR-126 (p<0.001) as a significant predictors of CAD. However, on multivariate analysis chest pain (p=0.017) and miR-126 (p=0.030) were found as a significant independent risk predictor of CAD. A study by Su T et al. 2019; reported that circulating miR-1 might be an important biomarker for early AMI diagnosis and may predict the prognosis of patients with chest pain.[29] Similarly, Fichtlscherer et al. in their study reported significantly reduced levels of endothelial expressed miR-126, -92a, and -17 in CAD cases compared to non-CAD cases.[30] A prospective population-based cohort study by Zampetaki et al. 2012 evaluated the predictive value of miRNA concerning myocardial infarction (MI). Multivariate cox regression analysis of miR-126, -197, and -223 showed predictive value for MI. The expression level of miR-126 was found to be positively associated with MI prediction while those of miR-197 and -223 were inversely associated with MI prediction.[31] A study by Zhu L 2017; reported that the presence of circulating miRNA-133a level in blood was a risk factor of CHD (OR: 2.565, 95% CI: 1.105-5.954, P = 0.028) and showed a positive correlation with miR-133a expression and Gensini score in patients with CHD (r = 0.303, P = 0.007).[32] In a recent study, Gao W et al. 2012 showed that plasma levels of miR-122 significantly increased in hyperlipidemic patients as compared to controls and were positively correlated with TC, TG, and LDL-C levels in both hyperlipidemic patients and controls. Multiple logistic regression analyses revealed the presence of CAD in patients with increased levels of miR-122.[33] A study by Wang YL et al. 2018 showed the significantly up-regulated level of miR-122 in patients with atherosclerotic lesions and these were positively correlated with cholesterol, triglycerides, and atherosclerotic severity.[34] A study by Pilbrow et al. 2014 reported that miR-652 was an independent predictor of heart failure after acute myocardial infarction (AMI).[35] However, the use of a combination of miRNAs or a combination of miRNAs with established prognostic markers such as BNP or cardiac troponin seems to improve the risk management in cardiovascular diseases.[35,36] In our study, the HbA1c level was significantly different between the CAD cases and non CAD cases indicating that patients with diabetes mellitus may affect the extracellular miRNA expressions. [37, 27, 28]
Several studies have evaluated the diagnostic values of circulating miRNAs and reported that miRNA values may have diagnostic potential in the identification and differentiation of coronary artery disease.[34, 35, 36] In a study by Zhong Z et al. 2018, analyzed miRNA-126-5p in unstable angina (UA) patients and ROC analysis revealed an AUC of 0.714 (95% CI: 0.555–0.873), however for ST-segment elevation myocardial infarction (STEMI) patients AUC was 0.703 (95% CI: 0.541–0.864).[38] In our study, ROC analysis of miR-122 and miR-126 showed significant diagnostic accuracy with an AUC of 0.805 and 0.806 respectively.
These results strongly indicate that serum miR-126 and miR-122 might be used as a novel, noninvasive, and risk predictive biomarkers for CAD patients. Likewise, many studies have found the association of miRNAs with restenosis-related processes, such as VSMC proliferation, migration, and neointima formation highlighting the great use for diagnosis, prognosis, therapeutic, or in additional clinical management. [39-41] Present study advocates the use of circulating miRNAs in the field of population-based risk assessment of CAD. However, to fully exploit the potential of miRNAs, there is a need to standardized, sample processing as well as to use advanced techniques for analysis. Our study is limited by a smaller sample size. Therefore, future studies on larger cohorts without CAD (CAD-ve) and patients with CAD (CAD +ve) are needed to broadly evaluate the miRNAs for risk prediction in comparison with other established cardiac markers. Our findings suggest that blood-based miRNAs may be sensitive and specific biomarkers for monitoring cardiovascular diseases, and for the evaluation of myocardial protection during cardiac surgery. Further, an in-depth evaluation of the role of these miRNAs in the pathogenesis and progression of CAD will contribute to our understanding of the disease process and lead to new therapeutic and preventive strategies.
Abbreviations used:
- BNP
Brain Natriuretic Peptide
- CAD
Coronary Artery disease
- Echo
Echocardiogram
- CT
Computed Tomography
- ECG
Electrocardiogram
- HDL
High density lipoproteins
- HF
Heart failure
- LDL
Low density lipoproteins
- miRNA
MicroRNA
- MI
myocardial infarction
- TG
Triglyceride
- VLDL
Very low-density lipoproteins
- sdLDL
Small dense low-density lipoproteins
- VEGF
Vascular Endothelial Growth Factor
REFERENCES
- 1.Latronico M V, Catalucci D, Condorelli G. MicroRNA and cardiac pathologies. Physiol Genomics 2008; 34:239-242. [DOI] [PubMed] [Google Scholar]
- 2.Braza-Boils A, Mari-Alexandre J, Molina P, Domingo D, Abellan Y, Sancho J, et al. P62 Role of microRNAs associated with non-alcoholic fatty liver disease in sudden cardiac death from coronary artery disease, Cardiovascular Res 2014; 103: S10-S10. [Google Scholar]
- 3.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 2005; 111: 3481-3488. [DOI] [PubMed] [Google Scholar]
- 4.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, et al. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation 2011; 9124(6):720-730. [DOI] [PubMed] [Google Scholar]
- 5.van Empel VP, De Windt LJ, da Costa Martins PA. Circulating miRNAs: reflecting or affecting cardiovascular disease. Current hypertension reports 2012; 14(6):498-509. [DOI] [PubMed] [Google Scholar]
- 6.Van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences 2008; 105(35): 13027-13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007; 116(3):258-267. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, et al. Altered microRNA expression in human heart disease. Physiol Genomics. 2007; 31(3):367-363. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda S, He A, Kong SW, Lu J, Bejar R, Bodyak N, et al. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol Cell Biol. 2009; 29(8):2193-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thum T. MicroRNA therapeutics in cardiovascular medicine. EMBO Mol Med. 2012;4:3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Zhang M, Sanagawa A, Mori C, Ito S, Iwaki S, et al. Circulating microRNA-126 in patients with coronary artery disease: correlation with LDL cholesterol. Thrombosis journal. 2012; 10(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 writing group to review new evidence and update the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention, writing on behalf of the 2005 writing committee, Circulation 2008; 117: 261-295. [DOI] [PubMed] [Google Scholar]
- 13.Mishra S, Rizvi A, Pradhan A, Perrone MA, Ali W. Circulating microRNA-126 &122 in patients with coronary artery disease: Correlation with small dense LDL. Prostaglandins & Other Lipid Mediators. 2021; 153:106536. [DOI] [PubMed] [Google Scholar]
- 14.Santulli G, Iaccarino G, De Luca N, Trimarco B, Condorelli G. Atrial fibrillation and microRNAs. Front physiol. 2014; 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol 2015; 213:60-83. [DOI] [PubMed] [Google Scholar]
- 16.Charan Reddy KV. Regulatory noncoding RNAs in cardiovascular disease: shedding light on ‘Dark Matter.’ J Cardiovasc Dis 2015; 3:301-307. [Google Scholar]
- 17.Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail 2012; 14:147-144. [DOI] [PubMed] [Google Scholar]
- 18.Long G, Wang F, Duan Q, Chen F, Yang S, Gong W, et al. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. Int J Biol Sci 2012; 8:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayed AS, Xia K, Li F, Deng X, Salma U, Li T, et al. The diagnostic value of circulating microRNAs for middle-aged (40–60-year-old) coronary artery disease patients. Clinics 2015; 70:257-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoekstra M, van der Lans CA, Halvorsen B, Gullestad L, Kuiper J, Aukrust P, et al. The peripheral blood mononuclear cell microRNA signature of coronary artery disease. Biochemical and biophysical research communications. 2010; 394(3):792-797. [DOI] [PubMed] [Google Scholar]
- 21.Melak T, Baynes HW. Circulating microRNAs as possible biomarkers for coronary artery disease: a narrative review. EJIFCC. 2019; 30(2):179. [PMC free article] [PubMed] [Google Scholar]
- 22.Szilágyi B, Fejes Z, Pócsi M, Kappelmayer J, Nagy B, Jr. Role of sepsis modulated circulating microRNAs. EJIFCC. 2019; 30(2):128. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Lian Y, Wen X, Guo J, Wang Z, Jiang S, et al. Expression of miR-126 and its potential function in coronary artery disease. African health sciences. 2017; 17(2):474-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li HY, Zhao X, Liu YZ, Meng Z, Wang D, Yang F, et al. Plasma microRNA-126-5p is associated with the complexity and severity of coronary artery disease in patients with stable angina pectoris. Cellular Physiology and Biochemistry. 2016; 39(3):837-836. [DOI] [PubMed] [Google Scholar]
- 25.Willeit P, Zampetaki A, Dudek K, Kaudewitz D, King A, Kirkby NS, et al. Circulating microRNAs as novel biomarkers for platelet activation. Circulation research. 2013; 112(4):595-600. [DOI] [PubMed] [Google Scholar]
- 26.Kaudewitz D, Lee R, Willeit P, McGregor R, Markus HS, Kiechl S, et al. Impact of intravenous heparin on quantification of circulating microRNAs in patients with coronary artery disease. Thrombosis and haemostasis. 2013; 110(09):609-615. [DOI] [PubMed] [Google Scholar]
- 27.Stratz C, Bomicke T, Younas I, et al. Comparison of Immature Platelet Count to Established Predictors of Platelet Reactivity During Thienopyridine Therapy. J Am Coll Cardiol 2016; 68: 286–293. [DOI] [PubMed] [Google Scholar]
- 28.Fejes Z, Póliska S, Czimmerer Z, Káplár M, Penyige A, Szabó GG, et al. Hyperglycaemia suppresses microRNA expression in platelets to increase P2RY12 and SELP levels in type 2 diabetes mellitus. Thrombosis and Haemostasis. 2017; 117(03):529-542. [DOI] [PubMed] [Google Scholar]
- 29.Su T, Shao X, Zhang X, Han Z, Yang C, Li X. Circulating microRNA-1 in the diagnosis and predicting prognosis of patients with chest pain: a prospective cohort study. BMC cardiovascular disorders. 2019; 19(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010; 107(5):677-674. [DOI] [PubMed] [Google Scholar]
- 31.Zampetaki A, Willeit P, Tilling L, Drozdov I, Prokopi M, Renard JM, et al. Prospective study on circulating MicroRNAs and risk of myocardial infarction. Journal of the American College of Cardiology. 2012; 60(4):290-299. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L. The correlations of circulating microRNA-133a with the risk and severity of coronary heart disease. International Journal of Clinical and Experimental Medicine. 2017; 10(1):972-978. [Google Scholar]
- 33.Gao W, He HW, Wang ZM, Zhao H, Lian XQ, Wang YS, et al. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids in health and disease. 2012; 11(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YL, Yu W. Association of circulating microRNA-122 with presence and severity of atherosclerotic lesions. Peer J. 2018; 6:e5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilbrow AP, Cordeddu L, Cameron VA, Frampton CM, Troughton RW, Doughty RN, et al. Circulating miR-323-3p and miR-652: Candidate markers for the presence and progression of acute coronary syndromes. Int J Cardiol. 2014; 176:375–375. [DOI] [PubMed] [Google Scholar]
- 36.Devaux Y, Vausort M, McCann GP, Kelly D, Collignon O, Ng LL, et al. A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction. PLoS One. 2013; 8:e70644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circulation research. 2010; 107(6):810-817. [DOI] [PubMed] [Google Scholar]
- 38.Zhong Z, Hou J, Zhang Q, Zhong W, Li B, Li C, et al. Circulating microRNA expression profiling and bioinformatics analysis of dysregulated microRNAs of patients with coronary artery disease. Medicine 2018; 97(27). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen LJ, Lim SH, Yeh YT, Lien SC, Chiu JJ. Roles of microRNAs in atherosclerosis and restenosis. Journal of biomedical science. 2012; 19(1):1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gareri C, De Rosa S, Indolfi C. MicroRNAs for restenosis and thrombosis after vascular injury. Circulation research. 2016; 118(7):1170-1184. [DOI] [PubMed] [Google Scholar]
- 41.Yamakuchi M. MicroRNAs in vascular biology. International journal of vascular medicine. 2012. 1; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


