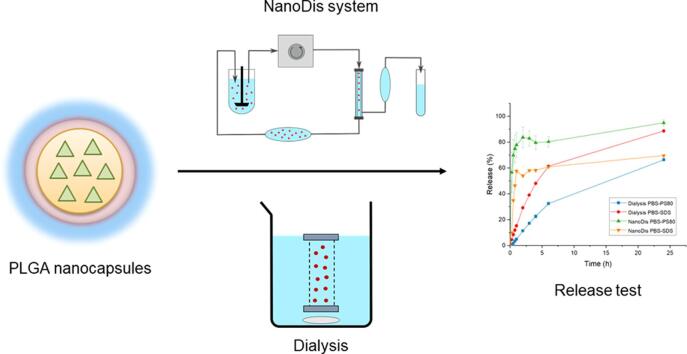
Graphical abstract
Keywords: PLGA nanocapsules, Nanoparticles, Release testing, Dialysis, Retinoic acid, Dissolution
Highlights
-
•
An innovative sample and separate method, the NanoDis system, was used.
-
•
Innovative NanoDis was compared to traditional dialysis approach.
-
•
The release profile of PLGA nanocapsules was characterized.
-
•
The NanoDis system was superior over dialysis.
-
•
The burst release of the nanocapsules could be accurately measured.
Abstract
One of the critical quality attributes of nanoparticle formulations is drug release. Their release properties should therefore be well characterized with predictive and discriminative methods. However, there is presently still no standard method for the release testing of extended release nanoformulations. Dialysis techniques are widely used in the literature but suffer from severe drawbacks. Burst release of formulations can be masked by slow permeation kinetics of the free drug through the dialysis membrane, saturation in the membrane, and absence of agitation in the membrane. In this study, the release profile of poly(lactic co-glycolic) (PLGA) nanocapsules loaded with all-trans retinoic acid was characterized using an innovative sample and separate set-up, the NanoDis System, and compared to the release profile measured with a dialysis technique. The NanoDis System showed clear superiority over the dialysis method and was able to accurately characterize the burst release from the capsules and furthermore discriminate between different all-trans retinoic acid nanoparticle formulations.
1. Introduction
Polyester nanoparticles, and more specifically poly(lactic acid) (PLA) and poly(lactic co-glycolic acid) (PLGA) nanoparticles, have been used abundantly in research during the last few decades due to their good biocompatibility properties (Makadia et al., 2011). These polymeric nanoparticles have been reported to have controlled and extended release properties thanks to their slow degradation kinetic. However, such nanoparticle formulations often suffer from burst release, wherein a large amount of the encapsulated drug is released within the first hours due to leakage of the API (Active Pharmaceutical Ingredient) located close to the particle surface (Rodrigues de Azevedo et al., 2017, Yoo and Won, 2020). A high burst release can lead to a toxic effect if the drug concentration exceeds the therapeutic window and must therefore be well characterized.
Until now there have been no standardized techniques for release testing of extended release nanoparticle systems. Current dissolution methodologies suffer from the inefficient separation of nanoparticles from the dissolution medium, independent of the equipment used for the dissolution studies.
Dialysis is one of the methods used in drug release testing of extended release nanoparticle formulations, ensuring the physical separation of the nanoparticles from the sampling compartment (Yang et al., 2016, Dinesh Kumar et al., 2015, Sant et al., 2005, Wang et al., 2011). The nanoparticles are dispersed in release medium and filled into a dialysis bag or tube, which acts as the donor compartment. The bag is stirred in a large volume of release medium —referred to as the acceptor compartment— which allows the diffusion of the released drug from the donor to the acceptor compartment due to the concentration difference. The released drug can then be measured by sampling from the acceptor compartment. Despite being widely used, dialysis techniques suffer from severe drawbacks such as the permeation kinetic of the free drug through the dialysis membrane, which often limits the measured release of the API (Modi and Anderson, 2013, Zambito et al., 2012). If the permeation kinetic of the free drug is slower than the release rate of the drug, the amount of API found in the acceptor compartment will not reflect the real release profile of the drug at this time point (D’Souza, 2014, Nothnagel and Wacker, 2018). The limited permeation of the drug through the membrane can also lead to non-sink conditions inside of the dialysis bag, thus impacting the release profile. The so-called sink conditions refer to experimental in vitro conditions where the maximum drug concentration in the bulk fluid should not exceed about 20–30% of the drug’s saturation concentration in the respective medium (Siepmann and Siepmann, 2020). In these conditions, the release from the particles is not limited by the concentration gradient or by saturation. If free drug accumulates inside the dialysis bag due to low permeation kinetics, saturation can be reached, leading to recrystallization and precipitation of the drug, thereby forming a new drug depot inside the bag instead of free API diffusing towards the acceptor compartment. Furthermore, due to the lack of agitation inside the bag, adsorption of excipients or precipitates of drug can easily occur on the membrane, further reducing the effective surface area for medium exchange and thus impacting the permeation kinetic and the measured release even more (Nothnagel and Wacker, 2018). Based on the aforementioned reasons, the measured release profile, and especially the profile of the burst release, are often underestimated when using dialysis techniques.
As an alternative to the dialysis set-up, sample and separate techniques can be used. In these methods, the nanoparticles are diluted in sink conditions in release medium. Samples are taken at various time points and separation techniques are used to isolate the free drug from the nanoparticles. To achieve separation, centrifugation, filtration techniques, or centrifugal ultrafiltration devices can be employed (Budhian et al., 2008, Hasan et al., 2007, Nimesh et al., 2006). When using centrifugation, the high centrifugal speed necessary to pellet the nanoparticles can disrupt sheer-sensitive particles and lead to forced release of the drug (D’Souza, 2014). As release can also continue during the centrifugation time, this technique is not applicable for early time points (D’Souza, 2014, Nothnagel and Wacker, 2018). Filtration techniques using syringe filters are fast and easy to set up, but must use low pore size filters to avoid permeation of nanoparticles through the membranes. Filter clogging or breakage can occur, and the sheer stress of this technique can also disrupt some fragile particles (Nothnagel and Wacker, 2018). When using centrifugal ultrafiltration devices, lower rotation speed can be used when compared with classical centrifugation, however the same issue of filter clogging and breakage can still occur together with the length of time needed for the ultrafiltration where the dissolution still occurs in the ultrafiltration device (Nothnagel and Wacker, 2018). The use of tangential flow filtration (TFF), also referred to as cross-flow filtration, can reduce clogging of the membranes. The nanoparticle dispersion feed is streamed parallel to the membrane face with one portion passing through the membrane (filtrate or permeate), whereas the remainder (retentate or concentrate) is circulated back to the feed reservoir (Dalwadi et al., 2005). The cross-flow prevents particle from clogging the membrane and reduces the sheer force on the nanoparticles. TFF is an efficient technique for the purification of nanoparticles and can also be employed for the separation of released drug from nanoparticles.
Recently, alternative techniques for dissolution test of nanoparticles have been developed, like in-situ techniques, measurements using Differential Scanning calorimetry (DSC), or automated sample and separate technique. In-situ techniques allow the dosage of the free API directly in the vessels, thus avoiding the need for sampling and loss of material. As the detection is done directly in the vessel, the free API needs to be detected independently of the API still encapsulated in the nanoparticles, and with minimum interference due to the nanoparticles or excipients present in the medium. A new device has been reported to be able to measure UV absorbance of the released drug in-situ without separation from the nanoparticles, the Sirius® inForm apparatus. This device can make adjustments for the amount of UV light lost by the scattering of the nanoparticles, using the Tyndall-Rayleigh scattering theory, and correct the absorbance measurement accordingly. However, this device remains sensitive to turbidity (Balzus et al., 2016). Potentiometric sensors or differential pulse polarography (DPP) have both been used for the in-situ measurements of nanoformulations. Potentiometric sensors provide accurate, reproducible, fast and selective determination of various ionic species, in a non-destructive manner and are thus only available for ionizable API (Kakhki, 2013). DPP is an electrochemical method and thus is only available for electroactive drugs, with a suitable redox potential (Kontoyannis and Douroumis, 2001, Charalampopoulos et al., 2003, Rosenblatt et al., 2007). DSC has also been used for the release testing of a variety of API loaded in lipid nanoparticles, as the crystallization temperature increased proportionally with the release of the loaded API (Roese and Bunjes, 2017). Finally, an innovative device for dissolution test of nanoparticles has been developed based on a sample and separate technique using TFF with hollow fiber membranes: the NanoDis System.
The NanoDis System is coupled with a USP II dissolution apparatus (paddle) and an autosampler. In the NanoDis System, the dissolution medium containing nanoparticles is pumped through TFF filters while the filtrate, free from particles, is collected with an autosampler. With the NanoDis System, nanoparticles are separated from dissolved API within minutes and independent from particle size since the molecular weight cut-off of the filters is selected accordingly to ensure the complete separation of nanoparticles from the dissolution medium.
In this study, the NanoDis System was used for determining the release profile of PLGA nanocapsules loaded with all-trans retinoic acid (RA). RA, a derivative of vitamin A, has proven to be an interesting molecule for stimulating the differentiation of neural stem cells (NSCs) in new neuronal cells, both in vitro and in vivo (Maia et al., 2011, Santos et al., 2012). Because NSCs can differentiate into new neural cells, including neurons, the regulation of their proliferation, differentiation and migration represents a promising regenerative and therapeutic strategy for central nervous system (CNS) diseases, like strokes or neurodegenerative diseases, such as Alzheimer’s disease. Loading nanoparticles with RA could increase RA cell uptake by NSCs and thus prove valuable in therapeutic applications.
To reach NSCs, nanoparticles should be able to cross the blood–brain barrier (BBB), a selective barrier surrounding the brain formed by the endothelial cells of the cerebral microvessels (Abbott et al., 2006, Sweeney et al., 2018). It was shown that coating polymeric nanoparticles with specific surfactants, like polysorbate 80 or poloxamer 188, increased their BBB crossing ability (Kreuter et al., 1995, Kreuter et al., 1997, Gelperina et al., 2010, Petri et al., 2007). Surfactant-coated nanoparticles can adsorb apolipoproteins in the blood on their surface and then cross the BBB through receptor-mediated transcytosis (Petri et al., 2007, Kreuter et al., 2002). However, for these formulations to be efficient, their cargo should not be released before the formulations have crossed the BBB. Their burst release should therefore be limited.
To summarize, there is still an unmet need for the development of an appropriate release testing technique for the accurate in vitro evaluation of release kinetics of nanoparticles. The innovative release testing technique should be capable of separating the free drug from the nanoparticles efficiently without imposing much stress on the particles during separation and should not be limited by permeation kinetics. In this work, the release from PLGA nanocapsules loaded with all-trans retinoic acid was studied using dialysis and the NanoDis System, to compare the ability of both methods to measure their release kinetic accurately.
2. Material and methods
2.1. Materials
PLGA Resomer® RG502H, was obtained from Evonik (Essen, Germany) and all-trans retinoic acid from Acros Organics (Waltham, USA). Tween® 80 (polysorbate 80) was purchased from Merck (Darmstadt, Germany), Kolliphor® P188 (poloxamer P188) and chitosan (50–190 kDa) from Sigma-Aldrich (Saint-Louis, USA), Kolliphor® SLS (sodium lauryl sulfate/sodium dodecyl sulfate) from BASF (Ludwigshafen, Germany), Span 80 (sorbitan monooleate) from Guangdong Runhua Chemistry Co. (Guangdong, China), and oleic acid from PanReac AppliChem (Darmstadt, Germany).
2.2. Nanocapsules production
The nanocapsules (NC) were produced with a continuous nanoprecipitation method in a confined chamber. Two formulations, P188 benchtop and PS80 benchtop, were produced benchtop by simply adding the solvent solutions in the non-solvent solutions under stirring. PLGA, oleic acid, retinoic acid and Span 80 dissolved in acetone were used as solvent solution (Table 1). Polysorbate 80 (PS80) or poloxamer 188 (P188) were dissolved in water to form the non-solvent solution. A solvent:non-solvent ratio of 1:2 was used for the production of the NC. To produce NC coated with chitosan, the same technique was used but chitosan was added in the non-solvent solution, dissolved in water with 1% v/v of acetic acid. The NC produced with this extra-layer of chitosan were labelled P188-C and PS80-C for NC coated with P188 and PS80 respectively. One formulation coated with chitosan and PS80 was prepared with higher amount of PLGA and oil and was labelled PS80-C high. The NC were purified by TFF using a 300 kD mPES Spectrum® MicroKros hollow fiber filter from Repligen (Waltham, USA) at 15 psi (∼1 bar), with 6 volumes of water, or acetic acid 1% v/v in water for chitosan-coated NC. NC size and zeta potential were measured by dynamic light scattering (DLS) with a Zetasizer NanoZS 90 from Malvern Instruments (Malvern, UK).
Table 1.
Solvent and non-solvent solutions composition for nanocapsules preparation.
| NC |
Solvent solution |
Non-solvent solution |
|||||
|---|---|---|---|---|---|---|---|
| PLGA (% w/v) | Oleic acid (% w/v) | Span 80 (% w/v) | Retinoic acid (% w/v) | Surfactant type | Surfactant (% w/v) | Chitosan (% w/v) | |
| P188 | 1.0 | 1.5 | 0.5 | 0.03 | Poloxamer 188 | 1.0 | 0 |
| P188 benchtop | 1.0 | 1.5 | 0.5 | 0.03 | Poloxamer 188 | 1.0 | 0 |
| PS80 | 1.0 | 1.5 | 0.5 | 0.03 | Polysorbate 80 | 1.0 | 0 |
| PS80 benchtop | 1.0 | 1.5 | 0.5 | 0.03 | Polysorbate 80 | 1.0 | 0 |
| P188-C | 0.3 | 0.9 | 0 | 0.03 | Poloxamer 188 | 1.0 | 0.05 |
| PS80-C | 0.3 | 0.9 | 0 | 0.03 | Polysorbate 80 | 1.0 | 0.05 |
| PS80-C high | 1.0 | 1.5 | 0 | 0.03 | Polysorbate 80 | 1.0 | 0.05 |
2.3. Cryo-TEM imaging
Cryo-TEM imaging of the P188 NC was conducted by placing a 3 µl droplet of the aqueous solution on a S147-4 holey carbon film (Plano, Germany) before blotting the liquid droplet to a thin film for 2 s and plunging into undercooled liquid ethane at T = 108 K using a Gatan (Pleasonton, USA) CP3 cryo plunger. The vitrified samples were transferred under liquid nitrogen to a Gatan model 914 cryo-TEM holder and imaged at T = 100 K using a JEOL (Akishima, Japan) JEM-2100 LaB6 TEM operating at an accelerating voltage of 200 kV under low-dose conditions. TEM micrographs were obtained using a Gatan Orius SC1000 CCD camera and an acquisition time of 4 s.
2.4. Encapsulation efficiency
NC were centrifuged for 5 min at 1,000 g through Nanosep® Centrifugal devices with Omega™ membrane (mPES) 300 kD from Pall Laboratory purchased from VWR (Darmstadt, Germany). The filtrates were measured with a spectrophotometer UV-1600PC from VWR (Darmstadt, Germany) at 345 nm. No RA could be measured in the filtrate, so the encapsulation efficiency (EE) was estimated based on the detection limit of RA in water with 1% w/v PS80 (0.1 µg/ml) using Equation (1).
| (1) |
2.5. RA solubility in release media
To measure RA solubility, 1 mg of RA was stirred in 2 ml of release medium for 24 h at 20 °C. Samples were taken at 3 and 24 h. The sample suspensions were centrifuged for 15 min at 16,000 g and the supernatants were measured with a UV spectrophotometer at 345 nm for PBS-PS80 media and 355 nm for PBS-SDS media. RA concentration was calculated from calibration curves in either PBS-PS80 or PBS-SDS.
2.6. Release testing
2.6.1. NanoDis method
To measure the NC release profile, a USP II dissolution apparatus 708-DS coupled with a NanoDis System and an 850-DS Sampling Station from Agilent (Santa Clara, USA) was used. The nanoparticles were diluted to reach 5 µg/ml of retinoic acid in the vessel and reach sink conditions in 750 ml of PBS-PS80 or PBS-SDS. The NanoDis System was fitted with 500 kD mPES Spectrum® MicroKros hollow fiber filters from Repligen. The NC were stirred at 50 rpm at 37 °C. At chosen time points, the suspension was automatically filtered through the filters by TFF. The filtrates were collected and the retentates were circulated back into the vessels (Fig. 1). Control of the lag time of filtration and permeation of the dissolved API were done with RA dissolved in release medium to check the permeation of free RA through the filters. The filtrates were measured with a UV spectrophotometer at 345 nm for PBS-PS80 and 355 nm for PBS-SDS. RA concentration was calculated from calibration curves in either PBS-PS80 or PBS-SDS. The release percentage was calculated using Equation (2) .
| (2) |
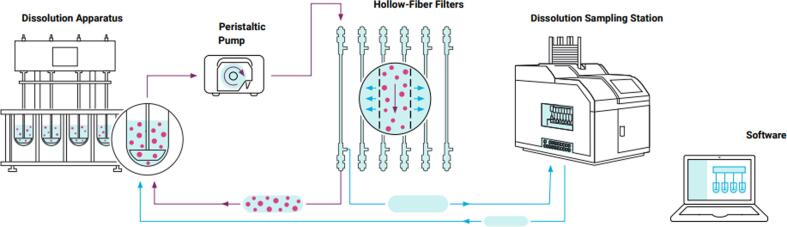
Fig. 1.
The NanoDis System. The NC are diluted in sink conditions in a USP II dissolution apparatus. At specific time points, the NC suspension is automatically filtered by TFF through hollow fiber filters with a peristaltic pump. The filtrate containing the released drug is sampled while the NC suspension is circulated back into the vessel. © Agilent Technologies, Inc. 2020. Reproduced with permission, courtesy of Agilent Technologies, Inc.
2.6.2. Dialysis method
The NC P188-span were diluted to reach 20 µg/ml of RA in release medium, either PBS-PS80 or PBS-SDS, to ensure sink conditions. 10 ml of the suspension was placed inside a 10 ml 300 kD cellulose ester Float-A-Lyzer® from Repligen. The tubes were stirred at 150 rpm in 150 ml of release medium at 37 °C in a drying oven. At chosen time points, 300 µl of medium from the acceptor compartment was sampled and replaced with 300 µl of fresh medium. At 6, 24 and 30 h, the complete medium from the acceptor compartment was replaced with fresh medium. The samples were measured with a UV spectrophotometer at 345 nm for PBS-PS80 and 355 nm for PBS-SDS. RA concentration was calculated from calibration curves in either PBS-PS80 or PBS-SDS. The release percentage was calculated using Equation (3).
| (3) |
3. Results and discussion
3.1. Nanoparticle production and characterization
The PLGA NC were produced with a continuous nanoprecipitation technique in a confined chamber. Depending on the formulations, NC of different sizes were produced. NC of size from 120 to 422 nm were produced, with a polydispersity index (PDI) from 0.09 to 0.32 (Table 2). NC prepared benchtop had larger sizes than the NC prepared using the continuous nanoprecipitation method despite having the same composition, due to differences in mixing properties. When comparing P188-NC and PS80-NC, PS80-NC had the smallest size (120.7 nm against 185.7 nm). As coating the NC with chitosan increased their size, to keep size in a comparable range, PLGA and oleic acid concentrations were reduced from 1 and 1.5% w/v to produce the chitosan-coated NC with a concentration of 0.3 and 0.9% w/v respectively, labelled PS80-C and P188-C. Reducing PLGA and oil concentrations allowed the production of 254.4 nm for P188-C NC and 137.2 nm for PS80-C NC, respectively. When keeping the PLGA and oleic acid concentrations at 1 and 1.5% w/v (PS80-C high), the chitosan-coated NC had a large size of over 400 nm. The NC had negative zeta potentials around −40 mV, except when coated with chitosan, where their zeta potentials turned positive to values between 25 and 30 mV.
Table 2.
Sizes, PDI and zeta potentials of PLGA nanocapsules
| Nanocapsules | Size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| P188 | 185.7 | 0.18 | −42.1 |
| P188 benchtop | 251.7 | 0.10 | −52.4 |
| PS80 | 120.7 | 0.23 | −38.5 |
| PS80 benchtop | 189.0 | 0.25 | −49.1 |
| P188-C | 254.4 | 0.09 | 25.5 |
| PS80-C | 137.2 | 0.32 | 30.4 |
| PS80-C high | 422.8 | 0.17 | 28.4 |
The encapsulation efficiency for all formulations was higher than 99%. This high encapsulation efficiency was possible due to the oleic acid core inside the NC. After several rinse cycles of the nanocapsules by TFF, the retinoic concentration of the NC stayed stable, confirming the high encapsulation efficiency of the NC. The size, PDI, and count rate of the NC were unchanged after TFF purification.
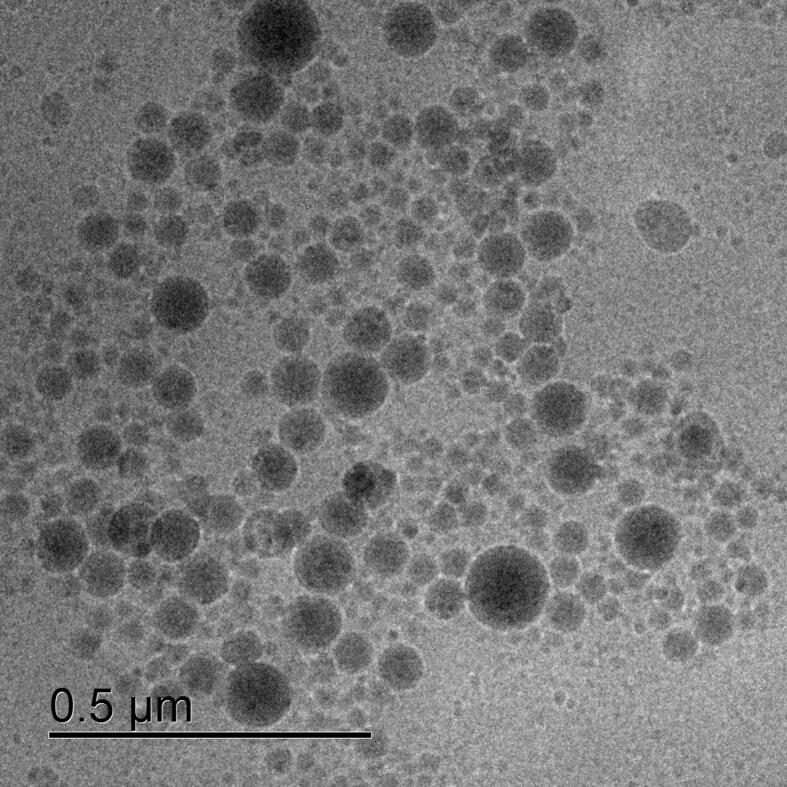
The P188 NC were imaged by cryo-TEM (Fig. 2). The nanocapsules were spherical and seemed to be smaller and have a larger polydispersity than what was measured by DLS, with diameters ranging from 20 to 130 nm. This size difference is to be expected as the hydrodynamic shell is not measured here by TEM.
Fig. 2.
Cryo-TEM picture of P188 NC.
3.2. Solubility in release media
Retinoic acid is a very hydrophobic molecule. Due to its poor solubility in water (<1 µg/ml), sink conditions for the tested concentrations could not be reached in PBS (Phosphate Buffered Saline). Surfactants had to be added to increase RA solubility in PBS pH 7.4. To do so, polysorbate 80 or SDS were added to PBS at 0.5% w/v. The surfactants increased RA solubility after 3 h of stirring, in PBS-PS80 and PBS-SDS to 92.3 ± 3.6 µg/ml and 84.6 ± 0.4 µg/ml respectively. RA solubility stayed constant after 24 h of stirring and was measured at 92.5 ± 2.1 µg/ml and 85.8 ± 2.9 µg/ml in PBS-PS80 and PBS-SDS respectively.
3.3. Dialysis release
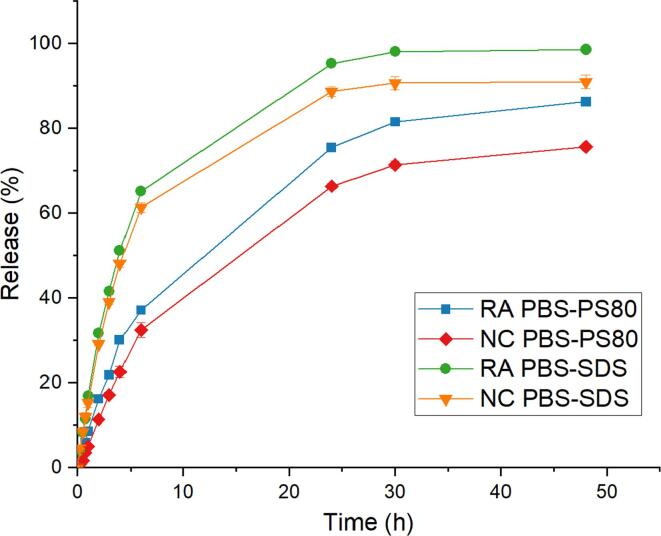
The release from the NC was tested by dialysis. Free RA dissolved in release medium was used as control to assess the permeation kinetic of RA through the dialysis membrane. Two release media were compared: PBS pH 7.4 supplemented with either polysorbate 80 or SDS. The permeation kinetic of RA through the membrane was slow, despite testing membranes with different MW cut-offs. A high MWCO dialysis membrane of 300 kDa was used, as when a lower MWCO (14 kD) dialysis membrane was used, dissolved RA was not able to not cross the membrane even after 48 h of stirring. It is very likely that the added surfactants formed micelles loading RA which prevented permeation of RA across the membrane despite the MWCO being, at least theoretically, sufficient for the permeation of this small molecule (MwRA = 300.44 g/mol).
In PBS-SDS and when using RA by itself, release took place over a time span of 30 h (Fig. 3). In PBS-PS80 media, only 85% of the full RA amount passed the membrane towards the acceptor compartment after 48 h. The longer release time in PS80 might again be due to the formation of PS80-micelles around RA, which inhibited the permeation of the RA molecule through membrane. There was no significant difference between the release profiles of RA encapsulated in PLGA and RA dissolved alone in the donor compartment, showing that the kinetic is controlled solely by the membrane permeation rather than the carriers. The release profile measured was therefore not representative of the actual release happening inside the dialysis tube. These results could have easily been misinterpreted if not for the control of the permeation kinetics of the free drug.
Fig. 3.
Release profile of P188 nanocapsules (NC) and dissolved retinoic acid (RA) in PBS-PS80 and PBS-SDS by dialysis. NC (n = 3) and RA (n = 1).
3.4. NanoDis release
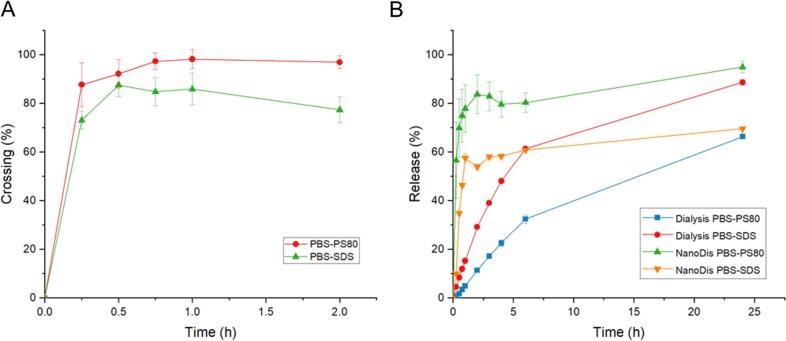
To determine the filter efficiency and the lag phase between the time the API is released and the time the API is found in the filtrate, a control sample of RA dissolved in release medium was used (Fig. 4.A). When using 500 kD filters, RA dissolved in medium could cross the filters after the first time point at a rate of 87% in PBS-PS80 and 82% in PBS-SDS. In PBS-PS80, 97% of the initial RA amount was found in the filtrate after the third time point (45 min) while in PBS-SDS, the crossing percentage remained stable at 80%. RA therefore demonstrated the ability to cross the hollow fiber filters in higher amount when dissolved in PBS-PS80 than in PBS-SDS, probably due to mild interaction between SDS and the filters.
Fig. 4.
A. Crossing of dissolved RA in PBS-PS80 and PBS-SDS through hollow fiber filters (n = 3); B. Comparison of release profile of P188 NC measured by dialysis or using the NanoDis System in PBS-PS80 and PBS-SDS (n = 3).
Next, NC-P188 was tested for drug release in the same release media in sink conditions. Release profiles using the NanoDis System were compared to the ones obtained by dialysis (Fig. 4.B). A high burst release of 80% and 60% after 1 h in PBS-PS80 and PBS-SDS respectively was observed with the NanoDis System, while only 5% and 15% release were measured by the dialysis method. With the NanoDis set-up, release was not limited by the permeation kinetic of the dialysis membrane, which allowed a much more accurate measurement of the burst release of the NC. The burst release of the NC was severely underestimated when using dialysis, particularly at short time points.
The measured release profile in PBS-SDS with the NanoDis System reached lower values than in PBS-PS80 at the same time, likely due to the interaction of RA micelles in SDS with the filters, as observed with the dissolved RA control. After 6 h, the measured dialysis release in PBS-SDS became higher than the one measured with the NanoDis. Thus, the selection of the release medium and its interaction with the filters should be carefully assessed before performing release experiments with the NanoDis System, as any interactions of media also impact the measured release profile of the NC and lead to an underestimation of the burst release, although less drastic than with the dialysis method.
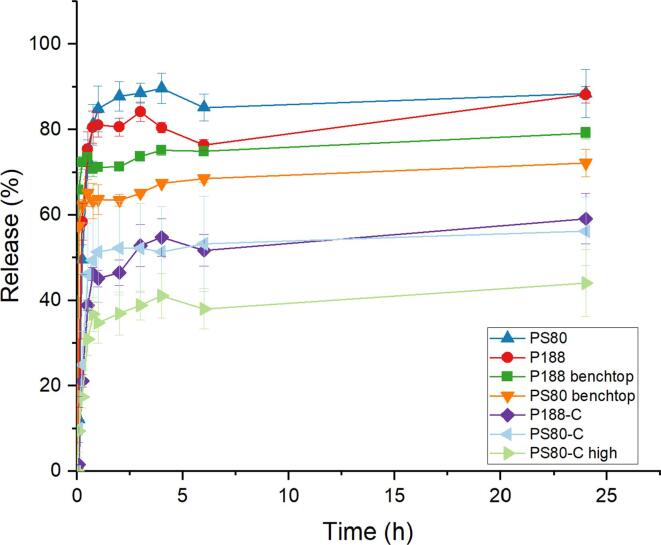
The release profiles of different NC with or without chitosan were measured using PBS-PS80 (Fig. 5) as media. NC prepared with either P188 or PS80 had similar release profiles with a high burst release of 85% after 1 h. When NC were coated with chitosan, burst release was markedly reduced to 50% for both NC prepared with PS80 or P188. As chitosan is positively charged, ionic interaction with retinoic acid —whose carboxylic group is negatively charged at neutral pH (pKa 4.76)— facilitates the control of the burst release. Increasing the amount of PLGA and oil in the chitosan-coated NC formulation decreased the burst release to 40% (PS80-C high). Increasing the polymer and oil contents resulted in a longer diffusion path for RA, due to the increase in size of the particles, and thereby decreased the burst release. Furthermore, increasing the PLGA and oil amount in the formulation increased the NC size, thus decreasing the surface/volume ratio. With a lower amount of RA exposed at the surface of the NC, a lower amount of RA could immediately dissolve in the medium, leading to a lower burst release. This reduction of burst release was also observed with the benchtop nanocapsules. Indeed, the NC prepared benchtop, with similar compositions but with larger size than their continuous-prepared counterparts, had a slightly lower burst release of 71% and 64% after 1 h, reaching 79% and 72% after 24 h, for P188 benchtop NC and PS80 benchtop NC respectively. Thus, diffusion seemed to have a slight impact in reducing the burst release. However, simply increasing the NC size, to even larger size than the nanocapsules coated with chitosan for PS80 NC, did not allow a reduction of the burst release to the same range as the NC coated with chitosan. Thus, the coating with chitosan seemed to be the driving force of the burst release reduction, rather than pure increase of the diffusion path.
Fig. 5.
Release profile of PLGA nanocapsules in PBS-PS80 using the NanoDis System (n = 3).
Reduction of the burst release from NC was therefore possible by coating them with chitosan. However, this change in surface charges might cause change in the protein corona forming around the NC, as the protein corona composition has been reported to be sensitive to size and charges (del Pino et al., 2014). The particles developed here were coated with surfactants to cross the BBB by receptor-mediated transcytosis, thanks to their protein corona composition enriched in apolipoproteins. Once coated with chitosan, the nanocapsules become positively charge and might then cross the BBB by adsorption-mediated transcytosis rather than by receptor-mediated transcytosis as initially planned. Furthermore, it was possible to further reduce the burst release of chitosan-coated NC by increasing their size. Larger NC might have more difficulty crossing the BBB by endocytosis. In conclusion, a compromise should be found between the parameters of the formulation to reduce burst release to a minimum while still conserving the ability of the NC to cross the BBB to deliver their cargo to the brain.
4. Conclusion
PLGA NC loaded with RA showed high burst release when measured using the NanoDis System, while this burst release could not be measured using a classic dialysis technique. The NanoDis System therefore showed clear superiority over dialysis for the accurate measurement of the release profile of nanoparticles. Optimization of the parameters of the experiment (membrane/filter type and MWCO, medium composition) should be carefully performed using appropriate control beforehand, to avoid misinterpretation of results and highly underestimate the burst release of the studied nanoformulations.
CRediT authorship contribution statement
Sonia M. Lombardo: Methodology, Investigation, Writing – original draft. Nazende Günday Türeli: Methodology, Writing – review & editing, Supervision, Project administration, Funding acquisition. Marcus Koch: Investigation. Marc Schneider: Methodology, Writing – review & editing, Supervision. Akif E. Türeli: Conceptualization, Methodology, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is part of the NANOSTEM project, a Marie Skłodowska-Curie Innovative Training Network (ITN). This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 764958.
References
- Makadia H.K., Siegel S.J., Makadia H.K., Siegel S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues de Azevedo C., von Stosch M., Costa M.S., Ramos A.M., Cardoso M.M., Danhier F., Préat V., Oliveira R. Modeling of the burst release from PLGA micro- and nanoparticles as function of physicochemical parameters and formulation characteristics. Int. J. Pharm. 2017;532(1):229–240. doi: 10.1016/j.ijpharm.2017.08.118. [DOI] [PubMed] [Google Scholar]
- Yoo J., Won Y.-Y. Phenomenology of the initial burst release of drugs from PLGA microparticles. ACS Biomater. Sci. Eng. 2020;6(11):6053–6062. doi: 10.1021/acsbiomaterials.0c01228. [DOI] [PubMed] [Google Scholar]
- Yang X., Trinh H.M., Agrahari V., Sheng Y.e., Pal D., Mitra A.K. Nanoparticle-based topical ophthalmic gel formulation for sustained release of hydrocortisone butyrate. AAPS PharmSciTech. 2016;17(2):294–306. doi: 10.1208/s12249-015-0354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh Kumar V., Verma P.R.P., Singh S.K. Development and evaluation of biodegradable polymeric nanoparticles for the effective delivery of quercetin using a quality by design approach. LWT – Food Sci. Technol. 2015;61(2):330–338. doi: 10.1016/j.lwt.2014.12.020. [DOI] [Google Scholar]
- Sant S., Nadeau V., Hildgen P. Effect of porosity on the release kinetics of propafenone-loaded PEG-g-PLA nanoparticles. J. Control. Release. 2005;107(2):203–214. doi: 10.1016/j.jconrel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang X., Yang J., Wang Y., Chen R., Wu J., Liu Y., Zhang N. Self-assembled nanoparticles of methotrexate conjugated o-carboxymethyl chitosan: preparation, characterization and drug release behavior in vitro. Carbohydr. Polym. 2011;86(4):1665–1670. doi: 10.1016/j.carbpol.2011.06.080. [DOI] [Google Scholar]
- Modi S., Anderson B.D. Determination of drug release kinetics from nanoparticles: overcoming pitfalls of the dynamic dialysis method. Mol. Pharmaceutics. 2013;10(8):3076–3089. doi: 10.1021/mp400154a. [DOI] [PubMed] [Google Scholar]
- Zambito Y., Pedreschi E., Di Colo G. is dialysis a reliable method for studying drug release from nanoparticulate systems?—A case study. Int. J. Pharm. 2012;434(1-2):28–34. doi: 10.1016/j.ijpharm.2012.05.020. [DOI] [PubMed] [Google Scholar]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms Available online: https://www.hindawi.com/journals/ap/2014/304757/ (accessed on 12 January 2021).
- Nothnagel L., Wacker M.G. How to measure release from nanosized carriers? Eur. J. Pharm. Sci. 2018;120:199–211. doi: 10.1016/j.ejps.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Siepmann J., Siepmann F. Sink conditions do not guarantee the absence of saturation effects. Int. J. Pharm. 2020;577:119009. doi: 10.1016/j.ijpharm.2019.119009. [DOI] [PubMed] [Google Scholar]
- Budhian A., Siegel S.J., Winey K.I. Controlling the in vitro release profiles for a system of haloperidol-loaded PLGA nanoparticles. Int. J. Pharm. 2008;346(1-2):151–159. doi: 10.1016/j.ijpharm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Hasan A.S., Socha M., Lamprecht A., Ghazouani F.E., Sapin A., Hoffman M., Maincent P., Ubrich N. Effect of the microencapsulation of nanoparticles on the reduction of burst release. Int. J. Pharm. 2007;344(1-2):53–61. doi: 10.1016/j.ijpharm.2007.05.066. [DOI] [PubMed] [Google Scholar]
- Nimesh S., Manchanda R., Kumar R., Saxena A., Chaudhary P., Yadav V., Mozumdar S., Chandra R. Preparation, characterization and in vitro drug release studies of novel polymeric nanoparticles. Int. J. Pharm. 2006;323(1-2):146–152. doi: 10.1016/j.ijpharm.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Dalwadi G., Benson H.A.E., Chen Y. Comparison of diafiltration and tangential flow filtration for purification of nanoparticle suspensions. Pharm. Res. 2005;22(12):2152–2162. doi: 10.1007/s11095-005-7781-z. [DOI] [PubMed] [Google Scholar]
- Balzus B., Colombo M., Sahle F.F., Zoubari G., Staufenbiel S., Bodmeier R. Comparison of different in vitro release methods used to investigate nanocarriers intended for dermal application. Int. J. Pharm. 2016;513(1-2):247–254. doi: 10.1016/j.ijpharm.2016.09.033. [DOI] [PubMed] [Google Scholar]
- Kakhki R.M. Application of nanoparticles in the potentiometric ion selective electrodes. Russ. J. Electrochem. 2013;49(5):458–465. doi: 10.1134/S1023193513050078. [DOI] [Google Scholar]
- Kontoyannis C.G., Douroumis D. Release study of drugs from liposomic dispersions using differential pulse polarography. Anal. Chim. Acta. 2001;449(1-2):135–141. doi: 10.1016/S0003-2670(01)01351-4. [DOI] [Google Scholar]
- Charalampopoulos N., Avgoustakis K., Kontoyannis C.G. Differential pulse polarography: a suitable technique for monitoring drug release from polymeric nanoparticle dispersions. Anal. Chim. Acta. 2003;491(1):57–62. doi: 10.1016/S0003-2670(03)00788-8. [DOI] [Google Scholar]
- Rosenblatt K.M., Douroumis D., Bunjes H. Drug release from differently structured monoolein/poloxamer nanodispersions studied with differential pulse polarography and ultrafiltration at low pressure. J Pharm Sci. 2007;96(6):1564–1575. doi: 10.1002/jps.20808. [DOI] [PubMed] [Google Scholar]
- Roese E., Bunjes H. Drug release studies from lipid nanoparticles in physiological media by a new DSC method. J. Control Release. 2017;256:92–100. doi: 10.1016/j.jconrel.2017.04.032. [DOI] [PubMed] [Google Scholar]
- Maia J., Santos T., Aday S., Agasse F., Cortes L., Malva J.O., Bernardino L., Ferreira L. Controlling the neuronal differentiation of stem cells by the intracellular delivery of retinoic acid-loaded nanoparticles. ACS Nano. 2011;5(1):97–106. doi: 10.1021/nn101724r. [DOI] [PubMed] [Google Scholar]
- Santos T., Ferreira R., Maia J., Agasse F., Xapelli S., Cortes L., Bragança J., Malva J.O., Ferreira L., Bernardino L. Polymeric nanoparticles to control the differentiation of neural stem cells in the subventricular zone of the brain. ACS Nano. 2012;6(12):10463–10474. doi: 10.1021/nn304541h. [DOI] [PubMed] [Google Scholar]
- Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood-brain barrier breakdown in alzheimer disease and other neurodegenerative disorders. Nature Rev. Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter J., Alyautdin R.N., Kharkevich D.A., Ivanov A.A. Passage of peptides through the blood-brain barrier with colloidal polymer particles (Nanoparticles) Brain Res. 1995;674(1):171–174. doi: 10.1016/0006-8993(95)00023-J. [DOI] [PubMed] [Google Scholar]
- Kreuter J., Petrov V.E., Kharkevich D.A., Alyautdin R.N. Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood-brain barrier using surfactant-coated nanoparticles. J. Control. Release. 1997;49(1):81–87. doi: 10.1016/S0168-3659(97)00061-8. [DOI] [Google Scholar]
- Gelperina S., Maksimenko O., Khalansky A., Vanchugova L., Shipulo E., Abbasova K., Berdiev R., Wohlfart S., Chepurnova N., Kreuter J. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur. J. Pharm. Biopharm. 2010;74(2):157–163. doi: 10.1016/j.ejpb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Petri B., Bootz A., Khalansky A., Hekmatara T., Müller R., Uhl R., Kreuter J., Gelperina S. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: revisiting the role of surfactants. J. Control. Release. 2007;117(1):51–58. doi: 10.1016/j.jconrel.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Kreuter J., Shamenkov D., Petrov V., Ramge P., Cychutek K., Koch-Brandt C., Alyautdin R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 2002;10(4):317–325. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- del Pino P., Pelaz B., Zhang Q., Maffre P., Ulrich Nienhaus G., Parak W.J. Protein corona formation around nanoparticles – from the past to the future. Mater. Horiz. 2014;1:301–313. doi: 10.1039/C3MH00106G. [DOI] [Google Scholar]