Summary
The differentiation of lymphatic progenitors is a crucial step in lymphangiogenesis. However, its underlying mechanism remains unclear. Here, we found that noncanonical protease-activated receptor 1 (par1) regulates the differentiation of lymphatic progenitors in zebrafish embryos. Loss of par1 function impaired lymphatic differentiation by downregulating prox1a expression in parachordal lymphangioblasts and caused compromised thoracic duct formation in zebrafish. Meanwhile, the G protein gnai2a, a par1 downstream effector, was selectively required for lymphatic development in zebrafish, and its mutation mimicked the lymphatic phenotype observed in par1 mutants. Interestingly, mmp13, but not thrombin, was required for lymphatic development in zebrafish. Furthermore, analyses of genetic interactions confirmed that mmp13b serves as a par1 upstream protease to regulate lymphatic development in zebrafish embryos. Mechanistically, par1 promotes flt4 expression and phospho-Erk1/2 activity in the posterior cardinal vein. Taken together, our findings highlight a function of par1 in the regulation of lymphatic differentiation in zebrafish embryos.
Subject areas: Biological sciences, Molecular biology, Immunology
Graphical abstract
Highlights
-
•
The Mmp13b-Par1-Gnai2a axis regulates lymphatic differentiation in zebrafish
-
•
Par1 mutant showed decreased prox1a expression in parachordal lymphangioblasts
-
•
Par1 promotes flt4 expression in the posterior cardinal vein of zebrafish embryos
Biological sciences; Molecular biology; Immunology
Introduction
The lymphatic system is a complex vasculature that originates from a preexisting embryonic vein. It has crucial functions in maintaining the interstitial fluid balance and retrieving water and macromolecules, taking up lipids, and also providing the major conduit for immune cells to take part in the immune surveillance system (Alitalo, 2011). Malformation of the lymphatic system can lead to many pathologies, such as tissue fluid accumulation, edema or lymphedema, cancer cell dissemination, and inflammation (Schulte-Merker et al., 2011).
During the development of the lymphatic system of mice, a sub-population of venous endothelial cells (VECs) expresses the transcription factor prospero homeobox protein 1 (PROX1) and differentiates into lymphatic progenitors in the cardinal vein (CV) at embryonic day 9.5 (E9.5) (Wigle et al., 2002; Wigle and Oliver, 1999). Ablation of prox1 in mice prevents the development of lymphatic vessels, and the ectopic expression of prox1 in endothelial cells (ECs) of blood vasculature upregulates lymphatic endothelial cell (LEC) markers (Kim et al., 2010; Wigle and Oliver, 1999). These studies suggest that prox1 is both necessary and sufficient to induce LEC fate (Hong et al., 2002; Wigle et al., 2002). In zebrafish embryos, lymphatic progenitors are induced within the ventral posterior cardinal vein (PCV) at an early stage, approximately 26 h postfertilization (hpf) (Nicenboim et al., 2015). The LECs show prox1a expression and move away to vacate the PCV in response to the sprouting process (Koltowska et al., 2015). These lymphatic progenitors migrate to the horizontal myoseptum (HM) region to form parachordal lymphangioblasts (PLs), a pool of lymphatic precursor cells, at about 48 hpf. Afterward, the PLs migrate in two directions, ventrally to form the thoracic duct (TD) and dorsally to form the dorsal longitudinal lymphatic vessel (DLLV); this is completed at around 5 days postfertilization (dpf) (Cha et al., 2012). Maternal and zygotic prox1a mutant leads to defective lymphatic development in zebrafish embryos, indicating its conserved role in the lymphatic vasculature. Recent studies have shown that a crucial role of Vegfc-Flt4 and its downstream effector Erk1/2 involves the induction of prox1a expression and LEC sprouting in zebrafish trunk (Koltowska et al., 2015; Shin et al., 2016).
Protease-activated receptor 1 (PAR1) is a G-protein-coupled receptor (GPCR), more specifically, a thrombin receptor (F2r) that plays a critical role in vascular biology (Alberelli and De Candia, 2014; Coughlin, 2005). It is activated through cleavage of the N-terminal exodomain by the serine protease thrombin (F2) at a canonical site (Vu et al., 1991). However, other PAR1 upstream proteases have been found to activate PAR1 (Alberelli and De Candia, 2014). For example, matrix metalloproteases (MMPs), especially MMP1 and MMP13, can also cleave and activate PAR1 at a noncanonical site, which leads to a signaling pattern that is distinct from that seen with F2 (Austin et al., 2013; Galt et al., 2002; Jaffré et al., 2012; Trivedi et al., 2009). Once irreversibly cleaved and activated by different proteases at either canonical or noncanonical sites, PAR1 can couple to and activate multiple heterotrimeric G protein subtypes including G12/13, G11/q, and GI . This results in a multitude of cellular signaling events including the activation of MAPK/ERK signaling (Soh et al., 2010; Zhao et al., 2014). Par1 has been reported to play a pivotal role in hematopoiesis (Yue et al., 2012) and cardiovascular development in zebrafish (Ellertsdottir et al., 2012). Although par1 is enriched in the PCV of zebrafish embryos at 1 dpf (Xu et al., 2011), its role within it remains unknown.
Here, we report that the Mmp13b-Par1-Gnai2a axis regulates the differentiation of lymphatic progenitors in zebrafish embryos. Mechanistically, we show that par1 promotes Erk1/2 activity and the expression of the lymphatic fate marker prox1a by regulating flt4 expression.
Results
par1 is required for trunk lymphatic development in zebrafish embryos
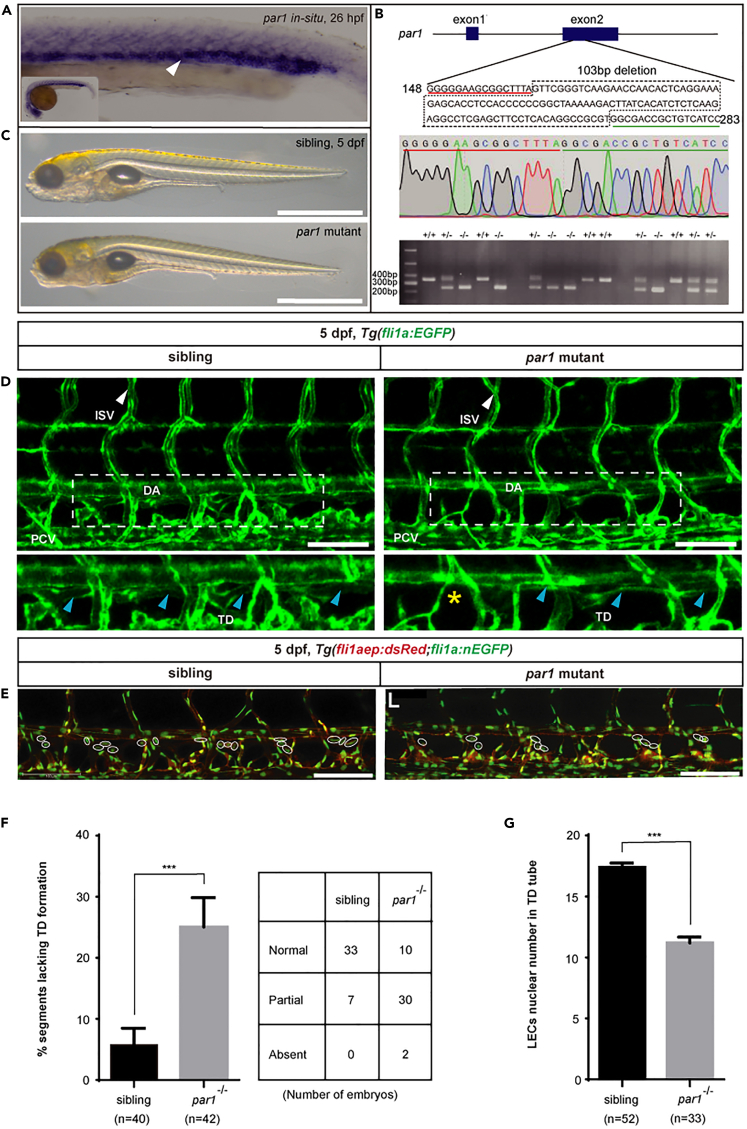
PCV acts as a source of trunk lymphatic progenitors in zebrafish embryos (Yaniv et al., 2006). Interestingly, our results from whole-mount in situ hybridization (WISH) showed that par1 is highly expressed in the PCV region of zebrafish embryos at 26 hpf (Figure 1A), consistent with a previous report (Xu et al., 2011). Hence, par1 could be involved in lymphatic development during embryogenesis in zebrafish. To investigate this, we successfully generated a zebrafish mutant of par1 harboring a 103 bp deletion within its exon 2 using CRISPR/Cas9 technology (Figure 1B). We found that F2-generation embryos of the mutants seemed to be normal without any severe defects (Figure 1C). However, at 5 dpf, they showed impaired TD formation (Figures 1D and 1F). In siblings, only 6% of somites lacked TDs (Figures 1D and 1F). In contrast, nearly 25% of somites in par1 zebrafish mutants lacked them (Figures 1D and 1F). In addition, mutants had an average of only 11.2 number of LEC nuclei/6 somites (Figures 1E and 1G), significantly less than 17.5 found in sibling embryos (Figures 1E and 1G).
Figure 1.
par1 mutant zebrafish embryos show defective TD formation
(A) WISH of par1 gene expression in zebrafish embryos at 26 hpf. The white arrowhead indicates the expression of the par1 gene in the PCV.
(B) Brightfield lateral views of sibling and par1 homozygous mutant zebrafish embryos at 5 dpf. Scale bars: 1 mm.
(C) Top row, schematic representation of the generated par1 zebrafish mutant; middle, results of sequencing for validating par1 mutants; bottom, DNA gel results for genotyping wildtype (+/+), heterozygous (+/−), and homozygous mutant (−/−) embryos.
(D) Confocal images showing TD formation of sibling and par1 homozygous mutants using the Tg(fli1a:EGFP) line at 5 dpf. Blue arrowheads indicate the presence of TD formation in each somite; yellow asterisks represent the absence of TD formation in each somite; DA and PCV areas are denoted. Scale bars: 100 μm.
(E) Confocal images showing the nuclear numbers of LECs in the TD tube in Tg(fli1aep:dsRed;fli1:nEGFP) siblings and par1 zebrafish mutants at 5 dpf. White circles indicate the presence of LECs nuclear numbers in the TD tube. Scale bars: 100 μm.
(F) Percentage of somites lacking TDs in siblings (n = 40 embryos) and par1 homozygous mutants (n = 42 embryos); 6 somites/embryos were used for quantification. Right is the table showing the number of embryos with normal TD, partial TD, and without TD, respectively.
(G) LECs nuclear number in the TD tube of siblings (n = 52 embryos) and par1 homozygous mutants (n = 33 embryos); 6 somites/embryos were used for quantification. In (F) and (G), values represent means ± SEMs. ∗p ≤ 0.001 in the Student's t test. See also Figure S1.
Taken together, these results confirmed that par1 is required for TD formation, which implies that it is involved in the development of the lymphatic trunk during zebrafish embryogenesis.
par1 regulates the differentiation of lymphatic progenitors in zebrafish
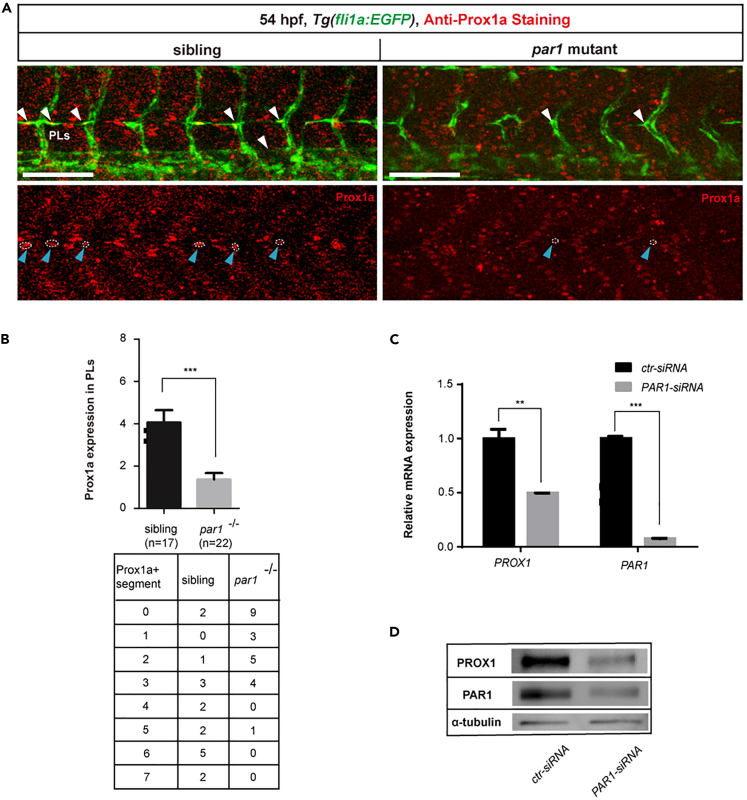
During the lymphatic development of zebrafish, Prox1a-positive endothelial cells sprout dorsally from the PCV at 30 hpf and form PLs as a pool of lymphatic progenitors at 48 hpf. At the same time, VECs sprout to form the venous intersegmental vessel (vISV) in parallel, which then fuses with the arterial intersegmental vessel (aISV) to establish a circulatory network with alternating arterial and venous connections (Mulligan and Weinstein, 2014; Semo et al., 2016). To determine whether par1 is involved in lymphatic differentiation, we first performed a whole embryo immunostaining assay to examine expression of Prox1a in PLs of Tg(fli1a:EGFP) line at 54 hpf. Compared with sibling embryos, par1 zebrafish mutants showed a comparable reduction of prox1a expression in PLs (Figures 2A and 2B). Concomitant with the role of par1 in regulating prox1a expression in zebrafish embryos, we knocked down the expression of PAR1 in human dermal lymphatic endothelial cells (HDLECs) using an siRNA method. Interestingly, the expression of PROX1 significantly decreased not only in terms of mRNA levels (Figure 2C) but also in terms of protein levels (Figure 2D). These results suggest a conserved role of PAR1 in regulating PROX1 expression in vivo and in vitro. To determine whether par1 is involved in venous and lymphatic sprouting in an earlier stage, we crossed par1 mutant with the Tg(lyve1b:dsRed;flk1:EGFP) line, in which lyve1b:dsRed labels both venous and lymphatic vessels. Sibling showed almost 100% venous and lymphatic sprouting from the PCV at 36 hpf (Figures S1A and S1B), whereas the par1 mutants showed only about 80% at the same stage (Figures S1A and S1B). Together, these results demonstrate that par1 is required for the differentiation of lymphatic progenitors in zebrafish embryos.
Figure 2.
par1 is required for lymphatic differentiation in zebrafish embryos
(A) Immunostaining of prospero homeobox protein-1a (Prox1a) in Tg(fli1a:EGFP) siblings and par1 homozygous mutants at 54 hpf. White arrowheads indicate the presence of parachordal lymphangioblasts (PLs) sprouting in each somite; blue arrowheads with white dashed lines represent positive Prox1a staining in PLs. Scale bars: 100 μm.
(B) Prox1a-positive PLs in siblings (n = 17 embyos) and par1 homozygous mutants (n = 22 embryos) at 54 hpf; 7 somites/embryos were used for quantification. Table shows the number of prox1a-positive segment per 7 somites in each embryo.
(C) Relative mRNA expression of PROX1 and PAR1 in human dermal lymphatic endothelial cells (HDLECs) after ctr-siRNA (control) and PAR1-siRNA transfection.
(D) Western blot analysis of PROX1 and PAR1 expression upon PAR1 knockdown in HDLECs. In (C and D), values represent means ± SEMs. ∗p ≤ 0.01, ∗∗p ≤ 0.001 in the Student's t test.
gnai2a is selectively required for trunk lymphatic development in zebrafish embryos
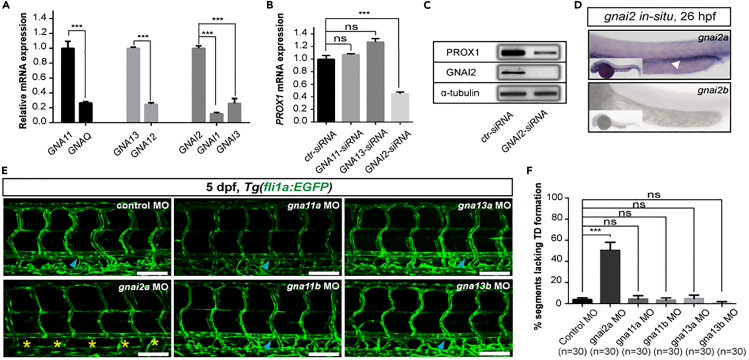
A number of different guanine-nucleotide-binding G-protein α-subunits function as downstream effectors of PAR1, including members of the GI , Gq/11, and G12/13 sub-families (Soh et al., 2010; Zhao et al., 2014). In seeking an alternative G protein involved in par1-mediated lymphangiogenesis in zebrafish embryos, we first performed quantitative real-time PCR (qPCR) analysis of HDLECs using seven G proteins from different subclasses including GNAQ, GNA11, GNA12, GNA13, GNAI1, GNAI2, and GNAI3. We found that only GNA11, GNA13, and GNAI2 had a relatively high mRNA expression level (Figure 3A). Then, we knocked down the expression of these three genes in HDLECs using an siRNA method and validated the expression level of PROX1. Surprisingly, the results showed that only knockdown of GNAI2 caused a significant decrease in PROX1 expression in terms of both mRNA (Figure 3B) and protein levels (Figure 3C). This indicates that GNAI2 is more likely to be involved in regulating lymphatic differentiation than other G proteins.
Figure 3.
gnai2a is selectively required for lymphatic development in zebrafish embryos
(A) Relative mRNA expression of different G-protein-coupled receptors in HDLECs.
(B) Relative mRNA expression of PROX1 in HDLECs upon transfection with ctr-siRNA (control), GNA11-siRNA, GNA13-siRNA, and GNAI2-siRNA.
(C) Western blot analysis of PROX1 and GNAI2 expression upon GNAI2 knockdown in HDLECs.
(D) WISH of gnai2a gene expression (upper) and gnai2b gene expression (below) at 26 hpf in zebrafish embryos. The white arrowhead indicates the PCV area.
(E) Confocal images showing TD formation in Tg(fli1a:EGFP) injected with 4 ng control MO, 4 ng gnai2a MO, 4 ng gnai11a MO, 4 ng gnai11b MO, 4 ng gna13a MO, and 4 ng gna13b MO at 5 dpf. Scale bars: 100 μm.
(F) Percentage of somites lacking TD formation. For each group, 30 embryos were quantified, and 6 somites/embryo were used for quantification. In (A), (B), and (F), values represent means ± SEMs. ∗p ≤ 0.001; ns, not significant in Student's t test.
Next, we analyzed the expression pattern of gnai2 homologous genes (gnai2a and gnai2b) in zebrafish embryos via WISH. The results showed that gnai2a, but not gnai2b, is highly expressed in the PCV region in which the lymphatic trunk originated at 26 hpf (Figure 3D). To support this result, we then carried out knockdown assays by injecting gna11a MO, gna11b MO, gna13a MO, gna13b MO, as well as gnai2a MO into embryos in the one-cell stage. We inspected TD formation at 5 dpf in each respective zebrafish morphant. Interestingly, we discovered that only gnai2a morphants exhibited defective TD formation, with more than 50% of somites lacking TDs (Figures 3E and 3F). In contrast, control embryos and gna11a, gna11b, gna13a, and gna13b morphants showed normal TD formation (Figures 3E and 3F). These results indicate that gnai2a is selectively required for lymphatic development in zebrafish embryos.
gnai2a recapitulates par1-mediated phenotypes during lymphatic development in zebrafish
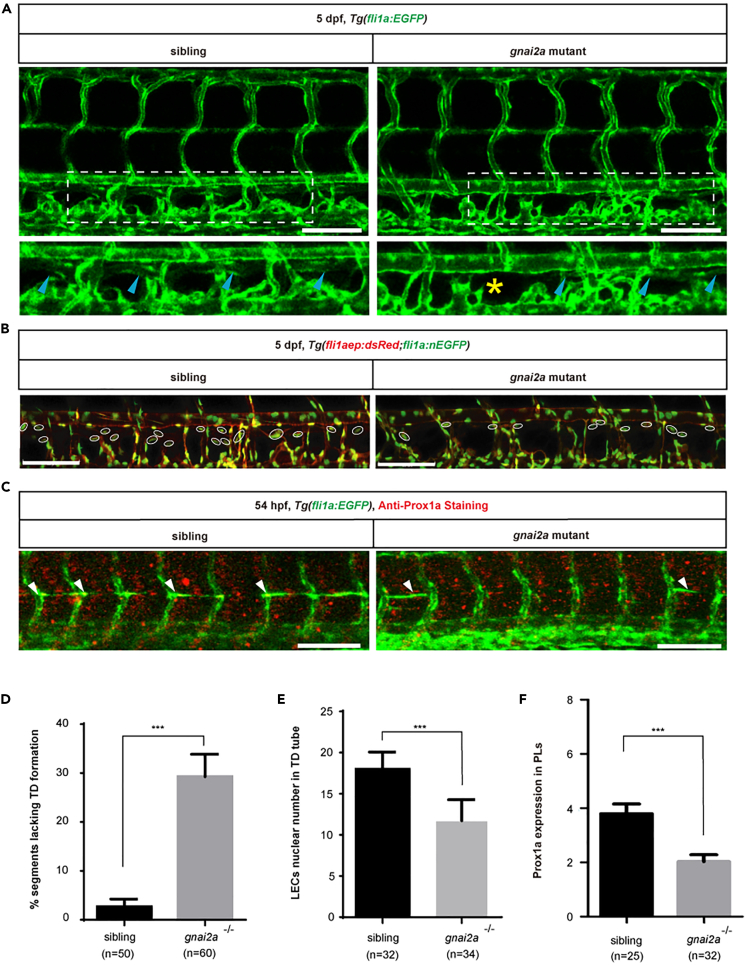
To investigate the role of gnai2a during zebrafish lymphangiogenesis, we used CRISPR/Cas9 technology to obtain a gnai2a mutant harboring a 68 bp deletion in its exon 3 (Figure S2A). The F2 generations of most mutants had normal phenotypes at 5 dpf (Figure S2B). Next, we examined TD formation at 5 dpf during embryogenesis. As shown in Figure 4, nearly 30% of somites lacked TDs in the mutants, significantly more than sibling embryos. In addition, we assessed the number of LEC nuclei/6 somites in TD tubes at 5 dpf using the transgenic zebrafish line Tg(fliaep:dsRed;fli1a:nEGFP). The mutants had notably decreased numbers, with an average of 11.7 compared with an average of 18.2 in sibling embryos (Figures 4B and 4E).
Figure 4.
gnai2a mutant mimics the lymphatic phenotypes observed in the par1 mutant
(A) Confocal images showing TD formation in Tg(fli1a:EGFP) siblings and gnai2a homozygous mutants at 5 dpf. Blue arrowheads indicate TD formation in each somite; yellow asterisks represent the absence of TD formation in each somite. Scale bars: 100 μm.
(B) Confocal images showing LECs nuclear numbers in the TD tube of siblings and gnai2a homozygous mutants in the Tg(fli1aep:dsRed;fli1:nEGFP) line at 5 dpf. White circles indicate the presence of LECs nuclear numbers in the TD tube. Scale bars: 100 μm.
(C) Immunostaining of Prox1a of siblings and gnai2a homozygous mutants in the Tg(fli1a:EGFP) line at 54 hpf. White arrowheads indicate positive Prox1a staining in PLs. Scale bars: 100 μm.
(D) Percentage of somites lacking TD formation in siblings (n = 50) and gnai2a homozygous mutants (n = 60 embryos); 6 somites/embryos were used for quantification.
(E) LEC nuclear number in the TD tube of siblings (n = 32) and gnai2a homozygous mutants (n = 34 embryos); 6 somites/embryos were used for quantification.
(F) Prox1a-positive PLs of siblings (n = 25) and gnai2a homozygous mutants (n = 32 embryos) at 54 hpf; 7 somites/embryos were used for quantification. In (D–F), values represent means ± SEMs. ∗p ≤ 0.001 in the Student's t test. See also Figures S2–S4.
To evaluate whether gnai2a is required to regulate lymphatic differentiation, we conducted an immunostaining assay using anti-Prox1 antibody at 54 hpf in Tg(fli1a:EGFP) embryos. We found that F2 generations of mutants had fewer prox1a-positive PLs than sibling embryos (Figures 4C and 4F). Meanwhile, we found that knockdown of gnai2a caused a partial failure in the formation of lympho-venous sprouting at 36 hpf, compared with control embryos with normal sprouting (Figures S3A and S3B). This suggests that gnai2a is required for lymphatic differentiation in zebrafish.
To further validate that gnai2a is the downstream effector of par1 in this process, we cross-bred par1 heterozygous mutant and gnai2a heterozygous mutant using the Tg(fli1a:EGFP) line and Tg(fli1aep:dsRed;fli1:nEGFP) line. We found that wild-type embryos, par1 heterozygous mutants, gnai2a heterozygous mutants, and cross-bred par1-gnai2a heterozygous mutants had normal morphologies (Figure S4E) and did not display a comparable difference in TD formation (Figures S4A and S4C). However, interestingly, we found that the average number of LEC nuclei/6 somites in the cross-bred mutants was 13.2, compared with 17.6 in wild-type embryos and about 15 in each par1 heterozygous mutant and gnai2a heterozygous mutant (Figures S4B and S4D). This indicates that there is a genetic interaction between par1 and gnai2a in cross-bred par1-gnai2a heterozygous zebrafish mutants, especially at the cellular level.
Taken together, these results show that the gnai2a zebrafish mutant mimics the phenotypes of par1 zebrafish mutants, indicating that gnai2a is the downstream effector of par1 in the regulation of zebrafish lymphangiogenesis.
Thrombin is not involved in zebrafish lymphangiogenesis
F2 was the first ligand to be reported to have the ability to cleave and activate PAR1 at a canonical site (Vu et al., 1991). To elucidate whether F2 is required for lymphatic development in zebrafish embryos, we injected F2 MO into embryos (4 ng/embryo) in the one-cell stage. At 5 dpf, we analyzed TD formation and unexpectedly found that both control and F2 morphants had normal TD formation (Figures S5A and S5C). To further validate this result, we treated zebrafish embryos with SCH79797, a specific PAR1 antagonist that blocks the interaction between F2 and PAR1. Consistent with the above results, both treated and vehicle control embryos exhibited normal TD formation at 5 dpf (Figures S5B and S5D), suggesting that par1 is independent of F2 in regulating lymphatic development in zebrafish. These results clearly indicate that F2 is not required for lymphatic development in zebrafish.
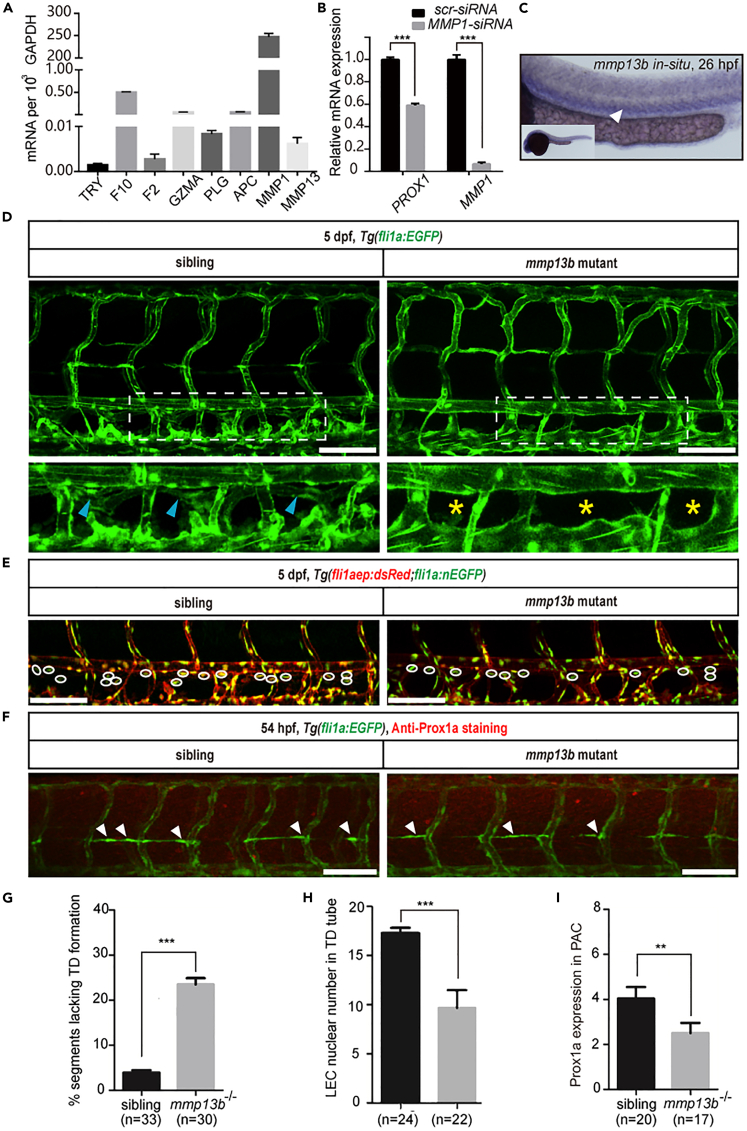
Noncanonical mmp13b is required for lymphatic development in zebrafish
Several proteases cleave and activate PAR1, including F2, plasmin, activated protein C, thrombocytin, factor Xa, factor VIIa, tryptase, trypsin, MMPs, and so on (Zhao et al., 2014). To search for candidate proteases involved in PAR1 regulation of lymphangiogenesis in zebrafish, we first conducted qPCR analyses of HDLECs and observed the relative mRNA expression of these proteases. Interestingly, MMP1 showed the highest relative mRNA expression (Figure 5A). Next, we transfected MMP1-siRNA into HDLECs and evaluated the expression of PROX1. Interestingly, knockdown of MMP1 caused a significant reduction of PROX1 mRNA expression, suggesting that MMP1 may be involved in lymphangiogenesis (Figure 5B).
Figure 5.
mmp13b mutant mimics the lymphatic phenotypes observed in the par1 mutant
(A) Relative mRNA expression of different PAR1 proteases in HDLECs.
(B) Relative mRNA expression of PROX1 and MMP1 in HDLECs after ctr-siRNA (control) and MMP1-siRNA transfection.
(C) WISH of mmp13b gene expression in zebrafish at 26 hpf. The white arrowhead indicates the expression of the mmp13b gene in the PCV area.
(D) Confocal images showing TD formation in Tg(fli1a:EGFP) siblings and mmp13b homozygous mutants at 5 dpf. Blue arrowheads indicate TD formation in each somite; yellow asterisks represent the absence of TD formation in each somite; DA and PCV areas are denoted. Scale bars: 100 μm.
(E) Confocal images showing LECs nuclear numbers in the TD tube of siblings and mmp13b homozygous mutants in the Tg(fli1aep: dsRed;fli1:nEGFP) line at 5 dpf. White circles indicate the presence of LECs nuclear numbers in the TD tube. Scale bars: 100 μm.
(F) Immunostaining of Prox1a of siblings and mmp13b homozygous mutants in the Tg(fli1a:EGFP) line at 54 hpf. White arrowheads indicate Prox1a-positive staining in the PLs. Scale bars: 100 μm.
(G) Percentage of somites lacking TD formation in siblings (n = 33 embryos) and mmp13b homozygous mutants (n = 30 embryos); 6 somites/embryos were used for quantification.
(H) LEC nuclear numbers in the TD tube of siblings (n = 24 embryos) and mmp13b homozygous mutants (n = 22 embryos); 6 somites/embryos were used for quantification.
(I) Prox1a-positive PLs in siblings (n = 20 embryos) and mmp13b homozygous mutants (n = 17 embryos) at 54 hpf; 7 somites/embryos were used for quantification. In (G–I), values represent means ± SEMs. ∗p ≤ 0.01, ∗∗p ≤ 0.001 in Student's t test. See also Figures S5 and S6.
In the human genome, there are three collagenases (MMP1, MMP8, and MMP13). However, two of them (Mmp1 and Mmp8) have no zebrafish homolog, and only MMP13 is present in duplicate (Mmp13a and Mmp13b) in the zebrafish genome (Wyatt et al., 2009). mmp13a is expressed in myeloid lineage cells but not in the vein (Qian et al., 2005). Therefore, we speculated that mmp13b could replace the function of the human MMP1 gene to regulate par1-mediated lymphatic development in zebrafish embryos. We used WISH to observe the expression patterns of mmp13b during zebrafish embryogenesis. Interestingly, we found that it is highly expressed in the PCV region at 26 hpf, suggesting that it is involved in lymphatics (Figure 5C). Next, we generated a zebrafish mmp13b mutant using CRISPR/Cas9 technology and obtained a stable mutant harboring a 7 bp deletion in its exon 4 (Figure S6). To validate the role of mmp13b in regulating lymphangiogenesis, we examined TD formation in the F2 generation of embryos. As observed in the par1 zebrafish mutant, mmp13b mutant embryos showed nearly 25% of somites lacking TD at 5 dpf, compared with siblings with normal TD formation (Figures 5D and 5G). We also checked the number of LEC nuclei in TD tube with transgenic zebrafish line Tg(fliaep:dsRed;fli1a:nEGFP) at 5 dpf and found that the mutant had significantly fewer numbers than sibling embryos (Figures 5E and 5H). Furthermore, immunostaining assays of Prox1a expression in PLs at 54 hpf indicated that mutants also showed significantly reduced Prox1a expression compared with siblings (Figures 5F and 5I). Together, those data demonstrate that mmp13b is required for lymphatic development in zebrafish.
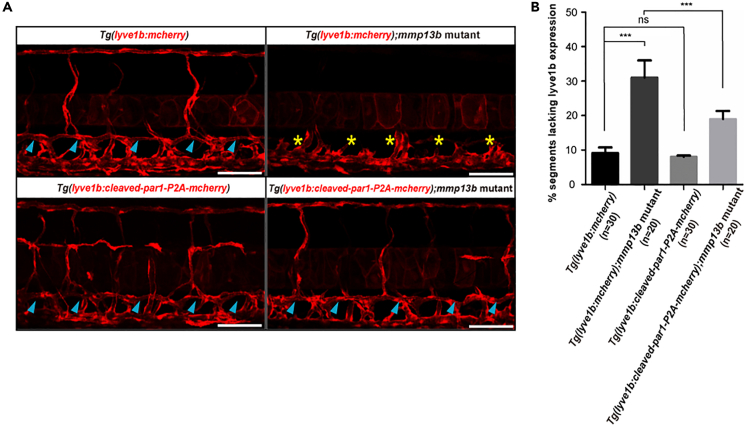
Overexpression of cleaved par1 recovers the phenotypes of mmp13b mutant in zebrafish lymphangiogenesis
As observed with the tethered ligand sequence (SFLLRN) in human PAR1, zebrafish Par1 also harbors a putative tethered ligand sequence (SFSGFFL) within the extracellular N-terminal region (Kim et al., 2009; Xu et al., 2011), which can induce thrombocyte activation (Kim et al., 2009). Meanwhile, MMP-13 can cleave PAR1 at the S42↓43FLLRN site, and cleaved PAR1 changes its conformation and activates downstream ERK1/2 signaling in cardiac cells (Jaffré et al., 2012). To confirm the function of mmp13b as an upstream par1 protease that regulates lymphatic development in zebrafish, we generated a zebrafish transgenic line, Tg(lyve1b:cleaved-par1-P2A-mCherry), in which cleaved Par1 at the S↓FSGFFL site (named cleaved-par1) was fused into cleaved-par1-P2A-mCherry and was driven by the venous and lymphatic promoter lyve1b. Then we crossed mmp13b mutant with the Tg(lyve1b:cleaved-par1-P2A-mCherry) or Tg(lyve1b:mCherry) line for rescue experiments. We examined TD formation at 5 dpf both for the mmp13b zebrafish mutant and siblings in these two transgenic lines. Compared with the normal TD formation of siblings in the Tg(lyve1b:mCherry) line at 5 dpf, the mmp13b zebrafish mutant lacked TD at 5 dpf (Figures 6A and 6B). Meanwhile, overexpression of par1 under the lyve1b promoter did not induce ectopic TD formation, and overexpression showed a normal TD morphology (Figures 6A and 6B). However, the mutant in the Tg(lyve1b:cleaved-par1-P2A-mCherry) background showed normal TD formation (Figures 6A and 6B), indicating that the overexpression of par1 in the mmp13b zebrafish mutant can recover the defect phenotype. Hence, mmp13b could serve as an upstream par1 protease that regulates lymphatic development in zebrafish embryos.
Figure 6.
Overexpression of cleaved par1 recovers the lymphatic phenotypes observed in mmp13b zebrafish mutants
(A) Confocal images showing TD formation of siblings and mmp13b homozygous mutants in the Tg(lyve1b:mcherry) line and Tg(lyve1b:cleaved-par1-P2A-mcherry) line at 5 dpf. Blue arrowheads indicate TD formation in each somite; yellow asterisks represent the absence of TD formation in each somite. Scale bars: 100 μm.
(B) Percentage of somites lacking TD formation in siblings with the Tg(lyve1b:mcherry) line (n = 30 embryos), mmp13b homozygous mutants with the Tg(lyve1b:mcherry) line (n = 20 embryos), siblings with the Tg(lyve1b:cleaved-par1-P2A-mcherry) line (n = 30 embryos), and mmp13b homozygous mutants with the Tg(lyve1b:cleaved-par1-P2A-mcherry) line (n = 20 embryos); 6 somites/embryos were used for quantification. In (B), values represent means ± SEMs. ∗p ≤ 0.01, ∗∗p ≤ 0.001 in the Student's t test.
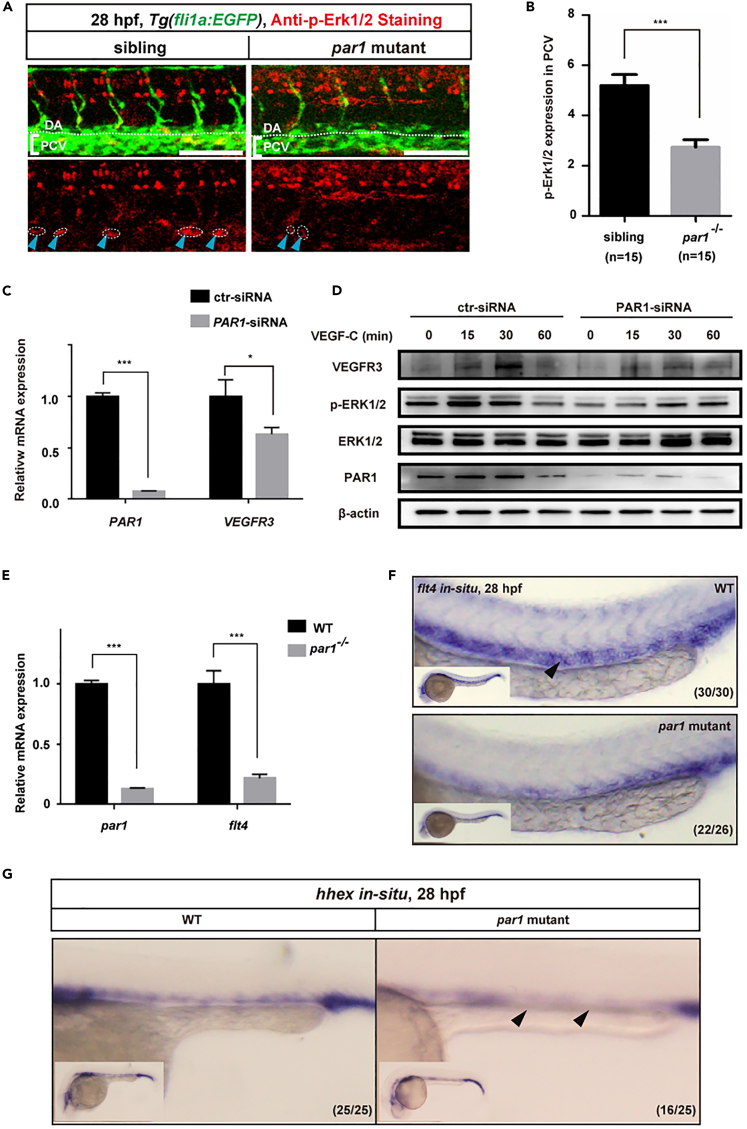
par1 promotes Erk1/2 activity and flt4 expression in zebrafish embryos
Vegfc and its receptor Flt4 activate p-Erk1/2 by inducing prox1a expression, ultimately regulating the differentiation of lymphatic progenitor cells in zebrafish embryos (Koltowska et al., 2015; Küchler et al., 2006; Shin et al., 2016). Based on our above results, we propose that par1 may promote Vegfc/Flt4/Erk signaling activity to regulate lymphangiogenesis in zebrafish embryos. To test this hypothesis, we performed a whole-embryo p-Erk1/2 immunostaining assay using the F2 generations of Tg(fli1a:EGFP) embryos. At 28 hpf, when lymphatic progenitors start migrating dorsally from the PCV, we observed clear p-Erk1/2 staining in the PCV of sibling embryos (Figures 7A and 7B). However, there was significantly less staining in the PCV of par1 zebrafish mutants (Figures 7A and 7B). Next, to confirm whether PAR1 promotes VEGFC-induced p-ERK1/2 activation, we performed PAR1 knockdown experiments in cultured HDLECs and found that knockdown of PAR1 caused a significant decrease in VEGFR3 mRNA level (Figure 7C). We then evaluated the effect of PAR1 on VEGFC-induced phosphor-ERK1/2 activity in HDLECs in vitro. In HDLECs transfected with control-siRNA (ctr-siRNA), a significant increase in ERK1/2 phosphorylation was detected at 15 min. In contrast, HDLECs transfected with PAR1-siRNA showed a significant decrease in p-ERK1/2 levels (Figure 7D). Meanwhile, knockdown of PAR1 also caused a compromised VEGFR3 expression at the protein level (Figure 7D).
Figure 7.
Loss of par1 function decreases p-Erk1/2 activity and flt4 expression in the PCV of zebrafish embryos
(A) Immunostaining of phosphoErk1/2 (p-Erk1/2) activity in Tg(fli1a:EGFP) siblings and par1 homozygous mutants at 28 hpf. Blue arrowheads with white dashed lines indicate positive p-Erk1/2 staining; DA and PCV (in brackets) area are noted. Scale bars: 100 μm.
(B) Quantification of p-Erk1/2 expression staining in the PCV of siblings (n = 15 embryos) and par1 mutants (n = 15 embryos); 8 somites/embryos were used for quantification.
(C) Relative mRNA expression of VEGFR3 in HDLECs after ctr-siRNA (control) and PAR1-siRNA transfection.
(D) Western blot analysis of p-ERK1/2 activity in HDLECs transfected with ctr-siRNA or PAR1-siRNA, followed by VEGFC treatment.
(E) Relative mRNA expression of the flt4 gene in siblings and par1 homozygous zebrafish embryo mutants.
(F) WISH of flt4 gene expression at 28 hpf in wild-type and par1 homozygous mutant embryos. The white arrowhead indicates the PCV area.
(G) WISH of hhex gene expression at 28 hpf in wild-type and par1 mutant embryos. The white arrowhead indicates the PCV area. In (B, C, and, E), values represent means ± SEMs. ∗p ≤ 0.05, ∗∗p ≤ 0.001, ∗∗∗p ≤ 0.0001 in the Student's t test.
In parallel, we also examined the expression of flt4 during zebrafish embryogenesis. The results from qPCR analysis showed that loss of par1 function decreased flt4 mRNA levels in zebrafish embryos (Figure 7E). Meanwhile, par1 mutants clearly showed a reduction in flt4 expression at 28 hpf, compared with sibling in the same stage (Figure 7F). Transcription factor hhex has been reported to regulate flt4 mRNA levels in the PCV during the lymphatic development of zebrafish (Gauvrit et al., 2018). To determine whether par1 regulates hhex expression in zebrafish embryos, we performed WISH tests. par1 mutants showed decreased hhex expression in the PCV at 28 hpf, compared with siblings (Figure 7F). Together, these results demonstrate that par1 promotes flt4 expression during lymphatic development in zebrafish.
Discussion
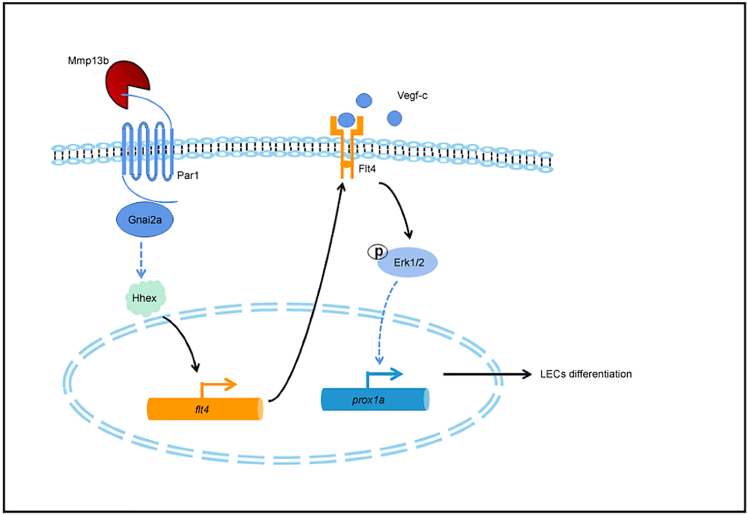
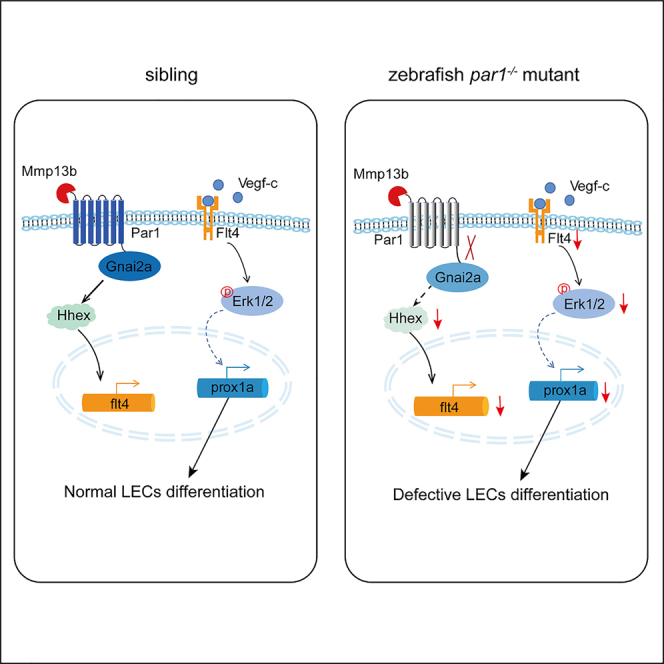
The PAR family, especially PAR1, is a critical mediator of vascular hemostasis, thrombosis, and inflammation (Alberelli and De Candia, 2014). Here, we found compelling evidence that the noncanonical Mmp13b-Par1-Gnai2a axis regulates the differentiation of trunk lymphatic progenitors by promoting flt4 expression in the PCV in the early stage (Figure 8).
Figure 8.
Work model
The mmp13b-Par1-Gnai2a axis regulates flt4 expression in the PCV in early stage zebrafish embryos probably through hhex transcription factor, therefore indirectly regulating prox1a expression and promoting the differentiation of lymphatic trunk progenitors.
In zebrafish embryos, trunk lymphatic progenitors originate from the PCV (Yaniv et al., 2006), suggesting that genes enriched in the PCV may be involved in lymphangiogenesis in zebrafish. We found several lines of evidence that par1 regulates lymphatic differentiation in zebrafish. First, par1 is highly enriched in the PCV at 26 hpf. Second, par1 regulates prox1 expression in vitro and in vivo. Third, par1 mutant shows decreased flt4 expression and compromised phospho-Erk1/2 activity in the PCV, both of which are crucial for lymphatic differentiation in zebrafish embryos (Koltowska et al., 2015; Shin et al., 2016). However, both defective venous-lymphatic sprouting and subsequent compromised TD formation are mild in the par1 mutant, whereas par1 homozygous mutants showed clear reduction in prox1a expression in PLs at 54 hpf and in the nucleus number of LECs in TD tubes at 5 dpf. These results suggest that par1 plays an important role in lymphatic differentiation rather than in lymphatic migration in zebrafish embryos. Consistent with this, prox1a mutant zebrafish embryos show normal TD formation (van Impel et al., 2014), whereas maternal and zygotic prox1a mutant zebrafish show a significant decrease in number of LEC nuclei (Koltowska et al., 2015).
Several G proteins (including GNA12/13, GNAQ/11, and GANI1/2/3) function as downstream effectors of PAR1 (Zhao et al., 2014). Here, we demonstrated that gnai2a is selectively required for par1-mediated lymphatic development in zebrafish embryos. Furthermore, gnai2a mutants mimicked the phenotypes observed in par1 mutants in terms of lymphatic development. We also performed genetic interaction analyses by cross-breeding par1 heterozygous zebrafish mutant and gnai2a heterozygous zebrafish mutants. The morphologies of the embryos did not show any significant differences at 5 dpf, compared with wild-type embryos. However, nucleus counting at 5 dpf indicated significantly fewer number of LEC nuclei in the par1-gnai2a heterozygous mutant embryos. This indicates that there is a genetic interaction at the cellular level between par1 and gnai2a.
Several PAR1 upstream proteases have been identified, such as F2, plasmin, factor Xa, factor VIIa, trypsin, MMPs, and others (Zhao et al., 2014). Surprisingly, canonical F2 was not involved in lymphatic development in zebrafish embryos. Although human MMP1 regulates PROX1 expression in HDLECs in vitro, our results strongly suggest that mmp13 serves as an upstream protease of par1 that regulates lymphatic development in zebrafish. In mice, there is no mmp1 gene, and MMP13 is thought to play the role of human MMP1, cleaving and activating PAR1 at the conserved S42↓43FLLRN site (Jaffré et al., 2012). In zebrafish, two orthologs of MMP13 (Mmp13a, Mmp13b) are present (Wyatt et al., 2009). mmp13a is expressed in myeloid lineage cells but not in the vein (Qian et al., 2005). Interestingly, we found that mmp13b is highly expressed in the PCV region of zebrafish embryos at 26 hpf. Furthermore, we found that zebrafish mmp13b mutants perfectly mimicked the phenotypes of par1 zebrafish mutants. Further analysis also revealed that the overexpression of cleaved par1 in mmp13b zebrafish mutants rescued the defective TD phenotype. These results suggest the mmp13b acts upstream of par1 to regulate lymphatic development in zebrafish embryos.
During LEC progenitors differentiation, these Prox1-positive cells migrate dorsally out of PCV, sprouting in response to signaling induced by VEGFC, through its receptor VEGFR3 or FLT4 to form the first lymphatic vessels (Haiko et al., 2008; Hogan et al., 2009a, 2009b; Karkkainen et al., 2004; Küchler et al., 2006). Disruption of vegfc or flt4 causes a severe defect in the lymphatic development of zebrafish and mice (Hogan et al., 2009a, 2009b; Karkkainen et al., 2004; Küchler et al., 2006; Villefranc et al., 2013; Yaniv et al., 2006). Further, Vegfc/Flt4 signaling can induce prox1a expression through Erk1/2, therefore initiating the differentiation and sprouting of lymphatic endothelial cells in the lymphatic trunk of zebrafish (Shin et al., 2016). In our study, we found several lines of evidence indicating that par1 regulates Vegfc/Flt4/Erk1/2 signaling during lymphatic development in zebrafish. First, loss of the par1 gene reduced flt4 expression. Second, the par1 zebrafish mutant showed decreased expression of hhex, an upstream transcription factor of flt4 in zebrafish embryos (Gauvrit et al., 2018). Third, disruption of par1 inhibited phospho-Erk1/2 activities in the PCV of zebrafish embryos and VEGFC-induced phospho-ERK1/2 activity in vitro. A high level of VEGFR3/FLT4 may be a crucial prerequisite for the sensitivity of LEC progenitors to the VEGF-C induction (Ducoli and Detmar, 2021). Consistent with this, our zebrafish par1 mutant showed significantly less prox1 expression and compromised flt4 expression. In contrast, disruption of the par1 gene caused a mild defect in venous-lymphatic sprouting and in subsequent TD formation. We speculate that high expression of flt4 is much more sensitive to lymphatic differentiation than that to venous-lymphatic sprouting in zebrafish embryos. Previous document reported that flt4 null mutant showed clear edema, whereas flt4Y1226/7Δ mutants have no edema (Shin et al., 2016). Further analysis revealed signaling through flt4 Y1226/7 and ERK is required for trunk lymphatic development but not for facial lymphatic formation. This indicated that facial lymphatic vessels, but not the thoracic duct, are initially dispensable for lymphatic function in zebrafish embryos. In this study, we found that par1 mutant, mmp13b mutant, and gnai2a mutant have no edema. It is possibility that par1 signaling mainly regulated trunk lymphatic development in zebrafish embryos through flt4 and Erk1/2 signaling, which supported the previous notion (Shin et al., 2016).
Taken together, our results uncover a mechanism in which the mmp13b-par1-gnai2a axis regulates the lymphangiogenic process in the lymphatic trunk of zebrafish by promoting Vegfc/Flt4/Erk1/2 signaling, which induces prox1a. expression.
Limitation of the study
In our study, we generated three mutants and found that mmmp13b-par1-gnai2a axis regulates the differentiation of lymphatic progenitors in zebrafish embryos. Mechanistically, par1 promotes flt4 expression and phospho-Erk1/2 activity in the posterior cardinal vein. However, the mechanism linking the mmp13b-par1-gnai2a pathway to regulation of flt4 would need to be further expanded.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Recombinant anti-PROX1 antibody | GeneTex | Cat#GTX128354;RRID:AB_2893482 |
| F2R rabbit pAb | ABclonal | Cat#A5641;RRID:AB_2766401 |
| GNAI2 rabbit pAb | ABclonal | Cat#A7676;RRID:AB_2769633 |
| Anti-alpha tubulin antibody | Abcam | Cat#ab18251;RRID:AB_2210057 |
| GAPDH (D16H11) XP® Rabbit mAb | Cell Signaling Technology | Cat#5174;RRID:AB_10622025 |
| p44/42 MAPK (Erk1/2) (137F5) Rabbit mAb | Cell Signaling Technology | Cat#4695;RRID:AB_390779 |
| Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) XP® Rabbit mAb | Cell Signaling Technology | Cat#4370;RRID:AB_2315112 |
| ß-actin | ZEN BIO | Cat#380624;RRID:AB_2893488 |
| Goat anti-Mouse IgG (H&L) (HRP conjugate) | ZEN BIO | Cat# 511103;RRID:AB_2893489 |
| Goat anti-rabbit IgG (H+L) secondary antibody 555 | ThermoFisher Scientific | Cat#A21434;RRID:AB_2535855 |
| Chemicals, peptides, and recombinant proteins | ||
| TritonX-100 | Sigma | T9284 |
| Bovine Serum Albumin | Sigma | A9647 |
| 4x protein loading buffer | Li-cor | 928-40004 |
| PTU | Sigma | 2954-52-1 |
| Tricaine | Sigma | 886-86-2 |
| SCH79797 dihydrochloride | MCE | HY-14994 |
| BM Purple AP | Roche | 11442074001 |
| RNA from yeast | Roche | 10109223001 |
| Proteinase K | MERCK | 39450-01-6 |
| Recombinant human VEGF-C (Cys156Ser) protein | R&D Systems | Cat# 752-VC-025 |
| Critical commercial assays | ||
| Tol2 kit | (Kwan et al., 2007) | N/A |
| LR Clonase II Plus Enzyme mix | Invitrogen | 12538200 |
| HiScribe T7 ARCA mRNA kit | NEB | #E2065S |
| DIG RNA Labeling Mix | Roche | 11277073910 |
| TB Green Fast RT-PCR Mix | TAKARA | RR430A |
| Experimental models: Cell lines | ||
| Human Primary Lymphatic Endothelial Cells | CellBiologics | H-6092 |
| Experimental models: Organisms/strains | ||
| Zebrafish: Tg(fli1a:EGFP)y1 | (Jin, 2005) | N/A |
| Zebrafish: Tg(fli1aep:DsRedEX)um13 | (Proulx et al., 2010) | N/A |
| Zebrafish: Tg(fli1a:nEGFP)y7 | (Roman et al., 2002) | N/A |
| Zebrafish: Tg(kdrl:mCherry) | (Proulx et al., 2010) | N/A |
| Zebrafish: Tg(lyve1b:TopazYFP)tsu | This paper | N/A |
| Oligonucleotides | ||
| sgRNA targeting sequence: par1 : GGGGGAAGCGGCTTTAGTTCGGG | This paper | N/A |
| sgRNA targeting sequence: gnai2a: GTGCAAGCAGTATCGAGCTG |
This paper | N/A |
| sgRNA targeting sequence: mmp13b: CCTCCTGGAATCGGCATTGG |
This paper | N/A |
| siRNA targeting sequence: PAR1: UAGAGUGUGAUGUAUGUGUAA |
This paper | N/A |
| siRNA targeting sequence: GNA11: CAGUGAUACUUGUAUAUUACA |
This paper | N/A |
| siRNA targeting sequence: GNA13: GGUGGUCAGAGAUCAGAAAGG |
This paper | N/A |
| siRNA targeting sequence:GNAI2: CCUUGAGCGCCUAUGACUUTT |
This paper | N/A |
| siRNA targeting sequence:MMP1: GCGUGUGACAGUAAGCUAACC |
This paper | N/A |
| Morpholino: MO-par1 CCGTCACCAACAGAACCCGCAACAT |
Gene Tools | N/A |
| Morpholino: MO-gnai2a GCTTCAGGGCGACGGATTTATGA |
Gene Tools | N/A |
| Morpholino: MO-gna11a TCCAGAGTCATCACCACAGCGTTTG |
Gene Tools | N/A |
| Morpholino: MO-gna11b GACTCTAAAGTCATCCCCACTGCTT |
Gene Tools | N/A |
| Morpholino: MO-gna13a AAATCCGCCATCTTTGTAGTAGCGA |
Gene Tools | N/A |
| Morpholino: MO-gna13b AGGAAATACGCCATCTTTGTGCAAC |
Gene Tools | N/A |
| Morpholino: MO-F2 GCTGCAAGCCTCGGACGTGCGCCAT |
Gene Tools | N/A |
| Probe for WISH, see Table S1 | This paper | N/A |
| Primers for XX, see Table S1 | This paper | N/A |
| Software and algorithms | ||
| Prism | GraphPad | 7.0e |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact: Yeqi Wang (yeqi.wang@cqu.edu.cn).
Materials availability
Transgenic zebrafish lines and mutants generated in this study are available from lead contact upon request.
Experimental model and subject details
Zebrafish
Zebrafish (Danio rerio) embryos were incubated in Holtfreter’s solution at 28.5°C(Wang et al., 2011). The following transgenic fish lines were used: Tg(fli1a:EGFP)y1 (ref. (Jin, 2005)), Tg(fli1aep:DsRedEX)um13 (ref. (Proulx et al., 2010)), Tg(fli1a:nEGFP)y7 (ref. (Roman et al., 2002)), Tg(kdrl:mCherry) (ref. (Proulx et al., 2010)), and Tg(lyve1b:TopazYFP)tsu (ref. [34]), which were gifted by Prof. Anming Meng from Tsinghua University, China, and were generated according to a previous report (Okuda et al., 2012). All zebrafish maintenance and experiments were carried out in accordance with the guidelines approved by the Ethics Committee of Chongqing University.
Method details
Generation of the zebrafish transgenic line
To generate the construct for Tg(lyve1:mCherry) and Tg(lyve1:cleaved-par1-P2A-mCherry), we used Gateway-compatible vectors of the Tol2kit (Kwan et al., 2007). For venous and lymphatic expression, a 5.2 kb fragment of the zebrafish lyve1 promoter (Okuda et al., 2012) was subcloned into p5E-MCS. Zebrafish par1 cDNA, which lacks 87 bp within its N-terminal, was amplified by PCR. Then this par1 fragment, P2A sequence, and mCherry sequence were subcloned into pME-cleaved-par1-P2A-mCherry plasmid. The p5E-lyvel1 plasmid was combined with pME-mCherry or pME-cleaved-par1-P2A-mCherry, the 3’ entry clone p3E-polyA, and the pDestTol2pA2 destination vector to create the pDest-lyve1:mCherry or pDest-lyve1:cleaved-par1-P2A-mCherry construct using LR Clonase II Plus Enzyme mix (Invitrogen, Cat#12538200). Embryos in the one-cell stage were injected with 25 ng/μL plasmid and 25 ng/μL Tol2 transposase RNA. Embryos injected with pTol2-lyve1:mCherry or pTol2-lyve1:cleaved-par1-P2A-mCherry were raised to adults and screened for founders.
Generation of zebrafish mutants using CRISPR/Cas9 technology
Zebrafish mutants were generated using CRISPR/Cas9 from Nanjing XinJia Medical Technology, Co., LTD, China. Briefly, the par1 zebrafish mutant was targeted to exon 2 of the par1 gene locus, with the target sequence 5’- GGGGGAAGCGGCTTTAGTTCGGG -3’; the gnai2a zebrafish mutant was targeted to exon 3 of the gnai2a gene locus, with the target sequence 5’-GTGCAAGCAGTATCGAGCTG-3’; and the mmp13b zebrafish mutant was targeted to exon 4 of the mmp13b gene locus, with the target sequence 5’-CCTCCTGGAATCGGCATTGG-3’. Cas9 mRNA (600 ng/μL) and par1, gnai2a, and mmp13b gRNA (300 ng/μL) were co-injected into wild-type embryos in the one-cell stage. Founder (F0) were grown into adulthood and then were identified by genomic PCR and sequencing. Next, to confirm germline-transmitted mutations of par1, gnai2a, and mmp13b, the identified F0 were crossed with wildtype fish to obtain heterozygous mutant zebrafish (F1). These were in-crossed to generate homozygous mutant embryos (F2) and used for the experimental analyses. Genotyping was carried out to distinguish between siblings (wild type), heterozygous mutants, and homozygous mutants after PCR amplification of target sites using caudal fin DNA (for adulthood) or whole embryo DNA (for embryonic stage); sequencing was used for verification. The primers for genotyping par1 zebrafish mutant, gnai2a zebrafish mutant, and mmp13b zebrafish mutant are listed in Table S1.
Microinjection of MOs
All MOs were ordered from Gene Tools LIC. Embryos in the one-cell stage were injected with 4 ng anti-sense MO/embryo. TD formation of embryos at 5 dpf was observed by via confocal microscopy (Leica, SP8, Germany). The MO sequences used are listed in key resources table.
Antagonist exposure
A stock solution of SCH79797 was dissolved in DMSO to 10 mM, and a working solution was freshly prepared by diluting the stock solution to 100 nM with E3 embryo medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4). Then 100 Tg(fli1a:EGFP) zebrafish embryos were collected and equally divided into two groups at random. One group was exposed to the SCH79797 working solution, and the other group was exposed to vehicle control. The solutions were used to treat embryos at 1 dpf and were changed once a day; dead embryos were removed using a stereoscopic microscope (Carl Zeiss, Jena, Germany). TD formation of zebrafish embryos at 5 dpf in both groups was observed via confocal microscopy (Leica, SP8, Germany).
Imaging
Zebrafish embryos were treated with 0.003% 1-phenyl-2-thiourea (PTU, Sigma) to prevent pigment formation from 24 hpf. And the embryos were mounted in 0.8% low-melting agarose (Sigma) from imaging. For imaging, Leica SP8 microscopy was setting as following: magnification 25× water objective, 0.75× zoom in, and a step size of 1.5 μm.
In situ hybridization
WISH of zebrafish embryos was following the standard procedure. Stained embryos were mounted in 5% methylcellulose and glycerol, and the images were captured unsing a microscope (ZEISS Stemi 2000-C) (Li et al., 2012). The probe primers of specific genes are listed in Table S1.
Whole mount immunostaining
Live embryos were fixed in fresh 4% paraformaldehyde at 4°C overnight. The embryos were washed three times and dehydrated through a series of washings with methanol in PBST. Then they were placed in 100% methanol at -20°C overnight and re-dehydrated through a series of washings with methanol in PBST. The samples were gradually warmed in Tris buffer (150 mM Tris-HCl, pH 9.0), placed in a water bath from 37°C to 70°C, and then kept for 30 min. This was followed by gradually cooling to room temperature. Then the embryos were treated with Proteinase K (30 μg/mL) for 40 min for Prox1 staining or with Proteinase K (10 μg/mL) for 5 min for p-Erk1/2 staining at room temperature. Next, we blocked the embryos for 3–4 h at 4°C and then incubated them with the primary antibody (anti-Prox1 antibody, GeneTex: GTX128354; p-Erk1/2 antibody, Cell Signaling Technology: #4370) in 1:500 dilution buffer at 4°C overnight. The embryos were washed and then incubated with goat anti-rabbit IgG (H+L) secondary antibody (Alexa 555-1:750 dilution in dilution buffer) for 1.5 h at room temperature under dark conditions. Finally, the embryos were washed in PBST-S and PBST at room temperature before being used for imaging.
Cell culture and western blotting
Human Primary Lymphatic Endothelial Cells (HDLECs) were obtained from CellBiologicsand cultured with endothelial cell medium (ECM) and 10% fetal calf serum (FBS). All siRNAs and antibodies used in this study are listed in key resource table. Recombinant human VEGF-C (Cys156Ser) protein was purchased from R&D Systems (Catalogue: 752-VC-025). HDLECs were growth to 60-80% confluence and transfected with siRNA using RNAiMAX (Invitrogen). 72 hours after transfection, the HDLECs were serum starved for 8 hours, followed by 100 ng/ml VEGF-C stimulation. Then the HDLECs were harvested for Western blotted with standard protocol (Cui et al., 2014).
Quantitative RT-PCR analysis
Quantitative real-time PCR (qPCR) was performed with TB Green Fast RT-PCR Mix (TAKARA, RR430A). The qPCR primers are listed in Table S1.
Quantification and statistical analysis
We used 6 somites/embryo, 6 somites/embryo, 8 ISVs/embryo, 7 somites/embryo, 7 somites/embryo, and 8 somites/embryo to quantify TD formation, LECs nuclear number, lympho-venous sprouting in the PCV, PL sprouting, Prox1 expression staining, and p-Erk1/2 expression staining in the PCV, respectively. All statistical analyses were done using GraphPad Prism software. The statistical significance of the difference between control and experimental groups was determined using the Student’s unpaired two-tailed t-test followed by one-way analysis of variance. Data are presented as mean ± SEM. P-values ≤ 0.05 were considered significant. ∗∗∗<0.0001, ∗∗<0.001, ∗<0.01, ns>0.01.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (31771599, 31971242), Chongqing Science and Technology Bureau (cstc2019jcyj-zdxmX0028), and the National Key Technology R&D Program of China (2016YFC1102305). The authors want to thank the assistance from the Chongqing Engineering Laboratory in Vascular Implants and the Public Experiment Center of State Bioindustrial Base (Chongqing), China.
Author contributions
Conceptualization, Y.W.; Methodology, D.L., M.A.R., X.Z., Y.M., X.Z., and L.W.; Investigation, Y.W., D.L., M.A.R., and X.Z.; Writing—Original Draft, Y.W., G.W., D.L., and M.A.R.; Writing—Review & Editing, Y.W., G.W., D.L., and M.A.R.; Funding Acquisition, Y.W. and G.W.; Resources, L.L., T.Z., and Y.L. Supervision, Y.W. and G.W. All authors approved the final version of the manuscript.
Declaration of interests
The authors declare that there is no conflict of interest.
Published: November 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103386.
Contributor Information
Guixue Wang, Email: wanggx@cqu.edu.cn.
Yeqi Wang, Email: yeqi.wang@cqu.edu.cn.
Supplemental information
Data and code availability
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request. This paper does not report original code.
References
- Alberelli M.A., De Candia E. Functional role of protease activated receptors in vascular biology. Vascul. Pharmacol. 2014;62:72–81. doi: 10.1016/j.vph.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Alitalo K. The lymphatic vasculature in disease. Nat. Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- Austin K.M., Nguyen N., Javid G., Covic L., Kuliopulos A. Noncanonical matrix metalloprotease-1-protease-activated receptor-1 signaling triggers vascular smooth muscle cell dedifferentiation and arterial stenosis. J. Biol. Chem. 2013;288:23105–23115. doi: 10.1074/jbc.M113.467019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y.R., Fujita M., Butler M., Isogai S., Kochhan E., Siekmann A.F., Weinstein B.M. Chemokine signaling directs trunk lymphatic network formation along the preexisting blood vasculature. Dev. Cell. 2012;22:824–836. doi: 10.1016/j.devcel.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S.R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- Cui H., Wang Y.Y., Huang H., Yu W., Bai M., Zhang L., Bryan B.A., Wang Y.Y., Luo J., Li D., et al. GPR126 protein regulates developmental and pathological angiogenesis through modulation of VEGFR2 receptor signaling. J. Biol. Chem. 2014;289:34871–34885. doi: 10.1074/jbc.M114.571000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducoli L., Detmar M. Beyond PROX1: Transcriptional, epigenetic, and noncoding RNA regulation of lymphatic identity and function. Dev. Cell. 2021;56:406–426. doi: 10.1016/j.devcel.2021.01.018. [DOI] [PubMed] [Google Scholar]
- Ellertsdottir E., Berthold P.R., Bouzaffour M., Dufourcq P., Trayer V., Gauron C., Vriz S., Affolter M., Rampon C. Developmental role of zebrafish protease-activated receptor 1 (PAR1) in the cardio-vascular system. PLoS One. 2012;7:e42131. doi: 10.1371/journal.pone.0042131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galt S.W., Lindemann S., Allen L., Medd D.J., Falk J.M., McIntyre T.M., Prescott S.M., Kraiss L.W., Zimmerman G.A., Weyrich A.S. Outside-in signals delivered by matrix metalloproteinase-1 regulate platelet function. Circ. Res. 2002;90:1093–1099. doi: 10.1161/01.RES.0000019241.12929.EB. [DOI] [PubMed] [Google Scholar]
- Gauvrit S., Villasenor A., Strilic B., Kitchen P., Collins M.M., Marín-Juez R., Guenther S., Maischein H.M., Fukuda N., Canham M.A., et al. HHEX is a transcriptional regulator of the VEGFC/FLT4/PROX1 signaling axis during vascular development. Nat. Commun. 2018;9:2074. doi: 10.1038/s41467-018-05039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiko P., Makinen T., Keskitalo S., Taipale J., Karkkainen M.J., Baldwin M.E., Stacker S.A., Achen M.G., Alitalo K. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol. Cell. Biol. 2008;28:4843–4850. doi: 10.1128/mcb.02214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan Benjamin M., Bos F.L., Bussmann J., Witte M., Chi N.C., Duckers H.J., Schulte-Merker S. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 2009;41:396–398. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- Hogan Benjamin M., Herpers R., Witte M., Heloterä H., Alitalo K., Duckers H.J., Schulte-Merker S. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development. 2009;136:4001–4009. doi: 10.1242/dev.039990. [DOI] [PubMed] [Google Scholar]
- Hong Y.K., Harvey N., Noh Y.H., Schacht V., Hirakawa S., Detmar M., Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Jaffré F., Friedman A.E., Hu Z., MacKman N., Blaxall B.C. β-Adrenergic receptor stimulation transactivates protease-activated receptor 1 via matrix metalloproteinase 13 in cardiac cells. Circulation. 2012;125:2993–3003. doi: 10.1161/CIRCULATIONAHA.111.066787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S.-W. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Karkkainen M.J., Haiko P., Sainio K., Partanen J., Taipale J., Petrova T.V., Jeltsch M., Jackson D.G., Talikka M., Rauvala H., et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Kim H., Nguyen V.P., Petrova T.V., Cruz M., Alitalo K., Dumont D.J. Embryonic vascular endothelial cells are malleable to reprogramming via Prox1 to a lymphatic gene signature. BMC Dev. Biol. 2010;10:17. doi: 10.1186/1471-213X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Carrillo M., Kulkarni V., Jagadeeswaran P. Evolution of primary hemostasis in early vertebrates. PLoS One. 2009;4:e8403. doi: 10.1371/journal.pone.0008403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltowska K., Lagendijk A.K., Pichol-Thievend C., Fischer J.C., Francois M., Ober E.A., Yap A.S., Hogan B.M. Vegfc regulates bipotential precursor division and Prox1 expression to promote lymphatic identity in zebrafish. Cell Rep. 2015;13:1828–1841. doi: 10.1016/j.celrep.2015.10.055. [DOI] [PubMed] [Google Scholar]
- Küchler A.M., Gjini E., Peterson-Maduro J., Cancilla B., Wolburg H., Schulte-Merker S. Development of the zebrafish lymphatic system requires vegfc signaling. Curr. Biol. 2006;16:1244–1248. doi: 10.1016/j.cub.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Kwan K.M., Fujimoto E., Grabher C., Mangum B.D., Hardy M.E., Campbell D.S., Parant J.M., Yost H.J., Kanki J.P., Chien C.B. The Tol2kit: A multisite gateway-based construction Kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang Y., Zhang M., Xu P., Huang H., Wu D., Meng A. The Amotl2 gene inhibits Wnt/β-catenin signaling and regulates embryonic development in zebrafish. J. Biol. Chem. 2012;287:13005–13015. doi: 10.1074/jbc.M112.347419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan T.S., Weinstein B.M. Emerging from the PAC: Studying zebrafish lymphatic development. Microvasc. Res. 2014;96:23–30. doi: 10.1016/j.mvr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Nicenboim J., Malkinson G., Lupo T., Asaf L., Sela Y., Mayseless O., Gibbs-Bar L., Senderovich N., Hashimshony T., Shin M., et al. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature. 2015;522:56–61. doi: 10.1038/nature14425. [DOI] [PubMed] [Google Scholar]
- Okuda K.S., Astin J.W., Misa J.P., Flores M.V., Crosier K.E., Crosier P.S. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development. 2012;139:2381–2391. doi: 10.1242/dev.077701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx K., Lu A., Sumanas S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev. Biol. 2010;348:34–46. doi: 10.1016/j.ydbio.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Qian F., Zhen F., Ong C., Jin S.W., Soo H.M., Stainier D.Y.R., Lin S., Peng J., Wen Z. Microarray analysis of zebrafish cloche mutant using amplified cDNA and identification of potential downstream target genes. Dev. Dyn. 2005;233:1163–1172. doi: 10.1002/dvdy.20444. [DOI] [PubMed] [Google Scholar]
- Roman B.L., Pham V.N., Lawson N.D., Kulik M., Childs S., Lekven A.C., Garrity D.M., Moon R.T., Fishman M.C., Lechleider R.J., et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129:3009–3019. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Sabine A., Petrova T.V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell. Biol. 2011;93:607–618. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semo J., Nicenboim J., Yaniv K. Development of the lymphatic system: New questions and paradigms. Development. 2016;143:924–935. doi: 10.1242/dev.132431. [DOI] [PubMed] [Google Scholar]
- Shin M., Male I., Beane T.J., Villefranc J.A., Kok F.O., Zhu L.J., Lawson N.D. Vegfc acts through ERK to induce sprouting and differentiation of trunk lymphatic progenitors. Development. 2016;144:3785–3795. doi: 10.1242/dev.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh U.J., Dores M.R., Chen B., Trejo J. Signal transduction by protease-activated receptors. Br. J. Pharmacol. 2010;160:191–203. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi V., Boire A., Tchernychev B., Kaneider N.C., Leger A.J., O’Callaghan K., Covic L., Kuliopulos A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137:332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Impel A., Zhao Z., Hermkens D.M.A., Roukens M.G., Fischer J.C., Peterson-Maduro J., Duckers H., Ober E.A., Ingham P.W., Schulte-Merker S., et al. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development. 2014;141:1228–1238. doi: 10.1242/dev.105031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villefranc J.A., Nicoli S., Bentley K., Jeltsch M., Zarkada G., Moore J.C., Gerhardt H., Alitalo K., Lawson N.D. A truncation allele in vascular endothelial growth factor c reveals distinct modes of signaling during lymphatic and vascular development. Development. 2013;140:1497–1506. doi: 10.1242/dev.084152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T.K.H., Hung D.T., Wheaton V.I., Coughlin S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-V. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li Z., Xu P., Huang L., Tong J., Huang H., Meng A. Angiomotin-like2 gene (amotl2) is required for migration and proliferation of endothelial cells during angiogenesis. J. Biol. Chem. 2011;286:41095–41104. doi: 10.1074/jbc.M111.296806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle J.T., Harvey N., Detmar M., Lagutina I., Grosveld G., Gunn M.D., Jackson D.G., Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle J.T., Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/S0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wyatt R.A., Keow J.Y., Harris N.D., Haché C.A., Li D.H., Crawford B.D. The zebrafish embryo: A powerful model system for investigating matrix remodeling. Zebrafish. 2009;6:347–354. doi: 10.1089/zeb.2009.0609. [DOI] [PubMed] [Google Scholar]
- Xu H., Echemendia N., Chen S., Lin F. Identification and expression patterns of members of the protease-activated receptor (par) gene family during zebrafish development. Dev. Dyn. 2011;240:278–287. doi: 10.1002/dvdy.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv K., Isogai S., Castranova D., Dye L., Hitomi J., Weinstein B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- Yue R., Li H., Liu H., Li Y., Wei B., Gao G., Jin Y., Liu T., Wei L., Du J., et al. Thrombin receptor regulates hematopoiesis and endothelial-to-hematopoietic transition. Dev. Cell. 2012;22:1092–1100. doi: 10.1016/j.devcel.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Zhao P., Metcalf M., Bunnett N.W. Biased signaling of protease-activated receptors. Front. Endocrinol. 2014;13:67. doi: 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request. This paper does not report original code.