Abstract
Between 2005–2009, several research groups identified a strikingly dense inhibitory input to midbrain dopamine neurons in a previously uncharted region posterior to the ventral tegmental area (VTA). This region is now denoted as either the rostromedial tegmental nucleus (RMTg) or the “tail of the VTA” (tVTA), and is recognized to express distinct genetic markers, encode negative “prediction errors” (inverse to dopamine neurons), and play critical roles in behavioral inhibition and punishment learning. RMTg neurons are also influenced by many categories of abused drugs, and may drive some aversive responses to such drugs, particularly cocaine and alcohol. However, despite much progress, many important questions remain about RMTg molecular/genetic properties, diversity of projection targets, and applications to addiction, depression, and other neuropsychiatric disorders.
Introduction:
By now, a vast literature has arisen describing roles of dopamine (DA) in motivated behavior, reward-seeking and addiction (Marsden, 2006; Wise and Robble, 2020). Much of this work has focused on downstream targets of dopamine in striatum, cortex, and other sites, while functions of upstream inputs to dopamine neurons have been much less investigated (Tian et al., 2016), despite their importance in regulating this critical neurotransmitter system. One striking example of just how much remained to be discovered occurred between 2005–2009, when multiple research groups simultaneously described a dense GABAergic input to dopamine neurons arising from an unmapped region posterior to the ventral tegmental area (VTA) and extending caudally the border of the acetylcholine-rich pedunculopontine nucleus (Figure 1) (Jhou, 2005; Perrotti et al., 2005; Colussi-Mas et al., 2007; Jhou et al., 2009a; Jhou et al., 2009b; Kaufling et al., 2009; Barrot et al., 2012; Bourdy and Barrot, 2012). Now denoted as either the rostromedial tegmental nucleus (RMTg), or the tail of the ventral tegmental area (tVTA), these GABA neurons are recognized as a genetically distinct group of neurons providing a major “brake” on motivated behavior. For example, RMTg inactivations or lesions produce marked hyperactivity and severe deficits in punishment learning (Bourdy et al., 2014; Lavezzi et al., 2015; Vento et al., 2017), along with a loss of conditioned avoidance responses to cocaine (Jhou et al., 2013). The RMTg also receives a prominent excitatory afferent from the lateral habenula (LHb), a region whose stimulation had been known to inhibit dopamine neurons (Christoph et al., 1986; Ji and Shepard, 2007), but which had been largely neglected until breakthrough recording studies of the LHb revealed that they encode motivational valence in a manner inverse to dopamine neurons (Matsumoto and Hikosaka, 2007; Geisler and Trimble, 2008; Matsumoto and Hikosaka, 2009). From these auspicious beginnings, the past decade has seen important new work characterizing the RMTg, particularly its genetic expression profiles, interactions with the LHb and other brain structures, encoding of aversive “prediction errors”, and responses to drugs of abuse. However, many challenges remain, including a need for further characterization of RMTg genetic and cellular properties, its downstream targets, its responses to drugs of abuse, and possible translational applications.
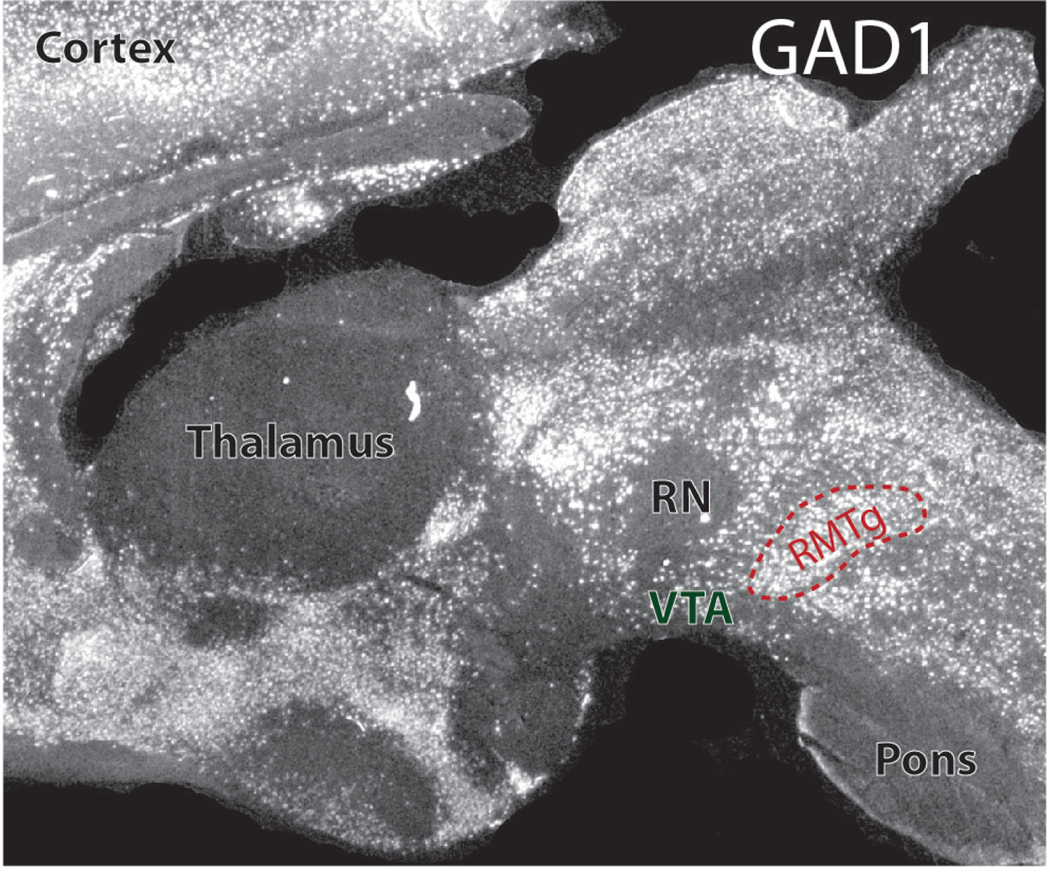
Figure 1.
Sagittal rat brain section (∼0.5mm from midline) showing in situ hybridization for GAD1. The RMTg is outlined in red, and other nearby landmarks are denoted, including the ventral tegmental area (VTA), red nucleus (RN) and pons. In rats, the stereotaxic coordinates of the RMTg center are roughly 7.5mm posterior to bregma, 7.4mm ventral to dura, and 0.6mm lateral from the midline, albeit with some variation between individual animals.
Early challenges: visualizing RMTg neurons
Early studies of the RMTg utilized a combination of immunohistochemical and tracing techniques to identify these neurons as a distinct population based on their expression of GABAergic markers, innervation of midbrain dopamine neurons, and expression of immediate early genes (IEGs) such as c-Fos after psychostimulant exposure (Scammell et al., 2000; Jhou, 2005; Perrotti et al., 2005; Jhou et al., 2009b; Kaufling et al., 2009; Kaufling et al., 2010). However, the variability and incompleteness of these methods posed considerable early challenges. For example, psychostimulant-induced cFos occurs in only a subset (typically 30–50%) of RMTg neurons in rats, and is largely ineffective in the commonly used C57BL6 mouse strain (Lavezzi and Zahm, 2011; Smith et al., 2019), while GABA markers are more expressed more universally within RMTg neurons, but are also present at high levels in adjacent regions. Hence, the exact boundaries of the RMTg were open to debate, including questions about whether the RMTg is distinct from classical VTA GABA interneurons.
An important advance in defining the RMTg occurred in 2015, when Partanen and colleagues at the University of Helsinki examined the distribution of various transcription factors in and around the VTA (Lahti et al., 2016). In general, transcription factors play critical roles in neuronal development and cell fate determination, and within the VTA and RMTg, different subsets of GABA neurons were found to express different subsets of these factors. In particular, Lahti et al. found three transcription factors (FoxP1, Sox2, and Sox14) expressed at high levels in RMTg neurons, relative to immediately adjacent regions. Among these, FoxP1 has been particularly useful in corroborating and refining earlier delineations of the RMTg (Figure 2), as it strongly labels almost all RMTg neurons, is strongly expressed in both rats and mice, and is readily detectable using commercially available antibodies and RNAscope probes (Lahti et al., 2016; Smith et al., 2019). The expression pattern of FoxP1 not only corroborates previous delineations of the RMTg, but reveals surprisingly sharp boundaries, as FoxP1 is found in >90% of neurons within these boundaries, and <5% of neurons immediately lateral (Smith et al., 2019). FoxP1 shows a very similar expression pattern in mice, and furthermore, in VGAT-Cre mice crossed to a ZsGreen reporter strain, essentially all (∼98%) of FoxP1 neurons within the RMTg boundaries expressed ZsGreen reporter (Smith et al., 2019), confirming an overwhelmingly, if not exclusively, GABAergic identity. FoxP1 is hence increasingly used across multiple labs to define the RMTg (Taylor et al., 2019; Castillo-Rolon et al., 2020; St Laurent et al., 2020; Zhao et al., 2020; Pradel et al., 2021).
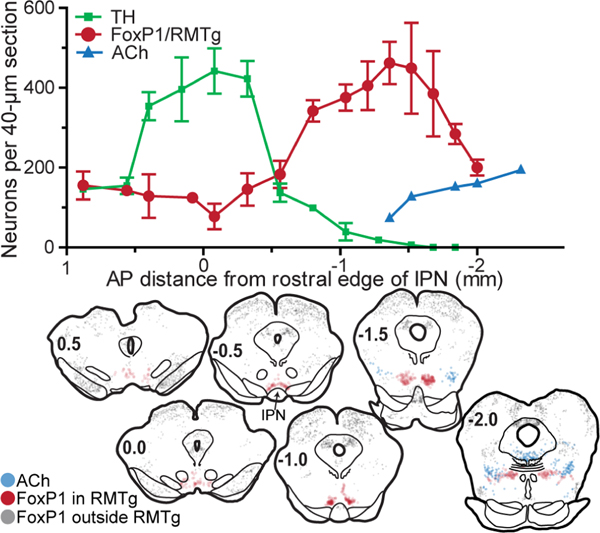
Figure 2.
Distribution of FoxP1/RMTg neurons at various rostro-caudal levels in coronal rat brain sections. (A) Counts of neurons in 40-micron sections plotted versus anterior-posterior distance from rostral edge of IPN. RMTg neurons, as identified by FoxP1 immunostaining, reside caudal to the majority of VTA dopamine neurons, identified via tyrosine hydroxylase (TH), and rostral to many cholinergic neurons of the pedunculopontine nucleus. (B) Coronal rat brain sections show distribution of FoxP1 neurons inside RMTg (red symbols), which are distinct from FoxP1 neurons outside the RMTg (grey symbols). Cholinergic neurons of the PPTg are denoted by blue symbols.
In addition to transcription factors noted above, RNA sequencing experiments identified dozens of additional genes enriched in RMTg relative to the adjacent VTA (Smith et al., 2019). Some of these genes, such as GABAergic markers, were already known, but others were novel, including genes encoding prepronociceptin (PNOC) and the serotonin 2C receptor (5HT2CR), which were previously shown to influence reward-seeking (Maillet et al., 2008; Parker et al., 2019), but whose function in RMTg is unexplored. Hence, these genes, among others, represent major areas for further investigation. Notably, genes encoding PNOC and FoxP1 are either absent or present at greatly reduced levels in VTA GABAergic interneurons, again suggesting differential origins and functions of RMTg versus classical VTA GABA neurons.
RMTg “brake” on dopamine firing and motor behavior:
Anatomic studies using light and electron microscopy show that even small anterograde tracer injections in the RMTg give rise to a remarkably dense innervation of dopamine neurons in VTA and substantia nigra (SN), and possibly the retrorubral dopamine fields as well (Jhou et al., 2009a; Balcita-Pedicino et al., 2011; Bourdy et al., 2014). Hence, the RMTg likely influences almost the entire dopaminergic system, with over half of all RMTg neurons innervating the VTA in rat, and nearly 70% innervating at least one of the VTA or SN (Smith et al., 2019). Surprisingly, the RMTg neurons innervating the SN are mostly distinct from those innervating the VTA, and arise preferentially from more lateral RMTg neurons (Smith et al., 2019). Hence, this suggests the possibility of differential modulation of different dopamine neuron subsets from different subsets of RMTg neurons.
Anatomic findings have been corroborated via electrophysiological studies showing that the RMTg indeed exerts a massive “braking” effect on dopamine neuron firing. Single brief pulses of electrical stimulation in the RMTg inhibit 95% and 94% of SN dopamine neurons in rat and monkey, respectively, with these stimulations typically causing total cessation of firing at short latency (Hong et al., 2011; Bourdy et al., 2014), indicating an overwhelming inhibitory influence. Stimulations of the LHb is similarly powerful, with single pulses of electrical stimulation inhibiting 85–97% of dopamine neurons in the SN, and 91–97% in the VTA of rats (Christoph et al., 1986; Ji and Shepard, 2007). Interestingly, one study found a lower proportion of VTA dopamine neurons inhibited by RMTg stimulation - 53% (Lecca et al., 2012), than after LHb stimulation.
In awake rats or monkeys, or urethane-anesthetized rats, RMTg neurons exhibit fast basal firing rates of 14–20 Hz (Jhou et al., 2009a; Hong et al., 2011; Lecca et al., 2011; Melis et al., 2014), that would presumably exert continuous ongoing inhibition of dopamine firing. Such a high “basal” activity is consistent with findings that acute inactivation of the RMTg increases both tonic and burst firing of SN dopamine neurons to roughly 200% of baseline (Bourdy et al., 2014), indicating a strong disinhibitory effect. Similarly, excitotoxic lesions of the RMTg produce long-lasting increases in SN dopamine firing rates, albeit to a slightly lower degree (148% of baseline) than acute inactivation (Bourdy et al., 2014). This tonic influence likely varies between individual dopamine neurons, as three different groups report that VTA dopamine neurons having slower basal firing rates exhibit more prolonged inhibitory responses to single-pulse RMTg stimulation (Lecca et al., 2012; Melis et al., 2014), and show larger increases in firing after RMTg inhibition via opioids (Jalabert et al., 2011). Hence, RMTg activation might have stronger inhibitory influences on some dopamine neurons than others, leading to lower basal firing in those neurons.
The inhibitory physiological RMTg influence on dopamine is also consistent with numerous behavioral finings. For example, whereas dopamine invigorates motoric activity, RMTg activation suppresses it (Jhou et al., 2013; Lavezzi et al., 2015), while conversely, RMTg lesions or inactivations can produce large increases in locomotion, reaching 2 to 10-fold over baseline (Jhou et al., 2013; Lavezzi et al., 2015; Vento et al., 2017). Notably, these increases appear much larger in novel environments than in animals’ home cages, where RMTg lesions have relatively less effect on locomotion (Jhou et al., 2009a). This would be consistent with a disinhibitory effect, in which removal of the RMTg “brake” is permissive for dopamine firing, but with such firing still requiring dopamine activation via other excitatory inputs driven by external stimuli.
What is the RMTg role in aversive learning and behavior?
Of course, dopamine neurons influence much more than locomotor behavior, with particular roles in learning of motivated behaviors. Over a century ago, Thorndike characterized such learning according to the “law of effect” (Thorndike, 1911), positing that animals’ actions that lead to desirable outcomes are reinforced, and hence more likely to occur in similar situations in the future. Conversely, actions leading to undesirable outcomes are punished, becoming less likely to occur. Increasing evidence suggests that dopamine and RMTg neurons appear to play opposing roles in this phenomenon. In particular, optogenetic activation of dopamine neurons reinforces operant behavior, increasing its likelihood of future occurrence (Adamantidis et al., 2011; Saddoris et al., 2015), while activation of the RMTg or of its LHb afferents just after an operant action biases animals’ choices away from that action (Shumake et al., 2010; Stamatakis and Stuber, 2012; Proulx et al., 2018; Elmer et al., 2019). Activation of the RMTg also produces aversive effects in non-operant tasks, such as condition place preference tests (Smith et al., 2019; St Laurent et al., 2020).
Although exogenous activation of the RMTg produces clear behavioral effects, inactivation studies are needed to assess whether these neurons are necessary for normal motivated behavior. And indeed, inactivations or lesions of the RMTg produce profound “resistance to punishment” in rats trained to lever press for food pellets followed by a very brief (30ms) footshock. Specifically, RMTg lesions produce a 3 to 4-fold increase in the shock amplitude required to suppress food-seeking (Vento et al., 2017), demonstrating a marked insensitivity to the suppressive effects of footshock punishment. Although this effect might be seen as secondary to the motoric disinhibition induced by RMTg impairment, several lines of evidence weigh against this interpretation. First, these animals show no increase in pressing of an adjacent inactive lever, nor do they show increased pressing during normal extinction learning (i.e. when food is no longer delivered). Hence, RMTg lesioned rats are not impaired in their ability to withhold behavior perse, but show a selective deficit in inhibiting behaviors that are followed by punishment (Vento et al., 2017). Additional evidence comes from optogenetic tests in which RMTg inhibition is confined to the few milliseconds of shock delivery itself. This very brief RMTg inactivation also increases shock breakpoints, without any motoric disinhibition at the time of lever pressing (Vento et al., 2017).
Despite evidence from multiple labs for an RMTg role in aversive processing, it would also be erroneous to equate RMTg activity with all aversion (just as dopamine activity is not equivalent to all reward). For example, RMTg-lesioned rats still run away from acute footshocks, and do so at similar latencies as intact rats (Vento et al., 2017; Elmer et al., 2019). RMTg lesioned rats also still exhibit defensive responses to predator odors (Jhou et al., 2009a), and are still motivated to avoid falling on a rotarod test, where they even show enhanced motoric performance (Bourdy et al., 2014). In general, despite the RMTg’s critical role in inhibitory responses to aversive stimuli, it does not appear necessary for many active responses to the same stimuli. In one particularly striking example, RMTg lesioned and unlesioned rats exposed to predator odor showed equal similar durations of defensive behavior, but intact rats almost exclusively exhibited behavioral inhibition (freezing), while lesioned rats almost exclusively exhibited active responses such as defensive treading/burying (Jhou et al., 2009a). Given these results, it might be tempting to ascribe to the RMTg a role in motoric behavior but not punishment or aversion, but this too would be an oversimplification, as RMTg lesioned rats are not globally disinhibited in all behaviors, and as noted above, they exhibit normal ablility to inhibit reward-seeking under non-punishment conditions, such as extinction. A parsimonious description for the RMTg role is that it mediates neither inhibition nor aversion exactly, but rather drives the learning that allows aversive stimuli to translate into subsequent behavioral inhibition. In other words, the RMTg is the “effector” in Thorndike’s law of effect, at least for punishment learning, a role symmetrically opposed to that of dopamine in reinforcement learning.
RMTg and dopamine phasic firing patterns:
The profound influence of RMTg in punishment and avoidance learning suggest that these neurons might encode information about the stimuli in such tasks. Again, RMTg encoding patterns are in many ways symmetrically opposite to dopamine firing. Of course, the latter is a complex topic, as dopamine neurons are increasingly recognized to encode complex and diverse types of information (Berke, 2018; Saddoris et al., 2018; Collins and Saunders, 2020), but considerable evidence from Pavlovian conditioning paradigms nonetheless indicates that dopamine activity encodes information about whether rewarding and aversive stimuli are “better” or “worse” than expected (Schultz, 2007, 2016). This encoding pattern became widely recognized in the mid-1990s, when a large proportion (albeit not all) of dopamine neurons were found to be phasically activated not by rewards per se, but by cues predicting upcoming rewards, or by rewards that are “surprisingly” delivered when none was expected (Montague et al., 1996; Schultz et al., 1997). Further, many dopamine neurons are phasically inhibited by cues predicting aversive outcomes, or by outcomes that are smaller than expected (or entirely absent). This response pattern was termed a “reward prediction error” (RPE), and was quickly recognized for its remarkable resemblance to learning signals developed years earlier in theoretical and computational learning models (Sutton, 1988; Tesauro, 1994; Montague et al., 1996; Schultz et al., 1997). This convergence of biology and theory infused new ideas into the study of dopamine function, including the recognition that unexpected outcomes are major drivers of learning, and that dopamine neurons are ideally poised to broadcast “teaching signals” that indicate such unexpectancy. Accumulating optogenetic evidence indicates that phasic dopamine activation indeed drives new learning about environmental stimuli that alters future behavior (Tsai et al., 2009; Adamantidis et al., 2011; Steinberg et al., 2013; Saddoris et al., 2015; Chang et al., 2016; Hamid et al., 2016). Conversely, inhibition of dopamine activity produces aversive learning in many paradigms (Shippenberg et al., 1991; Danjo et al., 2014), suggesting that dopamine can encode a bidirectional signal, with positive and negative changes in firing signaling that stimuli are “better” or “worse” than expected.
Consistent with opposing roles for RMTg and dopamine, many RMTg neurons across rats, mice, and non-human primates exhibit an inverted version of the canonical dopamine RPE signal (Hong et al., 2011; Li et al., 2019a; Li et al., 2019b) (Fig. 3). Among RMTg neurons that respond phasically to motivation-related stimuli, clear majorities (65–70%) are inhibited by reward-predictive cues and activated by negatively valenced stimuli such as shocks, shock-predictive cues, or unexpected reward omission (Jhou et al., 2009a; Hong et al., 2011; Li et al., 2019a; Li et al., 2019b). This aggregate pattern is inverse to the canonical dopamine response, and similar to patterns seen in the LHb, a major RMTg afferent (Matsumoto and Hikosaka, 2007, 2009; Hong et al., 2011). Hence, both RMTg and dopamine neurons encode a bidirectional valence signal indicating whether stimuli are “better” or “worse”, but in opposite directions.
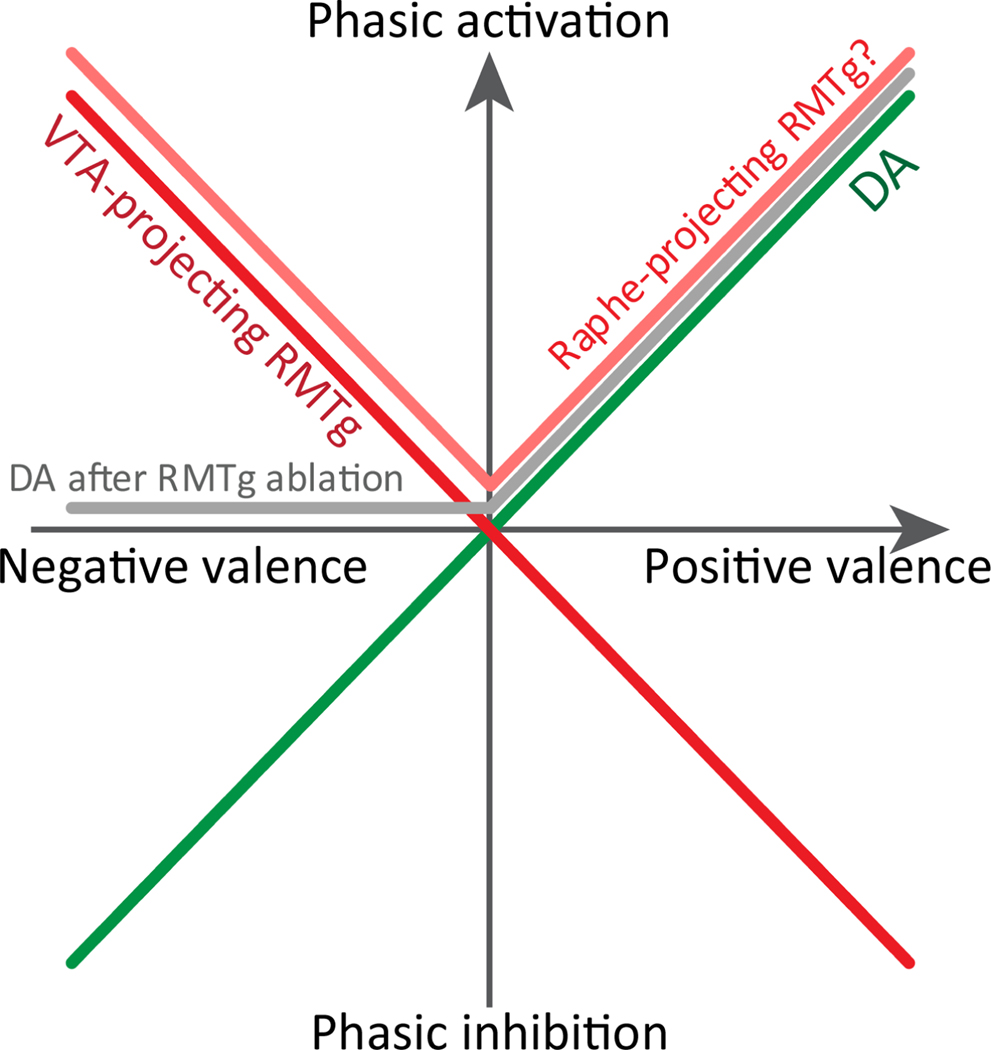
Figure 3.
Simplified depiction of RMTg and DA responses to stimuli of positive and negative valence. Responses of dopamine neurons are shown in both intact rats (green trace) and after ablation of RMTg (grey trace). Responses to RMTg neurons projecting to VTA versus raphe are adapted from (Li et al., 2019a).
However, not all aspects of the RMTg and dopamine signal are perfectly symmetric. For example, after selective ablation of RMTg projections to the VTA in rats, dopamine neurons in the VTA are no longer phasically inhibited by footshocks, shock-predictive cues, or reward omission, but are still activated by reward predictive cues (Li et al., 2019b) (Fig. 3). Hence, RMTg activation may drive dopamine phasic inhibition to negative motivational stimuli, but surprisingly, RMTg phasic inhibition by positive motivational stimuli does not seem to contribute to dopamine neuron activation (Li et al., 2019b). This may seem paradoxical, but is consistent with observations that RMTg inhibitions by reward predictive cues occur after dopamine activation by the same stimulus (Fig. 3). Hence, even though the RMTg encodes information about both positive and negative motivational stimuli, transmission of this information is preferentially weighted toward the latter.
“Surprising” differences between LHb and RMTg function:
As noted above, RMTg and LHb neurons show similar phasic responses to environmental stimuli, leading us and others to initially assume the LHb is the main driver of responses in RMTg. However, emerging evidence suggests this is true only in specific (but potentially very important) circumstances. Most surprisingly, after optogenetic inactivation of its LHb afferents, RMTg neurons are still strongly activated by footshock and shock-predictive cues, and inhibited by reward predictive cues (Li et al., 2019b). In contrast, LHb inactivation abolished RMTg activation by surprising reward omission (Li et al., 2019b), indicating a highly selective LHb influence on RMTg. Interestingly, this parallels the LHb influence on dopamine firing seen by Uchida and colleagues, in which LHb ablation impairs dopamine phasic inhibitions to reward omission, but not other motivational stimuli (Tian and Uchida, 2015).
Additional experiments suggest that the LHb influence on RMTg drives a broad signaling of “negatively surprising” events. For example, RMTg phasic activations by predicted footshocks are brief - about ∼30ms - but become prolonged to ∼100ms if the footshock is surprising, for example if it is given without prior predictive cues (Li et al., 2019b). LHb inactivation eliminates the surprise-driven prolongation, without affecting the initial 30ms response. Like the responses to footshock, RMTg responses to auditory cues predicting shock are also prolonged if they are surprising (not preceded by other cues), but become shorter if expected (due to a second preceding predictive cue). Again this prolongation depends on the LHb. Meanwhile, “positively surprising” events do not seem to depend on the LHb, and the LHb’s main contribution to RMTg firing may be to augment its responses to “surprisingly worse” stimuli, whether these are cues, shocks, or reward omission (Li et al., 2019b).
These differences in LHb and RMTg influences on dopamine have several implications. First, they indicate that despite the LHb being a particularly prominent input to the RMTg, other inputs should not be overlooked, and indeed, RMTg responses to footshocks and shock cues were found to depend on inputs from the parabrachial and prefrontal cortical regions, respectively (Li et al., 2019b) (Fig. 4). Secondly, these results could also explain some of the differences reported in LHb versus RMTg behavioral effects. Multiple labs have shown that LHb influences on behavior are particularly strong in tasks involving quickly changing outcomes, or during acquisition of new learning, or reversal learning, all situations that would generate particularly large prediction errors (Stopper and Floresco, 2014; Laurent et al., 2017; Li et al., 2019b; Trusel et al., 2019; Durieux et al., 2020). In contrast, the RMTg role in punishment tasks appears critical both in situations when outcomes are changing rapidly, or when they are static or changing only slowly (Vento et al., 2017).
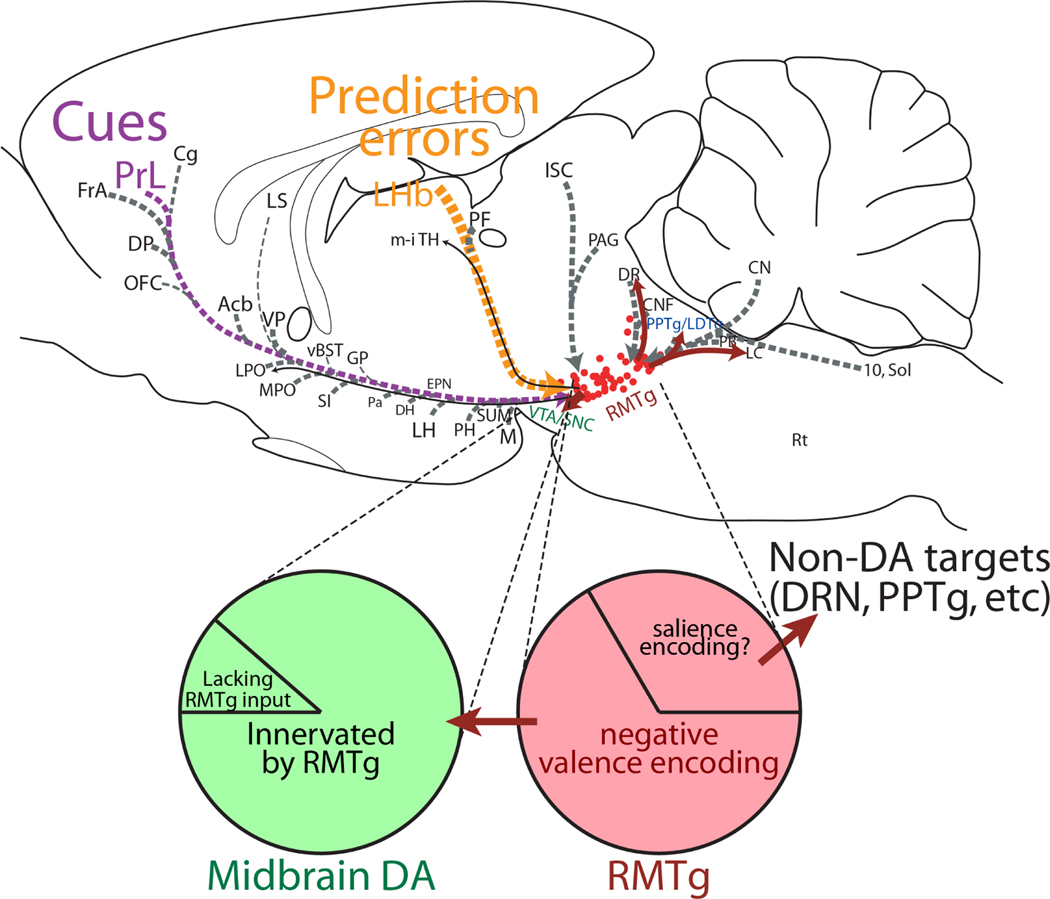
Figure 4.
Sagittal drawing of RMTg efferents (red) and afferents (grey). Afferents with verified electrophysiological influences on RMTg are indicated with colored lines and text: prelimbic cortex (PrL, purple), and lateral habenula (LHb, orange). RMTg and dopamine neuron populations are further dissected in pie chart diagram, showing diversity of neuron subtypes with proportions approximated by areas of pie chart. Roughly one-third of RMTg neurons may project to targets outside the midbrain dopamine system, while a minority of dopamine neurons may lack an RMTg input.
Astute readers may note that some RMTg responses described above deviate from mathematically idealized RPEs. In particular, whereas majorities of dopamine, LHb and RMTg neurons show no significant changes in firing to rewards that are fully predicted, RMTg neurons still respond, albeit more briefly, to fully predicted footshocks, and retain this response even after extensive training (Li et al., 2019b). This response deviates from an idealized error signal that should disappear entirely, rather than simply being attenuated, when an outcome is fully predicted by prior cues. While seemingly at odds with theory, similar deviations had been reported by Hikosaka and colleagues in the LHb, again for aversion-related but not reward-related RPEs (Matsumoto and Hikosaka, 2009), while VTA dopamine neurons also show attenuation, but not elimination, of inhibitory responses to fully predicted airpuffs (Tian and Uchida, 2015). Hence, all three types of neurons deviate from the idealized RPE signal for predicted aversive stimuli, but not predicted rewards. The significance of this deviation is not known, but given the roles of these phasic responses in associative learning, their selective persistence for predicted aversion but not predicted reward, even after extensive training, suggests a bias toward “loss aversion” over “reward-seeking”. Such an asymmetry has indeed been seen in human decision-making (Kahneman and Tversky, 1979), although it is unknown whether these decision biases are related to reported encoding biases.
Responses to cocaine and alcohol:
In addition to their responses to sensory stimuli, RMTg neurons are also strongly influenced by many drugs of abuse (Table 1). Acute withdrawal from cocaine, morphine, or alcohol markedly increases firing and IEG expression in the LHb and/or RMTg (Jhou et al., 2013; Glover et al., 2016; Sanchez-Catalan et al., 2016; Glover et al., 2019; Li et al., 2019a). These findings are consistent with long-standing observations that withdrawal from many drugs of abuse leads to suppression of dopamine activity (Rossetti et al., 1992; Diana et al., 1999; Melis et al., 2005), and suggests the possibility that this suppression is driven by RMTg activation. Notably, increases in RMTg activity often parallel the time course of aversive effects of abused drugs. For example, single intravenous cocaine infusions in rats produce an initial euphoric phase lasting ∼5–10 minutes followed by aversive effects beginning 15 minutes after infusions (Ettenberg et al., 1999; Jhou et al., 2013). Paralleling this time course, LHb and RMTg firing rates in awake behaving rats are suppressed for the first few minutes after single intravenous cocaine infusions, but show a rebound activation starting around 15 minutes later, paralleling cocaine’s aversive phase (Jhou et al., 2013; Li et al., 2019a; Parrilla-Carrero et al., 2021). Furthermore, optogenetic RMTg inactivation overlapping the aversive (but not rewarding) phase abolishes conditioned avoidance of cocaine in a runway operant cocaine-seeking task, indicating that this activation causally drives avoidance behavior (Jhou et al., 2013).
Table 1:
At least six categories of abused drugs strongly influence RMTg activity. Among these, cocaine is the most widely studied, but other drugs, particularly opioids and cannabinoids, appear to have large acute effects. In several cases, activity appears protective against acquisition of drug-seeking, but this topic is overall relatively understudied.
| Drug | RMTg neural response: | Relevant receptors | References |
|---|---|---|---|
| Cocaine | Biphasic: reduced firing for several minutes, followed by excitation in some neurons 15–30 min after exposure. Marked cFos seen at 1–2 hours. | CP-AMPARs, 5HT2CR | (Colussi-Mas et al., 2007; Geisler et al., 2008; Jhou et al., 2009; Lavezzi et al., 2010; Jhou et al., 2013; Li et al., 2019; Parrilla-Carrero et al., 2021) |
| Methamphetamine | Firing rate unknown, but cFos increased at 2 hours. | Unknown. | (Lecca et al., 2011) |
| Morphine | Large acute reduction in firing. Increased cFos during withdrawal in dependent rats. Morphine blocks RMTg’s ability to suppress DA firing. | Mu opioid receptor | (Jalabert et al., 2011; Lecca et al., 2011; Lecca et al., 2012; Kaufling and Aston-Jones, 2015) |
| Nicotine | Acute increase in firing. | Alpha-7 nicotinic receptors on presynaptic glutamate inputs to RMTg | (Lecca et al., 2011; Castillo-Rolon et al., 2020) |
| Alcohol | Modest acute firing increase in firing, large cFos increase during withdrawal in dependent rats. | Unknown | (Melis et al., 2014; Glover et al., 2016; Glover et al., 2019) |
| Cannabinoids | Large acute firing inhibition, also blocks RMTg-induced suppression of DA firing | CB1 receptors on RMTg and presynaptically on its projections to VTA | (Lecca et al., 2011; Lecca et al., 2012; Melis et al., 2014) |
Interestingly, avoidance responses to cocaine show considerable variability between individual rats, with some rats being “high-avoiders” of cocaine while others are “low-avoiders”. Furthermore, high-avoiders show greater RMTg “rebound” firing 15–30 minutes after individual cocaine infusions (Parrilla-Carrero et al., 2021), suggesting a possible protective effect of RMTg activity against acquisition of cocaine-seeking. At a cellular level, this differential RMTg activation by cocaine may be due to downregulated calcium-permeable AMPA receptors in RMTg neurons of “low-avoiders”, in concert with increased presynaptic release from glutamatergic afferents in “high-avoiders” (Parrilla-Carrero et al., 2021). It is not known why these neuroplastic changes occur in some rats but not others, but their elucidation could shed new light on differences in addiction vulnerability between individuals.
In addition to cocaine, several studies have also examined RMTg responses to ethanol. In one study comparing Sardinian alcohol-preferring (sP) versus non-preferring (sNP) rats, RMTg neurons (in anesthetized rats) showed a dose-dependent increase in firing in response to intravenous alcohol in sNP but not sP rats (Melis et al., 2014). Analogous to findings with cocaine, this result is consistent with a possible protective effect of RMTg activation in sNP rats, though that hypothesis remains to be directly tested. A separate study examined rats exposed to chronic intermittent ethanol (CIE) for 14 weeks via vapor chamber, and then withdrawan, and found marked RMTg cFos activation (Glover et al., 2019) peaking 12 hours after removal from vapor chambers, coinciding with the peak of withdrawal-induced reductions in reward sensitivity measured by intracranial self-stimulation (ICSS). RMTg inactivation via muscimol further reduced measures of withdrawal-induced anxiety-like behaviors on a battery of tests (Glover et al., 2019), although RMTg inactivation interestingly did not prevent reductions in reward sensitivity measured by ICSS. This study also did not examine influences of RMTg on alcohol consumption per se, but a different group examining depressive-like behavior after 48 hours of withdrawal from 6 weeks of alcohol drinking (2 bottle free-choice procedure) showed that RMTg inactivation relieved a variety of depressive-like behaviors, but did not reduce drinking in withdrawn rats (Fu et al., 2019). Numerous other studies indicate that lesions or inactivations of either the RMTg or LHb increase voluntary alcohol intake in non-withdrawn rats (Haack et al., 2014; Fu et al., 2016b; Fu et al., 2016a; Sheth et al., 2016; Fu et al., 2019).
In a preponderance of studies, increased RMTg activity is associated with reduced drug-seeking, suggesting a mechanism by which aversive effects of drugs protect against drug-seeking. However, aversive effects of abused drugs can also increase drug-seeking, as when the dysphoria of acute drug withdrawal drives individuals to alleviate the discomfort via resumed drug taking (Solomon and Corbit, 1974; Koob and Le Moal, 2008). This latter phenomenon is known as negative reinforcement, and evidence gathered so far indicates that the RMTg does not contribute to this effect, as inhibiting the RMTg during acute ethanol withdrawal does not reduce intake (Fu et al., 2019), and actually increases reinstated cocaine-seeking after extinction (Huff and LaLumiere, 2015). Interestingly, LHb inactivation reduces yohimbine-induced reinstatement of ethanol-seeking (Haack et al., 2014), suggesting the LHb may drive some negative reinforcement type behaviors that the RMTg does not (Stamatakis and Stuber, 2012; Trusel et al., 2019).
Responses to other drugs of abuse:
In addition to cocaine and alcohol, multiple labs have described RMTg responses to many other classes of abused drugs (Lecca et al., 2011). For example, in anesthetized rats, intravenous morphine inhibits RMTg firing to roughly half its baseline rate (Lecca et al., 2012), and in electrophysiological slices mu opioid agonists reduce by 60–75% the magnitude of IPSCs induced in dopamine neurons by RMTg optogenetic stimulation (Matsui et al., 2014; St Laurent et al., 2020). Both effects would be expected to acutely disinhibit dopamine release, producing rewarding effects. Interestingly, mu opioid-mediated disinhibition of dopamine had long been assumed to be mediated by VTA interneurons, but unexpectedly, mu opioid modulation of VTA interneuron effects on dopamine neurons were much weaker than those driven by the RMTg (Matsui et al., 2014). Hence, the prevailing wisdom may have been a case of mistaken identity – i.e. mu opioid effects thought to be mediated by VTA interneurons are more likely mediated by the RMTg residing immediately posterior. Consistent with these electrophysiological results, rats self-administer mu opioid agonist into the RMTg more avidly than into the VTA or other surrounding regions (Jhou et al., 2012), while RMTg inactivation entirely blocked the ability of intra-VTA morphine to disinhibit dopamine neurons (Jalabert et al., 2011). Because RMTg inactivation influences dopamine via disinhibition rather than direct activation, the ability of mu opioids to increase dopamine firing likely requires concomitant activation of glutamatergic or cholinergic inputs to these neurons (Jalabert et al., 2011; Steidl et al., 2017; Buie et al., 2020). Prolonged opioid withdrawal may also produce adaptations in RMTg projections, although the latter result varies between studies, as one study noted tolerance of RMTg responses to mu opioid agonists in morphine dependent animals (Matsui et al., 2014), while another did not (Kaufling and Aston-Jones, 2015).
Similarly to mu opioid agonists, agonists at CB1 cannabinoid receptors also directly inhibit RMTg firing to about half its baseline rate in anesthetized rats, while also markedly reducing by 78% the ability of RMTg electrical stimulation to inhibit dopamine firing (Lecca et al., 2011; Lecca et al., 2012). Again, both of these cannabinoid effects would disinhibit dopamine neurons, presumably producing rewarding effects. The latter of these effects is further consistent with presynaptic effects on RMTg axons in the VTA, and CB1 receptors at this location also mediate depolarization-induced suppression of inhibition (DSI), which could further disinhibit dopamine neurons (Melis et al., 2014). Interestingly, the latter effect was larger in Sardinian alcohol preferring (sP) relative to non-preferring rats (Melis et al., 2014), again suggesting that RMTg activation could be protective against drug-seeking in sNP rats, but again this has not been directly tested.
Lastly, nicotine appears to increase RMTg firing to about double its baseline firing in anesthetized rats (Lecca et al., 2011), an effect mediated by alpha 7 nicotinic receptor subunits, possibly located on glutamatergic inputs to the RMTg (Castillo-Rolon et al., 2020). The functional significance of this is unknown, but one obvious hypothesis is that it could contribute to aversive effects of nicotine, analogous to aversive effects of nicotine mediated via alpha 5 receptor subunits located on medial habenula circuits (Bierut et al., 2008; Fowler et al., 2011; Morton et al., 2018).
Future challenges:
After its initial description 15 years ago, the last decade has seen considerable progress in elucidation of RMTg function, but much work remains in both basic science and translational areas. RMTg roles in individual differences in addiction may be a particularly ripe area for study. For example, RMTg activation may protect against acquisition of drug-seeking, as animals with stronger RMTg activation by cocaine or alcohol are slower to acquire addiction-like behavior, while RMTg inactivation conversely accelerates intake of abused substances. Hence, potential therapeutic approaches might seek to selectively enhance RMTg activation by cocaine or alcohol.
In addition to translational goals, many basic science questions also remain unanswered. For example, inverse RPE-like encoding is present in 60–70% of cue-responsive RMTg neurons, a clear majority, but one that also leaves a substantial minority encoding other patterns that are not well understood. Similarly, almost 70% of RMTg neurons project to dopamine neurons in either the VTA or SN, but once again substantial minorities project to other targets, such as the dorsal raphe and pedunculopontine (PPTg) nuclei (Lavezzi et al., 2012), which are enriched in serotonin and acetylcholine neurons respectively. However, RMTg projections may not target serotonin or acetylcholine neurons directly (Lavezzi et al., 2012; Sego et al., 2014), and the function of these pathways is largely unexplored, aside from one study noting that RMTg projections to the raphe may encode salience rather than valence (Li et al., 2019a).
In addition to heterogeneity in the RMTg itself, there is likely heterogeneity in its influence on dopamine neurons. While RMTg axons innervate a very high percentage of midbrain dopamine neurons (likely >90%), they avoid minor but potentially important subsets of dopamine neurons in both the VTA and SN (Lecca et al., 2012; Vento et al., 2017). The significance of such heterogeneity is unknown, but would be consistent with a growing literature on diversity in dopamine anatomy and function (Ikemoto, 2007; Schultz, 2007; Brischoux et al., 2009; Root et al., 2014; Schultz, 2016; Berke, 2018; Gardner et al., 2018; Saddoris et al., 2018; Engelhard et al., 2019; Lee et al., 2019; Heymann et al., 2020; Hughes et al., 2020; Root et al., 2020).
Finally, in addition to roles in addiction, recent work has uncovered possible RMTg roles in an increasing range of neuropsychiatric disorders. For example, RMTg ablation alleviates motor deficits in a rat model of Parkinson’s disease (Faivre et al., 2020), alleviates learned helplessness in a depression model (Elmer et al., 2019), and also influences sleep-wake regulation (Yang et al., 2018) in a manner inverse to recently uncovered dopamine roles in sleep regulation (Eban-Rothschild et al., 2016). These models have received much less study relative to addiction, but could be highly fruitful given the strong dopaminergic influences on these disorders, particularly Parkinson’s disease and major depressive disorder (Belujon and Grace, 2017). Clearly there is much yet to do.
Highlights:
The RMTg provides major GABAergic inputs to midbrain dopamine neurons
Many RMTg neurons are selectively activated by aversive stimuli and their predictors
RMTg inactivation profoundly impairs punishment but not reward or extinction learning
RMTg neurons contribute to aversion during withdrawal from abused drugs
A minority of RMTg neurons project to non-dopamine targets of unknown function
Acknowledgements:
Work described in this article has been funded by NIDA R01DA037327, R03DA034431, and R21DA032898.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Tourino C, Bonci A, Deisseroth K, de Lecea L (2011) Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci 31:10829–10835,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR (2011) The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol 519:1143–1164, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC (2012) Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci 32:14094–14101,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA (2017) Dopamine System Dysregulation in Major Depressive Disorders. Int J Neuropsychopharmacol 20:1036–1046,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD (2018) What does dopamine mean? Nat Neurosci 21:787–793, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ et al. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165:1163–1171,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdy R, Barrot M (2012) A new control center for dopaminergic systems: pulling the VTA by the tail. Trends Neurosci 35:681–690, [DOI] [PubMed] [Google Scholar]
- Bourdy R, Sanchez-Catalan MJ, Kaufling J, Balcita-Pedicino JJ, Freund-Mercier MJ, Veinante P, Sesack SR, Georges F, Barrot M (2014) Control of the nigrostriatal dopamine neuron activity and motor function by the tail of the ventral tegmental area. Neuropsychopharmacology 39:2788–2798, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA (2009) Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A 106:4894–4899, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie N, Sodha D, Scheinman SB, Steidl S (2020) Rewarding effects of M4 but not M3 muscarinic cholinergic receptor antagonism in the rostromedial tegmental nucleus. Behav Brain Res 379:112340,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Rolon D, Ramirez-Sanchez E, Arenas-Lopez G, Garduno J, Hernandez-Gonzalez O, Mihailescu S, Hernandez-Lopez S (2020) Nicotine Increases Spontaneous Glutamate Release in the Rostromedial Tegmental Nucleus. Front Neurosci 14:604583,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Esber GR, Marrero-Garcia Y, Yau HJ, Bonci A, Schoenbaum G (2016) Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nat Neurosci 19:111–116,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS (1986) Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci 6:613–619, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Saunders BT (2020) Heterogeneity in striatal dopamine circuits: Form and function in dynamic reward seeking. J Neurosci Res 98:1046–1069,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colussi-Mas J, Geisler S, Zimmer L, Zahm DS, Berod A (2007) Activation of afferents to the ventral tegmental area in response to acute amphetamine: a double-labelling study. Eur J Neurosci 26:1011–1025, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S (2014) Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc Natl Acad Sci U S A 111:6455–6460,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Muntoni AL, Pistis M, Melis M, Gessa GL (1999) Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur J Neurosci 11:1037–1041, [DOI] [PubMed] [Google Scholar]
- Durieux L, Mathis V, Herbeaux K, Muller MA, Barbelivien A, Mathis C, Schlichter R, Hugel S, Majchrzak M, Lecourtier L (2020) Involvement of the lateral habenula in fear memory. Brain structure & function 225:2029–2044, [DOI] [PubMed] [Google Scholar]
- Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L (2016) VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci 19:1356–1366, 5519826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Palacorolla H, Mayo CL, Brown PL, Jhou TC, Brady D, Shepard PD (2019) The rostromedial tegmental nucleus modulates the development of stress-induced helpless behavior. Behav Brain Res 359:950–957, [DOI] [PubMed] [Google Scholar]
- Engelhard B, Finkelstein J, Cox J, Fleming W, Jang HJ, Ornelas S, Koay SA, Thiberge SY, Daw ND, Tank DW, Witten IB (2019) Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 570:509–513,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD (1999) Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav 64:507–512, [DOI] [PubMed] [Google Scholar]
- Faivre F, Sanchez-Catalan MJ, Dovero S, Bido S, Joshi A, Bezard E, Barrot M (2020) Ablation of the tail of the ventral tegmental area compensates symptoms in an experimental model of Parkinson’s disease. Neurobiol Dis 139:104818, [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ (2011) Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471:597–601,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Zuo W, Gregor D, Li J, Grech D, Ye JH (2016a) Pharmacological Manipulation of the Rostromedial Tegmental Nucleus Changes Voluntary and Operant Ethanol Self-Administration in Rats. Alcoholism, clinical and experimental research 40:572–582,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Chen X, Zuo W, Li J, Kang S, Zhou LH, Siegel A, Bekker A, Ye JH (2016b) Ablation of mu opioid receptor-expressing GABA neurons in rostromedial tegmental nucleus increases ethanol consumption and regulates ethanol-related behaviors. Neuropharmacology 107:58–67,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Zuo W, Shiwalkar N, Mei Q, Fan Q, Chen X, Li J, Bekker A, Ye JH (2019) Alcohol withdrawal drives depressive behaviors by activating neurons in the rostromedial tegmental nucleus. Neuropsychopharmacology 44:1464–1475,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MPH, Schoenbaum G, Gershman SJ (2018) Rethinking dopamine as generalized prediction error. Proc Biol Sci 285,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Trimble M (2008) The lateral habenula: no longer neglected. CNS Spectr 13:484–489, [DOI] [PubMed] [Google Scholar]
- Glover EJ, McDougle MJ, Siegel GS, Jhou TC, Chandler LJ (2016) Role for the Rostromedial Tegmental Nucleus in Signaling the Aversive Properties of Alcohol. Alcoholism, clinical and experimental research 40:1651–1661,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EJ, Starr EM, Chao Y, Jhou TC, Chandler LJ (2019) Inhibition of the rostromedial tegmental nucleus reverses alcohol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA (2014) Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One 9:e92701,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD (2016) Mesolimbic dopamine signals the value of work. Nat Neurosci 19:117–126,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann G, Jo YS, Reichard KL, McFarland N, Chavkin C, Palmiter RD, Soden ME, Zweifel LS (2020) Synergy of Distinct Dopamine Projection Populations in Behavioral Reinforcement. Neuron 105:909–920 e905,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O (2011) Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci 31:11457–11471, 3315151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, LaLumiere RT (2015) The rostromedial tegmental nucleus modulates behavioral inhibition following cocaine self-administration in rats. Neuropsychopharmacology 40:861–873,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN, Bakhurin KI, Petter EA, Watson GDR, Kim N, Friedman AD, Yin HH (2020) Ventral Tegmental Dopamine Neurons Control the Impulse Vector during Motivated Behavior. Current biology : CB 30:2681–2694 e2685,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56:27–78, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F (2011) Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A 108:16446–16450, 3182694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou T (2005) Neural mechanisms of freezing and passive aversive behaviors. J Comp Neurol 493:111–114, [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC (2009a) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61:786–800,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS (2009. b) The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol 513:566–596,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Xu SP, Lee MR, Gallen CL, Ikemoto S (2012) Mapping of reinforcing and analgesic effects of the mu opioid agonist Endomorphin-1 in the ventral midbrain of the rat. Psychopharmacology (Berl),. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S (2013) Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci 33:7501–7512,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD (2007) Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci 27:6923–6930, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A (1979) Prospect Theory: An Analysis of Decision under Risk. Econometrica 47:263–291, [Google Scholar]
- Kaufling J, Aston-Jones G (2015) Persistent Adaptations in Afferents to Ventral Tegmental Dopamine Neurons after Opiate Withdrawal. J Neurosci 35:10290–10303,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M (2009) Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol 513:597–621, [DOI] [PubMed] [Google Scholar]
- Kaufling J, Waltisperger E, Bourdy R, Valera A, Veinante P, Freund-Mercier MJ, Barrot M (2010) Pharmacological recruitment of the GABAergic tail of the ventral tegmental area by acute drug exposure. Br J Pharmacol 161:1677–1691, 3010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2008) Addiction and the brain antireward system. Annu Rev Psychol 59:29–53, [DOI] [PubMed] [Google Scholar]
- Lahti L, Haugas M, Tikker L, Airavaara M, Voutilainen MH, Anttila J, Kumar S, Inkinen C, Salminen M, Partanen J (2016) Differentiation and molecular heterogeneity of inhibitory and excitatory neurons associated with midbrain dopaminergic nuclei. Development 143:516–529, [DOI] [PubMed] [Google Scholar]
- Laurent V, Wong FL, Balleine BW (2017) The Lateral Habenula and Its Input to the Rostromedial Tegmental Nucleus Mediates Outcome-Specific Conditioned Inhibition. J Neurosci 37:10932–10942,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi HN, Zahm DS (2011) The mesopontine rostromedial tegmental nucleus: an integrative modulator of the reward system. Basal ganglia 1:191–200, 3233474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi HN, Parsley KP, Zahm DS (2012) Mesopontine rostromedial tegmental nucleus neurons projecting to the dorsal raphe and pedunculopontine tegmental nucleus: psychostimulant-elicited Fos expression and collateralization. Brain structure & function 217:719–734,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi HN, Parsley KP, Zahm DS (2015) Modulation of locomotor activation by the rostromedial tegmental nucleus. Neuropsychopharmacology 40:676–687,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M (2012) Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology 37:1164–1176,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M (2011) Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology 36:589–602, 3055682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Mattar MG, Parker NF, Witten IB, Daw ND (2019) Reward prediction error does not explain movement selectivity in DMS-projecting dopamine neurons. Elife 8,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Pullmann D, Cho JY, Eid M, Jhou TC (2019a) Generality and opponency of rostromedial tegmental (RMTg) roles in valence processing. Elife 8,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Vento PJ, Parrilla-Carrero J, Pullmann D, Chao YS, Eid M, Jhou TC (2019. b) Three Rostromedial Tegmental Afferents Drive Triply Dissociable Aspects of Punishment Learning and Aversive Valence Encoding. Neuron 104:987–999 e984,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet JC, Zhang Y, Li X, Zhang X (2008) PTEN-5-HT2C coupling: a new target for treating drug addiction. Prog Brain Res 172:407–420, [DOI] [PubMed] [Google Scholar]
- Marsden CA (2006) Dopamine: the rewarding years. Br J Pharmacol 147 Suppl 1:S136–144,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT (2014) Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 82:1346–1356,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2007) Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447:1111–1115, [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2009) Representation of negative motivational value in the primate lateral habenula. Nat Neurosci 12:77–84, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M (2005) The dopamine hypothesis of drug addiction: hypodopaminergic state. International review of neurobiology 63:101–154, [DOI] [PubMed] [Google Scholar]
- Melis M, Sagheddu C, De Felice M, Casti A, Madeddu C, Spiga S, Muntoni AL, Mackie K, Marsicano G, Colombo G, Castelli MP, Pistis M (2014) Enhanced endocannabinoid-mediated modulation of rostromedial tegmental nucleus drive onto dopamine neurons in Sardinian alcohol-preferring rats. J Neurosci 34:12716–12724,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ (1996) A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci 16:1936–1947, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton G, Nasirova N, Sparks DW, Brodsky M, Sivakumaran S, Lambe EK, Turner EE (2018) Chrna5-Expressing Neurons in the Interpeduncular Nucleus Mediate Aversion Primed by Prior Stimulation or Nicotine Exposure. J Neurosci 38:6900–6920,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE, Pedersen CE, Gomez AM, Spangler SM, Walicki MC, Feng SY, Stewart SL, Otis JM, Al-Hasani R, McCall JG, Sakers K, Bhatti DL, Copits BA, Gereau RW, Jhou T, Kash TJ, Dougherty JD, Stuber GD, Bruchas MR (2019) A Paranigral VTA Nociceptin Circuit that Constrains Motivation for Reward. Cell 178:653–671 e619, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrilla-Carrero J, Eid M, Li H, Chao YS, Jhou TC (2021) Synaptic Adaptations at the Rostromedial Tegmental Nucleus Underlie Individual Differences in Cocaine Avoidance Behavior. J Neurosci, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M (2005) DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci 21:2817–2824, [DOI] [PubMed] [Google Scholar]
- Pradel K, Drwiega G, Blasiak T (2021) Superior Colliculus Controls the Activity of the Rostromedial Tegmental Nuclei in an Asymmetrical Manner. J Neurosci 41:4006–4022, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx CD, Aronson S, Milivojevic D, Molina C, Loi A, Monk B, Shabel SJ, Malinow R (2018) A neural pathway controlling motivation to exert effort. Proc Natl Acad Sci U S A 115:5792–5797, 5984527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang HL, Hoffman AF, Lupica CR, Morales M (2014) Single rodent mesohabenular axons release glutamate and GABA. Nat Neurosci 17:1543–1551,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Barker DJ, Estrin DJ, Miranda-Barrientos JA, Liu B, Zhang S, Wang HL, Vautier F, Ramakrishnan C, Kim YS, Fenno L, Deisseroth K, Morales M (2020) Distinct Signaling by Ventral Tegmental Area Glutamate, GABA, and Combinatorial Glutamate-GABA Neurons in Motivated Behavior. Cell Rep 32:108094,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Diana M, Gessa GL (1992) Alcohol withdrawal in rats is associated with a marked fall in extraneuronal dopamine. Alcoholism, clinical and experimental research 16:529–532, [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Siletti KA, Stansfield KJ, Bercum MF (2018) Heterogeneous dopamine signals support distinct features of motivated actions: implications for learning and addiction. Learn Mem 25:416–424,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM (2015) Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol Psychiatry 77:903–911,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Catalan MJ, Faivre F, Yalcin I, Muller MA, Massotte D, Majchrzak M, Barrot M (2016) Response of the Tail of the Ventral Tegmental Area to Aversive Stimuli. Neuropsychopharmacology, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB (2000) Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 20:8620–8628, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (2007) Multiple dopamine functions at different time courses. Annual review of neuroscience 30:259–288, [DOI] [PubMed] [Google Scholar]
- Schultz W (2016) Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci 17:183–195,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275:1593–1599, [DOI] [PubMed] [Google Scholar]
- Sego C, Goncalves L, Lima L, Furigo IC, Donato J Jr., Metzger M (2014) Lateral habenula and the rostromedial tegmental nucleus innervate neurochemically distinct subdivisions of the dorsal raphe nucleus in the rat. J Comp Neurol 522:1454–1484, [DOI] [PubMed] [Google Scholar]
- Sheth C, Furlong TM, Keefe KA, Taha SA (2016) Lesion of the rostromedial tegmental nucleus increases voluntary ethanol consumption and accelerates extinction of ethanol-induced conditioned taste aversion. Psychopharmacology (Berl) 233:3737–3749,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Huber A, Herz A (1991) Neuroanatomical substrates mediating the aversive effects of D-1 dopamine receptor antagonists. Psychopharmacology (Berl) 103:209–214, [DOI] [PubMed] [Google Scholar]
- Shumake J, Ilango A, Scheich H, Wetzel W, Ohl FW (2010) Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J Neurosci 30:5876–5883, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Vento PJ, Chao YS, Good CH, Jhou TC (2019) Gene expression and neurochemical characterization of the rostromedial tegmental nucleus (RMTg) in rats and mice. Brain structure & function 224:219–238,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD (1974) An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev 81:119–145, [DOI] [PubMed] [Google Scholar]
- St Laurent R, Martinez Damonte V, Tsuda AC, Kauer JA (2020) Periaqueductal Gray and Rostromedial Tegmental Inhibitory Afferents to VTA Have Distinct Synaptic Plasticity and Opiate Sensitivity. Neuron 106:624–636 e624,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD (2012) Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci 15:1105–1107, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl S, Wasserman DI, Blaha CD, Yeomans JS (2017) Opioid-induced rewards, locomotion, and dopamine activation: A proposed model for control by mesopontine and rostromedial tegmental neurons. Neurosci Biobehav Rev 83:72–82,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH (2013) A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci 16:966–973,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB (2014) What’s better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat Neurosci 17:33–35, 4974073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS (1988) Learning to predict by the methods of temporal differences. Machine Learning 3:9–44, [Google Scholar]
- Taylor NE, Long H, Pei J, Kukutla P, Phero A, Hadaegh F, Abdelnabi A, Solt K, Brenner GJ (2019) The rostromedial tegmental nucleus: a key modulator of pain and opioid analgesia. Pain 160:2524–2534,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesauro G (1994) TD-Gammon, a Self-Teaching Backgammon Program, Achieves Master-Level Play. Neural Computation 6:215–219, [Google Scholar]
- Thorndike E (1911) Animal intelligence: McMillan. [Google Scholar]
- Tian J, Uchida N (2015) Habenula lesions reveal that multiple mechanisms underlie dopamine prediction errors. Neuron 87:1304–1316,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Huang R, Cohen JY, Osakada F, Kobak D, Machens CK, Callaway EM, Uchida N, Watabe-Uchida M (2016) Distributed and mixed information in monosynaptic inputs to dopamine neurons. Neuron 91:1374–1389,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusel M, Nuno-Perez A, Lecca S, Harada H, Lalive AL, Congiu M, Takemoto K, Takahashi T, Ferraguti F, Mameli M (2019) Punishment-Predictive Cues Guide Avoidance through Potentiation of Hypothalamus-to-Habenula Synapses. Neuron 102:120–127 e124, [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324:1080–1084, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento PJ, Burnham NW, Rowley CS, Jhou TC (2017) Learning from one’s mistakes: a dual role for the rostromedial tegmental nucleus in the encoding and expression of punished reward seeking. Biol Psychiatry 81:1041–1049,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Robble MA (2020) Dopamine and Addiction. Annu Rev Psychol 71:79–106, [DOI] [PubMed] [Google Scholar]
- Yang SR, Hu ZZ, Luo YJ, Zhao YN, Sun HX, Yin D, Wang CY, Yan YD, Wang DR, Yuan XS, Ye CB, Guo W, Qu WM, Cherasse Y, Lazarus M, Ding YQ, Huang ZL (2018) The rostromedial tegmental nucleus is essential for non-rapid eye movement sleep. PLoS Biol 16:e2002909, 5919677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YN, Yan YD, Wang CY, Qu WM, Jhou TC, Huang ZL, Yang SR (2020) The Rostromedial Tegmental Nucleus: Anatomical Studies and Roles in Sleep and Substance Addictions in Rats and Mice. Nat Sci Sleep 12:1215–1223,. [DOI] [PMC free article] [PubMed] [Google Scholar]