SUMMARY:
Defensive behavioral responses are contingent upon threat intensity, proximity, and context of exposure. Based on these factors, we developed a classical conditioning paradigm that elicits clear transitions between conditioned freezing and flight behavior within individual subjects. This model is crucial for the understanding the pathologies involved in anxiety, panic, and post-traumatic stress disorders.
Fear- and anxiety-related behaviors significantly contribute to an organism’s survival. However, exaggerated defensive responses to perceived threat are characteristic of various anxiety disorders, which are the most prevalent form of mental illness in the United States. Discovering the neurobiological mechanisms responsible for defensive behaviors will aid in the development of novel therapeutic interventions. Pavlovian fear conditioning is a widely used laboratory paradigm to study fear-related learning and memory. A major limitation of traditional Pavlovian fear conditioning paradigms is that freezing is the only defensive behavior monitored. We recently developed a modified Pavlovian fear conditioning paradigm that allows us to study both conditioned freezing and flight (also known as escape) behavior within individual subjects. This model employs higher intensity footshocks and a greater number of pairings between the conditioned stimulus and unconditioned stimulus. Additionally, this conditioned flight paradigm utilizes serial presentation of pure tone and white noise auditory stimuli as the conditioned stimulus. Following conditioning in this paradigm, mice exhibit freezing behavior in response to the tone stimulus, and flight responses during the white noise. This conditioning model can be applied to the study of rapid and flexible transitions between behavioral responses necessary for survival.
Keywords: Fear conditioning, freezing, flight, anxiety, fear, panic, defensive behavior
INTRODUCTION:
Fear is an evolutionarily conserved adaptive response to immediate threat1,2. While organisms possess innate defensive responses to a threat, learned associations are crucial to elicit appropriate defensive responses to stimuli predictive of danger3. Dysregulation in brain circuits controlling defensive responses is likely to contribute to maladaptive reactions associated with multiple debilitating anxiety disorders, such as post-traumatic stress disorder (PTSD), panic disorder4, and specific phobias5,6. The prevalence rate in the United States for anxiety disorders is 19.1% for adults and 31.9% in adolescents7,8. The burden of these illnesses is extremely high on the daily routine of individuals and negatively impacts quality of life.
Over the last several decades, Pavlovian fear conditioning has served as a powerful model system to gain tremendous insight into the neural mechanisms underlying fear-related learning and memory9-11. Pavlovian fear conditioning entails pairing a conditioned stimulus (CS, such as an auditory stimulus) with an aversive unconditioned stimulus (US; for example, an electrical footshock)12. Because freezing is the dominant behavior evoked and measured in standard Pavlovian conditioning paradigms, the neural control mechanisms of active forms of defensive behavior such as escape/flight responses remain largely unexplored. Previous studies show that different forms of defensive behavior, such as flight, are evoked depending upon the threat intensity, proximity and context13,14. Studying how the brain controls different types of defensive behavior may significantly contribute to our understanding of the neuronal processes that are dysregulated in fear and anxiety disorders.
To address this critical need, we developed a modified Pavlovian conditioning paradigm that elicits flight and escape jumps, in addition to freezing15. In this paradigm, mice are conditioned with a serial compound stimulus (SCS) consisting of a pure tone followed by white noise. Following two days of pairing the SCS with a strong electrical footshock, mice exhibit freezing in response to the tone component and flight during the white noise. Behavioral switches between conditioned freezing and flight behavior are rapid and consistent. Interestingly, mice exhibit flight behavior only when the white noise CS is presented in the same context as a previously delivered footshock (the conditioning context) but not in a neutral context. Instead, freezing responses dominate in this the neutral context, with significantly greater levels of freezing in response to the white noise compared to the tone. This is consistent with the role of context in modulating defensive response intensity and with the regulatory role of contextual information in fear-related learning and memory found in traditional threat conditioning paradigms16,17. This model allows for direct, within-subject comparisons of multiple defensive behaviors in a context-specific manner.
PROTOCOL:
We have conducted the following steps/procedures in accordance with institutional guidelines after approval from the Institutional Animal Care & Use Committee of Tulane University.
1. Preparation of mice
1.1. Male and/or female adult mice aged between 3-5 months can be used. In the present study, we used male C57BL/6J mice obtained from Jackson Laboratory, but any mouse strain from a reputable supplier can be used.
1.2. At least one week before the experiment, house all the mice individually on a 12:12 h light/dark cycle throughout the study. Provide the mice ad libitum access to food and water.
1.3. Perform all behavioral experiments during the light cycle. Perform all sessions at the same time of day within an individual cohort. For example, if you start your experiment at 9 AM on Day 1, continue starting at that time until the experiment is completed.
2. Preparation of study materials
2.1. Study contexts
2.1.1. Choose two different contexts to perform the experiments in.
2.1.2. Context A is a cylindrical chamber composed of clear Plexiglas (diameter 30 cm), with a smooth Plexiglas floor. The height of the chamber should be sufficient to prevent escape (at least 30 cm high).
2.1.3. Context B is a rectangular enclosure (25 X 30 cm) with an electrical grid floor used to deliver alternating current footshocks. The height of this chamber is very important and should be at least 35 cm. high. Alternatively, one can use a transparent roof (ensure that you are able to record video through this material).
Note: Use a chamber with smooth wall surfaces that can be easily cleaned.
2.1.4. Use a different cleaning solution to clean the contexts. For example, context A is cleaned with 1% acetic acid and context B with 70% ethanol. Clean the contexts before beginning the first session, between testing individual mice, and after completion of the day’s sessions. This is vital to remove the olfactory cues from previous mice. Thorough cleaning will also help prevent urine scaling on the shock grid, which will compromise conditioning sessions.
Note: The cleaning solutions also serve as an olfactory cue, therefore use the same cleaning liquid for a particular context.
2.1.5. Place context A or context B in a sound-attenuating box during respective study sessions.
2.2. Audio generator
2.2.1. Mount an overhead speaker above the contexts to deliver auditory stimuli at 75 dB.
2.2.2. Use a programmable audio generator to generate auditory stimuli on a pre-defined schedule.
2.2.3. The 7.5 kHz pure tone is a sound with a sinusoidal waveform, whereas the white noise is a random signal having equal intensity at different frequencies, ranging from 1-20,000 Hz.
2.2.4. Use TTL pulses to deliver auditory stimuli and shock signals with temporal precision.
Note: Before starting the experiments, measure the sound intensity output from the mounted speaker in each chamber using dB meter.
2.3. Shocker: Connect the shocker with the electrical grid floor which is used to deliver the 0.9 mA AC shock. Define the frequency, onset, and duration of shocks in a computer program. Each shock stimulus should be delivered at the end of each SCS for a duration of 1 sec, totaling five SCS-shock pairings per conditioning session.
3. Preparation of computer program and Video tracking
3.1. Generate behavioral protocols using coding in a software program.
3.2. In the program, define the serial compound stimulus (SCS). This stimulus is a serial presentation of a 10 sec pure tone (each pip is presented for 500 ms, at frequency of 7.5 kHz and rate of 1 Hz) and 10 sec white noise (500 ms pips at 1 Hz).
3.3. Define the inter-trial intervals (ITI) presented following each trial, pseudorandomly.
3.4. During the study, record all mouse behavior to video for subsequent analysis.
Note: Commercially available fear conditioning boxes may not be set up to record the behaviors through the top-mounted camera. This is very important since the recorded video is used to calculate vertical movement, speed and total distance travelled by the animal.
3.5. For setting the software tracking, place a test mouse in each relevant context, adjust the contour size, and define the center of gravity. This will ensure the acquisition of reliable data on relative position. In addition, define the whole context area accessible to the subject.
Note: The adjustment of contour size for both contexts is important as the change in brightness in different contexts will change the contour size.
3.6. Determine a calibration coefficient using the chambers’ known sizes and the camera’s pixel dimensions and calculate speed (cm/sec).
3.7. Synchronize the central computer’s event markers to their real-time occurrences.
4. Behavioral experiment
4.1. Turn on all the equipment –computers, fear conditioning box controller, shocker, and video recording software. Make sure all the switches of relevant instruments are properly switched on.
4.2. Check all the functions including tone, white noise, and shock delivery, and set up the system for the data acquisition.
4.3. Transport the animals from their storage room to the conditioning room. Allow them to acclimatize there for at least 10 min.
4.4. Take the animal out from the home cage, gently place it in the respective context, and then immediately activate the computer programs.
Note: The initialization of both fear conditioning system and data collection (timestamps, mouse tracking and video recording) software at a time can be synchronized using TTL pulse mediated activations.
4.5. Pre-conditioning/Pre-exposure
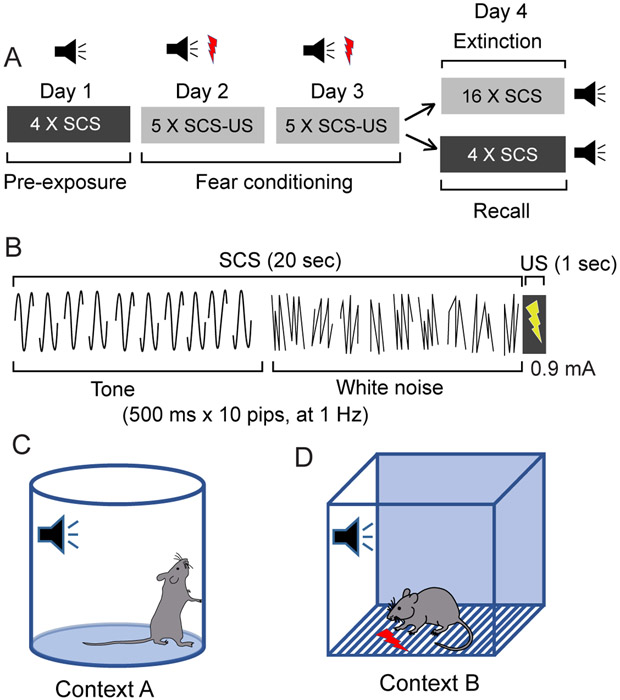
4.5.1. On Day 1, place the subject into context A (neutral context). Allow it to acclimate to the chamber for 3 min (the baseline period), and then expose it to 4 trials of a SCS of 20 sec total duration (Fig. 1A-B).
Figure 1:
Study design for flight paradigm. A) Diagram of the sessions of the behavioral conditioned flight paradigm. B) Diagram detailing the composition of the serial compound stimulus (SCS), as well as the timing of the US. C) Context A - served as a neutral context, and used during pre-exposure and recall sessions. D) Context B - used for fear conditioning. This figure has been modified from Fadok et al. 2017.
4.5.2. Maintain an 80 sec average pseudorandom intertrial interval (ITI) (range 60-100 sec).
4.5.3. The total duration of each pre-exposure session is 590 sec.
4.6. Fear conditioning
4.6.1. On Day 2 and Day 3, place the subject into Context B. Following a 3 min baseline period, expose the subject to five pairings of the SCS co-terminating with a 1 sec, 0.9 mA AC footshock.
4.6.2. Maintain a 120 sec average pseudorandom ITI (range 90-150 sec).
4.6.3. Each conditioning session lasts for 820 sec in total (Figure 1A).
4.6.4. Depending on the goal of your experiment, mice can be subjected on Day 4 to either a recall test (see 4.3) or to fear extinction (see 4.4).
4.7. Fear recall (to test context dependence)
4.7.1. On Day 4, place the subject into Context A. After the 3 min baseline period, present it with 4 trials of the SCS without footshock, over 590 sec.
4.7.2. Maintain an 80 sec average pseudorandom ITI (range 60-100 sec).
4.8. Fear extinction
4.8.1. On Day 4, place the subject into context B. Following the 3 min baseline period, present 16 trials of the SCS without footshock, over 1910 sec.
4.8.2. Maintain a 90 sec average pseudorandom ITI (range 60-120 sec).
4.9. Return the animal to its home cage and repeat the procedure for all the animals.
5. Quantification of behavior
5.1. An observer blind to the experiment should score the recorded videos for freezing behavior using automatic freezing detector thresholding followed by a frame-by-frame analysis of pixel changes.
Note: Other software packages can also be used to calculate freezing automatically by using 2 camera system. It is also possible for an observer to manually score freezing behavior.
5.2. Freezing is defined as a complete cessation of bodily movements, except for those required for respiration, for a minimum of 1 sec.
5.3. Jumps are scored when all 4 of the paws leave the floor, resulting in a vertical and/or horizontal movement.
5.4. Export the marked file with freezing, jump and event markers.
5.5. Extract relevant events (freezing and jumps) from defined time periods (e.g. 10 sec duration of pre-SCS, tone and white noise, for each trial).
5.6. Using the extracted start-stop durations of events in a spreadsheet file, calculate the duration of freezing (in sec) by subtracting start time from end time, from the respective trial periods.
5.7. One can represent this data trial-wise or day-wise by summing up freezing duration from all trials.
Note: Depending on the purpose of the study, the flight or freezing behaviors can be scored and calculated from any trial/duration from the study session.
5.8. The total number of jumps from a particular trial duration is summated.
5.9. Extract the file generated by mouse tracking coordinates from frame by frame X-Y axis movement of the center of gravity of mouse and calculate the speed of the mouse (cm/sec).
Note: The speed data may be present either in the cm/sec or pixel/sec format. Convert the pixel/sec unit to cm/sec by using pixel/inch or cm value defined in the video for that testing context (please see section 3.6).
5.10. After extracting speed data for frame by frame movement of the animal, based on frame rate of the video (preferably 30 frames/sec), calculate the average speed of the animal in a specific frame number bracket (multiply start and end times in sec with 30, you will get the start and end frame number).
5.11. Calculate the flight scores by dividing the average speed during each SCS by the average speed during the 10 sec pre-SCS (baseline, BL) and then adding 1 point for each escape jump (speedCS/speedBL + # of jumps).
5.12. A flight score of 1 therefore indicates no change in flight behavior from the pre-SCS period.
5.13. Videos can also be scored manually for other behaviors such as rearing and grooming.
6. Statistical analysis
6.1. Data is analyzed for statistical significance using statistical analysis software. For all tests, the definition of statistical significance is p<0.05.
6.2. Check the data for normal distribution using the Shapiro-Wilk normality test (α=0.05).
6.3. To test the effect of cues, carry out the pairwise comparisons using the appropriate parametric (paired t-test) or non-parametric (Wilcoxon signed-rank test) test.
6.4. To assess the 2-way interaction of factors (cue X trial), perform a 2-way ANOVA followed by post-hoc tests (e.g. Bonferroni’s multiple comparison test/Tukey’s test).
REPRESENTATIVE RESULTS:
As described in the diagram (Fig. 1A), the session starts with pre-exposure (Day 1), followed by fear conditioning (Days 2 and 3), then either extinction or retrieval (Day 4).
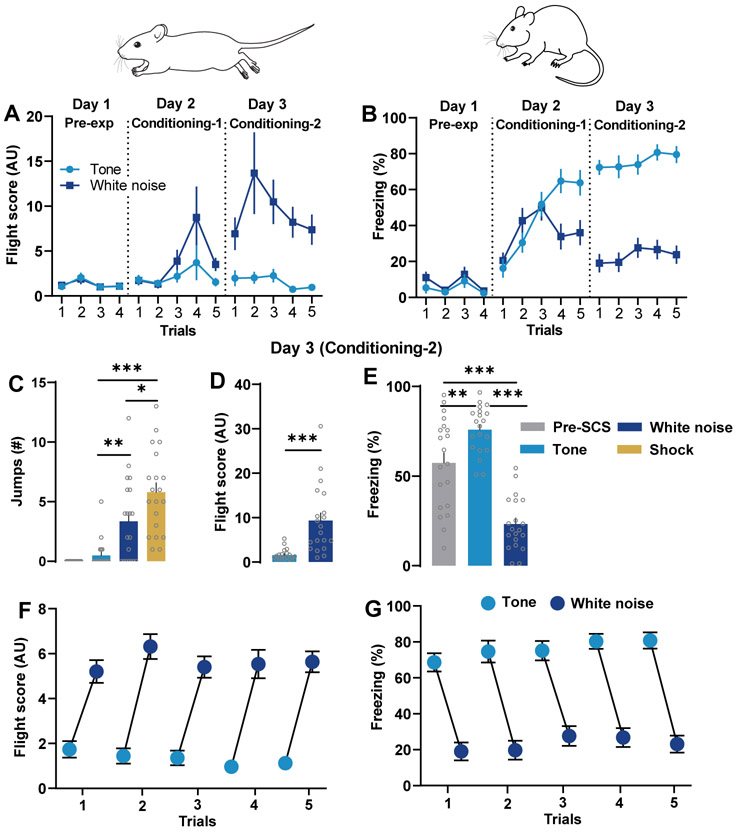
Presentations of the SCS in the pre-exposure (Day 1) session did not elicit flight or freezing response in the mice (Fig. 2A-B). Behavioral analysis during conditioning (Days 2 and 3) revealed that the tone component of the SCS significantly enhanced freezing compared to freezing during the pre-SCS (Fig. 2B,E). Flight scores changed significantly across sessions (Day 1 to Day 3, n = 20; Fig. 2A). Mice showed higher speed and more jumps, and thus greater flight scores, to the white noise cue compared to tone (Fig. 2C-D). Mice showed a clear transition of defensive behavior--exhibiting lower flight scores during the tone followed by higher flight scores during white noise (Fig 2F) and vice-versa for freezing responses (Fig. 2G).
Figure 2:
Conditioned flight response. A) Comparison of average trial-wise flight scores (n = 20) following presentation of the tone and white noise across Days 1-3. A significant change in the flight scores across sessions have been noted (Day 1 to Day 3; two-way repeated measures ANOVA, cue × trial interaction, F (13, 266) = 5.795; P<0.0001). Post-hoc Bonferroni’s multiple comparison test reveals a significant difference between tone and white noise induced flight scores at fear conditioning Day 1 (trial 4, P < 0.05) and Day 2 (trials 2-5, P < 0.001). B) Comparison of average trial-wise % freezing during the tone and white noise periods across Days 1-3. Note a statistically significant changes in % freezing across the sessions (Day 1 to Day 3, n = 20; two-way repeated-measures ANOVA, cue × trial interaction, F (13, 266) = 20.81; P < 0.001; Fig. 2B). Post-hoc Bonferroni’s multiple comparison test reveals a significant difference between tone and white noise induced freezing at fear conditioning Day 1 (trial 4 and 5, P < 0.001) and Day 2 (all trials, P < 0.001). C) Comparison of number of jump escape responses in during the pre-SCS, tone, white noise, and shock periods on Day 3. One-way ANOVA followed by Bonferroni's multiple comparisons test showed that escape jumps were significantly higher during white noise and shock as compared to tone period (P < 0.01 and P < 0.001, respectively). D) Comparison of flight scores during the presentation of tone and white noise on Day 3. Note a significantly higher flight scores on Day 3 during white noise period (P < 0.001, Wilcoxon matched-pairs signed-rank test). E) Comparison of % freezing during the pre-SCS, tone, and white noise on Day 3. Moreover, freezing behavior on Day 3 reveals significant effect of tone and white noise (one-way repeated-measures ANOVA, F = 56.82, P<0.01). Bonferroni's multiple comparisons test showed that presentation of tone significantly increases % freezing vs pre-SCS duration (P < 0.01), whereas % freezing was significantly reduced as compared pre-SCS and tone durations (both P < 0.001). The representative trial-wise data shows transitions of flight (F) and freezing (G) behavior following the presentation of tone and white noise in the mouse on Day 3. The represented values are means ± SEM. *p<0.05, **p<0.01, ***p <0.001. Pre-exp, Pre-exposure. Panels A-E are modified from Fadok et al., 2017.
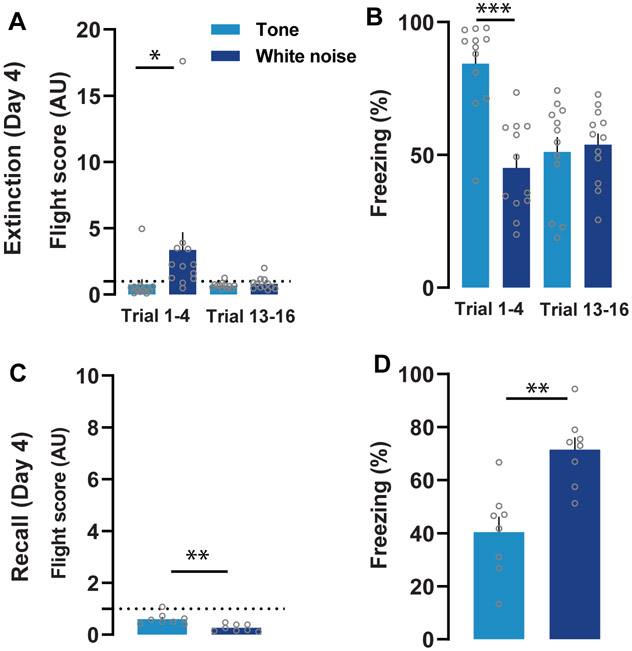
To test for the effect of threat proximity and context on conditioned flight, mice were split into two groups: one group underwent extinction training in the conditioning context (Fig. 3A-B), and another group was tested for fear memory recall by exposing them to the SCS in a neutral context (Fig. 3C-D). Mice subjected to the 16 trials of extinction training showed rapid extinction of conditioned flight (n = 12). Flight scores during the first block of four trials were higher during white noise as compared to the tone (Fig. 3A). Flight behavior was no longer elicited by either cue at the end of the extinction session. There was an overall decrease in tone-induced freezing and an increase in white noise-mediated freezing during the extinction session. Freezing for the first block of four trials was significantly higher to the tone compared to the white noise (Fig. 3B). This suggests imminence of the threat is vital for the flight response.
Figure 3:
Extinction and recall following flight conditioning (Day 4). A) Comparison of flight scores during extinction training showed rapid extinction of conditioned flight (n = 12; 16 trials, two-way repeated-measures ANOVA, cue × trial interaction, F(15,165) = 3.05, P < 0.01). Flight scores from first block of four trials (trial 1-4) of extinction observed significantly higher for white noise as compared to the tone (P < 0.05, Wilcoxon matched-pairs signed-rank test). B) Comparison of freezing showed a statically significant effect on freezing (%) following white noise (n = 12; 16 trials, two-way repeated-measures ANOVA, cue × trial interaction, F(15,165) = 3.55, P < 0.01). The freezing for the first block of four trials (trial 1-4) during extinction found to be significantly lower during white noise period as compared to the tone (Paired t-test, P < 0.01). C) Change in the context significantly affect the flight scores (n = 8; 4 trials, two-way repeated-measures ANOVA, cue × trial interaction, F(1,7) = 27.44, P < 0.01). Flight scores significantly reduced during white noise as compared to the tone period in the neutral context (two-tailed paired t-test, P < 0.01) D). Freezing responses across trials during retrieval were also significant (n = 8, 4 trials, two-way repeated-measures ANOVA, effect of cue F(1,7) = 27.67, P < 0.01). Exposure of WN in neutral context significantly increased the freezing responses as compared to the tone (two-tailed paired t-test, P < 0.001). The represented values are means ± SEM. *p<0.05, **p<0.01, ***p <0.001. Panels A-D are modified from Fadok et al., 2017.
The flight response was diminished in a context-dependent manner. Exposure to the white noise in the neutral context did not elicit flight (n = 8). Instead, white noise presentations in the neutral context elicited freezing responses which were higher than those elicited by the tone. (Fig. 3C-D). This demonstrates the importance of context in modulating defensive responding.
DISCUSSION:
The described sound and shock parameters are important elements of this protocol. It is critical, therefore, to test the shock amplitude and sound pressure level before starting the experiments. Fear conditioning studies typically use 70-80 dB sound pressure levels and 0.1-1 mA shock intensity18; thus, the described parameters are within the bounds of traditional fear conditioning paradigms. In a previous CS-only (no footshock) control experiment, we did not observe flight or freezing responses in the mice, indicating that the auditory stimuli are not aversive when presented as described15. Increasing the dB level of the white noise above 80 dB may induce innate aversion. However, noise stimuli presented at 75 dB do not elicit stress in the form of suppressed behavioral activity in mice19.
The auditory stimuli that comprise the SCS must be carefully selected. In our previous study, we determined that single-CS conditioning with white noise induces higher flight scores than conditioning with a pure tone15. This illustrates the importance of stimulus salience in this protocol (see 20). However, a recent study showed that conditioning with a reversal of the SCS sequence (white noise-tone) results in flight to the tone and freezing to the white noise21. These data endorse that the learned temporal relationship of the cues is also an important factor.
Because cage changes are a potential source of stress, it is recommended to start conditioning at least 2 days after the last most recent cage change. To further minimize the impact of stress in the mice undergoing study, appropriate care should be taken to reduce the olfactory cues remaining from previous subjects, including the smell of feces and urine. Therefore, cleaning the chamber before and after each mouse is crucial. To avoid other potential sources of disturbance, it is best to conduct this protocol in a room separated from any other ongoing experiments. Mice should exhibit very low baseline freezing15. To test the experimental conditions, each laboratory should conduct a pilot experiment to test baseline freezing in each context.
Other than the C57BL/6J and other transgenic lines used by Fadok et al. (2017)15, this method should be suitable for adaptation to other strains of mice and rats20,21. Our recent data (Borkar et al., 2020)22 suggest that both male and female mice show comparable flight responses, therefore the paradigm is suitable for both sexes. As mentioned in section 2.1.2, in response to high intensity shocks, mice jump very high, thus carefully select the height of the chamber to prevent the mice from escaping the context. It is also important to ensure the consistent and accurate timing of cues and shock stimuli. Both AC and DC shocks are effective; however, when using DC shocks, it may be necessary to increase footshock intensity to reach similar flight scores as that of AC shocks. Because DC shocks have a less detrimental effect on electrophysiological recordings, use of DC shock is recommended for studies that require electrophysiology data. It is important to note that decreasing the intensity of the footshock may decrease the intensity of the flight response.
As denoted in the Protocol section, flight scores are calculated by normalizing speed data during tone and white noise by dividing them with individual trial pre-SCS speed values. However, if a mouse exhibits extremely high levels of freezing during the pre-SCS, the resultant flight scores may be very high, thus increasing data variability. This can be circumvented by using a different baseline measurement, such as average speed data from the 3 min baseline period at the beginning of the session or using the average overall pre-SCS (average of 5 trials).
Flexible and rapid behavioral adaptation to threat is crucial for survival. Most classical fear conditioning protocols use conditions that induce freezing as a sole determinant of fear learning. The benefit of this protocol is that it allows for study of complex defensive state transitions within subjects. Previously, this model was used to discover that behavioral transitions are processed by local recurrent inhibitory circuits in the central amygdala15,23. This paradigm also enabled researchers to elucidate cortico-thalamic circuits for the selection of defensive behavior21. These studies demonstrate that this method will facilitate studies investigating neural circuit control of rapid transitions between defensive behaviors within a subject. This has potential applications for developing a better understanding of the neurobiological underpinnings of anxiety, panic disorder, or PTSD24,25.
ACKNOWLEDGMENTS:
This work was supported by the Louisiana Board of Regents through the Board of Regents support fund (LEQSF(2018-21)-RD-A-17) and the National Institute of Mental Health of the National Institutes of Health under award number R01MH122561. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES:
The authors have nothing to disclose.
REFERENCES
- 1.Gross CT, Canteras NS. The many paths to fear. Nature Reviews Neuroscience. 2012;13(9):651–658. doi: 10.1038/nrn3301 [DOI] [PubMed] [Google Scholar]
- 2.LeDoux J Rethinking the Emotional Brain. Neuron. 2012. doi: 10.1016/j.neuron.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maren S Neurobiology of Pavlovian fear conditioning. Annual review of neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- 4.Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural pathways underlying lactate-induced panic. Neuropsychopharmacology. 2008;33(9):2093–2107. doi: 10.1038/sj.npp.1301621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mobbs D, Marchant JL, Hassabis D, et al. From threat to fear: The neural organization of defensive fear systems in humans. Journal of Neuroscience. 2009;29(39):12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Münsterkötter AL, Notzon S, Redlich R, et al. Spider or no spider? neural correlates of sustained and phasic fear in spider phobia. Depression and Anxiety. 2015;32(9):656–663. doi: 10.1002/da.22382 [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Wai TC, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Survey NC. Generalized anxiety disorder definition prevalence of generalized anxiety disorder among adults generalized anxiety disorder with impairment among adults. 2017:3–8. [Google Scholar]
- 9.Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nature Neuroscience. 2014;17(12):1644–1654. doi: 10.1038/nn.3869 [DOI] [PubMed] [Google Scholar]
- 10.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16(6):317–331. doi: 10.1038/nrn3945 [DOI] [PubMed] [Google Scholar]
- 12.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301(5634):846–850. doi: 10.1126/science.1085818 [DOI] [PubMed] [Google Scholar]
- 13.Blanchard DC, Blanchard RJ. Defensive behaviors, fear, and anxiety. In: Handbook of Anxiety and Fear. Handbook of behavioral neuroscience. Blanchard D Caroline: Department of Genetics & Molecular Biology, John A Burns School of Medicine, & Pacific Biosciences Research Center, University of Hawaii at Manoa, 1993 East West Road, Honolulu, HI, US, 96822: Elsevier Academic Press; 2008:63–79. doi: 10.1016/S1569-7339(07)00005-7 [DOI] [Google Scholar]
- 14.Perusini JN, Fanselow MS. Neurobehavioral perspectives on the distinction between fear and anxiety. Learning and Memory. 2015;22(9):417–425. doi: 10.1101/lm.039180.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadok JP, Krabbe S, Markovic M, et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542(7639):96–99. doi: 10.1038/nature21047 [DOI] [PubMed] [Google Scholar]
- 16.Maren S Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behavioral Neuroscience. 1999;113(2):283–290. doi: 10.1037/0735-7044.113.2.283 [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Krabbe S, Gründemann J, et al. Distinct hippocampal pathways mediate dissociable roles of context in memory retrieval. Cell. 2016;167(4):961–972.e16. doi: 10.1016/j.cell.2016.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curzon P, Rustay NR BK. Chapter 2: Cued and contextual fear conditioning for rodents. In: JJ B, ed. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. [PubMed] [Google Scholar]
- 19.Mollenauer S, Bryson R, Robison M, Phillips C. Noise avoidance in the C57BL/6J mouse. Animal Learning & Behavior. 1992;20(1):25–32. doi: 10.3758/BF03199943 [DOI] [Google Scholar]
- 20.Hersman S, Allen D, Hashimoto M, Brito SI, Anthony TE. Stimulus salience determines defensive behaviors elicited by aversively conditioned serial compound auditory stimuli. eLife. 2020;9. doi: 10.7554/eLife.53803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong P, Wang H, Shen XF, et al. A novel cortico-intrathalamic circuit for flight behavior. Nature Neuroscience. 2019;22(6):941–949. doi: 10.1038/s41593-019-0391-6 [DOI] [PubMed] [Google Scholar]
- 22.Borkar CD, Dorofeikova M, Le QE, et al. Sex differences in behavioral responses during a conditioned flight paradigm. Behavioural Brain Research. 2020;389:112623. doi: 10.1016/j.bbr.2020.112623 [DOI] [PubMed] [Google Scholar]
- 23.Fadok JP, Markovic M, Tovote P, Lüthi A. New perspectives on central amygdala function. Current Opinion in Neurobiology. 2018;49:141–147. doi: 10.1016/j.conb.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 24.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canteras NS, Graeff FG. Executive and modulatory neural circuits of defensive reactions: Implications for panic disorder. Neuroscience and Biobehavioral Reviews. 2014. doi: 10.1016/j.neubiorev.2014.03.020 [DOI] [PubMed] [Google Scholar]