Abstract
Background
COVID-19 infection has recently become a pandemic disease around the world, and its risk factors have not fully evaluated. This study aimed to compare the serum vitamin D (Vit D) and zinc levels in patients infected with novel coronavirus and healthy volunteers (HVs).
Methods
This was a single-center, cross-sectional study conducted on 56 patients (32 severe cases and 24 nonsevere) admitted to the COVID-19 ward and 46 HVs living in Esfarayen City, North Khorasan Province of Iran. Serum levels of Vit D and zinc in admitted patients to the COVID-19 ward and HVs were measured.
Results
The average levels of serum Vit D in severe cases, nonsevere cases, and HVs were 31.03 ± 15.49, 37.25 ± 18.49, and 39.33 ± 14.83, respectively (P = 0.05). Moreover, the average concentrations of serum zinc in severe cases, nonsevere cases, and HVs were 31.03 ± 15.49, 37.25 ± 18.49, and 39.33 ± 14.83, respectively (P = 0.01). Mortality rate, reinfection (for 5 months), and length of hospital stay in severe cases were higher than in nonsevere cases (P > 0.05).
Conclusions
Results showed that severe cases had lower levels of Vit D than did other groups and were marginally significant. Also, severe cases had a significantly low level of zinc when compared with nonsevere cases and HVs. Levels of Vit D and zinc can affect the incidence of COVID-19 infection.
Key Words: COVID-19, SARS-CoV-2, novel coronaviruses, vitamin D, zinc
COVID-19 infection is considered a pandemic and causes the death of many people in the world (1,204,028 deaths until the third of November 2020).1 It can cause severe lower respiratory symptoms.2 This virus can be transmitted from person-to-person through droplets, airborne, and contact with surfaces contaminated by viruses.3 After being transmitted into the human body, it binds to angiotensin-converting enzyme 2 and induces internalization of the complex by the host cell. The enzyme is highly expressed in the lung, heart, ileum, kidney, and bladder, and therefore, these tissues are susceptible to novel coronavirus infection.4 The most significant physical symptoms are fever, cough, and dyspnea.5 Also, reverse-transcription polymerase chain reaction (RT-PCR) and chest computed tomography (CT) scan are used for diagnosis.6 Because of the novelty of 2019-nCoV and the uncertainty of its various aspects, it is necessary to identify the effective risk factors for disease incidence.7 Based on a Centers for Disease Control and Prevention report, older adults and people with underlying conditions such as diabetes, hypertension, and pulmonary disease are more likely to be infected with COVID-19.8 Besides, vitamin and mineral deficiencies can affect the incidence of COVID-19 and the disease severity.9
Researchers have recently focused on treating COVID-19 infection with vitamin D (Vit D) and zinc and have paid less attention to their effects on the onset of the disease. Vitamin D deficiency has been described as a pandemic and a great concern for medical staff.10 Also, it has main roles such as immune regulator, antiviral, and anti-inflammatory in the body.11 Previous studies have shown a direct relationship between severe pulmonary disease (tuberculosis, influenza, asthma) and Vit D deficiency. In other words, low levels of Vit D may be related to an increased risk of 2019-nCoV infection.12–14 Vitamin D can reduce the rate of viral replication and the concentration of inflammatory cytokines by inducing cathelicidin and defensin.11 Cathelicidin has antiviral action against respiratory viruses such as respiratory syncytial virus, influenza, and probably the COVID-19 infection.15
Another factor promoting the immune system against viral infections is zinc through intercellular adhesion molecule-1 action.16 In vitro studies have revealed zinc inhibits coronavirus (severe acute respiratory syndrome coronavirus) and hepatitis E infections.17,18 Results of a review study found zinc therapy has an effective role in the treatment of common cold, as a viral infection.19 Also, zinc balances oxidative stress and antioxidants. Antioxidants are the main factor in controlling viral diseases.20
After binding to the Vit D receptor, intracellular zinc levels enhance the function of Vit D in the cell. Therefore, many important functions in the body can be impaired by a low concentration of both Zn and Vit D. In addition, animal studies have found beneficial outcomes of Vit D on zinc increments.21
Both Vit D and zinc play a significant role in the treatment of viral infection, but their relation with the incidence of COVID-19 infection is not clear.16 Accordingly, the present study aimed to compare serum Vit D and zinc levels in novel coronavirus–infected patients and healthy volunteers (HVs) in northeastern Iran, 2020.
METHODS
We conducted a cross-sectional and descriptive-analytical research with 2 main groups (patients, 56; HVs, 44). This study approved by the Esfarayen Faculty of Medical Sciences research and ethics committees. Convenience sampling was used in the research to provide samples. The sampling of the patient and control groups was carried out simultaneously. Healthy volunteers were invited via virtual networks around the city of Esfarayen, and all eligible patients were selected to participate in research from the COVID-19 ward of the hospital. In fact, sampling was performed from patients newly admitted to the coronavirus ward of Imam Khomeini Hospital in Esfarayen from March 24 to May 5, 2020. Inclusion criteria were patients with a positive RT-PCR test result for COVID-19 and chest CT scan, admitted in the COVID-19 ward, who were alert, who were older than 18 years, and who had no thyroid, parathyroid, or chronic kidney disorder, or a history of dialysis. Finally, 56 patients were included in the study. Three patients who were younger than 18 years and 2 patients who had cancer and chronic renal failure were excluded from the research. Moreover, the patient group was divided into severe (radiological findings of pneumonia; respiratory distress [≥30 breaths/min] and oxygen saturation ≤93% at rest) and nonsevere cases using the seventh edition of Diagnosis and Treatment Protocol for COVID-19.22
The control group also consisted of HVs who were invited through local social networks (with >15,000 members) for the measurement of serum levels of Vit D and zinc. Inclusion criteria for this group were individuals without any chronic disease; with no symptoms such as fever, headache, sore throat, and myalgia over the last 4 months; and older than 18 years. In this group, 44 HVs were included in the final analysis. In addition, 4 individuals (3 people opted out of the study and 1 person was younger than 18 years) were excluded from the study.
Exclusion criteria for the 2 groups were the consumption of Vit D and zinc supplementation over the past 2 weeks and dissatisfaction for taking the blood sample.
To perform coronavirus testing on all newly hospitalized patients in the COVID-19 ward, a sample of nasopharyngeal swab was obtained by a laboratory science expert and sent to Corona Molecular Laboratory for the RT-PCR test. Results are usually reported after 24 to 48 hours. Also, patients underwent a chest CT scan during hospital admission. COVID-19 pneumonia was confirmed based on a radiologist report (presence of the pattern of multifocal peripheral ground-glass or mixed consolidation). Blood sample was sent to the medical diagnostic laboratory during patient admission for measurement of the serum levels of Vit D and zinc. Complete blood count and C-reactive protein (CRP) were analyzed in the hospital laboratory. Registration of HVs was performed virtually, and they were referred to the diagnostic laboratory for venipuncture sampling. Healthy volunteers who were reluctant to go to the laboratory for fear of infection with novel coronavirus, a member of the research team would take the sample at a location other than the laboratory. In the control group, only temperature and medical history were taken, and RT-PCR, because of COVID-19 testing kit shortages, was not obtained. Healthy individuals concurrent with taking the blood sample completed the demographic questionnaire. The mortality rate during hospitalization, reinfection (after 5 months from hospital discharging), and length of hospital stay in the patient group were reported.
The measurement of serum Vit D (25-hydroxyvitamin D, or 25(OH)D) level was performed with an HPLC Waters device and Chemilum Inensece method. The normal level of which was between 30 and 100 ng/mL for adults. Furthermore, a Spectr 20.AA device and the photometric method were used to check the zinc level. The normal levels were between 127 and 72 mg/dL for men and 70 to 114 mg/dL for women.
Statistical analysis was performed in SPSS 18 software. Kolmogorov-Smirnov test was applied for checking the data distribution.
The Mann-Whitney U and independent t tests were used for comparing the means of platelet, white blood cell (WBC) differential (lymphocyte, neutrophil), and length of hospital stay between the groups. The χ2 and Fisher exact tests were applied for comparing frequency, sex, place of residence, comorbidity (diabetes, hypertension, chronic obstructive pulmonary disease [COPD]), signs and symptoms (fever, sore throat, coughing, dyspnea, myalgia, headache, chest pain, vomiting), mortality, reinfection, CT scan changes, and CRP levels. Comparison levels of Vit D, zinc, body mass index (BMI), and age were performed in the control group, severe cases, and nonsevere cases using the Kruskal-Wallis test. Two-way analysis of variance for controlling the effect of sex and analysis of covariance for adjusting the effect of age in the patient and control groups were used. A P value of <0.05 was considered as the level of statistical significance.
RESULTS
The mean ages of the patient and control groups were 54.10 ± 2.07 and 46.65 ± 1.76 years, respectively, and were similar (P = 0.06, Table 1). Also, 2 groups were homogeneous in terms of sex, place of residence, and BMI (P > 0.05, Table 1). The serum CRP level in most of the patients was negative upon arrival at the hospital coronavirus ward (Table 2). The chest CT scan showed pulmonary change from normal condition in 49 (87.5%) patients and higher frequency in severe cases than in nonsevere cases (P < 0.05, Table 2). The most common patients' complaints were dyspnea (33; 58.9%), coughing (24; 42.9%), and fever (23; 41.1%), respectively (Table 2). Moreover, hypertension (26.8%) was the most comorbidity in the patient group (Table 2). There were higher levels of WBC and lymphocyte in severe cases and lower neutrophil levels than in nonsevere cases (P < 0.05, Table 2).
TABLE 1.
Comparison of 2 Groups Based on Some Demographic Variables
| Variables | Classification | Severe Cases, n (%) or Mean ± SD | Nonsevere Cases, n (%) or Mean ± SD | Control Group, n (%) of Mean ± SD | P |
|---|---|---|---|---|---|
| Sex | Male | 15 (46.9) | 16 (66.7) | 17 (38.64) | 0.09 |
| Female | 17 (53.1) | 8 (33.3) | 27 (61.36) | ||
| Place of residence | Urban | 20 (62.5) | 15 (62.5) | 26 (37.5) | 0.94 |
| Rural | 12 (37.5) | 9 (37.5) | 18 (40.91) | ||
| Age, y | — | 54.88 ± 25.40 | 54.31 ± 16.98 | 46.65 ± 17.66 | 0.14 |
| 54.10 ± 20.73 | 46.65 ± 17.66 | 0.06 | |||
| BMI, kg/m2 | — | 25.88 ± 1.77 | 25.64 ± 1.58 | 25.46 ± 1.89 | 0.52 |
TABLE 2.
Clinical and Paraclinical Information on the Patient Group
| Variables | Classification | Nonsevere Cases, n (%) or Mean ± SD | Severe Cases, n (%) or Mean ± SD | P |
|---|---|---|---|---|
| CRP | Negative | 20 (83.3) | 7 (21.9) | >0.001 |
| 1+ | 0 | 8 (25) | ||
| 2+ | 3 (12.5) | 8 (25) | ||
| 3+ | 1 (4.2) | 9 (28.1) | ||
| Pulmonary change in CT scan |
Yes | 17 (70.8) | 32 (100) | 0.001 |
| No | 7 (29.2) | 0 (0) | ||
| Fever | Yes | 14 (58.3) | 9 (28.13) | 0.02 |
| No | 10 (41.7) | 23 (71.87) | ||
| Sore throat | Yes | 10 (41.7) | 11 (34.37) | 0.57 |
| No | 14 (58.3) | 21 (65.63) | ||
| Coughing | Yes | 12 (50) | 12 (37.5) | 0.35 |
| No | 12 (50) | 20 (62.5) | ||
| Dyspnea | Yes | 0 | 32 (100) | >0.001 |
| No | 24 (100) | 0 (0) | ||
| Myalgia | Yes | 2 (8.3) | 6 (18.75) | 0.44 |
| No | 22 (91.7) | 26 (81.25) | ||
| Headache | Yes | 5 (20.8) | 2 (6.25) | 0.12 |
| No | 19 (79.2) | 30 (93.75) | ||
| Chest pain | Yes | 5 (20.8) | 3 (9.37) | 0.26 |
| No | 19 (79.2) | 29 (90.63) | ||
| Nausea/vomiting | Yes | 4 (16.7) | 3 (9.37) | 0.44 |
| No | 20 (83.3) | 29 (90.63) | ||
| Diarrhea | Yes | 3 (12.5) | 0 (0) | 0.07 |
| No | 21 (87.5) | 32 (100) | ||
| COPD | Yes | 1 (4.2) | 7 (21.87) | 0.12 |
| No | 23 (95.8) | 25 (78.13) | ||
| Hypertension | Yes | 3 (12.5) | 12 (37.5) | 0.03 |
| No | 21 (87.5) | 20 (62.5) | ||
| Diabetes | Yes | 2 (8.3) | 5 (15.62) | 0.69 |
| No | 22 (91.7) | 27 (84.38) | ||
| WBC | — | 6.82 ± 3.03 | 8.51 ± 4.42 | 0.11 |
| Lymphocyte | — | 28.59 ± 12.28 | 18.81 ± 10.54 | 0.005 |
| Neutrophil | — | 62.82 ± 13.88 | 72.84 ± 12.64 | 0.006 |
| Platelet | — | 215.12 ± 81.96 | 200.90 ± 62.54 | 0.74 |
Regarding the levels of Vit D, the results did not show a significant difference between the disease and control groups (33.69 ± 16.97 vs 39.33 ± 14.84, P = 0.08). However, means of the serum Vit D level in the severe, nonsevere, and HV groups were 31.03 ± 15.49, 37.25 ± 18.49, and 39.33 ± 14.83, respectively, and their differences were marginally significant (P = 0.05, Fig. 1). The results also showed that Vit D levels were significantly lower in patients with pulmonary involvement (n = 49) than in HVs (32.55 ± 17.14 vs 39.33 ± 14.83, P = 0.01).
FIGURE 1.
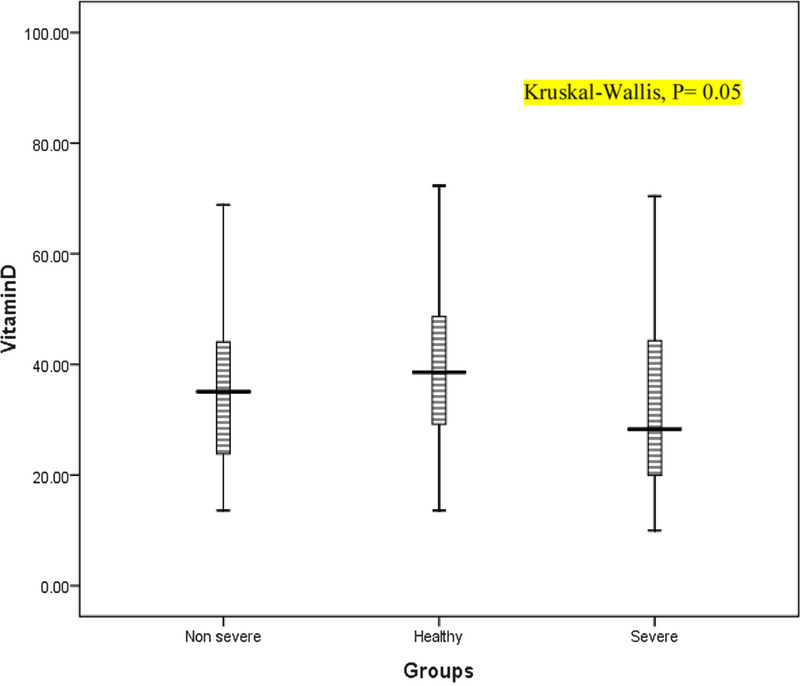
Box plots of Vit D in the patient (severe and nonsevere) and control groups.
Moreover, the findings did not indicate a significant difference between the disease and control groups in terms of zinc levels (75.99 ± 20.30 vs 32.10 ± 17.97, P = 0.07). However, the average of serum zinc levels in severe cases, nonsevere cases, and HVs were 72.10 ± 18.18, 78.72 ± 22.58, and 82.10 ± 17.96, respectively, and their differences were significant (P < 0.05, Fig. 2). The results also indicated that zinc levels were significantly lower in patients with pulmonary involvement (n = 49) than in HVs (74.29 ± 20.94 vs 82.10 ± 17.95, P = 0.02).
FIGURE 2.
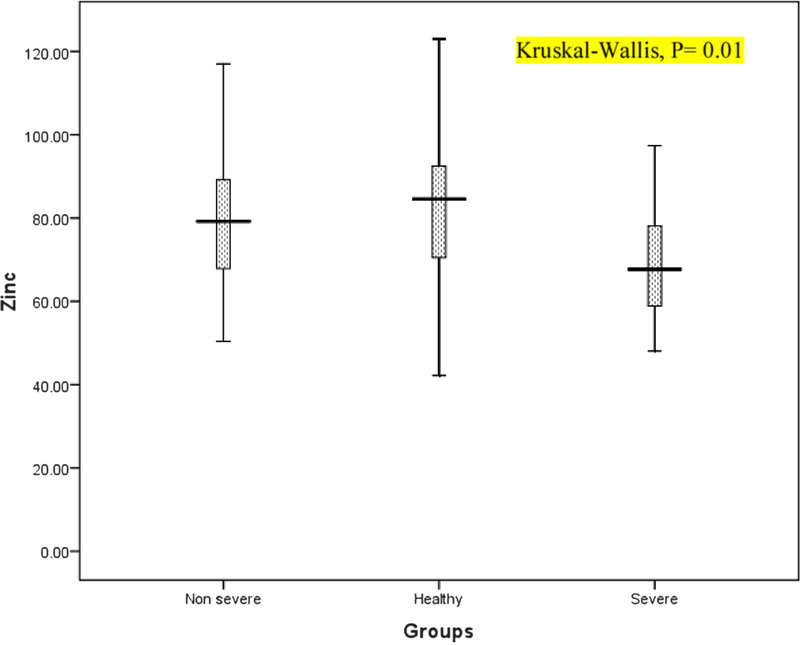
Box plots of zinc in the patient (severe and nonsevere) and control groups.
The 2-way analysis of variance did not indicate significant effects of group (P = 0.11), sex (P = 0.45), and their interaction (P = 0.70) on Vit D levels. Furthermore, analysis revealed the group had a significant effect on zinc concentration (P = 0.04), but sex (P = 0.54) and their interaction (P = 017) showed no significant effects.
Analysis of covariance for adjusting the effects of age (covariate) did not indicate a significant effect on the levels of Vit D in the groups (df2, 97, P = 0.55, partial η2 = 0.004). In addition, it did not show a significant effect on the levels of zinc in the groups (df2, 97, P = 0.20, partial η2 = 0.02).
The mortality rate, reinfection (for 5 months), and length of hospital stay in severe cases were higher than in nonsevere cases (P > 0.05, Table 3).
TABLE 3.
Comparison Severe and Nonsevere Cases in COVID-19–Infected Patients
| Variables | Sever Cases (n = 32), n (%) or Mean ± SD |
Nonsevere Cases (n = 24), n (%) or Mean ± SD | P |
|---|---|---|---|
| Mortality | 5 (15.62) | 0 | 0.06 |
| Length of hospital stay, d | 6.09 ± 3.64 | 4.58 ± 3.78 | 0.11 |
| Reinfection (after 5 mo) |
7 (21.87) | 1 (4.17) | 0.12 |
DISCUSSION
This cross-sectional study aimed to compare the serum levels of Vit D and zinc between patients infected with novel coronavirus and HVs. The findings showed the mean level of Vit D in severe cases was the lowest, and these differences were marginally significant between the groups. Also, the mean concentration of zinc was observed to be the lowest among severe cases, and it was statistically significant when compared with nonsevere cases and HVs.
Jain et al23 found that ill patients admitted to the intensive care unit had a lower Vit D level than did asymptomatic patients (14.35 ± 5.79 vs 27.89 ± 6.21) with COVID-19 infection, which was consistent with our finding. In Jain's study, mean levels of Vit D were lower than in the present study. It can be related to ultraviolet skin exposure and diet, which are some of the effective factors that affect levels of Vit D.24 We also compare the mean levels of Vit D and zinc, and the result showed the patient group had lower levels than did the HV group.
A study by Pletz et al25 showed Vit D deficiency (the activated form: 1,25 dihydroxyvitamin D) is related to the severity of community-acquired pneumonia. However, no relationship was found between the reservoir form (25(OH)D) and disease severity. In the present study, also, 25(OH)D levels were measured and were lower in the patient group than in the HV group. Low levels of Vit D can decrease physical barrier, and cellular natural and adaptive immunity in the body.16 Moreover, recent evidence has shown that a low level of Vit D can enhance cytokine storm through activation of interferon-γ in patients infected with novel coronavirus.11 In another study, Zhou et al26 showed the administration of the preventive high dose of Vit D has a positive effect on seasonal influenza. Also, another study showed a direct relationship between greater disease severity in patients infected with novel coronavirus and a low serum level of Vit D.27
Results of the case-control study showed a low level of Vit D is associated with a higher incidence of community-acquired pneumonia.28 In this study, the average level of Vit D was lower than the normal value (deficiency category) in the patient group, but in our study, it was in normal value (sufficient category) in the patient group. This difference can be attributed to the average age between the 2 patient groups of 2 studies (67.75 ± 10.25 vs 54.10 ± 2.07). Serum Vit D levels begin to decline with advancing age.29
In another study, Ghoneim et al30 revealed the mean of Vit D in the COPD group (n = 80) was significantly lower than in the healthy group (n = 40). Previous studies have shown negative effects of Vit D deficiency on pulmonary diseases with different mechanisms.31
Regarding the role of zinc among patients with pulmonary disease, Bhat et al32 performed a study to compare zinc serum levels between patients with pneumonia and healthy individuals. Results showed the average level of zinc serum in the patient group was significantly lower than in another group. Also, the results of our study showed the average level of zinc in the patient group was lower than in the control group. Zinc can impede viral replication by changing the proteolytic processing of replicase polyproteins, RNA-dependent RNA polymerase, and reducing the RNA-synthesizing activity in viral disease such as a novel coronavirus infection.33 A case-series study showed high-dose oral zinc improves clinical parameters among 4 patients infected with novel coronavirus.34 Zinc deficiency occurs for a number of reasons, including low intake of zinc-containing foods, malabsorption, and the presence of high phytates in the diet, which bind to zinc and reduce zinc absorption in the human body.21
In another study by Reda et al,35 serum levels of Vit D and zinc were lower in patients infected with hepatitis C virus (70 patients) than in the healthy group (50 people), which is consistent with the results of our study. This study found a negative relationship between the serum level of zinc and lowering of inflammatory responses such as interleukin-17. Besides, some pieces of evidence showed the relationship between zinc and reducing the activity of angiotensin-converting enzyme 2, as a receptor for novel coronavirus.36 The mortality rate in severe cases was higher than in nonsevere cases. In addition, patients with severe cases had lower levels of Vit D than did those with nonsevere cases. Findings of a research in Turkey showed that Vit D is associated with mortality in patients infected with COVID-19.37
In the present study, the levels of zinc and Vit D did not show statistically significant differences between the patients and the HV group. Using a new classification (based on the severity of the disease) and recomparing with HVs, the results indicated that patients with severe cases had the lowest levels and their differences were significant. Also, the result was significant when only patients with pulmonary involvement were compared with the HVs. Previous studies have confirmed that the severity of the disease in Vit D–deficient patients is significantly higher than in patients with normal levels.38
In the present study, patients were older than the healthy group, but this difference was not significant. The findings remained unchanged after adjusting for age. Also, given the division of patient group into the severe and nonsevere cases, with an almost equal average age, the results showed the levels of Vit D and zinc were the lowest in the severe cases, and this difference was significant when compared with the healthy group.
This study has some limitations including (1) inaccessibility to the outpatients cured in their home; (2) short duration of the study, but all eligible patients entered the research during the period of 1½ months; (3) RT-PCR test was not obtained for the control group because of COVID-19 testing kit shortages, and only temperature and medical history were taken; and (4) ultraviolet skin exposure and diet were not evaluated in this study.
CONCLUSIONS
The present study was performed to compare serum Vit D and zinc levels in patients infected with novel coronavirus and HVs. Finally, the results showed the average levels of Vit D and zinc in the patient group were lower than in the control group. The results of this study require confirmation in future studies. Next studies can use the design of this study with a larger sample size and evaluate the relationship between serum Vit D and zinc levels with inflammatory markers and long-term outcomes (reinfection, mortality death) in severe cases, nonsevere cases, and HVs. The effects of Vit D and zinc on the severity of disease should also be evaluated in a future study.
ACKNOWLEDGMENTS
We thank our colleagues at Esfarayen Imam Khomeini Hospital and Vali-asr Laboratory.
Footnotes
Author Contributions: S.J.H.: conceptualization and design, writing the original draft and data analysis, sample collection; M.F.: conceptualization and design, writing the original draft; B.M.: conceptualization and design, manuscript editing; M.M.: sample collection; A.A.F.: data collection; E.I.: manuscript editing; A.A.: manuscript editing.
This study was supported by a grant from the Esfarayen Faculty of Medical Sciences.
The authors have no conflicts of interest to disclose.
Ethical Approval: The Research and Ethics Committee of the Esfarayen Faculty of Medical Sciences (IR.ESFARAYENUMS.REC.1398.018) approved this study.
Contributor Information
Seyed Javad Hosseini, Email: s.j.hoseini2016@gmail.com.
Bagher Moradi, Email: moradib901@gmail.com.
Mahmood Marhemati, Email: marhamatim@gmail.com.
Ali Asghar Firouzian, Email: nursing85_8511136@yahoo.com.
Eshagh Ildarabadi, Email: ildarabadi@gmail.com.
Ali Abedi, Email: abedia1371@gmail.com.
REFERENCES
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. November 2020. Available at: https://covid19.who.int. Accessed November 3, 2020.
- 2.Meltzer DO Best TJ Zhang H, et al. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Network Open. 2020;3(9):e2019722-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng S-Q, Peng H-J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou X Chen K Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamidi Farahani R Gholami M Hazrati E, et al. Clinical features of ICU admitted and intubated novel corona virus–infected patients in Iran. Arch Clin Infect Dis. 2020;15(2):e103295. [Google Scholar]
- 6.Wu J Wu X Zeng W, et al. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. 2020;55(5):257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodise T, Tillotson GS. Managing bacterial infections in the era of COVID-19. Infect Dis Clin Pract. 2020;28(5):251–254. [Google Scholar]
- 8.Centers for Disease Control and Prevention . People with Certain Medical Conditions. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra precautions/people-with-medical-conditions.html. Accessed April 15, 2021.
- 9.Luo X Liao Q Shen Y, et al. Vitamin D deficiency is associated with COVID-19 incidence and disease severity in Chinese people [corrected]. J Nutr. 2021;151(1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kara M Ekiz T Ricci V, et al. ‘Scientific strabismus’ or two related pandemics: COVID-19 & vitamin D deficiency. Br J Nutr. 2020;1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant WB Lahore H McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali NS, Nanji K. A review on the role of vitamin D in asthma. Cureus. 2017;9(5):e1288-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talat N Perry S Parsonnet J, et al. Vitamin d deficiency and tuberculosis progression. Emerg Infect Dis. 2010;16(5):853–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruber-Bzura BM. Vitamin D and influenza-prevention or therapy. Int J Mol Sci. 2018;19(8):2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50(3):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rondanelli M Miccono A Lamburghini S, et al. Self-care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds-practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid Based Complement Alternat Med. 2018;2018:5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.te Velthuis AJW van den Worm SHE Sims AC, et al. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaushik N Subramani C Anang S, et al. Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J Virol. 2017;91(21):e00754–e00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemilä H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 2017;8(5):2054270417694291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camini FC da Silva Caetano CC Almeida LT, et al. Implications of oxidative stress on viral pathogenesis. Arch Virol. 2017;162(4):907–917. [DOI] [PubMed] [Google Scholar]
- 21.Shams B Afshari E Tajadini M, et al. The relationship of serum vitamin D and Zinc in a nationally representative sample of Iranian children and adolescents: the CASPIAN-III study. Med J Islam Repub Iran. 2016;30:430. [PMC free article] [PubMed] [Google Scholar]
- 22.National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (7th trial version). Available at: https://www.chinadaily.com.cn/pdf/2020/1.Clinical.Protocols.for.the.Diagnosis.and.Treatment.of.COVID-19.V7.pdf. Accessed February 15, 2021.
- 23.Jain A Chaurasia R Sengar NS, et al. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10(1):20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin G Hewison M Hopkin J, et al. Vitamin D and COVID-19: evidence and recommendations for supplementation. Royal Soc Open Sci. 7(12):201912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pletz MW Terkamp C Schumacher U, et al. Vitamin D deficiency in community-acquired pneumonia: low levels of 1,25(OH)2 D are associated with disease severity. Respir Res. 2014;15(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J Du J Huang L, et al. Preventive effects of vitamin d on seasonal influenza A in infants: a multicenter, randomized, open, controlled clinical trial. Pediatric Infect Dis J. 2018;37(8). [DOI] [PubMed] [Google Scholar]
- 27.Panagiotou G Tee SA Ihsan Y, et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin Endocrinol (Oxf). 2020;93(4):508–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamani M Muceli N Ghasemi Basir HR, et al. Association between serum concentration of 25-hydroxyvitamin D and community-acquired pneumonia: a case-control study. Int J Gen Med. 2017;10:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meehan M, Penckofer S. The role of vitamin D in the aging adult. J Aging Gerontol. 2014;2(2):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoneim AH Al-Azzawi MA Elmasry SA, et al. Association of vitamin D status in the pathogenesis of chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2015;64(4):805–812. [Google Scholar]
- 31.Gilbert CR, Arum SM, Smith CM. Vitamin D deficiency and chronic lung disease. Can Respir J. 2009;16(3):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhat MH Mudassir Rather AB, et al. Zinc levels in community acquired pneumonia in hospitalized patients; a case control study. Egypt J Chest Dis Tuberc. 2016;65(2):485–489. [Google Scholar]
- 33.Kumar A Kubota Y Chernov M, et al. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med Hypotheses. 2020;144:109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J Infect Dis. 2020;99:307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reda R Abbas AA Mohammed M, et al. The interplay between zinc, vitamin D and, IL-17 in patients with chronic hepatitis C liver disease. J Immunol Res. 2015;2015:846348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skalny AV Rink L Ajsuvakova OP, et al. Zinc and respiratory tract infections: perspectives for COVID-19 (review). Int J Mol Med. 2020;46(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karahan S, Katkat F. Impact of serum 25(OH) vitamin D level on mortality in patients with COVID-19 in Turkey. J Nutr Health Aging. 2021;25(2):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira M Dantas Damascena A Galvão Azevedo LM, et al. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;1–9. [DOI] [PubMed] [Google Scholar]


