Abstract
Peroxisome Proliferator-Activated Receptors (PPARs) are a family of nuclear receptors that play essential roles in modulating cell differentiation, inflammation, and metabolism. Three subtypes of PPARs are known: PPAR-alpha (PPARα), PPAR-gamma (PPARγ), and PPARbeta/delta (PPARβ/δ). PPARα activation reduces lipid levels and regulates energy homeostasis, activation of PPARγ results in regulation of adipogenesis, and PPARβ/δ activation increases fatty acid metabolism and lipolysis. PPARs are linked to various diseases, including but not limited to diabetes, non-alcoholic fatty liver disease, glaucoma and atherosclerosis.
In the past decade, numerous studies have assessed the functional properties of PPARs in the eye and key PPAR mechanisms have been discovered, particularly regarding the retina and cornea. PPARγ and PPARα are well established in their functions in ocular homeostasis regarding neuroprotection, neovascularization, and inflammation, whereas PPARβ/δ isoform function remains understudied. Naturally, studies on PPAR agonists and antagonists, associated with ocular pathology, have also gained traction with the development of PPAR synthetic ligands.
Studies on PPARs has significantly influenced novel therapeutics for diabetic eye disease, ocular neuropathy, dry eye, and age-related macular degeneration (AMD). In this review, therapeutic potentials and implications will be highlighted, as well as reported adverse effects.
Further investigations are necessary before any of the PPARs ligands can be utilized, in the clinics, to treat eye diseases. Future research on the prominent role of PPARs will help unravel the complex mechanisms involved in order to prevent and treat ocular diseases.
Keywords: Eye; Peroxisome proliferator-activated receptors; Diabetic retinopathy; Diabetic Keratopathy, Ocular neuropathy; Fibrates; Thiazolidinedione; Ocular disease
1. The human eye
The human eye is a complex sense organ consisting of three distinct anatomical layers. The outer layer consisting of the cornea and sclera; the middle layer composed of the ciliary body, iris, and choroid, and the inner layer known as the retina. There are three additional structures surround these layers: the aqueous humor, the vitreous, and the lens (Willoughby et al., 2010) (Fig. 1).
Figure 1:
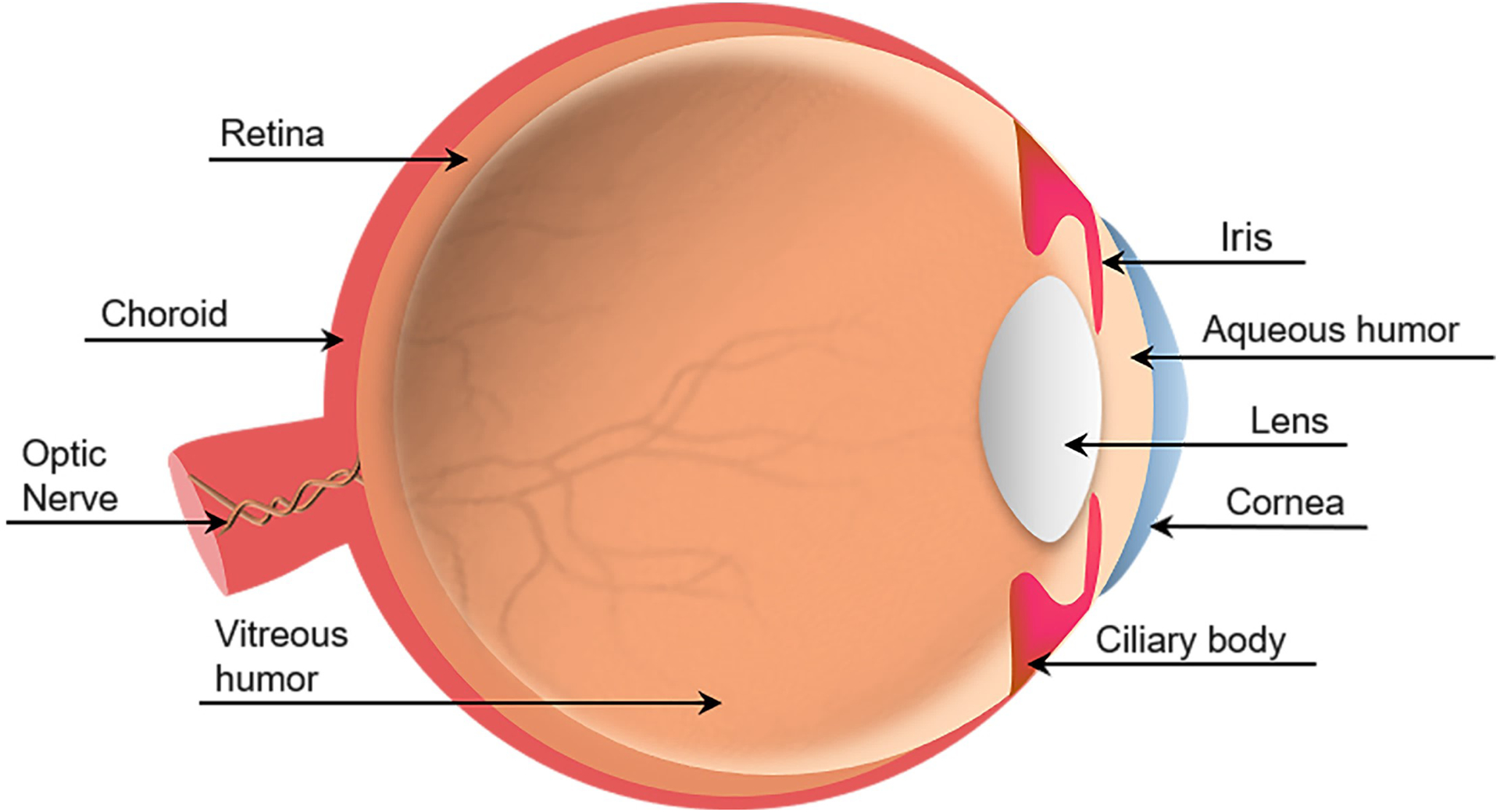
Illustration of the human eye.
The anatomy of the human eye is divided into two segments: the anterior segment corresponds to the front third-portion of the eye containing the aqueous humor fluid; whereas the posterior segment refers to the back two-thirds of the eye and contain the vitreous humor fluid. In this section, we will briefly discuss the components of the eye in each segment.
1.1. Anterior segment
1.1.1. Cornea
The cornea constitutes the outer layer of the eye which protects the inner structures against infection and serves an essential role in the vision process (Lavker et al, 2020). The cornea is convex, spherical, and consists of five distinct layers (Fig. 2): epithelium, Bowman’s membrane, stroma, Descemet’s membrane, and endothelium, spanning anterior to posterior (Sridhar, 2018). The epithelial layer serves as a barrier for the inner corneal layers from the external environment, with resident cells serving a lifespan of 7–10 days. The epithelial cells bind together via tight junctions, preventing the adjacent tear film from flooding into the stroma (Willoughby et al., 2010, Lavker et al, 2020).
Figure 2:
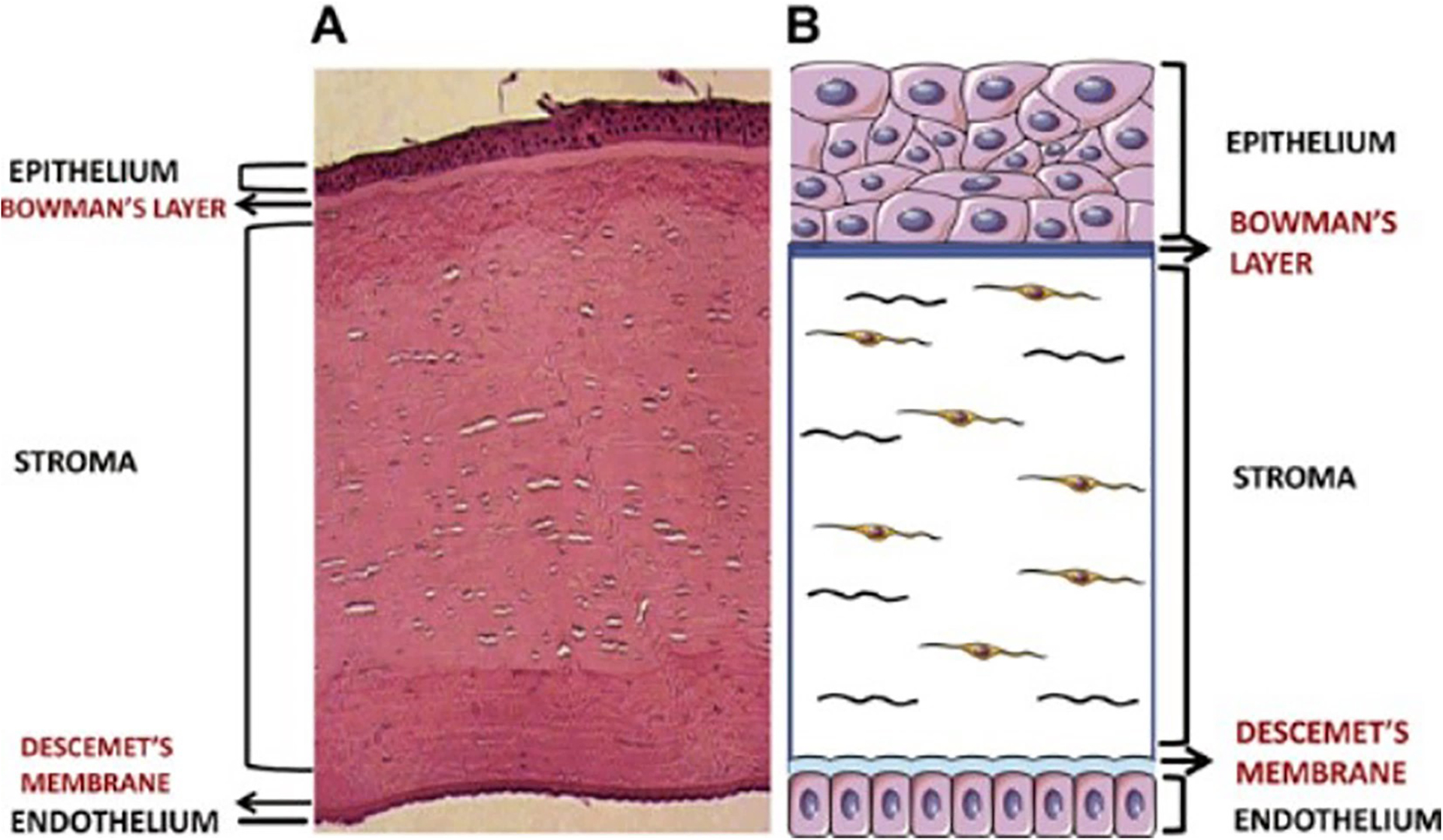
The human cornea. Both images show the five-layer structure of the cornea. (A) Histology of a cornea (B) Cornea illustration (not in scale). Source: Priyadarsini et al., (2020).
An acellular Bowman’s membrane separates the epithelium from the stroma. This thin and smooth membrane is composed of randomly arranged, type I and V collagen fibrils and proteoglycans (Sridhar, 2018). It is not considered a true membrane but rather a condensation of the most anterior portion of the stroma as it merges with the collagen lamellae of this layer (Wilson, 2020, Sridhar, 2018).
The stroma accounts for approximately 80–90% of corneal thickness. The structural makeup of the stroma is extremely well-organized to ensure strength and light transparency (Funderburgh et al., 2003). Resident cells, termed keratocytes, contain crystallins within their cell bodies, which defend them from self-inflicted light backscatter (Jester et al., 1999). The primary function of stromal keratocytes is to secrete and assemble extracellular matrix (ECM) components to maintain rigidity reducing the maximum amount of forwarding light scatter (Jester, 2008, Benedek, 1971). The main components that keratocytes produce are flattened layers of type I and type V collagen, known as lamellae (Hassell and Birk, 2010). The secreted collagen is organized into bundles parallel to one another and stacked at right angles, similar to a woven basket.
The cornea’s most posterior layer the endothelium, secretes an acellular Descemet’s membrane that separates it from the stroma. This thin membrane is composed of type IV and VIII collagens, and is organized in two main layers: The anterior layer with lamellae, collagen, and proteoglycans (Murphy, 1984); and the posterior layer which is deposited by the endothelial cells and grows thicker with age (de Oliveira and Wilson, 2020, Aurora, 1979). The actual endothelium is a monolayer of cells arranged in a honeycomb-like fashion, with a high density of Na+/K+ ATPase pumps to create an osmotic gradient for fluid to travel passively from the stroma and into the aqueous humor (Sridhar, 2018).
Because the cornea is directly exposed to the external environment, innervation is imperative to ensure that it can quickly, and actively respond to adverse/pathological stimuli. It is the most innervated tissue in the human body where almost all epithelial cells are in contact with nerve bundles (Yang, Chow, and Liu 2018).
1.1.2. Lens, iris, ciliary body, aqueous humor
The lens, iris, and ciliary bodies control the amount of light that enters the eye, protecting the retina and allowing light to properly focus onto the fovea (Hecht, 1987). The ciliary body is responsible for controlling light exposure while the lens aids in focusing light (Semo et al., 2014). The ciliary bodies are found on the anterior chamber and produce the aqueous humor, filling the space between the posterior cornea and the lens (Borges-Giampani and Giampani, 2013, Sridhar, 2018)). Essential to the avascular cornea (Willoughby et al, 2010), the aqueous humor contains proteins, metabolites, and neuropeptides that serve as nutrients and messengers for the anterior segment (Kiel et al., 2011). The aqueous humor is also responsible for removing waste products from bordering tissues (such as cornea and iris) through constant production and outflow. Fluid movement also helps maintain the eye’s shape, referred to as intraocular pressure (IOP). A drop or elevation of IOP can lead to debilitating effects to the optic nerve, ultimately leading to ocular diseases (Abu-Hassan et al., 2014).
The iris is a flat, ring-shaped membrane located within the anterior and posterior chamber. It is the only pigmented part of the eye and is directly connected to the ciliary bodies where it responds to their contractile function (Delamere, 2005). The iris is responsible, along with the pupil, for regulating the light that enters the eye (Chen and Kardon, 2013). The lens focuses light onto the retina and is a clear oval-shaped structure composed of crystallins (water-soluble structural proteins), responsible for lens transparency (Bloemendal et al., 2004).
1.2. Posterior segment
1.2.1. Retina
The retina, also known as the “neural portion” of the eye, is considered to be part of the central nervous system (Purves et al., 2001). Its primary function is to receive information from the external world (light stimulus) (Metea and Newman, 2007, Torborg and Feller, 2005) and translating it into perceivable information by the brain. It completes this task through an elegantly organized network of neurons, photoreceptors, and supporter cells which include: Müller, horizontal cells, bipolar cells, ganglions, and photoreceptors (Masland, 2001). Photoreceptors are the main cell-types of the retina (Masland, 2001), consisting of rods and cones, both are responsible for the initial phototransduction of photon to chemical signal (Kern, 2017). Photoreceptors connect to the ganglion cells via bipolar cells which are responsible for communicating the type of light stimulus (light or dark, i.e., rods or cones) to the ganglion cells (Euler and Masland, 2000). Rods are most sensitive to light and dark changes where cones are most sensitive to color vision, requiring bright light in order to send proper signals to the brain. Cones contribute to 95% of our vision, whereas rods contribute over extended periods at very low light levels (Lamb, 2016). In dark settings, these cells respond to the neurotransmitter, glutamate, which is released from the photoreceptors (Nawy and Jahr, 1991, 1990). Contrastingly, light stimulus has been reported to reduce glutamate production (Feher, 2012). Finally, ganglion cells provide the neural output inside the retina prior to transmitting information to the brain. The retina receives 90% of its oxygen supply from the choroid, whereas the remaining 10% is supplied by the retinal circulation (Ahmed et al., 1993).
1.2.2. Choroid
The choroid is the layer between the retina and the sclera that maintains temperature and volume and is responsible for supplying oxygen and nutrients to the posterior segment (Ehrlich et al., 2017). It directly connects to the ciliary bodies and extends back to the optic nerve; when described with the ciliary bodies and iris it is usually referred to as the “uvea”. The choroid has five distinct layers: Bruch’s membrane, choriocapillaris, two vascular layers (Haller’s and Sattler’s), and the suprachoroid (Hogan et al., 1971). Bruch’s membrane is a fibrous layer that serves as a basement membrane to both the choriocapillaris and the retinal pigmented epithelium (RPE) (Nickla and Wallman, 2010). The choroid deteriorates with age which manifests as thickening of Bruch’s membrane and thinning of the choriocapillaris and the lumen of capillaries in the choroid’s vascular layers. While this is a common occurrence, with age, it has significant implications in the pathophysiology of ocular diseases such as AMD (Spraul et al., 1999).
1.2.3. Vitreous humor
The vitreous humor fills the posterior segment. It is an ECM composed of 99% water with low collagen concentration compared to other ECMs found in the body (Le Goff and Bishop, 2008). Random spaces of collagen bundles and hyaluronan, form the gel-like composition that maintains the surrounding tissues’ shape and mechanical strength (Halfter et al., 2006). This collagen-hyaluronan composition also aids in maintaining the transparency of the vitreous humor allowing light to reach the retina unobstructed (Ahmed and Lutty, 2017). Vitreous liquefaction is characterized by thinning of the vitreous humor fluid that begins at age four and slowly progresses over a lifetime (Los et al., 2003). Free radical buildup and changes in concentrations of glycosaminoglycan and chondroitin sulfate are also attributes associated with vitreous liquefaction.
2. Overview of PPARs
PPARs are a group of transcription factors belonging to the superfamily of nuclear receptors (Issemann and Green, 1990). Upon activation, PPARs activate transcription of genes most commonly involved in metabolic pathways. Initially, PPARs were named for their ability to promote peroxisome biogenesis. PPARs are now well known to sense and interpret fatty acid signals derived from lipids and essential fatty acid metabolites. These functions made them key lipid metabolism regulators (Desvergne and Whali, 1999; Lemberger et al., 1996; Varga et al., 2011; Wahli et al., 1995). Further understanding of PPARs activity indicated that they also have diverse functions, including modulation of inflammatory responses, among others (Ju et al., 2019; Lee et al., 2011; Varga et al., 2011). The three PPARs isoforms identified, PPARα, PPARγ, and PPARβ/δ, modulate different signaling pathways. In this section, PPAR activation, regulation, gene expression, and isoforms are reviewed.
2.1. Activation of PPARs
PPARs are activated when bound by endogenous ligands such as fatty acids and synthetic xenobiotic ligands, common in pharmaceuticals (Table 1). As ligand-activated receptors, PPARs undergo a conformational change, leading to modulation of numerous regulatory pathways. Once activated, PPARs heterodimerize with the Retinoid X Receptor (RXR) to form a functional transcriptional unit (Fig. 3) (Tan et al., 2005; Yang et al., 2000). The PPARs-RXR heterodimer binds to PPAR Response Elements (PPREs) in the promoter regions, where they reside in target genes, and stimulate the gene expression by recruiting co-activators/cofactor (Yang et al., 2000) (Fig. 3). PPARs are known to modulate numerous processes such as energy metabolism, oxidative stress, inflammation, circadian rhythm, immune response, mitochondrial genesis, and cell differentiation (Kobayashi et al., 2005; Shirai et al., 2007; Tan et al., 2005; Varga et al., 2011). The actual PPARs binding mechanism can either be PPREs-dependent or independent. Mechanisms also exist where PPARs can modulate receptors activity in the absence of functional PPREs (Delerive et al., 2002; Ju et al., 2019). PPARs can interact directly with nuclear factor (NF)-kB and thus repress NF-kB genes (Fig. 3). A study by Delerive et al. showed the ability of PPARα to interact with the p65 subunit of NF-kB, resulting in the inhibition of NF-kB dependent transactivation (Delerive et al., 2002). This alternative pathway is essential for the repression of pro-inflammatory genes by PPARs.
Table 1:
PPARs distribution, ligands and transcriptional functions. H-Tissue: distribution of human PPARs. M-Tissue: distribution of rodent PPARs.
| Tissues | Endogenous Ligands | Synthetic Ligands | Transcriptional functions involved | References | |
|---|---|---|---|---|---|
| PPARα | Liver (H)(M) Skeletal muscle (H) Heart (H)(M) Kidney (H)(M) Adipose tissues(H)(M) Brain (H)(M) Intestinal mucosa (H)(M) Eye(H)(M) |
Saturated and unsaturated fatty acids Perfluorooctanoic acid Perfluorooctanoic sulfonate Prostaglandins derived from arachidonic acid Eicosanoid |
Pirinixic acid (WY14643) Clofibrate Fenofibrates Genofibrozil NSAIDS |
Lipid metabolism (Lipid transport, ketogenesis, lipogenesis, cholesterol metabolism, fatty acid transport and oxidation) Carbohydrate metabolism Adipogenesis Neonatal development Angiogenesis Inflammation Circadian rhythm |
(Wahli et al., 1995) (Desvergne and Whali, 1999) (Lemberger et al., 1996) (Kersten et al., 2001) (Tan et al., 2005) (Mukherjee et al., 1997) (Abbott, 2009) (Lee et al., 1995) (Mukherjee et al., 1994) (Shirai et al., 2007) |
| PPARγ | Liver (H) Adipose tissues (H) Heart (H) Bone marrow(H) Pancreas(H) Lung(H) Placenta (H) Skeletal muscle (H) Eye (H)(M) Kidney(H)(M) |
Only unsaturated fatty acids Prostaglandins derived from arachidonic acid Eicosanoid |
TZD MRL24 NSAID 15-PGJ2 |
Lipid metabolism (Cholesterol metabolism, lipogenesis, fatty acid transport and oxidation) Gluconeogenesis Adipogenesis Angiogenesis Inflammation Anti-fibrosis |
(Wahli et al., 1995) (Desvergne and Whali, 1999) (Lemberger et al., 1996) (Kobayashi et al., 2005) (Tan et al., 2005) (Mukherjee et al., 1997) (Tontonoz et al., 1994) (Mueller et al., 2002) (Choi et al., 2010) (Forman et al., 1995) (Wolf and Phil, 2004) (Ahmadian et al., 2013) (Moore et al., 2001) (Abbott, 2009) |
| PPARβ/δ | Liver (H)(M) Heart (H)(M) Skeletal muscle (H) Brain (H)(M) Placenta (H) Adipose tissues (H)(M) Small intestine(M) Keratinocytes (H)(M) Eye(H)(M) |
Saturated and unsaturated fatty acids | L165041 GW-501516 |
Lipid metabolism (Fatty acid transport and oxidation) Cell survival Ubiquitination Adipogenesis Inflammation |
(Abbott, 2009) (Wahli et al., 1995) (Desvergne and Whali, 1999) (Lemberger et al., 1996) (Tan et al., 2005) (Mukherjee et al., 1997) (Wu et al., 2017) |
Figure 3:
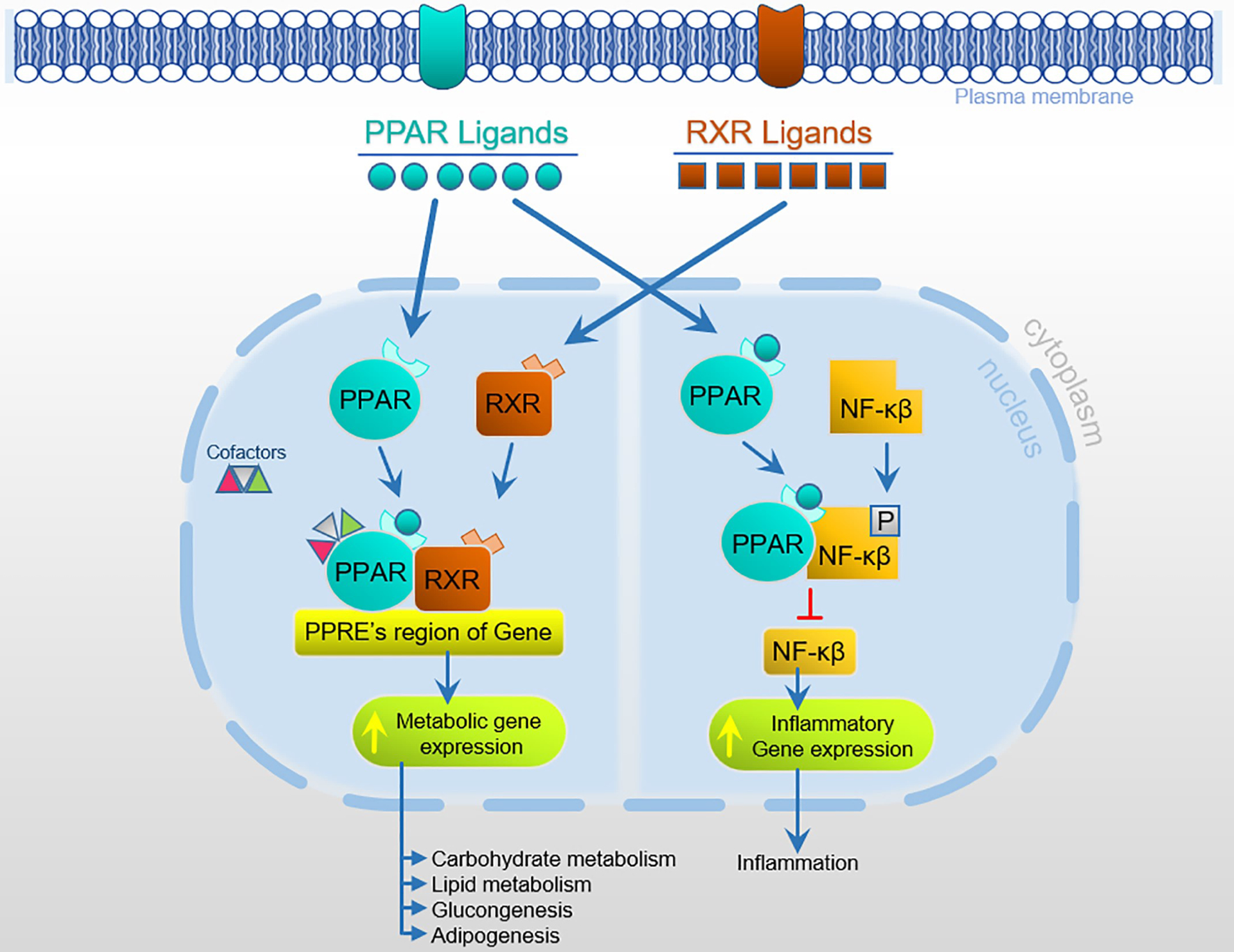
PPAR activation leading to multiple regulatory pathways.
2.2. Regulatory Pathways of PPARs
Each PPAR isoform has been identified in tissues and organs of rodent models and humans (Table 1). The three isoforms are critical modulators, stimulating diverse genes in the regulation of complex transcriptional functions such as lipid metabolism, cell survival, inflammation, angiogenesis, gluconeogenesis, and adipogenesis in a variety of tissues/organs (Table 1) (Mandard et al., 2004; Tan et al., 2005; Varga et al., 2011; Xin et al., 1999). It is therefore not surprising that the PPARs therapeutic implications, in different pathologies, have increased significantly including metabolic disorders, fatty liver, neurological disorders, cardiovascular diseases, and diabetes.
2.2.1. PPARα
PPARα expression has been found in skeletal muscle, liver, kidney, intestine, and heart of rodents and humans (Lee et al., 1995; Mukherjee et al., 1994). PPARα is most notably known for its involvement in lipid metabolism, including high- and low-density lipoprotein, cholesterol, apolipoprotein, triglyceride, phospholipids, and fatty acids (Berthou et al., 1996; Brautbar et al., 2013; Hertz et al., 1995; Lefebvre et al., 2006; Mandard et al., 2004; Motojima et al., 1998; Schoonjans et al., 1996). PPARα activation by fatty acids results in transcriptional activation of fatty acid catabolism and oxidation, highlighting PPARα therapeutic effects against non-alcoholic fatty liver disease (Montagner et al., 2016). In cardiovascular diseases, the activation of PPARα inhibits the development and progression of atherosclerosis by inhibiting the formation of thrombus, lipid buildup, and plaque rupture. (Kuusisto et al., 2007). In addition to lipid metabolism, PPARα also regulates carbohydrate metabolism (Edgar et al., 1998; Kersten et al., 2001). Specifically, Kersten et al. reported that activation of PPARα resulted in decreased enzyme expression in the deamination of amino acids and urea synthesis in the liver (Kersten et al., 2001).
The implication of PPARα with other various regulatory pathways is vast, but most notable are inflammation, hypertension, neuropathology, and circadian rhythm (Fidaleo et al., 2014; Lee et al., 2011; Shirai et al., 2007). In a study by Lee et al., activation of PPARα reduced the expression of pro-inflammatory mediators, including interleukin-6 (IL-6), an inflammatory cytokine, and renal expression of cyclooxygenase-2 (COX-2), leading to decreasing arterial pressure during salt-induced hypertension (Lee et al., 2011). PPARα’s prominent roles have also been focused on endothelial dysfunction, neovascularization, vasoregression, and vascular hyperpermeability (Cervantes-Perez et al., 2012; Ding et al., 2014; Moran and Ma, 2015; Wang et al., 2014). Lastly, PPARα activation was critical in the initial phase of inflammation during skin wound healing in mice (Michalik et al., 2001).
A study by Fidaleo et al., reported PPARα modulation of neurotransmission, neurogenesis, neuroinflammation, and glial cell proliferation/differentiation (Fidaleo et al., 2014). These observations have prompted extensive, active investigations on PPARα and its role in various neurological diseases, including Alzheimer’s and Parkinson’s disease (Barbiero et al., 2014; Brune et al., 2003; Hwang, 2013; Roy and Pahan, 2015; Sáez-Orellana et al., 2020).
In recent studies, PPARα therapeutic roles have been investigated in the context of eye complications due to diabetes (Chen et al., 2017; Cheng et al., 2016; Ding et al., 2014; Hu et al., 2013; Matlock et al., 2020; Moran et al., 2014). Our group recently showed that PPARα activation results in protection against corneal nerve damage caused by diabetes, for the first time ever (Matlock et al., 2020). Further information on PPARα functions and therapeutic potential in the eye will be discussed in sections 3 and 4.
2.2.2. PPARγ
PPARγ is found in adipose tissues, skeletal muscle, and liver in both humans and mice (Abbott, 2009; Ahmadian et al., 2013; Wolf and Phil, 2004). These tissues are essential in glucose homeostasis and fat metabolism, therefore, PPARγ has been mechanistically and strongly associated with lipid metabolism (Moore et al., 2001). A study by Choi and co-authors found that Cdk5-mediated phosphorylation of PPARγ plays a role in insulin-resistance, and discussed a future development of an anti-diabetic drug(s) through PPARγ. (Choi et al., 2010). These unique regulatory functions of PPARγ make it a prominent target of future therapeutics (Ahmadian et al., 2013; Choi et al., 2010; Forman et al., 1995).
PPARγ is also associated with inflammation, angiogenesis, neurological homeostasis, redox equilibrium, trophic factor production, and cellular differentiation (Barak et al., 1999; Ju et al., 2019; Kobayashi et al., 2005; Kotlinowski and Jozkowicz, 2016; Marx et al., 1998; Rosen and Spiegelman, 2001; Xin et al., 1999). PPARγ has been reported to inhibit the NF-kB pathway and directly neutralize oxidative stress (Croasdell et al., 2015; Heneka et al., 2000; Ju et al., 2019). A study by Ju et al. showed that activation of PPARγ resulted in suppression of pro-inflammatory mediators including, nitric oxide (NO), inducible NO synthase, COX-2, IL-6 and tumor necrosis factor-α (TNF-α), which was comparable to the PPARα findings by Ju et al (Ju et al., 2019). Adding to its involvement in tissue homeostasis a study by Vattulainen-Collanus et al. showed that lack of functional PPARγ, in pulmonary microvascular endothelial cells, leads to impaired angiogenesis in both humans and mice (Vattulainen-Collanus et al., 2016). Contrastingly, PPARγ activation resulted in inhibition of angiogenic factors, such as vascular endothelial growth factor (VEGF), and proliferation in endothelial cells and vascular smooth muscle cells (Biscetti et al., 2008; Kotlinowski et al., 2014; Kotlinowski and Jozkowicz, 2016; Vattulainen-Collanus et al., 2016). Lastly, PPARγ is shown to have neuroprotective roles, similar to its PPARα isoform, in pathologically progressive neurological disorders, including Alzheimer’s and Parkinson’s disease (Barrera et al., 2018; Chen et al., 2012; Garrido-Gil et al., 2012).
2.2.3. PPARβ/δ
Among the PPARs, PPARβ/δ is currently the least studied, however recent studies show its involvement in lipid and cholesterol metabolism. PPARβ/δ is, in fact, known for its role in the metabolic pathways of mitochondrial respiration, fatty acid metabolism (adipocyte physiology), and programming of muscle fiber type (Barak et al., 2002; Doktorova et al., 2017; Gan et al., 2011; Koh et al., 2017; Wang et al., 2003). Contrary to the PPARγ and PPARα isoform’s tissue-specific distribution, PPARβ/δ is generally distributed in all tissue/cell types but profoundly expressed in those related to lipid metabolism including the liver, colon, small intestine, and keratinocytes (Abbott, 2009; Girroir et al., 2008). PPARβ/δ, similar to PPARα, is shown to improve lipid profile homeostasis, and reduce adiposity. PPARβ/δ activation is shown to stimulate fat metabolism to prevent obesity (Bortolotto et al., 2007; Doktorova et al., 2017; Giordano Attianese and Desvergne, 2015; Le Garf et al., 2019; Palomer et al., 2018; Wang et al., 2003). Interestingly, Garf et al. reported that activation of PPARβ/δ had increasing immunometabolic effects, resulting in weight loss in obese female mice (Le Garf et al., 2019).
The role of PPARβ/δ in various cancers (i.e. colon, breast and prostate cancer) and tumor development, has also been reported displaying conditionally pro-cancerous and anti-cancerous effects through modulation of vasculature and inflammation (Liu et al., 2018; Martín-Martín et al., 2018; Wagner and Wagner, 2020; Wang et al., 2016). In addition to cancer development, Romero et al. reported that, when activated, PPARβ/δ exhibited effects related to hypertension, including vasodilation and vascular inflammation (Romero et al., 2016). Interestingly, PPARβ/δ also regulates skin wound healing inflammatory mechanisms by controlling keratinocyte proliferation (Man et al., 2008; Michalik et al., 2001; Peters et al., 2000; Tan et al., 2001). Lastly, PPARβ/δ is shown to play a role in the implantation and development of embryos (Barak et al., 2002).
3. PPARs in the eye
All three PPAR isoforms have been investigated in various parts of the eye correlating to specific isotypes (Fig. 4). The γ and α isoform have been well established in their function on ocular homeostasis, however, β/δ isoform remains largely understudied. This section will review studies focusing on PPAR-dependent pathways of each PPAR isoform.
Figure 4:
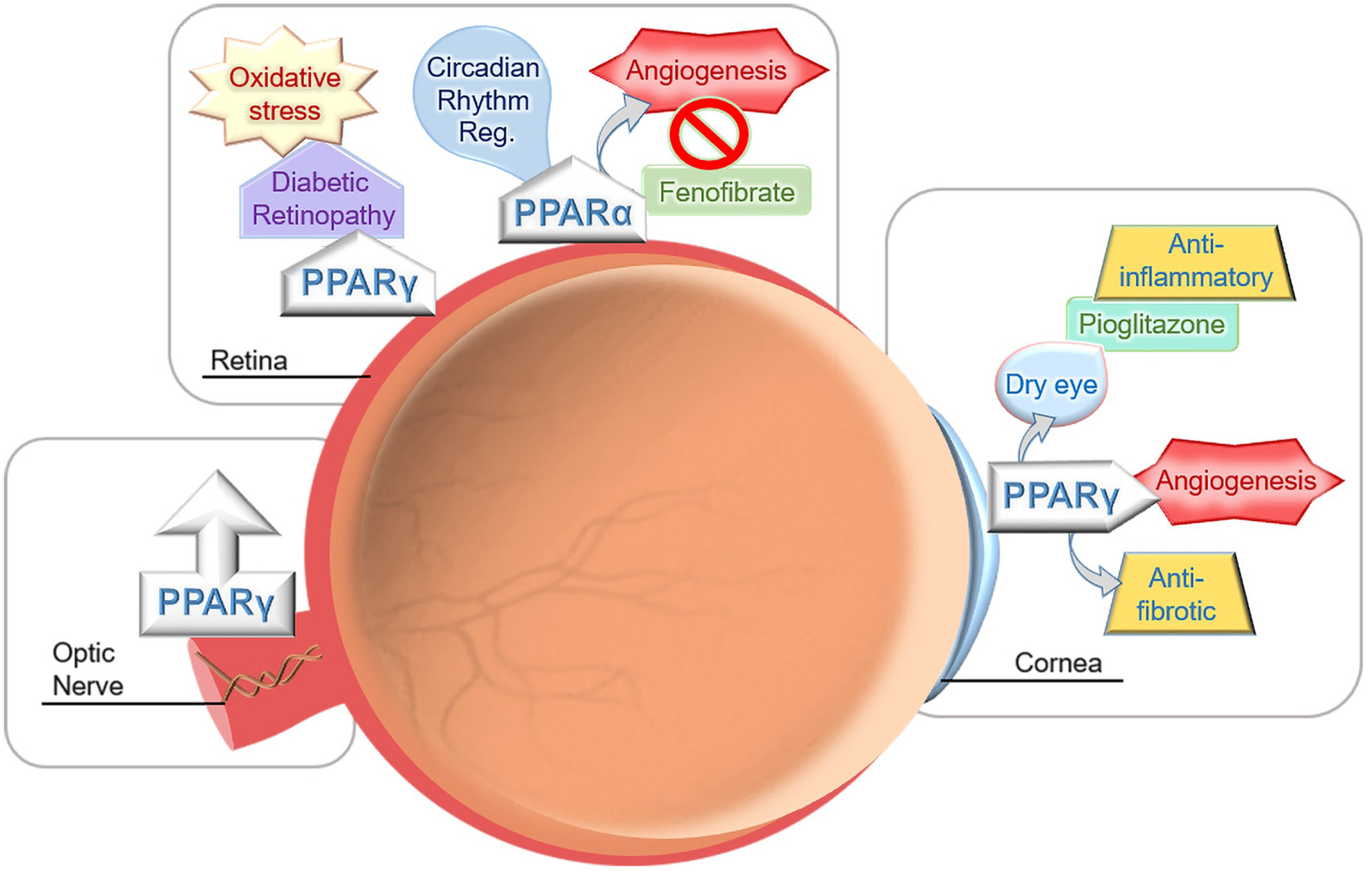
Illustration of the PPARs presence and modulation in different parts of the eye.
3.1. PPARα
PPARα has been extensively studied in the retina, where it has been found to be involved in the regulation of circadian rhythm, neuroprotection, and angiogenesis. Circadian rhythm is a biological, internal process that displays endogenous oscillations and is driven by a 24-hour circadian clock located in the central nervous system (Takahashi, 2017). The circadian rhythm regulates the sleep-wake cycle, also known as daily light-dark (LD) cycle conducted by a group of genes called clock genes (Chen and Yang, 2014). Light is an important factor that synchronizes and moves the clock through the retinohypothalamic tract (Teboul et al., 2009). In this scenario, PPARs appear to serve as molecular links between clock genes and specific rhythmic metabolic outputs (Charoensuksai and Xu, 2010). All PPARs were found to be rhythmically expressed in mammalian tissue when analyzed in mice (Yang et al., 2006), but PPARα and PPARγ seem to have a more direct role/interaction with the core clock related genes (Inoue et al., 2005; Wang et al., 2008). PPARα has been reported to help synchronize peripheral clocks (McNamara et al., 2001) and treat circadian rhythm sleep disorders associated with LD cycles (such as delayed sleep phase syndrome). Bezafibrate, a hypolipidemic drug, is a PPARα ligand that has exhibited interesting effects in the regulation of circadian behavioral rhythm when administered to mice (Shirai et al., 2007). Bezafibrate affects circadian rhythm by activating PPARα and advancing the active phase under long photoperiods conditions (12 to 18 hours of light) (Oishi et al., 2008). Authors, of this study, suggested that PPARα is critical to the photoperiod-dependent entrainment of the circadian clock (Oishi et al., 2008). In the context of diabetic retinopathy, maculopathy and diabetic neuropathy, the Fenofibrate Intervention in Event Lowering in Diabetes (FIELD) study, conducted in 9795 patients, showed a significant 30% reduction in laser therapy for patients with type 2 diabetic retinopathy (Keech et al., 2007). This study certainly represents a strong indicator of PPARα’s role in the diabetic retina. Our group has shown that PPARα activation by fenofibrate, reduces retinal vascular leakage, inflammation, pericyte dropout and acellular capillary formation in type 1 diabetic models, through a PPARα-dependent mechanism (Chen et al., 2013b; Ding et al., 2014). Additionally, ligand-dependent activation of PPARα attenuates ischemia-induced retinal neovascularization (Chen et al., 2013a). Furthermore, PPARα levels in the retina are significantly down-regulated in diabetic human donors and animal models. Delivery of PPARα ameliorates diabetic retinopathy manifestation (Hu et al., 2013) and decreases retinal degeneration induced by ischemia and diabetes (Moran et al., 2014; Pearsall et al., 2019). A neuroprotective PPARα role is thought to be through the regulation of mitochondrial function and lipid metabolism, as reported by Pearsall and co-authors. The authors, using a PPARα knockout (KO) mouse model, showed progressive electroretinogram response decline and retinal degeneration (Pearsall et al., 2017).
In the context of the cornea, the role of PPARα is somewhat unknown but neurotrophic and inflammatory effects have been reported. In a study by Zhang et al., the authors showed that PPARα activation (using PPAR ligand WY-14 643) induces pro-inflammatory and pro-angiogenic responses in corneal epithelial cells (Zhang and Ward, 2010). Recently, we evaluated the protective effect of PPARα against diabetic keratopathy and corneal neuropathy (Matlock et al., 2020). Our study showed that PPARα levels were down-regulated in both the human and rat, diabetic cornea (Fig. 5). A PPARα KO mouse showed corneal nerve degeneration (Fig. 6), decreased corneal sensitivity, and spontaneous corneal epithelial lesions (Fig. 7). On the other hand, PPARα activation prevented corneal epithelial lesions and delayed corneal nerve degeneration in diabetic mice. Protein analysis revealed partial restoration of neurotrophic factors in diabetic rats when stimulated with PPARα agonist, fenofibrate (Matlock et al., 2020).
Figure 5:
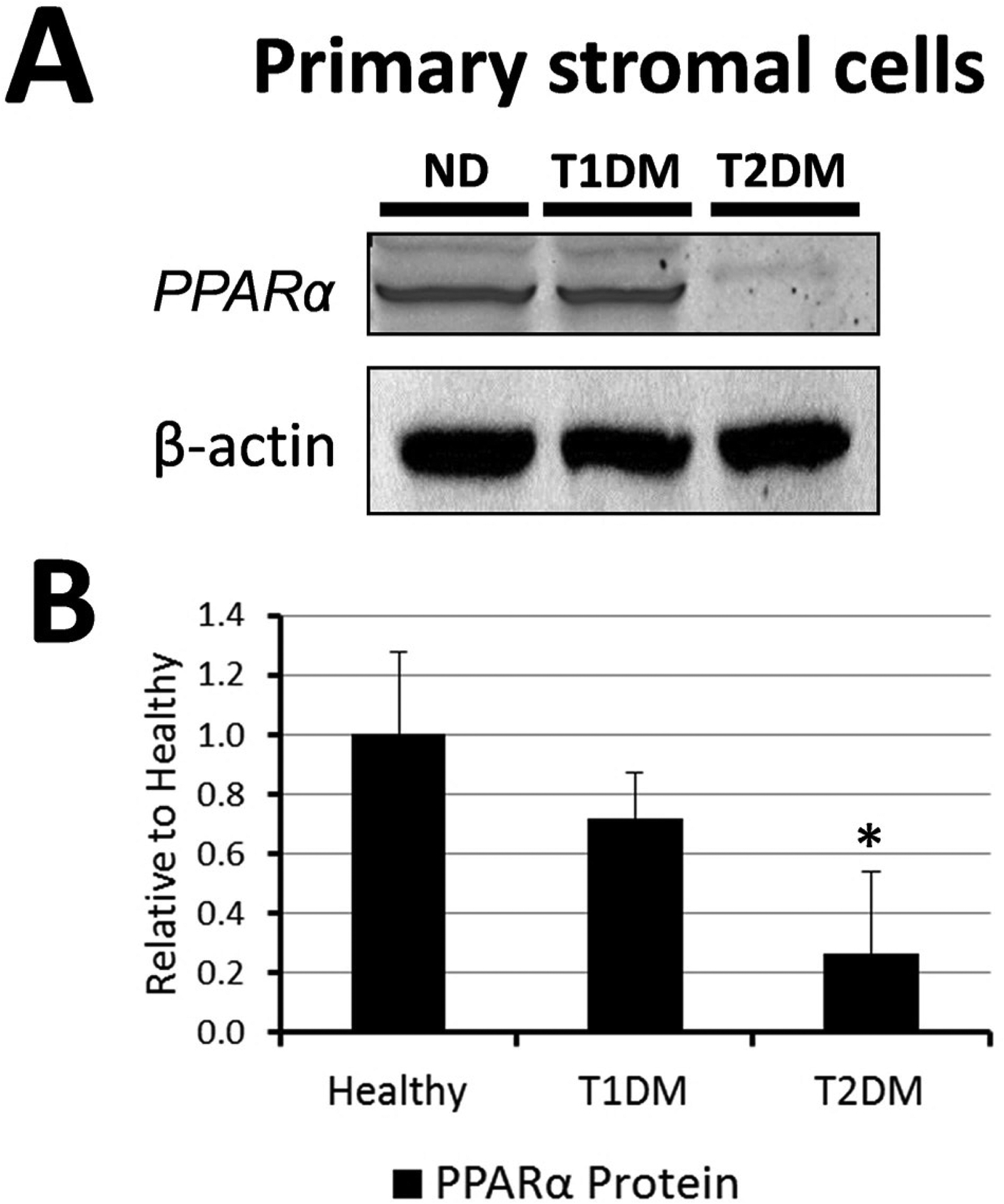
Cornea PPARα levels in non-diabetic and diabetic human donors. A) A representative PPARα Western blot image of cornea homogenates. B) Western blot image quantification showed PPARα levels were decreased in T2D cornea compared to healthy corneas. mean ± SEM; *P<0.05 rel. to ND; N=4. Modified from Matlock et al. (2020).
Figure 6:
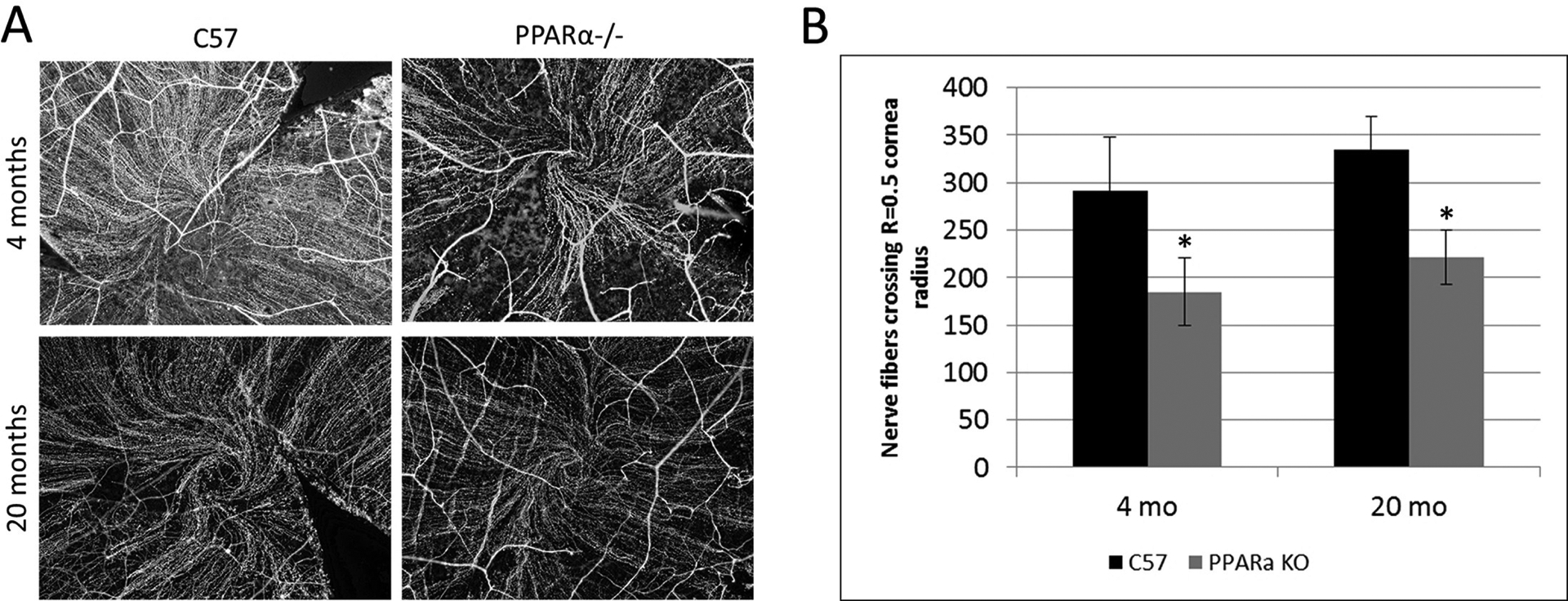
Decreased corneal nerve fibers in PPARα−/− (KO) mice compared to C57Bl/6J (WT). A) Corneal nerve fibers immuno-labeled with an antibody for beta-III neuron-specific tubulin. WT and KO nerve fiber densities were compared at 4 and 20 months of age by counting nerve fibers crossing 0.5 times the cornea radius. B) Maximum central corneal nerve fiber count: mean ± SEM; *P≤0.05, rel. to WT; 4 months N(WT)=7, N(KO)=6; 20 months N(WT)=10, N(KO)=9. Modified from Matlock et al. (2020).
Figure 7:
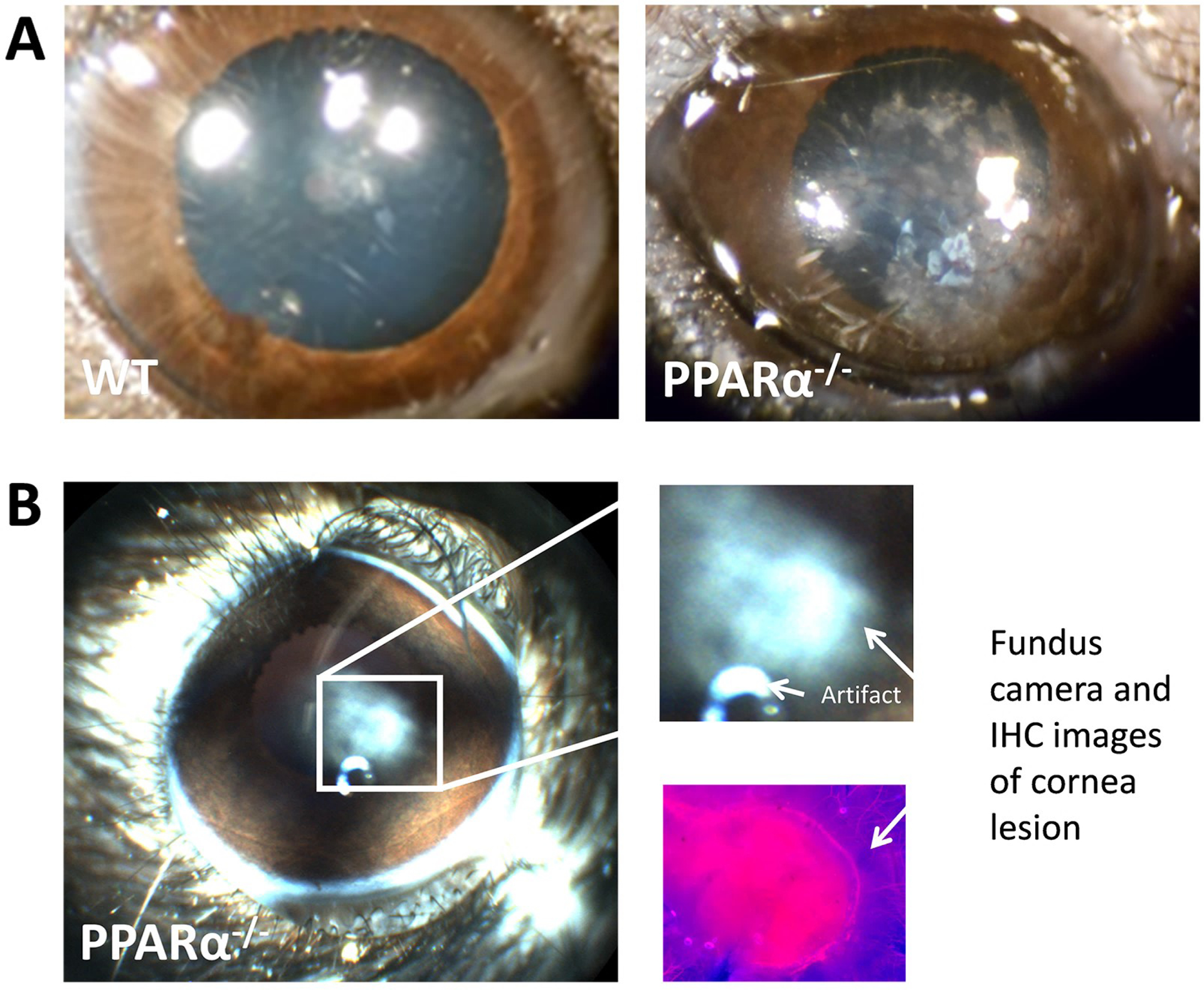
Cornea lesion in the PPARα KO mouse. A) Photographs of wild-type mouse cornea with normal opacity (left) and large PPARα KO mouse cornea lesion at 24 months of age. B) Fundus camera image of small PPARα KO cornea lesion in 24-month-old mouse (left), explode view (right), and neuron-specific beta-III tubulin immuno-labeling from the same lesion (lower right). Modified from Matlock et al. (2020).
3.2. PPARγ
The retina is a major metabolic tissue that can result in an accumulation of reactive oxygen species; it is crucial for the proper functioning of the retina to remove these and prevent cellular damage and reduce oxidative stress (Rohowetz et al., 2018). Oxidative stress is a strong component of multiple retinal diseases, including diabetic retinopathy, glaucoma, and AMD (Polvani et al., 2012; Williams, 2008). Retinal microvasculature produces a steady amount of NO to maintain proper blood flow. In diabetic vasculature a study showed stunted NO production, restored by PPARγ ligands in endothelial cells. (Sorrentino et al., 2007). The PPARγ down-regulation in diabetic retinas is linked to the interactions between NADPH oxidase 2 (NOX2) and NF-kB activation, implicating NOX2 as a major participant in the inflammatory regulation of PPARγ (Tawfik et al., 2009). Activation of PPARγ by PPARγ ligands is also linked to anti-inflammatory effects. Ebert and co-authors found that Docosahexaenoic acid (DHA), an n-3 long-chain polyunsaturated fatty acid, treatment of microglial retinal cells suppresses microglial activation through PPARγ regulation on NF-kB and promotes an anti-inflammatory response in vitro (Ebert et al., 2009). Interestingly, a study conducted by Li et al., found interactions between PPARγ, Vascular endothelial growth factor receptor 2 (VEGFR2), and arachidonate 15-Lipoxygenase (15-LOX-1) during neovascularization in oxygen-induced retinopathy (OIR) (Li et al., 2014). This study suggests that 15-LOX-1 gene transfer inhibits neovascularization in OIR via up-regulation of PPARγ and down-regulation of VEGFR2 (Li et al., 2014).
PPARγ has displayed neuroprotective effects against neurological diseases according to numerous studies (Escribano et al., 2009; Esposito and Cuzzocrea, 2011; Lee et al., 2011; Qi et al., 2010). Commonly, activation of PPARγ is evaluated in the context of retinal ganglion cell (RGC) protection in multiple CNS models. Under normal circumstances, glutamate acts as a neurotransmitter in the retina, but it can be neurotoxic when in excess (Lau and Tymianski, 2010; Otori et al., 1998). Aoun et al., proposed that there were neuroprotective effects mediated through antioxidative and not only PPARγ-dependent pathways stimulated by 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) and troglitazone; (PPARγ ligands inhibiting the cytotoxic effects of glutamate excess in the retina (Aoun et al., 2003).
The expression of PPARγ in the retina was studied in patients treated with optic nerve crush (ONC), an experimental technique often used to simulate retinal dysfunction. The studies found that PPARγ mRNA and protein levels increased in the retina when compared with patients that did not receive ONC treatment (Tang et al., 2011). Zhu et al., evaluated the survival of RGCs induced by ONC, in rats. Rats received doses of pioglitazone and were subjected to ONC. The results showed that PPARγ protects RGCs from ONC, via the reduction of Müller glial activation (Zhu et al., 2013).
Corneal neovascularization is typically associated with numerous inflammatory/infectious diseases representing a major public health concern (Azar, 2006). PPARγ activation, by PPARγ ligands, have been shown that can significantly inhibit corneal angiogenesis (Sarayba et al., 2005; Uchiyama et al., 2013; Usui et al., 2008). The inhibition of corneal neovascularization by PPARγ activation with PPAR ligand, 15d-PGJ2, was first observed in VEGR-induced angiogenesis in the rat cornea (Xin et al., 1999). Xin et al., showed that activation of PPARγ inhibits expression of VEGF receptors Flk/KDR, Flt-1 and protease uPA, leading to the suppression of endothelial cell differentiation and proliferation in vitro (Xin et al., 1999). Inhibitory effects were found to be mediated through PPARγ signaling, when Usui et al., utilized a PPARγ antagonist, GW9662, showing corneal neovascularization rescuing (Usui et al., 2008).
Disruption of the corneal tissue architecture is often related to the wound healing process and fibrosis. Agonist-activated PPARγ has exhibited major corneal anti-fibrotic properties associated with transforming growth factor-beta (TGF-β)-1, as measured by levels of smooth muscle actin (SMA), fibronectin and collagen (Zhou et al., 2016; Pan et al., 2009; Huxlin et al., 2013). Pan et al., conducted a study on rabbit corneal keratocytes treated with PPAR agonist, pioglitazone, showing that activation of PPARγ could be a key player to corneal scar formation and the wound healing cascade. The role of PPARγ ligands as a potential therapeutic target for fibrosis in ocular diseases will be described in section 4.
Expression of PPAR-γ is relatively low in the lacrimal gland (Chen et al., 2014), in contrast to what is seen in the RPE, the corneal endothelium and epithelium, (Castelli et al., 2018). The lacrimal gland is an accessory gland of the human eye located in the anterior, superotemporal orbit and consists of a bilobed, tear-shaped gland. Its primary function is in maintaining the ocular surface via secretion of the aqueous portion of the tear film. This tear film protects the ocular surface, helps remove debris, and provides a smooth surface in the interface between the air and the cornea (Machiele et al., 2020). Despite the fact that PPARγ is expressed at a low level in the lacrimal gland (Beauregard & Brandt, 2003), it appears to have an important role in the inflammatory process in dry eye syndrome (Zhang et al., 2015). It has been reported, in lacrimal gland acinar cells (rabbit lacrimal glands), that PPARγ agonists (pioglitazone) can inhibit endogenous interleukin-1 beta (IL-1β)-induced NO production (Chen et al., 2014; Omae et al., 2011). Pioglitazone is also linked to increased tear fluid production, reduction of ocular surface damage, and/or the improvement of tear film stability (Chen et al., 2014).
3.3. PPARβ/δ
A number of synthetic PPARβ/δ agonists have been described. Possible endogenous PPARβ/δ activators include fatty acids (Forman et al., 1997), triglycerides (Lee et al., 2006), and all-trans retinoic acid (ATRA) (Shaw et al., 2003). ATRA is derived from retinol, a high-level component in the eye (Luo et al., 2006) and can activate PPARβ/δ, leading to downstream effects. Nevertheless, there is still no formal evidence of this hypothesis and studies are lacking.
4. Therapeutic implications of PPARs in ocular diseases
The functional implications of PPARs in ocular pathology make them a target of interest in developing novel therapeutics. PPAR’s ligands can have beneficial effects on diabetic eye diseases such as diabetic retinopathy, diabetic macular edema, and diabetic keratopathy as outlined on Table 2. Additionally, PPAR’s ligands have promising therapeutic effects for conditions related to ocular neuropathy, dry eye, and AMD. This section will discuss the therapeutic implication of PPARs ligands and any harmful effects on ocular disease.
Table 2:
PPARs ligands therapeutic implications in disease eye.
| Diseases | Therapeutic Ligands | Therapeutic outcomes | References | |
|---|---|---|---|---|
| PPARα | Diabetic retinopathy Dry eye symptoms AMD Diabetic keratopathy |
Fibrates* (Fenofibrate) | Amelioration of retinal acellular capillary formation and pericyte loss Retinal microvascular repair Protect against corneal nerve loss Corneal neovascularization |
(Ding et al., 2014) (Bhatwadekar et al., 2017) (Shao et al., 2019) (Matlock et al., 2020) (Wang et al., 2020) (He et al., 2020) |
| PPARγ | Diabetic retinopathy Dry eye symptoms AMD |
TZD* (Rosiglitazone, pioglitazone, and troglitazone) 15-PGJ2 |
Prevention of diabetic retinal tissues form apoptosis Attenuate retinal vasculature Elevate tear film stability and tear production Anti-fibrotic properties in the cornea |
(Shen et al., 2008) (Hatanaka et al., 2012) (Cheng et al., 2008) (Jiang et al., 2014) (Thakran et al., 2015) (Muranaka et al., 2006) (Chen et al., 2014) (Murata et al., 2000) (Zhou et al., 2016) (Xin et al., 1999) |
| PPARβ/δ | AMD Ocular Angiogenesis Choroidal neovascularization Diabetic retinopathy |
GW0742* GSK0660** |
Agonist exacerbated pre-retinal vascularization Antagonist reduced pre-retinal vascularization and induce retinal leukostasis |
(Bishop-Bailey, 2008) (Capozzi et al., 2013) (Choudhary et al., 2016) (Savage et al., 2015) (Huang et al., 2011) (Joussen et al., 2009) |
PPARs agonists.
PPARs antagonists
4.1. PPARα agonists: Fibrates class
Fibrates are known to be hypolipidemic agents mainly used for hypercholesterolemia. Fibrates effectively reduce plasma triglyceride and cholesterol levels by promoting triglyceride catabolism in peripheral tissues and increasing lipoprotein lipase activity in adipose tissues (Catapano, 1992; Despres et al., 2004). Fibrates are PPARα ligands that lead to reduced synthesis of triglyceride and fatty acids. Additional to reducing triglycerides, PPARα ligands have been found to regulate inflammatory responses. Delerive et al. reported that the PPARα ligand’s fibrates decreased NF-kB activation, enhancing IL-1α expression, and exerting an anti-inflammatory reaction (Delerive et al., 2000). The expression of IFN-y, IL-6, and TNF-α were also inhibited in mice treated with a fibrate (Cunard et al., 2002). Cunard et al. noted higher expression of PPARα in B-cells than T-cells, although treatment with the PPARα ligand inhibited both B and T-cell proliferation (Cunard et al., 2002). Fibrates also play a role in Akt’s phosphorylation and endothelial NO synthase, leading to increased production of NO and the inhibition of cytokine-induced NF-kB activation, through AMP-activated protein kinase, in vascular endothelial cells (Okayasu et al., 2008).
Fenofibrate is a drug within the fibrate class used to treat dyslipidemia and cardiovascular disease but also improves glycemic control and decreases the risk of diabetic retinopathy in those with diabetes mellitus. In diabetic retinopathy, early retinal microvascular dysfunction progresses with pericyte degradation. In a study by Hu et al., it was reported that diabetic-induced PPARα down-regulation was critical to diabetic retinopathy development (Hu et al., 2013). Additionally, in a later study, streptozotocin (STZ)-induced diabetic mice treated with fenofibrate showed significant amelioration of retinal acellular capillary formation and pericyte loss through a PPARα-dependent mechanism (Ding et al., 2014). The protective effects of fenofibrate on diabetes-induced mice relate to the over-expression of PPARα, leading to NF-kB inhibition by the up-regulation of Ik-Bα and contributing to the anti-inflammatory and antioxidant effect, tightly linked to the protection of pericyte in diabetes (Ding et al., 2014). These results support earlier studies where PPARα ligand fenofibrate shown to inhibit cytokine-induced NF-kB activity, as well as monocyte chemoattractant-1 and intracellular adhesion molecule-1, in the diabetic retina (Chen et al., 2013). PPARα activation also inhibits the canonical Wnt signaling, known for its anti-inflammatory and anti-angiogenic impact (Cheng et al., 2016). In a more recent study, over-expression of micro RNA-21 is proposed to be at least partially responsible for the PPARα down-regulation observed in diabetic retinopathy (Chen et al., 2017).
Ischemia in the retina is a significant contributing factor to the development of diabetic retinopathy. Moran and co-authors showed fenofibrate antioxidant effects via regulation of hypoxia-inducible factor-a and nicotine oxidase-4 (Moran et al., 2014).
Correlation between retinal microvascular repair in diabetic retinopathy and endothelial progenitor cells, such as circulating angiogenic cells (CAC) and endothelial colony-forming cells (ECFC), has been reported (Bhatwadekar et al., 2017). Shao et al. reported down-regulation of PPARα in diabetic mice which led to decreased ECFC/CAC and mitochondrial dysfunction. In contrast, activation of PPARα, using fenofibrate, normalized the ECFC/CAC levels and mitochondrial function in diabetic mice (Shao et al., 2019).
The beneficial effects of fenofibrate in patients with type 2 diabetes were highlighted in two clinical trials, FIELD (Fenofibrate Intervention in Event Lowering in Diabetes) and ACCORD (Action to Control Cardiovascular Risk in Diabetes) (Katome et al., 2015; Keech et al., 2007; Khatol et al., 2018). Recent studies on fenofibrate’s has protective effects against corneal neovascularization and diabetic keratopathy, are also promising (Matlock et al., 2020; Wang et al., 2020). Matlock et al. administered oral fenofibrate treatment on STZ-induced diabetic rats and mice showing protective effects against corneal nerve degeneration. Similarly, Wang et al. showed the role of PPARα and corneal keratocytes. Specifically, results suggest that keratocytes could promote the expression of vascular endothelial growth factor receptor 3 (VEGFR3) and matrix metallopeptidase 13 (MMP13), and corneal neovascularization formation through PPARα downregulation. (Wang et al., 2020).
Fenofibrate has also been tested as a potential treatment for dry eye inflammation. A study by He et al. investigated the use of fenofibrate to the effects and mechanism on the formation of ocular surface squamous metaplasia induced by topical benzalkonium chloride. The study found that fenofibrate reduced TNF-α and IL-6 in the cornea, leading to the suppression of inflammation associated with ocular surface squamous metaplasia (He et al., 2020). Additionally, fenofibrate led to the restoration of microvilli morphology, and sleep deprivation-induced dry eye (Tang et al., 2018).
Adverse effects associated with fibrates treatment are considered minor and include gastrointestinal discomfort, musculoskeletal symptoms, and headaches; although there are some rare reports of severe cases of myopathy and rhabdomyolysis (Danis et al., 2010; Despres et al., 2004; Wang and Wang, 2018; Zhou et al., 2020). The risk factors for fenofibrate-induced rhabdomyolysis increases in cases with hypothyroidism, renal disease, and diabetes mellitus (Danis et al., 2010; Satarasinghe et al., 2007; Wang and Wang, 2018; Zhou et al., 2020).
4.2. PPARγ agonists: Thiazolidinedione (TZD) class
TZD’s are known as antidiabetic drugs that improve the cell’s responsiveness to insulin by regulating glucose and fatty acids metabolism, which in turn, reduces insulin within the blood. Beneficial treatment effects of TZDs on glycemic control in type 2 diabetes mellitus have been reported (Davidson et al., 2018; Saltiel and Olefsky, 1996). TZD’s mechanism works via a PPARs agonist, altering genes’ expression, which results in decreased insulin resistance in adipose tissue, muscle, and liver cells (Greenfield and Chisholm, 2004; Hauner, 2002). Troglitazone, pioglitazone, and rosiglitazone are drugs within the class TZD. Troglitazone and pioglitazone are potent, selective agonists to PPARγ and, to a lesser extent, to PPARα, where rosiglitazone is a ligand selective to PPARγ (Colca et al., 2014). In addition to the impacts on insulin resistance, TZDs have also displayed anti-inflammatory effects on the properties of PPARs ligands, which decrease NF-kB, stimulating the inflammatory pathways, and increase NF-kB inhibitor, suppressing the inflammatory pathways (Chawla et al., 2001; Jiang et al., 1998; Mohanty et al., 2004).
Rosiglitazone, pioglitazone, and troglitazone inhibit NF-kB-induced angiogenesis, as measured by in-vitro migration assay, in the choroid and RPE cells which have a fibrotic phenotype in proliferative diabetic retinopathy and proliferative vitreoretinopathy (Cheng et al., 2008; Hatanaka et al., 2012; Shen et al., 2008). Proliferation by epithelial-mesenchymal transition, implicates pathogenic TGF-β-linked fibrosis. Hatanaka et al. showed that pioglitazone inhibits monkey RPE cells’ fibrotic change by reducing phalloidin, SMA, and fibronectin through the suppression of TGF- β signaling (Hatanaka et al., 2012). PPARγ ligand troglitazone can ameliorate the fibrotic RPE phenotype, in proliferative retinopathies, by preventing TGF- β2 induced overexpression of collagen type I and fibronectin, and by delaying cell migration (Cheng et al., 2008). In 2014, a study Jeon et al., demonstrated the positive effects of PPARγ agonists, 15d-PGJ2, troglitazone, and rosiglitazone, in reducing myofibroblast differentiation driven by TGF-β-1 and SMA downregulation (Jeon et al., 2014). Tested in cultured cat corneal fibroblasts, these three agonists demonstrated to have in vitro anti-fibrotic effects (Jeon et al., 2014). Particularly rosiglitazone, has been successfully tested in vivo for corneal scarring by topical application (Huxlin et al., 2013). The results of this study showed an effective control of fibrosis, restoring corneal thickness and optics. A separate study indicated that fibrotic pathologies can be treated with ophthalmic solutions of PPARγ agonists in the cornea, following alkali burn injury (Uchiyama et al., 2013). One possible mechanism of stromal myofibroblast differentiation prevention is by blocking the TGF-β-1/SMAD-signaling pathway via the inhibition of p38 mitogen-activated protein kinase (MAPK) (Guo et al., 2018). Interestingly, PPARγ ligands act on the p38 MAPK pathway, blocking myofibroblast differentiation markers (alpha-SMA and TGF-β-1) (Jeon et al., 2015).
Extensive literature has shown that TZDs also play a role in retinal vasculature (Jiang et al., 2014; Omae et al., 2011; Thakran et al., 2015). Pioglitazone treatment was shown to restore insulin signaling transduction, in a diabetic rat retina model, by dilating retinal arterioles (Jiang et al., 2014). Specifically, authors used a type 2 diabetic rat model, as well as retinal endothelial cells (REC), and Müller cells cultured in normal glycemic and hyperglycemic conditions to investigate the effects of pioglitazone. The results showed that pioglitazone normalized insulin signaling transduction by reducing the levels of tumor necrosis factor-alpha (TNF-α) and suppression of cytokine signaling 3 (SOCS-3), inducing insulin receptor resistance and protecting the diabetic retinal tissues from apoptosis (Jiang et al., 2014). Pioglitazone treatment has also been shown to prevent insulin resistance and cell apoptosis by restoring insulin-like growth factor binding protein-3 (IGFBP-3) levels independently from TNF-α actions (Thakran et al., 2015). Thakran et al. found that Pioglitazone restores IGFBP-3 levels via activation of protein kinase A and DNA-dependent protein kinase, in RECs during hyperglycemic conditions. Muranala et al. treated diabetic rats with rosiglitazone and showed no changes in TNF-α or VEGF, but did restore the elevated NF-kB-regulated Intercellular adhesion molecule 1 (ICAM-1) expression to a healthy level (Muranaka et al., 2006). Together, these studies suggest that pioglitazone and rosiglitazone are good candidates for treating diabetic retinopathy (Jiang et al., 2014; Muranaka et al., 2006; Thakran et al., 2015). TZDs have also shown effects on AMD. Vision loss due to choroidal neovascularization via VEGF, is well established (Kvanta et al., 1996; Lopez et al., 1996). Murata et al. found that troglitazone and rosiglitazone inhibit the VEGF pathway leading to inhibition of choroidal angiogenesis and neovascularization. Additionally, troglitazone and rosiglitazone have been shown to inhibit NF-kB-induced angiogenesis, in the choroid and RPE, through in vitro migration assay (Park et al., 2009).
Limited literature exists on PPARγ ligands and its therapeutic effects on dry eye symptom. PPARγ agonists were reported to be able to inhibit TNF-α and IL-1β in dry eyes, suppressing inflammatory pathways and increasing the tear fluid tear production, while inhibiting NO (Beauregard and Brandt, 2003; Chen et al., 2014). In a study by Chen et al., scopolamine-induced mouse dry eye model were treated with pioglitazone leading to elevated tear film stability, increased tear production, and reduced damage to the ocular surface (Chen et al., 2014). Interestingly, PPARγ ligands are viewed as potential corneal angiogenesis modulators, by many (Giaginis et al., 2007; Margeli et al., 2003; Uchiyama et al., 2013; Usui et al., 2008; Xin et al., 1999). Pioglitazone, for example, has been studied as a possible drug for treating ocular neovascularization, and shown to decrease the density of angiogenesis in a corneal rat model (Sarayba et al., 2005).
Treatment with PPARγ ligands can also have adverse effects. TZDs have shown to worsen and possibly stimulate diabetic macular edema (Fong and Contreras, 2009; Idris et al., 2012; Saw et al., 2019). However, a longitudinal cohort and a cross-sectional study of type 2 diabetes patients found no association between TZDs, the progression of diabetic macular edema, and visual acuity (Ambrosius et al., 2010; Gower et al., 2018). Furthermore, TZDs have shown adverse cardiovascular effects related to fluid retention, leading to congestive heart failure and peripheral tissue edema from endothelial cell leakage (Duan et al., 2008; Nesto et al., 2004). Rosiglitazone was shown to promote endothelial cell migration and vascular permeability through Akt phosphorylation (Ku et al., 2017), suggesting a possible role of Akt phosphorylation in the fluid retention and peripheral tissue edema adverse effects.
4.3. PPARβ/δ agonists and antagonists
There is limited literature on PPARβ/δ agonist’s/antagonist’s therapeutic effects, related to ocular diseases/disorders. We know, from previous studies, that PPARβ/δ plays a role in ocular angiogenesis in AMD and ocular neovascularization (Bishop-Bailey, 2008; Capozzi et al., 2013; Choudhary et al., 2016; Tobita et al., 2020). Capozzi et al. studied the effects of PPARβ/δ agonist and antagonists in human retinal microvascular endothelial cells (HRMEC) and pre-retinal ocular neovascularization using a rat oxygen-induced retinopathy model. In that study, GW0742 was used as a highly selective PPARβ/δ agonist and GSK0660 as a PPARβ/δ antagonist, showing that PPARβ/δ agonist exacerbates pre-retinal ocular neovascularization and the PPARβ/δ antagonist reduces pre-retinal neovascularization (Capozzi et al., 2013). Similarly, in a separate study, treatment with PPARβ/δ antagonists resulted in the inhibition of experimentally induced choroidal neovascular lesions, associated with the down-regulation of VEGF-α, TGF-β1, and platelet-derived endothelial growth factor-beta known to be critical factors for ocular angiogenesis (Choudhary et al., 2016). In a more recent study, PPARβ/δ agonist (GW501516) was used to treat rat corneal alkali burn injuries, resulting in the suppression of inflammatory cytokines IL-6, IL-1β, TNF α, and NF-kB, promotion of neovascularization, by expressing of VEGF-α and stimulating infiltration of M2 macrophages, and vascular endothelial cell in the wound area (Tobita et al., 2020).
Choundary et al., demonstrated for the first time that selective targeting of PPARβ/δ may be a suitable strategy for treatment of different clinical sub-types of AMD(Choudhary et al., 2016). Additionally, Suarez et al. demonstrated that PPARβ/δ antagonist (GSK0660) may have a therapeutic effects against retinal hyperpermeability (Suarez et al., 2014). Suarez et al. treated HRMEC monolayer with VEGF to induce low transendothelial electrical resistance measurements, treated with GSK0660. Results showed that it completely reversed the transendothelial electrical resistance levels in the VEGF-treated HRMEC (Suarez et al., 2014). The data available on the role of PPARβ/δ in retinal inflammation and diabetic retinopathy is even less understood. The current hypothesis is that PPARβ/δ up-regulates TNF-α, which recruits leukocytes, increasing retinal inflammation, leading to the development of diabetic retinopathy (Huang et al., 2011; Joussen et al., 2009). A study by Savage et al. showed that TNF-α significantly up-regulates CCL5, CX3CL1, and CXCL10 cytokines known to be involved in leukocyte recruitment. This study also demonstrated that GSK0660 inhibits TNF-α, including CCL8, CCL17, and CXCL10, which induced retinal leukostasis in RECs (Savage et al., 2015).
5. Future directions and conclusions
PPARs tell an intriguing story, indeed. The potential uses in medicine are enormous, but currently are largely unexplored. PPARs history is rather interesting as they were originally identified in Xenopus frogs as receptors that induced proliferation of peroxisomes in cells (Dreyer et al., 1992). PPARα was discovered as part of a search for a group of agents referred to as peroxisome proliferators, because of their ability to increase peroxisomal numbers in rodent liver. Later on, when it turned out that PPARs played a more significant, and versatile, role in biology these agents were termed PPARs ligands.
By far, the most investigated PPAR is the γ isoform with implications and roles not only in the human eye, and eye diseases, but also numerous tissues/organs. On the other hand, the studies on β/δ isoform are rather limited. Perhaps this is not surprising given that its discovery came late, in 1992. In the context of ocular pathobiology, the α isoform has known effects in the retina, but not in the cornea, where only a handful of recent studies have tested its effects. What is rather clear, when looking at the current literature, is the absence of PPARs studies on ocular tissues outside the cornea and retina. This might be something to consider as we move forward, and collectively, the scientific community consider expanding the investigations to other ocular tissues.
It is clear that there is much work to be done before each isoform can be fully characterized and utilized as treatment(s) for ocular diseases. However, it is very encouraging that the PPARs-related studies are gaining traction and scientists are looking more and more into their action, mechanisms, and function.
Highlights.
PPAR ligands are gradually being recognized as potential new therapeutic options for ocular diabetes and neuropathy, dry eye, and age-related macular degeneration.
PPARα is known for its role in regulating circadian rhythm, retinal neuroprotection and angiogenesis, as well as diabetic keratopathy.
PPARγ is widely expressed in the corneal endothelium and epithelium, and known for its role in retinal oxidative stress.
PPARβ/δ increases fatty acids metabolism and lipolysis, an essential function in the prevention of adipogenesis.
Acknowledgements
The authors would like to thank the National Eye Institute (NEI) for providing research support (EY028949).
Abbreviations:
- PPARs
Peroxisome Proliferator-Activated Receptors
- ECM
Extracellular matrix
- AMD
Age-related macular degeneration
- RXR
Retinoid X Receptor
- PPREs
PPAR Response Elements
- NOX2
NADPH oxidase 2
- DHA
Docosahexaenoic acid
- VEGFR2
Vascular endothelial growth factor receptor 2
- VEGFR3
Vascular endothelial growth factor receptor 3
- 15-LOX-1
Arachidonate 15-Lipoxygenase
- RGC
Retinal ganglion cell
- ONC
Optic nerve crush
- MAPK
p38 mitogen-activated protein kinase
- SMA
Smooth muscle actin
- IL-1β
Interleukin-1 beta
- ATRA
All-trans retinoic acid
- CAC
Circulating angiogenic cells
- ECFC
Endothelial colony-forming cells
- MMP13
Matrix metallopeptidase 13
- TZD
Thiazolidinedione
- RPE
Retinal pigment epithelium
- REC
Retinal endothelial cells
- TNF-α
Tumor necrosis factor-alpha
- SOCS-3
Suppressor of cytokine signaling 3
- IGFBP-3
Insulin-like growth factor binding protein-3
- STZ
Streptozotocin
- ICAM-1
Intercellular adhesion molecule 1
- HRMEC
Human retinal microvascular endothelial cells
- FIELD
Fenofibrate Intervention in Event Lowering in Diabetes
- IOP
Intraocular pressure
- VEGF
Vascular endothelial growth factor
- KO
Knockout
- IL-6
Interleukin-6
- COX-2
Cyclooxygenase-2
- OIR
Oxygen-induced retinopathy
- NO
Nitric oxide
- LD
Light-dark
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
None
References
- Abbott BD, 2009. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPARγ) in rodent and human development. Reprod Toxicol 27, 246–257. [DOI] [PubMed] [Google Scholar]
- Abu-Hassan DW, Acott TS, Kelley MJ, 2014. The Trabecular Meshwork: A Basic Review of Form and Function. J. Ocul. Biol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta MC, Tan ME, Belmonte C, Gallar J, 2001. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci 42, 2063–2067. [PubMed] [Google Scholar]
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM, 2013. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med 19, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed J, Braun RD, Dunn R, Linsenmeier RA, 1993. Oxygen distribution in the macaque retina. Invest. Ophthalmol. Vis. Sci 34, 516–521. [PubMed] [Google Scholar]
- Ahmed Z, Lutty GA, 2017. Anti-Angiogenic Properties of Vitreous. Ref. Modul. Neurosci. Biobehav. Psychol [Google Scholar]
- Ambrosius WT, Danis RP, Goff DC, Greven CM, Gerstein HC, Cohen RM, Riddle MC, Miller ME, Buse JB, Bonds DE, Peterson KA, Rosenberg YD, Perdue LH, Esser BA, Seaquist LA, Felicetta JV, Chew EY, ACCORD Study Group, 2010. Lack of association between thiazolidinediones and macular edema in type 2 diabetes: the ACCORD eye substudy. arch ophthalmol 128, 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoun P, Simpkins JW, Agarwal N, 2003. Role of PPAR-γ Ligands In Neuroprotection against Glutamate-Induced Cytotoxicity in Retinal Ganglion Cells. Invest Ophthalmol Vis Sci 44, 2999–3004. [DOI] [PubMed] [Google Scholar]
- Aurora AL, 1979. Corneal blindness-- a review. Indian J Ophthalmol 27(1): p. 1–14. [PubMed] [Google Scholar]
- Azar DT, 2006. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc 104, 264–302. [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM, 2002. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A 99, 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM, 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4, 585–595. [DOI] [PubMed] [Google Scholar]
- Barbiero JK, Santiago RM, Persike DS, da Silva Fernandes MJ, Tonin FS, da Cunha C, Boschen SL, Lima MMS, Vital MABF, 2014. Neuroprotective effects of peroxisome proliferator-activated receptor alpha and gamma agonists in model of parkinsonism induced by intranigral 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine. Behav Brain Res 274, 390–399. [DOI] [PubMed] [Google Scholar]
- Barrera J, Subramanian S, Chiba-Falek O, 2018. Probing the role of PPARγ in the regulation of late-onset Alzheimer’s disease-associated genes. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard C, Brandt P, 2003. Peroxisome Proliferator-Activated Receptor Agonists Inhibit Interleukin-1β-Mediated Nitric Oxide Production in Cultured Lacrimal Gland Acinar Cells. J. Ocul. Pharmacol. Ther 19, 579–587. [DOI] [PubMed] [Google Scholar]
- Benedek GB, 1971. Theory of transparency of the eye. Appl Opt, 10(3): p. 459–73. [DOI] [PubMed] [Google Scholar]
- Berthou L, Duverger N, Emmanuel F, Langouet S, Auwerx J, Guillouzo A, Fruchart JC, Rubn E, Denèfle P, Staels B, Branellec D, 1996. Opposite regulation of human versus mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. J Clin Invest 97, 2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatwadekar AD, Duan Y, Korah M, Thinschmidt JS, Hu P, Leley SP, Caballero S, Shaw L, Busik J, Grant MB, 2017. Hematopoietic stem/progenitor involvement in retinal microvascular repair during diabetes: implications for bone marrow rejuvenation. Vision Res. 139, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscetti F, Gaetani E, Flex A, Aprahamian T, Hopkins T, Straface G, Pecorini G, Stigliano E, Smith RC, Angelini F, Castellot JJ Jr, Pola R, 2008. Selective activation of peroxisome proliferator-activated receptor (PPAR)alpha and PPAR gamma induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanism. Diabetes 57, 1394–1404. [DOI] [PubMed] [Google Scholar]
- Bishop-Bailey D, 2008. A Role for PPARbeta/delta in Ocular Angiogenesis. PPAR Res. 825970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A, 2004. Ageing and vision: structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol 86, 407–485. [DOI] [PubMed] [Google Scholar]
- Borges-Giampani SA, Giampani J, 2013. Anatomy of Ciliary Body, Ciliary Processes, Anterior Chamber Angle and Collector Vessels, in: Glaucoma - Basic and Clinical Aspects. pp. 3–14. [Google Scholar]
- Bortolotto JW, Margis R, Bersch Ferreira AC, Padoin AV, Mottin CC, Guaragna RM, 2007. Adipose Tissue Distribution and Quantification of PPARβ/δ and PPARγ1–3 mRNAs: Discordant Gene Expression in Subcutaneous, Retroperitoneal and Visceral Adipose Tissue of Morbidly Obese Patients. Obes. Surg 17, 934–940. [DOI] [PubMed] [Google Scholar]
- Brautbar A, Barbalic M, Chen F, Belmont J, Virani SS, Scherer S, Hegele RA, Ballantyne CM, 2013. Rare APOA5 promoter variants associated with paradoxical HDL cholesterol decrease in response to fenofibric acid therapy. J Lipid Res 54, 1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune S, Kolsch H, Ptok U, 2003. Polymorphism in the peroxisome proliferator-activated receptor ? gene influences the risk for Alzheimer’s disease. J Neural Transm. 110, 1041–1050. [DOI] [PubMed] [Google Scholar]
- Capozzi ME, McCollum GW, Savage SR, Penn JS, 2013. Peroxisome proliferator-activated receptor- β/δ regulates angiogenic cell behaviors and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 54, 4197–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli V, D’Angelo M, Antonosante A, Catanesi M, Benedetti E, Desideri G, Cimini A, 2018. Physiology and Pathophysiology of PPARs in the Eye. Nucl. Recept. Res 5. [Google Scholar]
- Catapano A, 1992. Mode of action of fibrates. Pharmacol Res 26, 331–340. [DOI] [PubMed] [Google Scholar]
- Cervantes-Perez LG, Ibarra-Lara M, Escalante B, 2012. Endothelial nitric oxide synthase impairment is restored by clofibrate treatment in an animal model of hypertension. Eur. J Pharm 658, 108–115. [DOI] [PubMed] [Google Scholar]
- Charoensuksai P, Xu W, 2010. PPARs in Rhythmic Metabolic Regulation and Implications in Health and Disease. PPAR Res. 242643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM, 2001. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med 7, 48–52. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang G, 2014. PPARs Integrate the Mammalian Clock and Energy Metabolism. PPAR Res. 653017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Qiu F, Zhou K, Matlock HG, Takahashi Y, Rajala RVS, Yang Y, Moran E, Ma JX, 2017. Pathogenic Role of microRNA-21 in Diabetic Retinopathy Through Downregulation of PPARα. Diabetes 66, 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, Mott R, Lyons TJ, Ma JX, 2013a. Therapeutic effects of PPARα agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62, 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kardon RH, 2013. Studying the Effect of Iris Mechanics on the Pupillary Light Reflex Using Brimonidine-Induced Anisocoria. Invest Ophthalmol Vis Sci 54, 2951–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Y H, M L, Jenkins AL, Keech AC, Mott R, Lyons TJ, Ma JX, 2013b. Therapeutic effects of PPARα agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62, 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang X, Yang L, Li M, Li B, Wang W, Sheng M, 2014. Decreased PPAR-γ expression in the conjunctiva and increased expression of TNF-α and IL-1β in the conjunctiva and tear fluid of dry eye mice. Mol Med Rep 9, 2015–2023. [DOI] [PubMed] [Google Scholar]
- Chen YC, Wu JS, Tsai HD, Huang CY, Chen JJ, Sun GY, Lin TN, 2012. Peroxisome proliferator-activated receptor gamma (PPAR-γ) and neurodegenerative disorders. Mol Neurobiol 46, 114–124. [DOI] [PubMed] [Google Scholar]
- Cheng HC, Ho TC, Chen SL, Lai HY, Hong KF, Tsao YP, 2008. Troglitazone suppresses transforming growth factor beta-mediated fibrogenesis in retinal pigment epithelial cells. Mol Vis 14, 95–104. [PMC free article] [PubMed] [Google Scholar]
- Cheng R, Ding L, He X, Takahashi Y, Ma JX, 2016. Interaction of PPARα With the Canonic Wnt Pathway in the Regulation of Renal Fibrosis. Diabetes 65, 3730–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertov AO, Holzhausen L, Kuok IT, Couron D, Parker E, Lindton JD, Sadilek M, Sweet IR, Hurley JB, 2011. Roles of glucose in photoreceptor survival. J Biol Chem 286, 34700–34711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M, Griffin PR, Spiegelman BM, 2010. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 466, 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary M, Ding JD, Qi X, Boulton ME, Yao PL, Peters JM, Malek G, 2016. PPARβ/δ Selectively Regulates Phenotypic Features of Age-Related Macular Degeneration. Aging (Albany. NY). 8, 1952–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca JR, Tanis SP, McDonald WG, Kletzien RF, 2014. Insulin sensitizers in 2013: new insights for the development of novel therapeutic agents to treat metabolic diseases. Expert Opin. Investig. Drugs 23, 1–7. [DOI] [PubMed] [Google Scholar]
- Croasdell A, Duffney PF, Kim N, Lacy SH, Sime PJ, Phipps RP, 2015. PPARγ and the Innate Immune System Mediate the Resolution of Inflammation. PPAR Res. 2015, 549691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunard R, Ricote M, DiCampli D, Archer DC, Kahn DA, Glass CK, Kelly CJ, 2002. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J Immunol 168, 2795–2802. [DOI] [PubMed] [Google Scholar]
- Danis R, Akbulut S, Ozmen S, Arikan S, 2010. Rhabdomyolysis-induced acute renal failure following fenofibrate therapy: a case report and literature review. Case Rep Med 2010, 537818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MA, Mattison DR, Azoulay L, Krewski D, 2018. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: past, present and future. Crit Rev Toxicol 48, 52–108. [DOI] [PubMed] [Google Scholar]
- de Oliveira RC, and Wilson SE, 2020. Descemet’s membrane development, structure, function, and regeneration. Exp Eye Res, 197: p. 108090. [DOI] [PubMed] [Google Scholar]
- Delamere NA, 2005. Ciliary Body and Ciliary Epithelium. Adv. Organ Biol 10, 127–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delerive P, De Bosscher K, Vanden Berghe W, Fruchart JC, Haegeman G, Staels B, 2002. DNA Binding-Independent Induction of IκBα Gene Transcription by PPARα. Mole Endocrinol 16, 1029–1039. [DOI] [PubMed] [Google Scholar]
- Delerive P, Gervois P, Fruchart JC, Staels B, 2000. Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem 275, 36703–36707. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I, Robins SJ, 2004. Role of fibric acid derivatives in the management of risk factors for coronary heart disease. Drugs 64, 2177–2198. [DOI] [PubMed] [Google Scholar]
- Desvergne D, Whali W, 1999. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev 20, 649–688. [DOI] [PubMed] [Google Scholar]
- Ding L, Cheng R, Hu Y, Takahashi Y, Jenkins AJ, Keech AC, Humphries KM, Gu X, Elliot MH, Xia X, Ma J, 2014. Peroxisome proliferator-activated receptor α protects capillary pericytes in the retina. Am J Pathol 184, 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doktorova M, Zwarts I, van Zutphen T, van Dijk TH, Bloks VW, Harkema L, de Bruin A, Downes M, Evans RM, Verkade HJ, Jonker JW, 2017. Intestinal PPARδ protects against diet-induced obesity, insulin resistance and dyslipidemia. Sci. Rep 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W, 1992. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68: 879–87. [DOI] [PubMed] [Google Scholar]
- Duan SZ, Usher MG, Mortensen RM, 2008. Peroxisome proliferator-activated receptor gamma-mediated effects in the vasculature. Circ Res 102, 283–294. [DOI] [PubMed] [Google Scholar]
- Ebert S, Weigelt K, Walczak Y, Drobnik W, Mauerer R, Hume DA, Weber BHF, Langmann T, 2009. Docosahexaenoic acid attenuates microglial activation and delays early retinal degeneration. J Neurochem 110, 1863–1875. [DOI] [PubMed] [Google Scholar]
- Edgar AD, Tomkiewicks C, Costet P, Legendre C, Aggerbeck M, Boughet J, Staels B, Guyomand C, Pineau T, Barouki R, 1998. Fenofibrate modifies transaminase gene expression via a peroxisome proliferator activated receptor alpha-dependent pathway. Toxicol Lett 98, 13–23. [DOI] [PubMed] [Google Scholar]
- Ehrlich R, Harris A, Wentz SM, Moore NA, Siesky BA, 2017. Anatomy and Regulation of the Optic Nerve Blood Flow`, in: Reference Module in Neuroscience and Biobehavioral Psychology. [Google Scholar]
- Escribano L, Simón AM, Pérez-Mediavilla A, Salazar-Colocho P, Del Río J, Frechilla D, 2009. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer’s disease mouse model. Biochem. Biophys. Res. Commun 379, 406–410. [DOI] [PubMed] [Google Scholar]
- Esposito E, Cuzzocrea S, 2011. Targeting the peroxisome proliferator-activated receptors (PPARs) in spinal cord injury. Expert Opin Ther Targets 15, 943–959. [DOI] [PubMed] [Google Scholar]
- Euler T, Masland RH, 2000. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 83, 1817–1829. [DOI] [PubMed] [Google Scholar]
- Fargo AA, McDermott ML, Soong HK, 2009. Corneal anatomy, physiology, and wound healing, in: Yanoff M, Duker JS (Eds.), Ophtalmology. Elsevier Inc, pp. 203–208. [Google Scholar]
- Feher J, 2012. Vision, in: Quantitative Human Physiology. [Google Scholar]
- Fidaleo M, Fanelli F, Ceru MP, Moreno S, 2014. Neuroprotective properties of peroxisome proliferator-activated receptor alpha (PPARα) and its lipid ligands. Curr Med Chem 21, 2803–2821. [DOI] [PubMed] [Google Scholar]
- Fong DS, Contreras R, 2009. Glitazone use associated with diabetic macular edema. Am J Ophthalmol 147, 583–586. [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM, 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci U S A 94, 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM, 1995. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83, 803–812. [DOI] [PubMed] [Google Scholar]
- Foster J, Gouveia R, Connon C, 2015. Low-glucose enhances keratocyte-characteristic phenotype from corneal stromal cells in serum-free conditions. Sci. Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL, Mann MM, Funderburgh ML, 2003. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J. Biol. Chem 278, 45629–45637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Z, Burkart-Hartman EM, Han DH, Finck B, Leone TC, Smith EY, Ayala JE, Holloszy J, Kelly DP, 2011. The nuclear receptor PPARβ/δ programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev 25, 2619–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Gil P, Joglar B, Rodriguez-Perez A, Guerra MJ, Labandeira-Garcia JL, 2012. Involvement of PPAR-γ in the neuroprotective and anti-inflammatory effects of angiotensin type 1 receptor inhibition: effects of the receptor antagonist telmisartan and receptor deletion in a mouse MPTP model of Parkinson’s disease. J. Neuroinflammation [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaginis C, Margeli A, Theocharis S, 2007. Peroxisome proliferator-activated receptor-γ ligands as investigational modulators of angiogenesis. Expert Opin. Investig. Drugs 16, 1561–1572. [DOI] [PubMed] [Google Scholar]
- Giordano Attianese GM, Desvergne B, 2015. Integrative and systemic approaches for evaluating PPARβ/δ (PPARD) function. Nucl Recept Signal 13, e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girroir EE, Hollingshead HE, He P, Zhu B, Perdew GH, Peters JM, 2008. Quantitative expression patterns of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) protein in mice. Biochem. Biophys. Res. Commun 371, 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower EW, Lovato JF, Ambrosius WT, Chew EY, Danis RP, Davis MD, Goff DC, Greven CM, Group AS, 2018. Lack of longitudinal association between thiazolidinediones and incidence and progression of diabetic eye disease: The ACCORD eye study. Am J Ophthalmol 187, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield JR, Chisholm DJ, 2004. Thiazolidinediones-mechanisms of action. Exp. Clin. Pharmacol 27, 67–70. [Google Scholar]
- Halfter W, Winzen U, Bishop PN, Eller A, 2006. Regulation of eye size by the retinal basement membrane and vitreous body. Invest. Ophthalmol. Vis. Sci 47, 3586–3594. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Birk DE, 2010. The molecular basis of corneal transparency. Exp Eye Res, 2010. 91(3): p. 326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka H, Koizumi N, Okumura N, Kay EP, Mizuhara E, Hamuro J, Kinoshita S, 2012. Epithelial-Mesenchymal Transition-Like Phenotypic Changes of Retinal Pigment Epithelium Induced by TGF-β Are Prevented by PPAR-γ Agonists. Invest Ophthalmol Vis Sci 53, 6955–6963. [DOI] [PubMed] [Google Scholar]
- Hauner H, 2002. The mode of action of thiazolidinediones. Diabetes Metab Res Rev 18, S10–S15. [DOI] [PubMed] [Google Scholar]
- He H, Liang M, Li L, Luo S, Xie F, He H, Xiao X, Wu H, Lin Z, 2020. PPARα agonist Fenofibrate suppressed the formation of ocular surface squamous metaplasia induced by topical benzalkonium chloride. Invest Ophthalmol Vis Sci 61, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht E, 1987. Optics, 2nd ed. Addison Wesley. [Google Scholar]
- Heneka MT, Klockgether T, Feinstein DL, 2000. Peroxisome Proliferator-Activated Receptor-γ Ligands Reduce Neuronal Inducible Nitric Oxide Synthase Expression and Cell Death In Vivo. J Neurosci 20, 6862–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz R, Bishara-Shieban J, Bar-Tana J, 1995. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J Biol Chem 270, 13470–13475. [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Alvarado JA, Weddell JE, 1971. Histology of the Human Eye.
- Hu Y, Chen Y, Ding L, He X, Takahashi Y, Gao Y, Shen W, Cheng R, Chen Q, Qi X, Boulton ME, Ma JX, 2013. Pathogenic role of diabetes-induced PPAR-α down-regulation in microvascular dysfunction. Proc Natl Acad Sci U S A 110, 15401–15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA, 2011. TNFalpha is required for late BRB breakdown in diabetic retinopathy and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci 52, 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxlin KR, Hindman HB, Jeon KI, Buhren J, MacRae S, DeMagistris M, Ciufo D, Sime PJ, and Phipps RP. 2013. ‘Topical rosiglitazone is an effective anti-scarring agent in the cornea’, PLoS One, 8: e70785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang O, 2013. Role of oxidative stress in Parkinson’s disease. Exp Neurobiol 22, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris I, Warren G, Donnelly R, 2012. Association Between Thiazolidinedione Treatment and Risk of Macular Edema Among Patients With Type 2 Diabetes. Arch Inter Med 172, 1005–1011. [DOI] [PubMed] [Google Scholar]
- Inoue I, Shinoda Y, Masaaki I, Hayashi K, Kanazawa K, Nomura M, Matsunaga Y, Xu H, Kawai S, Awata T, Komoda T, Katayama S, 2005. CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the Peroxisome Proliferator-Activated Receptor (PPAR) response element. J Alther. Thromb 12, 169–174. [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S, 1990. Activation of the member of the steroid hormone receptor superfamily by peroxisome proliferators. Comp. Study 347, 645–650. [DOI] [PubMed] [Google Scholar]
- Jeon KI, Kulkarni A, Woeller CF, Phipps RP, Sime PJ, Hindman HB, and Huxlin KR. 2014. ‘Inhibitory effects of PPARgamma ligands on TGF-beta1-induced corneal myofibroblast transformation’, Am J Pathol, 184: 1429–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon KI, Phipps RP, Sime PJ, Huxlin KR, 2015. Inhibitory effects of PPARγ ligands on TGF-β1-induced CTGF expression in cat corneal fibroblasts. Exp Eye Res 138, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J, 1999. The cellular basis of corneal transparency: evidence for “corneal crystallins”. J. Cell Sci 112, 613–622. [DOI] [PubMed] [Google Scholar]
- Jester JV, 2008. Corneal cyrstallins and the development of cellular transparency. Semin Cell Dev Biol, 19(2): p. 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B, 1998. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391, 82–86. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Thakran S, Bheemreddy R, Ye EA, He H, Walker RJ, Steinle JJ, 2014. Pioglitazone normalizes insulin signaling in the diabetic rat retina through reduction in tumor necrosis factor α and suppressor of cytokine signaling 3. J Biol Chem 289, 26395–26405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, Poulaki V, Semkova I, Kociok N, 2009. TNF-α mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis 15, 1418–1428. [PMC free article] [PubMed] [Google Scholar]
- Ju Z, Su M, Li D, Hong J, Im DS, Kim S, Kim EL, Jung JH, 2019. An Algal Metabolite-Based PPAR-γ Agonist Displayed Anti-Inflammatory Effect via Inhibition of the NF-κB Pathway. Mar Drugs 17, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katome T, Namekata K, Mitamura Y, Semba K, Egawa M, Naito T, Harada C, Harada T, 2015. Expression of intraocular peroxisome proliferator-activated receptor gamma in patients with proliferative diabetic retinopathy. J Diabetes Complicat. 29, 275–281. [DOI] [PubMed] [Google Scholar]
- Keech A, Mitchell P, Summanen PA, O’Day J, Davis TME, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamsom E, Merrifield A, Laatikainen LT, D’Emden MC, Crimet DC, O’Connell RLO, Colman PG, 2007. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370, 1687–1697. [DOI] [PubMed] [Google Scholar]
- Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TME, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, D’Emden MC, Crimet DC, O’Connell RLO, Colman PG, 2005. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366, 1849–1861. [DOI] [PubMed] [Google Scholar]
- Kern TS, 2017. Do photoreceptor cells cause the development of retinal vascular disease? Vision Res. 139, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Mandard S, Escher P, Gonzalez FJ, Tafuri S, Desvergne B, Wahli W, 2001. The peroxisome proliferator-activated receptor alpha regulated amino acid metabolism. FASEB 15, 1971–1978. [DOI] [PubMed] [Google Scholar]
- Khatol P, Saraf S, Jain A, 2018. Peroxisome proliferated activated receptors (PPARs): opportunities and challenges for ocular therapy. Crit Rev Ther Drug Carr. Syst 35, 65–97. [DOI] [PubMed] [Google Scholar]
- Kiel JW, Hollingsworth M, Rao R, Chen M, Reitsamer HA, 2011. Ciliary blood flow and aqueous humor production. Prog. Retin. Eye Res 30, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Ueki S, Mahemuti G, Chiba T, Oyamada H, Saito N, Kanda A, Kayaba H, Chihara J, 2005. Physiological levels of 15-deoxy-Delta12,14-prostaglandin J2 prime eotaxin-induced chemotaxis on human eosinophils through peroxisome proliferator-activated receptor-gamma ligation. J Immunol 175, 5744–5750. [DOI] [PubMed] [Google Scholar]
- Koh JH, Hancock CR, Terada S, Higashida K, Holloszy JO, Han DH, 2017. PPARβ Is Essential for Maintaining Normal Levels of PGC-1α and Mitochondria and for the Increase in Muscle Mitochondria Induced by Exercise. Cell Metab 25, 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinowski J, Grochot-Przeczek A, Taha H, Kozakowska M, Pilecki B, Skrypek K, Bartelik A, Derlacz R, Horrevoets AJG, Pap A, Nagy L, Dulak J, Jozkowicz A, 2014. PPARγ activation but not PPARγ haplodeficiency affects proangiogenic potential of endothelial cells and bone marrow-derived progenitors. Cardiovasc. Diabetol 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinowski J, Jozkowicz A, 2016. PPAR Gamma and Angiogenesis: Endothelial Cells Perspective. J Diab Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku YH, Cho BJ, Kim MJ, Lim S, Park YJ, Jang HC, Choi SH, 2017. Rosiglitazone increases endothelial cell migration and vascular permeability through Akt phosphorylation. BCM Pharmacol Toxicol 18, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusisto J, Andrulionyte L, Laakso M, 2007. Atherosclerosis and cardiovascular risk reduction with PPAR agonists. Curr Atheroscler Rep 9, 274–280. [DOI] [PubMed] [Google Scholar]
- Kvanta A, Algvere PV, Berglin L, Seregard S, 1996. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 37, 1929–1934. [PubMed] [Google Scholar]
- Lamb TD, 2016. Why rods and cones? Eye 30, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A, Tymianski M, 2010. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460, 525–542. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Kaplan N, Wang J, Peng H Corneal epithelial biology: Lessons stemming from old to new. Exp Eye Res, 2020. 198: p. 108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Garf S, Murdaca J, Mothe-Satney I, Sibille B, Le Menn G, Chinetti G, Neels JG, Rousseau AS, 2019. Complementary Immunometabolic Effects of Exercise and PPARβ/δ Agonist in the Context of Diet-Induced Weight Loss in Obese Female Mice. Int J Mol Sci 20, 5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff MM, Bishop PN, 2008. Adult vitreous structure and postnatal changes. Eye 22, 1214–1222. [DOI] [PubMed] [Google Scholar]
- Lee DL, Wilson JL, Duan R, Hudson T, El-Marakby A, 2011. Peroxisome Proliferator-Activated Receptor-α Activation Decreases Mean Arterial Pressure, Plasma Interleukin-6, and COX-2 While Increasing Renal CYP4A Expression in an Acute Model of DOCA-Salt Hypertension. PPAR Res. 2011, 502631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ, 1995. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15, 3012–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart JC, Staels B, 2006. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J Clin Invest 116, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberger D, Desvergne D, Whali W, 1996. Peroxisome proliferator-activated receptors: A nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol 12, 335–363. [DOI] [PubMed] [Google Scholar]
- Li Z, He T, Du K, Xing Y-Q, Run Y-M, Yan Y, Shen Y, 2014. Inhibition of Oxygen-Induced Ischemic Retinal Neovascularization with Adenoviral 15-Lipoxygenase-1 Gene Transfer via Up-Regulation of PPAR-γ and Down-Regulation of VEGFR-2 Expression. PLoS One 9, e85824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Colby JK, Zuo X, Jaoude J, Wei D, Shureiqi I, 2018. The Role of PPAR-δ in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int J Mol Sci 19, 3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR, 1996. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 37, 855–868. [PubMed] [Google Scholar]
- Los LI, van der Worp RJ, van Luyn MJ, Hooymans JM, 2003. Age-Related Liquefaction of the Human Vitreous Body: LM and TEM Evaluation of the Role of Proteoglycans and Collagen. Invest Ophthalmol Vis Sci 44, 2828–2833. [DOI] [PubMed] [Google Scholar]
- Luo T, Sakai Y, Wagner E, Dräger UC, 2006. Retinoids, eye development, and maturation of visual function. J. Neurobiol 66, 677–686. [DOI] [PubMed] [Google Scholar]
- Machiele R, Lopez MJ, Czyz CN, 2020. Anatomy, Head and Neck, Eye Lacrimal Gland, in: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing. [PubMed] [Google Scholar]
- Maena SA, Constantin A, Manda G, Sasson S, Manea A, 2015. Regulation of Nox enzymes expression in vascular pathophysiology: Focusing on transcription factors and epigenetic mechanisms. Redox Biol 5, 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man MQ, Barish GD, Schmuth M, Crumrine D, Barak Y, Chang S, Jiang Y, Evans RM, Elias PM, Feingold KR, 2008. Deficiency of PPARbeta/delta in the epidermis results in defective cutaneous permeability barrier homeostasis and increased inflammation. J Invest Dermatol 128, 370–377. [DOI] [PubMed] [Google Scholar]
- Mandard S, Müller M, Kersten S, 2004. Peroxisome proliferator-activated receptor alpha target genes. 2Cell Mol Life Sci 61, 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Cox J, Deek S, Dvorska L, 2010. Anatomy of the human corneal innervation. Exp Eye Res 90, 478–492. [DOI] [PubMed] [Google Scholar]
- Margeli A, Kouraklis G, Theocharis S, 2003. Peroxisome proliferator activated receptor-gamma (PPAR-gamma) ligands and angiogenesis. Angiogenesis 6, 165–169. [DOI] [PubMed] [Google Scholar]
- Martín-Martín N, Zabala-Letona A, Fernández-Ruiz S, Arreal L, Camacho L, Castillo-Martin M, Cortazar AR, Torrano V, Astobiza I, Zúñiga-García P, Ugalde-Olano A, Loizaga-Iriarte A, Unda M, Valcárcel-Jiménez L, Arruabarrena-Aristorena A, Piva M, Sánchez-Mosquera P, Aransay AM, Gomez-Muñoz A, Barrio R, Sutherland JD, Carracedo A, 2018. PPARδ Elicits Ligand-Independent Repression of Trefoil Factor Family to Limit Prostate Cancer Growth. Cancer Res 78, 399–409. [DOI] [PubMed] [Google Scholar]
- Marx N, Schonbeck U, Lazar MA, Libby P, Plutzky J, 1998. Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res 83, 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland R, 2001. The fundamental plan of the retina. Nat Neurosci 4, 877–886. [DOI] [PubMed] [Google Scholar]
- Matlock HG, Qiu F, Malechka V, Zhou K, Cheng R, Benyajati S, Whelchel A, Karamichos D, Ma J, 2020. Pathogenic role of PPARa down-regulated in corneal nerve degeneration and impaired corneal sensitivity in diabetes. Diabetes 69, 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA, 2001. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: A Humoral Mechanism to Reset a Peripheral Clock. Cell 105, 877–889. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA, 2007. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol 92, 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik L, Desvergne B, Tan NS, Basu-Modak S, Escher P, Rieusset J, Peters JM, Kaya G, Gonzalez FJ, Zakany J, Metzger D, Chambon P, Duboule D, Wahli W, 2001. Impaired skin wound healing in peroxisome proliferator–activated receptor (PPAR)α and PPARβ mutant mice. J. Cell Biol 154, 799–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P, 2004. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab 89, 2728–2735. [DOI] [PubMed] [Google Scholar]
- Montagner A, Polizzi A, Fouché E, Ducheix S, Lippi Y, Lasserre F, Barquissau V, Régnier M, Lukowicz C, Benhamed F, Iroz A, Bertrand-Michel J, Al Saati T, Cano P, Mselli-Lakhal L, Mithieux G, Rajas F, Lagarrigue S, Pineau T, Loiseau N, Postic C, Langin D, Wahli W, Guillou H, 2016. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 65, 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KJ, Rosen ED, Fitzgerald ML, Randow F, Andersson LP, Altshuler D, Milstone D. s., Mortensen RM, Spiegelman BM, Freeman MW, 2001. The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nat. Med 7, 41–47. [DOI] [PubMed] [Google Scholar]
- Moran E, Ding L, Wang Z, Cheng R, Chen Q, Moore R, Takahashi Y, Ma JX, 2014. Protective and Antioxidant Effects of PPARα in the Ischemic Retina. Invest Ophthalmol Vis Sci 55, 4568–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EP, Ma J, 2015. Therapeutic effects of PPAR alpha on neuronal death and microvascular impairment. PPAR Res. 595426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N, 1998. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem 273, 16710–16714. [DOI] [PubMed] [Google Scholar]
- Mueller E, Drori S, Aiyer A, Yie J, Sarraf P, Chen H, Hauser S, Rosen ED, Ge K, Roeder RG, Spiegelman BM, 2002. Genetic analysis of adipogenesis through peroxisome proliferator-activated receptor gamma isoforms. J Biol Chem 277, 41925–41930. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Jow L, Croston GE, Paterniti JR Jr, 1997. Identification, characterization, and tissue distribution of human peroxisome proliferator-activated receptor (PPAR) isoforms PPARgamma2 versus PPARgamma1 and activation with retinoid X receptor agonists and antagonists. J Biol Chem 272, 8071–8076. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Jow L, Noonan D, McDonell DP, 1994. Human and rat peroxisome proliferator activated receptors (PPARs) demonstrate similar tissue distribution but different responsiveness to PPAR activators. Comp. Study 51, 157–166. [DOI] [PubMed] [Google Scholar]
- Muranaka K, Yanagi Y, Tamaki Y, Usui T, Kubota N, Iriyama A, Terauchi Y, Kadowaki T, Araie M, 2006. Effects of Peroxisome Proliferator-Activated Receptor γ and Its Ligand on Blood–Retinal Barrier in a Streptozotocin-Induced Diabetic Model. Invest Ophthalmol Vis Sci 47, 4547–4552. [DOI] [PubMed] [Google Scholar]
- Murata T, He S, Hangai M, Ishibashi T, Xi XP, Kim S, Hsueh WA, Ryan SJ, Law RE, Hinton DR, 2000. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci 41, 2309–2317. [PubMed] [Google Scholar]
- Murphy C, Alvarado J, Juster R, 1984. Prenatal and postnatal growth of the human Descemet’s membrane. Invest Opthalmol Vis Sci, 25(12): p. 1402–15. [PubMed] [Google Scholar]
- Nawy S, Jahr CE, 1991. cGMP-gated conductance in retinal bipolar cells is suppressed by the photoreceptor transmitter. Neuron 7, 677–683. [DOI] [PubMed] [Google Scholar]
- Nawy S, Jahr CE, 1990. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature 346, 267–271. [DOI] [PubMed] [Google Scholar]
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R, 2004. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 27, 256–263. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wallman J, 2010. The multifunctional choroid. Prog. Retin. Eye Res 29, 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F, Carpenter S, Swenson R, 2008. The eye, in: Basic Human Anatomy. Dartmouth Medical School [Google Scholar]
- Ofri R, 2007. Neuro-Ophthalmology, in: Slatter’s Fundamentals of Veterinary Ophthalmology. Saunders. [Google Scholar]
- Oishi K, Shirai H, Ishida N, 2008. PPARα is involved in photoentrainment of the circadian clock. Neuroreport 19, 487–489. [DOI] [PubMed] [Google Scholar]
- Okayasu T, Tomizawa A, Suzuki K, Manaka K, Hattori Y, 2008. PPAR alpha activators up-regulate eNOS activity and inhibit cytokine-induced NF-kappaB activation through AMP-activated protein kinase activation. Life Sci 82, 884–891. [DOI] [PubMed] [Google Scholar]
- Oliveira-Soto L, Efron N, 2001. Morphology of corneal nerves using confocal microscopy. Cornea 20, 374–384. [DOI] [PubMed] [Google Scholar]
- Omae T, Nagaoka T, Tanano I, Yoshida A, 2011. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, induces dilation of isolated porcine retinal arterioles: role of nitric oxide and potassium channels. Invest Ophthalmol Vis SciOphthalmol 52, 6749–6756. [DOI] [PubMed] [Google Scholar]
- Otori Y, Wei JY, Barnstable CJ 1998. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 39(6):972–81. [PubMed] [Google Scholar]
- Palomer X, Barroso E, Pizarro-Delgado J, Peña L, Botteri G, Zarei M, Aguilar D, Montori-Grau M, Vázquez-Carrera M, 2018. PPARβ/δ: A Key Therapeutic Target in Metabolic Disorders. Int J Mol Sci 19, 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Chen J, Xu J, Chen M, Ma R, 2009. Antifibrotic effect by activation of peroxisome proliferator-activated receptor–γ in corneal fibroblasts. Mol Vis 15, 2279–2286. [PMC free article] [PubMed] [Google Scholar]
- Park BC, Thapa D, Lee JS, Park SY, Kim JA, 2009. Troglitazone inhibits vascular endothelial growth factor-induced angiogenic signaling via suppression of reactive oxygen species production and extracellular signal-regulated kinase phosphorylation in endothelial cells. J Pharmacol Sci 111, 1–12. [DOI] [PubMed] [Google Scholar]
- Pearsall E, R C, K Z, Takahashi Y, Matlock HG, Vadvalkar SS, Y S, Fredrick TW, Gantner ML, Meng S, Z F, Y G, M K, Humphries KM, Szweda L, Smith LEH, Ma JX, 2017. PPARα is essential for retinal lipid metabolism and neuronal survival. BMC Biol. 15, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall E, R C, Matsuzaki S, Zhou K, L D, B A, M K, Humphries KM, Quiambao a, Farjo R, Ma JX, 2019. Neuroprotective effects of PPARα in retinopathy of type 1 diabetes. PLoS One 14, e0208399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ, 2000. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol 20, 5119–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polvani S, Tarocchi M, Galli A, 2012. PPAR and oxidative stress: con(β) catenating NRF2 and FOXO. PPAR Res. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, 2001. The Retina, in: Neuroscience. Sinauer Associates, Sunderland (MA). [Google Scholar]
- Qi L, Jacob A, Wang P, Wu R, 2010. Peroxisome proliferator activated receptor-gamma and traumatic brain injury. Int J Clin Exp Med 3, 283–292. [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, 2009. Motor Autonomic Transmission, in: Squire, L.R.B.T.-E. of N. (Ed.),. Academic Press, Oxford, pp. 995–1000. 10.1016/B978-008045046-9.00700-2 [DOI] [Google Scholar]
- Rohowetz LJ, Kraus JG, Koulen P, 2018. Reactive Oxygen Species-Mediated Damage of Retinal Neurons: Drug Development Targets for Therapies of Chronic Neurodegeneration of the Retina. Int J Mol Sci 19, 3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M, Jiménez R, Toral M, León-Gómez E, Gómez-Gúzman M, Sánchez M, Zarzuelo MJ, Rodríguez-Gómez I, Rath G, Tamargo J, Pérez-Vizcaíno F, Dessy C, Duarte J, 2016. Vascular and Central Activation of Peroxisome Proliferator–Activated Receptor-β Attenuates Angiotensin II–Induced Hypertension: Role of RGS-5. J Pharmacol Exp Ther 358, 151–163. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM, 2001. PPAR gamma: a nuclear regulator of metabolism, differentiation and cell growth. J Biol Chem 276, 37731–37734. [DOI] [PubMed] [Google Scholar]
- Roy A, Pahan K, 2015. PPAR alpha signaling in the hippocampus: crosstalk between fat and memory. J Neuroimmune Pharmacol 10, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S Z, H G, N H, 2015. Role of Peroxisome Proliferator-Activated Receptor γ in Ocular Diseases. J. Ophtalmol 2015, 275435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez-Orellana F, Octave J, Pierrot N, 2020. Alzheimer’s disease, a lipid story: Involvement of peroxisome proliferator-activated receptor. Cells 9, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Olefsky JM, 1996. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 45, 1661–1669. [DOI] [PubMed] [Google Scholar]
- Sarayba MA, Li L, Tungsiripat T, Norman HL, Sweet PM, Patel AJ, Osann KE, Chittiboyina A, Benson SC, Pershadsingh A, Chuck RS, 2005. Inhibition of corneal neovascularization by a peroxisome proliferator-activated receptor-γ ligand. Exp Eye Res 80, 435–442. [DOI] [PubMed] [Google Scholar]
- Satarasinghe RL, Ramesh R, Riyaaz AAA, Gunarathne PAKG, de Silva AP, 2007. Hypothyroidism is a predisposing factor for fenofibrate-induced rhabdomyolysis--patient report and literature review. Drug Metab. Drug Interact 22, 279–283. [DOI] [PubMed] [Google Scholar]
- Savage SR, McCollum GW, Yang R, Penn JS, 2015. RNA-seq identifies a role for the PPAR inverse agonist GSK0660 in the regulation of TNF alpha-induced cytokine signaling in retinal endothelial cells. Mol Vis 21, 568–576. [PMC free article] [PubMed] [Google Scholar]
- Saw M, Wong V, Ho I, Liew G, 2019. New anti-hyperglycemic agents for type 2 diabetes and their effects on diabetic retinopathy. Eye 33, 1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J, 1996. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 15, 5336–5348. [PMC free article] [PubMed] [Google Scholar]
- Semo M, Gias C, Ahmado A, Vugler A, 2014. A role for the ciliary marginal zone in the melanopsin-dependentintrinsic pupillary light reflex. Exp Eye Res 119, 8–18. [DOI] [PubMed] [Google Scholar]
- Shao Y, Chen J, Dong L, He X, Cheng R, Zhou K, Liu J, Qiu F, Li X, Ma J, 2019. A protective effect of PPARα in endothelial progenitor cells through regulating metabolism. Diabetes 68, 2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N, Elholm M, Noy N, 2003. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor β/δ. J. Biol. Chem 278, 41589–41592. [DOI] [PubMed] [Google Scholar]
- Shen LQ, Child A, Weber GM, Folkman J, Aiello LP, 2008. Rosiglitazone and delayed onset of proliferative diabetic retinopathy. arch ophthalmol 126, 793–799. [DOI] [PubMed] [Google Scholar]
- Shirai H, Oishi K, Kudo T, Shibata S, Ishida N, 2007. PPARα is a potential therapeutic target of drugs to treat circadian rhythm sleep disorders. Biochem. Biophys. Res. Commun 357, 679–682. [DOI] [PubMed] [Google Scholar]
- Sorrentino SA, Bahlmann FH, Besler C, Müller M, Schulz S, Kirchhoff N, Doerries C, Horváth T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U, 2007. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation 116, 163–173. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE, Lang GK, 1999. Histologic and morphometric analysis of the choroid, bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv. Ophthalmol 44, 10–32. [DOI] [PubMed] [Google Scholar]
- Sridhar MS, 2018. Anatomy of cornea and ocular surface. Indian J. Ophthalmol 66, 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC, 2004. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res 78, 409–416. [DOI] [PubMed] [Google Scholar]
- Suarez S, McCollum GW, Bretz CA, Yang R, Capozzi ME, Penn JS, 2014. Modulation of VEGF-Induced Retinal Vascular Permeability by Peroxisome Proliferator-Activated Receptor-β/δ. Retin. Cell Biol 55, 8232–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J, 2017. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18, 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan NS, Michalik L, Desvergne B, Wahli W, 2005. Multiple expression control mechanisms of peroxisome proliferator-activated receptors and their target genes. J Steroid Biochem Mol Biol 93, 99–105. [DOI] [PubMed] [Google Scholar]
- Tan NS, Michalik L, Noy N, Yasmin R, Pacot C, Heim M, Flühmann B, Desvergne B, Wahli W, 2001. Critical roles of PPARβ/δ in keratinocyte response to inflammation. Genes Dev 15, 3262–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Wang X, Wu J, Li SM, Zhang Z, Wu S, Su T, Lin Z, Chen X, Liao X, Bai T, Qiu Y, Reinach PS, Li W, Chen Y, Liu Z, 2018. Sleep deprivation induces dry eye through inhibition of PPARα expression in corneal epithelium. Invest Ophthalmol Vis Sci 59, 5494–5508. [DOI] [PubMed] [Google Scholar]
- Tang Z, Zhang S, Lee C, Kumar A, Arjunan P, Li Y, Zhang F, Li X, 2011. An optic nerve crush injury murine model to study retinal ganglion cell survival. J. Vis. Exp 50, 2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik A, Sanders T, Kahook K, Akeel S, Elmarakby A, Al-Shabrawey M 2009. Suppression of retinal peroxisome proliferator-activated receptor gamma in experimental diabetes and oxygen-induced retinopathy: role of NADPH oxidase. Invest Ophthalmol Vis Sci. Feb;50(2):878–84. doi: 10.1167/iovs.08-2005. Epub 2008 Sep 20. [DOI] [PubMed] [Google Scholar]
- Teboul M, Gréchez-Cassiau A, Guillaumond F, Delaunay F, 2009. How nuclear receptors tell time. J Appl Physiol 107, 1965–1971. [DOI] [PubMed] [Google Scholar]
- Thakran S, Zhang Q, Morales-Tirado V, Steinle JJ, 2015. Pioglitazone restores IGFBP-3 levels through DNA PK in retinal endothelial cells cultured in hyperglycemic conditions. Invest Ophthalmol Vis Sci 56, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita Y, Arima T, Nakano Y, Uchiyama M, Shimizu A, Takahashi H, 2020. Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization. Int J Mol Sci 21, 5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F, Engel S, 2001. Interocular rivalry revealed in the human cortical blind-spot representation. Nature. Nature 411, 195–199. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM, 1994. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8, 1224–1234. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB, 2005. Spontaneous patterened retinal activity and the refinement of retinal projections. Prog Neurobiol, 76(4): p. 213–35. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Shimizu A, Masuda Y, Nagasaka S, Fukuda Y, Takashashi H, 2013. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol. Vis 19, 2135–2150. [PMC free article] [PubMed] [Google Scholar]
- Usui T, Sugisaki K, Iriyama A, Yokoo S, Yamagami S, Nagai N, Ishida S, Amano S, 2008. Inhibition of corneal neovascularization by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci 49, 4370–4376. [DOI] [PubMed] [Google Scholar]
- Varga T, Czimmerer Z, Nagy L, 2011. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochem. Biophys. Res. Commun 1812, 1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattulainen-Collanus S, Akinrinade O, Li M, Koskenvuo M, Li CG, Rao SP, Perez V, Yuan K, Sawada H, Koskenvuo JW, Alvira C, Rabinovitch M, Alastalo TP, 2016. Loss of PPARγ in endothelial cells leads to impaired angiogenesis. J Cell Sci 129, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N, Wagner KD, 2020. PPAR Beta/Delta and the Hallmarks of Cancer. Cells 9, 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W, Braissant O, Desvergne B, 1995. Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more… Chem. Biol 2, 261–266. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang Y, 2018. Fenofibrate monotherapy-induced rhabdomyolysis in a patient with hypothyroidism: a rare case report and literature review. Med. 97, e0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez F, Litwin SE, Yang T, 2008. Vascular PPARγ controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab 8, 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tang L, Zhang Z, Li W, Chen Y, 2020. Kertocytes promote corneal neovascularization through VEGFr3 induced by PPARα inhibition. Exp Eye Res 193, 107982. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang G, Shi Y, Sun L, Gorczynski R, Li Y-J, Xu Z, Spaner DE, 2016. PPAR-delta promotes survival of breast cancer cells in harsh metabolic conditions. Oncogenesis. Oncogenesis 5, e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM, 2003. Peroxisome-Proliferator-Activated Receptor δ Activates Fat Metabolism to Prevent Obesity. Cell 113, 159–170. [DOI] [PubMed] [Google Scholar]
- Wang Z, Moran E, Ding L, Cheng R, Xu X, Ma J, 2014. PPAR? regulates mobilization and homing of endothelial progenitor cells through the HIF-1?/SDF-1 pathway. Invest Ophthalmol Vis Sci 55, 3820–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA, 2003. Retinal oxygen: fundamental and clinical aspects. arch ophthalmol 121, 547–557. [DOI] [PubMed] [Google Scholar]
- Williams DL, 2008. Oxidative stress and the eye. Vet Clin North Am Small Anim Pr. 38, 179–192. [DOI] [PubMed] [Google Scholar]
- Willoughby CE, Ponzin D, Ferrari S, Lobo A, Landau K, Omidi Y, 2010. Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function – a review. Clin. Exp. Ophtalmol 38, 2–11. [Google Scholar]
- Winkler BS, Pourcho RG, Starnes C, Slocum J, Slocum N, 2003. Metabolic mapping in mammalian retina: a biochemical and 3H-2-deoxyglucose autoradiographic study. Exp Eye Res 77, 327–337. [DOI] [PubMed] [Google Scholar]
- Wolf G, Phil D, 2004. Tissue-specific Knockout Defines Peroxisome Proliferator-activated Receptor Gamma Function in Muscle and Liver. Nutr Rev 62, 253–255. [DOI] [PubMed] [Google Scholar]
- Wu C, Baiga TJ, Downes M, La Clair JJ, Atkins AR, Richard SB, Fan W, Stockley-Noel TA, Bowman ME, Noel JP, Evans RM, 2017. Structural basis for specific ligation of the peroxisome proliferator-activated receptor δ. PNAS 114, E2563–E2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Yang S, Kowalski J, Gerritsen ME, 1999. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J. Biol. Chem 274, 9116–9121. [DOI] [PubMed] [Google Scholar]
- Yang W, Rachez C, Freedman LP, 2000. Discrete roles for peroxisome proliferator-activated receptor gamma and retinoid X receptor in recruiting nuclear receptor coactivators. Mol Cell Biol 20, 8008–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM, 2006. Nuclear Receptor Expression Links the Circadian Clock to Metabolism. Cell 126, 801–810. [DOI] [PubMed] [Google Scholar]
- Yang AY, Chow J, Liu J, 2018. Corneal Innervation and Sensation: The Eye and Beyond. Yale J Biol Med, 91(1): p. 13–21. [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Ward KW 2010. WY-14 643, a selective PPAR{alpha} agonist, induces proinflammatory and proangiogenic responses in human ocular cells. Int J Toxicol. Sep-Oct;29(5):496–504. doi: 10.1177/1091581810376674. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang W, Bi M, Wu J, 2016. The molecular mechanisms of action of PPAR-γ agonists in the treatment of corneal alkali burns (Review). Int J Mol Med 38, 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li D, Cheng Q, 2020. Fenofibrate monotherapy-induced rhabdomyolysis in a patient with post-pancreatitis diabetes mellitus: A rare case report and a review of the literature. Med. 99, e20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhang J, Ji M, Gu H, Zu Y, Chen C, Hu N, 2013. The Role of Peroxisome Proliferator-Activated Receptor and Effects of Its Agonist, Pioglitazone, on a Rat Model of Optic Nerve Crush: PPARγ in Retinal Neuroprotection. PLoS One 8, e68935. [DOI] [PMC free article] [PubMed] [Google Scholar]


