Abstract
There is an urgent unmet medical need for novel HIV-1 inhibitors that are effective against a variety of NNRTI-resistance mutations. We report our research efforts aimed at discovering a novel chemotype of anti-HIV-1 agents with improved potency against a variety of NNRTI-resistance mutations in this paper. Structural modifications of the lead K-5a2 led to the identification of a potent inhibitor 16c. 16c yielded highly potent anti-HIV-1 activities and improved resistance profiles compared with the approved drug etravirine (ETR). The co-crystal structure revealed the key role of the water networks surrounding the NNIBP for binding and for resilience against resistance mutations, while suggesting further extension of 16c towards the NNRTI-adjacent site as lead development strategy. Furthermore, 16c demonstrated favorable pharmacokinetic and safety properties, suggesting the potential of 16c as a promising anti-HIV-1 drug candidate.
INTRODUCTION
Acquired immunodeficiency syndrome (AIDS) is caused by human immunodeficiency virus type 1 (HIV-1), becoming a pandemic health problem which globally affects nearly 38.0 million people and with 1.7 million newly infected with HIV in 2019.1 In the HIV-1 infection process, HIV-1 reverse transcriptase (RT) is responsible for the reverse transcription of single-stranded RNA into double-stranded DNA.2 With RT as target, eight nucleoside/nucleotide RT inhibitors (NRTIs/NtRTIs) and six non-nucleoside RT inhibitors (NNRTIs) have been approved by the U.S. Food and Drug Administration (FDA) so far.3,4 NRTIs are analogues of the natural substrate deoxynucleotide triphosphates and they inhibit RT as chain terminators, while NNRTIs do not compete for the natural substrate, they bind in the NNRTI binding pocket (NNIBP) about 10 Å away from the polymerase active site known and acted as allosteric inhibitors.5 Therein, NNRTIs are widely used in combination antiretroviral therapy (cART) with the advantage of their promising antiviral activity and higher selectivity. Currently FDA-approved NNRTIs in clinical use include the first-generation NNRTIs nevirapine (NVP), delavirdine (DLV), efavirenz (EFV), and the second generation NNRTIs etravirine (ETR), rilpivirine (RPV) and doravirine (DOR).3, 4 However, NNIBP residues are not involved in the polymerase binding site, and their mutations do not have significant effect on replication. Thus, the NNRTIs have a low genetic barrier and NNRTI resistance mutations appear relatively easy and fast.5 The first-generation NNRTIs quickly suffered from drug resistance, including single mutations (K103N and Y181C) and double mutation K103N+Y181C (RES056). Although the second generation exhibited these mutations sensitive to the first-generation NNRTIs, drug resistance and cross resistance still emerged with their clinical use. In addition, the adverse effects (such as hypersensitivity reactions) can also result in treatment failure of the second generation NNRTIs.6, 7 Therefore, there is a relevant need of novel NNRTIs with greater potency, improved drug-resistance profiles and safety.
Our previous efforts have prompted the discovery of novel piperidine-substituted thiophene[3,2-d]pyrimidine compounds 3 (K-5a2) and 4 (25a), exhibiting higher anti-HIV-1 potency and favorable resistance profiles compared with ETR and RPV.8, 9 The co-crystal structures revealed extensive hydrophobic interactions and a network of backbone hydrogen bonds formed between the NNRTIs and NNIBP, and explained why K-5a2 and 25a are resilient to NNRTI-resistance mutations in the NNIBP.10 Although K-5a2 and 25a developed extensive interactions with NNIBP and their central thiophene[3,2-d]pyrimidine core establishes nonpolar interactions with the alkyl chain of Glu138, the entrance channel gated by Glu138 in the p51 subunit and Lys101 in the p66 subunit is still an underexplored region in the NNIBP.
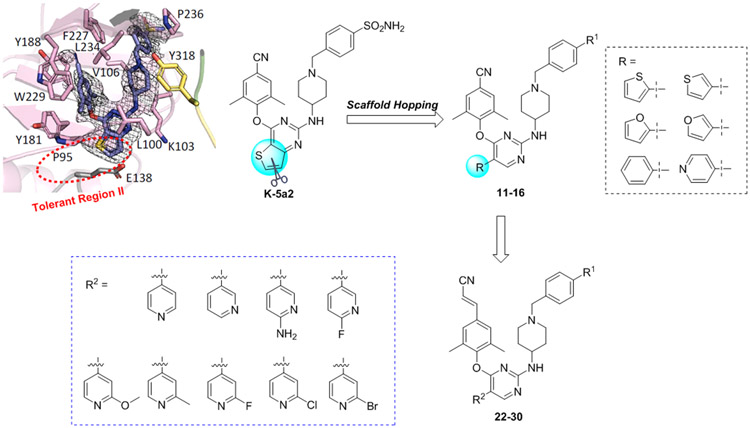
In this study, we have kept the privileged left wing and piperidine-substituted benzyl of K-5a2 unchanged,8, 11 and 18 novel 2,4,5-trisubstituted pyrimidine derivatives (11-16) with 2-thienyl, 3-thienyl, 2-furyl, 3-furyl, phenyl and 4-pyridyl linked to the C5 position of the central pyrimidines were designed utilizing a scaffold hopping strategy, to get a deeper insight of the structure-activity relationships (SARs) of the NNIBP Tolerant Region II (Figure 2).12 The preliminary results demonstrated that the disruption of the molecular planarity of the fused bicyclic thiophene[3,2-d]pyrimidine was a valuable strategy for the subsequent optimization of our lead compounds and the introduction of a pyridine ring in the Tolerant Region II is the most beneficial modification for the activity. Moreover, multiple substituent groups were introduced into the pyridine ring in the second-round structural modification, and yielded 27 novel derivatives. In addition, the privileged cyanovinyl group was merged into the left wing of the compounds,9, 13-15 to develop stronger π-π interactions with conserved amino acids F227 and W229 (Figure 2).
Figure 2.
Rational design of novel NNRTIs bearing the 2,4,5-trisubstituted pyrimidine scaffold utilizing scaffold hopping strategy (truncation of the fused ring).
CHEMISTRY
As depicted in Schemes 1 and 2, two short synthetic protocols that would allow the rapid optimization of the two variable points (R1 and Ar) were used to produce the newly designed compounds. At first, the commercially available material 2,4-dichloropyrimidine (5) was reacted with 4-hydroxy-3,5-dimethylbenzonitrile at room temperature, affording intermediate 6, which generated compound 7 with tert-butyl 4-aminopiperidine-1-carboxylate at 120°C via nucleophilic reaction. Compound 7 was treated with NIS and HOAc to form compound 8; subsequent treatment of which in the presence of trifluoroacetic acid afforded product 9. Then treatment of 9 with substituted benzyl chloride (bromine) at room temperature yielded the key intermediates 10a-c, which were finally coupled with boric acid substituents in the presence of potassium carbonate and Pd(PPh3)4 at 100°C via Suzuki reaction, to afford the corresponding target compounds 11-16. In an analogous way, target compounds 22-30 were prepared, only with the difference that the starting material 5 was treated with (E)-3-(4-hydroxy-3,5-dimethylphenyl)acrylonitrile.
Scheme 1.
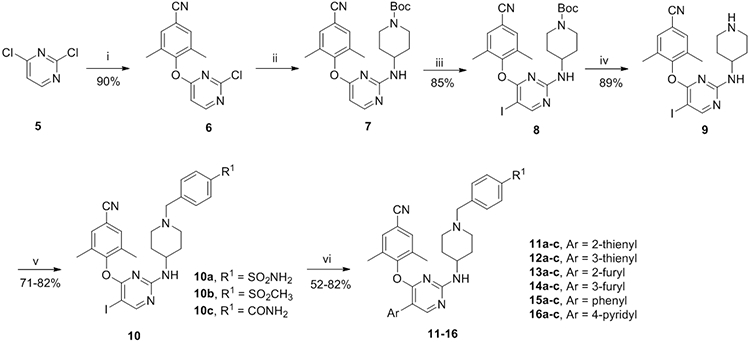
Synthesis of 11-13a
a Reagents and conditions: (i) K2CO3, DMF, 4-hydroxy-3,5-dimethylbenzonitrile, r.t.; (ii) K2CO3, DMF, tert-butyl 4-aminopiperidine-1-carboxylate, 120°C; (iii) NIS, HOAc, CH3CN, r.t.; (iv) TFA, DCM, r.t.; (v) K2CO3, DMF, r.t.; (vi) Pd(PPh3)4, K2CO3, DMF, H2O, 100°C.
Scheme 2.
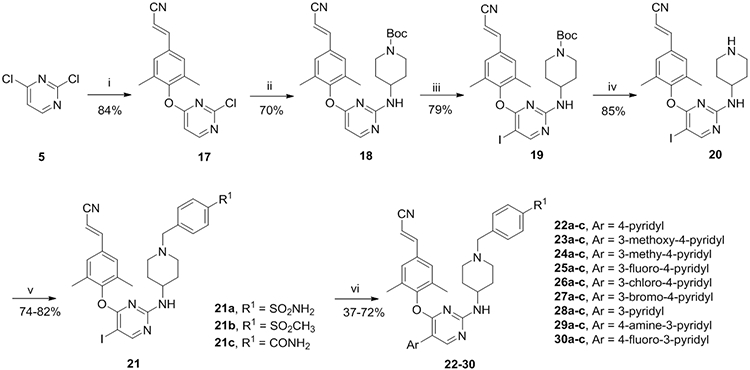
Synthesis of 22-30 a
a Reagents and conditions: (i) (E)-3-(4-hydroxy-3,5-dimethylphenyl)acrylonitrile, K2CO3, DMF, r.t.; (ii) K2CO3, DMF, tert-butyl 4-aminopiperidine-1-carboxylate, 120°C; (iii) NIS, HOAc, CH3CN, r.t.; (iv) TFA, DCM, r.t.; (v) K2CO3, DMF, r.t.; (vi) Pd(PPh3)4, K2CO3, DMF, H2O, 100°C.
BIOLOGY
HIV-1 RT Crystallization and Structure Determination
An engineered HIV-1 RT construct, RT52A, referred to as wild-type (WT) RT, was expressed and purified as described previously.16, 17 RT52A (20 mg mL−1) was incubated with 16c at a 1:1.5 protein:drug molar ratio at room temperature for 30 min. Co-crystals of RT with 16c were set up in hanging drops at 4°C with a 1:1 ratio of protein-ligand complex and reservoir (10%(v/v) PEG 8000, 4%(v/v) PEG 400, 100 mM MES pH 6.3, 10 mM spermine, 15 mM MgSO4, 100 mM ammonium sulfate, and 5 mM tris(2-carboxyethyl)phosphine) together with an experimentally optimized concentration of microseeds made by crushing previously obtained RT/rilpivirine crystals (pre-seeding). The crystals were cryo-protected by dipping them into reservoir with 25% ethylene glycol and plunge-frozen in liquid N2. X-ray data were collected from three of the plunge-frozen crystals at the APS 23-ID-B beamline. The crystallographic software packages HKL2000,18 Phenix,19 and COOT20 were used for data processing, structure refinement, and model building, respectively. 16c coordinates and restraints were generated with the Grade Web Server (http://grade.globalphasing.org). The structure was solved by molecular replacement using PDB ID 4G1Q as the template. The diffraction data and refinement statistics are summarized in Table S1.
RESULTS AND DISCUSSION
First Round of Structural Modification
All of the synthesized inhibitors possessing the 2,4,5-trisubstituted pyrimidine scaffold of the first round (11-16) were firstly screened for their biological activity in vitro against wild-type (WT) HIV-1 (IIIB), as well as against the NNRTI-resistant strain K103N+Y181C (RES056) using the MTT method according to previously reported procedures,21, 22 with the aim of getting the first insight into the SAR and to identify the most promising candidates for further optimization. The second-generation NNRTI drugs ETR and RPV were selected as controls. The values of EC50 (anti-HIV potency), CC50 (cytotoxicity), and SI (selectivity index, CC50/EC50 ratio) of the synthesized compounds were determined.
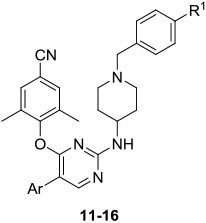
As shown in Table 1, 14a (EC50 = 2.80 nM) was demonstrated to be the most promising inhibitor against HIV-1 IIIB, being comparable to that of the approved drug ETR (EC50 = 2.80 nM). Moreover, all synthesized inhibitors exhibited high effective activity against the HIV-1 IIIB strain with EC50 values ranging from 2.51 to 5.93 nM. The substitution pattern of Ar and R1 did not significantly influence the compounds’ antiviral profile against HIV-1 IIIB. However, the Ar group located in the Tolerant Region II could result in significant differences in activity to mutant HIV-1 strain RES056. On the whole, most of the compounds experienced a dramatic activity drop to a micromolar level of inhibition against RES056. 15a-c (Ar = phenyl) exhibited the weakest potency, with EC50 values of 427-590 nM, being much inferior to that of ETR (EC50 = 37.6 nM) and RPV (EC50 = 10.7 nM). Changing the phenyl substituent of 15a-c to thienyl (11a-c and 12a-c) and furyl (13a-c and 14a-c) improved the compounds’ activity (EC50 = 115-415 nM). More specifically, 11a-c (EC50 = 103-134 nM) with 2-thienyl substituent exhibited more potent activity than those of 12a-c (EC50 = 209-415 nM) with 3-thienyl substituent, while 13a-c (EC50 = 218-254 nM) with 2-furyl substituent exhibited decreased activity compared to those of 14a-c (EC50 = 108-172 nM) with the 3-furyl substituent. Replacement of phenyl substituent of 15a-c with 4-pyridyl substituent also increased the compounds’ potency (16a-c, EC50 = 24.4-244 nM), especially, 16c (EC50 = 24.4 nM) yielded the greatest potency against mutant strain RES056, being superior to that of ETR (EC50 = 37.6 nM) and slightly inferior to RPV (EC50 = 10.7 nM). Notably, 16c displayed much lower cytotoxicity (CC50 = 36.0 μM) and higher SI values against HIV-1 IIIB and RES056 strain (SI = 9603 and 1474, respectively) compared to that of RPV (CC50 = 3.98 μM, SI = 3989 and 371, respectively). None of the tested compounds showed anti-HIV-2 ROD activity.
Table 1.
Anti-HIV activity, cytotoxicity and SI values of target compounds 11-16.
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Compds | Ar | R1 | EC50(nM)a |
CC50 (μM)b |
SIc |
|||
| IIIB | RES056 | ROD | IIIB | RES056 | ||||
| 11a |
|
SO2NH2 | 4.57±0.50 | 103±33.8 | ≥33410 | 53.8±41.9 | 11787 | 520 |
| 11b |
|
SO2CH3 | 4.01±0.73 | 134±29.8 | >8383 | 8.38±4.50 | 2091 | 62 |
| 11c |
|
CONH2 | 3.34±1.17 | 115±6.71 | >9263 | 9.26±3.75 | 2773 | 80 |
| 12a |
|
SO2NH2 | 5.09±1.50 | 209±40.7 | >6124 | 6.13±1.98 | 1205 | 29 |
| 12b |
|
SO2CH3 | 4.92±1.60 | 253±83.8 | >4252 | 4.25±1.45 | 863 | 17 |
| 12c |
|
CONH2 | 4.22±0.38 | 415±76.4 | >37332 | 37.3±4.31 | 8838 | 90 |
| 13a |
|
SO2NH2 | 4.40±1.26 | 218±100 | >11187 | 11.2±2.92 | 2539 | 51 |
| 13b |
|
SO2CH3 | 4.52±1.03 | 233±45.6 | >7459 | 7.45±3.27 | 1650 | 32 |
| 13c |
|
CONH2 | 5.78±1.45 | 254±66.4 | >7347 | 7.35±2.46 | 1271 | 29 |
| 14a |
|
SO2NH2 | 2.51±0.73 | 161±40.9 | >29105 | 29.1±8.99 | 11611 | 180 |
| 14b |
|
SO2CH3 | 4.75±0.94 | 172±9.51 | >55821 | 55.8±13.7 | 11748 | 323 |
| 14c |
|
CONH2 | 3.40±0.48 | 108±24.5 | >181990 | 181±7.10 | 53580 | 1685 |
| 15a |
|
SO2NH2 | 5.14±2.08 | 427±62.6 | >14067 | 14.1±4.72 | 2735 | 33 |
| 15b |
|
SO2CH3 | 4.93±1.75 | 590±103 | >12118 | 12.1±5.26 | 2456 | 21 |
| 15c |
|
CONH2 | 5.11±2.13 | 562±75.9 | >13367 | ≥13.37 | ≥2618 | ≥24 |
| 16a |
|
SO2NH2 | 2.59±0.46 | 111±35.9 | >5669 | 5.67±0.27 | 2189 | 51 |
| 16b |
|
SO2CH3 | 5.93±2.79 | 244±45.8 | >17197 | 17.2±4.09 | 2897 | 70 |
| 16c |
|
CONH2 | 3.75±0.40 | 24.4±3.06 | >35998 | 36.0±4.85 | 9603 | 1474 |
| ETR | - | - | 2.80±0.65 | 37.6±2.09 | >4594 | >4.59 | >1638 | >122 |
| RPV | 1.00±0.27 | 10.7±7.96 | - | 3.98 | 3989 | 371 | ||
EC50: concentration of compound required to achieve 50% protection of MT-4 cell cultures against HIV-1-induced cytopathicity, as determined by the MTT method.
CC50: concentration required to reduce the viability of mock-infected cell cultures by 50%, as determined by the MTT method.
SI: selectivity index, the ratio of CC50/EC50.
Moreover, all these compounds were further evaluated for their activity against single mutant strains L100I, K103N, Y181C, Y188L, and E138K, and double-mutant strain F227L + V106A. As displayed in Table 2, the most promising mutant HIV-1 strain RES056 inhibitor 16c also exhibited effective potency against all tested mutant strains, with EC50 values of 4.26 nM (L100I), 3.79 nM (K103N), 6.79 nM (Y181C), 6.79 nM (Y188L), 10.9 nM (E138K), and 10.4 nM (F227L+V106A), being equipotent to or superior to ETR and RPV in the same cellular assay. Specifically, in the case of Y188L and F227L+V106A mutant strains, 16c showed 11-fold and 7.8-fold activity enhancement over that of RPV (EC50 = 79.4 nM and 81.6 nM), respectively.
Table 2.
Activity against mutant HIV-1 strains of target compounds 11-16.
| Compds | EC50(nM)a |
|||||
|---|---|---|---|---|---|---|
| L100I | K103N | Y181C | Y188L | E138K | F227L+V106A | |
| 11a | 5.92±1.11 | 5.39±1.58 | 10.7±3.63 | 9.31±1.28 | 7.71±1.86 | 30.2±2.43 |
| 11b | 8.93±3.62 | 5.71±1.46 | 11.6±5.99 | 15.4±4.30 | 6.27±0.35 | 38.4±9.65 |
| 11c | 14.7±2.59 | 5.15±0.90 | 10.2±4.68 | 17.9±5.48 | 8.85±1.90 | 75.4±21.9 |
| 12a | 15.4±5.52 | 4.70±0.97 | 12.3±2.21 | 30.3±5.84 | 19.7±6.52 | 85.3±15.6 |
| 12b | 20.3±5.08 | 4.75±1.16 | 11.7±2.04 | 27.2±9.63 | 10.6±2.80 | 67.5±21.4 |
| 12c | 62.6±12.1 | 5.52±0.85 | 26.4±6.79 | 30.0±12.49 | 22.0±7.28 | 325±95.5 |
| 13a | 20.9±10.5 | 5.33±1.42 | 24.8±11.8 | 36.5±14.3 | 24.5±5.57 | 128±46.6 |
| 13b | 20.4±4.24 | 5.92±0.90 | 26.4±10.0 | 28.8±10.6 | 27.4±7.81 | 110±32.1 |
| 13c | 38.2±11.3 | 3.73±0.48 | 14.9±3.89 | 23.3±2.89 | 11.9±2.12 | 161±26.7 |
| 14a | 13.6±7.53 | 3.00±0.27 | 9.35±3.88 | 15.3±5.08 | 13.0±1.13 | 63.2±11.5 |
| 14b | 14.8±3.99 | 4.39±0.66 | 14.4±4.40 | 24.2±3.50 | 15.3±4.23 | 41.5±6.88 |
| 14c | 10.1±1.75 | 3.44±0.16 | 9.81±1.49 | 21.1±3.27 | 11.2±4.12 | 307±7.28 |
| 15a | 53.2±6.18 | 7.74±0.82 | 26.2±7.23 | 33.2±2.51 | 24.5±2.10 | 219±62.4 |
| 15b | 71.7±12.4 | 11.7±2.25 | 33.7±12.1 | 35.5±1.47 | 32.1±4.55 | 218±47.6 |
| 15c | 151±84.2 | 9.15±2.14 | 28.7±6.81 | 30.5±8.73 | 17.1±5.43 | 393±28.4 |
| 16a | 10.6±2.13 | 2.33±0.61 | 6.93±1.36 | 21.4±3.82 | 8.12±0.17 | 45.9±2.43 |
| 16b | 17.4±9.08 | 4.40±0.96 | 10.8±2.43 | 18.5±3.37 | 9.19±0.79 | 79.4±13.9 |
| 16c | 4.26±0.62 | 3.79±0.42 | 6.79±1.49 | 6.79±2.82 | 10.9±5.63 | 10.4±5.30 |
| ETR | 5.42±1.80 | 2.71±0.96 | 13.6±3.56 | 13.7±4.85 | 7.17±2.78 | 17.5±5.14 |
| RPV | 1.54±0.00 | 1.31±0.36 | 4.73±0.48 | 79.4±0.77 | 5.75±0.11 | 81.6±21.2 |
EC50: concentration of compound required to achieve 50% protection of MT-4 cell cultures against HIV-1-induced cytopathicity, as determined by the MTT method.
In addition, most compounds were potent inhibitors against K103N, Y181C, Y188L, and E138K strains, with EC50 values ranging from 2.33 nM to 35.5 nM. However, apart from 16c, all the compounds exhibited inferior potency (EC50 = 5.92-151 nM and 30.2-393 nM, respectively) compared to that of ETR (EC50 = 5.42 nM and 17.5 nM, respectively) against the L100I and F227L+V106A strains. The first round of SAR results indicated that, among all the employed substituents in the first round of this study, the 4-pyridyl group was the best choice for the NNIBP Tolerant Region II.
Second Round of Structural Modification
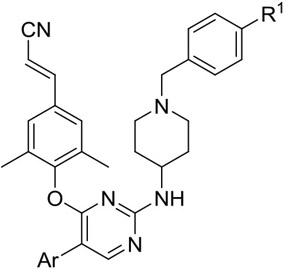
Based on the preliminarily established SARs, 27 novel 2,4,5-trisubstituted pyrimidine derivatives (22-30) were designed with the most promising inhibitor 16c as lead. These newly designed compounds retained the privileged piperidine-substituted benzyl as their right wing; the cyano group in the left wing was replaced with a cyanovinyl group, to develop stronger π-π interactions with the highly conserved residues F227 and W229;9 meanwhile, fluorine, chlorine, bromine, methyl, methoxy, and amino substituents were also introduced into the 4-pyridyl moiety located in Tolerant Region II, to explore the SARs of Tolerant Region II more comprehensively. All derivatives 22-30 from the second round were assayed in vitro for their activity against HIV-1 IIIB and RES056. The results of this evaluation are summarized in Table 3.
Table 3.
Anti-HIV activity, cytotoxicity and SI values of target compounds 22-30.
| |||||||
|---|---|---|---|---|---|---|---|
| Compds | R1 | R2 | EC50(nM)a |
CC50 (μM)b |
SIc |
||
| IIIB | RES056 | IIIB | RES056 | ||||
| 22a |
|
SO2NH2 | 4.60±1.14 | 22.9±7.07 | 9.25±5.17 | 2011 | 404 |
| 22b |
|
SO2CH3 | 5.74±1.38 | 26.3±6.99 | 7.20±5.41 | 1255 | 273 |
| 22c |
|
CONH2 | 4.19±0.45 | 23.3±7.67 | 10.2±6.69 | 2428 | 436 |
| 23a |
|
SO2NH2 | 5.75±1.27 | 34.3±13.2 | 5.50±1.98 | 957 | 160 |
| 23b |
|
SO2CH3 | 3.95±0.54 | 30.0±12.4 | 2.10±0.95 | 533 | 70 |
| 23c |
|
CONH2 | 7.28±1.27 | 40.3±10.7 | 6.27±1.21 | 861 | 156 |
| 24a |
|
SO2NH2 | 4.16±0.98 | 23.0±5.85 | 4.37±1.15 | 1105 | 190 |
| 24b |
|
SO2CH3 | 4.55±0.61 | 31.5±5.50 | 5.34±2.87 | 1174 | 169 |
| 24c |
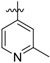
|
CONH2 | 3.53±0.38 | 18.1±0.30 | 6.51±2.82 | 1845 | 358 |
| 25a |
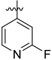
|
SO2NH2 | 6.37±1.27 | 38.8±12.6 | 8.46±2.08 | 1326 | 218 |
| 25b |
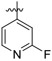
|
SO2CH3 | 4.75±1.19 | 25.1±7.23 | 5.03±1.72 | 1057 | 200 |
| 25c |
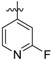
|
CONH2 | 8.26±1.79 | 53.4±30.9 | 15.5±5.32 | 1878 | 290 |
| 26a |
|
SO2NH2 | 4.30±0.54 | 20.9±4.14 | 3.89±1.01 | 904 | 185 |
| 26b |
|
SO2CH3 | 4.23±1.06 | 33.3±7.24 | 2.04±0.88 | 482 | 61 |
| 26c |
|
CONH2 | 4.14±0.43 | 81.7±61.7 | 19.1±1.66 | 4627 | 243 |
| 27a |
|
SO2NH2 | 4.42±0.52 | 26.9±12.1 | 6.60±2.69 | 1493 | 246 |
| 27b |
|
SO2CH3 | 4.52±0.55 | 33.2±9.31 | 3.21±0.61 | 711 | 97 |
| 27c |
|
CONH2 | 7.00±4.69 | 32.2±8.02 | 9.45±4.34 | 1351 | 293 |
| 28a |
|
SO2NH2 | 5.04±2.07 | 25.8±9.45 | 20.4±4.11 | 4063 | 793 |
| 28b |
|
SO2CH3 | 4.57±1.24 | 22.4±1.87 | 23.5±0.96 | 5151 | 1049 |
| 28c |
|
CONH2 | 4.78±2.57 | 23.1±3.80 | 25.3±1.84 | 5297 | 1092 |
| 29a |
|
SO2NH2 | 5.80±1.37 | 33.5±6.89 | 2.91±0.65 | 503 | 87 |
| 29b |
|
SO2CH3 | 5.29±2.14 | 26.2±4.80 | 2.36±0.93 | 447 | 90 |
| 29c |
|
CONH2 | 6.56±2.11 | 30.2±6.55 | 12.8±3.73 | 1956 | 424 |
| 30a |
|
SO2NH2 | 5.80±1.53 | 28.2±6.82 | 17.1±4.82 | 2956 | 607 |
| 30b |
|
SO2CH3 | 6.04±1.76 | 25.5±2.89 | 6.05±1.18 | 1003 | 237 |
| 30c |
|
CONH2 | 4.56±1.49 | 20.4±0.98 | 20.9±3.65 | 4586 | 1021 |
| ETR | - | - | 3.53±0.70 | 52.2±24.2 | >4.59 | >1300 | >88 |
| RPV | 1.0±0.27 | 10.7±7.96 | 3.98 | 3989 | 371 | ||
EC50: concentration of compound required to achieve 50% protection of MT-4 cell cultures against HIV-1-induced cytopathicity, as determined by the MTT method.
CC50: concentration required to reduce the viability of mock-infected cell cultures by 50%, as determined by the MTT method.
SI: selectivity index, the ratio of CC50/EC50.
The compounds generated in the second round contain the privileged 2,4,5-trisubstituted pyrimidine scaffold, and displayed nanomolar activity against HIV-1 IIIB with EC50 values of 3.95-8.26 nM, being comparable to that of ETR (EC50 = 3.53 nM). More encouragingly, their activity against the mutant HIV-1 strain RES056 was significantly increased, yielding EC50 values ranging from 20.4 nM to 40.3 nM, and being about 2-fold more potent than that of ETR (EC50 = 52.2 nM), with the exception of compounds 25c (EC50 = 53.4 nM) and 26c (EC50 = 81.7 nM). However, the introduction of a cyanovinyl group substituent resulted in these compounds having enhanced cytotoxicity (CC50 = 2.10-25.3 μM) compared to the lead 16c (CC50 = 36.0 μM). Although the cyanovinyl group was favorable for improving potency against mutant strains, it could result in potential covalent binding with nucleic acids or proteins as a “Michael acceptor” and cause an increase of cytotoxicity.21 When comparing the cytotoxicity of these target compounds, 28a-c tolerating the 3-pyridyl in the Tolerant Region II showed the lowest cytotoxicity, exhibiting CC50 values of 20.4-25.3 μM. However, harboring an amino group or fluorine atom on the 3-pyridyl substituent resulted in compounds 29a-c (CC50 = 2.39-12.8 μM) and 30a-c (CC50 = 6.05-20.9 μM), respectively, which resulted in increased cytotoxicity. Replacing the 3-pyridyl of 28a-c with 4-pyridyl resulted in compounds 22a-c (CC50 = 7.20-10.2 μM), which exhibited enhanced cytotoxicity. Also, the introduction of fluorine, chlorine, bromine, methyl, methoxy, or amino substituent on the 4-pyridyl moiety of 22a-c led to greater cytotoxicity.
Furthermore, eight compounds (22a, 22c, 28a-c, 29c, 30a, and 30c) that exhibited higher potency and lower cytotoxicity were further evaluated for their activity against HIV-1 viral isolates carrying a variety of NNRTI-resistance mutations. As depicted in Table 4, although most of the selected compounds demonstrated more potent activity against L100I, Y181C, E138K and F227L+V106A strains than did ETR, all of the compounds exhibited inferior potency (EC50 = 3.84-7.50 nM, and 22.7-54.2 nM, respectively) compared to ETR (EC50 = 3.77 and 1.31 nM, respectively) and RPV (EC50 = 18.8 and 5.75 nM, respectively) for the most common mutant strains K103N and Y188L. The anti-HIV-1 results of two rounds of structural modification led to the conclusion that compound 16c was the most potent among all the novel synthesized compounds, exhibiting higher potency against a panel of NNRTI-resistant strains than that of ETR and deserving further druggability evaluation.
Table 4.
Activity against mutant HIV-1 strains.
| Compds | EC50(nM)a |
|||||
|---|---|---|---|---|---|---|
| L100I | K103N | Y181C | Y188L | E138K | F227L+V106A | |
| 22a | 5.87±1.99 | 4.91±1.47 | 24.2±8.96 | 27.1±9.19 | 11.1±2.21 | 23.7±13.8 |
| 22c | 5.62±1.98 | 3.84±0.66 | 7.14±1.07 | 23.0±4.49 | 8.66±0.37 | 35.5±21.6 |
| 28a | 7.85±3.59 | 6.25±4.17 | 9.95±0.96 | 25.1±12.7 | 11.9±2.35 | 12.2±2.27 |
| 28b | 10.7±1.54 | 5.42±2.35 | 10.3±1.32 | 22.7±9.14 | 12.2±4.53 | 13.2±3.41 |
| 28c | 10.8±1.66 | 5.76±2.33 | 10.1±1.62 | 25.5±12.3 | 11.3±2.74 | 21.7±9.43 |
| 29c | 11.3±3.73 | 6.66±0.50 | 12.0±1.45 | 54.2±10.9 | 15.4±3.98 | 15.4±3.22 |
| 30a | 11.4±2.85 | 7.50±1.61 | 15.4±2.24 | 36.7±6.86 | 49.1±31.5 | 23.1±13.9 |
| 30c | 5.76±3.50 | 5.97±1.49 | 10.3±0.53 | 30.1±10.9 | 11.7±6.16 | 27.5±10.5 |
| ETR | 11.7±7.44 | 3.77±1.06 | 16.6±5.36 | 15.4±4.72 | 18.8±8.52 | 26.9±2.30 |
| RPV | 1.54±0.00 | 1.31±0.36 | 4.73±0.48 | 79.4±0.77 | 5.75±0.11 | 81.6±21.2 |
EC50: concentration of compound required to achieve 50% protection of MT-4 cell cultures against HIV-1-induced cytopathicity, as determined by the MTT method.
Inhibition of WT HIV-1 RT by the Representative Compounds.
Some representative compounds (14a, 16c, 22a, and 22c) were assayed in vitro for their ability to inhibit recombinant WT HIV-1 RT enzyme, and the results are summarized in Table 5. 14a, 16c, 22a, and 22c (IC50 = 0.092, 0.113, 0.143, and 0.102 μM, respectively) exhibited modest inhibitory activity against WT HIV-1 RT with IC50 values of 0.092, 0.113, 0.143, and 0.102 μM, being about 3.5-5.5-fold potent than that of NVP (IC50 = 0.510 μM). Although these novel inhibitors exhibited inferior activity compared to that of ETR (IC50 = 0.012 μM), the preliminary results validate their binding target is HIV-1 RT, and they are classical NNRTIs.
Table 5.
Inhibitory activity against WT HIV-1 RT
| Compds | IC50 (μM)a | Compds | IC50 (μM)a |
|---|---|---|---|
| 14a | 0.092±0.002 | 22a | 0.143±0.003 |
| 16c | 0.113±0.032 | 22c | 0.102±0.013 |
| NVP | 0.510±0.140 | ETR b | 0.011±0.000 |
IC50: inhibitory concentration required to inhibit biotin deoxyuridine triphosphate (biotin-dUTP) incorporation into WT HIV-1 RT by 50%.
Results from reference 11.
Crystal Structure of HIV-1 RT in Complex with 16c: Implications for Further Lead Development
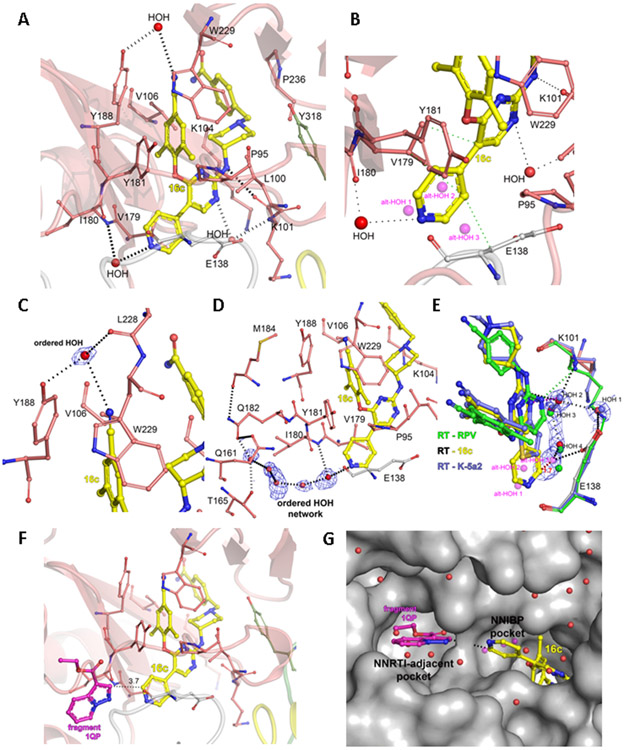
To observe the detailed interactions between 16c and the NNIBP (formed by three channels named tunnel, entrance and groove), the co-crystal structure of WT RT in complex with 16c was determined at 2.02 Å resolution (Table S1 and Figure S1). As expected, the structure waas almost identical to that of the related RT complexes with K-5a2 and 25a (Figure S1F). Figure 3A depicts the main interactions of 16c with RT. The left wing of 16c is snuggling into the tunnel via hydrophobic interactions with Y181 and Y188 at one side, and P95 and L234 at the other. Meanwhile, the right wing is anchored into the groove by a hydrogen bond with the main-chain carbonyl of K101 and through hydrophobic contacts with L100, Y318, and P236. The central pyrimidine moiety is located in the entrance, surrounded by E138 of p51, L100 (hydrophobic contacts), and K101 (hydrogen-bonded to the main-chain amino group through a water bridge).
Figure 3.
Crystal structure of HIV-1 RT in complex with 16c (PDB ID: 7KWU). (A) 16c binding in the NNIBP, with hydrogen bonding-interactions with RT and water molecules. (B) Detail of the binding of the 4-pyridyl substituent. (C-E) Water molecule and networks involved in binding of 16c. (F) Overlay of the RT-16c structure with RT-RPV-1QP fragment (bound to the NNRTI Adjacent site, PDB ID 4KFB), with the distance between nearer atoms of each. (G) Surface representation of (F), displaying the labeled pockets, with surrounding crystallographic water molecules from the RT-16c structure. Color legend for carbon atoms: (i) RT p66 subdomains: fingers in blue, palm in dark red, thumb in green, and connection in yellow; (ii) RT p51 subunit in white, and water molecules in red unless otherwise indicated.
The 4-pyridyl substituent, as envisioned, sits in the solvent-exposed area in the vicinity of the entrance channel, displacing three water molecules (modeled in the RT-16c structure as an alternate conformation) observed in the RT-RPV high resolution structure (Figures 3B, S1B and S1E).23 Moreover, it forms hydrophobic interactions with the side chains of V179 and Y181, and it is water-bridged to the main-chain carbonyl of I180 (Figure 3B). Notably, in all three RT structures with K-5a2, 25a, and RPV, their central ring forms a water-mediated hydrogen bonding network with the main-chain atoms of E138 and of I180.10, 23 Herein, the water molecule hydrogen-bonded to the central ring and E138 is displaced (alt-HOH 1 in Figure 3B), but the second water molecule hydrogen-bonded to I180 is conserved.
The RT-16c structure illustrates the importance of ordered water networks in NNRTI binding (Figures 3C-E). The left wing of 16c makes a hydrogen bond with an interfacial water that is in turn hydrogen-bonded to the side chain of Y188 and the main chain of L228 (Figure 3C). Next, as mentioned, the entrance channel displays another interfacial water, which is connected to a succession of water molecules that end up in a water molecule bridging Q161, T165, and Q182, the latter being hydrogen-bonded to the main chain of M184 (Figure 3D), a residue that is part of the catalytic motif YMDD.24 Both the left wing interfacial water and the ordered network near the entrance channel are observed in the RT-RPV high resolution structure (data not shown).23 Lastly, the right wing of 16c is also interacting with a water network bridging it to RT (Figure 3E). Central to it, HOH 2 connects the pyrimidine ring with the main chain of K101. At one end, the water network ends with HOH 1, hydrogen-bonded to p51’s E138 side chain. At the other end, the water network finishes with HOH 4, which would be in the right position to be bonded to alt-HOH 3, expelled by the binding. The last is hydrogen bonded to the other carboxylic oxygen of p51’s E138 side chain. This network is observed, albeit with some discontinuity, in the case of parent lead K-5a2. Interestingly, in the RT-RPV structure, there is a direct hydrogen bond between the main chain of K101 and the central ring, while the terminal amino group of the side chain of K101 overlaps with HOH 1 of RT-16c (present also in RT- K-5a2). Thus, in a different manner, all three complexes maintain a similar architecture in that part in between the entrance and the groove channels.
The RT-16c structure suggests that, not only the hydrogen bonds in the right wing and the several hydrophobic contacts all around the ligand, but also the water molecules (and networks) may anchor the compound in the NNIBP. While in the current structure the right-wing top amide group is not hydrogen-bonded (or with a polar interaction) with RT or has water molecules in the vicinity, both RPV (i.e., nitrile substituent of the right wing with the carbonyl group of H235) and K-5a2 (i.e., hydrogen bonds to K104 and V106) present one or the other. In the case of RT-RPV, molecular dynamics (MD) simulations suggest as well the existence of a water molecule hydrogen-bonded to the benzonitrile in the right wing, as this area is solvent exposed.10, 23
Regarding the SAR, the structure points to all the different compounds binding roughly identically in terms of both the two wings and the central core, as well as for the different Ar substituents. Indeed, this is supported by the very similar antiviral activity of all of the tested compounds against strain IIIB (Tables 1-4) and in the in vitro activity assay (Table 5). The Ar substituents may thus displace the alternative conformation water molecules and will be engaged in hydrophobic contacts with the side chains of V179 and Y181 (Figure 3B). Meanwhile, the water-bridged interaction with I180 is potentially possible in all the compounds bearing an electronegative atom in the ring, able to function as a hydrogen bond acceptor. However, the geometry and/or distance may not be ideal in the five-membered rings. This interaction may be critical in the case of mutant strain RES056, where just compound 16c can retain a similar antiviral activity as in the WT IIIB strain.
Structures of the K103N/Y181C mutant RT with RPV and compound 3 (25a) show that either the repositioning of the aromatic side chain of Y183 and/or maintaining the hydrogen-bonding interactions in the right wing may provide these adaptable compounds a similar tight interaction with WT RT.10 Nevertheless, these interactions are expected to occur as well for both 16c and the rest of compounds. Therefore, the interfacial water-mediated contact with I180 (connected to an extensive water network) may be the difference. This contact is not possible in compounds 15a-c, and likely suboptimal or not existing for compounds of the 11-14 series. What happens then with compounds 16a and 16b? The response is not apparent from this series, but from the second round of compounds designed after 16c (Table 3). Compounds 22-30, also resilient to K103N/Y181C mutations as 16c, differ from compounds of the 16 series in that the ring wing bears the cyanovinyl group substituent instead of the cyano.
Notably, our previous high-resolution RT/RPV structure, combined with infrared spectroscopy experiments and MD simulations of WT and K103N/Y181C mutant RT,23 shows that the conserved interaction of the cyanovinyl group with a water molecule contributes to the enhanced binding of RPV in both the WT and the mutant. This water molecule is conserved in the RT-16c structure, the first time this is observed with a left wing benzonitrile substituent. Thus, while the geometry is similar, the hydrogen bond is weaker in the current structure (2.7 Å in RT-RPV vs. 3.7 Å in RT-16c). We surmise that 16c, but not 16a or 16b, is the only compound of the first round series presenting this water molecule (which in the RT-RPV structure is seen connected to a water network extending towards residues 224-226). Accordingly, compounds 22-30, with the longer cyanovinyl group likely forming this water interaction, cope similarly well with the K103N/Y181C mutations irrespective of the R1 substituent.
Overall, the analysis of the RT-16c structure and related SAR put the spotlight on the key role of the water networks around the NNIBP for tight ligand binding to both WT and mutant RT. In relation to it, several recent papers, by combining MD simulations, high resolution X-ray and neutron crystallography, and isothermal titration calorimetry (ITC), have shown the paramount role of this kind of water networks in ligand binding, suggesting that “solvent structure is an evolutionary constraint on protein sequence that contributes to ligand affinity and selectivity.”25-27
We also have recognized that the 4-pyridyl substituent extends out of the NNIBP in the direction of fragment 1QP (Figures 3F-G) sitting on the NNRTI-adjacent site,16 which may allow fragment linking/merging approaches. To note that fragment 1QP displaces most of the water molecules forming the water network shown in Figure 3D. Taking into account that the 4-pyridyl substituent displaces the water molecules near the entrance channel, a compound targeting both pockets, as designed and tested by us recently, will displace all the water molecules out of these pockets and likely accounting for an entropic gain.28 Therefore, the further development of these kind of compounds is warranted. The second round of optimization also encourages us to further pursue this direction, as the substitutions assayed were probably oriented towards the solvent area (because of the likely penalty of facing the protein side, i.e., near residue I180 main chain), not resulting in any improvement in comparison to 16c.
In Vivo Pharmacokinetics Study
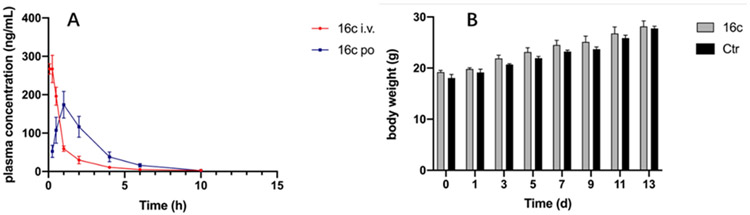
The in vivo pharmacokinetic profile of 16c was evaluated in Sprague Dawley rats (Table 6 and Figure 4A). After a single 2 mg/kg iv dose, compound 16c was characterized by a modest clearance (CL = 110 mL/min/kg) and half-life (T1/2 = 2.46 h), the maximum concentration (Cmax) could be up to 278 ng/mL. Absorption of 16c was evaluated after being dosed at 20 mg/kg, it reached maximum concentration (Tmax) at 1.00 h with a Cmax of 174 ng/mL and the half-life was 1.74 h. However, the oral bioavailability (F) was 15.3%, which need further improvement for a drug candidate.
Table 6.
Pharmacokinetic profile of 16ca
| Subject | T1/2 | Tmax | Cmax | AUC0-t | AUC0-∞ | CL | F |
|---|---|---|---|---|---|---|---|
| (h) | (h) | (ng/mL) | (h*ng/mL) | (h*ng/mL) | (mL/min/kg) | (%) | |
| 16c (iv)b | 2.46±0.56 | 0.033 | 278±31.2 | 293±32.7 | 303±31.1 | 110±10.9 | - |
| 16c (po)c | 1.74±0.65 | 1.00±0.00 | 174±12.5 | 460±296 | 466±294 | - | 15.3 |
PK parameter (mean ± SD, n = 3)
Dosed intravenously at 2 mg/kg
Dosed orally at 20 mg/kg.
Figure 4.
(A) The plasma concentration–time profiles of 16c in rats following oral administration (20 mg.kg−1) and intravenous administration (2 mg.kg−1). (B) The relative body weight changes of Kunming mice in different groups.
Safety Assessment
Assessment of Acute Toxicity
Next, the acute toxicity test of 16c was carried out. A total of 20 Kunming mice were randomly divided into two groups and given single oral doses of 0 mg/kg (control group) and 2000 mg/kg of 16c on the first day, respectively. The mice did not exhibit any toxic symptoms or mortality immediately during the subsequent two weeks. Additionally, there was no abnormality of body weight changed (Figure 4B). The results demonstrated that 16c was well-tolerated up to a dose of 2000 mg/kg with no acute toxicity.
Assessment of hERG activity
With the aim to estimate the potential risk for cardiotoxicity, 16c was evaluated for its activity against the hERG potassium channel using manual patch-clamp electrophysiology approach, and terfenadine was selected as reference drug. As shown in Figure 5, 16c exhibited an IC50 value of 1.19 μM, and demonstrated much reduced QT liability and lower hERG inhibition in comparison with that of the lead K-5a2 (IC50 = 0.130 μM) and the approved NNRTIs drug RPV (IC50 = 0.50 μM)29.
Figure 5.
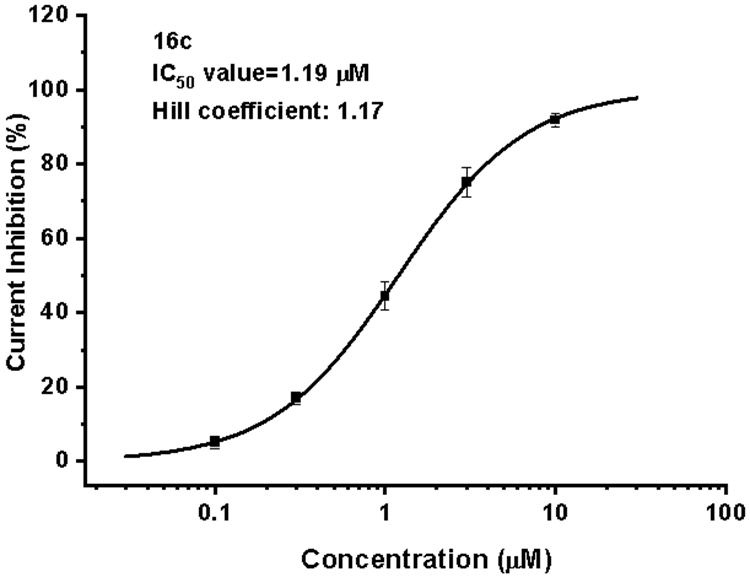
Activity of 16c against hERG potassium channel in HEK293 cells.
Conclusion
We have reported herein efforts to discover novel potent HIV-1 inhibitors by exploiting the underexplored Tolerant Region II of the NNIBP, and forty-five novel 2,4,5-trisubstituted pyrimidine derivatives were designed using a scaffold hopping approach. Notably, we could demonstrate that compound 16c, designed by introducing a 4-pyridyl group in the C5 position of the central pyrimidine scaffold, led to improved anti-HIV-1 potency against WT and mutant HIV-1 strains compared to that of ETR, with EC50 values of 3.75 nM (WT), 4.26 nM (L100I), 3.79 nM (K103N), 6.79 nM (Y181C), 6.79 nM (Y188L), 10.9 nM (E138K), 10.4 nM (F227L+V106A), and 24.4 nM (RES056) in MT-4 cells. Moreover, 16c exhibited lower cytotoxicity (CC50 = 36.0 μM), which contribute to its higher SI values toward WT and mutant HIV-1 strains. Additionally, 16c exhibited in vivo favorable pharmacokinetic properties in Sprague Dawley rats (F = 15.3%) and safety in Kunming mice (LD50 > 2000 mg/kg).
The co-crystal structure and the SAR analysis, on the basis of the previous crystallographic and spectroscopic experiments and MD simulations, revealed that the water networks surrounding the NNIBP, both on top and on the bottom, may work as a sort of molecular staples anchoring the NNRTIs and allowing them to “wiggle and jiggle” (i.e., conformational and positional adaptability) when resistance mutations arise. Furthermore, they provide a framework for an improved structure-based drug design of NNRTIs, especially those targeting simultaneously the NNIBP and the NNRTI Adjacent site.
EXPERIMENTAL SECTION
Chemistry
All melting points were determined on a micro melting point apparatus and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded in CDCl3 or DMSO-d6 on a Bruker AV-400 spectrometer with tetramethylsilane (TMS) as the internal standard. A G1313A Standard LC Autosampler (Agilent) was used to collect samples for measurement of mass spectra. All reactions were monitored by thin layer chromatography (TLC), and spots were visualized with iodine vapor or by irradiation with UV light. Flash column chromatography was performed on columns packed with Silica Gel (200-300 mesh). Solvents were of reagent grade and were purified by standard methods when necessary. Compounds purity was analyzed on a Shimadzu SPD-20A/20AV HPLC system with a Inertsil ODS-SP, 5 μm C18 column (150 mm × 4.6 mm). HPLC conditions: methanol/ water 80:20; flow rate 1.0 mL/min; UV detection from 210 to 400 nm; temperature, ambient; injection volume, 20 μL. Purity of all final compounds was >95%.
4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (6)
To a solution of 4-hydroxy-3,5-dimethylbenzonitrile (1.47 g, 10 mmol) and K2CO3 (1.70 g, 12 mmol) in DMF (30 mL) was added 2,4-dichloropyrimidine (1.50 g, 10 mmol), and the resultant mixture stirred at room temperature for 5 h. The precipitated white solid was collected by filtration, washed with ice-water (100 mL), and recrystallized in DMF-H2O to provide the intermediate 6 as a white solid in 90 % yield. 1H NMR (400 MHz, DMSO-d6) δ 8.70 (d, J = 5.7 Hz, 1H), 7.75 (s, 2H), 7.32 (d, J = 5.7 Hz, 1H), 2.10 (s, 6H). ESI-MS: m/z 260.3 [M + H]+. C13H10ClN3O (259.05).
tert-butyl4-((4-(4-cyano-2,6-dimethylphenoxy)pyrimidin-2-yl)amino)piperidine-1-carboxylate (7)
A solution of 6 (0.26 g, 1.0 mmol), N-Boc-4-aminopiperidine (0.24 g, 1.2 mmol), and anhydrous K2CO3 (0.28 g, 2 mmol) in DMF (5 mL) was heated at 120°C for 12 h under magnetic stirring. Then the mixture wad cooled to room temperature and ice-water (40 mL) was added. The resulting precipitate was collected by filtration, and dried to give crude product, which was recrystallized from ethyl acetate (EA)/petroleum ether (PE) to afford the target compound 7 as a white solid in 69 % yield. 1H NMR (400 MHz, DMSO-d6) δ 8.20 (d, J = 5.5 Hz, 1H), 7.68 (s, 2H), 6.39 – 6.01 (m, 1H), 3.85 (s, 3H), 2.81 (dd, J = 63.9, 1.4 Hz, 3H), 2.08 (s, 6H), 1.64 (d, J = 80.2 Hz, 2H), 1.38 (d, J = 1.5 Hz, 9H), 1.23 (s, 2H). ESI-MS: m/z 424.5 [M + H]+, 446.06 [M + Na]+. C23H29N5O3 (423.23). HPLC purity: 99.28%.
tert-butyl4-((4-(4-cyano-2,6-dimethylphenoxy)-5-iodopyrimidin-2-yl)amino) piperidine-1-carboxylate (8)
Intermediate 7 (0.42g, 1.0 mmol) was added to a suspension of HOAc (0.30 g, 5.0 mmol) and NIS (0.34 g, 1.5 mmol) in acetonitrile (20 mL). The mixture solution was stirred for 4 h at room temperature; then, 10% Na2CO3 (1.06 g, 10.0 mmol) was added and the mixture was stirred for another 20 min. The obtained sloid was filtered and dried to give crude product, which was recrystallized in EA to afford the target compound 8 as a white solid in 85 % yield. ESI-MS: m/z 550.4 [M + H]+. C23H28IN5O3 (549.12). HPLC purity: 97.26%.
4-((5-iodo-2-(piperidin-4-ylamino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (9)
To a solution of 8 (0.55 g, 1.0 mmol) in dichloromethane (DCM) (4 mL) was added trifluoroacetic acid (TFA) (0.74 mL, 10 mmol) at room temperature, and the mixture was stirred for 6 h (monitored by TLC). Then, the reaction solution was alkalized to pH 9 with saturated sodium bicarbonate solution and extracted with DCM. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give 9 as a white solid in 89% yield. 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H), 8.46 (s, 1H, C6-pyrimidine-H), 7.70 (s, 2H), 7.37 (s, 1H), 3.03 – 2.97 (m, 2H), 2.09 (d, 6H), 1.97 – 1.37 (m, 7H). ESI-MS: m/z 450.3 [M + H]+. C18H20IN5O (449.07). HPLC purity: 96.65%.
General procedure for the preparation of intermediates 10a-c
To a solution of 9 (0.45 g, 1.0 mmol) in anhydrous DMF (10 mL) was added anhydrous K2CO3 (0.28 g, 2.0 mmol) and substituted benzyl chloride (bromine) (1.2 mmol), and the solution was stirred for 4-7 h (monitored by TLC) at room temperature. The solvent was removed under reduced pressure and 30 mL water was added to the residue. Then the mixture solution was extracted with ethyl acetate and washed with saturated sodium chloride, purified by flash column chromatography and recrystallized from ethyl acetate (EA)/petroleum ether (PE) to afford the target compounds 10a-c.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-iodopyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (10a)
White solid, 76 % yield, mp 145–147°C. 1H NMR (400 MHz, DMSO-d6) δ 8.42 (s, 1H, C6-pyrimidine-H), 7.77 (d, J = 8.2 Hz, 2H), 7.69 (s, 2H), 7.45 (d, J = 8.1 Hz, 2H), 7.30 (s, 2H), 7.16 (s, 1H), 3.46 – 3.42 (m, 2H), 2.68 (s, 2H), 2.07 (s, 6H), 1.92 – 1.17 (m, 7H). ESI-MS: m/z 619.5 [M + H]+. C25H27IN6O3S (618.09). HPLC purity: 97.52%.
4-((5-iodo-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (10b)
White solid, 82 % yield, mp 152–154°C. 1H NMR (400 MHz, DMSO-d6) δ 8.42 (s, 1H, C6-pyrimidine-H), 7.87 (d, J = 8.0 Hz, 2H), 7.69 (s, 2H), 7.54 (d, J = 8.0 Hz, 2H), 7.16 (s, 1H), 3.50 – 3.42 (m, 2H), 3.20 (s, 3H), 2.74 (s, 2H), 2.07 (s, 6H), 1.90 – 1.16 (m, 7H). ESI-MS: m/z 618.4 [M + H]+. C26H28IN5O3S (617.10). HPLC purity: 99.02%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-iodopyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (10c)
White solid, 71 % yield, mp 136–138°C. 1H NMR (400 MHz, DMSO-d6) δ 8.42 (s, 1H, C6-pyrimidine-H), 7.81 (d, J = 7.9 Hz, 2H), 7.68 (s, 2H), 7.36 – 7.28 (m, 4H), 7.15 (s, 1H), 3.66 – 3.37 (m, 2H), 2.69 (s, 2H), 2.07 (s, 6H), 1.96 – 1.17 (m, 7H). ESI-MS: m/z 583.3 [M + H]+. C26H27IN6O2 (582.12). HPLC purity: 98.65%.
General procedure for the preparation of intermediates 11-16
Pd(PPh3)4 (0.05 mmol) and 2M Na2CO3 aqueous solution (2.00 mmol) were added to a mixture solution of 10a-c (1.00 mmol) and boric acid substituents (1.20 mmol) in DMF (10 mL). The mixture was stirred at 100°C for 6-10 h (monitoring with TLC) under N2. Then the mixture was diluted with 10 mL water, and the aqueous layer was extracted with EtOAc. The organic phase was dried over Na2SO4, filtered and evaporated under reduced pressure, purified by flash column chromatography and recrystallized from EA/PE to afford the target compounds 11-16.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(thiophen-2-yl)pyrimidin-2-yl)amino )piperidin-1-yl)methyl)benzenesulfonamide (11a)
11a was synthesized from 10a (602 mg, 1.0 mmol) and thiophen-2-ylboronic acid (153 mg, 1.2 mmol). White solid, 74% yield, mp 152–153°C. 1H NMR (400 MHz, DMSO-d6) δ 8.57 (s, 1H, C6-pyrimidine-H), 7.71 (d, J = 8.0 Hz, 2H, C3,C5-Ph-H), 7.63 (s, 2H, C3,C5-Ph’-H), 7.52 – 7.43 (m, 3H), 7.39 (d, J = 8.0 Hz, 2H, C2,C6-Ph-H), 7.24 (s, 2H, SO2NH2), 7.06 – 7.05 (m, 1H), 3.42 (s, 2H, N-CH2), 2.68 – 2.57 (m, 2H), 2.02 (s, 6H), 1.94 – 1.15 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 163.3, 160.4, 143.1, 135.3, 133.0, 129.4, 127.8, 126.9, 125.9, 123.9, 119.1, 108.7, 62.7, 61.9, 52.6, 31.3, 16.4. ESI-MS: m/z 575.6 [M + H]+, 597.5 [M + Na]+. C29H30N6O3S2 (574.18). HPLC purity: 95.98%.
3,5-dimethyl-4-((2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)-5-(thiophen-2-yl)pyrimidin-4-yl)oxy)benzonitrile (11b)
11b was synthesized from 10b (602 mg, 1.0 mmol) and thiophen-2-ylboronic acid (153 mg, 1.2 mmol). White solid, 82% yield, mp 168–170°C. 1H NMR (400 MHz, DMSO-d6) δ 8.58 (s, 1H, C6-pyrimidine-H), 7.81 (d, J = 7.9 Hz, 2H, C3,C5-Ph-H), 7.63 (s, 2H, C3,C5-Ph’-H), 7.61 – 7.59 (m, 1H), 7.48 (d, J = 7.8 Hz, 2H, C2,C6-Ph-H), 7.42 – 7.40 (m, 2H), 7.06 (s, 1H), 3.43 (s, 2H, N-CH2), 3.13 (s, 3H, SO2CH3), 2.68 – 2.60 (m, 2H), 2.02 (s, 6H), 1.94 – 1.24 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 163.3, 160.5, 145.4, 139.8, 135.3, 133.1, 132.5, 129.8, 127.8, 127.4, 125.4, 123.9, 119.1, 61.8, 52.8, 44.0, 31.3, 16.3. ESI-MS: m/z 574.2 [M + H]+, 596.2 [M + Na]+. C30H31N5O3S2 (573.19). HPLC purity: 98.32%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(thiophen-2-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (11c)
11c was synthesized from 10c (583 mg, 1.0 mmol) and thiophen-2-ylboronic acid (153 mg, 1.2 mmol). White solid, 70% yield, mp 155–157°C. 1H NMR (400 MHz, DMSO-d6) δ 8.58 (s, 1H, C6-pyrimidine-H), 7.85 (s, 1H), 7.75 (d, J = 7.8 Hz, 2H, C3,C5-Ph-H), 7.63 (s, 2H, C3,C5-Ph’-H), 7.48 – 7.45 (m, 2H), 7.27 (d, J = 7.8 Hz, 2H, C2,C6-Ph-H), 7.23 (s, 2H, CONH2), 7.06 (s, 1H), 3.38 (s, 2H, N-CH2), 2.68 – 2.59 (m, 2H), 2.03 (s, 6H), 1.94 – 1.15 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.2, 163.3, 160.4, 142.4, 135.3, 133.4, 133.0, 132.6, 128.9, 127.9, 125.5, 124.0, 121.5, 120.4, 119.1, 108.7, 62.1, 52.7, 31.7, 29.1, 16.3. ESI-MS: m/z 539.7 [M + H]+, 561.1 [M + Na]+. C30H30N6O2S (538.22). HPLC purity: 98.29%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(thiophen-3-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (12a)
12a was synthesized from 10a (602 mg, 1.0 mmol) and thiophen-3-ylboronic acid (153 mg, 1.2 mmol). White solid, 72% yield, mp 144–146°C. 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, C6-pyrimidine-H), 7.78 (d, J = 8.0 Hz, 2H, C3,C5-Ph-H), 7.67 (s, 2H, C3,C5-Ph’-H), 7.62 (s, 2H), 7.46 (d, J = 8.0 Hz, 2H, C2,C6-Ph-H), 7.35 – 7.30 (m, 3H), 7.02 (s, 1H, NH), 3.47 (s, 2H, N-CH2), 2.78 – 2.61 (m, 2H), 2.08 (s, 6H), 1.85 – 1.10 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 164.3, 155.0, 143.3, 133.8, 133.0, 129.4, 126.6, 126.0, 119.1, 108.5, 104.4, 61.9, 60.2, 52.6, 21.2, 16.3, 14.5. ESI-MS: m/z 575.5 [M + H]+, 597.3 [M + Na]+. C29H30N6O3S2 (574.18). HPLC purity: 99.11%.
3,5-dimethyl-4-((2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)-5-(thiophen-3-yl)pyrimidin-4-yl)oxy)benzonitrile (12b)
12b was synthesized from 10b (602 mg, 1.0 mmol) and thiophen-3-ylboronic acid (153 mg, 1.2 mmol). White solid, 66% yield, mp 159–161°C. 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, C6-pyrimidine-H), 7.88 (d, J = 7.9 Hz, 2H, C3,C5-Ph-H), 7.83 – 7.68 (m, 1H), 7.62 (s, 2H, C3,C5-Ph’-H), 7.67 – 7.60 (m, 2H), 7.55 (d, J = 8.0 Hz, 2H, C2,C6-Ph-H), 7.02 (s, 1H, NH), 3.43 (s, 2H, N-CH2), 3.20 (s, 3H, SO2CH3), 2.86 – 2.58 (m, 2H), 2.08 (s, 6H), 1.94 – 1.17 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 164.1, 155.2, 145.1, 143.6, 139.0, 133.3, 132.4, 129.7, 127.8, 119.1, 118.2, 108.6, 61.9, 52.7, 44.0, 31.3, 16.2. ESI-MS: m/z 574.3 [M + H]+, 596.6 [M + Na]+. C30H31N5O3S2 (573.19). HPLC purity: 98.66%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(thiophen-3-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (12c)
12c was synthesized from 10c (583 mg, 1.0 mmol) and thiophen-3-ylboronic acid (153 mg, 1.2 mmol). White solid, 74% yield, mp 140–142°C. 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, C6-pyrimidine-H), 7.92 (s, 1H), 7.82 (d, J = 8.0 Hz, 2H, C3,C5-Ph-H), 7.68 (s, 2H, C3,C5-Ph’-H), 7.62 (s, 2H), 7.33 (d, J = 7.9 Hz, 2H, C2,C6-Ph-H), 7.30 (s, 2H, CONH2), 7.02 (s, 1H), 3.44 (s, 2H, N-CH2), 2.64 – 2.59 (m, 2H), 2.09 (s, 6H), 2.00 – 1.20 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 164.2, 155.0, 143.7, 140.2, 133.1, 131.9, 129.1, 126.5, 122.8, 121.4, 119.8, 118.2, 115.8, 108.0, 61.9, 60.2, 52.5, 21.2, 16.3, 14.5. ESI-MS: m/z 539.4 [M + H]+, 561.3 [M + Na]+. C30H30N6O2S (538.22). HPLC purity: 96.59%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(furan-2-yl)pyrimidin-2-yl)amino) piperidin-1-yl)methyl)benzenesulfonamide (13a)
13a was synthesized from 10a (602 mg, 1.0 mmol) and furan-2-ylboronic acid (134 mg, 1.2 mmol). White solid, 77% yield, mp 140–142°C. 1H NMR (400 MHz, DMSO-d6) δ 8.61 (s, 1H, C6-pyrimidine-H), 7.78 (d, J = 7.9 Hz, 2H, C3,C5-Ph-H), 7.77 – 7.70 (m, 2H), 7.72 (s, 2H, C3,C5-Ph’-H), 7.46 (d, J = 8.0 Hz, 2H, C2,C6-Ph-H), 7.31 – 7.19 (m, 3H), 6.59 (s, 1H, NH), 3.47 (s, 2H, N-CH2), 2.51 – 2.02 (m, 2H), 2.02 (s, 6H), 1.95 – 1.13 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 170.8, 163.0, 160.2, 156.3, 147.1, 143.2, 142.1, 133.1, 129.4, 126.9, 126.0, 119.1, 112.3, 108.7, 61.9, 60.2, 52.7, 21.2, 16.1. ESI-MS: m/z 559.4 [M + H]+, 581.4 [M + Na]+. C29H30N6O4S (558.20). HPLC purity: 98.96%.
4-((5-(furan-2-yl)-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino) pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (13b)
13b was synthesized from 10b (602 mg, 1.0 mmol) and furan-2-ylboronic acid (134 mg, 1.2 mmol). White solid, 82% yield, mp 137–139°C. 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, C6-pyrimidine-H), 7.88 (d, J = 7.9 Hz, 2H, C3,C5-Ph-H), 7.72 – 7.63 (m, 3H), 7.55 (d, J = 7.8 Hz, 2H, C2,C6-Ph-H), 6.73 (d, J = 11.7 Hz, 1H), 7.21 – 7.19 (m, 1H), 6.59 (s, 1H, NH), 3.51 (s, 2H, N-CH2), 3.20 (s, 3H, SO2CH3), 2.83 – 2.57 (m, 2H), 2.02 (s, 6H), 1.94 – 1.18 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 163.0, 160.3, 156.0, 147.1, 145.3, 142.1, 139.8, 133.1, 132.5, 129.8, 128.5, 127.4, 119.1, 112.3, 107.7, 61.8, 52.7, 44.0, 31.2, 16.3. ESI-MS: m/z 558.6 [M + H]+, 580.4 [M + Na]+. C30H31N5O4S (557.21). HPLC purity: 97.52%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(furan-2-yl)pyrimidin-2-yl)amino )piperidin-1-yl)methyl)benzamide (13c)
13c was synthesized from 10c (583 mg, 1.0 mmol) and furan-2-ylboronic acid (134 mg, 1.2 mmol). White solid, 65% yield, mp 148–150°C. 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, C6-pyrimidine-H), 7.91 (s, 1H), 7.82 (d, J = 7.8 Hz, 2H, C3,C5-Ph-H), 7.71 (s, 2H, C3,C5-Ph’-H), 7.52 (s, 1H), 7.41 – 7.14 (m, 4H), 6.73 (d, J = 12.4 Hz, 1H), 6.58 (s, 1H, NH), 3.38 (s, 2H, N-CH2), 2.89 – 2.60 (m, 2H), 2.03 (s, 6H), 1.94 – 1.14 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.2, 163.0, 160.2, 154.2, 147.1, 142.1, 133.4, 133.0, 132.5, 128.9, 127.9, 119.1, 112.3, 108.7, 100.6, 62.1, 52.5, 31.2, 16.0. ESI-MS: m/z 523.3 [M + H]+. C30H30N6O3 (522.24). HPLC purity: 97.49%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(furan-3-yl)pyrimidin-2-yl)amino) piperidin-1-yl)methyl)benzenesulfonamide (14a)
14a was synthesized from 10a (602 mg, 1.0 mmol) and furan-3-ylboronic acid (134 mg, 1.2 mmol). White solid, 69% yield, mp 172–174°C. 1H NMR (400 MHz, DMSO-d6) δ 8.52 (s, 1H, C6-pyrimidine-H), 8.00 (s, 1H), 7.85 (s, 1H), 7.75 (d, J = 7.8 Hz, 2H, C3,C5-Ph-H), 7.68 (s, 1H), 7.62 (s, 2H, C3,C5-Ph’-H), 7.30 – 7.19 (m, 4H), 7.00 (s, 1H, NH), 3.39 – 3.32 (m, 2H, N-CH2), 2.77 – 2.52 (m, 2H), 2.01 (s, 6H), 1.81 – 1.07 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.2, 164.2, 160.4, 143.9, 140.0, 133.4, 133.1, 132.6, 128.9, 127.9,126.0, 119.1, 118.2, 108.9, 108.5, 62.1, 52.6, 39.4, 31.4, 16.2. ESI-MS: m/z 559.3 [M + H]+, 581.4 [M + Na]+. C29H30N6O4S (558.20). HPLC purity: 98.96%.
4-((5-(furan-3-yl)-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino) pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (14b)
14b was synthesized from 10b (602 mg, 1.0 mmol) and furan-3-ylboronic acid (134 mg, 1.2 mmol). White solid, 77% yield, mp 180–182°C. 1H NMR (400 MHz, DMSO-d6) δ 8.52 (s, 1H, C6-pyrimidine-H), 8.01 (s, 1H), 7.81 (d, J = 7.9 Hz, 2H, C3,C5-Ph-H), 7.70 – 7.66 (m, 1H), 7.62 (s, 2H, C3,C5-Ph’-H), 7.48 (d, J = 7.9 Hz, 2H, C2,C6-Ph-H), 7.27 (s, 1H), 7.00 (s, 1H, NH), 3.44 (s, 2H, N-CH2), 3.13 (s, 3H, SO2CH3), 2.75 –2.63 (m, 2H), 2.01 (s, 6H), 1.90 – 1.28 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 164.2, 145.4, 143.9, 140.0, 139.8, 133.1, 132.6, 129.7, 127.4, 119.1, 118.2, 108.8, 61.9, 52.7, 44.0, 31.4, 16.2. ESI-MS: m/z 558.5 [M + H]+, 580.2 [M + Na]+. C30H31N5O4S (557.21). HPLC purity: 99.01%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(furan-3-yl)pyrimidin-2-yl)amino) piperidin-1-yl)methyl)benzamide (14c)
14c was synthesized from 10c (583 mg, 1.0 mmol) and furan-3-ylboronic acid (134 mg, 1.2 mmol). White solid, 71% yield, mp 158–160°C. 1H NMR (400 MHz, DMSO-d6) δ 8.52 (s, 1H, C6-pyrimidine-H), 8.00 (s, 1H), 7.76 – 7.68 (m, 4H), 7.62 (s, 2H), 7.39 (d, J = 7.9 Hz, 2H, C2,C6-Ph-H), 7.24 (s, 2H), 7.00 (s, 1H, NH), 3.39 (s, 2H, N-CH2), 2.81 – 2.53 (m, 2H), 2.01 (s, 6H), 1.96 – 1.19 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 164.2, 143.9, 143.1, 140.0, 133.1, 132.4, 129.4, 126.0, 123.0, 121.9, 119.1, 118.2, 115.8, 108.8, 61.9, 60.2, 52.6, 21.2, 16.2, 14.5. ESI-MS: m/z 523.3 [M + H]+, 545.4 [M + Na]+. C30H30N6O3 (522.24). HPLC purity: 97.48%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-phenylpyrimidin-2-yl)amino) piperidin-1-yl)methyl)benzenesulfonamide (15a)
15a was synthesized from 10a (602 mg, 1.0 mmol) and phenylboronic acid (145 mg, 1.2 mmol). White solid, 79% yield, mp 162–164°C. 1H NMR (400 MHz, DMSO-d6) δ 8.34 (s, 1H, C6-pyrimidine-H), 7.78 (d, J = 8.0 Hz, 2H, C3,C5-Ph-H), 7.64 (s, 2H, C3,C5-Ph’-H), 7.64 – 7.49 (m, 3H), 7.45 – 7.39 (m, 3H), 7.32 (d, J = 8.0 Hz, 2H, C2,C6-Ph-H), 7.05 (s, 1H), 3.47 (s, 2H, N-CH2), 2.89 – 2.59 (m, 2H), 2.08 (s, 6H), 1.87 – 1.15 (m, 7H). 13C NMR (100 MHz, DMSO) δ 164.7, 161.0, 156.2, 154.4, 143.4, 143.1, 133.0, 132.5, 129.4, 129.0, 128.6, 127.3, 126.0, 119.1, 115.7, 112.4, 108.6, 62.0, 60.2, 52.7, 21.2, 16.4, 14.5. ESI-MS: m/z 569.2 [M + H]+. C31H32N6O3S (568.23). HPLC purity: 96.98%.
3,5-dimethyl-4-((2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)-5-phenylpyrimidin-4-yl)oxy)benzonitrile (15b)
15b was synthesized from 10b (602 mg, 1.0 mmol) and phenylboronic acid (145 mg, 1.2 mmol). White solid, 81% yield, mp 147–149°C. 1H NMR (400 MHz, DMSO-d6) δ 8.27 (s, 1H, C6-pyrimidine-H), 7.81 (d, J = 8.0 Hz, 2H, C3,C5-Ph-H), 7.64 – 7.53 (m, 4H), 7.48 (d, J = 7.9 Hz, 2H, C2,C6-Ph-H), 7.38 (t, J = 7.7 Hz, 2H), 7.26 (t, J = 7.6 Hz, 1H), 6.97 (s, 1H), 3.54 – 3.35 (m, 2H, N-CH2), 3.13 (s, 3H, SO2CH3), 2.68 – 2.62 (m, 2H), 2.01 (s, 6H), 1.90 – 1.17 (m, 7H). 13C NMR (100 MHz, DMSO) δ 164.3, 156.0, 153.8, 143.7, 142.8, 133.7, 132.5, 129.0, 128.6, 127.3, 125.2, 118.4, 115.6, 112.7, 108.0, 62.1, 52.7, 21.4, 16.3, 14.5. ESI-MS: m/z 568.4 [M + H]+, 590.5 [M + Na]+. C32H33N5O3S (567.23). HPLC purity: 98.59%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-phenylpyrimidin-2-yl)amino) piperidin-1-yl)methyl)benzamide (15c)
15c was synthesized from 10c (583 mg, 1.0 mmol) and phenylboronic acid (145 mg, 1.2 mmol). White solid, 64% yield, mp 150–152°C. 1H NMR (400 MHz, DMSO-d6) δ 8.34 (s, 1H, C6-pyrimidine-H), 7.92 (s, 1H), 7.83 (d, J = 7.8 Hz, 2H, C3,C5-Ph-H), 7.65 (d, J = 12.5 Hz, 4H), 7.45 (t, J = 7.6 Hz, 2H), 7.37 – 7.27 (m, 4H), 7.04 (s, 1H, NH), 3.38 (s, 2H, N-CH2), 2.73 – 2.57 (m, 2H), 2.08 (s, 6H), 2.02 – 1.17 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 164.2, 155.7, 152.6, 143.7, 142.4, 133.1, 131.7, 129.3, 125.8, 122.3, 119.3, 118.2, 115.8, 108.0, 61.7, 60.2, 52.5, 21.2, 16.4, 14.5. ESI-MS: m/z 533.7 [M + H]+, 555.2 [M + Na]+. C32H32N6O2 (532.26). HPLC purity: 98.96%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(pyridin-4-yl)pyrimidin-2-yl)amino) piperidin-1-yl)methyl)benzenesulfonamide (16a)
16a was synthesized from 10a (602 mg, 1.0 mmol) and pyridin-4-ylboronic acid (145 mg, 1.2 mmol). White solid, 53% yield, mp 174–176°C. 1H NMR (400 MHz, DMSO-d6) δ 8.60 – 8.54 (m, 3H), 8.18 (d, J = 5.4 Hz, 2H), 7.76 – 7.73 (m, 2H), 7.64 (s, 2H, C3,C5-Ph’-H), 7.46 (d, J = 8.0 Hz, 2H), 7.31 (s, 2H), 6.95 (s, 1H, NH), 3.47 (s, 2H, N-CH2), 2.87 – 2.59 (m, 2H), 2.09 (s, 6H), 2.03 – 1.22 (m, 7H). 13C NMR (100 MHz, DMSO) δ 164.5, 161.4, 156.4, 143.7, 143.0, 133.6, 129.4, 129.0, 128.5, 128.1, 127.3, 126.2, 119.0, 118.2, 115.7, 112.9, 108.3, 62.0, 60.7, 52.7, 21.2, 16.4, 14.5. ESI-MS: m/z 570.3 [M + H]+, 592.1 [M + Na]+. C30H31N7O3S (569.22). HPLC purity: 99.32%.
3,5-dimethyl-4-((2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)-5-(pyridin-4-yl)pyrimidin-4-yl)oxy)benzonitrile (16b)
16b was synthesized from 10b (602 mg, 1.0 mmol) and pyridin-4-ylboronic acid (145 mg, 1.2 mmol). White solid, 59% yield, mp 158–160°C. 1H NMR (400 MHz, DMSO-d6) δ 8.60 (d, J = 5.1 Hz, 2H), 8.55 (d, J = 6.2 Hz, 1H), 7.88 (d, J = 7.9 Hz, 2H), 7.74 (d, J = 5.1 Hz, 1H), 7.69 (s, 3H), 7.55 (d, J = 7.9 Hz, 2H), 7.04 (s, 1H), 3.55 – 3.48 (m, 2H, N-CH2), 3.20 (s, 3H), 2.92 – 2.60 (m, 2H), 2.09 (s, 6H), 2.02 – 1.22 (m, 7H). 13C NMR (100 MHz, DMSO) δ 164.2, 160.7, 156.9, 153.8, 143.7, 142.1, 133.7, 132.5, 128.2, 127.9, 125.8, 119.3, 118.3, 115.0, 112.7, 108.0, 62.3, 52.7, 21.4, 16.4, 14.5. ESI-MS: m/z 569.2 [M + H]+, 591.5 [M + Na]+. C31H32N6O3S (568.23). HPLC purity: 97.65%.
4-((4-((4-(4-cyano-2,6-dimethylphenoxy)-5-(pyridin-4-yl)pyrimidin-2-yl)amino) piperidin-1-yl)methyl)benzamide (16c)
16c was synthesized from 10c (583 mg, 1.0 mmol) and pyridin-4-ylboronic acid (145 mg, 1.2 mmol). White solid, 52% yield, mp 163–165°C. 1H NMR (400 MHz, DMSO-d6) δ 8.61 – 8.53 (m, 3H), 7.87 (d, J = 7.8 Hz, 2H), 7.74 – 7.67 (m, 4H), 7.69 (s, 2H), 7.55 (d, J = 7.9 Hz, 2H), 7.04 (s, 1H), 3.50 – 3.48 (m, 2H, N-CH2), 2.75 – 2.61 (m, 2H), 2.08 (s, 6H), 2.02 – 1.29 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 164.3, 160.1, 155.8, 152.6, 143.6, 142.3, 133.7, 132.1, 129.2, 127.4, 125.1, 122.2, 119.0, 118.5, 115.8, 108.7, 61.5, 60.2, 52.5, 21.2, 16.4, 14.5. ESI-MS: m/z 534.7 [M + H]+, 556.4 [M + Na]+. C31H31N7O2 (533.25). HPLC purity: 98.78%.
(E)-3-(4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (17)
The synthetic method was similar to that described for 6, except that the starting material 5 (1.50 g, 10 mmol) was reacted with (E)-3-(4-hydroxy-3,5-dimethylphenyl)acrylonitrile (2.07 g, 12 mmol). White solid, 84 % yield. 1H NMR (400 MHz, DMSO-d6) δ 8.67 (d, J = 5.7 Hz, 1H), 7.62 (d, J = 16.7 Hz, 1H), 7.52 (s, 2H), 7.25 (d, J = 5.7 Hz, 1H), 6.45 (d, J = 16.7 Hz, 1H), 2.07 (s, 6H). ESI-MS: m/z 286.2 [M + H]+. C15H12ClN3O (285.07). HPLC purity: 98.39%.
tert-butyl(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)pyrimidin-2-yl) amino)piperidine-1-carboxylate (18)
The synthetic method was similar to that described for 7, except that the starting material 17 (0.28 g, 1.0 mmol) was reacted with N-Boc-4-aminopiperidine (0.24 g, 1.2 mmol). White solid, 70% yield. 1H NMR (400 MHz, DMSO-d6) δ 8.17 (d, J = 5.6 Hz, 1H), 7.68 – 7.51 (m, 1H), 7.46 (d, J = 4.5 Hz, 2H), 6.40 (d, J = 16.6 Hz, 1H), 6.17 (s, 1H), 3.85 (s, 3H), 2.81 (d, J = 63.8 Hz, 2H), 2.06 (s, 6H), 1.91 – 1.52 (m, 3H), 1.41 (d, J = 6.0 Hz, 3H), 1.38 (s, 6H), 1.31 – 0.99 (m, 2H). ESI-MS: m/z 472.02 [M + Na]+. C25H31N5O3 (449.24). HPLC purity: 98.79%.
tert-butyl(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-iodopyrimidin-2-yl)amino)piperidine-1-carboxylate (19)
The synthetic method was similar to that described for 8, except that NIS (0.34 g, 1.5 mmol) was reacted with 18 (0.47 g, 1.0 mmol). White solid, 79% yield. ESI-MS: m/z 576.2 [M + H]+. C25H30IN5O3 (575.14). HPLC purity: 96.28%.
(E)-3-(4-((5-iodo-2-(piperidin-4-ylamino)pyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (20)
The synthetic method was similar to that described for 9. White solid, 85% yield. 1H NMR (400 MHz, DMSO-d6) δ 8.45 (s, 1H, C6-pyrimidine-H), 8.27 (s, 1H), 7.60 (d, J = 16.6 Hz, 1H, ArCH=), 7.47 (s, 2H), 7.32 (d, J = 13.5 Hz, 1H), 6.42 (d, J = 16.7 Hz, 1H, =CHCN), 3.23 – 2.71 (m, 5H), 2.06 (s, 6H), 1.94 – 1.26 (m, 4H). ESI-MS: m/z 476.2 [M + H]+, 498.5 [M + Na]+. C20H22IN5O (475.09). HPLC purity: 97.09%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-iodopyrimidin-2-yl) amino)piperidin-1-yl)methyl)benzenesulfonamide (21a)
The synthetic method was similar to that described for 10a-c. White solid, 75% yield, mp: 210-212°C. 1H NMR (400 MHz, DMSO-d6) δ 8.41 (s, 1H, C6-pyrimidine-H), 7.96 (s, 1H), 7.77 (d, J = 7.9 Hz, 2H), 7.68 – 7.55 (m, 2H), 7.46 (s, 2H), 7.35 – 7.26 (m, 2H), 7.15 (s, 1H, NH), 6.42 (d, J = 16.7 Hz, 1H, =CHCN), 3.41 (s, 2H, N-CH2), 2.74 (s, 2H), 2.04 (s, 6H), 1.86 – 1.08 (m, 7H). ESI-MS: m/z 645.7 [M + H]+, 667.2 [M + Na]+. C27H29IN6O3S (644.11). HPLC purity: 96.59%.
(E)-3-(4-((5-iodo-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino) pyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (21b)
The synthetic method was similar to that described for 10a-c. White solid, 82% yield, mp: 204-206°C. 1H NMR (400 MHz, DMSO-d6) δ 8.41 (s, 1H, C6-pyrimidine-H), 7.88 (d, J = 8.1 Hz, 2H), 7.68 – 7.58 (m, 2H), 7.45 (s, 2H), 7.29 – 7.27 (m, 1H), 7.15 (s, 1H, NH), 6.41 (d, J = 16.7 Hz, 1H, =CHCN), 3.47 – 3.45 (m, 2H), 3.20 (s, 3H, SO2CH3), 2.74 (s, 2H), 2.04 (s, 6H), 1.83 – 1.10 (m, 7H). ESI-MS: m/z 644.5 [M + H]+, 666.3 [M + Na]+. C28H30IN5O3S (643.11). HPLC purity: 99.02%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-iodopyrimidin-2-yl) amino)piperidin-1-yl)methyl)benzamide (21c)
The synthetic method was similar to that described for 10a-c. White solid, 74% yield, mp: 231-232°C. 1 1H NMR (400 MHz, DMSO-d6) δ 8.40 (s, C6-pyrimidine-H), 7.91 (s, 1H), 7.81 (d, J = 7.9 Hz, 2H), 7.45 (s, 2H), 7.38 – 7.08 (m, 5H), 6.42 (d, J = 16.5 Hz, 1H, =CHCN), 3.54 – 3.47 (m, 2H), 2.74 (s, 2H), 2.04 (s, 6H), 1.99 – 1.21 (m, 7H). ESI-MS: m/z 609.2 [M + H]+, 631.4 [M + Na]+. C28H29IN6O2 (608.14). HPLC purity: 99.25%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(pyridin-4-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (22a)
22a was synthesized from 21a (644 mg, 1.0 mmol) and pyridin-4-ylboronic acid (145 mg, 1.2 mmol). White solid, 59% yield, mp: 134-136°C. 1H NMR (400 MHz, DMSO-d6) δ 8.53 (s, 1H, C6-pyrimidine-H), 8.46 (d, J = 6.6 Hz, 2H), 7.71 (d, J = 8.1 Hz, 2H), 7.65 – 7.47 (m, 3H), 7.39 – 7.35 (m, 3H), 7.24 – 7.21 (m, 3H), 6.91 (s, 1H, NH), 6.35 (d, J = 17.3 Hz, 1H, =CHCN), 3.43 (s, 2H, N-CH2), 2.88 – 2.47 (m, 2H), 1.99 (s, 6H), 1.87 – 1.09 (m, 7H). 13C NMR (100 MHz, DMSO-d6): δ 170.6, 165.3, 161.6, 160.4, 150.7, 150.4, 143.2, 131.6, 129.5, 128.6, 128.1, 126.0, 123.0, 122.5, 119.4, 96.6, 90.8, 62.1, 52.8, 50.6, 31.6, 29.0, 16.5. ESI-MS: m/z 596.3 [M + H]+, 618.5 [M + Na]+. C32H33N7O3S (595.24). HPLC purity: 97.19%.
(E)-3-(3,5-dimethyl-4-((2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)-5-(pyridin-4-yl)pyrimidin-4-yl)oxy)phenyl)acrylonitrile (22b)
22b was synthesized from 21b (643 mg, 1.0 mmol) and pyridin-4-ylboronic acid (145 mg, 1.2 mmol). White solid, 63% yield, mp: 122-124°C. 1H NMR (400 MHz, DMSO-d6) δ 8.75 – 8.47 (m, 3H), 7.88 (d, J = 7.8 Hz, 2H), 7.75 – 7.69 (m, 2H), 7.66 – 7.49 (m, 3H), 7.46 (s, 2H), 6.91 (s, 1H, NH), 6.41 (d, J = 16.9 Hz, 1H, =CHCN), 3.48 – 3.45 (m, 2H, N-CH2), 3.20 (s, 3H, SO2CH3), 2.94 – 2.54 (m, 2H), 2.07 (s, 6H), 2.00 – 1.17 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.8, 165.2, 161.5, 150.6, 150.2, 145.5, 139.8, 131.6, 131.3, 129.7, 128.6, 128.2, 127.4, 122.6, 119.4, 105.6, 61.9, 52.8, 44.0, 31.7, 29.0, 16.6. ESI-MS: m/z 595.5 [M + H]+. C33H34N6O3S (594.2). HPLC purity: 98.54%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(pyridin-4-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (22c)
22c was synthesized from 21c (608 mg, 1.0 mmol) and pyridin-4-ylboronic acid (145 mg, 1.2 mmol). White solid, 50% yield, mp: 125-127°C. 1H NMR (400 MHz, DMSO-d6) δ 8.52 (s, 1H, C6-pyrimidine-H), 8.46 (d, J = 6.0 Hz, 2H), 7.85 (s, 1H), 7.76 (d, J = 7.7 Hz, 2H), 7.70 – 7.47 (m, 3H), 7.39 (s, 2H), 7.32 – 7.18 (m, 3H), 6.90 (s, 1H, NH), 6.34 (d, J = 16.7 Hz, 1H, =CHCN), 3.41 (s, 2H, N-CH2), 2.73 (s, 2H), 1.99 (s, 6H), 1.77 – 1.15 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.2, 161.6, 150.2, 142.5, 133.5, 131.7, 131.6, 128.9, 128.8, 127.9, 122.9, 122.6, 119.4, 96.5, 62.1, 52.8, 31.7, 29.0, 16.5. ESI-MS: m/z 560.3 [M + H]+, 682.4 [M + Na]+. C33H33N7O2 (559.27). HPLC purity: 97.44%.
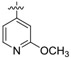
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(2-methoxypyridin-4-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (23a)
23a was synthesized from 21a (644 mg, 1.0 mmol) and (2-methoxypyridin-4-yl)boronic acid (183 mg, 1.2 mmol). White solid, 65% yield, mp: 132-134°C. 1H NMR (400 MHz, DMSO-d6) δ 8.51 (d, J = 6.1 Hz, 1H), 8.19 (d, J = 5.4 Hz, 1H), 7.78 (d, J = 7.7 Hz, 2H), 7.59 (d, J = 15.4 Hz, 1H, ArCH=), 7.46 – 7.42 (m, 5H), 7.37 –7.30 (m, 3H), 7.14 (s, 1H, NH), 6.50 – 6.34 (m, 1H, =CHCN), 3.88 (s, 3H, OCH3), 3.44 (s, 2H, N-CH2), 2.77 – 2.74 (m, 2H), 2.06 (s, 6H), 1.99 – 1.17 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.7, 164.5, 161.5, 155.2, 150.7, 147.3, 147.3, 143.4, 143.1, 131.6, 129.4, 128.6, 128.2, 126.0, 119.4, 116.9, 116.5, 109.1, 108.7, 96.4, 62.0, 53.5, 52.6, 31.2, 16.5. ESI-MS: m/z 626.4 [M + H]+, 643.7 [M + NH4]+. C33H35N7O4S (625.25). HPLC purity: 98.55%.
(E)-3-(4-((5-(2-methoxypyridin-4-yl)-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (23b)
23b was synthesized from 21b (643 mg, 1.0 mmol) and (2-methoxypyridin-4-yl)boronic acid (183 mg, 1.2 mmol). White solid, 65% yield, mp: 225-227°C. 1H NMR (400 MHz, DMSO-d6) δ 8.51 (d, J = 6.1 Hz, 1H), 8.24 – 8.11 (m, 1H), 7.88 (d, J = 7.8 Hz, 2H), 7.68 – 7.49 (m, 4H), 7.45 (d, J = 5.2 Hz, 2H), 7.36 – 7.29 (m, 1H), 7.13 (s, 1H, NH), 6.40 (d, J = 16.7, 1H, =CHCN), 3.87 (s, 3H, OCH3), 3.54 (s, 2H, N-CH2), 3.20 (s, 3H, SO2CH3), 2.75 – 2.60 (m, 2H), 2.05 (s, 6H), 1.96 – 1.12 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.7, 164.4, 161.6, 147.3, 145.5, 139.8, 137.4, 131.5, 129.7, 127.4, 119.4, 116.5, 108.7, 96.4, 61.9, 53.5, 52.6, 44.0, 31.7, 29.0, 16.7. ESI-MS: m/z 625.3 [M + H]+. C34H36N6O4S (624.25). HPLC purity: 97.90%.
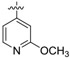
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(2-methoxypyridin-4-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (23c)
23c was synthesized from 21c (608 mg, 1.0 mmol) and (2-methoxypyridin-4-yl)boronic acid (183 mg, 1.2 mmol). White solid, 72% yield, mp: 170-172°C. 1H NMR (400 MHz, DMSO-d6) δ 8.51– 8.37 (m, 2H), 7.80 (d, J = 7.8 Hz, 2H), 7.59 (d, J = 16.4 Hz, 1H, ArCH=), 7.45 – 7.42 (m, 3H), 7.40 – 7.39 (m, 2H), 7.37 –7.30 (m, 3H), 7.14 (s, 1H, NH), 6.44 (d, J = 16.7 Hz, 1H, =CHCN), 3.87 (s, 3H, OCH3), 3.43 (s, 2H, N-CH2), 2.73 – 2.71 (m, 2H), 2.04 (s, 6H), 1.99 – 1.21 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.7, 154.5, 161.7, 155.2, 150.7, 147.5, 147.3, 143.4, 142.8, 131.6, 129.4, 128.6, 128.0, 126.5, 119.4, 116.8, 109.1, 108.7, 96.4, 62.2, 53.5, 52.6, 31.5, 16.7. ESI-MS: m/z 590.2 [M + H]+, 612.4 [M + Na]+. C34H35N7O3 (589.28). HPLC purity: 98.11%.
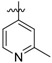
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(2-methylpyridin-4-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (24a)
24a was synthesized from 21a (644 mg, 1.0 mmol) and (2-methylpyridin-4-yl)boronic acid (164 mg, 1.2 mmol). White solid, 57% yield, mp: 138-140°C. 1H NMR (400 MHz, DMSO-d6) δ 8.40 (dd, J = 13.9, 7.8 Hz, 2H), 7.71 (d, J = 7.7 Hz, 2H), 7.58 – 7.40 (m, 3H), 7.38 (d, J = 6.5 Hz, 4H), 7.24 (d, J = 5.2 Hz, 2H), 6.88 (s, 1H, NH), 6.34 (d, J = 17.3, 1H, =CHCN), 3.43 (s, 2H, N-CH2), 3.20 (s, 3H, CH3), 2.68 (d, J = 12.1 Hz, 2H), 1.99 (s, 6H), 1.84 – 1.17 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 167.9, 165.2, 161.5, 160.3, 158.5, 152.1, 150.6, 150.4, 149.5, 143.4, 143.1, 142.0, 131.7, 129.4, 128.6, 128.2, 126.0, 122.1, 120.0, 119.4, 107.1, 96.4, 62.0, 52.5, 48.6, 31.7, 24.7, 16.5. ESI-MS: m/z 610.7 [M + H]+, 632.4 [M + Na]+. C33H35N7O3S (609.25). HPLC purity: 99.05%.
(E)-3-(3,5-dimethyl-4-((5-(2-methylpyridin-4-yl)-2-((1-(4-(methylsulfonyl)benzyl) piperidin-4-yl)amino)pyrimidin-4-yl)oxy)phenyl)acrylonitrile (24b)
24b was synthesized from 21b (643 mg, 1.0 mmol) and (2-methylpyridin-4-yl)boronic acid (164 mg, 1.2 mmol). White solid, 58% yield, mp: 125-127°C. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (dd, J = 14.3, 7.6 Hz, 2H), 7.88 (d, J = 7.8 Hz, 2H), 7.57 (dt, J = 25.1, 13.8 Hz, 5H), 7.46 (s, 2H), 6.89 (s, 1H, NH), 6.41 (d, J = 16.7 Hz, 1H, =CHCN), 3.54 – 3.42 (m, 2H, N-CH2), 3.20 (s, 3H, SO2CH3), 2.84 – 3.76 (m, 2H), 2.48 (s, 3H, CH3), 2.06 (s, 6H), 2.00 – 1.19 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 170.7, 167.2, 165.3, 161.5, 158.5, 150.8, 149.5, 145.5, 139.8, 131.5, 129.8, 127.4, 120.4, 105.9, 96.7, 61.9, 52.8, 44.0, 31.2, 24.7, 16.8. ESI-MS: m/z 609.7 [M + H]+, 626.5 [M + NH4]+. C34H36N6O3S (608.26). HPLC purity: 99.36%.
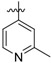
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(2-methylpyridin-4-yl) pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (24c)
24c was synthesized from 21c (608 mg, 1.0 mmol) and (2-methylpyridin-4-yl)boronic acid (164 mg, 1.2 mmol). White solid, 61% yield, mp: 127-129°C. 1H NMR (400 MHz, DMSO-d6) δ 8.40 (dd, J = 13.8, 7.4 Hz, 2H), 7.84 (s, 1H), 7.75 (d, J = 7.7 Hz, 2H), 7.50 – 7.44 (m, 3H), 7.39 (s, 2H), 7.32 – 7.14 (m, 3H), 6.89 (s, 1H, NH), 6.42 – 6.27 (m, 1H, =CHCN), 3.40 (s, 2H, N-CH2), 3.20 (s, 3H, CH3), 2.72 (d, J = 26.0 Hz, 2H), 1.96 (s, 6H), 1.91 – 1.12 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.2, 165.3, 158.4, 150.5, 149.5, 142.5, 133.4, 131.6, 128.8, 127.8, 121.6, 119.4, 114.4, 96.6, 62.3, 57.6, 52.8, 31.7, 24.7, 16.8. ESI-MS: m/z 574.5 [M + H]+, 596.3 [M + Na]+. C34H35N7O2 (573.29). HPLC purity: 98.77%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(2-fluoropyridin-4-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (25a)
25a was synthesized from 21a (644 mg, 1.0 mmol) and (2-fluoropyridin-4-yl)boronic acid (169 mg, 1.2 mmol). White solid, 59% yield, mp: 222-224°C. 1H NMR (400 MHz, DMSO-d6) δ 8.56 (d, J = 12.0 Hz, 1H), 8.19 (d, J = 5.3 Hz, 1H), 7.71 – 7.62 (m, 5H), 7.50 – 7.37 (m, 4H), 7.25 (d, J = 10.6 Hz, 2H), 6.89 (s, 1H, NH), 6.36 (d, J = 15.6 Hz, 1H, =CHCN), 3.58 – 3.28 (m, 2H, N-CH2), 2.69 (s, 2H), 2.00 (s, 6H), 1.90 – 1.20 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 165.1, 160.7, 156.0, 153.1, 151.9, 150.6, 150.4, 148.1, 143.4, 131.7 (d, JCF = 13 Hz), 129.3, 128.6, 128.2 (d, JCF = 8 Hz), 126.1, 121.3, 119.3, 104.6, 96.9, 52.5, 45.8, 29.0, 16.8. ESI-MS: m/z 614.4 [M + H]+, 636.5 [M + Na]+. C32H32FN7O3S (613.23). HPLC purity: 98.95%.
(E)-3-(4-((5-(2-fluoropyridin-4-yl)-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl) amino)pyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (25b)
25b was synthesized from 21b (643 mg, 1.0 mmol) and (2-fluoropyridin-4-yl)boronic acid (169 mg, 1.2 mmol). White solid, 57% yield, mp: 147-149°C. 1H NMR (400 MHz, DMSO-d6) δ 8.63 (d, J = 11.2 Hz, 1H), 8.31 – 8.22 (m, 1H), 7.88 (d, J = 7.9 Hz, 2H), 7.81 – 7.60 (m, 2H), 7.60 – 7.50 (m, 3H), 7.46 (d, J = 10.6 Hz, 2H), 6.70 (s, 1H, NH), 6.53 – 6.35 (m, 1H, =CHCN), 3.49 – 3.48 (m, 2H), 3.21 (s, 3H, SO2CH3), 2.94 – 2.55 (m, 2H), 2.07 (s, 6H), 1.95 – 1.05 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 165.3, 160.7, 147.9, 145.4, 139.8, 132.5, 132.0 (d, JCF = 10 Hz), 131.7, 131.6, 129.8 (d, JCF = 9 Hz), 129.2 (d, JCF = 11 Hz), 128.6, 128.2, 127.3, 120.8, 119.3, 107.4, 90.5, 52.7, 44.0, 31.7, 16.8. ESI-MS: m/z 613.4 [M + H]+, 635.3 [M + Na]+. C33H33FN6O3S (612.23). HPLC purity: 98.56%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(2-fluoropyridin-4-yl) pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (25c)
25c was synthesized from 21c (608 mg, 1.0 mmol) and (2-fluoropyridin-4-yl)boronic acid (169 mg, 1.2 mmol). White solid, 49% yield, mp: 173-175°C. 1H NMR (400 MHz, DMSO-d6) δ 8.63 (d, J = 11.1 Hz, 1H), 8.26 (d, J = 5.3 Hz, 1H), 8.04 – 7.54 (m, 5H), 7.46 (d, J = 10.6 Hz, 4H), 7.33 (d, J = 8.1 Hz, 2H), 6.70 (s, 1H, NH), 6.43 (d, J = 16.9 Hz, 1H, =CHCN), 3.41 (s, 2H, N-CH2), 2.74 (s, 2H), 2.07 (s, 6H), 1.99 – 1.28 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.2, 165.3, 158.8, 157.6, 152.7, 150.5, 148.1, 142.6, 133.2, 131.6, 127.9, 119.3, 115.0, 110.7, 107.0, 96.8, 52.5, 48.9, 29.0, 18.9, 16.5. ESI-MS: m/z 578.5 [M + H]+, 600.5 [M + Na]+. C33H32FN7O2 (577.26). HPLC purity: 97.77%.
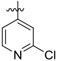
(E)-4-((4-((5-(2-chloropyridin-4-yl)-4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy) pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (26a)
26a was synthesized from 21a (644 mg, 1.0 mmol) and (2-chloropyridin-4-yl)boronic acid (188 mg, 1.2 mmol). White solid, 68% yield, mp: 133-135°C. 1H NMR (400 MHz, DMSO-d6) δ 8.53 (d, J = 14.2 Hz, 1H), 8.35 (d, J = 5.2 Hz, 1H), 7.75 – 7.70 (m, 4H), 7.55 (d, J = 16.7 Hz, 1H, ArCH=), 7.38 (d, J = 10.7 Hz, 4H), 7.24 (d, J = 6.8 Hz, 2H), 6.90 (s, 1H, NH), 6.35 (d, J = 16.6, 1H, =CHCN), 3.43 (s, 2H, N-CH2), 2.86 – 2.58 (m, 2H), 1.99 (s, 6H), 1.90 – 0.98 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.4, 150.6, 145.7, 143.3, 143.1, 131.7, 129.4, 128.6, 128.2, 126.0, 122.0, 119.4, 105.7, 96.8, 62.0, 52.5, 49.7, 31.2, 23.8, 16.5. ESI-MS: m/z 630.7 [M + H]+, 652.2 [M + Na]+. C32H32ClN7O3S (629.20). HPLC purity: 98.11%.
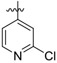
(E)-3-(4-((5-(2-chloropyridin-4-yl)-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (26b)
26b was synthesized from 21b (643 mg, 1.0 mmol) and (2-chloropyridin-4-yl)boronic acid (188 mg, 1.2 mmol). White solid, 51% yield, mp: 130-132°C. 1H NMR (400 MHz, DMSO-d6) δ 8.60 (d, J = 14.0 Hz, 1H), 8.42 (d, J = 5.2 Hz, 1H), 7.87 – 7.81 (m, 3H), 7.76 (d, J = 8.9 Hz, 2H), 7.67 – 7.49 (m, 2H), 7.45 (d, J = 8.9 Hz, 2H), 6.89 (s, 1H, NH), 6.50 – 6.32 (m, 1H, =CHCN), 3.48 (s, 2H, N-CH2), 3.20 (s, 3H, SO2CH3), 2.84 – 2.56 (m, 2H), 2.06 (s, 6H), 1.98 – 1.17 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.7, 165.3, 161.8, 160.4, 153.6, 151.2, 150.6, 150.3, 145.5, 139.8, 131.7, 129.8, 128.6, 128.2, 127.4, 122.3, 119.4, 112.6, 96.5, 61.9, 52.6, 44.0, 31.7, 29.0, 16.6. ESI-MS: m/z 629.6 [M + H]+, 646.2 [M + NH4]+. C33H33ClN6O3S (628.20). HPLC purity: 97.28%.
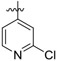
(E)-4-((4-((5-(2-chloropyridin-4-yl)-4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy) pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (26c)
26c was synthesized from 21c (608 mg, 1.0 mmol) and (2-chloropyridin-4-yl)boronic acid (188 mg, 1.2 mmol). White solid, 57% yield, mp: 123-127°C. 1H NMR (400 MHz, DMSO-d6) δ 8.53 (d, J = 13.4 Hz, 1H), 8.35 (d, J = 5.2 Hz, 1H), 7.84 (s, 1H), 7.75 (d, J = 7.9 Hz, 3H), 7.68 (d, J = 9.8 Hz, 1H), 7.54 (t, J = 17.3 Hz, 1H, ArCH=), 7.39 (s, 2H), 7.25 (dd, J = 14.0, 7.3 Hz, 3H), 6.89 (s, 1H, NH), 6.35 (d, J = 16.7 Hz, 1H, =CHCN), 3.40 (s, 2H, N-CH2), 2.87 – 2.63 (m, 2H), 1.99 (s, 6H), 1.93 – 1.12 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.2, 164.3, 161.7, 160.7, 152.4, 150.3, 145.5, 142.5, 133.4, 131.7, 128.9, 127.9, 122.4, 121.8, 119.3, 96.8, 62.3, 52.5, 31.2, 23.7, 16.5. ESI-MS: m/z 594.3 [M + H]+, 616.5 [M + Na]+. C33H32ClN7O2 (593.23). HPLC purity: 99.15%.
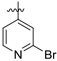
(E)-4-((4-((5-(2-bromopyridin-4-yl)-4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy) pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (27a)
27a was synthesized from 21a (644 mg, 1.0 mmol) and (2-bromopyridin-4-yl)boronic acid (241 mg, 1.2 mmol). White solid, 64% yield, mp: 183-185°C. 1H NMR (400 MHz, DMSO-d6) δ 8.56 (d, J = 11.4 Hz, 1H), 8.20 (d, J = 5.3 Hz, 1H), 7.71 – 7.62 (m, 3H), 7.57 – 7.55 (m, 2H), 7.50 – 7.37 (m, 4H), 7.25 (d, J = 8.6 Hz, 2H), 6.89 (s, 1H, NH), 6.36 – 6.35 (m, 1H, =CHCN), 3.58 – 3.28 (m, 2H, N-CH2), 2.69 (s, 2H), 2.00 (s, 6H), 1.90 – 1.20 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 170.2, 165.8, 161.5, 156.9, 153.9, 151.3, 144.5, 143.4, 137.7, 131.7, 129.4, 126.9, 126.2, 122.2, 107.9, 90.7, 52.6, 50.6, 45.8, 29.0, 16.5. ESI-MS: m/z 674.5 [M + H]+, 696.4 [M + Na]+. C32H32BrN7O3S (673.15). HPLC purity: 98.66%.
(E)-3-(4-((5-(2-bromopyridin-4-yl)-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (27b)
27b was synthesized from 21b (643 mg, 1.0 mmol) and (2-bromopyridin-4-yl)boronic acid (241 mg, 1.2 mmol). White solid, 57% yield, mp: 157-159°C. 1H NMR (400 MHz, DMSO-d6) δ 8.41 (s, 1H), 7.87 (d, J = 7.1 Hz, 2H), 7.65 – 7.49 (m, 4H), 7.45 (s, 2H), 7.28 – 7.14 (m, 2H), 6.70 (s, 1H, NH), 6.40 (d, J = 16.7 Hz, 1H, =CHCN), 3.56 – 3.52 (m, 2H), 3.19 (s, 3H, SO2CH3), 2.74 (s, 2H), 2.05 (s, 6H), 1.98 – 1.39 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 166.2, 161.4, 150.5, 145.4, 145.4, 139.8, 131.7, 131.6, 131.4, 131.1, 129.8, 129.7, 128.6, 127.3, 120.7, 119.3, 101.2, 96.2, 61.9, 52.7, 50.6, 29.0, 16.5. ESI-MS: m/z 673.5 [M + H]+, 695.2 [M + Na]+. C33H33BrN6O3S (672.15). HPLC purity: 99.54%.
(E)-4-((4-((5-(2-bromopyridin-4-yl)-4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy) pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (27c)
27c was synthesized from 21c (608 mg, 1.0 mmol) and (2-bromopyridin-4-yl)boronic acid (241 mg, 1.2 mmol). White solid, 55% yield, mp: 178-180°C. 1H NMR (400 MHz, DMSO-d6) δ 8.41 – 8.40 (m, 1H), 7.95 (s, 2H), 7.83 (d, J = 7.1 Hz, 2H), 7.69 – 7.54 (m, 3H), 7.46 (s, 2H), 7.30 – 7.28 (m, 3H), 6.71 (s, 1H, NH), 6.43 (d, J = 16.7 Hz, 1H, =CHCN), 3.42 (s, 2H, N-CH2), 2.75 (s, 2H), 2.10 (s, 6H), 1.97 – 1.12 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.2, 165.8, 161.3, 157.1, 151.4, 150.5, 146.1, 144.2, 142.6, 133.7, 131.7, 131.4, 131.0, 128.9, 127.9, 122.9, 119.3, 110.0, 100.4, 96.5, 52.7, 50.6, 29.0, 16.4. ESI-MS: m/z 638.4 [M + H]+, 655.4 [M + NH4]+. C33H32BrN7O2 (637.18). HPLC purity: 99.18%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(pyridin-3-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (28a)
28a was synthesized from 21a (644 mg, 1.0 mmol) and pyridin-3-ylboronic acid (147 mg, 1.2 mmol). White solid, 62% yield, mp: 148-150°C. 1H NMR (400 MHz, DMSO-d6) δ 8.78 (d, J = 18.1 Hz, 1H), 8.45 (dd, J = 4.8, 1.6 Hz, 1H), 8.34 (d, J = 3.3 Hz, 1H), 7.98 (s, 1H), 7.71 (d, J = 7.9 Hz, 2H), 7.51– 7.39 (m, 6H), 7.31 – 7.21 (m, 2H), 7.11 (d, J = 8.2 Hz, 1H), 6.34 (d, J = 17.5 Hz, 1H, =CHCN), 3.42 (s, 2H), 2.88 – 2.52 (m, 2H), 1.99 (s, 6H), 1.82 – 1.12 (m, 7H). 13C NMR (100 MHz, DMSO) δ 165.2, 161.4, 160.0, 159.5, 149.2, 148.2, 143.4, 143.1, 136.2, 131.6, 129.4, 128.1, 126.0, 124.0, 119.4, 113.7, 96.6, 91.2, 61.9, 52.8, 31.2, 29.0, 16.6. ESI-MS: m/z 596.3 [M + H]+, 618.5 [M + Na]+. C32H33N7O3S (595.24). HPLC purity: 98.99%.
(E)-3-(3,5-dimethyl-4-((2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)-5-(pyridin-3-yl)pyrimidin-4-yl)oxy)phenyl)acrylonitrile (28b)
28b was synthesized from 21b (643 mg, 1.0 mmol) and pyridin-3-ylboronic acid (147 mg, 1.2 mmol). White solid, 39% yield, mp: 193-195°C. 1H NMR (400 MHz, DMSO-d6) δ 8.78 (d, J = 17.4 Hz, 1H), 8.45 (dd, J = 4.7, 1.6 Hz, 1H), 8.34 (d, J = 3.3 Hz, 1H), 7.99 (d, J = 13.9 Hz, 1H), 7.81 (d, J = 7.9 Hz, 2H), 7.59 – 7.35 (m, 7H), 6.34 (d, J = 16.7 Hz, 1H, =CHCN), 3.41 (s, 2H), 3.14 (s, 3H, SO2CH3), 2.84 – 2.49 (m, 2H), 1.99 (s, 6H), 1.78 – 1.12 (m, 7H). 13C NMR (100 MHz, DMSO) δ 165.2, 161.4, 159.6, 154.7, 152.1, 150.6, 149.3, 148.2, 145.5, 139.8, 136.1, 135.8, 131.6, 131.5, 129.7, 127.4, 124.0, 119.4, 106.5, 96.7, 61.9, 52.6, 44.0, 31.3, 16.6. ESI-MS: m/z 595.5 [M + H]+, 617.4 [M + Na]+. C33H34N6O3S (594.24). HPLC purity: 98.41%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(pyridin-3-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (28c)
28c was synthesized from 21c (608 mg, 1.0 mmol) and pyridin-3-ylboronic acid (147 mg, 1.2 mmol). White solid, 37% yield, mp: 234-236°C. 1H NMR (400 MHz, DMSO-d6) δ 8.78 (d, J = 17.3 Hz, 1H), 8.50 – 8.41 (m, 1H), 8.34 (d, J = 3.0 Hz, 1H), 7.99 (d, J = 12.6 Hz, 1H), 7.86 (s, 1H), 7.76 (d, J = 7.8 Hz, 2H), 7.55 (d, J = 17.7 Hz, 1H), 7.46 – 7.35 (m, 3H), 7.29 – 7.25 (m, 3H), 7.10 (d, J = 7.1 Hz, 1H), 6.34 (d, J = 16.7 Hz, 1H, =CHCN), 3.41 (s, 2H), 2.84 – 2.60 (m, 2H), 1.99 (s, 6H), 1.87 – 1.12 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 168.2, 165.2, 161.4, 159.5, 150.5, 149.1, 148.2, 136.2, 133.4, 131.6, 131.5, 128.8, 127.8, 124.0, 119.4, 96.6, 90.7, 62.2, 52.7, 39.6, 16.7. ESI-MS: m/z 560.2 [M + H]+, 577.5 [M + NH4]+. C33H33N7O2 (559.27). HPLC purity: 97.55%.
(E)-4-((4-((5-(6-aminopyridin-3-yl)-4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy) pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (29a)
29a was synthesized from 21a (644 mg, 1.0 mmol) and (6-aminopyridin-3-yl)boronic acid (165 mg, 1.2 mmol). White solid, 51% yield, mp: 146-148°C. 1H NMR (400 MHz, DMSO-d6) δ 8.16 (d, J = 3.4 Hz, 1H), 8.06 (s, 1H), 7.71 (dd, J = 8.3, 2.7 Hz, 2H), 7.56 – 7.52 (m, 3H), 7.38 (d, J = 6.5 Hz, 4H), 7.25 (s, 2H), 6.45 (d, J = 8.6 Hz, 1H), 6.33 (d, J = 16.7 Hz, 1H, =CHCN), 5.93 (s, 2H), 3.40 (s, 2H, N-CH2), 2.87 – 2.74 (m, 2H), 1.97 (s, 6H), 1.82 – 1.02 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 165.2, 160.7, 159.1, 152.4, 150.6, 147.3, 143.1, 137.6, 131.6, 131.3, 129.4, 128.4, 126.0, 119.4, 118.1, 108.0, 96.4, 62.0, 52.7, 31.5, 16.7. ESI-MS: m/z 611.3 [M + H]+ , 633.7 [M + Na]+. C32H34N8O3S (610.25). HPLC purity: 98.63%.
(E)-3-(4-((5-(6-aminopyridin-3-yl)-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (29b)
29b was synthesized from 21b (643 mg, 1.0 mmol) and (6-aminopyridin-3-yl)boronic acid (165 mg, 1.2 mmol). White solid, 40% yield, mp: 209-211°C. 1H NMR (400 MHz, DMSO-d6) δ 8.23 (d, J = 3.4 Hz, 1H), 8.14 (s, 1H), 7.88 (d, J = 7.9 Hz, 2H), 7.62 – 7.57 (m, 3H), 7.53 (d, J = 7.7 Hz, 2H), 7.44 (s, 2H), 6.52 (d, J = 8.6 Hz, 1H), 6.40 (d, J = 16.7 Hz, 1H, =CHCN), 6.00 (s, 2H), 3.41 (s, 2H, N-CH2), 3.20 (s, 3H, SO2CH3), 2.80 – 2.67 (m, 2H), 2.06 (s, 6H), 1.87 – 1.21 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 165.2, 160.7, 159.1, 148.6, 147.3, 145.5, 139.8, 137.6, 131.6, 131.3, 129.8, 129.7, 127.4, 119.4, 118.1, 108.0, 61.9, 52.7, 44.0, 31.6, 16.7. ESI-MS: m/z 611.2 [M + H]+. C33H35N7O3S (609.25). HPLC purity: 98.39%.
(E)-4-((4-((5-(6-aminopyridin-3-yl)-4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy) pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (29c)
29c was synthesized from 21c (608 mg, 1.0 mmol) and (6-aminopyridin-3-yl)boronic acid (165 mg, 1.2 mmol). White solid, 47% yield, mp: 182-184°C. 1H NMR (400 MHz, DMSO-d6) δ 8.23 (d, J = 3.8 Hz, 1H), 8.13 (s, 1H), 7.96 (s, 1H), 7.84 (d, J = 7.8 Hz, 2H), 7.61 (d, J = 14.4 Hz, 3H), 7.44 (s, 2H), 7.40 – 7.27 (m, 4H), 6.53 (d, J = 8.6 Hz, 1H), 6.41 (d, J = 16.7 Hz, 1H, =CHCN), 6.02 (s, 2H), 3.41 (s, 2H, N-CH2), 2.81 – 2.58 (m, 2H), 2.05 (s, 6H), 1.82 – 1.10 (m, 7H). 13C NMR (100 MHz, DMSO) δ 172.4, 168.2, 165.1, 160.7, 159.1, 150.5, 147.3, 144.4, 137.7, 131.6, 130.1, 129.3, 127.9, 119.4, 118.0, 108.0, 96.5, 90.0, 53.8, 33.1, 29.5, 16.7. ESI-MS: m/z 575.3 [M + H]+, 597.1 [M + Na]+. C33H34N8O2 (574.28). HPLC purity: 98.06%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(6-fluoropyridin-3-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzenesulfonamide (30a)
30a was synthesized from 21a (644 mg, 1.0 mmol) and (6-fluoropyridin-3-yl)boronic acid (169 mg, 1.2 mmol). White solid, 71% yield, mp: 118-120°C. 1H NMR (400 MHz, DMSO-d6) δ 8.41 (d, J = 18.3 Hz, 1H), 8.33 (s, 1H), 8.18 (s, 1H), 7.71 (dd, J = 7.1, 3.6 Hz, 2H), 7.62 – 7.48 (m, 1H), 7.39 (d, J = 11.2 Hz, 4H), 7.28 – 7.17 (m, 4H), 6.34 (d, J = 16.5 Hz, 1H, =CHCN), 3.55 – 3.52 (m, 2H), 2.81 – 2.50 (m, 2H), 1.99 (s, 6H), 1.75 – 1.15 (m, 7H). 13C NMR (100 MHz, DMSO) δ 165.1, 161.5, 159.5, 152.5, 150.6, 148.5, 146.9 (JCF = 20 Hz), 143.4 (JCF = 23 Hz), 142.1, 131.6, 131.5, 128.7, 126.9, 126.0, 119.4, 110.0, 109.6, 104.4, 96.6, 62.0, 52.8, 31.7, 29.0, 16.8. ESI-MS: m/z 614.2 [M + H]+, 636.4 [M + Na]+. C32H32FN7O3S (613.23). HPLC purity: 99.23%.
(E)-3-(4-((5-(6-fluoropyridin-3-yl)-2-((1-(4-(methylsulfonyl)benzyl)piperidin-4-yl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (30b)
30b was synthesized from 21b (643 mg, 1.0 mmol) and (6-fluoropyridin-3-yl)boronic acid (169 mg, 1.2 mmol). White solid, 61% yield, mp: 115-117°C. 1H NMR (400 MHz, DMSO-d6) δ 8.42 (d, J = 17.7 Hz, 1H), 8.33 (s, 1H), 8.17 (d, J = 8.5 Hz, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.57 – 7.41 (m, 4H), 7.38 (s, 2H), 7.20 (dd, J = 8.6, 2.9 Hz, 1H), 6.34 (d, J = 16.7 Hz, 1H, =CHCN), 3.46 – 3.42 (m, 2H), 3.14 (s, 3H, SO2CH3), 2.79 – 2.49 (m, 2H), 1.99 (s, 6H), 1.76 – 1.06 (m, 7H). 13C NMR (100 MHz, DMSO) δ 165.1, 161.5, 161.2, 160.0, 150.6, 146.9 (JCF = 20 Hz), 145.5, 142.4, 139.8, 131.6 (JCF = 10 Hz), 129.8, 128.7, 128.1, 127.4, 124.5, 119.4, 109.9, 96.6, 61.9, 52.6, 44.0, 31.7, 16.6. ESI-MS: m/z 613.5 [M + H]+, 635.3 [M + Na]+. C33H33FN6O3S (612.23). HPLC purity: 97.95%.
(E)-4-((4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)-5-(6-fluoropyridin-3-yl)pyrimidin-2-yl)amino)piperidin-1-yl)methyl)benzamide (30c)
30c was synthesized from 21c (608 mg, 1.0 mmol) and (6-fluoropyridin-3-yl)boronic acid (169 mg, 1.2 mmol). White solid, 65% yield, mp: 113-115°C. 1H NMR (400 MHz, DMSO-d6) δ 8.41 (d, J = 17.7 Hz, 1H), 8.33 (s, 1H), 8.18 (s, 1H), 7.86 (s, 1H), 7.76 (d, J = 7.7 Hz, 2H), 7.60 – 7.47 (m, 1H), 7.38 (s, 2H), 7.31 – 7.09 (m, 5H), 6.34 (d, J = 16.7 Hz, 1H, =CHCN), 3.54 – 3.53 (m, 2H), 2.77 – 2.73 (m, 2H), 1.99 (s, 6H), 1.83 – 1.10 (m, 7H). 13C NMR (100 MHz, DMSO) δ 168.2, 165.1, 161.2, 159.4, 155.8, 150.4, 146.7, 142.4, 133.4, 131.6 (JCF = 10 Hz), 128.8, 127.8, 119.4, 109.9, 109.6, 96.6, 62.3, 52.8, 31.5, 29.0, 16.8. ESI-MS: m/z 578.6 [M + H]+, 600.4 [M + Na]+. C33H32FN7O2 (577.26). HPLC purity: 98.52%.
Supplementary Material
Figure 1.
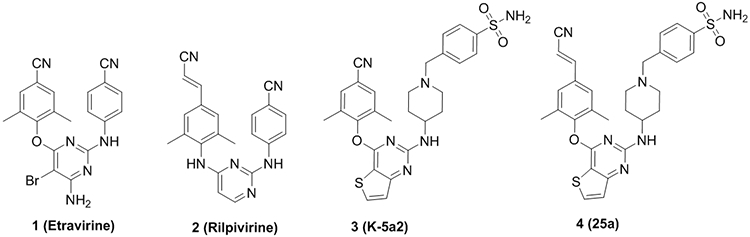
The chemical structures of ETR, RPV, and the piperidine-substituted thiophene[3,2-d]pyrimidine compounds K-5a2 and 25a.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from the National Natural Science Foundation of China (NSFC Nos. 81973181, 81903453), Shandong Provincial Key research and development project (Nos. 2019JZZY021011), Shandong Provincial Natural Science Foundation (ZR2019BH011, ZR2020YQ61, ZR2020JQ31), Foreign cultural and educational experts Project (GXL20200015001), Young Scholars Program of Shandong University (YSPSDU No. 2016WLJH32), National Science and Technology Major Projects for "Major New Drugs Innovation and Development" (2019ZX09301126), the Taishan Scholar Program at Shandong Province, KU Leuven (GOA 10/014), and NIH grant R01 AI027690 (to E.A.). The technical assistance of Mr. Kris Uyttersprot and Mrs. Kristien Erven, for the HIV experiments is gratefully acknowledged.
ABBREVIATIONS USED
- AIDS
acquired immune deficiency syndrome
- cART
combination antiretroviral therapy
- CC50
50% cytotoxicity concentration
- Cmax
maximum concentration
- DAPY
diarylpyrimidine
- DLV
delavirdine
- DOR
doravirine
- EFV
efavirenz
- ETV
etravirine
- EC50
the effective concentration causing 50% inhibition of viral cytopathogenicity
- FDA
U.S. Food and Drug Administration
- HIV
human immunodeficiency virus
- hERG
the human ether-à-go-go related gene
- NNIBP
NNRTI-binding pocket
- NNRTI
non-nucleoside RT inhibitor
- NRTI
nucleoside RT inhibitor
- NVP
nevirapine
- RF
fold-resistance
- RPV
rilpivirine
- RT
reverse transcriptase
- SAR
structure-activity relationship
- SI
selectivity index
- TLC
thin layer chromatography
- TMS
tetramethylsilane
- WT
wild type
Footnotes
Supporting Information includes:
In vitro assay of anti-HIV activities in MT-4 cells, recombinant HIV-1 reverse transcriptase (RT) inhibitory assays, pharmacokinetic methods, acute toxicity experiment, and assay procedures for hERG activity (PDF).
The authors declare that all experimental work complied with the institutional guidelines on animal studies (care and use of laboratory animals).
The authors declare no competing financial interest.
References
- 1. https://www.who.int/hiv/data/en/.
- 2.Bec G; Meyer B; Gerard M-A; Steger J; Fauster K; Wolff P; Burnouf D; Micura R; Dumas P; Ennifar E Thermodynamics of HIV-1 reverse transcriptase in action elucidates the mechanism of action of non-nucleoside inhibitors. J. Am. Chem. Soc 2013, 135, 9743–9752. [DOI] [PubMed] [Google Scholar]
- 3.Namasivayam V; Vanangamudi M; Kramer VG; Kurup S; Zhan P; Liu X; Kongsted J; Byrareddy SN The journey of HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) from lab to clinic. J. Med. Chem 2019, 62, 4851–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhuang C; Pannecouque C; De Clercq E; Chen F Development of non-nucleoside reverse transcriptase inhibitors (NNRTIs): our past twenty years. Acta Pharm. Sin. B 2020, 10, 961–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cilento ME , Kirby KA , & Sarafianos SG Avoiding drug resistance in hiv reverse transcriptase. Chem. Rev 2021. doi: 10.1021/acs.chemrev.0c00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyrer C; Pozniak A HIV drug resistance - an emerging threat to epidemic control. N. Engl. J. Med 2017, 377, 1605–1607. [DOI] [PubMed] [Google Scholar]
- 7.Battini L; Bollini M Challenges and approaches in the discovery of human immunodeficiency virus type-1 non-nucleoside reverse transcriptase inhibitors. Med. Res. Rev 2019, 39, 1235–1273. [DOI] [PubMed] [Google Scholar]
- 8.Kang D; Fang Z; Li Z; Huang B; Zhang H; Lu X; Xu H; Zhou Z; Ding X; Daelemans D; De Clercq E; Pannecouque C; Zhan P; Liu X Design, synthesis, and evaluation of thiophene[3,2-d]pyrimidine derivatives as HIV-1 non-nucleoside reverse transcriptase inhibitors with significantly improved drug resistance profiles. J. Med. Chem 2016, 59, 7991–8007. [DOI] [PubMed] [Google Scholar]
- 9.Kang D; Fang Z; Huang B; Lu X; Zhang H; Xu H; Huo Z; Zhou Z; Yu Z; Meng Q; Wu G; Ding X; Tian Y; Daelemans D; De Clercq E; Pannecouque C; Zhan P; Liu X Structure-based optimization of thiophene[3,2-d]pyrimidine derivatives as potent HIV-1 non-nucleoside reverse transcriptase inhibitors with improved potency against resistance-associated variants. J. Med. Chem 2017, 60, 4424–4443. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y; Kang D; Nguyen LA; Smithline ZB; Pannecouque C; Zhan P; Liu X; Steitz TA Structural basis for potent and broad inhibition of HIV-1 RT by thiophene[3,2-d] pyrimidine non-nucleoside inhibitors. eLife 2018, 7, No. e36340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang D; Zhang H; Wang Z; Zhao T; Ginex T; Luque FJ; Yang Y; Wu G; Feng D; Wei F; Zhang J; De Clercq E; Pannecouque C; Chen CH; Lee K-H; Murugan NA; Steitz TA; Zhan P; Liu X Identification of dihydrofuro[3,4-d]pyrimidine derivatives as novel HIV-1 non-nucleoside reverse transcriptase inhibitors with promising antiviral activities and desirable physicochemical properties. J. Med. Chem 2019, 62, 1484–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa M; Hashimoto Y Improvement in aqueous solubility in small molecule drug discovery programs by disruption of molecular planarity and symmetry. J. Med. Chem 2011, 54, 1539–1554. [DOI] [PubMed] [Google Scholar]
- 13.Kang D; Feng D; Sun Y; Fang Z; Wei F; De Clercq E; Pannecouque C; Liu X; Zhan P Structure-based bioisosterism yields HIV-1 NNRTIs with improved drug-resistance profiles and favorable pharmacokinetic properties. J. Med. Chem 2020, 63, 4837–4848. [DOI] [PubMed] [Google Scholar]
- 14.Kang D; Ruiz FX; Feng D; Pilch A; Zhao T; Wei F; Wang Z; Sun Y; Fang Z; De Clercq E; Pannecouque C; Arnold E; Liu X; Zhan P Discovery and characterization of fluorine-substituted diarylpyrimidine derivatives as Novel HIV-1 NNRTIs with highly improved resistance profiles and low activity for the hERG ion channel. J. Med. Chem 2020, 63, 1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang B; Chen W; Zhao T; Li Z; Jiang X; Ginex T; Vílchez D; Luque FJ; Kang D; Gao P; Zhang J; Tian Y; Daelemans D; De Clercq E; Pannecouque C; Zhan P; Liu X Exploiting the tolerant region I of the non-nucleoside reverse transcriptase inhibitor (NNRTI) binding pocket: discovery of potent diarylpyrimidine-typed HIV-1 NNRTIs against wild-type and E138K mutant virus with significantly improved water solubility and favorable safety profiles. J. Med. Chem 2019, 62, 2083–2098. [DOI] [PubMed] [Google Scholar]
- 16.Bauman JD; Das K; Ho WC; Baweja M; Himmel DM; Clark AD Jr.; Oren DA; Boyer PL; Hughes SH; Shatkin AJ; Arnold E Crystal engineering of HIV-1 reverse transcriptase for structure-based drug design. Nucleic Acids Res. 2008, 36, 5083–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das K; Bauman JD; Clark AD Jr.; Frenkel YV; Lewi PJ; Shatkin AJ; Hughes SH; Arnold E High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc.Natl. Acad. Sci. U. S. A 2008, 105, 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otwinowski Z; Minor W Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [DOI] [PubMed] [Google Scholar]
- 19.Adams PD; Afonine PV; Bunkoczi G; Chen VB; Davis IW; Echols N; Headd JJ; Hung LW; Kapral GJ; Grosse-Kunstleve RW; McCoy AJ; Moriarty NW; Oeffner R; Read RJ; Richardson DC; Richardson JS; Terwilliger TC; Zwart PH PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr., Sect. D: Biol. Crystallogr 2010, 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emsley P; Cowtan K Coot: model-building tools for molecular graphics. Acta Crystallogr., Sect. D: Biol. Crystallogr 2004, 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- 21.Kang D; Feng D; Ginex T; Zou J; Wei F; Zhao T; Huang B; Sun Y; Desta S; De Clercq E; Pannecouque C; Zhan P; Liu X Exploring the hydrophobic channel of NNIBP leads to the discovery of novel piperidine-substituted thiophene[3,2-d]pyrimidine derivatives as potent HIV-1 NNRTIs. Acta Pharm. Sin. B 2020, 10, 878–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang D; Sun Y; Murugan NA; Feng D; Wei F; Li J; Jiang X; De Clercq E; Pannecouque C; Zhan P; Liu X Structure-activity relationship exploration of NNIBP tolerant region I leads to potent HIV-1 NNRTIs. ACS Infect. Dis 2020, 6, 2225–2234. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda DG; Bauman JD; Challa JR; Patel D; Troxler T; Das K; Arnold E; Hochstrasser RM Snapshot of the equilibrium dynamics of a drug bound to HIV-1 reverse transcriptase. Nat. Chem 2013, 5, 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarafianos SG; Marchand B; Das K; Himmel DM; Parniak MA; Hughes SH; Arnold E Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J. Mol. Biol 2009, 385, 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darby JF; Hopkins AP; Shimizu S; Roberts SM; Brannigan JA; Turkenburg JP; Thomas GH; Hubbard RE; Fischer M Water networks can determine the affinity of ligand binding to proteins. J. Am. Chem. Soc 2019, 141, 15818–15826. [DOI] [PubMed] [Google Scholar]
- 26.Schiebel J; Gaspari R; Wulsdorf T; Ngo K; Sohn C; Schrader TE; Cavalli A; Ostermann A; Heine A; Klebe G Intriguing role of water in protein-ligand binding studied by neutron crystallography on trypsin complexes. Nat. Commun 2018, 9, 3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudling A; Orro A; Carlsson J Prediction of ordered water molecules in protein binding sites from molecular dynamics simulations: the impact of ligand binding on hydration networks. J. Chem. Inf. Model 2018, 58, 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huo Z; Zhang H; Kang D; Zhou Z; Wu G; Desta S; Zuo X; Wang Z; Jing L; Ding X; Daelemans D; De Clercq E; Pannecouque C; Zhan P; Liu X Discovery of novel diarylpyrimidine derivatives as potent HIV-1 NNRTIs targeting the "NNRTI adjacent" binding site. ACS Med. Chem. Lett 2018, 9, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang D; Huo Z; Wu G; Xu J; Zhan P; Liu X Novel fused pyrimidine and isoquinoline derivatives as potent HIV-1 NNRTIs: a patent evaluation of WO2016105532A1, WO2016105534A1 and WO2016105564A1. Expert Opin. Ther. Pat 2017, 27, 383–391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.