Abstract
Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the time the guidelines were drafted. Further controlled clinical studies may be needed to clarify aspects of this guideline. This guideline may be revised as necessary to account for changes in technology, new data, or other aspects of clinical practice. The recommendations in this document were based on reviewed studies using the GRADE and systematic review methodologies described in the Methods section.
This guideline is intended to be an educational device to provide information that may assist endoscopists in providing care to patients. This guideline is not a rule and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment. Clinical decisions in any particular case involve a complex analysis of the patient’s condition and available courses of action. Therefore, clinical considerations may lead an endoscopist to take a course of action that varies from these guidelines.
INTRODUCTION
Bile duct stones (choledocholithiasis) most frequently result from the migration of gallstones from the gallbladder into the biliary tree. Gallstones are the consequence of cholesterol supersaturation in bile, inadequate bile salt levels or function, and diminished contractility of the biliary epithelium because of the multifactorial effects of diet, hormones, and genetic predisposition. 1,2 Prospective population data reveal that 10% of American adults will develop symptomatic gallstones over the course of a decade.2 Greater than 700,000 will undergo outpatient cholecystectomy, and despite 436,000 being managed as outpatients, the annual cost exceeds 6.6 billion dollars.2,3 Among those with symptomatic cholelithiasis 10% to 20% have concomitant choledocholithiasis.4 An analysis using Diagnosis-Related Group (DRG); International Classification of Disease, 9th Revision (ICD-9); and Current Procedural Terminology (CPT) codes suggests that each episode of choledocholithiasis results in a cost of 9000 dollars.5 Furthermore, choledocholithiasis is the leading cause of acute pancreatitis, which results in 275,000 hospitalizations annually at a cost of 2.6 billion dollars.6
ERCP has transformed bile duct stone removal from a major operation to a minimally invasive procedure. Over the past 3 decades a number of strategies have been introduced to address even the most difficult bile duct stones, including large balloon papillary dilation and cholangioscopy-guided intraductal laser and electrohydraulic lithotripsy (EHL).7,8 However, a significant risk (6%-15%) of major adverse events associated with ERCP-guided treatment of bile duct stones has also been recognized.9,10 This has underscored the need to identify appropriate candidates for this procedure and to reserve biliary endoscopy for patients who have the highest probability of intraductal stones.
AIMS/SCOPE
The aim of this document is to provide evidence-based recommendations for the endoscopic evaluation and treatment of choledocholithiasis based on rigorous review and synthesis of the contemporary literature, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. The GRADE framework is a system for rating the quality of evidence and strength of recommendations that is comprehensive and transparent and has been recently adopted by the American Society for Gastrointestinal Endoscopy (ASGE).11 This document addresses the following 4 clinical questions:
What is the diagnostic utility of EUS versus MRCP to confirm choledocholithiasis in patients at intermediate risk of choledocholithiasis?
In patients with gallstone pancreatitis, what is the role of early ERCP?
In patients with large choledocholithiasis, is endoscopic papillary dilation after sphincterotomy favored over sphincterotomy alone?
What is the role of ERCP-guided intraductal therapy (EHL and laser lithotripsy) in patients with large and difficult choledocholithiasis?
Five additional clinical questions were addressed by the guideline panel using comprehensive literature review but not adhering to GRADE methodology: (1) Is same admission cholecystectomy necessary for patients with gallstone pancreatitis? (2) Are combinations of liver function tests, clinical characteristics, and transabdominal ultrasound (US) able to predict choledocholithiasis? (3) What is the optimal timing of ERCP for choledocholithiasis in patients undergoing cholecystectomy? (4) What is the role of ERCP in the management of Mirizzi syndrome and hepatolithiasis? (5) What is the role of bile duct stents in the management of choledocholithiasis?
METHODS
Overview
This article was prepared by a working group of the Standards of Practice (SOP) Committee of the ASGE in conjunction with a GRADE methodologist. This document includes a systematic review of available literature along with guidelines for the endoscopic diagnosis and management of choledocholithiasis. The panel members first formulated the relevant questions and agreed on patient-important outcomes for each question, which were subsequently approved by the ASGE Governing Board. The GRADE framework was used to develop clinical questions 1 to 4, systematically review the relevant evidence, rate the quality of evidence, and develop guidelines.12 All other clinical questions (5-9) were evaluated by comprehensive literature review, and recommendations were based on consensus opinion. All recommendations were drafted by the full panel during a face-to-face meeting on March 17, 2018 and approved by the SOP committee members and the ASGE Governing Board.
Panel composition and conflict of interest management
The panel was composed of a GRADE methodologist (S.S.), 4 content experts with expertise in systematic review and meta-analysis (J.L.B., S.A.F., B.J.Q., D.S.F.), a content expert independent of the SOP committee (P.Y.), a hepatobiliary surgeon (L.M.), committee chair (S.B.W.), and the other members of the SOP committee. The panel members disclosed possible intellectual and financial conflicts of interest in concordance with ASGE policies (https://www.asge.org/docs/default-source/about-asge/mission-and-governance/asge-conflict-of-interest-and-disclosure-policy.pdf).
Formulation of clinical questions
Nine clinical questions were developed by an iterative process on March 24, 2017 by the ASGE SOP Committee. Four of these questions were deemed to be amenable to a PICO approach. For each PICO question we identified the population (P), intervention (I), comparator (C), and outcomes of interest (O) (Table 1). Patient-important outcomes included confirmation and complete clearance of choledocholithiasis as well as associated adverse events. The clinical questions were approved by the ASGE Governing Board.
TABLE 1.
List of PICO questions addressed by Grading of Recommendations Assessment, Development and Evaluation methodology
| Population | Intervention | Comparator | Outcomes | Rating |
|---|---|---|---|---|
| 1. Patients with intermediate risk of choledocholithiasis | EUS | MRCP | 1) Confirmation of bile duct stones 2) Cost-effectiveness 3) Adverse events |
Critical Important Important |
| 2. Patients with gallstone pancreatitis | Early ERCP | Conservative management | 1) Local adverse events 2) Systemic adverse events 3) Mortality |
Critical Critical Critical |
| 3. Patients with large choledocholithiasis | Endoscopic papillary balloon dilation after endoscopic sphincterotomy | Endoscopic sphincterotomy | 1) Complete stone removal 2) Stone removal in 1 session 3) Adverse events 4) Procedure time 5) Need for mechanical lithotripsy |
Critical Important Important Important Important |
| 4. Patients with large and difficult choledocholithiasis | Intraductal therapy | Conventional lithotripsy | 1) Complete stone removal 2) Stone removal in 1 session 3) Adverse events 4) Procedure time |
Critical Important Important Important |
Literature search and study selection criteria
For each PICO question a comprehensive literature search for existing systematic reviews and meta-analyses was first performed. If no published review was identified, a systematic review and meta-analysis was performed. For PICO question one, two, and four, a librarian (LK) created and documented search strategies in the following bibliographic databases: Ovid Medline, Embase, Cochrane Library, and Web of Science on September 21, 2017. For PICO question three, a librarian (HS) created and documented search strategies in the following bibliographic databases: Ovid Medline, Embase, Cochrane Library, and Web of Science on November 16, 2017. A combination of subject headings (when available) and keywords were used for the concepts lithotripsy, balloon dilatation, sphincterotomy, and bile duct stones. No language or other limits were applied. See Supplementary Tables 3A-4D for full search strategies including database details. In an effort to capture unpublished studies LK and HS conducted searches in Google Scholar and ClinicalTrials.gov. Due to database constraints and lack of replicability, only the first 200 citations from Google Scholar were collected. Only English language citations were included. Cross-referencing and forward searches of the citation from articles fulfilling inclusion criteria were performed using the Web of Science. For PICO questions 2 and 3 only randomized controlled trials (RCTs) were included in the primary analyses. Given limitations in the available literature, randomized controlled and observational cohort studies were included in searches for PICO questions 1 and 4. Identified citations were imported into EndNote x7.7.1 (Clarivate Analytics, Philadelphia, Pa), duplicates remove by the Bramer method,13 and uploaded into Covidence (Melbourne, Australia).
Data extraction and statistical analysis
For questions that required meta-analysis, data extraction was performed by at least 2 independent reviewers. Pooled effects were derived using random effects models and the specific summary statistic depended on the relevant outcomes: overall diagnostic odds ratio (OR) for PICO 1, risk ratios for PICO 2, summary OR for PICO 3, and pooled proportions for PICO 4 using Stata 14.2 (Stata Corp, College Station, Tex). Indirect comparisons were used to estimate effect size and direction when direct comparisons were unavailable. Heterogeneity was quantified by the I2 statistic (I2) and evaluated by sensitivity analyses. Funnel plots and analyses stratified by study design were used to evaluate for publication bias and influence of study quality.
Certainty in evidence
Quality of evidence.
The certainty in the body of evidence (also known as quality of the evidence or confidence in the estimated effects) was assessed for each of the outcomes of interest, following the GRADE approach based on the following domains: risk of bias of individual studies, imprecision, inconsistency, indirectness of the evidence, and risk of publication bias. The certainty was categorized into 4 levels ranging from very low to high (Table 2).14 In this approach evidence from RCTs starts at high quality but can then be rated down based on assessment of above domains. On the other hand, evidence from observational studies starts at low quality and then is potentially downgraded based on the above variables or upgraded in case of dose–response relationship, large magnitude of effect, or confounding. For each PICO, an evidence profile or summary of findings table was created using the GRADEpro/GDT application (http://gdt.guidelinedevelopment.org/app).
TABLE 2.
Grading of Recommendations Assessment, Development and Evaluation categories of quality of evidence
| Categories | Symbols | Meaning | Interpretation |
|---|---|---|---|
| High | ⊕⊕⊕⊕ | We are confident that the true effect lies close to that of the estimate of the effect. | Further research is very unlikely to change our confidence in the estimate of the effect. |
| Moderate | ⊕⊕⊕ | We are moderately confident in the estimate of the effect; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. | Further research is likely to have an impact on our confidence in the estimate of the effect and may change the estimate. |
| Low | ⊕⊕ | Our confidence in the estimate of the effect is limited; the true effect may be substantially different from the estimate of the effect. | Further research is very likely to have an impact on our confidence in the estimate of the effect and is likely to change the estimate. |
| Very low | ⊕ | We have very little confidence in the estimate of the effect; the true effect is likely to be substantially different from the estimate of the effect. | Any estimate of the effect is very uncertain. |
Development of recommendations.
During an inperson meeting, the panel developed recommendations based on the following: the certainty in the evidence, the overall balance of benefits and harms, values and preferences associated with the decision, and available data on resource utilization and cost-effectiveness. The final wording of the recommendations (including direction and strength) was decided by consensus and was approved by all members of the panel. The recommendations are labeled as either “strong” or “conditional” according to the GRADE approach. The words “the guideline panel recommend” are used for strong recommendations and “suggest” for conditional recommendations. Table 3 provides the suggested interpretation of strong and conditional recommendations by patients, clinicians, and healthcare policymakers.
TABLE 3.
Interpretation of definitions of strength of recommendation using Grading of Recommendations Assessment, Development and Evaluation framework
| Implications for | Strong recommendation | Conditional recommendation |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | Most individuals in this situation would want the suggested course of action, but many would not. |
| Clinicians | Most individuals should receive the intervention. Formal decision aids are not likely to be needed to help individual patients make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with his or her values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their values and preferences. |
| Policymakers | The recommendation can be adopted as policy in most situations. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. | Policymaking will require substantial debate and involvement of various stakeholders. |
Patient values and preferences.
Few publications addressing choledocholithiasis have measured or addressed patient values and preferences. Single-step treatment (combined laparoscopic cholecystectomy and bile duct exploration [LC-BDE]) was associated with higher patient satisfaction scores than the strategy of ERCP before cholecystectomy.15 This was attributed to shortened hospital stay. In a trial of EUS/ERCP before cholecystectomy versus ERCP after cholecystectomy in patients with a positive intraoperative cholangiogram, quality of life outcomes were assessed using EuroQol Group, 5-level (EQ-5D-5L) scores.16 Although the latter strategy was associated with shorter hospital stay and less procedures, there was no statistically significant difference in the EQ-5D-5L scores for the 2 approaches.
Cost-effectiveness.
Limited data address the cost-effectiveness of evaluation and management strategies in patients with choledocholithiasis. The most extensive modeling study assessed the role of EUS and MRCP in patients at intermediate risk of choledocholithiasis. It appears that EUS and MRCP result in cost-saving by avoiding the expense and adverse events of ERCP.17-20 Cost-effectiveness models using the British National Health Service data revealed that use of MRCP rather than ERCP to evaluate patients at intermediate risk (37% likelihood of stones) resulted in an increase of 0.11 (range, 0-.30) quality-adjusted life-years and a savings of 149 British pounds per patient.21 A similar approach using Medicare costs for financial modeling revealed that EUS was more cost-effective than intraoperative cholangiography (IOC) and ERCP for patients with an intermediate (15%-45%) risk of bile duct stones.22 Scheiman et al17 compared the cost of MRCP versus EUS for patients at intermediate risk of stones using Medicare reimbursements as an equivalent for cost ($407 for MRCP vs $680 for EUS); when the cost of avoiding ERCP and related adverse events was included in the model, the cost per patient for EUS ($1111) was slightly less than MRCP ($1145). However, further analysis of this trial by the same authors in a subsequent publication revealed that if sensitivity of MRCP increased to .6 it would be the less costly strategy and if greater than .75 would dominate.20 In a study of intermediate- and high-risk patients that compared the cost of EUS before ERCP versus ERCP, the former strategy was more cost-effective.18
Several studies have also compared costs for single-step treatment (LC-BDE) for concomitant choledocholithiasis and cholelithiasis versus ERCP before or after cholecystectomy. In a randomized trial comparing LC-BDE versus ERCP followed by LC, Bansal et al15 determined that the former was less costly with an incremental cost-effectiveness ratio, measuring the difference in cost versus effect of the 2 approaches, of $1182.70. In a similar RCT Rogers et al23 found a trend toward lower total costs for LC-BDE versus ERCP before LC and significantly lower professional fees ($4820 vs $6139).
RESULTS
The recommendations and quality of evidence for the 4 clinical questions that were addressed using the GRADE framework are summarized in Table 4.
TABLE 4.
Summary of recommendations with strength of recommendation and quality of evidence derived by Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology
| Statement | Strength of recommendation |
Quality of evidence |
|---|---|---|
| 1. In patients with intermediate risk of choledocholithiasis we suggest either EUS or MRCP given high specificity; consider factors including patient preference, local expertise, and availability. | Conditional | Low |
| 2. In patients with gallstone pancreatitis without cholangitis or biliary obstruction/choledocholithiasis we recommend against urgent (<48 hours) ERCP. | Strong | Low |
| 3. In patients with large choledocholithiasis we suggest performing large balloon dilation after sphincterotomy rather than endoscopic sphincterotomy alone. | Conditional | Moderate |
| 4. For patients with large and difficult choledocholithiasis we suggest intraductal therapy or conventional therapy with papillary dilation; this may be impacted by local expertise, cost, patient and physician preferences. | Conditional | Very low |
Clinical questions for which the GRADE framework was used
Question 1: What is the diagnostic utility of EUS versus MRCP to confirm choledocholithiasis in patients at intermediate risk?
Recommendation: In patients with intermediate risk (10%-50%24) of choledocholithiasis, we suggest either EUS or MRCP to confirm the diagnosis; the choice of test should take into account factors such as patient preference, local expertise, and availability of resources (conditional recommendation, low quality of evidence).
Summary of the evidence.
The outcomes of interest for this clinical question included sensitivity and specificity of the 2 diagnostic modalities. No RCTs compared EUS with MRCP, but several prospective observational trials comparing MRCP and EUS were identified. The evidence for MRCP versus EUS for choledocholithiasis was evaluated by recent systematic review and meta-analysis by Meeralam et al.25 The evidence profiles for this question are presented in Tables 5A and 5B.
TABLE 5A.
PICO question 1A: Should EUS be used to diagnose choledocholithiasis in low to intermediate risk of disease?
| No. of studies and patients |
Factors that may decrease certainty of evidence |
Effect per 1000 patients tested |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | study design |
Risk of bias |
Indirectness | Inconsistency | Imprecision | Publication bias |
Pretest probability of 5% |
Pretest probability of 20% |
Pretest probability of 50% |
Test accuracy | |
| True positives (patients with [target condition]) | 5 studies 272 patients | Cross-sectional (cohort type accuracy study) | Not serious | Not serious | Serious* | Serious† | None | 49 (46-50) | 194 (182-198) | 485 (455-495) | ⊕⊕○○ LOW |
| False negatives (patients incorrectly classified as not having [target condition]) | 1 (0-4) | 6 (2-18) | 15 (5-45) | ||||||||
| True negatives (patients without [target condition]) | 5 studies 272 patients | Cross-sectional (cohort type accuracy study) | Not serious | Not serious | Serious* | Serious† | None | 855 (789-893) | 720 (664-752) | 450 (415-470) | ⊕⊕○○ LOW |
| False positives (patients incorrectly classified as having [target condition]) | 95 (57-161) | 80 (48-136) | 50 (30-85) | ||||||||
We rated down for inconsistency because the confidence intervals did not overlap and the I2 for EUS specificity was 54.2%.
We rated down for imprecision because of wide confidence intervals.
TABLE 5B.
PICO question1B: Should MRCP be used to diagnose choledocholithiasis in patients with low or intermediate risk for it?
| No. of studies and patients |
Factors that may decrease certainty of evidence |
Effect per 1000 patients tested |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Study design |
Risk of bias |
Indirectness | Inconsistency | Imprecision | Publication bias |
Pretest probability of 5% |
Pretest probability of 20% |
Pretest probability of 50% |
Test accuracy |
|
| True positives (patients with choledocholithiasis) | 5 studies 272 patients | Cross-sectional (cohort type accuracy study) | Not serious | Not serious | Serious* | Not serious | None | 44 (40-47) | 174 (160-186) | 435 (400-465) | ⊕⊕⊕○ MODERATE |
| False negatives (patients incorrectly classified as not having choledocholithiasis) | 6 (3-10) | 26 (14-40) | 65 (35-100) | ||||||||
| True negatives (patients without choledocholithiasis) | 5 studies 272 patients | Cross-sectional (cohort type accuracy study) | Not serious | Not serious | Serious* | Not serious | None | 874 (827-912) | 736 (696-768) | 460 (435-480) | ⊕⊕⊕○ MODERATE |
| False positives (patients incorrectly classified as having choledocholithiasis) | 76 (38-123) | 64 (32-104) | 40 (20-65) | ||||||||
We rated down for inconsistency; the I2 was 55.5% for MRCP sensitivity and 66.8% for specificity.
Meeralam et al25 included studies that directly compared MRCP with EUS and used a criterion standard for verification (ERCP or IOC and clinical follow-up of ≥3 months). The authors identified 5 prospective comparative studies (272 patients; Supplementary Table 1, available online at www.giejournal.org). The pooled sensitivity of EUS was higher compared with MRCP (.97 [95% confidence interval [CI], .91-.99], I2 = 15.1%, vs .87 [95% CI, .80-.93], I2 = 55.5, P = .006). However, there was no difference in specificity between EUS and MRCP (.90 [95% CI, .83-.94], I2 = 54.2%, vs .92 [95% CI, .87-.96], I2 = 68.8%, P = .42). The diagnostic OR was greater for EUS (162.5 [95% CI, 54.0-489.3], I2 = 0) than MRCP (79.0 [95% CI, 23.8-262.2], I2 = .22.3, P = .008).
The systematic review and meta-analysis did not formally address the outcome of cost-effectiveness. Among the 5 included studies, only Scheiman et al17 specifically addressed cost of EUS versus MRCP, although the financial analysis included patients with distal biliary strictures in addition to those with choledocholithiasis. As described previously in the cost-effectiveness section, EUS was favored over MRCP, but this did not take into account the cost of anesthesia. Additionally, this analysis assumed a very modest sensitivity of .4 for MRCP. MRCP was more cost-effective than EUS when the sensitivity of MRCP was assumed to be greater than .6.20 Additionally, the meta-analysis did not address adverse events. Among the included trials, 2 studies reported no serious adverse events associated with EUS or MRCP, and the rate of adverse events was not documented in other reports.17,26-29 Nevertheless, diagnostic EUS used to evaluate for choledocholithiasis is associated with a low but finite (.02%-.07%) risk of perforation.30
Certainty in the evidence.
Although the 5 trials were observational, they were prospective, comparative, and blinded (Supplementary Table 2, available online at www.giejournal.org). The authors used the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool to assess for risk of bias and found that none of the included trials had high likelihood of bias; 4 were intermediate and 1 low (Supplementary Fig. 1, available online at www.giejournal.org). The quality of evidence was rated down for inconsistency given the high I2 and for imprecision suggested by nonoverlapping CIs among the included studies (Tables 5A and 5B). Hence, the overall quality of evidence for the outcome was rated to be low for EUS but moderate for MRCP (rated down for inconsistency).
Considerations.
The current evidence indicates that EUS and MRCP have high specificity for choledocholithiasis, although EUS may be more sensitive. However, an important consideration is the cost of EUS, particularly if anesthesia services are used for sedation, and the fact that it is operator-dependent. Similarly, patient inconvenience related to the procedure may influence decisionmaking. The meta-analysis did not address cost, adverse events, and patient preferences for EUS versus MRCP. Additionally, the studies have variable inclusion criteria, and a significant number of patients were ineligible for 1 or both tests. Given the low quality of evidence supporting this recommendation, it is likely that further evidence on adverse events, cost, and patient experience may impact future recommendations.
Discussion.
EUS has a comparable accuracy with diagnostic ERCP for evaluation of choledocholithiasis and is associated with a significantly lower adverse event rate.31 Among patients at indeterminate risk, EUS before ERCP may obviate the need for the latter.31,32 MRCP overcomes the limitations of transabdominal US, particularly the obfuscation of the distal bile duct because of intraductal air.19 In the meta-analysis of head-to head studies by Meeralam et al,24 the specificities of both EUS and MRCP were very high (.97 vs .92), consistent with a Cochrane meta-analysis,33 which primarily used indirect comparison of the 2 tests. In the Cochrane review the sensitivity of MRCP and EUS were also comparable.33 However, in the meta-analysis of direct comparison studies by Meeralam et al24 the sensitivity of EUS was superior to MRCP. In the 2 individual studies with the largest discrepancy between the sensitivity of EUS and MRCP, the false-negative MRCPs were for small stones (6 mm in diameter).17,27 Kondo et al27 proposed that EUS be considered in those with a negative MRCP. Although this may not be necessary unless there is strong persistent clinical suspicion of choledocholithiasis, a tailored approach deserves additional study.
Nevertheless, the relative cost of EUS versus MRCP in the era in which monitored anesthesia care is frequently used for EUS is unknown. Furthermore, although low, the adverse event rate of EUS is not zero.30,31 Although more widely available, EUS is also not universally performed in community health centers, and requirement for travel to a referral center may render it inconvenient. Additionally, prospective studies reveals that learning curves for EUS are highly variable, with approximately one fourth not achieving competence at the end of advanced endoscopy training, highlighting the need for more standardized approaches to training and evaluation for EUS.34 The implications of this are that performance characteristics of EUS outside of the research setting are likely to be even more variable, leading to lower diagnostic test accuracy. Other considerations include patient-specific factors that may limit the feasibility of using a specific test, such as claustrophobia and pacemakers (which may preclude MRCP) or a history of GI bypass procedures (which may preclude EUS).
Question 2: In patients with gallstone pancreatitis, what is the role of early ERCP?
Recommendation: In patients with gallstone pancreatitis without cholangitis or biliary obstruction/choledocholithiasis we recommend against urgent (within 48 hours) ERCP (strong recommendation, low quality of evidence).
Summary of the evidence.
The patient-important outcomes for this clinical question were mortality and systemic and local adverse events of pancreatitis (critical). This question had been previously addressed in a Cochrane systematic review conducted by Tse and Yuan in 201235 in which the authors systematically reviewed the literature from inception until January 2012 for the Cochrane Database of Systematic Reviews. To inform this guideline, and based on our request, Tse and Yuan used their initial search strategy and carried it forward to January 2018. Their search revealed 991 additional references during this period. However, after abstract and manual review no studies fulfilling the inclusion criteria for their prior meta-analysis were identified. The evidence profile for this question is presented in Table 6A.
TABLE 6A.
PICO question 2: Early ERCP compared with conservative management for management of gallstone pancreatitis
| Certainty assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | study design |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations |
Early ERCP | Conservative management | Relative (95% CI) | Absolute (95% CI) | Certainty | Importance |
| Reduction in all-cause mortality | ||||||||||||
| 5 | Randomized trials | Serious* | Serious† | Not serious | Not serious | None | 19/326 (5.8%) | 19/318 (6.0%) | RR, .74 (.18-3.03) | 16 fewer per 1000 (from 49 fewer to 121 more) | ⊕⊕○○ LOW |
CRITICAL |
| Reduction in local adverse events (defined by the Atlanta classification) | ||||||||||||
| 4 | Randomized trials |
Serious* | Not serious | Not serious | Not serious | None | 35/262 (13.4%) | 38/255 (14.9%) | RR, .86 (.52-1.43) | 21 fewer per 1000 (from 72 fewer to 64 more) | ⊕⊕○○ MODERATE |
CRITICAL |
| Reduction in systemic adverse events (defined by the Atlanta classification) | ||||||||||||
| 4 | Randomized trials |
Serious* | Not serious | Not serious | Not serious | None | 17/200 (8.5%) | 31/206 (15.0%) | RR, .59 (.31-1.11) | 62 fewer per 1000 (from 104 fewer to 17 more) | ⊕⊕○○ MODERATE |
CRITICAL |
CI, Confidence interval; RR, risk ratio.
We rated down for bias given low Cochrane Collaboration RCT Bias score.
We rated down for inconsistency; I2 = 62% for mortality.
Five RCTs informed the mortality outcome and 7 RCTs informed the outcomes of systemic and local adverse events.35 Early ERCP does not reduce mortality relative to a conservative approach (risk ratio [RR], .74 [95% CI, .18-3.03], I2 = 62%). Early ERCP also did not diminish the risk of local (RR, .85 [95% CI, .52-1.43], I2 = 12%) or systemic adverse events (RR, .59 [95% CI, .31-1.11], I2 = 14%). Conservative treatment included analgesics, intravenous fluids, selective ERCP for cholangitis, rising bilirubin, or clinical deterioration.
To investigate heterogeneity for the main result addressing overall mortality, the authors performed several subgroup analyses. Initial trials suggested that early ERCP would benefit those with predicted severe but not mild pancreatitis.36,37 The meta-analysis did not show a reduction in mortality, systemic, or local adverse events for patients with predicted severe disease. However, subgroup analysis of studies, which included patients with cholangitis, revealed that early ERCP reduced mortality (RR, .2 [95% CI, .06-.68], I2 = 0), systemic (RR, .37 [95% CI, .18-.78], I2 = 0), and local adverse events (RR, .45 [95% CI, .20-.99], I2 = 0) in this patient population. The evidence profiles for studies that included patients with cholangitis are presented in Table 6B. Stratified analysis of studies that included patients with biliary obstruction demonstrated a trend toward decreased local (RR, .53 [95% CI, .26-1.07], I2 = 0) and systemic adverse events (RR, .56 [95% CI, .30-1.02], I2 = 10) but not mortality (RR, .38 [95% CI, .12-1.17], I2 = 11). With regard to adverse events of bleeding, there was no difference with early ERCP (RR, 1.58 [95% CI, .54-4.63], I2 = 0) compared with conservative therapy. No episodes of perforation or cholangitis were reported in these studies. No episodes of post-ERCP pancreatitis were reported, although it was acknowledged that this is difficult to measure in patients who already have pancreatitis.
TABLE 6B.
PICO question 2: Early ERCP compared with conservative management for management of gallstone pancreatitis and cholangitis
| Certainty assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations |
Early ERCP | Conservative management | Relative (95% CI) | Absolute (95% CI) | Certainty | Importance |
| Reduction in all-cause mortality | ||||||||||||
| 5 | Randomized Trials | Serious* | Not serious | Not serious | Not serious | None | 2/200 (1.0%) | 15/215 (7.0%) | RR, .20 (.06-.68) | 56 fewer per 1,000 (from 22 fewer to 66 fewer) | ⊕⊕○○ MODERATE |
CRITICAL |
| Reduction in local adverse events (defined by the Atlanta classification) | ||||||||||||
| 3 | Randomized Trials | Serious* | Not serious | Not serious | Not serious | None | 8/115 (7.0%) | 19/121 (15.7%) | RR, .45 (.20-.99) | 86 fewer per 1,000 (from 2 fewer to 126 fewer) | ⊕⊕○○ MODERATE |
CRITICAL |
| Reduction in systemic adverse events (defined by the Atlanta classification) | ||||||||||||
| 4 | Randomized trials | Serious* | Not serious | Not serious | Not serious | None | 8/179 (4.5%) | 25/184 (13.6%) | RR, .37 (.18-.78) | 86 fewer per 1000 (from 30 fewer to 111 fewer) | ⊕⊕⊕○ MODERATE |
CRITICAL |
CI, Confidence interval; RR, risk ratio.
We rated down for bias given low Cochrane Collaboration RCT Bias score.
Certainty in the evidence.
Although the included studies were RCTs, the quality of evidence was rated down given that all but 1 trial had an unclear or low risk of bias (Supplementary Fig. 2, available online at www.giejournal.org). Specifically, only 2 studies reported the use of random sequence generation for randomization, and a single trial reported the use of concealed allocation. For the outcome of mortality, we also rated down for inconsistency given the high I2. The certainty in the evidence was moderate for local and systemic adverse events.
Considerations.
Although the overall quality of evidence across outcomes was low, the panel members made a strong recommendation against early ERCP in those with gallstone pancreatitis (but without cholangitis or biliary obstruction) given the lack of benefit and potential for increased harm of ERCP. Studies included in the meta-analysis differed in how early ERCP was defined; some studies used time from admission to procedure time versus time from symptoms, whereas others used the time frame of 48 to 72 hours. The committee believed that early ERCP defined as within 48 hours was most appropriate given that urgent ERCP is of benefit in those with cholangitis with or without gallstone pancreatitis if done in the first 48 hours.38,39 There was also extensive panel discussion regarding early ERCP for patients with gallstone pancreatitis and concomitant biliary obstruction or choledocholithiasis given a favorable but nonsignificant trend. The panel voted to exclude patients with simultaneous biliary obstruction or choledocholithiasis and gallstone pancreatitis from the recommendation against early ERCP for gallstone pancreatitis.
Discussion.
The concept of early ERCP for gallstone pancreatitis originates from observational surgical reports that suggested operative relief of bile duct obstruction in gallstone pancreatitis decreased mortality.40,41 Those who underwent surgical exploration at >48 hours exhibited more severe histologic lesions than those who had ampullary gallstone impaction for ≤48 hours.40,41 In this multihit theory of gallstone pancreatitis it is postulated that passage of small calculi through the ampulla initiates acute pancreatitis and larger choledocholithiasis persistently obstructed at the papilla result in severe disease.42 However, an RCT of early surgery for gallstone pancreatitis demonstrated that early intervention resulted in increased morbidity and mortality.43 This favored an alternate “single-hit” hypothesis that gallstone pancreatitis results from passage of an initial gallstone through the ampulla and additional surgical or endoscopic manipulation of the region is more likely to exacerbate than alleviate inflammation. Additional supportive evidence for this approach is found in endoscopic series in which most patients with gallstone pancreatitis have negative cholangiography even among those with rising liver tests.44,45
In their meta-analysis, Tse and Yuan35 demonstrated that early ERCP does not decrease the mortality or adverse events of gallstone pancreatitis. The panel’s recommendation against ERCP was thus driven by the need to minimize risk and undue harm; ERCP carries a risk of harm in addition to cost and inconvenience without clear benefit. The results of the meta-analysis differed from the findings of the earlier trials by Fan et al36 and Neoptolemos et al.37 However, these earlier trials included patients with concomitant pancreatitis and cholangitis. These trials also demonstrated a greater benefit for those with predicted severe pancreatitis, which was not seen in later trials. However, they used predictive scoring systems such as Ranson’s and Glasgow whose components (ie, white blood cell count) are also elevated in cholangitis.46 Our recommendation against early ERCP does not apply to patients with gallstone pancreatitis and cholangitis, given the demonstrated benefit of ERCP in the setting of cholangitis.38,39 More recent reports by Oria et al47 and Folsch et al48 used more focused inclusion criterion, which enables a more nuanced application of their findings. Both studies excluded patients with cholangitis, which benefits from early endoscopic therapy.38,39 Folsch et al excluded patients with a bilirubin <5 mg/dL and instituted ERCP for patients who developed fever, an increase of bilirubin >3 mg/dL, and refractory biliary type pain.
One challenge in informing the recommendation for early ERCP for gallstone pancreatitis is that a method to diagnose post-ERCP pancreatitis in those with concomitant gallstone pancreatitis is lacking.35 Given this limitation, Tse and Yuan could not directly compare adverse events for early versus conservative management. Nevertheless, ERCP is associated with a significant 9.7% to 14.7% risk of post ERCP pancreatitis and .9% to 6% risk of other adverse events including hemorrhage, perforation, and cholangitis.49,50 Future trials would also be improved by adoption of consistent terminology to define inclusion criteria and score outcomes such as the Tokyo cholangitis criterion or Revised Atlanta Pancreatitis classification.51,52 These recommendations are consistent with the recent American Gastroenterological Association Institute Guidelines on Initial Management of Acute Pancreatitis that also suggest against routine use of urgent ERCP for gallstone pancreatitis.53
Question 3: In patients with large bile duct stones, is endoscopic papillary dilation after sphincterotomy favored over sphincterotomy alone?
Recommendation: In patients with large bile duct stones, we suggest performing endoscopic sphincterotomy followed by large balloon dilation (ES-LBD) rather than endoscopic sphincterotomy (ES) alone (conditional recommendation, moderate evidence).
Summary of the evidence.
The patient-important outcomes for this clinical question were bile duct clearance, adverse events, and the requirement for mechanical lithotripsy. The evidence profile is presented in Table 7.
TABLE 7.
PICO question 3: Large balloon papillary dilation Dsphincterotomy compared with sphincterotomy alone for large choledocholithiasis
| Certainty assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Papillary dilation + sphincterotomy |
Sphincterotomy alone |
Relative (95% CI) |
Absolute (95% CI) | Certainty | Importance |
| Overall clearance | ||||||||||||
| 9 | Randomized trials | Not serious | Not serious | Not serious | Serious* | None | 534/551 (96.9%) | 500/551 (90.7%) | OR 2.8 (1.4-5.7) | 57 more per 1000 (from 25 more to 75 more) | ⊕⊕⊕○ MODERATE |
CRITICAL |
| Need for mechanical lithotripsy | ||||||||||||
| 8 | Randomized trials | Not serious | Not serious | Not serious | Serious* | None | 50/551 (9.1%) | 144/551 (26.1%) | OR .27 (.16-.46) | 174 fewer per 1000 (from 121 fewer to 208 fewer) | ⊕⊕⊕○ MODERATE |
CRITICAL |
| All adverse events | ||||||||||||
| 8 | Randomized trials | Not serious | Not serious | Not serious | Serious* | None | 33/551 (6.0%) | 45/551 (8.2%) | OR .79 (.45-1.38) | 16 fewer per 1000 (from 28 more to 43 fewer) | ⊕⊕⊕○ MODERATE |
CRITICAL |
CI, Confidence interval; OR, odds ratio
We rated down for imprecision because of wide confidence intervals.
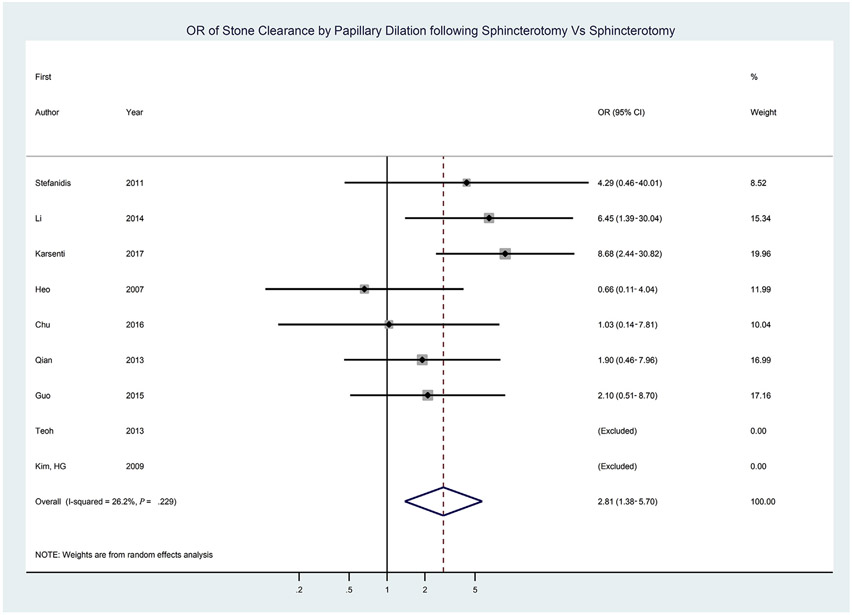
We conducted a systematic review and meta-analysis to evaluate these outcomes. A systematic search in collaboration with an information specialist revealed 4233 abstracts (Supplementary Table 3, available online at www.giejournal.org). Authors of the studies were contacted if there was concern for longitudinal publication of the same cohort and to obtain missing information. Studies that reported ES-LBD for stones of a wide range of diameters were not included unless the subset of results for stones ≥1 cm were reported. We identified 9 RCTs comparing ES-LBD versus ES alone. These studies reported on 551 patients who underwent ES-LBD and 551 patients who received ES alone. Based on random effects models, patients were more likely to have complete clearance of large stones by ES-LBD versus ES alone (pooled OR, 2.8 [95% CI, 1.4-5.7], I2 = 26%) (Fig. 1, Table 8). A funnel plot showed low likelihood of publication bias. No significant difference in first procedure clearance for ES-LBD versus ES (OR, 1.8 [95% CI, .9-3.7], I2 = 63%) was found. There was a decreased requirement for mechanical lithotripsy in those treated with ES-LBD versus ES (OR, .2 [95% CI, .1-.7], I2 = 82%) (Supplementary Fig. 3). For the outcome of adverse events, there was no difference in overall adverse events (OR, .8 [95% CI, .5-1.4], I2 = 0) or specific adverse events of cholangitis, pancreatitis, bleeding, or perforation.
Figure 1.
Forest plot of randomized trials comparing endoscopic sphincterotomy followed by large balloon dilation versus endoscopic sphincterotomy for stone clearance.
TABLE 8.
Procedural features of randomized trials comparing ES-LBD versus ES
| First Author |
Year | Stone size (mm) |
Maximum balloon size (mm) |
Extent of sphincter incision (%) |
Procedure cost: ES-LBD |
Procedure cost: ES |
Procedure duration: ES-LBD (min) |
Procedure duration: ES (min) |
|---|---|---|---|---|---|---|---|---|
| Heo60 | 2007 | 10-40 | 20 | 50 | — | — | — | — |
| Kim62 | 2009 | >15 | 18 | 50 | — | — | 18+12 | 19+13 |
| Stefanidis70 | 2011 | 12-20 | 20 | 100 | — | — | — | — |
| Teoh8 | 2013 | Subset >13 | 15 | 33-50 | — | — | 24.3+12.9* | 27.2+16.9* |
| Jun Bo65 | 2013 | >15 | 20 | 33 | — | — | 14.5+8.4 | 15.9+8.8 |
| Li59 | 2014 | Subset >12 | 18 | 33 | — | — | 38.6+15.5 | 47.1+20.2 |
| Guo136 | 2015 | >10 | 15 | 33-66 | — | — | 20+11 | 20+10 |
| Chu58 | 2017 | >10 | 20 | 33 | 18,021 (18,021-22,541) | 13,199 (13,199-17,719) | — | — |
| Karsenti57 | 2017 | >13 | 20 | 100 | 447 euros 447 euros | 449 euros 709 euros | 30 (22-48) 30 (22-48) | 35 (25-50) 45† |
ES-LBD, Endoscopic sphincterotomy followed by large balloon dilation; —, data not available.
Procedure durations are for entire published cohort, which includes smaller stones.
Procedure duration and cost when mechanical lithotripsy is used.
In a sensitivity analysis, we included the 22 observational comparative reports in addition to the 9 RCTs (ES-LBD, 1939 patients; ES alone, 2148 patients). There was greater overall clearance (OR, 2.33 [95% CI, 1.66-3.28], I2 = 30%) and first procedure clearance (OR, 2.09 [95% CI, 1.41-3.09], I2 = 66%) in the ES-LBD cohorts (Supplementary Figs. 4 and 5, available online at www.giejournal.org).
Certainty in the evidence.
There were no issues with risk of bias as summarized in Supplementary Figure 6. The quality of evidence was rated down for imprecision (Table 7). There did not appear to be serious indirectness or inconsistency. Overall certainty was determined to be moderate.
Considerations.
The panel had significant discussion about the overall quality of evidence and the balance between benefit and harm. There was acknowledgment that the heterogeneous classification of adverse events made it difficult to compare the proportions of patients who develop adverse events and, in particular, severe adverse events, combined with variability in techniques. The panel voted to make a conditional recommendation for ES-LBD over ES. Additional studies using well-characterized definitions of adverse events as well as more standardized balloon sizes and sphincterotomy extent may impact this recommendation. Furthermore, studies on cost and procedure times are also needed.
Discussion.
ES-LBD was developed to facilitate removal of large stones and to avoid the increased rates of pancreatitis seen when balloon dilation was performed without sphincterotomy for choledocholithiasis.54,55 Although the relative performance varies among the 9 RCTs comparing ES-LBD and ES alone, the summary effect demonstrated greater overall successful stone removal for ES-LBD. When all comparative trials (including observational studies) were included, a consistent finding was observed. A recent meta-analysis of RCT by Park et al56 reported greater first procedure clearance for ES-LBD than ES among those with large and small stone sizes. In contrast to the study by Park et al, we include 2 additional RCTs published in 201757,58 and only included the subsets of studies by Teoh and Li, which reported specific results for large stones (Table 8).8,59 Another important consideration was heterogeneity in the techniques of ES-LBD: The maximum size of the papillary dilation balloon ranged from 15 to 20 mm, some groups used a complete sphincterotomy from the biliary orifice to the horizontal fold, whereas others made an incision 33% to 66% of the distance. Also, the minimal stone size for inclusion varied from 10 to 15 mm.
Summary estimates suggest that adverse events for ES-LBD were comparable with ES alone. Nevertheless, their classification was highly variable. Although the Cotton Consensus criteria were ostensibly used in most studies, it was subjected to various “modifications.”60-63 Stefanidis et al70 reported a high rate of cholangitis with ES, but the cases were all mild and responded to conservative treatment. In a recent multicenter study, Karsenti et al57 reported comparable adverse events for ES-LBD versus ES but described that 2 patients in the former group developed life-threatening adverse events, whereas those after ES were mild. In a large multicenter retrospective series by Park et al,64 it was reported that 10% (95/946) of ES-LBD procedures were associated with adverse events. Multivariate analysis indicated that complete ES (to transverse fold) was associated with bleeding and long distal strictures associated with perforation. The authors advocate avoiding a complete ES before LBD, and the approach should be used with caution in those with distal biliary strictures. It was also recommended not to dilate to greater than the size of the bile duct. Standardized granular definitions of adverse events with specific classification by severity are needed to better compare these methods. Alternative approaches to ES-LBD such as laser lithotripsy may be a consideration in patients with specific anatomic features such as distal biliary stricture.
The RCTs provided little evidence regarding cost or length of hospitalization associated with these approaches. Jun Bo et al65 reported a shorter length of stay for those managed with ES-LBD versus ES (11 days vs 15 days). Nevertheless, the need for greater than a week of hospitalization in both groups is unclear.65 Relative procedural costs ranged from higher for ES-LBD,65 similar,57 or less particularly if ES was supplemented with mechanical lithotripsy.57 Although not limited to patients with large stones, Teoh et al8 reported that overall cost of hospitalization was less for ES-LBD, $ (U.S.) 5025 (interquartile range [IQR], 4150-5235), than ES, $6005 (IQR, 4462-5441). In an observational study of ES-LBD versus ES, Itoi et al75 reported shorter procedure duration (32 vs 40 minutes) and decreased fluoroscopy time (13 vs 22 minutes). The randomized trial by Li et al59 replicated these data but included patients with all stone sizes. Among individual trials of ES-LBD versus ES for large stones there were no significant differences in procedure time.57,65,66 However, variable definitions of procedure duration (ie, cannulation to drain placement vs time from scope introduction to removal) prevented quantitative pooling of the individual trials for this outcome. The trend toward greater first procedure clearance could be proposed as a surrogate of overall procedure time potentially in favor of ES-LBD. Trials examining cost, procedure time, and hospital length are needed to more comprehensively compare these approaches.
Question 4: What is the role of intraductal versus conventional therapy in patients with large and difficult choledocholithiasis?
Recommendation: For patients with difficult and large choledocholithiasis we suggest intraductal therapy or conventional therapy with papillary dilation. The choice of therapy may be impacted by local expertise, cost, and patient and physician preferences (conditional recommendation, very low quality of evidence).
Summary of the evidence.
The outcomes of interest for this clinical question were complete stone removal (critical), removal in the first session (important), and differences in adverse events (important) or procedure duration (important). Only 1 RCT addressed this question.67 Therefore, evidence from observational studies was also used. The evidence profile for this question is provided in Table 9.
TABLE 9.
PICO question 4: Intraductal therapy compared with conventional therapy for difficult bile duct stones
| Certainty assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations |
Intraductal therapy |
Conventional therapy |
Relative (95% CI) | Absolute (95% CI) | Certainty | Importance |
| Complete stone clearance | ||||||||||||
| 182 | Observational studies | Not serious* | Serious† | Serious‡ | Not serious | None | 2023/2204 (91.8%) | 10311/11384 (90.6%) | RR 1.01 (.96-1.06) | 9 more per 1000 (from 36 fewer to 54 more) | ⊕○○○ VERY LOW |
CRITICAL |
| Need for mechanical lithotripsy | ||||||||||||
| 121 | Observational studies | Serious* | Serious† | Serious‡ | Not serious | None | 125/1029 (12.1%) | 2620/9505 (27.6%) | RR, .44 (.32-.56) | 154 fewer per 1000 (from 121 fewer to 187 fewer) | ⊕○○○ VERY LOW |
CRITICAL |
| Overall adverse events | ||||||||||||
| 167 | Observational studies | Serious* | Serious* | Serious‡ | Not serious | None | 164/1891 (8.7%) | 1080/11080 (9.7%) | Not assessable | ⊕○○○ VERY LOW |
CRITICAL | |
| Complete stone clearance without balloon dilation | ||||||||||||
| 90 | Observational studies | Serious* | Serious† | Serious‡ | Not serious | None | 1873/2038 (91.9%) | 1932/2448 (78.9%) | RR 1.17 (1.09-1.24) | 134 more per 1000 (from 71 more to 189 more) | ⊕○○○ VERY LOW |
CRITICAL |
CI, Confidence interval; RR, risk ratio.
We rated down for bias given overall low scores on Newcastle-Ottawa score.
We rated down for inconsistency; the I2 was 91% for conventional therapy and 60% for intraductal therapy.
We down for indirectness given indirect comparison and calculations.
We conducted a systematic review and meta-analysis to compare intraductal versus conventional treatment for difficult and large choledocholithiasis. Intraductal therapy included cholangioscopy and fluoroscopically guided laser and EHL. Conventional therapy included mechanical lithotripsy, balloon extraction, and papillary dilation. In collaboration with a research librarian the extant literature from inception through October 2017 (Supplementary Table 4, available online at www.giejournal.org) was searched, and a total of 3257 abstract and 663 full text articles were identified. We reviewed 182 studies reporting on patients treated specifically for bile duct stones with diameter ≥1 cm or for which removal was characterized by authors of the report as difficult for other reasons (ie, anatomic considerations or impaction). The analytic set contained 123 cohort studies of conventional therapy, 57 cohort studies of intraductal therapy, and a single randomized trial that compared the 2 approaches. Included studies reported on a total of 13,588 patients, of whom 2204 (16%) were treated by intraductal and 11,384 (84%) by conventional approaches.
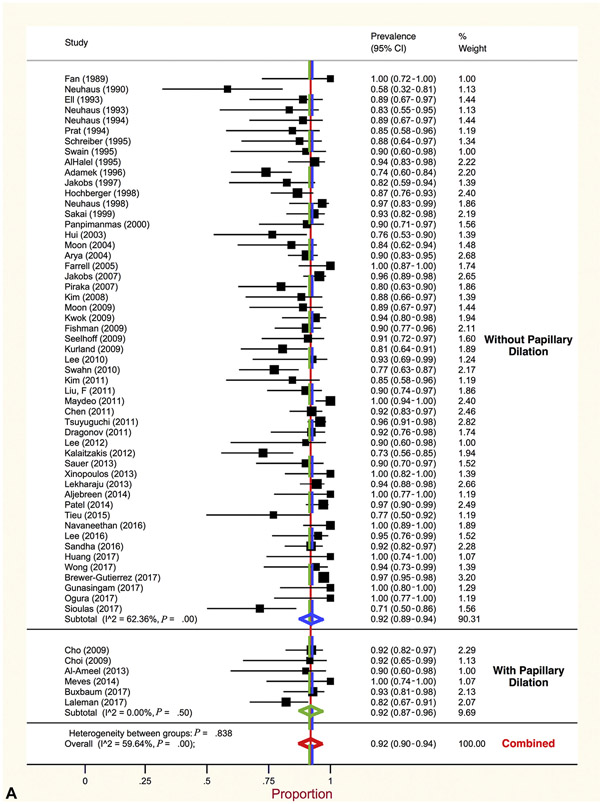
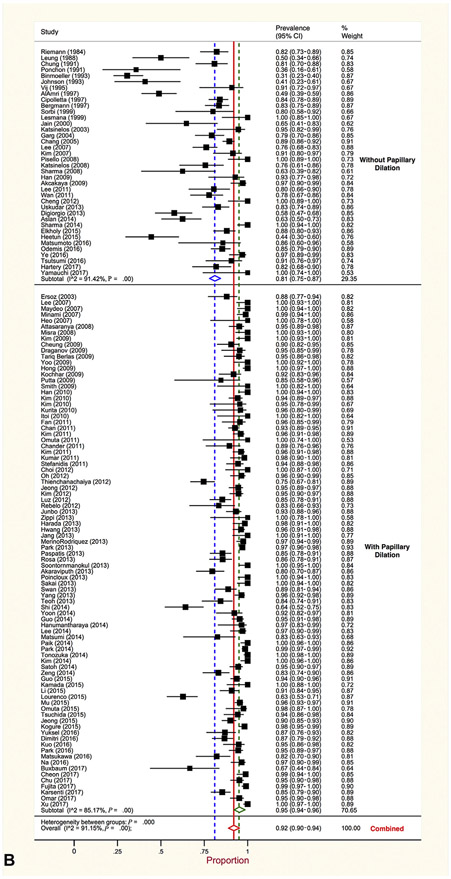
Overall, summary estimates of proportion of patients with complete stone clearance did not differ between the 2 therapeutic approaches. Generated using random effects models, pooled proportion of complete stone clearance for intraductal therapy was (summary estimates of proportion, .92 [95% CI, .90-.94], I2 = 60%). This was the same for patients treated with conventional approaches (summary estimates of proportion, .92 [95% CI, .90-.94], I2 = 91%). Stratified meta-analysis identified noteworthy differences in complete stone clearance between further subsets of studies (Table 10). Clearance was more likely after intraductal than conventional therapy in 3 subsets of studies: those published before 2007 (summary estimates of proportion, .89 [95% CI, .85-.93], vs summary estimates of proportion, .75% [95% CI, .64-.84]), those in which papillary dilation was not used (summary estimates of proportion, .92 [95% CI, .87-.96], vs summary estimates of proportion, .81 [95% CI, .75-.87]) (Fig. 2), and those conducted in Western countries (summary estimates of proportion, .91% [95% CI, .88-.94], vs summary estimates of proportion, .84 [95% CI, .78-.89]). Further analyses jointly stratified all 3 covariates and revealed that better clearance after intraductal therapy was largely confined to studies that did not use papillary dilation, regardless of year or geographic region (Table 10). Thus, time and geographic differences were largely because of variable use of papillary dilation. In 74.6% of studies that used papillary dilation the minimum size of the dilator balloon was ≥12 mm and was preceded by sphincterotomy (ES-LBD). In the 94 studies reporting on whether clearance was achieved in the first procedure, this was accomplished less frequently in patients managed by intraductal (summary estimates of proportion, .69 [95% CI, .62-.75]) versus conventional therapy (summary estimates of proportion, .81 [95% CI, .77-.84]) (Table 11). However, this distinction was restricted to studies in which papillary dilation was used.
TABLE 10.
Results of meta-analyses estimating summary prevalence of stone clearance by intraductal and conventional therapy for all studies and subgroups defined by attributes of studies and clinical features of patients
| Intraductal therapy |
Conventional therapy |
|||
|---|---|---|---|---|
| No. of contributing studies |
Summary estimates of proportion (95% confidence interval) |
No. of contributing studies | Summary estimates of proportion (95% confidence interval) |
|
| Overall results | ||||
| All studies | 58 | .92 (.90-.94) | 124 | .92 (.90-.94) |
| Subgroups of studies defined by single factors | ||||
| Year study conducted | ||||
| Before 2007 | 19 | .89 (.85-.93) | 17 | .75 (.64-.84) |
| 2007 and later | 39 | .93 (.91-.95) | 107 | .94 (.92-.95) |
| Use of papillary dilation | ||||
| Without | 52 | .92 (.89-.94) | 38 | .81 (.75-.87) |
| With | 6 | .92(.87-.96) | 86 | .95 (.94-.96) |
| Geographic region | ||||
| Western country* | 37 | .91 (.88-.94) | 34 | .84 (.78-.89) |
| Eastern country† | 21 | .94 (.91-.97) | 90 | .95 (.93-.96) |
| Study design | ||||
| Prospective cohorts | 8 | .91 (.84-.96) | 93 | .91 (.88-.94) |
| Retrospective cohorts | 50 | .92 (.90-.94) | 31 | .92 (.90-.94) |
| Type of report | ||||
| Full article | 46 | .92 (.89-.94) | 88 | .92 (.89-.94) |
| Abstract | 12 | .93 (.88-.96) | 36 | .93 (.89-.96) |
| Subgroups defined by use of papillary dilation, overall, and further stratified on year and geographic region of study | ||||
| Without papillary dilation | 52 | .92 (.89-.94) | 38 | .81 (.75-.87) |
| Before 2007 | 19 | .90 (.85-.93) | 16 | .74 (.63-.84) |
| 2007 and later | 33 | .93 (.90-.96) | 22 | .86 (.80-.93) |
| Western country* | 33 | .91 (.88-.94) | 15 | .71 (.58-.83) |
| Eastern country† | 19 | .94 (.90-.97) | 23 | .86 (.75-.87) |
| With papillary dilation | 6 | .92 (.87-.96) | 86 | .95 (.94-.96) |
| Before 2007 | 0 | — | 1 | .88 (.77-.94) |
| After 2007 | 6 | .92 (.87-.96) | 86 | .95 (.94-.96) |
| Western country* | 4 | .91 (.83-.98) | 20 | .90 (.86-.94) |
| Eastern country† | 2 | .93 (.85-.99) | 66 | .96 (.95-.97) |
—, Not applicable.
Europe, United States, Canada, Australia.
Asia, Latin America.
Figure 2.
A, Proportion of large and difficult stone clearance by intraductal therapy stratified by papillary dilation. B, Proportion of large and difficult stone clearance by conventional therapy stratified by papillary dilation.
TABLE 11.
Results of meta-analyses estimating summary proportions of clearance in first procedures, all adverse events, and specific adverse events
| Intraductal therapy |
Conventional therapy |
|||
|---|---|---|---|---|
| No. of contributing studies |
Summary estimates of proportion (95% confidence interval) |
No. of contributing studies |
Summary estimates of proportion (95% confidence interval) |
|
| Clearance in first procedure, all studies | 28 | .69 (.62-.75) | 66 | .81 (.77-.84) |
| Studies without papillary dilation | 24 | .68 (.60-.75) | 13 | .56 (.42-.69) |
| Studies with papillary dilation | 4 | .75 (.66-.83) | 53 | .85 (.82-.88) |
| Any adverse events | 49 | .08 (.06-.11) | 118 | .09 (.08-.11) |
| Studies without papillary dilation | 46 | .08 (.05-.10) | 35 | .11 (.07-.15) |
| Studies with papillary dilation | 3 | .11 (.01-.25) | 83 | .09 (.07-.10) |
| Pancreatitis | 49 | .00 (.00-.00) | 116 | .03 (.02-.04) |
| Studies without papillary dilation | 46 | .00 (. 00-.00) | 33 | .02 (.01-.04) |
| Studies with papillary dilation | 3 | .04 (.01-.09) | 83 | .03 (.02-.04) |
| Cholangitis | 49 | .01 (.00-.02) | 116 | .01 (.00-.01) |
| Studies without papillary dilation | 46 | .01 (.00-.02) | 33 | .03 (.01-.05) |
| Studies with papillary dilation | 3 | .02 (.00-.06) | 83 | .00 (.00-.00) |
| Bleeding | 49 | .01 (.00-.02) | 116 | .02 (.01-.03) |
| Studies without papillary dilation | 46 | .00 (.00-.01) | 33 | .02 (.01-.03) |
| Studies with papillary dilation | 3 | .03 (.00-.18) | 83 | .02 (.01-.03) |
| Sedation adverse event | 49 | .00 (.00-.00) | 116 | .00 (.00-.00) |
| Studies without papillary dilation | 46 | .00 (.00-.00) | 33 | .00 (.00-.00) |
| Studies with papillary dilation | 3 | .00 (.00-.01) | 83 | .00 (.00-.00) |
| Otder adverse event | 49 | .01 (.00-.02) | 116 | .00 (.00-.00) |
| Studies without papillary dilation | 46 | .01 (.00-.02) | 33 | .00 (.00-.01) |
| Studies with papillary dilation | 3 | .00 (.00-.01) | 83 | .00 (.00-.00) |
| Requirement for mechanical lithotripsy | 18 | .19 (.10-.29) | 93 | .29 (.23-.36) |
| Studies without papillary dilation | 15 | .17 (.08-.27) | 22 | .74 (.53-.91) |
| Studies with papillary dilation | 3 | .28 (.05-.58) | 72 | .18 (.15-.22) |
| Clearance with laser | 26 | .94 (.91-.96) | N/A | N/A |
| Studies with papillary dilation | 2 | .93 (.87-.98) | ||
| Studies without papillary dilation | 24 | .94 (.91-.97) | ||
| Clearance with electrohydraulic lithotripsy | 17 | .90 (.85-.95) | N/A | N/A |
| Studies with papillary dilation | 1 | .90 (.60-.98) | ||
| Studies without papillary dilation | 16 | .90 (.84-.85) | ||
N/A, Not applicable.
There was no difference in overall frequency of adverse events between intraductal and conventional therapy (summary estimates of proportion, .08 [95% CI, .06-.11], vs summary estimates of proportion, .09 [95% CI, .08-.11]). Mechanical lithotripsy was more frequently required with conventional than with intraductal therapy (summary estimates of proportion, .29 [95% CI, .23-.36], vs summary estimates of proportion, .19 [95% CI, .10-.29]) but less so for studies that used papillary dilation. Overall stone clearance for intraductal therapy with laser was not significantly different from EHL (summary estimates of proportion, .94 [95% CI, .91-.96], vs summary estimates of proportion, .91 [95% CI, .86-.95]).
Certainty in the evidence.
The quality of evidence was rated down to very low given that the observational studies were deemed to be at high risk of bias using the Newcastle Ottawa Scale Tool (Supplementary Table 5, available online at www.giejournal.org). We also rated down for inconsistency as reflected by the high I2 values and also indirectness given that an indirect comparison approach was required.
Considerations.
The panel agreed on a conditional recommendation that large or difficult bile duct stone may be managed either by intraductal therapy or by conventional therapy, which includes ES-LBD. There was extensive discussion regarding the potential high cost, procedure time, and inconvenience (referral to tertiary centers) related to cholangioscopy-guided therapy. It was also discussed that training in cholangioscopy and large balloon papillary dilation is needed. It was acknowledged that future studies would be enhanced by the development and implementation of a standardized lexicon to grade bile duct stones in a hierarchical manner based on size and objective features and that detailed cost-effectiveness, procedure time, and quality of life assessment may also impact future recommendations for this clinical question.
Discussion.
Large (>10 mm) size stones and those with unusual hardness or eccentric shapes may be difficult to remove.68 Additionally, the presence of an abnormal distal duct (oblique, narrowed, perivaterian), stone impaction, or high multiplicity may render stones refractory to extraction. The recent introduction of more evolved cholangioscopes, including those that are disposable and provide high-resolution images, has intensified interest in intraductal treatment of difficult choledocholithiasis using EHL and laser lithotripsy.57,69
Systematic review of the endoscopic management of difficult bile duct choledocholithiasis reveals similar proportions of successful clearance (.92 for both) with use of intraductal and conventional nonintraductal approaches. This is in contrast to the 1 randomized trial comparing intraductal versus conventional treatment of large choledocholithiasis that demonstrated greater clearance with intraductal therapy (.93 vs .67, P = .009).67 There are several explanations for this difference. When stratified by use of LBD the meta-analysis found that intraductal therapy was superior to conventional treatment when ES-LBD was not performed as part of conventional therapy. In the randomized trial, ES-LBD was potentially underutilized in that large (>12 mm) dilation was used in <20% of patients in the conventional arm. Additionally, the results may be impacted by discrepant enrollment criteria based on stone size.70,71 Other investigators studied intraductal therapy only in patients who had failed conventional (mechanical lithotripsy or papillary dilation) therapy.72,73 In the RCT by Buxbaum et al,67 randomization was stratified on whether the procedure was their first ERCP or whether than had undergone a previous ERCP in the prior 3 months. Increased success for intraductal versus conventional therapy was seen in those who had undergone prior ERCP (.90 versus .54), with no difference among those who had not undergone a prior procedure.
There was inconsistent reporting of procedure or fluoroscopy times for the 2 approaches among the observational studies included in the meta-analysis. In the RCT comparing intraductal and conventional approaches, the procedure time was longer for intraductal, 120.7 ± 40.5 minutes, compared with conventional therapy, 81.2 ± 49.3 minutes.67 There is also very limited study on the cost of difficult bile duct stone management. A recent publication modeled the use of cholangioscopy-guided laser lithotripsy after unsuccessful mechanical lithotripsy compared with repeat conventional approaches.74 Using cost data from a Belgian hospital and literature reports of success for intraductal therapy, they estimate a cost savings of 363 Euros per patient. Nevertheless, the high cost of digital cholangioscopes has resulted in administrative approval being required for their use in many tertiary care centers. Assessment of the extant literature underscores the need for a direct comparison of intraductal versus ES-LBD and accords with the current state of clinical equipoise. It also underlines the need for controlled study of management algorithms for specific stone types (ie, attempt first procedure clearance with ES-LBD followed by intraductal treatment if unsuccessful). Higher resolution cholangioscopy and more efficient ES-LBD may impact the performance of these approaches.7,75
Clinical questions for which a comprehensive review was used
The following clinical questions were addressed by the guideline panel on the basis of comprehensive literature review but not adhering to GRADE methodology.
Is same admission cholecystectomy necessary for patients with mild gallstone pancreatitis?
Recommendation: Same admission cholecystectomy is recommended for patients with mild gallstone pancreatitis.
Comprehensive review.
A recent technical review systematically assessed the role of same admission cholecystectomy for gallstone pancreatitis.76 Among the 120 citations revealed by the search the only RCT identified was the Pancreatitis of biliary origin, optimal timing of cholecystectomy (PONCHO) trial.77 This trial challenged the theory that inflammation increases the morbidity of cholecystectomy and other surgical procedures in gallstone pancreatitis. It had been postulated that the increased morbidity seen in surgery for patients with >3 Ranson’s criterion could be extrapolated to patients with mild disease.43 However, in a small (n = 50) randomized trial, Aboulian et al78 demonstrated that early <48 hours cholecystectomy among patients with mild acute gallstone pancreatitis (Ranson’s score <3) shortened mean hospitalization by 2 days compared with those who underwent cholecystectomy at a later time during the initial admission.
Before the PONCHO trial the investigators (Dutch Pancreatitis Study Group) performed a meta-analysis to assess the safety of cholecystectomy during the index admission for mild gallstone pancreatitis and the risk of biliary adverse events between discharge and cholecystectomy in those who did not undergo cholecystectomy during their initial hospitalization.79 The authors’ search of the extant literature between 1992 and 2010 revealed data on 948 patients: 483 patients who underwent same admission cholecystectomy and 515 who were managed with cholecystectomy a median of 40 days (IQR, 19-58) after discharge. Among the latter group 95 patients (18%) were readmitted before cholecystectomy; 43(8%) for recurrent pancreatitis, 35(7%) for biliary colic, and 17(3%) for acute cholecystitis. There were no differences in adverse events or conversion to open procedure among those who underwent index hospitalization or interval cholecystectomy. In the PONCHO trial, 266 patients from 23 Dutch centers with mild gallstone pancreatitis were randomized to same admission versus interval cholecystectomy.77 The primary outcome was gallstone-related adverse events requiring readmission, including cholangitis, biliary obstruction, recurrent pancreatitis, biliary colic, or mortality. Biliary adverse events occurred in 17% of patients in the interval versus 5% in the same admission cholecystectomy group (RR, .28 [95% CI, .12-.66]). There was no difference in adverse events or the proportion converted to open procedures. The panel recommended that same admission cholecystectomy be performed for patients presenting with gallstone pancreatitis. This recommendation concurs with the recent guideline statement from the American Gastroenterological Association.53
A related clinical question is whether ES protects against biliary adverse events in those in whom the gallbladder remains in situ. In their pre-PONCHO meta-analysis, the Dutch Pancreatitis Study Group found that among 136 patients with mild gallstone pancreatitis who underwent ERCP with sphincterotomy but not cholecystectomy 14 (10%) were readmitted for biliary adverse events and 2 (1%) for recurrent pancreatitis.77 In contrast, 48 of 197 patients (24%) who had not undergone ERCP or cholecystectomy were readmitted for biliary adverse events and 31 (16%) with recurrent pancreatitis. Nevertheless, in the PONCHO trial, the protective effect of same admission cholecystectomy was not attenuated by ES.77 Readmission for biliary adverse events occurred in 17% of patients who had undergone ES without cholecystectomy compared with 3% managed with same admission cholecystectomy and ES. These findings accord with previous randomized trials comparing ERCP with sphincterotomy as an alternative for cholecystectomy in patients at high risk for surgery.80-82 A Cochrane analysis of 662 patients from 5 RCTs revealed that a nonoperative approach after ES and bile duct clearance was associated with an increased risk of recurrent biliary pain (14.6 [95% CI 5.0-42.8]), jaundice or cholangitis (2.5 [1.1-5.9]), and mortality (1.8 [1.2-2.8]) versus prophylactic cholecystectomy.83 A very large recent cohort study compared 7330 patients who underwent ES alone with 4478 who underwent ES and cholecystectomy for choledocholithiasis, ascending cholangitis, or gallstone pancreatitis.84 Consistent with the PONCHO trial and the prior Cochrane meta-analysis, a greater proportion managed with ES alone, 39.3% developed recurrent adverse events, versus 18.0% managed with ES and cholecystectomy (adjusted OR, .38 [95% CI, .34-.42]). The panel agreed that ERCP with prophylactic sphincterotomy to prevent recurrent pancreatitis or other biliary adverse events should not be used as an alternative to cholecystectomy for patients with gallstone pancreatitis unless surgery is absolutely contraindicated (eg, recurrent pancreatitis in setting of end-stage liver disease).
Are combinations of liver function tests, clinical characteristics, and transabdominal US able to predict choledocholithiasis?
We suggest the following high-risk criteria for choledocholithiasis, which should directly prompt ERCP:
Common bile duct stone on US or cross-sectional imaging
Total bilirubin >4 mg/dL and dilated common bile duct
Ascending cholangitis
We suggest that patients with other criteria such as abnormal liver tests, age >55 years, and dilated common bile duct on US (intermediate risk for choledocholithiasis) undergo EUS, MRCP, or laparoscopic IOC or laparoscopic intraoperative US for further evaluation
Comprehensive review.
The 2010 ASGE Guideline for the Evaluation of Suspected Choledocholithiasis proposed an algorithm using clinical factors to predict the risk (high [>50%], intermediate [10%-50%], low [<10%]) of bile duct stones.105 These predictors were informed by the prospective McGill Laparoscopic Cholecystectomy Registry, several large cohort studies, and a meta-analysis by Abboud et al.85-89 Since that time, these guidelines have been the subject of validation studies using multiple clinical cohorts.90-94
Studies using ERCP or a composite of EUS, MRCP, and ERCP as reference standards have demonstrated that very strong and strong predictors were associated with a several-fold increase in the odds of choledocholithiasis (Table 12).90-94 The exception was that gallstone pancreatitis did correlate with increased risk of choledocholithiasis in these series.17,90-92 These studies have confirmed the intent of the guidelines, to identify patients with high-risk criterion who have >50%, intermediate 10% to 50%, and low <10% likelihood of choledocholithiasis. Nevertheless, ERCP for choledocholithiasis typically requires native papilla cannulation and is associated with a significant 6% to 15% rate in adverse events and 1% to 2% of severe adverse events categorized by death or prolonged (>10 day) hospitalization.9,95 Additionally, the techniques of EUS and MRCP have a diagnostic performance comparable with ERCP with much lower risk.96,97 Validation studies have also convincingly shown that the 2010 ASGE guidelines will result in performance of diagnostic ERCP in 20% to 30% of cases (Table 12).90,92 Assessment of the criterion in a small series of pediatric patients demonstrated similar findings; ongoing studies suggest a possible role for conjugated bilirubin in this population.98,99
TABLE 12.
Proportion of patients with choledocholithiasis by risk category104
| Cohort | Reference standard | High likelihood with stones |
High likelihood without stones |
Intermediate likelihood with stones |
Low likelihood with stones |
|---|---|---|---|---|---|
| Rubin90 2013 | ERCP | 189/264 | 75/264 | 102/249 | 2/8 |
| 72% | 28% | 35% | 25% | ||
| Adams 2015,91 first set labs* | EUS, MRCP, ERCP | 99/179 | 80/179 | 111/208 | |
| 55% | 45% | 35% | |||
| Adams 2015,91 second set labs* | EUS, MRCP, ERCP | 93/161 | 68/161 | 108/209 | |
| 58% | 42% | 34% | |||
| Magalhaes92 2015 | ERCP | 154/193 | 39/193 | 25/73 | 0/2 |
| 80% | 20% | 34% | 0% | ||
| Suarez 2016,93 first set labs* | EUS, MRCP, ERCP | 39/71 | 32/71 | 32/102 | |
| 55% | 45% | 31 | |||
| Suarez 2016,93 second set labs* | EUS, MRCP, ERCP | 33/58 | 25/58 | 25/76 | |
| 57% | 43% | 33% | |||
Excludes cholangitis.
Given the high risk and lack of benefit of diagnostic ERCP, there is a call for improvement. This reflects an increase in the threshold probability of choledocholithiasis required by endoscopists from historic levels of <50%.100 After excluding patients with cholangitis, Adams et al91 found that the 2010 ASGE criterion had an accuracy of only 62%, sensitivity of 47%, and specificity of 73% for choledocholithiasis or sludge (Table 13). Integration of a second set of liver laboratories did not markedly improve the performance characteristics. A second article using an ethnically and demographically distinct cohort yielded consistent results.93 In a very large cohort, He et al94 found that the existing guidelines had a specificity of 74% and positive predictive value of 64% (Tables 13 and 14). However, when revised to define high probability as the combined findings of total bilirubin >4 mg/dL and dilated duct or a stone on US, this improved the specificity to 94% and positive predictive value to 85% (Tables 13 and 14). Nevertheless, this approach improves specificity to the detriment of sensitivity, expands the intermediate category, and increases the need to arbitrate by EUS or MRCP.
TABLE 13.
Performance characteristics of the 2010 American Society for Gastrointestinal Endoscopy guidelines
| Cohort | Reference standard | Sensitivity (%) |
Specificity (%) |
Positive predictive value (%) |
Negative predictive value (%) |
|---|---|---|---|---|---|
| Adams 2015,91 first set labs | EUS, MRCP, ERCP | 47 | 73 | 56 | 65 |
| Adams 2015,91 second set labs | EUS, MRCP, ERCP | 46 | 76 | 58 | 66 |
| He 2017,94 Full ASGE criteria | EUS, MRCP, IOC, PTC, ERCP | 70 | 74 | 64 | 79 |
| He 2017,94 Bilirubin >4 mg/dL, common bile duct stone on US, or bilirubin level 1.8-4 mg/dL and common bile duct dilation | EUS, MRCP, IOC, PTC, ERCP | 64 | 85 | 74 | 78 |
| He 2017,94 bilirubin >4 mg/dL and common bile duct dilation or common bile duct stone on US | EUS, MRCP, IOC, PTC, ERCP | 55 (95% CI, 55-61) | 94 (95% CI, 93-95) | 85 (95% CI, 82-88) | 76 (95% CI, 74-78) |
| Suarez 2016,93 first set labs | EUS, MRCP, ERCP | 55 | 69 | 55 | 69 |
| Suarez 2016,93 second set of labs | EUS, MRCP, ERCP | 57 | 67 | 57 | 71 |
IOC, Intraoperative cholangiography; PTC, percutaneous transhepatic cholangiography; CI, confidence interval.
TABLE 14.
Test characteristics of individual predictors of common bile duct stones
| Stone US |
Cholangitis | Total bilirubin >4 mg /dL first set labs |
Total bilirubin >4 mg /dL second set labs |
Dilated common bile duct |
Bilirubin 1.8-4 mg/dL |
Abnormal liver function tests |
Age > 55 |
Gallstone pancreatitis |
Total bilirubin > 1.8 mg/dL + common bile duct dilation |
Total bilirubin > 4.0 mg/dL + common bile duct dilation |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adams91OR (95% CI) | 5.5 (2.7-11.1) | 2.0 (1.3-3.0) | 2.0 (1.3-3.0) | ||||||||
| Suarez93 MV OR (95% CI) | 6.4 (1.5-27.3) | 4.9 (1.8-12.9) | |||||||||
| He94 OR (95% CI) | 17.3 (12.6-23.8) | 3.1 (2.3-4.2) | 1.8 (1.5-2.2) | 1.8 (1.4-2.4) | 2.3 (1.9-2.9) | 1.4 (1.2-1.7) | .4 (.3-.6) | ||||
| Rubin90 OR (95% CI) | 6.7 (2.6-17.2) | 3.9 (1.3-11.6) | 2.7 (1.8-4.0) | 2.2 (1.5-3.1) | .9 (.6-1.3) | 2.9 (1.2-7.2) | 1.4 (.9-2.2) | .6 (.4-.9) | |||
| Magalhaes92 OR (95% CI) | 11.3 (5.3-23.8 | 6.5 (1.9-21.8) | 1.8 (1.0-3.1) | 5.1 (2.9-9.0) | 3.2 (1.6-6.1) | 2.4 (1.2-4.9) | 2.4 (1.4-4.2) | .6 (.3-1.0) | |||
| Adams91 sensitivity | 22% | 30% | 22% | 42% | 17% | ||||||
| Suarez93 sensitivity | 14% | 30% | 36% | 56% | 20% | ||||||
| He94 sensitivity (95% CI) | 44% (41-47) | 1% (0-2) | 22% (20-25) | 75% (72-77) | 44% (41-47) | 77% (75-80) | 60% (57-62) | 10% (8-12) | 36% (33-38) | 19% (17-22) | |
| Rubin90 sensitivity | 13% | 7% | 41% | 58% | 32% | 98% | 18% | 22% | |||
| Magalhaes92 sensitivity | 56% | 18% | 43% | 84% | 61% | 90% | 79% | 20% | |||
| Adams91 specificity | 94% | 83% | 86% | 69% | 93% | ||||||
| Suarez93 specificity | 97% | 84% | 90% | 76% | 94% | ||||||
| He94 specificity (95% CI) | 97% (95-98) | 99% (99-100) | 94% (92-95) | 63% (60-65) | 80% (78-82) | 50% (48-52) | 54% (51-56) | 85% (83-86) | 90% (89-91) | 96% (95-97) | |
| Rubin90 specificity | 98% | 98% | 79% | 61% | 63% | 7% | 86% | 69% | |||
| Magalhaes92 specificity | 90% | 97% | 71% | 49% | 67% | 21% | 38% | 70% | |||
| Adams91 positive predictive value | 71% | 56% | 53% | 44% | 59% | ||||||
| Suarez93 positive predictive value | 77% | 57% | 66% | 53% | 70% | ||||||
| He94 positive predictive value (95% CI) | 91% (89-94) | 56% (37-75) | 69% (54-74) | 57% (54-59) | 59% (55-62) | 50% (48-53) | 46% (43-48) | 29% (25-34) | 70% (66-74) | 78% (73-83) | |
| Rubin90 positive predictive value | 88% | 83% | 72% | 66% | 54% | 57% | 63% | 48% | |||
| Magalhaes92 positive predictive value | 92% | 92% | 75% | 77% | 75% | 70% | 72% | 57% | |||
| Adams91 negative predictive value | 62% | 60% | 60% | 67% | 58% | ||||||
| Suarez93 negative predictive value | 62% | 63% | 73% | 78% | 63% | ||||||
| He94 negative predictive value (95% CI) | 73% (71-75) | 61% (59-63) | 65% (63-67) | 79% (77-81) | 69% (66-71) | 77% (74-79) | 67% (64-69) | 59% (57-61) | 68% (66-70) | 58% (54-61) | |
| Rubin90 negative predictive value | 47% | 45% | 51% | 53% | 43% | 68% | 45% | 41% | |||
| Magalhaes92 negative predictive value | 50% | 37% | 38% | 60% | 51% | 51% | 48% | 30% |
US, Ultrasound; OR, odds ratio; CI, confidence interval; MV, multivariate.
Ideally, a more optimal group of clinical features could be identified to predict the presence of persistent choledocholithiasis. Jovanovic et al101 demonstrated that an artificial neural network could be developed to predict choledocholithiasis with 93% sensitivity and 68% specificity. Nevertheless, the reliable input data needed to fit complex exponential formulas might not be readily available at most centers, and it is unclear whether its performance changes with evolution in patient population. Sherman et al102 proposed a scoring system using ductal diameter, gamma-glutamyl transpeptidase, alkaline phosphatase, and total and direct bilirubin to predict persistent choledocholithiasis in patients with gallstone pancreatitis. The authors found that a score of 0 had a negative predictive value of 100% and score of 5 had a positive predictive value of 100%. Nevertheless, it is unclear whether these asymptotic scores applied to a significant portion of the population because those with scores of 1 to 4 required additional testing with IOC or MRCP.
It is possible that the protean and nonspecific causes of liver test and ultrasonic anomalies may limit the ultimate capability of these clinical features to predict choledocholithiasis. Although the performance of various clinical factors was retrospectively studied by He et al,94 in practice the group performed MRCP for 90% of patients who underwent ERCP. As a consequence, 97% of those who underwent ERCP were found to have stones in comparison with 72% to 80% of cohorts using only the clinical predictors recommended in the ASGE 2010 guidelines. Multiple controlled tandem and RCTs have shown that EUS before ERCP decreases the requirement for ERCP, lowers adverse events rates, and is not associated with higher rates of subsequent biliary adverse events because of “missed stones.”31,32,103,104
After reviewing the comprehensive contemporary evidence, the panel of experts suggested the 2010 criterion be revised to decrease the use of diagnostic ERCP, which has significant risk but minimal benefit. Given a lack of correlation, gallstone pancreatitis was removed as a criterion. Because 3 studies have shown improved specificity with a combination of total bilirubin >4 mg/dL and bile duct dilation, this was included as a high-risk criterion. Thus, the panel recommended the following high-risk criteria: cholangitis, stone on imaging, and the combination of total bilirubin >4 mg/dL and bile duct dilation (Table 15). The latter was defined as >6 mm in adults who have not undergone and 8 mm in those who have undergone cholecystectomy.94 Intermediate criterion were defined as abnormal liver biochemical tests, age >55 years, or bile duct dilation. It proposed that patients with any of the high-risk criteria proceed to ERCP and those with intermediate-risk criterion undergo EUS, MRCP, IOC, or intraoperative US. Those without clinical risk factors should undergo cholecystectomy with or without IOC or intraoperative US if indicated for symptomatic cholelithiasis. This stratification and management approach will require validation in future large prospective trials. Finally, specific guidelines for ERCP in pediatric patients with choledocholithiasis will likely require further research, and current adult guidelines may not be directly applicable.
TABLE 15.
Proposed strategy to assign risk of choledocholithiasis and manage patients with symptomatic cholelithiasis based on clinical predictors
| Probability | Predictors of choledocholithiasis | Recommended strategy |
|---|---|---|
| High | Common bile duct stone on US/cross-sectional imaging or Clinical ascending cholangitis or Total bilirubin >4 mg/dL and dilated common bile duct on US/cross-sectional imaging |
Proceed to ERCP |
| Intermediate | Abnormal liver biochemical tests or Age >55 years or Dilated common bile duct on US/cross-sectional imaging |
EUS, MRCP, laparoscopic IOC, or intraoperative US |
| Low | No predictors present | Cholecystectomy with/without IOC or intraoperative US |
US, Ultrasound; IOC, intraoperative cholangiography.
What is the optimal timing of ERCP for choledocholithiasis in patients undergoing cholecystectomy?
Recommendation: We suggest that pre- or postoperative ERCP or laparoscopic treatment be performed for patients at high risk of choledocholithiasis or positive IOC depending on local surgical and endoscopic expertise.
Comprehensive review.
There are several approaches to the management of choledocholithiasis when cholecystectomy is planned; they are frequently described as 1-step approaches when 1 combined surgical procedure is used versus a variety of 2-step approaches using surgery and a minimally invasive bile duct clearance procedure. One frequently used 2-step pathway is to perform ERCP for patients at high risk for choledocholithiasis before cholecystectomy. Rogers et al23 randomized 100 patients to this 2-step approach versus a 1-step LC-BDE and demonstrated comparable proportions of stone clearance 98% versus 88% as well as adverse events. Patients managed by the 1-step surgical approach had a shorter time from the first procedure to discharge compared with the 2-step algorithm. Subsequent RCTs have similarly demonstrated comparable success and adverse events for this comparison but longer hospitalization for ERCP before cholecystectomy.15,105
An alternative 2-step approach is to perform LC with IOC and subsequent postoperative ERCP for positive IOC.106 Rhodes et al106 compared this algorithm with the single-step LC-BDE and found comparable success and adverse events but a nonsignificant trend toward shorter hospitalization. Among those randomized to laparoscopic treatment, however, 23% required subsequent ERCP. Laparoscopic treatment is simpler in patients amenable to trancystic treatment compared with those who require a choledochotomy. Nathanson et al107 performed a RCT in which only 86 patients who failed laparoscopic transcystic bile duct stone clearance at time of LC were randomized to choledochotomy versus postoperative ERCP. There was comparable success for ERCP versus choledochotomy, (96% vs 98%), adverse events (13% vs 17%), hospital stay (7.7 vs 6.4 days), and need for reoperation (6.3% vs 7.3%). Given a postcholedochotomy bile leak rate of 14.6%, the authors recommended that this approach should be used with caution for inflamed ducts and those less than 7 mm in diameter.
A new algorithm was presented by Iranmanesh et al.16 The authors randomized 100 patients defined as intermediate risk for choledocholithiasis based on the 2010 ASGE guidelines to EUS with ERCP for positive endosonography followed by cholecystectomy versus cholecystectomy with intraoperative cholangiogram followed by intraoperative or postoperative ERCP if positive. The authors found that the latter strategy was associated with significantly decreased length of stay (5 [IQR, 5-8] versus 8 [IQR, 6-12] days). This was driven by a fairly low 21% prevalence of choledocholithiasis. Although all patient randomized to preprocedure EUS underwent the procedure, resulting in a median delay of 1.5 days (IQR, 1.5-3), only one fifth of patients assigned to the latter strategy required a postcholecystectomy ERCP.
What is the role of ERCP in the management for Mirizzi syndrome and hepatolithiasis?
Recommendations: For patients with Mirizzi syndrome, peroral cholangioscopic therapy may be an alternative to surgical management depending on local expertise; however, gallbladder resection is needed regardless of strategy. For hepatolithiasis we suggest a multidisciplinary approach including endoscopy, interventional radiology, and surgery.
Comprehensive review.
Approximately .3% to 1.4% of patients will develop Mirizzi syndrome in which biliary obstruction develops because of a cystic duct or gallbladder neck stone.108,109 ERCP is well established as a method to diagnose Mirizzi syndrome and temporize biliary obstruction with biliary stent placement before definitive surgical treatment. Cholangioscopy-guided intraductal laser and EHL appear to expand the role of endoscopic treatment.110-112 In a recent cohort study of patients with Mirizzi syndrome and symptomatic cystic duct stones, conventional ERCP techniques were successful in only 40% of patients (8/20); the addition of cholangioscopy-guided holmium laser enabled endoscopic clearance in the remaining 60% (12/20).110 Larger series revealed a success rate of 75% to 91% for cholangioscopy-guided intraductal approaches to treat Mirizzi syndrome.111,113 Nevertheless, if the gallbladder is not removed after endoscopic therapy, most patients develop additional bile duct adverse events, and even after cholecystectomy 10% may develop subsequent biliary problems.111,114 Experts advocate that cholangioscopy-guided therapy should be limited to type II Mirizzi syndrome because type I is difficult to approach using this technique and the surgical approach typically requires only a cholecystectomy without ductal exploration.111
Intrahepatic lithiasis complicates postoperative biliary strictures (ie, post-transplant), primary sclerosing cholangitis, progressive familial intrahepatic cholestasis, and recurrent pyogenic cholangitis.115-118 Recurrent pyogenic cholangitis is the most frequently reported origin of intrahepatic lithiasis in the literature and appears to result from a helminthic injury to the biliary epithelium, which favors subsequent bacterial infection and stone formation.119 Adverse events of intrahepatic lithiasis include recurrent cholangitis, cholangiocarcinoma, and atrophy of the affected hepatic lobe.120 Although studies are very limited, approximately two thirds of patients with intrahepatic biliary disease have favorable responses to conventional endoscopic approaches.121 Advances in peroral cholangioscopy, including the development of flexible, high-resolution endoscopes, have enabled successful endoscopic therapy in laser and electrohydraulic treatment in >85% of patients.7,69 Nevertheless, although not significant, there was a trend toward lower success (OR, 2.7 [95% CI, .6-12.6]) for intrahepatic disease in international multicenter cohort studies of cholangioscopic-guided stone treatment.7 There is also a role for percutaneous therapy. Akin to endoscopic approaches, this has been bolstered by percutaneous transhepatic cholangioscopic lithotripsy via a catheter or t tube. In a large series of patients with recurrent pyogenic cholangitis, 85.3% achieved clearance with this approach.122 However, in certain cases strictures and casts of stones may obviate clearance by either endoscopic or percutaneous approaches, and partial liver resection in those with good hepatic function enables success in >80% of patients with severe intrahepatic stone disease.123,124 Thus, a multidisciplinary approach is recommended including the endoscopist, radiologist, and surgeon for intrahepatic stone disease.115
What is the role of bile duct stents in the management of choledocholithiasis?
Recommendation: Plastic and covered metal stents may facilitate removal of difficult choledocholithiasis but require planned exchange or removal.
Comprehensive review.
Biliary stents are commonly used to maintain biliary drainage between ERCP in patients with difficult choledocholithiasis and signs of infection.125 However, it has also been proposed as a treatment strategy for difficult choledocholithiasis. Bergman et al125 studied long-term therapy using a 10F polyethylene stent, which was only exchanged for recurrent problems in 58 elderly patients (median age, 83 years). Although the strategy was initially successful, over time 38% developed recurrent cholangitis, and in 12% it was fatal. In a comparison of EHL versus permanent stent therapy for difficult stones, Hui et al126 demonstrated that EHL was associated with a much lower rate of recurrent cholangitis, 7.7%, than the latter, 63.2%. In a randomized comparison of duct clearance versus long-term biliary stent placement, Chopra et al127 consistently demonstrated that although procedural adverse events were higher for duct clearance, 16% versus 7%, it was associated with lower rates of long-term biliary adverse events, 14% versus 36%. The authors concluded that destination therapy of biliary stents for complex choledocholithiasis without planned exchanges are associated with high rates of recurrent cholangitis and are recommended only in patients with a very short life expectancy.
In contrast, temporary placement of biliary stents appears to be an effective therapy for chodocholithiasis. Cohort studies demonstrate that stent placement for difficult choledocholithiasis results in a significant decrease in stone burden and number.128-130 At the time of scheduled stent removal 2 to 6 months after initial placement, complete clearance was achieved in 65% to 93% of cases. Two investigators have also shown that placement of covered metal stents for a median of 6 and 8 weeks, respectively, enabled complete clearance during the ERCP in >80% of patients during the second ERCP.131,132 In the larger series the previously difficult stones could be removed by simple balloon sweep in 66%.132 The authors hypothesized that the stent favors removal of challenging choledocholithiasis by fragmentation by direct mechanical friction and by inducing papillary dilation.132
FUTURE DIRECTIONS
A systematic assessment of the literature pertaining to the diagnosis and management of bile duct stones has identified several areas that require further study. To favor accurate comparison of different therapies a more objective, hierarchical system is needed to categorize stones, that is, large but not giant stones may be amenable to specific treatment and should be identified using a reproducible system (Table 16).69 Additionally, international consensus definitions of adverse endoscopic events and their severity are needed to compare new therapeutic maneuvers with nontrivial risk profiles.133 Specific criteria to diagnose post-ERCP cholangitis in those with preexisting biliary problems and post-ERCP pancreatitis in those with recent gallstone pancreatitis would help to more completely categorize the safety profile of endoscopic therapy for choledocholithiasis. Development of this framework to characterize stone and adverse events of their removal will strengthen trials between contemporary modalities and evolving technology such as drug eluting stents.
TABLE 16.
Future directions
| Category | Specific needs |
|---|---|
| Classification systems | Predicted removal difficulty based on size, stone features, duct features |
| Standardized diagnostic criterion | Post-ERCP cholangitis |
| Post-ERCP pancreatitis in patients presenting with biliary pancreatitis | |
| Adverse event severity | |
| Clinical trials | Validation of 2018 risk stratification algorithm |
| Cost-effectiveness and quality of life studies for all aspects of choledocholithisis algorithms | |
| Comparative trials of ES-LBD versus intraductal therapy for difficult choledocholithisis | |
| Management of Mirizzi syndrome, intrahepatic stones | |
| Standardized training | EUS detection of choledocholithaisis |
| ES-LBD | |
| Intraductal (EHL, laser) therapy of difficulty choledocholithiasis |
ES-LBD; Endoscopic sphincterotomy followed by large balloon dilation; EHL, electrohydraulic lithotripsy.
Predicting the probability of persistent bile duct stones continues to be a controversial problem, and a high-fidelity algorithm using clinical features has not yet been identified.91,94 Because the use of more advanced radiographic and endoscopic testing is costly, a greater prospective multicenter effort is needed using predefined protocols and a systematic classification of stones. Furthermore, testing of algorithms that consider training and cost-effectiveness are needed to determine if and when EUS, MRCP, and additional studies should be used to evaluate patient in the intermediate-risk category.22,27,134
Direct comparative trials of intraductal and ES-LBD methods are needed to define an optimal approach for stones with specific features. Additionally, training and competency algorithms for large balloon dilation, cholangioscopy, and future technologies will need to be developed for trainees and endoscopists already in practice who encounter difficult bile choledocholithiasis as well as challenges such as Mirizzi syndrome and intrahepatic lithiasis. Future studies will also need to further define the interplay between evaluation, endoscopy, and surgery to optimize quality and cost in patients with biliary disease.135
SUMMARY AND CONCLUSIONS
GRADE methodology was used to develop practice guidelines for the diagnosis and treatment of bile duct stones. Furthermore, they adhere to the Institute of Medicine standards for guideline creation. These Guidelines use an evidence-based approach to inform a series of practical clinical questions encountered by those caring for patients with choledocholithiasis; these include the use of MRCP versus EUS for intermediate-risk patients, the role of early ERCP for gallstone pancreatitis, and the utility of papillary dilation after sphincterotomy and intraductal therapy for large and difficult concretions. Furthermore, the optimal timing of cholecystectomy, the use of endoscopy vis-a-vis surgery, and the role of endoscopy in difficult cases such as Mirizzi syndrome and intrahepatic lithiasis is addressed. A practical algorithm to risk stratify and manage patients has been developed. The aim of this guideline, as summarized in Table 17, is to enable the clinicians to gauge the available literature to provide the most informed care of patients with choledocholithiasis.
TABLE 17.
Summary of recommendations on the role of endoscopy in the evaluation and management of choledocholithiasis
| Clinical question | Recommendations based on GRADE methodology |
|---|---|
| 1. What is the diagnostic utility of EUS versus magnetic resonance cholangiopancreatography (MRCP) to confirm choledocholithiasis in patients with intermediate risk of choledocholithiasis? | 1. In patients with intermediate risk (10%-50%) of choledocholithiasis, we suggest either EUS or MRCP given high specificity; consider factors including patient preference, local expertise, and availability. |
| 2. In patients with gallstone pancreatitis, what is the role of early ERCP? | 2. In patients with gallstone pancreatitis without cholangitis or biliary obstruction/ choledocholithiasis, we recommend against urgent (<48 hours) ERCP. |
| 3. In patients with large choledocholithiasis, is endoscopic papillary dilation after sphincterotomy favored over sphincterotomy alone? | 3. In patients with large choledocholithiasis, we suggest performing large-balloon dilation after sphincterotomy rather than endoscopic sphincterotomy alone. |
| 4. What is the role of intraductal versus conventional therapy in patients with large and difficult choledocholithiasis? | 4. For patients with large and difficult choledocholithiasis, we suggest intraductal therapy or conventional therapy with papillary dilation. This may be impacted by local expertise, cost, and patient and physician preferences. |
| Clinical question | Recommendations based on comprehensive review |
| 5. Is same-admission cholecystectomy necessary for patients with mild gallstone pancreatitis? | 5. Same-admission cholecystectomy is recommended for patients with mild gallstone pancreatitis. |
| 6. Are combinations of liver function tests, clinical characteristics, and transabdominal ultrasound able to predict choledocholithiasis? | 6. In order to minimize the risk of diagnostic ERCP, we suggest the following HIGH-RISK criteria to directly prompt ERCP for suspected choledocholithiasis: (1) CBD stone on ultrasound or cross-sectional imaging or (2) Total bilirubin >4 mg/dL AND dilated common bile duct on imaging (>6 mm with gallbladder in situ)* or (3) Ascending cholangitis. In patients with INTERMEDIATE-RISK criteria of abnormal liver tests or age >55 years or dilated CBD on ultrasound, we suggest EUS, MRCP, laparoscopic intraoperative cholangiography (IOC), or laparoscopic intraoperative ultrasound for further evaluation.† For patients with symptomatic cholelithiasis without any of these risk factors, we suggest cholecystecomy without IOC. |
| 7. What is the optimal timing of ERCP for choledocholithiasis in patients undergoing cholecystectomy? | 7. We suggest that pre-operative or post operative ERCP or laparoscopic treatment be performed for patients at high risk of choledocholithiasis or positive intraoperative cholangiopancreaography depending on local surgical and endoscopic expertise. |
| 8. What is the role of ERCP in the management for Mirizzi syndrome and hepatolothiasis? | 8. For patients with Mirizzi syndrome, per-oral cholangioscopic therapy may be an alternative to surgical management depending on local expertise; however, gallbladder resection is needed regardless of strategy. For hepatolithiasis we suggest a multidisciplinary approach including endoscopy, interventional radiology, and surgery. |
| 9. What is the role of bile duct stents in the management of choledocholithiasis? | 9. Plastic and covered metal stents may facilitate removal of difficult choledocholithiasis but require planned exchange or removal. |
In the 2010 ASGE Choledocholithiasis Guideline,24 total bilirubin 1.8-4.0 mg/dL and bile duct dilation or total bilirubin >4 mg/dL alone qualified as high-risk criteria. In this revised Guideline, the presence of both total bilirubin >4 mg/dL and bile duct dilation are required to qualify as a high-risk criterion to directly prompt ERCP.
In contrast to the 2010 ASGE Choledocholithiasis Guideline,24 gallstone pancreatitis is no longer included as an intermediate-risk criterion.
Supplementary Material
Each year choledocholithiasis results in biliary obstruction, cholangitis, and pancreatitis in a significant number of patients. The primary treatment, ERCP, is minimally invasive but associated with adverse events in 6% to 15%. This American Society for Gastrointestinal Endoscopy (ASGE) Standard of Practice (SOP) Guideline provides evidence-based recommendations for the endoscopic evaluation and treatment of choledocholithiasis. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was used to rigorously review and synthesize the contemporary literature regarding the following topics: EUS versus MRCP for diagnosis, the role of early ERCP in gallstone pancreatitis, endoscopic papillary dilation after sphincterotomy versus sphincterotomy alone for large bile duct stones, and impact of ERCP-guided intraductal therapy for large and difficult choledocholithiasis. Comprehensive systematic reviews were also performed to assess the following: same-admission cholecystectomy for gallstone pancreatitis, clinical predictors of choledocholithiasis, optimal timing of ERCP vis-à-vis cholecystectomy, management of Mirizzi syndrome and hepatolithiasis, and biliary stent therapy for choledocholithiasis. Core clinical questions were derived using an iterative process by the ASGE SOP Committee. This body developed all recommendations founded on the certainty of the evidence, balance of risks and harms, consideration of stakeholder preferences, resource utilization, and cost-effectiveness. (Gastrointest Endosc 2019;89:1075-105.)
Abbreviations:
- ASGE
American Society for Gastrointestinal Endoscopy
- CI
confidence interval
- CPT
current procedural terminology
- DRG
diagnosis-related group
- EHL
electrohydraulic lithotripsy
- EQ-5D-5L
EuroQol Group, 5-level
- ES
endoscopic sphincterotomy
- ES-LBD
endoscopic sphincterotomy followed by large balloon dilation
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- I2
the I2 statistic
- ICD-9
International Classification of Disease, 9th Revision
- IOC
intraoperative cholangiography
- IQR
interquartile range
- LC
cholecystectomy
- LC-BDE
combined laparoscopic cholecystectomy and bile duct exploration
- OR
odds ratio
- PICO
Population, Intervention, Comparator, Outcome
- PONCHO trial
Pancreatitis of biliary origin, optimal timing of cholecystectomy trial
- PTC
percutaneous transhepatic cholangiography
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies-2
- RCT
randomized controlled trial
- RR
risk ratio
- SOP
Standards of Practice
- US
ultrasound
Footnotes
DISCLOSURE: The following authors disclosed financial relationships relevant to this publication: J. L. Buxbaum: Consultant for Olympus. S. A. Fehmi, P. Yachimski: Consultant for Boston Scientific. L. H. Jamil: Consultant for Aries Pharmaceutical; speaker for Aries Pharmaceutical. M. A. Khashab: Consultant for Boston Scientific Corp, Olympus, and Medtronic; medical advisory board for Boston Scientific Corp and Olympus. N. Thosani, S. B. Wani: Consultant for Boston Scientific Corp and Medtronic. All other authors disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Paumgartner G, Sauerbruch T. Gallstones: pathogenesis. Lancet 1991;338:1117–21. [DOI] [PubMed] [Google Scholar]
- 2.Figueiredo JC, Haiman C, Porcel J, et al. Sex and ethnic/racial-specific risk factors for gallbladder disease. BMC Gastroenterol 2017;17:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall MJ, Schwartzman A, Zhang J, et al. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. National Health Statistics Reports; 2017. Available at: https://www.cdc.gov/nchs/data/nhsr/nhsr102.pdf. [PubMed] [Google Scholar]
- 4.Frossard JL, Morel PM. Detection and management of bile duct stones. Gastrointest Endosc 2010;72:808–16. [DOI] [PubMed] [Google Scholar]
- 5.Sun SX, Kulaylat AN, Hollenbeak CS, et al. Cost-effective decisions in detecting silent common bile duct gallstones during laparoscopic cholecystectomy. Ann Surg 2016;263:1164–72. [DOI] [PubMed] [Google Scholar]
- 6.Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015;149:1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewer Gutierrez OI, Bekkali NLH, Raijman I, et al. Efficacy and safety of digital single-operator cholangioscopy for difficult biliary stones. Clin Gastroenterol Hepatol 2017;16:918–26. [DOI] [PubMed] [Google Scholar]
- 8.Teoh AYB, Cheung FKY, Hu B, et al. Randomized trial of endoscopic sphincterotomy with balloon dilation versus endoscopic sphincterotomy alone for removal of bile duct stones. Gastroenterology 2013;144:341–5. [DOI] [PubMed] [Google Scholar]
- 9.Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med 1996;335:909–18. [DOI] [PubMed] [Google Scholar]
- 10.Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007;102:1781–8. [DOI] [PubMed] [Google Scholar]
- 11.Wani S, Sultan S, Qumseya B, et al. The ASGE’S vision for developing clinical practice guidelines: the path forward. Gastrointest Endosc 2018;87:932–3. [DOI] [PubMed] [Google Scholar]
- 12.Andrews JC, Schunemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol 2013;66:726–35. [DOI] [PubMed] [Google Scholar]
- 13.Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016;104:240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansal VK, Misra MC, Rajan K, et al. Single-stage laparoscopic common bile duct exploration and cholecystectomy versus two-stage endoscopic stone extraction followed by laparoscopic cholecystectomy for patients with concomitant gallbladder stones and common bile duct stones: a randomized controlled trial. Surg Endosc 2014;28:875–85. [DOI] [PubMed] [Google Scholar]
- 16.Iranmanesh P, Frossard JL, Mugnier-Konrad B, et al. Initial cholecystectomy vs sequential common duct endoscopic assessment and subsequent cholecystectomy for suspected gallstone migration: a randomized clinical trial. JAMA 2014;312:137–44. [DOI] [PubMed] [Google Scholar]
- 17.Scheiman JM, Carlos RC, Barnett JL, et al. Can endoscopic ultrasound or magnetic resonance cholangiopancreatography replace ERCP in patients with suspected biliary disease? A prospective trial and cost analysis. Am J Gastroenterol 2001;96:2900–4. [DOI] [PubMed] [Google Scholar]
- 18.Buscarini E, Tansini P, Vallisa D, et al. EUS for suspected choledocholithiasis: do benefits outweigh costs? A prospective, controlled study. Gastrointest Endosc 2003;57:510–8. [DOI] [PubMed] [Google Scholar]
- 19.Romagnuolo J, Currie G. Noninvasive vs. selective invasive biliary imaging for acute biliary pancreatitis: an economic evaluation by using decision tree analysis. Gastrointest Endosc 2005;61:86–97. [DOI] [PubMed] [Google Scholar]
- 20.Carlos RC, Scheiman JM, Hussain HK, et al. Making cost-effectiveness analyses clinically relevant: the effect of provider expertise and biliary disease prevalence on the economic comparison of alternative diagnostic strategies. Acad Radiol 2003;10:620–30. [DOI] [PubMed] [Google Scholar]
- 21.Vergel YB, Chilcott J, Kaltenthaler E, et al. Economic evaluation of MR cholangiopancreatography compared to diagnostic ERCP for the investigation of biliary tree obstruction. Int J Surg 2006;4:12–9. [DOI] [PubMed] [Google Scholar]
- 22.Arguedas MR, Dupont AW, Wilcox CM. Where do ERCP, endoscopic ultrasound, magnetic resonance cholangiopancreatography, and intraoperative cholangiography fit in the management of acute biliary pancreatitis? A decision analysis model. Am J Gastroenterol 2001;96:2892–9. [DOI] [PubMed] [Google Scholar]
- 23.Rogers SJ, Cello JP, Horn JK, et al. Prospective randomized trial of LC+LCBDE vs ERCP/S+LC for common bile duct stone disease. Arch Surg 2010;145:28–33. [DOI] [PubMed] [Google Scholar]
- 24.ASGE Standards of Practice Committee; Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010;71:1–9. [DOI] [PubMed] [Google Scholar]
- 25.Meeralam Y, Al-Shammari K, Yaghoobi M. Diagnostic accuracy of EUS compared with MRCP in detecting choledocholithiasis: a meta-analysis of diagnostic test accuracy in head-to-head studies. Gastrointest Endosc 2017;86:986–93. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Esparrach G, Gines A, Sanchez M, et al. Comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the diagnosis of pancreatobiliary diseases: a prospective study. Am J Gastroenterol 2007;102:1632–9. [DOI] [PubMed] [Google Scholar]
- 27.Kondo S, Isayama H, Akahane M, et al. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol 2005;54:271–5. [DOI] [PubMed] [Google Scholar]
- 28.Aube C, Delorme B, Yzet T, et al. MR cholangiopancreatography versus endoscopic sonography in suspected common bile duct lithiasis: a prospective, comparative study. AJR Am J Roentgenol 2005;184:55–62. [DOI] [PubMed] [Google Scholar]
- 29.de Ledinghen V, Lecesne R, Raymond JM, et al. Diagnosis of choledocholithiasis: EUS or magnetic resonance cholangiography? A prospective controlled study. Gastrointest Endosc 1999;49:26–31. [DOI] [PubMed] [Google Scholar]
- 30.Early DS, Acosta RD, Chandrasekhara V, et al. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc 2013;77:839–43. [DOI] [PubMed] [Google Scholar]
- 31.Canto MI, Chak A, Stellato T, et al. Endoscopic ultrasonography versus cholangiography for the diagnosis of choledocholithiasis. Gastrointest Endosc 1998;47:439–48. [DOI] [PubMed] [Google Scholar]
- 32.Prat F, Amouyal G, Amouyal P, et al. Prospective controlled study of endoscopic ultrasonography and endoscopic retrograde cholangiography in patients with suspected common-bile duct lithiasis. Lancet 1996;347:75–9. [DOI] [PubMed] [Google Scholar]
- 33.Giljaca V, Gurusamy KS, Takwoingi Y, et al. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for common bile duct stones. Cochrane Database Syst Rev 2015:CD011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wani S, Keswani R, Hall M, et al. A prospective multicenter study evaluating learning curves and competence in endoscopic ultrasound and endoscopic retrograde cholangiopancreatography among advanced endoscopy trainees: the Rapid Assessment of Trainee Endoscopy Skills study. Clin Gastroenterol Hepatol 2017;15:1758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tse F, Yuan Y. Early routine endoscopic retrograde cholangiopancreatography strategy versus early conservative management strategy in acute gallstone pancreatitis. Cochrane Database Syst Rev 2012:CD009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan ST, Lai EC, Mok FP, et al. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med 1993;328:228–32. [DOI] [PubMed] [Google Scholar]
- 37.Neoptolemos JP, Carr-Locke DL, London NJ, et al. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet 1988;2:979–83. [DOI] [PubMed] [Google Scholar]
- 38.Navaneethan U, Gutierrez NG, Jegadeesan R, et al. Delay in performing ERCP and adverse events increase the 30-day readmission risk in patients with acute cholangitis. Gastrointest Endosc 2013;78:81–90. [DOI] [PubMed] [Google Scholar]
- 39.Khashab MA, Tariq A, Tariq U, et al. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin Gastroenterol Hepatol 2012;10:1157–61. [DOI] [PubMed] [Google Scholar]
- 40.Acosta JM, Rossi R, Galli OM, et al. Early surgery for acute gallstone pancreatitis: evaluation of a systematic approach. Surgery 1978;83:367–70. [PubMed] [Google Scholar]
- 41.Acosta JM, Pellegrini CA, Skinner DB. Etiology and pathogenesis of acute biliary pancreatitis. Surgery 1980;88:118–25. [PubMed] [Google Scholar]
- 42.Neoptolemos JP. The theory of “persisting” common bile duct stones in severe gallstone pancreatitis. Ann R Coll Surg Engl 1989;71:326–31. [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly TR, Wagner DS. Gallstone pancreatitis: a prospective randomized trial of the timing of surgery. Surgery 1988;104:600–5. [PubMed] [Google Scholar]
- 44.Chang L, Lo SK, Stabile BE, et al. Gallstone pancreatitis: a prospective study on the incidence of cholangitis and clinical predictors of retained common bile duct stones. Am J Gastroenterol 1998;93:527–31. [DOI] [PubMed] [Google Scholar]
- 45.Cohen ME, Slezak L, Wells CK, et al. Prediction of bile duct stones and complications in gallstone pancreatitis using early laboratory trends. Am J Gastroenterol 2001;96:3305–11. [DOI] [PubMed] [Google Scholar]
- 46.Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology 2012;142:1476–82; quiz e15-6. [DOI] [PubMed] [Google Scholar]
- 47.Oria A, Cimmino D, Ocampo C, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction: a randomized clinical trial. Ann Surg 2007;245:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folsch UR, Nitsche R, Ludtke R, et al. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med 1997;336:237–42. [DOI] [PubMed] [Google Scholar]
- 49.Chandrasekhara V, Khashab MA, Muthusamy VR, et al. Adverse events associated with ERCP. Gastrointest Endosc 2017;85:32–47. [DOI] [PubMed] [Google Scholar]
- 50.Kochar B, Akshintala VS, Afghani E, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc 2015;81:143–9. [DOI] [PubMed] [Google Scholar]
- 51.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- 52.Mayumi T, Takada T, Kawarada Y, et al. Tokyo guidelines for the management of acute cholangitis and cholecystitis. Proceedings of a consensus meeting, April 2006, Tokyo, Japan. J Hepatobil Pancreat Surg 2007;14:1–121. [PubMed] [Google Scholar]
- 53.Crockett SD, Wani S, Gardner TB, et al. American Gastroenterological Association institute guideline on initial management of acute pancreatitis. Gastroenterology 2018;154:1096–101. [DOI] [PubMed] [Google Scholar]
- 54.Ersoz G, Tekesin O, Ozutemiz AO, et al. Biliary sphincterotomy plus dilation with a large balloon for bile duct stones that are difficult to extract. Gastrointest Endosc 2003;57:156–9. [DOI] [PubMed] [Google Scholar]
- 55.Disario JA, Freeman ML, Bjorkman DJ, et al. Endoscopic balloon dilation compared with sphincterotomy for extraction of bile duct stones. Gastroenterology 2004;127:1291–9. [DOI] [PubMed] [Google Scholar]
- 56.Park CH, Jung JH, Nam E, et al. Comparative efficacy of various endoscopic techniques for the treatment of common bile duct stones: a network meta-analysis. Gastrointest Endosc 2018;87:43–57. [DOI] [PubMed] [Google Scholar]
- 57.Karsenti D, Coron E, Vanbiervliet G, et al. Complete endoscopic sphincterotomy with vs. without large-balloon dilation for the removal of large bile duct stones: randomized multicenter study. Endoscopy 2017;49:968–76. [DOI] [PubMed] [Google Scholar]
- 58.Chu X, Zhang H, Qu R, et al. Small endoscopic sphincterotomy combined with endoscopic papillary large-balloon dilation in the treatment of patients with large bile duct stones. Acta Chir Austriaca 2017;49:9–16. [Google Scholar]
- 59.Li G, Pang Q, Zhang X, et al. Dilation-assisted stone extraction: an alternative method for removal of common bile duct stones. Dig Dis Sci 2014;59:857–64. [DOI] [PubMed] [Google Scholar]
- 60.Heo JH, Kang DH, Jung HJ, et al. Endoscopic sphincterotomy plus large-balloon dilation versus endoscopic sphincterotomy for removal of bile-duct stones. Gastrointest Endosc 2007;66:720–71. [DOI] [PubMed] [Google Scholar]
- 61.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991;37:383–93. [DOI] [PubMed] [Google Scholar]
- 62.Kim HG, Cheon YK, Cho YD, et al. Small sphincterotomy combined with endoscopic papillary large balloon dilation versus sphincterotomy. World J Gastroenterol 2009;15:4298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapral C, Duller C, Wewalka F, et al. Case volume and outcome of endoscopic retrograde cholangiopancreatography: results of a nationwide Austrian benchmarking project. Endoscopy 2008;40:625–30. [DOI] [PubMed] [Google Scholar]
- 64.Park SJ, Kim JH, Hwang JC, et al. Factors predictive of adverse events following endoscopic papillary large balloon dilation: results from a multicenter series. Dig Dis Sci 2013;58:1100–9. [DOI] [PubMed] [Google Scholar]
- 65.Jun Bo Q, Li Hua X, Tian Min C, et al. Small endoscopic sphincterotomy plus large-balloon dilation for removal of large common bile duct stones during ERCP. Pakistan J Med Sci 2013;29:907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo S-B, Meng H, Duan Z-J, et al. Small sphincterotomy combined with endoscopic papillary large balloon dilation vs sphincterotomy alone for removal of common bile duct stones. World J Gastroenterol 2014;20:17962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buxbaum J, Sahakian A, Ko C, et al. Randomized trial of cholangioscopy-guided laser lithotripsy versus conventional therapy for large bile duct stones (with videos). Gastrointest Endosc 2018;87:1050–60. [DOI] [PubMed] [Google Scholar]
- 68.Garg PK, Tandon RK, Ahuja V, et al. Predictors of unsuccessful mechanical lithotripsy and endoscopic clearance of large bile duct stones. Gastrointest Endosc 2004;59:601–5. [DOI] [PubMed] [Google Scholar]
- 69.Navaneethan U, Hasan MK, Kommaraju K, et al. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: a multicenter clinical experience (with video). Gastrointest Endosc 2016;84:649–55. [DOI] [PubMed] [Google Scholar]
- 70.Stefanidis G, Viazis N, Pleskow D, et al. Large balloon dilation vs. mechanical lithotripsy for the management of large bile duct stones: a prospective randomized study. Am J Gastroenterol 2011;106:278–85. [DOI] [PubMed] [Google Scholar]
- 71.Chang W-H, Chu C-H, Wang T-E, et al. Outcome of simple use of mechanical lithotripsy of difficult common bile duct stones. World J Gastroenterol 2005;11:593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Binmoeller KF, Bruckner M, Thonke F, et al. Treatment of difficult bile duct stones using mechanical, electrohydraulic and extracorporeal shock wave lithotripsy. Endoscopy 1993;25:201–6. [DOI] [PubMed] [Google Scholar]
- 73.Maydeo A, Kwek BEA, Bhandari S, et al. Single-operator cholangioscopy-guided laser lithotripsy in patients with difficult biliary and pancreatic ductal stones (with videos). Gastrointest Endosc 2011;74:1308–14. [DOI] [PubMed] [Google Scholar]
- 74.Deprez PH, Garces Duran R, Moreels T, et al. The economic impact of using single-operator cholangioscopy for the treatment of difficult bile duct stones and diagnosis of indeterminate bile duct strictures. Endoscopy 2018;50:109–18. [DOI] [PubMed] [Google Scholar]
- 75.Itoi T, Itokawa F, Sofuni A, et al. Endoscopic sphincterotomy combined with large balloon dilation can reduce the procedure time and fluoroscopy time for removal of large bile duct stones. Am J Gastro 2009;104:560–5. [DOI] [PubMed] [Google Scholar]
- 76.Vege SS, DiMagno MJ, Forsmark CE, et al. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology 2018;154:1103–39. [DOI] [PubMed] [Google Scholar]
- 77.da Costa DW, Bouwense SA, Schepers NJ, et al. Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): a multicentre randomised controlled trial. Lancet 2015;386:1261–8. [DOI] [PubMed] [Google Scholar]
- 78.Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg 2010;251:615–9. [DOI] [PubMed] [Google Scholar]
- 79.van Baal MC, Besselink MG, Bakker OJ, et al. Timing of cholecystectomy after mild biliary pancreatitis: a systematic review. Ann Surg 2012;255:860–6. [DOI] [PubMed] [Google Scholar]
- 80.Lau JY, Leow CK, Fung TM, et al. Cholecystectomy or gallbladder in situ after endoscopic sphincterotomy and bile duct stone removal in Chinese patients. Gastroenterology 2006;130:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suc B, Escat J, Cherqui D, et al. Surgery vs endoscopy as primary treatment in symptomatic patients with suspected common bile duct stones: a multicenter randomized trial. French Associations for Surgical Research. Arch Surg 1998;133:702–8. [DOI] [PubMed] [Google Scholar]
- 82.Targarona EM, Ayuso RM, Bordas JM, et al. Randomised trial of endoscopic sphincterotomy with gallbladder left in situ versus open surgery for common bile duct calculi in high-risk patients. Lancet 1996;347:926–9. [DOI] [PubMed] [Google Scholar]
- 83.McAlister VC, Davenport E, Renouf E. Cholecystectomy deferral in patients with endoscopic sphincterotomy. Cochrane Database Syst Rev 2007:CD006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elmunzer BJ, Noureldin M, Morgan KA, et al. The impact of cholecystectomy after endoscopic sphincterotomy for complicated gallstone disease. Am J Gastroenterol 2017;112:1596–602. [DOI] [PubMed] [Google Scholar]
- 85.Barkun AN, Barkun JS, Fried GM, et al. Useful predictors of bile duct stones in patients undergoing laparoscopic cholecystectomy. McGill Gallstone Treatment Group. Ann Surg 1994;220:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Onken JE, Brazer SR, Eisen GM, et al. Predicting the presence of choledocholithiasis in patients with symptomatic cholelithiasis. Am J Gastroenterol 1996;91:762–7. [PubMed] [Google Scholar]
- 87.Peng WK, Sheikh Z, Paterson-Brown S, et al. Role of liver function tests in predicting common bile duct stones in acute calculous cholecystitis. Br J Surg 2005;92:1241–7. [DOI] [PubMed] [Google Scholar]
- 88.Prat F, Meduri B, Ducot B, et al. Prediction of common bile duct stones by noninvasive tests. Ann Surg 1999;229:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abboud PA, Malet PF, Berlin JA, et al. Predictors of common bile duct stones prior to cholecystectomy: a meta-analysis. Gastrointest Endosc 1996;44:450–5. [DOI] [PubMed] [Google Scholar]
- 90.Rubin MI, Thosani NC, Tanikella R, et al. Endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis: testing the current guidelines. Dig Liver Dis 2013;45:744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adams MA, Hosmer AE, Wamsteker EJ, et al. Predicting the likelihood of a persistent bile duct stone in patients with suspected choledocholithiasis: accuracy of existing guidelines and the impact of laboratory trends. Gastrointest Endosc 2015;82:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Magalhaes J, Rosa B, Cotter J. Endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis: From guidelines to clinical practice. World J Gastrointest Endosc 2015;7:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suarez AL, LaBarre NT, Cotton PB, et al. An assessment of existing risk stratification guidelines for the evaluation of patients with suspected choledocholithiasis. Surg Endosc 2016;30:4613–8. [DOI] [PubMed] [Google Scholar]
- 94.He H, Tan C, Wu J, et al. Accuracy of ASGE high-risk criteria in evaluation of patients with suspected common bile duct stones. Gastrointest Endosc 2017;86:525–32. [DOI] [PubMed] [Google Scholar]
- 95.Buxbaum J, Leonor P, Tung J, et al. Randomized trial of endoscopist-controlled vs. assistant-controlled wire-guided cannulation of the bile duct. Am J Gastroenterol 2016;111:1841–7. [DOI] [PubMed] [Google Scholar]
- 96.Romagnuolo J, Bardou M, Rahme E, et al. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med 2003;139:547–57. [DOI] [PubMed] [Google Scholar]
- 97.Tse F, Liu L, Barkun AN, et al. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008;67:235–44. [DOI] [PubMed] [Google Scholar]
- 98.Fishman D, Barth B, Orlando S. Predictors of choledocholithiasis at ERCP in pediatric patients: a report from the Pediatric ERCP Database Initiative (PEDI) [abstract]. Gastrointest Endosc 2016;83:AB167. [DOI] [PubMed] [Google Scholar]
- 99.Fishman DS, Chumpitazi BP, Raijman I, et al. Endoscopic retrograde cholangiography for pediatric choledocholithiasis: assessing the need for endoscopic intervention. World J Gastrointest Endosc 2016;8:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shea JA, Asch DA, Johnson RF, et al. What predicts gastroenterologists’ and surgeons’ diagnosis and management of common bile duct stones? Gastrointest Endosc 1997;46:40–7. [DOI] [PubMed] [Google Scholar]
- 101.Jovanovic P, Salkic NN, Zerem E. Artificial neural network predicts the need for therapeutic ERCP in patients with suspected choledocholithiasis. Gastrointest Endosc 2014;80:260–8. [DOI] [PubMed] [Google Scholar]
- 102.Sherman JL, Shi EW, Ranasinghe NE, et al. Validation and improvement of a proposed scoring system to detect retained common bile duct stones in gallstone pancreatitis. Surgery 2015;157:1073–9. [DOI] [PubMed] [Google Scholar]
- 103.Karakan T, Cindoruk M, Alagozlu H, et al. EUS versus endoscopic retrograde cholangiography for patients with intermediate probability of bile duct stones: a prospective randomized trial. Gastrointest Endosc 2009;69:244–52. [DOI] [PubMed] [Google Scholar]
- 104.Lee YT, Chan FK, Leung WK, et al. Comparison of EUS and ERCP in the investigation with suspected biliary obstruction caused by choledocholithiasis: a randomized study. Gastrointest Endosc 2008;67:660–8. [DOI] [PubMed] [Google Scholar]
- 105.Ding G, Cai W, Qin M. Single-stage vs. two-stage management for concomitant gallstones and common bile duct stones: a prospective randomized trial with long-term follow-up. J Gastrointest Surg 2014;18:947–51. [DOI] [PubMed] [Google Scholar]
- 106.Rhodes M, Sussman L, Cohen L, et al. Randomised trial of laparoscopic exploration of common bile duct versus postoperative endoscopic retrograde cholangiography for common bile duct stones. Lancet 1998;351:159–61. [DOI] [PubMed] [Google Scholar]
- 107.Nathanson LK, O'Rourke NA, Martin IJ, et al. Postoperative ERCP versus laparoscopic choledochotomy for clearance of selected bile duct calculi: a randomized trial. Ann Surg 2005;242:188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schafer M, Schneiter R, Krahenbuhl L. Incidence and management of Mirizzi syndrome during laparoscopic cholecystectomy. Surg Endosc 2003;17:1186–90; discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 109.Kulkarni SS, Hotta M, Sher L, et al. Complicated gallstone disease: diagnosis and management of Mirizzi syndrome. Surg Endosc 2017;31:2215–22. [DOI] [PubMed] [Google Scholar]
- 110.Bhandari S, Bathini R, Sharma A, et al. Usefulness of single-operator cholangioscopy-guided laser lithotripsy in patients with Mirizzi syndrome and cystic duct stones: experience at a tertiary care center. Gastrointest Endosc 2016;84:56–61. [DOI] [PubMed] [Google Scholar]
- 111.Tsuyuguchi T, Sakai Y, Sugiyama H, et al. Long-term follow-up after peroral cholangioscopy-directed lithotripsy in patients with difficult bile duct stones, including Mirizzi syndrome: an analysis of risk factors predicting stone recurrence. Surg Endosc 2011;25:2179–85. [DOI] [PubMed] [Google Scholar]
- 112.Binmoeller KF, Thonke F, Soehendra N. Endoscopic treatment of Mirizzi’s syndrome. Gastrointest Endosc 1993;39:532–6. [DOI] [PubMed] [Google Scholar]
- 113.Sepe PS, Berzin TM, Sanaka S, et al. Single-operator cholangioscopy for the extraction of cystic duct stones (with video). Gastrointest Endosc 2012;75:206–10. [DOI] [PubMed] [Google Scholar]
- 114.Tsuyuguchi T, Saisho H, Ishihara T, et al. Long-term follow-up after treatment of Mirizzi syndrome by peroral cholangioscopy. Gastrointest Endosc 2000;52:639–44. [DOI] [PubMed] [Google Scholar]
- 115.Pitt HA, Venbrux AC, Coleman J, et al. Intrahepatic stones. The transhepatic team approach. Ann Surg 1994;219:527–35; discussion 35-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yoshimoto H, Ikeda S, Tanaka M, et al. Choledochoscopic electrohydraulic lithotripsy and lithotomy for stones in the common bile duct, intrahepatic ducts, and gallbladder. Ann Surg 1989;210:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paik WH, Lee SH, Ryu JK, et al. Long-term clinical outcomes of biliary cast syndrome in liver transplant recipients. Liver Transpl 2013;19:275–82. [DOI] [PubMed] [Google Scholar]
- 118.Pan S, Li X, Jiang P, et al. Variations of ABCB4 and ABCB11 genes are associated with primary intrahepatic stones. Mol Med Rep 2015;11:434–46. [DOI] [PubMed] [Google Scholar]
- 119.Al-Sukhni W, Gallinger S, Pratzer A, et al. Recurrent pyogenic cholangitis with hepatolithiasis—the role of surgical therapy in North America. J Gastrointest Surg 2008;12:496–503. [DOI] [PubMed] [Google Scholar]
- 120.Su CH, Shyr YM, Lui WY, et al. Hepatolithiasis associated with cholangiocarcinoma. Br J Surg 1997;84:969–73. [DOI] [PubMed] [Google Scholar]
- 121.Sperling RM, Koch J, Sandhu JS, et al. Recurrent pyogenic cholangitis in Asian immigrants to the United States: natural history and role of therapeutic ERCP. Dig Dis Sci 1997;42:865–71. [PubMed] [Google Scholar]
- 122.Huang MH, Chen CH, Yang JC, et al. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol 2003;98:2655–62. [DOI] [PubMed] [Google Scholar]
- 123.Uenishi T, Hamba H, Takemura S, et al. Outcomes of hepatic resection for hepatolithiasis. Am J Surg 2009;198:199–202. [DOI] [PubMed] [Google Scholar]
- 124.Suzuki Y, Mori T, Yokoyama M, et al. Hepatolithiasis: analysis of Japanese nationwide surveys over a period of 40 years. J Hepatobil Pancreat Sci 2014;21:617–22. [DOI] [PubMed] [Google Scholar]
- 125.Bergman J, Rauws EAJ, Tijssen JGP,et al. Biliary endoprostheses in elderly patients with endoscopically irretrievable common bile-duct stones—report on 117 patients. Gastrointest Endosc 1995;42:195–201. [DOI] [PubMed] [Google Scholar]
- 126.Hui CK, Lai KC, Ng M,et al. Retained common bile duct stones: a comparison between biliary stenting and complete clearance of stones by electrohydraulic lithotripsy. Aliment Pharmacol Therap 2003;17:289–96. [DOI] [PubMed] [Google Scholar]
- 127.Chopra KB, Peters RA, O'Toole PA, et al. Randomised study of endoscopic biliary endoprosthesis versus duct clearance for bile duct stones in high-risk patients. Lancet 1996;348:791–3. [DOI] [PubMed] [Google Scholar]
- 128.Han J, Moon JH, Koo HC, et al. Effect of biliary stenting combined with ursodeoxycholic acid and terpene treatment on retained common bile duct stones in elderly patients: a multicenter study. Am J Gastroenterol 2009;104:2418–21. [DOI] [PubMed] [Google Scholar]
- 129.Horiuchi A, Nakayama Y, Kajiyama M, et al. Biliary stenting in the management of large or multiple common bile duct stones. Gastrointest Endosc 2010;71:1200–3. [DOI] [PubMed] [Google Scholar]
- 130.Jain SK, Stein R, Bhuva M, et al. Pigtail stents: an alternative in the treatment of difficult bile duct stones. Gastrointest Endosc 2000;52:490–3. [DOI] [PubMed] [Google Scholar]
- 131.Cerefice M, Sauer B, Javaid M, et al. Complex biliary stones: treatment with removable self-expandable metal stents: a new approach (with videos). Gastrointest Endosc 2011;74:520–6. [DOI] [PubMed] [Google Scholar]
- 132.Hartery K, Lee CS, Doherty GA, et al. Covered self-expanding metal stents for the management of common bile duct stones. Gastrointest Endosc 2017;85:181–6. [DOI] [PubMed] [Google Scholar]
- 133.Karsenti D, Coron E, Vanbiervliet G, et al. Complete sphincterotomy plus large balloon dilatation of sphincter of Oddi versus endoscopic sphincterotomy for large bile duct stones removal: a large prospective multicenter randomized study [abstract]. Gastrointest Endosc 2016;83:AB133. [Google Scholar]
- 134.Wani S, Cote GA, Keswani R, et al. Learning curves for EUS by using cumulative sum analysis: implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest Endosc 2013;77:558–65. [DOI] [PubMed] [Google Scholar]
- 135.Zuckerman RB, Sheingold SH, Orav EJ, et al. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med 2016;374:1543–51. [DOI] [PubMed] [Google Scholar]
- 136.Guo Y, Lei S, Gong W, et al. A preliminary comparison of endoscopic sphincterotomy, endoscopic papillary large balloon dilation, and combination of the two in endoscopic choledocholithiasis treatment. Med Sci Monit 2015;21:2607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.