Abstract
Chromatin regulators of the Polycomb group of genes are well-known by their activities as transcriptional repressors. Characteristically, their presence at genomic sites occurs with specific histone modifications and sometimes high-order chromatin structures correlated with silencing of genes involved in cell differentiation. However, evidence gathered in recent years, on flies and mammals, shows that in addition to these sites, Polycomb products bind to a large number of active regulatory regions. Occupied sites include promoters and also intergenic regions, containing enhancers and super-enhancers. Contrasting with occupancies at repressed targets, characteristic histone modifications are low or undetectable. Functions on active targets are dual, restraining gene expression at some targets while promoting activity at others. Our aim here is to summarize the evidence available and discuss the convenience of broadening the scope of research to include Polycomb functions on active targets.
Keywords: Polycomb, RING1B, active targets, enhancer, promoter, fine-tuning regulation, differentiation
1. Introduction
The genetic information encoded in eukaryotic DNA is managed through the intertwined actions of a large collection of transcription factors and chromatin regulators. Eukaryotic genomes are organized as long fibers of repeated nucleosomal units made of DNA wrapped around an octameric set of histone proteins, an evolutionary successful structure serving gene expression and replication functions. Transcription factors confer the specificity to regulate transcription by recognizing DNA sequences with various affinities. On the other hand, chromatin regulators, usually present as multiprotein complexes, modulate the interactions of transcription factors and transcriptional machinery with the DNA template, usually restricted by nucleosomes [1]. Chromatin regulators encompass a large collection of proteins and multiprotein complexes responsible for the so-called epigenetic regulation of the genome. The modified chromatin, the epigenome, resulting from histone modifications, location and mobility of nucleosomes, or DNA methylation include marks that sometimes can be inherited throughout cell divisions. As a whole, these modifications depict cell type-specific epigenomic landscapes with predictive properties about the presence and activity of DNA elements essential in transcription regulation, such as enhancers and promoters. For example, nucleosomes decorated with mono- or trimethylated forms of lysine 4 of histone H3 locate to enhancers and promoters, respectively. Also, the state of lysine 27 of histone H3, that in acetylated or trimethylated form corresponds with active enhancers or repressed enhancers and promoters, respectively [2]. Transcriptional activity, determined by communication between enhancers and promoters, can be affected by the organization of chromatin in mutually exclusive compartments of similar transcriptional activity (A, active and B, repressed). In addition, at a smaller size-scale, topologically associated domains (TADs) contain sequences that interact preferentially between themselves and not with those of other domains and may contain loop-folded structures mediated by architectural proteins (reviewed in [3,4]).
Among chromatin regulators, the machinery of the Polycomb group (PcG) of proteins is present throughout most eukaryotic groups. Identified first during the genetic analysis of the development of the fly Drosophila melanogaster [5] soon it was associated with transcriptional repression of developmentally relevant genes as the homeotic genes, an activity conserved in vertebrates and plants (summarized in [6,7]). Thus, it was only natural that the first biochemical assemblies of Polycomb proteins were termed Polycomb repressive complexes (PRCs) [8,9,10,11,12]. Accordingly, in the prevalent models used in the study of the Polycomb system, Drosophila tissues and mammalian embryonic stem cells (ESCs), Polycomb proteins localize at promoters and other regions of silent loci that, generally, undergo derepression upon the depletion of Polycomb components [13,14]. The interest on the Polycomb system was significantly encouraged not only by the key roles in differentiation of stem and oligopotent cells but also by their involvement in malignant transformation processes [15,16]. Our reference to their functions as transcriptional repressors will be only in passing, to provide some background on the best-known activities of components. Detailed descriptions can be found in a number of reviews [6,17,18,19,20].
Instead, we will focus our attention on a number of observations that, accumulating steadily, link unquestionably the Polycomb system, or at least some of its components, to the modulation of transcriptionally active loci. Within the conventional understanding Polycomb repressive functions are antagonized by the Trithorax system (the products of the antagonistic Trithorax group of genes [21,22]), acting on common control regions as in a two-sided system. The activities we will address, instead, deal with functions compatible with gene activity that Polycomb products modulate negatively at some loci, and positively, potentiating gene expression, at others. Compared to studies on repressive functions, it is clear that research efforts invested in roles on active targets reflect the larger attention commanded by canonical Polycomb activities. The relevance of recent genetic analysis in flies and mice, showing active Polycomb targets as part of tumor suppressor functions and of differentiation programs, led us to believe that this aspect of Polycomb activity merits further work.
2. The Polycomb System
The following is the overview of the biochemistry and functionality of the Polycomb system, most of which derives from studies aimed at understanding its role as chromatin regulators in gene repression. Biochemically, the system comprises a heterogeneous collection of unrelated proteins, that can assemble in a highly diverse set of protein complexes (schematized in Figure 1). The subset of subunits/complexes mentioned below have been listed in Table 1 for reference and clarity.
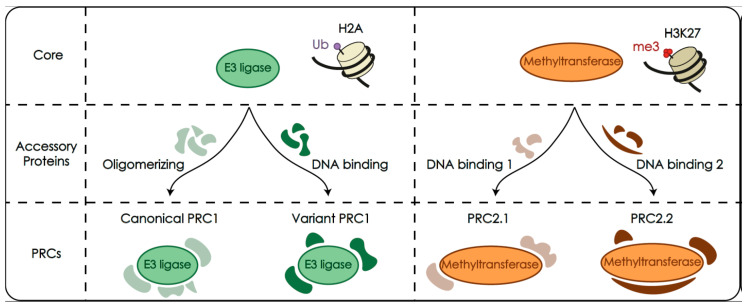
Figure 1.
Simplified architecture of Polycomb repressive complexes (PRCs). Assemblies contain catalytic cores that monoubiquitylate histone H2A (green) or methylate histone H3K27 (orange). These cores correspond to heterodimeric RING E3 ligases, or to a multiprotein complex containing a lysine methyltransferase, respectively, and define the two large classes of Polycomb complexes, PRC1 and PRC2. The catalytic activities, recruitment to targets and other functions, are largely determined by the presence of a variety of specific accessory subunits that result in a heterogenous collection of complexes expressed in a cell-context dependent manner. Subunits with oligomerizing abilities and other protein–protein interactions form the canonical class of PRC1 complexes, whereas DNA-binding subunits are found among the variant class of PRC1. Similarly, specific, exclusive subunits with DNA-binding abilities and other functions define the PRC2.1 and PRC2.2 types of PRC2 complexes.
Table 1.
Polycomb subunits mentioned in the text.
| Complex | Mammals | Flies | Protein Motifs | Functions |
|---|---|---|---|---|
| PRC1 core subunits | RING1/RING1A, RNF2/RING1B | Sce | RING finger, RAWUL domain | RING1-PCGF pairs as heterodimeric E3 ligases that monoubiquitylate H2A |
| PCGF1, PCGF2/MEL18, PCGF3, PCGF4/BMI1, PCGF5, PCGF6 | Psc, Su(z)2, L(3)73Ah | RING finger, RAWUL domain | ||
| canonical PRC1 | CBX2, CBX4, CBX6 CBX7, CBX8 | Pc | Chromobox | H3K27me3 recognition |
| PHC1, PHC2, PHC3 | Ph | SAM domain | Oligomerization, high order structures | |
| variant PRC1 | KDM2B | dKDM2 | CXXC motif, jmjC, Fbox, LRR | DNA binding |
| RYBP, YAF2 | dRYBP | Zn finger | H2AUb recognition | |
| MGA-MAX | (1) | heterodimeric DNA binding module | ||
| E2F-TFDP1 | (1) | |||
| L3MBTL2 | dSfmbt | MBT domains | ||
| PRC2 core subunits | EZH1/EZH2 | E(z) | SET domain | H3K27 methyltransferase |
| SUZ12 | Su(z)12 | Several | Allosteric integration, recruitment | |
| EED | esc | WD repeats | H3K27me recognition | |
| RBBP4, RBBP7 | Caf1 | WD repeats | H3, H4 recognition | |
| PRC2.1 | PCL1, PCL2, PCL3 | Pcl | DNA binding | |
| PALI1, PALI2, PALI3 | (1) | Protein-protein interactions | ||
| PRC2.2 | JARID2 | Jarid2 | JmjC, ARID | H2AUb recognition, recruitment |
| AEBP2 | Jing | Zn finger | DNA binding |
(1) No PcG-related homolog identified.
2.1. Polycomb Complexes PRC1 and PRC2 as Histone Modifiers
The biochemical complexity of Polycomb assemblies can be reduced to two large classes defined by the type of histone modification they produce: one group is that of complexes that modify the C-terminal end of histone H2A (PRC1 complexes), and the other that of complexes that modify the N-terminal of histone H3 (PRC2 complexes). Polycomb-induced H2A modification results in the monoubiquitylation of (predominantly) lysine 119 (H2AK119Ub) in mammals (lysine 118 in Drosophila), and is catalyzed by heterodimeric RING E3 ligases present in PRC1 complexes [23]. The RING1 half of the oligomer (RING1A or its paralog RING1B, in mammals; Sce in Drosophila) interacts with the ubiquitin-conjugating component (E2 ligase), acting as substrate adapter, together with the PCGF partner, as a nucleosome recognition module for H2A modification [24,25]. PRC2 complexes, on the other hand, methylate histone H3 at its lysine 27 (H3K27me1, me2 and me3), in a reaction catalyzed by EZH2 and paralog EZH1, lysine methyl transferases that use S-adenosylmethionine as a donor of methyl groups [9,10,11,12]. Additional enzymatic activities, associated to a few PRC1 subunits, i.e., SUMO E3 ligase of CBX4 [26] or histone H3K36 demethylase of KDM2B [27] are of restricted impact or of unclear relevance in gene control [28].
PRC1 and PRC2 can act together and also independently of each other [29,30,31]. For a long time, it was accepted that PRC1 was epistatic to PRC2. The current understanding, however, is that they can engage in a feed forward interaction that reinforce each other activities [32] and that on new targets PRC1 activity may precede that of PRC2 [33].
2.2. PRC1 and PRC2 Are Made of a Catalytic Module and Associated Accessory Subunits
Following an architectural design found in other chromatin modifiers, the variety of Polycomb assemble around a (relatively) invariant core, specific for each of the two classes of complexes. For PRC1, the heterodimeric catalytic core is formed by combinations of a RING1 protein, either RING1A or RING1B, with one of six paralogs of a family of RING finger proteins (Polycomb group RING finger proteins, PCGF1 to PCGF6; Drosophila homologs: Psc, Su(z)2, l(3)73Ah). Given that the presence of one or another PCGF seems to influence the association of accessory subunits, six groups of PRC1 complexes are considered, named after the PCGF component: PRC1.1 to PRC1.6 [34]. Moreover, the nature of the accessory subunits confers key differences in the biochemical activities of the complexes, such as the ability to participate in interactions mediating high-order chromatin structures. Thus, the presence of one of five chromobox (CBX) paralogs (CBX2, CBX4, CBX6, CBX7, and CBX8 in mammals), homologs of Polycomb (Pc) in Drosophila, the founder member of the Polycomb system and one of three Drosophila polyhomeotic homologs, PHC1 to PHC3 in PRC1.2 and PRC1.4, sets them apart in a category of canonical PRC1 complexes (thus termed because the original Polycomb function associated with the first biochemical isolates). The remaining complexes are known as non-canonical or variant PRC1 and share a RYBP subunit, or its paralog YAF2 that interact directly with RING1 subunits in a mutually exclusive fashion with CBX proteins [35].
The catalytic core of PRC2 consists of the methyltransferase EZH1 or EZH2, together with two solenoid-shaped WD40 proteins, EED and RBB4 (or paralog RBBP7), and SUZ12 [E(z), ESC, CAF1 and Su(z)12 homologs in Drosophila, respectively]. This assembly is exquisitely sensitive to interactions, for example with modified histone tails, that influence allosterically EZH2 catalytic activity [36,37,38]. As before, the mutually exclusive association of accessory subunits to this holocomplex determines two main groups of PRC2 assemblies: PRC2.1, with PALI homologs (PALI1,2) and Polycomb-like (PCL) homologs, PCL1 to PCL3, or PRC2.2 with AEBP2 and JARID2 subunits [39,40,41].
In addition to the ability of CBX and PHC subunits of canonical PRC1 to engage in high-order chromatin structure [42,43,44,45], other important activities are contributed by accessory subunits. For example, binding to DNA KDM2B, MGA-MAX, or E2F6-DPA in PRC1 [46,47,48,49], PCL homologs, JARID2 or AEBP2 in PRC2 [50,51,52,53,54], or specific recognition of histone tails (CBX subunits in PRC1 or EED and RBBP4,7 in PRC2 [36,55,56]. The pool of Polycomb complexes displayed in each cell type is determined by the expression levels of each of the subunits, an aspect of the system still poorly characterized. A quantitative study of PRC1 and PRC2 complexes displayed in ESCs and in neural progenitors (NPCs) derived from them, illustrates this point. The study describes dramatic changes in levels of PRC2 core subunits or exchange of PCGF and CBX subunits in PRC1 [57].
2.3. Catalytic Modifications of Histone Tails
PRC1 and PRC2 modify their substrate histones both in a ubiquitous and targeted manner. PRC1 monoubiquitylates histone H2A globally at a very low level and then, at a higher density in a focused manner, restricted to cis-regulatory sites such as promoter proximal sequences [58,59]. E3 ligases of variant PRC1 complexes are constitutively active whereas those of canonical PRC1 complexes are in an autoinhibited form from which they are released upon appropriate interaction with the nucleosome [60]. The enhanced E3 ligase activity of variant PRC1 complexes is related, at least in part, to paralogs RYBP and YAF2, present in all of them [61,62].
H3K27 methylation can affect large extensions of the genome as mono- and, preferentially, dimethyl stages [63,64]. The additional methyl group in H3K27me3—the characteristic Polycomb modification associated to gene repression—is superbly regulated in a combination of the kinetic requirement of the reaction [65] and allosteric changes of the catalytic core after sensing specific histone tail modifications. For example, the interaction of EED with a previously trimethylated tail of H3K27 in an adjacent nucleosome greatly activates EZH2 [36,66]. An alternative pathway implies H2AK119Ub stimulating the catalytic activity of complexes containing AEBP2 [32], confirmed by the impact on the extent of H3K27me3 in cells expressing inert RING1 E3 ligases [67]. In contrast, if the nucleosomal environment contains H3K36me3 the activities of EZH2 and E3 ligases of PRC2 and PRC1 are inhibited [68,69].
Within PRC1, the best-known interaction with histone tails is the recognition of H3K27me3 by some CBX subunits (CBX7, CBX8) for instance in canonical PRC1 [70,71]. Also relevant, in the recognition of H2AUb by PRC1 subunits RYBP-YAF2 [62,72], or JARID2 in PRC2 [73]. Of course, Polycomb-induced modifications are always in a balance with antagonistic activities of enzymatic complexes that remove them. Lysine-specific histone demethylases KDM6A and B or a subset of diverse deubiquitinases such as BAP1, USP16, or MYSM1, effectively modulate the extent of histone changes induced by PRC2 and PRC1, respectively [74,75,76,77].
Polycomb-induced histone modifications characteristically correlate with transcriptionally repressed states. Their function and relative impact on gene expression are loci and context-dependent, as in bivalent promoters—not active but poised for expression—recently re-defined as bistable [78] or large silent domains [67,79]. Thus, in ESCs, histone H2A monoubiquitylation seems essential to gene repression [67,80], whereas in other cell types, PRC1-dependent repression can take place in the absence of H2AK119Ub [81]. Importantly, nucleosomes decorated with H3K27me3 and H2AUb contribute to their propagation, on the same sites, throughout cell division [62,82,83].
2.4. Recruitment of Polycomb Complexes to Chromatin
Targeted localization, or increased residence times of complexes on chromatin, favors enzymatic modifications and functions involving protein–protein interactions. In contrast, non-targeted collisions leading to more transient contacts are probably the origin of global histone modifications mentioned above. Targeted recruitment can be direct, through DNA-binding subunits in the complex or, indirect, through contacts with non-Polycomb DNA binding proteins.
Cis-regulatory modules that mediate Polycomb repression (the so-called Polycomb response elements or PRE) were first identified in transgenic assays in Drosophila [84]. PREs sequences are enriched in sites for DNA-binding proteins [85]. Of these, only pleiohomeotic—a zinc finger protein homolog of vertebrate YY1 [86]—is encoded by a PcG gene. Recruiting of PRC complexes to PREs, however, depends on the concurrent activity of a varied set of DNA-binding proteins [87,88,89]. YY1, which is not part of Polycomb complexes, has a controverted role in recruiting in mammals [90,91]. Instead, PRC1 and PRC2 subunits with DNA binding activity can mediate localization to targets, in particular to those associated to non-methylated CpG-rich sequences (CpG islands, CGIs), a peculiar signature associated to a large number of mammalian promoters. Examples of DNA binding proteins stably associated to Polycomb complexes are KDM2B in variant PRC1.1 [46,47,48] and PCL homologs in PRC2.2 [50,51,52], that use as DNA binding motifs a CXXC-type of Zn finger and a winged helix, respectively. Interactions with modified histones, as H3K27me3 recognition by PRC1, at one time considered a key recruiting mechanism, is now considered to contribute only partially to Polycomb targeting [33,92]. Recruitment involving binding to RNAs is rather controversial and currently under active investigation [93,94].
Recently, single-cell tracking in vivo of Polycomb subunits has shed new light about association of PRC1 and PRC2 to chromatin. The new observations describe highly dynamic systems in which at any given time, only a small proportion of the subunits are found on chromatin, with brief residence times, and low rates of occupancy [95,96]. The rather unexpected stage, contrast with the view inferred from aggregated cell populations, more static derived from methodologies such as chromatin immunoprecipitation (ChIP).
2.5. High Order Chromatin Structures Mediated by Polycomb
Polycomb-anchored loops documented in Drosophila [97,98] and in mammalian cells [99] often encompass developmentally relevant genes, and are formed during development in a dynamically, discontinuous manner [98]. Polycomb-dependent long range promoter–promoter and promoter–enhancer contacts described in ESCs involve the Hox clusters and other genes encoding important developmental regulators [100] but also other genomic sites contributing to the overall 3D structure of the genome [101]. In mammalian cells, Polycomb mediated contacts between distal genomic regions are cell type specific, readily observed in ESCs but not in differentiated or tumoral cell types [102]. These long-range contacts withstand removal of cohesins [102], the molecules involved in chromatin organization through DNA loop extrusion [103,104] and, therefore, represent an independent element of chromatin organization. Some of these structures, are stabilized by PHC paralogs and other canonical PRC1 subunits containing a SAM domain, endowed with oligomerizing properties [42,43]. The requirement of H2AUb, however is controversial [67,101]. Also, despite the apparent correlation with gene repression, these contacts are separable of gene activity [101,105] as also suggested by their association with transcriptionally active loci (see below, [106]).
The presence, in a few PRC1 subunits, of so-called internally disordered sequences, allows them to bring genomic sites together, in condensates that separate them from other nuclear compartments. These phase-separated liquid structures are involved in a variety of cell biology processes [107]. Regarding gene expression, these condensates have been associated to specific transcriptional states such as those at active super-enhancers [108,109] or heterochromatic domains [110,111]. Mammalian CBX2 and Drosophila PSC are two of these subunits able to form these condensates in vitro [45,112,113,114] although evidence for their functional involvement in vivo is still preliminar.
3. Polycomb and Transcriptionally Active Loci
PRC1 loss-of-function mutations in mouse embryos lead to the identification of unexpected Polycomb activities sustaining gene expression. Examples illustrating this situation include the decreased levels of Hoxb1 mRNA in PCGF2-deficient embryos [115] or the downregulated mRNA-encoding homeobox protein Nkx2.5 in embryonic cardiomyocytes deficient in PHC1 [116]. Additionally, chromatin localization studies in flies and mice showed that the presence of Polycomb subunits does not always correlate with transcriptionally repressed states. Work with transgenic flies shows that at least certain PRE-containing templates can undergo transcription without eviction of Polycomb-bound proteins [89]. In natural scenarios, in Drosophila cell lines PRC1 subunits PC, PH, and PSC bind to Abdominal-B, one of the genes in the Bithorax complex (BX-C) of homeotic genes, regardless of transcriptional state [117]. Similarly, in Drosophila tissues, subunits from PRC2 and PhoRC complexes associate with Ultrabithorax (Ubx)—another gene in the BX-C complex—both in wing and halter imaginal discs where Ubx is active and inactive, respectively [118,119,120]. In mice, PRC1 subunits PHC1 and CBX2, but not RING1B locate to Hoxb8 sites both in anterior (active) and caudal (repressed) tissues of the embryos [121].
Recently, systematic analysis of Polycomb occupancies in mammalian cells and in Drosophila tissues show their presence on a large number of active loci (summarized in Table 2). The data listed refer only to subunits known or suspected to act together with other Polycomb subunits. Arbitrarily, we separate these instances from activities of Polycomb subunits that act with non-Polycomb proteins in functions that may include transcriptional activation. Examples are EZH2 acting as a positive cofactor with Androgen receptor in a subset of prostate cancer cell lines [122] or CBX8 in a non-PRC1 complex in mammary epithelial tumor cells [123].
Table 2.
Polycomb subunits that localize (ChIP) to active targets.
| PRC Subunits | Cell Type | Reference |
|---|---|---|
| RING1B, EZH2, SUZ12 | Murine ESCs | [125,126] |
| RING1B, CBX7, EZH2 | Murine quiescent B-cells | [127] |
| CBX8 | Murine neural progenitors | [128] |
| Pc, PSc, Ph | Drosophila imaginal discs | [129] |
| CBX6, 7, 8; RING1A, RING1B | Human fibroblast cell lines | [130] |
| RING1B | Postnatal mouse brain cells | [131] |
| EZH1, EZH2, EED, SUZ12 | Human differentiating erythroid cells | [132] |
| RING1B, PCGF2, CBX2, RYBP | Murine cardiac-mesoderm precursor cells | [133] |
| RING1B | Human melanoma cell lines | [134] |
| RING1B, PCGF2 | Neural progenitors | [57] |
| RING1A, RING1B, CBX2, PCGF1, KDM2B | Human erythroleukemic K562 cell line, AML patient cells | [135] |
| Pc, Ph | Drosophila embryo, imaginal discs | [124] |
| RING1B, PCGF4 | Human fibroblasts, K562 cells | [124] |
| RING1B, PCGF2 | Murine spermatogonia cells | [136] |
| Pc, Ph | Drosophila BG3 cell line | [137] |
| RING1B, RYBP, PCGF4, KDM2B, L3MBTL2 | Murine epidermal progenitors | [138,139] |
| RING1B | Human breast tumor cell lines MCF10A, T47D, MDA-MB-231; Human liver cancer cell line Hep G2, K562 cells | [140] |
| CBX4, PCGF2, PCGF4 | Human breast tumor cell lines | [140,141] |
| RING1B | Human leukemic cell line ME-1 | [142] |
The products encoded by Polycomb active targets somehow deviate from the conventional understanding of Polycomb functionality. In this traditional perspective, active Polycomb targets include genes encoding metabolic functions, cell proliferation, signaling, and cytoskeletal functions [124]. In a simplified view of Polycomb functions, conventional repressive roles would impact programs for alternative cell lineages, while functions on active loci would further progress along ongoing differentiation processes.
Although speculative, because of the rather limited data, a number of features identifiable in Polycomb regulation of active targets include:
Presence of PRC1 (invariable, RING1B in particular) and PRC2 subunits;
Low/undetectable levels of H3k27me3 or H2AK119Ub modifications;
Chromatin enrichment rates generally lower than at Polycomb-repressed domains;
In differentiated cell types, the ratio of active to silent targets larger than in cells of fly embryos or ESCs;
Enhancers and super-enhancers, in addition to promoters, among regulatory sites occupied by Polycomb products.
From a gene control perspective, the outcome of Polycomb regulation of active targets includes both negative and positive influences, rather than the steady negative role(s) when acting on silent targets. An additional function promoting gene activation can be identified in cell differentiation processes by which the Polycomb system is involved only during the turning on process, without further implication while the gene is active.
3.1. Polycomb Occupancy of Active Targets
Data available seem to suggest that PRC occupation of active sites (Table 2) seem to fall into two categories. In one PRC proteins locate predominantly at promoters as in ESCs, B-cells, fibroblasts, epidermal progenitors, or the erythroleukemic cell line K562 [125,127,130,135,138]. In the other one, occupied sites also contain a large number of intergenic and intragenic or distal sites, including enhancers and super-enhancers. Examples of this situation are found in Drosophila imaginal discs [106,137], murine neural progenitors [57], and human breast tumor cell lines [140]. It is possible, however, that the notion of distinctive patterns of PRC binding to active targets has to be revisited in the light that ChIP analysis, in some cases, may have missed low density occupancies at distal, intergenic sites that may have led to the over-representation of promoters among binding sites. For example, in K562 cells, while one report describes predominant PRC1 binding to promoters [135], a large number of super-enhancers are identified among RING1B bound sites in these cells by other report [140]. An interesting observation is the relatively high representation of cancer cells among cell types with Polycomb functions on active targets. It is possible that the number of normal cell types investigated so far is rather limited, while transformed types are found in abundance among tissue culture cell lines used in labs. However, the observation can be meaningful by itself, reflecting some property of cancer cells related to the differential number of repressed/active Polycomb targets in primitive cells and in differentiating cells indicated above. It is possible that the deregulated transcription programs, a feature of transformed cell types, and the associated transcriptional dependence on the expression of a number of regulators [141] makes them singularly suited cell contexts for positive functions of, for example RING1B, supporting gene activity.
Few studies follow genomic localization of Polycomb products through differentiation [i.e., embryo to larva, ESCs or hematopoietic stem/progenitor (HSPCs) to their differentiated progenies]. If a trend was to be identified, it would be that the ratio of repressed to active Polycomb targets in primitive cells is high whereas, in contrast, in differentiated cells types the ratio is tilted toward the set of active targets [57,124,140]. During cell-state transitions, Polycomb is not only evicted from repressed sites but also displaced to new sites which are often active enhancers and promoters (Figure 2). Localization to the new active sites is probably mediated by transcription factors. For instance, in K562 cells, there is a significant overlap between promoter and enhancer occupancies of RING1B and the transcription factors GATA1 and GATA2 [140]. Likewise, in human leukemic cell line ME-1 there is a significant overlap between genomic sites occupied by RING1B and DNA-binding RUNX1 [142]. These examples support an instructive mechanism, mediated by transcription factors, in Polycomb recruiting to active targets. Furthermore, in breast tumor cell lines, sequences at RINGB-bound super-enhancers are enriched in ERα and FOXA1/2 consensus binding sites [140]. In this scenario, the molecular logic suggests that the pioneer factor FOXA1/2 makes room in nucleosome-wrapped DNA to facilitate the binding of ERα, with RING1B association somewhere along the process. However, such a straightforward view appears as a large oversimplification, as subsequent work shows binding of RING1B to estrogen response elements in response to hormone presence in a highly dynamic manner, together with FOXA1 and ERα, including mutual influences between the three proteins [143].
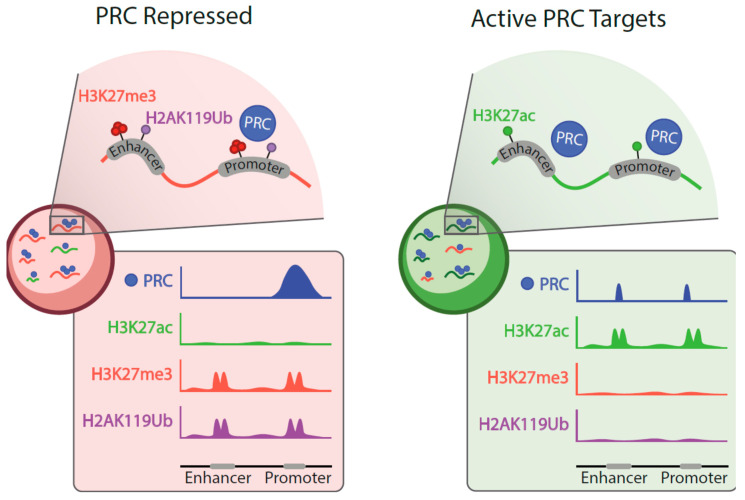
Figure 2.
Schematized characterization of repressed and active Polycomb targets. Genomic sites occupied by PRC proteins in undifferentiated, primitive cells (left), and in cells in more advanced stages of differentiation pathways (right). Color-coded repressed and active targets show a higher proportion of active targets in differentiated cells. Polycomb-induced modifications are prevalent on repressed targets. Below are simplified views of ChIP profiles showing distinctive Polycomb occupancies (PRC, encompassing all classes of assemblies) and its increased presence on active enhancers (H3K27ac).
Binding patterns of Polycomb products to active sites partially differ from those of repressed domains (Figure 2). In general, occupancies extend not as broadly in active sites, and often ChIP peaks are sharp as those of transcription factors [140]. Also, occupancy densities, inferred from the reads counts are lower at active targets (see genome browser screen shots in [124,132,140]). These features, together with an idealized redeployment of Polycomb subunits in differentiated cell types are schematized in Figure 2.
Just as Polycomb is involved in the formation of loops and other contacts between occupied, distant sites in repressed domains, active Polycomb targets are tied in similar structures. In imaginal discs, a higher frequency of short- or long-range contacts correlates with the presence of PRC1 components on active promoters and enhancers [106]. These PRC1-tied loops, involving active targets form in a developmentally dynamic fashion. Similar chromatin arrangements in murine neural progenitors [106] indicates a conserved regulatory strategy, for both Polycomb-repressed and active targets.
3.2. Polycomb Proteins on Active Targets
ChIP experiments studying the localization of PRC1 often include the core subunit RING1B, and variably other subunits from canonical and variant PRC1 complexes. The presence of PRC2 proteins, in contrast, is much less studied. Polycomb-induced histone modifications, H3K27me3 and H2AK119Ub, usually found at repressed targets are, generally, absent. This is documented for Drosophila cell lines and imaginal discs [124,129] or in the murine system, in lymphoid [127], germinal [136], epidermal cells [138,139], hematopoietic progenitors, and their derived erythroid cells [132,144]. Polycomb-bound active targets in transformed mammalian cell lines also lack H3K27me3 and H2AK119Ub [135,140]. In contrast, many of these active targets are enriched in H3K27ac, a modification that together with H3K4me1 is typically associated to active enhancers [95,145]. Nevertheless, exceptions showing H3K27me3 on select active targets have been reported in neural and mesodermal progenitors derived from ESCs [128,133]. The effects of enforced decreases of H3K27me3, trough depletion of PRC2 products, shows minimal impact on active targets in imaginal discs or epidermal progenitors [124,138,139]. However, the wide down-regulation effect seen in EZH1-defficient erythroid cells, is unrelated to H3K27me3 because the prevalent PRC2 complex in these cells (lacking EED) is enzymatically inert [132].
The presence of H2AUb is generally undetectable or detected at low levels on active targets. The relatively little impact of this modification has been tested in epidermal progenitors depleted of RING1A and RING1B. The accompanying down regulation of active Polycomb targets, however, can be rescued to a large extent by an inert form of RING1B occupying these targets, thus showing that the transcriptionally positive influence of RING1B on these targets is independent of its E3 ligase activity [138]. Nevertheless, analysis in Drosophila imaginal discs, shows that chromatin at a subset of active enhancers associated to Polycomb targets is decorated with H2AK118Ub [106]. The observation probably indicates that there is more into the correlation between these histone modifications and transcriptional states than what can be anticipated at this time.
Although incomplete, the current evidence on the association of Polycomb proteins to active sites suggests that it may not occur, necessarily, as part of the known, biochemically defined complexes. In imaginal discs, for example, PH and PC bind to active regulatory elements independently of each other, in contrast with their colocalization at repressive PREs [137]. In ESCs, PCGF paralogs PCGF3 and PCGF5 act in gene activation functions independently of RING1A and RING1B [146]. These PCGF homologs bind their targets through interactions with DNA binding proteins outside the Polycomb realm, such as Tex10 or USF1/2 [146,147]. But even if occurring under canonical conditions, the functional outcome could be altered (i.e., reversed) by the proximity of interacting/non-interacting proteins able to modify the immediate molecular environment, making it conducive to gene expression.
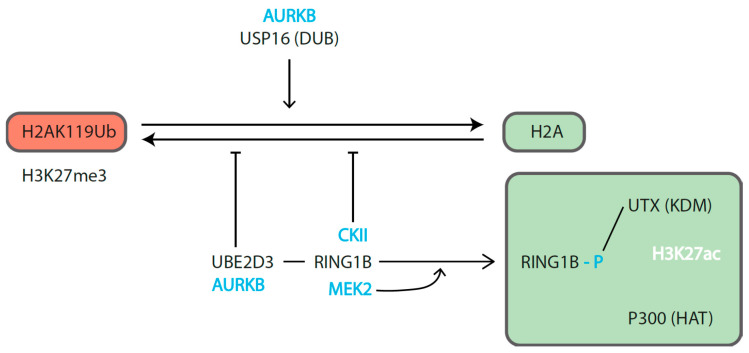
Kinases are among the regulators that can flip RING1B roles (Figure 3). RING1B phosphorylation can inhibit its E3 ligase activity or affect its ability to associate direct or indirectly with proteins. For example, in resting peripheral B cells, Aurora B kinase (AURBK)—a kinase well known by its involvement in mitosis—indirectly inhibits RING1B E3 ligase activity through phosphorylation of ubiquitin-conjugating enzyme UBE2D3 [127]. At the same time, the deubiquitinase USP16 is phosphorylated to promote its deubiquitinase activity. The overall effect can be one of the efficient reduction of H2AK119Ub on promoter proximal nucleosomes. Direct down modulation of RING1B E3 ligase activity is also associated to its phosphorylation (at S168) by casein kinase II (CKII), a subunit regularly present as part of PRC1.5 complexes that in some cells include AUTS2, a subunit that can recruit histone acetylase (HAT) p300 [131]. Alternatively, posttranslational modifications of RING1B can affect its ability to associate direct or indirectly with proteins involved in transcriptional activation such as HAT p300 and histone H3K27 demethylases (KDM6A/UTX), as seen by MEK1 phosphorylation of RING1B S41 [134].
Figure 3.
Posttranslational modifications of RING1B mediating opposite transcriptional states. Phosphorylation can accompany the activities of RING1B on repressed (left) and active (right) targets. The indicated kinases, in blue, correspond to reported examples where the outcome of its E3 catalytic activity is modulated directly (RING1B, UBE2D3) or indirectly (USP16). The continuous modification of H2A to oppose the action of DUBs, is needed to sustain the chromatin environment at PRC-repressed sites. Tilting the balance toward the non-ubiquitylated form of H2A may occur at the same time that RING1B phosphorylation (MEK2-induced) enhances its ability to associate with histone H3K27 demethylase (KDM) UTX-containing complexes. In turn, these facilitate the recruitment of histone acetyl transferase (HAT) p300, a well-known axis in enhancer activation.
Not only RING1B phosphorylation can modulate its functions, but also the alteration in composition of Polycomb subunits is what affects the activity of the modified complex in other cases. This is the case mentioned above of PRC2 complexes in differentiating murine erythroid cells. There, a repressive, enzymatically active EZH2-EED-SUZ12 core complex in primitive cells, turns during differentiation into an inert EZH1-SUZ12 complex located on active targets [132]. The selective absence of PCGF2 from Polycomb-occupied promoters at active targets, while present at repressed sites of germ cells [136], maybe another example of how complex composition can dictate a stage conducive to gene expression.
Attempts to discern between contributions of canonical or variant PRC1 complexes are hampered by the evidence available nowadays, which is fragmentary and sometimes contradictory. For instance, in epidermal progenitors, there is a trend for the association of canonical subunits (PCGF4 and CBX8) with repressed targets and subunits from variant complexes, such as L3MBTL2, RYBP, or KDM2B, enriched on active sites [138]. In contrast, in mesodermal progenitors, some active targets are enriched in canonical PCGF2/CBX7 subunits and others in variant subunit RYBP [133]. Regardless of the PRC1 class, it appears that Polycomb assemble at active targets as combinations of PRC1 and/or PRC2 subunits, in a target-specific and cell-context dependent manner [57,132,133], compatible with functionality modifiable by associated/nearby non-Polycomb proteins.
3.3. Polycomb Functions on Active Genes
Polycomb proteins regulate active genes through mechanisms still not fully understood. For some targets a negative influence is observed (i.e., dampening activity), identified by the upregulation that follows depletion of Polycomb subunits [124,125,137]. This activity contrasts with the positive influence exerted on other targets (i.e., facilitating activity), which respond to Polycomb inactivation with decreased expression [124,125,137]. The outcomes of Polycomb inactivation/downregulation on active targets, however, are of a lesser magnitude than those usually observed when acting under its conventional repression function.
Evidence connecting Polycomb subunits with regulators associated to gene expression has been accumulating steadily. Thus, subunits of histone acetylase complexes MOF and Tip60 appear in the characterization of Polycomb interactors [148,149,150]. Cohesins, proteins that form ring-shaped complexes on promoters and enhancers [26] are interactors of PRC1 subunits [151] and, in Drosophila, assist their recruitment to active targets [129]. This biochemical knowledge, however, has not been integrated yet in a comprehensive mechanism underlying Polycomb functions on active genes.
3.3.1. Dampening Gene Activity
The down modulation of active Polycomb targets is best documented in ESCs and in Drosophila imaginal discs. These studies are not readily comparable because of their inherently different resolution: single ESCs [152] and imaginal disc cell populations [124,137]. Nevertheless, active Polycomb targets differ in H3K27me3 modification, absent or very low in cells of imaginal discs. It is not clear whether the difference reflects distinct regulatory demands by the functional hardwiring of the targets, related to metabolic and signaling processes in ESCs [125], and to cell proliferation and polarity in imaginal discs [124]. Promoters, are occupied by the non-elongating, but active form of RNA polymerase II (RNApolII), which is phosphorylated at the serine 5 (S5P) in its C-terminal repeat domain at active targets, in contrast with the unphosphorylated, non-productive form of RNApolII of repressed targets [125,137,152].
Kinetic analysis of the activity of the Polycomb-bound active promoters in ESCs shows a lower frequency of transcriptional bursting than that of active genes, consistent with a switch between repressed and Polycomb-activated states [152]. These bursts represent the discontinuous nature of the general transcription process, so that the overall RNA output results of the combined effects of the size and frequency of bursts [153]. The highly variable expression per cell of Polycomb-bound active promoters could be due to Polycomb acting allelically, within every cell, or independently in every cell in the population, but it could not be determined because promoter activity was assessed as RNA level per cell [152]. The moderate increase in expression levels at Polycomb active targets in RING1A and RING1B-depleted ESCs has been correlated with a decrease in transcriptional noise, a parameter related to stochastic fluctuations in promoter activity.
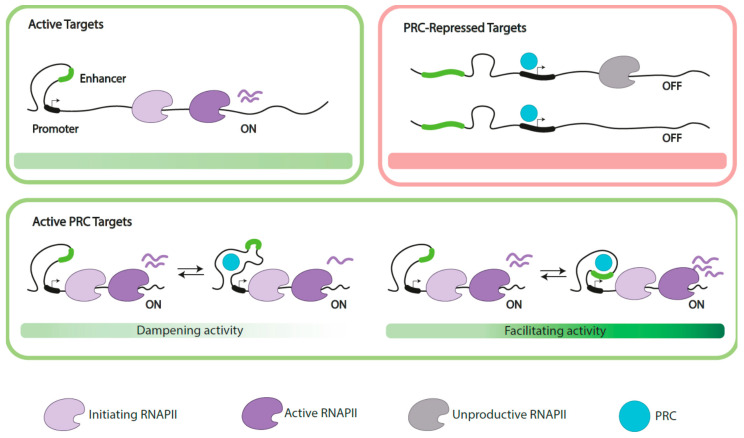
Polycomb-bound sites at active targets are enriched among genomic three-dimensional contacts, although less extensively than Polycomb-repressed promoters [100]. Moreover, the proportion of active promoters that interact with active enhancers is significantly higher than that of repressed promoters [152], which are involved in contacts with poised enhancers [100,152]. Interestingly, since the communication of enhancers and promoters influences transcriptional bursting [110,154] it would be feasible that the intervention of Polycomb products in such communication becomes crucial for its regulatory role (diagrammed in Figure 4). Polycomb-dependent dampening of gene expression through control of transcriptional bursting may have been selected for the fine tuning of gene expression of selected targets in specific cell contexts.
Figure 4.
Simplified representation of cis regulatory elements at extended PRC targets. Depicted scenarios include active, not PRC-regulated targets (top, left), and PRC-repressed targets (top, right) as extreme scenarios, showing promoter proximal and elongating forms of productive RNA polymerases (RNAPII), in contrast with low/undetectable levels of mRNA at PRC-repressed targets, where RNAPII is in an unproductive form. Below, two possibilities of active PRC targets. In both cases, expression dampened (left) or facilitated (right) is interpreted as the outcome of the abilities of PRC proteins, together with uncharacterized factors, to modulate the communication between enhancers and promoters. Fine tuning of expression at these targets is illustrated as switching scenarios where PRC impairs such contacts to decrease overall promoter output (dampening activity), in contrast with the promotion of the contacts leading to sustained expression (facilitating activity). Target identities, accessory factors and available Polycomb products (cell context) are probable determinants of one or another function on these active targets.
The analysis of active Polycomb targets in imaginal disc cells, instead, enlightens dampening events related to the transition between paused (arrested shortly after initiated) and elongating transcription [137]. An idea of the unresolved complexity of the system is given by the different outcomes arising from the downregulation of one or another PRC1 subunit. Thus, while Ph acts against the presence of phosphorylated forms of RNApolII (both initiating, S5P, and elongating, S2P), SCE and PC promote their presence. Another marker of productive transcription that decreases with SCE depletion is SPT5, one of the two subunits of DSIF (DRB sensitivity-inducing factor, an essential complex for RNApolII release and productive elongation). These alterations take place at active, but not at repressed Polycomb targets. Of interest, the localization of SPT5 at enhancers and PREs also depends on SCE. In turn, the presence of PRC1 proteins on active targets is promoted by cohesins [129]. Additional work is needed to establish whether these findings apply to Polycomb-dampened targets in other cell types.
3.3.2. Supporting Gene Expression
Examples of Polycomb-supported transcription are predominantly known in mammalian cells. Its identification comes from loss-of-function mutant experiments that results in decreased stationary mRNA and important alterations in differentiation programs of PRC1 mutant mouse lines. These experiments correlate with decreased expression of signature elements of cell lineage programs as is the case of those conducted on epidermis, germ cells, and early mesodermal cell types [127,133,136,138,139,155]. Cells where these targets have been identified, use Polycomb conventional-repressive activities in the silencing of alternate cell lineage programs, and Polycomb-promotion of gene expression in the progression of the ongoing differentiation.
The mechanism by which Polycomb supports transcription have not been elucidated yet. In general, depletion-associated gene downregulation is rather moderate, suggesting roles as positive cofactors. Following the same line of reasoning mentioned above, the influence on enhancer-promoter communication would serve to interpret Polycomb sustaining gene expression: by promoting communication; a higher activity at the promoter could be expected. Although there might be mechanistic differences, Polycomb subunits would be acting as coactivators. The accompanying histone modifications at regulatory sequences (i.e., H3K27ac enrichment) would buffer them against non-specific repressive influences. The fact that Polycomb subunits play a direct role sustaining gene activity is supported in epidermal progenitors by both the progressive decline in expression of active targets as a result of RING1A and RING1B depletion and the complementation of the defect by ectopic expression of RING1B [138].
The involvement of RING1B in the occurrence and maintenance of regulatory regions (assessed by Assay for Transposase-Accessible Chromatin (ATAC) experiments where accessible chromatin sites are evaluated through exposure to a transposase) has been documented in breast tumor cell lines [140]. The effects observed through manipulation of RING1B dosage are accompanied by changes in the activity of enhancers measured as variation in enhancer RNA (eRNA). Deregulation of active targets upon RING1B depletion varies with the tumor cell line, but in some cases, many undergo downregulation. Interestingly, chromatin accessibility alterations (gain and loss of ATAC peaks) associated to RING1B depletion occurs at enhancer and intergenic regions, but not at promoters [140]. Also, in cells with decreased levels of RING1B, variations in eRNA levels show both upregulation and downregulation of eRNAs, consistent with a dual role on enhancer function.
An entirely different approach, CRISPR-CAS mediated deletion of DNA sites occupied by PRC1, also demonstrates the involvement of PRC1 products in gene activation. These laborious experiments are loci-specific and do not involve changes in the levels of PRC1 products. In one case, the deletion of a promoter proximal site resulted in downregulation of the selector gene vestigial in the pouch of the wing imaginal disc, where it is normally expressed [156]. In a different study, deletions involve one or both of the PRC1-anchoring sites with contrasting effects on gene expression: while deletion of both sites decreases expression of dac, linked CG588 gene is upregulated. Alternatively, deletion of the 3’ site decreases CG588 expression with a much milder effect on dac [106]. In either manipulation, chromatin contacts are lost and hence is difficult to separate the contributions of the looped structure from that of the presence of PRC1 products.
3.3.3. Indirect (Positive) Role in Gene Activation
In certain cases, gene expression induced during differentiation/development processes requires the activity of Polycomb proteins. However, its involvement seems to be limited to the establishment of the active transcriptional state rather than to its maintenance; as active transcriptional states are maintained without Polycomb presence.
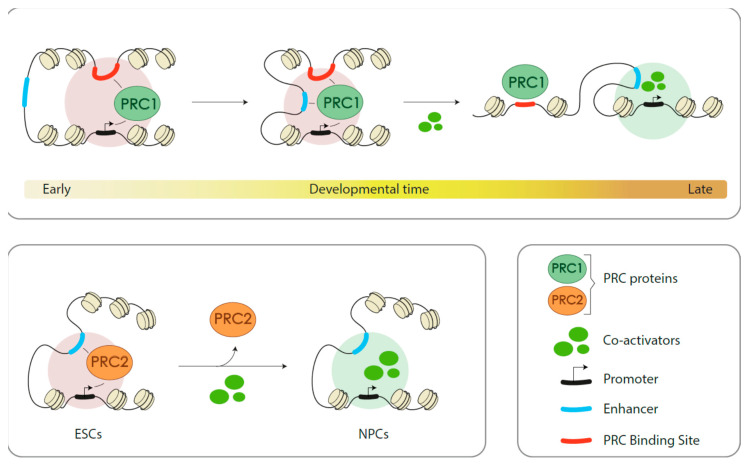
A well-studied example in mouse embryos is the spatiotemporal expression of Meis2; a developmentally important pleiotropic transcription factor. RING1B contributes positively (embryonic brain structures) and negatively (other tissues) to Meis2 expression. RING1B binds constitutively a site located 3’ to the end of the locus and its occupation at the promoter varies with developmental time and expression states (Figure 5). Early in development, immuno-fluorescent in situ hybridation (immuno-FISH) analysis in non-expressing tissues yield close signals for the promoter and the 3’ binding site. These signals become distant in the derepressed state and are accompanied with the absence of RING1B [157]. Signals for the enhancer responsible of Meis2 activation do not colocalize with those of RING1B-occupied sites. Later in development, this scenario changes so that, shortly before expression, signals for the three elements coalesce, in a RING1B-dependent manner. Subsequently, in the active site, signal proximity is observed only for promoter and enhancer [157]. The results are interpreted as RING1B playing a role in the communication between promoter and enhancer so that a chromatin conformation that prevents their interaction keeps silent Meis2, but that, later on, probably in cooperation with uncharacterized transcription factors, the eviction of RING1B from the promoter facilitates the interaction of the promoter with the enhancer inducing the activation of the Meis2 gene.
Figure 5.
PRC topological activities are required for gene activation. Two situations are depicted, corresponding to reported cases where gene activation along differentiation pathways depends on the presence of PRC complexes. One, at the top, corresponds to configurations of the embryonic Meis2 locus throughout development [157]. Three-dimensional contacts, dependent on RING1B, evolve from the repressed state, left, where the enhancer cannot interact with the promoter, to the active state, where communication enhancer-promoter is effective, progressing through an intermediate state previous to the (assumed) involvement of DNA binding proteins and other factors required for gene activation. The second example, below, corresponds to configurations of one of the loci encoding anterior neural genes [158]. A major difference with the previous scenario is that, in the repressed state, in ESCs, enhancers (poised) are contacting the promoter, in a PRC2-dependent manner. The contacts as such, are not relevant to the transcriptional state, however, these are required for effective response to differentiating cues NPCs.
Immuno-FISH lacks the resolution that can be acquired with Hi-C methods, but the correlation between overlapping signals and topological loops is widely accepted. Under this assumption, the structure proposed for the embryonic Meis2 locus is similar to the PRC1-dependent contacts between promoters and enhancers in Polycomb repressed targets in ESCs [100]. However, their role in promoting the concomitant activation of these targets, while suggestive, remains to be studied. On the contrary, evidence along these lines has been shown, for PRC2-mediated chromatin structures associated to silent loci [158]. Here, contacts between promoters and poised enhancers are required for the induction of a subset of developmental regulators expressed in anterior domains of embryonic neural structures (Figure 5). The deletion of candidate enhancer sequences, using a CRISPR-Cas approach, impairs the contacts with the promoters of selected targets and results in poor expression in an in vitro differentiation system [158]. Inactivation of the core PRC2 EED subunit leads to similar results, although at the ESC stage, targets are not derepressed and the loss of H3K27me3 at poised enhancers is not accompanied by a gain of H3K27ac. This is probably due to the absence of the developmental signals required for neural activation in ESCs. This contrasts with the effects of PRC1 depletion on HOX clusters in ESCs, where promoter-promoter, but not promoter-enhancer contacts are lost upon RING1A and RING1B depletion, and poised enhancers become active and targets derepressed [100]. The overall impact of this strategy, turning a repressive Polycomb activity into one that facilitates gene induction, is not known.
4. Conclusions and Perspectives
Evidence for the activity of Polycomb proteins modulating the expression of active targets is incontrovertible. Nevertheless, it is a minor part of the current understanding about the Polycomb system, overwhelmed by the volume of work that supports functions on gene repression. How Polycomb proteins bind their active targets, probably not as the assemblies identified when acting in repressive functions, and how do they modulate transcriptional activity are areas in need of progress. Of particular interest is the finding of active enhancers among PRC1 sites. It is likely that mechanisms pertinent to the communication between regulatory elements, enhancer–promoter, are relevant to gaining insight in the role(s) of Polycomb proteins on active targets. It also underlines the relevance of the quantitative regulation of gene expression, in opposition to alternative on and off states. In this regard, Polycomb regulation of active genes is dual, either restricting transcription output, in some cases or, in others, supporting expression. Phenotypic consequences of defective regulation when dampening gene expression are not known. In contrast, decreased expression levels resulting from loss-of-function mutations are more easily correlated with impaired differentiation processes. It is clear that conventional approaches in the genetic analysis, downregulating/depleting any given subunit, lack the needed resolution. Instead, the use of allelic variants, as those being used to dissect catalytic from other activities, will be necessary.
Further studies are required to measure the impact of functions on active genes along cell lineage differentiation pathways. The current evidence, however, points at a large number of targets, including some important in the maintenance of cellular homeostasis. The detection of PRC1 products, RING1B in particular, on enhancers and super enhancer in tumor cell lines is intriguing. It appears, that as it has been previously observed for co-repressors [159], Polycomb, the system formerly known only as repressor, can handle additional identities in gene control.
Funding
Natalia Giner is supported by an ESR fellowship from ARCH H2020-MSCA-ITN-2018, grant 813091.
Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Klemm S.L., Shipony Z., Greenleaf W.J. Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 2019;20:207–220. doi: 10.1038/s41576-018-0089-8. [DOI] [PubMed] [Google Scholar]
- 2.Ernst J., Kheradpour P., Mikkelsen T.S., Shoresh N., Ward L.D., Epstein C.B., Zhang X., Wang L., Issner R., Coyne M., et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabo Q., Bantignies F., Cavalli G. Principles of genome folding into topologically associating domains. Sci. Adv. 2019;5:eaaw1668. doi: 10.1126/sciadv.aaw1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowley M.J., Corces V.G. Organizational principles of 3D genome architecture. Nat. Rev. Genet. 2018;19:789–800. doi: 10.1038/s41576-018-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis E.B. A gene complex controlling segmentation in Drosophila Genes. Dev. Cancer Life Work Edward B Lewis. 2007;276:229–242. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 6.Schuettengruber B., Bourbon H.-M., Di Croce L., Cavalli G. Genome Regulation by Polycomb and Trithorax: 70 Years and Counting. Cell. 2017;171:34–57. doi: 10.1016/j.cell.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Whitcomb S.J., Basu A., Allis C.D., Bernstein E. Polycomb Group proteins: An evolutionary perspective. Trends Genet. 2007;23:494–502. doi: 10.1016/j.tig.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Shao Z., Raible F., Mollaaghababa R., Guyon J.R., Wu C.-T., Bender W., Kingston R.E. Stabilization of Chromatin Structure by PRC1, a Polycomb Complex. Cell. 1999;98:37–46. doi: 10.1016/S0092-8674(00)80604-2. [DOI] [PubMed] [Google Scholar]
- 9.Kuzmichev A., Nishioka K., Erdjument-Bromage H., Tempst P., Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czermin B., Melfi R., McCabe D., Seitz V., Imhof A., Pirrotta V. Drosophila Enhancer of Zeste/ESC Complexes Have a Histone H3 Methyltransferase activity that marks chromosomal polycomb sites. Cell. 2002;111:185–196. doi: 10.1016/S0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 11.Plath K., Fang J., Mlynarczyk-Evans S.K., Cao R., Worringer K.A., Wang H., De La Cruz C.C., Otte A.P., Panning B., Zhang Y. Role of Histone H3 Lysine 27 Methylation in X Inactivation. Science. 2003;300:131–135. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- 12.Müller J., Hart C.M., Francis N.J., Vargas M.L., Sengupta A., Wild B., Miller E.L., O’Connor M., Kingston R.E., Simon J.A. Histone Methyltransferase Activity of a Drosophila Polycomb Group Repressor Complex. Cell. 2002;111:197–208. doi: 10.1016/S0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 13.Boyer L.A., Plath K., Zeitlinger J., Brambrink T., Medeiros L.A., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K., et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 14.Endoh M., Endo T.A., Koseki H., Endoh T., Fujimura Y.-I., Ohara O., Toyoda T., Otte A.P., Okano M., Brockdorff N., et al. Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development. 2008;135:1513–1524. doi: 10.1242/dev.014340. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs J.J.L., Kieboom K., Marino S., DePinho R.A., Van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 16.Koppens M., Van Lohuizen M. Context-dependent actions of Polycomb repressors in cancer. Oncogene. 2015;35:1341–1352. doi: 10.1038/onc.2015.195. [DOI] [PubMed] [Google Scholar]
- 17.Mozgova I., Hennig L. The Polycomb Group Protein Regulatory Network. Annu. Rev. Plant Biol. 2015;66:269–296. doi: 10.1146/annurev-arplant-043014-115627. [DOI] [PubMed] [Google Scholar]
- 18.Vidal M., Starowicz K., Starowicz K. Polycomb complexes PRC1 and their function in hematopoiesis. Exp. Hematol. 2017;48:12–31. doi: 10.1016/j.exphem.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Huang C., Nogales E., Ciferri C. Molecular Architecture of the Polycomb Repressive Complex 2. Polycomb Group Proteins. 2017;45:165–189. [Google Scholar]
- 20.Aranda S., Martín G.M., Di Croce L.D. Regulation of gene transcription by Polycomb proteins. Sci. Adv. 2015;1:e1500737. doi: 10.1126/sciadv.1500737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingston R.E., Tamkun J.W. Transcriptional Regulation by Trithorax-Group Proteins. Cold Spring Harb. Perspect. Biol. 2014;6:a019349. doi: 10.1101/cshperspect.a019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassis J.A., Kennison J.A., Tamkun J.W. Polycomb and Trithorax Group Genes in Drosophila. Genetics. 2017;206:1699–1725. doi: 10.1534/genetics.115.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H., Wang L., Erdjument-Bromage H., Vidal M., Tempst P., Jones R.S., Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- 24.Bentley M.L., Corn J.E., Dong K.C., Phung Q., Cheung T.K., Cochran A.G. Recognition of UbcH5c and the nucleosome by the Bmi1/Ring1b ubiquitin ligase complex. EMBO J. 2011;30:3285–3297. doi: 10.1038/emboj.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGinty R.K., Henrici R.C., Tan S. Crystal structure of the PRC1 ubiquitylation module bound to the nucleosome. Nature. 2014;514:591–596. doi: 10.1038/nature13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagey M.H., Melhuish T.A., Wotton D. The Polycomb Protein Pc2 Is a SUMO E3. Cell. 2003;113:127–137. doi: 10.1016/S0092-8674(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 27.Tsukada Y.-I., Fang J., Erdjument-Bromage H., Warren M.E., Borchers C.H., Tempst P., Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2005;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 28.Turberfield A.H., Kondo T., Nakayama M., Koseki Y., King H.W., Koseki H., Klose R.J. KDM2 proteins constrain transcription from CpG island gene promoters independently of their histone demethylase activity. Nucleic Acids Res. 2019;47:9005–9023. doi: 10.1093/nar/gkz607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeb M., Pasini D., Novatchkova M., Jaritz M., Helin K., Wutz A. Polycomb complexes act redundantly to repress genomic repeats and genes. Genes Dev. 2010;24:265–276. doi: 10.1101/gad.544410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn T.G., Dorafshan E., Schultheis D., Zare A., Stenberg P., Reim I., Pirrotta V., Schwartz Y.B. Interdependence of PRC1 and PRC2 for recruitment to Polycomb Response Elements. Nucleic Acids Res. 2016;44:10132–10149. doi: 10.1093/nar/gkw701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zepeda-Martinez J.A., Pribitzer C., Wang J., Bsteh D., Golumbeanu S., Zhao Q., Burkard T.R., Reichholf B., Rhie S.K., Jude J., et al. Parallel PRC2/cPRC1 and vPRC1 pathways silence lineage-specific genes and maintain self-renewal in mouse embryonic stem cells. Sci. Adv. 2020;6:eaax5692. doi: 10.1126/sciadv.aax5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalb R., Latwiel S., Baymaz H.I., Jansen P.W.T.C., Müller C.W., Vermeulen M., Müller J., Müller C.W. Histone H2A monoubiquitination promotes histone H3 methylation in Polycomb repression. Nat. Struct. Mol. Biol. 2014;21:569–571. doi: 10.1038/nsmb.2833. [DOI] [PubMed] [Google Scholar]
- 33.Blackledge N.P., Farcas A.M., Kondo T., King H.W., McGouran J., Hanssen L.L., Ito S., Cooper S., Kondo K., Koseki Y., et al. Variant PRC1 Complex-Dependent H2A Ubiquitylation Drives PRC2 Recruitment and Polycomb Domain Formation. Cell. 2014;157:1445–1459. doi: 10.1016/j.cell.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Z., Zhang J., Bonasio R., Strino F., Sawai A., Parisi F., Kluger Y., Reinberg D. PCGF Homologs, CBX Proteins, and RYBP Define Functionally Distinct PRC1 Family Complexes. Mol. Cell. 2012;45:344–356. doi: 10.1016/j.molcel.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R., Taylor A.B., Leal B.Z., Chadwell L.V., Ilangovan U., Robinson A.K., Schirf V., Hart P.J., Lafer E., Demeler B., et al. Polycomb Group Targeting through Different Binding Partners of RING1B C-Terminal Domain. Structure. 2010;18:966–975. doi: 10.1016/j.str.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margueron R., Justin N., Ohno K., Sharpe M.L., Son J., Iii W.J.D., Voigt P., Martin S.R., Taylor W.R., De Marco V., et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461:762–767. doi: 10.1038/nature08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Justin N., Wilson J.R., Gamblin S.J. Comment on “Structural basis of histone H3K27 trimethylation by an active polycomb repressive complex 2”. Science. 2016;354:1543. doi: 10.1126/science.aaf6236. [DOI] [PubMed] [Google Scholar]
- 38.Lee C.-H., Yu J.-R., Kumar S., Jin Y., Leroy G., Bhanu N., Kaneko S., Garcia B.A., Hamilton A.D., Reinberg D. Allosteric Activation Dictates PRC2 Activity Independent of Its Recruitment to Chromatin. Mol. Cell. 2018;70:422–434.e6. doi: 10.1016/j.molcel.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holoch D., Margueron R. Mechanisms Regulating PRC2 Recruitment and Enzymatic Activity. Trends Biochem. Sci. 2017;42:531–542. doi: 10.1016/j.tibs.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Conway E., Jerman E., Healy E., Ito S., Holoch D., Oliviero G., Deevy O., Glancy E., Fitzpatrick D.J., Mucha M., et al. A Family of Vertebrate-Specific Polycombs Encoded by the LCOR/LCORL Genes Balance PRC2 Subtype Activities. Mol. Cell. 2018;70:408–421.e8. doi: 10.1016/j.molcel.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Healy E., Mucha M., Glancy E., Fitzpatrick D.J., Conway E., Neikes H.K., Monger C., Van Mierlo G., Baltissen M.P., Koseki Y., et al. PRC2.1 and PRC2.2 Synergize to Coordinate H3K27 Trimethylation. Mol. Cell. 2019;76:437–452.e6. doi: 10.1016/j.molcel.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Isono K., Endo T.A., Ku M., Yamada D., Suzuki R., Sharif J., Ishikura T., Toyoda T., Bernstein B.E., Koseki H. SAM Domain Polymerization Links Subnuclear Clustering of PRC1 to Gene Silencing. Dev. Cell. 2013;26:565–577. doi: 10.1016/j.devcel.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Wani A.H., Boettiger A.N., Schorderet P., Ergun A., Münger C., Sadreyev R.I., Zhuang X., Kingston R.E., Francis N.J. Chromatin topology is coupled to Polycomb group protein subnuclear organization. Nat. Commun. 2016;7:10291. doi: 10.1038/ncomms10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatavosian R., Duc H.N., Huynh T.N., Fang N., Schmitt B., Shi X., Deng Y., Phiel C., Yao T., Zhang Z., et al. Live-cell single-molecule dynamics of PcG proteins imposed by the DIPG H3.3K27M mutation. Nat. Commun. 2018;9:2080. doi: 10.1038/s41467-018-04455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plys A.J., Davis C.P., Kim J., Rizki G., Keenen M.M., Marr S.K., Kingston R.E. Phase separation of Polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 2019;33:799–813. doi: 10.1101/gad.326488.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farcas A.M., Blackledge N.P., Sudbery I., Long H.K., McGouran J., Rose N.R., Lee S., Sims D., Cerase A., Sheahan T.W., et al. KDM2B links the Polycomb Repressive Complex 1 (PRC1) to recognition of CpG islands. eLife. 2012;1:00205. doi: 10.7554/eLife.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He J., Shen L., Wan M., Taranova O., Wu H., Zhang Y. Kdm2b maintains murine embryonic stem cell status by recruiting PRC1 complex to CpG islands of developmental genes. Nat. Cell Biol. 2013;15:373–384. doi: 10.1038/ncb2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X., Johansen J.V., Helin K. Fbxl10/Kdm2b Recruits Polycomb Repressive Complex 1 to CpG Islands and Regulates H2A Ubiquitylation. Mol. Cell. 2013;49:1134–1146. doi: 10.1016/j.molcel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 49.Stielow B., Finkernagel F., Stiewe T., Nist A., Suske G. MGA, L3MBTL2 and E2F6 determine genomic binding of the non-canonical Polycomb repressive complex PRC1.6. PLoS Genet. 2018;14:e1007193. doi: 10.1371/journal.pgen.1007193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi J., Bachmann A.L., Tauscher K., Benda C., Fierz B., Müller J. DNA binding by PHF1 prolongs PRC2 residence time on chromatin and thereby promotes H3K27 methylation. Nat. Struct. Mol. Biol. 2017;24:1039–1047. doi: 10.1038/nsmb.3488. [DOI] [PubMed] [Google Scholar]
- 51.Li H., Liefke R., Jiang J., Kurland J.V., Tian W., Deng P., Zhang W., He Q., Patel D.J., Bulyk M.L., et al. Polycomb-like proteins link the PRC2 complex to CpG islands. Nature. 2017;549:287–291. doi: 10.1038/nature23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perino M., Van Mierlo G., Karemaker I.D., Van Genesen S., Vermeulen M., Marks H., Van Heeringen S.J., Veenstra G.J.C. MTF2 recruits Polycomb Repressive Complex 2 by helical-shape-selective DNA binding. Nat. Genet. 2018;50:1002–1010. doi: 10.1038/s41588-018-0134-8. [DOI] [PubMed] [Google Scholar]
- 53.Pasini D., Cloos P.A.C., Walfridsson J., Olsson L., Bukowski J.-P., Johansen J.V., Bak M., Tommerup N., Rappsilber J., Helin K. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature. 2010;464:306–310. doi: 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- 54.Asenjo H.G., Gallardo A., López-Onieva L., Tejada I., Martorell-Marugán J., Carmona-Saez P., Landeira D. Polycomb regulation is coupled to cell cycle transition in pluripotent stem cells. Sci. Adv. 2020;6:eaay4768. doi: 10.1126/sciadv.aay4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Min J., Zhang Y., Xu R.-M. Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev. 2003;17:1823–1828. doi: 10.1101/gad.269603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nowak A.J., Alfieri C., Stirnimann C.U., Rybin V., Baudin F., Ly-Hartig N., Lindner D., Müller C.W. Chromatin-modifying complex component Nurf55/p55 associates with histones H3 and H4 and polycomb repressive complex 2 subunit Su(z)12 through partially overlapping binding sites. J. Biol. Chem. 2011;286:23388–23396. doi: 10.1074/jbc.M110.207407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kloet S., Makowski M.M., Baymaz H.I., Van Voorthuijsen L., Karemaker I.D., Santanach A., Jansen P.W., Croce L.D., Vermeulen M. The dynamic interactome and genomic targets of Polycomb complexes during stem-cell differentiation. Nat. Struct. Mol. Biol. 2016;23:682–690. doi: 10.1038/nsmb.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee H.-G., Kahn T.G., Simcox A., Schwartz Y.B., Pirrotta V. Genome-wide activities of Polycomb complexes control pervasive transcription. Genome Res. 2015;25:1170–1181. doi: 10.1101/gr.188920.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fursova N.A., Blackledge N.P., Nakayama M., Ito S., Koseki Y., Farcas A.M., King H.W., Koseki H., Klose R.J. Synergy between Variant PRC1 Complexes Defines Polycomb-Mediated Gene Repression. Mol. Cell. 2019;74:1020–1036.e8. doi: 10.1016/j.molcel.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taherbhoy A.M., Huang O.W., Cochran A.G. BMI1–RING1B is an autoinhibited RING E3 ubiquitin ligase. Nat. Commun. 2015;6:7621. doi: 10.1038/ncomms8621. [DOI] [PubMed] [Google Scholar]
- 61.Rose N.R., King H.W., Blackledge N.P., Fursova N.A., Ember K.J., Fischer R., Kessler B.M., Klose R.J. RYBP stimulates PRC1 to shape chromatin-based communication between Polycomb repressive complexes. eLife. 2016;5:524. doi: 10.7554/eLife.18591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao J., Wang M., Zhu B., Li G., Chang L., Yu J., Song A., Liu C., Huang W., Zhang T., et al. RYBP/YAF2-PRC1 complexes and histone H1-dependent chromatin compaction mediate propagation of H2AK119ub1 during cell division. Nat. Cell Biol. 2020;22:439–452. doi: 10.1038/s41556-020-0484-1. [DOI] [PubMed] [Google Scholar]
- 63.Ferrari K.J., Scelfo A., Jammula S., Cuomo A., Barozzi I., Stützer A., Fischle W., Bonaldi T., Pasini D. Polycomb-Dependent H3K27me1 and H3K27me2 Regulate Active Transcription and Enhancer Fidelity. Mol. Cell. 2014;53:49–62. doi: 10.1016/j.molcel.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 64.Oksuz O., Narendra V., Lee C.-H., Descostes N., Leroy G., Raviram R., Blumenberg L., Karch K., Rocha P.P., Garcia B.A., et al. Capturing the Onset of PRC2-Mediated Repressive Domain Formation. Mol. Cell. 2018;70:1149–1162.e5. doi: 10.1016/j.molcel.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCabe M.T., Graves A.P., Chen S.B., Della Pietra A., Dul E., Hughes A.M., Gilbert S.A., Thrall S.H., Tummino P.J., Kruger R.G., et al. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27) Proc. Natl. Acad. Sci. USA. 2012;109:2989–2994. doi: 10.1073/pnas.1116418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poepsel S., Kasinath V., Nogales E. Cryo-EM structures of PRC2 simultaneously engaged with two functionally distinct nucleosomes. Nat. Struct. Mol. Biol. 2018;25:154–162. doi: 10.1038/s41594-018-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blackledge N.P., Fursova N.A., Kelley J.R., Huseyin M.K., Feldmann A., Klose R.J. PRC1 Catalytic Activity Is Central to Polycomb System Function. Mol. Cell. 2020;77:857–874.e9. doi: 10.1016/j.molcel.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan W., Xu M., Huang C., Liu N., Chen S., Zhu B. H3K36 Methylation Antagonizes PRC2-mediated H3K27 Methylation. J. Biol. Chem. 2011;286:7983–7989. doi: 10.1074/jbc.M110.194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan G., Ma B., Yuan W., Zhang Z., Chen P., Ding X., Feng L., Shen X., Chen S., Li G., et al. Histone H2A Ubiquitination Inhibits the Enzymatic Activity of H3 Lysine 36 Methyltransferases. J. Biol. Chem. 2013;288:30832–30842. doi: 10.1074/jbc.M113.475996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhen C.Y., Tatavosian R., Huynh T.N., Duc H.N., Das R., Kokotovic M., Grimm J.B., Lavis L.D., Lee J., Mejia F.J., et al. Live-cell single-molecule tracking reveals co-recognition of H3K27me3 and DNA targets polycomb Cbx7-PRC1 to chromatin. eLife. 2016;5:731. doi: 10.7554/eLife.17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Connelly K.E., Weaver T.M., Alpsoy A., Gu B.X., Musselman C.A., Dykhuizen E.C. Engagement of DNA and H3K27me3 by the CBX8 chromodomain drives chromatin association. Nucleic Acids Res. 2019;47:2289–2305. doi: 10.1093/nar/gky1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arrigoni R., Alam S.L., Wamstad J.A., Bardwell V.J., Sundquist W.I., Schreiber-Agus N. The Polycomb-associated protein Rybp is a ubiquitin binding protein. FEBS Lett. 2006;580:6233–6241. doi: 10.1016/j.febslet.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 73.Cooper S., Grijzenhout A., Underwood E., Ancelin K., Zhang T., Nesterova T.B., Anil-Kirmizitas B., Bassett A., Kooistra S.M., Agger K., et al. Jarid2 binds mono-ubiquitylated H2A lysine 119 to mediate crosstalk between Polycomb complexes PRC1 and PRC2. Nat. Commun. 2016;7:13661. doi: 10.1038/ncomms13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agger K., Cloos P.A.C., Christensen J., Pasini D., Rose S., Rappsilber J., Issaeva I., Canaani E., Salcini A.E., Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 75.Zhu P., Zhou W., Wang J., Puc J., Ohgi K.A., Erdjument-Bromage H., Tempst P., Glass C.K., Rosenfeld M.G. A Histone H2A Deubiquitinase Complex Coordinating Histone Acetylation and H1 Dissociation in Transcriptional Regulation. Mol. Cell. 2007;27:609–621. doi: 10.1016/j.molcel.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Joo H.-Y., Zhai L., Yang C., Nie S., Erdjument-Bromage H., Tempst P., Chang C., Wang H. Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature. 2007;449:1068–1072. doi: 10.1038/nature06256. [DOI] [PubMed] [Google Scholar]
- 77.Campagne A., Lee M.-K., Zielinski D., Michaud A., Le Corre S., Dingli F., Chen H., Shahidian L.Z., Vassilev I., Servant N., et al. BAP1 complex promotes transcription by opposing PRC1-mediated H2A ubiquitylation. Nat. Commun. 2019;10:348. doi: 10.1038/s41467-018-08255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sneppen K., Ringrose L. Theoretical analysis of Polycomb-Trithorax systems predicts that poised chromatin is bistable and not bivalent. Nat. Commun. 2019;10:2133. doi: 10.1038/s41467-019-10130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwartz Y.B., Kahn T.G., Nix D.A., Li X.-Y., Bourgon R., Biggin M., Pirrotta V. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat. Genet. 2006;38:700–705. doi: 10.1038/ng1817. [DOI] [PubMed] [Google Scholar]
- 80.Tamburri S., Lavarone E., Fernández-Pérez D., Conway E., Zanotti M., Manganaro D., Pasini D. Histone H2AK119 Mono-Ubiquitination Is Essential for Polycomb-Mediated Transcriptional Repression. Mol. Cell. 2020;77:840–856.e5. doi: 10.1016/j.molcel.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsuboi M., Kishi Y., Yokozeki W., Koseki H., Hirabayashi Y., Gotoh Y. Ubiquitination-Independent Repression of PRC1 Targets during Neuronal Fate Restriction in the Developing Mouse Neocortex. Dev. Cell. 2018;47:758–772.e5. doi: 10.1016/j.devcel.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 82.Coleman R.T., Struhl G. Causal role for inheritance of H3K27me3 in maintaining the OFF state of aDrosophilaHOX gene. Science. 2017;356:eaai8236. doi: 10.1126/science.aai8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moussa H.F., Bsteh D., Yelagandula R., Pribitzer C., Stecher K., Bartalska K., Michetti L., Wang J., Zepeda-Martinez J.A., Elling U., et al. Canonical PRC1 controls sequence-independent propagation of Polycomb-mediated gene silencing. Nat. Commun. 2019;10:1931. doi: 10.1038/s41467-019-09628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simon J., Chiang A., Bender W., Shimell M.J., Connor M.O. Elements of the Drosophila Bithorax Complex That Mediate Repression by Polycomb Group Products. Dev. Biol. 1993;158:131–144. doi: 10.1006/dbio.1993.1174. [DOI] [PubMed] [Google Scholar]
- 85.Orsi G.A., Kasinathan S., Hughes K.T., Saminadin-Peter S., Henikoff S., Ahmad K. High-resolution mapping defines the cooperative architecture of Polycomb response elements. Genome Res. 2014;24:809–820. doi: 10.1101/gr.163642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown J., Mucci D., Whiteley M., Dirksen M.-L., Kassis J.A. The Drosophila Polycomb Group Gene pleiohomeotic Encodes a DNA Binding Protein with Homology to the Transcription Factor YY1. Mol. Cell. 1998;1:1057–1064. doi: 10.1016/S1097-2765(00)80106-9. [DOI] [PubMed] [Google Scholar]
- 87.Brown J.L., Sun M.-A., Kassis J.A. Global changes of H3K27me3 domains and Polycomb group protein distribution in the absence of recruiters Spps or Pho. Proc. Natl. Acad. Sci. USA. 2018;115:E1839–E1848. doi: 10.1073/pnas.1716299115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kassis J.A., Brown J.L. Polycomb Group Response Elements in Drosophila and Vertebrates. Genet. Genom. Fish Phenomics. 2013;81:83–118. doi: 10.1016/b978-0-12-407677-8.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erokhin M., Georgiev P., Chetverina D. Drosophila DNA-Binding Proteins in Polycomb Repression. Epigenomes. 2018;2:1. doi: 10.3390/epigenomes2010001. [DOI] [Google Scholar]
- 90.Mendenhall E.M., Koche R.P., Truong T., Zhou V.W., Issac B., Chi A.S., Ku M., Bernstein B.E. GC-Rich Sequence Elements Recruit PRC2 in Mammalian ES Cells. PLoS Genet. 2010;6:e1001244. doi: 10.1371/journal.pgen.1001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Basu A., Wilkinson F.H., Colavita K., Fennelly C., Atchison M.L. YY1 DNA binding and interaction with YAF2 is essential for Polycomb recruitment. Nucleic Acids Res. 2013;42:2208–2223. doi: 10.1093/nar/gkt1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Højfeldt J.W., Laugesen A., Willumsen B.M., Damhofer H., Hedehus L., Tvardovskiy A., Mohammad F., Jensen O.N., Helin K. Accurate H3K27 methylation can be established de novo by SUZ12-directed PRC2. Nat. Struct. Mol. Biol. 2018;25:225–232. doi: 10.1038/s41594-018-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brockdorff N. Noncoding RNA and Polycomb recruitment. RNA. 2013;19:429–442. doi: 10.1261/rna.037598.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davidovich C., Cech T.R. The recruitment of chromatin modifiers by long noncoding RNAs: Lessons from PRC2. RNA. 2015;21:2007–2022. doi: 10.1261/rna.053918.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Creyghton M.P., Cheng A.W., Welstead G.G., Kooistra T., Carey B.W., Steine E.J., Hanna J.H., Lodato M.A., Frampton G.M., Sharp P.A., et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huseyin M.K., Klose R.J. Live-cell single particle tracking of PRC1 reveals a highly dynamic system with low target site occupancy. bioRxiv. 2020 doi: 10.1038/s41467-021-21130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eagen K.P., Aiden E.L., Kornberg R.D. Polycomb-mediated chromatin loops revealed by a subkilobase-resolution chromatin interaction map. Proc. Natl. Acad. Sci. USA. 2017;114:8764–8769. doi: 10.1073/pnas.1701291114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ogiyama Y., Schuettengruber B., Papadopoulos G.L., Chang J.-M., Cavalli G. Polycomb-Dependent Chromatin Looping Contributes to Gene Silencing during Drosophila Development. Mol. Cell. 2018;71:73–88.e5. doi: 10.1016/j.molcel.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 99.Kundu S., Ji F., Sunwoo H., Jain G., Lee J.T., Sadreyev R.I., Dekker J., Kingston R.E. Polycomb Repressive Complex 1 Generates Discrete Compacted Domains that Change during Differentiation. Mol. Cell. 2017;65:432–446.e5. doi: 10.1016/j.molcel.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schoenfelder S., Sugar R., Dimond A., Javierre B.M., Armstrong H., Mifsud B., Dimitrova E., Matheson L., Tavares-Cadete F., Furlan-Magaril M., et al. Polycomb repressive complex PRC1 spatially constrains the mouse embryonic stem cell genome. Nat. Genet. 2015;47:1179–1186. doi: 10.1038/ng.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boyle S., Flyamer I.M., Williamson I., Sengupta D., Bickmore W.A., Illingworth R.S. A central role for canonical PRC1 in shaping the 3D nuclear landscape. Genes Dev. 2020;34:931–949. doi: 10.1101/gad.336487.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rhodes J.D., Feldmann A., Hernández-Rodríguez B., Díaz N., Brown J.M., Fursova N.A., Blackledge N.P., Prathapan P., Dobrinic P., Huseyin M.K., et al. Cohesin Disrupts Polycomb-Dependent Chromosome Interactions in Embryonic Stem Cells. Cell Rep. 2020;30:820–835.e10. doi: 10.1016/j.celrep.2019.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davidson I.F., Bauer B., Goetz D., Tang W., Wutz G., Peters J.-M. DNA loop extrusion by human cohesin. Science. 2019;366:1338–1345. doi: 10.1126/science.aaz3418. [DOI] [PubMed] [Google Scholar]
- 104.Fudenberg G., Imakaev M., Lu C., Goloborodko A., Abdennur N., Mirny L.A. Formation of Chromosomal Domains by Loop Extrusion. Cell Rep. 2016;15:2038–2049. doi: 10.1016/j.celrep.2016.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheutin T., Cavalli G. Loss of PRC1 induces higher-order opening of Hox loci independently of transcription during Drosophila embryogenesis. Nat. Commun. 2018;9:3898. doi: 10.1038/s41467-018-05945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loubiere V., Papadopoulos G.L., Szabo Q., Martinez A.-M., Cavalli G. Widespread activation of developmental gene expression characterized by PRC1-dependent chromatin looping. Sci. Adv. 2020;6:eaax4001. doi: 10.1126/sciadv.aax4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Banani S.F., Lee H.O., Hyman A.A., Rosen M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boija A., Klein I.A., Sabari B.R., Dall’Agnese A., Coffey E.L., Zamudio A.V., Li C.H., Shrinivas K., Manteiga J.C., Hannett N.M., et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell. 2018;175:1842–1855.e16. doi: 10.1016/j.cell.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sabari B.R., Dall’Agnese A., Li C.H., Guo Y.E., Day D.S., Schuijers J., Vasile E., Malik S., Hnisz D., Lee T.I., et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018;361:eaar3958. doi: 10.1126/science.aar3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larson A.G., Elnatan D., Keenen M.M., Trnka M.J., Johnston J.B., Burlingame A.L., Agard D.A., Redding S., Narlikar G.J. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature. 2017;547:236–240. doi: 10.1038/nature22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strom A.R., Emelyanov A.V., Mir M., Fyodorov D.V., Darzacq X., Karpen G.H. Phase separation drives heterochromatin domain formation. Nature. 2017;547:241–245. doi: 10.1038/nature22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grau D.J., Chapman B., Garlick J.D., Borowsky M., Francis N.J., Kingston R.E. Compaction of chromatin by diverse Polycomb group proteins requires localized regions of high charge. Genes Dev. 2011;25:2210–2221. doi: 10.1101/gad.17288211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lau M.S., Schwartz M.G., Kingston R.E., Kundu S., Savol A.J., Wang P.I., Marr S.K., Grau D.J., Schorderet P., Sadreyev R.I., et al. Mutation of a nucleosome compaction region disrupts Polycomb-mediated axial patterning. Science. 2017;355:1081–1084. doi: 10.1126/science.aah5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tatavosian R., Kent S., Brown K., Yao T., Duc H.N., Huynh T.N., Zhen C.Y., Ma B., Wang H., Ren X. Nuclear condensates of the Polycomb protein chromobox 2 (CBX2) assemble through phase separation. J. Biol. Chem. 2018;294:1451–1463. doi: 10.1074/jbc.RA118.006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Graaff W., Tomotsune D., Oosterveen T., Takihara Y., Koseki H., Deschamps J. Randomly inserted and targeted Hox/reporter fusions transcriptionally silenced in Polycomb mutants. Proc. Natl. Acad. Sci. USA. 2003;100:13362–13367. doi: 10.1073/pnas.2237046100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shirai M., Osugi T., Koga H., Kaji Y., Takimoto E., Komuro I., Hara J., Miwa T., Yamauchi-Takihara K., Takihara Y. The Polycomb-group gene Rae28 sustains Nkx2.5/Csx expression and is essential for cardiac morphogenesis. J. Clin. Investig. 2002;110:177–184. doi: 10.1172/JCI0214839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Breiling A., O’Neill L.P., D’Eliseo D., Turner B.M., Orlando V. Epigenome changes in active and inactive Polycomb-group-controlled regions. EMBO Rep. 2004;5:976–982. doi: 10.1038/sj.embor.7400260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kwong C., Adryan B., Bell I., Meadows L., Russell S., Manak J.R., White R. Stability and Dynamics of Polycomb Target Sites in Drosophila Development. PLoS Genet. 2008;4:e1000178. doi: 10.1371/journal.pgen.1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Papp B., Muller J. Histone trimethylation and the maintenance of transcriptional ONand OFF states by trxG and PcG proteins. Genes Dev. 2006;20:2041–2054. doi: 10.1101/gad.388706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Langlais K.K., Brown J.L., Kassis J.A. Polycomb Group Proteins Bind an engrailed PRE in Both the “ON” and “OFF” Transcriptional States of engrailed. PLoS ONE. 2012;7:e48765. doi: 10.1371/journal.pone.0048765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fujimura Y.-I., Vidal M., Koseki H., Isono K.-I., Endoh M., Kajita H., Mizutani-Koseki Y., Takihara Y., Van Lohuizen M., Otte A., et al. Distinct roles of Polycomb group gene products in transcriptionallyrepressed and active domains of Hoxb8. Development. 2006;133:2371–2381. doi: 10.1242/dev.02405. [DOI] [PubMed] [Google Scholar]
- 122.Xu K., Wu Z.J., Groner A.C., He H.H., Cai C., Lis R.T., Wu X., Stack E.C., Loda M., Liu T., et al. EZH2 Oncogenic Activity in Castration-Resistant Prostate Cancer Cells Is Polycomb-Independent. Science. 2012;338:1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chung C.-Y., Sun Z., Mullokandov G., Bosch A., Qadeer Z.A., Cihan E., Rapp Z., Parsons R., Aguirre-Ghiso J.A., Farias E.F., et al. Cbx8 Acts Non-canonically with Wdr5 to Promote Mammary Tumorigenesis. Cell Rep. 2016;16:472–486. doi: 10.1016/j.celrep.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Loubiere V., Delest A., Thomas A., Bonev B., Schuettengruber B., Sati S., Martinez A.-M., Cavalli G. Coordinate redeployment of PRC1 proteins suppresses tumor formation during Drosophila development. Nat. Genet. 2016;48:1436–1442. doi: 10.1038/ng.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morey L., Aloia L., Cozzuto L., Benitah S.A., Di Croce L. RYBP and Cbx7 Define Specific Biological Functions of Polycomb Complexes in Mouse Embryonic Stem Cells. Cell Rep. 2013;3:60–69. doi: 10.1016/j.celrep.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 126.Brookes E., De Santiago I., Hebenstreit D., Morris K.J., Carroll T., Xie S.Q., Stock J.K., Heidemann M., Eick D., Nozaki N., et al. Polycomb Associates Genome-wide with a Specific RNA Polymerase II Variant, and Regulates Metabolic Genes in ESCs. Cell Stem Cell. 2012;10:157–170. doi: 10.1016/j.stem.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frangini A., Sjöberg M., Roman-Trufero M., Dharmalingam G., Haberle V., Bartke T., Lenhard B., Malumbres M., Vidal M., Dillon N. The Aurora B Kinase and the Polycomb Protein Ring1B Combine to Regulate Active Promoters in Quiescent Lymphocytes. Mol. Cell. 2013;51:647–661. doi: 10.1016/j.molcel.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 128.Creppe C., Palau A., Malinverni R., Valero V., Buschbeck M. A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation. PLoS Genet. 2014;10:e1004851. doi: 10.1371/journal.pgen.1004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schaaf C.A., Misulovin Z., Gause M., Koenig A., Gohara D.W., Watson A., Dorsett D. Cohesin and Polycomb Proteins Functionally Interact to Control Transcription at Silenced and Active Genes. PLoS Genet. 2013;9:e1003560. doi: 10.1371/journal.pgen.1003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pemberton H., Anderton E., Patel H., Brookes S., Chandler H., Palermo R., Stock J., Rodriguez-Niedenführ M., Racek T., De Breed L., et al. Genome-wide co-localization of Polycomb orthologs and their effects on gene expression in human fibroblasts. Genome Biol. 2014;15:R23. doi: 10.1186/gb-2014-15-2-r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao Z., Lee P., Stafford J.M., Von Schimmelmann M., Schaefer A., Reinberg D. An AUTS2–Polycomb complex activates gene expression in the CNS. Nature. 2014;516:349–354. doi: 10.1038/nature13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu J., Shao Z., Li D., Xie H., Kim W., Huang J., Taylor J.E., Pinello L., Glass K., Jaffe J.D., et al. Developmental control of polycomb subunit composition by GATA factors mediates a switch to non-canonical functions. Mol. Cell. 2015;57:304–316. doi: 10.1016/j.molcel.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morey L., Santanach A., Blanco E., Aloia L., Nora E.P., Bruneau B.G., Di Croce L. Polycomb Regulates Mesoderm Cell Fate-Specification in Embryonic Stem Cells through Activation and Repression Mechanisms. Cell Stem Cell. 2015;17:300–315. doi: 10.1016/j.stem.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 134.Rai K., Akdemir K.C., Kwong L.N., Fiziev P., Wu C.-J., Keung E.Z., Sharma S., Samant N.S., Williams M., Axelrad J.B., et al. Dual Roles of RNF2 in Melanoma Progression. Cancer Discov. 2015;5:1314–1327. doi: 10.1158/2159-8290.CD-15-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boom V.V.D., Maat H., Geugien M., López A.R., Sotoca A.M., Jaques J., Brouwers-Vos A.Z., Fusetti F., Groen R.W.J., Yuan H., et al. Non-canonical PRC1.1 Targets Active Genes Independent of H3K27me3 and Is Essential for Leukemogenesis. Cell Rep. 2016;14:332–346. doi: 10.1016/j.celrep.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 136.Maezawa S., Hasegawa K., Yukawa M., Sakashita A., Alavattam K.G., Andreassen P.R., Vidal M., Koseki H., Barski A., Namekawa S.H. Polycomb directs timely activation of germline genes in spermatogenesis. Genes Dev. 2017;31:1693–1703. doi: 10.1101/gad.302000.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pherson M., Misulovin Z., Gause M., Mihindukulasuriya K.A., Swain A., Dorsett D. Polycomb repressive complex 1 modifies transcription of active genes. Sci. Adv. 2017;3:e1700944. doi: 10.1126/sciadv.1700944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cohen I., Zhao D., Zheng D., Ezhkova E. 1358 PRC1 fine-tunes gene repression and activation to safeguard skin epithelium development and stem cell specification. J. Investig. Dermatol. 2018;138:S231. doi: 10.1016/j.jid.2018.03.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cohen I., Zhao D.-J., Menon G., Nakayama M., Koseki H., Zheng D., Ezhkova E. PRC1 preserves epidermal tissue integrity independently of PRC2. Genes Dev. 2018;33:55–60. doi: 10.1101/gad.319939.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chan H.L., Beckedorff F., Zhang Y., Garcia-Huidobro J., Jiang H., Colaprico A., Bilbao D., Figueroa M.E., Lacava J., Shiekhattar R., et al. Polycomb complexes associate with enhancers and promote oncogenic transcriptional programs in cancer through multiple mechanisms. Nat. Commun. 2018;9:3377. doi: 10.1038/s41467-018-05728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y., Chan H.L., Garcia-Martinez L., Slingerland J.M., Karl D.L., Verdun R.E., Morey L. Estrogen induces dynamic ERα and RING1B recruitment to control gene and enhancer activities in luminal breast cancer. Sci. Adv. 2020;6:7249–7254. doi: 10.1126/sciadv.aaz7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bradner J.E., Hnisz D., Young R.A. Transcriptional Addiction in Cancer. Cell. 2017;168:629–643. doi: 10.1016/j.cell.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vo L.T., Kinney M.A., Liu X., Zhang Y., Barragan J., Sousa P.M., Jha D.K., Han A., Cesana M., Shao Z., et al. Regulation of embryonic haematopoietic multipotency by EZH1. Nature. 2018;553:506–510. doi: 10.1038/nature25435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rada-Iglesias Á., Bajpai R., Swigut T., Brugmann S.A., Flynn R.A., Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2010;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scelfo A., Fernández-Pérez D., Tamburri S., Zanotti M., Lavarone E., Soldi M., Bonaldi T., Ferrari K.J., Pasini D. Functional Landscape of PCGF Proteins Reveals Both RING1A/B-Dependent-and RING1A/B-Independent-Specific Activities. Mol. Cell. 2019;74:1037–1052.e7. doi: 10.1016/j.molcel.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao W., Huang Y., Zhang J., Liu M., Ji H., Wang C., Cao N., Li C., Xia Y., Jiang Q., et al. Polycomb group RING finger proteins 3/5 activate transcription via an interaction with the pluripotency factor Tex10 in embryonic stem cells. J. Biol. 2017;292:21527–21537. doi: 10.1074/jbc.M117.804054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cordonnier G., Mandoli A., Cagnard N., Hypolite G., Lhermitte L., Verhoeyen E., Asnafi V., Dillon N., MacIntyre E., Martens J.H., et al. CBFβ-SMMHC Affects Genome-wide Polycomb Repressive Complex 1 Activity in Acute Myeloid Leukemia. Cell Rep. 2020;30:299–307.e3. doi: 10.1016/j.celrep.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 148.Illingworth R.S., Botting C.H., Grimes G.R., Bickmore W.A., Eskeland R. PRC1 and PRC2 Are Not Required for Targeting of H2A.Z to Developmental Genes in Embryonic Stem Cells. PLoS ONE. 2012;7:e34848. doi: 10.1371/journal.pone.0034848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kang H., Jung Y.L., McElroy K.A., Zee B.M., Wallace H.A., Woolnough J.L., Park P.J., Kuroda M.I. Bivalent complexes of PRC1 with orthologs of BRD4 and MOZ/MORF target developmental genes inDrosophila. Genes Dev. 2017;31:1988–2002. doi: 10.1101/gad.305987.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dou Y., Milne T., Tackett A.J., Smith E.R., Fukuda A., Wysocka J., Allis C.D., Chait B.T., Hess J.L., Roeder R.G. Physical Association and Coordinate Function of the H3 K4 Methyltransferase MLL1 and the H4 K16 Acetyltransferase MOF. Cell. 2005;121:873–885. doi: 10.1016/j.cell.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 151.Strübbe G., Popp C., Schmidt A., Pauli A., Ringrose L., Beisel C., Paro R. Polycomb purification by in vivo biotinylation tagging reveals cohesin and Trithorax group proteins as interaction partners. Proc. Natl. Acad. Sci. USA. 2011;108:5572–5577. doi: 10.1073/pnas.1007916108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kar G., Kim J.K., Kolodziejczyk A.A., Natarajan K.N., Triglia E.T., Mifsud B., Elderkin S., Marioni J.C., Pombo A., Teichmann S.A. Flipping between Polycomb repressed and active transcriptional states introduces noise in gene expression. Nat. Commun. 2017;8:36. doi: 10.1038/s41467-017-00052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lenstra T.L., Rodriguez J., Chen H., Larson D.R. Transcription Dynamics in Living Cells. Annu. Rev. Biophys. 2016;45:25–47. doi: 10.1146/annurev-biophys-062215-010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fukaya T., Lim B., Levine M. Enhancer Control of Transcriptional Bursting. Cell. 2016;166:358–368. doi: 10.1016/j.cell.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yan Y., Zhao W., Huang Y., Tong H., Xia Y., Jiang Q., Qin J. Loss of Polycomb Group Protein Pcgf1 Severely Compromises Proper Differentiation of Embryonic Stem Cells. Sci. Rep. 2017;7:46276. doi: 10.1038/srep46276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ahmad K., Spens A.E. Separate Polycomb Response Elements control chromatin state and activation of the vestigial gene. PLoS Genet. 2019;15:e1007877. doi: 10.1371/journal.pgen.1007877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kondo T., Isono K., Kondo K., Endo T.A., Itohara S., Vidal M., Koseki H. Polycomb Potentiates Meis2 Activation in Midbrain by Mediating Interaction of the Promoter with a Tissue-Specific Enhancer. Dev. Cell. 2014;28:94–101. doi: 10.1016/j.devcel.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 158.Cruz-Molina S., Respuela P., Tebartz C., Kolovos P., Nikolic M., Fueyo R., Van Ijcken W., Grosveld F., Frommolt P., Bazzi H., et al. PRC2 Facilitates the Regulatory Topology Required for Poised Enhancer Function during Pluripotent Stem Cell Differentiation. Cell Stem Cell. 2017;20:689–705.e9. doi: 10.1016/j.stem.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 159.Mikulski P., Komarynets O., Fachinelli F., Weber A.P., Schubert D. Characterization of the Polycomb-Group Mark H3K27me3 in Unicellular Algae. Front. Plant Sci. 2017;8:505–512. doi: 10.3389/fpls.2017.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]