Abstract
Foods and beverages provide a source of fluoride exposure in Mexico. While high fluoride concentrations are neurotoxic, recent research suggests that exposures within the optimal range may also pose a risk to the developing brain. This prospective study examined whether dietary fluoride intake during pregnancy is associated with toddlers’ neurodevelopment in 103 mother-child pairs from the PROGRESS cohort in Mexico City. Food and beverage fluoride intake was assessed in trimesters 2 and 3 using a food frequency questionnaire and Mexican tables of fluoride content. We used the Bayley-III to evaluate cognitive, motor, and language outcomes at 12 and 24 months of age. Adjusted linear regression models were generated for each neurodevelopment assessment time point (12 and 24 months). Mixed-effects models were used to consider a repeated measurement approach. Interactions between maternal fluoride intake and child sex on neurodevelopmental outcomes were tested. Median (IQR) dietary fluoride intake during pregnancy was 1.01 mg/d (0.73, 1.32). Maternal fluoride intake was not associated with cognitive, language, or motor outcomes collapsing across boys and girls. However, child sex modified the association between maternal fluoride intake and cognitive outcome (p interaction term = 0.06). A 0.5 mg/day increase in overall dietary fluoride intake was associated with a 3.50-point lower cognitive outcome in 24-month old boys (95% CI: −6.58, −0.42); there was no statistical association with girls (β = 0.07, 95% CI: −2.37, 2.51), nor on the cognitive outcome at 12-months of age. Averaging across the 12-and 24-month cognitive outcomes using mixed-effects models revealed a similar association: a 0.5 mg/day increase in overall dietary fluoride intake was associated with a 3.46-point lower cognitive outcome in boys (95% CI: −6.23, −0.70). These findings suggest that the development of nonverbal abilities in males may be more vulnerable to prenatal fluoride exposure than language or motor abilities, even at levels within the recommended intake range.
Keywords: fluoride, foods, beverages, neurodevelopment, prospective cohort, infants
1. Introduction
Fluoride has been well established as an effective dental caries preventing agent (Everett 2011). The dietary reference value for fluoride from all sources is 3 mg/d for women as set by the U.S. Institute of Medicine (IOM 2006) compared with 2.45 mg/d as set for the Mexican population (Bourges et al. 2004) This value was chosen to prevent caries without causing moderate dental fluorosis and is the same for pregnant and non-pregnant women. However, the usefulness of recommending an “optimal” level of fluoride ingestion is debated, especially for pregnant women (Buzalaf 2018; Martinez-Mier 2018; Mejare 2018; Warren et al. 2009). Fluoride is not an essential nutrient and it is possible to achieve caries prevention through the topical application of fluoride (Featherstone 2000; Pizzo et al. 2007; Warren and Levy 2003). Moreover, fluoride ingestion in pregnancy does not strengthen enamel during tooth formation in the fetus (Takahashi et al., 2017), but has been associated with increased risk of neurotoxicity, even at optimal exposure levels (Bashash et al. 2017; Green et al. 2019).
In 2020, the National Toxicology Program (NTP) conducted a systematic review on the impact of fluoride on neurodevelopmental outcomes (NTP, 2020). In 2020, the National Toxicology Program (NTP) reviewed a growing body of epidemiological studies conducted in endemic and non-endemic areas (Choi et al., 2015; Ding et al., 2011; Rocha-Amador et al., 2007; Seraj et al., 2012; Sudhir et al., 2009), including three high-quality prospective birth cohort studies (Bashash et al., 2018, 2017; Green et al., 2019; Till et al., 2020; Valdez Jimenez et al., 2017). This review found consistent evidence showing an association between fluoride exposure and adverse neurodevelopmental outcomes. However, their report – while still in draft form – was based primarily on evidence showing neurotoxicity of fluoride at exposure levels that are greater than 1.5 mg/L in drinking water; less information is available for lower exposure levels. Ecological studies conducted in New Zealand (Broadbent et al., 2015) and in Sweden (Aggeborn and Ohman, 2017) did not find an effect of optimal levels of fluoride in drinking water on cognitive development. However, these studies did not measure fluoride exposure in pregnancy and are limited by their lack of individualized measures due to the ecological design of the study. A study conducted in pregnant rats showed that fetal exposure to fluoride was associated with changes to the hippocampal proteomic profile of offspring rats, including decrease antioxidant capacity (Ferreira et al., 2020; Zhang et al., 2008). Furthermore, perinatal rat exposure to sodium fluoride reported sex-specific alterations in learning, memory and motor coordination (Bera et al., 2007) in rat offspring.
Fluoride intake and excretion is higher in children and adults living in communities with community fluoridation programs (i.e. water, salt or milk) (Green et al. 2020b; Horowitz 2000; Till et al. 2018). Foods and beverages are the main source of fluoride intake (Martinez-Mier et al. 2003), especially when prepared with fluoridated water or salt. Fluoride content may vary due to intrinsic factors that depend on the season, as well as soil type (e.g. tea), feed regimen (animal products) (Murray 1986), and extrinsic factors such as pesticides (EPA 2010), food storage containers (Teflon-coated containers) (Full and Parkins 1975), or food packaging (migration of perfluorochemicals into food) (Schaider et al. 2017; Trier et al. 2011). Previous studies conducted in pregnant women and their offspring measured fluoride exposure in maternal urine samples and municipal drinking water (Green et al. 2019; Jimenez-Zabala et al. 2018), but did not evaluate maternal fluoride intake from dietary sources.
This study evaluated the association between dietary fluoride intake during pregnancy and neurodevelopmental outcomes in offspring at 12 and 24 months of age. We also assessed the potential for sex-specific effects based on a recent literature review suggesting a higher male-specific vulnerability to prenatal fluoride exposure (Green et al. 2020a).
2. Materials and Methods
2.1. Participants and study design
The Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) cohort included 948 mother-child pairs from Mexico City. Details on the methods of the cohort are provided elsewhere (Braun et al. 2014). Briefly, pregnant women were enrolled through the Mexican Social Security System between 2007–2011; women had to be at least 18 years old, with less than 20 weeks of gestation, had completed primary education, and planned to reside in Mexico City for the following three years. Women were excluded if they had a history of infertility, diabetes or psychosis, heart or kidney disease, used steroids or anti-epilepsy drugs, or consumed alcohol (>1 drink/day). After a detailed explanation of the project protocols, during each study visit women provided written informed consent. Women were assessed at two times during pregnancy (second and third trimester). Mother-infant pairs were invited for post-partum assessments at the National Institute of Perinatology “Isidro Espinoza de los Reyes” at 1, 6, 12, and 24 months of childreńs age, with a response rate of 63% and 59% at 12 and 24 months respectively.
From the 948 mother-infant pairs enrolled in PROGRESS, assessment of childś neurodevelopment is available at 12 months (n=593) and 24 months (n=556). For the current study, we selected offspring for whom neurodevelopmental testing was conducted and their mothers had available valid dietary data during pregnancy (second or third trimester) (n=103). Of the 103 mother-infant pairs, 72 (70%) completed neurodevelopmental testing at both 12 and 24 months, whereas 31 (30%) completed testing at either the 12 or 24 months visit (see Supplemental Figure 1 for flowchart of included participants). To assess the generalizability (internal validity) of our study sample, we compared maternal and child demographic characteristics of the participants who were included in our analytical sample to those who were excluded (Supplemental Table 1).
Study protocols were approved by the institutional review boards of the Icahn School of Medicine at Mount Sinai Hospital (protocol number 17-00751), Harvard T. H. Chan School of Public Health, the National Institute of Public Health in Mexico (project #560), and the National Institute of Perinatology in Mexico. A parent/guardian provided written informed consent for child participation.
2.2. Fluoride intake (foods and beverages)
To assess fluoride intake during pregnancy, a validated food frequency questionnaire (FFQ) was administered by trained personnel to mothers in the second and third trimester using standardized procedures (Hernandez-Avila et al. 1998). The data collected included the number of days, times per day, serving size, and number of servings consumed of each food and drink listed, during the month prior to the interview. To process the dietary information, the quantity of each food and drink was obtained by multiplying the number of days by the times per day, by the portion size [grams (g), milliliters (mL)], and by the number of portions or pieces consumed on each occasion. Total g and mL were divided by seven days to obtain the average daily intake. After the quantity of each food and beverage (in g and mL, respectively) included in the list was estimated, the total fluoride intake per day (mg/d) was calculated using the Fluoride Content Tables in Foods and Beverages from Mexico City (Cantoral et al. 2019). As a brief description of the mentioned tables, the most consumed foods and beverages reported in the Mexican National Health and Nutrition Survey (2016) were purchased in the largest supermarket chains and local markets in Mexico City (Cantoral et al. 2019). Samples were analyzed for fluoride, at least in duplicate, using a modification of the hexamethyldisiloxane micro-diffusion method reported previously by our research group (Martinez-Mier et al. 2011). Given that salt is fluoridated in Mexico (at 250 ppm) (SSA 1995), the FFQ also asked about the practice of adding salt (via salt-shaker) to meals as yes/no.
2.3. Neurodevelopment (Bayley-III)
We assessed developmental functioning of infants and toddlers at 12 and 24 months using the Spanish version of the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) (Bayley 2006). Children were assessed by trained personnel on the following domains: cognitive (e.g. sensorimotor development, memory, problem solving, exploration and manipulation), language (expressive and receptive communication), and motor (gross and fine motor) development (Bayley 2006). The supervising psychologist performed quality control checks by reviewing videotaped evaluations. We derived age- and sex-normed scaled scores for each domain and used the composite (integrated) scores for the language and motor scale [mean = 100; standard deviation (SD) = 15]. Examiners were blinded to maternal fluoride intake levels.
2.4. Covariates
Covariates included motheŕs age, and education collected in a general questionnaire, and socioeconomic status (SES) of the family assessed using a validated questionnaire for categorizing SES in Mexican families (AMAI). Additionally, we included calcium intake (mg/d) during the second and third trimester as a covariate because calcium can reduce fluoridés intestinal absorption and therefore reduce the extent of fetal fluoride exposure (Martinez-Mier 2012; Mulualem et al. 2021). We included the following child variables in the analyses: birth weight, child sex, breastfeeding practices (none, at least one month, up to 12 months), and Z-scores for weight-for-age.
2.5. Statistical analysis
Characteristics of mother-child pairs were summarized using means ± SDs for continuous variables and n (%) for categorical variables. T-test or Fisheŕs exact tests were used to test differences between boys and girls. Dietary variables were described as medians and interquartile ranges by trimester of pregnancy as these variables presented a skewed distribution, and comparison between boys and girls was done using Wilcoxon test. For each woman, an average of all her available fluoride intake values was computed (maximum 2 values and minimum 1 value), and the correlation between fluoride intake at the second and third trimester was run using Spearman correlation. Bayley-III composite scores at ages 12 and 24 months were summarized as means and SDs.
We generated separate linear regression models to estimate the associations between maternal fluoride intake and the three composite scores (cognitive, language, and motor development) at each time point (12 and 24 months). Models were estimated using maternal fluoride intake measured at trimester 2, trimester 3, and averaged across both trimesters if two values were measured (or at either trimester 2 or 3 if only one value was measured); we also assessed the potential modifying role of child sex using interactions. Regression diagnostics were conducted and confirmed that model assumptions were met.
To examine the association between maternal fluoride intake and neurodevelopmental functioning across 12 and 24 months of age, we generated mixed effects linear regression models for longitudinal data. This approach allowed us to consider an unbalanced panel design using the individual as the unit of analysis for clustering. All models were adjusted using the same covariates as used in the linear models and we also considered an interaction term between fluoride intake and child sex. To aid interpretation, we adjusted the regression coefficients so that they represent the predicted difference in Bayley-III outcome per 0.5 mg/d of maternal dietary fluoride intake; 0.5 mg/d fluoride intake corresponds to the IQR (difference between the 25th and 75th percentiles) of dietary fluoride across pregnancy.
Significance for main effects was considered with a p-value of < 0.05 and significance for the interaction was considered with a p-value <0.10. All data analyses were performed with Stata 15.0 (StataCorp 2017).
3. Results
There were no statistically significant differences between participants included in the current study sample (n=103) and the remaining cohort (n=844) on maternal and child demographic characteristics, including gestational age, child sex, birth weight, family SES, and maternal age and educational level (Supplemental Table 1). The comparison of the sample of infants regarding neurodevelopmental testing at 12 or 24 months versus the overall cohort showed that both groups were comparable on all of the Bayley-III outcomes, with two exceptions: the analytic sample had significantly lower cognitive outcomes at 12 months [93.7 (11.1) vs. 96.6 (9.8), p < 0.01], but higher language outcomes at 24 months relative to the overall sample [92.2 (9.3) vs, 89.3 (9.1), p < 0.01].
Descriptive statistics for maternal and child characteristics of the study sample and by child sex are shown in Table 1. On average, mothers were 27.2 years of age at delivery and had a mean (SD) of 12 (2.8) years of school education. More than 80% were married or living with a partner. Mean (SD) birth weight and gestational age among offspring (50.5% female) were 3.1 (0.4) kg and 38.1 (1.9) weeks, respectively. Twenty-three percent of mothers breastfed their babies until 12 months of age. We did not observe any significant differences in relevant covariates between sexes.
Table 1.
Demographic characteristics, fluoride intake, and Bayley-III outcomes for mother-child pairs
| All participants (n=103) | Boys (n=51) | Girls (n=52) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p value | |
| Maternal Characteristics | |||||||
| Age (years) | 27.5 | 5.7 | 27.7 | 5.9 | 27.2 | 5.6 | 0.66 |
| Education (years) | 12 | 2.8 | 12.1 | 2.8 | 11.9 | 2.8 | 0.77 |
| Socioeconomic Status** | |||||||
| Low | 46 (45) | 22(43) | 24 (46) | 0.09 | |||
| Middle | 47 (45) | 27(53) | 20 (39) | ||||
| High | 10 (10) | 2 (4) | 8 (15) | ||||
| Marital status** | |||||||
| Married | 83 (81) | 42 (82) | 41 (79) | 0.80 | |||
| Single | 20 (19) | 9 (18) | 11 (21) | ||||
| Child Characteristics | |||||||
| Delivery | |||||||
| Birth weight (Kg) | 3.1 | 0.4 | 3.1 | 0.4 | 3.0 | 0.3 | 0.14 |
| Z-score (birth weight/age) | −0.5 | 0.9 | −0.5 | 0.9 | −0.5 | 0.8 | 0.95 |
| Gestational age (weeks) | 38.1 | 1.9 | 37.8 | 2.1 | 38.3 | 1.6 | 0.15 |
| Breastfeeding (months) ** | |||||||
| Never/ < 1st month | 66 (64) | 35 (68) | 31 (60) | ||||
| Exclusive 1st month | 13 (12) | 6 (12) | 7 (13) | 0.18 | |||
| Non-exclusive up to 1 year | 11 (11) | 2 (4) | 9 (17) | ||||
| Exclusive up to 1 year | 13 (13) | 8 (16) | 5 (10) | ||||
| Bayley-III at 12 months of age (n=98) | |||||||
| Cognitive | 93.7 | 11.1 | 93.4 | 11.5 | 93.9 | 10.7 | 0.82 |
| Language | 81.2 | 10.9 | 78.5 | 11.0 | 83.6 | 10.3 | 0.02 |
| Motor | 87.8 | 10.5 | 86.9 | 11.0 | 88.7 | 10.0 | 0.39 |
| Bayley-III at 24 months of age (n=77) | |||||||
| Cognitive | 93.6 | 9.3 | 92.6 | 9.0 | 94.7 | 9.6 | 0.30 |
| Language | 92.2 | 9.3 | 89.2 | 8.4 | 95.3 | 9.2 | 0.003 |
| Motor | 93.6 | 9.3 | 92.2 | 9.5 | 95.1 | 9.1 | 0.18 |
p value for t-test or Fisher exact test
n (%)
Among the 103 women who had valid dietary fluoride intake data, 64 women completed the FFQ at trimester 2 and 91 completed the FFQ at trimester 3 (Supplemental Figure 1). The median (IQR) dietary intake of fluoride was 1.12 mg/d (0.54) across pregnancy (range: 0.38 to 3.07 mg/d), and 1.13 mg/d (0.64) and 1.10 mg/d (0.59) in the second and third trimester, respectively (Table 2). Among the women who had completed the FFQ at two time points (n=58), dietary fluoride intake at trimester 2 was moderately correlated with fluoride intake at trimester 3 (Spearman r = 0.38, p = .006). Only 5 (3.8%) participants consumed more than 3 mg of fluoride per day from food and beverages (i.e., exceeding the US dietary reference value). The practice of adding salt to a meal was reported by 27% of the participants.
Table 2.
Maternal dietary information
| All | Boys | Girls | |||||
|---|---|---|---|---|---|---|---|
| Mother’s diet | Median | IQR | Median | IQR | Median | IQR | p value* |
| 2nd trimester (n=64) | |||||||
| Fluoride (mg/d) | 0.95 | 0.64, 1.45 | 1.04 | 0.62, 1.47 | 0.92 | 0.67, 1.36 | 0.63 |
| Calcium (mg/d) | 1198.3 | 850.1, 1517.7 | 1177.3 | 878.1, 1435.0 | 1217.6 | 819.1, 1838.8 | 0.77 |
| Energy (Kcal/d) | 2011.1 | 1582.3, 2781.2 | 2080.4 | 1582.3, 2847.0 | 1967.9 | 1580.5, 2664.4 | 0.69 |
| 3rd trimester (n=91) | |||||||
| Fluoride (mg/d) | 0.98 | 0.70, 1.26 | 0.92 | 0.69, 1.18 | 1.000 | 0.79, 1.27 | 0.19 |
| Calcium (mg/d) | 1308.0 | 1045.2, 1666.1 | 1224.5 | 1045.2, 1589.4 | 1374.3 | 1015.9, 1749.6 | 0.19 |
| Energy (Kcal/d) | 2131.2 | 1776.2, 292 4.2 | 2028.9 | 1617.4, 2924.2 | 2337.8 | 1853.3, 2960.5 | 0.17 |
| Average (n=103) | |||||||
| Fluoride (mg/d) | 1.01 | 0.73, 1.32 | 1.01 | 0.69, 1.33 | 1.03 | 0.78, 1.31 | 0.46 |
| Calcium (mg/d) | 1238.8 | 979.4, 1702.4 | 1223.1 | 1009.1, 1666.1 | 1305.7 | 977.0, 1764.3 | 0.26 |
| Energy (Kcal/d) | 2218.0 | 1803.8, 2746.1 | 2191.1 | 1801.6, 2746.1 | 2259.3 | 1833.0, 2773.5 | 0.70 |
| Added salt with salt-shaker: yes: n (%) | 27 (28) | 24 (12) | 30 (16) | 0.60 | |||
p value for Wilcoxon test comparing boys and girls
At 12 months, mean cognitive and motor scores fell within the average range (93.7 ± 11.1 and 87.8 ± 10.5, respectively) whereas the mean language score fell below the average range (81.2 ± 10.9) (Table 1). However, at 24 months, mean scores on all domains fell within the average range. Language scores were significantly lower in boys compared with girls at both 12 months (78.5 ± 11.0 vs 83.6 ± 10.3) and 24 months (89.2 ± 8.4 vs. 95.3 ± 9.2). Boys and girls did not significantly differ on the cognitive and motor scores at both time points.
Using linear regression, higher dietary fluoride intake in the second trimester, third trimester, and across pregnancy was not associated with cognitive, language and motor outcomes at 12 and 24 months (Table 3). There were no significant interactions between sex and dietary fluoride intake at either time point (interaction p value > 0.10), except for the model at 24 months which used average dietary fluoride intake to predict cognitive score (p-value for interaction = 0.06). Specifically, a 0.5 mg/day increase in dietary fluoride intake was associated with a statistically significant lower cognitive score among boys (β = −3.50, 95% CI: −6.58, −0.42; p = .02), but not girls (β = 0.07, 95% CI: −2.37, 2.51; p = 0.95) at 24 months of age. This sex-interaction was probed further in the mixed-effect model given the larger sample size when combining the 12 and 24 month Bayley-III scores.
Table 3.
Adjusted regression coefficients (95% confidence interval) from linear regression models for the time-specific associations between fluoride intake (0.5 mg/d) in pregnancy and Bayley-III neurodevelopmental outcomes at 12 (n=98) and 24 months (n=77).
| 12 months (n=98) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cognitive | Language | Motor | |||||||
| Fluoride intake (mg/d) | β | 95% CI | β | 95% CI | β | 95% CI | |||
| 2nd Trimester (n=61) | −0.63 | −3.03 | 1.79 | −0.74 | −3.22 | 1.74 | −0.90 | −3.07 | 1.28 |
| 3rd trimester (n=86) | −1.31 | −3.64 | 1.03 | −1.24 | −3.57 | 1.10 | −1.02 | −3.36 | 1.32 |
| Average in pregnancy (n=98) | −1.85 | −4.18 | 0.49 | −0.98 | −3.21 | 1.25 | −1.10 | −3.28 | 1.08 |
| Boys* | −2.43 | −6.12 | 1.27 | −1.04 | −4.90 | 2.83 | −0.72 | −4.52 | 3.09 |
| Girls* | −0.71 | −3.50 | 2.09 | −1.05 | −3.95 | 1.84 | −1.77 | −4.62 | 1.08 |
| P interaction term* | 0.43 | 0.99 | 0.65 | ||||||
| 24 months (n=77) | |||||||||
| Cognitive | Language | Motor | |||||||
| Fluoride intake (mg/d) | β | 95% CI | β | 95% CI | β | 95% CI | |||
| 2nd Trimester (n=43) | −0.62 | −3.58 | 2.34 | −0.92 | −3.90 | 2.07 | −1.65 | −4.42 | 1.12 |
| 3rd trimester (n=71) | −1.26 | −3.31 | 0.79 | 0.15 | −1.93 | 2.24 | −0.21 | −2.29 | 1.87 |
| Average in pregnancy (n=77) | −1.14 | −3.26 | 0.99 | −0.22 | −2.34 | 1.91 | −0.88 | −3.02 | 1.26 |
| Boys* | −3.50 | −6.58 | −0.42 | −1.77 | −5.02 | 1.47 | −2.36 | −5.67 | 0.95 |
| Girls* | 0.07 | −2.37 | 2.51 | 0.70 | −1.90 | 3.31 | 0.40 | −2.24 | 3.04 |
| P interaction term* | 0.06 | 0.23 | 0.19 | ||||||
Models adjusted for motheŕs age, motheŕs education, SES, calcium intake (concurrent), added salt, gestational age, birth weight (z score), sex, breastfeeding practices.
Beta coefficients are presented for model with the interaction term sex*fluoride intake (3rd trimester), adjusted for motheŕs age, motheŕs education, SES, calcium intake (concurrent), added salt, gestational age, birth weight (z score), breastfeeding practices.
Bold font indicates p-value of interaction <0.10
In the mixed-effects longitudinal model, we observed a statistically significant negative association between dietary fluoride intake in pregnancy and cognitive score (averaged across both time points) in boys, but not girls (interaction p value = 0.07) (Table 4). Specifically, a 0.5 mg increase in dietary fluoride intake during the third trimester and across pregnancy (i.e. trimesters 2 and 3) was associated with a 3.10-point (95% CI: −5.67, −0.53) and 3.46-point (95% CI: −6.23, −0.70) lower cognitive score in boys, respectively. Although the effect estimates were in the expected direction, maternal fluoride intake was not significantly associated with language or motor scores, nor was there a significant fluoride intake by sex interaction for these outcomes. The adjusted margin effects and 95% confidence intervals of the cognitive scores according to fluoride intake in pregnancy and sex are presented in Figure 1.
Table 4.
Adjusted associations estimated from longitudinal mixed effects linear regression models of 0.5 mg/day fluoride intake in pregnancy and Bayley-III domains (12 and 24 months) with the effect modification of sex
| Fluoride intake (mg/d) | Cognitive | Language | Motor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trimester 2 (n=64)a | β | 95% CI | β | 95% CI | β | 95% CI | ||||
| Boys | −1.72 | −4.00 | 0.55 | −0.34 | −3.02 | 2.33 | −2.01 | −4.31 | 0.28 | |
| Girls | 0.24 | −2.30 | 2.78 | −2.32 | −5.31 | 0.67 | −0.41 | −2.94 | 2.13 | |
| P interaction term | 0.24 | 0.31 | 0.34 | |||||||
| Trimester 3 (n=91)b | Boys | −3.10 | −5.67 | −0.53 | −1.84 | −4.67 | 1.00 | −1.70 | −4.60 | 1.20 |
| Girls | −0.28 | −2.26 | 1.70 | 0.10 | −2.09 | 2.29 | −0.16 | −2.39 | 2.06 | |
| P interaction term | 0.07 | 0.26 | 0.38 | |||||||
| Averaged (n=103) | Boys | −3.46 | −6.23 | −0.70 | −0.81 | −3.65 | 2.03 | −2.12 | −5.00 | 0.76 |
| Girls | −0.33 | −2.53 | 1.88 | −0.67 | −2.95 | 1.61 | −0.51 | −2.80 | 1.78 | |
| P interaction term | 0.08 | 0.94 | 0.40 | |||||||
Sample for 2nd trimester may consist of a woman whose child was assessed at one (n=6) or both time points (n=58).
Sample for 3rd trimester may consist of a woman whose child was assessed at one (n=33) or both time points (n=58).
Models adjusted for Motheŕs: age, education, SES, calcium intake (concurrent); Infantś: gestational age and birth weight (z score), breastfeeding practices
Interaction: Sex (reference is boys) × Fluoride intake (mg/d), bolds indicates p-values <0.05
Figure 1.
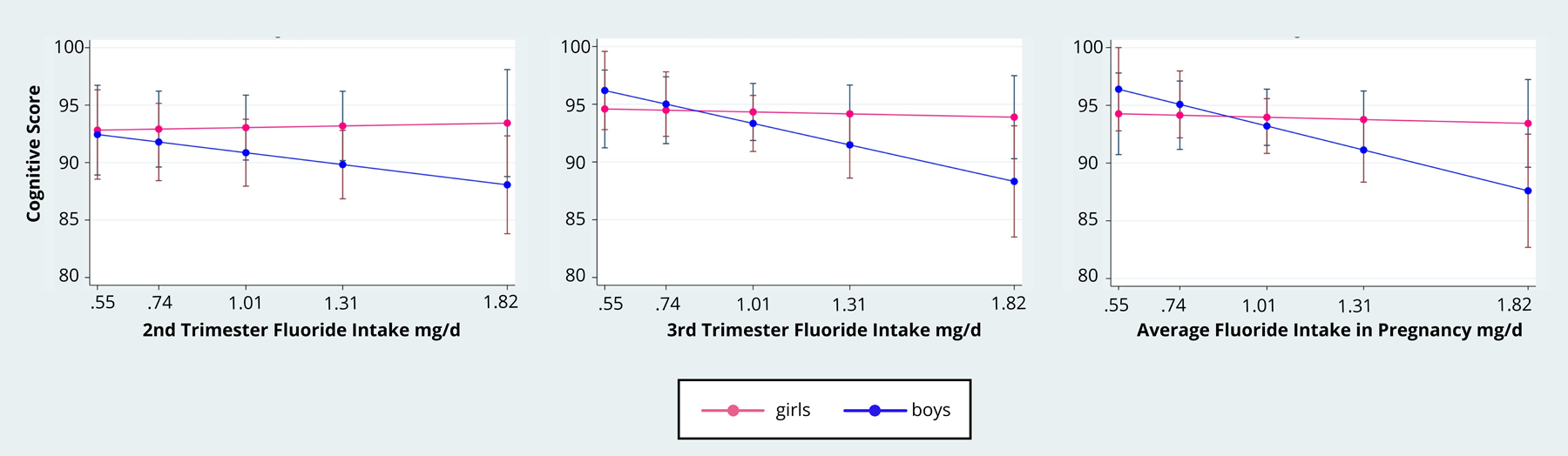
Adjusted Predictive Margins of the association between fluoride intake in pregnancy and Bayley-III Cognitive Score with the effect modification of sex
4. Discussion
In our sample of mother-infant pairs from Mexico City, dietary fluoride intake in pregnancy was not associated with cognitive, language, or motor outcomes collapsing across boys and girls. However, we observed sex-specific associations on the cognitive scale. Specifically, higher overall dietary fluoride intake predicted lower cognitive scores among boys, but not girls at 24-months of age. Averaging across the longitudinal data (i.e. both 12- and 24-month time points), the mixed effects regression model showed that an increment of 0.5 mg fluoride per day was associated with a reduction of 3.1-to-3.5 points on the cognitive scale in boys; 0.5 mg/day is the approximate interquartile range for dietary fluoride intake in our sample. This association was observed when dietary fluoride intake was averaged across trimesters 2 and 3, and specifically for the third trimester. The Bayley-III cognitive scale estimates general cognitive functioning on the basis of nonverbal activities involving memory, problem solving and manipulation of objects. These findings suggest that the development of nonverbal abilities in males may be more vulnerable to prenatal fluoride exposure than language or motor abilities, even at levels within the recommended intake range.
To our knowledge, this is the first prospective and longitudinal study to examine associations between maternal fluoride intake from food and beverages during pregnancy and offspring neurodevelopment. Our findings are consistent with two other prospective cohort studies from Mexico that measured urinary fluoride levels in pregnancy. Among 65 Mexican mother-infant pairs living in areas with high water fluoride levels (i.e. >2 mg/L), elevated maternal urinary fluoride levels in the first and second trimester of pregnancy were associated with significantly lower scores of infant mental development, but not psychomotor development (Valdez Jimenez et al. 2017). In another pregnancy cohort from Mexico City (ELEMENT), an increase of 1-mg/L maternal urinary fluoride concentration was associated with 5–6 points lower IQ scores at 4 years in 287 children and 6–12 years in 211 children (Bashash et al. 2018; Bashash et al. 2017). Another study conducted in Canada (MIREC) found a 1-mg/L increase in maternal urinary fluoride concentration (adjusted for specific gravity) was associated with a 4.5-point lower Full Scale IQ score in 3-to-4 year old boys, but not girls (Green et al. 2019). Moreover, among the boys in the MIREC study, the association between prenatal fluoride exposure and child IQ was significant for non-verbal IQ, but not verbal IQ. Thus, the magnitude of effect and specificity of the associations in the current study are consistent with recent prospective birth cohort studies conducted in areas receiving optimally fluoridated water or salt. Past studies examining other neurotoxicants, such as lead, have also reported non-verbal abilities as being more sensitive to neurotoxic effects than language abilities (Bellinger et al. 1991; Dietrich et al. 1991; Jusko et al. 2008). In early childhood, fluid (i.e. non-verbal) abilities are more influenced by genetics and biology whereas language is more likely to be shaped by experience (Asbury et al. 2005; Luster and Dubow 1992).
Our finding of a male-specific vulnerability to prenatal fluoride was observed on the cognitive scale of the Bayley-III, but not the language and motor scales. An effect of fetal exposure to fluoride has been observed in both animal and human studies, though conclusions about sex-specific effects of prenatal fluoride exposure remain limited by the small number of epidemiologic and animal studies that have tested for sex-specific effects (Green et al. 2020a). Other environmental exposures, such as lead and BPA, have also been associated with sex-specific effects on neurodevelopment (Braun et al. 2017; Desrochers-Couture et al. 2018; Singh et al. 2018). For example, in the Swedish Environmental Longitudinal, Mother and child, Asthma and allergy study, 26 prenatal endocrine disruptors were assessed, showing an impact on IQ at 7 years of age in boys (Tanner et al. 2020). Prior evidence suggests that the brain development of males may be more susceptible to environmental perturbations encountered in utero when compared to female offspring (Alves et al. 2020; Argente-Arizon et al. 2018; Schulz et al. 2011). Studies from endemic fluorosis areas and animal studies show that fluoride exposure can affect thyroid levels (Wang et al. 2020), as well as neurotransmitters and brain function in the offspring. In addition, differences in sex hormones (e.g. estradiol) and toxicokinetics between sexes may also contribute to sex-specific neurotoxic effects (Gandhi et al. 2004). Further studies are needed to understand mechanisms that may contribute to sex-specific vulnerabilities to fluoride.
The current study builds upon our prior work evaluating the fluoride content in the most representative foods and beverages consumed in Mexico (Cantoral et al. 2019). In Mexico, fast foods and processed foods (e.g. sausage, pork rinds) were found to have high levels of fluoride, likely due to the fluoridated salt used in their preparation and through food packaging as a source of fluoride. Other major dietary sources of fluoride include marine fish (Cantoral et al. 2019), and tea (Das et al. 2017; Rodriguez et al. 2020; Waugh et al. 2016), which can contain considerable amounts of fluoride (1.5–3.7 mg/L) depending on the orgin of tea and the type of water used. While the current study measured fluoride intake from the main dietary sources known to contain fluoride, other sources of fluoride exposure (e.g. fluoridated dental products) could have also contributed to total fluoride intake in pregnant women.
Dietary guidelines on fluoride intake have discrepancies between countries and organizations. For example, the U.S. Institute of Medicine (1997), the Mexican guidelines (2004), and the Institute of Nutrition of Central America and Panama (1996) established 3 mg/d as the recommended dietary intake for adults, the WHO (2004). In contrast, the Nordic countries (NNR, 2004), the Scientific Committee for Food (SCF, 1993), and the Netherlands Food and Nutrition Council (1992) do not have a dietary reference value because fluoride is not an essential nutrient (EFSA and NDA 2013). Dietary guidelines for fluoride are derived based on intakes that have been shown to maximally reduce the occurrence of caries. In pregnancy, however, a Cochrane review of randomized control trials found no evidence that fluoride supplementation is effective in preventing caries in the fetus (Takahashi et al. 2017). Consistent with this conclusion, the Center for Disease Control and Prevention does not recommend the use of fluoride supplements during pregnancy (CDC 2001).
One limitation of the study was the small sample size and possibility of selection bias by including only participants who had a valid FFQ and neurodevelopmental data. Relative to the overall (PROGRESS) participants from which our study sample was drawn, ours consisted of children who had significantly lower cognitive outcomes at 12 months. It is possible that our sample represented a group of higher-risk infants at baseline relative to the overall sample; however, this pattern was no longer observed at 24 months (instead, the study sample demonstrated higher language outcomes relative to the original cohort). Also, the FFQ is not the ideal method for assessing dietary fluoride intake because it depends on motheŕs recall. A better method would be to use a duplicate plate to assess specific exposure levels of foods that are consumed because fluoride content may depend on how the food was prepared (e.g. type of water used) and how much salt is added (Cantoral et al. 2019). However, limitations related to maternal recall and measurement of dietary fluoride intake would contribute to non-differential exposure misclassification since women were not aware of fluoride-diet sources. While we cannot confirm that dietary patterns remained stable throughout pregnancy, a large study of dietary patterns conducted in over 12,000 pregnant women in the United Kingdom support the idea of stable diet in pregnancy (Crozier et al. 2009). In addition, a diet that is high in salt is more likely to be an overall “unhealthy” diet (Seo et al. 2020); thus, a high fluoride diet in pregnancy may be confounded by other unhealthy habits that may affect fetal neurodevelopment (Krzeczkowski et al. 2020). Another limitation is that we were unable to adjust for some important predictors of child cognitive abilities, specifically parental cognitive abilities (IQ) and the caregiving environment, but we used maternal education (years of school) as a proxy. Quality of caregiving and stimulation in the home environment have been shown to modify developmental outcomes in young children (Horton et al. 2012; Till et al. 2019; Walkowiak et al. 2001) exposed prenatally to neurotoxins, including lead, PCBs, and chlorpyrifos. Important strengths of this study are the prospective design, assessment of fluoride intake at multiple time points using a validated FFQ, and repeated and blinded assessment of offspring neurodevelopment at 12 and 24 months using a standardized measure.
5. Conclusion
In this prospective cohort study, higher exposure to fluoride from food and beverage consumption in pregnancy was associated with reduced cognitive outcome, but not with language and motor outcome in male offspring over the first two years of life. Given the ubiquity of fluoride in food and beverages, it will be important to develop recommendations for how vulnerable populations, such as pregnant women, may limit dietary fluoride intake to minimize potential adverse health risks of the unborn fetus. More human studies are needed to monitor and quantify fluoride intake and exposure from all sources, including water, salt, foods, beverages, and dental products, and to test for long-term health impacts of this exposure.
Supplementary Material
Highlights.
Mexican guidelines recommended a dietary reference intake for fluoride of 2.45 mg/d for adult and pregnant women.
Mexico has a salt fluoridation program.
High fluoride concentrations are neurotoxic
Median fluoride intake through foods and beverages was estimated to be 1.01 mg/d (0.73, 1.32) in a Mexican pregnancy cohort (subsample of PROGRESS cohort)
Higher fluoride intake from foods and beverages during pregnancy is associated with lower cognitive neurodevelopment in male offspring.
Acknowledgments
This research was funded by the National Institutes of Health (NIH)/National Institute of Environmental Health Science (NIEHS)(grant numbers: P30ES023515, R01ES014930, R01ES021357, R24ES028522, R21ES027044). We thank the Centro Médico ABC and the National Institute of Perinatology, México, for their support with the facilities and staff
Abbreviations
- Bayley-III
Bayley Scales of Infant and Toddler Development, Third Edition
- FFQ
Food Frequency Questionnaire
- PROGRESS
Programming Research in Obesity, Growth, Environment and Social Stressors
- SES
Socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Aggeborn L, Ohman M (2017) The Effects of Fluoride In The Drinking Water. Institute for Housing and Urban Research (IBF) & Department of Women’s and Children’s Health at Uppsala University, Uppsala, Sweden [Google Scholar]
- Alves JM, Luo S, Chow T, Herting M, Xiang AH, Page KA (2020) Sex differences in the association between prenatal exposure to maternal obesity and hippocampal volume in children. Brain Behav 10(2):e01522 doi: 10.1002/brb3.1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMAI Asociación Mexicana de Agencias de Investigación de Mercado. In. http://www.amai.org/
- Argente-Arizon P, et al. (2018) The Hypothalamic Inflammatory/Gliosis Response to Neonatal Overnutrition Is Sex and Age Dependent. Endocrinology 159(1):368–387 doi: 10.1210/en.2017-00539 [DOI] [PubMed] [Google Scholar]
- Asbury K, Wachs TD, Plomin R (2005) Environmental moderators of genetic influence on verbal and nonverbal abilities in early childhood. Intelligence 33(6):18 doi: 10.1016/j.intell.2005.03.008 [DOI] [Google Scholar]
- Bashash M, et al. (2018) Prenatal fluoride exposure and attention deficit hyperactivity disorder (ADHD) symptoms in children at 6–12years of age in Mexico City. Environ Int 121(Pt 1):658–666 doi: 10.1016/j.envint.2018.09.017 [DOI] [PubMed] [Google Scholar]
- Bashash M, et al. (2017) Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6–12 Years of Age in Mexico. Environ Health Perspect 125(9):097017 doi: 10.1289/EHP655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N (2006) Bayley Scales of Infant and Toddler Development. Pearson, San Antonio, TX [Google Scholar]
- Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C (1991) Low-level lead exposure and children’s cognitive function in the preschool years. Pediatrics 87(2):219–27 [PubMed] [Google Scholar]
- Bera I, et al. (2007) Neurofunctional effects of developmental sodium fluoride exposure in rats. Eur Rev Med Pharmacol Sci 11(4):211–24 [PubMed] [Google Scholar]
- Bourges H, Casanueva E, Rosaldo JL (2004) I. Vitaminas y nutrimentos inorgánicos. Médica Panamericana [Google Scholar]
- Braun JM, et al. (2014) Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ Health 13(1):50 doi: 10.1186/1476-069X-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. (2017) Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 62:192–199 doi: 10.1016/j.neuro.2017.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent JM, et al. (2015) Community Water Fluoridation and Intelligence: Prospective Study in New Zealand. Am J Public Health 105(1):72–76 doi: 10.2105/AJPH.2013.301857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzalaf MAR (2018) Review of Fluoride Intake and Appropriateness of Current Guidelines. Adv Dent Res 29(2):157–166 doi: 10.1177/0022034517750850 [DOI] [PubMed] [Google Scholar]
- Cantoral A, et al. (2019) Fluoride Content in Foods and Beverages From Mexico City Markets and Supermarkets. Food Nutr Bull 40(4):514–531 doi: 10.1177/0379572119858486 [DOI] [PubMed] [Google Scholar]
- CDC (2001) Recommendations for using fluoride to prevent and control dental caries in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep 50(RR-14):1–42 [PubMed] [Google Scholar]
- Choi AL, et al. (2015) Association of lifetime exposure to fluoride and cognitive functions in Chinese children: a pilot study. Neurotoxicol Teratol 47:96–101 doi: 10.1016/j.ntt.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM (2009) Women’s dietary patterns change little from before to during pregnancy. J Nutr 139(10):1956–63 doi: 10.3945/jn.109.109579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, de Oliveira LM, da Silva E, Liu Y, Ma LQ (2017) Fluoride concentrations in traditional and herbal teas: Health risk assessment. Environ Pollut 231(Pt 1):779–784 doi: 10.1016/j.envpol.2017.08.083 [DOI] [PubMed] [Google Scholar]
- Desrochers-Couture M, et al. (2018) Prenatal, concurrent, and sex-specific associations between blood lead concentrations and IQ in preschool Canadian children. Environ Int 121(Pt 2):1235–1242 doi: 10.1016/j.envint.2018.10.043 [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Succop PA, Berger OG, Hammond PB, Bornschein RL (1991) Lead exposure and the cognitive development of urban preschool children: the Cincinnati Lead Study cohort at age 4 years. Neurotoxicol Teratol 13(2):203–11 doi: 10.1016/0892-0362(91)90012-l [DOI] [PubMed] [Google Scholar]
- Ding Y, et al. (2011) The relationships between low levels of urine fluoride on children’s intelligence, dental fluorosis in endemic fluorosis areas in Hulunbuir, Inner Mongolia, China. J Hazard Mater 186(2–3):1942–6 doi: 10.1016/j.jhazmat.2010.12.097 [DOI] [PubMed] [Google Scholar]
- EFSA, NDA (2013) Scientific opinion on Dietary Reference Values for fluoride ((EFSA Panel on Dietetic Products, Nutrition and Allergies). EFSA Journal 11(8):3332 doi:doi: 10.2903/j.efsa.2013.3332 [DOI] [Google Scholar]
- EPA (2010) Fluoride: Exposure and Relative Source Contribution Analysis United States Environmental Protection Agency, Washington DC [Google Scholar]
- Everett ET (2011) Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res 90(5):552–60 doi: 10.1177/0022034510384626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone JD (2000) The science and practice of caries prevention. J Am Dent Assoc 131(7):887–99 doi: 10.14219/jada.archive.2000.0307 [DOI] [PubMed] [Google Scholar]
- Ferreira MKM, et al. (2020) Fluoride exposure during pregnancy and lactation triggers oxidative stress and molecular changes in hippocampus of offspring rats. Ecotoxicol Environ Saf 208:111437 doi: 10.1016/j.ecoenv.2020.111437 [DOI] [PubMed] [Google Scholar]
- Full CA, Parkins FM (1975) Effect of cooking vessel composition on fluoride. J Dent Res 54(1):192 doi: 10.1177/00220345750540012501 [DOI] [PubMed] [Google Scholar]
- Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF (2004) Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 44:499–523 doi: 10.1146/annurev.pharmtox.44.101802.121453 [DOI] [PubMed] [Google Scholar]
- Green R, et al. (2019) Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr doi: 10.1001/jamapediatrics.2019.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Rubenstein J, Popoli R, Capulong R, Till C (2020a) Sex-Specific Neurotoxic Effects of Early-Life Exposure to Fluoride: a Review of the Epidemiologic and Animal Literature. Current Epidemiology Reports [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, et al. (2020b) Associations between Urinary, Dietary, and Water Fluoride Concentrations among Children in Mexico and Canada. Toxics 8(4) doi: 10.3390/toxics8040110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila M, Romieu I, Parra S, Hernandez-Avila J, Madrigal H, Willett W (1998) Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex 40(2):133–40 doi: 10.1590/s0036-36341998000200005 [DOI] [PubMed] [Google Scholar]
- Horowitz HS (2000) Decision-making for national programs of community fluoride use. Community Dent Oral Epidemiol 28(5):321–9 doi: 10.1034/j.1600-0528.2000.028005321.x [DOI] [PubMed] [Google Scholar]
- Horton MK, Kahn LG, Perera F, Barr DB, Rauh V (2012) Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicol Teratol 34(5):534–41 doi: 10.1016/j.ntt.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (2006) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. The National Academies Press, Washington, DC [Google Scholar]
- Jimenez-Zabala A, et al. (2018) [Fluoride intake through consumption of water from municipal network in the INMA-Gipuzkoa cohort]. Gac Sanit 32(5):418–424 doi: 10.1016/j.gaceta.2017.02.008 [DOI] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL (2008) Blood lead concentrations < 10 microg/dL and child intelligence at 6 years of age. Environ Health Perspect 116(2):243–8 doi: 10.1289/ehp.10424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzeczkowski JE, et al. (2020) Maternal Pregnancy Diet Quality Is Directly Associated with Autonomic Nervous System Function in 6-Month-Old Offspring. J Nutr 150(2):267–275 doi: 10.1093/jn/nxz228 [DOI] [PubMed] [Google Scholar]
- Luster T, Dubow E (1992) Home Environment and Maternal Intelligence as Predictors of Verbal Intelligence: A Comparison of Preschool and School-Age Children. Merrill-Palmer Quarterly 38(2):25 [Google Scholar]
- Martinez-Mier EA (2012) Fluoride: Its Metabolism, Toxicity, and Role in Dental Health. Journal of Evidence-Based Complementary & Alternative Medicine 17(1):28–32 [Google Scholar]
- Martinez-Mier EA (2018) Guidelines for Fluoride Intake: First Discussant. Adv Dent Res 29(2):177–178 doi: 10.1177/0022034517750590 [DOI] [PubMed] [Google Scholar]
- Martinez-Mier EA, et al. (2011) Development of gold standard ion-selective electrode-based methods for fluoride analysis. Caries Res 45(1):3–12 doi: 10.1159/000321657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Mier EA, Soto-Rojas AE, Urena-Cirett JL, Stookey GK, Dunipace AJ (2003) Fluoride intake from foods, beverages and dentifrice by children in Mexico. Community Dent Oral Epidemiol 31(3):221–30 doi: 10.1034/j.1600-0528.2003.00043.x [DOI] [PubMed] [Google Scholar]
- Mejare I (2018) Current Guidance for Fluoride Intake: Is It Appropriate? Adv Dent Res 29(2):167–176 doi: 10.1177/0022034517750589 [DOI] [PubMed] [Google Scholar]
- Mulualem D, Hailu D, Tessema M, Whiting SJ (2021) Efficacy of Calcium-Containing Eggshell Powder Supplementation on Urinary Fluoride and Fluorosis Symptoms in Women in the Ethiopian Rift Valley. Nutrients 13(4) doi: 10.3390/nu13041052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JJ (1986) Appropriate Use of Fluorides for Human Health. In: Organization WH (ed) Organization, International Dental Federation & WK Kellogg Foundation [Google Scholar]
- NTP (2020) Revised draft National Toxicology Program monograph on the systematic review of fluoride exposure and neurodevelopmental and cognitive health effects. In: Sciences NIoEH (ed) Research Triangle Park, NC. U.S. Department of Health and Human Services [Google Scholar]
- Pizzo G, Piscopo MR, Pizzo I, Giuliana G (2007) Community water fluoridation and caries prevention: a critical review. Clin Oral Investig 11(3):189–93 doi: 10.1007/s00784-007-0111-6 [DOI] [PubMed] [Google Scholar]
- Rocha-Amador D, Navarro ME, Carrizales L, Morales R, Calderon J (2007) Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad Saude Publica 23 Suppl 4:S579–87 doi: [DOI] [PubMed] [Google Scholar]
- Rodriguez I, et al. (2020) Human exposure to fluoride from tea (Camellia sinensis) in a volcanic region-Canary Islands, Spain. Environ Sci Pollut Res Int doi: 10.1007/s11356-020-10319-9 [DOI] [PubMed] [Google Scholar]
- Schaider LA, et al. (2017) Fluorinated Compounds in U.S. Fast Food Packaging. Environ Sci Technol Lett 4(3):105–111 doi: 10.1021/acs.estlett.6b00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, et al. (2011) Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiol Behav 104(2):340–7 doi: 10.1016/j.physbeh.2011.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y, Jeong YS, Koo KA, Yang JI, Park YK (2020) Maternal nutrition intervention focused on the adjustment of salt and sugar intake can improve pregnancy outcomes. Food Sci Nutr 8(7):3900–3911 doi: 10.1002/fsn3.1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraj B, et al. (2012) Effect of high water fluoride concentration on the intellectual development of children in makoo/iran. J Dent (Tehran) 9(3):221–9 [PMC free article] [PubMed] [Google Scholar]
- Singh G, Singh V, Sobolewski M, Cory-Slechta DA, Schneider JS (2018) Sex-Dependent Effects of Developmental Lead Exposure on the Brain. Front Genet 9:89 doi: 10.3389/fgene.2018.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SSA (1995) Norma oficial Mexicana nom-040-ssa-1–1993. Sal yodatadaysalfluorada [in Spanish]. In: Health Mo (ed). Diario Oficial de la Federación, Mexico, p 12–27 [Google Scholar]
- StataCorp (2017) Stata Statistical Software. In: 15 R (ed). StataCorp LLC, College Station, TX [Google Scholar]
- Sudhir KM, Chandu GN, Prashant GM, Subba Reddy VV (2009) Effect of fluoride exposure on Intelligence Quotient {IQ) among 13–15 year old school children of known endemic area of fluorosis, Nalgonda District, Andhra Pradesh. J Indian Assoc Public Health Dent 7(13):6 [Google Scholar]
- Takahashi R, et al. (2017) Fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women for preventing dental caries in the primary teeth of their children. Cochrane Database Syst Rev 10:CD011850 doi: 10.1002/14651858.CD011850.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner EM, et al. (2020) Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environ Int 134:105185 doi: 10.1016/j.envint.2019.105185 [DOI] [PubMed] [Google Scholar]
- Till C, et al. (2019) Caregiving and infants’ neurodevelopment in rural Costa Rica: Results from the Infants’ Environmental Health Study (ISA). Neurotoxicology 74:100–107 doi: 10.1016/j.neuro.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Till C, et al. (2020) Fluoride exposure from infant formula and child IQ in a Canadian birth cohort. Environ Int 134:105315 doi: 10.1016/j.envint.2019.105315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till C, et al. (2018) Community Water Fluoridation and Urinary Fluoride Concentrations in a National Sample of Pregnant Women in Canada. Environ Health Perspect 126(10):107001 doi: 10.1289/EHP3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier X, Granby K, Christensen JH (2011) Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res Int 18(7):1108–20 doi: 10.1007/s11356-010-0439-3 [DOI] [PubMed] [Google Scholar]
- Valdez Jimenez L, et al. (2017) In utero exposure to fluoride and cognitive development delay in infants. Neurotoxicology 59:65–70 doi: 10.1016/j.neuro.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Walkowiak J, et al. (2001) Environmental exposure to polychlorinated biphenyls and quality of the home environment: effects on psychodevelopment in early childhood. Lancet 358(9293):1602–7 doi: 10.1016/S0140-6736(01)06654-5 [DOI] [PubMed] [Google Scholar]
- Wang M, et al. (2020) Thyroid function, intelligence, and low-moderate fluoride exposure among Chinese school-age children. Environ Int 134:105229 doi: 10.1016/j.envint.2019.105229 [DOI] [PubMed] [Google Scholar]
- Warren JJ, Levy SM (2003) Current and future role of fluoride in nutrition. Dent Clin North Am 47(2):225–43 doi: 10.1016/s0011-8532(02)00098-8 [DOI] [PubMed] [Google Scholar]
- Warren JJ, Levy SM, Broffitt B, Cavanaugh JE, Kanellis MJ, Weber-Gasparoni K (2009) Considerations on optimal fluoride intake using dental fluorosis and dental caries outcomes--a longitudinal study. J Public Health Dent 69(2):111–5 doi: 10.1111/j.1752-7325.2008.00108.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh DT, Potter W, Limeback H, Godfrey M (2016) Risk Assessment of Fluoride Intake from Tea in the Republic of Ireland and its Implications for Public Health and Water Fluoridation. Int J Environ Res Public Health 13(3) doi: 10.3390/ijerph13030259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang A, Xia T, He P (2008) Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-kappaB in primary cultured rat hippocampal neurons. Toxicol Lett 179(1):1–5 doi: 10.1016/j.toxlet.2008.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


