Abstract
Class A GPCRs have evolved to recognize ligands ranging from small molecule odorants to proteins. Although among the most diverse membrane receptors in eukaryotic organisms, they possess a highly conserved core within their seven-transmembrane helix framework. The conservation of the transmembrane core has led to the idea of a common mechanism by which ligand-binding is coupled to the outward rotation of helix H6, the hallmark of an active receptor. Nevertheless, there is still no consensus on the mechanism of coupling or on the roles of specific residues within the core. Recent insights from crystallography and NMR spectroscopy provide a way to decompose the core into its essential structural and functional elements that shed new light on this important region.
Keywords: rhodopsin, ligand-activated, microswitches, visual receptors, olfactory receptors
A common transmembrane core in class A GPCRs
G protein-coupled receptors (GPCRs; see Glossary) control the ability to sense our external environment by acting as receptors for vision, smell, and taste [1–3]. They also exert a strong influence over our internal environment through modulation of neuronal responses and control of key physiological processes. Correspondingly, they have emerged as a major target for pharmaceuticals [4–6]. In humans, there are five main GPCR classes: rhodopsin (class A), secretin (class B1), adhesion (class B2), glutamate (class C), and frizzled-taste2 (class F) [7] (see also https://gpcrdb.org). The only significant sequence identity between the different GPCR classes is between the class B1 and B2 receptors. However, a seven-transmembrane (TM) helix architecture along with the ability to bind and activate G proteins are common features throughout all classes [8, 9].
Class A is the largest, most extensively studied GPCR family in humans with over 800 members [7]. This class of receptors is defined by a conserved set of motifs within the TM helices that couple extracellular ligand-binding to the opening of a G protein binding site on the intracellular side of the receptor [10, 11] (Figure 1). Within the class A receptors, there are 11 subfamilies that exhibit unique sequence and structural differences associated with their ability to recognize distinct types of ligands (https://gpcrdb.org). Well-known class A subfamilies include the visual, olfactory, aminergic, and peptide hormone receptors.
Figure 1. GPCR structure and the conserved TM core.
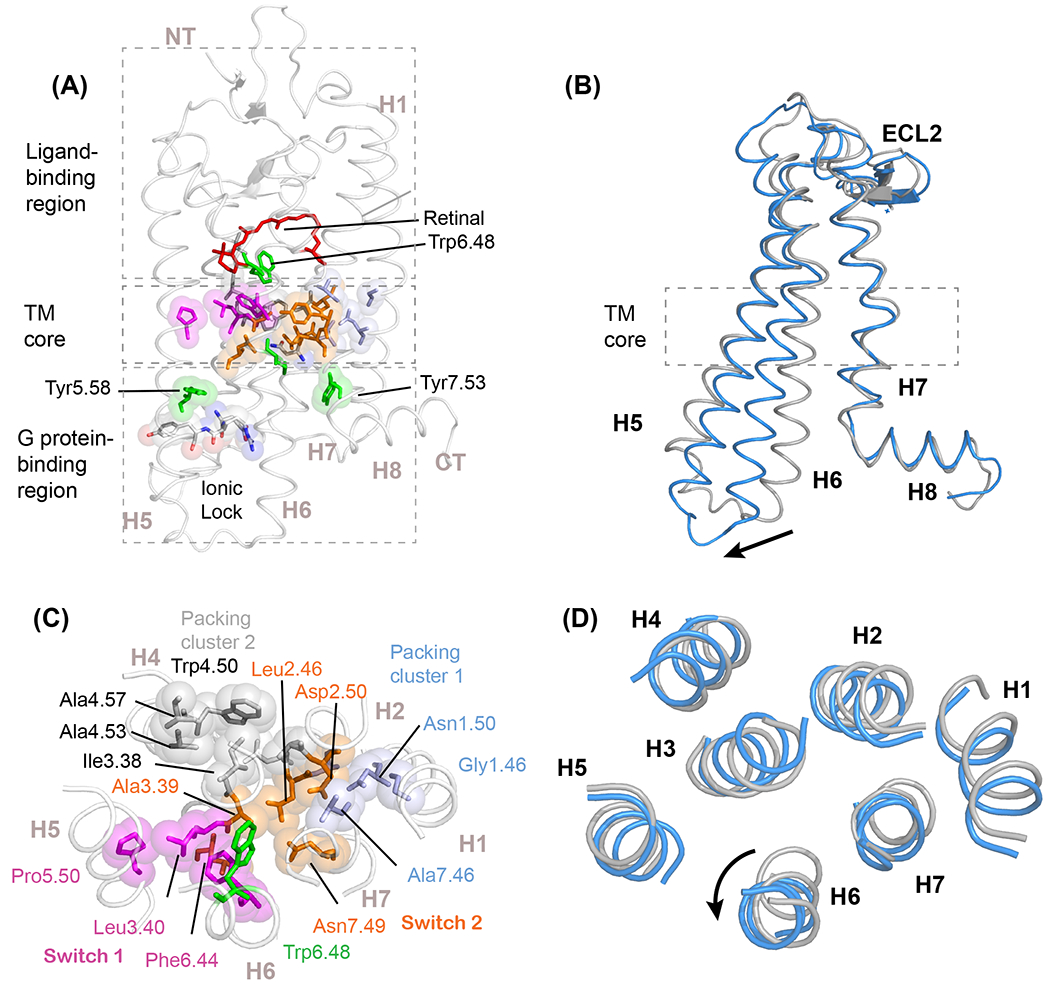
(A) Structure of rhodopsin illustrating the three functional regions of the visual receptor: the extracellular ligand-binding region, the transmembrane (TM) core, and the intracellular G-protein binding region (PDB-ID 1GZM). Trp6.48 contacts the 11-cis retinal chromophore at the boundary with the TM core, and is a key residue in controlling the dynamics of TM helix H6. (B) The comparison of inactive (grey) and active (blue, PDB-ID 3PQR) structures illustrates the outward motion of the intracellular end of H6. This disrupts an ionic lock formed by the E/DRY motif on the end of TM helix H3. Two conserved tyrosines, Tyr5.58 and Tyr7.53, stabilize the active receptor structure [76]. (C) Cross section through the TM core of rhodopsin showing conserved positions contributing to two activation switches and two packing clusters. Activation switch 1 (magenta) is formed by the PIF motif. In rhodopsin this involves Pro5.50, Leu3.40 (typically conserved as isoleucine) and Phe6.44 on helices H5, H3, and H6, respectively. Activation switch 2 (orange) is centered on two strongly polar residues: Asp2.50 of the conserved S/NLAxAD motif on H2 and Asn7.49 of the conserved NPxxY motif on H7. The small side chain conserved residues mediate close packing of helices H1-H2 (packing cluster 1, light blue) and H3-H4 (packing cluster 2, grey) and facilitate interhelical hydrogen-bonding interactions. (D) Cross section of the TM core of inactive and active rhodopsin. The major conformational changes occur in helices H6 and H7.
The seven-TM bundle can conceptually be divided into three regions [12, 13] (Figure 1A). The extracellular ligand-binding region, which is formed by the N-terminus, extracellular loops, and extracellular ends of the TM helices, has evolved to recognize a diverse range of signals. The intracellular region, which is formed by the C-terminus, intracellular loops, and intracellular ends of the TM helices, is the site of binding of the heterotrimeric G proteins, GPCR kinases, and arrestins [2]. The TM core of class A GPCRs can be seen as the transducer to convert an extracellular signal into one or more intracellular signals. It spans 2-3 helical turns in the middle of the TM domain and contains many of the residues that have high sequence identity and homology across the class. The high conservation has led to the idea that these receptors have a common mechanism for receptor signaling [11, 13–15]. An overarching question in the field involves how this transducer works in the class A receptors, and whether the same mechanism(s) exist in the other GPCR classes.
The first crystal structure of an inactive GPCR was solved of bovine rhodopsin, a receptor in the visual receptor subfamily of class A GPCRs [16]. This structure revealed the location of conserved residues in the class A GPCRs, but only offered limited clues as to how the receptor was activated. The largest structural change upon GPCR activation, first observed by electron paramagnetic resonance (EPR) spectroscopy [17], is an outward rotation of the intracellular end of TM helix H6 (Figure 1B, D). Crystal structures of class A GPCRs in both inactive and active conformations have since been determined for receptors in many of the class A subfamilies [16, 18–31]. A challenge for crystallography is that these receptors have both rigid and dynamic features [2, 32–34]. Nevertheless, the large database of the GPCR structures solved to date has been used to identify the residue contacts that change upon activation [11, 14, 35]. These changes, which are mostly associated with the motion of H6, highlight the links between conserved motifs within the receptor. From these studies, a mechanistic picture of TM signal transduction has emerged in which ligand-binding triggers conformational changes within the TM core that are coupled to changes in the conserved motifs on the intracellular side of the receptor. Recent NMR studies build on this picture to address how ligand-binding modulates local receptor dynamics to produce the observed global conformational changes between inactive and active receptors [36–41].
We have previously proposed a framework that deconstructs the TM core into two ‘rigid’ packing clusters and two ‘dynamic’ activation switches on the basis of packing and mutational studies [15] (Figure 1C). The packing clusters involve TM helices H1, H2, H3, and H4 and consist of conserved residues that mediate the assembly of the seven TM helix bundle. The activation switches affect the more loosely packed helices H5, H6, and H7 and allow H6 to reorient upon activation [15]. The sections below focus on the visual receptor rhodopsin to further decompose the class A GPCR TM core into key residue types (e.g. aromatic, polar, and hydrophobic residues) that mediate structure and dynamics. This description forms a framework to understand the possible mechanisms of TM signaling that operate in the other classes of GPCRs.
Prolines can function as switches and hinges
Prolines are the most conserved residues on helices H5, H6, and H7 [13] (https://gpcrdb.org). The function of these prolines is determined by the orientation of the free carbonyl group associated one helical turn away from the backbone proline nitrogen [36] (Figure 2). The free carbonyls associated with the conserved prolines on helices H5 (residue 5.46; Figure 2A) and H7 (residue 7.46; Figure 2C) are oriented toward the TM bundle and form interhelical hydrogen-bonds in many class A GPCRs. A non-hydrogen-bonded backbone C=O is estimated to be unfavorable in a hydrophobic environment by ~4-5 kcal/mol [42]. Binding of agonist ligands can trigger the rearrangement of these hydrogen-bonding interactions to stabilize the active receptor conformation or shift the free C=O into an environment where it is not able to hydrogen-bond allowing the conserved proline to function as a hinge. In contrast, the free carbonyl associated with the conserved proline on H6 (Pro6.50) is oriented away from the helix bundle with only the possibility of forming a hydrogen-bonding contact with bound water (Figure 2B). This predisposes this region to act as a flexible hinge that would allow for the outward rotation of the intracellular end of H6 [17, 43] (Figure 2D). Recent NMR studies argue that a key function of the TM core is to constrain this hinge in an inactive receptor and to allow it to sample a range of conformational states upon activation [38] (see below).
Figure 2. Prolines can function as switches and flexible hinges.
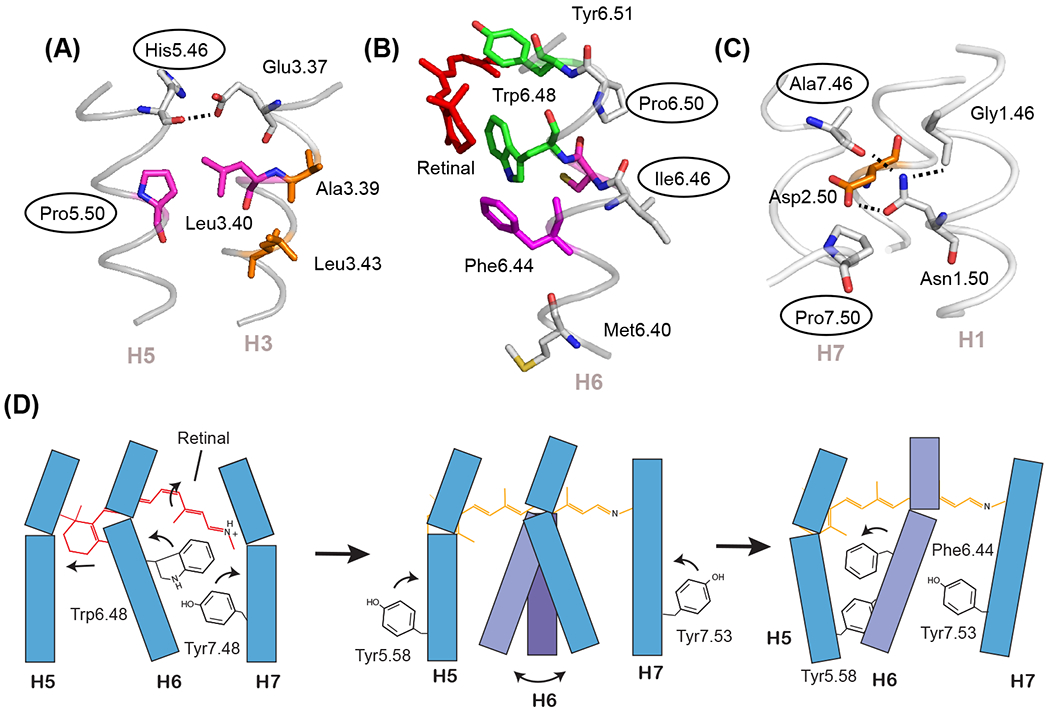
The most conserved residues on TM helices H5, H6, and H7 are prolines that are associated with free carbonyls at positions 5.46, 6.46, and 7.46. (A) In the inactive state of rhodopsin (PDB-ID 1GZM), the His5.46 backbone carbonyl hydrogen-bonds with Glu3.37 on H3. Upon activation, there is a switch in hydrogen-bonding partners to form direct side chain His5.46 - Glu3.37 contacts. The side chain at residue 5.46 is subfamily specific (e.g. in the adrenergic receptors this residue is part of a conserved SSxxS motif that hydrogen-bonds to hydroxyl groups on the catechol ring of agonist ligands). (B) The free C=O group at position 6.46 associated with Pro6.50 is oriented toward the surrounding lipids and consequently is not in a position to form interhelical hydrogen-bonds. This region is predisposed to function as a flexible hinge. (C) The free C=O group at position 7.46 is conserved as a small residue (typically Ala or Ser) in class A GPCRs and serves to orient the side chain of Asn1.50. (D) Schematic showing key steps in rhodopsin activation. Helices H1-H4 (not shown) form a structural scaffold on which helices H5-H7 pack. Retinal isomerization (left panel) releases packing constraints on Trp6.48 which disrupts the Trp6.48-Tyr7.48 interaction and imparts flexibility at the H6 proline hinge (middle panel). An outward orientation of H6 is stabilized by the rotation and repacking of three aromatic and hydrophobic residues. Key residues are Phe6.44, Tyr5.58, and Tyr7.53 (right panel).
Aromatic residues modulate the H6 hinge
Aromatic residues are conserved in the TM core and intracellular region of class A GPCRs and are very often conserved within subfamilies in the extracellular ligand-binding region [13]. Aromatic stacking and the rearrangement of aromatic stacking interactions prominently occur in ligand-activated GPCRs to translate ligand-binding energy into large conformational changes [44] (Figure 3). Tyrosine and tryptophan have the added feature of a polar functional group that can orient the side chain through hydrogen-bonding interactions.
Figure 3. Aromatic residues in receptor activation.
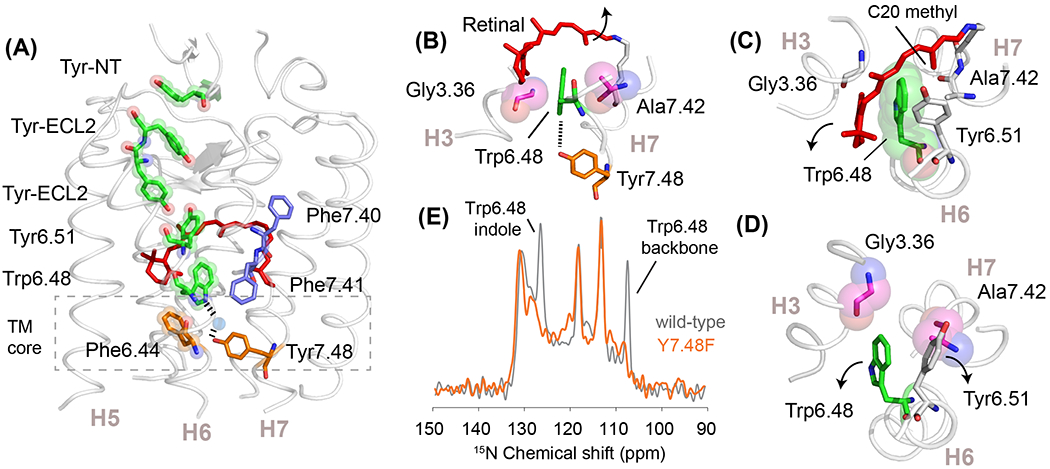
(A) Structure of the visual receptor rhodopsin (PDB-ID 1GZM) highlighting a string of aromatic residues that stretch from the N-terminus into the TM core. The last residue in this string is Phe6.44, which is oriented toward the center of the TM core. (B) Trp6.48 is packed between Gly3.36 and Ala7.42 in rhodopsin. Its indole NH forms a water-mediated hydrogen-bond with Tyr7.48. (C) View from the extracellular surface of inactive rhodopsin showing the packing of Trp6.48 with Tyr6.51 and the retinal C20 methyl group. Retinal isomerization moves the C20 methyl group away and causes a shift of Tyr6.51 that removes its packing interaction with Trp6.48 [36]. (D) Same view as in (C) of active metarhodopsin II (PDB-ID 3PQR) showing the relative changes in the positions of Trp6.48 and Tyr6.51. This motion breaks the hydrogen-bonding interaction with Tyr7.48, which is part of the switch 2 hydrogen-bonding network. (E) Solid-state 15N NMR spectrum of rhodopsin containing 15N-labeled tryptophan. Mutation of Tyr7.48 to phenylalanine results in complete loss in the intensity of the Trp6.48 indole NH and backbone NH resonances, consistent with a high degree of dynamics [38]. Similar changes are observed with the A7.42V night blindness mutation and to a lesser extent with the G3.36A mutation [38].
The central residue in the TM core on H6 is a conserved phenylalanine, Phe6.44. This phenylalanine is part of activation switch 1 (Figure 1C). Together with Pro5.50 and Ile3.40, it forms the conserved “PIF” motif, a group of hydrophobic residues on helices H3, H5, and H6 that were initially observed to rearrange in the β2-adrenergic receptor [45]. Phe6.44 is two residues away from the free carbonyl group associated with Pro6.50 (see Figure 2B) and in the inactive state packs against Trp6.48, the conserved tryptophan in the CWxP motif on H6.
Trp6.48 is located at the interface between the TM core and the ligand-binding pocket. It often interacts directly with the ligand and subfamily-conserved residues [46]. Tyr6.51 is one of the most highly conserved residues in the visual receptor subfamily and packs on the extracellular side of Trp6.48 [13] (Figures 2B and 3A). Additionally, the indole ring of the Trp6.48 is in contact with the retinal C20 methyl group and is sandwiched between Gly3.36 and Ala7.42 (Figure 3B–D). The indole NH of Trp6.48 is hydrogen-bonded via water to Tyr7.48. Together, these interactions immobilize Trp6.48 in the inactive state and restrict motion of the Pro6.50 hinge. Upon activation, solid-state NMR studies on the wild-type receptor show a loss of intensity in the backbone 15N NMR resonance of Trp6.48, suggesting an increase in dynamics upon activation [47]. The first steps in activation involve motion of the retinal C20 methyl group away from Trp6.48 and motion of Tyr6.51 towards the Schiff base end of the retinal [36] (Figure 3C). This releases the packing constraints on the extracellular side of the Trp6.48 indole ring. Experiments designed to probe hydrogen-bonding interactions with Tyr7.48 yield an unexpected, but revealing, result [38]. Specifically, mutation of Tyr7.48 to phenylalanine results in a complete loss of both the Trp6.48 indole NH and backbone NH resonances (Figure 3E). These data are indicative of substantial dynamics emerging at Trp6.48 consistent with unraveling of the helical backbone in this region. A similar result is obtained by mutating Ala7.42 to valine [47], an activating mutation in congenital stationary night blindness [38].
Recent solution NMR studies targeting leucine residues in the β2-adrenergic receptor suggest that the same mechanism operates in the ligand activated GPCRs [39]. In these studies, selective deuteration of leucine residues provides probes for following the influence of ligands with different efficacies. Three of the four largest NMR chemical shift differences (> 0.4 ppm) in 1H-15N solution NMR spectra that occur upon ligand-binding are found for Leu5.51, Leu6.46, and Leu6.49. Leu5.51 is adjacent to Pro5.50 of the PIF motif, while Leu6.46 and Leu6.49 are in the region of conserved Trp6.48 in the CWxP motif. The intensities of the NMR resonances are sensitive to molecular motions. Strikingly, the intensity of the Leu6.46 1H-15N resonance decreases dramatically with the full agonists isoproterenol and formoterol compared to ligands that were either partial agonists or antagonists, consistent with agonist-induced mobility of this region.
Different variations of aromatic packing interactions triggering activation are observed in many of the ligand activated GPCRs. While the amine receptor subfamily, which includes the β2-adrenergic receptor, has a conserved phenylalanine at position 6.51, the neuropeptide Y receptors have a leucine at this position. In these receptors, the last residue of the neuropeptide Y agonist is a tyrosine which is believed to snake down into the ligand-binding pocket and pack against Trp6.48 [40, 48], thereby triggering activation.
Polar residues in switch 2 are coupled to conformational changes of Trp6.48
Polar residues are present in both the packing clusters and activation switches. Asn1.50 on helix H1 is the most conserved residue in the class A GPCRs [13]. The Asn side chain hydrogen-bonds with Asp2.50, the most conserved residue on H2 and a central residue in switch 2 [13] and can form a water-mediated hydrogen-bond with Tyr7.53 (see below). Switch 2 is characterized by a network of hydrogen-bonded residues that stretch from the indole NH of Trp6.48 to Asn1.50. A cross section through the TM core highlights the residues in switch 2 in rhodopsin (Figure 4A). Along with Asn1.50 and Asp2.50, switch 2 includes Asn7.49 of the conserved NPxxY sequence. Structural water molecules mediate interactions between Asn7.49, Asn1.50, Asp2.50, and Trp6.48 [49]. Early studies on rhodopsin found that activation weakens hydrogen-bonding of the Trp6.48 indole NH [47] and strengthens hydrogen-bonding of Asp2.50 [50]. The rearrangement of the hydrogen-bonding interactions in switch 2 is accompanied in rhodopsin by a large change in the position and interactions of Tyr7.48 and Tyr7.53 upon activation (Figure 4C,D).
Figure 4. Polar residues serve both structural and functional roles in the TM core.
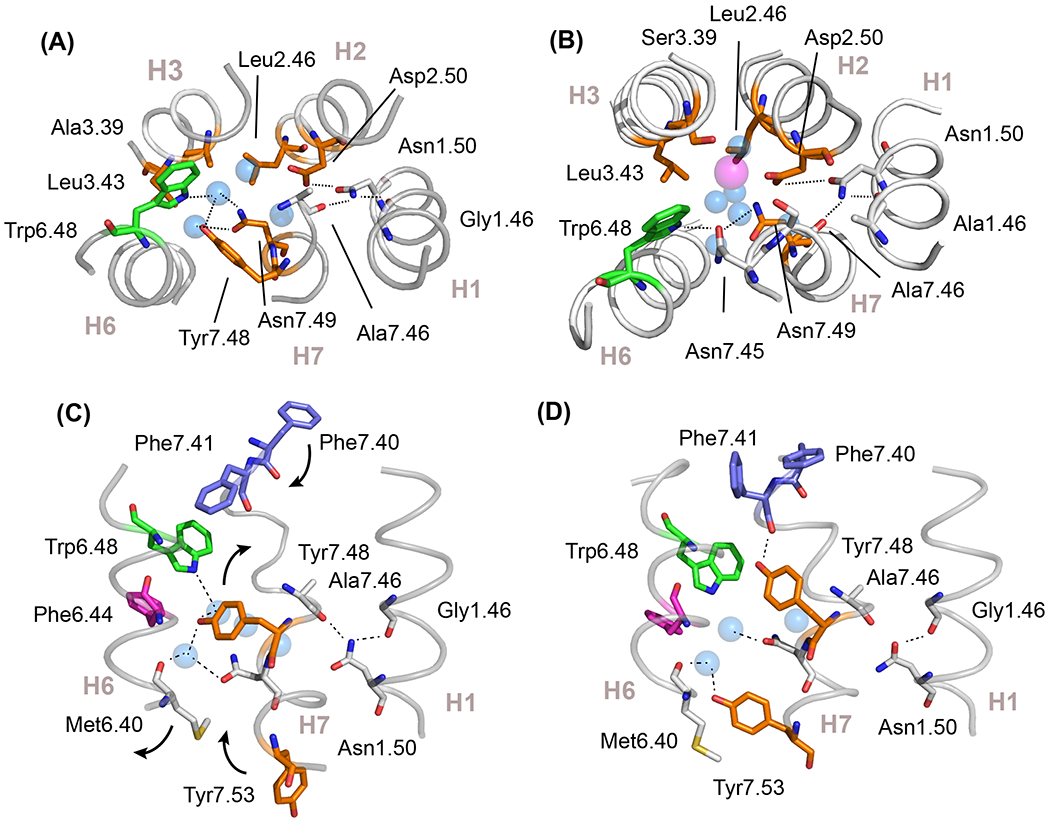
Cross section of activation switch 2 in rhodopsin (A) (PDB-ID 1GZM) and in the adenosine A2a receptor (B) (PDB-ID 4EIY) showing the location of key conserved residues and structural waters (blue spheres). In the A2a receptor, activation switch 2 includes a bound Na+ ion (magenta sphere) that functions as a negative allosteric modulator. The hydrogen-bonding network in switch 2 stretches from the indole NH of Trp6.48 to the backbone C=O of Ala7.46. The Ala7.46 C=O, along with the backbone Gly1.46 C=O, serve structural roles by orienting the side chain of Asn1.50, the most conserved residue in the class A GPCRs. The residue hydrogen bonding with the indole NH of Trp6.48 is subfamily specific; it is Asn7.45 in the nucleotide subfamily and Tyr7.48 in the visual receptor subfamily. (C,D) Activation leads to a rearrangement of the hydrogen-bonding network within switch 2. Panels (C) and (D) show the inactive (PDB-ID 1GZM) and active (PDB-ID 3PQR) states of rhodopsin, respectively. Release of constraints on Trp6.48 allows Tyr7.48 to form a new hydrogen bond with the backbone C=O of Phe7.41, stabilizing the H7 helix. The outward rotation of H6 allows Tyr7.53 to rotate inward and pack in the position vacated by Met6.40. While Tyr7.53 forms hydrophobic packing interactions with residues in the TM core, it also hydrogen bonds with the structural water coordinated by Met6.40.
In many of the other ligand-activated receptors, the hydrogen-bonding network comprising switch 2 accommodates a Na+ ion, which acts as a negative allosteric modulator [51] (Figure 4B). Agonist binding leads to release of the sodium and conversion to an active receptor conformation [51]. Recent NMR studies on the adenosine A2a receptor, which targeted the tryptophan indole 15N-1H resonances, revealed coupling between the ligand-binding site, Trp6.48, and Asp2.50 [41]. In these studies, chemical shift changes in Trp6.48 correlated with ligand efficacy and were influenced by mutation of Asp2.50 to asparagine, a substitution which completely abolishes G protein activation. Together these studies show that the polar network in switch 2 constrains the H6 proline hinge in an inactive conformation, and may contribute to constraining Tyr7.53 in an inactive conformation as well [49].
Small group-conserved residues mediate the H1-H4 scaffold
The conserved polar, aromatic, and proline residues garner the most attention in studies focused on understanding the mechanism of GPCR activation. However, residues with small sidechains (Gly, Ala, Ser, Cys) that have high sequence identity or high homology (i.e. group-conserved) are the glue that stabilizes helix-helix contacts in both the core and the intracellular region of GPCRs.
The TM helices in membrane proteins typically tilt relative to neighboring helices to provide better van der Waals contact between the sidechains and/or the peptide backbone [52, 53]. Two common membrane protein packing motifs are leucine-zipper-like motifs and GxxxG-like motifs [54]. The GxxxG-like motifs involve small (group-conserved) residues (e.g. Gly, Ala, Ser, Cys) at the interface where neighboring helices cross [54, 55]. Small residues at the cross-points allow close helix association, which in turn facilitates interhelical hydrogen-bond formation between polar groups.
Group-conserved residues with small sidechains mediate tight packing of helices H1 - H2 and helices H3 - H4 within the TM core [15]. In packing cluster 1, Ala2.47 on H2 is part of a conserved S/NLAxAD motif and is at the crossing point with H1. Close helix packing allows Asn1.50 to hydrogen-bond with Asp2.50 (Figure 5A). In packing cluster 2, small residues are conserved at positions 4.53 and 4.57 at the H3-H4 crossing point [15]. These residues allow Trp4.50 to pack against a conserved small residue at position 3.38 and to hydrogen-bond with a polar amino acid, typically Asn or Ser, at position 2.45 (Figure 5A, B) [15]. The Trp4.50 interactions that bridge helices H2, H3, and H4 appear to stabilize the overall structural scaffold [15]. NMR studies on rhodopsin [47] and the neuropeptide Y2 receptor [40] show that Trp4.50 does not undergo changes in chemical shift upon activation consistent with a stable packing network.
Figure 5. Small residues mediate helix-helix interactions.
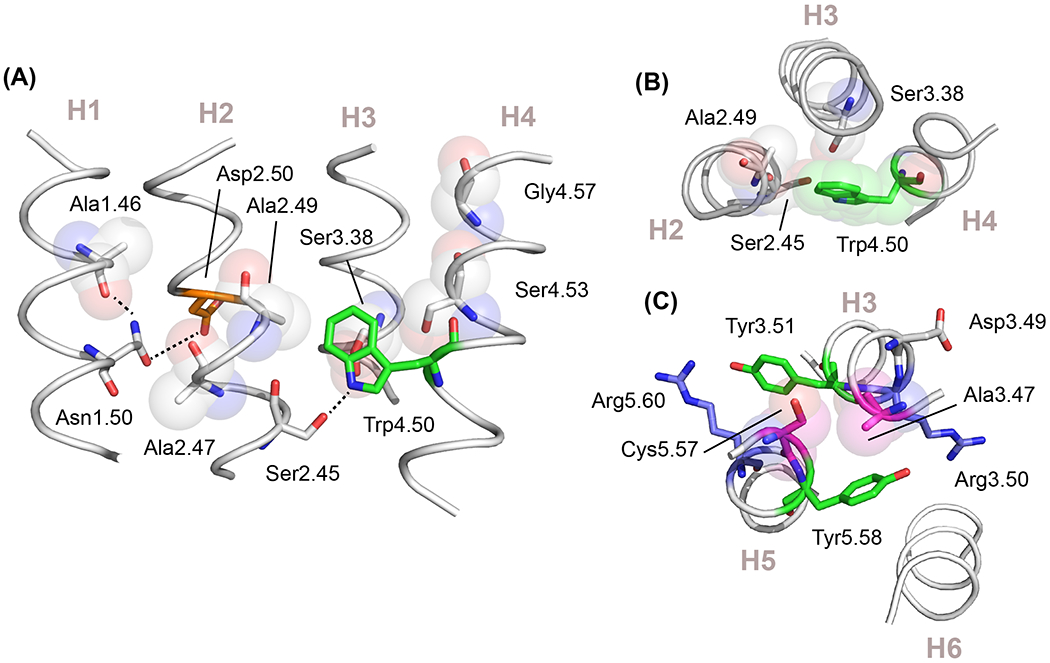
(A) TM helices H1 to H4 form a structural scaffold mediated by small side-chain amino acids that are conserved across the class A GPCRs. These residues occur at helix-crossing angles and facilitate interhelical hydrogen-bonding. The structure of H1-H4 of the adenosine A2a receptor (PDB-ID 4EIY) is shown. Ala1.46 and Ala2.47 contribute to packing cluster 1 and allow interhelical hydrogen-bonding of Asn1.50 and Asp2.50. Gly4.57 and Ser4.53 are group-conserved small residues at the crossing angle of H3-H4 in packing cluster 2. The geometry of these two helices allow Trp4.50 to form an interhelical hydrogen-bond with Ser2.45. (B) View from the extracellular surface of the adenosine A2a receptor showing the packing of Trp4.50 onto residue 3.38, which is a small side chain group-conserved site in the class A GPCRs. (C) Conserved small residues mediate packing interactions of H3 and H5. Although not in the TM core, Ala3.47 and Cys5.57 are residues with conserved small sidechains that play an important role in both structure and function. The structure shown is of the neuropeptide Y receptor (PDB-ID 7DDZ). Ala3.47 has a high conservation as either an alanine or serine. It packs against residue 5.57 and serves to orient the side chain of Tyr5.58 toward Arg3.50 of the conserved E/DRY sequence. This packing geometry orients Tyr3.51 toward the surrounding lipids where it often interacts with a polar side chain.
There are also a number of key contact points that are mediated by small residues outside of the TM core and are essential for stabilizing receptor structure. Figure 5C highlights the packing interactions between Ala3.47 and Cys5.57 at the intracellular crossing point of helices H3 and H5 in the neuropeptide Y2 receptor. Position 3.47 has the highest level of conservation as a residue with a small side chain in the class A (99%) and forms a notch for orienting Tyr5.58 in active state GPCRs. In the neuropeptide Y2 receptor, this active-like orientation is observed for the inactive apo protein and NMR studies indicate that this region does not change upon activation [40]. This stable interaction involving small residue packing at the H3-H5 crossing point in inactive and active receptors implies that the rotation of the intracellular end of H5 is not a component of transmembrane signaling in class A GPCRs.
Hydrophobic residues underlie the conversion from active to inactive receptors
The model that arises from the NMR studies described above for receptor activation focuses on the release of constraints on the proline hinge and Trp6.48 by ligand-binding. There is evidence for multiple conformational states involving H6 with a predominant conformation often observed in active state crystal structures [33, 39]. The last category of conserved residues in the TM core that has not been discussed encompasses the large hydrophobic residues, which appear to stabilize the active state conformation of H6.
Conserved hydrophobic residues occur in both switch 1 and switch 2. In switch 1, Phe6.44 and Ile3.40 were discussed above in connection with the PIF motif [45, 56]. Motion of Trp6.48 changes the packing interactions with Phe6.44, which repacks against Pro5.50 and Ile3.40 in the active conformation (Figure 6A, B) [45, 56]. In switch 2, there are three hydrophobic residues at positions 2.46, 3.43, and 6.40 that lie below the polar hydrogen-bonding network and change packing interactions upon activation. Leu2.46 is one of the most highly conserved residues in the class A GPCRs [13]. This residue is part of the S/NLAxAD motif on TM helix H2 and has contacts with H3, H6, and H7 (Figure 6C, D). Following conversion to an active conformation, the sidechains of Leu2.46 and Leu3.43 provide the surface for packing of Tyr7.53 of the conserved NPxxY motif [19]. The hydrophobic packing of Tyr7.53 is further stabilized by conserved water-mediated hydrogen-bonds [49, 57], although hydrophobic interactions appear to dominate [58].
Figure 6. Hydrophobic residue packing in active conformations of switch 1 and 2.
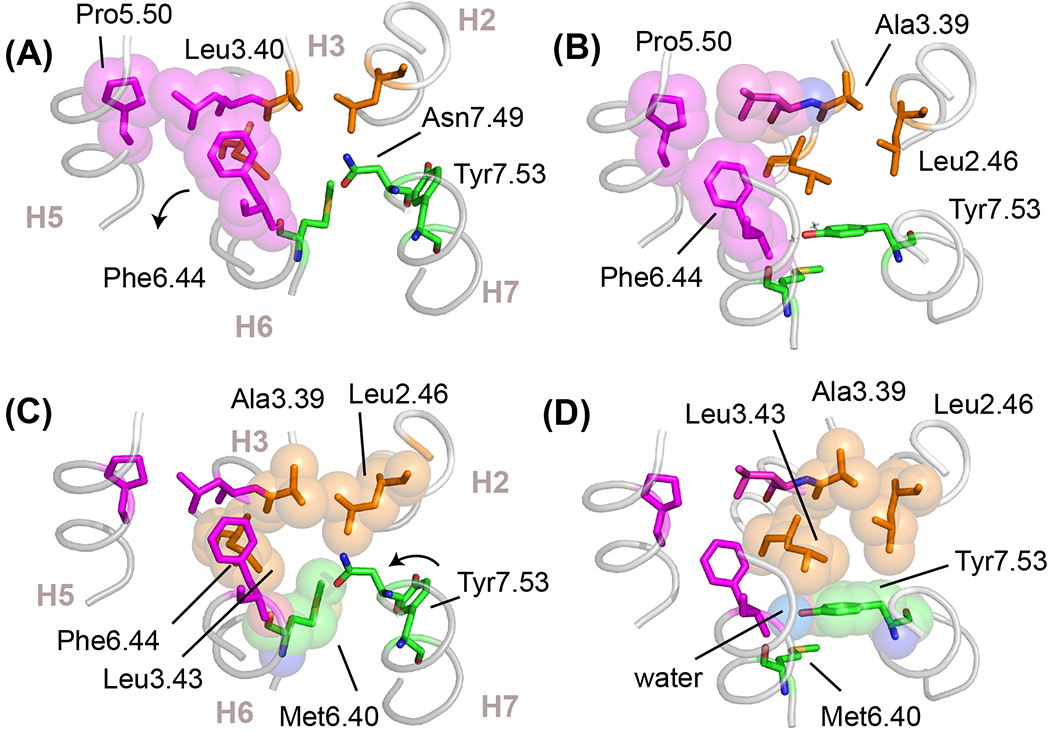
(A,C) Structure of the inactive state of rhodopsin (PDB-ID 1GZM) highlighting hydrophobic packing interactions in switch 1 and switch 2, shown in magenta and orange spheres, respectively. In the inactive state, Phe6.44 in switch 1 stacks with Trp6.48 (see Figure 2B) and Met6.40 in switch 2 packs against Leu2.46, Leu3.43 and Asn7.49. Position 6.40 varies in different subfamilies, but is often found (>50%) as a valine or isoleucine. In rhodopsin, this hydrophobic interaction stabilizes the inactive conformation [59]. (B,D) Structure of the active state of rhodopsin (PDB-ID 3PQR) showing the changes in hydrophobic packing interactions. In switch 1, Phe6.44 rotates to form a packing contact with Pro5.50. In switch 2, the outward rotation of H6 disrupts the packing contact formed between Met6.40 and the other residues in switch 2. The conserved Tyr7.53 of the NPxxY motif swings up and packs into the space vacated by Met6.40 and packs against Leu2.46 and Leu3.43. The Cζ-OH of Tyr7.53 forms a water-mediated hydrogen bond with a conserved water molecule that bridges the backbone C=O residues of Met6.40 and Leu3.43. Water is shown as a blue van-der Waals sphere in panel (D).
Leu3.43 along with conserved hydrophobic residues at positions 6.40 and 6.41 form what has been termed a hydrophobic lock [11]. The importance of the hydrophobic lock is highlighted in the visual receptor rhodopsin where residue 6.40 is a methionine. Mutation of Met6.40 to any other residue except leucine results in constitutive receptor activity and allows the apoprotein opsin to be activated by adding the “agonist” all-trans retinal [59]. That is, a single mutation at position 6.40 effectively converts rhodopsin from a light-activated to a ligand-activated receptor. Methionine has generally been found to contribute to ligand-binding and modulate the orientation of key conserved aromatic residues during activation [60].
Conserved water-mediated interactions in class A GPCRs
Conserved water molecules within the TM core of GPCRs are often overlooked as key elements that stabilize the inactive and/or active-state conformations. By comparing the positions of water molecules in crystal structures with molecular dynamics (MD) simulations, Dror and colleagues [49] describe a number of conserved water-mediated interactions in the TM core. Stable water molecules are observed associated with the kink in H6, between Asp1.50 and Asn2.50, and between Asn7.45 and Asn7.49 of the NPxxY motif. There are also conserved waters that are dependent on the conformation. Tyr7.53 can interact with waters associated with Asn1.50 and Asp2.50 in the inactive conformation, but forms water-meditated interactions with Tyr5.58, Met6.40 and Leu3.43 in the active conformation (Figure 4D and 6D).
Insights on the TM core from visual receptors and olfactory receptors
Within the class A receptors, perhaps the two most unusual subfamilies are the visual and olfactory receptors. The visual receptors have a covalently linked ligand and are designed to have extremely low basal activity in the inactive state [13]. As a result, mutations that result in activity of the dark-state receptor or mutations that shift the equilibrium between active and inactive conformations are revealing in terms of the mechanism of activation. Conversely, the olfactory receptors respond to numerous small, volatile ligands [61]. They are often excluded from comparative studies across the class A receptors since they lack several of the well-known motifs associated with the two activation switches.
The apo-protein opsin and rhodopsin both exhibit low (~1%) basal activity [59]. There are two loci of mutations that result in constitutive activity and/or dark activity (with 11-cis retinal bound). The first locus involves mutations that release constraints on the position of Trp6.48. Rhodopsin formed with retinal lacking the C20 methyl group exhibits increased dark activity [62], consistent with its packing against Trp6.48. The Trp6.48 indole ring is sandwiched between two residues that have small sidechains: Gly3.36 and Ala7.42 (Figure 3B, C). Residues with larger sidechains at these positions lead to activation, presumably by steric interactions that disrupt its interaction with Tyr7.48 in switch 2 [38, 63, 64]. Moreover, deprotonation of the PSB linkage is required for activation and mutations that neutralize or alter the environment of the PSB counterion (Glu3.28) impart constitutive activity. For example, the E3.28Q mutation is constitutively activity (23%), which is attributed to a change in the position of Tyr6.51 and release of packing constraints on Trp6.48 [59]. The second locus involves mutations that destabilize H6 in the inactive state and/or stabilize H6 in the active state. The M6.40Y mutation discussed in the previous section results in ~33% constitutive activity [59]. Combining the E3.28Q/M6.40Y mutations results in over 80% activity [59], consistent with the idea that there are two switches required for rhodopsin activation [36]. The first switch releases constraints on the Pro6.50 hinge, while the second switch involves the repacking of hydrophobic residues in both switch 1 and switch 2.
The olfactory receptors are members of the class A GPCRs on the basis of the conservation of many of the motifs described above [61]. Nevertheless, these receptors largely lack the CWxP and PIF motifs that are associated with the two activation switches. The absence of these well-known motifs emphasizes the key roles played by those residues and motifs that are conserved. First, the conserved residues forming the H1-H4 scaffold are retained in the olfactory receptors. These receptors contain either tryptophan or tyrosine at position 4.50. They also retain Asn1.50 on H1 and small residues at positions 2.47, 2.49, 3.38, 4.53, and 4.57. Ikegami et al. [65] found that mutation of residue 4.53 resulted in endoplasmic reticulum (ER) retention of the Olfr539 olfactory receptor, which they attributed to loss of receptor stability.
While the olfactory receptors largely lack Pro5.50 on H5, on the intracellular side of the receptor they retain Ser5.57 and Tyr5.58 on H5, as well as Ala3.47 and the DRY sequence on H3. The conservation of these residues indicates that the packing interactions involving H3 and H5 described in Figure 5C are conserved. The NPxxY sequence on H7 is also conserved along with Leu2.46 on H2. Together, these conserved residues argue that H6 in the activated state of these receptors is stabilized by the same interactions in the TM core and intracellular region as the other class A receptors.
Two residues that are highly conserved only in the TM core of the olfactory receptor subfamily are a glutamate at position 3.39 and a histidine at position 6.40 [66]. The glutamate replaces a serine residue in switch 2 often observed to coordinate Na+ in many ligand-activated GPCRs. The histidine is at a position similar to Met6.40 in rhodopsin. The H6.40A mutation results in loss of function [67]. This alignment places a conserved tyrosine at position 6.48, which has been found to be sensitive to odorant binding [67, 68]. The conservation of the Glu-His pair argues for electrostatic interactions within switch 2 as the trigger for activation, and as in rhodopsin suggests that switch 2 is the primary activation switch in the olfactory receptors.
Concluding remarks
Recent studies that probe class A GPCR dynamics using NMR spectroscopy shed light on the role of the conserved residues in the TM core. Decomposing the conserved core into different residue types is used here to describe a common mechanism of TM signaling shared across the class A receptors. Overall, the TM core appears to have three functions. The first function is to guide the folding and stability of helices H1-H4. Small group-conserved and polar residues on these helices act together to create a structural scaffold on which helices H5, H6, and H7 pack. The second function is to control flexibility of the Pro6.50 hinge. Release of constraints on the position of Trp6.48 allows H6 to adopt a range of orientations and conformations. There may be several intermediate structures in the pathways between inactive and active conformations [32, 39, 69] and the energy landscape reflected in these structures is dependent on the nature of the bound ligand [70–73]. The third function is to stabilize H6 in an active conformation via repacking of hydrophobic residues in switch 1 and switch 2.
Nevertheless, there remain open questions (see Outstanding questions) about the structure and function of the TM core. Many of these questions involve how the unique ligands associated with different class A subfamilies couple ligand-binding to the release of constraints within the core. A second set of questions involves the nature of local dynamics within the core and global dynamics of the TM helices along the activation pathway. The answers to these questions will reveal how the simple seven-TM framework has given rise to the largest and most diverse receptor family.
Outstanding Questions.
How do residues that are conserved within individual subfamilies impart variations on the common mechanism of GPCR signaling?
What is the nature of the intermediate states that lie between stable inactive and active crystal structure conformations?
How do local and global dynamics change in the absence of bound ligands or G proteins?
How does the transmembrane domain couple extracellular ligand binding to intracellular conformational changes in the other GPCR classes?
Importantly, the deconstruction of the TM core in class A GPCRs provides a framework for dissecting the core of other GPCR classes. For example, the TM core of class B receptors appears to retain residues that mediate the stability of an H1-H4 scaffold. In these receptors a highly conserved tryptophan (Trp4.50) on H4 packs against a conserved small side chain on H3 and hydrogen-bonds with a conserved asparagine residue on H2, similar to that in the class A receptors. The class B receptors also have a conserved proline on helix H6 that is oriented outward such that the backbone carbonyls associated with this proline are not able to form interhelical hydrogen-bonds. Activation results in unraveling of the helix and a large outward motion of its intracellular end [8, 74, 75]. The mechanism of stabilizing H6 in an active orientation, however, is different than in the class A receptors. In class B, the conserved Pro6.47-xx-Gly6.50 motif on H6 rotates inward allowing stabilizing hydrogen-bonds to form between the free backbone C=O groups and polar residues on helices H3, H5 and H7.
Highlights.
G protein coupled receptors (GPRCs) have a transmembrane (TM) core that converts extracellular signals into one or more intracellular signals.
Decomposition of the conserved TM core of class A GPCRs in terms of different residue types suggests that the core has three functions: to form a structural scaffold, to modulate the dynamics of the Pro6.50 hinge, and to stabilize the inactive and active conformations of helix 6 (H6).
Analysis of the conserved residues in the olfactory receptors provides insights into the essential functions of the TM core in these receptors and how different class A subfamilies have evolved to accommodate their unique ligands.
Understanding the function of the TM core of class A receptors provides a foundation for analyzing other GPCR classes.
Acknowledgements.
This work was supported by a grant from the National Institutes of Health (GM-129012).
Glossary
- Ballesteros-Weinstein number convention
Generalized numbering scheme where the number 50 is given for the most conserved amino acid in a TM helix. Pro6.50 indicates that proline is the most conserved residue on helix H6 in class A GPCRs, whereas the designation Trp6.48 indicates that tryptophan has the highest conservation at a position two residues toward the N-terminus from Pro6.50.
- Electron paramagnetic resonance spectroscopy
Magnetic resonance technique using spin labels (with unpaired electrons) to measure distances and dynamics. This method is most often applied to GPCRs having native or substituted cysteine residues modified with spin labels.
- G protein-coupled receptors
Seven transmembrane helix receptors that bind extracellular ligands and activate intracellular heterotrimeric G proteins or β-arrestins. There are over 800 unique human GPCRs that are involved in a wide range of physiological processes.
- NMR chemical shift
Resonant frequency of an NMR-active nucleus relative to a standard reference compound. 13C, 15N and 1H are common NMR active nuclei. The chemical shift is sensitive to chemical structure and the surrounding chemical environment (e.g. electrostatic and hydrogen bonding interactions).
- NMR resonance
Absorption band in an NMR spectrum. The position of the NMR resonance is defined by the NMR chemical shift. The width and intensity of the resonance are sensitive to molecular motion and can be used as probes of local dynamics.
- Solid-state NMR spectroscopy
NMR methods that target molecules with slow or no molecular motions. GPCRs are large membrane proteins whose overall dynamics are not ideal for high resolution solution spectroscopy. Magic angle spinning is a solid-state NMR technique in which the sample is mechanically spun at high frequencies to yield high resolution NMR spectra of GPCRs or GPCR complexes.
- Solution NMR spectroscopy
NMR methods that target molecules with rapid molecular motions. Solution NMR provides atomic resolution information of both GPCR structure and dynamics. Selective deuteration of amino acids or specific solution NMR techniques (such as TROSY) provide approaches for obtaining high resolution NMR spectra of GPCRs.
- TM core
A highly conserved region that spans 2-3 helical turns within the center of the seven TM bundle of class A GPCRs. The TM core lies between the extracellular ligand binding site and the intracellular G protein binding pocket and plays an important role in both receptor structure and function.
- Visual receptors
A subfamily of class A GPCRs that bind the 11-cis isomer of retinal (vitamin A). Humans have four different visual receptors. Rhodopsin is found in rod cells and is responsible for dim-light vision. There are three additional visual receptors found in cone cells that are responsible for color vision.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erlandson SC et al. (2018) Structural basis for G protein-coupled receptor signaling. Ann. Rev. Biophys 47, 1–18 [DOI] [PubMed] [Google Scholar]
- 2.Weis WI and Kobilka BK (2018) The molecular basis of G protein-coupled receptor activation. Ann. Rev. Biochem 87, 897–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wootten D et al. (2018) Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nature Rev. Molec. Cell Biol 19, 638–653 [DOI] [PubMed] [Google Scholar]
- 4.Lagerström MC and Schiöth HB (2008) Structural diversity of G protein-coupled receptors and significance for drug discovery. Nature Rev. Drug Discovery 7, 339–357 [DOI] [PubMed] [Google Scholar]
- 5.Hauser AS et al. (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nature Rev. Drug Discovery 16, 829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser AS et al. (2018) Pharmacogenomics of GPCR drug targets. Cell 172, 41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredriksson R et al. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol 63, 1256–72 [DOI] [PubMed] [Google Scholar]
- 8.Hilger D et al. (2020) Structural insights into differences in G protein activation by family A and family B GPCRs. Science 369, 523–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulte G and Kozielewicz P (2020) Structural insight into Class F receptors - What have we learnt regarding agonist-induced activation? Basic Clin. Pharmacol. Toxicol 126, 17–24 [DOI] [PubMed] [Google Scholar]
- 10.Deupi X and Standfuss J (2011) Structural insights into agonist-induced activation of G-protein-coupled receptors. Curr. Opin. Struct. Biol 21, 541–551 [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q et al. (2019) Common activation mechanism of class A GPCRs. eLife 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madabushi S et al. (2004) Evolutionary trace of G protein-coupled receptors reveals clusters of residues that determine global and class-specific functions. J. Biol. Chem 279, 8126–8132 [DOI] [PubMed] [Google Scholar]
- 13.Smith SO (2010) Structure and activation of the visual pigment rhodopsin. Ann. Rev. Biophys 39, 309–328 [DOI] [PubMed] [Google Scholar]
- 14.Venkatakrishnan AJ et al. (2013) Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194 [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Reyes OB et al. (2017) G protein-coupled receptors contain two conserved packing clusters. Biophys. J 112, 2315–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palczewski K et al. (2000) Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 17.Farrens DL et al. (1996) Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274, 768–770 [DOI] [PubMed] [Google Scholar]
- 18.Li J et al. (2004) Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol 343, 1409–1438 [DOI] [PubMed] [Google Scholar]
- 19.Park JH et al. (2008) Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 454, 183–187 [DOI] [PubMed] [Google Scholar]
- 20.Scheerer P et al. (2008) Crystal structure of opsin in its G-protein-interacting conformation. Nature 455, 497–502 [DOI] [PubMed] [Google Scholar]
- 21.Granier S and Kobilka B (2012) A new era of GPCR structural and chemical biology. Nat. Chem. Biol 8, 670–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katritch V et al. (2013) Structure-function of the G protein-coupled receptor superfamily. Ann. Rev. Pharm. Tox 53, 531–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang KH et al. (2014) Structure of the human P2Y(12) receptor in complex with an antithrombotic drug. Nature 509, 115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng Y et al. (2018) 5-HT2C receptor structures reveal the structural basis of GPCR polypharmacology. Cell 172, 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li XT et al. (2019) Crystal structure of the human cannabinoid receptor CB2. Cell 176, 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J et al. (2020) Determination of the melanocortin-4 receptor structure identifies Ca2+ as a cofactor for ligand binding. Science 368, 428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehl A et al. (2018) Structure of the mu-opioid receptor-G(i) protein complex. Nature 558, 547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda S et al. (2019) Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 364, 552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spoerri PM et al. (2019) Conformational plasticity of human protease-activated receptor 1 upon antagonist- and agonist-binding. Structure 27, 1517–1526 [DOI] [PubMed] [Google Scholar]
- 30.Huang WJ et al. (2020) Structural insights into mu-opioid receptor activation. Nature 584, 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang WJ et al. (2020) Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579, 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manglik A et al. (2015) Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 161, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latorraca NR et al. (2017) GPCR dynamics: structures in motion. Chem. Rev 117, 139–155 [DOI] [PubMed] [Google Scholar]
- 34.Hilger D (2021) The role of structural dynamics in GPCR-mediated signaling. FEBS J 288, 2461–2489 [DOI] [PubMed] [Google Scholar]
- 35.Lans I et al. (2015) Helix 3 acts as a conformational hinge in class A GPCR activation: An analysis of interhelical interaction energies in crystal structures. J. Struct. Biol 192, 545–553 [DOI] [PubMed] [Google Scholar]
- 36.Kimata N et al. (2016) Retinal orientation and interactions in rhodopsin reveal a two-stage trigger mechanism for activation. Nat. Commun 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimata N et al. (2016) Free backbone carbonyls mediate rhodopsin activation. Nat. Struct. Mol. Biol 23, 738–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pope AL et al. (2020) A conserved proline hnge mediates helix dynamics and activation of rhodopsin. Structure 28, 1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imai S et al. (2020) Structural equilibrium underlying ligand-dependent activation of beta(2)-adrenoreceptor. Nat. Chem. Biol 16, 430–439 [DOI] [PubMed] [Google Scholar]
- 40.Krug U et al. (2020) The conformational equilibrium of the neuropeptide Y2 receptor in bilayer membranes. Angew. Chem.-Int. Edit 59, 23854–23861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eddy MT et al. (2018) Allosteric coupling of drug binding and Intracellular signaling in the A(2A) adenosine receptor. Cell 172, 68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White SH and Wimley WC (1999) Membrane protein folding and stability: Physical principles. Annu. Rev. Biophys. Biomol. Struct 28, 319–365 [DOI] [PubMed] [Google Scholar]
- 43.Sansom MSP and Weinstein H (2000) Hinges, swivels and switches: The role of prolines in signalling via transmembrane α-helices. Trends Pharmacol. Sci 21, 445–451 [DOI] [PubMed] [Google Scholar]
- 44.Shi L et al. (2002) β2 adrenergic receptor activation - Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J. Biol. Chem 277, 40989–40996 [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen SGF et al. (2011) Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torrice MM et al. (2009) Probing the role of the cation-π interaction in the binding sites of GPCRs using unnatural amino acids. Proc. Natl. Acad. Sci. U.S.A 106, 11919–11924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel AB et al. (2005) Changes in interhelical hydrogen bonding upon rhodopsin activation. J. Mol. Biol 347, 803–812 [DOI] [PubMed] [Google Scholar]
- 48.Kaiser A et al. (2015) Unwinding of the C-terminal residues of neuropeptide Y is critical for Y-2 receptor binding and activation. Angew. Chem.-Int. Edit 54, 7446–7449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venkatakrishnan AJ et al. (2019) Diverse GPCRs exhibit conserved water networks for stabilization and activation. Proc. Natl. Acad. Sci. U.S.A 116, 3288–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fahmy K et al. (1993) Protonation states of membrane-embedded carboxylic acid groups in rhodopsin and metarhodopsin II: A Fourier-transform infrared spectroscopy study of site-directed mutants. Proc. Natl. Acad. Sci. U.S.A 90, 10206–10210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katritch V et al. (2014) Allosteric sodium in class A GPCR signaling. Trends Biochem. Sci 39, 233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowie JU (1997) Helix packing angle preferences. Nat. Struct. Biol 4, 915–917 [DOI] [PubMed] [Google Scholar]
- 53.Lee SY and Chirikjian GS (2004) Interhelical angle and distance preferences in globular proteins. Biophys. J 86, 1105–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eilers M et al. (2002) Comparison of helix interactions in membrane and soluble α-bundle proteins. Biophys. J 82, 2720–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eilers M et al. (2000) Internal packing of helical membrane proteins. Proc. Natl. Acad. Sci. U.S.A 97, 5796–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobilka BK (2011) Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol. Sci 32, 213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ragnarsson L et al. (2019) Mutations in the NPxxY motif stabilize pharmacologically distinct conformational states of the α(1B)- and β(2)-adrenoceptors. Sci. Signal 12, 1–12 [DOI] [PubMed] [Google Scholar]
- 58.Gabilondo AM et al. (1996) Mutations of Tyr326 in the β2-adrenoceptor disrupt multiple receptor functions. Eur. J. Pharmacol 307, 243–250 [DOI] [PubMed] [Google Scholar]
- 59.Han M et al. (1998) Constitutive activation of opsin by mutation of methionine 257 on transmembrane helix 6. Biochemistry 37, 8253–8261 [DOI] [PubMed] [Google Scholar]
- 60.Cordomi A et al. (2013) Sulfur-containing amino acids in 7TMRs: molecular gears for pharmacology and function. Trends Pharmacol. Sci 34, 320–331 [DOI] [PubMed] [Google Scholar]
- 61.Buck L and Axel R (1991) A novel multigene family may encode odorant receptors - a molecular-basis for odor recognition. Cell 65, 175–187 [DOI] [PubMed] [Google Scholar]
- 62.Ebrey T et al. (1980) Light activation of bovine rod phosphodiesterase by non-physiological visual pigments. FEBS Lett. 116, 217–219 [DOI] [PubMed] [Google Scholar]
- 63.Han M et al. (1996) The effects of amino acid replacements of glycine 121 on transmembrane helix 3 of rhodopsin. J. Biol. Chem 271, 32330–32336 [DOI] [PubMed] [Google Scholar]
- 64.Han M et al. (1997) The C9 methyl group of retinal interacts with glycine-121 in rhodopsin. Proc. Natl. Acad. Sci. U.S.A 94, 13442–13447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikegami K et al. (2020) Structural instability and divergence from conserved residues underlie intracellular retention of mammalian odorant receptors. Proc. Natl. Acad. Sci. U.S.A 117, 2957–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bushdid C et al. (2019) Mammalian class I odorant receptors exhibit a conserved vestibular-binding pocket. Cell. Mol. Life Sci 76, 995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de March CA et al. (2018) Odorant receptor 7D4 activation dynamics. Angew. Chem.-Int. Edit 57, 4554–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bushdid C et al. (2018) Agonists of G-protein-coupled odorant receptors are predicted from chemical features. J. Phys. Chem. Lett 9, 2235–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dror RO et al. (2011) Activation mechanism of the β2-adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A 108, 18684–18689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seifert R et al. (2001) Functional differences between full and partial agonists: evidence for ligand-specific receptor conformations. J. Pharm. Exp. Thera 297, 1218–1226 [PubMed] [Google Scholar]
- 71.Ghanouni P et al. (2001) Functionally different agonists induce distinct conformations in the G protein coupling domain of the β2 adrenergic receptor. J Biol. Chem 276, 24433–24436 [DOI] [PubMed] [Google Scholar]
- 72.Deupi X and Kobilka BK (2010) Energy landscapes as a tool to integrate GPCR structure, dynamics, and function. Physiology 25, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casiraghi M et al. (2019) NMR analysis of GPCR conformational landscapes and dynamics. Mol. Cell. Endocrinol 484, 69–77 [DOI] [PubMed] [Google Scholar]
- 74.Liang YL et al. (2018) Cryo-EM structure of the active, G(s)-protein complexed, human CGRP receptor. Nature 561, 492–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang YL et al. (2018) Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature 555, 121–125 [DOI] [PubMed] [Google Scholar]
- 76.Goncalves JA et al. (2010) Highly conserved tyrosine stabilizes the active state of rhodopsin. Proc. Natl. Acad. Sci. U.S.A 107, 19861–19866 [DOI] [PMC free article] [PubMed] [Google Scholar]


