Abstract
Background and Aims
Locoregional therapies, including yttrium‐90 radioembolization, play an important role in the treatment of unresectable HCC. The aim of the LEGACY (Local radioEmbolization using Glass Microspheres for the Assessment of Tumor Control with Y‐90) study was to evaluate objective response rate (ORR) and duration of response (DoR) in patients with solitary unresectable HCC treated with yttrium‐90 glass microspheres.
Approach and Results
LEGACY is a multicenter, single‐arm, retrospective study conducted at three sites that included all eligible, consecutive patients with HCC treated with radioembolization between 2014 and 2017. Eligibility criteria included solitary HCC ≤ 8 cm, Child‐Pugh A cirrhosis, and Eastern Cooperative Oncology Group performance status 0‐1. Primary endpoints were ORR and DoR based on modified Response Evaluation Criteria in Solid Tumors in the treated area (localized), as evaluated by blinded, independent, central review. Radioembolization was performed with intent of ablative‐level dosimetry in a selective fashion when possible. Overall survival was evaluated using Kaplan‐Meier and multivariate Cox proportional hazards. Among the 162 patients included, 60.5% were Eastern Cooperative Oncology Group 0, and the median tumor size was 2.7 cm (range: 1‐8) according to blinded, independent, central review. Radioembolization served as neoadjuvant therapy for transplantation or resection in 21.0% (34 of 162) and 6.8% (11 of 162) of patients, respectively, and as primary treatment for all others. Median follow‐up time was 29.9 months by reverse Kaplan‐Meier. ORR (best response) was 88.3% (CI: 82.4‐92.4), with 62.2% (CI: 54.1‐69.8) exhibiting a DoR ≥ 6 months. Three‐year overall survival was 86.6% for all patients and 92.8% for those neoadjuvant patients with resected or transplanted liver.
Conclusions
In this multicenter study of radioembolization, clinical meaningful response rates and prolonged DoR were observed in the treatment of unresectable, solitary HCC ≤ 8 cm.
Abbreviations
- AE
adverse event
- BCLC
Barcelona Clinic Liver Cancer
- BICR
blinded, independent, central review
- CR
complete response
- DoR
duration of response
- ECOG
Eastern Cooperative Oncology Group
- LEGACY
Local radioEmbolization using Glass Microspheres for the Assessment of Tumor Control with Y‐90
- LT
liver transplantation
- mRECIST
modified Response Evaluation Criteria in Solid Tumors
- NE
not evaluable
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SAE
serious adverse event
- TTP
time‐to‐progression
- 90Y
yttrium‐90
Patients with solitary HCC have several curative treatment options, including liver transplantation (LT), surgical resection, and thermal ablation; however, many patients are not candidates due to tumor size, location, or comorbidities. Although LT may be the most ideal option, many patients require bridging due to prolonged wait times, or downstaging to achieve Milan criteria. Surgical resection is considered curative in patients with solitary tumor, normal bilirubin, and absence of portal hypertension; many patients do not meet these criteria. Thermal ablation is also considered potentially curative, but is limited to small tumors in favorable locations.( 1 )
Although radioembolization has traditionally been used to treat advanced HCC, recent refinements in technique have shown promising response rates in solitary HCC.( 2 ) Rather than using a conventional strategy of lobar yttrium‐90 (90Y) infusion, selective radioembolization of the tumor‐bearing hepatic segment, termed “radiation segmentectomy,” spares most of the noninvolved hepatic parenchyma. With a segmental treatment, higher radiation doses can be safely delivered, translating to robust and consistent response rates.( 3 , 4 , 5 , 6 , 7 ) The duration of response (DoR), however, a concept thoroughly investigated with immunotherapies, is understudied in radioembolization. DoR takes into account objective response and provides information relating to the time component and durability of the response, variables of significant interest to patients and clinicians. Recently, DoR has served as a key parameter leading to regulatory approval of several immunotherapy agents.
The purpose of this retrospective, multicenter study was to assess the response rates and DoR following treatment with 90Y glass microspheres in patients with unresectable, solitary HCC.
Materials and Methods
LEGACY (Local radioEmbolization using Glass Microspheres for the Assessment of Tumor Control with Y‐90) is a retrospective, single‐arm, multicenter HCC study conducted across three US sites. The study was approved by each site’s institutional review board. Consecutively treated patients with HCC who underwent radioembolization using 90Y glass microspheres between January 1, 2014, and December 31, 2017, were evaluated for study enrollment. Inclusion criteria included age ≥ 18 years, a solitary tumor with largest diameter ≤ 8 cm, treatment with lobar or selective hepatic radioembolization, Child‐Pugh A cirrhosis, and Eastern Cooperative Oncology Group (ECOG) performance score 0‐1. Exclusion criteria included prior LT or resection, prior locoregional or systemic therapy, vascular invasion, extrahepatic metastases, clinically significant ascites, or hepatic encephalopathy. The Food and Drug Administration (FDA) agreed with key parameters of the LEGACY protocol, which served as the basis for Premarket Approval (PMA) of TheraSphere (Boston Scientific Corp., Marlborough, MA) for local tumor control of unresectable, solitary HCC < 8 cm.
Treatment
All patients underwent radioembolization using TheraSphere glass microspheres. This was performed according to standard practices, which included a mapping angiogram with lung shunt fraction determination followed by 90Y microsphere infusion. During the mapping procedure, selective hepatic angiography and C‐arm CT were performed to determine tumor arterial supply. Technetium macroaggregated albumin was infused into the tumor‐bearing lobe, and the lung shunt fraction was determined. Perfused volume was measured retrospectively using either the treatment cone beam CT or post–Y90 single‐proton emission computerized tomography/CT. The absorbed dose to the perfused volume was then calculated based on the infused Y90 activity and the perfused volume. This is standard unicompartment dosimetry and is consistent with the package insert.
Data Collection
Data were abstracted from medical records by study team members at each site and entered into the electronic Clinical Report Form within the main study database; sites were monitored on‐site periodically to ensure accuracy of data entry. Collected patient data included demographics, medical history, disease characteristics (etiology, Barcelona Clinic Liver Cancer [BCLC] stage, Child‐Pugh status), clinical assessments (ECOG performance status, ascites, encephalopathy), laboratory assessments (bilirubin, albumin, international normalized ratio, alpha‐fetoprotein, hematology panel [white blood cell count, hemoglobin, platelet count]), and tumor assessment and liver volume estimation based on diagnostic imaging. Serious adverse events (SAEs) and nonserious adverse events (AEs) within 60 days following treatment were recorded; only those possibly related to treatment or procedure were collected thereafter (through 12 months). AE severity was graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 5.0.( 8 ) Whether the SAEs and AEs were possibly or probably related to device use or administration was determined by each site investigator.
Response Assessment
Imaging follow‐up was performed per standard of care guidelines every 2‐3 months. Radiologic images were reviewed and evaluated using localized modified Response Evaluation Criteria in Solid Tumors (mRECIST) and RECIST 1.1 criteria by a blinded, independent, central review (BICR). Localized mRECIST was defined as the response/progression within the radioembolization‐treated region. mRECIST and RECIST assessed the treated segment for response, but also included new nodules and extrahepatic metastases as progression. The BICR consisted of two independent radiologists who assessed responses on follow‐up imaging, blinded to visit sequence for the primary read. Discordance was adjudicated by a third independent reviewer. To compensate for the limitations inherent in a retrospective analysis, the BICR reads were performed blinded to dosing data, clinical outcomes, and subsequent HCC treatments.
Outcomes
There were co‐primary endpoints. In agreement with the FDA, the study was considered successful if both of the following criteria were met: a lower limit of the 95% CI for confirmed objective response rate (ORR) by localized mRECIST > 40% and, DoR by localized mRECIST > 6 months for ≥ 60% of responders. Best and confirmed ORRs were reported, the latter declared when a subsequent scan ≥ 4 weeks later confirmed the initial response.( 9 ) Secondary endpoints included ORR/DoR by mRECIST/RECIST 1.1, time to progression (TTP), progression‐free survival (PFS), and overall survival (OS).( 10 )
Statistical Analysis
For the hypothesized 50% localized mRECIST ORR with 90Y, 100 patients would be required to achieve a CI of 40%‐60%; it would narrow to 43%‐57% with 200 patients based on the method of Wilson.( 11 ) Hence, a minimum sample size of 100 was targeted. Baseline data were analyzed using descriptive statistics. ORR was summarized as counts and percentages, with CIs. DoR was computed as the time between the first localized tumor response and progressive disease (PD) observed. If PD was not observed, the DoR was computed using the date of the last imaging assessment before any further treatment was administered within the treatment area. DoR was summarized using descriptive statistics, and time‐to‐endpoint analyses were performed using Kaplan‐Meier.( 12 ) Incidence of AEs and SAEs were reported as counts and percentages and coded according to the Medical Dictionary for Regulatory Activities.( 13 )
Results
Baseline Characteristics
A total of 162 patients were enrolled (Fig. 1). Patient characteristics are listed in Table 1. The median age was 66 (range: 21‐90); most were male (75.9%, 123 of 162), afflicted hepatitis C (69.1%, 112 of 162), and BCLC A (60.5%, 98 of 162). Cirrhosis was present in 92.0% (149 of 162). On the basis of cancer‐related symptoms/pain, 39.5% were ECOG 1 and staged as BCLC C. The median tumor size was 2.7 cm (range: 1.0‐8.1 cm), according to BICR.
FIG. 1.
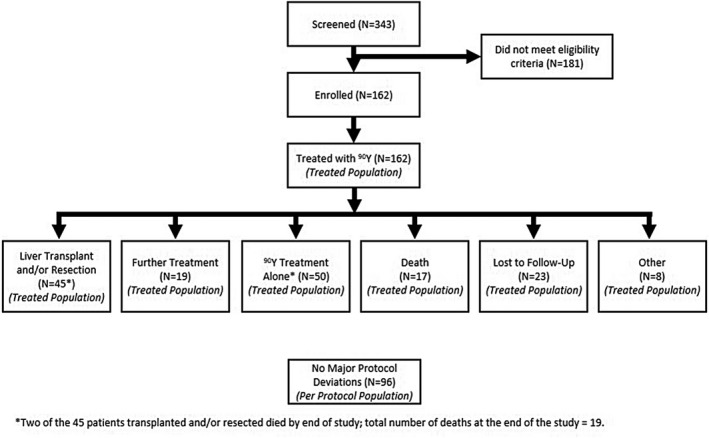
Flow diagram of patient study status.
TABLE 1.
Baseline Characteristics
| Treated Population (n = 162), n (%) | ||
|---|---|---|
| Age | 18‐64 | 69 (42.6%) |
| 65‐74 | 64 (39.5%) | |
| ≥75 | 29 (17.9%) | |
| Gender | Male | 123 (75.9%) |
| Female | 39 (24.1%) | |
| Race | White | 80 (49.4%) |
| Black or African‐American | 16 (9.9%) | |
| Asian | 13 (8.0%) | |
| Native American, Alaska Native | 2 (1.2%) | |
| Native Hawaiian, Pacific Islander | 2 (1.2%) | |
| Not reported | 49 (30.2%) | |
| HCC etiology | HBV | 15 (9.3) |
| HCV | 112 (69.1%) | |
| NASH | 23 (14.2%) | |
| Autoimmune disease | 3 (1.9%) | |
| Alcohol | 48 (29.6%) | |
| Unknown | 4 (2.5%) | |
| Other | 1 (0.6%) | |
| Tumor size | <3 cm | 100 (61.7%) |
| 3‐5 cm | 50 (30.9%) | |
| >5‐8 cm | 9 (5.6%) | |
| Missing | 3 (1.9%) | |
| BCLC/ECOG score | BCLC A (ECOG 0) | 98 (60.5%) |
| BCLC C (ECOG 1) | 64 (39.5%) | |
| Child‐Pugh score | A5 | 108 (66.7%) |
| A6 | 54 (33.3%) |
Treatment Characteristics
Treatment intent for initial radioembolization included definitive tumor treatment (66.7%, 108 of 162), bridging to transplantation or surgery (24.7%, 40 of 162), and other/unknown (8.6%, 14 of 162) (Table 2). Most patients received selective infusions (95.7%, 155 of 162); 1.9% (3 of 162) received lobar segmental infusions; and 2.5% (4 of 162) received mixed treatments. The median absorbed dose to the treated liver volume was 410.1 Gy (interquartile range: 199.7, 797.7).
TABLE 2.
Y‐90 Treatment Characteristics
| Treated Population (n = 162), n (%) | ||
|---|---|---|
| Treatment approach and goal | Radiation segmentectomy | 95 (58.6%) |
| Radiation lobectomy | 4 (2.5%) | |
| Downsizing to surgery | 4 (2.5%) | |
| Bridge to LT | 36 (22.2%) | |
| Treat to local tumor control | 9 (5.6%) | |
| Other | 1 (0.6%) | |
| Unknown | 13 (8.0%) | |
| Lung shunt fraction mean (SD), median [IQR] | 4.5, (3.4), | |
| 4.0 [2.3, 5.] | ||
| Total number of vials administered (first treatment) | 1 | 118 (72.8%) |
| 2 | 38 (23.5%) | |
| 3 | 6 (3.7%) | |
| Type of infusion | Selective | 155 (95.7%) |
| Lobar | 3 (1.9%) | |
| Mixed | 4 (2.5%) | |
| Absorbed dose to treated liver volume (Gy), mean (SD), median [IQR] (n = 155) | 578.6 (540.1), | |
| 410 [199.7, 797.7] | ||
| Number of 90Y treatments | 1 | 130 (80.2%) |
| ≥ 2 | 32 (19.8%) |
Abbreviation: IQR, interquartile range.
ORR and DoR
Localized mRECIST
The best ORR and confirmed ORRs were 88.3% (CI: 82.4‐92.4) and 72.2% (CI: 64.9‐78.5), respectively, with a median time‐to‐best confirmed response of 3.9 months (CI: 3.5‐4.1) (Table 3 and Fig. 2). There were no local progressors by BICR. Median DoR for confirmed response was 11.8 months, with 76.1% exhibiting DoR ≥ 6 months. Figure 3 shows a swimmer’s plot detailing imaging/response/transplant/resection status, and last follow‐up, by confirmed response. Figure 4 and Supporting Fig. S1 shows a spider plot detailing percent change from baseline in target lesion by localized mRECIST (confirmed response).
TABLE 3.
Response to Y‐90 Treatment
| Localized mRECIST, n (%) | mRECIST, n (%) | RECIST 1.1, n (%) | |
|---|---|---|---|
| ORR, confirmed response, n (%) [95% CI] | 117 (72.2%) [64.9%, 78.5%] | 111 (68.5%) [61.0%, 75.2%] | 75 (46.3%) [38.8%, 54.0%] |
| ORR, best response, n (%) [95% CI] | 143 (88.3%) [82.4%, 92.4%] | 140 (86.4%) [80.3%, 90.9%] | 102 (63.0%) [55.3%, 70.0%] |
| Best overall response | |||
| CR | 136 (84%) | 133 (82.1%) | 13 (8.0%) |
| PR | 7 (4.3%) | 7 (4.3%) | 89 (54.9%) |
| Stable disease | 0 | 0 | 38 (23.5%) |
| PD | 0 | 3 (1.9%) | 3 (1.9%) |
| NE | 19 (11.7%) | 19 (11.7%) | 19 (11.7%) |
| No imaging assessments after day 46 | 5 (3.1%) | 5 (3.1%) | 5 (3.1%) |
| No imaging assessments after day 46 due to LT or resection | 9 (5.6%) | 9 (5.6%) | 9 (5.6%) |
| Other reasons | 5 (3.1%) | 5 (3.1%) | 5 (3.1%) |
| DoR* in months, mean (SD), median | 15.1 (11.2), 11.8 | 14.0 (10.5), 10.6 | 13.8 (10.4), 10.6 |
| DoR* ≥ 6 months, n (%) [95% CI] | 89 (76.1%) [67.6%, 82.9%] | 83 (74.8%) [66.0%, 81.9%] | 54 (72%) [61.0%, 80.9%] |
DoR based the number of confirmed responders by BICR (n = 117 for localized mRECIST, n = 111 for mRECIST, and n = 75 for RECIST.
FIG. 2.
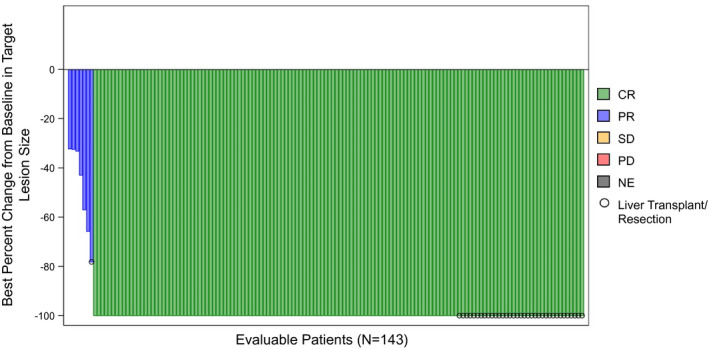
Waterfall plot of greatest decrease in target lesion size and best overall tumor response by localized mRECIST among evaluable patients in the LEGACY study (n = 143). Abbreviations: CR, complete response; NE, not evaluable; PR, partial response; SD, stable disease.
FIG. 3.
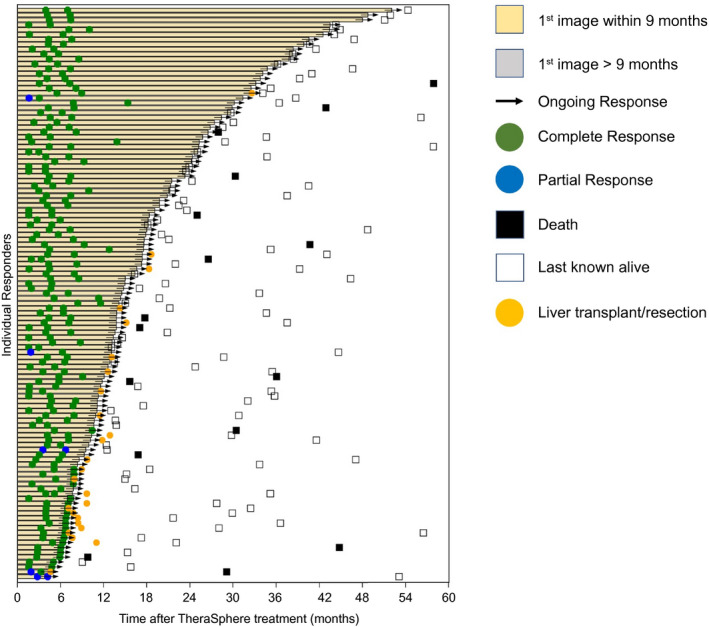
Swimmer plot of TTR and DoR by localized mRECIST (confirmed response).
FIG. 4.
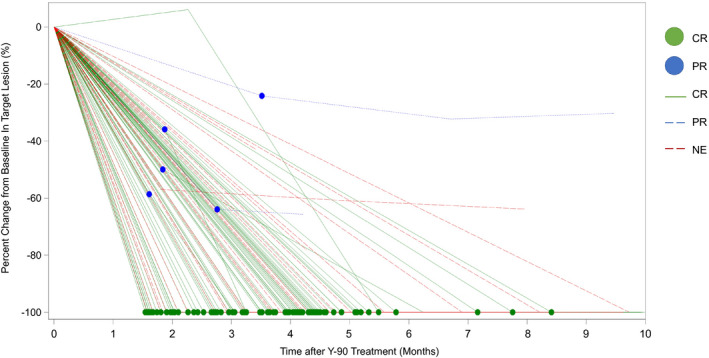
Detailed spider plot of percent change from baseline in target lesion by localized mRECIST (confirmed response).
mRECIST
The best ORR and confirmed ORRs were 86.4% (CI: 80.3‐90.9) and 68.5% (CI: 61.0‐75.2), respectively. The summary statistics median DoR for confirmed response was 10.6 months, with 74.8% exhibiting DoR ≥ 6 months.
RECIST 1.1
The best ORR and confirmed ORRs were 63.0% (CI: 55.3‐70.0) and 46.3% (CI: 38.8‐54.0), respectively. The summary statistics median DoR for confirmed response was 10.6 months, with 72% exhibiting DoR ≥ 6 months.
BCLC A patients exhibited a best ORR of 89.8% (CI: 82.2‐94.4), with 65.9% (CI: 55.5‐75.0) exhibiting a DoR of ≥ 6 months. BCLC C patients exhibited a best ORR of 85.9% (CI: 75.4‐92.4), with 56.4% (CI: 43.3‐68.6) exhibiting a DoR of ≥ 6 months.
TTP and PFS
Median TTP by BICR was not reached.
Localized mRECIST
At 24 months, the local progression rate was 0, and PFS rate was 93.9%. Median PFS was 57.9% (CI: 40.7, 57.9).
mRECIST/RECIST 1.1
A total of 84.1% (CI: 72.6‐91.0) and 82.0% (CI:71.6‐88.9) experienced no progression at 24 months, respectively (Fig. 5A). PFS rates were 78.8% and 76.7%, respectively (Fig. 5B). Median PFS was 40.7 (CI: 36.0, not evaluable [NE]) for both mRECIST and RECIST.
FIG. 5.
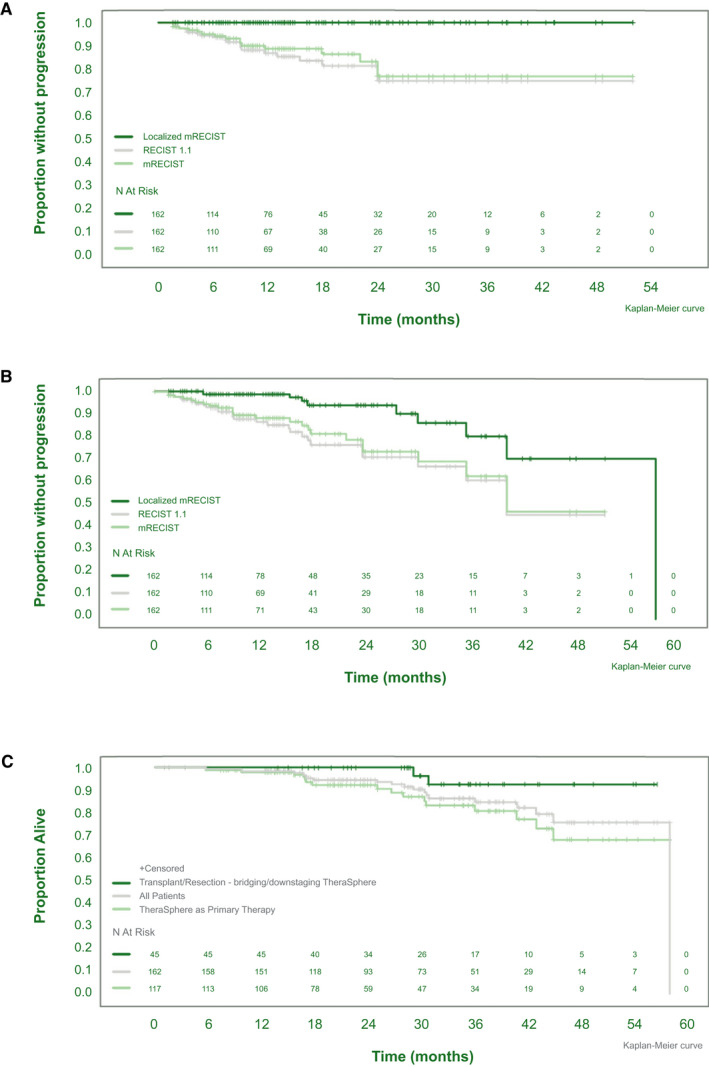
Kaplan‐Meier analysis of TTP (A), PFS (B), and OS (C) by transplantation/resection status.
OS
Median OS was reached (57.9; 95% CI: NE, NE). For the entire group, OS was 94.8% (CI: 89.5‐97.5) and 86.6% (CI: 78.2‐92.0) at 24 and 36 months, respectively (Fig. 5C). Thirteen patients had >36 months of follow‐up, up to 52 months. The 45 patients who went on to LT or resection exhibited an OS of 100.0% (CI: 100.0‐100.0) and 92.8% (CI: 74.2‐98.2) at 24 and 36 months, respectively (Fig. 5C).
ECOG and Milan Criteria
Using a complete case analysis, performance status was maintained or improved in 69 of 77 (89.6%), 41 of 47 (87.2%), and 14 of 17 (82.4%) of patients at 6, 12 and 24 months, respectively. Milan criteria was maintained in 116 of 122 (95.1%), 77 of 86 (89.5%), and 29 of 34 (85.3%) at 6, 12 and 24 months, respectively.
Safety Outcomes
Grade 3 events occurred in 31 of 162 patients (19.1%), including anemia (n = 1); gastrointestinal disorders (n = 7); fatigue (n = 1); pyrexia (n = 1); hepatobiliary disorders (ascites [n = 3], gallbladder obstruction [n = 1], and portal vein thrombosis [n = 1]); infection (n = 4); increase in bilirubin (n = 3) and international normalized ratio (n = 2); decrease in lymphocyte count (n = 12), platelets (n = 1), and white blood cells (n = 1); hypoalbuminemia (n = 1); musculoskeletal and connective tissue disorders (n = 2); nervous system disorder (n = 1); dyspnea (n = 1); and pulmonary embolism (n = 1). Note that the number of AEs adds up to > 31 as patients could have > 1 AE. Grade 4 (worsened platelet count) and grade 5 (cerebrovascular accident) AEs occurred in 1 patient each; neither were related to radioembolization. Most AEs resolved during the course of the study period. SAEs occurred in 9.9% of patients; 5.6% were at least possibly related to radioembolization. No patient experienced radiation‐induced liver disease. There were 19 deaths (11.7%), 12 of which were attributable to nontarget disease progression, and none were related to treatment. There was one death in the transplant group (multisystem organ failure at 3 years), and one in the resection group (HCC recurrence at 3 years).
Discussion
Over the last few years, there has been an increasing role for immunotherapy in the management of HCC, with several studies demonstrating antitumoral activity translating to ORR and prolonged DoR. While these observations have mostly been reported for systemic therapies, there is a dearth of such data in the locoregional therapy (LRT) space. Radioembolization with 90Y is an LRT applied to HCC across all stages, including the curative setting in BCLC A, downstaging and bridging in the transplant patient, and palliation in BCLC B/C.( 2 , 14 , 15 , 16 ) LEGACY is a study that investigates ORR/DoR in the homogenous patient population as defined by solitary HCC < 8 cm with preserved performance status. The study was considered positive, surpassed the clinically meaningful co‐primary endpoint thresholds, and establishing 90Y as clinically efficacious in this patient population.( 17 , 18 )
The ORR observed suggests that focal tumors amenable to catheterization respond well to arterially delivered 90Y radiation. Although localized mRECIST and mRECIST best responses replicate prior less‐controlled investigations, the strength of the observation is reinforced with the confirmatory scan.( 19 ) LEGACY is the first to report on 90Y confirmatory response as reported by a BICR. The local control of 100% by mRECIST also imparts radiation segmentectomy the status of “ablative treatment,” with progressive disease manifesting solely as new distant lesions or metastases. Furthermore, in conjunction with an elevated response rate, those responses were noted to be durable, translating to a prolonged clinical patient benefit.
PFS by localized mRECIST was 93.9% at 2 years. Local tumor recurrence rates were low and competitive to outcomes of thermal ablation.( 2 , 20 , 21 , 22 ) Prolonged TTP in the treated tumor area is critical for patients being bridged to LT, given the mandated 6‐month waiting period.( 20 , 23 , 24 , 25 , 26 ) In LEGACY, the high rates of tumor response achieved using radioembolization allowed bridging to LT and/or resection in 45 patients. The remaining patients who did not receive a LT/resection exhibited OS similar to other curative‐intent treatments.( 2 , 27 , 28 )
Advancements in the understanding of administration technique, optimal dosing, and the segmental approach has led to changes in practice with radioembolization. The LEGACY study included patients treated using the strategy of high‐dose radiation delivery to the tumor, with the goal of improved tumor response; median absorbed dose to the treated liver volume was 410 Gy. In fact, in a subset dose‐pathology correlation of resection/transplantation, LEGACY patients exhibited complete pathological necrosis when their dose exceeded 400 Gy to the tumor‐bearing tissue, establishing this as the new “threshold dose” for an ablative effect.( 21 ) This approach is supported by a phase 2 study, in which investigators determined a significant difference in tumor dose of responders versus nonresponders (490 Gy vs. 275 Gy).( 29 ) Another prospective trial using photon emission tomography to determine tumor dose after radioembolization also showed that responders had a higher median dose to the tumor compared with nonresponders (225 gray vs. 83 gray).( 30 ) Finally, in DOSISPHERE, a prospective randomized trial, the median OS survival was more than doubled, from 10.7 to 26.6 months, when personalized multicompartment dosimetry was applied. The amalgamation of these findings further points to personalized and optimized dosimetry representing the next area of clinical research in 90Y.
Limitations include the retrospective design, lack of a control arm, and limited sample size compared with systemic therapy trials. Strengths include the multicenter nature, long‐term, comprehensive follow‐up, and strict independent monitoring/auditing. Interestingly, the outcomes were similar across the study institutions. Also, all images were reviewed using a BICR, blinded to treatment information. LEGACY evaluated tumor response using three different criteria (localized mRECIST, mRECIST, and RECIST 1.1), permitting cross‐comparison and providing evaluation of local tumor control, and liver and systemic extent of HCC following 90Y. Localized mRECIST was the designated primary endpoint, given that Y90 is a local treatment. Eighteen percent of the patients enrolled were >75 years of age, in accordance with the contemporary focus on geriatric oncology and inclusion of elderly patients in clinical trials.( 31 ) The focus on DoR is of interest, given its established role and purported benefit in immuno‐oncology therapies. The applicability of a loco‐regional therapy such as 90Y in BCLC C is of particular interest in the context of liver‐limited disease. OS is a critical endpoint that contextualizes the entire patient cohort and was reported in accordance with oncology standards. Although OS could have been considered the primary endpoint, it is well‐known that BCLC‐A studies are challenging, given the long natural history and effective post‐progression treatments; surrogate endpoints are therefore integral to the advancement of HCC therapies in early disease. Finally, the co‐primary endpoints exceeded the hypothesis and generated compelling data that are sufficient for the first PMA of its kind, highlighting a successful collaboration among investigators, industry, and regulatory bodies to advance the field of HCC.
In conclusion, the multicenter LEGACY study demonstrates that radioembolization provides a strong and durable response in solitary unresectable HCC, with ORR and DoR observations that are significant and clinically meaningful. Additionally, OS and PFS compared favorably between patients receiving radioembolization as definitive treatment and those who underwent curative therapies. Radioembolization for solitary HCC is safe and efficacious when used either as neoadjuvant to transplant or resection, or as a stand‐alone treatment.
Author Contributions
All authors performed all of those tasks.
Supporting information
Fig S1
Acknowledgment
The authors thank Binal Patel for the statistical support, Evelyn Schnuerer for the scientific expertise, Joanne MacFie for clinical trial management, and Alexandra J. Greenberg‐Worisek, Ph.D., M.P.H., for medical writing assistance (all from Boston Scientific Corp.). They also acknowledge the efforts of research coordinators Carlene del Castillo (Northwestern), Ellen Weiss (Mount Sinai), and Ann Wilson (University of Washington), in addition to the efforts of Ahmed Gabr, M.D., research fellow (Northwestern).
Supported by Boston Scientific Corporation.
Potential conflict of interest: Dr. Boucher is employed and owns stock in Boston Scientific. Dr. Bishay consults for Boston Scientific. Dr. Padia consults for Boston Scientific and Bristol‐Myers Squibb. He received grants from Varian. Dr. Fowers is employed and owns stock in Boston Scientific. Dr. Riaz consults for Boston Scientific. Dr. Salem consults for Eisai, Boston Scientific, Sirtex, AstraZeneca, Genentech, Cook, Bard, and QED. Dr. Kim consults, advises, and is on the speakers’ bureau for Boston Scientific. He advises Sirtex. Dr. Johnson consults for Boston Scientific, Genentech, and AstraZeneca. Dr. Lewandowski consults for Boston Scientific.
SEE EDITORIAL ON PAGE 2333
References
- 1. Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. EASL Clinical Practice Guidelines management of hepatocellular carcinoma. J Hepatol 2018;69:182‐236. [DOI] [PubMed] [Google Scholar]
- 2. Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology 2018;287:1050‐1058. [DOI] [PubMed] [Google Scholar]
- 3. Padia SA, Kwan SW, Roudsari B, Monsky WL, Coveler A, Harris WP. Superselective yttrium‐90 radioembolization for hepatocellular carcinoma yields high response rates with minimal toxicity. J Vasc Interv Radiol 2014;25:1067‐1073. [DOI] [PubMed] [Google Scholar]
- 4. Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, et al. Segmental Yttrium‐90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single‐center, retrospective, propensity score‐matched study. J Vasc Interv Radiol 2017;28:777‐785.e771. [DOI] [PubMed] [Google Scholar]
- 5. Biederman DM, Titano JJ, Korff RA, Fischman AM, Patel RS, Nowakowski FS, et al. Radiation segmentectomy versus selective chemoembolization in the treatment of early‐stage hepatocellular carcinoma. J Vasc Interv Radiol 2018;29:30‐37.e32. [DOI] [PubMed] [Google Scholar]
- 6. Biederman DM, Titano JJ, Bishay VL, Durrani RJ, Dayan E, Tabori N, et al. Radiation segmentectomy versus TACE combined with microwave ablation for unresectable solitary hepatocellular carcinoma up to 3 cm: a propensity score matching study. Radiology 2017;283:895‐905. [DOI] [PubMed] [Google Scholar]
- 7. Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology‐pathology correlation and survival of radiation segmentectomy. Hepatology 2014;60:192‐201. [DOI] [PubMed] [Google Scholar]
- 8.. Common Terminology Criteria for Adverse Events (CTCAE), Version 5. Bethesda, MD: US Department of Health and Human Services; 2018. [Google Scholar]
- 9. Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol 2011;22:265‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. In: Seminars in Liver Disease. New York, NY: Thieme Medical Publishers; 2010:52‐60. [DOI] [PubMed] [Google Scholar]
- 11. Wilson E. Calculating a confidence interval of a proportion. J Am Stat Assoc 1927;22:209‐212. [Google Scholar]
- 12. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457‐481. [Google Scholar]
- 13. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999;20:109‐117. [DOI] [PubMed] [Google Scholar]
- 14. Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920‐1928. [DOI] [PubMed] [Google Scholar]
- 15. Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000‐patient 15‐year experience. Hepatology 2019;68:1429‐1440. [DOI] [PubMed] [Google Scholar]
- 16. Abouchaleh N, Gabr A, Ali R, Al Asadi A, Mora RA, Kallini JR, et al. (90)Y radioembolization for locally advanced hepatocellular carcinoma with portal vein thrombosis: long‐term outcomes in a 185‐patient cohort. J Nucl Med 2018;59:1042‐1048. [DOI] [PubMed] [Google Scholar]
- 17. Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, et al. REFLECT‐a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol 2020;55:113‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yau T, Kang YK, Kim TY, El‐Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol 2020;6:e204564. 10.1001/jamaoncol.2020.4564. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Memon K, Kulik L, Lewandowski RJ, Wang E, Riaz A, Ryu RK, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology 2011;141:526‐535.e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gabr A, Kulik L, Mouli S, Riaz A, Ali R, Desai K, et al. Liver transplantation following Yttrium‐90 radioembolization: 15‐year experience in 207‐patient cohort. Hepatology 2021;73:998‐1010. [DOI] [PubMed] [Google Scholar]
- 21. Gabr A, Riaz A, Johnson GE, Kim E, Padia S, Lewandowski RJ, et al. Correlation of Y90‐absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging 2021;48:580‐583. [DOI] [PubMed] [Google Scholar]
- 22. Gabr A, Polineni P, Mouli SK, Riaz A, Lewandowski RJ, Salem R. Neoadjuvant radiation lobectomy as an alternative to portal vein embolization in hepatocellular carcinoma. Semin Nucl Med 2019;49:197‐203. 10.1053/j.semnuclmed.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 23. Roberts JP, Venook A, Kerlan R, Yao F. Hepatocellular carcinoma: ablate and wait versus rapid transplantation. Liver Transpl 2010;16:925‐929. [DOI] [PubMed] [Google Scholar]
- 24. Halazun KJ, Patzer RE, Rana AA, Verna EC, Griesemer AD, Parsons RF, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology 2014;60:1957‐1962. [DOI] [PubMed] [Google Scholar]
- 25. Mehta N, Heimbach J, Lee D, Dodge JL, Harnois D, Burns J, et al. Wait time of less than 6 and greater than 18 months predicts hepatocellular carcinoma recurrence after liver transplantation: proposing a wait time “sweet spot”. Transplantation 2017;101:2071‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehta N, Yao FY. What are the optimal liver transplantation criteria for hepatocellular carcinoma? Clin Liv Dis 2019;13:20‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahn KS, Kang KJ. Appropriate treatment modality for solitary small hepatocellular carcinoma: radiofrequency ablation vs. resection vs. transplantation? Clin Mol Hepatol 2019;25:354‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eilard MS, Naredi P, Helmersson M, Hemmingsson O, Isaksson B, Lindell G, et al. Survival and prognostic factors after transplantation, resection and ablation in a national cohort of early hepatocellular carcinoma. HPB 2021;23:394‐403. [DOI] [PubMed] [Google Scholar]
- 29. Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium‐90 radioembolization for intermediate‐advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57:1826‐1837. [DOI] [PubMed] [Google Scholar]
- 30. Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, et al. Prospective trial using internal pair‐production positron emission tomography to establish the Yttrium‐90 radioembolization dose required for response of hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2018;101:358‐365. [DOI] [PubMed] [Google Scholar]
- 31. Swaminathan D, Swaminathan V. Geriatric oncology: problems with under‐treatment within this population. Cancer Biol Med 2015;12:275‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


