Abstract
Members of the integrin family of adhesion receptors mediate both cell-cell and cell-matrix interactions and have been shown to play vital roles in embryonic development, wound healing, metastasis, and other biological processes. The integrin α9β1 is a receptor for the extracellular matrix proteins osteopontin and tenacsin C and the cell surface immunoglobulin vascular cell adhesion molecule-1. This receptor is widely expressed in smooth muscle, hepatocytes, and some epithelia. To examine the in vivo function of α9β1, we have generated mice lacking expression of the α9 subunit. Mice homozygous for a null mutation in the α9 subunit gene appear normal at birth but develop respiratory failure and die between 6 and 12 days of age. The respiratory failure is caused by an accumulation of large volumes of pleural fluid which is rich in triglyceride, cholesterol, and lymphocytes. α9−/− mice also develop edema and lymphocytic infiltration in the chest wall that appears to originate around lymphatics. α9 protein is transiently expressed in the developing thoracic duct at embryonic day 14, but expression is rapidly lost during later stages of development. Our results suggest that the α9 integrin is required for the normal development of the lymphatic system, including the thoracic duct, and that α9 deficiency could be one cause of congenital chylothorax.
Integrins are heterodimeric receptors for extracellular matrix and cell surface counterreceptors, which play important roles in embryonic development, inflammation, wound healing, and tumorigenesis (8, 9, 15). The integrin β1 subunit pairs with at least 12 α subunits, forming the largest subfamily of integrins. Ablation of the β1 gene produces early embryonic lethality (3, 17), and most null mutations described for individual β1-associated α subunits cause severe but individually distinct developmental phenotypes (10).
α9β1 is a member of the β1 family that recognizes tenascin C (23, 26), osteopontin (16, 24), and vasular cell adhesion molecule-1 (VCAM-1) (20) as ligands. Immunostaining of mouse tissue has shown that this integrin is expressed in skeletal and cardiac muscle, visceral smooth muscle, hepatocytes, airway epithelium, squamous epithelium, and choroid plexus epithelium (13, 21). Wound healing experiments performed on mouse corneas (18, 19) suggest that α9 may play a role in corneal epithelial migration and differentiation. In mouse embryos, α9 expression was not detected prior to embryonic day 12.5 (E12.5) (21), suggesting that this integrin is unlikely to play a general role in the earliest stages of tissue morphogenesis.
In vitro experiments demonstrate that α9β1 mediates cell adhesion as well as cell migration on all three known ligands (16, 20, 26). In addition, α9-transfected SW480 cells use α9β1 to proliferate on tenascin-C (25), and these effects are associated with phosphorylation of the integrin signaling intermediates focal adhesion kinase and paxillin and with activation of the mitogen-activated protein kinase isoform, erk2. However, the in vivo function(s) of α9β1 is unknown.
In this study, we have generated mice homozygous for a null mutation of the α9 subunit gene to directly examine the role(s) of α9 integrins in vivo. α9−/− mice were born at the expected Mendelian frequency but developed bilateral chylothorax within 6 to 12 days after birth and died of respiratory failure. The presence of edema and extravascular lymphocytes surrounding the thoracic duct and other lymphatic vessels suggested a defect in lymphatic development. These results suggest that α9β1 plays a critical role in development of the thoracic duct and other lymphatic vessels and that mutations in the α9 subunit gene could be one cause of congenital chylothorax.
MATERIALS AND METHODS
Constructing the mouse α9 targeting vector.
A 240-bp fragment of murine α9 cDNA was amplified from RNA obtained from murine liver using the degenerate integrin α subunit PCR primers A14F and A2AR that we have previously described (2, 14). This fragment was cloned into pBluescript and completely sequenced from both strands. The resultant sequence was used to design the murine α9-specific PCR primers, mα9-1F (5′-TCCTCCTTGTGTGCAGTCGACC-3′) and mα9-3R (5′-TCTTGAATTCTCATCTCTGATCTCAGAA-3′). These primers were used to identify an approximately 80-kb genomic P1 clone (clone 2532; Genome System, Inc., St. Louis, Mo.) containing the mouse α9 gene. A 7.4-kb BglII fragment containing two exons from this clone was used for making a replacement targeting vector. The vector contained a neomycin resistance gene inserted into the first of the two exons in the clone and a thymidine kinase gene at the 5′ end.
Detection of recombinant clones by Southern blotting.
As described previously (7), RF8 embryonic stem (ES) cells were grown in ES complete medium. The targeting vector was linearized at a unique SacII site and electroporated into RF8 ES cells. Selection medium containing G418 plus 1-(2′-deoxy-2′-fluoro-β-d-arabinofuranosyl)-5-iodouracil (FIAU) was used to obtain resistant clones. Individual colonies were screened by Southern blotting. Genomic DNA digested with SacI was blotted with two different probes, one specific for mouse α9 and the other for the neo gene. Only clones with single integration were used for blastocyst injection.
Generation of germ line chimeras.
Chimeras were generated as described by Bradley (1). Targeted ES cells were injected into C57BL/6 blastocysts, and injected embryos were transferred into the uteri of pseudopregnant recipients. Male chimeras were mated with C57BL/6 females. Agouti offspring were tested for the targeted α9 gene by PCR and Southern blotting.
Reverse transcription-PCR (RT-PCR).
Total RNA was extracted from freshly isolated mouse tissue using TRIzol solution (Gibco/BRL) according to the company's recommendations. Single-stranded cDNA was transcribed from RNA using the superscript cDNA synthesis system and random hexamers (Gibco/BRL). PCR was performed with the α9-specific primers mα9-1F and mα9-3R to show that the transcription of α9 was disrupted in α9−/− mice.
Western blot analysis.
Freshly isolated mouse liver was minced and then ultrasonicated in lysis buffer (150 mM NaCl, 50 mM Tris, 1% Triton X-100, 0.1% sodium dodecyl sulfate). The homogenates were centrifuged, and the supernatant was saved. Eighty micrograms of total protein from both α9+/+ and α9−/− lysate was separated on a 7.5% polyacrylamide gel and transferred onto Immobilon membrane (Millipore, Bedford, Mass.) using a Hoefer transfer apparatus. The membrane was blocked with 5% milk overnight at 4°C (or 2 h at room temperature) and blotted with rabbit anti-α9 antiserum 1057, raised against a portion of the cytoplasmic domain of human α9 (13). The membrane was exposed to film after a brief incubation in Luminol (Amersham, Arlington Heights, Ill.).
Histology and immunohistochemistry.
For histology, mouse tissue was fixed in 10% formalin for 48 h and embedded in paraffin. Sections (5 μm thick) were stained with hematoxylin and eosin (H&E). For immunohistochemistry, freshly isolated organs were embedded in OCT and quick frozen in liquid nitrogen. Unfixed sections were air dried for 30 min and washed twice with phosphate-buffered saline (PBS). Sections were blocked for endogenous peroxidase and biotin activities with Peroxoblock solution (Zymed Labs) and an avidin-biotin blocking kit (Vector) at room temperature. After rinsing, sections were preblocked with 3% normal goat serum in PBS for 15 min and then incubated overnight at 4°C (or 1 h at room temperature) in primary antibody. After being rinsed in PBS, sections were incubated in biotin-labeled secondary antibody for 1 h, which was followed by incubation in ABC avidin-peroxidase reagent (Vector Labs) for another hour at room temperature. Chromagen was developed using the DAB Plus kit (Zymed Labs). Finally, sections were dehydrated, cleared, and coverslipped using Permount.
RESULTS
Generation of α9-deficient mice.
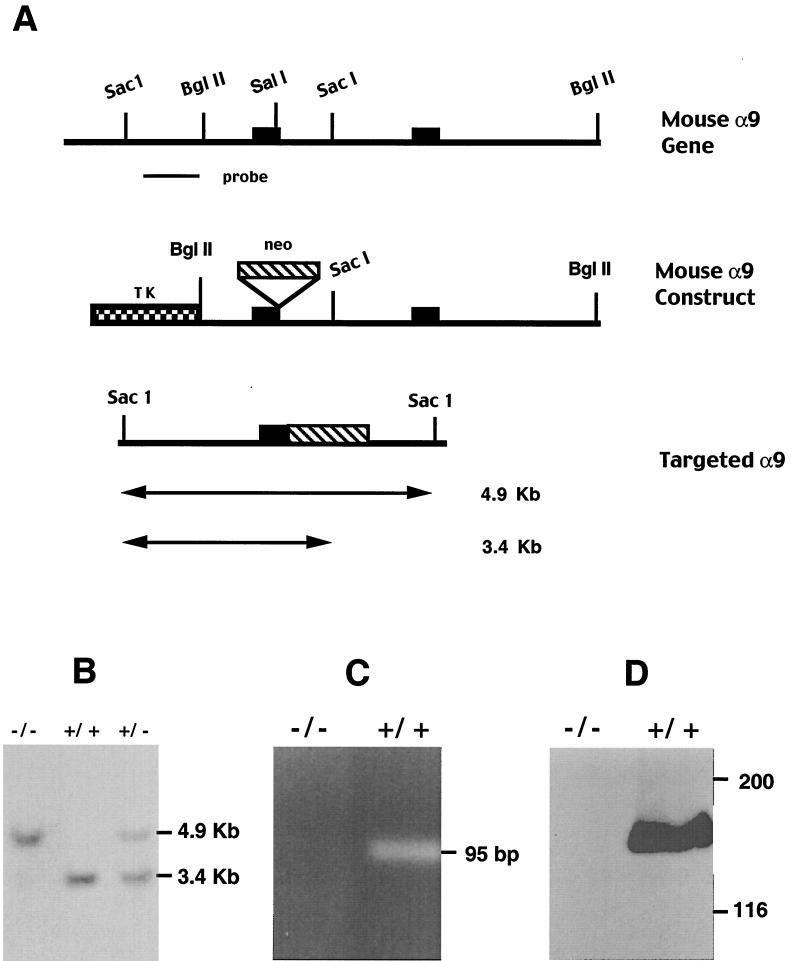
The targeting strategy to inactivate the α9 subunit gene is outlined in Fig. 1A. The linearized construct was electroporated into RF8 embryonic stem cells and the resultant targeted ES cell clones were used to generate α9-deficient mice. Offspring were tested for the targeted α9 gene by Southern blotting (Fig. 1B) and PCR.
FIG. 1.
Disruption of the mouse α9 gene by homologous recombination. (A) Structures of mouse α9 wild-type allele, targeting vector, and targeted allele. Two exons are shown as solid boxes. The expected fragment size after SacI digestion is 3.4 kb for the wild-type allele and 4.9 kb for the targeted allele. (B) Southern blot analysis of genomic DNA from mouse tail digested with SacI and hybridized with the external specific probe of mouse α9 indicated in panel A. (C) RT-PCR analysis of mRNA from α9+/+ and α9−/− mice. Total RNA was extracted from mouse liver and transcribed to complementary DNA (cDNA). A 95-bp fragment was amplified from α9+/+ mouse but not from α9−/− mouse using primers specific for wild-type α9 cDNA. (D) Western blotting of cell lysate of mouse liver with a polyclonal antiserum against α9. A band of the appropriate size to be α9 is demonstrated in α9+/+ but not in α9−/− mice. The positions of molecular mass markers (in kilodaltons) are shown to the right.
α9 mRNA and protein expression were also examined. RT-PCR analysis of RNA derived from mouse liver showed that an expected 95-bp fragment was amplified from the cDNA of α9+/+ mice, but no amplification product was detectable from the cDNA of α9−/− mice (Fig. 1C). To confirm that α9−/− mice did not produce α9 protein, we performed Western blot analysis using a polyclonal antiserum against the cytoplasmic domain of human α9. The α9 antibody detected a band of the appropriate molecular mass to be α9 in α9+/+ mouse liver homogenates; however, no band was detected in α9−/− mouse liver (Fig. 1D).
α9-deficient mice are born alive but develop bilateral chylothorax.
Cross-breeding of heterozygous α9+/− mice gave rise to viable homozygous offspring in the expected Mendelian distribution (α9+/+, 25.5%; α9+/−, 51.8%; α9−/−, 22.7%), indicating that α9 integrins are not essential for intrauterine development. For the first 4 to 6 days after birth, α9−/− mice appeared normal. However, by 6 to 8 days of age the α9−/− mice appeared smaller than control littermates and began to show signs of respiratory distress. The body weights of 6- to 8-day-old α9−/− mice were less than those of wild-type littermates (α9−/−, 4.98 ± 0.43 g; α9+/+, 6.12 ± 0.17 g).
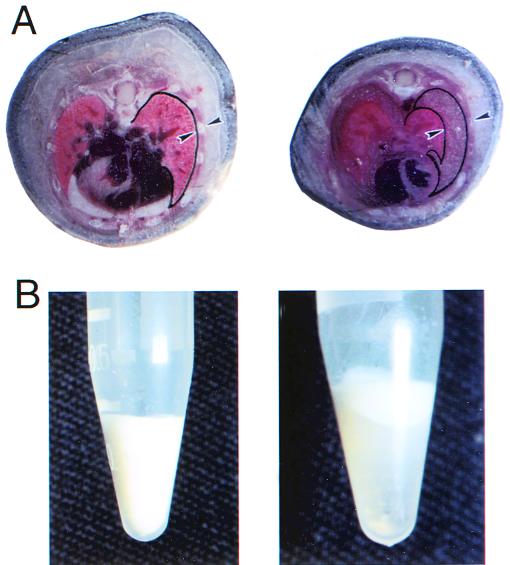
Between 6 and 12 days of age, 100% of α9−/− mice showed signs of respiratory distress and were physically inactive. α9−/− mice died within 2 days of the onset of respiratory distress. To determine the cause of death, the whole mouse body was embedded and frozen in OCT, and cross sections were made. Sections through the thorax of α9−/− mice revealed a marked increase in the space between the outer surface of the lung and the chest wall, and this space was filled with opaque milky fluid (Fig. 2A). This pleural fluid was separated into three layers (Fig. 2B) by centrifugation. Cell differential counts showed more than 90% of the cells in this fluid were lymphocytes. Analysis of pleural fluid demonstrated a very high concentration of both triglycerides and cholesterol, confirming that these mice had developed chylothorax (Table 1).
FIG. 2.
Appearance of pleural effusion in α9−/− mice. (A) Cross section through mouse thorax shows fluid filling an expanded pleural space (demarcated by arrowheads) in an α9−/− mouse compared to the absence of any identifiable space in an α9+/+ littermate. Arrowheads indicate locations of visceral pleura and the external surface of the ribs. (B) Fluid collected from the pleural space of α9−/− mice is milky prior to centrifugation (left) and can be separated into three layers by centrifugation (right): top, white lipid layer; middle, transparent aqueous layer; bottom, cell pellet, typical of chylous effusion.
TABLE 1.
Measurement of cholesterol and tryiglycerides in pleural fluid
| Pleural fluid sample | Cholesterol (mg/dl) | Triglyceride (mg/dl) |
|---|---|---|
| 1 | 380 | 1,956 |
| 2 | 530 | 3,262 |
| 3 | 780 | 1,129 |
To date, from more than 100 α9−/− mice analyzed not a single mouse has survived from either a 129/C57BL/6 mixed or pure 129 inbred genetic background, suggesting that a strain-specific modifier is not the cause of the defect in α9−/− mice. By draining 50 to 200 μl of chyle daily with a syringe, we were able to postpone respiratory failure and extend the life of α9−/− mice up to 21 days, confirming that respiratory failure from chylothorax contributed to their death. However, treated mice failed to gain weight and eventually died, presumably as a consequence of malnutrition.
Because we have previously reported that α9 is expressed in skeletal muscle, visceral smooth muscle, hepatocyte, squamous epithelium, and airway epithelium (13, 21), we analyzed the effects of α9 deficiency on all of the organs in which we have detected α9 expression. However no gross or microscopic abnormalities were found in these tissues.
Lymphatic vessels in α9−/− mice.
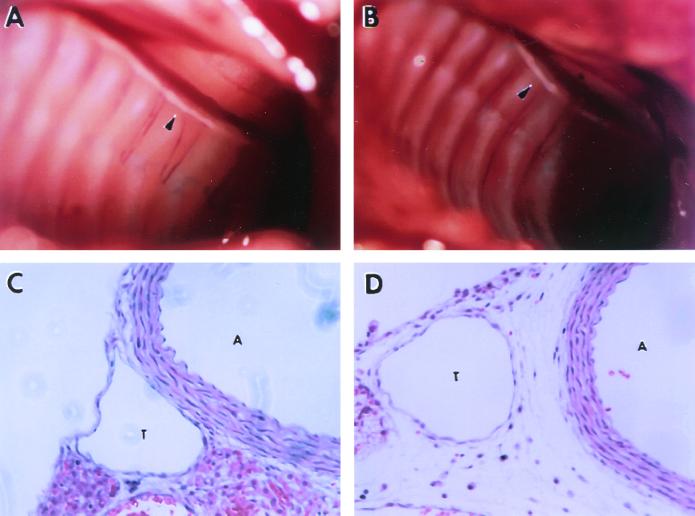
A common cause of chylothorax is leakage of chyle from the thoracic duct. We therefore examined the thoracic duct, grossly and microscopically, in wild-type and α9−/− mice. The thoracic duct was easily visualized in 6- to 10-day-old wild type and α9−/− mice on the basis of its white color, and no gross defects were apparent in α9−/− mice in either the integrity or diameter of this structure (Fig. 3A and B).
FIG. 3.
Anatomy and histology of thoracic duct in α9−/− mouse and a wild-type littermate at 8 days of age. Presented are photographs of the opened thorax of α9+/+ (A) and α9−/− (B) mice, showing the presence of a visible thoracic duct in both groups (the white tubular structure along the spine denoted by arrowheads). (C and D) H&E-stained sections of the region including the thoracic duct (T) and the adjacent aorta (A) in α9+/+ (C) and α9−/− mice (D), demonstrating edema and extravascular lymphocytes surrounding the thoracic duct in α9−/− but not in α9+/+ mice.
Microscopically (Fig. 3C and D), we were able to identify the thoracic duct in H&E-stained sections from both wild-type and α9−/− mice as a thin-walled structure immediately to the right of the posterior portion of the aorta. This structure was composed of a tubular endothelial layer with no luminal red blood cells surrounded by a thin layer of smooth muscle. The microscopic structure of the thoracic duct was not consistently different between α9−/− and α9+/+ mice. However, in several α9−/− mice obvious edema and extravascular lymphocytes were present in the tissue surrounding the thoracic duct, findings that were not observed in sections from wild-type mice.
α9−/− mice also develop edema and inflammatory cell accumulation in the chest wall.
To determine whether the apparent extravasation of fluid from the thoracic duct in α9−/− mice reflects a more generalized defect in lymphatic development, we also examined the morphology of the lymphatics in the chest wall. Sections from the chest wall of α9−/− but not wild-type mice demonstrated numerous areas of tissue edema and lymphocyte accumulation, and extravascular lymphocytes were most prominent in close proximity to lymphatic vessels (Fig. 4A and B). Such accumulations of lymphocytes could be seen throughout the skeletal muscle of the chest wall (Fig. 4C and D) and even within the dermis (Fig. 4F).
FIG. 4.
Lymphocyte infiltration in α9 null mice. Presented are H&E-stained cross sections from the chest wall of α9−/− mice, showing mononuclear cell infiltration around lymphatic vessels adjacent to the parietal pleura (A and B), within skeletal muscle and interstitial tissue (C and D), and within the dermis (F) in the α9−/− mice. No such findings were ever observed in these locations in α9+/+ mice. One such example is shown for the dermis (E).
Transient α9 expression in the developing thoracic duct.
Because no previous reports have described expression of α9 in the thoracic duct, the phenotype of α9−/− mice was unexpected. Our initial efforts to identify expression of this integrin subunit in the thoracic duct of adult and newborn animals were also unsuccessful. We therefore undertook a comprehensive evaluation of thoracic expression of α9 during mouse embryonic development. We performed immunohistochemistry with affinity-purified rabbit polyclonal anti-α9 antiserum on E12, E13, E14, E15, E17, and E19 embryos and on mice at 1, 6, 8, or 60 days after birth. α9 was clearly expressed from day E12 onward in the smooth muscle cells of the aorta, immediately adjacent to the developing thoracic duct, raising the possibility that normal thoracic duct development might be dependent on an inductive signal from aortic smooth muscle cells. At only a single time point, E14, did the cluster of cells adjacent to the aorta that ultimately form the thoracic duct clearly demonstrate α9 immunoreactivity (Fig. 5A and B). This staining, as well as the immunoreactivity in the adjacent aorta, was specific, since no such staining was seen in corresponding tissue from any α9−/− E14 embryo (data not shown). α9 expression in the developing thoracic duct was transient, since immunoreactivity was not seen in this region at any other time during embryonic or postnatal development (Fig. 5A).
FIG. 5.
Transient α9 expression in the primordial thoracic duct and adjacent aorta of E14 mouse embryo. Frozen sections fixed with acetone were stained with affinity-purified anti-α9 polyclonal antiserum (brown staining). (A) Section of an α9+/+ E19 embryo (original magnification, ×200), demonstrating staining of the aorta but not of the adjacent thoracic duct (Td). (B) Section of an α9+/+ E14 embryo at the same magnification, demonstrating staining of both the aorta and the adjacent tissue that will ultimately form the thoracic duct. Airway smooth muscle (ASM) also demonstrates α9 immunoreactivity. (C) Enlargement of the same section shown in panel B.
By comparing the pattern of α9 expression in α9−/− and wild-type mice, we were able to confirm the specificity of our immunodetection methods and our previously reported cell and tissue distribution of α9. By both immunohistochemistry and Western blotting, α9 immunoreactivity was never seen in α9−/− mice, and was found in a pattern similar to those described in our previous reports in wild-type mice (13, 21).
DISCUSSION
In this report, we describe a novel and completely unexpected phenotype in mice expressing a null mutation of the integrin α9 subunit, congenital chylothorax and defective development of the thoracic duct and other lymphatics. Our finding that α9 is transiently expressed during the early stages of thoracic duct development suggests that an α9-containing integrin, presumably α9β1, plays some temporally restricted critical role in formation of a functionally intact thoracic duct. Alternatively, given the expression of α9 in the adjacent aortic smooth muscle before and during the period of thoracic duct development, it is possible that an α9-containing integrin is required for the expression of an inductive signal arising from aortic smooth muscle. Interestingly, despite considerable indirect evidence of abnormal leakage of chylous fluid and lymphocytes from the thoracic duct and other lymphatics in these animals, we could not identify obvious structural abnormalities in these vessels, suggesting that the developmental defect is subtle.
These results, while surprising, are consistent with a pattern that has emerged from other reports of the phenotypes of mice expressing null mutations of individual integrin subunits. Despite in vitro evidence demonstrating considerable functional overlap among integrin heterodimers, in virtually every case, inactivation of individual integrins in mice has produced unique phenotypes (10). These findings suggest that the large numbers of integrins seen in mammals has evolved, in part, to support an array of unique functions. The molecular mechanisms responsible for this specificity are only beginning to emerge.
As noted in the introduction, three proteins, tenascin C, osteopontin, and VCAM-1, have been described as ligands for α9β1. The effects of null mutations in each of these α9β1 ligands have been reported. Mice expressing a null mutation of the tenascin C gene have been described by two groups (4, 5) and have either a minimal phenotype or subtle changes in behavior, but do not develop chylothorax. Mice expressing a null mutation of the osteopontin gene are similarly viable and fertile, with no thoracic or lymphatic pathology (11). Inactivation of the VCAM-1 gene does produce a dramatic phenotype with usually lethal defects in the development of both the placenta and the heart (6). The allantois of VCAM-1 knockout mice fails to fuse to the chorion, resulting in a defective vasculature. A strikingly similar phenotype is seen in mice expressing a null mutation in the integrin α4 subunit gene (22). These data suggest that interactions between VCAM-1 and the integrin α4β1 play a critical role in vascular development. However, a small number of VCAM-deficient embryos survive, and they can become viable and fertile adults without chylothorax or other described lymphatic abnormalities (6). Taken together, these results suggest that the abnormality in lymphatic development we describe is not explained by an interaction of α9β1 with any single ligand thus far reported.
A somewhat surprising finding from the current study was the absence of any phenotype in nonlymphatic tissues in which α9 expression is temporally and/or spatially regulated during development, including squamous and airway epithelium, airway and gut smooth muscle, choroid plexus, and liver (13, 21). However, this result is also consistent with results seen with many other integrin knockouts and is probably explained, at least in part, by the functional redundancy among integrins alluded to above. Because of the early mortality of α9−/− mice, it was not possible to examine the potential functional consequences of loss of this integrin on organ physiology or in disease models requiring adult animals.
The results of the current study provide little insight into the mechanism(s) by which an α9-containing integrin(s) contributes to lymphatic development. Our inability to identify any discrete structural abnormality compounds this problem, as does the scarcity of information from previous studies on the general mechanisms underlying lymphatic development. Nonetheless, our results suggest a potential genetic cause of chylothorax. Congenital chylothorax is a rare disorder in humans and has thus far been largely unexplained (12). The results of the present study raise the possibility that some of these cases might be due to inactivating or null mutations in the integrin α9 subunit.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants HL/AI33259, HL47412, HL53949, and HL56385 (D.S.).
REFERENCES
- 1.Bradley A. Teratocarcinomas and embryonic stem cells. In: Robertson E J, editor. Production and analysis of chimaeric mice. Oxford, United Kingdom: IRL Press; 1987. pp. 113–151. [Google Scholar]
- 2.Erle D J, Sheppard D, Breuss J, Rüegg C, Pytela R. Novel integrin α and β subunit cDNAs identified using the polymerase chain reaction. Am J Respir Cell Mol Biol. 1991;5:170–177. doi: 10.1165/ajrcmb/5.2.170. [DOI] [PubMed] [Google Scholar]
- 3.Fässler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 4.Forsberg E, Hirsch E, Fröhlich L, Meyer M, Ekblom P, Aszodi A, Werner S, Fässler R. Skin wounds and severed nerves heal normally in mice lacking tenascin-C. Proc Natl Acad Sci USA. 1996;93:6594–6599. doi: 10.1073/pnas.93.13.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukamauchi F, Mataga N, Wang Y J, Sato S, Youshiki A, Kusakabe M. Abnormal behavior and neurotransmissions of tenascin gene knockout mouse. Biochem Biophys Res Commun. 1996;221:151–156. doi: 10.1006/bbrc.1996.0561. [DOI] [PubMed] [Google Scholar]
- 6.Gurtner G C, Davis V, Li H, McCoy M J, Sharp A, Cybulsky M I. Targeted disruption of the murine VCAM-1 gene in chorioallantoic fusion and placentation. Genes Dev. 1995;9:1–14. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Huang X Z, Wu J F, Cass D, Erle D J, Corry D, Young S G, Farese R V, Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynes R O. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 9.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 10.Hynes R O. Targeted mutations in cell adhesion genes: what have we learned from them? Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- 11.Liaw L, Birk D E, Ballas C B, Whitsitt J S, Davidson J M, Hogan B L. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Investig. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moerman P, Vandenberghe K, Devlieger H, Van Hole C, Fryns J P, Lauweryns J M. Congenital pulmonary lymphangiectasis with chylothorax: a heterogeneous lymphatic vessel abnormality. Am J Med Genet. 1993;47:154–158. doi: 10.1002/ajmg.1320470112. [DOI] [PubMed] [Google Scholar]
- 13.Palmer E L, Rüegg C, Ferrando R, Pytela R, Sheppard D. Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. J Cell Biol. 1993;123:1289–1297. doi: 10.1083/jcb.123.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pytela R, Suzuki S, Breuss J, Erle D, Sheppard D. PCR cloning with degenerate primers: homology based identification of novel adhesion molecules. Methods Enzymol. 1994;245:420–451. doi: 10.1016/0076-6879(94)45022-6. [DOI] [PubMed] [Google Scholar]
- 15.Ruoslahti E, Pierschbacher M D. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 16.Smith L L, Cheung H-K, Ling L E, Chen J, Sheppard D, Pytela R, Giachelli C M. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by α9β1 integrin. J Biol Chem. 1996;271:28485–28491. [PubMed] [Google Scholar]
- 17.Stephens L E, Sutherland A E, Klimanskaya I V, Andrieux A, Meneses J, Pedersen R A, Damsky C H. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 18.Stepp M A, Zhu L. Upregulation of alpha 9 integrin and tenascin during epithelial regeneration after debridement in the cornea. J Histochem Cytochem. 1997;45:189–201. doi: 10.1177/002215549704500205. [DOI] [PubMed] [Google Scholar]
- 19.Stepp M A, Zhu L, Sheppard D, Cranfill R L. Localized distribution of alpha 9 integrin in the cornea and changes in expression during corneal epithelial cell differentiation. J Histochem Cytochem. 1995;43:353–362. doi: 10.1177/43.4.7534781. [DOI] [PubMed] [Google Scholar]
- 20.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha 9 beta 1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang A, Patrone L, McDonald J A, Sheppard D. Expression of the integrin subunit alpha 9 in the murine embryo. Dev Dynam. 1995;204:421–431. doi: 10.1002/aja.1002040408. [DOI] [PubMed] [Google Scholar]
- 22.Yang J T, Rayburn H, Hynes R O. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 23.Yokosaki Y, Matsuura N, Higashiyama S, Murakami I, Obara M, Yamakido M, Shigeto N, Chen J, Sheppard D. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin type III repeat of tenascin-C. J Biol Chem. 1998;273:11423–11428. doi: 10.1074/jbc.273.19.11423. [DOI] [PubMed] [Google Scholar]
- 24.Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y, Sheppard D. The integrin α9β1 binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 25.Yokosaki Y, Monis H, Chen J, Sheppard D. Differential effects of the integrins α9β1, αvβ3 and αvβ6 on cell proliferative responses to tenascin: roles of the β subunit extracellular and cytoplasmic domains. J Biol Chem. 1996;271:24144–24150. doi: 10.1074/jbc.271.39.24144. [DOI] [PubMed] [Google Scholar]
- 26.Yokosaki Y, Palmer E L, Prieto A L, Crossin K L, Bourdon M A, Pytela R, Sheppard D. The integrin α9β1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]