Abstract
Background
Migraine is associated with depression as well as negative impact on quality of life and work productivity. Fremanezumab, a fully humanized monoclonal antibody (IgG2Δa), selectively targets the calcitonin gene‐related peptide and has proven efficacy for the preventive treatment of migraine.
Objective
In this open‐label extension (OLE) of the phase 3b FOCUS study, we assessed patient‐reported outcomes (PROs) over time.
Methods
Patients with episodic migraine (EM) and chronic migraine (CM) completing the 12‐week, double‐blind (DB) period of the FOCUS trial entered the 12‐week OLE and received three monthly doses of fremanezumab (225 mg). PROs included the Migraine‐Specific Quality of Life (MSQoL) questionnaire (role function—restrictive [RFR], role function—preventive [RFP], and emotional function [EF] domains), EuroQol‐5‐Dimension‐5‐Level (EQ‐5D‐5L) questionnaire, Patient Global Impression of Change (PGIC) assessment, Work Productivity and Activity Impairment (WPAI) questionnaire, and 9‐Item Patient Health Questionnaire (PHQ‐9).
Results
A total of 838 patients were randomized in the DB period, 807 entered the OLE at 3 months, and 772 were still enrolled at 6 months. At 6 months, patients in the quarterly fremanezumab, monthly fremanezumab, and placebo DB randomization groups, respectively, reported improvements in RFR (mean [standard deviation] change from baseline: 24.6 [21.9]; 22.9 [21.3]; 20.8 [26.5]), RFP (19.6 [20.0]; 18.3 [19.7]; 16.0 [19.9]), and EF (22.5 [24.2]; 19.1 [23.6]; 17.2 [24.7]) domains of the MSQoL questionnaire, the EQ‐5D‐5L questionnaire (8.0 [19.6]; 7.3 [21.1]; 6.6 [21.0]), all four domains of the WPAI questionnaire, and the PHQ‐9 (−2.4 [5.3]; −1.6 [5.5]; −2.0 [4.9]); 77.1% (209/271), 75.4% (205/272), and 68.8% (181/263) of patients were identified as PGIC responders.
Conclusion
Among patients with EM or CM and prior inadequate response to multiple migraine‐preventive medication classes, progressive improvements in MSQoL, depression, and work productivity were achieved during 6 months of fremanezumab treatment.
Keywords: calcitonin gene‐related peptide, migraine, Migraine‐Specific Quality of Life, monoclonal antibody, Work Productivity and Activity Impairment
Abbreviations
- CGRP
calcitonin gene‐related peptide
- CM
chronic migraine
- DB
double‐blind
- EF
emotional function
- EM
episodic migraine
- EQ‐5D‐5L
EuroQol‐5‐Dimension‐5‐Level
- HRQoL
health‐related quality of life
- LSM
least‐squares mean
- mITT
modified intent‐to‐treat
- MSQoL
Migraine‐Specific Quality of Life
- OLE
open‐label extension
- PGIC
Patient Global Impression of Change
- PHQ‐9
9‐Item Patient Health Questionnaire
- PRO
patient‐reported outcome
- RFP
role function—preventive
- RFR
role function—restrictive
- SD
standard deviation
- SE
standard error
- WPAI
Work Productivity and Activity Impairment
INTRODUCTION
Migraine carries a substantial disease burden, including social and economic burdens, functional impairments, and negative impact on health‐related quality of life (HRQoL), with a higher burden for patients with prior inadequate response to migraine‐preventive treatments or more frequent headaches. 1 , 2 , 3 , 4 In a large cross‐sectional study, patients with migraine reported significantly lower EuroQol‐5‐Dimension‐5‐Level (EQ‐5D‐5L) health utility scores than the nonmigraine controls. 5 Similarly, Migraine‐Specific Quality of Life (MSQoL) scores indicate greater functional impairment, with domain and total scores being significantly lower among patients with migraine. 6 In the United States, approximately 113 million workdays are lost annually as a result of migraine attacks. 7 Migraine prevalence is highest during the most productive years of life; as such, lost workdays may have a significant negative impact on the overall career trajectory of individuals with migraine. 4 Furthermore, the presence of comorbid health conditions may further impact patients’ work productivity. 8 Depression was among the most commonly reported comorbid conditions in a survey of patients with migraine (63.8%). 9 Longitudinal research demonstrates that depression among migraine patients is associated with a 56% greater risk of moderate/severe migraine‐related disability. 10 Consequently, patient‐reported outcomes (PROs) are a critical component of evaluating the effects of new treatments on HRQoL, emotional and psychological well‐being, disability status, and occupational functioning. 11 Calcitonin gene‐related peptide (CGRP) plays a major role in the pathophysiology of migraine. Biologic therapies that target the CGRP pathway are the first preventive treatments for migraine specifically designed to target the underlying disease pathophysiology. 12 Fremanezumab is a fully humanized monoclonal antibody (IgG2Δa) that selectively targets the CGRP pathway and is approved as a migraine‐preventive treatment in adults. 13 In previous double‐blind (DB), placebo‐controlled trials, fremanezumab was safe, effective, and generally well tolerated in patients with episodic migraine (EM) and chronic migraine (CM). 14 , 15 , 16
In the 12‐week, randomized, DB period of the phase 3b FOCUS study (ClinicalTrials.gov Identifier: NCT03308968), fremanezumab demonstrated efficacy and tolerability as a quarterly or monthly migraine‐preventive treatment in adults with EM or CM and documented prior inadequate response to two to four migraine‐preventive medication classes. 16 Furthermore, compared with the placebo group, patients receiving fremanezumab (quarterly or monthly) had significantly greater improvements from baseline in all prespecified exploratory PROs evaluated, including disability scores (6‐Item Headache Impact Test and Migraine Disability Assessment), HRQoL, health status, patient satisfaction, work productivity and impairment, and patient‐reported depression status. 16 The objective of the open‐label extension (OLE) of the FOCUS study was to further evaluate the efficacy and tolerability of fremanezumab in this patient population during a longer observational period. By analyzing PRO data from this patient population over the 6‐month course of the FOCUS study (3‐month DB period; 3‐month OLE), the present study aimed to evaluate the long‐term efficacy, tolerability, and impact on disease burden of fremanezumab treatment.
METHODS
Study design and participants
The international, multicenter, randomized, placebo‐controlled, phase 3b FOCUS study has been described in detail previously. 16 Briefly, the study consisted of a 12‐week DB treatment period and a 12‐week OLE, with a final follow‐up of 6 months after the last dose of fremanezumab (Figure 1). The FOCUS study enrolled adults ≤70 years of age, with a diagnosis of EM or CM at or before 50 years of age, for ≥12 months prior to the screening visit. Adults with EM had a headache on ≥6 but <15 days per month, with ≥4 days fulfilling criteria from the International Classification of Headache Disorders, 3rd edition, beta version for migraine, probable migraine, or use of triptans or ergot derivatives to treat an established headache. Adults with CM had headache on ≥15 days per month, with ≥8 days fulfilling the International Classification of Headache Disorders, 3rd edition, beta version criteria for migraine, probable migraine, or use of triptans or ergot derivatives to treat an established headache. 17
FIGURE 1.
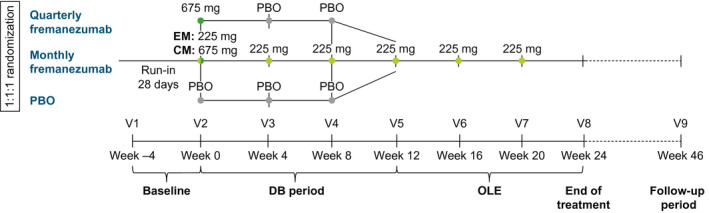
FOCUS study design. CM, chronic migraine; DB, double‐blind; EM, episodic migraine; OLE, open‐label extension; PBO, placebo; V, visit
Inclusion criteria included documented, prior, inadequate response to two to four classes of migraine‐preventive medications within the past 10 years (anticonvulsants, angiotensin II receptor antagonists, beta‐blockers, calcium channel blockers, tricyclic antidepressants, onabotulinumtoxinA, or valproic acid). Inadequate response was defined as one of the following: treatment contraindicated/unsuitable for migraine prevention for the patient, poor tolerability, or lack of efficacy. Eligible patients were randomized (1:1:1) to receive subcutaneously administered placebo or fremanezumab quarterly (675 mg/placebo/placebo) or monthly (EM: 225/225/225 mg; CM: 675/225/225 mg). All patients who completed the DB period were eligible to enter the 12‐week OLE and receive three monthly doses (225 mg) of fremanezumab.
This study was conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guidelines for Good Clinical Practice and applicable national and local laws and regulations. All patients provided written informed consent. Independent ethics committees or institutional review boards of all participating institutions approved the study protocol and informed consent form.
Outcomes
The MSQoL is a 14‐item questionnaire that assesses the impact of migraine and migraine treatment on a patient's quality of life in the preceding 4 weeks and has been validated for use in patients with CM and EM. 18 Specifically, the MSQoL measures the degree to which performance of normal activities is limited by migraine (role function—restrictive [RFR] domain), the degree to which the performance of normal activities is prevented by migraine (role function—preventive [RFP] domain), and the emotional effects of migraine (emotional function [EF] domain), with scores ranging from 0 to 100 and higher scores indicating better HRQoL. 18 The MSQoL questionnaire was completed at Week 0 (baseline), 1 month, 3 months (end of DB period), and 6 months (end of OLE). For within‐group analyses, the minimally important difference was 5.0 for RFR, 5.0 to 7.9 for RFP, and 8.0 to 10.6 for EF. 19
The EQ‐5D‐5L questionnaire consists of two parts. For the first part, patients used a 5‐point categorical scale to rate their health state in the five domains: mobility, self‐care, usual activities, pain/discomfort, and mood. Scores are described as follows: 1, no problems; 2, slight problems; 3, moderate problems; 4, severe problems; or 5, extreme problems. For the second part, patients rated their health state on a continuous, 100‐mm visual analog scale, with 0 representing the worst imaginable health state and 100 representing the best imaginable health state. 20 The EQ‐5D‐5L questionnaire was completed at Week 0 (baseline), 3 months (end of DB period), and 6 months (end of OLE).
The Patient Global Impression of Change (PGIC) assessment uses a 7‐point categorical scale to describe the impact of a patient's migraine on their general quality of life and health status since the beginning of treatment. Scores are described as follows: 1, no change or worsening of the condition; 2, almost the same; 3, a little better; 4, somewhat better; 5, moderately better; 6, better; or 7, a great deal better. 21 PGIC responders were defined as those with a score between 5 and 7. The proportion of PGIC responders was summarized as counts and percentages at the end of the DB period and OLE, according to DB randomization group. The PGIC was completed at 1 month, 3 months (end of DB period), and 6 months (end of OLE).
The Work Productivity and Activity Impairment (WPAI) questionnaire measures the overall effect of health on productivity at work and daily activities; the specific health problem version of the questionnaire allows investigators to attribute productivity and activity impairment issues to specific health conditions. Responses to the WPAI questionnaire are based on an 11‐point categorical scale, with scores ranging from 0 (no impairment) to 10 (complete impairment). WPAI outcomes are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity. 22 The WPAI questionnaire was completed at Week 0 (baseline), 3 months (end of DB period), and 6 months (end of OLE).
In the 9‐Item Patient Health Questionnaire (PHQ‐9), each item corresponds to the diagnostic criteria for major depressive disorder (Diagnostic and Statistical Manual for Mental Disorders, 4th edition). Items are scored categorically as follows, based on the frequency of symptoms during the past 2 weeks: 0, not at all; 1, several days; 2, more than half the days; or 3, nearly every day. Patients’ responses are summed to create a score that indicates minimal (1–4), mild (5–9), moderate (10–14), moderately severe (15–19), or severe (20–27) depressive symptoms. 23 The PHQ‐9 questionnaire was completed at Week 0 (baseline), 3 months (end of DB period), and 6 months (end of OLE).
The MSQoL, EQ‐5D‐5L, PGIC, WPAI, and PHQ‐9 were prespecified exploratory outcomes in the FOCUS study.
Statistical analysis
Results from the OLE were reported according to the randomization group for the DB period. All randomly assigned participants who received ≥1 dose of study drug were included in the safety analysis set. The modified intent‐to‐treat (mITT) analysis set for the DB period included patients who received ≥1 dose of study drug and had ≥10 days of postbaseline efficacy assessments on the primary endpoint. The mITT analysis set for the OLE included patients who received ≥1 dose of study drug during the OLE and had ≥10 days of postbaseline diary entries during the OLE treatment period. Demographics, baseline characteristics, and PROs were summarized descriptively by DB randomization group; categorical measures were reported as frequency (n) and percentage (%), and continuous variables were reported as the mean and standard deviation (SD) or standard error (SE) of the mean. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patients and baseline characteristics
Of the 838 patients randomized in the DB period, 96.3% (807/838) entered the OLE at 3 months, and 92.1% (772/838) were still enrolled at 6 months. Baseline characteristics for patients in the OLE were similar across treatment groups and similar to those in the DB period. 16 Most patients were female (83.5% [674/807]). The mean (SD) age was 46.4 (11.0) years, and the mean (SD) time since migraine diagnosis was 24.3 (13.3) years. More patients had CM (61.2% [494/807]) than EM (38.8% [313/807]). Baseline scores for the MSQoL, EQ‐5D‐5L, WPAI, and PHQ‐9 questionnaires were also similar across the treatment groups (Table 1).
TABLE 1.
Demographic and baseline characteristics according to DB randomization (OLE safety analysis set)
| Placebo a (n = 262) | Quarterly fremanezumab a (n = 271) | Monthly fremanezumab a (n = 274) | |
|---|---|---|---|
| Age, mean (SD), years | 46.9 (11.2) | 46.0 (11.0) | 46.1 (11.0) |
| Female sex, n (%) | 218 (83.2) | 226 (83.4) | 230 (83.9) |
| Race, n (%) | |||
| White | 247 (94.3) | 258 (95.2) | 254 (92.7) |
| Black/African American | 1 (0.4) | 1 (0.4) | 4 (1.5) |
| Asian | 1 (0.4) | 0 | 2 (0.7) |
| American Indian or Alaskan Native | 0 | 0 | 1 (0.4) |
| Other | 1 (0.4) | 2 (0.7) | 1 (0.4) |
| Not reported | 12 (4.6) | 10 (3.7) | 12 (4.4) |
| Weight, mean (SD), kg | 71.3 (13.9) | 70.5 (13.3) | 71.1 (13.8) |
| Height, mean (SD), cm | 167.6 (9.0) | 167.6 (7.9) | 167.4 (7.6) |
| Body mass index, mean (SD), kg/m2 | 25.3 (4.1) | 25.0 (4.1) | 25.3 (4.4) |
| Years since initial migraine diagnosis, mean (SD) | 24.3 (13.4) | 24.4 (12.9) | 24.3 (13.7) |
| Migraine classification, n (%) | |||
| EM | 105 (40.1) | 102 (37.6) | 106 (38.7) |
| CM | 157 (59.9) | 169 (62.4) | 168 (61.3) |
| Number of prior preventive medications failed, n (%) | |||
| 2 | 131 (50) | 138 (51) | 129 (47) |
| 3 | 77 (29) | 82 (30) | 94 (34) |
| 4 | 54 (21) | 49 (18) | 49 (18) |
| Baseline MSQoL scores, mean (SD) b | |||
| RFR | 47.9 (18.3) | 47.5 (17.4) | 49.5 (17.1) |
| RFP | 64.4 (19.9) | 63.7 (19.3) | 65.1 (19.7) |
| EF | 61.2 (24.2) | 58.8 (24.6) | 63.4 (23.0) |
| Baseline EQ‐5D‐5L score, mean (SD) b | 68.8 (21.5) | 70.1 (20.2) | 70.3 (20.7) |
| Baseline WPAI scores, mean (SD) b | |||
| Percentage work missed due to health | 10.0 (19.2) | 13.8 (23.1) | 12.8 (21.9) |
| Percentage impairment while working due to health | 36.6 (23.2) | 39.7 (23.0) | 39.8 (21.8) |
| Percentage overall work impairment due to health | 40.4 (25.8) | 44.6 (25.5) | 44.7 (24.5) |
| Percentage activity impairment due to health | 46.0 (23.5) | 46.5 (23.4) | 44.9 (23.3) |
| Baseline PHQ‐9 total score, mean (SD) b | 3.9 (5.3) | 4.3 (5.6) | 3.5 (5.1) |
Abbreviations: CM, chronic migraine; DB, double‐blind; EF, emotional function; EM, episodic migraine; EQ‐5D‐5L, EuroQol‐5‐Dimension‐5‐Level; mITT, modified intent‐to‐treat; MSQoL, Migraine‐Specific Quality of Life; OLE, open‐label extension; PHQ‐9, 9‐Item Patient Health Questionnaire; RFP, role function—preventive; RFR, role function—restrictive; SD, standard deviation; WPAI, Work Productivity and Activity Impairment.
All patients in the OLE received fremanezumab 225 mg monthly.
OLE mITT analysis set.
MSQoL
Mean baseline scores for the MSQoL questionnaire across the DB treatment groups for the RFR, RFP, and EF domains are presented in Table 1. During the last 4 weeks of the DB period, least‐squares mean (LSM; SE) increases from baseline in MSQoL scores were greater in the quarterly and monthly fremanezumab groups compared with the placebo group for the RFR (DB quarterly fremanezumab, 15.7 [1.5]; DB monthly fremanezumab, 17.5 [1.5]; placebo, 6.9 [1.5]), 16 RFP (DB quarterly fremanezumab, 11.9 [1.4]; DB monthly fremanezumab, 14.4 [1.4]; placebo, 6.2 [1.4]), and EF (DB quarterly fremanezumab, 13.4 [1.7]; DB monthly fremanezumab, 15.6 [1.7]; placebo, 4.4 [1.7]) domains (all p < 0.001; Figure 2A). At 6 months, patients receiving monthly fremanezumab reported improvements in the RFR (mean [SD] and percentage increase from baseline: DB quarterly fremanezumab, 24.6 [21.9] and 51.8%; DB monthly fremanezumab, 22.9 [21.3] and 46.3%; DB placebo, 20.8 [26.5] and 43.4%), RFP (DB quarterly fremanezumab, 19.6 [20.0] and 30.8%; DB monthly fremanezumab, 18.3 [19.7] and 28.1%; DB placebo, 16.0 [19.9] and 24.8%), and EF (DB quarterly fremanezumab, 22.5 [24.2] and 38.3%; DB monthly fremanezumab, 19.1 [23.6] and 30.1%; DB placebo, 17.2 [24.7] and 28.1%) domains of the MSQoL, regardless of DB randomization group (Figure 2B). At 6 months, these increases in MSQoL domain scores from baseline exceeded the threshold for minimal clinically important differences.
FIGURE 2.
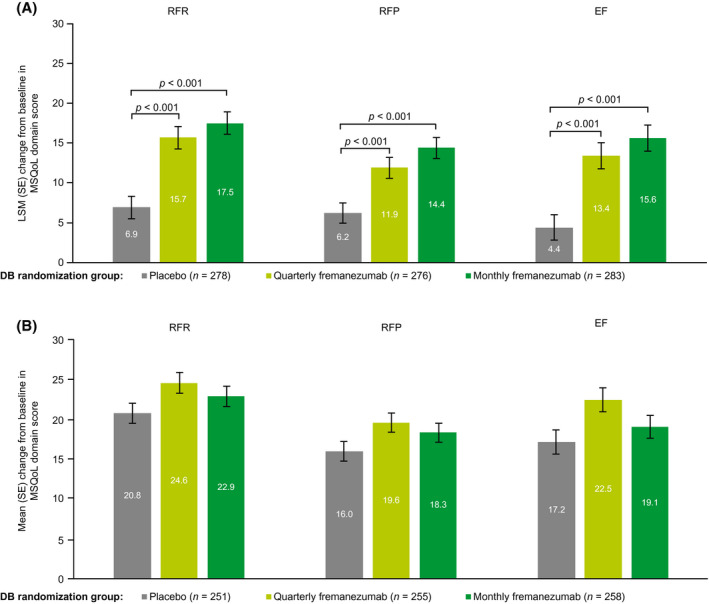
Change from baseline in MSQoL domain scoresa (A) during the last 4 weeks of the DB period and (B) at 6 months (mITT).b aScore range: 0 to 100; higher scores indicate better HRQoL. bAll patients in the OLE of the FOCUS study received fremanezumab 225 mg monthly. DB, double‐blind; EF, emotional function; LSM, least‐squares mean; mITT, modified intent‐to‐treat; MSQoL, Migraine‐Specific Quality of Life; OLE, open‐label extension; RFP, role function—preventive; RFR, role function—restrictive; SE, standard error
EQ‐5D‐5L
Mean (SD) EQ‐5D‐5L scores at baseline were similar across the DB quarterly fremanezumab, monthly fremanezumab, and placebo treatment groups: 70.1 (20.2), 70.3 (20.7), and 68.8 (21.5), respectively (Table 1). During the last 4 weeks of the DB period, LSM (SE) increases from baseline in EQ‐5D‐5L scores were greater in the quarterly and monthly fremanezumab groups (4.7 [1.4] and 7.2 [1.4], respectively) compared with the placebo group (1.6 [1.4]; p = 0.043 and p < 0.001, respectively; Figure 3A). 16 At 6 months, patients receiving fremanezumab reported substantial improvement in EQ‐5D‐5L scores (mean [SD] and percentage increase from baseline: DB quarterly fremanezumab, 8.0 [19.6] and 11.4%; DB monthly fremanezumab, 7.3 [21.1] and 10.4%; DB placebo, 6.6 [21.0] and 9.6%; Figure 3B).
FIGURE 3.
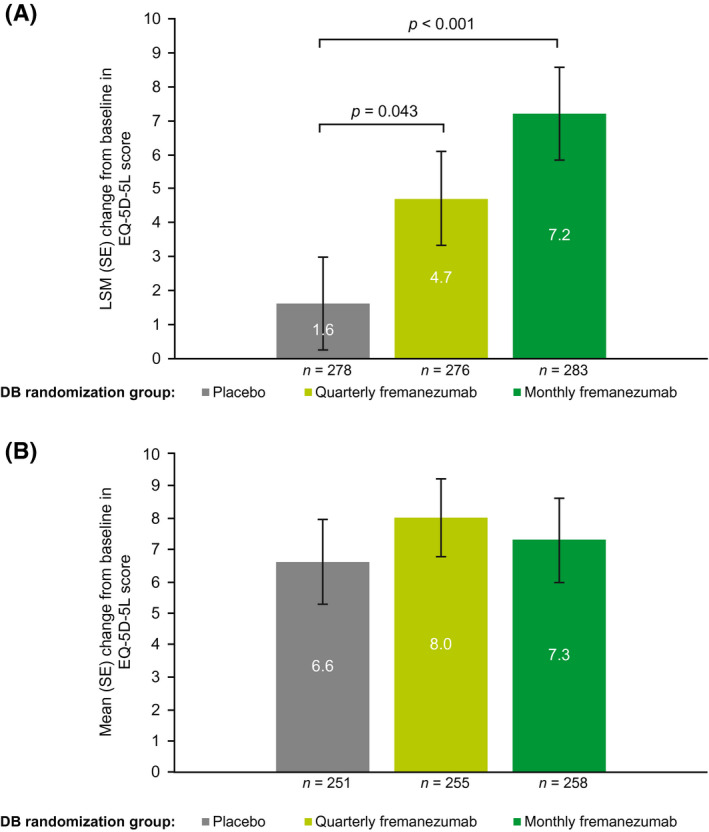
Change from baseline in EQ‐5D‐5La score (A) during the last 4 weeks of the DB period 16 and (B) at 6 months (mITT).b aPatients rated their health state from 0, the worst imaginable health state, to 100, the best imaginable health state. bAll patients in the OLE of the FOCUS study received fremanezumab 225 mg monthly. DB, double‐blind; EQ‐5D‐5L, EuroQol‐5‐Dimension‐5‐Level; LSM, least‐squares mean; mITT, modified intent‐to‐treat; OLE, open‐label extension; SE, standard error
PGIC
At 3 months, the percentages of PGIC responders were higher in the DB quarterly and monthly fremanezumab groups compared with the DB placebo group (58.0% [160/276] and 64.3% [182/283] vs. 29.1% [81/278]; both p < 0.001; Figure 4). 16 There were higher percentages of PGIC responders in all three treatment groups (DB quarterly fremanezumab, 77.1% [209/271]; DB monthly fremanezumab, 75.4% [205/272]; DB placebo, 68.8% [181/263]) at 6 months compared with the percentages of PGIC responders at 3 months.
FIGURE 4.
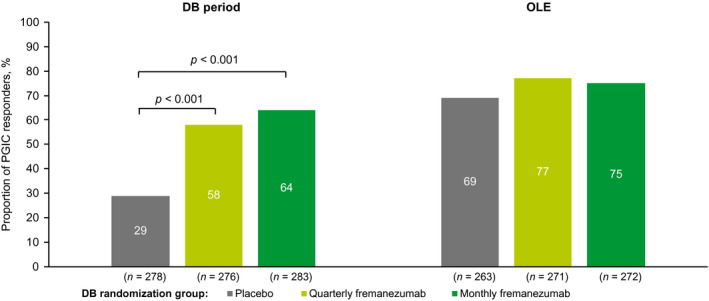
Proportion of PGIC respondersa at the end of the DB period 16 and at 6 months (mITT).b aResponders were defined as those with a score between 5, moderately better, and 7, a great deal better. bAll patients in the OLE of the FOCUS study received fremanezumab 225 mg monthly. DB, double‐blind; mITT, modified intent‐to‐treat; OLE, open‐label extension; PGIC, Patient Global Impression of Change
WPAI
Baseline WPAI scores for the DB treatment groups were similar across all four domains; the mean score for percentage work missed due to health ranged from 10.0% to 13.8%, the mean score for percentage impairment while working due to health ranged from 36.6% to 39.8%, the mean score for percentage overall work impairment due to health ranged from 40.4% to 44.7%, and the mean score for percentage activity impairment due to health ranged from 44.9% to 46.5% (Table 1). During the last 4 weeks of the DB period, improvements in WPAI scores were greater in the quarterly and monthly fremanezumab groups compared with the placebo group for percentage work missed due to health (LSM [SE] decreases from baseline: DB quarterly fremanezumab, −4.7 [2.2]; DB monthly fremanezumab, −5.3 [2.1]; DB placebo, −0.5 [2.2]; p = 0.058 and p = 0.030, respectively), 16 percentage impairment while working due to health (DB quarterly fremanezumab, −13.9 [2.3]; DB monthly fremanezumab, −15.2 [2.3]; DB placebo, −7.0 [2.4]; p = 0.005 and p < 0.001, respectively), percentage overall work impairment due to health (DB quarterly fremanezumab, −14.3 [2.6]; DB monthly fremanezumab, −16.2 [2.6]; DB placebo, −6.4 [2.7]; p = 0.004 and p < 0.001, respectively), and percentage activity impairment due to health (DB quarterly fremanezumab, −13.7 [1.9]; DB monthly fremanezumab, −16.0 [1.9]; DB placebo, −5.1 [1.9]; both p < 0.001; Figure 5A). At 6 months, patients receiving fremanezumab reported substantial improvements in percentage work missed due to health (mean [SD] decrease from baseline: DB quarterly fremanezumab, −4.9 [28.3]; DB monthly fremanezumab, −6.9 [23.3]; DB placebo, −4.0 [22.5]), percentage impairment while working due to health (DB quarterly fremanezumab, −18.5 [26.1]; DB monthly fremanezumab, −17.1 [27.8]; DB placebo, −13.4 [28.3]), percentage overall work impairment due to health (DB quarterly fremanezumab, −20.0 [28.3]; DB monthly fremanezumab, −19.1 [30.6]; DB placebo, −14.5 [30.5]), and percentage activity impairment due to health (DB quarterly fremanezumab, −19.5 [28.0]; DB monthly fremanezumab, −18.0 [29.3]; DB placebo, −15.4 [27.5]) domains of the WPAI (Figure 5B).
FIGURE 5.
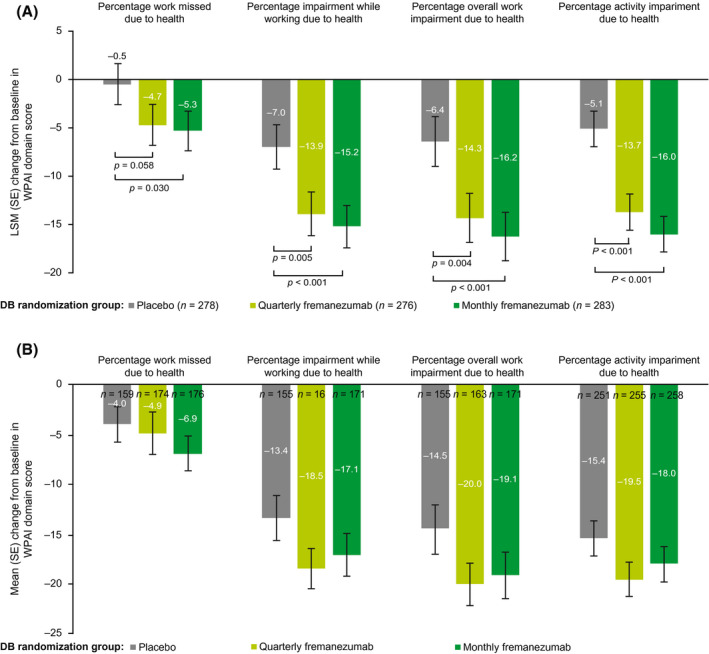
Change from baseline in WPAI domain scoresa (A) during the last 4 weeks of the DB period and (B) at 6 months (mITT).b aOutcomes expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity. bAll patients in the OLE of the FOCUS study received fremanezumab 225 mg monthly. DB, double‐blind; LSM, least‐squares mean; mITT, modified intent‐to‐treat; OLE, open‐label extension; SE, standard error; WPAI, Work Productivity and Activity Impairment
PHQ‐9
Mean (SD) PHQ‐9 scores at baseline were in the minimal to mild categories across the DB quarterly fremanezumab, monthly fremanezumab, and placebo treatment groups: 4.3 (5.6), 3.5 (5.1), and 3.9 (5.3), respectively (Table 1). During the last 4 weeks of the DB period, LSM (SE) decreases from baseline in PHQ‐9 scores were greater in the quarterly and monthly fremanezumab groups (−1.3 [0.4] and −1.8 [0.3], respectively) compared with the placebo group (−0.7 [0.3]; p = 0.082 and p = 0.004, respectively; Figure 6A), although the difference between quarterly fremanezumab and placebo did not reach statistical significance. 16 At 6 months, patients receiving fremanezumab reported improvement in PHQ‐9 scores (mean [SD] and percentage decrease from baseline: DB quarterly fremanezumab, −2.4 [5.3] and 55.8%; DB monthly fremanezumab, −1.6 [5.5] and 45.7%; DB placebo, −2.0 [4.9] and 51.3%; Figure 6B).
FIGURE 6.
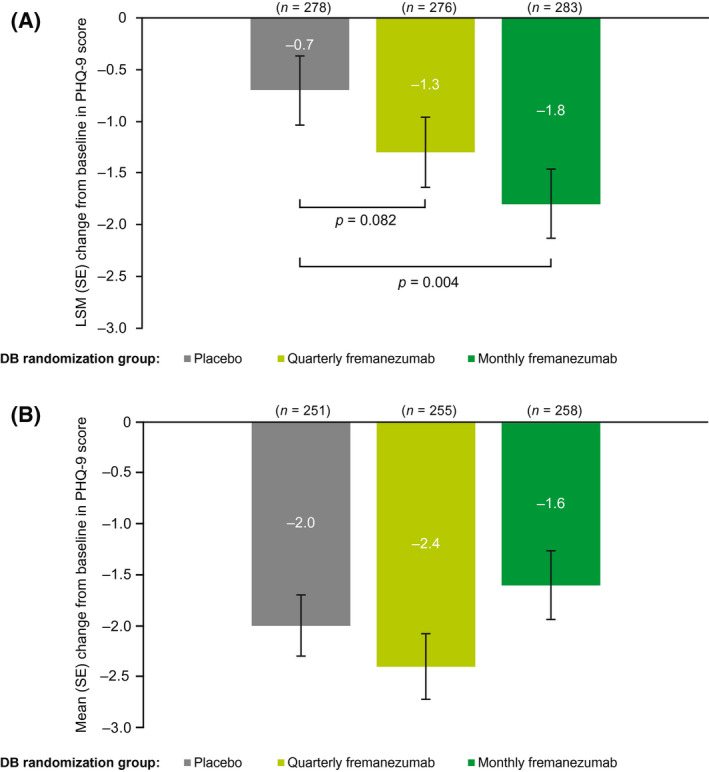
Change from baseline in PHQ‐9 scoresa (A) during the last 4 weeks of the DB period 16 and (B) at 6 months (mITT).b aScore range: 0, minimal depression, to 27, severe depression. bAll patients in the OLE of the FOCUS study received fremanezumab 225 mg monthly. DB, double‐blind; LSM, least‐squares mean; mITT, modified intent‐to‐treat; OLE, open‐label extension; PHQ‐9, 9‐Item Patient Health Questionnaire; SE, standard error
DISCUSSION
Migraine has a substantial burden of disease that impairs many aspects of HRQoL, especially for patients with more frequent headache. 1 , 2 , 3 , 10 , 24 , 25 A retrospective US claim analysis of adults with CM showed that persistence of oral migraine‐preventive medication was poor at 6 months (25%) and declined further at 12 months (14%). 26 This observation highlights the substantial unmet need for patients with migraine. As previously stated, biologic therapies targeting the CGRP pathway represent the first preventive treatments designed specifically to target the underlying pathophysiology of migraine. 12 PROs are critical tools for measuring the effects of treatment on patient HRQoL and, as such, are vital to fully understand the benefits of CGRP pathway–targeted, migraine‐preventive treatments. 11 , 27
The positive impact of preventive medications targeting the CGRP pathway on PROs evaluating HRQoL has been demonstrated previously. In phase 2, DB, placebo‐controlled trials in patients with EM 28 and CM, 29 patients receiving erenumab 70 mg (EM and CM) or 140 mg (CM) for 3 months showed improvements in the RFR, RFP, and EF domains of the MSQoL relative to baseline. 29 Similarly, improvements in MSQoL domain scores in patients with migraine have also been reported with eptinezumab 30 or galcanezumab 31 , 32 treatment. In the HALO CM study, improvements from baseline at 3 months of treatment were significantly greater with both dosing regimens of fremanezumab compared with placebo across all MSQoL domain scores, EQ‐5D‐5L score, WPAI overall work impairment, and PGIC responder rates. 33 In the FOCUS study, baseline scores for PRO measures were indicative of substantial limitations to patients’ daily lives, including daily social activities and work‐related activities. In the OLE, all three DB treatment groups received monthly fremanezumab for 3 months. At 6 months, across all treatment groups, patients receiving monthly fremanezumab reported mean improvements from baseline ranging from approximately 21 to 23 in the RFR, 16 to 20 in the RFP, and 17 to 23 in the EF domains of the MSQoL; these improvements were generally greater than those observed for the fremanezumab‐treated groups during the DB period of the study. 16
In addition to the improvements in quality of life as assessed by the MSQoL at 6 months, patients also exhibited substantial improvements in other measures of health status. Patients reported improvements of 9.6%–11.4% in their health state as measured by the EQ‐5D‐5L questionnaire. Patients also reported reduced impairment in their ability to work on the WPAI compared with baseline; in particular, reductions in percentage work missed due to health. Given the significant loss in productivity related to migraine‐related absenteeism and presenteeism, results from this study suggest that this therapy can help mitigate work loss among people with migraine. 8 Additionally, depression is highly comorbid with migraine 9 and has been shown to be associated with moderate/severe migraine‐related disability and sometimes, but not always, associated with worse treatment outcomes among people with migraine. 10 , 34 The present study demonstrated that patient‐reported depression symptoms at 6 months, as assessed by the PHQ‐9, decreased by 45.7% to 55.8%. Thus, in this context, fremanezumab provided benefit of reduction in patient‐reported symptoms of depression. Taken together, these results support previous findings from the HALO CM study. 33 Furthermore, they highlight the clinical and practical significance of fremanezumab. Specifically, these results suggest that fremanezumab treatment may help reduce functional impairment in patients with migraine and reduce their associated social and economic hardships.
This study had several limitations. Although PRO results from the DB period demonstrated significant improvements with fremanezumab (quarterly and monthly), the OLE had an open‐label, uncontrolled design and no placebo group or active comparator. Furthermore, longer‐term treatment beyond 6 months has not been evaluated in this population.
CONCLUSIONS
Among patients with EM or CM and prior inadequate response to two to four migraine‐preventive treatment classes, treatment with fremanezumab resulted in improvements in HRQoL compared with baseline, which were maintained throughout the 6‐month study period. In the OLE of the phase 3b FOCUS study, greater improvements were observed at 6 months than those observed at 3 months. These findings indicate substantial, long‐term improvements in multiple aspects of patients’ lives, including both disease‐specific and general quality‐of‐life measures. In conclusion, according to multiple PRO measures, fremanezumab is associated with a range of benefits, including improvements in HRQoL and reduction in disability for up to 6 months, in patients who previously did not respond to and/or could not tolerate up to four classes of migraine‐preventive medications.
INSTITUTIONAL REVIEW BOARD APPROVAL
Independent ethics committees or institutional review boards of all participating institutions approved the study protocol and informed consent form.
CONFLICT OF INTEREST
E.L.H. Spierings was an investigator on the FOCUS study and received research grants from Teva Pharmaceuticals; he is also a member of the company's speakers bureau. X. Ning, V.R. Campos, J.M. Cohen, and S. Barash are employees of Teva Pharmaceuticals. D.C. Buse has received grant support and honoraria from Allergan, Amgen, Avanir, Biohaven, Lilly, Promius, and Teva Pharmaceuticals. D.C. Buse is on the editorial board of Current Pain and Headache Reports.
AUTHOR CONTRIBUTIONS
Study concept and design: Egilius L. H. Spierings, Xiaoping Ning, Verena Ramirez Campos, Joshua M. Cohen, Steve Barash, Dawn C. Buse. Acquisition of data: Xiaoping Ning, Verena Ramirez Campos, Joshua M. Cohen, Steve Barash. Analysis and interpretation of data: Egilius L. H. Spierings, Xiaoping Ning, Verena Ramirez Campos, Joshua M. Cohen, Steve Barash, Dawn C. Buse. Drafting of the manuscript: Egilius L. H. Spierings, Xiaoping Ning, Verena Ramirez Campos, Joshua M. Cohen, Steve Barash, Dawn C. Buse. Revising it for intellectual content: Egilius L. H. Spierings, Xiaoping Ning, Verena Ramirez Campos, Joshua M. Cohen, Steve Barash, Dawn C. Buse. Final approval of the completed manuscript: Egilius L. H. Spierings, Xiaoping Ning, Verena Ramirez Campos, Joshua M. Cohen, Steve Barash, Dawn C. Buse.
CLINICAL TRIALS REGISTRATION NUMBER
ClinicalTrials.gov Identifier: NCT03308968.
ACKNOWLEDGMENTS
We thank the patients who participated in this study and their families, all investigators, site personnel, and the coordinating investigators. Medical writing support was provided by Dan Jackson, PhD, of MedErgy (Yardley, PA, USA), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by Teva Pharmaceutical Industries (Petach Tikva, Israel).
Spierings ELH, Ning X, Ramirez Campos V, Cohen JM, Barash S, Buse DC. Improvements in quality of life and work productivity with up to 6 months of fremanezumab treatment in patients with episodic and chronic migraine and documented inadequate response to 2 to 4 classes of migraine‐preventive medications in the phase 3b FOCUS study. Headache. 2021;61:1376–1386. 10.1111/head.14196
Funding information
This study was funded by Teva Pharmaceutical Industries Ltd., Petach Tikva, Israel
REFERENCES
- 1. Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention study. Headache. 2012;52:1456‐1470. [DOI] [PubMed] [Google Scholar]
- 2. Martelletti P, Schwedt TJ, Lanteri‐Minet M, et al. My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018;19:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 4. Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. Value Health. 2013;16:31‐38. [DOI] [PubMed] [Google Scholar]
- 5. Vo P, Fang J, Bilitou A, Laflamme AK, Gupta S. Patients’ perspective on the burden of migraine in Europe: a cross‐sectional analysis of survey data in France, Germany, Italy, Spain, and the United Kingdom. J Headache Pain. 2018;19:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ford JH, Foster SA, Nichols RM, et al. A real‐world analysis of patient‐reported outcomes in patients with migraine by preventive treatment eligibility status in the US and Europe. J Patient Rep Outcomes. 2020;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunter P. New migraine therapies promise prevention: a new generation of drugs could avert migraine attacks rather than merely relieve symptoms. EMBO Rep. 2016;17:797‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munakata J, Hazard E, Serrano D, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2009;49:498‐508. [DOI] [PubMed] [Google Scholar]
- 9. Malone CD, Bhowmick A, Wachholtz AB. Migraine: treatments, comorbidities, and quality of life, in the USA. J Pain Res. 2015;8:537‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipton RB, Seng EK, Chu MK, et al. The effect of psychiatric comorbidities on headache‐related disability in migraine: results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. Headache. 2020;60(8):1683‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agosti R. Migraine burden of disease: from the patient's experience to a socio‐economic view. Headache. 2018;58:17‐32. [DOI] [PubMed] [Google Scholar]
- 12. Silberstein SD, Cohen JM, Yeung PP. Fremanezumab for the preventive treatment of migraine. Expert Opin Biol Ther. 2019;19:763‐771. [DOI] [PubMed] [Google Scholar]
- 13. Tepper SJ. History and review of anti‐calcitonin gene‐related peptide (CGRP) therapies: from translational research to treatment. Headache. 2018;58:238‐275. [DOI] [PubMed] [Google Scholar]
- 14. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113‐2122. [DOI] [PubMed] [Google Scholar]
- 15. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319:1999‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double‐blind, placebo‐controlled, phase 3b trial. Lancet. 2019;394:1030‐1040. [DOI] [PubMed] [Google Scholar]
- 17. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 18. Bagley CL, Rendas‐Baum R, Maglinte GA, et al. Validating Migraine‐Specific Quality of Life questionnaire v2.1 in episodic and chronic migraine. Headache. 2012;52:409‐421. [DOI] [PubMed] [Google Scholar]
- 19. Cole JC, Lin P, Rupnow MF. Minimal important differences in the Migraine‐Specific Quality of Life questionnaire (MSQ) version. Cephalalgia. 2009;29:1180‐1187. [DOI] [PubMed] [Google Scholar]
- 20. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20:1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dodick DW, Silberstein S, Saper J, et al. The impact of topiramate on health‐related quality of life indicators in chronic migraine. Headache. 2007;47:1398‐1408. [DOI] [PubMed] [Google Scholar]
- 22. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a Work Productivity and Activity Impairment instrument. Pharmacoeconomics. 1993;4:353‐365. [DOI] [PubMed] [Google Scholar]
- 23. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self‐report version of PRIME‐MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737‐1744. [DOI] [PubMed] [Google Scholar]
- 24. Buse DC, Reed ML, Fanning KM, et al. Comorbid and co‐occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the Migraine in America Symptoms and Treatment (MAST) study. J Headache Pain. 2020;21:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buse DC, Reed ML, Fanning KM, Bostic RC, Lipton RB. Demographics, headache features, and comorbidity profiles in relation to headache frequency in people with migraine: results of the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2020;60:2340‐2356. [DOI] [PubMed] [Google Scholar]
- 26. Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37:470‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tassorelli C, Diener H‐C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38:815‐832. [DOI] [PubMed] [Google Scholar]
- 28. Dodick DW, Ashina M, Brandes JL, et al. ARISE: a Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026‐1037. [DOI] [PubMed] [Google Scholar]
- 29. Lipton RB, Tepper SJ, Reuter U, et al. Erenumab in chronic migraine: patient‐reported outcomes in a randomized double‐blind study. Neurology. 2019;92:e2250‐e2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene‐related peptide, for the prevention of frequent episodic migraine: a randomised, double‐blind, placebo‐controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100‐1107. [DOI] [PubMed] [Google Scholar]
- 31. Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double‐blind, placebo‐controlled REGAIN study. Neurology. 2018;91:e2211‐e2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE‐2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38:1442‐1454. [DOI] [PubMed] [Google Scholar]
- 33. Lipton RB, Cohen JM, Gandhi SK, Yang R, Yeung PP, Buse DC. Effect of fremanezumab on quality of life and productivity in patients with chronic migraine. Neurology. 2020;95:e878‐e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seng EK, Holroyd KA. Psychiatric comorbidity and response to preventative therapy in the treatment of severe migraine trial. Cephalalgia. 2012;32:390‐400. [DOI] [PubMed] [Google Scholar]


