Abstract
Background:
Emerging evidence links ambient air pollution with coronavirus 2019 (COVID-19) disease, an association that is methodologically challenging to investigate.
Objectives:
We examined the association between long-term exposure to air pollution with SARS-CoV-2 infection measured through antibody response, level of antibody response among those infected, and COVID-19 disease.
Methods:
We contacted 9,605 adult participants from a population-based cohort study in Catalonia between June and November 2020; most participants were between 40 and 65 years of age. We drew blood samples from 4,103 participants and measured immunoglobulin M (IgM), IgA, and IgG antibodies against five viral target antigens to establish infection to the virus and levels of antibody response among those infected. We defined COVID-19 disease using self-reported hospital admission, prior positive diagnostic test, or more than three self-reported COVID-19 symptoms after contact with a COVID-19 case. We estimated prepandemic (2018–2019) exposure to fine particulate matter [PM with an aerodynamic diameter of ()], nitrogen dioxide (), black carbon (BC), and ozone () at the residential address using hybrid land-use regression models. We calculated log-binomial risk ratios (RRs), adjusting for individual- and area-level covariates.
Results:
Among those tested for SARS-CoV-2 antibodies, 743 (18.1%) were seropositive. Air pollution levels were not statistically significantly associated with SARS-CoV-2 infection: Adjusted RRs per interquartile range were 1.07 (95% CI: 0.97, 1.18) for , 1.04 (95% CI: 0.94, 1.14) for , 1.00 (95% CI: 0.92, 1.09) for BC, and 0.97 (95% CI: 0.89, 1.06) for . Among infected participants, exposure to and were positively associated with IgG levels for all viral target antigens. Among all participants, 481 (5.0%) had COVID-19 disease. Air pollution levels were associated with COVID-19 disease: adjusted (95% CI: 1.00, 1.29) for and 1.17 (95% CI: 1.03, 1.32) for . Exposure to was associated with a slightly decreased risk (; 95% CI: 0.83, 1.03). Associations of air pollution with COVID-19 disease were more pronounced for severe COVID-19, with (95% CI: 0.89, 1.79) for and 1.51 (95% CI: 1.06, 2.16) for .
Discussion:
Exposure to air pollution was associated with a higher risk of COVID-19 disease and level of antibody response among infected but not with SARS-CoV-2 infection. https://doi.org/10.1289/EHP9726
Introduction
As of September 2021, coronavirus 2019 (COVID-19) disease has affected more than 230 million persons globally (Johns Hopkins CRC 2021), and many more have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) but were undetected asymptomatic cases or were not recorded (Angulo et al. 2021). Factors associated with infection have been well established, particularly proximity to infected persons in indoor spaces through airborne transmission (Azimi et al. 2021). Individual factors including age, sex, ethnicity, obesity, and specific chronic diseases, together with contextual factors, such as deprivation have been related to disease severity (Williamson et al. 2020).
Several biological pathways have been proposed whereby exposure to outdoor air pollution may relate to transmission, host susceptibility, and disease severity (Woodby et al. 2021; Stieb et al. 2021). Air pollution has been postulated to affect the viability and transport of viral particles in the air (Frontera et al. 2020; Martelletti and Martelletti 2020) and within the respiratory tract. Prior long-term exposure (on the order of years) could increase the risk of infection by altering host defenses to infection through suppression of mucociliary clearance, phagocytosis of viral particles by alveolar macrophages, and up-regulation of the angiotensin-converting enzyme 2 (ACE-2) receptor or altered recognition of the virus–particulate matter complexes by the ACE-2 receptor (Woodby et al. 2021). SARS-CoV-2 uses ACE-2 receptors for host cell entry and the transmembrane protease serine 2 (TMPRSS2) for spike full protein (S) priming; studies in mice have shown that ozone inhalation affects the expression levels of TMPRSS2 (Vo et al. 2020). Finally, air pollution could increase severity of COVID-19 through its contribution to chronic conditions—such as chronic respiratory disease, diabetes, and heart disease—and through long-term effects on immune system function (Bourdrel et al. 2021).
Several ecological studies (Wu et al. 2020; Lipsitt et al. 2021; Bourdrel et al. 2021) and a small number of individual-level studies (Elliott et al. 2021; López-Feldman 2021; Bowe et al. 2021) have reported associations between long-term exposure to air pollution prior to the pandemic and incident COVID-19 disease, hospital admission, and case fatality. Nearly all previous studies have been based on confirmed cases and deaths based on diagnostic testing data and have missed the majority of asymptomatic infected persons. Test-seeking behavior can vary across locations with varying levels of air pollution (e.g., test seeking is more likely in urban areas), and previous studies have shown associations between the probability of being tested and air pollution levels (Chadeau-Hyam et al. 2020). Thus, studies of the role of air pollution on COVID-19 incidence based on cases identified through testing alone are prone to selection bias (Villeneuve and Goldberg 2020). To our knowledge, no previous study has investigated the relationship between air pollution and incident disease in a large cohort based on cases confirmed by antibody serology or investigated the association between air pollution and immune response after infection.
We examined the association between long-term exposure to air pollution with infection with SARS-CoV-2 (measured through antibody response), level of antibody response among those infected, and COVID-19 disease in a general population cohort of adults in Catalonia, northeast Spain. Catalonia was one of the most affected regions in Spain by the pandemic in 2020 and its capital, Barcelona, has among the highest air pollution levels in cities in Western Europe. We also evaluated factors that may modify the effect of air pollution on COVID-19 disease.
Materials and Methods
Study Design, Participating Cohorts, and Participants
The COVID-19 cohort in Catalonia (COVICAT study) aims to characterize the health impact of the COVID-19 pandemic on the population in Catalonia, Spain. It builds on five preexisting adult cohort studies that were established before the outbreak. The cohorts are not family based and there were no shared residences. The largest cohort is the Genomes for Life (GCAT) cohort. We included four additional smaller cohorts that enrich the study with populations of older ages and rural residences [the Multi Case-Control (MCC)–Spain study, the European Community Respiratory Health Survey (ECRHS), the Urban Training cohort, and the Acute Kidney Injury in Agricultural Workers in Spain: Risk Factors and Long Term Effects (LeRAgs) cohorts].
The largest proportion of participants were sourced from the GCAT study (). The GCAT cohort study includes middle-aged participants (40–65 years of age) who are residents in Catalonia, and recruitment started in 2015. Most participants were enrolled from blood donors invited through the Blood and Tissue Bank, a public agency. Valid contact information (email or telephone) was available for 15,245 participants and, of those, 8,923 completed an online COVICAT questionnaire or, a small proportion, responded to a computer-assisted telephone questionnaire (Obón-Santacana et al. 2018). MCC-Spain () includes only the population controls of a population-based multicase-control study launched in 2008 to evaluate the influence of environmental factors in common tumors in 12 provinces in Spain (Castaño-Vinyals et al. 2015)). Population controls were selected at random from the roster of people registered in primary health care centers within the catchment areas of the hospitals where cases were recruited. For the COVICAT study, we recontacted controls living in the provinces of Barcelona and Girona. The European Community Respiratory Health Study () is a population-based study initiated in 1991–1993 to assess the prevalence of asthma and allergic disease in Europe (Burney et al. 1994). Young adults (20–44 years of age) were randomly selected from available population-based registers from 25 countries and 56 centers across Europe. Participants completed a detailed questionnaire at baseline (ECRHS 1) and in two follow-up surveys (ECRHS 2, ECRHS 3) taken 10 y apart. For the COVICAT study, we recontacted participants in the provinces of Barcelona. To increase the proportion of older participants and rural area residents () we drew participants from the Urban Training and LeRAgs studies. Urban Training is a multicenter randomized controlled trial (NCT01897298) on chronic obstructive pulmonary disease in five Catalan municipalities (Arbillaga-Etxarri et al. 2018). For the COVICAT study, we recontacted participants from both intervention arms who, according to medical care records, were known to be alive and cognitively able. The LeRAgs is a cross-sectional study that includes agricultural workers of crops from different climatic conditions in three provinces of Spain. For the COVICAT study, we contacted participants in the Tarragona province.
After the pandemic outbreak in Spain in March 2020, we harmonized the data of all the cohorts, and contacted participants and asked them to respond to a questionnaire and donate a blood sample. Questionnaires and blood sample collection to determine the SARS-CoV-2 seroprevalence started in late May 2020 and ended in November 2020.
Participants were contacted via email or telephone and invited to participate in COVICAT (Figure S1). Participants without registered email addresses were contacted through the telephone. The overall response rate among eligible participants (defined as all individuals who participated in the baseline recruitment out of those participating at cohort inception) was 61.6% (). We evaluated air pollution exposure among 16,900 responders and nonresponders for whom we had valid prepandemic addresses. Exposures tended to be slightly higher among responders (Table S1). The average nitrogen dioxide () exposure for responders was 34.1 vs. in nonresponders; the corresponding levels for fine particulate matter [PM with an aerodynamic diameter of ()] exposure were 16.25 vs. . For the GCAT cohort, the largest cohort in COVICAT, a prepandemic follow-up was done in 2018–2019 (), and over 90% of participants who participated in the 2018–2019 follow-up, participated in COVICAT. The smaller cohorts had not done a recent follow-up, and a similar percentage could not be calculated. Overall, among the 10,862 participants, 10,087 (92.9%) completed the questionnaire. Data collection was primarily completed on a study website where participants filled in the questionnaire. We administered the questionnaire via telephone when participants were not comfortable with online study participation (5.5% of the sample). All participants contacted from the cohort studies had consented in the past to be recontacted. Ethical approval for COVICAT was obtained from the Parc de Salut Mar Ethics Committee (CEIM-PS MAR, no. 2020/9307/I) and Hospital Universitari Germans Trias i Pujol Ethics Committee (CEI no. PI-20-182). All participants provided informed consent.
Outcomes
Our three primary outcomes were a) quantitative serologically confirmed infection to SARS-CoV-2, examined in the population with serology; b) antibody response among those infected based on levels of either immunoglobulin M (IgM), IgA, and IgG to viral antigens, examined in the population infected; and c) COVID-19 disease examined separately in the total population, the population with serology, and the population infected (Figure S1).
Epidemiological curves of the pandemic overtime.
In Spain the first recognized COVID-19 case was in 25 February 2020. Lockdown was applied on March 14 and lasted until the end of May 2020 during the period of the first wave of the pandemic. The second wave started in late October 2020. The evolution of the pandemic overtime in the different areas where the COVICAT study took place is shown in Figure S2 (SARS-CoV-2 positive tests over time), together with the time period when sampling for blood samples took place in the study. With the exception of one specific area (Lleida, 5.9% of the serological sample), the first wave of COVID-19 had already finished in all other areas by the time the first sampling period took place.
SARS-CoV-2 serology.
All participants were invited to participate in the serological study and 8,906 agreed (92.7%). We collected blood samples from 4,103 participants randomly selected from those agreeing to participate. Blood was drawn at the Blood and Tissue Biobank of Barcelona (85%) or through finger prick at the Barcelona Institute for Global Health (ISGlobal) or the participants’ residences (15%). Blood samples were processed within 24 h of collection and were analyzed at the ISGlobal Immunology laboratory in Barcelona. Levels of IgM, IgA, and IgG were assessed by high-throughput multiplex quantitative suspension array technology, including, as SARS-CoV-2 antigens, the spike full protein (S) [aa 1-1213 expressed in Expi293 expression system (ThermoFisher) and polyhistidine affinity tag (His-tag) purified] and the S2 fragment (S2; purchased from SinoBiologicals), the receptor-binding domain (RBD; donated by the Krammer laboratory, Mount Sinai, NY), the nucleocapsid full protein (NFL) and the nucleocapsid C-terminal region (NCt) (expressed in Escherichia coli and His-tag purified). Assay performance was previously established as 100% specificity and 95.78% sensitivity for seropositivity 14 d after symptoms onset (Dobaño et al. 2021). Assay positivity cutoffs specific for each isotype and analyte were calculated as described by Karachaliou et al. (2021).
Covid-19.
We defined cases of COVID-19 disease () as those reporting any of the following: COVID-19 hospital admission (); prior positive diagnostic test for SARS-CoV-2 infection (polymerase chain reaction, antigen test, or serology test, which during the first 3 months of the pandemic were nearly exclusively done among persons with symptoms (, not including hospitalized cases); or four COVID-19 related symptoms combined with being in contact with a diagnosed COVID-19 case (, not including cases in any in the previous two categories). This COVID-19 case definition was correlated with presence of antibodies by quantitative suspension array technology. We detected SARS-CoV-2 antibodies in 70% of self-reported cases and in 90% of participants reporting prior COVID-19 hospital admission. Severe COVID-19 disease ( in the total population; among serologically tested) was defined as having been admitted to the hospital or intensive care unit (ICU) or having had oxygen therapy without having been admitted to the hospital.
Covariates
Information on basic characteristics (age, sex, and educational level) was available from earlier contacts and verified in the COVICAT questionnaire (available in Spanish at http://www.gcatbiobank.org/media/upload/arxius/COVICAT/encuesta%20COVICAT.pdf). We recorded lifestyle factors, including smoking and physical activity and changes from prepandemic habits; work during the pandemic; use of masks; medical history, including prior diagnosis of any chronic disease including a list of several major diseases—such as cardiovascular, respiratory, diabetes, and kidney- and immune-related diseases—and admission to an ICU; as well as an open question on any other disease; height and weight; and mental health symptoms during the pandemic. We collected changes in residential address from the prepandemic period. We geocoded all prepandemic residential addresses to estimate air pollution exposure and link with the census tract-level deprivation index based on the 2011 census (Duque et al. 2021), and population density and degree of urbanization of the census tract of residence, using information from the 2011 census. The population average was 1,488.93 and the households average was 770.07 per census tract in Catalonia 2011 (INE 2011). Population density differs widely within Catalonia (https:www.idescat.cat), with an overall average density of in 2018 and a density of in Barcelona county (Barcelonès). Population density was categorized in quintiles. We estimated exposure to green spaces (percentage of greenness) in a buffer zone around the residence using the third version of the Land Cover Map of Catalonia (https://www.creaf.uab.es/mcsc/index_usa.htm; Ibàñez and Burriel 2010).
Air Pollution Exposure
We estimated exposure to , black carbon (BC), , and ozone () for the period 2018–2019 at participants’ prepandemic residential addresses using models developed by the Effects of Low-Level Air Pollution: A Study in Europe (ELAPSE) project (http://www.elapseproject.eu/). The model development and validation have been described in detail previously (de Hoogh et al. 2018). Briefly, models were Europe-wide hybrid land-use regression models incorporating air pollution monitoring data, satellite observations, dispersion model estimates, land use, and traffic variables as predictors. For , , and (warm season), models were based on 2010 measurements in the AirBase database maintained by the European Environmental Agency. For BC, models were developed and evaluated based on the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) monitoring data (Eeftens et al. 2012). The model was evaluated using 5-fold hold-out validation in random subsets of the monitoring data stratified by type of measurement and region of Europe. Models explained the following fractions of measured spatial variation in annual average concentrations in hold-out validation: Sixty-six percent for , 52% for BC, 58% for , and 63% for . Participants were assigned the annual average 2010 concentration based on predicted surfaces () from the ELAPSE model. We then applied a temporal correction to estimate exposures for the years 2018 and 2019 following protocols for temporal extrapolation developed in the ESCAPE project. Briefly, we used daily time-series data from the official routine monitoring network and calculated the ratios between the 2018–2019 period and 2010 for , nitrogen oxides (NOx), , and . We used NOx to adjust BC values given that BC is not currently measured at routine monitoring stations and that it is a primary combustion pollutant from traffic emissions with pollutant behavior similar to that of NOx. We used the average of 2018–2019 for each pollutant as our exposure metrics.
Statistical Analysis
We applied log-binomial regression models to estimate risk ratios (RRs) and 95% confidence intervals (CIs) separately for infection and COVID-19 disease. We used linear regression models to estimate the association between air pollution and antibody levels among SARS-CoV-2 infected participants. We applied multinomial regression models that allow for nominal dependent variables with more than two categories to estimate risk ratios ratios (RRRs) and 95% CIs between air pollution and COVID-19 severity (https://www.stata.com/manuals13/rmlogit.pdf). We identified confounders based on directed acyclic graphs (Figure S3). In the primary model, we included age, sex, educational level (less than primary school; primary school; secondary school; university and other) as a measure of socioeconomic position, an urban vulnerability index to measure census tract-level deprivation index (continuous variable between 0 and 1) and population density per kilometer squared. We adjusted for type of interview (online/telephone) to account for potential unidentified confounders associated with the assessment mode and cohort (assessment mode and cohort were correlated). In a second model, we also included prepandemic smoking habits (ever smoked at least 100 cigarettes or of tobacco vs. none) and physical activity [low, moderate, and high (according to the 2015 International Physical Activity Questionnaire categories)]. We estimated the association of air pollution on COVID-19 disease in the total population, as well as in two subgroups: those who provided blood samples and those with serologically confirmed SARS-CoV-2 infection. Participants with missing covariates were excluded from the complete-case analysis models.
For exposure–response analyses, we examined departures from linearity using generalized additive models (GAMs) with 2 degrees of freedom. We explored potential effect modification for factors that have been shown to be related with COVID-19 severity (Williamson et al. 2020), examining models by age (), sex, noncommunicable diseases (NCDs) (yes, no; self-reported prior diagnosis of cardiovascular, respiratory, diabetes, kidney, and immune-related diseases), obesity [prepandemic body mass index (BMI) ) and socioeconomic position [high (university) vs. low education (less than primary school)], area-based deprivation, degree of urbanization and population density (categorized into two strata). We calculated -values for interaction using a likelihood ratio test comparing models with and without the interaction term but with the same covariates. We defined a conventional and did not adjust for multiple comparisons. We performed all statistical analyses using Stata/SE (version 16; StataCorp LLC.).
Results
Study Population
Among the 10,097 participants who responded to the questionnaire, 9,926 (98%) had complete address information, of whom 9,605 (97%) had complete information on COVID-19 disease. From this total study population, 8,906 (92.7%) agreed to provide blood samples for serological testing and 4,103 participants were randomly selected and provided blood samples. Of those, 743 were infected with SARS-CoV-2. A description of the total population, the population with serological data and those infected is shown in Table 1. The distribution of demographic-, contextual-, and health-related factors were similar in the three populations, with the exception of COVID-19 disease, which was more frequent among those infected (16.2%) compared with the other two populations (5% and 4.5%), which included both infected and noninfected participants. Among the 9,605 participants, 9,035 (94%) were living in the same municipality prepandemic and during the pandemic. Among the 4,103 with serology, 3,920 (95.5%) did not change municipality.
Table 1.
Description of total COVICAT population, Catalonia, and subgroups with serological testing and positive antibodies for SARS-CoV-2 infection.
| Categories | Total population () | Participants with serology () | Participants with SARS-CoV-2 infection () |
|---|---|---|---|
| (%) | (%) | (%) | |
| Current age {y [mean (SD)]} | 55.3 (7.9) | 56 (8.1) | 56.4 (8) |
| Sex [female ()] | 5,656 (58.9) | 2,381 (58) | 433 (58.3) |
| Type of survey [telephone ()] | 556 (5.8) | 337 (8.2) | 64 (8.6) |
| SES [deprivation index (quintiles)] | — | — | — |
| 1 (least deprived) | 1,925 (20) | 893 (21.8) | 172 (23.1) |
| 2 | 1,917 (20) | 855 (20.8) | 165 (22.2) |
| 3 | 1,921 (20) | 839 (20.4) | 133 (17.9) |
| 4 | 1,926 (20.1) | 782 (19.1) | 151 (20.3) |
| 5 (most deprived) | 1,916 (19.9) | 734 (17.9) | 122 (16.4) |
| Educational level [ (%)] | — | — | — |
| Less than primary | 137 (1.4) | 59 (1.4) | 17 (2.3) |
| Primary | 1,013 (10.5) | 391 (9.5) | 68 (9.2) |
| Secondary | 4,001 (41.7) | 1,700 (41.4) | 314 (42.3) |
| University | 4,454 (46.4) | 1,953 (47.6) | 344 (46.3) |
| Smoking status before lockdown [ (%)] | — | — | — |
| Never smoker | 3,994 (41.6) | 1,709 (41.7) | 299 (40.4) |
| Ex-smoker | 4,049 (42.2) | 1,798 (43.9) | 348 (47) |
| Smoker | 1,548 (16.1) | 588 (14.4) | 93 (12.6) |
| Missing | 14 | 8 | 3 |
| Physical activity according to 2015 IPAQ categories [ (%)] | — | — | — |
| Low | 1,712 (18.8) | 719 (18.3) | 123 (17.4) |
| Moderate | 4,110 (45.2) | 1,825 (46.4) | 331 (47) |
| High | 3,280 (36) | 1,386 (35.3) | 251 (35.6) |
| Missing | 503 | 173 | 38 |
| Any chronic disease {yes [ (%)]} | 3,267 (34) | 1,427 (34.8) | 259 (34.9) |
| Respiratory, cardiometabolic, kidney, and immune-related chronic disease {yes [ (%)]} | 1,725 (18) | 754 (18.4) | 140 (18.8) |
| BMI before lockdown { [mean (SD)]} | 26.2 (4.3) | 26.2 (4.2) | 26.5 (4.3) |
| Missing | 19 | 6 | 1 |
| Obesity before lockdown {BMI | 1,631 (17) | 681 (16.6) | 130 (17.5) |
| Missing | 19 | 6 | 1 |
| COVID-19 disease {yes [ (%)]} | 481 (5) | 185 (4.5) | 121 (16.3) |
Note: —, no data available; BMI, body mass index; COVICAT, COVID-19 cohort in Catalonia study; IPAQ, International Physical Activity Questionnaire categories; SD, standard deviation; SES, socioeconomic status.
See text for the detailed COVID-19 disease definition, which was based on hospitalizations, positive tests, or four or more COVID-19 symptoms and contact with a diagnosed COVID-19 case.
Among the 9,605 participants in the total study population, 481 (5.0%) were classified as COVID-19 cases (Table S2). Cases were slightly younger than noncases and included women to a slightly higher proportion. There were no differences in education, area-level deprivation, or physical activity, and there were statistically significant but small differences in current smoking. Cases reported more prepandemic chronic diseases (22.7 vs. 17.7%) and were more likely to be obese (23.8 vs. 16.7%) than noncases (Table S2). The geographic distribution of the residence of COVID-19 cases and noncases in Catalonia did not show any marked differences (Figure S4).
The prevalence of serologically confirmed SARS-CoV-2 infection among participants providing blood samples () was 18.1% (IgM, 3.7%; IgA, 14.7%; IgG, 9.1%). Among those tested, 11.5% had an undetermined status, defined as a marginal positive response to one antigen–isotype combination. We considered participants with undetermined or seronegative status as not infected. Among participants tested for SARS-CoV-2 antibodies by the quantitative suspension array technology, the geographic distribution of the residence in Catalonia of those with COVID-19 disease compared with those who were nondiseased did not show any marked differences (Figure S5). Prevalence of infection was higher among obese persons (19.1%) compared with those with a BMI of (16.8%). Areas with higher population density had a slightly higher prevalence of infection (18.3%) compared with the least dense areas (17.6%), but the differences were modest (Table S3). The 699 participants who did not agree to participate in the serological study differed in several aspects from the 8,906 who agreed to provide blood samples although air pollution levels were similar (Table S4). Average levels were for nonparticipants compared with for participants, and the corresponding levels of were 16.3 vs. . Nonparticipants were older than participants (57.2 vs. 55.3 years of age), included a lower percentage of women (56.4% vs. 59.1%), were less likely to have higher education (40.2% vs. 46.9% in participants), had a higher prevalence of NCDs although they did not differ by several lifestyle factors, such as smoking, physical activity, and BMI.
Air Pollution Exposure
Mean exposure during 2018–2019 in the total study population was [] for and () for (Table 2). Pollution levels at residence were correlated with correlations between and , for and BC, as well as for and BC, and for and with (Table 2).
Table 2.
Distributions and Spearman correlation coefficients of air pollution concentrations (2018–2019 average) at the residence (), COVICAT study, Catalonia.
| Distributions/Spearman correlation coefficients | Mean (SD) | GM (95% CI) | P25–P75 |
|---|---|---|---|
| Air pollutants () | |||
| 34.14 (9.16) | 32.55 (32.33, 32.77) | 28.69–40.31 | |
| 16.25 (1.48) | 16.18 (16.15, 16.21) | 15.43–17.29 | |
| BC | 1.82 (0.39) | 1.77 (1.76, 1.78) | 1.62–2.06 |
| 65.00 (6.95) | 64.65 (64.52, 64.79) | 60.63–68.19 | |
| Spearman correlation coefficients | |||
| BC | |||
| 1 | — | — | |
| 0.816 | 1 | — | |
| BC | 0.786 | 0.726 | 1 |
Note: —, no data available; BC, black carbon; CI, confidence interval; COVICAT, COVID-19 cohort in Catalonia study; , nitrogen dioxide; , ozone; P, percentile; , particulate matter; SD, standard deviation.
Air Pollution and SARS-CoV-2 Infection
Among the 4,103 participants tested for SARS-CoV-2 infection, exposure to air pollutants was not associated with infection (Table 3). The adjusted RRs per interquartile range (IQR) were 1.07 (95% CI: 0.97, 1.18) for , 1.04 (95% CI: 0.94, 1.14) for , 1.00 (95% CI: 0.92, 1.09) for BC, and 0.97 (95% CI: 0.89, 1.06) for . Adjustment for lifestyle factors (i.e., physical activity and smoking) made a minimal difference to the effect estimates (Table 3). In the GAMs, we did not observe a departure from linearity for any of the four pollutants (Figure S6). Sensitivity analyses (Table S5) among participants without changes in residence, limiting analyses to those providing samples before July 31, and excluding participants with indeterminate serological results or adjusting for green spaces, did not provide a different pattern than what was observed overall (Table 3).
Table 3.
Risk ratios (RRs) and 95% confidence intervals (CIs) from log-binomial regression models, between air pollution at residence and SARS-CoV-2 infection determined through serology, COVICAT cohort, Catalonia. Associations reported per interquartile range.
| Air pollutant | RR (95% CI)a | RR (95% CI)b |
|---|---|---|
| 1.07 (0.97, 1.18) | 1.05 (0.95, 1.16) | |
| 1.04 (0.94, 1.14) | 1.02 (0.93, 1.13) | |
| BC | 1.00 (0.92, 1.09) | 0.98 (0.90, 1.08) |
| 0.97 (0.89, 1.06) | 0.98 (0.90, 1.07) |
Note: BC, black carbon; COVICAT, COVID-19 cohort in Catalonia study; , nitrogen dioxide; , ozone; , particulate matter.
Adjusted for age, sex, education (less than primary/primary/secondary/university), deprivation index (quintiles), population density, and type of survey (online/telephone).
Adjusted for age, sex, education (less than primary/primary/secondary/university), deprivation index (quintiles), population density, smoking (never/ex-smoker/smoker), physical activity (low/moderate/high), and type of survey (online/telephone).
Air Pollution and Antibody Response
Among those who were seropositive (), we observed a positive association for and , with IgG levels against all five viral target antigens and a negative association for (Figure 1; Table S6). We observed the strongest associations for anti-spike (S and S2) over anti-nucleocapsid responses (NFL and NCt). Results were inconsistent for IgM and IgA antibody levels (Figure 1; Table S6). An analysis by time since infection among seropositives with reported symptoms stratifying at 120 d (an approximate time for when IgA and IgG antibody levels may level off) did not show any systematic pattern in effect estimates for air pollution by latency (Table S7).
Figure 1.
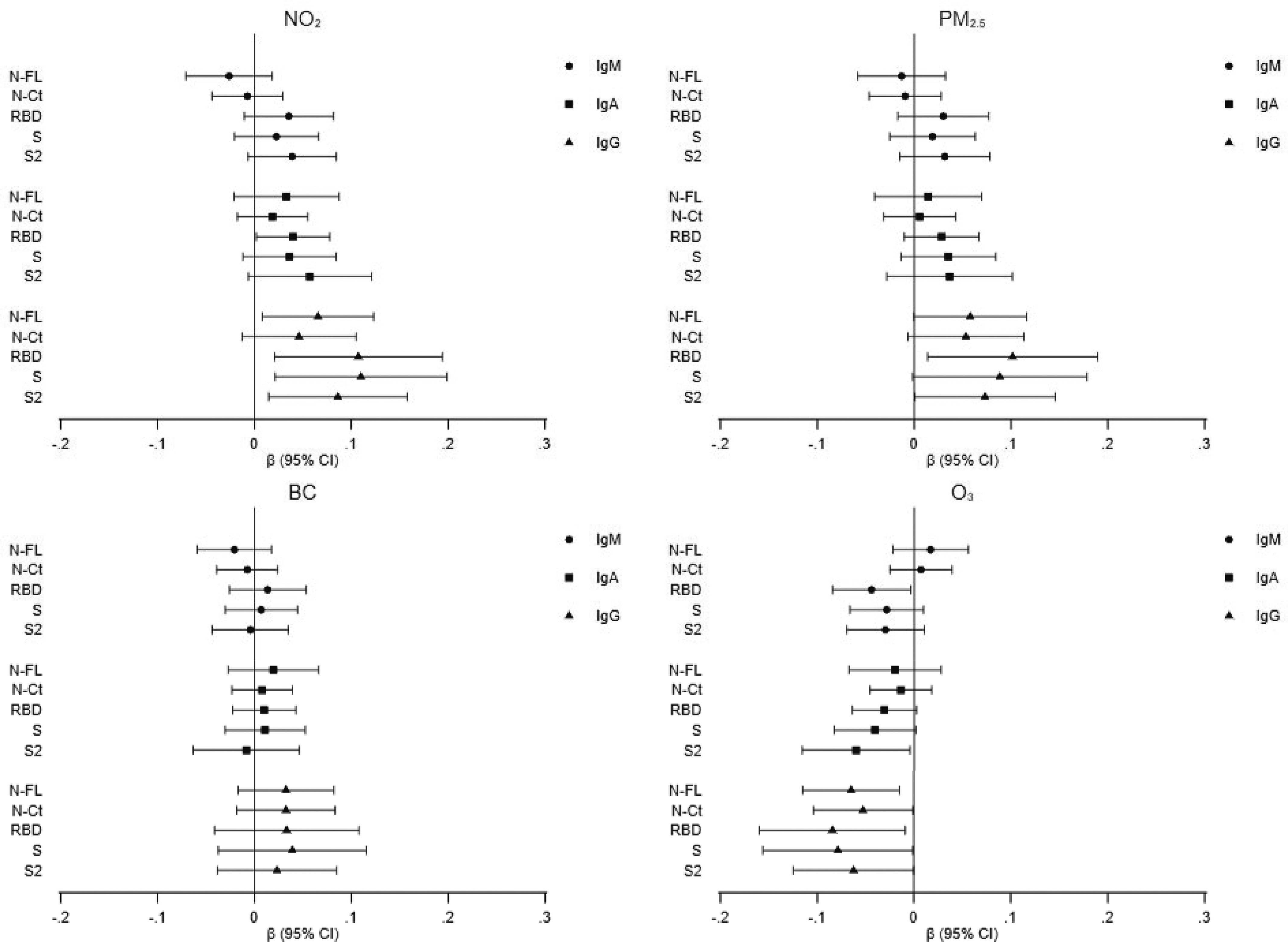
Association of air pollutants—(A) , (B) , (C) BC, and (D) )—with levels of IgM, IgA, and IgG against five viral target antigens among participants of the COVICAT study who were seropositive (). Linear regression beta coefficients and 95% CIs were adjusted for potential confounders. The model was adjusted for age, sex, education (less than primary/primary/secondary/university), deprivation index (quintiles), population density, type of survey (online/telephone), and batch. Precise numerical values are shown in Table S6. Note: BC, black carbon; CI, confidence interval; COVICAT, COVID-19 cohort in Catalonia study; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; NCt), nucleocapsid C-terminal region; NFL, nucleocapsid full protein; , nitrogen dioxide; , ozone; , fine particulate matter; RBD, receptor-binding domain; S, spike full protein; S2, S2 fragment.
Air Pollution and COVID-19 Disease
Table 4 shows associations between air pollution exposure and COVID-19 disease among a) the total study population (, b) those tested for antibodies to SARS-CoV-2 (), and c) those with serologically confirmed infection with SARS-CoV-2 (). We observed an overall pattern of positive associations between and exposure with COVID-19 disease in the total population, with statistically significant associations for [ (95% CI: 1.00, 1.29) per IQR] and [ (95% CI: 1.03, 1.32)]. There was no association with BC. Exposure to was associated with slightly decreased risk of COVID-19 disease [ (95% CI: 0.83, 1.03). In the GAMs, we observed a positive exposure–response and did not observe a departure from linearity (Figure S7). Among those with serology, the pattern of associations was similar to that with all participants and the magnitude of associations was larger: [ (95% CI: 1.07, 1.62) for ] and [ (95% CI: 1.06, 1.60)]. Associations were similar among those with serologically confirmed infection with SARS-CoV-2 compared with participants with serology although results were less precise. Additional adjustment for smoking and physical activity (Table 4) or proximity to green spaces (Table S8) made minimal difference.
Table 4.
Risk ratios (RRs) and 95% confidence intervals (CIs) from log-binomial regression models, between air pollution at residence and COVID-19 disease among the total COVICAT population and subgroups with serological testing and positive antibodies for SARS-CoV-2 infection.
| Population/air pollutant | RR (95% CI)a | RR (95% CI)b |
|---|---|---|
| Total population () | 9,605 | 9,088 |
| 1.14 (1.00, 1.29) | 1.16 (1.01, 1.32) | |
| 1.17 (1.03, 1.32) | 1.16 (1.02, 1.32) | |
| BC | 0.99 (0.89, 1.12) | 1.00 (0.89, 1.13) |
| 0.92 (0.83, 1.03) | 0.91 (0.82, 1.02) | |
| Participants with serology () | 4,103 | 3,922 |
| 1.31 (1.07, 1.62) | 1.35 (1.10, 1.67) | |
| 1.30 (1.06, 1.60) | 1.29 (1.05, 1.60) | |
| BC | 0.98 (0.81, 1.18) | 0.99 (0.82, 1.19) |
| 0.81 (0.68, 0.98) | 0.80 (0.66, 0.97) | |
| Participants with SARS-CoV-2 infection () | 743 | 702 |
| 1.23 (0.96, 1.56) | 1.26 (0.99, 1.60) | |
| 1.19 (0.93, 1.52) | 1.13 (0.90, 1.43) | |
| BC | 1.07 (0.86, 1.32) | 1.07 (0.86, 1.32) |
| 0.89 (0.72, 1.10) | 0.88 (0.71, 1.09) |
Note: Associations reported per interquartile range. BC, black carbon; COVICAT, COVID-19 cohort in Catalonia study; , nitrogen dioxide; , ozone; , particulate matter.
Adjusted for age, sex, education (less than primary/primary/secondary/university), deprivation index (quintiles), population density, and type of survey (online/telephone).
Adjusted for age, sex, education (less than primary/primary/secondary/university), deprivation index (quintiles), smoking (never/ex-smoker/smoker), physical activity (low/moderate/high), population density, and type of survey (online/telephone).
The association of air pollution with COVID-19 was stronger for severe disease that included mostly hospitalized cases and those in the ICU as compared with mild disease (Table 5), a pattern seen for the overall population and the subpopulation of those serologically tested. Similar to the overall results (Table 4), the strongest associations with severe disease were observed for exposure to and .
Table 5.
Risk ratios ratios (RRRs) and 95% confidence intervals (CIs) from multinomial logistic regression models between air pollution at residence and severity of COVID-19 disease among the total COVICAT population and subgroups with serological testing and positive antibodies for SARS-CoV-2 infection.
| Population/air pollutant | Mild cases | Severe/critical cases |
|---|---|---|
| RRR (95% CI)a | RRR (95% CI)a | |
| Total population (: 9,124 noncases, 413 mild cases, 68 severe/critical cases) | ||
| 1.12 (0.97, 1.30) | 1.26 (0.89, 1.79) | |
| 1.13 (0.98, 1.30) | 1.51 (1.06, 2.16) | |
| BC | 1.02 (0.90, 1.17) | 0.84 (0.62, 1.15) |
| 0.94 (0.83, 1.06) | 0.81 (0.59, 1.11) | |
| Participants with serology (: 3,918 noncases, 160 mild cases, 25 severe/critical cases) | ||
| 1.27 (1.01, 1.61) | 1.83 (1.01, 3.31) | |
| 1.24 (0.98, 1.57) | 2.12 (1.13, 3.96) | |
| BC | 0.95 (0.77, 1.17) | 1.21 (0.70, 2.09) |
| 0.84 (0.68, 1.04) | 0.59 (0.33, 1.06) | |
| Participants with SARS-CoV-2 infection (: 622 noncases, 100 mild cases, 21 severe/critical cases) | ||
| 1.18 (0.85, 1.65) | 1.84 (0.94, 3.58) | |
| 1.13 (0.80, 1.59) | 2.03 (0.99, 4.17) | |
| BC | 1.05 (0.79, 1.39) | 1.21 (0.68, 2.16) |
| 0.94 (0.70, 1.25) | 0.59 (0.31, 1.14) | |
Note: Associations reported per interquartile range. Severe COVID-19 was defined as having been admitted to the hospital or ICU, or having had oxygen therapy without having been admitted to the hospital. Mild cases included any other COVID-19 case. Noncases were the reference group. BC, black carbon; COVICAT, COVID-19 cohort in Catalonia study; ICU, intensive care unit; , nitrogen dioxide; , ozone; , particulate matter.
Adjusted for age, sex, education (less than primary/primary/secondary/university), 2011 census deprivation index (quintiles), population density and type of survey (online/telephone).
Factors Affecting the Association of Air Pollution with Infection, Level of Antibody Response, and COVID-19 Disease
Table 6 presents associations between air pollution and COVID-19 stratified by age, sex, individual-level education, area-level deprivation, previous diagnosis of chronic disease, obesity, and population density. Associations with (but not for other air pollutants) were stronger among participants of age, men, those of low education (), those living in more deprived census tracts, and among obese compared with nonobese participants; however, the interactions were not statistically significant. Similarly, no clear pattern was observed regarding effect modification for any of the characteristics examined for infection to SARS-CoV-2 (Table S9) or level of antibody response (Table S10 for IgG-RBD and Table S11 for IgG-NFL).
Table 6.
Risk ratios (RRs) and 95% confidence intervals (CIs) from log-binomial regression models, between prepandemic air pollution levels at residence and COVID-19 disease stratified by participant characteristics, COVICAT study.
| Category/air pollutant | RR (95% CI)a | RR (95% CI)a | -Valueb |
|---|---|---|---|
| Age [y ()] | (6,823) | (2,782) | — |
| 1.12 (0.98, 1.29) | 1.21 (0.91, 1.61) | 0.625 | |
| 1.14 (0.99, 1.30) | 1.33 (0.99, 1.79) | 0.317 | |
| BC | 1.02 (0.90, 1.16) | 0.89 (0.70, 1.12) | 0.281 |
| 0.94 (0.83, 1.05) | 0.86 (0.67, 1.10) | 0.529 | |
| Sex () | Female (5,656) | Male (3,949) | — |
| 1.16 (1.00, 1.35) | 1.09 (0.89, 1.33) | 0.575 | |
| 1.15 (0.99, 1.33) | 1.20 (0.98, 1.48) | 0.705 | |
| BC | 0.97 (0.84, 1.10) | 1.06 (0.88, 1.28) | 0.399 |
| 0.88 (0.77, 1.01) | 1.00 (0.84, 1.19) | 0.244 | |
| Educational level ()c | High (4,454) | Low (5,151) | — |
| 1.06 (0.89, 1.27) | 1.19 (1.01, 1.40) | 0.319 | |
| 1.04 (0.87, 1.23) | 1.28 (1.09, 1.51) | 0.065 | |
| BC | 0.96 (0.82, 1.13) | 1.02 (0.88, 1.19) | 0.570 |
| 0.95 (0.82, 1.11) | 0.90 (0.78, 1.03) | 0.542 | |
| Area-level deprivation ()d | High (3,842) | Low (5,763) | — |
| 1.04 (0.84, 1.28) | 1.17 (1.01, 1.35) | 0.347 | |
| 1.12 (0.91, 1.38) | 1.18 (1.02, 1.36) | 0.682 | |
| BC | 0.95 (0.81, 1.12) | 1.02 (0.89, 1.18) | 0.509 |
| 0.97 (0.81, 1.16) | 0.91 (0.80, 1.04) | 0.561 | |
| Previous diagnosis of chronic disease ()e | No (7,880) | Yes (1,725) | — |
| 1.18 (1.02, 1.35) | 0.97 (0.76, 1.24) | 0.166 | |
| 1.17 (1.02, 1.34) | 1.13 (0.88, 1.45) | 0.818 | |
| BC | 1.01 (0.89, 1.14) | 0.94 (0.76, 1.18) | 0.606 |
| 0.89 (0.79, 1.01) | 1.06 (0.87, 1.30) | 0.137 | |
| Obesity [ ()] | No (7,955) | Yes (1,631) | — |
| 1.12 (0.97, 1.29) | 1.15 (0.91, 1.46) | 0.862 | |
| 1.13 (0.98, 1.30) | 1.28 (1.00, 1.63) | 0.368 | |
| BC | 0.97 (0.86, 1.10) | 1.07 (0.85, 1.34) | 0.455 |
| 0.93 (0.82, 1.05) | 0.92 (0.75, 1.13) | 0.938 | |
| Degree of urbanization () | Suburb or rural (955) | City (8,650) | — |
| 0.98 (0.59, 1.61) | 1.17 (1.01, 1.36) | 0.495 | |
| 1.00 (0.70, 1.43) | 1.21 (1.05, 1.40) | 0.332 | |
| BC | 0.80 (0.43, 1.49) | 0.99 (0.88, 1.12) | 0.502 |
| 1.11 (0.82, 1.50) | 0.89 (0.78, 1.02) | 0.186 | |
| Population density ()f | Low (5,763) | High (3,842) | — |
| 1.13 (0.98, 1.30) | 1.15 (0.86, 1.52) | 0.923 | |
| 1.13 (0.98, 1.30) | 1.29 (0.99, 1.68) | 0.391 | |
| BC | 1.02 (0.89, 1.16) | 0.87 (0.68, 1.11) | 0.275 |
| 0.93 (0.82, 1.04) | 0.92 (0.72, 1.19) | 0.980 |
Note: Associations reported per interquartile range. —, no data available; BC, black carbon; BMI, body mass index; COVICAT, COVID-19 cohort in Catalonia study; , nitrogen dioxide; , ozone; , particulate matter; Q, quintile.
Adjusted for age, sex, education (less than primary/primary/secondary/university), deprivation index (continuous), population density, and type of survey (online/telephone).
-Value for likelihood ratio test for interaction.
University is considered as high educational level.
Low deprivation: Q3–Q5 of deprivation score; high deprivation: Q1–Q2 of deprivation score.
Previous diagnosis of any of the following: respiratory, cardiometabolic, kidney, or immune-related diseases.
Low density: Q1–Q3 of population density; high density: Q4–Q5 of population density.
Discussion
Our analysis, based on a well-characterized cohort including reliable and valid serological testing for infection measured through antibody response, resulted in several key findings. First, long-term exposure to prepandemic outdoor air pollution levels was not associated with SARS-COV-2 infection measured by antibody response. Second, air pollution exposure was positively associated with the magnitude of antibody response among seropositive participants. Third, exposure to and were positively associated with COVID-19 disease and with severity of the disease, with consistent patterns across the total study population and subgroups.
A key strength of the COVICAT study is the testing for SARS-CoV-2 antibodies in a large population using a comprehensive, sensitive, and specific test. Ascertainment of COVID-19 cases was based on hospital admissions and self-reported symptoms and testing, and this, similar to other studies, may have led to misclassification of COVID-19 disease as an outcome, with some mild cases not being identified. Extensive serological testing is particularly important given that a significant proportion of individuals infected with SARS-CoV-2 are asymptomatic [40% in our population, which is slightly higher than other reports indicating 30%; see, e.g., the review by Oran and Topol(2021)]. This allowed us to evaluate the association between air pollution and infection and to verify the association of air pollution on COVID-19 disease among those with confirmed infection. The prospective cohort study design, the high response rate among those invited for blood sample collection, the availability of individual information on potential confounders, and the robustness of the results across participant groups (e.g., all participants, participants with serology, participants who were seropositive) indicate that our results may be less affected by selection bias or confounding compared with previous studies. These sources of bias have been identified as important potential threats to validity in studies relating air pollution and COVID-19 outcomes (Villeneuve and Goldberg 2020; Griffith et al. 2020).
Although there are plausible biological mechanisms that could link long-term exposure to air pollution with SARS-COV-2 infection (Woodby et al. 2021), our results based on serological data did not provide support for this hypothesis. We applied a well-validated multiplex serology test measuring 15 isotype–antigen responses, which minimized the likelihood of undetectable infections. Our results do not indicate that long-term exposure to air pollution increases host susceptibility to infection, and recent evidence indicates that air pollutants are unlikely to be significant factors in the transmission of the virus (WMO 2021). This may be because other factors such as individual behaviors (e.g., social contacts, mask wearing, travel behavior) and public health control measures are much stronger determinants of transmission compared with air pollution.
Attenuation of antibody levels with time is physiologically expected due to decay of immune responses and transition of immunoglobulin production from short- to long-lived plasma cells. This is unlikely to have affected our results. Compared with most epidemiological studies that used IgG responses to only one antigen, we did simultaneous testing for multiple isotype–antigen combinations. Moreover, our own results (Dobaño et al. 2021; Ortega et al. 2021) and those of other studies (Dorigatti et al. 2021) have shown long-term persistence of seropositivity. For example, results from a study in the Italian town of Vo showed that 98.8% of seropositive people in the first wave still showed detectable levels of antibodies to at least one SARS-CoV-2 antigen 9 months later, regardless of whether they were symptomatic or not (Dorigatti et al. 2021). Furthermore, when we restricted our analysis to participants of the first sampling period (76% of all participants with serology sampled between June 23 and 31 July 2020), who would have been less likely to have lost antibodies, we still did not detect an association between air pollution and SARS-CoV-2 infection.
We observed positive associations between exposure to and and levels of IgG, which indicate a stronger humoral immune response. Toxicological evidence suggests exposure to particulate matter may contribute to disease severity by affecting respiratory immunity and host defense (Bauer et al. 2012), promoting viral replication, preventing uptake of infected cells by macrophages, and suppressing the antiviral adaptive immune response (Woodby et al. 2021). Particles have been associated with higher total IgG levels (Leonardi et al. 2000; Zhao et al. 2013). However, to our knowledge, there is scarce evidence in the wider scientific literature on the effects of air pollution on antibody levels of specific infections. We found that air pollution exposure is related to COVID-19 disease and disease severity, as well as to antibody levels, among seropositive individuals. The magnitude of antibody responses is strongly related to the severity of infection, with higher antibody levels observed among those experiencing more severe infection (Karachaliou et al. 2021).
Our results regarding the association between long-term exposure to air pollution and COVID-19 disease are comparable with the small number of individual-level studies linking long-term exposure to air pollution and incidence of COVID-19 disease using study designs that reduce the risk of selection bias due to asymptomatic infections (as reviewed by Vandenbroucke 2020). Results from the COVICAT cohort are consistent with the broader literature linking air pollution exposure with hospital admission for other viral respiratory infections, such as influenza and pneumonia (Croft et al. 2019; Domingo and Rovira 2020).
Air pollution could also be related to COVID-19 disease through its contribution to chronic conditions that increase susceptibility to more severe COVID-19 disease (Williamson et al. 2020). Long-term exposure to air pollution has been linked to incident diabetes (Zou et al. 2021), as well as cardiovascular (Yang et al. 2020) and chronic respiratory (Park et al. 2021) and neurodegenerative disease (Power et al. 2016). Owing to sample size limitations, we were not able to quantify the mediating role of these conditions in the air pollution-COVID-19 disease relationship. This should be a priority in future studies with larger sample sizes. However, our exploration of effect modification suggests a slightly stronger association between and COVID-19 disease among obese compared with nonobese participants, although the interaction term was not statistically significant. We also observed stronger associations between and COVID-19 among participants with low compared with high education. Prior evidence indicates individuals with lower socioeconomic position are more vulnerable to the adverse health effects of long-term exposure to (Di et al. 2017).
We did not perform phylogenetic analyses of viral genomes to confirm whether distinct strains were involved. Time-scaled phylogeny of sequences sampled in Europe, including Spain, up to the end of November 2020, shows that variant 20E (EU1) was identified in Spain in early summer 2020 (Hodcroft et al. 2021). This variant reached around 50% prevalence within a month of the first sequence detected and rose to 80%. There was no evidence of transmission advantage for the specific variant. Other variants occurring in the end of 2020 or later do not correspond to the period covered in this study.
Key strengths of our study are the use of individual-level data and include serological testing, which allowed us to avoid many sources of bias that are concerns in previous studies linking air pollution and COVID-19 disease. We adjusted for several individual-level confounders and for area-level deprivation together with population density and showed that associations with prepandemic levels of outdoor air pollution were observed even after adjusting for confounders. We did not evaluate the role of indoor environments, which have been shown to be a major factor for the spread of the infection, nor did we evaluate outdoor air pollution during the pandemic. Some, but not all, pollutants decreased considerably during the first months of the pandemic (Tobías et al. 2020), leading to complex relationships between short-term exposure to air pollution and health (Achebak et al. 2021).
The main limitations of our study include the relatively narrow age range of participants, which included few young or elderly adults, and the low response rates at first COVICAT contact for the full population although not for the selection of the subpopulation tested for SARS-CoV-2. Although we covered a wide age range, most participants were between 40 and 65 years of age. Lack of a broader age range limited our ability to test for effect modification by age. Among those contacted to participate in the study, 62% agreed to participate. The response rate was above 90% calculated based on the most recent prepandemic follow-up of the primary cohort contributing to COVICAT and above 90% for the serological study. Bias from nonresponse would occur if individuals with more symptoms were more likely to participate and if participation was related to prepandemic air pollution levels (or a correlate of this exposure). An evaluation of the spatial distribution of participants according to COVID-19 disease status or serological test for SARS-CoV-2 did not indicate pronounced spatial differences. The evaluation of those willing to participate in the serological study compared with the who indicated they would not provide blood samples did not indicate any significant differences in air pollution levels. However, we observed some differences in contextual and medical variables. Some of the differences were statistically significant (due to large numbers), but in absolute terms, differences were minor.
In summary, in this large cohort study, we examined prepandemic long-term exposure to air pollution in relation to infection with SARS-CoV-2, antibody response among those who were seropositive, and COVID-19 disease using individual-level exposure, covariate, and outcome data. Our results indicate that long-term exposure to air pollution was not associated with prevalence of infection with SARS-CoV-2 but that it was associated with stronger antibody response among infected individuals, probably reflecting higher viral exposure and disease severity. Indeed, our results add to the handful of previous studies based on individual-level data reporting a positive association between air pollution and COVID-19 disease. Our results, based on long-term exposure to prepandemic levels of air pollution, provide additional support for broad public health benefits of reducing levels of outdoor air pollution; nonetheless, it has been shown that SARS-CoV-2 transmits mostly between people at close range through inhalation (Tang et al. 2021) and, consequently, that the risk of transmission in outdoor environments is lower than those in indoor environments. Finally, our results add to the growing literature focused on the impact of environmental toxicants on the risk of infection and the interaction of environmental and infectious agents in the development of disease.
Supplementary Material
Acknowledgments
We extend our gratitude to all the Genomes for Life (GCAT) Study volunteers who participated in the study and to all Banc de Sang i Teixits workers for sample recruitment. We thank L. Mayer and J. Chi, who assisted with the antibody analyses. We also thank D. Casabonne and V. Moreno (Institut Català d’Oncologia, Barcelona) for their contribution to the Multi Case-Control (MCC)–Spain study.
We acknowledge support from the Incentius a l’Avaluació de Centres CERCA (in_CERCA); European Institute of Health and Technology [BP2020-20873 (to M.K.)], Certify.Health; Fundació Privada Daniel Bravo Andreu; Spanish Ministry of Science and Innovation [PID2019-110810RB-I00 (to L.I.)], the Spanish State Research Agency and Ministry of Science and Innovation through the Centro de Excelencia Severo Ochoa 2019–2023 Program [CEX2018-000806-S (to M.K.)], the Instituto de Salud Carlos III (ISCIII) [PI17/01555 (to C.O-G.)], and the Generalitat de Catalunya through the CERCA Program. This study made use of data generated by the GCAT study). The cohort study of the Genomes of Catalonia, Fundacio [Germans Trias i Pujol Research Institute (IGTP)]. IGTP is part of the CERCA Program/Generalitat de Catalunya. GCAT is supported by Acción de Dinamización del ISCIII-MINECO and by the Department of Health of the Generalitat of Catalunya (ADE 10/00026); the Agència de Gestió d’Ajuts Universitaris i de Recerca (2017-SGR 529). G.M. had the support of the Department of Health, Catalan Government (SLT006/17/00109). Development of SARS-CoV-2 reagents was partially supported by the National Institutes of Health National Institute of Allergy and Infectious Diseases Centers of Excellence for Influenza Research and Surveillance contract HHSN272201400008C. B.C. was supported by ISCIII national grant PI18/01512. The full list of the investigators who contributed to the generation of the GCAT data is available at http://www.genomesforlife.com.
References
- Achebak H, Petetin H, Quijal-Zamorano M, Bowdalo D, Pérez García-Pando C, Ballester J. 2021. Trade-offs between short-term mortality attributable to NO2 and O3 changes during the COVID-19 lockdown across major Spanish cities. Environ Pollut 286:117220, PMID: , 10.1016/j.envpol.2021.117220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo FJ, Finelli L, Swerdlow DL. 2021. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw Open 4(1):e2033706, PMID: , 10.1001/jamanetworkopen.2020.33706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, Balcells E, Benet M, Borrell E, et al. . 2018. Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (Urban Training) to increase physical activity in patients with COPD: a randomised controlled trial. Eur Respir J 52(4):1800063, PMID: , 10.1183/13993003.00063-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi P, Keshavarz Z, Cedeno Laurent JG, Stephens B, Allen JG. 2021. Mechanistic transmission modeling of COVID-19 on the Diamond Princess cruise ship demonstrates the importance of aerosol transmission. Proc Natl Acad Sci U S A 118(8):e2015482118, PMID: , 10.1073/pnas.2015482118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RN, Diaz-Sanchez D, Jaspers I. 2012. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain–like receptors. J Allergy Clin Immunol 129(1):14–26, PMID: , 10.1016/j.jaci.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdrel T, Annesi-Maesano I, Alahmad B, Maesano CN, Bind MA. 2021. The impact of outdoor air pollution on COVID-19: a review of evidence from in vitro, animal, and human studies. Eur Respir Rev 30(159):200242, PMID: , 10.1183/16000617.0242-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B, Xie Y, Gibson AK, Cai M, van Donkelaar A, Martin RV, et al. . 2021. Ambient fine particulate matter air pollution and the risk of hospitalization among COVID-19 positive individuals: cohort study. Environ Int 154:106564, PMID: , 10.1016/j.envint.2021.106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney PG, Luczynska C, Chinn S, Jarvis D. 1994. The European Community Respiratory Health Survey. Eur Respir J 7(5):954–960, PMID: , 10.1183/09031936.94.07050954. [DOI] [PubMed] [Google Scholar]
- Castaño-Vinyals G, Aragonés N, Pérez-Gómez B, Martín V, Llorca J, Moreno V, et al. . 2015. Population-based multicase-control study in common tumors in Spain (MCC-Spain): rationale and study design. Gac Sanit 29(4):308–315, PMID: , 10.1016/j.gaceta.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Chadeau-Hyam M, Bodinier B, Elliott J, Whitaker MD, Tzoulaki I, Vermeulen R, et al. . 2020. Risk factors for positive and negative COVID-19 tests: a cautious and in-depth analysis of UK Biobank data. Int J Epidemiol 49(5):1454–1467, PMID: , 10.1093/ije/dyaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DP, Zhang W, Lin S, Thurston SW, Hopke PK, Masiol M, et al. . 2019. The association between respiratory infection and air pollution in the setting of air quality policy and economic change. Ann Am Thorac Soc 16(3):321–330, PMID: , 10.1513/AnnalsATS.201810-691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoogh K, Chen J, Gulliver J, Hoffmann B, Hertel O, Ketzel M, et al. . 2018. Spatial PM2.5, NO2, O3 and BC models for Western Europe—evaluation of spatiotemporal stability. Environ Int 120:81–92, PMID: , 10.1016/j.envint.2018.07.036. [DOI] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. . 2017. Air pollution and mortality in the Medicare population. N Engl J Med 376(26):2513–2522, PMID: , 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaño C, Ramírez-Morros A, Alonso S, Vidal-Alaball J, Ruiz-Olalla G, Vidal M, et al. . 2021. Persistence and baseline determinants of seropositivity and reinfection rates in health care workers up to 12.5 months after COVID-19. BMC Med 19(1):155, PMID: , 10.1186/s12916-021-02032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaño C, Vidal M, Santano R, Jiménez A, Chi J, Barrios D, et al. . 2021. Highly sensitive and specific multiplex antibody assays to quantify immunoglobulins M, A, and G against SARS-CoV-2 antigens. J Clin Microbiol 59(2):e01731-20, PMID: , 10.1128/JCM.01731-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL, Rovira J. 2020. Effects of air pollutants on the transmission and severity of respiratory viral infections. Environ Res 187:109650, PMID: , 10.1016/j.envres.2020.109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigatti I, Lavezzo E, Manuto L, Ciavarella C, Pacenti M, Boldrin C, et al. . 2021. SARS-CoV-2 antibody dynamics and transmission from community-wide serological testing in the Italian municipality of Vo’. Nat Commun 12(1):4383, PMID: , 10.1038/s41467-021-24622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque I, Domínguez-Berjón MF, Cebrecos A, Prieto-Salceda MD, Esnaola S, Calvo Sánchez M, et al. . 2021. Índice de privación en españa por sección censal en 2011. [In Spanish.] Gac Sanit 35(2):113–122, PMID: , 10.1016/j.gaceta.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, et al. . 2012. Development of land use regression models for PM2.5, PM2.5 absorbance, PM10 and PMcoarse in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 46(20):11195–11205, PMID: , 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- Elliott J, Bodinier B, Whitaker M, Delpierre C, Vermeulen R, Tzoulaki I, et al. . 2021. COVID-19 mortality in the UK Biobank cohort: revisiting and evaluating risk factors. Eur J Epidemiol 36(3):299–309, PMID: , 10.1007/s10654-021-00722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera A, Martin C, Vlachos K, Sgubin G. 2020. Regional air pollution persistence links to COVID-19 infection zoning. J Infect 81(2):318–356, PMID: , 10.1016/j.jinf.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith GJ, Morris TT, Tudball MJ, Herbert A, Mancano G, Pike L, et al. . 2020. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun 11(1):5749, PMID: , 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodcroft EB, Zuber M, Nadeau S, Vaughan TG, Crawford KHD, Althaus CL, et al. . 2021. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature 595(7869):707–712, PMID: , 10.1038/s41586-021-03677-y. [DOI] [PubMed] [Google Scholar]
- Ibàñez JJ, Burriel JA. 2010. Mapa de cubiertas del suelo de Cataluña: características de la tercera edición y relación con SIOSE. [In Spanish.] In: La Información Geográfica al servicio de los ciudadanos: de lo global a lo local. 14th National Congress of Geographic Infomration Technolgoiges. Ojeda Zújar J, Vallejo Villata I, López MFP, eds. Seville, Spain: Secretariado de Publicaciones de la Universidad de Sevilla, 179–198. [Google Scholar]
- INE (Instituto Nacional de Estadística). 2011. Population and housing census. https://www.ine.es/en/censos2011_datos/cen11_datos_inicio_en.htm [accessed 27 October 2021].
- Johns Hopkins CRC (Johns Hopkins Coronavirus Resource Center). 2021. Cases/deaths by country/region/sovereignty table. Last updated 27 September 2021. https://coronavirus.jhu.edu/map.html [accessed 27 September 2021].
- Karachaliou M, Moncunill G, Espinosa A, Castaño-Vinyals G, Jiménez A, Vidal M, et al. . 2021. SARS-CoV-2 seroprevalence and characteristics of post-infection immunity in a general population cohort study in Catalonia, Spain. Res Sq. Preprint posted online 19 May 2021, 10.21203/rs.3.rs-448363/v1. [DOI] [Google Scholar]
- Leonardi GS, Houthuijs D, Steerenberg PA, Fletcher T, Armstrong B, Antova T, et al. . 2000. Immune biomarkers in relation to exposure to particulate matter: a cross-sectional survey in 17 cities of Central Europe. Inhal Toxicol 12(suppl 4):1–14, PMID: , 10.1080/08958370050164833. [DOI] [PubMed] [Google Scholar]
- Lipsitt J, Chan-Golston AM, Liu J, Su J, Zhu Y, Jerrett M. 2021. Spatial analysis of COVID-19 and traffic-related air pollution in Los Angeles. Environ Int 153:106531, PMID: , 10.1016/j.envint.2021.106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Feldman A, Heres D, Marquez-Padilla F. 2021. Air pollution exposure and COVID-19: a look at mortality in Mexico City using individual-level data. Sci Total Environ 756:143929, PMID: , 10.1016/j.scitotenv.2020.143929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelletti L, Martelletti P. 2020. Air pollution and the novel Covid-19 disease: a putative disease risk factor. SN Compr Clin Med 2:383–387, PMID: , 10.1007/s42399-020-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obón-Santacana M, Vilardell M, Carreras A, Duran X, Velasco J, Galván-Femenía I, et al. . 2018. GCAT|Genomes for Life: a prospective cohort study of the genomes of Catalonia. BMJ Open 8:e018324, PMID: , 10.1136/bmjopen-2017-018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oran DP, Topol EJ. 2021. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. [Video.] Ann Intern Med 174(5):655–662, PMID: , 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega N, Ribes M, Vidal M, Rubio R, Aguilar R, Williams S, et al. . 2021. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nat Commun 12(1):4740, PMID: , 10.1038/s41467-021-24979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim H-J, Lee C-H, Lee CH, Lee HW. 2021. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Environ Res 194:110703, 3341–7909, 10.1016/j.envres.2020.110703. [DOI] [PubMed] [Google Scholar]
- Power MC, Adar SD, Yanosky JD, Weuve J. 2016. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology 56:235–253, PMID: , 10.1016/j.neuro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb DM, Evans GJ, To TM, Lakey PSJ, Shiraiwa M, Hatzopoulou M, et al. . 2021. Within-City variation in reactive oxygen species from fine particle air pollution and COVID-19. Am J Respir Crit Care Med 204(2):168–177, PMID: , 10.1164/rccm.202011-4142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JW, Marr LC, Li Y, Dancer SJ. 2021. Covid-19 has redefined airborne transmission. BMJ 373:n913. 14n913, PMID: , 10.1136/bmj.n913. [DOI] [PubMed] [Google Scholar]
- Tobías A, Carnerero C, Reche C, Massagué J, Via M, Minguillón MC, et al. . 2020. Changes in air quality during the lockdown in Barcelona (Spain) one month into the SARS-CoV-2 epidemic. Sci Total Environ 726:138540, PMID: , 10.1016/j.scitotenv.2020.138540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke JP, Brickley EB, Vandenbroucke-Grauls CMJE, Pearce N. 2020. A test-negative design with additional population controls can be used to rapidly study causes of the SARS-CoV-2 epidemic. Epidemiology 31(6):836–843, PMID: , 10.1097/EDE.0000000000001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve PJ, Goldberg MS. 2020. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ Health Perspect 128(9):95001, PMID: , 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo T, Paudel K, Choudhary I, Patial S, Saini Y. 2020. Ozone exposure upregulates the expression of host susceptibility protein TMPRSS2 to SARS-CoV-2. bioRxiv. Preprint posted online 11 November 2020, PMID: , 10.1101/2020.11.10.377408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. . 2020. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821):430–436, PMID: , 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WMO (World Meteorological Organization). 2021. First Report of the WMO COVID-19 Task Team. Review on Meteorological and Air Quality Factors Affecting the COVID-19 Pandemic. WMO No. 1262. Geneva, Switzerland: World Meteorological Organization. https://www.preventionweb.net/publication/first-report-wmo-covid-19-task-team-review-meteorological-and-air-quality-factors [accessed 27 October 2021]. [Google Scholar]
- Woodby B, Arnold MM, Valacchi G. 2021. SARS-CoV-2 infection, COVID-19 pathogenesis, and exposure to air pollution: what is the connection? Ann N Y Acad Sci 1486(1):15–38, PMID: , 10.1111/nyas.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Nethery RC, Sabath MB, Braun D, Dominici F. 2020. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci Adv 6(45):eabd4049, PMID: , 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B-Y, Fan S, Thiering E, Seissler J, Nowak D, Dong G-H, et al. . 2020. Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ Res 180:108817, PMID: , 10.1016/j.envres.2019.108817. [DOI] [PubMed] [Google Scholar]
- Zhao J, Gao Z, Tian Z, Xie Y, Xin F, Jiang R, et al. . 2013. The biological effects of individual-level PM2.5 exposure on systemic immunity and inflammatory response in traffic policemen. Occup Environ Med 70(6):426–431, PMID: , 10.1136/oemed-2012-100864. [DOI] [PubMed] [Google Scholar]
- Zou L, Zong Q, Fu W, Zhang Z, Xu H, Yan S, et al. . 2021. Long-term exposure to ambient air pollution and myocardial infarction: a systematic review and meta-analysis. Front Med (Lausanne) 8:616355, PMID: , 10.3389/fmed.2021.616355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


