Abstract
OBJECTIVES:
To investigate potential mechanisms underlying the well-established relationship of diabetes and obesity with cognitive decline, among older adults participating in a population-based study.
DESIGN/SETTING:
10-year population-based cohort study.
PARTICIPANTS:
478 individuals aged 65+.
MEASUREMENTS:
We assayed fasting blood for markers of glycemia (glucose, HbA1c), insulin resistance (insulin, HOMA-IR), obesity (resistin, adiponectin, GLP-1), and inflammation (C-reactive protein). We modeled these indices as predictors of the slope of decline in global cognition, adjusting for age, sex, education, APOE*4 genotype, depressive symptoms, waist:hip ratio (WHR), and systolic blood pressure, in multivariable regression analyses of the entire sample and stratified by sex-specific median WHR. We then conducted WHR-stratified machine-learning (Classification and Regression Tree, CART) analyses of the same variables.
RESULTS:
In multivariable regression analyses, in the entire sample, HbA1c was significantly associated with cognitive decline. After stratifying by median WHR, HbA1c remained associated with cognitive decline in those with higher WHR. No metabolic indices were associated with cognitive decline in those with lower WHR. Cross-validated WHR-stratified CART analyses selected no predictors in participants older than 87–88 years. Faster cognitive decline was associated , in lower WHR participants <87 years, with adiponectin ≥ 11; and in higher WHR participants <88 years, with HbA1c ≥ 6.2%.
CONCLUSIONS:
Our population-based data suggest that, in individuals <88 years with central obesity, even modest degrees of hyperglycemia might independently predispose to faster cognitive decline. In contrast, among those <87 years without central obesity, adiponectin may be a novel independent risk factor for cognitive decline.
Keywords: hyperglycemia, abdominal obesity, adiponectin, epidemiology
INTRODUCTION
As life expectancy increases, chronic diseases of aging become more prevalent. Three conditions which are increasing in prevalence and co-occur more often than expected by chance are obesity, diabetes, and dementia.1 Several reviews and investigations have been undertaken to explain the mechanisms potentially underlying their relationships.2–4 Long-term prospective epidemiological studies have consistently demonstrated a pattern in which diabetes, high blood pressure (BP), and high body mass index (BMI) in midlife predict dementia in late life.5, 6 In contrast, when measured in later life, it is low BP and low BMI that are associated with the development of dementia in the near future. 7, 8 This paradox is inadequately understood but is assumed to reflect decreases in weight and BP that accompany the disease processes that causes dementia. 9 However, diabetes, whether measured in midlife or later life, is a consistent risk factor for dementia and cognitive decline.10, 11 It has been suggested that all three conditions should be examined together in evaluating their contributions to adverse cognitive outcomes.12 This purpose would be well-served by also examining indices of metabolic syndrome, including central obesity and insulin resistance (IR).13
In an ongoing prospective population-based study of older adults followed for ten years, we examined indices of diabetes, insulin resistance, obesity, inflammation, and BP in relation to the rate of cognitive decline. Given the paradoxical relationship of obesity with cognition and dementia, we investigated the associations of the indices of interest with cognitive decline separately among those with and without central obesity.
METHODS
The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) is a population-based cohort of individuals aged 65+ years, recruited during 2006–2008 by age-stratified random sampling from the voter registration lists for a group of contiguous small towns in southwestern Pennsylvania. The study is focused on the epidemiology of cognitive decline and dementia in a post-industrial area that has yet to recover from the economic devastation caused by the collapse of the steel manufacturing industry in the 1970s. Participants were assessed at study entry and at annual follow-up.14 The laboratory measures reported here were conducted on fasting blood samples obtained during 2014–2015 from individuals still alive and participating in the study.
Cognitive Outcome
At study entry and during annual follow-up of study participants, we administered a panel of neuropsychological tests tapping the domains of attention/processing speed, executive function, memory, language, and visuospatial function.15 We calculated global cognitive composite scores by averaging standardized composite scores for each domain at each annual assessment. We defined the slope of each participant’s global decline over time using subject-specific random slopes estimated from an unadjusted linear mixed model with random slope and intercept terms.16 Time was treated as a continuous variable, measured in years from baseline, and modeled as a linear fixed effect term.
Note that the cognitive outcome here is the slope of global cognitive decline throughout each individual’s duration of participation in the study, starting at study entry (baseline). We have not observed participants for a sufficient period since the blood draw to examine only subsequent cognitive decline. All 478 participants who provided fasting blood samples had at least 6 complete global cognitive composite scores at each assessment (at baseline and up to 9 annual follow-up assessments).
Predictors and Covariates
At study entry and annually, we measured blood pressure (BP), Body Mass Index (BMI) , waist:hip ratio (WHR),17 and depressive symptoms using the modified Center for Epidemiologic Studies Depression scale (mCES-D).18 Consenting participants provided blood samples for APOE genotyping. Fasting blood samples collected during 2014–2015 were assayed for glucose, insulin (calculating insulin resistance, HOMA-IR), HbA1c, resistin, adiponectin, glucagon-like peptide (GLP-1), and C-reactive protein (CRP). Laboratory assay methods and their coefficients of variation are detailed in Supplementary Text 1.
Statistical Analyses
We examined the distribution of all key variables at the time of blood draw, including age, sex, race (white vs nonwhite), education (≤ high school (HS) vs. > HS), APOE*4 allele carrier status, mCES-D score, BMI, WHR, systolic blood pressure (SBP) and the following laboratory assay variables: CRP, glucose, HbA1c, insulin, HOMA-IR, resistin, adiponectin, and GLP-1. Since metabolic variables were right-skewed, we used natural log-transformed values in the linear regression models. Additionally, all quantitative variables used in the linear regression analyses (age, mCES-D score, BMI, WHR, SBP, log-transformed metabolic indices, global cognitive decline slope) were centered and scaled to have mean zero and unit standard deviation.
Using linear regression models, we examined the relationships of each of the assay values to global cognitive decline, first individually and then jointly, unadjusted and adjusted for age, sex, education, APOE*4 carrier status, mCES-D score, WHR, and SBP. We then re-fit the models after stratifying by WHR, using sex-specific medians to equalize sample sizes and statistical power across groups.
Since a large proportion of the original 1982 participants recruited between 2006–2008 had died or dropped out before fasting blood was drawn between 2014–2015, and not all surviving participants consented to blood draw, we needed to address potential survivor (attrition) bias and nonresponse bias. We first compared demographics and APOE*4 frequencies of the subgroup with fasting blood to all remaining participants, using Welch’s t-test for continuous variables and Fisher’s exact test for categorical variables. We then refit the main multivariable regression models with inverse probability weighting (IPW) to account for potential attrition and nonresponse biases, using multiple imputations to address missing data where applicable. (See supplemental Text 2 for detailed methods and results.)
Finally, we used the machine learning approach of Classification and Regression Tree (CART) modeling19, stratified by sex-specific median WHR, to identify variables and their optimal respective thresholds (cutpoints) for predicting slope of cognitive decline, treating the outcome as continuous. Since CART is based on binary splits in the data, we used untransformed variables. We included all of the covariates and laboratory assay variables listed above. We performed 100 iterations of 5-fold cross-validation to choose the minimum terminal node size (selecting between 5 and 150, increasing in units of 5) and the optimal complexity parameter for each minimum terminal node size. We chose the minimum terminal node size based on the lowest average cross-validation error over the 100 iterations. We then applied the CART algorithm to the entire dataset and pruned it according to the cross-validation result. We calculated percent variance explained by the CART model. We used the R package rpart20 to fit the CART models and rattle21 to visualize the results.
All analyses were performed using R version 3.5.3 (R Core Team, Vienna, Austria).
RESULTS
Among 1982 participants who were recruited and underwent full assessment at baseline during 2006–2008, approximately eight years later we were able to obtain fasting blood samples from 478 individuals, with the characteristics shown in Table 1.
Table 1.
Characteristics of MYHAT cohort at time of fasting blood draw
| Covariates | Na | Mean (SD) | Median (25th, 75th percentile) |
|---|---|---|---|
| Age (years) | 478 | 81.9 (6.3) | 82 (76, 87) |
| Depression symptoms (mCES-D scoreb) | 477 | 1.89 (2.85) | 1 (1, 1) |
| Body Mass Index (BMI) | 417 | 28.3 (5.1) | 27.6 (24.9, 31.3) |
| Waist:Hip Ratio (WHR) | 411 | 0.90 (0.09) | 0.89 (0.84, 0.96) |
| Systolic blood pressure (SBP) mm Hg | 465 | 132.0 (14.1) | 130 (122, 140) |
| C-reactive protein (CRP) (mg/L) | 477 | 3.8 (5.5) | 1.9 (1.0, 4.1) |
| Na | n (%) | ||
| Female sex | 478 | 319 (66.7) | |
| Non-white race | 478 | 16 (3.3) | |
| Education > High School | 478 | 234 (49.0) | |
| APOE*4 carriage | 456 | 88 (19.3) | |
| Predictors of interest (metabolic indices) | Na | Mean (SD) | Median (25th, 75th percentile) |
| Glucose (mg/dL) | 477 | 103.4 (29.7) | 95 (87, 108) |
| Glycosulated hemoglobin (HbA1c) (%) | 467 | 6.3 (1.0) | 6.1 (5.8, 6.6) |
| Insulin (µU/mL) | 469 | 19.4 (20.4) | 14.5 (11.1, 20.6) |
| Insulin resistance (HOMA-IR ) | 469 | 5.3 (7.0) | 3.5 (2.5, 5.3) |
| Resistin (ng/mL) | 475 | 18.4 (10.7) | 16.4 (12.0, 22.2) |
| Adiponectin (µg/mL) | 477 | 13.1 (6.7) | 11.5 (8.7, 16.2) |
| Glucagon-like peptide-1 (GLP-1) (pg/mL) | 441 | 17.0 (71.8) | 2.2 (1.1, 6.2) |
number of participants with complete data
mCES-D score range: 0 to 20
Abbreviations: SD: standard deviation, HS: high school, mCES-D: Modified Center for Epidemiology Depression Scale. HOMA-IR: Homeostatic Model Assessment of Insulin Resistance.
Sample Characteristics
These 478 participants’ median age was 82 years; 66.7% were women; 96.7% white; 49.0% had greater than high school education (Table 1). Compared to the 1504 original participants without fasting blood data, at baseline, these 478 were significantly younger (74.6 years vs. 78.6 years, p<0.001); more likely to be women (66.7% vs. 59.2%, p=0.004); more likely to be of European descent (96.7% vs. 94.1%, p<0.001); more likely to have at least high school education (49.0% vs. 38.6%, p<0.001); but about equally likely to be APOE*4 carriers (19.3% vs. 21.5%, p=0.350).
Associations of Individual Predictors and Covariates with Cognitive Outcome
In unadjusted analyses in the sample as a whole (Table 2), faster cognitive decline was associated with greater age, less education, APOE*4 carriage, higher depression symptom (mCES-D) score, and higher adiponectin level There was no effect of sex and we had insufficient non-white participants to examine the effect of race. After adjustment for covariates (Table 2), only higher HbA1c was associated with cognitive decline in the fully adjusted model. Therefore, in subsequent multivariable analyses, we used HbA1c as the sole variable representing glycemia. Since CRP was not associated with the cognitive outcome in the unadjusted or adjusted models, we did not adjust for CRP in multivariable models in the interests of parsimony and statistical power.
Table 2.
Associations of predictor variables with global cognitive decline slope
| Unadjusted | Adjusted for age, sex, education, APOE*4, mCES-D score, WHR, and SBP | |||||
|---|---|---|---|---|---|---|
| Variable | Coef | 95% CI | p | Coef | 95% CI | p |
| age | −0.379 | (−0.462, −0.295) | <0.001 | |||
| female sex | 0.041 | (−0.150, 0.232) | 0.673 | |||
| education >HS | 0.212 | (0.033, 0.391) | 0.020 | |||
| APOE*4 carriage | −0.346 | (−0.581, −0.112) | 0.004 | |||
| mCES-D score | −0.131 | (−0.220, −0.042) | 0.004 | |||
| BMI | 0.067 | (−0.012, 0.147) | 0.096 | |||
| WHR | 0.009 | (−0.073, 0.090) | 0.835 | |||
| SBP | −0.017 | (−0.099, 0.065) | 0.682 | |||
| CRP | −0.009 | (−0.099, 0.081) | 0.846 | |||
| glucose | −0.031 | (−0.121, 0.060) | 0.505 | −0.055 | (−0.140, 0.030) | 0.206 |
| HbA1c | −0.034 | (−0.126, 0.058) | 0.469 | −0.093 | (−0.179, −0.008) | 0.033 |
| insulin | 0.045 | (−0.047, 0.136) | 0.337 | −0.001 | (−0.086, 0.083) | 0.974 |
| HOMA-IR | 0.026 | (−0.065, 0.118) | 0.574 | −0.020 | (−0.105, 0.066) | 0.652 |
| resistin | 0.009 | (−0.082, 0.099) | 0.851 | −0.011 | (−0.093, 0.071) | 0.791 |
| adiponectin | −0.098 | (−0.188, −0.008) | 0.032 | −0.008 | (−0.091, 0.076) | 0.860 |
| GLP-1 | −0.050 | (−0.146, 0.046) | 0.307 | −0.041 | (−0.124, 0.042) | 0.333 |
Note: Laboratory assay variables (CRP, glucose, HbA1c, insulin, HOMA-IR, resistin, adiponectin, GLP-1) were first natural log-transformed; then these and other quantitative variables (age, mCES-D score, BMI, WHR, SBP, global cognitive decline slope) were standardized to have mean zero and unit standard deviation.
Abbreviations: Coef: coefficient, CI: confidence interval, HS: high school, mCES-D: Modified Center for Epidemiology Depression Scale, BMI: body mass index, WHR: waist-hip ratio, SBP: systolic blood pressure, CRP: C-reactive protein, HbA1c: hemoglobin A1c, HOMA-IR: Homeostatic Model Assessment of Insulin Resistance, GLP-1: glucagon-like peptide-1.
Joint Associations of Predictors with Cognitive Outcome
We then examined the joint associations of resistin, adiponectin, GLP-1, and HbA1c with global cognitive decline slope in multivariable models: unadjusted (Supplemental Table S1) and adjusted for covariates (Table 3). Adiponectin was only associated with cognitive decline in the unadjusted model. HbA1c was the only significant metabolic predictor in the fully adjusted model. Age (coefficient: −0.26, SE: 0.04, p<0.001) and APOE*4 carriage (coefficient: −0.25, SE: 0.11, p=0.023) were also significant covariates in the fully adjusted model.
Table 3.
Joint associations of metabolic predictors with global cognitive decline slope, overall and stratified by WHR group
| Overall sample (n = 359) |
Low WHR (n = 184) |
High WHR (n = 175) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Coef | SE | p | Coef | SE | p | Coef | SE | p |
| resistin | −0.006 | 0.044 | 0.895 | 0.012 | 0.072 | 0.863 | −0.014 | 0.052 | 0.790 |
| adiponectin | −0.043 | 0.047 | 0.366 | −0.073 | 0.075 | 0.334 | −0.036 | 0.058 | 0.535 |
| GLP-1 | −0.039 | 0.042 | 0.361 | −0.058 | 0.064 | 0.361 | −0.008 | 0.055 | 0.884 |
| HbA1c | −0.105 | 0.047 | 0.027 | −0.064 | 0.084 | 0.450 | −0.148 | 0.053 | 0.006 |
Note: Models are adjusted for age, sex, education, APOE*4, mCES-D score, WHR, and SBP. Participants with missing data for any metabolic predictors or covariates (see Table 1) were excluded. Laboratory assay variables (resistin, adiponectin, GLP-1, HbA1c) were first natural log-transformed; then these and other quantitative variables (age, mCES-D score, BMI, WHR, SBP, global cognitive decline slope) were standardized to have mean zero and unit standard deviation.
Abbreviations: Coef: coefficient, SE: standard error, mCES-D: Modified Center for Epidemiology Depression Scale, WHR: waist-hip ratio, SBP: systolic blood pressure, HbA1c: hemoglobin A1c, GLP-1: glucagon-like peptide-1.
WHR-Stratified Joint Associations of Predictors with Cognitive Outcome
Since WHR measures central or abdominal obesity which is part of pre-diabetes/metabolic syndrome, we used WHR rather than BMI as our measure by which to determine whether obesity influences the associations of the metabolic indices with cognitive decline. In our sample, the median WHRs were 0.97 for men and 0.86 for women, consistent with the WHO definitions of abdominal obesity as WHR of ≥0.9 for men and ≥0.85 for women.22 We repeated the above regression analyses stratifying by WHR (sex-specific median split), (Table 3, Supplemental Tables S1, S2A, S2B).
In the lower WHR group, age, education, and APOE*4 carriage were associated with cognitive decline, but no other covariates or metabolic indices were associated in either the unadjusted or the adjusted models (Supplemental Table S2A). No metabolic indices were significant in the unadjusted (Supplemental Table S1) or adjusted (Table 3) multivariable models assessing their joint associations with cognitive decline. Only age (coefficient: −0.32, SE: 0.07, p<0.001) and APOE*4 carriage (coefficient: −0.49, SE: 0.16, p=0.002) were significant covariates in the adjusted model.
In the higher WHR group, age, but not education or APOE*4 carriage, was associated with cognitive decline. Additionally, higher glucose and HbA1c were associated with faster cognitive decline in both unadjusted and adjusted models (Supplemental Table S2B). In the models assessing the joint associations of predictors with cognitive decline, higher HbA1c was significantly associated with faster cognitive decline both when unadjusted (coefficient: −0.11, SE:0.05, p=0.032; Supplemental Table S1) and adjusted (coefficient: −0.15, SE: 0.05, p=0.006; Table 3); age was also a significant covariate in the adjusted model (coefficient: −0.18, SE: 0.06, p=0.002).
Addressing Potential Attrition and Nonresponse Bias
After using inverse probability weighting to account for attrition and nonresponse bias (See Supplemental Tables S5–S7), and multiple imputation to address missing data, the fully-adjusted association of HbA1c with cognitive decline remained significant only in the high WHR group (coefficient: −0.10, SE: 0.05, p=0.048) but not in the low WHR group or in the overall sample (Supplemental Table S6).
Classification and Regression Tree (CART) Models
In the CART analyses (Figure 1), stratifying by WHR (sex-specific median split) and including the same variables as the fully-adjusted multivariable regression models, results differed by both WHR (selected a priori ) and age (selected by the models)
Figure 1. Classification and regression tree (CART) results.
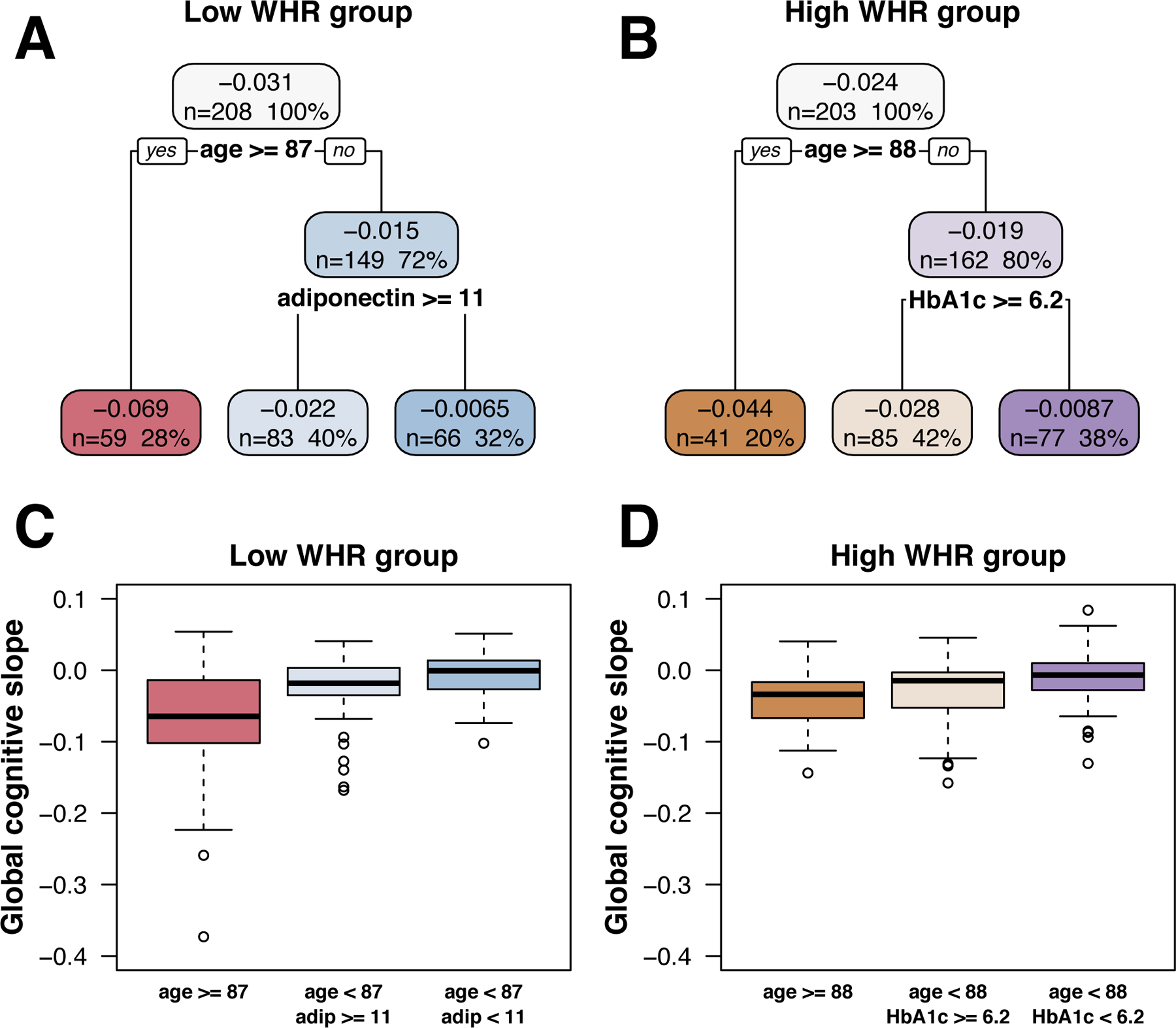
Top row shows the CART models predicting slope of global cognitive decline in (A) low WHR and (B) high WHR groups.
Topmost value in each box represents the mean global cognition slope (change in global composite score per year) for that subgroup, and, below that, the number and percentage of participants in that subgroup.
Boxplots in bottom row show the distributions of observed global cognition slopes in each corresponding CART subgroup for the low WHR (C) and high WHR (D) groups.
WHR: waist:hip ratio, age: age at time of fasting blood draw, adip: adiponectin, hgba1c: hemoglobin A1c.
In the lower WHR group, among those aged 87+, no covariates beyond age explained variance in slope of cognitive decline. In those aged less than 87, adiponectin ≥ 11 predicted faster decline (Figure 1, left). This model explained 21.0% of the variance in global cognition slope.
In the higher WHR group, in those aged 88+, no covariates beyond age explained variance in slope of cognitive decline. In those aged less than 88, HbA1c ≥ 6.2 % (44 mmol/mol) predicted faster decline (Figure 1, right). This model explained 11.6% of the variance.
See Supplemental Figure S1 for cross-validation results.
DISCUSSION
In this older population-based sample, predictors of the slope of cognitive decline varied by both age and abdominal obesity. Over and above the expected effects of aging and the APOE*4 genotype, certain subgroups in our study were at additional risk of faster cognitive decline up until their mid-80s. Among individuals younger than age 87–88 years, and with lower WHR, adiponectin ≥ 11 was associated with faster decline; while in the higher WHR group, HbA1c ≥ 6.2% predicted faster decline, according to the CART analysis. In the multiple regression analysis, the significant association of HbA1c with cognitive decline persisted after adjustment for age, sex, education, APOE*4 genotype5, systolic BP23, inflammation24, and depressive symptoms25.
Further, the CART analysis revealed that none of our examined variables predicted cognitive decline among those in their late 80s or older. Interestingly, the CART model empirically selected, for the lower and higher WHR groups. the age thresholds of 87 years and 88 years, which is consistent with the median age at dementia onset we reported earlier from our study. 14 In fact, no late-life vascular or metabolic risk have yet been reported for dementia among the “oldest old”. 14, 26, 27 This lack has been attributed to the increasing age-related disassociation of cognitive functioning with neuropathology, reflecting the highly mixed and heterogeneous nature of brain disease in this age group28, including not only cerebrovascular disease but also hippocampal sclerosis and TDP-43 proteinopathy 29.
Diabetes is a well-established risk factor for cognitive decline and dementia, but it remains unclear which of the many diabetes-related metabolic, biochemical, and hormonal influences on the brain drive that association. Prevailing opinion focuses on three potential pathophysiologic mechanisms: recurrent/frequent hypoglycemia, chronic hyperglycemia, and insulin resistance/obesity, as discussed below.
In a large observational study, severe hypoglycemia was found to be associated with accelerated cognitive decline in patients with Type 2 diabetes.30 However, hypoglycemia did not increase the risk of incident cognitive dysfunction in almost 12,000 patients with Type 2 diabetes in the ORIGIN trial.31 As only 30 (6.2%) of our participants had fasting glucose levels <80 mg/dL, we could not investigate this possibility in our data.
The second potential pathophysiologic mechanism is chronic hyperglycemia associated with poor glycemic control. In our study, HbA1c was the only variable significantly associated with cognitive decline among those with abdominal obesity. This finding is consistent with the abundant evidence implicating glycemic exposure as the fundamental pathophysiologic mechanism involved in diabetic microangiopathy, which is the pathognomonic feature of all diabetes-related microvascular complications, including retinopathy, nephropathy, and neuropathy.
Chronic hyperglycemia is also a factor in accelerated atherogenesis, the pathologic foundation of diabetic macrovascular disease which includes cardiovascular, peripheral vascular, and cerebrovascular disease. Absent neuroimaging data, we cannot determine how much of the cognitive decline observed in our cohort might be attributable to infarcts or significant white matter disease. However, an important risk factor for cerebrovascular disease is hypertension32; but higher SBP was not significantly associated with cognitive decline in this cohort whose mean age was 82 years This likely reflects the well-established observation that low, rather than high, systolic pressure in late life is associated with adverse cognitive outcomes.8 We do not, of course, know what our participants’ BPs were in midlife.
Our results allow us to postulate cautiously that microvascular disease was more likely than macrovascular disease to be the basis for the cognitive decline in our cohort. Cognitive decline was not significantly correlated with either WHR or BMI, the two most powerful predictors of insulin resistance, which is the foundation for accelerated atherogenesis in diabetes, and the other diabetes-related pathophysiologic mechanism implicated in cognitive decline. If anything, it was the reverse, because adiponectin, a well-established negative correlate of obesity, IR, and DM 33, was not significant in the high WHR group, while HbA1c was. Although there is no direct evidence that microvascular disease mediates neuronal dysfunction in the cerebral cortex, there is evidence that neuronal damage in the retina precedes clinical evidence of diabetic retinopathy.34 Taken together, these findings lend support to the notion that chronic hyperglycemia, rather than underlying metabolic state (obesity and IR), was the likely pathophysiologic mechanism underlying cognitive decline in our cohort of older adults. Since IR is a precursor to diabetes, its effects in older adults might be overwhelmed or superseded by the downstream manifestations of chronic hyperglycemia, which acts as a metabolic ‘toxin’ causing microvascular disease. IR, on the other hand, may be more potent as a macrovascular enabler.2, 35, 36
Some authorities37 have discussed the effect of insulin signaling on neurons and glia, and the concept of “brain insulin resistance” which is arguably intrinsic to AD and related dementias. We have no brain data, but, given the lack of association between peripheral IR and cognitive decline in our human population-based cohort, it is plausible that we would also not have found an association with brain IR, given the relatively advanced age of our cohort. Additionally, inflammation, oxidative stress, and mitochondrial dysfunction are common to both diabetes and AD and may even be part of a mechanism linking metabolic dysfunction to neurodegeneration. However, our inflammation measure, CRP, was not associated with slope of cognitive decline. 38
Obesity in midlife, but not late life,39 is a risk factor for dementia. In the current study we have no midlife data, and in our late-life data we found no association between WHR or BMI and slope of cognitive decline. However, higher adiponectin level predicted steeper cognitive decline in the lower WHR group. This finding potentially reflects loss of body fat, which is known to be associated with aging and also with increased risk of dementia.7, 39 Adiponectin which mobilizes fat has been found associated with AD and all-cause dementia in women.40 Another potential explanation for age-associated weight decrements in individuals with diabetes is loss of muscle mass. Myokines secreted by skeletal muscle are involved in insulin resistance41, which, as noted earlier, might play a role in neurodegeneration. We have no data on muscle with which to explore this mechanism in our study, and it would not explain our finding regarding adiponectin.
Our large, well-characterized, population-based cohort, with ten years of prospectively collected cognitive data, allows us to cautiously generalize our results to the type of communities from which the cohort was drawn. However, we were only able to collect fasting blood specimens (necessary to measure indices of hyperglycemia and insulin resistance) in a subgroup, approximately eight years into the study, raising concerns about both survival bias and some degree of response bias. At baseline, those who eventually provided specimens were younger, better educated, more likely to be women, and more likely to be white, than the rest of the cohort. Using inverse probability weighting to correct for survival and response bias, our multiple regression analyses findings were slightly attenuated but still remained significant. Although we were able to examine participants’ slope of cognitive decline throughout the study, ideally, these questions should be addressed in a cohort starting in midlife. We lack neuroimaging or retinal data that could have directly assessed the amount of cerebrovascular and neurodegenerative damage in our study participants. Finally, our study cohort was of largely European descent, representing the race/ethnicity of the older adults in the targeted communities. Our findings should therefore be replicated in other cohorts with larger minority representation.
In conclusion, our population-based data suggest that, among individuals <87 years without central obesity, adiponectin may be a novel independent risk factor for cognitive decline; the underlying mechanism warrants further investigation. In contrast, in individuals <88 years with central obesity, even modest degrees of hyperglycemia are associated with, and might independently predispose, to faster cognitive decline. This information may help target different strategies to different subgroups of older adults for the prevention of cognitive decline.
Supplementary Material
SUPPORTING INFORMATION in online supplementary files.
Additional Supporting Information may be found in the online version of this article.
Supplementary Text 1. Methods: Laboratory measures
Supplementary Table S1. Unadjusted joint associations of metabolic predictors with global cognitive decline slope, overall and stratified by WHR group
Supplementary Table S2A. Associations with global cognitive decline slope: Low WHR group (n = 208)
Supplementary Table S2B. Associations with global cognitive decline slope: High WHR group (n = 203)
Supplementary Text 2. Multiple imputation and inverse probability weighting methods.
Supplementary Table S3. Baseline variables used in IPW logistic regression model (n = 1982)
Supplementary Table S4. Estimates for logistic regression model of being in the fasting blood sample group (n = 1982)
Supplementary Table S5. Variables used in IPW multivariable regression models assessing the joint associations of metabolic predictors with cognitive decline slope (n = 478)
Supplementary Table S6. Joint associations of metabolic predictors with cognitive decline slope, overall and stratified by WHR group, with imputation for missing data and inverse probability weighting to generalize to the entire MYHAT cohort
Supplementary Figure S1. CART cross-validation results
ACKNOWLEDGMENTS
The authors are grateful to all participants in the MYHAT study. They also thank all MYHAT project staff. They particularly thank Ms. Beth Hauth who performed the chemistry assays in the Heinz Laboratory, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, and Ms. Erin Jacobsen for assistance with manuscript formatting.
Funding Source:
The work reported here was supported in part by research grant R01AG023651 from the National Institute on Aging. The sponsor played no role in the design, methods, subject recruitment, data collections, analysis and preparation of this paper.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
REFERENCES
- 1.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018;14:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbagallo M, Dominguez LJ. Type 2 diabetes mellitus and Alzheimer’s disease. World J Diabetes 2014;5:889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochim Biophys Acta 2009;1792:470–481. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman RF, Albert MS, Alonso A et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol 2017;74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–281. [DOI] [PubMed] [Google Scholar]
- 7.Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc 2008;56:111–116. [DOI] [PubMed] [Google Scholar]
- 8.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology 2003;61:1667–1672. [DOI] [PubMed] [Google Scholar]
- 9.Singh-Manoux A, Dugravot A, Shipley M et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement 2018;14:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuligenga RH, Dugravot A, Tabak AG et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol 2014;2:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes 2009;58:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74. [DOI] [PubMed] [Google Scholar]
- 13.Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Gerontology 2009;55:379–386. [DOI] [PubMed] [Google Scholar]
- 14.Ganguli M, Lee CW, Snitz BE, Hughes TF, McDade E, Chang CC. Rates and risk factors for progression to incident dementia vary by age in a population cohort. Neurology 2015;84:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganguli M, Snitz BE, Lee CW, Vanderbilt J, Saxton JA, Chang CC. Age and education effects and norms on a cognitive test battery from a population-based cohort: the Monongahela-Youghiogheny Healthy Aging Team. Aging Ment Health 2010;14:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamboh MI, Fan KH, Yan Q et al. Population-based genome-wide association study of cognitive decline in older adults free of dementia: identification of a novel locus for the attention domain. Neurobiol Aging 2019; [DOI] [PMC free article] [PubMed]
- 17.Liu Z, Yang H, Chen S, Cai J, Huang Z. The association between body mass index, waist circumference, waist-hip ratio and cognitive disorder in older adults. J Public Health (Oxf) 2019;41:305–312. [DOI] [PubMed] [Google Scholar]
- 18.Ganguli M, Gilby J, Seaberg E, Belle S. Depressive Symptoms and Associated Factors in a Rural Elderly Population: The MoVIES Project. Am J Geriatr Psychiatry 1995;3:144–160. [DOI] [PubMed] [Google Scholar]
- 19.Breiman LFJ, Olshen RA, Stone CJ. Classification and Regression Trees. Belmont, CA: Wadsworth, 1984. [Google Scholar]
- 20.Therneau TAB, Ripley B. rpart: Recursive Partitioning and Regression Trees. 2018.
- 21.GJ W Data Mining with Rattle and R: The Art of Excavating Data for Knowledge Discovery: Springer, 2011.
- 22.Organization WH. Waist circumference and waist-hip ratio: Report of a WHO expert consultation. Geneva: Organization WH, 2008. [Google Scholar]
- 23.Skoog I, Lernfelt B, Landahl S et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 24.Engelhart MJ, Geerlings MI, Meijer J et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 2004;61:668–672. [DOI] [PubMed] [Google Scholar]
- 25.Katon W, Lyles CR, Parker MM, Karter AJ, Huang ES, Whitmer RA. Association of depression with increased risk of dementia in patients with type 2 diabetes: the Diabetes and Aging Study. Arch Gen Psychiatry 2012;69:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner RC, Valcour V, Yaffe K. Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimers Res Ther 2013;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia 2006;49:2015–2023. [DOI] [PubMed] [Google Scholar]
- 28.Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: the 90+ study. Curr Alzheimer Res 2012;9:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson PT, Dickson DW, Trojanowski JQ et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 2019;142:1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feinkohl I, Aung PP, Keller M et al. Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2014;37:507–515. [DOI] [PubMed] [Google Scholar]
- 31.Cukierman-Yaffe T, Bosch J, Jung H, Punthakee Z, Gerstein HC. Hypoglycemia and Incident Cognitive Dysfunction: A Post Hoc Analysis From the ORIGIN Trial. Diabetes Care 2019;42:142–147. [DOI] [PubMed] [Google Scholar]
- 32.Arvanitakis Z, Capuano AW, Lamar M et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology 2018;91:e517–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev 2005;6:13–21. [DOI] [PubMed] [Google Scholar]
- 34.Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol 2008;586:4401–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyle PJ. Diabetes mellitus and macrovascular disease: mechanisms and mediators. Am J Med 2007;120:S12–17. [DOI] [PubMed] [Google Scholar]
- 36.Hyperglycemia Laakso M. and cardiovascular disease in type 2 diabetes. Diabetes 1999;48:937–942. [DOI] [PubMed] [Google Scholar]
- 37.Arnold SE, Arvanitakis Z, Macauley-Rambach SL et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 2018;14:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wennberg AMV, Hagen CE, Machulda MM, Knopman DS, Petersen RC, Mielke MM. The Cross-Sectional and Longitudinal Associations Between IL-6, IL-10, and TNFalpha and Cognitive Outcomes in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci 2018; [DOI] [PMC free article] [PubMed]
- 39.Fitzpatrick AL, Kuller LH, Lopez OL et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol 2009;66:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Himbergen TM, Beiser AS, Ai M et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: results from the Framingham Heart Study. Arch Neurol 2012;69:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckardt K, Gorgens SW, Raschke S, Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia 2014;57:1087–1099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION in online supplementary files.
Additional Supporting Information may be found in the online version of this article.
Supplementary Text 1. Methods: Laboratory measures
Supplementary Table S1. Unadjusted joint associations of metabolic predictors with global cognitive decline slope, overall and stratified by WHR group
Supplementary Table S2A. Associations with global cognitive decline slope: Low WHR group (n = 208)
Supplementary Table S2B. Associations with global cognitive decline slope: High WHR group (n = 203)
Supplementary Text 2. Multiple imputation and inverse probability weighting methods.
Supplementary Table S3. Baseline variables used in IPW logistic regression model (n = 1982)
Supplementary Table S4. Estimates for logistic regression model of being in the fasting blood sample group (n = 1982)
Supplementary Table S5. Variables used in IPW multivariable regression models assessing the joint associations of metabolic predictors with cognitive decline slope (n = 478)
Supplementary Table S6. Joint associations of metabolic predictors with cognitive decline slope, overall and stratified by WHR group, with imputation for missing data and inverse probability weighting to generalize to the entire MYHAT cohort
Supplementary Figure S1. CART cross-validation results


