Abstract
Background:
Raised low-density lipoprotein cholesterol (LDL-C) in young adulthood (ages 18–39 years) is associated with atherosclerotic cardiovascular disease (ASCVD) later in life. Most young adults with elevated LDL-C do not currently receive lipid-lowering treatment.
Objectives:
We aimed to estimate the prevalence of elevated LDL-C in ASCVD-free U.S. young adults and the cost-effectiveness of lipid-lowering strategies for raised LDL-C in young adulthood compared with standard care.
Methods:
Prevalence of raised LDL-C was examined in the U.S. National Health and Nutrition Examination Survey. The CVD Policy Model projected lifetime quality-adjusted life years (QALYs), healthcare costs, and incremental cost-effectiveness ratios (ICERs) for lipid-lowering strategies. Standard care was statin treatment for adults aged ≥40 years based on LDL-C, ASCVD risk, or diabetes plus young adults with LDL-C ≥190 mg/dL. Lipid-lowering incremental to standard care with moderate-intensity statins or intensive lifestyle interventions was simulated starting when young adult LDL-C was either ≥160 mg/dL or ≥130 mg/dL.
Results:
Around 27% of ASCVD-free young adults have LDL-C ≥130 mg/dL, and 9% have LDL-C ≥160 mg/dL. The model projected that young adult lipid-lowering with statins or lifestyle interventions would prevent lifetime ASCVD events and increase QALYs compared with standard care. ICERs were $31,000/QALY for statins in young adult men with LDL-C ≥130 mg/dL, and $106,000/QALY for statins in young adult women with LDL-C ≥130 mg/dL. Intensive lifestyle intervention was more costly and less effective than statin therapy.
Conclusion:
Statin treatment for LDL-C ≥130 mg/dL is highly cost-effective in young adult men and intermediately cost-effective in young adult women.
Keywords: Young adulthood, cholesterol, cardiovascular disease, statins, cost-effectiveness
CONDENSED ABSTRACT:
Raised low-density lipoprotein cholesterol (LDL-C) in young adulthood is associated with atherosclerotic cardiovascular disease (ASCVD) later in life. Most young adults with elevated LDL-C do not receive lipid-lowering treatment. We used the CVD Policy Model to estimate cost-effectiveness of lipid lowering with moderate-intensity statins or intensive lifestyle interventions for raised LDL-C in young adulthood compared with standard care. Statin treatment for LDL-C ≥130 mg/dL in young adulthood was projected to be highly cost-effective in men and intermediately cost-effective in women. Intensive lifestyle interventions were more costly and less effective than statins for young adult lipid lowering.
Introduction
Raised low density-lipoprotein cholesterol (LDL-C) in young adulthood (ages 18 to 39 years) increases risk of atherosclerotic cardiovascular disease (ASCVD) later in life (1–3). At present, only a small proportion of young adults with raised LDL-C are recommended for lipid-lowering treatment.
HMG Co-A reductase inhibitors (‘statins’) substantially lower LDL-C, are cost-effective for primary ASCVD prevention in adults aged 40–74 years, and are available in low-cost, generic formulations (4,5). Intensive lifestyle interventions involving face-to-face or group-based behavioral counseling to improve diet, physical activity, or both, alongside regular primary care significantly reduce LDL-C, blood pressure, and body mass index (6).
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) clinical guideline strongly recommended statins to prevent ASCVD in all adults with LDL-C ≥190 mg/dL, and those aged 40–74 years with 10-year ASCVD risk ≥7.5% or diabetes (7). The guideline recommended lifestyle modifications for all young adults with elevated LDL-C but restricted statin treatment to young adults with LDL-C ≥190 mg/dL. The United States Preventive Services Task Force has made no specific recommendation for the management of raised LDL-C in young adults (8).
Expanding statin or intensive lifestyle intervention eligibility for young adults could substantially improve population health (9). We compared the clinical and economic impact of initiating lipid-lowering strategies for raised LDL-C in young adulthood to 2018 ACC/AHA guideline-recommended standard care using a mathematical model that simulated long-term ASCVD effects of LDL-C exposures and lipid-lowering interventions.
Methods
We obtained Columbia University Institutional Review Board approval to perform secondary analysis on individual participant data from National Institutes of Health observational cohort studies. All other data sources were publicly accessible or published summary data.
Number of Treatment-Eligible Young Adults
To project the number of U.S. young adults eligible for lipid-lowering therapies, we examined the age-stratified distribution of LDL-C (<100 mg/dL, 100–129 mg/dL, 130–159 mg/dL, 160–189 mg/dL, and ≥190 mg/dL) among ASCVD-free U.S. adults in the National Health and Nutrition Examination Survey (NHANES) 1999–2014, scaled to 2020 U.S. population estimates (10,11). We also examined self-reported cholesterol screening rates to contextualize awareness of raised LDL-C (Supplemental Appendix).
CVD Policy Model
A microsimulation version of the CVD Policy Model was developed in TreeAge Pro, version 2020 (TreeAge Software, Inc., Williamstown, Massachusetts). The model estimated lifetime health-related quality of life and direct healthcare costs (2020 USD) for LDL-C-reduction strategies in a nationally representative cohort of ASCVD-free U.S. young adults. The CVD Policy Model uses time-varying ASCVD risk factor exposures to simulate an individual’s lifetime risk of ASCVD. As individuals progress through the model, they accumulate healthcare costs and quality-adjusted life years (QALYs; Supplemental Tables 1–5).
Individuals start the simulation without ASCVD and, each year, are at risk of coronary heart disease (CHD), stroke, combined CHD and stroke, and ASCVD or non-ASCVD death. Incident ASCVD and non-ASCVD mortality risks are estimated using competing risk survival models developed in the National Heart, Lung, and Blood Institute Pooled Cohorts Study (NHLBI-PCS) dataset (Supplemental Figure 1) (12–15).
Probabilities of first ASCVD event and probability of non-ASCVD mortality are operationalized in the model using logistic risk functions (Supplemental Table 1, Equation 1). Predictors include race (African American or non-African American), current age, LDL-C, high-density lipoprotein cholesterol, systolic blood pressure (SBP), body mass index, smoking status (current, former, never), cigarettes per day, diabetes status, and estimated glomerular filtration rate. Both LDL-C and SBP are incorporated in the risk functions as time-weighted averages (TWA) which are updated each year from age 18 to present. Individuals with higher lifetime exposure to LDL-C are at greater risk of experiencing CHD and stroke (Central Illustration). Interaction effects with age reduce the effect of the TWA variables over time.
Central Illustration. Conceptual diagram, CVD Policy Model and time-weighted average risk factors.
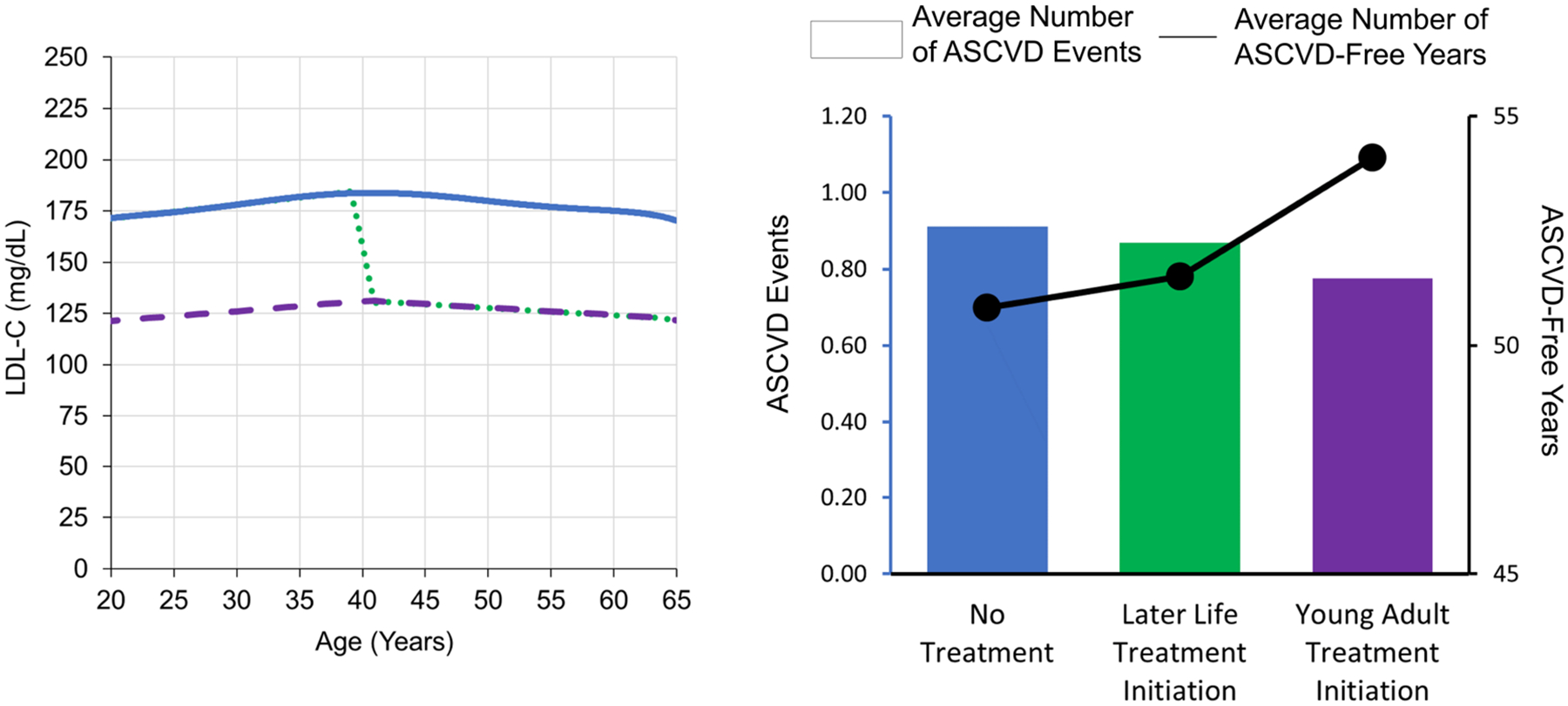
Panel A (left): Potential LDL-C trajectories for a 20-year-old young adult with raised LDL-C (172 mg/dL) are shown: no treatment (blue), later life statin treatment (green), and lifetime statin treatment (purple). Panel B (right): Results are shown for 100,000 simulations of the three LDL-C trajectories in Panel A while holding other risk factors constant. Mean number of ASCVD events (left axis) was lower and mean ASCVD-free years (right axis) was greater with treatment initiation in young adulthood.
Equation 1.
Logistic risk function predicting event k, incorporating underlying rate of event k in population (α), coefficients determining risk factor effect on risk (β), time-weighted average (TWA) LDL-C and SBP from age 18 to present, and remaining ASCVD risk factors (RFs)
Risk functions were recalibrated so that modeled event rates matched contemporary U.S. ASCVD incidence and total event, ASCVD-related mortality, and all-cause mortality rates (Supplemental Figure 2). Results were recalibrated with data from a previously validated, population simulation version of the CVD Policy Model and U.S. Centers for Disease Control and Prevention data. Recalibration was conducted separately for women and men.
Simulation Cohort
The simulated cohort was created by sampling ASCVD-free young adult participants from U.S. NHANES 1999–2014 exams. Participants were excluded if they reported a diagnosis of heart failure, stroke, or CHD. Lifetime (ages 18 to 89 years) ASCVD risk factor trajectories were assigned to each NHANES participant by randomly matching them 1:1 to NHLBI-PCS participants for whom trajectories were previously defined (Supplemental Table 6, Supplemental Figure 3) (1,13,14). We repeatedly sampled with replacement the NHANES-NHLBI-PCS matched participants to create 100 cohorts, each consisting of 250,000 young adult men and 250,000 young adult women.
Simulated Treatment Strategies
Every individual was simulated through the model from their age at NHANES observation until age 89 years or death. Lipid screening commenced at the start of the simulation and repeated every five years throughout an individual’s life while untreated and without ASCVD. To avoid unnecessary inconvenience and testing costs for patients unlikely to require lipid management, screening was not continued for young adults with an LDL-C ≥10 mg/dL below the treatment initiation threshold at first screen. For these individuals, screening recommenced at age 40.
Lifetime ASCVD, survival, and cost-effectiveness outcomes were projected for different LDL-C treatment strategies. The reference ‘standard care’ strategy was defined according to the 2018 AHA/ACC guideline (Supplement) (16). Standard care was statin treatment for adults aged ≥40 years based on LDL-C, ASCVD risk, or diabetes plus young adults with LDL-C ≥190 mg/dL. Each young adult treatment strategy was supplemental to standard care.
Four treatment strategies were compared with standard care in the primary analysis. The first two initiated moderate-intensity statins in young adults with LDL-C ≥160 mg/dL or ≥130 mg/dL. The second two initiated intensive lifestyle modification in young adults with LDL-C ≥160 mg/dL or ≥130 mg/dL. All individuals were treated according to ACC/AHA 2018 guidelines after age 40 years. A secondary analysis included moderate intensity statins plus intensive lifestyle intervention in young adults with LDL-C ≥160 mg/dL and LDL-C ≥130 mg/dL as a comparator.
Treatment Inputs
Treatment input parameters are outlined in Table 1.
Table 1.
Simulation parameters
| Parameter | Base case | Distribution for PSA | Lower Bound | Upper Bound | Source |
|---|---|---|---|---|---|
| RR per 1.0 mmol/L time-weighted average LDL-C reduction | |||||
| CHD | 0.740 | Beta | 0.710 | 0.780 | (13) |
| Stroke | 0.900 | Beta | 0.810 | 0.990 | (13) |
| RR per 1.0 mmol/L LDL-C reduction from statin therapy in adulthood | |||||
| CHD | 0.760 | Beta | 0.730 | 0.790 | (5) |
| Stroke | 0.850 | Beta | 0.800 | 0.890 | (5) |
| Statin LDL-C reduction (% change from baseline) | |||||
| Moderate-intensity | 29.0 | Beta | 14.0 | 38.0 | (17) |
| High-intensity | 43.0 | Beta | 39.0 | 46.0 | (17) |
| Statin-related adverse events | |||||
| Statin-induced diabetes, absolute annual risk increase (%) | 0.500 | Log-normal | 0.000 | 1.00 | (43) |
| Pill-taking disutility | 0.002 | Beta | 0.000 | 0.004 | (23) |
| Probability minor adverse event (%) | 4.7 | Beta | 0.0 | 9.4 | (23) |
| Probability major adverse event (%) | 0.006 | Beta | 0.000 | 0.012 | (23) |
| Cost minor adverse event ($) | 178.53 | Gamma | 133.90 | 223.16 | (23) |
| Cost major adverse event ($) | 7,033.00 | Gamma | 5,274.75 | 8,791.25 | (23) |
| Statin treatment adherence (%) | |||||
| Year 1 | 67.0 | Beta | 50.0 | 84.0 | (21) |
| Year 2 | 53.0 | Beta | 40.0 | 66.0 | (21) |
| Subsequent Years | 50.0 | Beta | 38.0 | 63.0 | (21) |
| Young adult intensive lifestyle intervention effects | |||||
| LDL-C reduction (mmol/L) | 0.05 | Beta | 0.00 | 0.11 | (18) |
| SBP reduction (mm Hg) | 1.80 | Beta | 0.89 | 2.21 | (18) |
| Relative risk incident diabetes | 0.67 | Beta | 0.51 | 0.87 | (18) |
| Duration of effect (years) | 5.00 | n/a | 2.00 | 8.00 | (18) |
| Annual intervention costs ($) | |||||
| Moderate-intensity (median; Medical Expenditure Panel Survey) | 102.52 | Gamma | 41.18 | 163.85 | (44) |
| High-intensity (median; Medical Expenditure Panel Survey) | 151.34 | Gamma | 65.70 | 236.98 | (44) |
| Statin dispensing fees | 30.00 | Gamma | 0.00 | 60.00 | Assumption |
| Group-based intensive lifestyle change intervention in primary care setting | 512.83 | Gamma | 384.63 | 641.04 | (26) |
| Checkup and screening visit costs ($ per visit) | |||||
| Checkup visit, on treatment | 81.83 | Gamma | 59.96 | 99.93 | (45) |
| Screening visit, no treatment | 81.83 | Gamma | 59.96 | 99.93 | (45) |
| Cardiovascular prevention behavioral visit (15 minutes) | 33.07 | Gamma | 24.23 | 40.38 | (45) |
| Other costs ($) | |||||
| Lipid panel test | 23.92 | Gamma | 17.94 | 29.89 | (46) |
| Liver panel test | 1.47 | Gamma | 1.10 | 1.84 | (46) |
| Weighted statin-induced diabetes cost | 9.75 | Gamma | 7.32 | 12.19 | (46) |
| Office visit frequency | |||||
| Years between screening visits | 5.00 | n/a | 4.00 | 6.00 | (16) |
| Primary care check-up while on statin treatment (years) | 1.25 | Gamma | 0.00 | 3.00 | (16) |
| Behavioral visits in treatment years 2 to 5 | 4.00 | Gamma | 1.00 | 7.00 | Assumption |
| Discount rate (%) | |||||
| Health | 3.0 | n/a | 0.0 | 6.0 | (29) |
| Costs | 3.0 | n/a | 0.0 | 6.0 | (29) |
LDL-C – low-density lipoprotein cholesterol; PSA – probabilistic sensitivity analysis; RR – relative risk; SBP – Systolic blood pressure
Footnote: This table contains a list of the parameters employed to simulate cholesterol screening, statin therapy, and lifestyle interventions in our analysis. Probabilistic distributions are provided, which were used to stochastically sample these parameters. Lower and upper bounds are provided, which detail ranges employed in one-way sensitivity analyses.
For statin treatment strategies, the magnitude of clinical benefit was a function of the magnitude of LDL-C reduction. Moderate- and high-intensity statin therapy reduced LDL-C by 29% and 43%, respectively (17). For adults aged ≥40 years, we applied the relative risk (RR) of statins on ASCVD per 1.0 mmol/L (38.67 mg/dL) reduction in LDL-C from clinical trials (5). For young adults, lipid-lowering benefit was modelled through the counterfactual of lower ASCVD risk with lower time-weighted average LDL-C observed in longitudinal cohort data (13).
For lifestyle interventions, the magnitude of clinical benefit corresponded to LDL-C and SBP reductions (6). Intensive lifestyle interventions reduced LDL-C by 0.05 mmol/L (2.1 mg/dL) and SBP by 1.9 mm Hg, which were derived from a U.S. Preventive Services Task Force meta-analysis of behavioral counselling for ASCVD prevention (6). Lifestyle interventions also reduced risk of incident diabetes (RR 0.67) throughout the course of treatment.
Clinical benefit was greater for individuals receiving lipid-lowering in young adulthood because earlier intervention results in lower cumulative LDL-C exposure over the adult life course. To benchmark this assumption, we compared the simulated CHD RRs of young adult and later life LDL-C lowering with the RR of 0.78 per 17 mg/dL lifetime lower LDL-C observed in a Mendelian randomization study (PCSK9 R46L loss-of-function mutation, Supplemental Table 7) (18,19). As expected, the simulated RRs for CHD per 17 mg/dL of LDL-C lowering in young adulthood and in later life were higher, 0.86 and 0.92, respectively.
Adherence to statin therapy (i.e., proportion continuing with treatment beyond persistence observed in clinical trials) was assumed to be 67%, 53%, and 50% in the first, second, and subsequent years of treatment, respectively (20). Persistent statin users experienced slight increases in diabetes risk, annual pill-taking utility decrements, and follow-up physician visits (7,21,22). While statins are generally well-tolerated (23), costs were also included for minor and major statin-related adverse events which occurred in 4.7% and 0.006% of statin users, respectively (22). Based on expert opinion, treatment effect for intensive lifestyle interventions did not persist past five years. Low adherence or abbreviated treatment attenuated LDL-C reduction, side effect risks, and treatment-related costs. In a sensitivity analysis, we examined outcomes when the duration of treatment effect with full adherence was varied from 1 to 22 years.
The annual cost of statins was calculated as the median cost of statin therapy to all payers, including patient out-of-pocket costs, in the 2017 Medical Expenditure Panel Survey (Supplemental Table 8) (24). The cost of lifestyle intervention was the median cost of group therapy from a systematic review of economic evaluations of diet and physical activity promotion programs for the prevention of type-2 diabetes (25). Few behavioral intervention studies extend beyond one year and optimal regularity of long-term behavioral visits with physicians has not been established. Based on expert opinion, we determined that patients receiving lifestyle modification treatment would incur costs for four annual cardiovascular prevention behavioral visits in years subsequent to treatment initiation.
Main Analysis
Primary model outcomes were lifetime QALYs gained, direct healthcare costs in 2020 U.S. dollars, and incremental cost-effectiveness ratios (ICERs), stratified by sex. The mean and 95% uncertainty interval (95% UI; i.e., the 2.5th to 97.5th percentile interval of the means) were calculated for model outcomes from 100 probabilistic simulations in which model inputs were sampled from pre-specified distributions. Strategies were classified as highly cost-effective (ICER <$50,000/QALY gained), intermediately cost-effective (ICER ≥$50,000/QALY but <$150,000/QALY gained), or not cost-effective (ICER ≥$150,000/QALY gained) in accordance with the ACC/AHA Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance Measures (26,27). We performed all analyses from a U.S. healthcare sector perspective, which includes all formal healthcare costs (i.e., treatment-related, ASCVD, and non-ASCVD healthcare costs) regardless of payer (e.g., patients, insurance companies, etc.). Future QALYs and costs were discounted 3.0% annually (28).
Sensitivity Analysis
One-way sensitivity analyses estimated the effect of changing the model inputs (Table 1) one at a time across the upper and lower uncertainty bounds while holding all other parameters constant (Supplement). In a further sensitivity analysis, we examined outcomes when the duration of treatment effect with full adherence was varied from 1 to 22 years.
This study followed the Consolidated Health Economic Evaluation Reporting Standards reporting guideline (Supplemental Table 9).
Results
Number of Treatment-Eligible Young Adults
An estimated 26.3 million (27%) U.S. young adults without ASCVD have LDL-C ≥130 mg/dL, and over 8.5 million (9%) have LDL-C ≥160 mg/dL (Figure 1, Table 2). Around half (56%) of young adults have had their cholesterol checked. In comparison, 87% of adults aged 40–74 years have had their cholesterol checked.
Figure 1. Distribution of untreated LDL-C in ASCVD-free, U.S. adults.
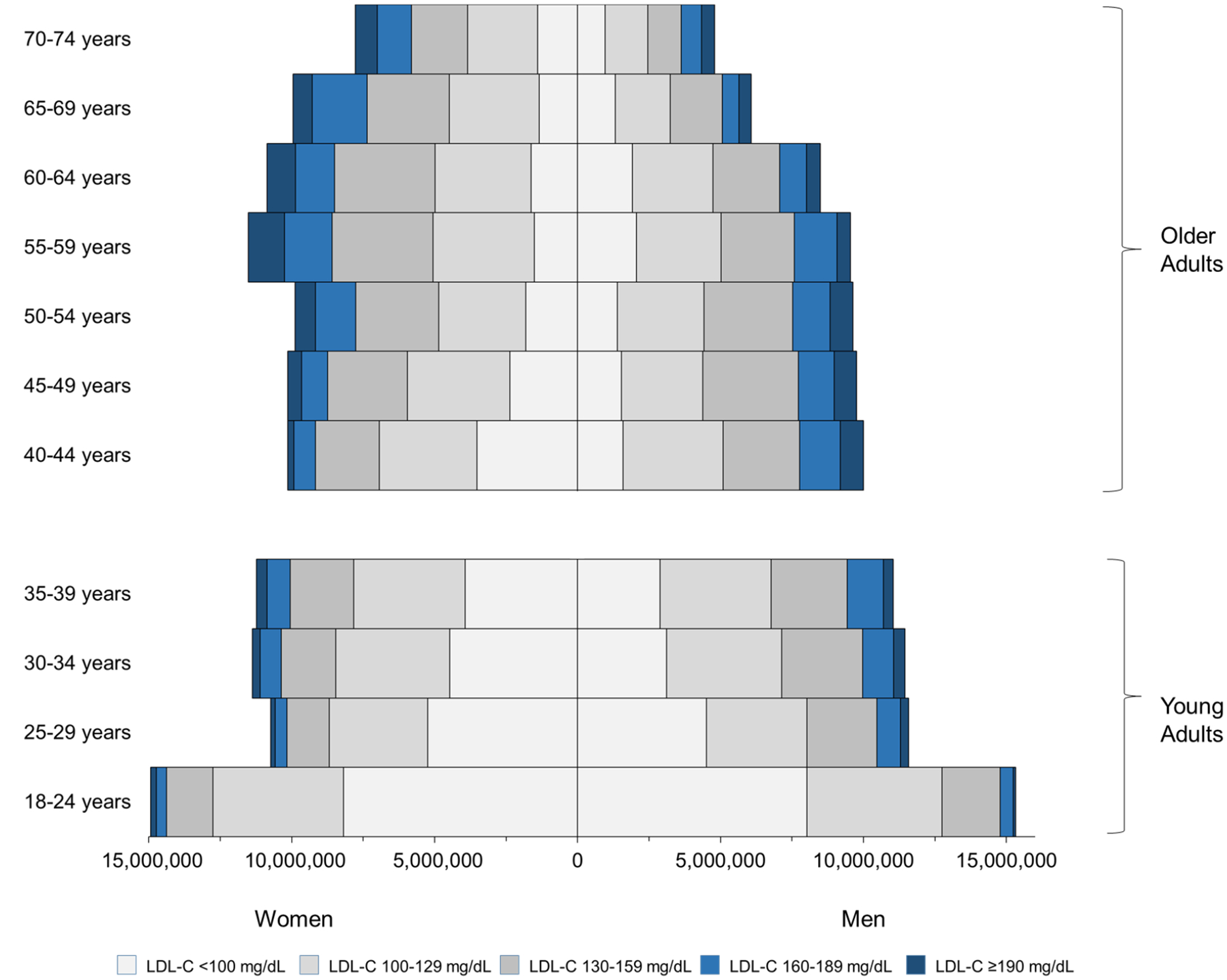
Analysis conducted in the National Health and Nutrition Examination Survey. LDL-C – low-density lipoprotein cholesterol. Notes: The LDL-C distribution was estimated from the 1999 to 2014 NHANES cycles and projected onto 2020 population estimates (method in Supplemental Methods; Table 2).To convert LDL-C from mg/dL to mmol/L, divide by 38.67.
Table 2.
Prevalence of untreated LDL-C in ASCVD-free U.S. adults
| Total Number ASCVD-Free | Ever had LDL-C Screened? N (%, 95% UI) | LDL-C screened within 5 years N (%, 95% UI) | Number (%, 95% UI) in low-density lipoprotein cholesterol group | |||||
|---|---|---|---|---|---|---|---|---|
| 0–99 mg/dL | 100–129 mg/dL | 130–159 mg/dL | 160–189 mg/dL | ≥190 mg/dL | ||||
| Women | ||||||||
| Young Adults (Aged 18–39) | 47,904,000 | 26,747,000 (56, 95% UI: 54–58) |
24,285,000 (51, 95% UI: 49–53) |
20,905,000 (44, 95% UI: 41–46) |
15,959,000 (33, 95% UI: 31–35) |
7,510,000 (15, 95% UI: 14–17) |
2,517,000 (5.3, 95% UI: 4.4–6.2) |
1,013,000 (2.1, 95% UI: 1.5–2.8) |
| Older Adults (Aged 40–74) | 63,366,000 | 55,345,000 (87, 95% UI: 86–88) |
51,735,000 (82, 95% UI: 80–83) |
12,829,000 (20, 95% UI: 19–22) |
20,713,000 (33, 95% UI: 31–34) |
17,659,000 (28, 95% UI: 26–30) |
7,874,000 (12, 95% UI: 11–14) |
4,291,000 (6.8, 95% UI: 5.9–7.8) |
| Men | ||||||||
| Young Adults (Aged 18–39) | 49,385,000 | 23,100,000 (47, 95% UI: 44–49) |
20,392,000 (41, 95% UI: 39–44) |
17,951,000 (36, 95% UI: 34–49) |
16,220,000 (33, 95% UI: 31–35) |
10,232,000 (21, 95% UI: 19–23) |
3,824,000 (7.7, 95% UI: 6.7–8.9) |
1,158,000 (2.3, 95% UI: 1.7–3.2) |
| Older Adults (Aged 40–74) | 58,091,000 | 48,613,000 (84, 95% UI: 82–85) |
44,866,000 (77, 95% UI: 75–79) |
10,341,000 (18, 95% UI: 16–19) |
18,746,000 (32, 95% UI: 30–34) |
17,344,000 (30, 95% UI: 28–32) |
7,612,000 (13, 95% UI: 12–15) |
4,048,000 (7.0, 95% UI: 5.9–8.1) |
| Combined | ||||||||
| Young Adults (Aged 18–39) | 97,289,000 | 49,847,000 (51, 95% UI: 50–53) |
44,677,000 (46, 95% UI: 44–48) |
38,856,000 (40, 95% UI: 38–42) | 32,179,000 (33, 95% UI: 31–35) | 17,742,000 (18, 95% UI: 17–19) | 6,341,000 (6.5, 95% UI: 5.8–7.3) | 2,171,000 (2.2, 95% UI: 1.8–2.8) |
| Older Adults (Aged 40–74) | 121,457,000 | 103,958,000 (86, 95% UI: 85–87) |
96,601,000 (80, 95% UI: 78–81) |
23,170,000 (19, 95% UI: 18–21) | 39,459,000 (32, 95% UI: 31–34) | 35,003,000 (29, 95% UI: 28–30) | 15,486,000 (13, 95% UI: 12–14) | 8,339,000 (6.9, 95% UI: 6.2–7.6) |
Total number of ASCVD-free individuals was derived by combining ASCVD-free proportion of NHANES age-groups onto 2020 U.S.
Census Bureau population estimates (46). Proportion of untreated LDL-C levels were derived from cross-sectional analysis of NHANES 1999–2014, using fasting blood sampling weights to produce representative cohort. Uncertainty intervals were derived using the incomplete beta function with sample size based on the estimated variance of the proportion.
LDL-C – low-density lipoprotein cholesterol; 95% UI – 95% uncertainty interval
Main Cost-Effectiveness Analysis
Baseline characteristics of the simulation cohorts are provided in Supplemental Table 10. Compared to women, on average men had higher mean LDL-C, lower mean HDL-C, higher mean SBP, and higher smoking rates.
For both men and women, the simulated model projected that adding statin treatment for young adults with LDL-C ≥130 mg/dL to standard care would prevent the most ASCVD events and gain the most QALYs in the population (Table 3, Figure 2, Supplemental Table 11).
Table 3.
Incremental cost-effectiveness outcomes for lipid-lowering strategies in U.S. young adult men and young adult women
| Policy | ASCVD Events Prevented (95% UI) | Discounted QALYs (95% UI) | Discounted total costs, thousands of 2020 $US (95% UI) | Incremental cost-effectiveness ratio ($/QALY) |
|---|---|---|---|---|
| Women | ||||
| Standard care* | Reference | Reference | Reference | Reference |
| Standard care + young adult LDL-C ≥160 mg/dL (lifestyle)** | 32 (11–57) | 130 (55–225) | 13,800 (12,500–22,100) | Strictly Dominated |
| Standard care + young adult LDL-C ≥160 mg/dL (statins) | 86 (38–150) | 167 (2–424) | 8,600 (2,580–17,100) | 51,500 |
| Standard care + young adult LDL-C ≥130 mg/dL (lifestyle)** | 98 (43–165) | 510 (256–778) | 77,800 (39,300–131,000) | Strictly Dominated |
| Standard care + young adult LDL-C ≥130 mg/dL (statins) | 412 (214–688) | 601 (−174–1,590) | 54,500 (24,800–101,000) | 106,000 |
| Men | ||||
| Standard care* | Reference | Reference | Reference | Reference |
| Standard care + young adult LDL-C ≥160 mg/dL (lifestyle)** | 164 (90–244) | 384 (215–639) | 25,200 (13,400–43,500) | Strictly Dominated |
| Standard care + young adult LDL-C ≥160 mg/dL (statins) | 491 (275–827) | 817 (274–1,630) | 10,800 (−3,190–28,300) | 13,200 |
| Standard care + young adult LDL-C ≥130 mg/dL (lifestyle)** | 405 (255–594) | 1,040 (643–1,530) | 101,000 (53,000–179,000) | Strictly Dominated |
| Standard care + young adult LDL-C ≥130 mg/dL (statins) | 1,530 (820–2,470) | 2,250 (592–4,470) | 55,200 (9,000–121,000) | 31,000 |
Mean and 95% uncertainty interval (95% UI; i.e., the 2.5th to 97.5th percentile interval of the means) were calculated for model outcomes from 100 probabilistic simulations in which model inputs were sampled from pre-specified distributions.
Treatment according to ACC/AHA 2018 guidelines; “standard care” strategy consisted of later life (age ≥40 years) statin treatment if clinical ASCVD, diabetes, LDL-C ≥190 mg/dl, or ten-year ASCVD risk ≥7.5% and young adult statin treatment if LDL-C ≥190 mg/dl.
Individuals with LDL-C ≥190 mg/dL also received moderate-intensity statins
LDL-C – low-density lipoprotein cholesterol; UI = uncertainty interval based on probabilistic analysis. To convert LDL-C from mg/dL to mmol/L, divide by 38.67.
Figure 2. Cost-effectiveness plane for lipid-lowering strategies in U.S. young adults.
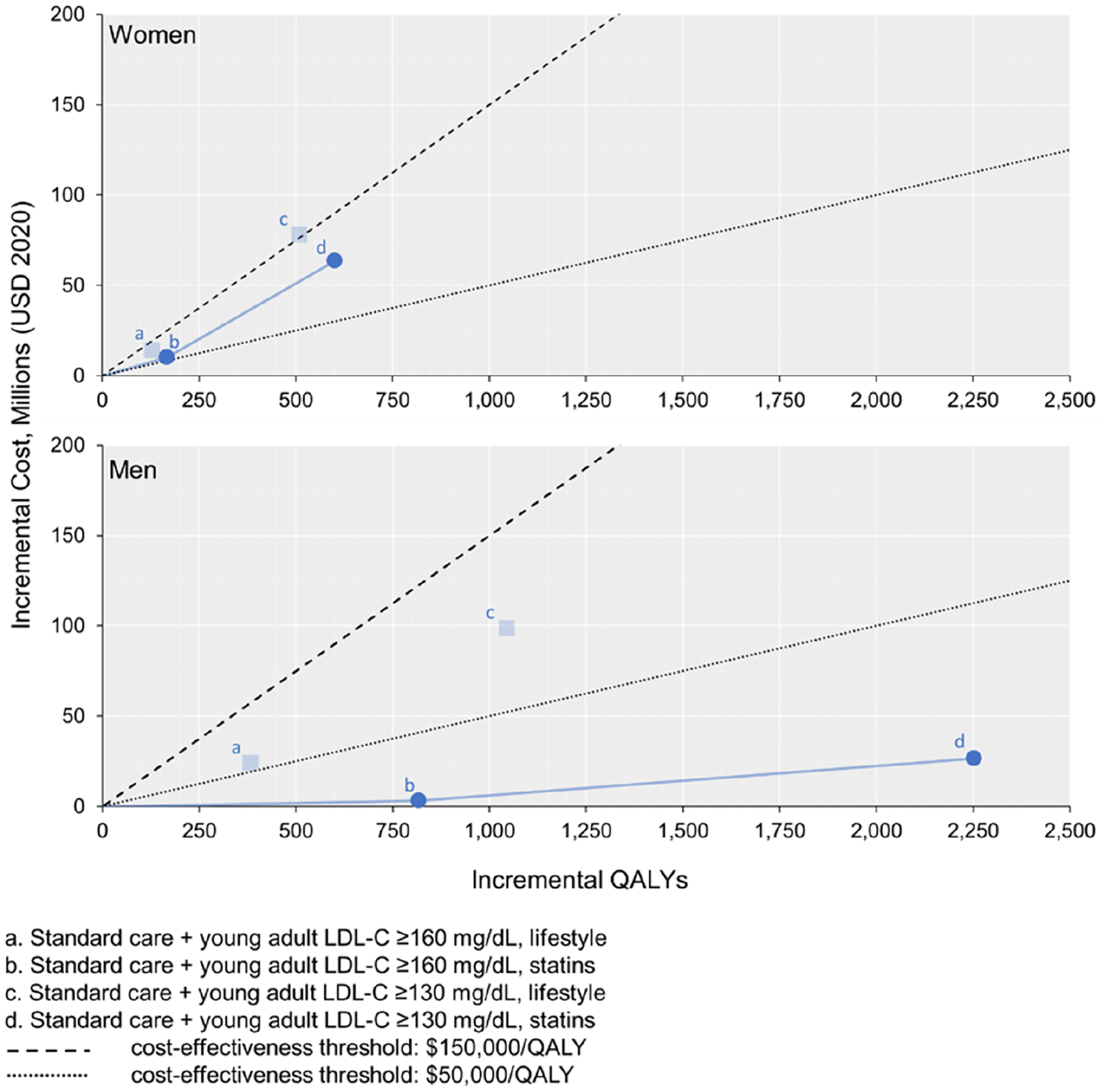
The transparent points illustrated indicate that the strategy costs more and is less effective than another strategy (i.e., strictly dominated). The solid blue lines represent strategies that are eligible to be considered the preferred treatment (i.e., the cost-effectiveness frontier, which comprises the non-dominated strategies ranked by increasing effectiveness). A strategy is considered cost-effective if the slope of the blue line connecting it to the next least effective strategy (i.e., the incremental cost-effectiveness ratio) is lower than the slope of the cost-effectiveness threshold line. The preferred strategy is defined as the treatment that results in the greatest QALY gains and is cost-effective. LDL-C - low-density lipoprotein cholesterol; QALYs – quality-adjusted life years. To convert LDL-C from mg/dL to mmol/L, divide by 38.67.
For young adult men with LDL-C ≥160 mg/dL, statin therapy was estimated to produce an ICER of $13,200/QALY compared to standard care. Further expanding statin eligibility to young adult men with LDL-C ≥130 mg/dL was estimated to produce an ICER of $31,000/QALY compared with statins for LDL-C ≥160 mg/dL. For young adult women with LDL-C ≥160 mg/dL, the model projected that statins would produce an ICER of $51,500/QALY compared to standard care. Further expanding statin eligibility to young adult women with LDL-C ≥130 mg/dL produced an ICER of $106,000/QALY. Young adult lifestyle treatment strategies were all predicted to be more costly and less effective than statin treatment strategies (i.e., strictly dominated).
For men, statin therapy for individuals with LDL-C ≥130 mg/dL was projected to be the preferred strategy in 72% of probabilistic simulations at a cost-effectiveness threshold of $50,000/QALY. At a cost-effectiveness threshold of $50,000/QALY, standard care had the highest probability (51%) of being the preferred strategy for women (Supplemental Figure 4). At a higher cost-effectiveness threshold of $150,000/QALY, however, statin therapy with LDL-C ≥130 mg/dL was projected to be the preferred treatment strategy for both men (95%) and women (59%).
In the secondary analysis, the model projected that statins plus intensive lifestyle intervention for young adults with LDL-C ≥160 mg/dL had a higher ICER and less QALYs gained than statin therapy alone for both men and women (i.e., statins plus intensive lifestyle strategies were extendedly dominated) (Supplemental Table 12). Statins plus intensive lifestyle intervention for young adults with LDL-C ≥160 mg/dL were projected to produce ICERs of $59,300/QALY and $137,000/QALY compared to statin therapy alone for men and women, respectively.
Sensitivity Analysis
In sensitivity analyses, the cost-effectiveness of statin strategies was most sensitive to changes in the discount rate, statin efficacy, and magnitude of effect of cumulative exposure to LDL-C on CHD risk (Supplemental Figure 5). The cost-effectiveness of statin therapy could be improved for men and women by using low-price statin formulations, improving patient adherence, reducing required check-up visits for persistent statin users, and averting patient pill-taking disutility. Results for the intensive lifestyle interventions strategies were sensitive to changes in the effect of such interventions on young adult LDL-C, discount rate, and number of behavioral visits required in years subsequent to treatment initiation.
All treatments were projected to become more effective compared to standard care as duration of treatment effect increased (Supplemental Figure 6). Even when treatment effects were sustained for ≥10 years, statin treatment strategies were more cost-effective than intensive lifestyle interventions (Supplemental Figure 7).
Discussion
In this mathematical modelling study, early statin treatment starting in young adulthood at lower-than- current-guideline-recommended LDL-C thresholds was projected to prevent or delay ASCVD events and improve population health. Statin therapy was projected to be highly cost-effective for young adult men with LDL-C ≥130 mg/dL and intermediately cost-effective for young adult women with LDL-C ≥130 mg/dL. The advent of generic pricing has rendered statin treatment highly cost-effective for tens of millions of young adults with raised LDL-C. This result echoes previous findings regarding increased cost-effectiveness of statin therapy following price reductions (4,22,29).
To our knowledge, this is the first cost-effectiveness analysis of LDL-C treatment in young adults. Though the efficacy of young adult statin treatment has never been tested in clinical trials, the relationship between cumulative LDL-C exposure earlier in life and later life ASCVD has been established extensively in observational cohort studies. Cumulative exposure to high LDL-C in young adulthood and middle-age have both been shown to significantly increase risk of ASCVD in later life (3,13,30). When clinical trial evidence is unavailable, simulating decades-long “trials” is a feasible approach to estimating the effectiveness and cost-effectiveness of long-term statin treatment in young adults with raised LDL-C. Our benchmarking exercise found that the dose-response we estimated for young adult statin treatment lies between the observed effects of lifelong lower LDL-C (from a Mendelian randomization study comparing PCSK9 loss-of-function mutation carriers to non-carriers) and later-life LDL-C lowering (from randomized controlled trials of statin versus placebo).
Our NHANES analysis found that almost half of U.S. young adults have never had their cholesterol screened. This aligns with previous estimates of young adult LDL-C screening rates (31,32). In the U.S., many young adults lack health insurance; for those with employer-based or government insurance, lipid screening is not generally reimbursed. Insurance and payment reforms could incentivize young adult lipid screening and treatment. For example, Medicare could reimburse private insurers for treating raised LDL-C in young adults due to anticipated avoided ASCVD events and cost savings when these patients become Medicare eligible. Young adult lipid screening could be expanded efficiently by offering lower cost screening tests and engaging with community centers or using team-based care to introduce home, work site, or community-based lipid screening. Additionally, selectively screening young adults with obesity, diabetes, or genetic testing for family history consistent with familial hypercholesterolemia may increase screening and treatment among those at highest risk of ASCVD. However, before investing in expanded statin treatment to young adults, it is important to consider that our previous analysis found treating “borderline” risk adults aged ≥40 years (10-year ASCVD risk 5.0–7.4%) is even more cost-effective incremental to standard care than treating young adults with raised LDL-C (Supplemental Table 13) (4).
Greater cost-effectiveness of statin treatment for men has been observed in previous analyses (4). This differential cost-effectiveness occurred in our analysis because men are at greater lifetime risk of ASCVD than women (33) and young adult men are more likely to have elevated LDL-C than young adult women. Both LDL-C levels and cholesterol screening rates vary across racial subgroups of U.S. young adults (Supplemental Table 14). Further research should establish the impact of young adult lipid-lowering screening and treatment strategies on racial disparities in health.
Study Limitations
Our results suggest that intensive intervention to lower LDL-C through individual lifestyle behavior change is not cost-effective. However, we did not evaluate the alternative of population-wide diet and lifestyle programs that have been highly effective in lowering LDL-C and improving population health (34). Alternative approaches that systematically optimize behavioral ‘treatment packages’ may help establish more cost-effective and sustainable behavioral treatments for LDL-C lowering (35,36).
Though young adult lipid-lowering could lead to lifetime health gains for eligible patients, there may be long-term statin-related adverse event risks that we have not accounted for. In middle-aged adults, long-term statins present a favorable profile when risks are weighed against benefits (37–39). Our analysis accounted for the risk of statin-induced diabetes, pill-taking disutility, and costs related to other major and minor statin-related adverse events. In sensitivity analyses, our results were robust to assumptions regarding the incidence and impact of statin-related adverse events. Nonetheless, we may have underestimated how intolerable daily pill-taking is for young adults. While there is limited evidence on statin adherence in ASCVD-free young adults, our estimate aligns with reported adherence rates in young adults with high LDL-C and individuals with who experienced ‘extremely premature’ ASCVD (40,41). Statin adherence rates may be lower in young adults compared with older adults and we explored alternative adherence rates in sensitivity analyses. A practical and more patient-centered approach to young adult lipid-lowering may require the development of once- or twice-yearly treatments (as seen with newer, experimental PCSK9 inhibitor agents) and making them low-cost.
Conclusions
Around one quarter of U.S. young adults have elevated LDL-C. This mathematical modelling study found that initiating statin treatment in young adults with LDL-C ≥130 mg/dL is highly cost-effective in men and intermediately cost-effective in women.
Supplementary Material
Clinical Perspectives.
Competency in Systems-Based Practice: Treating elevated LDL-cholesterol (LDL-C) is intermediately cost-effective for young women and highly cost-effective for young men. Translational Outlook: Given the challenges and potential risks of beginning lifetime statin treatment in young adulthood, additional research is needed to develop efficacious and sustainable lifestyle interventions to lower LDL-C.
Funding/Support:
This study was supported by grants R01-HL107475 and R01-HL141823 from the United States National Heart, Lung, and Blood Institute (NHLBI) (Dr Moran); grant DTP-1522025 from the Medical Research Council, Swindon, United Kingdom (Dr Kohli-Lynch), and NHLBI grant K01-HL140170 (Dr Bellows).
Conflict of Interest Disclosures:
Dr Kazi reported receiving economic support from the Institute for Clinical and Economic Review outside the submitted work. Dr Moran reported receiving grants from the National Heart, Lung, and Blood Institute during the conduct of the study. No other disclosures were reported.
Role of the Funder/Sponsor:
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
LIST OF ABBREVIATIONS
- ACC/AHA
American College of Cardiology/American Heart Association
- ASCVD
Atherosclerotic Cardiovascular Disease
- CHD
Coronary Heart Disease
- ICER
Incremental Cost-Effectiveness Ratio
- INMB
Incremental Net Monetary Benefits
- LDL-C
Low-Density Lipoprotein Cholesterol
- NHLBI-PCS
National Heart, Lung, and Blood Institute Pooled Cohorts Study
- NHANES
National Health and Nutrition Examination Survey
- QALY
Quality-Adjusted Life Year
- RR
Relative Risk
- SBP
Systolic Blood Pressure
- TWA
Time-Weighted Averages
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pletcher MJ, Vittinghoff E, Thanataveerat A, Bibbins-Domingo K, Moran AE Young Adult Exposure to Cardiovascular Risk Factors and Risk of Events Later in Life: The Framingham Offspring Study. PLOS ONE 2016;11(5):e0154288. Doi: 10.1371/journal.pone.0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen NB, Siddique J, Wilkins JT, et al. Blood Pressure Trajectories in Early Adulthood and Subclinical Atherosclerosis in Middle Age. JAMA 2014;311(5):490–7. Doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navar-Boggan AM, Peterson ED, D’Agostino RB, Neely B, Sniderman AD, Pencina MJ Hyperlipidemia in Early Adulthood Increases Long-Term Risk of Coronary Heart Disease. Circulation 2015;131(5):451–8. Doi: 10.1161/CIRCULATIONAHA.114.012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohli-Lynch CN, Bellows BK, Thanassoulis G, et al. Cost-effectiveness of Low-density Lipoprotein Cholesterol Level–Guided Statin Treatment in Patients With Borderline Cardiovascular Risk. JAMA Cardiol 2019. Doi: 10.1001/jamacardio.2019.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380(9841):581–90 p 588. Doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connor EA, Evans CV, Rushkin MC, Redmond N, Lin JS. Behavioral Counseling to Promote a Healthy Diet and Physical Activity for Cardiovascular Disease Prevention in Adults With Cardiovascular Risk Factors: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2020;324(20):2076. Doi: 10.1001/jama.2020.17108. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Stone NJ, Bailey A, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019. June 25;73(24):e285–e350. [DOI] [PubMed] [Google Scholar]
- 8.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016;316(19):1997–2007. Doi: 10.1001/jama.2016.15450. [DOI] [PubMed] [Google Scholar]
- 9.Miedema MD, Nauffal VD, Singh A, Blankstein R. Statin therapy for young adults: A long-term investment worth considering. Trends in Cardiovascular Medicine 2020;30(1):48–53. Doi: 10.1016/j.tcm.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 10.2019 Population Estimates by Age, Sex, Race and Hispanic Origin. Washington D.C.: United States Census Bureau; 2020. [Google Scholar]
- 11.National Health and Nutrition Examination Survey Data. Centers for Disease Control and Prevention. National Center for Health Statistics. Hyattsville, MD: U.S. Department of Health and Human Services. Centers for Disease Control and Prevention; 1999. [Google Scholar]
- 12.Oelsner EC, Balte PP, Cassano P, et al. Harmonization of Respiratory Data From Nine US Population-Based Cohorts: The NHLBI Pooled Cohorts Study. Am J Epidemiol 2018. Doi: 10.1093/aje/kwy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Vittinghoff E, Pletcher MJ, et al. Associations of Blood Pressure and Cholesterol Levels During Young Adulthood With Later Cardiovascular Events. J Am Coll Cardiol 2019;74(3):330–41. Doi: 10.1016/j.jacc.2019.03.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeki Al Hazzouri A, Vittinghoff E, Zhang Y, et al. Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course. Int J Epidemiol 2019;48(3):1004–13. Doi: 10.1093/ije/dyy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Statistics in Medicine 2007;26(11):2389–430. Doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Stone NJ, Bailey AL, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018:25709. Doi: 10.1016/j.jacc.2018.11.003. [DOI] [Google Scholar]
- 17.Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010;376(9753):1670–81. Doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators. Coding Variation in ANGPTL4, LPL, and SVEP1 and the Risk of Coronary Disease. N Engl Med 2016;374(12):1134–44. Doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol 2010;55(25):2833–42. Doi: 10.1016/j.jacc.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Greving JP, Visseren FLJ, Wit G de, Algra A. Statin treatment for primary prevention of vascular disease: whom to treat? Cost-effectiveness analysis. BMJ 2011;342:d1672. Doi: 10.1136/bmj.d1672. [DOI] [PubMed] [Google Scholar]
- 21.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375(9716):735–42 p 741. Doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 22.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-Year Risk Thresholds for Initiation of Statin Therapy for Primary Prevention of Cardiovascular Disease. JAMA 2015;314(2):142–50. Doi: 10.1001/jama.2015.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrett E, Williamson E, Brack K, et al. Statin treatment and muscle symptoms: series of randomised, placebo controlled n-of-1 trials. BMJ 2021;372:n135. Doi: 10.1136/bmj.n135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medical Expenditure Panel Survey 2015. 2015.
- 25.Li R, Qu S, Zhang P, et al. Economic Evaluation of Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann Intern Med 2015;163(6):452. Doi: 10.7326/M15-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson JL., Heidenreich PA, Barnett PG, et al. ACC/AHA Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance Measures. Circulation 2014;129(22):2329–45. Doi: 10.1161/CIR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 27.Vanness DJ, Lomas J, Ahn H. A Health Opportunity Cost Threshold for Cost-Effectiveness Analysis in the United States. Ann Intern Med 2020. Doi: 10.7326/M20-1392. [DOI] [PubMed] [Google Scholar]
- 28.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016;316(10):1093–103. Doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 29.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-Effectiveness of Statin Therapy for Primary Prevention in a Low-Cost Statin Era. Circulation 2011. [DOI] [PubMed] [Google Scholar]
- 30.Vasan RS, Massaro JM, Wilson PWF, et al. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation 2002;105(1):48–53. [DOI] [PubMed] [Google Scholar]
- 31.Fang J, Ayala C, Loustalot F, Dai S. Prevalence of Cholesterol Screening and High Blood Cholesterol Among Adults — United States, 2005, 2007, and 2009. MMWR 2012;61(35):697–702. [PubMed] [Google Scholar]
- 32.Grant DSL, Scott RD, Harrison TN, et al. Trends in Lipid Screening Among Adults in an Integrated Health Care Delivery System, 2009–2015. JMCP 2018;24(11):1090–101. Doi: 10.18553/jmcp.2018.18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of Lifetime Risk for Cardiovascular Disease by Risk Factor Burden at 50 Years of Age. Circulation 2006;113(6):791–8. Doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 34.Vartiainen E, Laatikainen T, Tapanainen H, Puska P. Changes in Serum Cholesterol and Diet in North Karelia and All Finland. Glob Heart 2016;11(2):179–84. Doi: 10.1016/j.gheart.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Spring B. Sound health care economics: Provide the treatment needed (not less, not more). Health Psychol 2019;38(8):701–4. Doi: 10.1037/hea0000782. [DOI] [PubMed] [Google Scholar]
- 36.Spring B, Pfammatter AF, Marchese SH, et al. A Factorial Experiment to Optimize Remotely Delivered Behavioral Treatment for Obesity: Results of the Opt-IN Study. Obesity 2020;28(9):1652–62. Doi: 10.1002/oby.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford I, Murray H, McCowan C, Packard CJ. Long-Term Safety and Efficacy of Lowering Low-Density Lipoprotein Cholesterol With Statin Therapy. Circulation 2016;133(11):1073–80. Doi: 10.1161/CIRCULATIONAHA.115.019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388(10059):2532–61. Doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 39.Wood FA, Howard JP, Finegold JA, et al. N-of-1 Trial of a Statin, Placebo, or No Treatment to Assess Side Effects. N Engl J Med 2020;383(22):2182–4. Doi: 10.1056/NEJMc2031173. [DOI] [PubMed] [Google Scholar]
- 40.Mahtta D, Ramsey DJ, Al Rifai M, et al. Evaluation of Aspirin and Statin Therapy Use and Adherence in Patients With Premature Atherosclerotic Cardiovascular Disease. JAMA Netw Open 2020;3(8):e2011051. Doi: 10.1001/jamanetworkopen.2020.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colantonio LD, Rosenson RS, Deng L, et al. Adherence to Statin Therapy Among US Adults Between 2007 and 2014. Journal of the American Heart Association 2019;8(1):e010376. Doi: 10.1161/JAHA.118.010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


