Abstract
Study Objectives
Delivery prior to full term affects 37% of US births, including ~400,000 preterm births (<37 weeks) and >1,000,000 early term births (37–38 weeks). Approximately 70% of cases of shortened gestation are spontaneous—without medically-indicated cause. Elucidation of modifiable behavioral factors would have considerable clinical impact.
Methods
This study examined the role of depressive symptoms and sleep quality in predicting the odds of spontaneous shortened gestation among 317 women (135 black, 182 white) who completed psychosocial assessment in mid-pregnancy.
Results
Adjusting for key covariates, black women had 1.89 times higher odds of spontaneous shortened gestation compared to White women (OR [95% CI] = 1.89 [1.01, 3.53], p = 0.046). Women who reported only poor subjective sleep quality (PSQI > 6) or only elevated depressive symptoms (CES-D ≥ 16) exhibited no statistically significant differences in odds of spontaneous shortened gestation compared to those with neither risk factor. However, women with comorbid poor sleep and depressive symptoms exhibited markedly higher odds of spontaneous shortened gestation than those with neither risk factor (39.2% versus 15.7% [OR (95% CI) = 2.69 (1.27, 5.70)], p = 0.01). A higher proportion of black women met criteria for both risk factors (23% of black women versus 11% of white women; p = 0.004), with a lower proportion experiencing neither risk factor (40.7% of black versus 64.3% of white women; p < 0.001).
Conclusions
Additive effects of poor subjective sleep quality and depressive symptoms were observed with markedly higher odds of spontaneous shortened gestation among women with both risk factors. Racial inequities in rates of comorbid exposure corresponded with inequities in shortened gestation. Future empirical studies and intervention efforts should consider the interactive effects of these commonly co-morbid exposures.
Keywords: sleep, depression, depressive symptoms, mental health, pregnancy, shortened gestation, early term birth, preterm birth, racial inequities
Statement of Significance.
This study provides novel evidence that poor subjective sleep quality and depressive symptoms show synergistic effects on gestational length.
Introduction
Delivery prior to full term (<39 weeks) affects 37% of US deliveries per year, including ~400,000 preterm births (<37 weeks) and >1,000,000 early term births (37–386/7 weeks). Preterm birth (PTB) has received extensive empirical focus for decades. However, early term birth (ETB) was only established as a clinical designation in 2013. At this time, the American College of Obstetricians and Gynecologists (ACOG) redefined “full term pregnancy” as birth between 390/7 and 406/7 weeks due to mounting evidence that the lowest risk for adverse neonatal outcomes are seen in that period, accordingly births occurring at 37–386/7 weeks were redefined as early term birth (ETB) to more accurately describe the risks associated with this birth timing.[1]
Delivery at <39 weeks (shortened gestation) increases risk for hypoglycemia, intravenous fluids, treatment with IV antibiotics, mechanical ventilation/intubation, NICU admission, and other adverse events [1–13], as well as poorer childhood academic achievement [2–4], childhood asthma [14, 15], and neonatal, postnatal, infant, childhood, and young adult mortality [7, 10]. While PTB confers greater risk along this continuum, ETB affects nearly three times as many deliveries. Overall, the public health implications of shortened gestation are substantial.
Approximately 30% of cases of shortened gestation are medically-indicated, initiated by the provider to protect maternal/fetal health (e.g. in context of preeclampsia, intrauterine growth restriction, etc.). The remaining 70% are spontaneous, following onset of labor or rupture of membranes [11]. The two strongest predictors of spontaneous shortened gestation are black race and history of shortened gestation [16]. Neither provides insight as to causal mechanisms that can be addressed to mitigate risk. Beyond these two factors, clinical prediction is remarkably limited. Although spontaneous cases are both more common and unpredictable, data disproportionately focuses on medically-indicated cases. While women at risk for medically-indicated delivery due to conditions such as preeclampsia or intrauterine growth restriction can easily be identified prior to delivery, studies of spontaneous occurrence of shortened gestation require prospective enrollment. This methodological hurdle partially contributes to a comparative lack of data on predictors and mechanisms [2].
There are remarkable and intractable racial inequities in shortened gestation in the United States; 40.6% of black births occur prior to full term (13.1% PTB and 27.5% ETB) compared to a rate of 33.1% among white births (8.9% PTB and 24.2% ETB) [17]. This racial inequity is not explained by SES; a black woman in the United States with college education has a greater risk for PTB than a white woman with <8th grade [18]. It is not explained by alcohol use, smoking, or access to early prenatal care [19–30]. Refuting a genetic explanation—birth weight increases across generations in white/European immigrants, while it decreases in those of African descent [31–33]. Remarkably, this effect is observable after only one generation spent in the United States, implicating a social rather than genetic underpinning. Thus, the role of psychosocial stress and related sequelae are receiving increasing attention [4].
Meta-analyses show that the presence of depressive symptoms is associated with relative risk of PTB of 1.39 [34]. However, studies to-date generally lack consideration of risk for shortened gestation inclusive of early term birth. Moreover, despite compelling evidence that short sleep contributes substantially to chronic disease, all-cause mortality, and racial inequities in health [35–37], until recently, sleep has received limited attention in relation to birth outcomes. Emerging data now link sleep parameters, including short sleep duration and poor subjective sleep quality, with risk for shortened gestation [38–44]. Given the common co-occurrence of depression and poor sleep quality, data on the independent versus additive effects of these exposures is needed.
Regardless of race, depressed pregnant women tend to experience poorer sleep quality than non-depressed pregnant women [45, 46]. However, black women may be at an increased risk to experience an exacerbated comorbidity of depression and poor sleep quality contributing to PTB and ETB, as they are both more likely to experience depressive symptoms [47] and to report poorer sleep quality during pregnancy when compared to white women [48]. Exposure to racial discrimination substantially contributes to these racial inequities [48–56]. Furthermore, black women may be less likely to seek treatment due to stigma and failure of the medical system to instill trust [57]. While research specific to the comorbidity between depression and sleep quality experienced by black women during pregnancy is limited, Field et al. [58] has reported that for black women during pregnancy, depressive symptoms were associated with disturbed sleep during late pregnancy and postpartum. Black women with elevated depressive symptoms also had a higher incidence of premature delivery prior to 37 weeks [58]. Further research clarifying the roles of depressive symptoms and sleep quality as mechanisms contributing to racial inequities in shortened gestation is warranted.
Addressing compelling gaps in the literature, we examined effects of maternal depressive symptoms and sleep on risk for spontaneous occurrence of shortened gestation among black and white women in the United States. We examined independent and additive effects of these exposures. It was hypothesized that women experiencing both high depressive symptoms and poor subjective sleep quality would exhibit the greatest risk for shortened gestation. In addition, we hypothesized that increased risk for shortened gestation among black women would be in part accounted for by increased exposure to these behavioral stressors.
Methods
Study design
This study was a prospective observational design. These analyses drew from data collected across five study protocols involving assessment of maternal psychosocial factors and birth outcomes. These included an observational longitudinal study (R21 HD067670) [59], a study of maternal oral health (Seed Grant, OSU College of Dentistry) [60], two protocols examining maternal immune responses to influenza virus vaccine (R01 NR01366) [61, 62], and one study on physiological reactivity to an acute laboratory stressor (R21 HD061644) [63]. Although the focal point of studies varied, all protocols had in common the element of inclusion of the same core questionnaire measures of sleep and depressive symptoms, permitting data to be pooled across studies for the current analyses. Across these protocols, data were collected from a total of 575 women between 2009−2018. Participants for these studies were recruited from The Ohio State University Wexner Medical Center and the surrounding community of Columbus, Ohio. All studies were approved by The Ohio State University Biomedical Institutional Review Board. All participants signed a written informed consent to at least one of the aforementioned research protocols and all were compensated for their participation.
Participants
The current analyses focused on racial inequities among black women as compared to white women in pregnancy. Women of other races/ethnicities were excluded, as were those endorsing both black and white race. Due to the known variability in sleep and mood across trimesters [64], these analyses focused specifically on women assessed in mid-pregnancy (14–28 weeks gestation), thus women assessed only at other points in pregnancy were excluded. Analyses focused on occurrence of spontaneous birth at early term or preterm, thus women with medically-indicated or elective births prior to full term were excluded as were those without adequate information available to define their birth outcomes.
Participants were also excluded from analyses if one or more of the following exclusion criteria were present. First, women with stillbirth or miscarriage, multiple fetal gestation, lack of dating ultrasound, elective abortion, progesterone use, illicit or controlled opioid use (methodone/suboxone), use of medications indicated for depression and/or use of medications indicated for sleep were excluded. Women who participated in two different studies during the same pregnancy (n = 13) were included once in the combined dataset. After applying these exclusionary criteria, the final analytic sample was 317.
Demographic, behavioral, and clinical characteristics
Demographic characteristics, including maternal race, marital status, and annual household income, were assessed by self-report. Tobacco use was also assessed per self-report. Pre-pregnancy body mass index (BMI) was calculated according to self-reported pre-pregnancy weight and height as measured at first study visit (kg/m2). Gravidity and parity were determined via medical record.
Maternal sleep and depressive symptoms
The Pittsburgh Sleep Quality Index (PSQI) was used to assess maternal sleep in the past month in all study participants. The PSQI has high diagnostic sensitivity and specificity in distinguishing good and poor sleepers [65]. Global scores as well as subscale scores show high test-retest reliability across short intervals in adults with insomnia [66]. The PSQI has good construct validity and reliability for assessing sleep in pregnant women, as well as predictive validity for perinatal mental and physical health outcomes [67–70]. A score >5 is indicative of poor subjective sleep quality in the general population [65]. However, a 2018 meta-analysis suggested that a higher cutoff of >6 may be appropriate in perinatal populations in accordance with higher overall symptoms reporting [64]. Thus, in these analyses a cutoff of >6 was used to operationalize poor subjective sleep quality.
The Center for Epidemiological Studies Depression Scale (CES-D) was used to assess depressive symptoms [71, 72]. This 20-item measure has predictive validity for birth outcomes and inflammation in pregnancy [73–77], and is recommended as a screening tool for perinatal depression by ACOG [78]. A score ≥16 is indicative of clinically significant depressive symptoms, including among pregnant populations. For example, Tandon et al. estimated an AUC of 0.94 for prediction of DSM-IV-determined perinatal depression using this cut off among a sample of pregnancy and postpartum black American women [79].
Birth outcomes
Births were classified as preterm (<37 weeks), early term birth (37−386/7 weeks), full-term (39−416/7 weeks), or post-term (≥42 weeks) based on medical record review. Cases of shortened gestation (<39 weeks) were classified as medically-indicated, spontaneous, or elective per medical records. Spontaneous cases were defined as spontaneous rupture of the chorioamniotic membranes and/or preterm labor characterized by contractions. Medically-indicated births included both inductions of labor and cesarean deliveries for reasons other than ruptured membranes and/or preterm labor including gestational hypertension, preeclampsia/ eclampsia, cardiac or renal disease, pre/gestational diabetes, and intrauterine growth restriction. Only one delivery prior to full term was classified as elective (i.e. induction of labor or cesarean delivery for non-medical reasons).
Statistical analyses
Differences in patient characteristics between black and white women were assessed using chi-square tests (or Fisher’s exact test when appropriate) for categorical variables. Binary logistic regression models were used to assess associations with the occurrence of shortened gestation versus full-term birth. Separate multivariable binary logistic regression models for binary CES-D (≥16 versus <16) and binary PSQI (>6 versus ≤6) were then fit including race (black, white) and the covariates such as maternal age (<20, 20–34, ≥35), income (<$15,000, $15,000–29,999, ≥$30,000), and pre-pregnancy BMI (underweight, normal, overweight, obese). Next, the additive effects of these exposures were tested by creating a variable describing exposure to neither behavioral stressor, disturbed sleep only (PSQI > 6), depressive symptoms only (CES-D ≥ 16), or both behavioral stressors. Race, maternal age, income, and pre-pregnancy BMI were then added to the combined risk factor model. All statistical tests were evaluated at the α = 0.05 significance level and completed in SAS, Version 9.4.[80] Main results are presented below. For greater detail, see supplementary tables.
Results
Participant characteristics
In total, 317 participants were included in these analyses. Participant characteristics are summarized in Table 1. This sample included 64 (20.2%) with spontaneous shortened gestation (15 preterm, 39 early term) and 253 term deliveries (237 full-term, 16 late-term). Women were assessed between 141/7and 28 weeks gestation (mean = 153.6 ± 22.4 days). In this sample, 42.6% of participants were black (n = 135) and 57.4% were white (n = 182). Black women had an average age of 25.1(SD: 4.4) and white women had an average age of 27.0 (SD: 4.8) years old, with an average pre-pregnancy BMI of 27.6 kg/m2 (SD: 7.2) and 26.0 kg/m2 (SD: 6.6), respectively. Women from lower socioeconomic backgrounds were overrepresented, with 82.2% of black women and 42.3% of white women reporting an annual household income <$30,000.
Table 1.
Participant characteristics
| Variable | Total (n = 317) |
Black (n = 135) |
White (n = 182) |
p-value* |
|---|---|---|---|---|
| Maternal age | 0.06 | |||
| <20 | 20 (6.3%) | 5 (3.7%) | 15 (8.2%) | |
| 20–34 | 286 (90.2%) | 128 (94.8%) | 158 (86.8%) | |
| ≥35 | 11 (3.5%) | 2 (1.5%) | 9 (5.0%) | |
| Annual household income | <0.001 | |||
| <$15,000 | 120 (37.9%) | 76 (56.3%) | 44 (24.2%) | |
| $15,000–29,999 | 68 (21.5%) | 35 (25.9%) | 33 (18.1%) | |
| ≥$30,000 | 129 (40.7%) | 24 (17.8%) | 105 (57.7%) | |
| Prepregnancy BMI | 0.07 | |||
| <18.5 | 10 (3.2%) | 6 (4.4%) | 4 (2.2%) | |
| 18.5–24.9 | 149 (47.0%) | 53 (39.3%) | 96 (52.8%) | |
| 25.0–29.9 | 85 (26.8%) | 38 (28.2%) | 47 (25.8%) | |
| ≥30.0 | 73 (23.0%) | 38 (28.2%) | 35 (19.2%) | |
| CES-D, total | <0.001 | |||
| <16 | 241 (76.0%) | 89 (65.9%) | 152 (83.5%) | |
| ≥16 | 76 (24.0%) | 46 (34.1%) | 30 (16.5%) | |
| PSQI, total | 0.001 | |||
| ≤6 | 197 (62.2%) | 70 (51.9%) | 127 (69.8%) | |
| >6 | 120 (37.8%) | 65 (48.1%) | 55 (30.2%) | |
| Shortened gestation | 64 (20.2%) | 38 (28.2%) | 26 (14.3%) | 0.002 |
*Black women compared to white women, chi-square test (Fisher’s exact test was used for maternal age and prepregnancy BMI).
Racial differences in odds of shortened gestation
In this sample, 28.2% of black women had spontaneous shortened gestation compared to 14.3% of white women. Adjusting for maternal age, income, and pre-pregnancy BMI, race remained a significant predictor of higher odds of spontaneous shortened gestation (p = 0.046). Specifically, black women were almost 2 times more likely to have spontaneous shortened gestation compared to white women [odds ratio (OR) 95% CI = 1.89 (1.01, 3.53)].
Maternal sleep and odds of shortened gestation
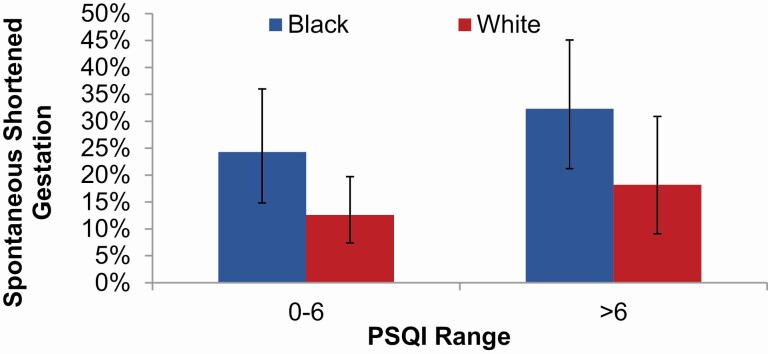
First, we examined the role of maternal sleep in predicting odds of spontaneous occurrence of shortened gestation. As detailed above, women were classified based on the PSQI cutoff of >6 to indicate presence of poor subjective sleep quality. In univariate analysis, compared to women with good subjective sleep quality, those with poor subjective sleep quality exhibited marginally higher odds of spontaneous shortened gestation [OR (95% CI) = 1.73 (1.00, 3.01), p = 0.0522]. In a model including PSQI and race, after adjusting for age, income, and BMI, compared to women with good subjective sleep quality, those with poor subjective sleep quality did not differ significantly in odds of spontaneous shortened gestation (Figure 1 (OR = 1.44 [95% CI] = 0.80, 2.57), p = 0.22]. In this model, race was marginally associated with spontaneous shortened gestation (p = 0.057), with black women having 1.84 times higher odds compared to white women (95% CI = 0.98, 3.44).
Figure 1.
Sleep and rates of shortened gestation by race. In a model with PSQI and race and adjusting for age, income, and BMI, compared to women with PSQI ≤ 6, those with PSQI > 6 did not differ significantly in risk for spontaneous shortened gestation (OR [95% CI] = 1.44 [0.80, 2.57], p = 0.22). In this model, Black women were marginally more likely to have higher risk of spontaneous shortened gestation (p = 0.057) (bars indicate 95% confidence intervals).
Maternal depressive symptoms and odds of shortened gestation
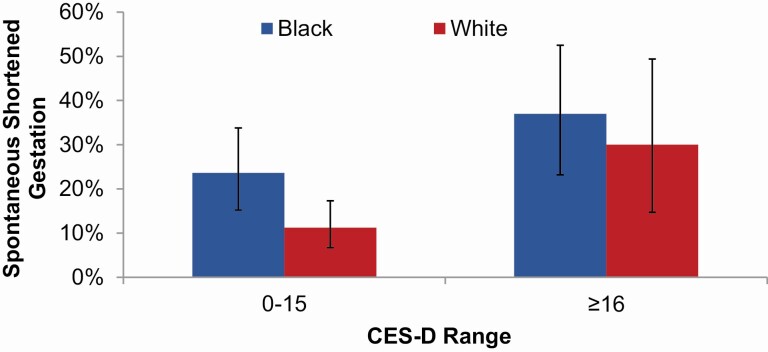
Next, the predictive value of depressive symptoms for odds of spontaneous shortened gestation was examined. Women were categorized based on presence of clinically significant depressive symptoms using the CES-D cutoff of ≥16. The odds of spontaneous shortened gestation was higher among women with significant depressive symptoms. Specifically, in univariate analysis, compared to women without significant depressive symptoms, those with CES-D scores ≥16 exhibited 2.78 times higher odds (95% CI = 1.54, 5.00; p = 0.001).
In a model including CES-D and race and adjusting for age, income, and BMI, black women exhibited 1.76 times higher odds of spontaneous shortened gestation (Figure 2; 95% CI = 0.93, 3.33; p = 0.08). In this model, depressive symptoms remained predictive; compared to women without significant depressive symptoms, those with presence of clinically significant depressive symptoms were 2.28 times more likely to have spontaneous shortened gestation (OR [95% CI] = 2.28 [1.20, 4.33]; p = 0.01).
Figure 2.
Depressive symptoms and rates of shortened gestation by race. In a model with CES-D and race and adjusting for age, income, and BMI, compared to women with CES-D <16, those with CES-D ≥ 16 had significantly greater risk for shortened gestation (OR [95% CI] = 2.28 [1.20, 4.33]; p = 0.01). In this model, Black women had 1.76 times higher odds of spontaneous shortened gestation (95% CI = 0.93, 3.33; p = 0.08) (bars indicate 95% confidence intervals).
Combined risk factor variable
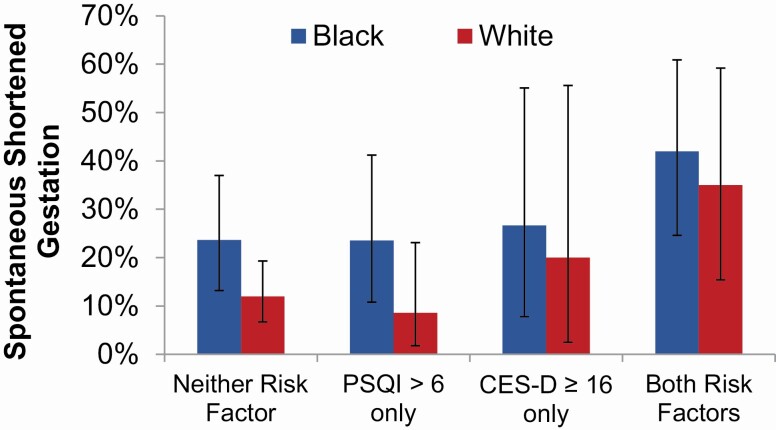
As expected, a strong, positive correlation was observed between continuous scores on the CES-D and PSQI (Pearson correlation coefficient (ρ) = 0.51, p < 0.001). We next examined the effects of unique versus co-occurring indication of clinically significant depressive symptoms (CES-D ≥ 16) and poor sleep quality (PSQI > 6) in a model including race and adjusting for maternal age, income, and pre-pregnancy BMI. Compared to those with neither risk factor, women who reported only disturbed sleep exhibited no increased risk for spontaneous shortened gestation (Figure 3; OR [95% CI] = 0.84 [0.38, 1.87], p = 0.67). Similarly, those with only elevated depressive symptoms did not differ significantly in risk as compared to those with no risk factor (OR [95% CI] = 1.26 [0.43, 3.67], p = 0.67). In contrast, as compared to those with neither risk factor, women with both disturbed sleep and elevated depressive symptoms exhibited significantly higher odds of spontaneous shortened gestation (39.2% versus 15.7%, OR [95% CI] = 2.69 [1.27, 5.70], p = 0.01). In this model black women had 1.80 times higher odds of spontaneous shortened gestation than white women (95% CI = 0.95, 3.41; p = 0.07).
Figure 3.
Sleep, depressive symptoms, and rates of shortened gestation by race. Race and risk factor categorization were included in the same model along with covariate controls. Compared to women with neither risk factor, women with both poor sleep and significant depressive symptoms had higher odds of spontaneous shortened gestation (OR [95% CI] = 2.69 [1.27, 5.70], p = 0.01). After accounting for these covariates, black race was not statistically significantly associated with spontaneous shortened gestation in this model (OR [95% CI] = 1.80 [0.95–3.41], p = 0.07) (bars indicate 95% confidence intervals).
Racial inequities in psychosocial risk exposure
As shown in Table 2, compared to white women, a higher proportion of black women met criteria for both poor subjective sleep quality and elevated depressive symptoms (23% of black women versus 11% of white women; p = 0.004), with a lower proportion experiencing neither risk factor (40.7% of black versus 64.3% of white women; p < 0.001).
Table 2.
Distribution of combined risk factor
| Risk factors | All subjects (n = 317) | Black (n = 135) |
White (n = 182) |
p-value versus all other risk factor levels* |
|---|---|---|---|---|
| Both | 51 (16.1%) | 31 (23.0%) | 20 (11.0%) | 0.004 |
| PSQI > 6 only | 69 (21.8%) | 34 (25.2%) | 35 (19.2%) | 0.20 |
| CES-D ≥ 16 only | 25 (7.9%) | 15 (11.1%) | 10 (5.5%) | 0.07 |
| Neither | 172 (54.3%) | 55 (40.7%) | 117 (64.3%) | <0.001 |
aOverall (omnibus) chi-square test for difference in risk factor level distribution by race: X2(3) = 19.2, p < 0.001.
Discussion
In this study of a racially diverse cohort assessed at mid-gestation, we examined independent and additive effects of poor sleep quality and depressive symptoms on birth outcomes. These data showed higher risk in association with depressive symptoms. Notably, when examined in a combined risk factor model, effects of depressive symptoms alone were no longer significant. However, markedly higher odds of spontaneous occurrence of shortened gestation (inclusive of preterm and early term birth) were observed among women with concurrent exposure to both poor sleep and elevated depressive symptoms. These data suggest that assessment of both sleep quality and depressive symptoms adds considerable predictive value for identifying risk for adverse birth outcomes.
Notably, black women in this sample had markedly higher rates of shortened gestation than did white women, with rates of 28.2% and 14.3%, respectively. Possible mediating effects of co-occurring poor sleep and depressive symptoms were suggested. Specifically, black women were markedly over-represented among participants with co-occurrence of both risk factors, with 23% of black versus 11% of white participants represented in this category. In addition, in the combined risk factor model, race was no longer a statistically significant predictor of risk for spontaneous shortened gestation.
While mediating effects were supported, this study was not adequately powered to examine moderating effects of race (i.e. differential risk for adverse birth outcome following the same behavioral exposure). Prior data from our group which suggest that upon similar exposure to poor subjective sleep quality, black women show enhanced vulnerability to inflammatory immune dysregulation than white women, as well as increased risk for shortened gestation [42, 44, 81]. The potential for heightened physiological sensitivity to stressors due to chronic stress remains an important area of investigation.
In this study, birth categories were rigorously defined, to permit for specific examination of the spontaneous occurrence of shortened gestation. There is considerable debate with regard to separating versus pooling spontaneous and medically-indicated cases in studies of etiology [82]. The central argument for pooling is that both types may share common underlying causes, particularly inflammation and vascular dysfunction [83], thus, pooling increases statistical power. Conversely, arguments in favor of the separation of subtypes contend that distinctive pathways will be obscured by pooling. There is currently no consensus on this issue. However, data specific to spontaneous cases is notably underrepresented in the literature.
An additional novel aspect of the current study is the focus on shortened gestation inclusive of preterm and early term birth. As detailed, early term birth is a relatively new designation. As such, efforts to date have focused almost exclusively on reducing occurrence of elective delivery prior to 39 weeks [9, 84–86]. As described, early term birth confers two times greater risk for both subsequent preterm birth and repeated early term birth [16], suggesting that these are best understood as a continuum of the same condition: shortened gestation. Early term birth affects more than twice as many deliveries as does preterm birth. Therefore, focus on shortened gestation inclusive of preterm and early term birth has the considerable benefit of increasing statistical power to detect effects of behavioral and biological exposures. In fact, if early term birth is instead combined with “term” deliveries, it meaningfully reduces the ability to detect pathways that contribute to adverse birth outcomes. Thus, the role of spontaneous early term birth should be fully considered in future studies. In addition, greater data are needed on potential racial differences in adverse outcomes in relation to shorter gestation, as some data suggests that black women may have a shorter physiological gestational length which may affect the rates of adverse outcomes observed when delivery occurs prior to 40 weeks [87].
Despite the common co-occurrence of depression and poor sleep quality, the independent and additive role of these exposures has not been adequately considered in the existing literature. As compared to depression [34], data on sleep and perinatal health is more limited, but growing. Among 166 women, poor sleep quality per self-report was associated with risk for preterm birth, particularly sleep in early pregnancy for which every one point increase on the PSQI corresponded with a 25% increase in the odds [39]. Demonstrating similar effects, an epidemiological analysis of 1,091 women demonstrated a significant association between both maternal snoring (indicating possible sleep apnea/hypoxia) in late pregnancy and risk for fetal growth restriction, as well as sleep deprivation in late pregnancy (as defined by ≤5 h of sleep on average) and risk for preterm birth [40]. Moreover, recent retrospective data in 672 women with diagnosed insomnia or sleep apnea per medical record review showed 1.3 times greater odds of preterm birth (PTB) in cases of insomnia and 1.5 times greater odds among cases with sleep apnea as compared to 1:1 healthy matched controls.[43] As demonstrated by the current data, consideration of only sleep or only depression provides an incomplete understanding of risk.
The current study focused on women assessed at mid-pregnancy. This is important, in that sleep quality and depressive symptoms change considerably across the course of pregnancy [88]. Therefore, limiting assessment to mid-gestation increases the signal-to-noise ratio in statistical analyses. However, longitudinal data would be informative for explicating the temporal relationship between depressive symptoms and sleep. In addition, longitudinal data would permit for examining the extent to which timing and chronicity of exposures to these behavioral factors affect risk for adverse outcomes.
The current investigation examined subjective sleep quality. Both sleep quality and sleep duration have strong predictive validity for a variety of health outcomes in pregnant and non-pregnant adults [89–92]. However, duration is more robustly associated with disease, disability, and death, with stronger effects for sleep duration measured objectively (i.e. via wrist actigraphy) [90, 93]. Sleep duration per self-report and actigraphy tend to be only modestly associated [94]. While subjective reports often reflect a person’s time in bed rather than time spent asleep, actigraphy-based sleep duration calculates actual sleep time, excluding difficulty in falling asleep and staying asleep (i.e. sleep disturbances), which are captured as sleep onset latency (SOL) and wake after sleep onset (WASO). Thus, future studies utilizing objective measures of sleep in addition to self-report would be informative.
In addition, in this study, depressive symptoms were assessed. However the presence of clinical diagnoses of mood disorders was not determined per a structured clinical interview. In the broader literature, a meta-analysis of studies of depression and birth outcomes found that only 3 of 29 studies examined clinical diagnoses [34]. A broad range of prior studies have examined other types of distress, such as perceived racial discrimination or general perceived stress [95]. While these data have empirical value, screening for racial discrimination, general perceived stress, or other types of stress in the clinic setting is unlikely due to unclear treatment options. In contrast, clinical mood diagnoses are well-defined with empirically-supported treatments [96, 97]. As such, clinical disorders present targets suitable for screening in the obstetric setting. Relatedly, while this study focused on depressive symptoms, anxiety disorders are highly comorbid with depressive symptoms and also highly treatable [98]. The studies aggregated to allow for the current analyses did not uniformly assess perinatal anxiety, prohibiting determination of the relative predictive value of depressive symptoms versus anxiety for birth outcomes. Future focus on clinical diagnoses inclusive of anxiety disorders and examining comparative predictive validity will help to advance translation to clinical application.
During the period of data collection (2009–2018), there was a decrease in preterm birth rates in the United States from 12.16% in 2009 to 9.57% in 2014 followed by a gradual rise to 10.02% in 2018 [99–101]. The decreases were largely driven by successful efforts to decrease elective c-sections prior to 39 weeks completed gestation and thus have minimal, if any, impact within the current study which focuses on spontaneous shortened gestation. Racial inequities in preterm birth rates persisted across the period of assessment.
In the current investigation, we were unable to explore analyses related to racial discrimination because different assessment tools for discrimination were used in different protocols of the contributing studies. Of note, a systematic review of 17 studies of discrimination and sleep found that 15 focused on individual experiences of discrimination [102]. However, racism contributes to differential exposures to social determinants of health across multiple levels (individual, interpersonal, community/societal) and domains (behavioral, physical environment, and sociocultural environment). Future studies should examine multi-level and multi-domain indicators of racial discrimination in relation to depressive symptoms and sleep [48, 51–55].
Furthermore, while health disparities between white versus black adults in the United States are well-documented, data on the antecedents and consequences among black people are sparse. It is increasingly recognized that “comparison studies, however necessary to establish inequities, are not sufficient to advance the science of diversity” [103]. Thus, for future studies to explore racial inequities, utilizing within participants designs is crucial. The social construct of race is characterized by multiple complex and inter-rated exposures that cannot be meaningfully disentangled statistically in comparative models (i.e. blacks versus whites).[103, 104]
In conclusion, the current study provides novel evidence that effects of poor subjective sleep quality and depressive symptoms show synergistic effects on gestational lengths, whereby women experiencing both risk factors exhibit markedly elevated odds of shortened gestation as compared to those with no risk factors, or only one of these two risk factors. These data also implicate increased rates of co-occurrence of poor sleep and depressive symptoms among black women as a factor contributing to racial inequities in birth outcomes. Future studies focusing on depression and sleep should include assessment and analyses examining the independent, additive, and interactive effects of these exposures. As empirically supported treatments are available for both sleep health and depressive symptoms, addressing these behavioral factors during pregnancy may provide considerable benefit for maternal health and birth outcomes.
Supplementary Material
Acknowledgments
We appreciate the contributions of our Clinical Research Assistants and students to data collection. We would like to thank our study participants and the staff at the OSU Clinical Research Center and Wexner Medical Center Prenatal Clinic.
Funding
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HD067670 and HD061644) and the National Institute for Nursing Research (NINR) (NR01366). This study was also supported by Award Number UL1TR001070 from the National Center for Advancing Translational Sciences (NCATS). This study was supported by the Roessler Medical Student Research Scholarship at The Ohio State University College of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosure Statement
None declared.
References
- 1. The American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion No. 579: definition of term pregnancy. Obstet Gynecol. 2013;122:1139–1140. [DOI] [PubMed] [Google Scholar]
- 2. Chan E, et al. . School performance at age 7 years in late preterm and early term birth: a cohort study. Arch Dis Child Fetal Neonatal Ed. 2014;99(6):F451–F457. [DOI] [PubMed] [Google Scholar]
- 3. Quigley MA, et al. . Early term and late preterm birth are associated with poorer school performance at age 5 years: a cohort study. Arch Dis Child Fetal Neonatal Ed. 2012;97(3):F167–F173. [DOI] [PubMed] [Google Scholar]
- 4. Noble KG, et al. . Academic achievement varies with gestational age among children born at term. Pediatrics. 2012;130(2):e257–e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spong CY. Defining “term” pregnancy: recommendations from the defining “Term” Pregnancy Workgroup. JAMA. 2013;309(23):2445–2446. [DOI] [PubMed] [Google Scholar]
- 6. Melamed N, et al. . Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114(2 Pt 1):253–260. [DOI] [PubMed] [Google Scholar]
- 7. Reddy UM, et al. . Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011;117(6):1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hibbard JU, et al. . Respiratory morbidity in late preterm births. J Am Med Assoc. 2010;304:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tita AT, et al. ; Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crump C, et al. . Early-term birth (37−38 weeks) and mortality in young adulthood. Epidemiology. 2013;24(2):270–276. [DOI] [PubMed] [Google Scholar]
- 11. Goldenberg RL, et al. . Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moster D, et al. . Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359(3):262–273. [DOI] [PubMed] [Google Scholar]
- 13. Behrman RE, et al. eds. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC), 2007. [PubMed] [Google Scholar]
- 14. Harju M, et al. . The burden of childhood asthma and late preterm and early term births. J Pediatr. 2014;164(2):295–299.e1. [DOI] [PubMed] [Google Scholar]
- 15. Edwards MO, et al. . Early-term birth is a risk factor for wheezing in childhood: A cross-sectional population study. J Allergy Clin Immunol. 2015;136(3):581–587.e2. [DOI] [PubMed] [Google Scholar]
- 16. Yang J, et al. . Recurrence of preterm birth and early term birth. Obstet Gynecol. 2016;128(2):364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Department of Health and Human Services, Centers for Disease Control and Prevention (CDC), and National Center for Health Statistics. CDC Natality Information: Natality for 2007-2015, CDC WONDER Online Database. 2017. [Google Scholar]
- 18. US Department of Health and Human Services, Centers for Disease Control and Prevention (CDC), and National Center for Health Statistics. Final Natality Data: Racial and Ethnic Disparities, CDC WONDER Online Database. 2017. [Google Scholar]
- 19. Hamilton BE, et al. . Births: final data for 2014. Natl Vital Stat Rep. 2015;64(12):1–64. [PubMed] [Google Scholar]
- 20. Okun N, et al. . Antibiotics for bacterial vaginosis or Trichomonas vaginalis in pregnancy: a systematic review. Obstet Gynecol. 2005;105(4):857–868. [DOI] [PubMed] [Google Scholar]
- 21. Carey JC, et al. . Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342(8):534–540. [DOI] [PubMed] [Google Scholar]
- 22. Goldenberg RL, et al. . Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317–1324. [DOI] [PubMed] [Google Scholar]
- 23. Ebrahim SH, et al. . Alcohol consumption by pregnant women in the United States during 1988−1995. Obstet Gynecol. 1998;92(2):187–192. [DOI] [PubMed] [Google Scholar]
- 24. Serdula M, et al. . Trends in alcohol-consumption by pregnant-women − 1985 through 1988. J Am Med Assoc. 1991;265:876–79. [PubMed] [Google Scholar]
- 25. McGrady GA, et al. . Preterm delivery and low birth weight among first-born infants of black and white college graduates. Am J Epidemiol. 1992;136(3):266–276. [DOI] [PubMed] [Google Scholar]
- 26. Collins JW Jr, et al. . Racial differences in post-neonatal mortality in Chicago: what risk factors explain the black infant’s disadvantage? Ethn Health. 1997;2(1-2):117–125. [DOI] [PubMed] [Google Scholar]
- 27. Shiono PH, et al. . Ethnic differences in birthweight: the role of lifestyle and other factors. Am J Public Health. 1997;87(5):787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schoendorf KC, et al. . Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992;326(23):1522–1526. [DOI] [PubMed] [Google Scholar]
- 29. CDC. Births: final data for 2003. 54(2). Natl Vital Stat Rep. 2005:4. [PubMed] [Google Scholar]
- 30. Klebanoff MA, et al. . Anemia and spontaneous preterm birth. Am J Obstet Gynecol. 1991;164(1 Pt 1):59–63. [DOI] [PubMed] [Google Scholar]
- 31. David RJ, et al. Differing birth weight among infants of US-born blacks, African-born blacks, and US-born whites. New Engl J Med. 1997;337:1209–14. [DOI] [PubMed] [Google Scholar]
- 32. DeSisto CL, et al. . Deconstructing a disparity: explaining excess preterm birth among U.S.-born black women. Ann Epidemiol. 2018;28(4):225–230. [DOI] [PubMed] [Google Scholar]
- 33. Collins JW Jr, et al. . Differing intergenerational birth weights among the descendants of US-born and foreign-born Whites and African Americans in Illinois. Am J Epidemiol. 2002;155(3):210–216. [DOI] [PubMed] [Google Scholar]
- 34. Grote NK, et al. . A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grandner MA, et al. . Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Y, et al. . Sleep duration and chronic diseases among U.S. adults age 45 years and older: evidence from the 2010 Behavioral Risk Factor Surveillance System. Sleep. 2013;36(10):1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gallicchio L, et al. . Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–158. [DOI] [PubMed] [Google Scholar]
- 38. Strange LB, et al. . Disturbed sleep and preterm birth: a potential relationship? Clin Exp Obstet Gynecol. 2009;36(3):166–168. [PubMed] [Google Scholar]
- 39. Okun ML, et al. . Poor sleep quality is associated with preterm birth. Sleep. 2011;34(11):1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Micheli K, et al. . Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;22(5):738–744. [DOI] [PubMed] [Google Scholar]
- 41. Samaraweera Y, et al. . Maternal sleep deprivation, sedentary lifestyle and cooking smoke: risk factors for miscarriage: a case control study. Aust N Z J Obstet Gynaecol. 2010;50(4):352–357. [DOI] [PubMed] [Google Scholar]
- 42. Blair LM, et al. . Poor sleep quality and associated inflammation predict preterm birth: heightened risk among African Americans. Sleep. 2015;38(8):1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Felder JN, et al. . Sleep disorder diagnosis during pregnancy and risk of preterm birth. Obstet Gynecol. 2017;130(3):573–581. [DOI] [PubMed] [Google Scholar]
- 44. Christian LM, et al. . Polyunsaturated Fatty Acid (PUFA) status in pregnant women: associations with sleep quality, inflammation, and length of gestation. PLoS One. 2016;11(2):e0148752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Field T, et al. . Sleep disturbances in depressed pregnant women and their newborns. Infant Behav Dev. 2007;30(1):127–133. [DOI] [PubMed] [Google Scholar]
- 46. Ahmed AH, et al. . Relationship between sleep quality, depression symptoms, and blood glucose in pregnant women. West J Nurs Res. 2019;41(9):1222–1240. [DOI] [PubMed] [Google Scholar]
- 47. Orr ST, et al. . Racial disparities in elevated prenatal depressive symptoms among black and white women in eastern north Carolina. Ann Epidemiol. 2006;16(6):463–468. [DOI] [PubMed] [Google Scholar]
- 48. Francis B, et al. . Racial discrimination and perinatal sleep quality. Sleep Health. 2017;3(4):300–305. [DOI] [PubMed] [Google Scholar]
- 49. Schulz AJ, et al. . Discrimination, symptoms of depression, and self-rated health among African American women in Detroit: results from a longitudinal analysis. Am J Public Health. 2006;96(7):1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Canady RB, et al. . Discrimination and symptoms of depression in pregnancy among African American and White women. Womens Health Issues. 2008;18(4):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tomfohr L, et al. . Racial differences in sleep architecture: the role of ethnic discrimination. Biol Psychol. 2012;89(1):34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grandner MA, et al. . Perceived racial discrimination as an independent predictor of sleep disturbance and daytime fatigue. Behav Sleep Med. 2012;10(4):235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beatty DL, et al. . Unfair treatment is associated with poor sleep in African American and Caucasian adults: Pittsburgh SleepSCORE project. Health Psychol. 2011;30(3):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bethea TN, et al. . Perceived racial discrimination and risk of insomnia among middle-aged and elderly Black women. Sleep. 2020;43:zsz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hoggard LS, et al. . Examining how racial discrimination impacts sleep quality in African Americans: is perseveration the answer? Behav Sleep Med. 2018;16(5):471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ertel KA, et al. . Racial discrimination, response to unfair treatment, and depressive symptoms among pregnant black and African American women in the United States. Ann Epidemiol. 2012;22(12):840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. O’Mahen HA, et al. . Stigma and depression during pregnancy: does race matter? J Nerv Ment Dis. 2011;199:257–62. [DOI] [PubMed] [Google Scholar]
- 58. Field T, et al. . Depressed pregnant black women have a greater incidence of prematurity and low birthweight outcomes. Infant Behav Dev. 2009;32(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gillespie SL, et al. . Adaptation of the inflammatory immune response across pregnancy and postpartum in Black and White women. J Reprod Immunol. 2016;114:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Christian LM, et al. . Self-rated health among pregnant women: associations with objective health indicators, psychological functioning, and serum inflammatory markers. Ann Behav Med. 2013;46(3):295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Christian LM, et al. . Serum proinflammatory cytokine responses to influenza virus vaccine among women during pregnancy versus non-pregnancy. Am J Reprod Immunol. 2013;70(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Christian LM, et al. . Effects of prior influenza virus vaccination on maternal antibody responses: implications for achieving protection in the newborns. Vaccine. 2017;35(39):5283–5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Christian LM, et al. . Stress-induced inflammatory responses in women: effects of race and pregnancy. Psychosom Med. 2013;75(7):658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sedov ID, et al. . Sleep quality during pregnancy: a meta-analysis. Sleep Med Rev. 2018;38:168–176. [DOI] [PubMed] [Google Scholar]
- 65. Buysse DJ, et al. . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 66. Backhaus J, et al. . Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. [DOI] [PubMed] [Google Scholar]
- 67. Qiu C, et al. . Construct validity and factor structure of the Pittsburgh Sleep Quality Index among pregnant women in a Pacific-Northwest cohort. Sleep Breath. 2016;20(1):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Coo Calcagni S, et al. . The relationship between sleep and mood in first-time and experienced mothers. Behav Sleep Med. 2012;10(3):167–179. [DOI] [PubMed] [Google Scholar]
- 69. Okun ML, et al. . Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord. 2011;130(3):378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Skouteris H, et al. . Assessing sleep during pregnancy: a study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Womens Health Issues. 2009;19(1):45–51. [DOI] [PubMed] [Google Scholar]
- 71. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 72. Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, eds. Measuring Patient Changes in Mood, Anxiety, and Personality Disorders. Washington D.C.: American Psychological Association; 1997. [Google Scholar]
- 73. Li D, et al. . Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. 2009;24(1):146–153. [DOI] [PubMed] [Google Scholar]
- 74. Phillips GS, et al. . Prepregnancy depressive symptoms and preterm birth in the Black Women’s Health Study. Ann Epidemiol. 2010;20(1):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Orr ST, et al. . Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797–802. [DOI] [PubMed] [Google Scholar]
- 76. Christian LM, et al. . Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 2009;23(6):750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Christian LM, et al. . Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain Behav Immun. 2010;24(1):49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Practice CoO. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268. [DOI] [PubMed] [Google Scholar]
- 79. Tandon SD, et al. . A comparison of three screening tools to identify perinatal depression among low-income African American women. J Affect Disord. 2012;136(1-2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. The Guilford Press. [Google Scholar]
- 81. Christian LM, et al. . Associations of postpartum sleep, stress, and depressive symptoms with LPS-stimulated cytokine production among African American and White women. J Neuroimmunol. 2018;316:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Savitz DA, et al. . Should spontaneous and medically indicated preterm births be separated for studying aetiology? Paediatr Perinat Epidemiol. 2005;19(2):97–105. [DOI] [PubMed] [Google Scholar]
- 83. Klebanoff MA, et al. . Top down, bottom up and inside out: reflections on preterm birth. Paediatr Perinat Epidemiol. 1995;9(2):125–129. [DOI] [PubMed] [Google Scholar]
- 84. Oshiro BT, et al. ; Women and Newborn Clinical Integration Program. Decreasing elective deliveries before 39 weeks of gestation in an integrated health care system. Obstet Gynecol. 2009;113(4):804–811. [DOI] [PubMed] [Google Scholar]
- 85. Snowden JM, et al. . Oregon’s hard-stop policy limiting elective early-term deliveries: limitations of before-and-after studies in evaluating obstetric policies reply. Obstet Gynecol. 2017;129:754–54. [DOI] [PubMed] [Google Scholar]
- 86. Richards JL, et al. . Temporal trends in late preterm and early term birth rates in 6 high-income countries in North America and Europe and association with clinician-initiated obstetric interventions. JAMA. 2016;316(4):410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Loftin R, et al. . Racial differences in gestational age-specific neonatal morbidity: further evidence for different gestational lengths. Am J Obstet Gynecol. 2012;206(3):259.e1–259.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Christian LM, et al. . Sleep quality across pregnancy and postpartum: effects of parity and race. Sleep Health. 2019;5(4):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Palagini L, et al. . Chronic sleep loss during pregnancy as a determinant of stress: impact on pregnancy outcome. Sleep Med. 2014;15(8):853–859. [DOI] [PubMed] [Google Scholar]
- 90. Cappuccio FP, et al. . Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Matthews KA, et al. . Do reports of sleep disturbance relate to coronary and aortic calcification in healthy middle-aged women?: Study of women’s health across the nation. Sleep Med. 2013;14(3):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hoevenaar-Blom MP, et al. . Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34(11):1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Irwin MR, et al. . Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Herring SJ, et al. . Do pregnant women accurately report sleep time? A comparison between self-reported and objective measures of sleep duration in pregnancy among a sample of urban mothers. Sleep Breath. 2013;17(4):1323–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Christian LM. At the forefront of psychoneuroimmunology in pregnancy: implications for racial disparities in birth outcomes. Part 1: Behavioral risks factors. Neurosci Biobehav Rev. 2020;117:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carroll JE, et al. . Maternal sleep in pregnancy and postpartum. Part II: Biomechanisms and intervention strategies. Curr Psychiatry Rep. 2019;21(3):19. [DOI] [PubMed] [Google Scholar]
- 97. Christian LM, et al. . Maternal sleep in pregnancy and postpartum. Part I: Mental, physical, and interpersonal consequences. Curr Psychiatry Rep. 2019;21(3):20. [DOI] [PubMed] [Google Scholar]
- 98. Kircanski K, et al. . Investigating the nature of co-occurring depression and anxiety: comparing diagnostic and dimensional research approaches. J Affect Disord. 2017;216:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Martin JA, et al. . Births: final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–70. [PubMed] [Google Scholar]
- 100. Hamilton BE, et al. . National vital statistics reports. Natl Vital Stat Rep. 2009;57. [Google Scholar]
- 101. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep. 2019;68. [PubMed] [Google Scholar]
- 102. Slopen N, et al. . Discrimination and sleep: a systematic review. Sleep Med. 2016;18:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Whitfield KE, et al. . Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? J Gerontol B Psychol Sci Soc Sci. 2008;63(5):P301–P308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Corwin EJ, et al. . Protocol for the Emory University African American Vaginal, oral, and gut microbiome in pregnancy cohort study (vol 17, 161, 2017). BMC Pregnancy Childb. 2017;17:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.