Abstract
Study Objectives
To evaluate how change in menopausal status related to spectral analysis and polysomnographic measures of sleep characteristics.
Methods
The Study of Women’s Health Across the Nation (SWAN) Ancillary Sleep Study evaluated sleep characteristics of 159 women who were initially pre- or early perimenopausal and repeated the assessment about 3½ years later when 38 were pre- or early perimenopausal, 31 late perimenopausal, and 90 postmenopausal. Participants underwent in-home ambulatory polysomnography for two to three nights. Average EEG power in the delta and beta frequency bands was calculated during NREM and REM sleep, and sleep duration, wake after sleep onset (WASO), and apnea hypopnea index (AHI) were based on visually-scored sleep.
Results
The women who transitioned to postmenopause had increased beta NREM EEG power at the second assessment, compared to women who remained pre-or early premenopausal; no other sleep measures varied by change in menopausal status. In multivariate models the associations remained; statistical controls for self-reported hot flashes did not explain findings. In secondary analysis, NREM beta power at the second assessment was greater among women who transitioned into the postmenopause after adjustments for initial NREM beta power.
Conclusions
Sleep duration and WASO did not vary by menopause transition group across assessments. Consistent with prior cross-sectional analysis, elevated beta EEG power in NREM sleep was apparent among women who transitioned to postmenopause, suggesting that independent of self-reported hot flashes, the menopausal transition is associated with physiological hyperarousal during sleep.
Keywords: spectral analysis, polysomnography, sleep, beta power, menopause, midlife women
Statement of Significance.
This longitudinal study is unique in showing that independent of self-reported hot flashes the transition to postmenopause is associated with increased EEG NREM beta power without concomitant changes in PSG-assessed indices of sleep duration or continuity. The pattern of increased cortical arousal absent differences in visually-scored sleep is similar to sleep patterns often observed in patients with insomnia.
Introduction
The menopausal transition is a reproductive milestone in women marked by changes in reproductive hormones and menstrual patterns, culminating in the complete cessation of menses. The transition is often accompanied by increased vasomotor symptoms, negative mood, and sleep complaints [1]. Women report increasing sleep disturbance, defined as trouble falling asleep, waking up several times, and waking up earlier than planned during the transition [2–4]. However, increasing sleep disturbance during the transition is not universal. A longitudinal analysis from the Study of Women’s Health Across the Nation (SWAN) showed that about 15% of women reported increasing problems staying asleep at least three times per week around the natural (i.e. non-surgical) menopause, the time of follicular exhaustion and the cessation of menses, although other women in the analysis showed either stable sleep symptoms over the transition or linear increases across the follow-up period, consistent with age-related increases in sleep complaints [5].
Sleep patterns measured by polysomnography (PSG) paint a more complex picture. In the Wisconsin Sleep Study, postmenopausal women had more slow-wave sleep and longer sleep time, compared to premenopausal women, but also reported being more dissatisfied with their sleep [6]. In 60 premenopausal Finnish women followed for 6 years, elevated follicle stimulating hormone (FSH), a marker of menopausal status, was related to increased slow wave sleep but no other PSG parameters [7]. Similarly, rapid increases in FSH in the seven years prior to the SWAN Ancillary Sleep Study were associated with greater slow wave sleep and longer total sleep time, yet poorer self-reported sleep quality [8]. A cross-sectional analysis in the SWAN Ancillary Sleep Study compared menopausal groups on delta and beta power based on spectral analysis of the electroencephalogram (EEG) [9]. Women who were late perimenopausal or postmenopausal had greater EEG beta power than premenopausal women, in part due to vasomotor symptoms, but the groups did not differ in percent time in various sleep stages, total sleep time, or EEG delta power.
Women enrolled in the SWAN Ancillary Sleep Study were invited to participate in a second sleep assessment approximately 3½ years after their initial assessment. Given the paucity of longitudinal data using PSG-assessed sleep measured during the menopausal transition, we evaluated changes in sleep parameters among the women who were initially pre- or early peri-menopause in relation to their menopausal status at the second assessment. We hypothesized that compared to women who remained pre- or early peri-menopausal at the second assessment, women who transitioned to late perimenopause or to postmenopause would exhibit increased EEG beta power but would not differ in EEG delta power, sleep duration, or sleep continuity. In addition, we explored whether race/ethnicity impacted the pattern of results.
Methods
Study sample
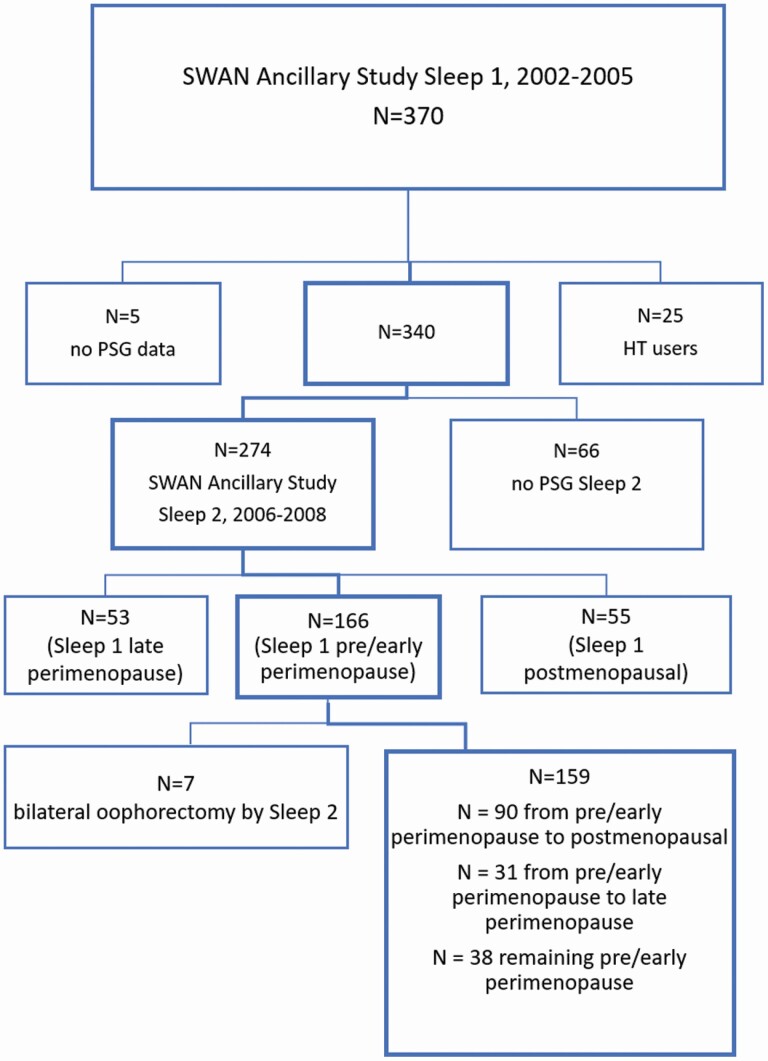
Women were participants in the Study of Women’s Health Across the Nation (SWAN), a longitudinal study of midlife women’s transition through the menopause [10]. Each of seven sites recruited White women and a minority group; of the 7 sites, 4 sites participated in the SWAN Ancillary Sleep Study [11]. These sites recruited Black, Chinese, and White women. In 2002–2005, 370 women were recruited to participate in a month-long study of sleep, with 344 women providing PSG data while not using hormone therapy. Exclusion criteria were having had a hysterectomy or bilateral oophorectomy, use of hormone therapy, ongoing cancer treatment, current oral corticosteroid use, regular consumption of >4 alcoholic drinks/day, noncompliance with core SWAN procedures, and regular night shift work. Approximately 3½ years later, in 2006–2008, 274 of these women with PSG data agreed to participate in a second sleep assessment. There were no exclusionary criteria for the second assessment. The present sample focused on 159 women who had PSG data at both time periods, were not using hormone therapy at either time point, were categorized as pre- (N = 17) or early peri-menopause (N = 142) at the first sleep assessment, and did not have a bilateral oophorectomy after the first sleep assessment (see Figure 1).
Figure 1.
Flow diagram of participants in the analytic sample.
Ancillary sleep study protocol
Subjective and objective sleep data were collected using questionnaires, daily diaries, actigraphy, and PSG [8, 11]. For women who were still menstruating, the sleep protocol began within 7 days of the onset of a menstrual period. For three consecutive days, PSG data were recorded at the participant’s home with a Vitaport 3 PSG recorder (Temec; Keerkade, Netherlands). All three nights the PSG montage included EEG recorded at C3 and C4 referred to linked mastoids, bilateral electrooculogram (EOG), and bipolar submental electromyogram (EMG). Night 1 included additional measures to evaluate sleep disordered breathing with oral-nasal temperature probes evaluating airflow, impedance plethysmography measuring chest and abdominal effort, and finger-tip oximetry measuring oxygen saturation. To eliminate possible first night effects, only PSG from nights 2 and 3 were used and averaged if both available (135 women had two nights, 24 had 1 night) [12]. A similar protocol was used at the second sleep study assessment, including assessment of sleep-disordered breathing on night 1, but only two nights of PSG data were recorded. The second night of PSG was used in the analysis for 150 women, and the first night for nine women (who did not have a second night.)
The Vitaport 3 digitized the EEG at 256 Hz. Low frequency hardware filters were set at 0.3 Hz, and high frequency hardware filters were set at 70 Hz. Digitized data were decimated to 128 Hz and then analyzed with fast Fourier transformation with 4-second Hanning tapered windows. Four-second windows with artifacts identified by methods of Brunner et al. were eliminated [13]. EEG power was averaged separately across all artifact-free epochs of NREM and REM sleep for the delta (0.5–4 Hz) band and the beta (16–32 Hz) band. These bands were chosen based on our previous cross-sectional analyses [9]. Data collected at the C3 electrode were used in the analysis across both assessments.
Based on visual scoring of each 20-s epoch, total sleep time and minutes awake after sleep onset (WASO) were calculated and averaged across the two nights at each of the two assessments. The apnea-hypopnea index (AHI) was determined by the number of apneas and hypopneas on night 1 and because of the distributions and precedent were coded into categories of 0 to <15 and ≥15.
Diaries were completed in the morning upon awakening and evening prior to bedtime. The diaries included questions about whether or not night sweats or hot flashes had occurred. Based on PSG nights only recorded in the morning diary, data were coded into any reports of vasomotor symptoms, yes/no. Women completed a 13- item insomnia questionnaire, which yields a clinical score, yes/no insomnia symptoms [14].
Menopausal status and other participant characteristics
Menopausal status was defined using menstrual bleeding criteria and was determined at the annual visit closest to the time of the sleep studies. Premenopause was defined as menstrual bleeding in the prior three months with no change in regularity; early perimenopause as menstrual bleeding in the prior three months with change in regularity; perimenopause as menstrual bleeding within the last year but not in the prior three months; and postmenopause as 12 months or more of amenorrhea not due to surgical menopause. Note that the SWAN categories were based on the definitions of perimenopause and best measurement approaches available in 1995 when SWAN began [15]. These definitions preceded those later established by 2001 Stages of Reproductive Aging Workshop (STRAW), which were updated in 2011, and differ somewhat from STRAW that relied principally on bleeding patterns and regularity and included supportive biochemical and physiological parameters and symptoms typically associated with stages of the menopausal transition [16].
As part of the core SWAN visits, a serum sample was obtained in the morning after an overnight fast targeted for days 2–7 of a menstrual cycle; if a timed sample could not be obtained a fasting sample was taken within 90 days of the core visit. Hormones assays were conducted using an ACS-180 automated analyzer (Bayer Diagnostics Corp, Norwood, MA) at the University of Michigan. For follicular stimulating hormone (FSH) a two-site chemiluminometric immunoassay was employed; the inter-assay coefficient of variation was 12.0%, the intra-assay coefficient of variation was 6.0%. For estradiol, assays were conducted in duplicate, with inter-assay coefficient of variation of 10.6% and intra-assay coefficient of variation of 6.4%.
At the core visits, women were asked their age, race/ethnicity, and educational attainment. At each annual visit they reported number of health problems on a specified list and current medication use. The latter was also updated at the sleep study visits and coded yes/no any medications that affect sleep during the sleep study protocol. These included any of the following medication classes identified by the World Health Organization Anatomical Therapeutic Chemical classifications: opioids (NO2A), antiepileptics (NO3A), anxiolytics (NO5B), hypnotics and sedatives (NO5C), antidepressives (NO6A), and antihistamines for systemic use (NO6A) (www.whocc.no/atcddd. Accessed December 10, 2007). Body mass index (BMI) was measured by stadiometer and scales in clinic. Center for Epidemiologic Studies Depression Scale (CES-D) was administered [17]; the single item measuring restless sleep was removed for formal analysis. At each core visit, sleep complaints were reported, including difficulty initiating and maintaining sleep, and early awakening; data were recoded into yes/no reporting any of the sleep complaints at least three nights per week.
Statistical approach
Because of skewness, WASO was log (ln) transformed, as done in the prior cross-sectional analysis [9]. EEG delta and beta power were transformed by log10. Participants’ characteristics at the first sleep assessment were compared according to the menopausal transition group by analysis of variance and chi squares. The primary analysis was a multiple regression mixed model with AR1 covariance structure predicting level of the sleep characteristic at the first and second assessment with a trichotomous variable. The three groups were (1) women who transitioned from pre-/early peri-menopause to late perimenopause (N = 31); (2) women who transitioned from pre-/early peri-menopause to postmenopause (N = 90); and (3) (the referent group) women who were pre-/early peri-menopausal and did not transition (i.e. remained in that group at the second assessment (N = 38). The basic models included menopause transition group, duration between the two sleep assessments, site, race/ethnicity, age at first sleep study, within-woman sleep characteristic at each assessment (time) and the interaction of transition group by time.
Only when the interaction term was a significant predictor, the next step was to address whether any potential covariates accounted for the associations. Potential covariates were a priori identified based on prior SWAN analyses and the sleep literature. Those covariates that were associated with sleep characteristics, p < 0.20, in models that included duration between the two assessments, site, race/ethnicity, age at first sleep study, and sleep characteristic at time 1 and 2 were added to the basic model. The potential time-varying covariates included measures collected at the closest SWAN core visits: number of health problems, BMI, CES-D scores (without the sleep item); and during the ancillary sleep protocol, any vasomotor symptoms reported in diaries concurrent with PSG assessment nights, any medications that affect sleep, and AHI ≥15 (yes/no). Exploratory analyses tested for interactions between race/ethnicity, transition group, and time in Black and White non-Hispanic women only; there were too few Chinese women in each transition group to conduct these analyses.
Because 22–24 women were missing EEG beta and delta power data (due to artifacts in the sleep EEG), we compared the women with EEG power data to those without and found no differences in site, race/ethnicity, menopausal status group, duration between assessments, BMI, depressive symptoms, insomnia, AHI group, sleep duration, and WASO. Women without EEG data were about a year younger and reported less than one fewer health problem.
Comparison of the 159 participants with the 51 women who were pre- (N = 3) or early perimenopausal (N = 48) at the first sleep assessment and did not participate in the second sleep assessment showed that the two groups had similar proportions of each race/ethnic group, and similar ages, BMI, number of health problems, sleep duration, depression, and insomnia scores. The 159 participants had nonsignificantly lower WASO scores, p = 0.06, and higher NREM, p = 0.06, and REM beta power, p = 0.09. Analyses were conducted using SAS 9.4.
Results
Table 1 shows the women’s characteristics at the first sleep assessment and Table 2 shows the women’s sleep characteristics during the sleep 1 and 2 assessments. The sample was around 51 years of age at the first assessment and was comprised of a majority of white, followed by Black, and Chinese women. The women had few health problems and moderate depressive symptoms (including the one item regarding restless sleep) and were overweight. On average women slept over 6 h and had almost 50 min of WASO, as previously reported [11]. Percentage of women with AHI scores ≥ 15 was 12.5%, and 36% reported any vasomotor symptoms concurrent with PSG studies.
Table 1.
Characteristics at the first sleep assessment of the full sample when all women were pre- or early peri-menopausal and for each menopause transition group based on menopausal status at the second sleep assessment
| N | Full sample |
Pre/early peri-menopause (N = 38) | Pre/early peri-menopause to late peri-menopause (N = 31) | Pre/early peri-menopause to postmenopause (N = 90) | p-value for group differences at first assessment | |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 159 | 50.95 (1.86) | 50.37 (1.68) | 50.32 (1.72) | 51.41 (1.86) | 0.001 |
| Racial group, N (%) | 159 | 0.47 | ||||
| White | 80 (50.31) | 23 (60.53) | 16 (51.61) | 41 (45.56) | ||
| Black | 51 (32.08) | 8 (21.05) | 11 (35.48) | 32 (35.56) | ||
| Chinese | 28 (17.61) | 7 (18.42) | 4 (12.90) | 17 (18.89) | ||
| E2 pg/ml | 149 | 80.58 (97.26) | 82.50 (92.79) | 65.60 (74.28) | 84.51 (105.74) | 0.59 |
| FSH IU/l | 150 | 40.65 (39.40) | 17.07 (16.64) | 39.32 (35.33) | 51.36 (43.28) | <0.001 |
| Body mass index, mean (SD) | 153 | 29.14 (7.15) | 27.80 (5.12) | 30.50 (6.30) | 29.23 (8.09) | 0.30 |
| No. health problems, mean (SD) | 159 | 0.78 (1.05) | 0.47 (0.65) | 0.77 (1.02) | 0.91 (1.18) | 0.10 |
| CES-Depression scores, mean (SD) | 157 | 7.05 (7.50) | 5.21 (5.81) | 7.72 (7.36) | 7.61 (8.09) | 0.14 |
| CES-Depression scores 16 or greater, N (%) | 157 | 22 (14.01) | 3 (7.89) | 4 (13.79) | 15 (16.67) | 0.47 |
| Use of medications that affect sleep, yes, N (%) | 158 | 46 (29.11) | 11 (29.73) | 9 (29.03) | 26 (28.89) | 0.99 |
| Hot flashes during PSG protocol, yes, N (%) | 156 | 56 (35.90) | 7 (18.92) | 10 (32.26) | 39 (44.32) | 0.02 |
| Any sleep complaints, yes N (%) | 157 | 65 (41.4) | 10 (26.3) | 15 (48.4) | 40 (44.9) | 0.09 |
| Insomnia, yes N(%) | 158 | 20 (12.7) | 5 (13.2) | 2 (6.5) | 13 (14.6) | 0.58 |
| AHI 15 or greater, N (%) | 152 | 19 (12.50) | 7 (18.42) | 2 (6.67) | 10 (11.90) | 0.38 |
Table 2.
Sleep characteristics at the first and second assessment of the full sample when all women were pre- or early peri-menopausal and for each menopause transition group based on menopausal status at the second sleep assessment
| Time | N | Full sample |
Pre/early peri-menopause (N = 38) | Pre/early peri-menopause to late peri-menopause (N = 31) | Pre/early peri-menopause to postmenopause (N = 90) | p-value for group differences at first assessment | |
|---|---|---|---|---|---|---|---|
| Sleep duration, mean hours (SD) | 1 | 159 | 6.39 (0.89) | 6.67 (0.84) | 6.41 (0.76) | 6.26 (0.92) | 0.06 |
| 2 | 159 | 6.42 (1.02) | 6.50 (1.20) | 6.62 (1.15) | 6.32 (0.88) | ||
| Wake after sleep onset, mean minutes (SD) | 1 | 159 | 47.41 (30.22) | 45.92 (26.53) | 46.25 (27.03) | 48.43 (32.86) | 0.99 |
| 2 | 159 | 60.27 (43.93) | 49.66 (30.85) | 58.25 (38.72) | 65.44 (49.48) | ||
| Log NREM Delta EEG power, mean (SD) | 1 | 136 | 2.19 (0.19) | 2.18 (0.18) | 2.22 (0.24) | 2.19 (0.17) | 0.68 |
| 2 | 136 | 2.22 (0.22) | 2.20 (0.20) | 2.21 (0.25) | 2.23 (0.22) | ||
| Log NREM Beta EEG power, mean (SD) | 1 | 136 | 0.51 (0.20) | 0.46 (0.17) | 0.58 (0.28) | 0.51 (0.17) | 0.07 |
| 2 | 136 | 0.57 (0.20) | 0.50 (0.16) | 0.55 (0.23) | 0.61 (0.21) | ||
| Log REM Delta EEG power, mean (SD) | 1 | 133 | 1.58 (0.15) | 1.55 (0.13) | 1.61 (0.16) | 1.58 (0.16) | 0.26 |
| 2 | 133 | 1.61 (0.20) | 1.58 (0.20) | 1.59 (0.21) | 1.62 (0.19) | ||
| Log REM Beta EEG power, mean (SD) | 1 | 135 | 0.55 (0.19) | 0.54 (0.21) | 0.57 (0.17) | 0.55 (0.19) | 0.83 |
| 2 | 135 | 0.63 (0.20) | 0.63 (0.24) | 0.61 (0.21) | 0.64 (0.18) |
Comparison of the three transition groups showed no significant differences at the first assessment, with three exceptions. Post hoc tests showed that women who subsequently transitioned to postmenopause were about a year older and more likely to report vasomotor symptoms during the PSG protocol; they also had higher FSH but not estradiol levels relative to the other groups. There were no significant group differences in the length of time between the two assessments, p = 0.38; mean (SD) length of time for the sample was 3.58 years (SD = 0.72). Paired t-tests on the (unadjusted) sleep characteristics showed that WASO, REM delta power, and NREM and REM beta power increased across time, ps <0.05.
Influence of menopausal transition group on PSG-assessed sleep
In the models including age at first sleep assessment, site, race/ethnicity, duration between assessments, transition groups, time, and the interaction of transition groups by time, the transition groups did not differ in the levels of sleep duration, WASO, EEG delta power during NREM or REM sleep, and EEG beta power during REM sleep from the first to second sleep assessment (Table 3).
Table 3.
Estimates (standard errors) from the mixed models predicting the relationships with PSG-assessed sleep and menopause transition groups
| Sleep duration | WASO | NREM EEG delta power | NREM EEG beta power | REM EEG delta power | REM EEG beta power | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | p value | Estimate (SE) | p value | Estimate (SE) | p value | Estimate (SE) | p value | Estimate (SE) | p value | Estimate (SE) | p value | |
| Age at sleep 1 | −0.042 (0.033) | 0.216 | 0.037 (0.022) | 0.090 | −0.013 (0.009) | 0.140 | −0.008 (0.008) | .291 | −0.013 (0.007) | .072 | −0.005 (0.009) | 0.565 |
| Race (White referent) | 0.016 | 0.017 | 0.096 | .476 | .280 | 0.936 | ||||||
| Black | −0.325 (0.148) | 0.030 | 0.281 (0.097) | 0.015 | −0.063 (0.040) | 0.118 | −0.004 (0.037) | .919 | −0.046 (0.035) | .187 | 0.013 (0.042) | 0.765 |
| Chinese | −0.381 (0.200) | 0.059 | −0.054 (0.131) | 0.680 | −0.076 (0.050) | 0.135 | −0.057 (0.047) | .226 | −0.040 (0.044) | .367 | 0.011 (0.053) | 0.836 |
| Duration between sleep studies | 0.104 (0.104) | 0.318 | 0.038 (0.071) | 0.592 | 0.053 (0.017) | 0.002 | −0.002 (0.022) | .927 | 0.015 (0.017) | .380 | −0.020 (0.015) | 0.182 |
| Sleep 1, sleep 2 assessment (time) | −0.560 (0.429) | 0.193 | −0.086 (0.293) | 0.770 | −0.170 (0.068) | 0.014 | 0.042 (0.089) | .639 | −0.021 (0.069) | .767 | 0.163 (0.059) | 0.006 |
| Transition group (referent, remaining pre−-/early perimenopause) | 0.301 | 0.905 | 0.497 | .030 | .177 | 0.903 | ||||||
| Late perimenopause | −0.223 (0.225) | 0.323 | −0.038 (0.151) | 0.803 | 0.058 (0.052) | 0.266 | 0.126 (0.052) | .018 | 0.084 (0.046) | .074 | 0.043 (0.053) | 0.419 |
| Postmenopausal | −0.319 (0.183) | 0.084 | −0.058 (0.123) | 0.634 | 0.030 (0.040) | 0.452 | 0.053 (0.041) | .193 | 0.051 (0.036) | .156 | 0.020 (0.041) | 0.626 |
| Interaction between time × transition group | 0.329 | 0.427 | 0.413 | .033 | .228 | 0.173 |
Note. Site is included in all models.
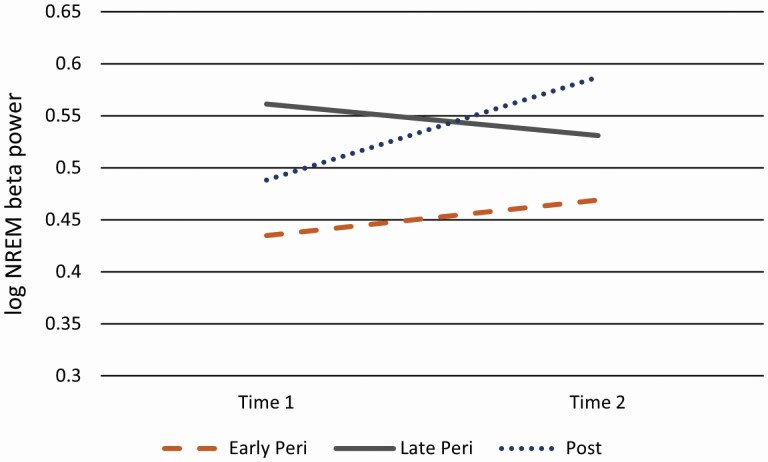
On the other hand, the transition group by time interaction term was significant for EEG beta power during NREM sleep. Further evaluation of the interaction term revealed that the group differences were apparent at the second sleep assessment, F = 4.249, p = 0.016. At the second sleep assessment, women who transitioned to postmenopause had elevated EEG beta power, estimate = 0.117 (0.04), p = 0.005, compared to women remaining pre/early perimenopausal, whereas women who transitioned to late perimenopause did not differ from the referent group, estimate = 0.061 (0.053), p = 0.251 (see Figure 2). Adding to the basic model the only covariate related to NREM EEG beta power at the threshold of p ≤ 0.20, any vasomotor symptoms during the PSG study, the pattern of results remained the same. The overall effect for transition group by time remained significant, F = 3.867, p = 0.02. The interaction effect was apparent at the second sleep assessment, F = 3.945, p = 0.02, with the estimate of NREM EEG beta power for women who transitioned to postmenopause greater relative to the referent group, estimate = 0.118 (0.042), p = 0.006.
Figure 2.
Estimated means for NREM beta (log) at the first and second assessment by menopausal transition groups.
We conducted a secondary analysis predicting time 2 NREM EEG beta power, adjusting for time 1 NREM EEG beta power, age, race/ethnicity, site, and time interval between assessments. Results showed an overall effect for transition group, F = 3.222, p = 0.04, with only the group who transitioned to postmenopause showing higher NREM EEG beta power at the second assessment, compared to the referent group of women who remained pre- or early peri-menopause, estimate (SE) = 0.089 (0.04), p = 0.029.
Influence of race/ethnicity on sleep measures
Consistent with a previous report from the first sleep study [11], across both assessments, Black women had shorter sleep duration and more WASO compared to Whites; Chinese women tended to have shorter sleep duration than Whites (Table 3). Tests for the three-way interaction with Black/White, transition group, and time showed one significant three-way interaction term for REM delta, p = 0.01, in the full model including AHI as a covariate because it was related to REM delta, p < 0.20. Further evaluation showed that the three-way effect was apparent at the second sleep assessment: Whites had higher REM delta values than Blacks in the late perimenopausal group only.
Discussion
The present paper evaluated PSG-assessed sleep characteristics at two timepoints of 159 women, all who were pre- or early peri-menopausal at the first assessment, according to whether they transitioned to late perimenopause or postmenopause or remained pre- or early peri-menopause at the second assessment approximately 3½ years later. Consistent with our primary hypotheses, women who transitioned to postmenopause had elevated NREM EEG beta power at the second assessment compared to women who remained pre- or early perimenopausal. The association with NREM EEG beta power was independent of age, race/ethnicity, site, BMI, and duration between assessments. Further adjustment for self-reported vasomotor symptoms during the sleep study did not change the overall results. The secondary analysis that predicted residualized NREM EEG beta power scores at follow-up (adjusted for time 1 scores) showed the same pattern of results. We did not find that the women who transitioned to late perimenopause had elevated NREM EEG beta power, in contrast to our prior cross sectional analyses [9].
Consistent with expectations, women who became late perimenopausal or postmenopausal by the time of the second assessment did not differ from those who remained pre- or early-perimenopausal in sleep duration, sleep continuity, or NREM delta power. These null findings are consistent with previous cross-sectional analyses based on the SWAN Ancillary Sleep Study [8, 9] and the longitudinal analyses from the Finnish study [7]. On the other hand, the Wisconsin Sleep Study [6] found that postmenopausal women had longer sleep duration, which is not consistent with our null finding. That study also reported a menopause-associated increase in visually scored slow wave sleep, in contrast to the null menopause-associated relationship for NREM EEG delta power found in this cross-sectional and longitudinal spectral analyses. Our results for visually scored sleep are similar to our prior report of the same women plus others in later stages of the transition who participated in our actigraphy protocol. In our prior report, women had no change in actigraphy measures of sleep duration, WASO, and sleep latency across 3 years, but after 12 years of follow-up, sleep duration and sleep midpoint had increased and WASO declined [18]. Perhaps evaluation of women’s sleep in the late postmenopause, defined as starting at least 5 years after the FMP, rather than the transition to early postmenopause may yield different results when using PSG measures. Taken together, these results suggest that during the transition to early postmenopause, PSG measures of sleep duration and continuity change little, on average.
As a whole, these results show that NREM EEG beta power is higher in women further along the menopausal transition, whether measured cross-sectionally between menopausal status groups in our prior study [9], or evaluated across the menopausal transition in our present longitudinal study. These results suggest that the menopausal transition may be associated with increased cortical arousal during NREM sleep, despite similar PSG-assessed indices of sleep duration or continuity. The pattern of elevated NREM EEG beta power has been observed in patients with insomnia in some, but not all, studies [19–21], an effect which may be stronger in female compared to male patients affected with insomnia [22]. Of interest is that in experimental work the increased NREM beta power localized in the central-posterior brain area was also associated with subjective underestimation of sleep duration, which could help explain why women subjectively report worse sleep across the transition at the same time little change in sleep duration or continuity [23]. Our findings may inform clinical care by affirming the concerns of women who report nonrefreshing sleep but who do not endorse sleep insufficiency or disruption. While low-risk lifestyle modifications to increase sleep opportunity may be proposed, we recognize that such interventions have not been studied for this type of sleep dysfunction. This type of sleep dysfunction may reflect a more physiological basis rather than perceived sleep. Nonetheless, emerging evidence suggests that cognitive-behavioral therapy for insomnia may normalize beta power in older adults with insomnia [24, 25]. Our findings support the need for future work to better understand the effects of sleep interventions on EEG parameters in older adults.
It is notable that the association between transition group and NREM EEG beta power was minimally affectedly by statistical adjustment for self-reported vasomotor symptoms. These findings suggest that women may report nonrefreshing sleep in the absence of self-reported vasomotor symptoms may benefit from therapies targeting their sleep. However, our study did not include objective measures of nighttime vasomotor symptoms, which are associated with increased autonomic arousal during sleep as measured by cardiac vagal inhibition [26].
As in our previous cross-sectional report, Blacks had more WASO and shorter sleep duration than Whites, and Chinese had shorter sleep duration than Whites [11, 27–29]. There was little evidence that race/ethnicity impacted the magnitude of change in sleep among women who transitioned to the postmenopause. Given that the number of women was small in some of the transition groups, it is necessary to replicate these findings in larger samples prior to asserting a differential effect of race/ethnicity on sleep response during the menopausal transition.
The study has several strengths. This is one of the first longitudinal studies that compared PSG-assessed sleep characteristics of pre- or early peri-menopausal women who subsequently changed in their menopausal status relative to women who remained pre- or early peri-menopausal. In-home PSG assessment enhances the study’s ecological validity and provides objective indices of sleep duration and continuity as well as EEG-assessed indices of cortical arousal and sleep depth. Finally, we had the potential to statistically adjust for factors known to influence sleep in midlife women, such as sociodemographic characteristics (age, race/ethnicity), BMI and mental health complaints (symptoms of depression, number of medical problems, use of medications that affect sleep), as well as nocturnal vasomotor symptoms.
The study has several limitations. Observational studies, like the present study, cannot establish causality but only can describe temporal associations. The proportion of women who agreed to the follow-up PSG studies was 76%, although they were similar in many characteristics to pre- or peri-menopausal women who did not participate in the follow-up. It did not address the timing of the PSG studies in relation to ovulation or reproductive hormones. Despite a relatively large racially diverse sample of midlife women, the racial/ethnic subgroups were of small sample size, especially the Chinese women. Although the sample included Black and Chinese women, it did not include other racial or ethnic groups including Hispanic women. We were unable to track changes across a longer follow-up period than 3½ years and had only one follow-up assessment. The facts that the majority of pre- and early peri-menopausal women in the analysis transitioned to a later stage, and that the average age of the sample at the first sleep study was 51 years, the average age of FMP, suggest that our study documented sleep changes late in the transition into the early postmenopausal period.
In summary, levels of PSG-assessed NREM EEG beta were higher at follow-up in midlife women who transitioned to post-menopause compared to those who remained pre- or early peri-menopausal, whereas indices of sleep duration, continuity, and depth were not related to change in menopausal status. Increased EEG beta power during NREM sleep may reflect increased hyperarousal in association with the menopausal transition and offer intervention opportunities that improve the quality of life for those affected women.
Funding
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), Department of Health and Human Services (DHHS), through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). Funding for the Study of Women’s Health Across the Nation Sleep Study was provided by the National Institute on Aging (NIA) (grants R01AG019360, R01AG019361, R01AG019362, R01AG019363 and K12HD103085). Sleep data were processed with the support of grant RR024153. Support and training for M. Evans was provided by T32 HL07560 and HL082610. Other sources of funding for this publication include K23HL122461 (Swanson), R01AG53838 (Joffe). The content of this article manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. We thank the study staff at each site and all the women who participated in SWAN.
Clinical Centers
University of Michigan, Ann Arbor – Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Sherri-Ann Burnett-Bowie, PI 2020–present; Joel Finkelstein, PI 1999–2020; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL – Imke Janssen, PI 2020–present; Howard Kravitz, PI 2009–2020; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser – Elaine Waetjen and Monique Hedderson, PIs 2020–present; Ellen Gold, PI 1994–2020; University of California, Los Angeles – Arun Karlamangla, PI 2020–present; Gail Greendale, PI 1994–2020; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA – Rebecca Thurston, PI 2020–present; Karen Matthews, PI 1994–2020.
NIH Program Office
National Institute on Aging, Bethesda, MD – Rosaly Correa-de-Araujo 2020–present; Chhanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory
University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center
University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA – Sonja McKinlay, PI 1995–2001.
Steering Committee
Susan Johnson, Current Chair; Chris Gallagher, Former Chair
Data Availability Statement
The data underlying this article are not shared publicly because of confidentiality. The data will be shared on reasonable request to the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu and will require a formal data use agreement between the applicant’s institution and the University of Pittsburgh.
Financial Disclosure
Consulting by Joffe with NeRRe/KaNDy, Merck, Sojarnix, Eisai, and Jazz Pharmaceutical.
Non-financial Disclosure
None.
References
- 1. El Khoudary SR, et al. . The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause. 2019;26(10):1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kravitz HM, et al. . Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 3. Xu Q, et al. . A systematic review of the longitudinal relationships between subjective sleep disturbance and menopausal stage. Maturitas. 2014;79(4):401–412. [DOI] [PubMed] [Google Scholar]
- 4. Woods NF, et al. . Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33(4):539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kravitz HM, et al. . Sleep trajectories before and after the final menstrual period in the Study of Women’s Health Across the Nation (SWAN). Curr Sleep Med Rep. 2017;3(3):235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Young T, et al. . Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26(6):667–672. [DOI] [PubMed] [Google Scholar]
- 7. Lampio L, et al. . Sleep during menopausal transition: a 6-year follow-up. Sleep. 2017;40. [DOI] [PubMed] [Google Scholar]
- 8. Sowers MF, et al. . Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31(10):1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell IG, et al. . Evaluation of the association of menopausal status with delta and beta EEG activity during sleep. Sleep. 2011;34(11):1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sowers M, et al. . SWAN: a multi-center, multi-ethnic community-based cohort study of women and the menopausal transition. In: Lobo R, Marcus R, Kelsey J, eds. Menopause:Biology and Pathology. New York: Academic Press; 2000. [Google Scholar]
- 11. Hall MH, et al. . Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 12. Agnew HW Jr, et al. . The first night effect: an EEG study of sleep. Psychophysiology. 1966;2(3):263–266. [DOI] [PubMed] [Google Scholar]
- 13. Brunner E, et al. . Childhood social circumstances and psychosocial and behavioural factors as determinants of plasma fibrinogen. Lancet. 1996;347(9007):1008–1013. [DOI] [PubMed] [Google Scholar]
- 14. Okun ML, et al. . Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J Clin Sleep Med. 2009;5(1):41–51. [PMC free article] [PubMed] [Google Scholar]
- 15. Brambilla DJ, et al. . Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol. 1994;140(12):1091–1095. [DOI] [PubMed] [Google Scholar]
- 16. Harlow SD, et al. ; STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18. Matthews KA, et al. . Does midlife aging impact women’s sleep duration, continuity, and timing?: a longitudinal analysis from the Study of Women’s Health Across the Nation. Sleep. 2020;43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perlis ML, et al. . Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleeper controls. J Sleep Res. 2001;10(2):93–104. [DOI] [PubMed] [Google Scholar]
- 20. Spiegelhalder K, et al. . Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91(3):329–333. [DOI] [PubMed] [Google Scholar]
- 21. Wu YM, et al. . EEG power during waking and NREM sleep in primary insomnia. J Clin Sleep Med. 2013;9(10):1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buysse DJ, et al. . EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31(12):1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lecci S, et al. . EEG changes associated with subjective under- and overestimation of sleep duration. Sleep. 2020;43. [DOI] [PubMed] [Google Scholar]
- 24. Hogan SE, et al. . Slow-oscillation activity is reduced and high frequency activity is elevated in older adults with insomnia. J Clin Sleep Med. 2020;16(9):1445–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cervena K, et al. . Effect of cognitive behavioural therapy for insomnia on sleep architecture and sleep EEG power spectra in psychophysiological insomnia. J Sleep Res. 2004;13(4):385–393. [DOI] [PubMed] [Google Scholar]
- 26. Thurston RC, et al. . Changes in heart rate variability during vasomotor symptoms among midlife women. Menopause. 2016;23(5):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matthews KA, et al. . Racial/ethnic disparities in women’s sleep duration, continuity, and quality, and their statistical mediators: Study of Women’s Health Across the Nation. Sleep. 2019;42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruiter ME, et al. . Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med. 2011;12(3):209–214. [DOI] [PubMed] [Google Scholar]
- 29. Whinnery J, et al. . Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37(3):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are not shared publicly because of confidentiality. The data will be shared on reasonable request to the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu and will require a formal data use agreement between the applicant’s institution and the University of Pittsburgh.