Abstract
Background
Opioid analgesics play a key role in pain management but providing access while mitigating risk of misuse and dependence remains a challenge. Tracking global consumption of all opioids over time can help identify emerging patterns and drivers of use.
Methods
Prescription opioid analgesic consumption was estimated for 76 countries between 2009 and 2019 using IQVIA MIDAS data. We reported country-level consumption trends in morphine milligram equivalents (MMEs), assessed differences in consumption between high-income (HICs), upper-middle income (UMICs), and low- and lower-middle income countries (LMICs), and identified country-level socioeconomic drivers of consumption using fixed-effects panel regression models.
Findings
Global opioid consumption rate declined from 216·3 to 151·5 morphine milligram equivalents per 1,000 inhabitants per day (MID) between 2009 and 2019, with consumption declines in the US and Germany. Overall, consumption rates increased in HICs by a median 36·6 MID (IQR, -7·5 -124·5) with substantial heterogeneity between countries. Median consumption rates were lower in UMICs (23·6 MID) and LMICs (8·3 MID) compared to HICs (345·1 MID) and increased by median 10·4 and 3·7 MID from 2009-2019, respectively. Consumption rates were associated with income (coefficient 18·84, 95% confidence interval 3·8-33·9) and trade (13·59, 1·3-25·8) in UMICs, and physician density (1·95, 1·2-2·7) in LMICs. Tramadol consumption rate increased in the study period and accounted for a relatively large proportion of total opioid volume consumed across all country-income groups.
Interpretation
Substantial heterogeneity in global opioid consumption patterns reflect the challenges involved with providing adequate access to opioid treatment while avoiding potential misuse.
Research in context.
Evidence before this study
Prior to undertaking the analysis, a rapid review of the literature was conducted to survey existing evidence on global opioid analgesic use. Relevant publications included nine studies that compared opioid consumption between a limited number of countries and reports from the International Narcotics Control Board (INCB), Organisation for Economic Cooperation and Development, and the European Monitoring Centre for Drugs and Drug Addiction.
Added value of this study
Our study is the first to assess the global consumption of all prescription opioid analgesics using a pharmaceutical sales database. We find that the consumption rate of opioids not tracked by the INCB such as tramadol has increased over the study period and account for a relatively large proportion of the total opioid volume consumed. The association between the opioid consumption rate and country-level socioeconomic factors varied by country-income group.
Implications of all the available evidence
This study underscores how evaluating the global consumption of all prescription opioid analgesic products allows for a better understanding of patterns of use to support formulating international guidelines for the management of pain. The growing consumption of opioids not scheduled by international conventions must be carefully monitored to ensure appropriate use. However, the large gap between the level of opioid consumption in high-income and low-income countries persists, so care must be taken to avoid unintended consequences from any restrictive measures.
Alt-text: Unlabelled box
1. Introduction
Pain is recognised as a global health problem affecting an estimated 20% of adults [1]. Untreated pain, especially chronic pain, has significant physical, psychological, social and economic consequences, and a profound impact on quality of life [2]. While there is a high prevalence of pain in high income countries, [1,3,4] the burden of untreated pain falls heavily on the developing world [5,6]. Opioids are used to treat moderate to severe pain with evidence supporting short-term efficacy [7] and they have been considered essential for pain management by the World Health Organisation (WHO) since 1986 [2]. However, there are well documented risks associated with opioid medication including misuse, dependence (opioid use disorder) and deaths due to overdose [7,8]. From 1999 to 2011, the opioid-analgesic poisoning death rate in the US nearly quadrupled due to misuse and abuse of prescription opioid analgesics [9]. Although this problem was most acute in the US, other countries like Canada, Sweden, Norway, Ireland, and the UK also saw a surge in opioid-analgesic poisoning deaths, indicative of a growing health crisis driven by opioid addiction and misuse [10].
The opioid crisis of the 2000s challenged attitudes towards pain management, prompting several countries to develop regulatory measures and recommendations to limit the consumption of opioids, including the establishment of dosage and duration thresholds, the removal of select opioid drugs from drug schedules, the implementation of prior authorisation requirements by payers, the increase in availability and accessibility to non-opioid pain treatment, and the intensification of prescription monitoring [11]. Some of these measures had harmful unintended consequences resulting from the misapplication of guidelines and sudden opioid discontinuation [12].Concurrently, the WHO published guidelines to increase the accessibility and availability of opioids in low- and middle-income countries. However, these guidelines were retracted after the discovery that experts involved in their development had conflicts of interest, and because disputed evidence on the low-risk of developing opioid dependence was originally included [13]. The uncertainty around these guidelines and recommendations highlights the global challenge of getting pain management right; providing access to pharmaceutical treatments while also balancing concerns around opioid misuse is difficult.
Global surveillance of country-level opioid use is essential to identify emerging patterns and drivers of consumption, assess country and regional variations in use and inform global efforts to optimise pain care while simultaneously minimising opioid-related risks. Previous efforts to assess opioid analgesic consumption globally have relied on data collected by the International Narcotics Control Board (INCB), based on information reported by government authorities on opioids available for retail distribution, medical use or scientific research [14], [15], [16]. However, there are some limitations to this data. Only substances regulated by the Single Convention on Narcotic Drugs are included in the INCB database [17]. The INCB invited governments to report consumption data on psychotropic substances through which it started receiving data on buprenorphine consumption in 2016. However, governments have no obligation to report these statistics and due to limitations in the data, the INCB does not include buprenorphine consumption in their estimates of total opioid consumption [18]. Therefore, the consumption of opioids such as tramadol, tapentadol, nalbuphine, and buprenorphine were excluded from previous studies, which may underestimate the opioid consumption rate. Emerging evidence has highlighted the risk of dependency associated with opioids such as tramadol, and thus it is important that global consumption analyses include these opioids [19,20]. Additionally, it is not possible to identify the indication for which opioids were used based on INCB data, so opioids used for non-analgesic purposes (e.g., anaesthesia) are captured in consumption estimates.
In order to address these limitations of previous efforts to estimate global opioid consumption and to more comprehensively characterise the global consumption patterns of all prescription opioid analgesic products (referred to as opioid consumption throughout this paper), including opioids not tracked by the INCB, we assessed opioid analgesic sales data in hospital and retail settings for 76 countries. We reported on opioid consumption in morphine milligram equivalents (MME) to allow equianalgesic comparisons from 2009 to 2019, a decade characterised by significant changes in pain management. We evaluated changes in global patterns of opioid analgesic use and identified socioeconomic drivers of consumption.
2. Methods
2.1. Data sources and outcomes
We used the IQVIA MIDAS database to obtain data on opioid consumption. This database has been used previously to evaluate global antibiotic consumption and the same methodology is utilised by IQVIA to collect data on opioid analgesics [21,22]. IQVIA MIDAS data is collected in the supply chain, at the individual country level, predominately via shipments to dispensing locations such as pharmacies, clinics and hospitals from wholesalers, distributors and manufacturers. In some circumstances, data is collected as consumption in terms of distribution to patients. The exact point of data collection varies by the healthcare system and distribution model within each country. Over-the-counter opioid sales are also captured by MIDAS. Most data are collected electronically and reported monthly apart from a small number of countries that report data quarterly. We obtained quarterly sales data in hospital and retail settings from 2009 to 2019 for 76 countries. The data for French-speaking west Africa (Benin, Burkina Faso, Cameroon, Chad, Côte d'Ivoire, Republic of Congo, Guinea, Mali, Niger, Senegal, and Togo) and Central America (Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, and Panama) were available as two individual aggregated groups.
We had complete data for the entire study period for 73 countries (Supplementary material Table S1). For Bosnia and Serbia, we had data from 2011, and for Ukraine, from 2010 onwards. By sector, data from the retail setting was available for all countries, and data from the hospital setting was available for 49 countries. However, amongst the 27 countries for which we did not have hospital sector data, the retail sector accounted for more than 70% of the prescription opioid market share in 21 countries (Supplementary material, Table S1). Therefore, the missing data for the hospital sector is unlikely to substantially impact the opioid consumption rate estimates we calculated for these countries. However, to assess the sensitivity of our findings to the missing hospital sector data, we used the retail sector opioid consumption volume and the estimated opioid sales market share of the retail and hospital sectors in each country (Supplementary material, Table S1) to impute the missing hospital sector opioid consumption volumes.
We included all opioids used for pain management based on the European Pharmaceutical Marketing Research Association (EphMRA) Anatomical Therapeutic Chemical (ATC3) classification codes N2A narcotic analgesics and N2B non-narcotic analgesics. We excluded opioids used for opioid dependence treatment (classification code N7F). Because we were not able to distinguish use of methadone for pain management from that for opioid substitution based on formulation, we excluded methadone from the analysis. However, we present the trends in global methadone consumption in the supplementary material, Table S2 & Figure A1.
There is a wide range of opioids with different potencies. We use morphine milligram equivalents (MMEs) as the consumption metric for opioid analgesics because it measures the total morphine equivalency exposure. MMEs account for the potency of the opioids consumed, therefore stronger opioids will be emphasised more compared to an alternative metric such as defined daily dose (DDD). Sales were converted into MMEs by applying morphine equivalency factors to unit volume for each unique molecule-formulation-strength combination by calendar quarter. The morphine equivalency factors are based on standards used by the Centers for Disease Control and Prevention (CDC), available from the CDC National Drug Code and Oral MME File, in addition to other published conversion factors in the literature [23,24].
Although it is likely that not all opioids sold are consumed, we estimated opioid consumption as the total of sales expressed in MMEs. Annual opioid consumption for each country was calculated as total MMEs per year. A country's annual consumption rate in MMEs per 1,000 inhabitants per day was calculated using population estimates from the World Bank DataBank [25]. Opioid consumption was compared between groups of countries based on their 2014 World Bank income classification as high-income countries (HICs), upper-middle income countries (UMICs), and low- and lower-middle income countries (LMICs) [26].
2.2. Statistical analysis
The primary comparison evaluated differences in opioid consumption in terms of: 1) absolute changes in the annual consumption rate from 2009 to 2019 for each country; and 2) differences in the median annual consumption rate trends across the three income groups during the study period. We also evaluated the differences in the consumption of individual opioids across the income groups as a proportion of total opioids consumed. Country-level consumption rate trends for the most consumed opioids globally were subsequently compared. Differences in trends were assessed using the Wald chi-square test. All statistical testing was two-sides with an a priori level of significance of P<·05.
We used fixed-effects panel regression models for the three income groups to estimate the association between annual opioid consumption and country-level socioeconomic indicators. The panel was indexed by country and year, where annual opioid consumption in MME per capita was the outcome variable. The indicators were chosen based on a previous analysis of global antibiotic consumption and collected from the World Bank World Development Indicators[21]. We included gross domestic product (GDP) per capita in current international dollars converted by purchasing power parity (PPP) conversion factor; imports of goods and services as a percentage of GDP (measure of trade); physician density per 1,000 people; and the proportion of the population living in urban areas (measure of health care system access). Robust standard errors were clustered by country to account for serial correlation. Statistical analyses were performed using R statistical software, version 3·6·2 (R Foundation).
Ethical approval was not required for this study and was confirmed by the National Health Service Health Research Authority ethics review tool for health research in the UK.
2.3. Role of the funding source
The funding source had no role in any aspect of this study including study design, data collection, analysis and interpretation, and the decision to submit for publication. SJ, AC, MA and EM had full access to the data and all authors decided to submit for publication.
3. Results
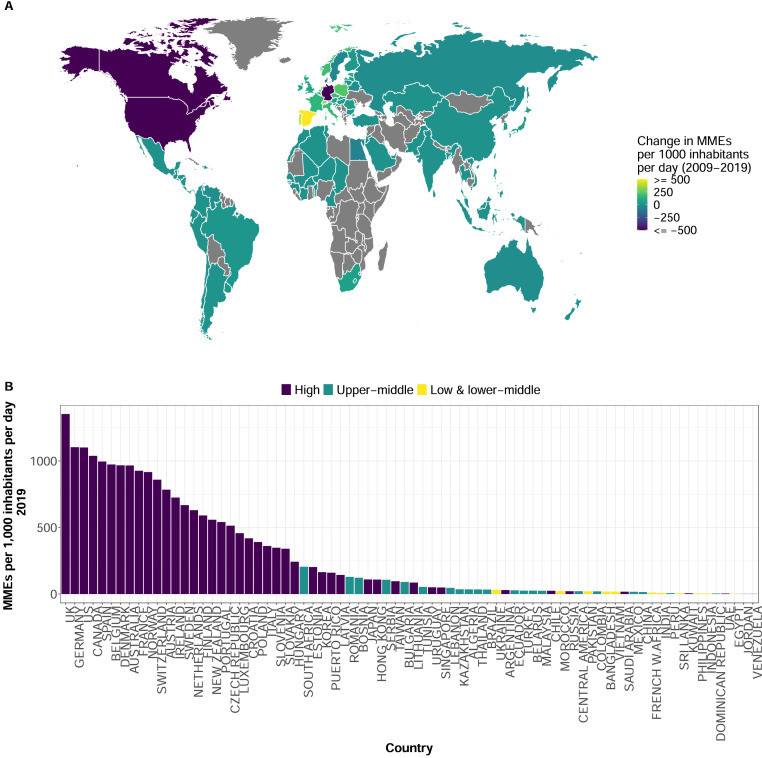
The global opioid consumption rate declined by 30% from 216·3 to 151·5 MME per 1,000 inhabitants per day (MID) between 2009 and 2019. The reduction in global consumption was primarily driven by decreased opioid consumption in the US and Germany. In 2009, Germany had the highest consumption rate (2649 MID), followed by the US (2119 MID), and Canada (1645 MID) (Supplementary material, Figure A2). The consumption rate declined by 58·3% in Germany, 48·0% in the US, and 36·8% in Canada from 2009 to 2019 (Figure 1A and Supplementary material, Table S3). In 2019, these three countries were still among those with the highest consumption rates in the world, but the UK had the highest rate (Figure 1B). However, there was heterogeneity in the consumption rate among HICs, even between the Group of Seven (G7) countries such as Japan (109 MID in 2019), Italy (360 MID), and France (926 MID). Countries in the Arabian Peninsula were among the HICs with the lowest opioid consumption rates (Figure 1B).
Figure 1.
Global opioid consumption by country:2009-2019. (A) Change in the national opioid consumption rate between 2009 and 2019 in morphine milligram equivalents (MME) per 1,000 inhabitants per day. Colour scale is continuous with darker shades indicating negative values and lighter shades indicating positive values. Countries with no data shaded in grey. (B) Opioid consumption rate by country for 2019 in MMEs per 1,000 inhabitants per day. Colours represent the 2014 World Bank income classification of high, upper-middle, and low- and lower-middle income countries.
Considerably lower opioid consumption rates were observed in UMICs and LMICs. From 2009 to 2019, the median opioid consumption rate in HICs was 345·1[interquartile range (IQR), 73·7 – 749·5] MID compared with 23·6[IQR, 10·6 – 46·4] MID for UMICs, and 8·3[IQR, 4·4 – 15·1] MID for LMICs. Among UMICs, the highest consumption rates in 2009 were observed in South Africa (142·0 MID), Romania (73·7 MID), and Lebanon (62·5 MID) (Supplementary material, Figure A2), and in 2019 the consumption rates in these countries were still well below the rates observed among most HICs included in the study (Figure 1B). The consumption rates were even lower in LMICs where the highest opioid consumption rate observed in 2019 was 32·7 MID (Ukraine) (Figure 1B).
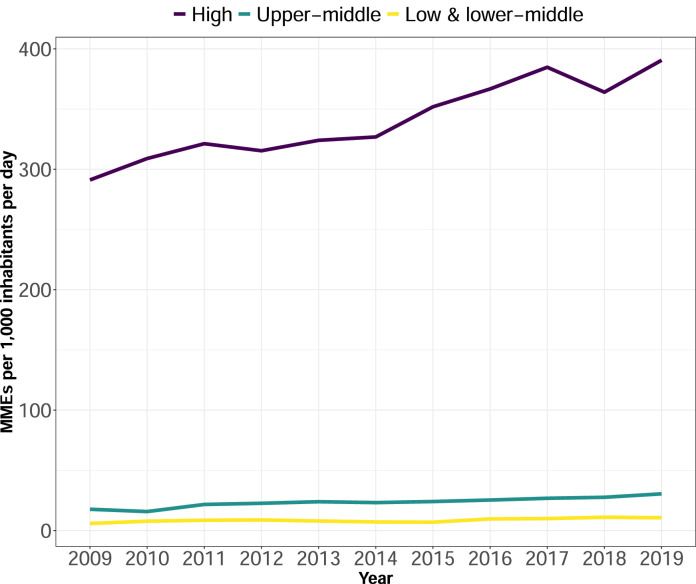
Despite the sharp declines in the consumption rate observed in countries like the US, Germany, and Canada, the opioid consumption rate in HICs increased by a median of 36·6 [IQR, -7·5 – 124·5] MID from 2009-2019 (Figure 2). Spain, Portugal, Switzerland, the Netherlands, Poland, and Norway sharply increased their opioid consumption rate between 2009 and 2019 (Figure 1A and Supplementary material, Table S3). In contrast, the opioid consumption rate in UMICs increased by a median of 10·4 [IQR, 2·5 – 15·0] MID, and in LMICs, the rate only increased by a median of 3·7 [IQR, 0·9 – 8·1] MID. The MME consumption rate trends from 2009 to 2019 significantly differed between LMICs, UMICs, and HICs [Wald chi-square test, HIC and LMIC: chi2=55·93; p value <·001, HIC and UMIC: chi2=44·99; p value <·001, UMIC and LMIC: chi2=44·99; p value = 0·003].
Figure 2.
Median opioid consumption rate in morphine milligram equivalents per 1,000 inhabitants per day by country income classification from 2009 to 2019. Lines represent the annual median opioid consumption rate. Colours represent the 2014 World Bank income classification of high, upper-middle, and low- and lower-middle income countries.
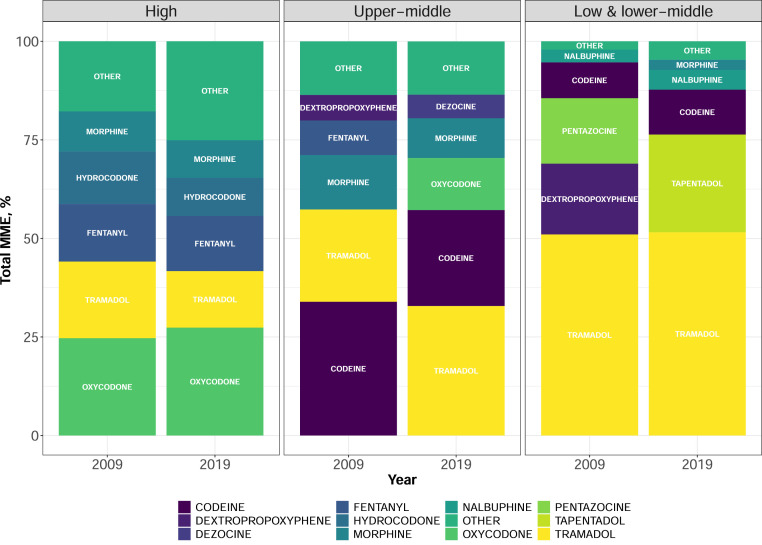
Oxycodone was the most consumed opioid based on MME volume in HICs in 2019, representing 27·3% of total opioid MME consumed within this income group (Figure 3). Tramadol, codeine, and oxycodone were the most consumed opioids in UMICs in 2019, accounting for 70% of total opioid MME volume consumed. Within LMICs, fewer opioids were available compared to UMICs and HICs; in 2019, tramadol, tapentadol, and codeine accounted for 87·7% of total MME consumption in LMICs.
Figure 3.
Proportion of the individual opioids consumed out of total opioid consumption in morphine milligram equivalents (MME) by country income classification in 2009 and 2019. Panels represent the 2014 World Bank income classification of high, upper-middle, and low- and lower-middle income countries. Colours represent the opioid consumed and the bars indicate the percentage consumed out of total MME opioid consumption in 2009 and 2019. Opioids in OTHER category listed in Supplementary material, Appendix 1. The US was the only major consumer of hydrocodone.
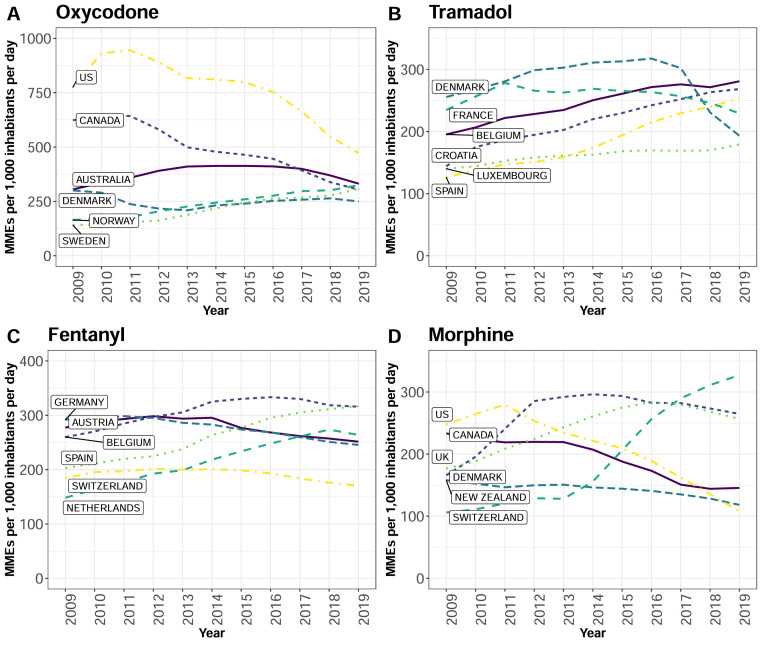
The median consumption rate for oxycodone, tramadol, and fentanyl in the HIC group increased between 2009 and 2019 (Table 1). Relatively high oxycodone consumption was observed in Scandinavia with consumption rates in Sweden and Norway increasing by a mean (SD) of 16·7(10·7) and 16·0(7·6), respectively, from 2009 to 2019, but the US still had the highest oxycodone consumption rate in the world (Figure 4A). Tramadol consumption rates steadily increased in several European countries (Figure 4B) but notably, the rate steeply declined in Germany at a mean (SD) of 155·6 (269·3) during the last decade. Six European countries had the highest fentanyl consumption rates in 2019, where sharp increases were observed in Spain and the Netherlands over the study period (Figure 4C).
Table 1.
Consumption rates (MID) for the top four most consumed opioids by country income group - 2009 & 2019.
| 2009 |
2019 |
|||||
|---|---|---|---|---|---|---|
| Median | IQR | 95% CI | Median | IQR | 95% CI | |
| High income | ||||||
| Oxycodone | 26.7 | 5.8 – 95.9 | 11.4 - 71.3 | 42.9 | 6.2-130.0 | 17.1 - 87.0 |
| Tramadol | 69.1 | 19.2 – 139.0 | 33.6 - 94.8 | 82.8 | 33.2 – 131.0 | 48.6 – 114.9 |
| Fentanyl | 57.6 | 16.6 – 137.0 | 22.4 – 121.4 | 71.5 | 23.0 – 122.0 | 36.5 – 92.1 |
| Morphine | 13.5 | 7.0 – 75.0 | 8.3 – 30.9 | 12.2 | 6.1 – 51.3 | 9.0 – 26.9 |
| Upper-middle | ||||||
| Tramadol | 3.9 | 3.0 – 9.1 | 2.8 – 7.8 | 16.6 | 6.6 – 22.8 | 9.7 – 22.4 |
| Codeine | 1.4 | 0.4 – 6.1 | 0.6 – 4.5 | 3.3 | 0.7 – 20.1 | 0.8 – 16.7 |
| Oxycodone | 0.1 | 0.0 – 0.4 | 0.0 – 0.4 | 1.1 | 0.3 – 4.8 | 0.3 – 4.6 |
| Morphine | 0.9 | 0.0 - 3.0 | 0.0 – 1.9 | 2.0 | 0.8 – 4.2 | 0.8 – 4.0 |
| Low & lower-middle | ||||||
| Tramadol | 1.8 | 1.3 – 2.4 | 1.2 – 2.5 | 4.3 | 1.5 – 5.8 | 0.8 – 6.7 |
| Tapentadol | NA | NA | NA | 3.3 | 1.8 – 8.2 | 0.3 – 13.1 |
| Codeine | 1.1 | 0.9 – 3.0 | 0.1 – 4.2 | 4.1 | 1.1 – 5.5 | 0.7 – 7.5 |
| Nalbuphine | 0.1 | 0 – 0.7 | 0.0 – 0.8 | 0.3 | 0.0 – 1.7 | 0.0 – 2.7 |
CI - bootstrapped confidence intervals.
Figure 4.
Opioid consumption trends of the high-income countries with the top six highest consumption rates in 2019, expressed in morphine milligram equivalents per 1,000 inhabitants per day (MID). Hydrocodone trend not shown because the US was the only major consumer. (A) Oxycodone consumption rate trends:2009-2019. (B) Tramadol consumption rate trends:2009-2019. (C) Fentanyl consumption rate trend: 2009-2019. (D) Morphine consumption rate trend: 2009-2019. Line colours and patterns represent the six countries with the highest consumption rate for each opioid in 2019. The data points represent the annual consumption rate in MID for each opioid.
In UMICs, the median consumption rate of tramadol increased between 2009 and 2019 (Table 1), driven by increasing tramadol consumption rates in Romania, South Africa, and Bosnia (Supplementary material, Figure A3). The consumption rates of codeine, oxycodone, and morphine only increased modestly between 2009 and 2019 in UMICs (Table 1). In LMICs, the median tramadol consumption rate increase from 1·8 to 4·3 MID, and codeine from 1·1 to 4·1 between 2009 and 2019, well below the rates observed in HICs (Table 1).
The summary statistics for the socioeconomic factors assessed in the regression model are presented in Supplementary material, Table S4. We found a significant positive association between physician density and the opioid consumption rate in LMICs (Table 2). Holding other factors constant, an increase in the physician density per 1,000 people by 1 is associated with the opioid consumption rate increasing by 2 MME per capita. In UMICs, GDP per capita and imports as a percentage of GDP were positively associated with the opioid consumption rate. A 1% increase in GDP per capita is associated with the opioid consumption rate increasing by 0·2 MME per capita. Similarly, a 1% increase in imports as a percentage of GDP is associated with a 0·1 MME per capita increase in the opioid consumption rate. No statistically significant association was found between the HIC opioid consumption rates and the indicators assessed in the regression model.
Table 2.
Fixed-effects regression analysis of factors associated with global opioid consumption (MME per capita): 2009-2019.
| Factor | Low & lower-middle | Upper middle | High | |||
|---|---|---|---|---|---|---|
| Coefficient(95% CI) | P value | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | |
| GDP per capita(log) | 0.15(-11.0 to 11.2) | 0.977 | 18.84(3.8 to 33.9) | 0.017 | -39.74(-152.0 to 72.5) | 0.478 |
| Imports as percentage of GDP (log) | 6.04(-2.6 to 14.7) | 0.150 | 13.59(1.3 to 25.8) | 0.031 | 23.73(-64.5 to 112.0) | 0.590 |
| Physicians (per 1,000 people) | 1.95(1.2 to 2.7) | 0.000 | 1.19(-3.9 to 6.3) | 0.631 | 0.36(-21.0 to 21.6) | 0.973 |
| Urban population (% of total population) | 0.44(-0.6 to 1.4) | 0.348 | -0.07(-1.0 to 0.9) | 0.872 | 12.36(-6.1 to 31.0) | 0.185 |
| Number of countries | 11 | 22 | 41 | |||
| Number of observations | 121 | 242 | 451 | |||
Robust standard errors, clustered by country.
The annual opioid consumption rates in 2009 and 2019 for each country, median opioid consumption rates by country income classification, and fixed-effects regression analysis results after imputing the missing hospital sector data are reported in Supplementary material, Appendix 2. The results were similar to the analyses conducted without imputation.
4. Discussion
The global opioid consumption volume was lower in 2019 compared to 2009, mostly due to declines in consumption in the US, Germany, and Canada. However, increasing opioid consumption rates were observed in most other HICs, but with substantial variation in consumption rates and types of opioids consumed. These trends were not associated with the socioeconomic indicators we assessed. UMIC and LMIC opioid consumption rates were much lower and only saw modest increases from 2009-2019. Increases in opioid consumption in UMICs were associated with rising incomes and trade, suggesting potential growth in future consumption, barring any policy interventions. However, this was not the case for LMICS, where only physician density was associated with consumption. This heterogeneity in global opioid consumption patterns demonstrates the complexity of pain management – there is no international consensus on optimal opioid prescribing levels to treat pain adequately while simultaneously mitigating risks of opioid dependence, overdose, and related crises.
With growing concern around the opioid epidemic in the US over the last two decades, public and industry-led policies aimed at reversing the crisis have been implemented. These included changes to pain management regulations and recommendations, improved accessibility of addiction treatment and recovery services, and research on pain, addiction and alternative methods for pain management [11]. These efforts likely influenced the 48% decline in the US opioid consumption rate observed over the study period. Canada, which also saw steep declines in consumption, implemented similar policies and initiatives to address its own opioid crisis [11].
Over the study period, the US and Canada saw large declines in oxycodone consumption. Oxycodone was heavily marketed in the 1990s and early 2000s as a ‘less-addictive’ painkiller despite company knowledge of the contrary [27]. In the US, after the oxycodone brand OxyContin became the most abused prescription opioid, the original medication was reformulated with abuse-deterrent properties in 2010 and the Food and Drug Administration (FDA) stopped accepting applications for generic versions of the original OxyContin formulation. The FDA has made additional efforts to minimise the consequences of controlled release opioids; they have implemented requirements for pharmaceutical companies to provide physicians with safe prescribing educational materials, changed labelling so that correct prescribing, risks and alternative medications are listed, and made long-term post-marketing studies to assess any long-term risks of these medications compulsory [28,29]. Canada has also taken policy action to combat the opioid epidemic in its country: in 2012, all but three of Canada's provinces removed OxyContin and OxyNeo (the reformulated, abuse-deterrent formulation) from drug formularies, which meant that OxyContin formulations would only be available in rare circumstances to patients with special exemptions [30]. In 2017, Ontario – the most populous province in Canada – delisted additional long-acting prescription opioid formulations [31].
The decline in German opioid consumption was driven by a sharp reduction in the tramadol MME volume consumed. In the 2000s, there was a significant increase in prescriptions of tramadol in relatively higher doses, [32] but prescriptions subsequently declined, and thus the overall opioid consumption rate in Germany decreased. This may be attributable to national guidelines introduced in 2010 recommending non-opioid analgesics as first-line treatment for back pain – the most common indication for tramadol prescriptions in Germany during this time – and to only prescribe tramadol when non-opioid analgesics failed [33].
Despite the declines seen in the above three countries, opioid consumption rates increased in many other HICs over the same period. Although the opioid consumption rate has gradually declined in the UK since 2016, it had the highest MME rate in the world in 2019. The INCB assigned the UK a global ranking of 19 for the total average consumption of narcotic drugs (in DDDs for statistical purposes per million inhabitants per day) from 2017 to 2019 [18]. However, this estimated total for the UK did not include codeine, tramadol, buprenorphine, and tapentadol. In 2019, these opioids accounted for 53% of total MME opioid consumption in the UK [codeine, 32%; tramadol, 12%; buprenorphine (for analgesic use) 7%; tapentadol, 2%]. Previous research has noted high levels of opioid use in the UK, [34] and discussed concern about an incipient opioid crisis. However, many factors blamed for the crisis in the US do not prevail in the UK context (in the UK centralised oversight is relatively strong, there is not a consumerist approach to health care delivery, and financial incentives to enhance customer satisfaction are relatively absent), [35] suggesting that other mechanisms are in play.
Some HICs with low overall opioid consumption at the study start, such as Spain and Portugal, also displayed sharp increases. In Spain, concerns around increasing fentanyl use, mainly driven by rapid-release formulations, [36] triggered the Ministry of Health to update fentanyl prescribing guidelines in 2018 [37]. Significant shifts in opioid prescribing patterns in Scandinavian countries (Denmark, Norway and Sweden) were also seen, with oxycodone use increasing across all three. These increases may be associated with aging populations and age-related health conditions in the region [38]. In Norway, reimbursement changes may also have influenced shifts in the profile of opioids prescribed [38].
Social and cultural attitudes towards opioids and pain management may also partially influence the heterogeneity in opioid consumptions rates. For example, opioid use in Japan is growing, but in 2019 it remained 90% less than that of the US. The experience of ill health, pain and discomfort varies significantly between cultures, as does the expression of distress and help-seeking behaviours. Innumerable other social and cultural norms may impact prescribing – including patient expectations, the degree to which opioids are associated with dependence and criminality, and the availability of alternative culturally acceptable treatments [39]. In addition, responses given by competent national authorities to global surveys conducted by the INCB on factors limiting the availability of controlled substances include the lack of training and awareness of health professionals, onerous regulations and fear of prosecution or sanctions [17]. Understanding these factors is key in determining optimal opioid prescribing and consumption practices for pain management in different contexts.
Like previous literature has highlighted, [15] our analysis underscored that consumption rates are low in LMICs and remained relatively stagnant over the study period despite international calls for increases in pharmaceutical pain management [5]. Physician density was positively associated with the opioid consumption rate in LMICs. Countries with less capacity for early detection of disease which will have more patients at advanced stages who require opioid analgesics[15] have less access to pain management options – exacerbating existing health inequities. Opioid choice also remained limited across LMICs over time. Tramadol accounted for more than 50% of MME opioid consumption in LMICS in 2009 and through to 2019. Notably, tapentadol, which was just emerging on the market at the start of the study period, was not part of LMIC opioid consumption in 2009 but accounted for about a quarter of it in MME by 2019. The increasing consumption of both tramadol and tapentadol may have been partially driven by the lack of restrictions on both of these opioids in some countries [40]. The 2018 WHO critical review of tramadol recommended against the international scheduling of this drug, even though several countries had national controls in place [41]. However, recent studies have called for reclassification of tramadol and caution in prescribing it [19]; and with growing concern around misuse, abuse and dependency, several jurisdictions have introduced restrictions on tramadol [41]. This calls into question the assumption that forms of opioids classified as ‘weak’ are also safer [19]. In India, concerns over growing bodies of evidence on abuse of and dependence on tapentadol have led to calls for restrictions [40].
The WHO has expressed concern about the low level of access to medication for moderate and severe pain in LMICs and is currently revising its pain management guidelines [13]. These efforts should seek to produce evidence-based guidance on effective, appropriate and safe opioid prescribing for the optimal treatment of pain. Additionally, these should account for vulnerabilities in health-care systems and the potential for pharmaceutical companies to influence policy decisions that could lead to crises similar to that which occurred in the US [9]. Reports suggest that the pharmaceutical industry has shifted its marketing strategy to new economies in Latin America, Asia, the Middle East and Africa [42]. using aggressive marketing practices similar to those that precipitated the opioid epidemic in the US [27]. Notably, the use of oxycodone has increased in UMICs and is now the third most consumed opioid in these countries after tramadol and codeine.
Several limitations should be considered when interpreting our analysis. Data were not available for all countries – especially those in sub-Saharan Africa; however, the countries included in our study account for more than 80% of the global population. The analysis was based on national-level sales data, and thus it neither reflected variation in opioid consumption within countries nor did it discern appropriate from inappropriate prescribing. Previous studies have highlighted that opioid consumption can widely differ within countries and is often associated with socioeconomic status and/or geographic location [34]. Additionally, when weak opioids are converted to MME, the estimated consumption volume of these opioids will be lower relative to strong opioids, when compared with an alternative metric such as DDD, where weak opioids will account for a higher proportion of the consumption volume if more doses are dispensed than strong opioids. Another limitation was that we did not have patient-level data including information on dosage and duration of opioid prescriptions, and therefore we could not evaluate the adequacy of opioid analgesic use. Lastly, due to the smaller number of countries in the LMIC group, the regression estimates could be biased and may not reflect the true relationship between the opioid consumption rate and socioeconomic factors due to potential model overfit.
Despite these limitations, our study shows a complex global picture of opioid consumption patterns that underline the challenge of providing adequate access to opioid treatment while also balancing concerns around potential misuse. The growing consumption of opioids not scheduled by international conventions must be carefully monitored to ensure appropriate use. Care must be taken to make sure that any restrictive measures do not have unintended consequences that force patients in need of opioids to turn to the illicit market [43]. Multiple factors are implicated in opioid consumption levels and while a ‘one-size-fits-all’ policy approach is unlikely to be successful, countries can learn from one another and translate policy lessons to local contexts. Continuous global tracking of opioid consumption rates is important to inform these policies.
Declaration of Competing Interest
AC and MA are employed by IQVIA. All other authors declare no competing interests.
Acknowledgments
Funding
This work was supported by LSE Health at the Department of Health Policy, London School of Economics. IQVIA did not fund this project.
Contributors
SJ, RF, CW, AC, MA, and EM conceived of and designed the study. AC and MA acquired the data. SJ conducted the statistical analyses. All authors interpreted the data. SJ, RF, and CW drafted the manuscript, and all authors critically reviewed and contributed revisions to the final version of the paper. SJ, AC, MA and EM accessed and verified the data. All authors approved the final version of the manuscript. The views expressed by SB & CJ are their own and do not necessarily reflect the views of the United Nations.
Data sharing
Data can be provided to the journal for evaluation of reported analyses, with a signed data access agreement.
Acknowledgements
The authors would like to thank Nuha Bazeer for research assistance.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101198.
Appendix. Supplementary materials
References
- 1.Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King NB, Fraser V. Untreated Pain, Narcotics Regulation, and Global Health Ideologies. PLOS Medicine. 2013;10 doi: 10.1371/journal.pmed.1001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16:769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmer Z, Zajacova A. Grol-Prokopczyk H. Trends in Pain Prevalence among Adults Aged 50 and Older across Europe, 2004 to 2015. J Aging Health. 2020;32:1419–1432. doi: 10.1177/0898264320931665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knaul FM, Farmer PE, Krakauer EL, et al. Alleviating the access abyss in palliative care and pain relief—an imperative of universal health coverage: the Lancet Commission report. The Lancet. 2018;391:1391–1454. doi: 10.1016/S0140-6736(17)32513-8. [DOI] [PubMed] [Google Scholar]
- 6.Bonnie RJ, Schumacher MA, Clark JD, Kesselheim AS. Pain Management and Opioid Regulation: Continuing Public Health Challenges. Am J Public Health. 2019;109:31–34. doi: 10.2105/AJPH.2018.304881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65:1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 9.Kolodny A, Courtwright DT, Hwang CS, et al. The Prescription Opioid and Heroin Crisis: A Public Health Approach to an Epidemic of Addiction. Annu Rev Public Health. 2015;36:559–574. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 10.OECD. Addressing Problematic Opioid Use in OECD Countries. 2019. https://www.oecd-ilibrary.org/content/publication/a18286f0-en.
- 11.Häuser W, Schug S, Furlan AD. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: a perspective from different continents. PAIN Reports. 2017;2:e599. doi: 10.1097/PR9.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowell D, Haegerich T, Chou R. No Shortcuts to Safer Opioid Prescribing. New England Journal of Medicine. 2019 doi: 10.1056/NEJMp1904190. published online April 24. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyer O. WHO retracts opioid guidelines after accepting that industry had an influence. BMJ. 2020;368 doi: 10.1136/bmj.m105. DOI. [DOI] [PubMed] [Google Scholar]
- 14.Seya M-J, Gelders SFAM, Achara OU, Milani B, Scholten WK. A First Comparison Between the Consumption of and the Need for Opioid Analgesics at Country, Regional, and Global Levels. Journal of Pain & Palliative Care Pharmacotherapy. 2011;25:6–18. doi: 10.3109/15360288.2010.536307. [DOI] [PubMed] [Google Scholar]
- 15.Berterame S, Erthal J, Thomas J, et al. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. The Lancet. 2016;387:1644–1656. doi: 10.1016/S0140-6736(16)00161-6. [DOI] [PubMed] [Google Scholar]
- 16.Scholten WK, Christensen A-E, Olesen AE, Drewes AM. Quantifying the Adequacy of Opioid Analgesic Consumption Globally: An Updated Method and Early Findings. Am J Public Health. 2019;109:52–57. doi: 10.2105/AJPH.2018.304753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.INCB . Vienna: International Narcotics Control Board; 2018. Progress in ensuring adequate access to internationally controlled substances for medical and scientific purposes. https://www.incb.org/incb/en/publications/annual-reports/annual-report-supplement-2018.html. [Google Scholar]
- 18.INCB . Vienna: INCB; 2020. Narcotic Drugs: Estimated World Requirements for 2021. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2020/Narcotic_Drugs_Technical_publication_2020.pdf. [Google Scholar]
- 19.Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. doi: 10.1136/bmj.l1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:265–269. doi: 10.15585/mmwr.mm6610a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein EY, Milkowska-Shibata M, Tseng KK, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. The Lancet Infectious Diseases. 2021;21:107–115. doi: 10.1016/S1473-3099(20)30332-7. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiology and Drug Safety. 2016;25:733–737. doi: 10.1002/pds.3945. [DOI] [PubMed] [Google Scholar]
- 24.CDC . Centers for Disease Control and Prevention; 2019. Analyzing Opioid Prescription Data and Oral Morphine Milligram Equivalents (MME) [Google Scholar]
- 25.World Bank . The World Bank Group; 2020. Population estimates and projections.https://databank.worldbank.org/home.aspx accessed Dec 15, 2020. [Google Scholar]
- 26.World Bank . World Bank; 2014. World Bank Country and Lending Groups.https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Google Scholar]
- 27.DeWeerdt S. Tracing the US opioid crisis to its roots. Nature. 2019;573:S10–S12. doi: 10.1038/d41586-019-02686-2. [DOI] [PubMed] [Google Scholar]
- 28.Larochelle MR, Zhang F, Ross-Degnan D, Wharam JF. Rates of Opioid Dispensing and Overdose After Introduction of Abuse-Deterrent Extended-Release Oxycodone and Withdrawal of Propoxyphene. JAMA Internal Medicine. 2015;175:978–987. doi: 10.1001/jamainternmed.2015.0914. [DOI] [PubMed] [Google Scholar]
- 29.Reisfield GM. OxyContin, the FDA, and Drug Control. AMA Journal of Ethics. 2014;16:279–283. doi: 10.1001/virtualmentor.2014.16.04.pfor1-1404. [DOI] [PubMed] [Google Scholar]
- 30.Fischer B, Keates A. Opioid Drought’, Canadian-style? Potential implications of the ‘natural experiment’ of delisting Oxycontin in Canada. International Journal of Drug Policy. 2012;23:495–497. doi: 10.1016/j.drugpo.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Fischer B, Vojtila L, Kurdyak P. ‘Delisting’ OxyContin® to reduce prescription opioid-related harms in Ontario (Canada)—gauging effects 5 years later. Pharmacoepidemiology and Drug Safety. 2017;26:1040–1043. doi: 10.1002/pds.4253. [DOI] [PubMed] [Google Scholar]
- 32.Hedenmalm K, Slattery J, Skibicka-Stepien I, Kurz X, Morales D. Prescribing patterns of tramadol in adults in IMS® primary care databases in France and Germany between 1 January 2006 and 30 June 2016. Eur J Clin Pharmacol. 2019;75:707–716. doi: 10.1007/s00228-018-02622-9. [DOI] [PubMed] [Google Scholar]
- 33.Tholen K, Hoffmann F. High use of tramadol in Germany: an analysis of statutory health insurance data: HIGH USE OF TRAMADOL IN GERMANY. Pharmacoepidemiol Drug Saf. 2012;21:1013–1021. doi: 10.1002/pds.3266. [DOI] [PubMed] [Google Scholar]
- 34.Jani M, Yimer BB, Sheppard T, Lunt M, Dixon WG. Time trends and prescribing patterns of opioid drugs in UK primary care patients with non-cancer pain: A retrospective cohort study. PLOS Medicine. 2020;17 doi: 10.1371/journal.pmed.1003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisberg DF, Becker WC, Fiellin DA, Stannard C. Prescription opioid misuse in the United States and the United Kingdom: cautionary lessons. Int J Drug Policy. 2014;25:1124–1130. doi: 10.1016/j.drugpo.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 36.González-Bermejo D, Rayón-Iglesias P, Rodríguez-Pascual A, et al. Drug utilization study on immediate release Fentanyl in Spain. Prevalence, incidence, and indication. Pharmacoepidemiology and Drug Safety. 2021 doi: 10.1002/pds.5118. n/aDOI. [DOI] [PubMed] [Google Scholar]
- 37.Spanish Ministry of Health. Fentanilo de liberación inmediata: importancia de respetar las condiciones de uso autorizadas.;2021: 4.
- 38.Neutel CI, Skurtveit S, Berg C, Sakshaug S. Trends in prescription of strong opioids for 41-80 year old Norwegians, 2005-2010. Eur J Pain. 2014;18:438–446. doi: 10.1002/j.1532-2149.2013.00374.x. [DOI] [PubMed] [Google Scholar]
- 39.Onishi E, Kobayashi T, Dexter E, Marino M, Maeno T, Deyo RA. Comparison of Opioid Prescribing Patterns in the United States and Japan: Primary Care Physicians’ Attitudes and Perceptions. J Am Board Fam Med. 2017;30:248–254. doi: 10.3122/jabfm.2017.02.160299. [DOI] [PubMed] [Google Scholar]
- 40.Basu D, Mahintamini T, Ghosh A, et al. Tapentadol, the new kid on the block in India: Is it time to worry? Indian Journal of Psychiatry. 2020;62:697. doi: 10.4103/psychiatry.IndianJPsychiatry_332_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO Critical Review Report: Tramadol. https://www.who.int/medicines/access/controlled-substances/Tramadol.pdf (accessed March 2, 2021).
- 42.Ryan H, Girion L, Glover S. OxyContin goes global — “We're only just getting started”. www.latimes.com. http://www.latimes.com/projects/la-me-oxycontin-part3/(accessed June 6, 2020).
- 43.Martin J, Cunliffe J, Décary-Hétu D, Aldridge J. Effect of restricting the legal supply of prescription opioids on buying through online illicit marketplaces: interrupted time series analysis. BMJ. 2018;361:k2270. doi: 10.1136/bmj.k2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.