Abstract
Since its first use as a bone void filler at the end of the 19th century, calcium sulphate products have been adapted in different ways to aid orthopaedic surgeons. Calcium sulphate local antibiotic delivery systems offer a promising solution in the delivery of high antibiotic concentrations locally for an extended period of time. Over the years, multiple centres have reported side effects such as wound drainage, heterotrophic ossification and hypercalcaemia. This study was carried out to assess the risk of wound drainage in prosthetic joints after implantation of antibiotic-impregnated calcium sulphate beads. Two reviewers searched the literature in three online databases using the Cochrane methodology for systematic reviews. The search of databases yielded 182 articles. The studies without reported post-operative complications, mainly drainage outcomes, were excluded. After screening, seven articles were deemed suitable and selected. Out of the 1,112 cases identified, 43 joints developed wound drainage after calcium sulphate bead placement. This complication was resolved in all these cases by either conservative or operative approaches. The factors implicated in the development of wound drainage include the volume of the product used, procedural placement and host factors. The result of this systematic review shows that calcium sulphate products can be used for treatment and prophylaxis in prosthetic joints with a risk of post-procedural wound drainage. This risk, however, is lesser with the use of synthetic calcium sulphate products as compared with conventional calcium sulphate products.
Keywords: wound leakage, total hip replacement (thr), total knee replacement (tkr), pji, calcium sulphate beads
Introduction and background
In England and Wales, there are about 160,000 joint replacements performed in a year [1]. This number is up to one million in the United States [2,3] and is expected to increase to four million a year by 2030 [4]. In recent times, calcium sulphate beads have been effectively used in septic and aseptic conditions. The incidence of prosthetic joint infection (PJI) is 1%-2% after primary replacement, with a weighted mean of 0.97% in total hip replacement (THR) and 1.03% in total knee replacement (TKR) from multiple national registries [5]. Prosthetic joint infection (PJI) is a serious limb and life-threatening complication of joint replacement surgery. The current protocol for PJI includes debridement and antibiotic therapy. Surgeons have been increasingly using calcium sulphate beads to deliver antibiotics to the joint space. Calcium sulphate beads are traditionally used as bone filling agents [6]. However, they have been found to be effective in treating PJI as the beads are completely absorbed from the joint space and therefore do not need to be surgically removed. Moreover, because of the beads getting absorbed, 100% of the antibiotic load is released [7].
There is limited literature on the risk associated with using calcium sulphate beads; however, one reported developing wound drainage as a risk of using calcium sulphate beads. The incidence of wound drainage is reported to range from up to 51% [8]. The consequences of wound drainage include a longer hospital stay for the patient, wound care and immobilisation. If wound drainage persists, washout and closure may be indicated. Persistent wound drainage may increase the risk of infection [9]; however, Ferguson et al. found no infections in those treated non-surgically for wound drainage [10]. Wound drainage has been associated with higher volumes of calcium sulphate beads, particularly volumes greater than 20 cc [11]. Wound drainage is also linked to a deeper subcutaneous insertion site and a McPherson systemic host score of C [12].
The aim of this study is to determine the risk of developing wound drainage when using calcium sulphate beads in any prosthetic joint surgery. We aim to determine the factors that increase the risk of developing wound drainage and what measures can be taken to treat wound drainage.
Review
Methodology
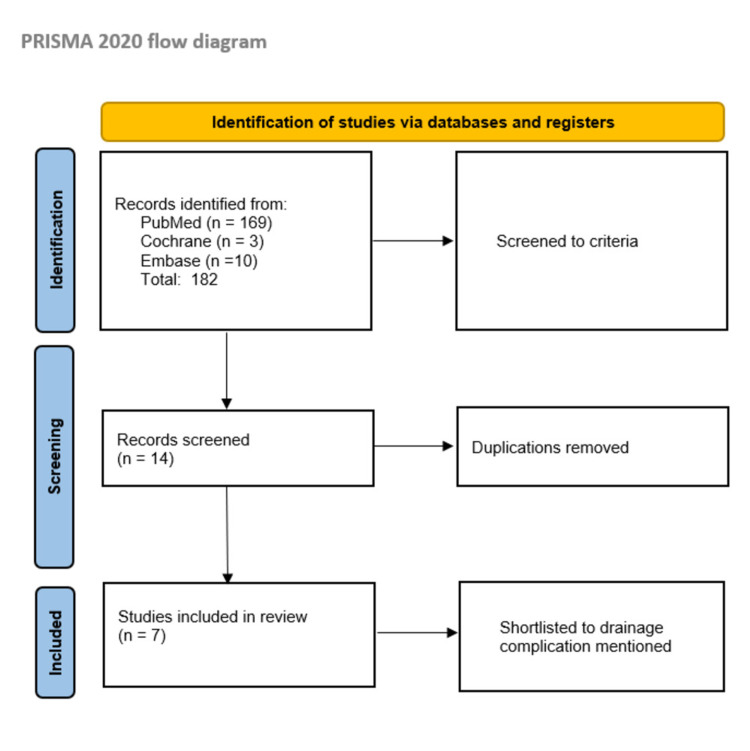
A literature search of three online databases, namely, MEDLINE (1946 to present), Embase (1974 to present) and Cochrane CENTRAL (1988 to present), using the Cochrane methodology for systematic review was conducted using the keywords 'prosthetic joint', 'total knee replacement' and 'total hip replacement' and cross-searched with 'calcium sulphate beads'. The inclusion criteria selected was any study that has reported complications in any prosthetic joint surgery regardless of the indication and regardless of the language in which the study was published. Studies without reported post-operative drainage outcomes were excluded. All full texts were retrieved and reviewed where open access was not available. Search screening and article shortlisting were performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology (Figure 1) [13].
Figure 1. Study methodology used for the systematic review (PRISMA).
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
The search yielded a total of 182 articles. All shortlisted studies were reviewed by the junior and senior study members. After review, seven articles were selected in the study as per the inclusion criteria (Table 1). Data from the selected studies, including the type of calcium sulphate beads and the dose used, and drainage outcomes, are tabulated for a detailed review (Table 1).
Table 1. Type of calcium sulphate derivatives used in the joints, incidence of wound drainage and management.
S/C: subcutaneous, G/A: general anaesthetic
| Author | Patients with Prosthetic Joint Procedures (N) | Calcium Sulphate Bead Derivative and Dosage | Space Calcium Sulphate Beads Used In | Wound Closure After Calcium Sulphate Insertion | Wound Leakage | Management of Wound Leakage |
| Sandiford [17] | 29 | Stimulan, 20–40 mL (mean: 25 mL) | Intracapsular compartment of hip joints, none in S/C tissue | Capsule closed over beads | 1/29 (3.4%) | Re-exploration under G/A + closure of soft tissue defect |
| Kallala and Haddad [18] | 15 | Stimulan, 10–40 mL | Around the hip/knee joints/prosthesis or spacer, none in S/C tissue | Wound closure methods not reported | 0/15 (0%) | N/A |
| Kallala et al. [19] | 755 | Stimulan, 5–80 cc (mean: 23.39 cc) | Knees – medial and lateral gutters | Wound closure methods not reported | 32/755 (4.2%) | Drainage >5 days post-operatively/serous/serosanguineous – wound redressed and anticoagulants stopped |
| Hips – placed deeply, inferior to the acetabulum and around the proximal femur | Drainage >5 days post-operatively/sanguineous discharge – washout under G/A | |||||
| McPherson et al. [20] | 250 | Stimulan, 5–50 cc | Knees – medial and lateral gutters, none in S/C tissue | Drain placed in the lateral gutter, wound closed in mid-flexion + - S/C drains | 3.2% overall | Lavage, debridement, wound VAC placement and/or compressive dressing |
| 3.5% knee wound drainage | ||||||
| Stimulan, 5–70 cc | Hips – deep hip space, inferior to the acetabulum and around the proximal femur, none placed in S/C tissue | Drain placed under TFL layer + - S/C drains, tension-free closure | 2.8% hip wound drainage | |||
| Menon et al. [21] | 3 | Stimulan (volume not reported specifically for PJI cases) | Not reported | Not reported for PJI | 1/3 wound patients had wound drainage | Re-exploration |
| Lum et al. [22] | 56 | Stimulan (volume not reported) | Not reported | Calcium sulphate beads placed into the wound during final closure | 1/56 (1.7%) | Resolved without surgical intervention |
| Agarwal et al. [23] | 4 | Stimulan (maximum volume: 20 cc) | Within the joint space and medullary canal | Not reported | 1/4 persistent culture negative discharge | Managed with regular dressing changes |
Discussion
In our review, across seven studies, we identified 1,112 patients who underwent prosthetic joint surgeries for various aseptic and septic indications (Table 2) with calcium sulphate bead implantation. Out of these 1,112 cases, 43 (3.8%) joints developed wound drainage as a complication of calcium sulphate bead use. Out of the selected studies, 578 patients had a prosthetic joint infection, with 237 hip PJI and 341 knee PJI. Five out of the seven selected studies have specified drainage in the PJI cohort; however, two studies (Kallala et al. [19] and Lum et al. [22]) have not specified drainage complications in the PJI patient group (Table 3). The diagnosis of PJI is commonly made using the criteria proposed by the Musculoskeletal Infection Society (Table 4) [14]. A combination of conservative and operative strategies was reported by the authors, which eventually resulted in resolution in all cases.
Table 2. Demographics, antibiotic combinations and procedures performed with follow-up details of the selected articles.
N: number of patients; THA: total hip arthroplasty
| Author | Study Method | Study Type | Mean Age | Sex (Male:Female) | Antibiotics Used | Procedures Calcium Sulphate Beads Were Used In | Follow-Up |
| Sandiford [17] | Prospective | Case series | 67 | 13:16 | Vancomycin (n = 28) | Single-staged THA revision (n = 7) | Six weeks |
| First-stage THA revision (n = 4) | |||||||
| Second-stage THA revision (n = 9) | |||||||
| Gentamicin (n = 27) | DAIR (n = 6) | ||||||
| Amikacin (n = 1) | Excision hip arthroplasty (n = 1) | ||||||
| Caspofungin (n = 1) | Periprosthetic hip fracture (n = 2) | ||||||
| Kallala and Haddad [18] | Retrospective | Case series | 64.8 | 8:7 | Vancomycin + gentamicin (n = 15) | Single-stage revision (resurfacing arthroplasty to THR) (n = 2) | Mean 16 months (12–22) |
| Primary THA revisions (n = 3) | |||||||
| Infected THA revisions (n = 3) | |||||||
| Proximal femoral replacement revision (n = 1) | |||||||
| Primary TKA revisions (n = 3) | |||||||
| Infected TKA revisions (n = 3) | |||||||
| Kallala et al. [19] | Prospective | Case series | 63 | 374:381 | Vancomycin + tobramycin (n = 755) | TKA revision (n = 456) | Mean 35 months (0–78) |
| (Amphotericin B included in fungal infections) (n = not reported) | THA revision (n = 299) | ||||||
| McPherson et al. [20] | Retrospective | Case series | Not reported | Not reported | Vancomycin + tobramycin (n = 250) | Aseptic TKA revision (n = 66) | Minimum of three months for all patients (maximum of 12 months post-operatively) |
| DECRA TKA (n = 16) | |||||||
| TKA resection (n = 35) | |||||||
| TKA re-implantation (n = 25) | |||||||
| Aseptic THA revision (n = 58) | |||||||
| DECRA THA (n = 8) | |||||||
| THA resection (n = 24) | |||||||
| THA re-implantation (n = 18) | |||||||
| Menon et al. [21] | Retrospective | Case series | 51 | 28:11 | Vancomycin (n = 17) | Total prosthetic joint infections (n = 3), staged revision: debridement, biopsy +- implant removal | Minimum period of six months for all cases |
| Colistin (n = 11) | |||||||
| Vancomycin + colistin (n = 8) | |||||||
| Vancomycin + gentamicin (n = 4) | Revision for infected TKR (n = 2) | ||||||
| Voriconazole (n = 1) | Revision for infected bipolar hemiarthroplasty (n = 1) | ||||||
| Lum et al. [22] | Retrospective | Case series | Not reported | Not reported | Tobramycin + vancomycin + cefazolin (n = 56) | Primary TKA (n = 6) | Two weeks, six weeks, 12 weeks and one year |
| Clean TKA revision (n = 12) | |||||||
| Infected TKA revision (n = 8) | |||||||
| Primary THAs (n = 5) | |||||||
| Clean THA revision (n = 19) | |||||||
| Infected THA revision (n = 6) | |||||||
| Agarwal et al. [23] | Retrospective | Case series | Not reported | Not reported | Vancomycin (n = 3) | Single-stage TKR revision (n = 1) | Six weekly intervals, average follow-up 19 months |
| Knee fusion using antegrade nail (n = 1) | |||||||
| Daptomycin (n = 1) | Single-stage hip revision (n = 1) | ||||||
| Cemented hip replacement (n = 1) |
Table 3. Wound leakage reported in patients with prosthetic joint infections in the selected studies.
| Study Name | Prosthetic Joint infection | Hips (n) | Knees (n) | Leakage (Hips) | Leakage (Knees) |
| Sandiford [17] | 29 | 29 | 0 | 1 | 0 |
| Kallala and Haddad [18] | 15 | 9 | 6 | 0 | 0 |
| Kallala et al. [19] | 387 | 140 | 247 | Not specified in PJI (overall: 32 in 755) | |
| McPherson et al. [20] | 126 | 50 | 76 | 2 | 0 |
| Menon et al. [21] | 3 | 1 | 2 | 0 | 0 |
| Lum et al. [22] | 14 | 6 | 9 | Not specified in PJI (overall: 1 in 56) | |
| Agarwal et al. [23] | 4 | 2 | 2 | 1 | 0 |
Table 4. Musculoskeletal Infection Society (MSIS) definition of prosthetic joint infection [14].
| Prosthetic Joint Infection |
| 1. There is a sinus tract communicating with the prosthesis; or |
| 2. A pathogen is isolated by culture from at least two separate tissue or fluid samples obtained from the affected prosthetic joint; or |
| 3. Four of the following six criteria exist: |
| 1. Elevated serum erythrocyte sedimentation rate (ESR) and serum C-reactive protein (CRP) concentration, |
| 2. Elevated synovial leukocyte count, |
| 3. Elevated synovial neutrophil percentage (PMN%), |
| 4. Presence of purulence in the affected joint, |
| 5. Isolation of a microorganism in one culture of periprosthetic tissue or fluid, or |
| 6. Greater than five neutrophils per high-power field in five high-power fields observed from histologic analysis of periprosthetic tissue at ×400 magnification. |
While the efficacy of local antibiotic delivery by calcium sulphate beads is well documented, potential side effects have also been recognised, which include excessive wound drainage requiring intervention, heterotrophic calcification, reactive synovitis and transient hypercalcaemia. Chronic infections in prostheses are characterised by biofilms that are not easily eradicated by conventional antibiotic therapy [15]. Any residual biofilm after implant removal can serve as a focus for persistent infection and morbidity. Conventional methods including parenteral antibiotics fail to achieve the desired concentration in local tissue for reliable and effective eradication of infection. Local antibiotic delivery systems offer a promising solution to this problem. Kanellakopoulou et al. showed that an antibiotic-soaked calcium sulphate delivery system (Stimulan) can provide concentrations more than 300 times the minimum inhibitory concentration of causative organisms in studies on rabbits [16]. This method of delivery not only promises effective antimicrobial activity but also avoids the systemic toxicity of systemic antibiotics.
The pathophysiology of wound drainage due to calcium sulphate is well established but poorly understood. This adverse effect is thought to occur due to accelerated absorption of calcium sulphate beads, which then lead to the development of a calcium-rich fluid. This calcium-rich fluid is suspected to cause increased wound drainage via two possible mechanisms. Firstly, the calcium-rich hyperosmolar fluid exerts an osmotic effect and promotes fluid sequestration in the surgical planes where the beads have been planted. Secondly, it is thought to trigger a direct inflammatory response that leads to fluid collection [24,25]. Both these mechanisms may contribute to the wound drainage seen in patients after the use of calcium sulphate products. However, the exact mechanism is still unknown.
McPherson et al. proposed that this inflammatory process was due to the derivation of prior calcium sulphate products from gypsum, a naturally derived compound [20]. Stimulan, a commercially pure, synthetic calcium sulphate compound, offered a less harsh atmosphere to the synovial joint environment and was a promising answer to this problem [20]. This was reinforced by their study that showed that only 3.2% of the cases developed significant wound leakage that required intervention. Furthermore, they attributed these cases to excessive use of the product, quality of local tissue and health of the subjects. They recommended the use of Stimulan to be restricted to less than 30 cc to avoid this complication (Table 1) [20].
In cases where wound drainage was significant enough to warrant intervention, a variety of methods were employed. McPherson et al. used lavage with debridement wound VAC placement or application of compressive dressing, which resulted in eventual resolution [20]. Kallala et al. stopped anticoagulants if the drainage was found within five days with redressing or if it occurred after five days and thorough washout was done under general anaesthesia [19]. The single case of wound drainage in Sandiford's series was treated by re-exploration upon which a defect was found in the insertion of abductors, closure of which resulted in resolution [17]. Other studies reported resolution of drainage with conservative management such as redressing and observation [22,23].
Wound drainage is also thought to be related to the procedural placement of calcium sulphate beads. Subcutaneous placement is thought to lead to increased wound drainage and is avoided by watertight closure of deeper spaces [21]. McPherson et al. [20] and Kallala et al. [18,19] planted Stimulan beads in the medial and lateral gutters in knee arthroplasty. The deep hip space inferior to the acetabulum was used in the hip cases. Agarwal et al. used the bigger beads within the joint space with smaller bead insertion into the medullary cavity (Table 1) [23]. Subcutaneous positing of beads was actively avoided in all studies, which is also recommended by the manufacturers.
Several factors are implicated in the development of wound drainage after calcium sulphate bead use. These include the volume of beads used, their subcutaneous placement and the overall medical condition of the host. Variable volumes of Stimulan have been employed, ranging from 5 to 50 cc in knee cases and 5 to 70 cc in hip procedures by McPherson et al. [20], a mean volume of 24 cc by Kallala et al. [19], a maximum volume of 20 cc by Agarwal et al. [23] and 5-30 cc by Menon et al. [21]. McPherson et al. reported that all wound drainage tended to be present when Stimulan with a volume greater than 30 cc was used (Table 1) [20]. However, a statistical correlation between the volume of beads and drainage is yet to be established.
As explained previously, physical phenomena are thought to promote fluid sequestration and trigger inflammation, which is responsible for excessive wound drainage [20,24,25]. This mechanism is thought to be exaggerated in patients with existing medical conditions. McPherson et al. reported that most of their cases with wound drainage were MSIS class B and C cases [20]. Kallala et al. also reported that the rate of complications was greater in patients with medical conditions [19]. More studies are needed to establish and elaborate on this correlation. The rate of wound drainage varies with different calcium sulphate derivatives used and can also be affected by the type of surgery and the indication for intervention. This ranges from 3.6% in a study conducted by Kelly, who used OsteoSet (Wright Medical Technology Inc., Arlington, USA) [26], to 51% as reported by Ziran et al. using calcium sulphate combined with demineralised bone matrix [8].
The use of synthetic calcium sulphate products has offered a potentially promising solution to a problem faced by orthopaedic surgeons worldwide. Over the years, the use of this calcium sulphate antibiotic delivery system has increased. However, clarity regarding the efficacy of this product and its potential side effects is lacking. Side effects such as wound drainage, heterotrophic ossification and hypercalcaemia are well documented [17-23].
Conclusions
Our study has shown that a small number of patients developed wound leakage after calcium sulphate bead implantation in prosthetic joint surgeries. However, the correlation of these complications with host factors, product volume and placement in surgery is not well understood. More studies are needed to understand this causality and the factors that play a role in complications arising from the use of calcium sulphate beads.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Joint replacement statistics. https://www.njrcentre.org.uk/njrcentre/Patients/Joint-replacement-statistics 2020
- 2.Williams SN, Wolford ML, Bercovitz A. Hyattsville, MD: NCHS Data Brief: National Center for Health Statistics; 2015. Hospitalization for total knee replacement among inpatients aged 45 and over: United States, 2000-2010. [PubMed] [Google Scholar]
- 3.Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Wolford ML, Palso K, Bercovitz A. https://pubmed.ncbi.nlm.nih.gov/25714040/ NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 4.Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 5.Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Springer BD, Cahue S, Etkin CD, Lewallen DG, McGrory BJ. Arthroplast Today. 2017;3:137–140. doi: 10.1016/j.artd.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antibiotic-impregnated calcium sulfate use in combat-related open fractures. Helgeson MD, Potter BK, Tucker CJ, Frisch HM, Shawen SB. Orthopedics. 2009;32:323. doi: 10.3928/01477447-20090501-03. [DOI] [PubMed] [Google Scholar]
- 7.Elution profiles of tobramycin and vancomycin from high-purity calcium sulphate beads incubated in a range of simulated body fluids. Cooper JJ, Florance H, McKinnon JL, Laycock PA, Aiken SS. J Biomater Appl. 2016;31:357–365. doi: 10.1177/0885328216663392. [DOI] [PubMed] [Google Scholar]
- 8.Use of calcium-based demineralized bone matrix/allograft for nonunions and posttraumatic reconstruction of the appendicular skeleton: preliminary results and complications. Ziran BH, Smith WR, Morgan SJ. J Trauma. 2007;63:1324–1328. doi: 10.1097/01.ta.0000240452.64138.b0. [DOI] [PubMed] [Google Scholar]
- 9.Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. Saleh K, Olson M, Resig S, et al. J Orthop Res. 2002;20:506–515. doi: 10.1016/S0736-0266(01)00153-X. [DOI] [PubMed] [Google Scholar]
- 10.The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. Ferguson JY, Dudareva M, Riley ND, Stubbs D, Atkins BL, McNally MA. Bone Joint J. 2014;96-B:829–836. doi: 10.1302/0301-620X.96B6.32756. [DOI] [PubMed] [Google Scholar]
- 11.Calcium sulfates: what is the evidence? Beuerlein MJ, McKee MD. J Orthop Trauma. 2010;24 Suppl 1:0–51. doi: 10.1097/BOT.0b013e3181cec48e. [DOI] [PubMed] [Google Scholar]
- 12.Periprosthetic total hip infection: outcomes using a staging system. McPherson EJ, Woodson C, Holtom P, et al. Clin Orthop Relat Res. 2002;403:8–15. [PubMed] [Google Scholar]
- 13.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Liberati A, Altman DG, Tetzlaff J, et al. J Clin Epidemiol. 2009;62:0–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 14.New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Parvizi J, Zmistowski B, Berbari EF, et al. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacterial biofilms: a common cause of persistent infections. Costerton JW, Stewart PS, Greenberg EP. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 16.Treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with a synthetic carrier of calcium sulphate (Stimulan) releasing moxifloxacin. Kanellakopoulou K, Galanopoulos I, Soranoglou V, et al. Int J Antimicrob Agents. 2009;33:354–359. doi: 10.1016/j.ijantimicag.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Complication rates are low with the use of Stimulan calcium sulphate based antibiotic delivery system in the management of patients with hip-related PJI: early results of a consecutive case series. Sandiford NA. Hip Int. 2020;30:3–6. doi: 10.1177/1120700020925093. [DOI] [PubMed] [Google Scholar]
- 18.Hypercalcaemia following the use of antibiotic-eluting absorbable calcium sulphate beads in revision arthroplasty for infection. Kallala R, Haddad FS. Bone Joint J. 2015;97-B:1237–1241. doi: 10.1302/0301-620X.97B9.34532. [DOI] [PubMed] [Google Scholar]
- 19.Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty: safety profile and complication rates. Kallala R, Harris WE, Ibrahim M, Dipane M, McPherson E. Bone Joint Res. 2018;7:570–579. doi: 10.1302/2046-3758.710.BJR-2017-0319.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dissolvable antibiotic beads in treatment of periprosthetic joint infection and revision arthroplasty-the use of synthetic pure calcium sulfate (Stimulan®) impregnated with vancomycin & tobramycin. McPherson E, Dipane M, Sherif S. Reconstructive Review. 2013;3 [Google Scholar]
- 21.Careful interpretation of the wound status is needed with use of antibiotic impregnated biodegradable synthetic pure calcium sulfate beads: series of 39 cases. Menon A, Soman R, Rodrigues C, Phadke S, Agashe VM. J Bone Jt Infect. 2018;3:87–93. doi: 10.7150/jbji.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Local bio-absorbable antibiotic delivery in calcium sulfate beads in hip and knee arthroplasty. Lum ZC, Pereira GC. J Orthop. 2018;15:676–678. doi: 10.1016/j.jor.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The use of antibiotic impregnated absorbable calcium sulphate beads in management of infected joint replacement prostheses. Agarwal S, Healey B. J Arthrosc Jt Surg. 2014;1:72–75. [Google Scholar]
- 24.Adverse reactions to OsteoSet bone graft substitute: the incidence in a consecutive series. Lee GH, Khoury JG, Bell JE, Buckwalter JA. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1888376/ Iowa Orthop J. 2002;22:35–38. [PMC free article] [PubMed] [Google Scholar]
- 25.Inflammatory reactions associated with a calcium sulfate bone substitute. Robinson D, Alk D, Sandbank J, Farber R, Halperin N. https://www.annalsoftransplantation.com/download/index/idArt/497494. Ann Transplant. 1999;4:91–97. [PubMed] [Google Scholar]
- 26.The use of a surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Kelly CM, Wilkins RM, Gitelis S, Hartjen C, Watson JT, Kim PT. https://journals.lww.com/clinorthop/Fulltext/2001/01000/The_Use_of_a_Surgical_Grade_Calcium_Sulfate_as_a.8.aspx. Clin Orthop Relat Res. 2001:42–50. doi: 10.1097/00003086-200101000-00008. [DOI] [PubMed] [Google Scholar]