Abstract
Neurological and functional recovery is limited following central nervous system injury and severe injury to the peripheral nervous system. Extracellular matrix (ECM)-mimetic hydrogels are of particular interest as regenerative scaffolds for the injured nervous system as they provide three-dimensional bioactive interfaces that modulate cellular response to the injury environment and provide naturally degradable scaffolding for effective tissue remodeling. In this review, three unique ECM-mimetic hydrogels used in models of neural injury are reviewed: fibrin hydrogels, which rely on a naturally occurring enzymatic gelation, hyaluronic acid hydrogels, which require chemical modification prior to chemical crosslinking, and elastin-like polypeptide (ELP) hydrogels, which exhibit a temperature-sensitive gelation. The hydrogels are reviewed by summarizing their unique biological properties, their use as drug depots, and their combination with other biomaterials, such as electrospun fibers and nanoparticles. This review is the first to focus on these three ECM-mimetic hydrogels for their use in neural tissue engineering. Additionally, this is the first review to summarize the use of ELP hydrogels for nervous system applications. ECM-mimetic hydrogels have shown great promise in preclinical models of neural injury and future advancements in their design and use will likely lead to viable treatments for patients with neural injury.
Keywords: hydrogels, fibrin, hyaluronic acid, elastin-like polypeptides, neural tissue engineering, extracellular matrix
Graphical Abstract
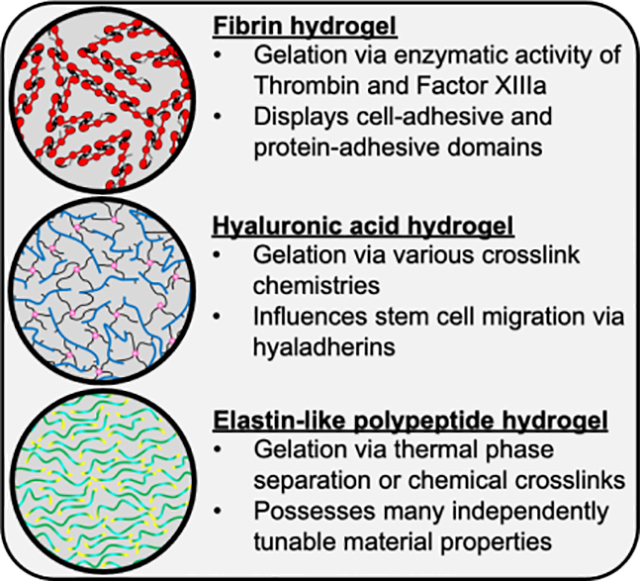
Fibrin, hyaluronic acid (HA), and elastin-like polypeptide (ELP) hydrogels are mimetic of the extracellular matrix but have different gelation schemes and bioactive properties. Fibrin has found a niche in spinal cord injury models while HA has become popular for ischemia models. ELP hydrogels have rarely been integrated into neural in vivo models but provide a promising substrate for future use.
1. Introduction
Injury to the nervous system frequently results in a neuroinhibitory environment in the central nervous system (CNS) and incomplete tissue remodeling in the peripheral nervous system (PNS). Spinal cord injury (SCI) affects nearly 18,000 individuals in the United States annually, of which less than 1% recover completely at the time of discharge.[1] Traumatic brain injury (TBI) affects 2.8 million individuals in the United States annually, where the majority of survivors still present moderate to severe disabilities five years after injury.[2] SCI and TBI often require rehospitalization and significantly impact employment status, life expectancy, mental health, and overall quality of life.[1–3] In fact, multiple studies link traumatic neural injury to an increased risk of neurodegenerative disease.[4,5] Stroke affects nearly 800,000 individuals in the United States annually and is the foremost cause of disability.[6,7] Severe peripheral nerve injury (PNI) also significantly impacts a patient’s quality of life.[8–10] Unique to each system is the series of specific cellular responses that ensue after the injury that requires a multi-faceted approach to enable and expedite tissue regeneration and functional recovery. One treatment modality that is being investigated for neural injury repair is hydrogels due to their three-dimensional structure, their potential to provide a minimally-invasive treatment via injections, and the wide selection of gelation materials. Hydrogels produced from extracellular matrix (ECM)-mimetic materials are of particular interest as they often represent the environment surrounding neural tissue and provide a degradable and bioactive substrate to facilitate tissue regeneration. In this review, the pathophysiology of CNS and PNS tissue damage will be briefly outlined. Then, making use of literature primarily from the last decade, three categories of extracellular matrix (ECM)-mimetic hydrogels will be summarized for neural tissue regeneration applications, namely fibrin hydrogels, hyaluronic acid (HA) hydrogels, and elastin-like polypeptide (ELP) hydrogels. The databases Scopus by Elsevier, PubMed, and Google Scholar were used to find relevant literature primarily from the last decade using keywords pertaining to neural tissue engineering and the three ECM-mimetic hydrogels. While some cornerstone articles published before 2010 were still included, this time range limits the included articles to more advanced developments in the HA and fibrin hydrogel field and encompasses most all literature regarding ELPs and neural tissue. To our knowledge, this is the first comprehensive review summarizing ELP hydrogels for neural tissue engineering.
1.1. CNS injury and pathophysiology
A traumatic CNS insult destroys glia and neurons at the site of injury. The insult also ruptures blood vasculature, compromising the integrity of the blood brain barrier (BBB), which enables the influx of blood serum components into neural tissue.[11] During this acute injury phase, neutrophils and cytokines penetrate the CNS parenchyma, triggering an inflammatory response. BBB rupture instigates immediate local hypoxia which further exacerbates cell death and the inflammatory response.[12–14] Sustained inflammation, leading to further glial and neuronal apoptosis, is a hallmark of the secondary injury response.[3,11,15] Polarization of nearby glia and accumulation of infiltrating cells leads to glial scar formation which further induces neuronal and neuroglial apoptosis, prevents axonal penetration, and ultimately inhibits functional recovery.[16]
Many cells contribute to neural tissue damage during the secondary phase of injury.[11,16] Resident microglia and infiltrating macrophages are polarized and recruited to the injury site where they clear cellular debris, produce axon repulsive molecules that incite axonal dieback, and further enhance the inflammatory response.[17,18] Chemokine release from these immune cells often induces astrocyte polarization towards a neurotoxic phenotype.[19] Polarized astrocytes proliferate and produce a filamentous scar around the injury site and release neuroinhibitory chondroitin sulfate proteoglycans (CSPGs). Axon contact with CSPGs results in dystrophic growth cone end bulbs, stopping axonal extension.[11,20] Increased metabolic activity, resulting from increased cellular reactivity, amplifies the production and secretion of reactive oxygen species (ROS) in and around the glial scar which leads to oxidative damage of neural tissue.[21] However, studies show that removing the glial scar completely does not improve regeneration, as scar removal induces influx of macrophages and activation of the underlying astrocyte layer.[22–24] The injury sequence highlighted above is representative of both SCI and TBI pathophysiology.[16,18] Similar cellular responses ensue after ischemic injury due to stroke including immune cell recruitment, glial cell activation, and chemokine and ROS production.[25] For a more detailed review on the cellular processes involved in CNS traumatic and ischemic injuries, we refer readers to reviews from Kong and Gao, Liddelow and Barres, and Kim and Cho.[17,24,25]
1.2. PNS injury and pathophysiology
PNS injury displays a pathophysiology that, unlike the CNS, results in greater functional recovery after traumatic injury.[26] Nevertheless, the cellular and molecular response is still complex and involves multiple cell types.[10] After the initial injury, Schwann cells at the lesion edge decouple from axons and attain a non-myelinating and repair-supportive phenotype with altered protein expression and increased proliferation.[27] The repair Schwann cells excrete proteins to support neuron survival and produce Bands of Büngner which provide topographical and chemotactic guidance for regenerating axons.[9,28] If the blood-nerve barrier is compromised, Schwann cells excrete chemokines to recruit macrophages.[29] Recruited macrophages promote Wallerian degeneration while removing cellular debris and neuroinhibitory ECM proteins. However, depending on the severity of PNS injury, hemorrhaging and the presence of disorganized fibrous tissue trap regenerating axons which results in incomplete recovery without surgical or therapeutic intervention.[9,30] In fact, current scaffolding biomaterials only address PNS injuries less than 10 mm in length for rats or 30 mm for humans and primates,[31,32] so more research is needed to develop materials that can promote regeneration across larger gap distances. For a more detailed review on the cellular and molecular dynamics following PNS injury, we refer the reader to reviews written by Jessen and Mirsky as well as Zigmond and Echevarria.[29,33]
1.3. ECM-mimetic Hydrogels for Neural Tissue Engineering
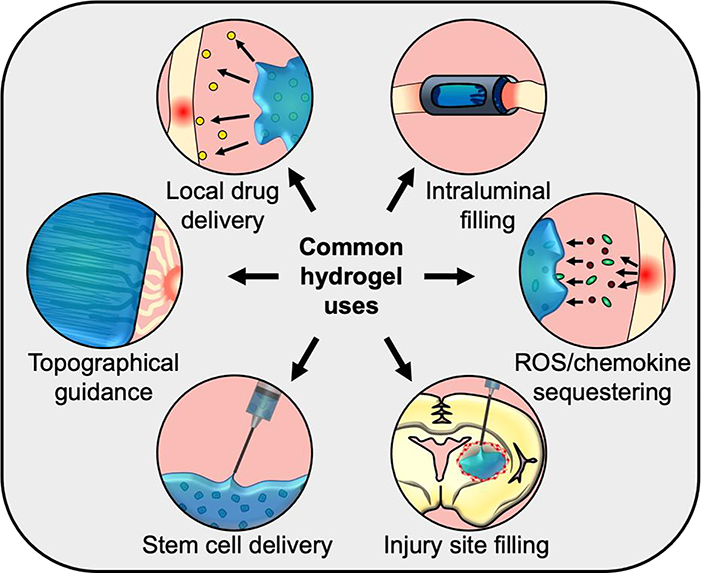
Many different biomaterials have been developed to modify the neural injury environment and promote subsequent axonal regeneration for the purpose of enabling functional recovery.[34–40] Hydrogels can be injected to fill irregular injury sites, used to deliver or sequester various molecules, and influence the activity of neural cells.[34,35,39] Hydrogels can also be implemented as intraluminal fillings in nerve guidance conduits to facilitate uniform cell infiltration as well as provide structural support to prevent conduit collapse.[10,41] Additionally, hydrogels can be tuned to match the elastic moduli of neural tissue which ranges from 0.1–2 kPa in mammalian tissue[42–45]; in vitro studies with hydrogels have demonstrated that this range facilitates robust neurite extension and stem cell proliferation and differentiation.[46–49] These hydrogel properties and applications frequently overlap to produce a multi-faced hydrogel that enhances neural tissue regeneration and ultimately functional recovery. Figure 1 outlines the different approaches hydrogels can be employed to promote regeneration. ECM-mimetic molecules are of particular interest for neural tissue engineering applications as they provide a naturally degradable substrate that has bioactive properties not present in the synthetic polymers commonly used for biomedical applications.[50,51] Additionally, 3D scaffolding better mimics the tissue environment and can enhance neurogenesis and normal physiological function between neighboring neurons.[52] Therefore, ECM-mimetic polymer-based hydrogels may provide a bioactive scaffolding that can facilitate robust neural tissue regeneration and functional recovery after injury.
Figure 1.
Hydrogels can serve many purposes regarding neural tissue engineering. This encompasses depots for drug delivery or sequestering toxic chemical signals, fillers for nerve guidance conduits or tissue cavities, cell delivery, and guidance cues for regenerating neurons. More often than not, a combination of these aspects is used to address multiple biological deficiencies after neural tissue injury.
While there are numerous hydrogels currently under development for neural tissue engineering, this review provides a summary on the ECM-mimetic biopolymers fibrin, HA, and ELPs. These polymers are considered to be mimetic as they not only consist of peptides or polysaccharides found in the native ECM but also are capable of innately modulating genetic expression, attracting and retaining nearby proteins, or providing sites for cell adhesion and cell-mediated degradation. While other hydrogel platforms can possess these same properties, they often require supplementation in the form of cell-adhesive ligands, enzyme-cleavable cross-linkers, or other molecules to modulate differentiation or retain loaded therapeutics. For example, poly(ethylene glycol), polyacrylamide, and poly(methacrylate) hydrogel derivatives may be able to degrade via hydrolysis and facilitate cell adhesion via cationic properties, but none of these components, in their raw form, bear bioactive motifs that can influence biological processes such as stem cell migration, differentiation, robust neurite extension, and matrix remodeling.[35,39] Other natural hydrogel platforms such as alginate, chitosan, gellan gum, and cellulose derivatives are not mimetic of the mammalian ECM, and therefore lack many of these bioactive properties.[35] This review focuses on three different types of ECM-mimetic hydrogels that innately bear bioactive properties without supplementation and are commonly used for neural tissue engineering applications: 1) fibrinogen which requires enzymatic cleavage to produce insoluble fibrin; 2) HA which requires chemical modifications and subsequent crosslinking for gelation; and 3) ELP’s which are completely synthetic but provide a tunable backbone that mimics elastin. Fibrin, HA, and elastin are all natural ECM components found near mammalian neural tissue during normal or pathological conditions[53–57]. However, elastin is not commonly used as a hydrogel for neural tissue engineering applications; instead, ELP hydrogels have recently become popular in this field due to their temperature triggered gelation and tunable material properties.[58] While collagen is an additional ECM-mimetic molecule commonly tested within neural tissue injury preclinical models, it is not summarized here since it encompasses a broad family of 29 proteins with diverse and, in some cases, inhibitory functions.[59–62][63–66] For example, collagens I and IV are highly prevalent in healthy neural tissue,[61,62]but collagen I was shown to induce astrogliosis after SCI in mice[64] whereas collagen IV was shown to be permissive to axonal regrowth after SCI in rats.[67] For a more detailed review on the various types of collagen, their prevalence in neural tissue, and their normal and pathophysiological roles in the PNS and CNS, please see Hubert et al.[61]
For each ECM-mimetic hydrogel, innate and supplemented cell-adhesion properties will be reviewed first. This includes the natural cell-adhesivity of the biopolymer, if any, as well as the integration of cell-adhesive ligands such as laminins (IKVAV, YIGSR, PPFLMLLKGSTR motifs) and fibronectin (RGD motif). This is important since the presence of ECM ligands can support exogenous cellular transplants or the integration of surrounding endogenous glia and neurons.[68] Next, the use of ECM-mimetic hydrogels to act as drug depots for delivery of proteins or synthetic therapeutics will be reviewed. Frequently, neurotrophins such as nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), and neurotrophin-3 (NT3) are incorporated into hydrogels to enhance the regeneration of neural tissue.[69] Lastly, ECM-mimetic hydrogels complexed with other material types will be reviewed. This encompasses the addition of materials such as other biopolymers, nanoparticles, and electrospun fibers. Implementing these materials can enhance hydrogel mechanical strength,[70] better protect cells during injection,[71] provide topographical cues for regenerating axons,[72] and sequester ROS.[73] These various hydrogel categories will be covered for each biopolymer in subsequent sections.
2. Fibrin hydrogels
Fibrinogen is a well-defined protein composed of two sets of three polypeptide chains with numerous disulfide bridges.[74,75] During coagulation, thrombin enzymatically cleaves fibrinogen exposing a polypeptide site that initiates polymerization with other fibrinogen molecules to produce the fibrin network. With the assistance of Ca2+ ions, Thrombin activates factor XIII protein, a transglutaminase, which further stabilizes the fibrin network, via crosslinks at specific polypeptide domains, and renders the network insoluble.[76] Therefore, fibrin hydrogels are formed via the combination of fibrinogen, thrombin, factor XIII, and Ca2+ ions in suspension. The innate bioactivity of fibrinogen and the concurrent enzymatic steps used to produce fibrin hydrogels are summarized in Figure 2. Careful selection of enzyme and fibrinogen precursor concentrations creates unique hydrogels with distinct gelation windows, enabling injectability, or tunable hydrogel moduli. Though fibrinogen is found in blood plasma, Golanov et al. demonstrated that after neural hemorrhage, neurons and astrocytes produce all fibrinogen constituents.[53] This, plus evidence of neurodegeneration in the presence of abnormal fibrin deposition, suggests that fibrinogen may play a pivotal role in normal and pathological neuronal processes.[53,54] While recent advances have created techniques to produce fibrinogen recombinantly, most of the fibrinogen used for research or medical applications is sourced from human or bovine plasma, which must be extensively purified/processed for use in vivo.[77] Recent work has focused on developing fibrin from other sources. One example of this is the use of salmon-derived fibrinogen, which does not contain human viruses or prions, yields greater recovery following SCI in a rat model, and facilitates greater serotonergic innervation compared to rats receiving human-sourced fibrin.[78,79] Regardless of the source, fibrinogen presents cell-adhesive properties that can promote the regeneration of neural tissue in vivo.
Figure 2.
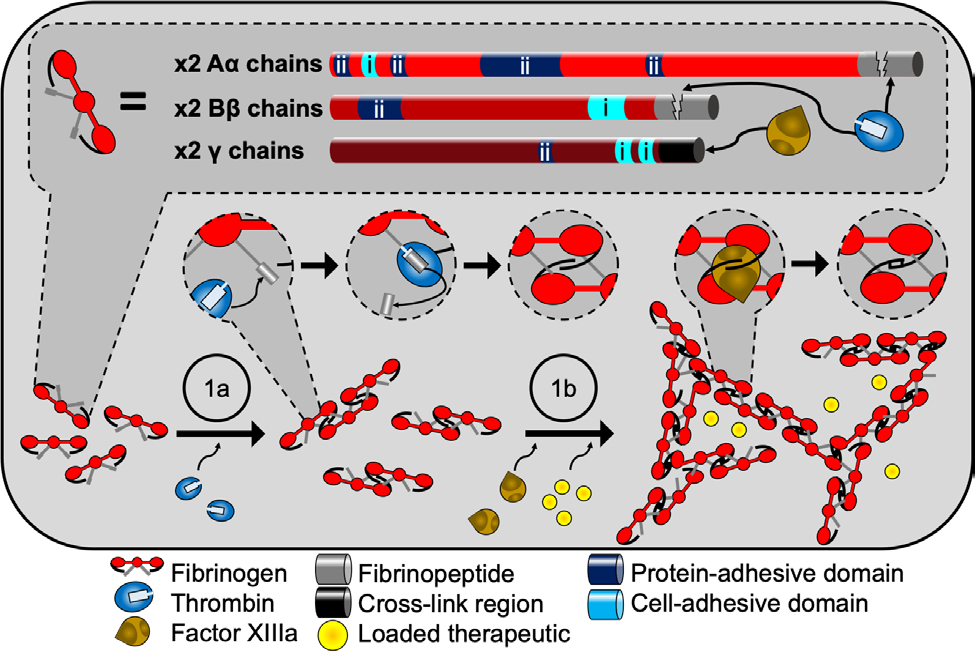
Fibrin hydrogels provide multiple bioactive interfaces. Fibrinogen is composed of two sets of three peptide chains called Aα Bβ, and γ (top). On each chain exists various binding sites for both cells (i) and several ECM proteins (ii). Fibrin hydrogel formation (bottom) is most often carried out in a batch solution containing fibrinogen, calcium, and the enzymes thrombin and factor XIIIa. First (1a), thrombin cleaves off the fibrinopeptides on chains Aα and Bβ which initiates polymerization and fibril formation via intermolecular association between the cleaved site of one molecule and a binding site from another. Next (1b), factor XIIIa covalently links the fibrinogen units together via overlapping γ chains. If therapeutics are used, they are included in the batch mixture before gelation.
2.1. Fibrin hydrogel bioactivity and combination with cell adhesive peptides
Fibrin is used in neural tissue engineering applications due to its physiological prevalence near neural tissue and its innate bioactive properties. These properties facilitate stem cell adhesion, induce lineage-specific differentiation, and sustain cell proliferation. Mooney et al. investigated the survival of cortical cultures, which were determined to be primarily neural progenitor cells (NPCs) and immature neurons, while altering concentrations of fibrinogen and thrombin precursor hydrogel constituents.[80] Tuning these variables affected cell proliferation and NPC process extension within fibrin hydrogels. Lower concentrations of fibrinogen and thrombin enhanced cell proliferation and process extension. NPCs were more likely to differentiate into neuronal lineages instead of astrocytic lineages regardless of the precursor concentrations, but neuronal maturation into cholinergic or dopaminergic neurons was dependent on the hydrogel composition; increased concentrations of fibrinogen or thrombin favored dopaminergic differentiation whereas reduced concentrations favored cholinergic differentiation.[80] With the assistance of various growth factors in cell culture media, Willerth et al. demonstrated that fibrin hydrogels could support NPC differentiation into oligodendrocytes.[81] Neuronal and oligodendrocyte differentiation in fibrin hydrogels was induced through the use of sonic hedgehog and NT3 growth factors, while NPC viability in the hydrogels was enhanced using basic fibroblast growth factor (bFGF) and platelet-derived growth factor. An optimal concentration of each growth factor was determined in a combinatorial approach to yield the greatest fraction of neuron and oligodendrocyte differentiation in the fibrin hydrogel.[81] However, even without growth factors, Asmani et al. demonstrated that fibrin hydrogels enhance the viability of seeded oligodendrocyte precursor cells (OPCs) when compared to two-dimensional (2D) fibrin matrices.[82]
In addition to sustaining cell survival and differentiation of neural and glial progenitors, fibrin hydrogels with distinct mechanical properties influence neurite extension from immature neurons. Man et al. adjusted fibrinogen concentration and salt supplementation to yield fibrin hydrogels with different compressive moduli.[83] Multiple hydrogels were produced with moduli ranging from 2 to 80 kPa. Hydrogels with lower moduli, those that had reduced concentrations of fibrinogen or salt, yielded greater neurite extensions from P1–3 mouse dorsal root ganglia (DRG) compared to hydrogels produced using higher concentrations of fibrinogen or salt.[83] Yao et al. enhanced neurite extension by using an electrospinning technique to produce aligned fibrillar fibrin (AFG) hydrogels, which was compared to plain fibrin hydrogels (no electrospinning) in the following studies.[84–86] Seeding P1 rat DRG on top of this aligned hydrogel yielded neurite extension along the aligned fibrillar plane. In the same study, reverse transcription-polymerase chain reaction was used to show that interaction with these aligned fibrillar fibrin hydrogels caused mesenchymal stem cells (MSCs) to downregulate the expression of adipogenic, chondrogenic, myogenic, and osteogenic markers but upregulate the expression of neural markers compared to tissue culture plates. Additionally, implantation within a 4-mm T9 rat hemisection SCI model induced neuronal cell invasion and axonal extension in both aligned and non-aligned hydrogels. However, the AFG produced a neural network oriented parallel to the long axis of the spinal cord whereas the randomly aligned hydrogels produced randomly organized tissue.[84] In a follow-up study, Yao et al. seeded these aligned scaffolds with MSCs before implantation into a 4-mm T9 rat transection SCI model.[85] This MSC-seeded material not only facilitated host neuronal migration into the gel but also induced neuronal differentiation of the donor MSCs, which produced axonal bundles that crossed the injury site. Additional electrophysiological examination and analysis of locomotor recovery using Basso−Beattie−Bresnahan (BBB) scoring showed that injured rats treated with MSC-seeded AFG experienced better functional recovery (BBB score of 12) after 12 weeks than rats treated with MSCs alone or AFGs alone (BBB scores of <9).[85,87] BBB scoring is a common technique that ranks functional recovery after SCI from 0 to 21 based on joint motion, ability to bear weight, and coordination, where a score of 21 indicates complete functional recovery.[87] Liu et al also improved functional recovery after SCI using MSCs within fibrin hydrogels. When MSC-seeded fibrin hydrogels were injected into a T10 rat transection SCI model, they reduced cellular apoptosis near the lesion site and improved neurite outgrowth and functional recover (BBB score of 7) compared to rats treated with fibrin hydrogels alone (BBB score of 5).[88] Du et al. demonstrated that AFGs can also be used as an intraluminal filling within the PNS.[86] In a 10-mm rat sciatic nerve transection injury model, AFGs significantly enhanced the density of nerve fibers that reached the distal end of the sciatic nerve after 12 weeks compared to rats treated with plain fibrin hydrogels. Additionally, axon myelination and electrophysiological recovery were significantly greater using AFGs; in fact, the recovery of rats treated with these hydrogels was comparable to the recovery observed in rats treated with autografts.[86]
While all the previously mentioned studies show that fibrin hydrogels alone induce neural regeneration, adhesion motifs can be integrated into the fibrin hydrogel to enhance axonal extension. King et al. produced a fibrin hydrogel containing the cell adhesive protein fibronectin by mixing the protein with fibrinogen before adding thrombin.[89] Fibrinogen has a binding site that helps retain fibronectin. This material was injected into a 2-mm rat T8 ventral resection SCI model and facilitated greater axonal infiltration compared to fibrin and fibronectin hydrogels alone 4 weeks after injury.[89] Silva et al. evaluated the use of multiple bioactive peptide sequences (indicated in Table 1) identified to facilitate integrin-mediated cell adhesion via the α6β integrin, a neural precursor integrin.[90] The covalent conjugation of these polypeptides to a fibrin hydrogel was mediated by the enzymatic activity of transglutaminase. Incorporation of the HYD1 sequence into the fibrin hydrogel facilitated greater neurite extension from embedded E18 rat DRG when compared to unmodified fibrin hydrogels. Implanting the HYD1-modified fibrin hydrogel into a 4-mm T8 rat transection SCI model increased axonal regeneration density and significantly improved functional recovery (BBB score of 9) compared to rats treated with unmodified fibrin hydrogels (BBB score of 4) 9 weeks after injury.[90] While these studies identify the advantage of using fibrin hydrogels for enhancing cell-transplant survival or axonal penetration in vivo, fibrin hydrogels have also been extensively used for controlled delivery of therapeutics.
Table 1.
Recent in vivo neural tissue engineering studies using fibrin hydrogels.
| Material | Supplement | Modulus [kPa] b) | Animal model | Significant finding(s) |
|---|---|---|---|---|
|
| ||||
| Fibrin | Fibronectin | - | Rat SCI | Fibronectin supplement better supports axon ingrowth compared to fibrin or fibronectin hydrogels alone[89] |
| Fibrin a) | chABC | - | Rat SCI | Fibrin prolongs chABC release which reduces glycosaminoglycan content compared to chABC injections[96] |
| Fibrin | - | - | Rat SCI | MSC-loaded hydrogel improved neurite extension and functional recovery[88] |
| Fibrin | chABC-loaded lipid microtubes and NEP1–40 loaded PLGA microspheres | - | Rat SCI | Drug-loaded composite improved axon growth, reduced astrocyte scarring, and reduced the presence of CSPGs around the injury site. |
| Fibrin a) | Laminin, VEGF, and NGF | - | Rat cortical implant Rat hemi-Parkinson |
Protein-immobilized hydrogels recruit endogenous neuronal cells and enhance neurite ingrowth Protein-immobilized hydrogels correct parkinsonian behavior[95] |
| AFG | - | 1.6 | Rat SCI | AFGs promote neuronal cell migration and guide axonal invasion[84] |
| AFG | - | 1.6 | Rat PNI | AFGs advance injury gap bridging and improve functional recovery[86] |
| Fibrin a) | T1, HYD1, or A5G81 synthetic ligands | 0.25 – 0.32 | Rat SCI | HYD1-immobilization boosts growth cone formation and functional recovery [90] |
| Fibrin | Tacrolimus-loaded PLGA microparticles | - | Rat PNI | Slowed drug release from microparticles improves neurogenesis[99] |
| AFG | - | 1.6 | Rat SCI | MSC-loaded AFGs produce nerve bundles that cross the injury site and improve functional recovery[85] |
inclusion of aprotinin, an enzyme inhibitor that reduces fibrin degradation
storage (elastic) modulus.
2.2. Fibrin hydrogels as drug and protein reservoirs
Promoting neural tissue regeneration has frequently been approached through the controlled release of growth factors from fibrin hydrogels. Multiple studies have used fibrin scaffolds to release neurotrophic factors by incorporating heparin. Sakiyama-Elbert and Hubbell first used this approach to slow the release of NGF from fibrin hydrogels.[91] Heparin inclusion within the hydrogel was facilitated by covalently linking peptides bearing heparin-binding domains to fibrin via transglutaminase. NGF’s affinity to heparin significantly reduced the initial burst release by 50%, enabled sustained NGF release for more than 16 days, and significantly enhanced neurite extension from E8 chick DRGs compared to fibrin hydrogels containing NGF without the heparin modification. Similar results were found using BDNF and NT3 neurotrophins within the heparin-modified fibrin hydrogel.[91] Bhang et al. also observed a slowed release of NGF when heparin was mixed into the fibrinogen precursor, but additionally demonstrated that the release profile could be tuned by altering fibrinogen and thrombin hydrogel precursor concentrations.[92] The inclusion of heparin with higher concentrations of thrombin or fibrinogen reduced the initial burst release of NGF, due to increased crosslink density, and sustained bioactive NGF release in a manner that enhanced both the number of neurites and the cell viability of PC12 cells compared to cells that received daily doses of similar NGF concentrations.[92] Lee et al. also demonstrated that 3D printed fibrin hydrogels mixed with heparin sustained release of bioactive vascular endothelial growth factor (VEGF) to enhance neural stem cell (NSC) migration and proliferation[93]. Willerth et al. investigated the use of custom-made peptides within fibrin hydrogels to alter NGF affinity to the scaffold.[94] Conjugation of these synthetic peptides was again made possible using transglutaminase activity. Neurite extension from E10 chick DRGs seeded within these hydrogels was altered depending on the conjugated peptide and the loaded NGF concentration, which demonstrated fine-tuned control over neurotrophin release using synthetic peptide methods.[94] Clark et al. covalently bound VEGF, NGF, and laminin to implantable fibrin hydrogels to recruit and influence the differentiation of endogenous NPCs from the subventricular zone in a rat model.[95] Immunohistochemistry results showed that long neurites extended within the implanted fibrin hydrogel and favored dopaminergic neuronal differentiation. The growth factor-loaded hydrogel also corrected drug-induced asymmetric rotation of hemi-Parkinson model rats, whereas hydrogels lacking VEGF and NGF did not correct the asymmetric rotation.[95]
While growth factors are beneficial in facilitating neuronal differentiation and axonal extension, fibrin hydrogel depots can alternately be loaded with unique molecules to reduce additional neural damage and digest neuroinhibitory products. Hyatt et al. loaded fibrin hydrogels with chondroitinase ABC (chABC), a bacterial enzyme capable of degrading inhibitory glycosaminoglycans (GAGs) that are produced after traumatic neural tissue injury.[96] In vitro, the hydrogel enabled sustained release of bioactive chABC for 15 days, and in a C4 rat lesion SCI model, chABC remained present in and near the lesion site three weeks post-implantation. In fact, three weeks after implantation chABC concentrations were significantly greater and CSPG concentrations were reduced in rats treated with the loaded hydrogel compared to rats that received an intraspinal injection of the same amount of chABC that was loaded into the hydrogels.[96] In another study, Ma et al. used fibrin hydrogels as reservoirs for oxygen to alleviate acute implantation-associated ischemic damage.[97] In this study, biologically inert perfluorotributylamine (PFTBA) was integrated into the fibrin hydrogel to enhance its oxygen solubility. Schwann cells were then exposed to hypoxic conditions in fibrin hydrogels with or without PFTBA supplementation for 48 hours. Results showed that inclusion of the PFTBA in the fibrin hydrogel significantly reduced Schwann cell apoptosis and restored near-normal neurotrophin production compared to cells cultured with fibrin hydrogels lacking PFTBA.[97] While many of these studies focus on the integration of therapeutics to enhance cell viability, additional biopolymers can be integrated to enhance hydrogel properties.
2.3. Fibrin hydrogels combined with other biopolymers
Due to the complexity associated with any type of neural tissue damage, a multipurpose hydrogel is often desired to address two or more challenges associated with the neural injury. This is possible through the development of composites where fibrin hydrogels are supplemented with another biomaterial. One example of a fibrin hydrogel composite is when fibrinogen is complexed with other native neural ECM biopolymers. Arulmoli et al. integrated HA and laminin into fibrin hydrogels seeded with neural stem/progenitor cells (NSPCs).[78] In this study, HA was used to reduce cell-mediated hydrogel degradation while laminin improved the attachment of neural cells. This composite maintained its original mechanical properties after 30 days, which better supported NSPCs and concurrently produced more robust neurite outgrowth compared to fibrin hydrogels alone. Additionally, co-culturing endothelial cells with the NSPCs produced greater vessel formation in the composite hydrogels compared to fibrin-only hydrogels.[78] While this study demonstrates how an additional biopolymer can enhance hydrogel degradation, synthetic polymers can also be embedded into fibrin hydrogels in the form of microparticles.
2.4. Fibrin hydrogels combined with microparticles
Microparticle formulation is often integrated into hydrogels to slow the local release of therapeutics. Tajdaran et al. produced a fibrin hydrogel containing poly lactic-co-glycolic acid (PLGA) mircoparticles loaded with tacrolimus, an immunosuppressant drug shown to enhance neurogenesis.[98] When implanted into a rat PNI model, tacrolimus could be detected at the injury site for 28 days after implantation and improved neurogenesis compared to rats that were treated with drug-loaded fibrin hydrogels lacking PLGA microparticles or hydrogels containing microparticles devoid of drug. In a biodistribution assay, minimal amounts of drug were found outside nervous tissue, indicating the composite’s ability to reduce systemic circulation in vivo.[99] Wilems and Sakiyama-Elbert also developed a microparticle-loaded fibrin hydrogel using chABC-loaded lipid microtubes and PLGA microspheres loaded with the peptide NEP1–40, which competitively binds to axon receptors to prevent association with neuroinhibitory myelin-associated inhibitors (MAIs).[100] These microparticles not only slowed the release of their respective therapeutics from the fibrin hydrogel, but also preserved the bioactivity of loaded chABC and released enough NEP1–40 to significantly increase neurite extension from dissociated DRG in the presence of inhibitory MAI substrates in vitro. The drug-loaded hydrogel was then implanted into a T8 rat transection SCI model, where it increased axonal extension and reduced astrocyte reactivity around the injury site compared to fibrin composites devoid of therapeutics. The composite additionally reduced the presence of CSPGs around the injury site compared to fibrin composites devoid of therapeutics and untreated control animals.[100] Synthetic polymers can also be integrated into fibrin hydrogels to promote directional cell growth.
2.5. Fibrin hydrogels combined with electrospun fibers
Fibrin hydrogels may also be complexed with topographical factors to generate composites that directionally enhance neurite growth. Omidinia-Anarkoli et al. electrospun PLGA fibers containing superparamagnetic iron oxide nanoparticles (SPIONs).[101] The fibers were cut into 60 μm long segments and suspended in a fibrinogen solution where subsequent exposure to a magnetic field influenced the SPIONs to align the fibers. This aligned network became fixed in place once the fibrin hydrogel formed. Both E9–10 chick DRG and dissociated neuron cultures were seeded into the aligned fibrous hydrogels where they produced longer neurite extensions in a unidirectional growth pattern compared to hydrogels without fibers and hydrogels with randomly oriented fibers that were not exposed to a magnetic field. Unidirectional signal propagation was also identified in the aligned fibrous hydrogels.[101] Similarly, Johnson et al. rolled SPION-loaded PLLA fibers into large-scale injectable conduits 3000–5000 μm in length.[102] These conduits were coated with laminin and suspended in a fibrinogen and thrombin solution, passed through a 22-gauge needle, and oriented using a magnetic field before complete gelation of the fibrin hydrogel. The study showed that fibrin could be used as a support matrix for the conduit. The conduit provided topographical cues and supported unidirectional neurite extension from P2 rat DRGs. Similar results were observed using a collagen hydrogel.[102] These injectable magneto-responsive composites are impactful as they provide a method for minimally-invasive placement and alignment of topographical guidance cues within a neuronal growth-supportive hydrogel. Hasanzadeh et al. also used electrospinning techniques to produce polyurethane (PU) fibers that contained multi-wall carbon nanotubes (MWCNT).[103] MWCNT integration increased the conductivity of these fibers by almost 9-fold compared to PU fibers alone. Additionally, suspension of these fibers into a fibrin hydrogel significantly enhanced MSC viability compared to fibrin hydrogels alone. While this study does not perform any other neural relevant experiments, the impact of the material is great, as it provides neural cell support via the fibrin matrix and holds the potential to transfer neural signals or permit electrical stimulation via MWCNTs in future studies.[103]
While fibrin hydrogels have drawn much attention for their use as scaffolding materials for neural regeneration, endogenous fibrinogen can trigger neuroinflammation and neurodegeneration.[104,105] Through careful selection of loaded therapeutics and conjugated constituents, the positive effects of fibrin hydrogels may alleviate the possible negative effects of fibrinogen. Fibrinogen also provides multiple benefits to tissue that are absent in other hydrogels; this includes enzyme-mediated degradation, multiple protein- and cell-adhesive domains, and tunable gelation properties to permit injectability. Nevertheless, evaluating inflammatory responses to implanted fibrin matrices should be studied to assess possible negative influences of fibrinogen. Key findings utilizing fibrin scaffolds in vivo are summarized in Table 1.
3. Hyaluronic acid hydrogels
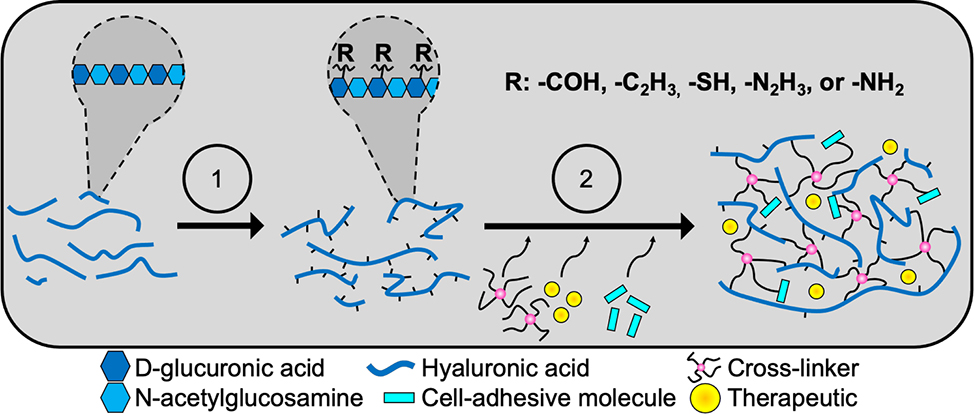
HA is a glycosaminoglycan composed of alternating units of D-glucuronic acid and N-acetylglucosamine and is an abundant ECM constituent in developing and mature neural tissue.[106,107] While HA does not promote cell adhesion like fibrinogen, it is known to impact cellular function via signaling through multiple receptors known as hyaladherins, including toll-like receptors (TLRs), receptor for HA-mediated motility (RHAMM or CD168), and CD44.[107–110] The molecular weight of HA has been shown to regulate glial migration, proliferation, maturation, and inflammatory responses.[55,111–114] HA hydrogel formation is possible using multiple techniques including hydrazide, thiol, furan, and acrylate modifications to the glycan backbone.[73,115–118] The type and extent of modification provides the opportunity to alter hydrogel properties such as the modulus, swelling ratio, injectability and therapeutic conjugations. Figure 3 provides an overview of the general synthetic steps to produce HA hydrogels. While HA is acquired from multiple sources including rooster comb and bovine eye, microbial fermentation is currently the most economically viable source of HA.[119] However, enzymatic synthesis, which is not yet at the commercial scale, is a promising technology for producing HA with tunable molecular weights without contaminants or variation between batches.[120] Regardless of the HA source, these hydrogels often require supplemental cell-adhesive peptides.
Figure 3.
Hyaluronic acid hydrogels provide a base scaffolding that can be modified to address multiple facets. Hyaluronic acid is a polysaccharide composed of alternating units of D-glucuronic acid and N-acetylglucosamine. Hydrogel fabrication is most often carried out in two stages. First, the polysaccharide backbone undergoes some form of modification (1) to provide sites for subsequent crosslinking (2). Different modifications can be used to create injectable hydrogels, alter hydrogel hydrophilicity, and enable chemical conjugation of other materials such as cell adhesive entities into the hydrogel. Therapeutics can additionally be mixed into the solution before crosslinking.
3.1. Hyaluronic acid hydrogels combined with cell adhesive molecules
Hyaluronic acid hydrogels have gained a foothold in the neural tissue engineering field not only due to HA’s prevalence in neural ECM, but also due to its presence in NSPC migratory pathways within the brain.[107] Since HA lacks cell-adhesive domains found in other ECM molecules, it is often combined with laminin for neural applications. Spearman et al. demonstrated that HA hydrogels required laminin and collagen to facilitate neurite extension from neonatal rat DRGs.[118] However, the lack of neurite extension in this study may be due to the stiffness (2–5 kPa) of the hydrogel since others show successful neurite extension in HA hydrogels (400 Pa) without additional ECM-derived molecules.[47] Addington et al. investigated the bioactive interplay between laminin-loaded HA hydrogels and NSPC response to the chemokine stromal cell-derived factor-1⍺ (SDF-1⍺), which is heavily expressed by glia after neural tissue injury.[121] HA hydrogels upregulated NSPC expression of CXCR4, a receptor for SDF-1⍺, via the NSPC hyaladherin CD44. Blocking CD44 prevented NSPC interaction with HA and resulted in the downregulation of CXCR4 which reduced NSPC response to SDF-1⍺ signaling. This study demonstrated that NSPC interaction with HA permits NSPC migration through the hydrogel in response to a chemokine signal. However, blocking NSPC association with HA yielded significantly reduced NSPC migration, similar to control hydrogels not exposed to a chemokine gradient.[121] In a follow-up study, Addington et al. found similar results in vivo when laminin-supplemented HA hydrogels were injected into the rat brain.[122] The hydrogels enhanced NSPC retention and migratory response to SDF-1⍺ compared to NSPC injections without the hydrogel. When the hydrogels were injected with a CD44 inhibitor, NSPC migration was significantly hindered, similar to bolus injections of NSPCs without the hydrogel.[122] However, it should be noted that these hydrogels failed to support NSPC migration without the addition of laminin[121].
While laminin is a commonly conjugated cell adhesion protein for HA hydrogels, successful modification of HA hydrogels has been achieved using other cell binding molecules. Tarus et al. demonstrated that integration of RGD ligands into an HA hydrogel affected NPC neurite density in a ligand concentration-dependent manner; the lowest tested weight fraction of ligand (3 wt%) resulted in the most significant increase in neurite density compared to hydrogels without ligands.[47] Using horseradish peroxidase enzymatic activity, Zaviskova et al. produced an injectable HA hydrogel containing RGD peptides and cell adhesive fibrinogen to enhance MSC survival and proliferation.[123] The integration of both adhesive molecules improved MSC proliferation compared to HA hydrogels with neither or only one of the cell adhesive molecules. The multi-component hydrogel was injected with MSCs into a 2-mm T8 rat hemisection SCI model where in situ crosslinking created a scaffold that enhanced axonal growth across the injury site compared to hydrogels injected without MSCs. While the MSCs did not survive past 8 weeks, it was hypothesized that they released various factors responsible for regulating axonal growth and host immune response.[123] Similarly, Adil et al. developed an injectable HA hydrogel containing both RGD domains for enhanced neuronal cell attachment and heparin to enhance cell maturation and survival via the presence of neurotrophin adhesive domains.[124] While RGD integration enhanced neurite extension from seeded NPCs, the inclusion of heparin provided additional enhancement of neurite extension and facilitated greater NPC dopaminergic differentiation compared to hydrogels without heparin. The modified hydrogel enhanced survival of these differentiated neurons after transplantation into the rat striatum compared to cells injected without the hydrogel.[124]
In an effort to optimize ligand concentrations, Lam et al. used a statistical design of experiments (DOE) approach to simultaneously optimize concentrations of RGD, IKVAV, and YIGSR bioactive motifs in an HA hydrogel. The optimized and combined ligand concentrations created an HA hydrogel that promoted greater neuronal differentiation of NPCs compared to HA hydrogels with either equimolar ligand concentrations or only RGD ligands.[48] Nih et al. also integrated multiple bioactive peptides into an injectable microporous HA hydrogel.[125] The hydrogel included RGD ligands for cell adhesion, matrix-metalloproteinase (MMP)-sensitive peptides to permit matrix degradation, and two transglutaminase susceptible peptides to facilitate the production of microporous hydrogels, as opposed to the conventional nano-porous structure of most HA hydrogels. Injection of microporous HA hydrogels into a mouse ischemic model significantly reduced microglia and astrocyte-mediated scarring, enhanced astrocyte infiltration, and therefore facilitated a greater migration of NPCs into the injury site compared to nano-porous hydrogels or no hydrogel.[125] Li et al. used a laminin-derived cell adhesion motif, PPFLMLLKGSTR, to enhance MSC survival and functional recovery in a 1.5-mm T9–10 rat transection SCI model.[126] While this peptide-modified HA hydrogel was not compared to other motifs or HA alone, injection of MSC-loaded, peptide-tethered hydrogels significantly enhanced MSC transplant survival and rat BBB scores (BBB score of 9) compared to implantation of MSCs devoid of a hydrogel scaffold or peptide-tethered hydrogels transplanted without MSCs (BBB scores of 7).[126] While much of this research demonstrates the benefit of cell-adhesive molecules, similar regenerative facets can be achieved via drug loading of the HA hydrogel.
3.2. Hyaluronic acid hydrogels as drug and protein reservoirs
HA hydrogels are of particular interest for their use as a drug depot for growth factor delivery. Multiple HA composites were designed to prolong the release of BDNF from HA hydrogels, which will be seen here and in a section 3.4. Nakaji-Hirabayashi et al. tethered recombinant BDNF to HA hydrogels using zinc chelation and a peptide-association method.[127] While implementation of BDNF into the HA hydrogel reduced the bioactivity of BDNF, higher loading concentrations were able to differentiate NSCs to neurons. This biomaterial enhanced the survival of neuronal cell cultures compared to HA hydrogels devoid of BDNF. Tethering of BDNF to the HA hydrogel also significantly slowed and prolonged the release of BDNF in that only 33% of loaded BDNF was released after 12 days compared to 90% release from hydrogels containing unbound BDNF.[127]
Similar to fibrin hydrogels, heparin can be incorporated into HA hydrogels to slow the release of loaded growth factors. Moshayedi et al. carried out an elaborate statistical DOE scheme to optimize MMP-degradable HA-heparin hydrogels with multiple cell adhesive ligands (RGD, IKVAV, and YIGSR) and growth factors (bone morphogenic protein 4 (BMP-4) and BDNF).[128] As a result, an HA hydrogel composite was developed that contained optimized concentrations of all ligands and heparin-bound growth factors; interestingly, the optimization hydrogel contained no BDNF. This optimized composite sustained angiogenesis, permitted matrix remodeling/degradation, and facilitated NPC survival and proliferation 2 weeks after implantation into a murine brain ischemia model. However, the non-optimized HA composite, which had no growth factors and equimolar concentrations of ligands, was able to induce neuronal differentiation more efficiently compared to the optimized hydrogel. The optimized hydrogel significantly enhanced NPC differentiation to an astrocytic lineage in vivo.[128]
While growth factors can facilitate neural cell proliferation or neurite extension, some groups have delivered other types of compounds to target a specific aspect of the injury environment. Tian et al. produced an HA hydrogel with covalently conjugated Nogo-66 antibodies to block the Nogo-66 receptor, an MAI known to inhibit the extension of axons.[129–131] The antibody loaded hydrogel enabled sustained antibody release for over 2.5 weeks, depending on the local pH, while retaining antibody bioactivity in rat brain tissue.[131] In follow up studies by Ma et al. and Wei et al., hydrogels were implanted into a rat cerebral ischemia model and a rat T8-T9 SCI model. In both studies, axons better infiltrated the antibody-loaded hydrogels compared to all controls.[132,133] Agrawal et al. also addressed the Nogo-receptor mechanism by chemically immobilizing antisense single-stranded DNA onto an HA backbone.[134] Antisense oligonucleotides with various binding affinities were used to control the release of HA hydrogel-loaded anti-Nogo receptor single-stranded RNA from 7 to 28 days. This RNA was shown to facilitate neurite extension from dissociated rat DRG in the presence of inhibitory MAIs at both time points.[134] Wei et al. additionally included poly-L-lysine (PLL) into the hydrogel and highlighted the hydrogel’s potential to block inhibitory aspects of the glial scar while supporting angiogenesis.[133] While therapeutic delivery can enhance neurogenesis or cell migration, toxic molecules can persist. Shi et al. developed an ROS-sensitive hydrogel by combining HA with poly(vinyl alcohol) (PVA) via boronic ester bonds.[135] The injectable hydrogel was self-healing and responded to the presence of ROS. The hydrogel composite enhanced NPC survival when exposed to ROS compared to HA only, PVA only, and Matrigel controls. Drug release from the hydrogel, doxorubicin in this instance, was accelerated by ROS, indicating its potential use as a smart drug delivery depot.[135]
3.3. HA hydrogels combined with other biopolymers
HA hydrogels are widely used to support regeneration of neural tissue. Supplementing HA hydrogels with other biopolymers, such as gelatin, can further enhance these regenerative properties. Zarei-Kheirabadi et al. used a combinatorial approach where gelatin was combined with a commercially available HA hydrogel kit, HyStem®-C Cell culture scaffold kit, to enhance the mechanical strength and present cell adhesive domains.[70] NSC-loaded hydrogels were implanted in a T10–11 rat contusion SCI model and subsequently reduced the cavity and glial scar area compared to hydrogels alone or NSCs alone. Additionally, the gelatin-HA hydrogel enhanced NSC survival and differentiation towards an oligodendrocyte lineage, whereas NSCs devoid of scaffolding favored an astrocytic lineage. Lastly, NSC-seeded gelatin-HA hydrogels increased the rat survival rate and significantly enhanced BBB scores (BBB score of 13) compared to rats treated with cells or hydrogels alone (BBB scores of 10 and 12, respectively).[70] Similarly, Geissler et al. used a collagen-HA hydrogel combined with laminin, which enhanced NPC differentiation towards an oligodendrocyte lineage in vitro compared to collagen alone and collagen combined with only HA or laminin[136]. When implanted in a C4 rat contusion SCI model, the NPC-loaded collagen-HA hydrogel significantly improved functional recovery compared to hydrogels implanted without exogenous NPCs or NPCs alone[136].
In addition to advancing the regenerative properties of HA, biopolymers can be integrated to improve their drug-delivery potential. Cook et al. achieved BDNF release from injectable HA-collagen hydrogels for over three weeks in both striatal and cortical mouse stroke models.[137] The sustained release led to significant improvements in motor function compared to injections of free BDNF which only elevated BDNF levels in the peri-infarct region for 1 week. Additionally, the released BDNF migrated 2 cm from the infarct region in a non-human primate model, a distance appropriate for therapeutic efficacy in humans.[137] Zhang et al. developed an injectable chitosan-HA composite where NGF release was sustained for 28 days after which 80% of loaded NGF was released. Subsequent culture with MSCs revealed that the hydrogel stabilized the NGF structure, which induced greater MSC proliferation after only 3 days compared to groups treated with a similar dose of NGF not released from the hydrogel.[138] A follow-up study by Xu et al. observed similar results using Schwann cells but additionally demonstrated that alterations to the chitosan-HA ratio and the concentration of loaded NGF can more precisely fine-tune NGF release. Furthermore, PC12 cell culture experiments showed that NGF release from chitosan-HA hydrogels facilitated neurite outgrowth for over 21 days, indicating the hydrogel’s ability to protect the loaded NGF.[139]
3.4. HA hydrogels combined with nanoparticles or microparticles
Integration of synthetic nanoparticles into HA hydrogels can slow the release of loaded therapeutics. Wang et al. was able to prolong the release of BDNF by loading the neurotrophin into PLGA microparticles which were subsequently loaded into an HA hydrogel.[140] This composite enabled sustained BDNF release for over 4 days in which only 12–13% of the loaded neurotrophin was released. Hydrogel-released BDNF prevented neuron apoptosis in a neurotoxicity assay, which demonstrated its preserved bioactivity in the hydrogel. Furthermore, this composite sustained NSCs in culture. Similar results were observed using VEGF as the growth factor being released from the PLGA nanoparticles in HA hydrogels.[140] Using click chemistry, Führmann and colleagues developed an injectable HA hydrogel and tested BDNF release from loaded PLGA nanoparticles. Nearly 77% of the loaded neurotrophin was released after 28 days. By analyzing neurite outgrowth from E17 rat DRG, the bioactivity of BDNF was preserved by the HA hydrogels over the entire 28 days.[141] Ju et al. combined features in previously-mentioned studies where HA hydrogels contained Nogo-66 receptor antibodies and PLGA microparticles loaded with angiogenic factors VEGF and Ang1.[142] Release curves showed the cumulative release of VEGF and Ang1 to reach 27% and 10% release over 13 days, respectively. The hydrogel composites supported NSC proliferation in vitro and enhanced endothelial cell proliferation compared to hydrogels lacking PLGA loaded growth factors. These hydrogels were subsequently implanted into mouse brain ischemic models where they reduced gliosis, enhanced mouse forelimb use, and induced greater angiogenesis in the peri-infarct region compared to HA hydrogels lacking growth factor-loaded PLGA microparticles.[142]
A number of studies have facilitated therapeutic delivery from hydrogels composed of HA and methylcellulose (MC), an injectable composite which has been shown to reduce inflammation and improve functional recovery after SCI.[143,144] Wang et al. combined this hydrogel with epidermal growth factor (EGF)-loaded PLGA nanoparticles and erythropoietin-loaded microparticles composed of a PLGA core and a poly(sebacic acid) (PSA) coating to delay erythropoietin release.[145] This composite sustained EGF release for the first 7 days followed by erythropoietin release for the subsequent 14 days in vitro and in a mouse stroke model. In the model, this resulted in reduced subventricular cell death and neuroregenerative properties 18 days after injury.[145] Caicco et al. integrated Cyclosporin-containing PLGA microparticles into the same HA/MC hydrogel system.[146] This composite could sustain release of the immunosuppressant drug for up to 28 days in vitro. When injected on the cortices of mice, diffused drug could be detected up to 24 days at the subventricular region after injection.[146] Tuladhar et al. combined the cyclosporin and erythropoietin-loaded PLGA microparticles into HA/MC hydrogels and injected the composite onto the cortices of stroke model rats. Both therapeutics could be detected in the subventricular region for 32 days and dually improved tissue regeneration and functional recovery compared to composites loaded with only one of the therapeutics.[147] Obermeyer et al. produced HA/MC hydrogels in which PLGA nanoparticles electrostatically slowed the release of loaded BDNF. Injection of the composite onto cortices in a rat stroke model sustained BDNF release for over 21 days and improved functional recovery.[148] Similar studies by Stanwick et al. successfully controlled the release of NT3 and the NogoA antibody from PLGA nanoparticles loaded into HA/MC hydrogels, though these materials were not evaluated in vivo.[149,150] Please see the review by Ho et al. for additional research encompassing the HA/MC composite and neural tissue regeneration.
A study by Jian et al. used nanoparticles composed of chitosan, chondroitin sulfate (CS), and heparin sulfate (HS) to control SDF-1α and bFGF delivery via MMP degradation within an HA hydrogel.[151] Depending on the nanoparticle formulation, SDF-1α or bFGF electrostatically interacted with the GAGs in the nanoparticles. In vitro nanoparticle-release kinetics was modeled by the addition of various concentrations of collagenase, where release could be sustained for 8 (more enzyme) to more than 14 days (less enzyme). When implanted into a rat brain ischemia model, the nanoparticle-loaded hydrogel recruited and increased the proliferation of endogenous NSCs compared to injections of hydrogels or nanoparticles alone. The HA scaffold also reduced astrogliosis, compared to nanoparticles alone, and facilitated neurogenesis and angiogenesis in vivo.[151]
Some studies investigated the use of various nanoparticles within HA hydrogels to enhance cellular growth and the electrophysiology of neurons. Shin et al. successfully produced an electroconductive HA hydrogel composite using conductive carbon nanotubes and polypyrrole.[152] Gelation was made possible via catechol modification of the HA backbone, subsequent π-π interactions, and oxidative crosslinking via sodium periodate. The inclusion of nanotubes and polypyrrole reduced the material impedance of HA hydrogels, accelerated neuronal differentiation, and reduced astrocytic differentiation of NSPCs in vitro. Varying the ratio of the conductive components within the hydrogel allowed for some control over neuronal, astrocytic and oligodendrocytic differentiation. Additionally, the presence of these conductive materials influenced neuronal differentiation since the neurons upregulated the expression of voltage-gated Ca+ channels. As a result, glutamate-induced stimulation resulted in greater intracellular Ca+ influx compared to HA hydrogels lacking these conductive components.[152] A study by Tay et al. investigated calcium influx in neurons cultured in an HA hydrogel sensitive to magnetic fields. Magnetic microparticles were chemically integrated into an HA scaffold and were subsequently exposed to a magnetic field, which together facilitated neurite growth from E18 rat DRG-sourced neurons and induced a calcium influx through overexpressed PIEZO2 and TRPV4 ion channels.[153]
Nanoparticles can also be integrated in HA hydrogels to sequester ROS. Li et al. developed an ROS-absorptive HA hydrogel where MnO2 nanoparticles were integrated into HA hydrogels modified with a laminin motif (PPFLMLLKGSTR).[73] In culture, the hydrogel composite reduced the presence of ROS species and enhanced MSC survival in ROS conditions compared to HA hydrogels lacking the MnO2 nanoparticles. Similar results were observed in a 0.5-mm T9–10 rat transection SCI model where the composite hydrogel reduced intracellular ROS concentration, lipid peroxidation products, and DNA damage markers. Additionally, the MnO2-loaded HA hydrogels improved functional recovery (BBB score of 10) and reduced astrogliosis compared to hydrogels lacking MnO2 nanoparticles (BBB score of 7).[73]
3.5. HA hydrogels combined with electrospun fibers
As previously stated in the drug depot section, synthetic polymers are used in HA hydrogel composites to slow and better control the release of loaded proteins. However, Richard McMurtrey used a synthetic polymer to provide topographical guidance cues within HA hydrogels.[72] Aligned polycaprolactone fibers, coated with either gelatin or laminin, were imbedded into HA hydrogels seeded with neuronal cells. Hydrogels loaded with the laminin-coated fibers increased the tracking efficiency of neurites along fibers compared to hydrogels loaded with uncoated fibers. This composite also increased average neurite length compared to 2D cultures with or without fibers and HA hydrogels alone.[72]
HA is a promising hydrogel component that is of particular interest for use in brain tissue. While HA hydrogels are often supplemented with other polymers or ligands, HA itself does have a biological role in normal and pathological neural tissue function.[108] While many of HA hydrogel-mediated mechanisms still require elucidation, HA hydrogels are a very promising biomaterial for cell implantation and drug delivery. HA hydrogel development must account for multiple design parameters including appropriate HA molecular weight, type of chemical modification for crosslinking or injectability, ligand complementation, and therapeutic conjugations. Important in vivo findings using HA hydrogels for neural tissue engineering are outlined in Table 2. For a more detailed review on the biological function of HA and its broad material applications for neural tissue, please refer to the review by Khaing and Seidlits[108].
Table 2.
Recent in vivo neural tissue engineering studies using hyaluronic acid (HA) hydrogels.
| Material(s) | Supplement(s) | Inj. | Modulus [kPa] g) | Model(s) | Significant finding(s) |
|---|---|---|---|---|---|
|
| |||||
| HA a) | Nogo-66 antibody, PLL |

|
- | Rat SCI | Antibody-immobilization enhances axon ingrowth[133] |
| HA a) | Nogo-66 antibody, VEGF and Ang1-loaded PLGA microspheres |

|
- | Mouse brain ischemia | Protein-loaded composites improve angiogenesis and functional recovery[142] |
| HA a) + MC | EGF-loaded PLGA nanoparticles and erythropoietin-loaded PLGA/PSA microparticles |

|
1 | Mouse stroke | Two distinct protein-release profiles observed due to particle formulation Released proteins reduce cell death and improve neuroregeneration after stroke[145] |
| HA a) + MC | Cyclosporin-loaded PLGA nanoparticles |

|
- | Mouse cortical implant | Composite sustained local drug release to the subventricular zone[146] |
| HA b) | BDNF-loaded PLGA nanoparticles |

|
0.048 | Rat SCI | Modified HA scaffold is biocompatible in intrathecal space[141] |
| HA c) | RGD, YIGSR, and IKVAV motifs, heparin, BMP-4, BDNF |

|
0.35 | Mouse brain ischemia/NPC transplant | DOE-optimization of bioactive molecules can be used to enhance stem cell transplant survival and control differentiation[128] |
| HA d) | Laminin |

|
1.02 | Mouse cortical/NSPC transplant | Stem cell interaction with HA facilitates migration in response to chemokine signaling[122] |
| HA a) | PPFLMLLKGSTR motif |

|
0.18 | Mouse SCI/MSC transplant | Laminin-derived motif improves stem cell transplant survival and functional recovery[126] |
| HA d) + collagen d) | BDNF |

|
- | Mouse stroke Monkey stroke |
BDNF-loaded hydrogel improves axonal sprouting and functional recovery Diffusion distance of released BDNF is appropriate for functional recovery[137] |
| HA e) | heparin, RGD motif |

|
0.35 | Rat cortical/neuron transplant | Hydrogel improves neuron transplant survival[124] |
| Microporous HA c) | RGD motif |

|
1.5 | Mouse stroke | Microporosity enhances vascularization, facilitates NPC migration/proliferation, and reduces inflammation[125] |
| HA f) | SDF-1⍺-loaded chitosan/HS nanoparticles, bFGF-loaded chitosan/CS nanoparticles |

|
0.1 | Rat brain ischemia | Composite hydrogel recruits endogenous stem cells, enhances neurogenesis and angiogenesis, and reduces glial scarring[151] |
| HA f) | Fibrinogen, RGD motif |

|
0.1 | Rat SCI/MSC transplant | Supplements dually improve MSC proliferation MSC-seeded hydrogel improves axonal ingrowth[123] |
| HA + collagen | Laminin |

|
Rat SCI/NPC transplant | NPC-seeded hydrogel improves functional recovery, oligodendrocyte differentiation, and reduces glial scarring[136] | |
| HA a, f) | MnO2 nanoparticles, PPFLMLLKGSTR motif |

|
- | Rat SCI/MSC transplant | Nanoparticle composite sequesters ROS, reduces scar formation, and improves functional recovery[73] |
| HA a) + MC | Erythropoietin-loaded PLGA microparticles and Cyclosporin-loaded PLGA microparticles |

|
- | Rat stroke | Particle-loaded therapeutics dually improved tissue regeneration and functional recovery[147] |
| HA d) + gelatin d) |

|
- | Rat SCI/NSC transplant | Hydrogel improves NSC survival, oligodendrocyte differentiation, functional recovery, and reduces glial scarring[70] | |
hydrazide modified
furan modified
acrylate or methacrylate modified
thiol modified
cyclooctyne modified
aldehyde modified
storage (elastic) modulus.
4. Elastin-like polypeptide (ELP) hydrogels
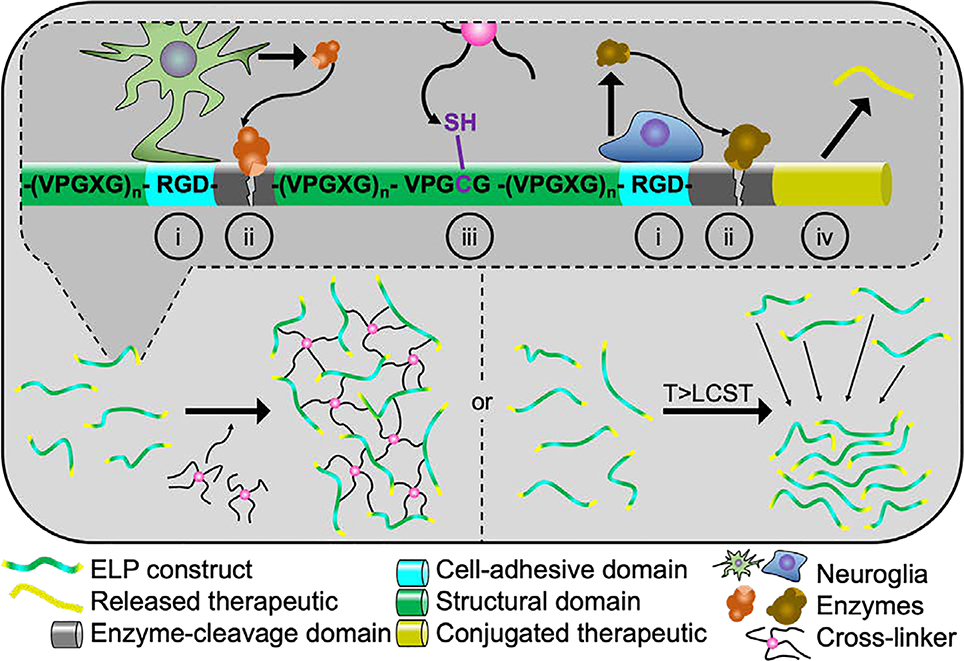
Elastin naturally occurs in the connective tissues surrounding axons and nerve bundles and can be produced by glia.[56,57] ELPs are recombinant polypeptides modeled from a structural motif found in elastin. This motif consists of the amino acid sequence (valine-proline-glycine-X-glycine)n where X denotes any amino acid except proline, which disrupts the native folding behavior, and n indicates the number of pentapeptide sequences. ELPs were attractive drug delivery vehicles due to their unique and reversible phase separation above a transition temperature or lower critical solution temperature (LCST).[154] This property can be tuned by varying ELP concentration, polypeptide length, and the guest residue (X). The transition temperature allows for a simple purification technique where hot-cold centrifugation cycles are used to recover pure ELP or protein-conjugated ELP from bacterial culture.[155–157] ELPs are a promising hydrogel constituent as they can be recombinantly produced with cell adhesive ligands, small proteins, enzyme-cleavable sites, and tunable transition temperatures. Additionally, multiple studies have identified that they are biocompatible and elicit no immune response.[158–160] Hydrogels are formed by producing a custom-made ELP containing ligands or proteins followed by crosslinking, which can occur via multiple mechanisms.[161–165] Due to the unique aggregation characteristic of ELPs above their LCST, many ELP derivates rely on phase separation to facilitate gelation, as highlighted in Figure 4. Since various ligands can be integrated into the backbone of the biopolymer, there is rarely a need to supplement ELP hydrogels with other bioactive molecules.
Figure 4.
Elastin-like polypeptide hydrogels can provide many tunable properties. ELPs can be expressed bearing multiple bioactive and functional molecules (top) including cell-adhesive ligands (i), various enzyme-cleavable domains (ii), cross-linkable residues (iii), and peptide/protein therapeutics (iv). ELP hydrogel formation can be carried out using two methods. First, ELPs can be expressed with thiol- or amine-bearing residues that permit chemical crosslinking without prior modification (bottom left). Second, a hydrophobic phase separation can be induced by raising the solution temperature above the tunable lower critical solution temperature or LCST (bottom right).
4.1. ELP hydrogels containing cell adhesive peptides
Similar to fibrin and hyaluronic acid hydrogels, ELPs often contain cell adhesive polypeptides and bioactive molecules. However, ELPs can be bacterially expressed with these conjugations in place, minimizing the use of complex synthesis schemes employed to integrate bioactive compounds into fibrin or HA hydrogels. Straley and Heilshorn first demonstrated the versatility of ELP hydrogels for neural tissue engineering applications using multiple bioactive peptides within the ELP backbone.[165] To facilitate neuronal degradation of the material, the ELP was constructed with different cleavage sites, each of which was susceptible to different degrees of degradation by urokinase plasminogen activator (upa), an enzyme specifically secreted from neuronal growth cones. The ELP was created to also contain cell adhesive RGD ligands. Lastly, a fifth of all ELP guest residues was substituted with lysine to provide sites for amine crosslinks. From this work, ELP hydrogel degradation was tunable from 24 to >164 hours, depending on the selected cleavage site, and was independent of initial mechanical properties. Additionally, the inclusion of various cleavage sites did not affect cell adhesion of PC12 cells, meaning that cell adhesivity could be tuned independently of hydrogel degradation. RGD concentration was tuned to facilitate cell adhesion similar to that of bovine serum albumin, a common blocking agent for promoting cell adhesion, or PLL, a common supplement for enhancing cell adhesion, demonstrating the tunable properties of ELP. Proliferation and neurite outgrowth from PC12 cells could be controlled by varying the concentration of ELPs containing RGD sequences.[165] This seminal study provided the initial framework for the use of ELPs in neural tissue engineering as it revealed the ability to finely tune many properties independently of each other to enhance neurite outgrowth. This tunable nature is not seen in the previously covered biopolymers.
Later work by Lampe et al. used a similar ELP, which contained lysine residues and RGD domains, to produce hydrogels with varying moduli depending on the extent of crosslinking.[49] ELP hydrogels were produced with moduli ranging from 0.5 kPa to 2 kPa, which was achieved independent of RGD density. Subsequent culture of DRG within these 3D scaffolds showed greater neurite extension in the presence of RGD ligands and in hydrogels with lower moduli, though neurite extension was also possible without the presence of RGD ligands.[49] A follow-up study by Romano et al. placed DRGs in an ELP hydrogel that was exposed to an NGF gradient via a microfluidic device. A high RGD ligand concentration created an environment where extending neurites ignored the gradient and extended uniformly throughout the hydrogel, whereas lower concentrations of RGD permitted neurites to preferentially grow in the direction of the NGF source, demonstrating a compromise between ligand density and neurite outgrowth by chemotaxis.[166]
While assessing the ability of ELPs to induce neurite extension is helpful in their development towards being utilized within in vivo injury environments, ELP hydrogels are also investigated for their potential as scaffolds for stem cells. Madl et al. varied crosslink density to produce ELP hydrogels with various degradation rates.[167] This study demonstrated that the initial hydrogel elastic modulus did not influence NPC stemness; however, the degradation rate of the ELP hydrogel did. Hydrogels that degraded quickly maintained NPC stemness whereas slowly degrading hydrogels caused NPCs to lose their stemness, which was shown through Sox2 and Nestin mRNA expression. Additional experiments verified that ECM degradation via ADAM9 protease was ultimately responsible for the maintenance of NPC stemness. Matrix remodeling also significantly impacted the proliferation of NPCs and their potential to differentiate into neurons and astrocytes after retinoic acid induction. Without the induction, NPCs showed no lineage-specific preferences when cultured with ELP alone.[167] A subsequent study by Madl et al. further identified that remodeling was vital for differentiating NPCs. The hydrogels that experienced NPC remodeling for only 1 day failed to differentiate NPCs under neuronal differentiation medium or astrocyte differentiation medium. But after remodeling for 7 days, these mediums successfully differentiated NPCs into their intended lineages.[168] Through both studies it was identified that ELP hydrogel remodeling enabled cell-cell cadherin interaction, which subsequently influenced β-catenin signaling to control the downstream process of maintaining NPC stemnesss.[167,168] Meco et al. seeded OPCs into ELP hydrogels containing urokinase plasminogen activator degradable sequences.[169] While the presence of these enzyme-degradable sequences did not appear to affect OPC proliferation or metabolic activity, they did affect the maturation of the cells. Genetic analysis showed that ELP hydrogels containing the degradable sequence appeared to improve the maturation of OPCs.[169]
Similar to the previous-mentioned fibrin and HA hydrogels, RGD sequences are not the only cell-adhesive ligands incorporated into ELPs. Paiva dos Santos et al. first conjugated, not expressed, the laminin-derived sequence IKVAV into an ELP hydrogel using a poly(ethylene glycol)-based crosslinker.[170] The conjugation supported MSC survival and enhanced neurite outgrowth from adult rat DRG neurons in a ligand concentration-dependent manner. Subcutaneous implantation of the hydrogel into mice initiated angiogenesis around the hydrogel and subsequent innervation within 11 days.[170] In the same year, Paiva dos Santos and colleagues also bacterially expressed ELPs with IKVAV dispersed throughout the polypeptide backbone.[157] However, these IKVAV-containing ELPs were unable to undergo a reversable phase transition. Subsequent experiments identified that the ELPs possess a thermodynamic equilibrium that favored characteristics similar to amyloid-like fibrils. Nevertheless, integration of this ELP into a poly(ethylene glycol) hydrogel significantly enhanced neurite extension from sensory neurons compared to hydrogels with scrambled sequences.[157] This study sheds light on an important aspect regarding ELPs and their relation to amyloid fibrils, CNS assemblies associated with Alzheimer’s disease[171]. Ma et al. demonstrated that soluble and large ELPs, >30 pentapeptide sequences, enhanced amyloid β (Aβ) protein production in an ovary cell line. Subsequent testing in mice showed that intravenous treatment with the large ELPs reduced normal cognitive function and increased the presence of Aβ peptides throughout the brain[172]. Therefore, ELP research regarding TBI and degenerative diseases may need to consider smaller ELP sequences and should evaluate these smaller ELP constructs in relevant animal models. Similar to fibrin hydrogels, specific therapeutic or ligand conjugation may outweigh this negative effect from ELPs.
4.2. ELPs as drug or protein reservoirs or carriers
Use of ELPs as drug depots for neural applications was first investigated by Shamji et al. where the ELP’s unique thermal transition behavior provided a medium for sustained drug delivery.[173] Perineural injection into rats showed that soluble ELPs retained a half-life of 5.5 hours at the injection site, whereas thermally phase separated ELP hydrogels increased the half-life to 39 hours. It was found that the aggregated depot reduced systemic circulation of ELP compared to soluble ELP depots. This study laid the groundwork for using ELP aggregation as a drug depot for neural tissue.[173] Sinclair et al. demonstrated the ability of ELPs to hold and release anti-inflammatory curcumin to target tumor necrosis factor alpha (TNF⍺), a cytokine that mediates the inflammatory response after traumatic injury and ultimately leads to cellular death. Curcumin was chemically functionalized via degradable carbamate linkages onto ELPs, which enhanced curcumin’s solubility and demonstrated efficacy in protecting against TNF⍺-mediated cell death in vitro. When injected near a mouse sciatic nerve, plasma samples showed that the curcumin-loaded ELP depot significantly decreased systemic release of curcumin compared to injections of curcumin alone. Tissue analysis showed that 48 hours after injection, greater concentrations of curcumin were detected near the sciatic nerve when injected with the ELP-curcumin gel compared to soluble curcumin, demonstrating its ability to extend curcumin release locally.[174]
Currently, no other studies have investigated ELPs as depots for drug or protein delivery for neural tissue engineering applications. Nevertheless, multiple studies have conjugated neural-relevant therapeutics onto ELP backbones. Shamji et al. published another study where ELPs were bacterially expressed conjugated to soluble TNF receptor Type II (sTNFRII) to attenuate TNF⍺ signaling. In vitro cultures of microglia, astrocytes, and DRG showed that the ELP therapeutic reversed the inflammatory effects of TNF⍺.[175] Hearst et al. expressed ELPs with the TRTK12 peptide, an antagonist to glial-secreted S100B.[176] S100B signaling increases intracerebral ROS and facilitates neurodegeneration.[177] The TRTK12-ELP therapeutic mitigated ROS-mediated damage to neurons and reduced cellular uptake of S100B.[176] In another study, Hearst et al. expressed ELPs conjugated to a protein kinase A (PKA) inhibitory polypeptide. This ELP therapeutic attenuated PKA-mediated phosphorylation of the ATXN1 gene, a toxic mechanism believed to facilitate neurodegeneration.[178] Lastly, Johnson and Koria bacterially expressed ELPs with either NGF or BDNF. These ELPs retained the bioactivity of the conjugated neurotrophins, shown by their ability to enhance neurite extensions from PC12 cells and induce phosphorylation of TrkB receptors, a pathway instrumental in neuronal survival.[179] All of the ELP therapeutics presented here could be modified to exhibit relevant (<37˚C) thermal transition temperatures, resulting in injectable hydrogels. Covalent crosslinks could also be functionalized onto the polypeptide backbone to slow ELP degradation rates and slow the release of conjugated or entrapped therapeutics. Table 3 summarizes significant findings from ELPs bacterially expressed with neural-relevant therapeutics.
Table 3.
Studies that have produced ELP constructs bearing neural-specific therapeutics.
| Guest Residue(s) | Conjugated molecule(s) a) | LCST [˚C] | Experiment(s) | Significant finding(s) |
|---|---|---|---|---|
|
| ||||
| V60 | sTNFRII | 32 | Astrocyte culture Microglia culture DRG culture |
Therapeutic mitigates cellular stresses induced by the inflammatory cytokine TNF⍺[175] |
| - | TRTK12 peptide, cpp | 39 – 42 | Neuronal culture Rat model |
Therapeutic reduces downstream ROS-mediated intercellular damage[176] |
| - | PKA inhibitory peptide, cpp | - | Neuronal culture Rat model |
Therapeutic reduces cytotoxic phosphorylation[178] |
| C2V40 | BDNF or NGF | <37 | PC12 cell culture | Therapeutics retain neurotrophin bioactivity and facilitate neurite outgrowth[179] |
cpp – cell-penetrating peptide.
4.3. ELP hydrogel composites
In efforts to synthesize an injectable hydrogel, Wang et al. produced an ELP-HA composite hydrogel where both components were crosslinked using hydrazone bonds.[71] The dynamic covalent chemistry of hydrazone bonding allowed the composite to crosslink ex situ and then self-heal after injection while undergoing thermal assembly. In vitro studies showed that this material significantly improved MSC survival and improved their potential to differentiate after injection with the ELP-HA composite compared to saline-only controls.[71]
While the use of ELP hydrogels for neural applications is not studied as well as fibrin and HA hydrogels, the initial studies presented here clearly demonstrate the potential of ELP hydrogels in fostering recovery after neural injury. ELPs are cheaply produced to provide bioactive interfaces such as cell adhesion motifs, enzyme specific degradation sequences, and short amino acid proteins. Some studies have begun to investigate the complexations of ELP with nucleic acids and conjugations to polysaccharides.[180,181] Additionally, ELP fiber production via electrospinning may eventually be integrated into ELP hydrogels to provide topographical cues similar to the aligned fibrillar fibrin hydrogels.[182–184] Furthermore, just as multiple crosslink mechanisms exist for HA hydrogels, unique crosslink methods have been proposed for ELP hydrogels, including catalyst-free click chemistry and vapor crosslinking[183,162,184]. Lastly, ELPs were investigated for intranasal drug delivery and may provide a means for sustained drug delivery to neural tissue via intranasal gelation.[178,185] Important findings using ELP hydrogels for neural applications are outlined in Table 4. For a more detailed review of ELPs and their broad biomedical applications, see Yeo et al. and Mbundi et al.[186–188]
Table 4.
Neural tissue engineering studies using elastin-like polypeptide hydrogels.
| Guest Residue(s) a) | Conjugated Molecule(s) b) | Supplement | LCST [˚C] | Modulus [kPa] f) | Experiment(s) | Significant finding(s) |
|---|---|---|---|---|---|---|
|
| ||||||
| K12I48 | RGD, adp, and upa motifs | - | 31 – 33 | 46 – 63 | PC12 culture | Hydrogel degradability, modulus, and cell adhesivity can be tuned independently of each other Hydrogel can enhance cell proliferation and neurite extension[165] |
| V120, V8A64G56 | - | - | 29, 78 | - | Rat model | Aggregated ELP improves perineural half-life and reduces systemic circulation[173] |
| K12I48 | RGD and adp motifs | - | 33 | 0.5 – 2.1 | DRG culture | Hydrogel properties can be tuned to alter rate, length, and density of neurite outgrowth[49] |
| V16I48E16 | - | Curcumin chemical conjugation | 29 | - | Toxicity assay Rat model |
Released curcumin attenuates TNF⍺ toxicity Injected depot sustains curcumin release and reduces systemic circulation[174] |
| K12I48 | RGD and adp motifs | NGF | 33 | 0.5 | DRG culture | There is a tradeoff between the chemotactic response and adhesivity of nerites[166] |
| K12I48 c) + HAd) | RGD and adp motifs | - | 26 | 1 | MSC culture | HA-ELP hydrogel protects cells during injection[71] |
| K12I48 | RGD and adp motifs | - | 33 | 0.5 – 50 | NPC culture | Hydrogel degradability is responsible for NPC stemness Degradation permits cell-cell contact which affects cell maintenance[167] |
| K12I48 | RGD and adp motifs | BMP-4 or FGF2 in media | 33 | 0.5 – 1.5 | NPC culture | Hydrogel degradability enhances neuronal and astrocytic differentiation potential[168] |
| M10V30 e) | - | IKVAV motif | 30 | 1.5 | Neuronal culture Mouse model |
Ligand-conjugated hydrogel enhances neurite extension Hydrogel elicits no inflammatory response and facilitates angiogenesis and innervation in vivo[170] |
| M4I8V8 | IKVAV motif | - | 23 | 0.9 – 1.6 | Neuronal culture Mouse model |
Ligand-integrated hydrogel enhances neurite extension Hydrogel elicits no inflammatory response[157] |
| K12I48 | RGD and upa motifs | - | 24 – 27 | 1.2 | OPC culture | Presence of upa motif improved OPC maturation[169] |
Subscripts indicate the number of pentapeptide repeats containing the respective amino acid
adp – ADAM9 protease degradable sequence; upa – urokinase plasminogen activator degradable sequence
hydrazine modified
aldehyde modified
alkene modified
storage (elastic) modulus.
5. Future Perspective
All three of the reviewed ECM-mimetic hydrogels have unique characteristics that provide bioactive interfaces appropriate for neural tissue engineering. Fibrin not only provides sites for protein and cell attachment, but also undergoes a simple gelation scheme that can conjugate synthetic molecules using the hemostatic enzyme Factor XIIIa. The recent development of aligned fibrillar fibrin hydrogels is particularly interesting as it provides 3D topographical guidance for regenerating axons without implementing other materials, and it has been shown to improve functional recovery in rat SCI models. This recovery is further improved by integrating MSCs or cell adhesive molecules into the injectable fibrin scaffolding. While many advancements have been made regarding fibrin, integration of nano and microparticles should be further investigated to better control therapeutic delivery of immunosuppressants and growth factors to improve the injury environment.
While HA does not present cell or protein-adhesive domains like fibrin, it is highly prevalent in neural tissue and influences NSPC migration via hyaladherins. For this reason, HA is frequently supplemented with laminins and other cell adhesive molecules and is implemented for stem cell transplants. HA hydrogels have also been more heavily investigated regarding nanoparticle and drug integration and have been more frequently implemented into stroke and ischemia models. However, electrospun fibers have not been incorporated into HA hydrogels for neural tissue engineering. HA composites composed of electrospun fibers should be further investigated since HA can already influence stem cell migration and aligned fibers can be used to guide migrating progenitor cells in SCI and PNI.
ELP hydrogels have not been implemented into as many in vivo studies as HA and fibrin hydrogels, but preliminary studies illustrate the impact that its independently tunable material properties may have in future studies. While the majority of ELP hydrogel research for neural engineering focuses on the integration of cell adhesive and enzyme-cleavable motifs, some studies have demonstrated the benefit of synthetically conjugating various protein therapeutics to the ELP backbone. Additional studies should conjugate new therapeutics and induce gel formation of these ELP-conjugated proteins to provide sustained release in the injury environment. ELP hydrogels may also benefit from the integration of other biopolymers, nanoparticles, and electrospun fibers.
All three ECM-mimetic components covered in this paper have already demonstrated success in clinical or pre-clinical models for various applications. A handful of fibrin glues/sealants have already been FDA approved for hemostatic applications[189,190] and many more clinical trials are expanding its application for additional surgical procedures and treatment of various diseases ([191]Other terms: fibrin). Similarly, numerous HA products have been FDA-approved for dermal injections in cosmetic applications and for intraarticular injections to treat arthritic pain.[192] While there are currently no FDA-approved ELP products, ELP therapeutics are actively being investigated clinically regarding treatment of cancers, diabetes, and heart disease.[193,194] However, fibrin, HA, and ELP biomaterials have not been FDA-approved for use in neural tissue engineering applications.
ECM-mimetic hydrogel research must continue to evolve, as complete functional recovery after neural tissue injury has yet to be achieved by any biomaterial. The advantages and disadvantages of each of the three hydrogels covered in this paper are compared in Table 5. Another rapidly evolving hydrogel research topic is the use of decellularized matrix/tissue, which contains many types of ECM components including collagens, proteoglycans and laminins.[195,196] Recently, decellularized tissue hydrogels have been shown to improve functional outcomes in rat PNI and SCI models.[197–199] The integration of this material into the hydrogels reviewed in this paper may produce a matrix with even greater regenerative properties and may move the research community a step closer to achieving complete functional recover after neural tissue injury.
Table 5.
Advantages and disadvantages of using fibrin, HA, and ELP hydrogels for neural regeneration applications.
| Hydrogel Material | Advantages | Disadvantages |
|---|---|---|
| Fibrin | Simple gelation scheme that requires the batch combination of fibrinogen and hemostatic enzymes Has innate bioactive entities including multiple integrin receptors and an RGD domain Provides sites for protein attachment, including fibronectin, tPA, and TGFβ |
Recruits an inflammatory response which can influence glial scarring and impact neuronal regeneration Often requires extensive purification to remove potential prions Sourced from Mammalia or salmon plasma |
| Hyaluronic acid | Abundant in the neural ECM and involved in many cellular processes including regeneration and angiogenesis Improves biological activity via non-integrin receptors such as CD44, RHAMM, TLR-2, and TLR-4 |
Often requires chemical modifications to provide sites for crosslinking Often requires supplementation to enhance growth factor retention and cell-adhesion Expensive and polydisperse |
| Elastin-like polypeptide | Provides a dynamic polymer with independently tunable parameters Can be produced bearing many bioactive polypeptide motifs Can be produced with enzyme-cleavable therapeutics Provides a facile gelation mechanism via a tunable LCST Cheaply produced with minimal polydispersity and polymorphism |
Most often expressed with degradation motifs since elastase is not highly prevalent in neural tissue |
6. Conclusion
While many biomaterials have the potential to aid in the regeneration of neural tissue, hydrogels provide 3D environments that better mimic the neural niche and can uniquely fill amorphous injury sites. They can be used to address general tissue engineering facets such as the ability to sequester cytotoxic chemicals and sustain local drug delivery, as well as address neural tissue-specific aspects such as intraluminal fillings for nerve guidance conduits and fiber integration to guide neurite extension. Hydrogels constructed from ECM-mimetic biopolymers are promising as they can not only address these same facets, but also degrade naturally, do not require or produce cytotoxic components, and often maintain the bioactivity of imbibed proteins. Fibrin and HA hydrogels were used frequently over the past few decades within experimental in vivo studies regarding neural injury. ELP hydrogels have demonstrated initial feasibility for neural tissue engineering applications as they provide greater control over various hydrogel properties and functionalities than is obtainable from other hydrogel designs. For this reason, it’s expected that ELP hydrogels will soon prove themselves to be as useful, if not more, as the well-developed fibrin and HA hydrogels. Whether they are solely functionalized with cell adhesive components, used as drug depots, or complexed with nanoparticles or fibers, ECM-mimetic biopolymer hydrogels will be further developed and investigated. Findings from these future studies will likely yield constructs that may significantly benefit those with neural injury.
Table 6.
List of acronyms and their definitions.
| Acronym | Definition |
|---|---|
|
| |
| AFG | Aligned fibrillar fibrin |
| BBB | Blood brain barrier |
| BBB score | Basso−Beattie−Bresnahan score |
| BDNF | Brain-derived neurotrophic factor |
| bFGF | Basic fibroblast growth factor |
| BMP-4 | Bone morphogenic protein 4 |
| chABC | Chondroitinase ABC |
| CNS | Central nervous system |
| CSPGs | Chondroitin sulfate proteoglycans |
| DOE | Design of experiments |
| DRG | Dorsal root ganglia |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ELP | Elastin-like polypeptide |
| GAG | Glycosaminoglycan |
| HA | Hyaluronic acid |
| HS | Heparin sulfate |
| LCST | Lower critical solution temperature |
| MAI | Myelin-associated inhibitor |
| MC | Methylcellulose |
| MMP | Matrix-metalloproteinase |
| MSC | Mesenchymal stem cell |
| MWCNT | Multi-wall carbon nanotube |
| NGF | Nerve growth factor |
| NPC | Neural progenitor cell |
| NSC | Neural stem cell |
| NSPC | Neural stem/progenitor cell |
| NT3 | Neurotrophin-3 |
| OPC | Oligodendrocyte precursor cell |
| PFTBA | Perfluorotributylamine |
| PKA | Protein kinase A |
| PLGA | Poly lactic-co-glycolic acid |
| PLL | Poly-L-lysine |
| PNI | Peripheral nerve injury |
| PNS | Peripheral nervous system |
| PSA | Poly(sebacic acid) |
| PU | Polyurethane |
| PVA | Poly(vinyl alcohol) |
| RHAMM | Receptor for HA-mediated motility |
| ROS | Reactive oxygen species |
| SCI | Spinal cord injury |
| SDF-1⍺ | Stromal cell-derived factor-1⍺ |
| SPION | Superparamagnetic iron oxide nanoparticle |
| sTNFRII | Soluble TNF receptor Type II |
| TBI | Traumatic brain injury |
| TLR | Toll-like receptor |
| TNF⍺ | Tumor necrosis factor ⍺ |
| upa | Urokinase plasminogen activator |
| VEGF | Vascular endothelial growth factor |
Acknowledgements
Funding for this work was provided by National Institutes of Health (NIH) R01 Grant (#NS092754), New York State Spinal Cord Injury Research Board (NYSCIRB) Institutional Support Grant (#C32245GG), the Veterans Affairs (I01 RX003502-01A1) awarded to R.J.G., and NIH T32 Grant (#AG057464) supporting D.W.N.
Author Biographies

Derek Nelson graduated from the University of New Mexico in 2019 with a B.S. in chemical and biological engineering and is pursuing his PhD in biomedical engineering at Rensselaer Polytechnic Institute. His previous research at Sandia National Laboratories encompassed smart self-assembling materials, thin film fabrication, and photovoltaics. His current research focuses on developing biomaterials for modulating the release of novel therapeutics to treat neural tissue injury and degeneration. This includes the fabrication of aligned electrospun fibers and elastin-like polypeptide thermo-gels.

Ryan Gilbert is a professor of biomedical engineering at Rensselaer Polytechnic Institute and a research scientist at the Albany Stratton Veterans Affairs medical center. He received his B.S.E. in chemical engineering at the University of Michigan – Ann Arbor, and received his PhD in biomedical engineering from Case Western Reserve University. Dr. Gilbert and members of his laboratory pursue research in areas of nervous system injury and biomaterial synthesis and characterization for neural applications. Dr. Gilbert is a standing member and ad hoc reviewer for several federal/international grant funding agencies. He is currently editor-in-chief of the journal Cells Tissues Organs.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].National Spinal Cord Injury Statistical Center, “Facts and Figures at a Glance,” can be found under https://www.nscisc.uab.edu/, 2020.
- [2].Traumatic Brain Injury Model Systems National Data and Statistical Center, “The Traumatic Brain Injury Model Systems,” can be found under https://www.tbindsc.org/, 2017.
- [3].Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG, Nature Reviews Disease Primers 2017, 3, 1. [DOI] [PubMed] [Google Scholar]
- [4].Cruz-Haces M, Tang J, Acosta G, Fernandez J, Shi R, Transl Neurodegener 2017, 6, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Amanat M, Vaccaro AR, Salehi M, Rahimi-Movaghar V, Informatics in Medicine Unlocked 2019, 16, 100245. [Google Scholar]
- [6].“Stroke Facts | cdc.gov,” can be found under https://www.cdc.gov/stroke/facts.htm, 2020.
- [7].Virani Salim S, Alonso Alvaro, Benjamin Emelia J, Bittencourt Marcio S, Callaway Clifton W, Carson April P, Chamberlain Alanna M, Chang Alexander R, Cheng Susan, Delling Francesca N, et al. , Circulation 2020, 141, e139. [DOI] [PubMed] [Google Scholar]
- [8].Wojtkiewicz DM, Saunders J, Domeshek L, Novak CB, Kaskutas V, Mackinnon SE, Hand (N Y) 2015, 10, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alvites R, Rita Caseiro A, Santos Pedrosa S, Vieira Branquinho M, Ronchi G, Geuna S, Varejão ASP, Colette Maurício A, Cogent Medicine 2018, 5, DOI 10.1080/2331205X.2018.1466404. [DOI] [Google Scholar]
- [10].Carvalho CR, Oliveira JM, Reis RL, Front. Bioeng. Biotechnol. 2019, 7, DOI 10.3389/fbioe.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J, Experimental Neurology 2014, 253, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yan EB, Hellewell SC, Bellander B-M, Agyapomaa DA, Morganti-Kossmann MC, J Neuroinflammation 2011, 8, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yan EB, Satgunaseelan L, Paul E, Bye N, Nguyen P, Agyapomaa D, Kossmann T, Rosenfeld JV, Morganti-Kossmann MC, J Neurotrauma 2014, 31, 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hernandez-Gerez E, Fleming IN, Parson SH, Cell Death Dis 2019, 10, DOI 10.1038/s41419-019-2104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kaur P, Sharma S, Curr Neuropharmacol 2018, 16, 1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bradbury EJ, Burnside ER, Nat Commun 2019, 10, 3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kong X, Gao J, J Cell Mol Med 2017, 21, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Karve IP, Taylor JM, Crack PJ, British Journal of Pharmacology 2016, 173, 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, et al. , Nature 2017, 541, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jones LL, Margolis RU, Tuszynski MH, Experimental Neurology 2003, 182, 399. [DOI] [PubMed] [Google Scholar]
- [21].Chen Y, Qin C, Huang J, Tang X, Liu C, Huang K, Xu J, Guo G, Tong A, Zhou L, Cell Prolif 2020, 53, DOI 10.1111/cpr.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sofroniew MV, Nat Rev Neurosci 2015, 16, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV, Nature 2016, 532, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liddelow SA, Barres BA, Immunity 2017, 46, 957. [DOI] [PubMed] [Google Scholar]
- [25].Kim E, Cho S, Experimental Neurology 2021, 335, 113508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Menorca RMG, Fussell TS, Elfar JC, Hand Clin 2013, 29, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nocera G, Jacob C, Cell. Mol. Life Sci. 2020, DOI 10.1007/s00018-020-03516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Belanger K, Dinis TM, Taourirt S, Vidal G, Kaplan DL, Egles C, Macromolecular Bioscience 2016, 16, 472. [DOI] [PubMed] [Google Scholar]
- [29].Jessen KR, Mirsky R, Front Cell Neurosci 2019, 13, DOI 10.3389/fncel.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].SUNDERLAND S, Brain 1951, 74, 491. [DOI] [PubMed] [Google Scholar]
- [31].Du J, Chen H, Qing L, Yang X, Jia X, Biomater. Sci. 2018, 6, 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kornfeld T, Vogt PM, Radtke C, Wien Med Wochenschr 2019, 169, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zigmond RE, Echevarria FD, Progress in Neurobiology 2019, 173, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ziemba AM, Gilbert RJ, Front. Pharmacol. 2017, 8, DOI 10.3389/fphar.2017.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kornev VA, Grebenik EA, Solovieva AB, Dmitriev RI, Timashev PS, Computational and Structural Biotechnology Journal 2018, 16, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zuidema JM, Gilbert RJ, Gottipati MK, Cells Tissues Organs 2018, 205, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Puhl DL, D’Amato AR, Gilbert RJ, Brain Research Bulletin 2019, 150, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Funnell JL, Balouch B, Gilbert RJ, Front Bioeng Biotechnol 2019, 7, DOI 10.3389/fbioe.2019.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Madhusudanan P, Raju G, Shankarappa S, J. R. Soc. Interface 2020, 17, 20190505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Puhl DL, Funnell JL, Nelson DW, Gottipati MK, Gilbert RJ, Bioengineering 2021, 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Daly W, Yao L, Zeugolis D, Windebank A, Pandit A, Journal of The Royal Society Interface 2012, 9, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Budday S, Nay R, de Rooij R, Steinmann P, Wyrobek T, Ovaert TC, Kuhl E, J Mech Behav Biomed Mater 2015, 46, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Spedden E, White JD, Naumova EN, Kaplan DL, Staii C, Biophys J 2012, 103, 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lu Y-B, Franze K, Seifert G, Steinhäuser C, Kirchhoff F, Wolburg H, Guck J, Janmey P, Wei E-Q, Käs J, et al. , PNAS 2006, 103, 17759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rosso G, Guck J, APL Bioengineering 2019, 3, 036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wu S, Xu R, Duan B, Jiang P, J. Mater. Chem. B 2017, 5, 3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tarus D, Hamard L, Caraguel F, Wion D, Szarpak-Jankowska A, van der Sanden B, Auzély-Velty R, ACS Appl. Mater. Interfaces 2016, 8, 25051. [DOI] [PubMed] [Google Scholar]
- [48].Lam J, Carmichael ST, Lowry WE, Segura T, Advanced Healthcare Materials 2015, 4, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lampe KJ, Antaris AL, Heilshorn SC, Acta Biomaterialia 2013, 9, 5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Murphy NP, Lampe KJ, J. Mater. Chem. B 2015, 3, 7867. [DOI] [PubMed] [Google Scholar]
- [51].Hwang J, Sullivan MO, Kiick KL, Front. Bioeng. Biotechnol. 2020, 8, DOI 10.3389/fbioe.2020.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ribeiro A, Vargo S, Powell EM, Leach JB, Tissue Engineering Part A 2011, 18, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Golanov EV, Sharpe MA, Regnier-Golanov AS, Del Zoppo GJ, Baskin DS, Britz GW, Front. Neurosci. 2019, 13, DOI 10.3389/fnins.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Norris EH, Strickland S, Neuron 2017, 96, 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE, J. Neural Eng. 2011, 8, 046033. [DOI] [PubMed] [Google Scholar]
- [56].Neumann C, Yu A, Welge-Lüssen U, Lütjen-Drecoll E, Birke M, Invest. Ophthalmol. Vis. Sci. 2008, 49, 1464. [DOI] [PubMed] [Google Scholar]
- [57].Tassler PL, Dellon AL, Canoun C, The Journal of Hand Surgery: British & European Volume 1994, 19, 48. [DOI] [PubMed] [Google Scholar]
- [58].Boni R, Ali A, Shavandi A, Clarkson AN, J Biomed Sci 2018, 25, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gregorio I, Braghetta P, Bonaldo P, Cescon M, Dis. Model. Mech. 2018, 11, dmm032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ring C, Hassell J, Halfter W, Developmental Biology 1996, 180, 41. [DOI] [PubMed] [Google Scholar]
- [61].Hubert T, Grimal S, Carroll P, Fichard-Carroll A, Cell. Mol. Life Sci. 2009, 66, 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Koopmans G, Hasse B, Sinis N, in International Review of Neurobiology, Academic Press, 2009, pp. 363–379. [DOI] [PubMed] [Google Scholar]
- [63].Neo S, Tang B, Neural Regen Res 2017, 12, 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y, et al. , Nat Med 2017, 23, 818. [DOI] [PubMed] [Google Scholar]
- [65].Koutsopoulos S, Zhang S, Acta Biomaterialia 2013, 9, 5162. [DOI] [PubMed] [Google Scholar]
- [66].Chernousov MA, Stahl RC, Carey DJ, J. Neurosci. 2001, 21, 6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Joosten EAJ, Dijkstra S, Brook GA, Veldman H, Bär PR, Journal of Neuroscience Research 2000, 62, 686. [DOI] [PubMed] [Google Scholar]
- [68].Wilems T, Vardhan S, Wu S, Sakiyama-Elbert S, Brain Research Bulletin 2019, 148, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Houlton J, Abumaria N, Hinkley SFR, Clarkson AN, Front. Neurosci. 2019, 13, DOI 10.3389/fnins.2019.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zarei-Kheirabadi M, Sadrosadat H, Mohammadshirazi A, Jaberi R, Sorouri F, Khayyatan F, Kiani S, International Journal of Biological Macromolecules 2020, 148, 1118. [DOI] [PubMed] [Google Scholar]
- [71].Wang H, Zhu D, Paul A, Cai L, Enejder A, Yang F, Heilshorn SC, Advanced Functional Materials 2017, 27, 1605609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McMurtrey RJ, J. Neural Eng. 2014, 11, 066009. [DOI] [PubMed] [Google Scholar]
- [73].Li L, Xiao B, Mu J, Zhang Y, Zhang C, Cao H, Chen R, Patra HK, Yang B, Feng S, et al. , ACS Nano 2019, 13, 14283. [DOI] [PubMed] [Google Scholar]
- [74].Mosesson MW, Journal of Thrombosis and Haemostasis 2005, 3, 1894. [DOI] [PubMed] [Google Scholar]
- [75].Mosesson MW, Doolittle RF, Science 1984, 224, 52.17783519 [Google Scholar]
- [76].Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É, Physiological Reviews 2011, 91, 931. [DOI] [PubMed] [Google Scholar]
- [77].Hirashima M, Imamura T, Yano K, Kawamura R, Meta A, Tokieda Y, Nakashima T, J Biochem 2016, 159, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Arulmoli J, Wright HJ, Phan DTT, Sheth U, Que RA, Botten GA, Keating M, Botvinick EL, Pathak MM, Zarembinski TI, et al. , Acta Biomaterialia 2016, 43, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sharp KG, Dickson AR, Marchenko SA, Yee KM, Emery PN, Laidmåe I, Uibo R, Sawyer ES, Steward O, Flanagan LA, Experimental Neurology 2012, 235, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mooney R, Tawil B, Mahoney M, Tissue Engineering Part A 2010, 16, 1607. [DOI] [PubMed] [Google Scholar]
- [81].Willerth SM, Faxel TE, Gottlieb DI, Sakiyama-Elbert SE, STEM CELLS 2007, 25, 2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Asmani MN, Ai J, Amoabediny G, Noroozi A, Azami M, Ebrahimi-Barough S, Navaei-Nigjeh M, Ai A, Jafarabadi M, Cell Biology International 2013, 37, 1340. [DOI] [PubMed] [Google Scholar]
- [83].Man AJ, Davis HE, Itoh A, Leach JK, Bannerman P, Tissue Engineering Part A 2011, 17, 2931. [DOI] [PubMed] [Google Scholar]
- [84].Yao S, Liu X, Yu S, Wang X, Zhang S, Wu Q, Sun X, Mao H, Nanoscale 2016, 8, 10252. [DOI] [PubMed] [Google Scholar]
- [85].Yao S, He F, Cao Z, Sun Z, Chen Y, Zhao H, Yu X, Wang X, Yang Y, Rosei F, et al. , ACS Biomater. Sci. Eng. 2020, 6, 1165. [DOI] [PubMed] [Google Scholar]
- [86].Du J, Liu J, Yao S, Mao H, Peng J, Sun X, Cao Z, Yang Y, Xiao B, Wang Y, et al. , Acta Biomaterialia 2017, 55, 296. [DOI] [PubMed] [Google Scholar]
- [87].Basso DM, Beattie MS, Bresnahan JC, Experimental Neurology 1996, 139, 244. [DOI] [PubMed] [Google Scholar]
- [88].Liu J, Chen Q, Zhang Z, Zheng Y, Sun X, Cao X, Gong A, Cui Y, He Q, Jiang P, Cells Tissues Organs 2013, 198, 35. [DOI] [PubMed] [Google Scholar]
- [89].King VR, Alovskaya A, Wei DYT, Brown RA, Priestley JV, Biomaterials 2010, 31, 4447. [DOI] [PubMed] [Google Scholar]
- [90].Silva J, Bento AR, Barros D, Laundos TL, Sousa SR, Quelhas P, Sousa MM, Pêgo AP, Amaral IF, Acta Biomaterialia 2017, 59, 243. [DOI] [PubMed] [Google Scholar]
- [91].Sakiyama-Elbert SE, Hubbell JA, Journal of Controlled Release 2000, 69, 149. [DOI] [PubMed] [Google Scholar]
- [92].Bhang SH, Jeon O, Choi CY, Kwon YHK, Kim B-S, Journal of Biomedical Materials Research Part A 2007, 80A, 998. [DOI] [PubMed] [Google Scholar]
- [93].Lee Y-B, Polio S, Lee W, Dai G, Menon L, Carroll RS, Yoo S-S, Experimental Neurology 2010, 223, 645. [DOI] [PubMed] [Google Scholar]
- [94].Willerth SM, Johnson PJ, Maxwell DJ, Parsons SR, Doukas ME, Sakiyama-Elbert SE, Journal of Biomedical Materials Research Part A 2007, 80A, 13. [DOI] [PubMed] [Google Scholar]
- [95].Clark AR, Carter AB, Hager LE, Price EM, Stem Cells and Development 2016, 25, 1109. [DOI] [PubMed] [Google Scholar]
- [96].Hyatt AJT, Wang D, Kwok JC, Fawcett JW, Martin KR, Journal of Controlled Release 2010, 147, 24. [DOI] [PubMed] [Google Scholar]
- [97].Ma T, Wang Y, Qi F, Zhu S, Huang L, Liu Z, Huang J, Luo Z, Biomaterials 2013, 34, 10016. [DOI] [PubMed] [Google Scholar]
- [98].Tajdaran K, Shoichet MS, Gordon T, Borschel GH, Biotechnology and Bioengineering 2015, 112, 1948. [DOI] [PubMed] [Google Scholar]
- [99].Tajdaran K, Chan K, Shoichet MS, Gordon T, Borschel GH, Acta Biomaterialia 2019, 96, 211. [DOI] [PubMed] [Google Scholar]
- [100].Wilems TS, Sakiyama-Elbert SE, Journal of Controlled Release 2015, 213, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Omidinia-Anarkoli A, Boesveld S, Tuvshindorj U, Rose JC, Haraszti T, Laporte LD, Small 2017, 13, 1702207. [DOI] [PubMed] [Google Scholar]
- [102].Johnson CDL, Ganguly D, Zuidema JM, Cardinal TJ, Ziemba AM, Kearns KR, McCarthy SM, Thompson DM, Ramanath G, Borca-Tasciuc DA, et al. , ACS Appl. Mater. Interfaces 2019, 11, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hasanzadeh E, Ebrahimi-Barough S, Mirzaei E, Azami M, Tavangar SM, Mahmoodi N, Basiri A, Ai J, Journal of Biomedical Materials Research Part A 2019, 107, 802. [DOI] [PubMed] [Google Scholar]
- [104].Ryu JK, Rafalski VA, Meyer-Franke A, Adams RA, Poda SB, Rios Coronado PE, Pedersen LØ, Menon V, Baeten KM, Sikorski SL, et al. , Nat Immunol 2018, 19, 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Petersen MA, Ryu JK, Akassoglou K, Nat Rev Neurosci 2018, 19, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Baier C, Baader SL, Jankowski J, Gieselmann V, Schilling K, Rauch U, Kappler J, Matrix Biology 2007, 26, 348. [DOI] [PubMed] [Google Scholar]
- [107].Lindwall C, Olsson M, Osman AM, Kuhn HG, Curtis MA, Brain Research 2013, 1503, 62. [DOI] [PubMed] [Google Scholar]
- [108].Khaing ZZ, Seidlits SK, J. Mater. Chem. B 2015, 3, 7850. [DOI] [PubMed] [Google Scholar]
- [109].Liu Y, Han SSW, Wu Y, Tuohy TMF, Xue H, Cai J, Back SA, Sherman LS, Fischer I, Rao MS, Developmental Biology 2004, 276, 31. [DOI] [PubMed] [Google Scholar]
- [110].Nagy J, Hacking J, Frankenstein U, Turley E, J. Neurosci. 1995, 15, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Back SA, Tuohy TMF, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, et al. , Nat Med 2005, 11, 966. [DOI] [PubMed] [Google Scholar]
- [112].Bourguignon LYW, Gilad E, Peyrollier K, Brightman A, Swanson RA, Journal of Neurochemistry 2007, 101, 1002. [DOI] [PubMed] [Google Scholar]
- [113].Turley EA, Noble PW, Bourguignon LYW, Journal of Biological Chemistry 2002, 277, 4589. [DOI] [PubMed] [Google Scholar]
- [114].Noble PW, Matrix Biology 2002, 21, 25. [DOI] [PubMed] [Google Scholar]
- [115].Shu XZ, Liu Y, Palumbo F, Prestwich GD, Biomaterials 2003, 24, 3825. [DOI] [PubMed] [Google Scholar]
- [116].Xu SA,Q, McMichael P, Gao Y, Li X, Wang X, Greiser U, Zhou D, Wang W, Chem. Commun. 2018, 54, 1081. [DOI] [PubMed] [Google Scholar]
- [117].Nimmo CM, Owen SC, Shoichet MS, Biomacromolecules 2011, 12, 824. [DOI] [PubMed] [Google Scholar]
- [118].Spearman BS, Agrawal NK, Rubiano A, Simmons CS, Mobini S, Schmidt CE, Journal of Biomedical Materials Research Part A 2020, 108, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Liu L, Liu Y, Li J, Du G, Chen J, Microb Cell Fact 2011, 10, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Boeriu CG, Springer J, Kooy FK, van den Broek LAM, Eggink G, International Journal of Carbohydrate Chemistry 2013, 2013, 1. [Google Scholar]
- [121].Addington CP, Heffernan JM, Millar-Haskell CS, Tucker EW, Sirianni RW, Stabenfeldt SE, Biomaterials 2015, 72, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Addington CP, Dharmawaj S, Heffernan JM, Sirianni RW, Stabenfeldt SE, Matrix Biology 2017, 60–61, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zaviskova K, Tukmachev D, Dubisova J, Vackova I, Hejcl A, Bystronova J, Pravda M, Scigalkova I, Sulakova R, Velebny V, et al. , Journal of Biomedical Materials Research Part A 2018, 106, 1129. [DOI] [PubMed] [Google Scholar]
- [124].Adil MM, Vazin T, Ananthanarayanan B, Rodrigues GMC, Rao AT, Kulkarni RU, Miller EW, Kumar S, Schaffer DV, Biomaterials 2017, 136, 1. [DOI] [PubMed] [Google Scholar]
- [125].Nih LR, Sideris E, Carmichael ST, Segura T, Advanced Materials 2017, 29, 1606471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Li L-M, Han M, Jiang X-C, Yin X-Z, Chen F, Zhang T-Y, Ren H, Zhang J-W, Hou T-J, Chen Z, et al. , ACS Appl. Mater. Interfaces 2017, 9, 3330. [DOI] [PubMed] [Google Scholar]
- [127].Nakaji-Hirabayashi T, Kato K, Iwata H, Biomaterials 2009, 30, 4581. [DOI] [PubMed] [Google Scholar]
- [128].Moshayedi P, Nih LR, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE, Segura T, Carmichael ST, Biomaterials 2016, 105, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Fournier AE, GrandPre T, Strittmatter SM, Nature 2001, 409, 341. [DOI] [PubMed] [Google Scholar]
- [130].GrandPré T, Li S, Strittmatter SM, Nature 2002, 417, 547. [DOI] [PubMed] [Google Scholar]
- [131].Tian WM, Zhang CL, Hou SP, Yu X, Cui FZ, Xu QY, Sheng SL, Cui H, Li HD, Journal of Controlled Release 2005, 102, 13. [DOI] [PubMed] [Google Scholar]
- [132].Ma J, Tian W-M, Hou S-P, Xu Q-Y, Spector M, Cui F-Z, Biomed. Mater. 2007, 2, 233. [DOI] [PubMed] [Google Scholar]
- [133].Wei Y-T, He Y, Xu C-L, Wang Y, Liu B-F, Wang X-M, Sun X-D, Cui F-Z, Xu Q-Y, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2010, 95B, 110. [DOI] [PubMed] [Google Scholar]
- [134].Agrawal NK, Allen P, Song YH, Wachs RA, Du Y, Ellington AD, Schmidt CE, Acta Biomaterialia 2020, 102, 315. [DOI] [PubMed] [Google Scholar]
- [135].Shi W, Hass B, Kuss MA, Zhang H, Ryu S, Zhang D, Li T, Li Y, Duan B, Carbohydrate Polymers 2020, 233, 115803. [DOI] [PubMed] [Google Scholar]
- [136].Geissler SA, Sabin AL, Besser RR, Gooden OM, Shirk BD, Nguyen QM, Khaing ZZ, Schmidt CE, J. Neural Eng. 2018, 15, 025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Cook DJ, Nguyen C, Chun HN, L Llorente I, Chiu AS, Machnicki M, Zarembinski TI, Carmichael ST, J Cereb Blood Flow Metab 2017, 37, 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Zhang L, Chen Y, Xu H, Bao Y, Yan X, Li Y, Li Y, Yin Y, Wang X, Qiu T, et al. , J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2016, 31, 1401. [Google Scholar]
- [139].Xu H, Zhang L, Bao Y, Yan X, Yin Y, Li Y, Wang X, Huang Z, Xu P, Journal of Bioactive and Compatible Polymers 2017, 32, 146. [Google Scholar]
- [140].Wang Y, Wei YT, Zu ZH, Ju RK, Guo MY, Wang XM, Xu QY, Cui FZ, Pharm Res 2011, 28, 1406. [DOI] [PubMed] [Google Scholar]
- [141].Führmann T, Obermeyer J, Tator CH, Shoichet MS, Methods 2015, 84, 60. [DOI] [PubMed] [Google Scholar]
- [142].Ju R, Wen Y, Gou R, Wang Y, Xu Q, Cell Transplant 2014, 23, 83. [DOI] [PubMed] [Google Scholar]
- [143].Gupta D, Tator CH, Shoichet MS, Biomaterials 2006, 27, 2370. [DOI] [PubMed] [Google Scholar]
- [144].Ho MT, Teal CJ, Shoichet MS, Brain Research Bulletin 2019, 148, 46. [DOI] [PubMed] [Google Scholar]
- [145].Wang Y, Cooke MJ, Sachewsky N, Morshead CM, Shoichet MS, Journal of Controlled Release 2013, 172, 1. [DOI] [PubMed] [Google Scholar]
- [146].Caicco MJ, Cooke MJ, Wang Y, Tuladhar A, Morshead CM, Shoichet MS, Journal of Controlled Release 2013, 166, 197. [DOI] [PubMed] [Google Scholar]
- [147].Tuladhar A, Obermeyer JM, Payne SL, Siu RCW, Zand S, Morshead CM, Shoichet MS, Biomaterials 2020, 235, 119794. [DOI] [PubMed] [Google Scholar]
- [148].Obermeyer JM, Tuladhar A, Payne SL, Ho E, Morshead CM, Shoichet MS, Tissue Engineering Part A 2019, 25, 1175. [DOI] [PubMed] [Google Scholar]
- [149].Stanwick JC, Baumann MD, Shoichet MS, International Journal of Pharmaceutics 2012, 426, 284. [DOI] [PubMed] [Google Scholar]
- [150].Stanwick JC, Baumann MD, Shoichet MS, Journal of Controlled Release 2012, 10. [DOI] [PubMed] [Google Scholar]
- [151].Jian W-H, Wang H-C, Kuan C-H, Chen M-H, Wu H-C, Sun J-S, Wang T-W, Biomaterials 2018, 174, 17. [DOI] [PubMed] [Google Scholar]
- [152].Shin J, Choi EJ, Cho JH, Cho A-N, Jin Y, Yang K, Song C, Cho S-W, Biomacromolecules 2017, 18, 3060. [DOI] [PubMed] [Google Scholar]
- [153].Tay A, Sohrabi A, Poole K, Seidlits S, Carlo DD, Advanced Materials 2018, 30, 1800927. [DOI] [PubMed] [Google Scholar]
- [154].MacEwan SR, Chilkoti A, Journal of Controlled Release 2014, 190, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].MacEwan SR, Hassouneh W, Chilkoti A, J Vis Exp 2014, DOI 10.3791/51583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Meyer DE, Chilkoti A, Nat Biotechnol 1999, 17, 1112. [DOI] [PubMed] [Google Scholar]
- [157].Paiva dos Santos B, Garbay B, Pasqua M, Chevron E, Chinoy ZS, Cullin C, Bathany K, Lecommandoux S, Amédée J, Oliveira H, et al. , Journal of Biotechnology 2019, 298, 35. [DOI] [PubMed] [Google Scholar]
- [158].Urry DW, Parker TM, Reid MC, Gowda DC, Journal of Bioactive and Compatible Polymers 1991, 6, 263. [Google Scholar]
- [159].Rincón AC, Molina-Martinez IT, de las Heras B, Alonso M, Baílez C, Rodríguez-Cabello JC, Herrero-Vanrell R, Journal of Biomedical Materials Research Part A 2006, 78A, 343. [DOI] [PubMed] [Google Scholar]
- [160].Ibáñez-Fonseca A, Ramos TL, de Torre IG, Sánchez-Abarca LI, Muntión S, Arias FJ, del Cañizo MC, Alonso M, Sánchez-Guijo F, Rodríguez-Cabello JC, Journal of Tissue Engineering and Regenerative Medicine 2018, 12, e1450. [DOI] [PubMed] [Google Scholar]
- [161].Asai D, Xu D, Liu W, Garcia Quiroz F, Callahan DJ, Zalutsky MR, Craig SL, Chilkoti A, Biomaterials 2012, 33, 5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].González de Torre I, Santos M, Quintanilla L, Testera A, Alonso M, Rodríguez Cabello JC, Acta Biomaterialia 2014, 10, 2495. [DOI] [PubMed] [Google Scholar]
- [163].Lim DW, Nettles DL, Setton LA, Chilkoti A, Biomacromolecules 2007, 8, 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Nagapudi K, Brinkman WT, Leisen JE, Huang L, McMillan RA, Apkarian RP, Conticello VP, Chaikof EL, Macromolecules 2002, 35, 1730. [Google Scholar]
- [165].Straley KS, Heilshorn SC, Soft Matter 2008, 5, 114. [Google Scholar]
- [166].Romano NH, Lampe KJ, Xu H, Ferreira MM, Heilshorn SC, Small 2015, 11, 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Madl CM, LeSavage BL, Dewi RE, Dinh CB, Stowers RS, Khariton M, Lampe KJ, Nguyen D, Chaudhuri O, Enejder A, et al. , Nature Mater 2017, 16, 1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Madl CM, LeSavage BL, Dewi RE, Lampe KJ, Heilshorn SC, Advanced Science 2019, 6, 1801716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Meco E, Zheng WS, Sharma AH, Lampe KJ, Biomacromolecules 2020, 21, 4724. [DOI] [PubMed] [Google Scholar]
- [170].Paiva dos Santos B, Garbay B, Fenelon M, Rosselin M, Garanger E, Lecommandoux S, Oliveira H, Amédée J, Acta Biomaterialia 2019, 99, 154. [DOI] [PubMed] [Google Scholar]
- [171].Ow S-Y, Dunstan DE, Protein Sci 2014, 23, 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ma C, Su J, Sun Y, Feng Y, Shen N, Li B, Liang Y, Yang X, Wu H, Zhang H, et al. , Angewandte Chemie International Edition 2019, 58, 18703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Shamji MF, Whitlatch L, Friedman AH, Richardson WJ, Chilkoti A, Setton LA, Spine (Phila Pa 1976) 2008, 33, 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Sinclair SM, Bhattacharyya J, McDaniel JR, Gooden DM, Gopalaswamy R, Chilkoti A, Setton LA, Journal of Controlled Release 2013, 171, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Shamji MF, Jing L, Chen J, Hwang P, Ghodsizadeh O, Friedman AH, Richardson WJ, Setton LA, Journal of Neurosurgery: Spine 2008, 9, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Hearst SM, Walker LR, Shao Q, Lopez M, Raucher D, Vig PJS, Neuroscience 2011, 197, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H, Journal of Biological Chemistry 2000, 275, 40096. [DOI] [PubMed] [Google Scholar]
- [178].Hearst SM, Shao Q, Lopez M, Raucher D, Vig PJS, Journal of Neurochemistry 2014, 131, 101. [DOI] [PubMed] [Google Scholar]
- [179].Johnson T, Koria P, BioDrugs 2016, 30, 117. [DOI] [PubMed] [Google Scholar]
- [180].Bravo-Anaya LM, Garbay B, Nando-Rodríguez JLE, Carvajal Ramos F, Ibarboure E, Bathany K, Xia Y, Rosselgong J, Joucla G, Garanger E, et al. , Journal of Colloid and Interface Science 2019, 557, 777. [DOI] [PubMed] [Google Scholar]
- [181].Xiao Y, Chinoy ZS, Pecastaings G, Bathany K, Garanger E, Lecommandoux S, Biomacromolecules 2020, 21, 114. [DOI] [PubMed] [Google Scholar]
- [182].Huang L, McMillan RA, Apkarian RP, Pourdeyhimi B, Conticello VP, Chaikof EL, Macromolecules 2000, 33, 2989. [Google Scholar]
- [183].Benitez PL, Sweet JA, Fink H, Chennazhi KP, Nair SV, Enejder A, Heilshorn SC, Advanced Healthcare Materials 2013, 2, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].González de Torre I, Ibáñez-Fonseca A, Quintanilla L, Alonso M, Rodríguez-Cabello J-C, Acta Biomaterialia 2018, 72, 137. [DOI] [PubMed] [Google Scholar]
- [185].McGowan JW, Shao Q, Vig PJ, Bidwell GL, Drug Des Devel Ther 2016, 10, 2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Yeo GC, Aghaei-Ghareh-Bolagh B, Brackenreg EP, Hiob MA, Lee P, Weiss AS, Advanced Healthcare Materials 2015, 4, 2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Mbundi L, González-Pérez M, González-Pérez F, Juanes-Gusano D, Rodríguez-Cabello JC, Front. Mater. 2021, 7, DOI 10.3389/fmats.2020.601795. [DOI] [Google Scholar]
- [188].Rodríguez-Cabello JC, Arias FJ, Rodrigo MA, Girotti A, Advanced Drug Delivery Reviews 2016, 97, 85. [DOI] [PubMed] [Google Scholar]
- [189].C. for B. E. and Research, FDA 2019. [Google Scholar]
- [190].Janmey PA, Winer JP, Weisel JW, J R Soc Interface 2009, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].“Home - ClinicalTrials.gov,” can be found under https://clinicaltrials.gov/ct2/home, n.d.
- [192].Bowman S, Awad ME, Hamrick MW, Hunter M, Fulzele S, Clin Transl Med 2018, 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Despanie J, Dhandhukia JP, Hamm-Alvarez SF, MacKay JA, J Control Release 2016, 240, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].PhaseBio, “Pipeline | PhaseBio,” can be found under https://phasebio.com/pipeline/, 2019.
- [195].Zhang W, Du A, Liu S, Lv M, Chen S, Regenerative Therapy 2021, 18, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Buckenmeyer MJ, Meder TJ, Prest TA, Brown BN, Methods 2020, 171, 41. [DOI] [PubMed] [Google Scholar]
- [197].Bai Y-R, Lai B-Q, Han W-T, Sun J-H, Li G, Ding Y, Zeng X, Ma Y-H, Zeng Y-S, Neural Regeneration Research 2021, 16, 2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Li R, Xu J, Rao Z, Deng R, Xu Y, Qiu S, Long H, Zhu Q, Liu X, Bai Y, et al. , Tissue Engineering Part A 2021, 27, 771. [DOI] [PubMed] [Google Scholar]
- [199].Cornelison RC, Gonzalez-Rothi EJ, Porvasnik SL, Wellman SM, Park JH, Fuller DD, Schmidt CE, Biomed. Mater. 2018, 13, 034110. [DOI] [PMC free article] [PubMed] [Google Scholar]