Abstract
Retroviral infection requires reverse transcription, and the reverse transcriptase (RT) uses cellular tRNA as its primer. In humans, the TRMT6-TRMT61A methyltransferase complex incorporates N1-methyladenosine modification at tRNA position 58 (m1A58); however, the role of m1A58 as an RT-stop site during retroviral infection has remained questionable. Here, we constructed TRMT6 mutant cells to determine the roles of m1A in HIV-1 infection. We confirmed that tRNA3Lys m1A58 was required for in vitro plus-strand strong-stop by RT. Accordingly, infectivity of VSV-G pseudotyped HIV-1 decreased when the virus contained m1A58-deficient tRNA3Lys instead of m1A58-modified tRNA3Lys. In TRMT6 mutant cells, the global protein synthesis rate was equivalent to that of wild-type cells. However, unexpectedly, plasmid-derived HIV-1 expression showed that TRMT6 mutant cells decreased accumulation of HIV-1 capsid, integrase, Tat, Gag, and GagPol proteins without reduction of HIV-1 RNAs in cells, and fewer viruses were produced. Moreover, the importance of 5,2′-O-dimethyluridine at U54 of tRNA3Lys as a second RT-stop site was supported by conservation of retroviral genome-tRNALys sequence-complementarity, and TRMT6 was required for efficient 5-methylation of U54. These findings illuminate the fundamental importance of tRNA m1A58 modification in both the early and late steps of HIV-1 replication, as well as in the cellular tRNA modification network.
INTRODUCTION
Post-transcriptional RNA modifications play pivotal roles in maintaining RNA structural integrity, function and metabolism (1). To date, more than 150 RNA modifications have been identified in the three domains of life, and the majority of RNA modifications exist in tRNAs (2). tRNA modifications are pivotal for life, and mutations or aberrations in over 50 tRNA modification enzyme genes are associated with diseases that most frequently manifest as brain dysfunction, mitochondrial diseases, and cancer (3,4).
N1-methyladenosine at position 58 of tRNAs (m1A58) (Figure 1A, B) is thought to be a primordial RNA modification based on its widespread presence in all domains of life, as well as the ability of ribozymes to incorporate m1A58 (2,5). m1A58 forms a reverse Hoogsteen base pair with 5-methyluridine at tRNA position 54 (m5U54) (Figure 1A, C) and strengthens the A58–U54 interaction to stabilize the L-shaped tRNA tertiary structure (6–8). In the thermophilic bacterium Thermus thermophilus, m5U54 is further modified to 5-methyl-2-thiouridine (m5s2U54) (9). In this bacterium, a tRNA modification network exists, including m1A58 formation promoted by m5U54, as well as 2-thiolation of m5U54 promoted by m1A58 (10,11). However, such a modification network of m1A58 and m5U54 has not been reported in eukaryotes.
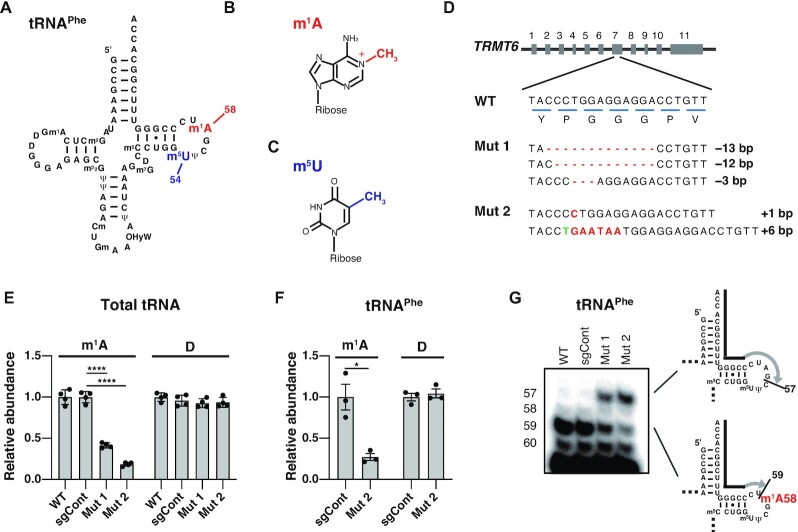
Figure 1.
Construction of TRMT6 mutant cell lines. (A) Secondary structure of the human cytoplasmic tRNAPhe with modified nucleosides: N1-methyladenosine (m1A), 5-methyluridine (m5U), N2-methylguanosine (m2G), dihydrouridine (D), N2,N2-dimethylguanosine (m22G), pseudouridine (Ψ), 2′-O-methylcytidine (Cm), 2′-O-methylguanosine (Gm), hydroxywybutosine (OHyW), 7-methylguanosine (m7G), and 5-methylcytidine (m5C). (B) Chemical structure of m1A. (C) Chemical structure of m5U. (D) TRMT6 alleles in TRMT6 mutant cells. Red lines indicate deletions. Red letters indicate insertions. Green letter indicates base exchange. Detection of three TRMT6 alleles in TRMT6 mutant #1 may be because HEK293FT cell is a near-triploid cell. (E) LC–MS analysis of total tRNA nucleosides from cells. Dihydrouridine level is shown as a control. WT indicates wild-type HEK293FT cells. sgCont indicates a control HEK293FT cell line that was generated using negative control sgRNA that does not target the human genome. Mut 1 and Mut 2 are TRMT6 mutant cell lines illustrated in Figure 1D. Relative abundance indicates LC-MS peak area relative to WT measurements. Means ± standard error of means (s.e.m.) from n = 4 biological replicates. (F) LC–MS analysis of nucleosides made by digestion of purified tRNAPhe. Dihydrouridine level is shown as a control. Relative abundance indicates LC-MS peak area relative to sgCont measurements. Means ± s.e.m. from n = 3 biological replicates. (G) Detection of hypomodified m1A58 using primer extension of HEK293FT cell line RNA samples. The primers are shown as black lines, and nascent cDNAs synthesized from the primers are depicted as gray lines. For reverse transcription, in addition to dATP, dTTP and dGTP, dideoxyCTP (ddCTP) was used to terminate reverse transcription at tRNAPhe G57. ****P < 0.0001, *P < 0.05 by Welch's t-test.
In human cells, the majority of cytoplasmic tRNAs bear the m1A58 modification (12). TRMT61A and TRMT6 are responsible for m1A58 modification of cytoplasmic tRNAs (13,14). TRMT61A and TRMT6 form a heterotetramer, in which two subunits of TRMT61A function as the catalytic methyltransferase subunits and two subunits of TRMT6 are required to bind to the substrate tRNA (15). siRNA-mediated knockdown of TRMT61A and TRMT6 results in a slow-growth phenotype and cell death, as well as a decrease in initiator tRNAMet levels (14,16). Moreover, the cytoplasmic tRNA m1A58 is hypothesized to play a role in the replication of various retroviruses (17).
Retroviruses, including HIV-1 and Moloney murine leukemia virus (M-MLV), are positive-strand RNA viruses (18). After retroviral entry into the host cell, reverse transcription initiates at the 18-nt primer binding site on the viral RNA, using a cellular cytoplasmic tRNA as the primer, and completes the synthesis of the minus-strand DNA copy (detailed in Supplementary Figure S1) (17,19). HIV-1 uses the cytoplasmic tRNA3Lys as the primer, and M-MLV uses cytoplasmic tRNAPro as the primer (18,20,21). RNaseH then degrades much of the viral RNA, leaving two short polypurine tracts of the viral RNA, which are used as the primer for plus-strand DNA synthesis (22–24). Plus-strand synthesis initially terminates after proceeding 18 nt into the 3′ end of the tRNA, generating the so-called ‘plus-strand strong-stop’ DNA (25). Next, the plus-strand strong-stop DNA transfers to the 3′ end of the template minus-strand DNA, and the RT completes the plus-strand DNA synthesis (17). Without the plus-strand strong-stop, RT continues to copy additional tRNA sequences. The additional sequences are not complementary to the minus-strand DNA, resulting in an inability to complete plus-strand synthesis and DNA integration into the host genome (Supplementary Figure S1) (26).
Since the plus-strand strong-stop occurs one nucleotide before m1A58 of the primer tRNA, it has been hypothesized that plus-strand strong-stop occurs due to the presence of methylation in m1A58 (17). However, this hypothesis remains unresolved. Several groups have provided evidence for this hypothesis by performing in vitro reconstitution of the plus-stand strong-stop and plus-strand transfer and comparing cellular modified tRNA3Lys to T7 RNA polymerase-synthesized unmodified tRNA3Lys (26–28). In these experiments, the effect of other tRNA3Lys modifications which may alter the local and overall tRNA structures, cannot be excluded. These modifications include dihydrouridine at tRNA positions 16, 20 and 47, pseudouridine at positions 27, 39 and 55, mcm5s2U at position 34, ms2t6A at position 37 and m7G at position 46. Another group supported this hypothesis by using A58U mutant tRNA3Lys (29). This substitution does not prove the role of m1A58, because the A-to-U mutation itself may affect the plus-strand strong-stop. Another group performed a genome-scale RNAi screen that detected TRMT6 as one of 311 host factors required for HIV-1 replication, but no further analysis was performed (30). The hypothesis has been opposed by a group that used an orthogonal HIV-1 expression system to express Escherichia coli tRNALys in human cells (31), but did not test for the presence of m1A58 in the ectopically expressed E. coli tRNALys. Therefore, whether m1A58 plays an essential role in plus-strand strong-stop DNA synthesis remains in question for decades.
MATERIALS AND METHODS
Cell culture
HEK293FT, HeLa and MEF cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, at 37°C under a humidified atmosphere with 5% CO2.
Construction of TRMT6 mutant cells
TRMT6 mutant cells were generated using the CRISPR/Cas9 system essentially as described previously (32). Briefly, sense and antisense oligonucleotides encoding a single guide RNA (sgRNA) (Supplementary Table S1) were cloned into the BsmBI sites of lentiCRISPR v2 Blast plasmid (Addgene #83480). Lentiviruses were generated using the sgRNA sequence-containing lentiCRISPR v2 Blast plasmid, psPAX2 (Addgene #12260), and pMD2.G (Addgene #12259), and HEK293FT cells were transduced with the generated viruses. After blasticidin selection of the transduced cells, single clones were acquired by diluting the cells in 96-well plates. The target region of the genome in each clone was PCR-amplified and sequenced. For sgControl cell line, human genome non-targeting oligos were designed as previously described (33).
HIV-1 plasmids
For production of VSV-G pseudotyped HIV-1, pRRLSIN.cPPT.PGK-GFP.WPRE (Addgene #12252) and pCMV delta R8.2 (Addgene #12263) were used. Vesicular stomatitis virus envelope protein G (VSV-G) was expressed from pMD2.G plasmid (Addgene #12259) to produce pseudotyped HIV-1 that can infect HeLa cells.
Production and transduction (infection) of VSV-G pseudotyped HIV-1
HEK293FT (WT, sgCont, Mut #1 and Mut #2) cells were transfected with pCMV delta R8.2, pMD2.G and pRRLSIN.cPPT.PGK-GFP.WPRE using TransIT-LT1 (Mirus Bio, Madison, WI, USA). One day post-transfection, the medium was changed. The medium was collected at 48 and 72 h post-transfection. The cells were lysed and analyzed by western blotting. The supernatants were filtered with a 0.45 μm filter and used for HIV-1 p24 quantification and transduction of HeLa cells. Concentration of the HIV-1 in the supernatants was quantified by HIV-1 p24 ELISA (Rimco, Uruma, Japan). HeLa cells were transduced with VSV-G pseudotyped HIV-1 equivalent to 5 ng of HIV-1 p24. Two days after transduction, the cells were collected, and the ratio of GFP-positive cells was counted by flow cytometry SH800S (Sony Biotechnology, Tokyo, Japan).
HIV-1 RNA collection
HEK293FT (WT, sgCont, Mut #1 and Mut #2) cells were transfected with pCMV delta R8.2 using TransIT-LT1 (Mirus Bio). Two days after transfection, RNA was collected as described below. The RNA was treated with DNase I (Invitrogen, Carlsbad, CA, USA) and used for qRT-PCR.
RNA extraction and tRNA isolation
Total RNA from each cell was prepared using the TRI Reagent (MRC, Cincinnati, OH, USA), according to the manufacturer's protocol. Total tRNA was collected from total RNA by electrophoresis using denaturing 7 M urea/TBE/10% PAGE, SYBR Gold staining, and gel excision of the tRNA band. For isolation of cytoplasmic tRNA3Lys or tRNAPhe from total RNA, 2 nmol of 3′ biotinylated DNA probe (Supplementary Table S1) was first bound to streptavidin beads (GE Healthcare, Milwaukee, WI, USA) at room temperature in binding buffer [100 mM NaCl, 10 mM HEPES–KOH (pH 7.5), and 5 mM EDTA] for 1 h and washed with the binding buffer and hybridization buffer [1200 mM NaCl, 30 mM HEPES–KOH (pH 7.5) and 7.5 mM EDTA]. The probe-bound beads were mixed with 100 μg of total RNA in hybridization buffer and incubated at 65°C for 1 h with occasional agitation. The beads were washed 10 times with wash buffer [600 mM NaCl, 30 mM HEPES–KOH (pH 7.5), and 7.5 mM EDTA] at 65°C, and eluted with elution buffer [20 mM NaCl, 0.5 mM HEPES–KOH (pH 7.5) and 0.25 mM EDTA] at 65°C. Subsequently, the eluate was subjected to 7 M urea/TBE/10% PAGE, followed by SYBR Gold staining and gel excision of the tRNA band.
RNA mass spectrometry
RNA was digested to nucleosides, and the nucleosides were subjected to RNA nucleoside mass spectrometry as previously described (34).
Primer extension
Primer extension was conducted in the same way as described previously (35), with the following modifications. In the primer extension experiment that used HIV-1 RT instead of M-MLV RT (Invitrogen), 10 units of HIV-1 RT (Merck) with an additional 3 mM MgCl2 were used. The primers used to detect tRNAPhe, tRNAPro, tRNA3Lys and mitochondrial (mt) tRNAMet are listed in Supplementary Table S1. The d/ddNTP mix consisted of dATP, dTTP, dGTP and ddCTP for tRNAPhe m1A58; dATP, dTTP and ddGTP for tRNAPro m1A58; dATP, dCTP, dTTP and ddGTP for tRNA3Lys m1A58; dATP, dGTP and ddCTP for tRNA3Lys m5Um54; and dATP, dTTP, dGTP and ddCTP for mt tRNAMet Ψ55.
Quantitative reverse-transcription real-time PCR (qRT-PCR)
qRT-PCR was performed as previously described (36). The primers are listed in Supplementary Table S1. For quantification of HIV-1 RNA, primers p1 and p3-10 were used as described previously (37,38). Primer p2 was designed based on pCMV delta R8.2 sequence.
35S-methionine labeling of nascent proteins
Cells grown in 6 cm dishes were briefly washed using 37°C DMEM without Met, Cys and Gln (Gibco, Paisley, Scotland). A total of 2 ml of 37°C preincubation medium (DMEM without Met, Cys and Gln, supplemented with 2% FBS, 2 mM Gln, 0.2 mM Cys) was added to the cells, and the cells were incubated in 37°C CO2 incubator for 15 min. The medium was then exchanged with 1.25 ml of incubation medium (1.25 ml of pre-incubation medium containing 3.7 MBq of 35S-labeled methionine), and the cells were incubated in 37°C CO2 incubator for 60 min. Subsequently, the medium was removed and the cells were washed with PBS and collected using trypsin and DMEM containing 10% FBS. Cells were lysed and protein concentration was measured using BCA Protein Assay Kit (Pierce, Rockford, IL, USA), and 40 μg of total proteins were run on Tricine PAGE gel (NOVEX, Carlsbad, CA, USA). The gel was stained using coomassie brilliant blue (CBB) staining solution (Bio-Rad, Hercules, California, USA), dried on gel dryer, photographed and the radiation image was acquired using an imaging plate and imager (Fujifilm, Fuji, Japan).
Western blotting
Western blotting was performed essentially as described previously (36). The antibodies and their used conditions are listed in the Supplementary Table S2.
Statistical analysis
All numerical data were analyzed by GraphPad Prism 9 software. Welch's t-test was used to assess differences between the two groups. A two-tailed P-value of 0.05 was considered significant. Data are presented as means ± standard error of means (s.e.m.).
RESULTS
Loss of m1A58 decreases 5-methylation of m5U54 and m5Um54 modifications
To explore the roles of tRNA m1A58 modification in human cells and retrovirus replication, we initially attempted to knock out TRMT61A or TRMT6 using the CRIPSR/Cas9 system in HEK293FT cells. Although we obtained knockout cells of other tRNA modification enzyme genes that we were simultaneously generating (data not shown), we did not obtain TRMT61A or TRMT6 null cell lines. TRMT61A or TRMT6 null cell lines could not be obtained even after repeated trials, suggesting that TRMT61A and TRMT6 are essential for cell viability. However, we obtained two TRMT6 mutant cell lines (Figure 1D). TRMT6 mutant cell line #1 had 13 bp deleted in its first allele, 12 bp deleted in the second allele and 3 bp deleted in the third allele. Thus, the second allele produces TRMT6 protein with a 4 amino acid deletion and the third allele produces TRMT6 with a 1 amino acid deletion. The second TRMT6 mutant cell line, TRMT6 mutant #2, had an allele with a 1 bp insertion and another allele with a 6 bp insertion, thus mutant #2 produces TRMT6 protein with a two amino acids insertion. Using the crystal structure information of the human TRMT6–TRMT61A–tRNA complex (15), homology modeling predicted that these mutant TRMT6 amino acid deletion/insertions likely alter the association interface of TRMT6 and tRNA anticodon stem (Supplementary Figure S2).
We then isolated total cellular tRNA fraction by gel excision, digested the tRNAs to nucleosides, and analyzed the m1A level by liquid chromatography–mass spectrometry (LC–MS) (Figure 1E). In the total tRNA nucleosides, we observed a 59% decrease of m1A in TRMT6 mutant #1 and 82% decrease of m1A in TRMT6 mutant #2. m1A is present not only in the cytoplasmic tRNA at position 58, but also in the cytoplasmic tRNAAsp at position 9, and mitochondrial tRNA at positions 9 and 58, which are incorporated by TRMT10B, TRMT10C and TRMT61B, respectively (35,39–41). In addition, m1A is present at the tRNAPhe position 14; the enzyme responsible for this modification has not been reported (3,4). The remaining m1A in the TRMT6 mutant cell total tRNA may have resulted from the m1A present in these tRNA species as well as residual TRMT6 activity, with TRMT6 mutant #2 retaining less m1A58 modification activity than TRMT6 mutant #1. To further clarify m1A status, we isolated tRNAPhe from TRMT6 mutant #2 using a biotinylated oligo DNA probe complementary to tRNAPhe, digested tRNAPhe to nucleosides, and subjected to LC–MS analysis. As a result, we observed a 73% reduction of m1A in tRNAPhe in TRMT6 mutant #2 (Figure 1F). To confirm the m1A reduction at position 58, primer extension analysis of tRNAPhe was performed. It showed a reduction in tRNAPhe m1A58 in TRMT6 mutant cells, especially in TRMT6 mutant #2 (Figure 1G).
To elucidate the potential role of m1A58 modification in the formation of other tRNA modification(s), we quantified tRNA modifications by reanalyzing LC-MS data from Figure 1E (Figure 2A). Among the various tRNA modifications, we noticed that 5-methyluridine (m5U), which exists at tRNA position 54 within various tRNAs, decreased in TRMT6 mutant cells in correlation with the degree of m1A reduction (Figure 2A). Moreover, a decrease of 5,2′-O-dimethyluridine modification (m5Um) was also observed in TRMT6 mutant cells (Figure 2A). m5Um is a ribose 2′-O-methylation derivative of m5U (Figure 2B), and m5Um exists at the position 54 in a subset of tRNA species including mammalian tRNA3Lys (Figure 2C) (2,42). These results strongly suggest that TRMT6-mediated m1A58 modification is required for m5U54 and m5Um54 modifications. To further confirm these results, we re-analyzed the LC-MS result of tRNAPhe in Figure 1F, and observed 65% decrease of m5U in tRNAPhe, while other modifications except for m1A were not decreased in tRNAPhe (Figure 2D). These results strongly suggest that TRMT6/61A-mediated m1A58 modification promotes 5-methylation of U54 to produce m5U54.
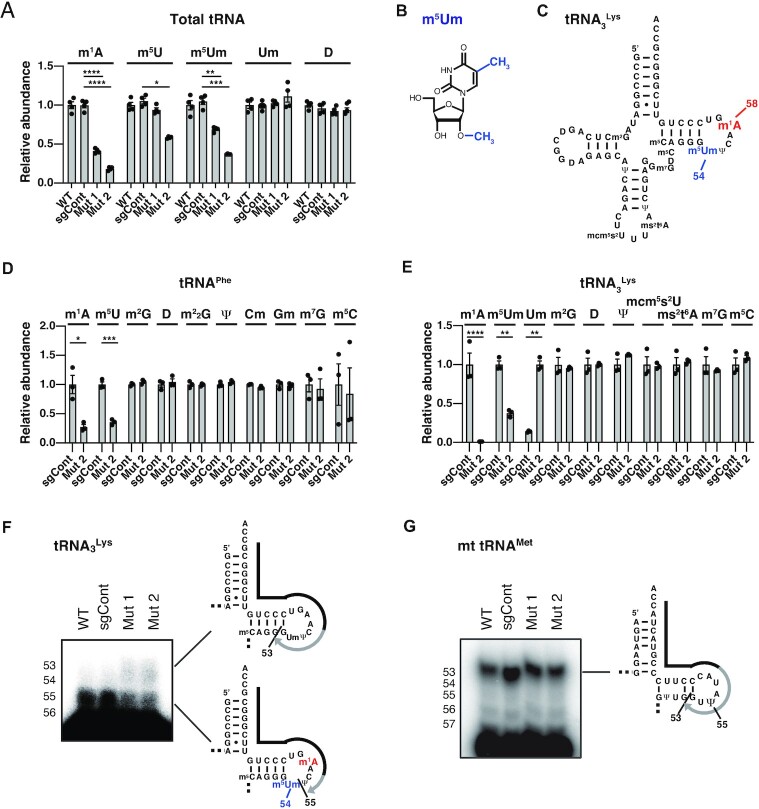
Figure 2.
Requirement of TRMT6 for 5-methylation of tRNA m5U54 and m5Um54 modifications. (A) LC–MS analysis of nucleosides produced by digestion of total cellular tRNAs. The LC–MS result in Figure 1E was reanalyzed. Dihydrouridine level is shown as a control. Relative abundance indicates LC–MS peak area relative to WT measurements. Means ± s.e.m. from n = 4 biological replicates. (B) Chemical structure of 5,2′-O-dimethyluridine (m5Um). (C) Secondary structure of human cytoplasmic tRNA3Lys with modified nucleosides not depicted in Figure 1A: 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U) and 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A). (D) LC–MS analysis of nucleosides made by digestion of purified tRNAPhe. The LC–MS result in Figure 1F was reanalyzed. Relative abundance indicates LC–MS peak area relative to sgCont measurements. Means ± s.e.m. from n = 3 biological replicates. (E) LC–MS analysis of nucleosides made by digestion of purified tRNA3Lys. Means ± s.e.m. from n = 3 biological replicates. (F) Detection of hypomodified m5Um54 using primer extension. The primers are shown as black lines, and nascent cDNAs synthesized from the primers are depicted as gray lines. In addition to dATP and dGTP, ddCTP was used to terminate reverse transcription at tRNA3Lys G53. (G) Primer extension analysis of mitochondrial (mt) tRNAMet of HEK293FT cell lines to confirm that reverse transcription-stop does not occur at Ψ55. In addition to dATP and dTTP, ddCTP was used to terminate reverse transcription at mt tRNAMet G53. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 by Welch's t-test.
To analyze m5Um status in detail, we isolated tRNA3Lys from total RNA of control cells or TRMT6 mutant #2 and subjected tRNA3Lys nucleosides to LC-MS analysis. We observed a complete loss of m1A in tRNA3Lys, as well as 62% decrease of m5Um, in TRMT6 mutant #2 (Figure 2E). In addition, we observed a marked increase of 2′-O-methyluridine (Um) in TRMT6 mutant #2, which was found only at a low level in control cell tRNA3Lys (Figure 2E). Collectively, these results strongly suggest that TRMT6/61A-mediated m1A58 modification promotes 5-methylation of Um54 to produce m5Um54.
Based on a primer extension experiment using cellular tRNA3Lys in a previous study, another group detected the presence of a reverse-transcription stop at one nucleotide before m5Um54, implying that m5Um54 may terminate reverse transcription (27). To confirm the position specificity of m5Um, we performed primer extension of tRNA3Lys, and observed an RT-stop at one nucleotide before position 54 in control cells (Figure 2F), in accordance with the previous study. This RT-stop was reduced in TRMT6 mutants and increased RT read-through was observed (Figure 2F), strongly suggesting that TRMT6-mediated m1A58 modification promotes m5Um modification at position 54. This RT-stop is unlikely to be due to pseudouridine at position 55 (Ψ55) as previously proposed (43), because primer extension of mitochondrial tRNAMet, which has Ψ55 and not m5Um54 (44), did not stop reverse transcription at Ψ55 (Figure 2G). Taken together, these data suggest that m1A58 modification by TRMT61A-TRMT6 complex promotes m5U54 formation in the cell.
Host cellular tRNA m1A58 is required for the retroviral RT to make a plus-strand strong-stop in vitro
Retroviruses use host cellular tRNA as the primer to initiate minus strand DNA synthesis. During plus-strand DNA synthesis, the plus-strand strong-stop occurs one nt before m1A58 (Supplementary Figure S1) (17). However, the role of tRNA m1A58 modification in plus-strand strong-stop has remained in question for decades, due to the use of inadequate tRNA materials. To formally investigate the role of host tRNA m1A58 in plus-strand strong-stop, we started by investigating whether M-MLV RT stops reverse transcription at its primer tRNA m1A58 in vitro. M-MLV, a mouse retrovirus, uses tRNAPro to prime reverse transcription. We decided that tRNAPro from the wild-type and TRMT6 mutant HEK293FT cells could be used, since mouse and human proline tRNAs have the same sequences. Using a primer extension in vitro reverse transcription experiment, we observed a halt of reverse transcription by M-MLV RT at one nt before position 58 of tRNAPro of wild-type cells. This halt was substantially alleviated in m1A58-lacking tRNAPro from TRMT6 mutant cells, especially in TRMT6 mutant cell #2 (Figure 3A). Thus, plus-strand strong-stop was recapitulated in vitro using a minimal substrate-enzyme system.
Figure 3.
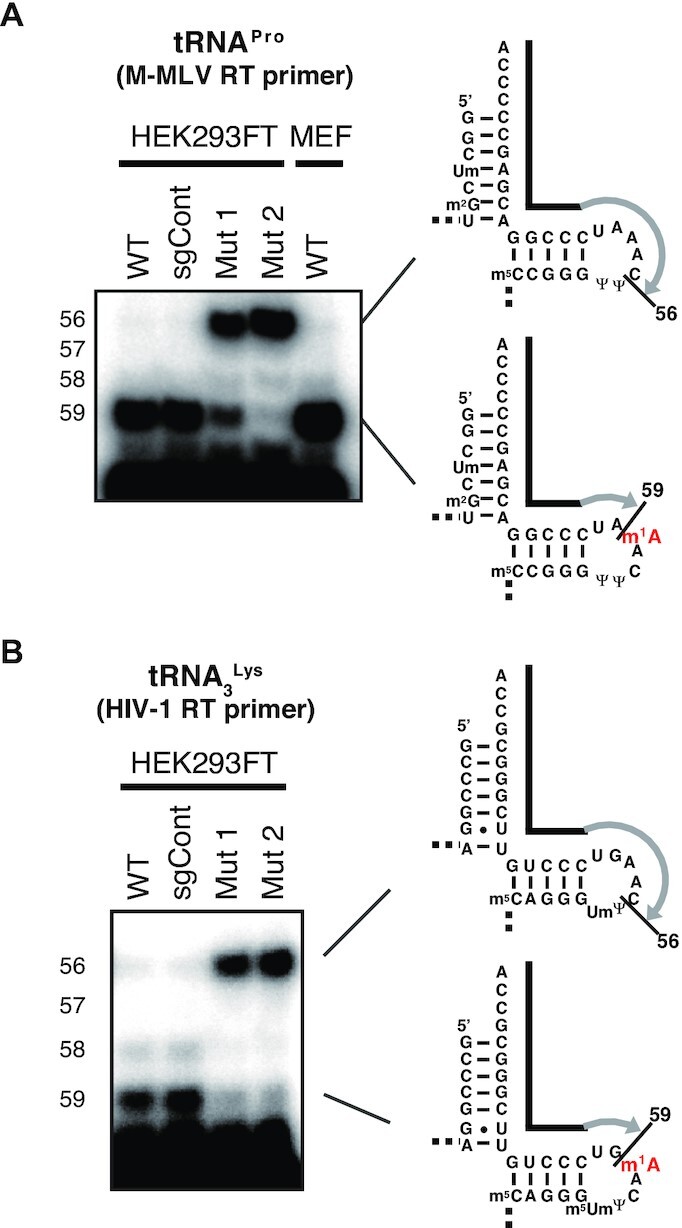
Requirement of tRNA m1A58 for plus-strand strong-stop by M-MLV RT and HIV-1 RT using retroviral primer tRNAs in vitro. (A) Investigation of the requirement for m1A58 in tRNAPro for reverse transcription-stop during plus-strand cDNA synthesis by M-MLV RT on primer tRNAProin vitro. In addition to dATP and dTTP, ddGTP was used to terminate reverse transcription at tRNAPro C56. Mouse embryonic fibroblast (MEF) RNA was used to confirm that reverse-transcription stops at m1A58 of wild-type mouse tRNAPro. (B) Investigation of the necessity of m1A58 in the HIV-1 primer tRNA3Lys for reverse transcription-stop during plus-strand cDNA synthesis by HIV-1 RT in vitro. In addition to dATP, dTTP and dCTP, ddGTP was used to terminate reverse transcription at tRNA3Lys C56.
Similar to M-MLV, HIV-1 uses tRNA3Lys as the primer to initiate its minus strand DNA synthesis. Using the tRNA3Lys primer and HIV-1 RT, we observed that reverse transcription indeed stopped at one nt before position 58 of tRNA3Lys of wild-type cells, but did not stop at one nt before position 58 of m1A58-lacking tRNA3Lys from TRMT6 mutant cells (Figure 3B). The only tRNA modifications absent in the TRMT6 mutant cell-derived tRNA3Lys were m1A58 and m5Um54 (Figure 2E), thus it is likely that much of the secondary and tertiary structures of tRNA3Lys in TRMT6 mutant cells were maintained.
Collectively, these results demonstrate that the host tRNA m1A58 is indeed required for retroviral RTs to make the plus-strand strong-stop during the synthesis of plus-strand DNA in vitro.
TRMT6 is required for the early steps of HIV-1 replication
The primer tRNA3Lys for HIV-1 replication, which is incorporated into the HIV-1 particles, derives from the virus-producer cell (20). After HIV-1 infection, the virus-producer cell-derived tRNA3Lys is used as the primer to initiate HIV-1 minus-strand DNA synthesis in the infected cell, followed by the synthesis of plus-strand DNA copy of the HIV-1 genome and integration of the double-stranded DNA into the host genome (17).
We next decided to investigate whether tRNA m1A58 modification in the virus-producer cell affects the early steps of HIV-1 replication, which are the steps between viral entry and HIV-1 DNA integration in the infected cells. To this end, control cells or TRMT6 mutant cells were transfected with plasmids to produce a vesicular stomatitis virus envelope protein G (VSV-G) pseudotyped HIV-1 containing genomic RNAs encoding the GFP gene. VSV-G pseudotyped HIV-1 from the control cells or TRMT6 mutant cell lines were quantified, and the same concentration of the HIV-1 was used to transduce HeLa cells. Subsequently, the genome integration rate was evaluated by the number of GFP-positive cells (Figure 4A). Instead of HIV-1 Envelope (Env), VSV-G was used as the envelope protein, to enable HIV-1 infection of the HeLa cells. Upon transduction (infection) of equal concentration of VSV-G pseudotyped HIV-1 from TRMT6 mutant cell #1, we observed a 54% decrease of GFP-positive cells compared to control cells (Figure 4B). Upon infection of VSV-G pseudotyped HIV-1 from TRMT6 mutant cell #2, we observed a 73% decrease of GFP-positive cells (Figure 4B), demonstrating that HIV-1 genome integration into the host cells substantially decreased when the virus was produced from TRMT6 mutant cells. Thus, TRMT6 in virus-producer cells is required for the early steps of HIV-1 replication.
Figure 4.
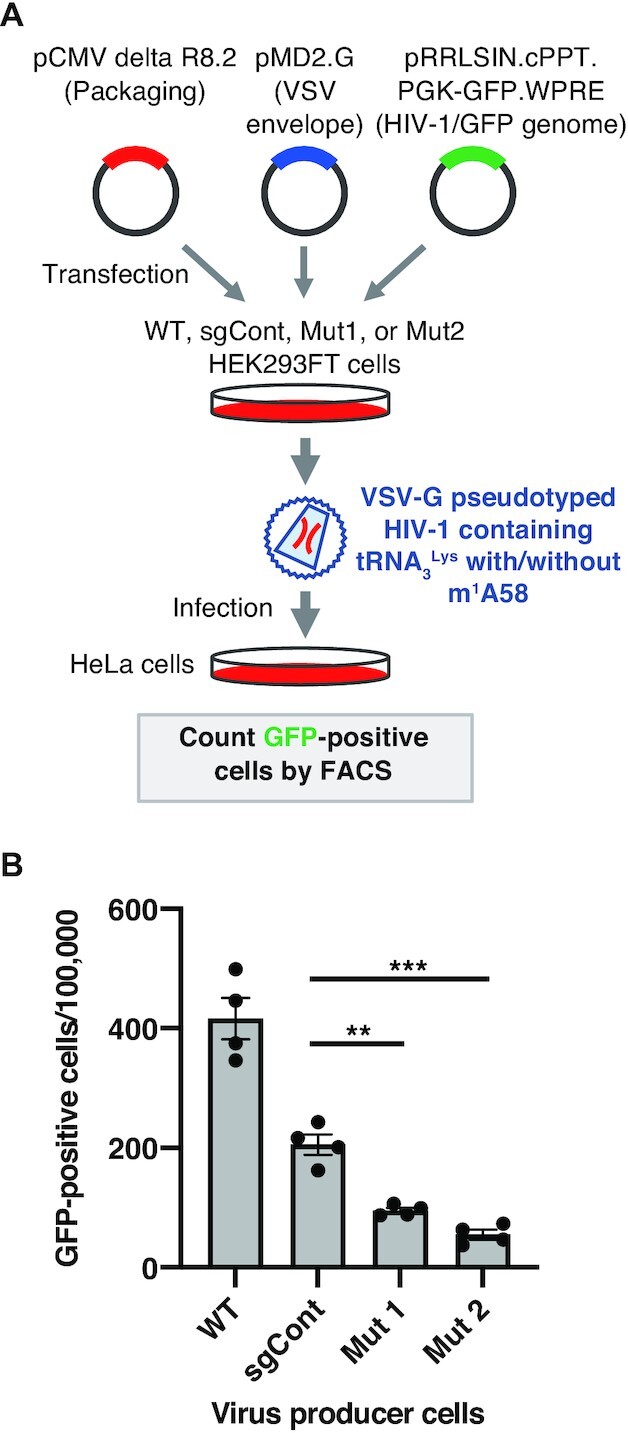
Requirement of TRMT6 for the early steps of HIV-1 replication in vivo. (A) Outline of VSV-G pseudotyped HIV-1 production and transduction experiment. (B) After transduction of HeLa cells using equal concentrations of the HIV-1 particles (equivalent to 5 ng of HIV-1 p24) produced from WT, sgCont, TRMT6 mutant #1, or TRMT6 mutant #2 HEK293FT cells, HeLa cells in which the HIV-1 GFP gene was integrated were quantified. Means ± s.e.m. from n = 4 biological replicates. ***P < 0.001, **P < 0.01 by Welch's t-test.
Requirement of TRMT6 for the late steps of HIV-1 replication
During the HIV-1 infection experiment shown in Figure 4, we realized that upon transfection of the plasmids that produce VSV-G pseudotyped HIV-1, TRMT6 mutant cells produced less HIV-1 than control cells. This result was unexpected, since other than the possibility of tRNA m1A58 modification stopping reverse-transcription, no other role has been reported. Repeated quantification of HIV-1 capsid protein p24 in the supernatant of the transfected cells showed that the amount of virus released from the cells was reduced in the TRMT6 mutant cell supernatant (Figure 5A, B). The amount of virus in the cell culture supernatant of TRMT6 mutant #2 was less than that of TRMT6 mutant #1 (Figure 5B), which correlates with the reduced amount of tRNA m1A58 in TRMT6 mutant #2 compared to TRMT6 mutant #1 (Figure 1E).
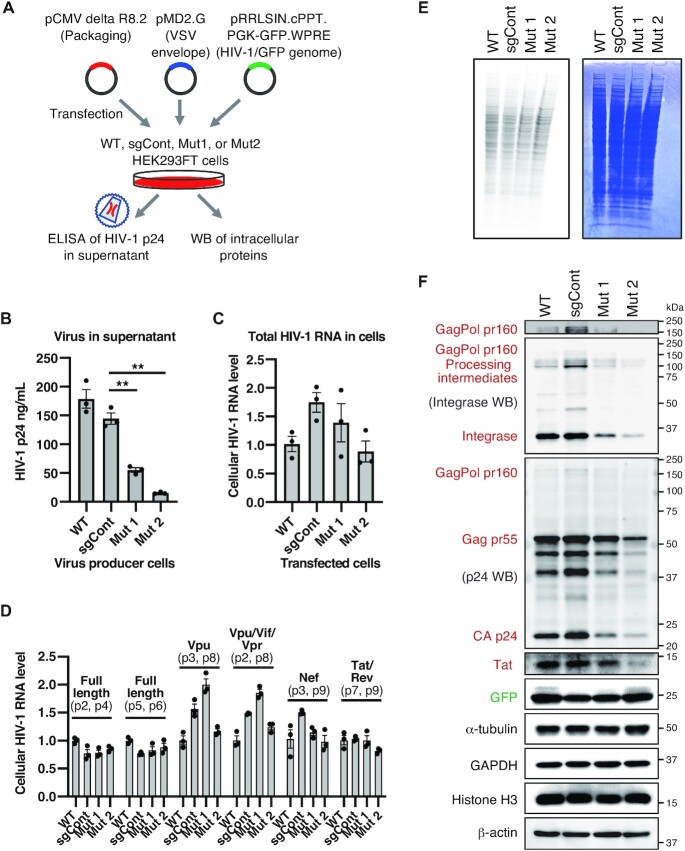
Figure 5.
Requirement of TRMT6 for efficient HIV-1 protein accumulation and virus production. (A) Outline of VSV-G pseudotyped HIV-1 production experiment. (B) Quantification of produced HIV-1 particles. HIV-1 particle capsid p24 protein in the cell culture supernatant was analyzed by ELISA to quantify the HIV-1 particles. Means ± s.e.m from n = 3 biological replicates, **P < 0.01 by Welch's t-test. (C) qRT-PCR quantification of total HIV-1 RNA in HEK293FT cells after transfection of HEK293FT cells with pCMV delta R8.2 plasmid. Primers 1 and 10 in Supplementary Figure S3 were used. RNA levels were normalized by GAPDH mRNA levels. Means ± s.e.m from n = 3 independent experiments. (D) qRT-PCR quantification of individual spliced HIV-1 RNA in HEK293FT cells after transfection of pCMV delta R8.2 plasmid. Primer locations (e.g. p2, p3) are depicted in Supplementary Figure S3. Each RNA level was normalized by the total HIV-1 RNA level in Figure 5C. Means ± s.e.m. from n = 3 independent experiments. (E) Nascent cellular protein synthesis observed by pulse-labeling using 35S-methionine that was added to the cell culture media for 1 h. Coomassie brilliant blue (CBB)-stained gel is shown on the right as a loading control and to show the status of steady-state protein levels. (F) Western blot analysis of HIV-1 proteins using HIV-1 integrase antibody, HIV-1 p24 capsid antibody and HIV-1 Tat antibody. Equal amounts of total cellular proteins from the virus producer cells were electrophoresed, and α-tubulin, GAPDH, Histone H3 and β-actin were used as loading controls and cellular proteins whose abundance remained unchanged in TRMT6 mutant cells. Western blotting of integrase or p24 proteins also detected their precursor Gag, GagPol and processing intermediates. HIV-1 proteins expressed from pCMV delta R8.2 plasmid are indicated in red and GFP expressed from pRRLSIN.cPPT.PGK-GFP.WPRE plasmid is indicated in green. The molecular mass marker (kDa) is shown on the right. The top panel is the same image of the region corresponding to the GagPol bands of the second panel, with its contrast changed to visualize GagPol bands. HIV-1 genome and precursor protein structures are shown in Supplementary Figure S3. Independent experiments were performed (n = 3: Tat, Integrase, GFP, α-Tubulin, GAPDH, Histone H3 and β-actin; n = 4: p24, Gag and GagPol). Quantified values are provided in Supplementary Figure S4.
HIV-1 RNA levels were similar in TRMT6 mutant cells and control cells transfected with the HIV-1 RNA expression plasmid pCMV delta R8.2 (Figure 5C, D; Supplementary Figure S3). Thus, it was unlikely that the decreased viral production observed in TRMT6 mutant cells was caused at the RNA level. In human cells, m1A58 is present in almost all cytoplasmic tRNA species (12). Generally, loss of a tRNA modification that critically affects tRNA function or stability results in decreased translational efficiency of the mRNA codons translated by that particular tRNA. To monitor the cellular global protein synthesis rate, we performed pulse-labeling of nascent proteins by adding 35S-methionine into the cell culture medium for 60 min. A comparison of cellular proteins in the TRMT6 mutant cells and control cells showed that the overall cellular protein synthesis rate and steady-state level of cellular proteins were unaffected in the mutant cells (Figure 5E). In addition, the steady-state levels of endogenous proteins such as α-Tubulin, GAPDH, Histone H3 and β-actin were unchanged with or without transfection of HIV-1 proteins-encoded plasmids (Figure 5F, Supplementary Figure S4A).
Although the overall nascent protein synthesis and the steady-state protein levels did not show observable changes (Figure 5E, F, Supplementary Figure S4A), it is still possible that the protein synthesis rate of individual proteins or translation fidelity of individual amino acids can be affected by loss of tRNA m1A58. To investigate possible changes in HIV-1 protein accumulation, the level of HIV-1 viral capsid protein p24, transcriptional activator Tat, as well as the viral integrase and their precursor and processing intermediates within the virus-producer cells were observed and quantified using western blot analysis (Figure 5F, Supplementary Figures S3 and S4B). As a result, we observed a reduction of the protein level of HIV-1 capsid p24 and its precursors GagPol pr160 and Gag pr55 in the TRMT6 mutant cell lines. Moreover, substantial reductions of the HIV-1 integrase, as well as its precursor GagPol pr160 and the processing intermediates, were also observed. In addition, we observed a reduction of Tat in TRMT6 mutant cell lines, although the house-keeping protein levels were unchanged within the same amount of total cellular protein (Figure 5F, Supplementary Figure S4B). Accumulation of GFP protein expressed from the pRRLSIN.cPPT.PGK-GFP.WPRE plasmid confirms that the four cell lines retained translational capacity even upon transfection of HIV-1 proteins expression plasmids (Figure 5F, Supplementary Figure S4B). The reductions of HIV-1 capsid p24, integrase and their precursors and processing intermediates, as well as Tat, were more prominent in the TRMT6 mutant #2 than in TRMT6 mutant #1, correlating with the greater reduction of tRNA m1A in the TRMT6 mutant #2 (Figure 1E). Together, these data indicate that TRMT6 is required for efficient HIV-1 protein accumulation and virus production in the late steps of HIV-1 replication.
DISCUSSION
The role of tRNA m1A58 in stopping reverse transcription has been proposed several decades ago, but has remained unclear, due to the lack of definitive methodologies and materials used in the previous studies. In the present study, we have confirmed that m1A58 is indeed required for RT to make a plus-strand strong-stop in vitro and have also shown that TRMT6 is required for the early steps of HIV-1 replication in vivo (Figure 6B, middle).
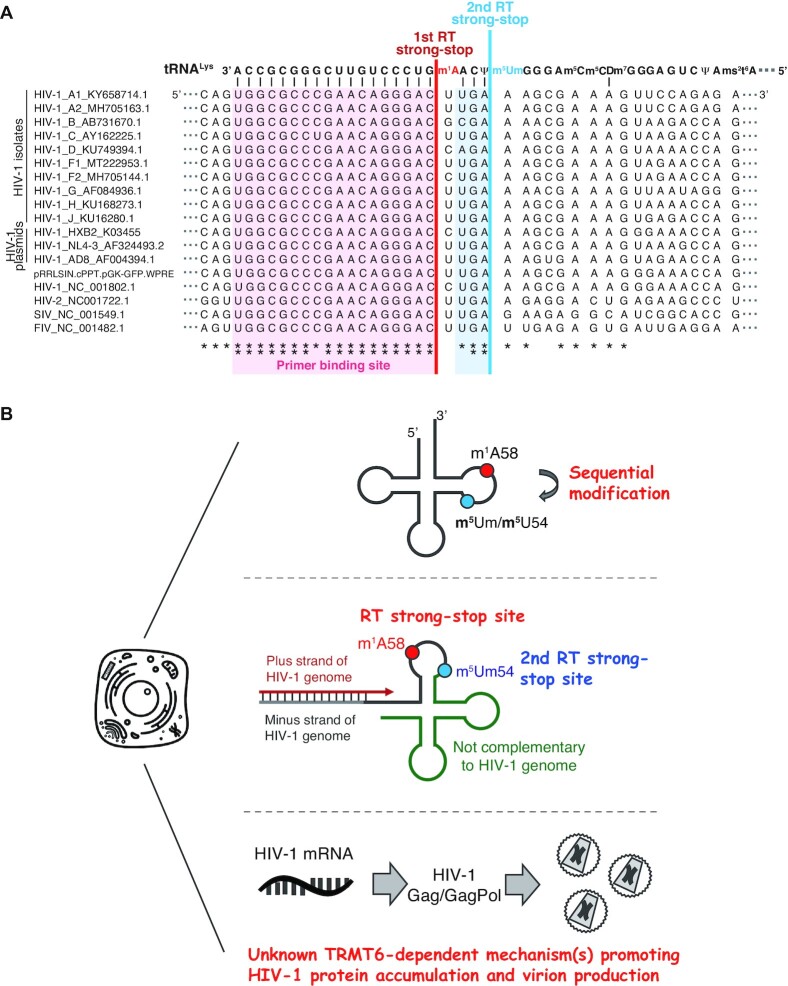
Figure 6.
Model of TRMT6-mediated regulation of m5U54 modification and HIV-1 replication. (A) Sequence alignment of tRNALys and the genome RNA of HIV-1, HIV-2, simian immunodeficiency virus (SIV), and feline immunodeficiency virus (FIV), all of which use tRNALys as their primers. tRNALys sequences are common in human, macaque, and cat. ** and * indicate 100% and >80% conservation, respectively. (B) TRMT6-mediated m1A58 modification has three roles: 1) TRMT6-mediated m1A58 modification promotes 5-methylation of m5Um54 and m5U54 modifications. 2) In the early steps of HIV-1 replication, TRMT6-mediated m1A58 halts reverse transcription at m1A58, and m5Um54 functions as the 2nd reverse transcription stop site when m1A58 modification is absent. Without an RT-stop at either of these positions, plus-strand cDNA synthesis continues beyond tRNA position 54, and the cDNA becomes uncomplementary to the other stand of HIV-1 DNA-copy after plus-strand transfer and cannot be integrated into the host genome (Supplementary Figure S1). 3) In the late steps of HIV-1 replication, TRMT6 is required for efficient accumulation of HIV-1 proteins and virus particles.
Moreover, we unexpectedly found that apart from the early steps of the HIV-1 replication, TRMT6 is also required for the late steps of HIV-1 replication. TRMT6 mutant cell lines accumulated smaller levels of HIV-1 proteins such as the precursor GagPol pr160 and Gag pr55, integrase, capsid p24 and Tat proteins, and produced substantially smaller levels of the virus particles (Figure 6B, bottom).
A mechanistic understanding of HIV-1 protein reduction awaits elucidation. Tat is the transcriptional activator of HIV-1, but a decrease in Tat protein levels is unlikely to be the cause of corresponding decrease in HIV-1 protein levels in our system. Tat activates transcription from the natural HIV-1 LTR promoter, but the HIV-1 plasmid (pCMV delta R8.2) does not have an LTR and instead uses the CMV promoter to drive transcription. Accordingly, HIV-1 RNA levels were similar in all four cell lines (Figure 5C, D). While HIV-1 RNA is known to contain the N6-methyladenosine (m6A) modification (45), it has been confirmed in a previous study using RNA LC-MS that the m1A modification is absent in packaged HIV-1 genomic RNA (46). Thus, it is possible that instead, the tRNA m1A58 modification is important for proper translational rate or fidelity during the translation of specific codons by specific tRNA species. The codon usage of HIV-1 late genes is significantly different from the codon usage of the host (47), and the proteins made from HIV-1 late genes include the long GagPol pr160 and Gag pr55. However, reduction of Tat, an HIV-1 early gene also occurred in TRMT6 mutant cells (Figure 5F, Supplementary Figure S4B), showing that codon frequency bias within HIV-1 genes may not be the cause of HIV-1 protein reduction. One possibility is that translation of specific cellular factors that are important for HIV-1 protein accumulation may be regulated by TRMT6/61A-mediated m1A modification within tRNAs, or mRNAs with tRNA T-loop-like structures (48). To objectively investigate the role of m1A in translation, the use of ribosome profiling and tRNA-sequencing would be useful. These techniques can detect the critical loss of tRNA function or stability, as well as translational decrease of specific codons and mRNAs at the level of individual codons, mRNA, and tRNA (49–51).
Although m1A58 modification of tRNA3Lys is almost completely absent in TRMT6 mutant #2 (Figures 2E and 3B), we observed some residual transduction ability of the VSV-G pseudotyped HIV-1 produced from TRMT6 mutant cell #2 (Figure 4B). This activity may be due to a second reverse-transcription stop caused by residual m5Um54. A previous in vitro study indicated that the HIV-1 RT stops at m5Um54 (27). Although the m5Um54 level decreased in TRMT6 mutants, about 40% of the m5Um54 in tRNA3Lys remained compared to control cells (Figure 2E). The RT stop at remaining m5Um54 might account for the residual infectivity of the HIV-1 produced from TRMT6 mutant cells (Figure 6B, middle). Since the RT product that extends to one nucleotide before position 54 of tRNA3Lys is still complementary to the HIV-1 genome, this RT product can be used for plus-strand transfer (Figure 6A).
Since both TRMT6 and TRMT61A mRNA levels are substantially lower in whole blood compared to various organs (52), it is possible that m1A58 levels are low in HIV-1 host macrophage and CD4+ T cells. In such cells, the remaining m5Um54 may promote reverse-transcription stop and HIV-1 infection. The importance of the second RT-stop site is supported by the sequence conservation and tRNA3Lys complementarity of the retroviral RNA genome in not only the 18-nt primer binding site, but also until one nt before the m5Um54 (Figure 6A). The conservation of the second RT strong-stop site extends from various HIV-1 strains to HIV-2, and simian and feline retroviruses, suggesting the evolutionary importance of the second RT strong-stop site (Figure 6A).
Among the hydrogen bond network within tRNAs, the reverse-Hoogsteen base pair between A58-U54 is one of the most conserved interactions that support the L-shaped tRNA tertiary structure (53). The m1A modification at position 58 stabilizes the A58-U54 reverse Hoogsteen base pair (7). The A58–U54 base pair stacks with the conserved G53–C61 base pair, and the increased hydrophobicity resulting from 5-methylation of m5U54 is thought to increase base stacking with G53 (53). Unlike many tRNA modification enzymes for which knockout cells can be generated without difficulty using the CRISPR/Cas9 system, we did not obtain TRMT61A or TRMT6 knockout cells, implying that TRMT61A and TRMT6 may be essential for human cells. In the present study, we demonstrated that the TRMT6 mutation greatly reduced m5U54 modification. TRMT6-mediated promotion of m5U54 methylation may support tRNA maturation in two ways. First, 5-methylation in m5U54 may increase the stacking of G53 and U54. Second, TRMT2A, the m5U54 methyltransferase, might promote proper tRNA folding. Both the E. coli and yeast m5U54 methyltransferases are postulated to be tRNA chaperones (54,55). Distinguishing between the two possible roles of m5U54 merit investigation in human, to further understand the role of TRMT6.
The role of TRMT6 in promoting m5U54 formation, however, cannot explain the possible essentiality of TRMT61A and TRMT6, because m5U54 methyltransferase TRMT2A can be knocked out in human cells (56). Thus, there is likely other mechanism(s) that relies on m1A58 to support tRNA function or stability. In yeast, the Trm61–Trm6 complex is required to prevent degradation of initiator methionine tRNA (tRNAiMet) (57,58). Also, in human cells, siRNA-mediated knock down of TRMT61A and TRMT6 reduced tRNAiMet (16). However, since tRNAiMet functions in translation initiation, a decrease in tRNAiMet should affect all proteins and cannot account for the specific decrease in HIV-1 proteins (Figure 5E, F, Supplementary Figure S4B). Thus, there must be other functions than tRNAiMet stabilization, such as stabilization of other cellular tRNAs. To elucidate basic cellular m1A58 function in tRNA stabilization and translation, unbiased methods such as AlkB-tRNA sequencing to monitor the level of all tRNAs (12) and ribosome-profiling to reveal the translation level of all codons (59) would be useful in future studies.
In summary, we first confirmed that m1A58 in tRNA is required for in vitro plus-strand strong-stop by the HIV-1 RT. Accordingly, infection of VSV-G pseudotyped HIV-1 that contained m1A58-less tRNA3Lys primers caused poor HIV-1 genome integration. Moreover, TRMT6 mutant cells decreased accumulation of HIV-1 capsid, integrase, their precursor proteins and Tat in the cells, followed by poor virus production from the cells, revealing unexpected roles for TRMT6 in HIV-1 protein and viral particle accumulation. In addition, TRMT6-mediated m1A58 promoted tRNA m5U54 formation. These findings illuminate the fundamental importance of m1A58 modification in retrovirus replication and tRNA modification network (Figure 6).
DATA AVAILABILITY
All data and cell resources presented in this study are available upon reasonable request.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Yasuo Ariumi and the members of the Tomizawa lab for fruitful discussions, Yuka Watanabe and Yuka Tashiro for technical assistance and Natalie DeWitt for critical reading and language editing. We also thank the Kumamoto University Radioisotope Center at which primer extension experiments and pulse-labeling experiment were performed. The cell illustration in Figure 6 was obtained from Lab icons (https://lab-icons.com).
Contributor Information
Hiroyuki Fukuda, Department of Molecular Physiology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Kumamoto 860-8556, Japan.
Takeshi Chujo, Department of Molecular Physiology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Kumamoto 860-8556, Japan.
Fan-Yan Wei, Department of Molecular Physiology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Kumamoto 860-8556, Japan; Department of Modomics Biology and Medicine, Institute of Development, Aging and Cancer, Tohoku University, Sendai, Miyagi 980-8575, Japan.
Sheng-Lan Shi, Department of Molecular Physiology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Kumamoto 860-8556, Japan.
Mayumi Hirayama, Department of Molecular Physiology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Kumamoto 860-8556, Japan.
Taku Kaitsuka, Department of Molecular Physiology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Kumamoto 860-8556, Japan; School of Pharmacy at Fukuoka, International University of Health and Welfare, Okawa, Fukuoka 831-8501, Japan.
Takahiro Yamamoto, Department of Molecular Physiology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Kumamoto 860-8556, Japan.
Hiroyuki Oshiumi, Department of Immunology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Kumamoto 860-8556, Japan.
Kazuhito Tomizawa, Department of Molecular Physiology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Kumamoto 860-8556, Japan.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Japan Society for the Promotion of Science KAKENHI [18H02865 and 17KT0054 to K.T., 20H03187 to T.C.]; Sasakawa Scientific Research Grant from the Japan Science Society (to H.F.); Fusion Oriented REsearch for Disruptive Science and Technology (FOREST) [JPMJFR204Z to T.C.]. Funding for open access charge: Japan Society for the Promotion of Science KAKENHI [20H03187 to T.C.].
Conflict of interest statement. None declared.
REFERENCES
- 1. Frye M., Jaffrey S.R., Pan T., Rechavi G., Suzuki T.. RNA modifications: what have we learned and where are we headed?. Nat. Rev. Genet. 2016; 17:365–372. [DOI] [PubMed] [Google Scholar]
- 2. Boccaletto P., Machnicka M.A., Purta E., Piatkowski P., Baginski B., Wirecki T.K., de Crecy-Lagard V., Ross R., Limbach P.A., Kotter A.et al.. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018; 46:D303–D307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chujo T., Tomizawa K.. Human transfer RNA modopathies: diseases caused by aberrations in transfer RNA modifications. FEBS J. 2021; 10.1111/febs.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Crecy-Lagard V., Boccaletto P., Mangleburg C.G., Sharma P., Lowe T.M., Leidel S.A., Bujnicki J.M.. Matching tRNA modifications in humans to their known and predicted enzymes. Nucleic Acids Res. 2019; 47:2143–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheitl C.P.M., Ghaem Maghami M., Lenz A.K., Hobartner C.. Site-specific RNA methylation by a methyltransferase ribozyme. Nature. 2020; 587:663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim S.H., Suddath F.L., Quigley G.J., McPherson A., Sussman J.L., Wang A.H., Seeman N.C., Rich A.. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974; 2:435–440. [DOI] [PubMed] [Google Scholar]
- 7. Oliva R., Cavallo L., Tramontano A.. Accurate energies of hydrogen bonded nucleic acid base pairs and triplets in tRNA tertiary interactions. Nucleic Acids Res. 2006; 34:865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zagryadskaya E.I., Doyon F.R., Steinberg S.V.. Importance of the reverse Hoogsteen base pair 54-58 for tRNA function. Nucleic Acids Res. 2003; 31:3946–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watanabe K., Oshima T., Saneyoshi M., Nishimura S.. Replacement of ribothymidine by 5-methyl-2-thiouridine in sequence GTYC in tRNA of an extreme thermophile. FEBS Lett. 1974; 43:59–63. [DOI] [PubMed] [Google Scholar]
- 10. Shigi N., Suzuki T., Terada T., Shirouzu M., Yokoyama S., Watanabe K.. Temperature-dependent biosynthesis of 2-thioribothymidine of Thermus thermophilus tRNA. J. Biol. Chem. 2006; 281:2104–2113. [DOI] [PubMed] [Google Scholar]
- 11. Yamagami R., Tomikawa C., Shigi N., Kazayama A., Asai S., Takuma H., Hirata A., Fourmy D., Asahara H., Watanabe K.et al.. Folate-/FAD-dependent tRNA methyltransferase from Thermus thermophilus regulates other modifications in tRNA at low temperatures. Genes Cells. 2016; 21:740–754. [DOI] [PubMed] [Google Scholar]
- 12. Clark W.C., Evans M.E., Dominissini D., Zheng G., Pan T.. tRNA base methylation identification and quantification via high-throughput sequencing. RNA. 2016; 22:1771–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozanick S., Krecic A., Andersland J., Anderson J.T.. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. RNA. 2005; 11:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saikia M., Fu Y., Pavon-Eternod M., He C., Pan T.. Genome-wide analysis of N1-methyl-adenosine modification in human tRNAs. RNA. 2010; 16:1317–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finer-Moore J., Czudnochowski N., O’Connell J.D. 3rd, Wang A.L., Stroud R.M.. Crystal structure of the human tRNA m(1)A58 methyltransferase-tRNA(3)(Lys) complex: refolding of substrate tRNA allows access to the methylation target. J. Mol. Biol. 2015; 427:3862–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macari F., El-Houfi Y., Boldina G., Xu H., Khoury-Hanna S., Ollier J., Yazdani L., Zheng G., Bieche I., Legrand N.et al.. TRM6/61 connects PKCalpha with translational control through tRNAi(Met) stabilization: impact on tumorigenesis. Oncogene. 2016; 35:1785–1796. [DOI] [PubMed] [Google Scholar]
- 17. Gilboa E., Mitra S.W., Goff S., Baltimore D.. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979; 18:93–100. [DOI] [PubMed] [Google Scholar]
- 18. Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M.. Nucleotide sequence of the AIDS virus, LAV. 1985; 40:9–17. [DOI] [PubMed] [Google Scholar]
- 19. Jin D., Musier-Forsyth K.. Role of host tRNAs and aminoacyl-tRNA synthetases in retroviral replication. J. Biol. Chem. 2019; 294:5352–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang M., Mak J., Lahda A., Cohen E., Klein M., Rovinski B., Kleiman L.. Identification of tRNAs incorporated into wild-type and mutant human immunodeficiency virus type 1. J. Virol. 1993; 67:3246–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters G., Dahlberg J.E.. RNA-directed DNA synthesis in Moloney murine leukemia virus: interaction between the primer tRNA and the genome RNA. J. Virol. 1979; 31:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charneau P., Clavel F.. A single-stranded gap in human immunodeficiency virus unintegrated linear DNA defined by a central copy of the polypurine tract. J. Virol. 1991; 65:2415–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huber H.E., Richardson C.C.. Processing of the primer for plus strand DNA synthesis by human immunodeficiency virus 1 reverse transcriptase. J. Biol. Chem. 1990; 265:10565–10573. [PubMed] [Google Scholar]
- 24. Omer C.A., Faras A.J.. Mechanism of release of the avian retrovirus tRNATrp primer molecule from viral DNA by Ribonuclease H during reverse transcription. Cell. 1982; 30:797–805. [DOI] [PubMed] [Google Scholar]
- 25. Roth M.J., Schwartzberg P.L., Goff S.. Structure of the termini of DNA intermediates in the integration of retroviral DNA: dependence on IN function and terminal DNA sequence. Cell. 1989; 58:47–54. [DOI] [PubMed] [Google Scholar]
- 26. Burnett B.P., McHenry C.S.. Posttranscriptional modification of retroviral primers is required for late stages of DNA replication. PNAS. 1997; 94:7210–7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Auxilien S., Keiths G., Le Grice S.F., Darlix J.L.. Role of post-transcriptional modifications of primer tRNALys,3 in the fidelity and efficacy of plus strand DNA transfer during HIV-1 reverse transcription. J. Biol. Chem. 1999; 274:4412–4420. [DOI] [PubMed] [Google Scholar]
- 28. Ben-Artzi H., Shemesh J., Zeelon E., Amit B., Kleiman L., Gorecki M., Panet A.. Molecular analysis of the second template switch during reverse transcription of the HIV RNA template. Biochemistry. 1996; 35:10549–10557. [DOI] [PubMed] [Google Scholar]
- 29. Renda M.J., Rosenblatt J.D., Klimatcheva E., Demeter L.M., Bambara R.A., Planelles V.. Mutation of the methylated tRNA(Lys)(3) residue A58 disrupts reverse transcription and inhibits replication of human immunodeficiency virus type 1. J. Virol. 2001; 75:9671–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou H., Xu M., Huang Q., Gates A.T., Zhang X.D., Castle J.C., Stec E., Ferrer M., Strulovici B., Hazuda D.J.et al.. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008; 4:495–504. [DOI] [PubMed] [Google Scholar]
- 31. Yu W., McCulley A., Morrow C.D.. Mutations in the TΨC loop of E. coli tRNALys3 have varied effects on in trans complementation of HIV-1 replication. Virol. J. 2007; 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shalem S., Sanjana N.E., Hartenian E., Shi X., Scott D.A., Mikkelsen T.S., Heckl D., Ebert B.L., Root D.E., Doench J.G.et al.. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014; 343:84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Y., Shi J., Liu X., Feng L., Gong Z., Koppula P., Sirohi K., Li X., Wei Y., Lee H.et al.. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018; 20:1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hirayama M., Wei F.Y., Chujo T., Oki S., Yakita M., Kobayashi D., Araki N., Takahashi N., Yoshida R., Nakayama H.et al.. FTO demethylates cyclin D1 mRNA and controls cell-cycle progression. Cell reports. 2020; 31:107464. [DOI] [PubMed] [Google Scholar]
- 35. Chujo T., Suzuki T.. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012; 18:2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takesue Y., Wei F.Y., Fukuda H., Tanoue Y., Yamamoto T., Chujo T., Shinojima N., Yano S., Morioka M., Mukasa A.et al.. Regulation of growth hormone biosynthesis by Cdk5 regulatory subunit associated protein 1-like 1 (CDKAL1) in pituitary adenomas. Endocr. J. 2019; 66:807–816. [DOI] [PubMed] [Google Scholar]
- 37. Baeyens A., Naessens E., Van Nuffel A., Weening K.E., Reilly A.M., Claeys E., Trypsteen W., Vandekerckhove L., Eyckerman S., Gevaert K.et al.. HIV-1 Vpr N-terminal tagging affects alternative splicing of the viral genome. Sci. Rep. 2016; 6:34573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Houzet L., Paillart J.C., Smagulova F., Maurel S., Morichaud Z., Marquet R., Mougel M.. HIV controls the selective packaging of genomic, spliced viral and cellular RNAs into virions through different mechanisms. Nucleic Acids Res. 2007; 35:2695–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howell N.W., Jora M., Jepson B.F., Libach P.A., Jackman J.E.. Distinct substrate specificities of the human tRNA methyltransferases TRMT10A and TRMT10B. RNA. 2019; 25:1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vilardo E., Amman F., Toth U., Kotter A., Helm M., Rossmanith W.. Functional characterization of the human tRNA methyltransferases TRMT10A and TRMT10B. Nucleic Acids Res. 2020; 48:6157–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vilardo E., Nachbagauer C., Buzet A., Taschner A., Holzmann J., Rossmanith W.. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase–extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012; 40:11583–11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gross H.J., Simsek M., Raba M., Limburg K., Heckman J., Raj Bhandary U.L.. 2′-O-Methyl ribothymidine: a component of rabbit liver lysine transfer RNA. Nucleic Acids Res. 1974; 1:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu T., Guo J., Bess J., Henderson L.E., Levin J.. Molecular requirements for human immunodeficiency virus type 1 plus-strand transfer: analysis in reconstituted and endogenous reverse transcription systems. J. Virol. 1999; 73:4794–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suzuki T., Yashiro Y., Kikuchi I., Ishigami Y., Saito H., Matsuzawa I., Okada S., Mito M., Iwasaki S., Ma D.et al.. Complete chemical structures of human mitochondrial tRNAs. Nat. Commun. 2020; 11:4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen S., Kumar S., Espada C.E., Tirumuru N., Cahill M.P., Hu L., He C., Wu L.. N6-methyladenosine modification of HIV-1 RNA suppresses type-I interferon induction in differentiated monocytic cells and primary macrophages. PLoS Pathogens. 2021; 17:e1009421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simonova A., Svojanovska B., Trylcova J., Hubalek M., Moravcik O., Zavrel M., Pavova M., Hodek J., Weber J., Cvacka J.et al.. LC/MS analysis and deep sequencing reveal the accurate RNA composition in the HIV-1 virion. Sci. Rep. 2019; 9:8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Weringh A., Ragonnet-Cronin M., Pranckeviciene E., Pavon-Eternod M., Kleiman L., Xia X.. HIV-1 modulates the tRNA pool to improve translation efficiency. Mol. Biol. Evol. 2011; 28:1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Safra M., Sas-Chen A., Nir R., Winkler R., Nachshon A., Bar-Yaacov D., Erlacher M., Rossmanith W., Stern-Ginossar N., Schwartz S.. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017; 551:251–255. [DOI] [PubMed] [Google Scholar]
- 49. Chen C.W., Tanaka M.. Genome-wide translation profiling by ribosome-bound tRNA capture. Cell Rep. 2018; 23:608–621. [DOI] [PubMed] [Google Scholar]
- 50. Lin S., Liu Q., Lelyveld V.S., Choe J., Szostak J.W., Gregory R.I.. Mettl1/Wdr4-mediated m(7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol. Cell. 2018; 71:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagayoshi Y., Chujo T., Hirata S., Nakatsuka H., Chen C.W., Takakura M., Miyauchi K., Ikeuchi Y., Carlyle B.C., Kitchen R.R.et al.. Loss of Ftsj1 perturbs codon-specific translation in the brain and is associated with X-linked intellectual disability. Sci. Adv. 2021; 7:eabf3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gamazon E.R., Segre A.V., van de Bunt M., Wen X., Xi H.S., Hormozdiari F., Ongen H., Konkashbaev A., Derks E.M., Aguet F.et al.. Using an atlas of gene regulation across 44 human tissues to inform complex disease- and trait-associated variation. Nat. Genet. 2018; 50:956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roovers M., Droogmans L., Grosjean H.. Post-transcriptional modifications of conserved nucleotides in the T-loop of tRNA: a tale of functional convergent evolution. Genes (Basel). 2021; 12:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Johansson M.J., Bystrom A.S.. Dual function of the tRNA (m5U54) methyltransferase in tRNA maturation. RNA. 2002; 8:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keffer-Wilkes L.C., Soon E.F., Kothe U.. The methyltransferase TrmA facilitates tRNA folding through interaction with its RNA-binding domain. Nucleic Acids Res. 2020; 48:7981–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carter J.M., Emmett W., Mozos I.R., Kotter A., Helm M., Ule J., Hussain S.. FICC-Seq: a method for enzyme-specified profiling of methyl-5-uridine in cellular RNA. Nucleic Acids Res. 2019; 47:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anderson J., Phan L., Cuesta R., Carlson B.A., Pak M., Asano K., Bjork G.R., Tamame M., Hinnebusch A.G.. The essential Gcd10p–Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes & Development. 1998; 12:3650–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kadaba S., Krueger A., Trice T., Krecic A.M., Hinnebusch A.G., Anderson J.. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 2004; 18:1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ingolia N.T., Ghaemmaghami S., Newman J.R., Weissman J.S.. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009; 324:218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and cell resources presented in this study are available upon reasonable request.