Abstract
Background
The influence of type 2 diabetes mellitus (T2D) duration on cancer incidence remains poorly understood.
Methods
We prospectively followed for cancer incidence 113 429 women in the Nurses’ Health Study (1978-2014) and 45 604 men in the Health Professionals Follow-up Study (1988-2014) who were free of diabetes and cancer at baseline. Cancer incidences were ascertained by review of medical records.
Results
In the multivariable-adjusted model incident, T2D was associated with higher risk of cancers in the colorectum, lung, pancreas, esophagus, liver, thyroid, breast, and endometrium. The pooled hazard ratios (HRs) ranged from 1.21 (95% confidence interval [CI] = 1.06 to 1.38) for colorectal cancer to 3.39 (95% CI = 2.24 to 5.12) for liver cancer. For both composite cancer outcomes and individual cancers, the elevated risks did not further increase after 8 years of T2D duration. The hazard ratio for total cancer was 1.28 (95% CI = 1.17 to 1.40) for T2D duration of 4.1-6.0 years, 1.37 (95% CI = 1.25 to 1.50) for 6.1-8.0 years, 1.21 (95% CI = 1.09 to 1.35) for 8.1-10.0 years, and 1.04 (95% CI = 0.95 to 1.14) after 15.0 years. In a cross-sectional analysis, a higher level of plasma C-peptide was found among participants with prevalent T2D of up to 8 years than those without T2D, whereas a higher level of HbA1c was found for those with prevalent T2D of up to 15 years.
Conclusions
Incident T2D was associated with higher cancer risk, which peaked at approximately 8 years after diabetes diagnosis. Similar duration-dependent pattern was observed for plasma C-peptide. Our findings support a role of hyperinsulinemia in cancer development.
The US Center for Disease Control estimated that more than 34 million Americans had diabetes mellitus in 2020, accounting for 10.5% of the US population (1). A growing body of evidence indicates a close link between the presence of type 2 diabetes mellitus (T2D) and risk of certain types of cancer (2). However, few previous studies simultaneously addressed multiple methodological challenges in the associations between T2D and cancer risk, including ascertainment bias because of more frequent screening following T2D diagnosis, reverse causality (such as pancreatic cancer), and influence of cancer screening (3). Moreover, the evidence regarding the associations of T2D with other cancers such as kidney and lung cancer remains inconclusive (2). In addition, the association between T2D duration and cancer risk remains poorly understood. Specifically, little is known about whether the increased cancer risk following T2D diagnosis would persist in patients with longer T2D duration or gradually diminish over time as endogenous production of insulin decreases (4). Also, whether such duration-related patterns vary by cancer types is yet to be determined. Characterizing the cancer risk trajectory following T2D diagnosis not only is critical to assess the long-term influence of diabetes on cancer incidence but also directly pertains to the assessment of cancer latency period, an important factor to elucidate the role of T2D in tumorigenesis.
The current study aimed to evaluate the association between incident T2D, T2D duration, and cancer incidence for multiple composite cancer outcomes as well as individual cancers while addressing aforementioned methodological issues. To gain mechanistic insights, we also assessed plasma levels of C-peptide and HbA1c, 2 most commonly used clinical measures for endogenous insulin secretion and long-term blood glucose concentration (5,6), respectively, according to diabetes duration. We leveraged data from Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS), 2 large well-characterized cohort studies with disease status and lifestyle factors repeatedly assessed over 3 decades of follow-up.
Methods
Study Population
The NHS cohort was initiated in 1976 when 121 700 female registered nurses aged 30-55 years were recruited, and the HPFS cohort was established in 1986 and included 51 529 male health professionals aged 40-75 years. Participants were followed biennially through mailed questionnaires inquiring medical and lifestyle information (7,8).
We set as baseline for the current study the first follow-up cycle for NHS (1978) and HPFS (1988) to identify patients with incident T2D. Prevalent diabetes in 1976 (n = 1955) and 1986 (n = 1640) were excluded because their diabetes diagnosis dates were not available. We also excluded participants with prevalent cancer at baseline (n = 4350 in NHS; n = 2652 in HPFS) and those who answered only the baseline questionnaire (n = 932 in NHS; n = 771 in HPFS). For incident T2D cases without known diagnosis date, we set the diagnosis date as the return date of the follow-up questionnaire in which participants first reported T2D diagnosis. After exclusion, we had 113 429 participants from NHS and 45 604 participants from HPFS.
This study was approved by the institutional review board at Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating registries as required. The return of a completed questionnaire was considered informed consent. We obtained written consent to acquire medical records to document cases of cancer.
Assessment of Type 2 Diabetes
Participants who reported a diagnosis of T2D were mailed a supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy. For cases diagnosed before 1998, T2D was confirmed using the National Diabetes Data Group criteria (9), and for cases of T2D identified after 1998, the cutoff point for elevated fasting plasma glucose concentrations was lowered to 7.0 mmol/L according to the American Diabetes Association criteria. We further considered HbA1c of 6.5% and higher in the diagnosis criteria for confirming T2D cases identified after January 2010 (10). Validation studies in the NHS and HPFS demonstrated the validity of using our supplementary questionnaire to adjudicate T2D diagnosis (Supplementary Methods, available online) (11,12).
Assessment of Cancer Incidence
Total cancer involved all types of cancer with International Classification of Diseases–9 code between 140 and 239 except nonmelanoma skin cancers and nonfatal prostate cancer. Nonmelanoma skin cancers were excluded from total cancer because of their high incidence and extremely low degree of malignancy, which would strongly influence the results. Nonfatal prostate cancer was not considered either, because of its consistent inverse association with T2D and potential detection bias resulting from prostate-specific antigen screening, all of which would introduce substantial heterogeneity to the risk estimates. Obesity-related cancer and diabetes-related cancer were defined according to previous studies (13,14). Cancer diagnosis was confirmed by medical record review among participants who reported a diagnosis of cancer on the follow-up questionnaires. For the current analysis, we counted only the first documented cancer for participants diagnosed with multiple cancers.
Assessment of Diet, Physical Activity, Screening, and Medication Use
A validated semiquantitative food frequency questionnaire was administered to collect information of diet and alcohol intake approximately every 4 years since 1980 in NHS and 1986 in HPFS. We used the alternative health eating index score to quantify overall diet quality (15). In both cohorts, information on demographic profiles, lifestyle characteristics, and body weight were collected at baseline and in biennial questionnaires. Physical activity was self-reported via a validated questionnaire (16). Starting from 1988 and every 2 years thereafter, the follow-up questionnaires asked participants whether they had had a colonoscopy and/or sigmoidoscopy in the previous 2 years in the 2 cohorts. Similarly, the information regarding fasting glucose examination was collected from 1998 in the NHS and 2000 in the HPFS. From 1988, women were asked whether they underwent a mammography screening during the previous 2 years. The information on insulin use and oral hypoglycemic drug use were collected from 1982 in NHS, but such data were not available in HPFS.
Assessment of C-Peptide and HbA1c
Blood samples were collected from subpopulations of the NHS (n = 32 826) in 1989-1990 and HPFS (n = 18 225) from 1993 to 1995. Detailed information on the blood collection has been described elsewhere (17). In these subpopulations, multiple biomarkers including C-peptide and HbA1c were measured from several nested case-control studies for various outcomes. Data were pooled from 2 cohorts to increase statistical power, and a total of 10 896 (362 prevalent diabetes patients) and 11 105 (1689 prevalent diabetes patients) participants were included in the analysis for C-peptide and HbA1c, respectively (Supplementary Methods, available online).
Statistical Analysis
To capture the dynamic characteristics for participants with and without T2D during the follow-up, the demographic, lifestyle, and dietary information was presented according to the person-time of T2D status. For each participant, follow-up time was counted from the return date of the baseline questionnaire to the date of cancer diagnosis, death date, date of return of last available follow-up questionnaire, or the end of follow-up (June 2014 for NHS and January 2014 for HPFS), whichever happened first. T2D duration was defined in years as the difference between the questionnaire return date in each follow-up cycle and the diabetes diagnosis date. We first analyzed the 2 cohorts separately and then combined the estimates using a fixed-effect meta-analysis.
A multivariable Cox proportional hazards model with age (months) as the time scale was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Given the importance of body mass index (BMI) for both T2D and cancer, we additionally adjusted for the cumulative-averaged BMI in the multivariable model. The proportional hazards assumption was tested by including the interaction terms between T2D duration and follow-up time for all models, and no statistically significant violations were detected.
We calculated the hazard ratios according to categories of T2D duration using prespecified cutoffs: no more than 2 years, 2.1-4.0 years, 4.1-6.0 years, 6.1-8.0 years, 8.1-10.0 years, 10.1-15 years, and more than 15 years. We also employed the restricted cubic spline analysis (18,19) to test for potential nonlinear relationships between T2D duration and cancer risk. Three knots were specified for diabetes duration of 0, 6, and 10 years based on the results of the categorical analysis. To assess potential effect modification, we performed stratified analyses according to age (60 years or younger, older than 60 years), cumulative-averaged BMI (<25 kg/m2, 25-30 kg/m2, ≥30 kg/m2), and smoking status (never smoker, ever smoker). Product terms between T2D status and dichotomized age, smoking status, and continuous BMI were additionally included in the models, and Wald test was used to calculate the P value for interaction.
In a secondary analysis, we conducted a cross-sectional analysis by calculating the least square geometric means of C-peptide and HbA1c by diabetes duration to further understand the nonlinear association between T2D duration and cancer risk in the primary analysis. The diabetes duration was calculated as year of blood draw (1990 for NHS and 1994 for HPFS) minus the year of T2D diagnosis. The multiple linear regression model was adjusted for age at blood draw, aspirin and/or NSAID use, presence of chronic diseases or conditions (hypercholesterinemia, hypertension, cancer, osteoporosis, rheumatoid arthritis, stroke, and myocardial infarction), physical activity, smoking status, case-control status, sex, and BMI (20,21).
Because participants may change their lifestyle and dietary behaviors following a diagnosis of T2D, the results might have been attenuated by overadjustment for these changes. Therefore, we conducted a sensitivity analysis by using baseline values for covariate adjustment. All statistical tests were 2-sided with a statistical significance level of .05 and were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
The age-standardized characteristics of participants are shown according to T2D status in Table 1. In both cohorts, compared with participants without T2D during the follow-up, participants diagnosed with T2D had older age, higher BMI, higher proportion of non-White race, higher prevalence of hypertension and hypercholesterinemia, less physical activity and alcohol intake, and higher proportion of family history of diabetes but slightly lower proportion of family history of cancer. Women participants diagnosed with T2D were also more likely to use oral contraceptive but less so for postmenopausal hormone. Similar diet quality and total energy intake were found for participants with and without diabetes.
Table 1.
Age-standardized characteristics of study participants in Nurses’ Health Study (1978-2014) and Health Professionals Follow-up Study (1988-2014) according to person-years by type 2 diabetesa
| Characteristics | Diagnosis of T2D |
|||
|---|---|---|---|---|
| NHS |
HPFS |
|||
| No | Yes | No | Yes | |
| Person-years | 3 156 447 | 181 931 | 896 795 | 58 905 |
| Age, yb | 58.7 (11.9) | 68.0 (9.6) | 64.5 (11.0) | 71.3 (9.4) |
| Body mass index, kg/m² | 25.6 (4.9) | 30.6 (6.5) | 25.9 (3.6) | 28.7 (5.0) 2 |
| Race | ||||
| White, % | 96.9 | 94.3 | 95.1 | 91.6 |
| African American, % | 1.0 | 2.0 | 2.3 | 3.1 |
| Asian, % | 1.0 | 1.9 | 1.7 | 3.2 |
| Others, % | 1.0 | 1.8 | 0.9 | 2.0 |
| Hypertension, % | 32.2 | 66.0 | 36.5 | 68.2 |
| High cholesterol, % | 35.6 | 58.1 | 44.4 | 68.4 |
| Smoking status | ||||
| Never-smokers, % | 45.1 | 44.5 | 45.5 | 37.3 |
| Past smokers, % | 37.9 | 40.9 | 48.2 | 54.7 |
| Current smokers, % | 17.0 | 14.6 | 6.4 | 8.0 |
| Family history of diabetes, % | 24.4 | 49.1 | 21.1 | 38.8 |
| Family history of cancer, % | 45.6 | 43.9 | 33.5 | 31.9 |
| Multivitamin use, % | 39.5 | 38.2 | 43.2 | 41.4 |
| Oral contraceptive use, % | 44.5 | 50.7 | — | — |
| Hormone use | ||||
| Premenopausal, % | 30.1 | 29.0 | — | — |
| Postmenopausal-never, % | 22.1 | 24.4 | — | — |
| Postmenopausal-current, % | 22.5 | 17.8 | — | — |
| Postmenopausal-past, % | 25.3 | 28.7 | — | — |
| Physical activity, median (IQR), MET-h/wk | 9.8 (3.2-22.7) | 6.7 (2.0-16.5) | 23.6 (10.0-45.0) | 18.1 (7.0-37.7) |
| Alcohol consumption, median (IQR), g/day | 2.0 (0.2-8.2) | 0.6 (0.0-2.7) | 6.5 (1.2-15.5) | 4.0 (0.7-11.7) |
| Alternative healthy eating index | 50.7 (10.3) | 49.4 (9.4) | 54.4 (10.6) | 53.2 (10.1) |
| Total energy intake (Kcal/d) | 1691 (539) | 1699 (546) | 1987 (634) | 1960 (662) |
Baseline characteristics based on analysis for total cancer incidence. IQR = interquartile range; MET-h/wk = Metabolic-equivalent-hours per week.
Value is not age adjusted.
During 4 294 078 person-years of follow-up, we documented in the 2 cohorts a total of 43 849 cancer cases of which 21 977 (50.1%) were obesity-related cancer and 26 904 (61.4%) were diabetes-related cancer. After adjusting for demographic, lifestyle, dietary factors, and BMI, T2D was associated with statistically significantly increased risk of all 3 composite cancer outcomes (Table 2). The pooled hazard ratios were 1.21 (95% CI = 1.16 to 1.26) for total cancer, 1.28 (95% CI = 1.21 to 1.35) for obesity-related cancer, and 1.25 (95% CI = 1.19 to 1.32) for diabetes-related cancer. For individual cancers, T2D was statistically significantly associated with increased risk of colorectal cancer (HR = 1.21, 95% CI = 1.06 to 1.38), lung cancer (HR = 1.27, 95% CI = 1.12 to 1.45), pancreatic cancer (HR = 2.07, 95% CI = 1.70 to 2.52), esophagus cancer (HR = 1.85, 95% CI = 1.28 to 2.69), liver cancer (HR = 3.39, 95% CI = 2.24 to 5.12), thyroid cancer (HR = 1.49, 95% CI = 1.03 to 2.15), breast cancer (HR = 1.26, 95% CI = 1.17 to 1.37), and endometrial cancer (HR = 1.26, 95% CI = 1.06 to 1.50). No statistically significant heterogeneity was found between the 2 cohorts. The associations for postmenopausal breast cancer, estrogen receptor– and progesterone receptor (ER/PR)–positive breast cancer, and ER/PR-negative breast cancer were similar to that for total breast cancer (Supplementary Table 1, available online).
Table 2.
Association between incident type 2 diabetes and cancer incidence (NHS: 1978-2014; HPFS: 1988-2014)
| Cancer outcomes | Women (NHS) |
Men (HPFS) |
Meta-analyzed HR (95% CI)a |
P heterogeneity | ||
|---|---|---|---|---|---|---|
| No diabetes | Diabetes | No diabetes | Diabetes | |||
| Composite cancer outcomes | ||||||
| Total cancer | ||||||
| Case/person-year | 32 147/3 156 447 | 3182/181 931 | 7755/896 795 | 765/58 905 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.24 (1.19 to 1.28) | (Referent) | 1.17 (1.09 to 1.26) | 1.22 (1.18 to 1.27) | .21 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.29 (1.24 to 1.35) | (Referent) | 1.20 (1.11 to 1.29) | 1.27 (1.22 to 1.32) | .09 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.22 (1.17 to 1.28) | (Referent) | 1.16 (1.07 to 1.25) | 1.21 (1.16 to 1.26) | .22 |
| Obesity-related cancerd | ||||||
| Case/person-year | 16 956/3 168 448 | 1820/182 934 | 2888/900 763 | 313/59 225 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.31 (1.25 to 1.37) | (Referent) | 1.42 (1.26 to 1.60) | 1.32 (1.27 to 1.39) | .22 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.37 (1.29 to 1.46) | (Referent) | 1.46 (1.29 to 1.65) | 1.39 (1.32 to 1.47) | .39 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.26 (1.18 to 1.34) | (Referent) | 1.37 (1.21 to 1.55) | 1.28 (1.21 to 1.35) | .23 |
| Diabetes-related cancere | ||||||
| Case/person-year | 20 807/3 165 042 | 2021/182 782 | 3677/900 047 | 399/59 163 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.28 (1.22 to 1.34) | (Referent) | 1.33 (1.20 to 1.47) | 1.28 (1.23 to 1.34) | .50 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.34 (1.27 to 1.42) | (Referent) | 1.32 (1.19 to 1.47) | 1.34 (1.27 to 1.41) | .79 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.25 (1.18 to 1.32) | (Referent) | 1.25 (1.13 to 1.40) | 1.25 (1.19 to 1.32) | .94 |
| Individual cancers | ||||||
| Colorectal cancer | ||||||
| Case/person-year | 2245/3 181 603 | 229/184 360 | 1030/902 189 | 109/59 345 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.24 (1.08 to 1.42) | (Referent) | 1.44 (1.17 to 1.76) | 1.30 (1.16 to 1.45) | .23 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.20 (1.01 to 1.42) | (Referent) | 1.45 (1.19 to 1.78) | 1.30 (1.14 to 1.48) | .16 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.13 (0.95 to 1.35) | (Referent) | 1.32 (1.08 to 1.63) | 1.21 (1.06 to 1.38) | .25 |
| Lung cancer | ||||||
| Case/person-year | 2679/3 181 921 | 261/184 406 | 776/902 687 | 98/59 386 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.03 (0.91 to 1.17) | (Referent) | 1.41 (1.14 to 1.75) | 1.12 (1.00 to 1.25) | .01 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.12 (0.96 to 1.31) | (Referent) | 1.42 (1.14 to 1.76) | 1.21 (1.07 to 1.38) | .09 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.20 (1.03 to 1.41) | (Referent) | 1.42 (1.14 to 1.77) | 1.27 (1.12 to 1.45) | .23 |
| Melanoma | ||||||
| Case/person-year | 1019/3 182 641 | 66/184 515 | 1136/902 084 | 82/59 366 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 0.86 (0.67 to 1.11) | (Referent) | 0.83 (0.66 to 1.04) | 0.84 (0.71 to 1.00) | .82 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.01 (0.74 to 1.39) | (Referent) | 0.89 (0.71 to 1.12) | 0.93 (0.77 to 1.12) | .52 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 0.95 (0.69 to 1.30) | (Referent) | 0.89 (0.70 to 1.12) | 0.91 (0.75 to 1.10) | .75 |
| Non-Hodgkin lymphoma | ||||||
| Case/person-year | 1274/3 182 442 | 114/184 473 | 812/902 383 | 66/59 387 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.01 (0.83 to 1.22) | (Referent) | 1.02 (0.79 to 1.31) | 1.01 (0.87 to 1.18) | .95 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.00 (0.78 to 1.28) | (Referent) | 1.02 (0.78 to 1.31) | 1.01 (0.84 to 1.20) | .93 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.01 (0.79 to 1.30) | (Referent) | 0.99 (0.76 to 1.29) | 1.00 (0.84 to 1.20) | .91 |
| Pancreatic cancer | ||||||
| Case/person-year | 556/3 183 276 | 111/184 521 | 318/902 980 | 64/59 414 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 2.05 (1.66 to 2.52) | (Referent) | 2.16 (1.64 to 2.84) | 2.09 (1.77 to 2.46) | .76 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 2.00 (1.54 to 2.60) | (Referent) | 2.29 (1.73 to 3.04) | 2.13 (1.76 to 2.58) | .49 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.87 (1.43 to 2.44) | (Referent) | 2.34 (1.76 to 3.12) | 2.07 (1.70 to 2.52) | .26 |
| Bladder cancer | ||||||
| Case/person-year | 574/3 182 974 | 63/184 515 | 718/902 425 | 63/59 385 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.18 (0.91 to 1.54) | (Referent) | 0.98 (0.76 to 1.28) | 1.08 (0.89 to 1.30) | .34 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.25 (0.90 to 1.73) | (Referent) | 0.92 (0.71 to 1.20) | 1.04 (0.85 to 1.28) | .16 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.21 (0.87 to 1.68) | (Referent) | 0.91 (0.69 to 1.18) | 1.02 (0.82 to 1.25) | .19 |
| Kidney cancer | ||||||
| Case/person-year | 430/3 183 183 | 59/184 512 | 292/902 867 | 30/59 417 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.52 (1.15 to 2.00) | (Referent) | 1.28 (0.88 to 1.88) | 1.43 (1.14 to 1.79) | .49 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.48 (1.05 to 2.07) | (Referent) | 1.22 (0.83 to 1.80) | 1.36 (1.05 to 1.76) | .48 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.18 (0.83 to 1.66) | (Referent) | 1.18 (0.80 to 1.75) | 1.18 (0.91 to 1.53) | .99 |
| Esophagus cancer | ||||||
| Case/person-year | 114/3 183 447 | 14/184 561 | 138/903 025 | 25/59 422 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.21 (0.69 to 2.12) | (Referent) | 2.15 (1.39 to 3.33) | 1.73 (1.23 to 2.45) | .11 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.66 (0.89 to 3.12) | (Referent) | 2.13 (1.36 to 3.33) | 1.96 (1.36 to 2.82) | .53 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.71 (0.90 to 3.26) | (Referent) | 1.93 (1.22 to 3.05) | 1.85 (1.28 to 2.69) | .76 |
| Leukemia | ||||||
| Case/person-year | 268/3 183 353 | 32/184 554 | 262/902 904 | 19/59 422 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.37 (0.95 to 1.99) | (Referent) | 0.83 (0.52 to 1.33) | 1.13 (0.84 to 1.51) | .10 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.32 (0.83 to 2.08) | (Referent) | 0.86 (0.53 to 1.38) | 1.07 (0.77 to 1.49) | .20 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.18 (0.74 to 1.88) | (Referent) | 0.79 (0.49 to 1.28) | 0.97 (0.69 to 1.36) | .25 |
| Liver cancer | ||||||
| Case/person-year | 82/3 183 470 | 32/184 552 | 43/903 100 | 12/59 433 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 3.94 (2.59 to 5.99) | (Referent) | 2.82 (1.47 to 5.41) | 3.57 (2.51 to 5.08) | .40 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 4.12 (2.50 to 6.78) | (Referent) | 3.11 (1.59 to 6.10) | 3.73 (2.50 to 5.56) | .51 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 3.80 (2.27 to 6.35) | (Referent) | 2.77 (1.39 to 5.51) | 3.39 (2.24 to 5.12) | .47 |
| Myeloma | ||||||
| Case/person-year | 281/3 183 299 | 30/184 540 | 183/902 968 | 10/59 429 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.25 (0.85 to 1.82) | (Referent) | 0.61 (0.32 to 1.16) | 1.03 (0.75 to 1.44) | .06 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.51 (0.97 to 2.34) | (Referent) | 0.62 (0.33 to 1.19) | 1.14 (0.80 to 1.65) | .03 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.44 (0.92 to 2.26) | (Referent) | 0.54 (0.28 to 1.04) | 1.06 (0.73 to 1.53) | .02 |
| Thyroid cancer | ||||||
| Case/person-year | 354/3 183 171 | 40/184 529 | 70/903 047 | 8/59 430 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.63 (1.16 to 2.27) | (Referent) | 1.74 (0.82 to 3.67) | 1.64 (1.21 to 2.23) | .88 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.62 (1.08 to 2.44) | (Referent) | 1.65 (0.77 to 3.55) | 1.63 (1.14 to 2.34) | .97 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.50 (0.99 to 2.27) | (Referent) | 1.45 (0.66 to 3.17) | 1.49 (1.03 to 2.15) | .94 |
| Gallbladder cancer | ||||||
| Case/person-year | 136/3 183 451 | 19/184 560 | 51/903 083 | 8/59 432 | ||
| Age-adjusted model, HR (95% CI) | (Referent) | 1.45 (0.89 to 2.36) | (Referent) | 1.79 (0.84 to 3.82) | 1.54 (1.02 to 2.32) | .65 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.22 (0.66 to 2.24) | (Referent) | 2.01 (0.92 to 4.38) | 1.47 (0.91 to 2.39) | .32 |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.05 (0.56 to 1.96) | (Referent) | 1.94 (0.88 to 4.29) | 1.33 (0.81 to 2.17) | .23 |
| Sex-specific cancers | ||||||
| Breast cancer | ||||||
| Case/person-year | 12 064/3 172 217 | 1013/183 570 | — | — | — | — |
| Age-adjusted model, HR (95% CI) | (Referent) | 1.16 (1.09 to 1.24) | — | — | — | — |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.30 (1.20 to 1.41) | — | — | — | — |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.26 (1.17 to 1.37) | — | — | — | — |
| Endometrial cancer | ||||||
| Case/person-year | 1799/3 181 864 | 241/184 344 | — | — | — | — |
| Age-adjusted model, HR (95% CI) | (Referent) | 1.88 (1.63 to 2.15) | — | — | — | — |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.79 (1.51 to 2.13) | — | — | — | — |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.26 (1.06 to 1.50) | — | — | — | — |
| Ovarian cancer | — | — | — | — | ||
| Case/person-year | 1090/3 182 610 | 70/184 504 | ||||
| Age-adjusted model, HR (95% CI) | (Referent) | 0.85 (0.67 to 1.09) | — | — | — | — |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 0.84 (0.61 to 1.15) | — | — | — | — |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 0.81 (0.59 to 1.11) | — | — | — | — |
| Fatal prostate cancer | ||||||
| Case/person-year | — | — | 721/902 446 | 45/59 397 | — | — |
| Age-adjusted model, HR (95% CI) | — | — | (Referent) | 0.94 (0.69 to 1.28) | — | — |
| Multivariable adjusted model, HR (95% CI)b | — | — | (Referent) | 1.02 (0.75 to 1.39) | — | — |
| Additionally adjusted for BMI, HR (95% CI)c | — | — | (Referent) | 0.98 (0.72 to 1.33) | — | — |
Estimates were meta-analyzed using random effect model. BMI = body mass index; CI = confidence interval; HPFS = Health Professionals Follow-up Study; HR = hazard ratio; NHS = Nurses’ Health Study.
Adjusting for age (month), ethnicity (White, African American, Asian, others), smoking status (never smoked, past smoker, currently smoke 1-14 cigarettes per day, 15-24 cigarettes per day, or ≥25 cigarettes per day), alcohol intake (0, 0.1-4.9, 5.0-9.9, 10.0-14.9, 15.0-29.9, and ≥30.0 g/d), multivitamin use (yes, no), physical activity (quintiles), total energy (quintiles), alternative healthy eating index (quintiles), family history of diabetes (yes, no), family history of cancer (yes, no), endoscopy screening (yes, no), and fasting glucose screening (yes, no). For women, insulin use (yes, no), oral hypoglycemic drug use (yes, no), mammography screening, postmenopausal hormone use (never, former, or current hormone use, or missing), and oral contraceptive use were further adjusted.
Cumulative-averaged BMI (calculated as weight in kilograms divided by height in meters squared) (<21.0, 21.0-22.9, 23.0-24.9, 25.0-26.9, 27.0-29.9, 30.0-32.9, 33.0-34.9, or ≥35.0 kg/m2) was additionally adjusted.
Including esophagus cancer, liver cancer, kidney cancer, myeloma, pancreatic cancer, colorectal cancer, gallbladder cancer, postmenopausal breast cancer, ovarian cancer, and thyroid cancer.
Including thyroid cancer, breast cancer, liver cancer, pancreatic cancer, endometrial cancer, esophagus cancer, colorectal cancer, kidney cancer, gallbladder cancer, ovarian cancer, non-Hodgkin lymphoma, leukemia, and bladder cancer.
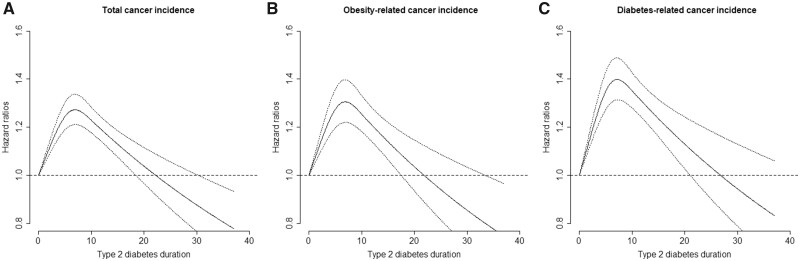
The cancer risks were statistically significantly elevated right after T2D diagnosis, and such risk elevation did not further increase after 8 years of diabetes duration. This risk trajectory was observed for both composite cancer outcomes and most individual cancers that were associated with T2D listed in Table 3. For example, the hazard ratio for developing any cancer increased from 1.29 (95% CI = 1.19 to 1.38) in patients with 0-2.0 years of diabetes duration to 1.37 (95% CI = 1.25 to 1.50) in patients having diabetes for 6.1-8.0 years. The total cancer risk then decreased to 1.21 (95% CI = 1.09 to 1.35) for 8.1-10.0 years of diabetes duration and further down to 1.04 (95% CI = 0.95 to 1.14) after 15.0 years of diabetes duration. The spline analysis revealed statistically significant (P < .05 for all included cancer outcomes) nonlinear relation between diabetes duration and cancer risks; cancer risk appeared to culminate around 6.5-7.5 years after T2D diagnosis and then gradually decreased afterward (Figure 1;Supplementary Figure 1, available online).
Table 3.
Pooled association between diabetes duration and cancer incidence (NHS: 1978-2014; HPFS: 1988-2014)a
| Cancer outcomes | Diabetes duration |
|||||||
|---|---|---|---|---|---|---|---|---|
| No diabetes | 0-2.0 years | 2.1-4.0 years | 4.1-6.0 years | 6.1-8.0 years | 8.1-10.0 years | 10.1-15.0 years | >15.0 years | |
| Total cancer | ||||||||
| Case/person-year | 39 902/4 052 767 | 742/47 747 | 503/35 168 | 524/31 214 | 503/27 103 | 380/22 500 | 681/40 008 | 614/37 058 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.34 (1.25 to 1.45) | 1.18(1.08 to 1.30) | 1.34 (1.23 to 1.47) | 1.44 (1.32 to 1.58) | 1.28 (1.15 to 1.42) | 1.23 (1.13 to 1.33) | 1.10 (1.01 to 1.20) |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.29 (1.19 to 1.38) | 1.13 (1.03 to 1.24) | 1.28 (1.17 to 1.40) | 1.37 (1.25 to 1.50) | 1.21 (1.09 to 1.35) | 1.16 (1.07 to 1.26) | 1.04 (0.95 to 1.14) |
| Obesity-related cancerd | ||||||||
| Case/person-year | 19 844/4 068 736 | 433/48 003 | 278/35 340 | 287/31 399 | 274/27 256 | 192/22 634 | 358/40 226 | 311/37 262 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.53 (1.39 to 1.68) | 1.29 (1.14 to 1.46) | 1.47 (1.30 to 1.65) | 1.59 (1.40 to 1.80) | 1.31 (1.13 to 1.52) | 1.32 (1.18 to 1.47) | 1.15 (1.02 to 1.30) |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.42 (1.28 to 1.56) | 1.19 (1.05 to 1.34) | 1.35 (1.19 to 1.52) | 1.45 (1.28 to 1.65) | 1.19 (1.03 to 1.39) | 1.20 (1.07 to 1.34) | 1.05 (0.92 to 1.19) |
| Diabetes-related cancere | ||||||||
| Case/person-year | 24 484/4 064 614 | 497/47 943 | 302/35 324 | 331/31 364 | 310/27 237 | 223/22 612 | 400/40 195 | 357/37 232 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.49 (1.36 to 1.63) | 1.20 (1.06 to 1.34) | 1.44 (1.28 to 1.61) | 1.52 (1.35 to 1.70) | 1.29 (1.13 to 1.48) | 1.25 (1.12 to 1.39) | 1.14 (1.01 to 1.28) |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.40 (1.28 to 1.53) | 1.12 (1.00 to 1.26) | 1.34 (1.20 to 1.50) | 1.41 (1.26 to 1.59) | 1.21 (1.05 to 1.38) | 1.16 (1.04 to 1.29) | 1.05 (0.94 to 1.18) |
| Colorectal cancer | ||||||||
| Case/person-year | 3275/4 083 317 | 66/48 325 | 41/35 547 | 48/31 608 | 45/27 450 | 42/22 767 | 48/40 484 | 48/37 485 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.34 (1.05 to 1.72) | 1.10 (0.80 to 1.51) | 1.43 (1.07 to 1.92) | 1.50 (1.10 to 2.03) | 1.66 (1.20 to 2.28) | 1.14 (0.85 to 1.54) | 1.08 (0.79 to 1.48) |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.25 (0.98 to 1.60) | 1.02 (0.75 to 1.40) | 1.33 (0.99 to 1.79) | 1.39 (1.02 to 1.89) | 1.54 (1.12 to 2.12) | 1.05 (0.78 to 1.42) | 1.00 (0.73 to 1.38) |
| Lung cancer | ||||||||
| Case/person-year | 3455/4 084 133 | 62/48 353 | 44/35 550 | 49/31 616 | 42/27 461 | 36/22 788 | 71/40 483 | 55/37 500 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.23 (0.95 to 1.58) | 1.13 (0.83 to 1.52) | 1.35 (1.01 to 1.81) | 1.31 (0.96 to 1.79) | 1.25 (0.90 to 1.76) | 1.28 (1.00 to 1.65) | 0.99 (0.75 to 1.32) |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.28 (0.99 to 1.65) | 1.18 (0.87 to 1.60) | 1.43 (1.07 to 1.92) | 1.37 (1.00 to 1.87) | 1.32 (0.94 to 1.85) | 1.36 (1.06 to 1.75) | 1.05 (0.79 to 1.40) |
| Pancreatic cancer | ||||||||
| Case/person-year | 874/4 085 780 | 39/48 368 | 15/35 575 | 25/31 640 | 21/27 479 | 20/22 800 | 24/40 516 | 31/37 520 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 2.91 (2.10 to 4.04) | 1.43 (0.85 to 2.40) | 2.48 (1.64 to 3.73) | 2.36 (1.51 to 3.68) | 2.48 (1.57 to 3.92) | 1.52 (0.99 to 2.33) | 1.76 (1.17 to 2.65) |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 2.82 (2.03 to 3.92) | 1.37 (0.81 to 2.31) | 2.38 (1.58 to 3.61) | 2.30 (1.47 to 3.60) | 2.41 (1.52 to 3.81) | 1.46 (0.95 to 2.25) | 1.67 (1.11 to 2.52) |
| Breast cancer | ||||||||
| Case/person-year | 12 064/3 172 217 | 211/34 162 | 134/25 748 | 147/23 168 | 129/20 327 | 69/17 051 | 170/30 982 | 153/32 132 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 1.44 (1.26 to 1.66) | 1.21 (1.01 to 1.44) | 1.48 (1.25 to 1.75) | 1.47 (1.23 to 1.76) | 0.93 (0.73 to 1.19) | 1.26 (1.06 to 1.48) | 1.13 (0.94 to 1.34) |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.40 (1.22 to 1.61) | 1.17 (0.98 to 1.40) | 1.44 (1.21 to 1.71) | 1.43 (1.19 to 1.71) | 0.91 (0.71 to 1.16) | 1.22 (1.04 to 1.44) | 1.10 (0.92 to 1.31) |
| Endometrial cancer | ||||||||
| Case/person-year | 1799/3 181 864 | 55/34 343 | 34/25 834 | 30/23 283 | 30/20 422 | 24/17 101 | 43/31 095 | 25/32 266 |
| Multivariable adjusted model, HR (95% CI)b | (Referent) | 2.25 (1.71 to 2.97) | 1.82 (1.28 to 2.59) | 1.74 (1.19 to 2.54) | 1.92 (1.31 to 2.80) | 1.81 (1.18 to 2.77) | 1.68 (1.20 to 2.36) | 0.97 (0.63 to 1.50) |
| Additionally adjusted for BMI, HR (95% CI)c | (Referent) | 1.63 (1.24 to 2.15) | 1.30 (0.91 to 1.86) | 1.22 (0.84 to 1.78) | 1.34 (0.91 to 1.96) | 1.24 (0.81 to 1.90) | 1.14 (0.81 to 1.60) | 0.65 (0.42 to 1.01) |
Only cancers that showed associations with incident type 2 diabetes in Table 2 were presented. Estimates were meta-analyzed using a random-effect model. BMI = body mass index; CI = confidence interval; HPFS = Health Professionals Follow-up Study; HR = hazard ratio; NHS = Nurses’ Health Study.
Adjusting for age (month), ethnicity (White, African American, Asian, others), smoking status (never smoked, past smoker, currently smoke 1-14 cigarettes per day, 15-24 cigarettes per day, or ≥25 cigarettes per day), alcohol intake (0, 0.1-4.9, 5.0-9.9, 10.0-14.9, 15.0-29.9, and ≥30.0 g/d), multivitamin use (yes, no), physical activity (quintiles), total energy (quintiles), alternative healthy eating index (quintiles), family history of diabetes (yes, no), family history of cancer (yes, no), endoscopy screening (yes, no), and fasting glucose screening (yes, no). For women, insulin use (yes, no), oral hypoglycemic drug use (yes, no), mammography screening, postmenopausal hormone use (never, former, or current hormone use, or missing), and oral contraceptive use were further adjusted.
Cumulative-averaged BMI (calculated as weight in kilograms divided by height in meters squared) (<21.0, 21.0-22.9, 23.0-24.9, 25.0-26.9, 27.0-29.9, 30.0-32.9, 33.0-34.9, or ≥35.0 kg/m2) was additionally adjusted.
Including esophagus cancer, liver cancer, kidney cancer, myeloma, pancreatic cancer, colorectal cancer, gallbladder cancer, postmenopausal breast cancer, ovarian cancer, and thyroid cancer.
Including thyroid cancer, breast cancer, liver cancer, pancreatic cancer, endometrial cancer, esophagus cancer, colorectal cancer, kidney cancer, gallbladder cancer, ovarian cancer, non-Hodgkin lymphoma, leukemia, and bladder cancer.
Figure 1.
Dose-response relationship between duration of type 2 diabetes and risk of cancer incidence. Data were combined from 2 cohorts. Spline regression adjusted for age (month), ethnicity (White, African American, Asian, others), smoking status (never smoked; past smoker; currently smoke 1-14 cigarettes per day, 15-24 cigarettes per day, or ≥25 cigarettes per day), alcohol intake (0, 0.1-4.9, 5.0-9.9, 10.0-14.9, 15.0-29.9, and ≥30.0 g/d), multivitamin use (yes, no), physical activity (quintiles), total energy (quintiles), alternative healthy eating index (quintiles), family history of diabetes (yes, no), family history of cancer (yes, no), endoscopy screening (yes, no), and fasting glucose screening (yes, no). For women, insulin use (yes, no), oral hypoglycemic drug use (yes, no), mammography screening (yes, no), postmenopausal hormone use (never, former, or current hormone use, or missing), and oral contraceptive use (yes, no) were further adjusted. P value for nonlinearity <.001 for panels A, B, and C. A) Total cancer incidence; (B) obesity-related cancer incidence; (C) diabetes-related cancer incidence.
In the stratified analysis (Supplementary Table 2, available online), men younger than 60 years had statistically significantly (Pinteraction = .04) higher risk (HR = 1.75, 95% CI = 1.11 to 2.75) of developing obesity-related cancer than their older counterparts (HR = 1.36, 95% CI = 1.18 to 1.56). However, such effect modification was not found for women. No other statistically significant interactions were detected. The sensitivity analysis that adjusted for the baseline covariates produced similar estimates as in the main models (Supplementary Table 3, available online).
In the secondary analysis (Table 4), we observed that, compared with participants without T2D, participants with prevalent T2D of 8 years or less had higher C-peptide levels, whereas those with longer duration of T2D had lower C-peptide level. The least square geometric mean of plasma C-peptide for participants with T2D duration of 4.1-8.0 years was 2.85 ng/mL (95% CI = 2.43 to 3.34) but 1.73 ng/mL (95% CI = 1.48 to 2.03) in those with 8.1-10.0 years. On the contrary, the HbA1c level was consistently higher among participants with longer diabetes duration, although a slightly lower level was observed for participants with a diabetes duration of more than 15.0 years compared with those with 10-15 years.
Table 4.
Least square geometric means of plasma C-peptide and HbA1c level by diabetes duration in NHS and HPFSa
| Diabetes duration | C-peptide |
HbA1c |
||
|---|---|---|---|---|
| No. | Least square geometric mean (95% CI), ng/ml | No. | Least square geometric mean% (95% CI) | |
| No diabetes | 9710 | 2.54 (2.26 to 2.85) | 7714 | 5.55 (5.43 to 5.68) |
| 0-2.0 y | 75 | 3.32 (2.81 to 3.93) | 201 | 7.18 (7.02 to 7.34) |
| 2.1-4.0 y | 46 | 2.67 (2.20 to 3.24) | 160 | 7.15 (6.99 to 7.32) |
| 4.1-8.0 y | 95 | 2.85 (2.43 to 3.34) | 275 | 7.69 (7.55 to 7.84) |
| 8.1-10.0 y | 96 | 1.73 (1.48 to 2.03) | 184 | 7.65 (7.49 to 7.81) |
| 10.1-15.0 y | 34 | 1.99 (1.60 to 2.46) | 209 | 7.92 (7.77 to 8.08) |
| ≥ 15.0 y | 34 | 2.09 (1.68 to 2.59) | 225 | 7.21 (7.06 to 7.36) |
Data were combined from 2 cohorts. Multiple linear regression adjusted for age at blood draw, aspirin/NSAIDs use, presence of chronic diseases or conditions (hypercholesterinemia, hypertension, cancer, osteoporosis, rheumatoid arthritis, stroke, and myocardial infarction), physical activity (continuous), current smoking status (yes, no), case-control status, sex, and body mass index (continuous). CI = confidence interval; HPFS; Health Professionals Follow-up Study; NHS = Nurses’ Health Study.
Discussion
Leveraging data from 2 large prospective cohorts, we found around 21% increased risk of total cancer, 28% increased risk of obesity-related cancer, and 25% increased risk of diabetes-related cancer comparing participants with and without diabetes. For individual cancers, incident T2D was associated with increased incidence of colorectal cancer, lung cancer, pancreatic cancer, thyroid cancer, esophagus cancer, breast cancer, and endometrial cancer. The cancer risks reached highest levels at 4-8 years after T2D diagnosis after which the risk elevation was no longer increased. Through analyzing key diabetes-related biomarkers, we found that C-peptide level was on average lower among participants with more than 8 years of diabetes duration than diabetes patients with shorter disease duration, whereas HbA1c level was generally higher for patients with longer diabetes duration. With careful adjustment of important confounders in all analyses, these 2 lines of evidence collectively favor the insulin resistance and hyperinsulinemia hypothesis that indicates T2D as a direct risk factor in the cancer etiology, rather than a spurious association confounded by common risk factors between T2D and cancer.
Results from the current study were largely in line with a previous meta-analysis of 12 cohorts consisting of 257 222 participants, which found 10% increased risk of cancer incidence among diabetes patients compared with those without diabetes (22). We observed a slightly stronger association for cancer incidence, which could result from the exclusion of prostate cancer, the only type of cancer inversely associated with T2D. In addition, to our knowledge, this is the first study to specifically examine the association between T2D and obesity-related cancer as a single group. However, our data suggest that not all included individual obesity-related cancers were positively associated with T2D. The positive associations were primarily driven by colorectal cancer, pancreatic cancer, thyroid cancer, esophagus cancer, liver cancer, breast cancer, and endometrial cancer. Additional adjustment for BMI attenuated associations for all obesity-related cancer, suggesting that overall body fatness could partially explain the increased cancer risk among diabetics.
Through categorical modeling and spline analysis of diabetes duration, our study demonstrated that the cancer risk gradually reduced after 8.0 years of diabetes duration when the risks were highest during the diabetes duration. Ascertainment bias was unlikely to explain the positive association because similar increased cancer risks were found between 0-2.0 years and 4.1-6.0 or 6.1-8.0 years of diabetes duration. It is notable that even though different cancers have distinct etiologic factors, the pattern of relationship with T2D duration was remarkably similar for individual cancers (colorectal, breast, endometrial, pancreatic), suggesting shared underlying biological mechanisms linking T2D to these cancers. These findings may imply that hyperinsulinemia in early diabetes plays a greater role than hyperglycemia in promoting cancer development because the hazard ratios of cancer decreases after reaching a certain diabetes stage when endogenous insulin secretion is gradually depleted because of exhaustion of β cell function. Our data on C-peptide and HbA1c support this hypothesis because we found the decline of the C-peptide level approximately coincided with the decrease of cancer risk, whereas the HbA1c level keeps increasing during the extended course of diabetes.
The strengths of the current study included a large sample size with long term of follow-up, use of incident T2D only, comprehensive repeated assessments of lifestyle and dietary factors, and a high follow-up rate. We also explicitly addressed the reverse causality, ascertainment bias, and influence of cancer screening. Our study also had some limitations. First, although our analysis included several major types of cancer, we did not have data to examine the associations for certain uncommon cancers such as bile duct cancers, which have been associated with T2D (23). Second, despite meticulous adjustment for potential confounders, we could not rule out residual confounding in our analysis particularly for BMI, which is closely related with T2D. However, given the consistent positive associations observed for most included cancers, residual confounding is unlikely to fully account for these statistically significant associations. Third, despite that we adjusted for the hypoglycemic drug use, detailed information on specific drug use such as sulphonylurea, metformin, and thiazolidinediones were lacking. However, it is difficult to assess the influence of such incomplete adjustment on the diabetes-cancer associations because of substantial heterogeneity in the effects of different hypoglycemic drugs on cancer risk (24–26). Additional long-term prospective cohort studies with comprehensive information on insulin and oral hypoglycemic drug use are needed to clarify the associations between individual diabetes drugs and cancer risk. Fourth, we were unable to directly examine the prospective association between circulating insulin level and diabetes duration because the repeatedly measured insulin was not available in our cohorts. Nonetheless, our biomarker analysis on C-peptide and HbA1c could still provide some insights to further understand the mechanisms underlying the diabetes-cancer associations. Finally, because the study participants were mostly White health professionals, whether our findings could be generalized to other populations remains unclear. However, the association between diabetes and major cancers incidence did not appear to differ substantially by ethnicity groups (27–29).
In conclusion, the cancer risks were statistically significantly increased after diabetes diagnosis, but the increased risk appeared to plateau or decrease after 8 years of diabetes duration, which coincided with the change of plasma C-peptide level over time. Our findings support a role of hyperinsulinemia in cancer development.
Funding
The cohorts were supported by grants UM1 CA186107, P01 CA87969, and UM1 CA167552 from the National Institutes of Health. This work was supported by the American Cancer Society Mentored Research Scholar Grant (MRSG-17-220-01 - NEC to MS), American Cancer Society Research Scholar Grant (RSG NEC-130476, to XZ), and by the US National Institutes of Health grants (R00 CA215314 to MS; K07, CA188126, R21CA238651 to XZ).
Notes
Role of the funders: The National Institutes of Health had no role in the design, conduct, analysis, or reporting of this study. The funding sources did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure: None disclosed.
Acknowledgments: We would like to thank the participants and staff of the NHS and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Author contributions: Drs Hu and Song had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Giovannucci, Song. Acquisition, analysis, or interpretation of data: Hu, Giovannucci, Song. Drafting of the manuscript: Hu. Critical revision of the manuscript for important intellectual content: Zhang, Ma, Yuan, Wang, Wu, Tabung, Tobias, Frank B. Hu. Statistical analysis: Hu. Obtained funding: Giovannucci, Song. Administrative, technical, or material support: Giovannucci, Song. Supervision: Giovannucci, Song.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed September 28, 2020.
- 2. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60(4):207–221. [DOI] [PubMed] [Google Scholar]
- 3. Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG; on behalf of the Diabetes and Cancer Research Consortium. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 2012;55(6):1607–1618. [DOI] [PubMed] [Google Scholar]
- 4. Carstensen B, Witte DR, Friis S.. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia. 2012;55(4):948–958. [DOI] [PubMed] [Google Scholar]
- 5. Jones AG, Hattersley AT.. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30(7):803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pai JK, Cahill LE, Hu FB, Rexrode KM, Manson JE, Rimm EB.. Hemoglobin A1c is associated with increased risk of incident coronary heart disease among apparently healthy, nondiabetic men and women. J Am Heart Assoc. 2013;2(2):e000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–468. [DOI] [PubMed] [Google Scholar]
- 8. Colditz GA, Manson JE, Hankinson SE.. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 9.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–1057. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association, .Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(S1):S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manson JE, Stampfer MJ, Colditz GA, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. [DOI] [PubMed] [Google Scholar]
- 12. Hu FB, Leitzmann MF, Stampfer MJ, Colditz G. A, Willett WC, Rimm EB.. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–1548. [DOI] [PubMed] [Google Scholar]
- 13. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K.. Body fatness and cancer-viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA.. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350(1):g7607. [DOI] [PubMed] [Google Scholar]
- 15. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun Q, Townsend MK, Okereke OI, Franco OH, Hu FB, Grodstein F.. Physical activity at midlife in relation to successful survival in women at age 70 years or older. Arch Intern Med. 2010;170(2):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351(25):2599–2610. [DOI] [PubMed] [Google Scholar]
- 18. Govindarajulu US, Malloy EJ, Ganguli B, Spiegelman D, Eisen EA.. The comparison of alternative smoothing methods for fitting non-linear exposure-response relationships with Cox models in a simulation study. Int J Biostat. 2009;5(1):Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Durrleman S, Simon R.. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 20. Tabung FK, Nimptsch K, Giovannucci EL.. Postprandial duration influences the association of insulin-related dietary indexes and plasma C-peptide concentrations in adult men and women. J Nutr. 2019;149(2):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nimptsch K, Brand-Miller JC, Franz M, Sampson L, Willett WC, Giovannucci E.. Dietary insulin index and insulin load in relation to biomarkers of glycemic control, plasma lipids, and inflammation markers. Am J Clin Nutr. 2011;94(1):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noto H, Tsujimoto T, Sasazuki T, Noda M.. Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Endocr Pract. 2011;17(4):616–628. [DOI] [PubMed] [Google Scholar]
- 23. Jing W, Jin G, Zhou X, et al. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev. 2012;21(1):24–31. [DOI] [PubMed] [Google Scholar]
- 24. Coyle C, Cafferty FH, Vale C, Langley RE.. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27(12):2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reiff S, Fava S.. All-cause mortality in patients on sulphonylurea monotherapy compared to metformin monotherapy in a nation-wide cohort. Diabetes Res Clin Pract. 2019;147:62–66. [DOI] [PubMed] [Google Scholar]
- 26. Du R, Lin L, Cheng D, et al. Thiazolidinedione therapy and breast cancer risk in diabetic women: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2018;34(2):e2961. [DOI] [PubMed] [Google Scholar]
- 27. Li D, Tang H, Hassan MM, Holly EA, Bracci PM, Silverman DT.. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22(2):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Setiawan VW, Hernandez BY, Lu SC, et al. Diabetes and racial/ethnic differences in hepatocellular carcinoma risk: the Multiethnic Cohort. J Natl Cancer Inst. 2014;106(12):dju326–dju326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He J, Stram DO, Kolonel LN, Henderson BE, Le Marchand L, Haiman CA.. The association of diabetes with colorectal cancer risk: the Multiethnic Cohort. Br J Cancer. 2010;103(1):120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.