Abstract
Background
Treatment for polycystic ovary syndrome (PCOS) usually initiates with a series of lifestyle modifications such as diet, weight loss, and exercise.
Aims
We, therefore, conducted this meta-analysis to systematically review and evaluate the possible benefits of LGD on a range of anthropometric, clinical, and biochemical parameters in women with PCOS.
Methods
We performed a systematic search through major indexing databases, including Scopus, Pubmed/Medline, ISI web of science, Embase, Cochrane central, and CINAHL (1966–April 30, 2021) using key concepts of PCOS.
Results
Of 935 initial publications, 542 remain after duplicates removal. Then, 141 records were removed at the title and abstract screening level. After excluding 392 literatures, we finally included 8 articles. The final selected studies included 412 overweight and obese individuals with PCOS (207 cases in LGID group and 205 patients in comparators) with a mean age of 21–32 years. Measured emotional health (3 studies, 132 participants, SMD: -1.97; 95%CI:-3.54, -0.40, P = 0.01, I2 = 89%) and body hair (3 studies, 132 participants, SMD: -0.40; 95%CI:-0.46, -0.35, P < 0.0001, I2 = 0%), were found to be significantly lower in women in LGD vs control diet groups. Moreover, infertility (3 studies, 132 participants, SMD: 1.45; 95%CI: 0.30, 2.61, P = 0.01, I2 = 79%) was significantly higher in women in LGD vs control diet groups.
Conclusion
The present meta-analysis has shown that LGD may play a significant role in reducing the risk and improving the clinical and biochemical features of PCOS. So far the evidences for choosing the best dietary modalities for PCOS are not strong to make a definite recommendation.
Keywords: Glycemic index, Low-GI diets, Endocrine parameters, Blood lipids, PCOSQ domains
Glycemic index; Low-GI diets; Endocrine parameters; Blood lipids; PCOSQ domains.
1. Introduction
Polycystic ovary syndrome (PCOS) is a widespread disorder that affects women during the reproductive age and is one of the most common causes of menstrual irregularity, hyper-androgenism, and infertility among young women [1]. PCOS is one of the most common endocrine disorders with cardio-metabolic risk affecting women [2]. This disease is of great importance both clinically and in terms of community health, because its prevalence is very high affecting 18–22% of women at reproductive age [3]. PCOS is a disease of unknown etiology is associated with many clinical symptoms, among which missed or irregular menstruation, ovarian cyst, excessive facial and body hair (hirsutism), and hyperpigmentation [4]. In addition, the disease includes a set of other symptoms such as excessive confusion and depression [5].
Endocrine disruption and increasing androgens such as testosterone and dehydroepiandrosterone (DHEA) associated with PCOS could have a negative impact on ovarian function and follicular development and growth; therefore, PCOS can lead to infertility, or increasing endometrial hyperplasia and cancers [6]. Another factor is the increased chance of developing diseases, especially in older age, such as obesity, insulin resistance, type 2 diabetes, high blood pressure, and impaired lipid status or uterine cancer [7, 8]. In PCOS increasing the pulsatility of luteinizing hormone (LH) levels regarding both frequency and amplitude, increases theca cell production of androgens, while relatively low FSH secretion impairs follicle maturation and consequently ovulation [9]. Thus, the increasing incidence of PCOS among young women of reproductive age and its complications, such as infertility, has necessitated further studies on this disease.
Treatment for PCOS usually initiates with a series of lifestyle modifications such as diet, weight loss, and exercise. Losing weight is one of the most effective measures to regulate the menstrual cycle and improve the symptoms of PCOS [10]. In low glycemic diets (LGD), the glycemic index (GI) is used to determine which foods have the least significant effect on blood sugar levels; thus, LGD may help weight loss [11].
Previous evidence found that a LGD may have benefits for individuals undergoing in vitro fertilization (IVF) [12] and natural fertility [13]. Other studies revealed that LGD reduced body fat and BMI and improved pregnancy outcomes [14, 15]. So far several meta-analyses have assessed the effect of nutritional intervention on many aspects of PCOS such as insulin resistance [16, 17, 18, 19, 20, 21], Biochemical parameters [22, 23, 24], and androgenic profiles [25, 26]. We, therefore, conducted this meta-analysis to systematically review and evaluate the possible benefits of LGD on a range of anthropometric, clinical, and biochemical parameters in women with PCOS.
2. Methods
This meta-analysis was conducted following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [27].
2.1. Search strategy
We comprehensively searched major indexing databases, including Scopus, Pubmed/Medline, ISI web of science, Embase, Cochrane central, and CINAHL using keywords: (“polycystic ovary syndrome” OR “PCOS”) AND ("Glycemic Index"[Mesh] OR "Glycemic Load"[Mesh] OR "Dietary Carbohydrates"[Mesh] OR "Dietary Sucrose"[Mesh] OR "Dietary Sugars"[Mesh] OR “GI” OR “GL” OR "Diet, Diabetic"[Mesh]) from Jan 1, 1980, to April 26, 2021, with no language restrictions. Also, two major clinical trial registries, including clinicaltrials.gov and the WHO clinical trials search portal were searched. We also performed hand searching through collections of records cited by the formerly found articles.
2.2. Inclusion and exclusion criteria
All randomized clinical trials (RCTs) of I week or longer in people with PCOS, using LGD compared with the control diet, measuring anthropometric, clinical, and biochemical parameters were included. Review articles, animal studies, letter to the editors, commentaries, case reports, observational studies, using LGD in non-PCOS people, were excluded.
2.3. Outcome measures
We collected information regarding all outcome measures. The first outcome of interest includes the assessment of anthropometric measures. Second outcomes were cardio-metabolic risk profile and biochemical assessments, including Serum total cholesterol (TC) and triglyceride (TG), Serum high-density lipoprotein cholesterol (HDL-C), Serum low-density lipoprotein cholesterol (LDL-C) levels, TC/HDL ratio, luteinizing hormone (LH), follicle-stimulating hormone (FSH), total testosterone (TT), sex-hormone binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEA-S), and prolactin. Third and most important outcomes were quality of life (QOL) according to the PCOS Health-Related Quality of Life Questionnaire (PCOSQ) in term of five domains, including emotional health, body hair, infertility, weight, and menstrual problems scoring range from 1 (a lower QOL) to 7 (a higher QOL).
2.4. Study selection
Two authors (FR and FH) independently performed the title and abstract screening. Any disagreement was resolved either by double-check the reference paper or discussion with a third author.
2.5. Methodological quality assessment
Two authors (FR and FH) independently conducted the methodological quality assessment with special consideration to potential sources of risk of bias. We used the Cochrane Collaboration's quality assessment tool for risk of bias assessment in RCTs [28]. Any disagreement was resolved either by double-check the reference paper or discussion with a third author. We rated the quality of the evidence as low to moderate according to GRADE criteria, as most information is from studies judged to be at unclear risk of bias [29].
2.6. Data extraction
Data extraction was performed by one reviewer (FH) and double-checked by another author (NS). Authors extracted data, including author's name, publication year, country, intervention, comparators, and outcomes of interest. In case the outcomes of interest were missing, we contact the authors three times; Also, if the outcomes were only presented in figures, we used WebPlotDigitizer to extract the data [30]. Median and range were converted to mean and standard deviation (SD) using the standard formula.
2.7. Data analysis
We used RevMan 5.3 software for data analysis, as well as used standardized mean difference as effect size. If data were present as median and range, we used Wan et al. methods to estimate the mean and standard deviation [31]. The Biochemical units such as LDL and TC were transformed from mg/dL to mmol/L as appropriate. Heterogeneity was described as the total variability (I2). The significant heterogeneity was tested by χ2 test. Low heterogeneity was indicated as I2 < 40%. In case the heterogeneity was significant (I2 > 75%), the source of heterogeneity was detected before meta-analysis. We conducted sub-group analyses based on various comparators. To assess publication bias we used funnel plots.
3. Results
3.1. Characteristics of included studies
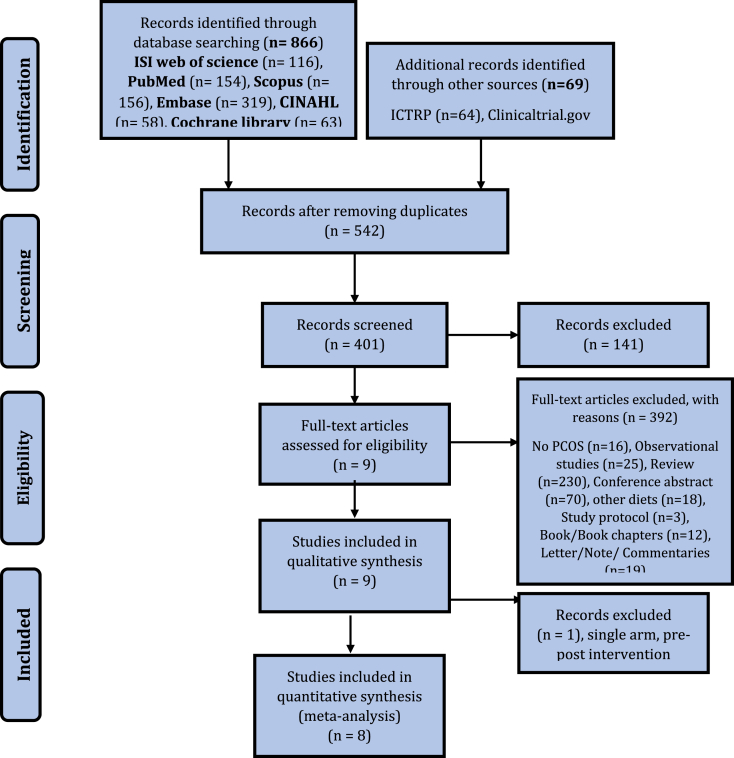
Of 935 initial publications, 542 remain after duplicates removal. Then, 141 records were removed at the title and abstract screening level. After excluding 392 literatures, we finally included 9 articles. We then removed Bar et al. study due to missing comparators [32]. Finally, eight articles were included in qualitative and quantitative analyses (Figure 1). The final selected studies included 412 overweight and obese individuals with PCOS (207 cases in the LGD group and 205 patients in comparators) with a mean age of 21–32 years (Table 1) [33-40]. Studies varied in length from 3 to 12 months, comparing LGID against high glycemic index diet, low-calorie diet, normal glycemic diet, low-fat diet, therapeutic lifestyle changes, and conventional hypocaloric diet.
Figure 1.
PRISMA flow diagram.
Table 1.
Characteristics of included studies.
| Study ID | Country | Study type length | No. of patients |
BMI category | Age (Year) | PCOS diagnosis | Intervention type | Comparators | Outcome | Quality of evidence | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | ||||||||||
| Marsh et al. 2010 [35] | Australia | Parallel 3 months | 50 | 46 | Overweight and obese | 18–40 | Rotterdam criteria [41] | LGID | CHD | The beneficial rule of LGID in the management of PCOS | Low∧% |
| Mehrabani et al. 2012 [36] | Iran | Parallel 3 months | 30 | 30 | Overweight and obese | 20–40 | Rotterdam criteria and mF-G score [42] | LGID + MHCD | CHCD | Significantly led to reduced body weight and androgen levels | Moderate% |
| Asemi et al. 2014 [33] | Iran | Parallel 3 months | 27 | 27 | Overweight and obese | 18–40 | Rotterdam criteria and mF-G score | LGID DASH-style diet | CD | Significantly reduction in LDL and increase in TAC and GSH levels | Moderate% |
| Panico et al. 2014 [37] | Italy | Cross-over 3 months | 15 | 15 | Overweight | 18–40 | Rotterdam criteria | LGID | HGID | Improves insulin resistance and serum androgen levels | High |
| Turner-McGrievy et al. 2014 [39] | USA | Parallel 6 months | 9 | 9 | Overweight and obese | 18–35 | Rotterdam criteria | LGID-vegan | LCD | Effective for promoting short-term weight loss | Moderate% |
| Sordia-Hernández et al. 2016 [38] | Mexico | Parallel 3 months | 20 | 20 | Overweight | 18–35 | Rotterdam criteria | LGID | NGID | Improves insulin resistance and serum androgen levels | Low∧% |
| Wong et al. 2016 [40] | USA | Parallel 3 months | 9 | 10 | Overweight and obese | 13–21 | Rotterdam criteria | LGID | LFD | Beneficial for weight control but did not attenuate biochemical hyperandrogenism | Moderate% |
| Kazemi et al. 2019 [34] | Canada | Parallel 12 months | 47 | 48 | Overweight and obese | 18–35 | Rotterdam criteria | LGID pulse-based diet, | TLC | Improve cardio-metabolic disease risk factors | High |
LGID, Low glycemic index diet; CHD, conventional healthy diet; CHCD, conventional hypocaloric diet; MHCD, modified hypocaloric diet; mF-G score, Modified Ferriman–Gallwey score; DASH, Dietary Approaches to Stop Hypertension; CD, Control diet; insulin, triglycerides and low-density lipoprotein cholesterol (LDL-c); TAC, plasma total antioxidant capacity; GSH, total glutathione; HGID, High glycemic index diet; LCD, Low calorie diet; NGID, normal glycemic diet; LFD, Low fat diet; TLC, Therapeutic Lifestyle Changes; Factors downgrading any specific evidence: ∗ Limitations (risk of bias), $ Inconsistency of results, # indirectness of results, % Imprecision, ^ Publications bias; GRADE of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
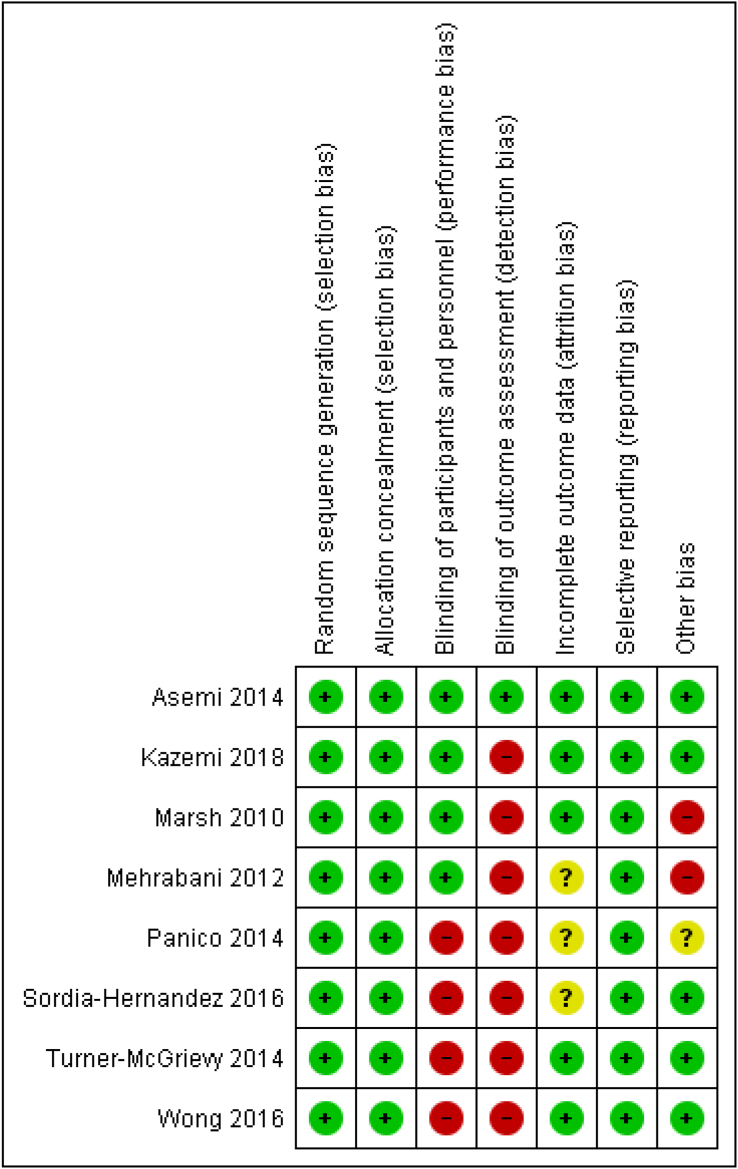
3.2. Quality of included studies
We found a substantial risk of bias mostly due to the wide-ranging problems of blinding. The method of randomization was mostly described (Figure 2).
Figure 2.
The risk of bias of included studies.
Glycemic and insulinemic control: We observed a statistically significant lowering of 2-hour insulin (4 studies, 177 participants, SMD: -0.79; 95%CI:-1.33, -0.24, P = 0.005, I2 = 57%) and HOMA-IR (6 studies, 293 participants, SMD: -0.36; 95%CI:-1.33, -0.24, P = 0.005, I2 = 57%) as a consequence of LGD compared to control diets (Table 2). We also did not find any statistically significant differences in terms of fasting glucose (FG), fasting insulin (FI), 2-hour glucose, HbA1c, and HOMA2-IS between LGD and control diets (Table 2).
Table 2.
Outcomes of meta-analyses (SMD [95% CI).
| Outcome | Studies | Participants | Model | I2 | p-values | Effect Estimate |
|---|---|---|---|---|---|---|
| Insulin sensitivity measures, endocrine parameters, and lipid profile | ||||||
| Fasting Glucose (mmol/L) | 7 | 280 | Random | 96% | 0.27 | -0.79 [-2.17, 0.60] |
| 2-hour glucose (mmol/L) | 4 | 177 | Random | 92% | 0.16 | -0.92 [-2.22, 0.38] |
| Fasting Insulin (μIU/mL) | 5 | 192 | Random | 52% | 0.47 | -0.16 [-0.60, 0.28] |
| 2-hour insulin (μIU/mL) | 4 | 177 | Random | 57% | 0.005 | -0.79 [-1.33, -0.24] |
| HOMA2-IR | 6 | 293 | Fixed | 0% | 0.003 | -0.36 [-0.59, -0.12] |
| HOMA2-IS (%) | 2 | 138 | Fixed | 0% | 0.13 | -0.26 [-0.59, 0.08] |
| HbA1c (%) | 2 | 114 | Random | 80% | 0.65 | 0.27 [-0.90, 1.44] |
| LDL-c (mmol/L) | 5 | 307 | Fixed | 0% | 0.05 | -0.22 [-0.01, -0.45] |
| TG (mmol/L) | 6 | 337 | Fixed | 0% | 0.009 | -0.02 [-0.50, -0.07] |
| Total cholesterol (TC) (mmol/L) | 6 | 337 | Random | 68% | 0.04 | -0.48 [-0.11, -1.07] |
| HDL-c (mmol/L) | 5 | 307 | Random | 59% | <0.0001 | -0.92 [-0.53, -1.31] |
| TC/HDL-c | 4 | 177 | Fixed | 0% | 0.03 | -0.07 [-0.14, -0.01] |
| hsCRP (mg/L) | 4 | 178 | Fixed | 9% | 0.54 | 0.09 [-0.20, 0.39] |
| Fasting insulin/glucose ratio | 4 | 190 | Random | 88% | 0.46 | -0.37 [-1.34, 0.61] |
| Total insulin AUC (μIU/ml∗ min) | 2 | 80 | Random | 81% | 0.92 | 0.07 [-1.16, 1.29] |
| Total glucose AUC (mmol/L∗ min) | 2 | 80 | Random | 82% | 0.83 | 0.14 [-1.15, 1.42] |
| Incremental glucose AUC (mmol/L∗ min) | 2 | 80 | Random | 70% | 0.57 | 0.28 [-0.69, 1.25] |
| SHBG (nmol/L) | 3 | 117 | Random | 67% | 0.04 | -0.72 [-1.42, -0.02] |
| FAI (%) | 2 | 98 | Random | 0% | <0.0001 | 1.16 [0.72, 1.59] |
| LH (IU/L) | 4 | 215 | Fixed | 27% | 0.03 | -0.30 [-0.57, -0.03] |
| FSH (IU/L) | 4 | 215 | Fixed | 0% | 0.0002 | 0.19 [-0.65, 1.04] |
| Testosterone (ng/dl) | 6 | 329 | Random | 42% | 0.0009 | -0.52 [-0.83, -0.22] |
| Androstenedione (A4) (ng/dl) | 2 | 63 | Fixed | 0% | 0.73 | 0.09 [-0.41, 0.58] |
| DHEAS (ng/ml) or ug/dl | 4 | 193 | Random | 78% | 0.34 | 0.33 [-0.35, 1.00] |
| Prolactin (ng/ml) |
3 |
165 |
Random |
68% |
0.16 |
-0.43 [-1.02, 0.17] |
| Anthropometrics, body composition measures, and physiologic measures | ||||||
| BMI (kg/m2) | 8 | 258 | Fixed | 22% | 0.83 | -0.03 [-0.27, 0.22] |
| Weight (kg) | 8 | 298 | Random | 83% | 0.17 | -0.43 [-1.05, 0.18] |
| Waist circumference (cm) | 2 | 110 | Fixed | 0% | 0.002 | -6.16 [-10.12, -2.20] |
| Trunk fat mass (kg) | 2 | 80 | Random | 76% | 0.77 | 0.16 [-0.93, 1.25] |
| Percent body fat (%) | 3 | 129 | Random | 78% | 0.77 | 0.52 [-2.99, 4.03] |
| Total body fat mass (kg) | 2 | 110 | Fixed | 0% | <0.0001 | -2.90 [-3.63, -2.17] |
| Systolic blood pressure (mm Hg) | 2 | 80 | Fixed | 0% | 1.00 | 0.00 [-0.44, 0.44] |
| Diastolic blood pressure (mm Hg) | 2 | 80 | Random | 46% | 0.72 | -0.77 [-4.99, 3.44] |
| Total body lean mass (kg) |
2 |
110 |
Fixed |
0% |
0.06 |
-0.37 [-0.75, 0.01] |
| Dietary parameters | ||||||
| Energy (kcal/day) | 7 | 258 | Random | 82% | 0.19 | -0.44 [-1.11, 0.22] |
| Fat (% of total Energy) | 6 | 240 | Random | 53% | 0.40 | 0.17 [-0.23, 0.56] |
| Total fat (g) | 3 | 123 | Random | 78% | 0.50 | 0.30 [-0.58, 1.18] |
| Protein (% of total energy) | 6 | 240 | Random | 94% | 0.10 | 1.08 [-0.20, 2.36] |
| Total protein (g) | 3 | 123 | Random | 87% | 0.08 | -1.14 [-2.44, 0.15] |
| Carbohydrate (% of total energy) | 7 | 258 | Random | 92% | 0.009 | -1.44 [-2.52, -0.35] |
| Total carbohydrate (g) | 3 | 123 | Fixed | 39% | 0.78 | 0.05 [-0.31, 0.41] |
| Glycemic index | 4 | 143 | Random | 95% | 0.04 | -2.87 [-5.58, -0.16] |
| Glycemic load | 4 | 143 | Random | 91% | 0.04 | -1.58 [-3.09, -0.06] |
| Saturated fatty acid (SFA) gr/day | 4 | 141 | Random | 96% | 0.26 | -1.66 [-4.53, 1.22] |
| MUFA (g/day) | 3 | 124 | Fixed | 32% | 0.11 | 0.27 [-0.22, 0.79] |
| PUFA (g/day) | 4 | 163 | Random | 96% | 0.10 | 1.68 [-0.30, 3.65] |
| SFA (g/day) | 4 | 197 | Random | 96% | 0.003 | -0.49 [-0.81, -0.17] |
| Fiber (g) | 5 | 191 | Random | 90% | 0.02 | 2.81 [0.42, 5.21] |
|
Cholesterol intake (mg/day) |
6 |
231 |
Random |
84% |
0.01 |
-0.76 [-1.48, -0.046] |
| Clinical outcomes (Change in PCOSQ domains) | ||||||
| Emotional health | 3 | 132 | Random | 89% | 0.01 | -1.97 [-3.54, -0.40] |
| Body hair | 3 | 132 | Fixed | 0% | <0.0001 | -0.40 [-0.46, -0.35] |
| Fertility | 3 | 132 | Random | 79% | 0.01 | 1.45 [0.30, 2.61] |
| Menstrual concerns | 3 | 132 | Random | 96% | 0.19 | -1.58 [-3.94, 0.77] |
Endocrine parameters: Total testosterone level improved significantly (reduced) in LGD group against comparators with a SMD of -0.52 (95% CI: -0.83 to -0.22, P = 0.0009, I2 = 42%). Also, LH levels were improved significantly (reduced) in the LGD group compared with comparator groups, with a SMD of -0.30 (95% CI: -0.57 to -0.03, P = 0.03, I2 = 27%). Measures of FSH levels were found to be improved significantly (decreased) in LGD group vs. comparator groups, with an SMD of -0.52 (95% CI: -0.79 to -0.25, P = 0.0002, I2 = 0%). SHBG levels were increased non-significantly in women with PCOS who used LGD intervention vs. comparator groups, with a SMD of 0.6 (95% CI: -0.14 to 1.34, P = 0.11, I2 = 77%). Measures of DHEA-S levels were found to be improved non-significantly (increased) in LGD group vs. comparator groups, with an SMD of 0.33 (95% CI: -0.35 to 1.00, P = 0.34, I2 = 78%). Prolactin levels were decreased non-significantly in women with PCOS used LGD intervention vs. comparator groups, with a SMD of -0.43 (95% CI: -01.02 to 0.17, P = 0.16, I2 = 68%) (Table2).
Lipid profile: We observed a statistically significant lowering TC (6 studies, 337 participants, SMD: -0.48; 95%CI:-0.11, -1.07, P = 0.04, I2 = 68%), TG (6 studies, 337 participants, SMD: -0.02; 95%CI:-0.50, -0.07, P = 0.04, I2 = 0%), LDL (5 studies, 307 participants, SMD: -0.22; 95%CI:-0.01, -0.45, P = 0.05, I2 = 0%), and HDL (5 studies, 307 participants, SMD: -0.92; 95%CI:-0.53, -1.31, P < 0.0001, I2 = 59%) as a consequence of LGD compared to control diets (Table 2).
Anthropometrics and body composition measures: Overall, the included studies failed to confirm a statistically significant difference between LGD and control diets in lowering anthropometrics and body composition measures (Table 2). Only measures that decreased significantly were waist circumference (2 studies, 110 participants, SMD: -6.16; 95%CI:-10.12, -2.20, P = 0.002, I2 = 0%) and total body fat mass (2 studies, 110 participants, SMD: -2.90; 95%CI:-3.63, -2.17, P < 0.0001, I2 = 59%) (Table 2).
Physiologic measures: Overall, the included studies failed to confirm a statistically significant difference between LGD and control diets in lowering systolic and diastolic blood pressure (Table 2).
Dietary parameters: We found a superior lowering carbohydrate (7 studies, 258 participants, SMD: -1.44; 95%CI:-2.52, -0.35, P = 0.009, I2 = 92%), GI (4 studies, 143 participants, SMD: -2.87; 95%CI:-5.58, -0.16, P = 0.04, I2 = 95%), GL (4 studies, 143 participants, SMD: -1.58; 95%CI:-3.09, -0.06, P = 0.04, I2 = 91%), and cholesterol intake (6 studies, 231 participants, SMD: -0.76; 95%CI:-1.48, -0.046, P = 0.01, I2 = 84%) as a consequence of LGD compared to control diets (Table 2).
Clinical outcomes (Change in PCOSQ domains): Measured emotional health (3 studies, 132 participants, SMD: -1.97; 95%CI:-3.54, -0.40, P = 0.01, I2 = 89%) and body hair (3 studies, 132 participants, SMD: -0.40; 95%CI:-0.46, -0.35, P < 0.0001, I2 = 0%), were found to significantly lower in women in LGD vs control diet groups. Moreover, fertility (3 studies, 132 participants, SMD: 1.45; 95%CI: 0.30, 2.61, P = 0.01, I2 = 79%) was significantly higher in women in LGD vs control diet groups (Table 2). Though menstrual concerns were lower in women who received LGD vs control diet, the estimation was not statistically significant.
3.3. Sensitivity analyses
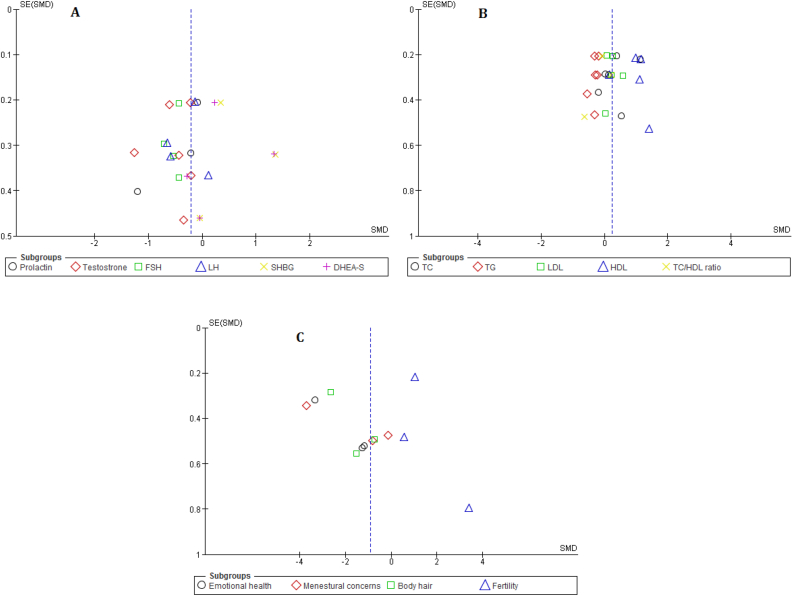
There was a substantial variation in the comparators of included studies, thus, we performed a sensitivity analysis of the effects of various comparator diets on study outcomes. We found no statistically significant differences between LGD and control diets in the sensitivity analysis. We did not observe any publication bias using funnel plots for insulin sensitivity measures, endocrine parameters, lipid profile, and clinical outcomes (Change in PCOSQ domains), in which all plots appeared to be symmetrical, with no obvious publication bias (Figure 3).
Figure 3.
Publication bias funnel plots for the effect of low glycemic diet on endocrine parameters, and lipid profile (A and B) and clinical outcomes (Change in PCOSQ domains) in patient with PCOS.
4. Discussion
The present meta-analysis has shown that LGD can play a significant role in reducing the risk and improving the clinical and biochemical features of PCOS. The best treatment for PCOS is yet to be recognized, but evidence supports a multifactorial approach, including any combination of one or more of the interventions such as diet and lifestyle management, use of pharmaceuticals (oral contraceptives and cyclic progestins, antiestrogens, gonadotropins, and insulin sensitizers), and surgery. In one evaluation of 138 endocrinologists and 172 gynecologists that was carried out by Cussons et al, the majority of respondents recommended that the first line of treatment for all presentations of PCOS should be diet and exercise [43].
As a whole, the two primary strategies for controlling the disease include weight control, insulin production, and insulin resistance [44]. Generally, insulin resistance is present in both obese and non-obese patients with PCOS [45]. Because insulin plays an important role in PCOS and its metabolic features, controlling the disease with diet is the best step for people who want to manage PCOS and its associated symptoms [46, 47]. Approximately, 50% of people with PCOS develop diabetes or pre-diabetes before turning 40 years age [48]; therefore, following a special lifestyle and diets that maintain body weight and eventually increases good insulin levels, reduces the risk of the disease and its associated adverse events [49, 50, 51, 52, 53, 54].
Hence, the recommended diet composition for PCOS patients is drawn from the available recommendations for the dietary management of type 2 diabetes. Of note, carbohydrates have been reported to influence PCOS primarily via their impact on insulin concentrations in the blood. Studies demonstrate that a high-fiber, low-glycemic-index diet such as the DASH diet will result in an overall weight loss and a reduction in insulin resistance [33, 55]. A 6-month, low-carbohydrate, ketogenic diet was used by Mavropoulos et al to investigate the influence of a low intake of carbohydrates on obese and overweight patients with PCOS. They observed the amelioration in weight, free testosterone, LH: FSH ratio, and fasting insulin [56]. Serum androgens were also reported to be lowered in individuals who had been consuming a high-fat diet, then began taking an isocaloric, high-fiber, low-fat diet for 8 weeks [57].
Proper diets for weight loss and improving the concentration of lipid and metabolic profiles place great emphasis on the effects of dietary carbohydrates and fats. The results of studies indicate that if the fat intake is reduced from the total energy intake, the consumption of carbohydrates will increase [58, 59]. Patients with PCOS, just like other people must eat, therefore, following special diets such as LGD with potentially positive effects on blood glucose and insulin may reduce the risk of the disease and its associated adverse events [60]. In this context, the improvements were seen in insulin and HOMA-IR may suggest a potential improvement in insulin sensitivity following the use of LGD. In line with our findings, a meta-analysis was conducted by Zafar et al. to evaluate the hypothesis that LGD may lead to lowering measures of blood glucose control in individuals with both types of diabetes. They suggested a potential improvement in insulin sensitivity with LGD compared with other dietary interventions [61].
Recently, Kazemi et al conducted a similar systematic review and meta-analysis of RCTs to review evidence on the effects of GI or GL index diet on cardio-metabolic and reproductive profiles of women with PCOS [62]. They included seven RCTs, including cardio-metabolic and reproductive profiles. Our meta-analysis included eight RCTs and presents clinical outcomes based on PCOSQ domains, including emotional health, body hair, menstrual concerns, and fertility. Having PCOS does not mean the patient can't get pregnant, because this syndrome is among the most common, but treatable, causes of infertility in women. In women with PCOS, the hormonal imbalance interferes with ovulation, so PCOS significantly increases the risk of infertility [63]. The earlier interventions have failed or can be offered as a treatment of the first choice in selective causes that impair the occurrence of pregnancy for the woman with PCOS; therefore, choosing an effective treatment option addresses not only subfertility, anovulation, and fertility plays an important role as a holistic approach [64].
Overall, whether in combination with pharmacotherapy or as a standalone treatment, diet and lifestyle modifications should be recommended in the treatment of women with PCOS. Women with PCOS who are overweight should lose weight through diet and exercise. Their diet should contain high-fiber complex carbohydrates, moderate levels of protein, and a sufficient amount of fat to meet essential fatty acid requirements. It should also focus on omega-3 fatty acids and monounsaturated fatty acids and contain limited amounts of trans and saturated fats, as recommended by available evidence. These dietary modifications should significantly ameliorate many of the symptoms that co-exist with PCOS. Because symptoms of PCOS usually occur with menarche, it is also essential that adolescents who are at risk be screened, and dietary modifications for the prevention of PCOS and related comorbidities be carried out.
4.1. Limitations
This study contains some limitations. There was insufficient data available for any outcome to see if there was a dose-response relationship e.g. the greater the difference in GI between the low GI and the control, the greater the effect on the outcome. Also, there was insufficient evidence to determine how soon the effects were seen, or if longer interventions were more effective.
5. Conclusion
The present meta-analysis has shown that LGD may play a significant role in reducing the risk and improving the clinical and biochemical features of PCOS. PCOS has a negative impact on the general health of many women, although most women who suffer from this metabolic disease came to the doctors for treatment of menstrual irregularity, hirsutism, and infertility. As a health-care provider, the ultimate goal for the treatment of PCOS should be the improvement of fundamental health issues and consequently general health status. Also, other goals include preventing long-life complications of metabolic disorders due to PCOS like increased cardio-metabolic risks or obesity, instead of just treatment of disease symptoms. In this manner, if we could find interventions that improve general health status, as the consequence, symptoms like menstrual irregularity, hirsutism and infertility might get better or even be cured. With such dietary modalities, the risk of endometrial cancer might be omitted. So far the pieces of evidence for choosing the best diet modalities for PCOS are not strong to make definite recommendations.
Declarations
Author contribution statement
Fakher Rahim: Conceived and designed the analysis; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Fatemeh Haidari and Najmieh Saadati: Conceived and designed the experiments; Performed the experiments.
Mojgan Barati and Roshan Nikbakht: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Golshan Mirmomeni: Analyzed and interpreted the data.
Funding statement
This work was supported by "deputy of research and technology affairs of Ahvaz Jundishapur University of Medical Sciences (Reference number: FIRC-0017, Ethics approval Code: IR.AJUMS.REC.1400.329)".
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to give our thanks to our colleagues who supported us in the whole process of this paper work.
References
- 1.Ndefo U.A., Eaton A., Green M.R. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P t. 2013;38(6):336–355. [PMC free article] [PubMed] [Google Scholar]
- 2.Osibogun O., Ogunmoroti O., Michos E.D. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends in Cardiovascular Medicine. 2020;30(7):399–404. doi: 10.1016/j.tcm.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Fauser B.C.J.M., et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertility and Sterility. 2012;97(1):28–38. doi: 10.1016/j.fertnstert.2011.09.024. e25. [DOI] [PubMed] [Google Scholar]
- 4.Tzalazidis R., Oinonen K.A. Continuum of symptoms in polycystic ovary syndrome (PCOS): links with sexual behavior and unrestricted sociosexuality. J Sex Res. 2021;58(4):532–544. doi: 10.1080/00224499.2020.1726273. [DOI] [PubMed] [Google Scholar]
- 5.Scaruffi E., et al. Personality and psychiatric disorders in women affected by polycystic ovary syndrome. Frontiers in endocrinology. 2014;5:185. doi: 10.3389/fendo.2014.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenfield R.L., Ehrmann D.A. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocrine reviews. 2016;37(5):467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jalilian A., et al. Prevalence of polycystic ovary syndrome and its associated complications in Iranian women: a meta-analysis. Iranian journal of reproductive medicine. 2015;13(10):591–604. [PMC free article] [PubMed] [Google Scholar]
- 8.Barthelmess E.K., Naz R.K. Polycystic ovary syndrome: current status and future perspective. Frontiers in bioscience (Elite edition) 2014;6:104–119. doi: 10.2741/e695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burt Solorzano C.M., et al. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77(4):332–337. doi: 10.1016/j.steroids.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim S.S., et al. Lifestyle changes in women with polycystic ovary syndrome. The Cochrane database of systematic reviews. 2019;3(3):CD007506. doi: 10.1002/14651858.CD007506.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ojo O., et al. The effect of dietary glycaemic index on glycaemia in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10(3):373. doi: 10.3390/nu10030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noli S.A., et al. Dietary carbohydrate intake, dietary glycemic load and outcomes of in vitro fertilization: findings from an observational Italian cohort study. Nutrients. 2020;12(6):1568. doi: 10.3390/nu12061568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins G.G., Rossi B.V. The impact of lifestyle modifications, diet, and vitamin supplementation on natural fertility. Fertility Research and Practice. 2015;1(1):11. doi: 10.1186/s40738-015-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes E.T., et al. Effects of a low-glycemic load diet in overweight and obese pregnant women: a pilot randomized controlled trial. The American journal of clinical nutrition. 2010;92(6):1306–1315. doi: 10.3945/ajcn.2010.30130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goletzke J., et al. Effect of a low-glycemic load diet intervention on maternal and pregnancy outcomes in obese pregnant women. Nutrients. 2021;13:748. doi: 10.3390/nu13030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassar S., et al. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod. 2016;31(11):2619–2631. doi: 10.1093/humrep/dew243. [DOI] [PubMed] [Google Scholar]
- 17.Heshmati J., et al. The effects of supplementation with chromium on insulin resistance indices in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Horm Metab Res. 2018;50(3):193–200. doi: 10.1055/s-0044-101835. [DOI] [PubMed] [Google Scholar]
- 18.Łagowska K., Bajerska J., Jamka M. The role of vitamin D oral supplementation in insulin resistance in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10(11) doi: 10.3390/nu10111637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porchia L.M., et al. Diets with lower carbohydrate concentrations improve insulin sensitivity in women with polycystic ovary syndrome: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;248:110–117. doi: 10.1016/j.ejogrb.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Sadeghi A., et al. Effect of omega-3 fatty acids supplementation on insulin resistance in women with polycystic ovary syndrome: meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2017;11(2):157–162. doi: 10.1016/j.dsx.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Shang Y., et al. Effect of diet on insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2020;105(10) doi: 10.1210/clinem/dgaa425. [DOI] [PubMed] [Google Scholar]
- 22.Abdolahian S., et al. Effect of lifestyle modifications on anthropometric, clinical, and biochemical parameters in adolescent girls with polycystic ovary syndrome: a systematic review and meta-analysis. BMC Endocr Disord. 2020;20(1):71. doi: 10.1186/s12902-020-00552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akbari M., et al. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2018;50(4):271–279. doi: 10.1055/s-0044-101355. [DOI] [PubMed] [Google Scholar]
- 24.Heydarpour F., et al. Effects of cinnamon on controlling metabolic parameters of polycystic ovary syndrome: a systematic review and meta-analysis. J Ethnopharmacol. 2020;254:112741. doi: 10.1016/j.jep.2020.112741. [DOI] [PubMed] [Google Scholar]
- 25.Azadi-Yazdi M., et al. The effect of vitamin D supplementation on the androgenic profile in patients with polycystic ovary syndrome: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2017;49(3):174–179. doi: 10.1055/s-0043-103573. [DOI] [PubMed] [Google Scholar]
- 26.Carmina E., et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104(7):2875–2891. doi: 10.1210/jc.2018-02548. [DOI] [PubMed] [Google Scholar]
- 27.Moher D., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J.P.T., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt G.H., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed.) 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohatgi A. 2021 27 April. WebPlotDigitizer. [Google Scholar]
- 31.Wan X., et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology. 2014;14(1):135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barr S., et al. An isocaloric low glycemic index diet improves insulin sensitivity in women with polycystic ovary syndrome. J Acad Nutr Diet. 2013;113(11):1523–1531. doi: 10.1016/j.jand.2013.06.347. [DOI] [PubMed] [Google Scholar]
- 33.Asemi Z., et al. Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized clinical trial. Nutrition. 2014;30(11-12):1287–1293. doi: 10.1016/j.nut.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Kazemi M., et al. A comparison of a pulse-based diet and the therapeutic lifestyle changes diet in combination with exercise and health counselling on the cardio-metabolic risk profile in women with polycystic ovary syndrome: a randomized controlled trial. Nutrients. 2018;10(10) doi: 10.3390/nu10101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh K.A., et al. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92(1):83–92. doi: 10.3945/ajcn.2010.29261. [DOI] [PubMed] [Google Scholar]
- 36.Mehrabani H.H., et al. Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J Am Coll Nutr. 2012;31(2):117–125. doi: 10.1080/07315724.2012.10720017. [DOI] [PubMed] [Google Scholar]
- 37.Panico A., et al. Effects of an isocaloric low-glycemic-load diet in polycystic ovary syndrome. Nutritional Therapy & Metabolism. 2014;32:85–92. [Google Scholar]
- 38.Sordia-Hernández L.H., et al. Effect of a low glycemic diet in patients with polycystic ovary syndrome and anovulation - a randomized controlled trial. Clin Exp Obstet Gynecol. 2016;43(4):555–559. [PubMed] [Google Scholar]
- 39.Turner-McGrievy G.M., et al. Low glycemic index vegan or low-calorie weight loss diets for women with polycystic ovary syndrome: a randomized controlled feasibility study. Nutr Res. 2014;34(6):552–558. doi: 10.1016/j.nutres.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Wong J.M., et al. A randomized pilot study of dietary treatments for polycystic ovary syndrome in adolescents. Pediatr Obes. 2016;11(3):210–220. doi: 10.1111/ijpo.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.workshop, R.E.A.-S.P.c. and group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 42.Ilagan M., et al. The modified ferriman-gallwey score and hirsutism among Filipino women. Endocrinol Metab (Seoul) 2019;34(4):374–381. doi: 10.3803/EnM.2019.34.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cussons A.J., et al. Polycystic ovarian syndrome: marked differences between endocrinologists and gynaecologists in diagnosis and management. Clin Endocrinol (Oxf) 2005;62(3):289–295. doi: 10.1111/j.1365-2265.2004.02208.x. [DOI] [PubMed] [Google Scholar]
- 44.Abdalla M.A., et al. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2020;11 doi: 10.1177/2042018820938305. 2042018820938305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morales A.J., et al. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81(8):2854–2864. doi: 10.1210/jcem.81.8.8768842. [DOI] [PubMed] [Google Scholar]
- 46.Diamanti-Kandarakis E., Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocrine reviews. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rojas J., et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. International journal of reproductive medicine. 2014;2014:719050. doi: 10.1155/2014/719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang E.T., et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstetrics and gynecology. 2011;117(1):6–13. doi: 10.1097/AOG.0b013e31820209bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiofalo B., et al. Fasting as possible complementary approach for polycystic ovary syndrome: hope or hype? Med Hypotheses. 2017;105:1–3. doi: 10.1016/j.mehy.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Farsinejad-Marj M., et al. Clinical and metabolic responses to magnesium supplementation in women with polycystic ovary syndrome. Biol Trace Elem Res. 2020;196(2):349–358. doi: 10.1007/s12011-019-01923-z. [DOI] [PubMed] [Google Scholar]
- 51.Geier L.M., Bekx M.T., Connor E.L. Factors contributing to initial weight loss among adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2012;25(6):367–370. doi: 10.1016/j.jpag.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Hernández I., et al. Hypothalamic dopaminergic tone and prolactin bioactivity in women with polycystic ovary syndrome. Arch Med Res. 2000;31(2):216–222. doi: 10.1016/s0188-4409(00)00059-x. [DOI] [PubMed] [Google Scholar]
- 53.Pasquali R., Gambineri A. Role of changes in dietary habits in polycystic ovary syndrome. Reprod Biomed Online. 2004;8(4):431–439. doi: 10.1016/s1472-6483(10)60927-3. [DOI] [PubMed] [Google Scholar]
- 54.Shahdadian F., et al. Association between major dietary patterns and polycystic ovary syndrome: evidence from a case-control study. Appl Physiol Nutr Metab. 2019;44(1):52–58. doi: 10.1139/apnm-2018-0145. [DOI] [PubMed] [Google Scholar]
- 55.Stein K. Polycystic ovarian syndrome: what it is and why registered dietitians need to know. J Am Diet Assoc. 2006;106(11):1738–1741. doi: 10.1016/j.jada.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Mavropoulos J.C., et al. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond) 2005;2:35. doi: 10.1186/1743-7075-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C., et al. Low-fat high-fiber diet decreased serum and urine androgens in men. J Clin Endocrinol Metab. 2005;90(6):3550–3559. doi: 10.1210/jc.2004-1530. [DOI] [PubMed] [Google Scholar]
- 58.Pereira E.V., Costa Jde A., Alfenas Rde C. Effect of glycemic index on obesity control. Arch Endocrinol Metab. 2015;59(3):245–251. doi: 10.1590/2359-3997000000045. [DOI] [PubMed] [Google Scholar]
- 59.Brand-Miller J.C., et al. Glycemic index and obesity. Am J Clin Nutr. 2002;76(1):281s–285s. doi: 10.1093/ajcn/76/1.281S. [DOI] [PubMed] [Google Scholar]
- 60.Liepa G.U., Sengupta A., Karsies D. Polycystic ovary syndrome (PCOS) and other androgen excess-related conditions: can changes in dietary intake make a difference? Nutr Clin Pract. 2008;23(1):63–71. doi: 10.1177/011542650802300163. [DOI] [PubMed] [Google Scholar]
- 61.Zafar M.I., et al. Low-glycemic index diets as an intervention for diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2019;110(4):891–902. doi: 10.1093/ajcn/nqz149. [DOI] [PubMed] [Google Scholar]
- 62.Kazemi M., et al. Effects of dietary glycemic index and glycemic load on cardiometabolic and reproductive profiles in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2021;12(1):161–178. doi: 10.1093/advances/nmaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dennett C.C., Simon J. The role of polycystic ovary syndrome in reproductive and metabolic health: overview and approaches for treatment. Diabetes spectrum : a publication of the American Diabetes Association. 2015;28(2):116–120. doi: 10.2337/diaspect.28.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sawant S., Bhide P. Fertility treatment Options for women with polycystic ovary syndrome. Clinical medicine insights. Reproductive health. 2019;13 doi: 10.1177/1179558119890867. 1179558119890867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.