Abstract
Intrinsic brain tumors often occur within functional neural networks, leading to neurological impairment and disability of varying degrees. Advances in our understanding of tumor-network integration, human cognition and language processing, and multiparametric imaging, combined with refined intraoperative tumor resection techniques, have enhanced surgical management of intrinsic brain tumors within eloquent areas. However, cognitive symptoms impacting health-related quality of life, particularly processing speed, attention, concentration, working memory, and executive function, often persist after the postoperative recovery period and treatment. Multidisciplinary cognitive rehabilitation is the standard of care for addressing cognitive impairments in many neurological diseases. There is promising research to support the use of cognitive rehabilitation in adult brain tumor patients. In this review, we summarize the history and usefulness of postacute cognitive rehabilitation for adult brain tumor patients.
Keywords: Glioma, Brain tumor, Eloquent area brain tumors, Cognitive outcomes, Neurocognitive outcomes, Cognitive rehabilitation, Primary brain tumor, Cognitive rehab
ABBREVIATIONS
- FLAIR
fluid-attenuated inversion recovery
- HGG
high-grade glioma
- HRQOL
health-related quality of life
- KPS
Karnofsky Performance Score
- LGG
low-grade glioma
- Mets
metastases
- MNG
meningioma
- MRI
magnetic resonance imaging
- PA
postacute outpatient
- VR
virtual reality
Approximately 100 000 people are diagnosed with a primary brain tumor each year, with gliomas comprising 72% of malignant brain tumors and 21% of all central nervous system tumors in adults.1,2 Survival outcomes for diffuse glioma patients are improving due to advances in neurosurgical techniques (particularly as it relates to awake mapping for eloquent tumors), molecular characterization, advanced radiotherapy, and chemotherapy.3-5 Median survival across all molecular subtypes ranges from 1.5 to 15 yr.3,5 Despite these diagnostic and treatment advances, patients often experience significant decrements in cognitive and emotional functioning that impact their health-related quality of life (HRQOL) and survival.6-9
The reported prevalence of cognitive impairments in glioma varies widely10 and ranges from 19% to 83%.10-12 At presentation, 31% to 81% of patients report cognitive impairments, which is more than any other cancer type,11,13-15 with high-grade glioma demonstrating greater rates of cognitive impairment (69%)11 when compared to low-grade glioma. Prevalence of cognitive impairments may vary across the disease trajectory. Some reports suggest that cognition improves 3 to 6 mo following tumor resection.16-18 However, other data indicate that many patients remain impaired, or decline further, as a consequence of subsequent treatment with radiation and chemotherapy, and/or tumor progression.9,11 The nonstatic waxing and waning disease trajectory experienced by brain tumor patients is not unique to cancer, and is also experienced by patients with movement disorders such as Parkinson's disease and stroke.
Gliomas are commonly localized in the frontal and temporal lobes19-22 and associated with cognitive impairments in executive function,12,13,18,23 language,24-26 learning, and memory.11,12,27 When gliomas are located in the dominant hemisphere, verbal learning and verbal intelligence are often impacted in addition to langauge.24 Furthermore, left hemisphere gliomas demonstrate more impaired verbal recall compared to their counterparts with intraventricular and posterior fossa tumors.28 From a language perspective, worsened lexical retrieval (object naming and verbal fluency) is commonly seen in dominant hemisphere tumors.26 Executive function-29 and memory-related4 processes in eloquent areas are felt to be more resistant in returning to premorbid baselines, despite the use of awake functional mapping with language preservation.
While surgical treatments may lead to more focal impairments, chemotherapy and radiotherapy are more likely to impact distributed networks.7,19,24,30 Impaired processing speed,11,12,20,30 attention and concentration,20,23 and motivation31 are associated with vasculopathy and white matter injury that occurs postradiation.32,33 Disruptions in learning can be seen with chemotherapy as treatment can reduce hippocampal neurogenesis by producing asynchronous communication between functionally related structures.34
Improved survival of adult brain tumor patients with persistent cognitive impairments that negatively impact their HRQOL supports the application of a chronic disease model for management starting in the perioperative period.3,35,36 Cognitive impairments are associated with lower rates of return to full-time work capacity (24%), strained personal relationships (23%), reduced HRQOL, and reduced independence (26%), despite high Karnofsky Performance Scores (KPS > 90).37 Additionally, anxiety and depression contribute to cognitive impairment, improve variably postsurgery,4 and are also linked to reduced HRQOL.22 These challenges call for a multidisciplinary approach to care,3,38-40 and there is emerging evidence in support of cognitive rehabilitation strategies.5,12,41 Cognitive rehabilitation is a collection of treatment strategies designed to address problems with attention, memory, learning, planning, judgment, and perception that are brought about by neurological disorders and injuries.
However, postacute cognitive rehabilitation has not been broadly applied in surgical neuro-oncology. In comparison with noncancer neurological diseases, utilization remains low (27.7%), and care is often fragmented.42 In the postacute setting, only 50% of patients receive rehabilitation in the first 6 mo following diagnosis, and furthermore, only 9% of that subset receive language or cognitive rehabilitation.42 Reasons for underuse include lack of identifying functional impairments, limited access to care, and poor understanding of rehabilitation's benefits for patients with brain tumors.43
There is an urgent need to integrate rehabilitative services with a validated referral protocol for treatment by health care professionals.44 The goal of this manuscript is to review cognitive rehabilitation principles, discuss the evidence for cognitive rehabilitation in brain tumor patients, and provide guidance regarding the referral process, with the aim of increasing provider knowledge.
THE PRINCIPLES OF COGNITIVE REHABILITATION
The history of cognitive rehabilitation dates back to World Wars I and II, when methods of rehabilitation were proposed to meet the needs of soldiers returning home from overseas combat.45 Modern cognitive rehabilitation includes 2 main interventional approaches: (1) retraining and (2) functional compensation.46-50 Retraining strengthens impaired cognitive skills through repeated practicing of cognitive tasks,49 while functional compensation focuses on improving daily functioning through environmental modifications and/or approaches to achieve their goals.47,51 Using compensatory strategies and retraining skills that closely align with functional tasks has greater transfer effects to daily life, and improves likelihood of meeting HRQOL goals.50-52
The mechanisms of cognitive rehabilitation are based on principles of neural plasticity,53,54 including the thought that cortical areas have greater affinity for functional reorganization compared to white matter tracts.55-58 Cognitive plasticity underlies neurotypical development and learning, and protects against the effects of aging and recovery from injury.55,57,59,60 Furthermore, research on mechanisms of cognitive rehabilitation indicates that active rewiring and impulse strengthening are crucial for neuronal recovery.55,60 Neuroimaging studies suggest 2 mechanisms of functional reorganization in eloquent areas: (1) recruitment of residual structures from the dominant hemisphere that were premorbidly involved in cognitive processing and (2) recruitment of nondominant hemisphere accessory regions.61 Animal models demonstrate that cortical plasticity is mediated at least in part by cholinergic systems of the basal forebrain that, when disrupted, can negatively impact cortical reorganization and learning.62 These findings were extended to brain tumors by Miotto et al,63 who demonstrated contralateral functional reorganization in patients aftercognitive rehabilitation.
At the outset of cognitive rehabilitation, particularly in the outpatient postacute setting, comprehensive neuropsychological assessment allows the development of a treatment plan by identifying cognitive and emotional vulnerabilities and strengths,27,39,51,52,64 which are compared to preillness baseline and healthy peer estimates.64,65 This evaluation is more sensitive and specific for assessing cognitive changes compared to screening tools such as the Montreal Cognitive Assessment66 or clinical interviews.64,67 Furthermore, task performance may reveal cognitive changes that precede tumor progression seen on magnetic resonance imaging (MRI).68 Neuropsychological assessments can be further used to advocate for workplace accommodations,67 and identify appropriate cognitive rehabilitation interventions.
Treatment is approached in 3 phases using the Triple A model,49,51 which educates patients and caregivers so that they may more effectively understand and manage the patient's cognitive and behavioral symptoms (see Figure 1 for practical case example)51,69:
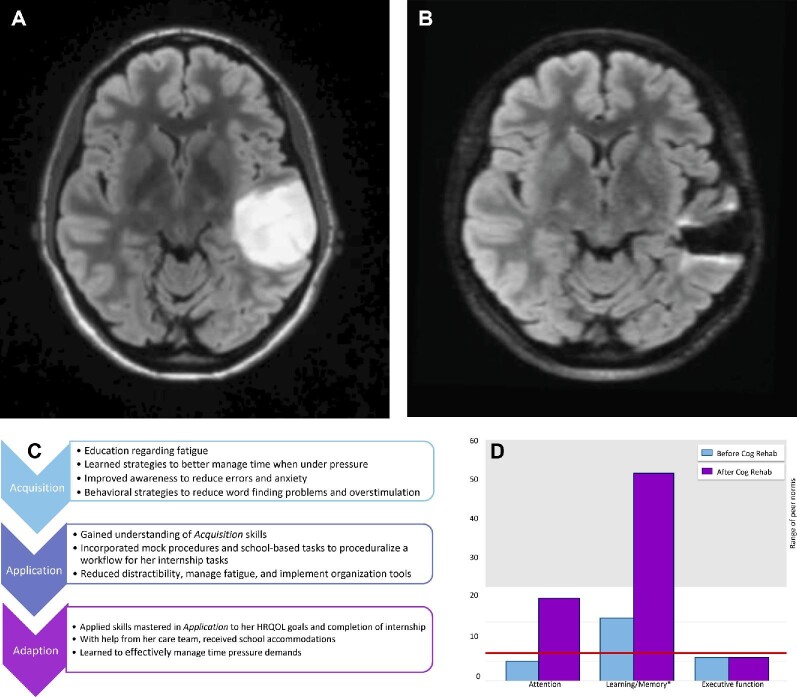
FIGURE 1.
Case example of a 29-yr-old right-handed Caucasian woman who presented with a generalized seizure and 10 yr of word finding difficulty and forgetfulness, and was found to have a nonenhancing left temporal mass. A, Axial MRI demonstrating T2/FLAIR hyperintense mass in the left temporal lobe prior to initial surgery. She underwent gross total resection with intraoperative awake mapping with pathology confirming diffuse astrocytoma, IDH mutant, with anomia of low-frequency words and expressive dysphasia that improved with occupational and speech therapy. She underwent a second surgery 12 mo later and postoperatively developed distractibility, forgetfulness, and reduced multitasking. She was referred for neuropsychological evaluation, with symptoms negatively impacting her schoolwork. B, Axial MRI demonstrating minimal T2/FLAIR hyperintense lesion in the left temporal lobe at time of referral to cognitive rehabilitation. C, She underwent 8 sessions of cognitive rehabilitation via telehealth over 4 mo, using the Triple A model. D, Baseline testing (blue) revealing mild-to-moderate vulnerabilities in distractibility, thinking speed, word finding, verbal memory, and executive functioning. Postintervention testing (purple) revealed improved auditory attention, working memory, and verbal memory. The asterisk denotes clinical significance at one standard deviation. The red line is the cutoff for clinical impairment and the gray-shaded area depicts the range of adjusted peer normative values. She went on to successfully pass her internship and licensing exam, and at the end of treatment, was working part-time in her preferred field (presented with patient permission). FLAIR, fluid-attenuated inversion recovery.
Acquisition: Patients are educated about cognitive vulnerabilities and strengths, and begin to learn compensatory strategies to improve their daily functioning.
Application: Patients practice previously learned compensatory strategies via in-session exercises to improve self-awareness, and begin building self-efficacy for managing cognitive impairments.
Adaptation: Patients begin to generalize compensation strategies learned outside of the rehabilitation sessions and integrate rehearsed skills into daily functioning to optimize self-efficacy of HRQOL goals and foster community integration.
Cognitive rehabilitation incorporates education, strategy instruction, practice, and environmental interventions47,49-52,70 with the goal of improving autonomy, self-awareness, emotional coping, acceptance, and management of cognitive impairments.71 By addressing cognitive needs along the disease trajectory, patients and caregivers may gain a sense of control over neuropsychiatric and behavioral changes.64 It remains important to consider, however, that individual patient perspectives, needs, interests, motivations, and investment in pursuing rehabilitation may differ.72-74
COGNITIVE REHABILITATION OUTCOMES FOR ADULT BRAIN TUMOR PATIENTS
There is emerging evidence regarding the use of cognitive rehabilitation in adult brain tumor patients (Table). Although most of the data for rehabilitation are in the acute inpatient setting, there are also promising results from the outpatient, postacute setting. Below, we discuss cognitive rehabilitation of patients with brain tumors in (1) the acute postsurgical75 and inpatient setting,76-78 and (2) the outpatient, postacute setting.63,79-84 Given the lack of guidelines for implementing cognitive rehabilitation in brain tumor patients, in addition to the complexity of these patients’ symptoms and needs, we also discuss practical considerations for referral and a proposed model for post-acute cognitive rehabilitation.
TABLE.
Evidence for Cognitive Rehabilitation in Brain Tumor
| Timing | Tumor histology | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | A | PA | Intervention | LGG | HGG | MNG | Mets | Other |
| Attention | ||||||||
| Gehring et al., 200981 | X | 2 h weekly iPad sessions | X | X | ||||
| Zucchella et al., 201375 | X | 4 h weekly for 16 sessions | X | X | X | X | ||
| Maschio et al., 201583 | X | 1 h weekly retraining for 10 sessions | X | X | X | X | ||
| Yang et al., 201484 | X | 1.5 h weekly virtual reality retraining | X | X | X | X | X | |
| Verbal learning and memory | ||||||||
| Gehring et al, 200981 | X | 2 h weekly iPad sessions | X | X | ||||
| Zucchella et al, 201375 | X | 4 h weekly for 16 sessions | X | X | X | X | ||
| Hassler et al, 201094 | X | 90 min in 10 sessions of holistic mnemonic training | X | |||||
| Miotto et al, 201395 and 201463 | X | 30 min of strategic semantic organizational training | X | X | ||||
| Maschio et al, 201583 | X | 1 h weekly retraining for 10 sessions | X | X | X | X | ||
| Yang et al, 201484 | X | 1.5 h weekly virtual reality retraining | X | X | X | X | X | |
| Executive function | ||||||||
| Richard et al, 201982 | X | 2 h weekly of Goal Management Training or Brain Health Program | X | X | X | X | ||
| Processing speed | ||||||||
| Richard et al, 201982 | X | 2 h weekly of Goal Management Training or Brain Health Program | X | X | X | X | ||
A, acute inpatient; HGG, high-grade glioma (anaplastic and glioblastoma); LGG, low-grade glioma; Mets, metastases; MNG, meningioma; PA, postacute outpatient.
Cognitive Rehabilitation in the Acute Setting
Though research investigating cognitive rehabilitation in the acute, postsurgical setting is limited, it holds promise.76,77,85-89 Adult brain tumor patients demonstrate greater recovery in cognitive gains75,87 compared to traumatic brain injury patients.79 A small study of patients with primary brain tumors (high- and low-grade gliomas and meningiomas), who received cognitive rehabilitation within 2 wk postsurgery, demonstrated improvements in visual attention and verbal memory at 1 mo compared to a group of matched controls.75 Marciniak et al studied patients with primary and metastatic brain tumors, noting improved cognition at discharge following acute cognitive rehabilitation.87 However, others were unable to demonstrate improvements in cognitive outcomes following rehabilitation.76,90 These conflicting results may be explained by differences in tumor location and a mixed patient cohort with respect to histologies. The impact of tumor location on cognitive gains remains controversial in the acute setting, as some studies suggest greater improvement in patients with left hemispheric lesions,85 while others fail to correlate tumor laterally with gains.76,91 Most data support similar trajectories of improvement across tumor histologies, without significant differences between glioma and meningioma, and with an average length of stay in acute rehabilitation of 24 d (vs 75 d for stroke recovery).90
Cognitive Rehabilitation Outcomes in the Outpatient, Postacute Setting
Compared with cognitive rehabilitation immediately following surgery, there is more mechanistic support for improving cognitive outcomes in the outpatient, postacute setting. Cognitive rehabilitation based on learning mechanisms60 synergizes with spontaneous and intervention-based recovery.53 Outpatient, postacute cognitive rehabilitation benefits partially recovered neuroplastic networks92 by potentiating and reinforcing the healthy functional connectivity selection and stabilization that underlies cognition.53 Additionally, there is emerging evidence that neuroinflammation (such as edema) may limit the process of plasticity,93 which underlies the mechanisms of cognitive rehabilitation and further supports its application in the stable, postacute setting.
Positive outcomes across several cognitive domains (memory and attention in particular) are seen with cognitive rehabilitation in patients with brain tumors (Table).63,81,83,84,94,95 A large randomized controlled trial, which enrolled patients with grade II and III gliomas for 6 weekly cognitive rehabilitation sessions (2 h of combined retraining and compensatory strategy training) vs a wait-list control group, demonstrated improvements in brief attention and verbal memory at 6 mo postintervention.81 Studies of internal strategy training (semantic categorization) in patients with tumors of the eloquent prefrontal cortex demonstrated significant improvement in memory and increased contralateral activation as seen on functional MRI.63,95 Improvements in verbal learning were also noted in anaplastic gliomas and glioblastoma after 10 wk of internal strategy training (mnemonics).94 Since the 1980s, virtual reality (VR) has been increasingly used and represents a promising technology for neurorehabilitation. VR has also been shown to improve attention and memory when applied in a mixed cohort of adult brain tumor patients (meningioma, glioma, and brain metastases).84
Executive functioning is also noted to improve after cognitive rehabilitation in brain tumor patients.82,96,97 A study including mixed histologies (low- and high-grade glioma, meningioma, and other tumor types) found significant improvements in executive functioning and processing speed following 8 wk of compensatory metacognitive strategy training (focusing on awareness, self-monitoring, and attentional control), when compared to brain tumor education alone.82 An additional study of gliomas and meningiomas found that 88% of patients described interventional problem-solving strategies as helpful, with the majority of patients continuing to use the learned approaches 3 mo posttreatment.98
Although promising research supports the feasibility and positive cognitive outcomes of cognitive rehabilitation in patients with brain tumors, results should be interpreted cautiously. The current literature is limited by methodological variability and lack of cognitive test battery uniformity.11,99,100 Also, sample heterogeneity and varied assessment intervals complicate a complete understanding of cognitive improvement with these interventions.18,101 Comparisons between different tumor grades, molecular subtypes, and histologies are important areas for future research.
Referral Considerations for Cognitive Rehabilitation for Adult Brain Tumor Patients
The optimal timing for cognitive rehabilitation along the brain tumor illness trajectory remains undefined. In the acute, postsurgical setting, there are limited studies supporting positive outcomes for patients with brain tumors, though the longer term durability of these gains remains unclear.86,89 While some data support cognitive rehabilitation in the acute, postsurgical setting,75,77 adjuvant treatments, such as radiotherapy, may further compromise cognition.
In the outpatient clinic, patients are considered for cognitive rehabilitation in the context of their medical status, rehabilitation needs, tolerance for intervention, HRQOL goals, and other factors.102 Patients who are less aware of their impairments and experience greater functional impact (which is more common in the acute, postsurgical setting) may underestimate the need for rehabilitation, thus complicating engagement in treatment.69,74,103 Suboptimal treatment intensity can also limit recovery, leading to frustration and psychological distress. Fatigue and stamina levels in patients with brain tumors mediate the relationship between treatment intensity and achievement of rehabilitation goals.104 Therefore, outpatient, postacute cognitive rehabilitation should be considered for medically stable patients who are (1) able to tolerate a full baseline neuropsychological battery, (2) have clearly defined goals, and (3) demonstrate a readiness to engage in additional services to address these issues.69,74
PROPOSED MODEL FOR AN OUTPATIENT, POSTACUTE COGNITIVE REHABILITATION
Current practice guidelines39 support the use of neuropsychological, multidisciplinary informed care for patients with brain tumors,105,106 though there is little guidance for its practical implementation into neuro-oncology practice.39,40 Below, we propose a model for integrating multidisciplinary cognitive rehabilitation into outpatient, postacute care (Figure 2).
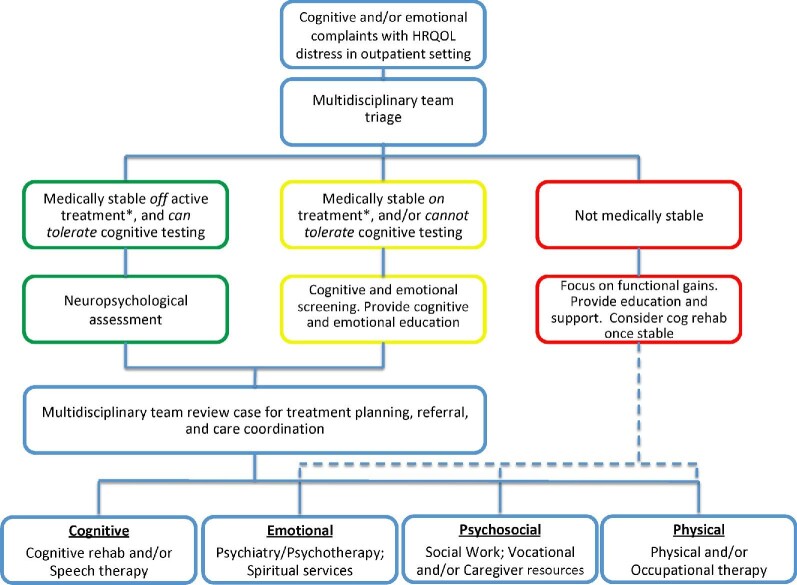
FIGURE 2.
A proposed model for a postacute cognitive rehabilitation in neuro-oncology practice.
The goal of the proposed model is to optimize cognitive care across the disease trajectory from a multidisciplinary, patient-centered perspective. This includes a team of clinicians caring for brain tumor patients alongside neuropsychologists, social workers, nurses, and mental health experts. To assess each patient consistently, we propose systematic cognitive and emotional screening across the disease trajectory to detect cognitive and psychiatric symptoms early. This approach also enhances communication among providers and tracks changes over time.
Cognitive and emotional symptoms that are identified through screening, and determined to negatively impact HRQOL, are then triaged by the multidisciplinary care team and used to inform recommended intervention strategy. Strategies for cognitive intervention are based on the patient's medical stability, fatigue, patient and caregiver goals, engagement in treatments, and ability to participate in rehabilitation. Of note, patients with new tumor progression, changes in therapy, and/or poorly controlled symptoms (eg, seizures) may be best suited for a lower intensity assessment and rehabilitation intervention. In these cases, formalized cognitive screening, tailored education, and support are provided with possible future referral for higher intensity cognitive services once stabilized. Furthermore, high emotional/psychiatric distress, reduced self-awareness and/or denial of cognitive impairments, and poor motivation and/or noncompliance are crucial elements that can negatively impact outcomes of cognitive rehabilitation.51,69,74,107 Prioritizing treatment of comorbid factors (such as debilitating depression, anxiety, or psychosis) prior to, or in conjunction with, cognitive rehabilitation is encouraged.74,108-111
After patients complete the appropriate assessments, the multidisciplinary team reviews the findings to inform the treatment plan, coordinate appropriate referrals, and provide feedback to the patient and caregiver regarding the recommendations, which focus on optimizing HRQOL. For patients whom cognitive rehabilitation is recommended, interventions are tailored to help both the patients and caregivers more effectively understand and manage the patient's cognitive and behavioral symptoms; the interventions are also time limited, and include specific and measurable treatment goals designed to optimize the patient's autonomy and HRQOL.
At the conclusion of the recommended treatment, findings are brought back to the multidisciplinary team and incorporated into ongoing care needs. The treatment team continues systematic clinical monitoring of cognitive and emotional symptoms, with the intent of reapproaching the multidisciplinary team if additional interventions (eg, repeated assessment, “booster” sessions, caregiver training) are needed in the future. This proposed multidisciplinary model is an area of active research investigation.
CONCLUSION
Despite diagnostic and treatment advances, intrinsic brain tumor patients often experience significant decrements in cognitive and emotional functioning, which negatively impact their HRQOL. There is evidence supporting the feasibility and efficacy of cognitive rehabilitation in the acute and postacute settings, with overall positive outcomes for primary and metastatic brain tumors. However, methodological concerns, low rates of systematic cognitive screening, lack of awareness of cognitive rehabilitation in the brain tumor population, and rehabilitation provider scarcity remain barriers to care. By encouraging providers to (1) screen for cognitive symptoms over the disease trajectory and (2) refer patients for neuropsychological assessment for diagnosis and rehabilitation planning, patients can experience improved cognitive outcomes. A multidisciplinary approach to cognitive rehabilitation is supported by brain tumor clinical practice guidelines. Recently proposed cognitive rehabilitation models for adult brain tumor patients serve as a translational bridge between behavioral neuroscience investigation and clinical practice.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors’ research work is supported by the LoGlio Collective (all authors); the Sheri Sobrato Brisson Brain Cancer Fund (C.W.J., S.H.J.); Robert Wood Johnson Foundation 74259 (S.H.J.); and NINDS K08 110919-01 (S.H.J.). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
We would also like to thank Melissa Lau for her editorial contributions
Contributor Information
Christina Weyer-Jamora, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California; Department of Psychiatry, Zuckerberg San Francisco General Hospital, San Francisco, California.
Melissa S Brie, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California; Department of Psychiatry, Zuckerberg San Francisco General Hospital, San Francisco, California.
Tracy L Luks, Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California.
Ellen M Smith, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California.
Shawn L Hervey-Jumper, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California.
Jennie W Taylor, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California; Department of Neurology, University of California San Francisco, San Francisco, California, USA.
REFERENCES
- 1. de Robles P, Fiest KM, Frolkis ADet al. . The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro Oncol. 2015;17(6):776-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15(7):405-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4(9):1254-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Racine CA, Li J, Molinaro AM, Butowski N, Berger MS. Neurocognitive function in newly diagnosed low-grade glioma patients undergoing surgical resection with awake mapping techniques. Neurosurgery. 2015;77(3):371-379. [DOI] [PubMed] [Google Scholar]
- 5. Chang SM, Cahill DP, Aldape KD, Mehta MP. Treatment of adult lower-grade glioma in the era of genomic medicine. Am Soc Clin Oncol. 2016;36:75-81. [DOI] [PubMed] [Google Scholar]
- 6. Klein M, Heimans J, Aaronson Net al. . Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet North Am Ed. 2002;360(9343):1361-1368. [DOI] [PubMed] [Google Scholar]
- 7. Laack NN, Brown PD, Ivnik RJet al. . Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study. Int J Radiat Oncol. 2005;63(4):1175-1183. [DOI] [PubMed] [Google Scholar]
- 8. Douw L, Klein M, Fagel SSet al. . Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810-818. [DOI] [PubMed] [Google Scholar]
- 9. Wolf J, Campos B, Bruckner T, Vogt L, Unterberg A, Ahmadi R. Evaluation of neuropsychological outcome and “quality of life” after glioma surgery. Langenbecks Arch Surg. 2016;401(4):541-549. [DOI] [PubMed] [Google Scholar]
- 10. van Loon EM, Heijenbrok-Kal MH, van Loon WSet al. . Assessment methods and prevalence of cognitive dysfunction in patients with low-grade glioma: a systematic review. J Rehabil Med. 2015;47(6):481-488. [DOI] [PubMed] [Google Scholar]
- 11. van Kessel E, Baumfalk AE, van Zandvoort MJE, Robe PA, Snijders TJ. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134(1):9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boone M, Roussel M, Chauffert B, Le Gars D, Godefroy O. Prevalence and profile of cognitive impairment in adult glioma: a sensitivity analysis. J Neurooncol. 2016;129(1):123-130. [DOI] [PubMed] [Google Scholar]
- 13. Noll KR, Ziu M, Weinberg JS, Wefel JS. Neurocognitive functioning in patients with glioma of the left and right temporal lobes. J Neurooncol. 2016;128(2):323-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt JE, Beckjord E, Bovbjerg DHet al. . Prevalence of perceived cognitive dysfunction in survivors of a wide range of cancers: results from the 2010 LIVESTRONG survey. J Cancer Surviv. 2016;10(2):302-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324-334. [DOI] [PubMed] [Google Scholar]
- 16. Barzilai O, Ben Moshe S, Sitt R, Sela G, Shofty B, Ram Z. Improvement in cognitive function after surgery for low-grade glioma. J Neurosurg. 2019;130(2):426-434. [DOI] [PubMed] [Google Scholar]
- 17. Hoffermann M, Bruckmann L, Mahdy Ali K, Zaar K, Avian A, von Campe G. Pre- and postoperative neurocognitive deficits in brain tumor patients assessed by a computer based screening test. J Clin Neurosci. 2017;36(Feb):31-36. [DOI] [PubMed] [Google Scholar]
- 18. Ng JCH, See AAQ, Ang TY, Tan LYR, Ang BT, King NKK. Effects of surgery on neurocognitive function in patients with glioma: a meta-analysis of immediate post-operative and long-term follow-up neurocognitive outcomes. J Neurooncol. 2019;141(1):167-182. [DOI] [PubMed] [Google Scholar]
- 19. Correa DD, DeAngelis LM, Shi W, Thaler HT, Lin M, Abrey LE. Cognitive functions in low-grade gliomas: disease and treatment effects. J Neurooncol. 2006;81(2):175-184. [DOI] [PubMed] [Google Scholar]
- 20. Hendriks EJ, Habets EJJ, Taphoorn MJBet al. . Linking late cognitive outcome with glioma surgery location using resection cavity maps. Hum Brain Mapp. 2018;39(5):2064-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Habets EJJ, Hendriks EJ, Taphoorn MJBet al. . Association between tumor location and neurocognitive functioning using tumor localization maps. J Neurooncol. 2019;144(3):573-582. [DOI] [PubMed] [Google Scholar]
- 22. Noll KR, Bradshaw ME, Weinberg JS, Wefel JS. Relationships between neurocognitive functioning, mood, and quality of life in patients with temporal lobe glioma. Psychooncology. 2017;26(5):617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cochereau J, Herbet G, Duffau H. Patients with incidental WHO grade II glioma frequently suffer from neuropsychological disturbances. Acta Neurochir. 2016;158(2):305-312. [DOI] [PubMed] [Google Scholar]
- 24. Scheibel RS, Meyers CA, Levin VA. Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. J Neurooncol. 1996;30(1):61-69. [DOI] [PubMed] [Google Scholar]
- 25. Lilja A, Brun A, Salford LGet al. . Neuropsychological indexes of a partial frontal syndrome in patients with nonfrontal gliomas. Neuropsychology. 1992;6(4):315-326. [Google Scholar]
- 26. Antonsson M, Jakola A, Longoni Fet al. . Post-surgical effects on language in patients with presumed low-grade glioma. Acta Neurol Scand. 2018;137(5):469-480. [DOI] [PubMed] [Google Scholar]
- 27. Wefel JS, Kayl AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer. 2004;90(9):1691-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miotto EC, Silva Junior A, Silva CCet al. . Cognitive impairments in patients with low grade gliomas and high grade gliomas. Arq Neuropsiquiatr. 2011;69(4):596-601. [DOI] [PubMed] [Google Scholar]
- 29. Santini B, Talacchi A, Squintani G, Casagrande F, Capasso R, Miceli G. Cognitive outcome after awake surgery for tumors in language areas. J Neurooncol. 2012;108(2):319-326. [DOI] [PubMed] [Google Scholar]
- 30. Taphoorn MJ, Klein M.. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168. [DOI] [PubMed] [Google Scholar]
- 31. Swennen MHJ, Bromberg JEC, Witkamp ThD, Terhaard CHJ, Postma TJ, Taphoorn MJB. Delayed radiation toxicity after focal or whole brain radiotherapy for low-grade glioma. J Neurooncol. 2004;66(3):333-339. [DOI] [PubMed] [Google Scholar]
- 32. Schatz J, Kramer J, Ablin A, Matthay KK. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189-200. [DOI] [PubMed] [Google Scholar]
- 33. Monje ML, Palmer T.. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16(2):129-134. [DOI] [PubMed] [Google Scholar]
- 34. Nokia MS, Anderson ML, Shors TJ. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur J Neurosci. 2012;36(11):3521-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chambers SK, Grassi L, Hyde MK, Holland J, Dunn J. Integrating psychosocial care into neuro-oncology: challenges and strategies. Front Oncol. 2015;5(Feb):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. National Institute for Health and Clinical Excellence . Improving Outcomes for People with Brain and Other CNS Tumours. Cancer service guideline [CSG10]; 2006. [Accessed February 25, 2020]. Available from: https://www.nice.org.uk/guidance/csg10/resources/improving-outcomes-for-people-with-brain-and-other-central-nervous-system-tumours-update-27841361437. [Google Scholar]
- 37. Liu R, Solheim K, Polley M-Yet al. . Quality of life in low-grade glioma patients receiving temozolomide. Neuro Oncol. 2009;11(1):59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Houillier C, Wang X, Kaloshi Get al. . IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75(17):1560-1566. [DOI] [PubMed] [Google Scholar]
- 39. Kim W-J, Novotna K, Amatya B, Khan F. Clinical practice guidelines for the management of brain tumours: a rehabilitation perspective. J Rehabil Med. 2019;51(2):89-96. [DOI] [PubMed] [Google Scholar]
- 40. Coomans MB, van der Linden SD, Gehring K, Taphoorn MJB. Treatment of cognitive deficits in brain tumour patients: current status and future directions. Curr Opin Oncol. 2019;31(6):540-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. IJzerman-Korevaar M, Snijders TJ, de Graeff A, Teunissen S, de Vos FYF. Prevalence of symptoms in glioma patients throughout the disease trajectory: a systematic review. J Neurooncol. 2018;140(3):485-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pace A, Villani V, Parisi Cet al. . Rehabilitation pathways in adult brain tumor patients in the first 12 months of disease. A retrospective analysis of services utilization in 719 patients. Support Care Cancer. 2016;24(11):4801-4806. [DOI] [PubMed] [Google Scholar]
- 43. McCartney A, Butler C, Acreman S. Exploring access to rehabilitation services from allied health professionals for patients with primary high-grade brain tumours. Palliat Med. 2011;25(8):788-796. [DOI] [PubMed] [Google Scholar]
- 44. Turner-Stokes L, Sykes N, Silber E. Long-term neurological conditions: management at the interface between neurology, rehabilitation and palliative care. Clin Med. 2008;8(2):186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boake C. A history of cognitive rehabilitation of head-injured patients, 1915 to 1980. J Head Trauma Rehabil. 1989;4(3):1-8. [Google Scholar]
- 46. Bäckman L, Dixon RA. Psychological compensation: a theoretical framework. Psychol Bull. 1992;112(2):259-283. [DOI] [PubMed] [Google Scholar]
- 47. Dixon RA, Garrett DD, Bäckman L.. Principles of compensation in cognitive neurorehabilitation. In: Stuss DT, Winocur G, Robertson IH, eds. Cognitive Neurorehabilitation. New York: Cambridge University Press; 1999:59-72. [Google Scholar]
- 48. Fawcett JW, Rosser AE, Dunnett SB, Wilson B, Robertson IH, eds. Brain Damage, Brain Repair. Oxford: Oxford University Press; 2001:289-297. [Google Scholar]
- 49. Sohlberg MM, Mateer CA.. Introduction to Cognitive Rehabilitation: Theory and Practice. New York: Guilford Press; 1989. [Google Scholar]
- 50. Wilson BA, Gracey F, Evans JJ, Bateman A. Neuropsychological Rehabilitation: Theory, Models, Therapy and Outcome. Cambridge University Press; 2009. [Google Scholar]
- 51. Haskins EC. Cognitive Rehabilitation Manual: Translating Evidence-Based Recommendations into Practice. Reston: ACRM Publishing; 2014. [Google Scholar]
- 52. Cicerone KD, Langenbahn DM, Braden Cet al. . Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92(4):519-530. [DOI] [PubMed] [Google Scholar]
- 53. Hara Y. Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch. 2015;82(1):4-13. [DOI] [PubMed] [Google Scholar]
- 54. Robertson IH, Murre JMJ.. Rehabilitation of brain damage: brain plasticity and principles of guided recovery. Psychol Bull. 1999;125(5):544-575. [DOI] [PubMed] [Google Scholar]
- 55. Kleim JA, Jones TA.. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225-S239. [DOI] [PubMed] [Google Scholar]
- 56. Prosperini L, Piattella MC, Giannì C, Pantano P. Functional and structural brain plasticity enhanced by motor and cognitive rehabilitation in multiple sclerosis. Neural Plast. 2015;2015:481574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duffau H. The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex. 2014;58(Sep):325-337. [DOI] [PubMed] [Google Scholar]
- 58. Picart T, Herbet G, Moritz-Gasser S, Duffau H. Iterative surgical resections of diffuse glioma with awake mapping: how to deal with cortical plasticity and connectomal constraints? Neurosurgery. 2019;85(1):105-116. [DOI] [PubMed] [Google Scholar]
- 59. Kolb B, Whishaw IQ. Brain plasticity and behavior. Annu Rev Psychol. 1998;49:43-64. [DOI] [PubMed] [Google Scholar]
- 60. Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: John Wiley & Sons; 1949. [Google Scholar]
- 61. LaPointe LL, Stierwalt JAG.. Aphasia and Related Neurogenic Language Disorders. 5th ed. Thieme Publishers; 2018. [Google Scholar]
- 62. Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38(5):819-829. [DOI] [PubMed] [Google Scholar]
- 63. Miotto EC, Balardin JB, Vieira Get al. . Right inferior frontal gyrus activation is associated with memory improvement in patients with left frontal low-grade glioma resection. PLoS One. 2014;9(8):e105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Noll KR, Bradshaw ME, Parsons MW, Dawson EL, Rexer J, Wefel JS. Monitoring of neurocognitive function in the care of patients with brain tumors. Curr Treat Options Neurol. 2019;21(7):33. [DOI] [PubMed] [Google Scholar]
- 65. Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5th ed. New York: Oxford University Press; 2012. [Google Scholar]
- 66. Meyers CA, Wefel JS.. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol. 2003;21(19):3557-3558. [DOI] [PubMed] [Google Scholar]
- 67. Block CK, Johnson-Greene D, Pliskin N, Boake C. Discriminating cognitive screening and cognitive testing from neuropsychological assessment: implications for professional practice. Clin Neuropsychol. 2017;31(3):487-500. [DOI] [PubMed] [Google Scholar]
- 68. Meyers CA, Hess KR.. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. Neuro Oncol. 2003;5(2):89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dams-O’Connor K, Gordon WA.. Role and impact of cognitive rehabilitation. Psychiatr Clin North Am. 2010;33(4):893-904. [DOI] [PubMed] [Google Scholar]
- 70. Stewart E, Ownsworth T.. Making Sense of Brain Tumour: A Practical Guide for Therapists. Australasian Society for the Study of Brain Impairment; 2014. [Google Scholar]
- 71. Tsaousides T, Gordon WA. Cognitive rehabilitation following traumatic brain injury: assessment to treatment. Mt Sinai J Med. 2009;76(2):173-181. [DOI] [PubMed] [Google Scholar]
- 72. Pinquart M, Sörensen S.. Associations of caregiver stressors and uplifts with subjective well-being and depressive mood: a meta-analytic comparison. Aging Ment Health. 2004;8(5):438-449. [DOI] [PubMed] [Google Scholar]
- 73. Sherwood PR, Given BA, Given CWet al. . Predictors of distress in caregivers of persons with a primary malignant brain tumor. Res Nurs Health. 2006;29(2):105-120. [DOI] [PubMed] [Google Scholar]
- 74. Boele F, Rooney A, Grant R, Klein M. Psychiatric symptoms in glioma patients: from diagnosis to management. Neuropsychiatr Dis Treat. 2015;11(Jun):1413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zucchella C, Capone A, Codella Vet al. . Cognitive rehabilitation for early post-surgery inpatients affected by primary brain tumor: a randomized, controlled trial. J Neurooncol. 2013;114(1):93-100. [DOI] [PubMed] [Google Scholar]
- 76. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390. [DOI] [PubMed] [Google Scholar]
- 77. Bartolo M, Zucchella C, Pace Aet al. . Early rehabilitation after surgery improves functional outcome in inpatients with brain tumours. J Neurooncol. 2012;107(3):537-544. [DOI] [PubMed] [Google Scholar]
- 78. Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehabil. 2001;80(5):346-350. [DOI] [PubMed] [Google Scholar]
- 79. Sherer M, Meyers CA, Bergloff P. Efficacy of postacute brain injury rehabilitation for patients with primary malignant brain tumors. Cancer. 1997;80(2):250-257. [PubMed] [Google Scholar]
- 80. Khan F, Amatya B, Drummond K, Galea M. Effectiveness of integrated multidisciplinary rehabilitation in primary brain cancer survivors in an Australian community cohort: a controlled clinical trial. J Rehabil Med. 2014;46(8):754-760. [DOI] [PubMed] [Google Scholar]
- 81. Gehring K, Sitskoorn MM, Gundy CMet al. . Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712-3722. [DOI] [PubMed] [Google Scholar]
- 82. Richard NM, Bernstein LJ, Mason WPet al. . Cognitive rehabilitation for executive dysfunction in brain tumor patients: a pilot randomized controlled trial. J Neurooncol. 2019;142(3):565-575. [DOI] [PubMed] [Google Scholar]
- 83. Maschio M, Dinapoli L, Fabi A, Giannarelli D, Cantelmi T. Cognitive rehabilitation training in patients with brain tumor-related epilepsy and cognitive deficits: a pilot study. J Neurooncol. 2015;125(2):419-426. [DOI] [PubMed] [Google Scholar]
- 84. Yang S, Chun MH, Son YR. Effect of virtual reality on cognitive dysfunction in patients with brain tumor. Ann Rehabil Med. 2014;38(6):726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. O’Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534. [DOI] [PubMed] [Google Scholar]
- 86. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335. [DOI] [PubMed] [Google Scholar]
- 87. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463. [DOI] [PubMed] [Google Scholar]
- 88. Han EY, Chun MH, Kim BR, Kim HJ. Functional improvement after 4-week rehabilitation therapy and effects of attention deficit in brain tumor patients: comparison with subacute stroke patients. Ann Rehabil Med. 2015;39(4):560-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Giordana MT, Clara E. Functional rehabilitation and brain tumour patients. A review of outcome. Neurol Sci. 2006;27(4):240-244. [DOI] [PubMed] [Google Scholar]
- 90. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573. [DOI] [PubMed] [Google Scholar]
- 91. Bilgin S, Kose N, Karakaya J, Mut M. Traumatic brain injury shows better functional recovery than brain tumor: a rehabilitative perspective. Eur J Phys Rehabil Med. 2014;50(1):17-23. [PubMed] [Google Scholar]
- 92. Duffau H, Capelle L, Denvil Det al. . Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatry. 2003;74(7):901-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Golia MT, Poggini S, Alboni Set al. . Interplay between inflammation and neural plasticity: both immune activation and suppression impair LTP and BDNF expression. Brain Behav Immun. 2019;81(Oct):484-494. [DOI] [PubMed] [Google Scholar]
- 94. Hassler MR, Elandt K, Preusser Met al. . Neurocognitive training in patients with high-grade glioma: a pilot study. J Neurooncol. 2010;97(1):109-115. [DOI] [PubMed] [Google Scholar]
- 95. Miotto EC, Savage CR, Evans JJet al. . Semantic strategy training increases memory performance and brain activity in patients with prefrontal cortex lesions. Clin Neurol Neurosurg. 2013;115(3):309-316. [DOI] [PubMed] [Google Scholar]
- 96. Stamenova V, Levine B.. Effectiveness of Goal Management Training® in improving executive functions: a meta-analysis. Neuropsychol Rehabil. 2019;29(10):1569-1599. [DOI] [PubMed] [Google Scholar]
- 97. Levine B, Schweizer TA, O’Connor Cet al. . Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5(Feb):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Locke DEC, Cerhan JH, Wu Wet al. . Cognitive rehabilitation and problem-solving to improve quality of life of patients with primary brain tumors: a pilot study. J Support Oncol. 2008;6(8):9. [PubMed] [Google Scholar]
- 99. Wefel JS, Noll KR, Rao G, Cahill DP. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncol. 2016;18(12):1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Talacchi A, Santini B, Savazzi S, Gerosa M. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103(3):541-549. [DOI] [PubMed] [Google Scholar]
- 101. Giovagnoli AR. Investigation of cognitive impairments in people with brain tumors. J Neurooncol. 2012;108(2):277-283. [DOI] [PubMed] [Google Scholar]
- 102. Barisa M, Bison K, Beck K, Reese C. Neurosurgical rehabilitation. In: Neurosurgical Neuropsychology. Elsevier; 2019:281-302. [Google Scholar]
- 103. Andrewes HE, Drummond KJ, Rosenthal M, Bucknill A, Andrewes DG. Awareness of psychological and relationship problems amongst brain tumour patients and its association with carer distress. Psychooncology. 2013;22(10):2200-2205. [DOI] [PubMed] [Google Scholar]
- 104. Kos N, Kos B, Benedicic M. Early medical rehabilitation after neurosurgical treatment of malignant brain tumours in Slovenia. Radiol Oncol. 2016;50(2):139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. National Institute for Health and Clinical Excellence . Improving Supportive and Palliative Care for Adults with Cancer: The Manual. 2004. Available from: https://www.nice.org.uk/guidance/csg4/resources/improving-supportive-and-palliative-care-for-adults-with-cancer-pdf-773375005. Accessed April 9, 2020. [Google Scholar]
- 106. Cancer Council Australia, Australian Cancer Network, Clinical Oncological Society of Australia, Australian Cancer Network Adult Brain Tumour Guidelines Working Party . Clinical Practice Guidelines for the Management of Adult Gliomas: Astrocytomas and Oligodendrogliomas. Sydney: Cancer Council Australia; 2009. [Google Scholar]
- 107. Choi J, Twamley EW.. Cognitive rehabilitation therapies for Alzheimer's disease: a review of methods to improve treatment engagement and self-efficacy. Neuropsychol Rev. 2013;23(1):48-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Armstrong TS, Gilbert MR.. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro Oncol. 2012;14(Suppl 4):iv65-iv72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hart SL, Hoyt MA, Diefenbach Met al. . Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst. 2012;104(13):990-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pilling S, Anderson I, Goldberg D, Meader N, Taylor C. on behalf of the two guideline development groups. Depression in adults, including those with a chronic physical health problem: summary of NICE guidance. BMJ. 2009;339(Oct):b4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rooney AG, Brown PD, Reijneveld JC, Grant R. Depression in glioma: a primer for clinicians and researchers. J Neurol Neurosurg Psychiatry. 2014;85(2):230-235. [DOI] [PubMed] [Google Scholar]