Abstract
The nuclear envelope and nucleoskeleton are emerging as signaling centers that regulate how physical information from the extracellular matrix is biochemically transduced into the nucleus, affecting chromatin and controlling cell function. Bone is a mechanically driven tissue that relies on physical information to maintain its physiological function and structure. Disorder that present with musculoskeletal and cardiac symptoms, such as Emery-Dreifuss muscular dystrophies and progeria, correlate with mutations in nuclear envelope proteins including Linker of Nucleoskeleton and Cytoskeleton (LINC) complex, Lamin A/C, and emerin. However, the role of nuclear envelope mechanobiology on bone function remains underexplored. The mesenchymal stem cell (MSC) model is perhaps the most studied relationship between bone regulation and nuclear envelope function. MSCs maintain the musculoskeletal system by differentiating into multiple cell types including osteocytes and adipocytes, thus supporting the bone’s ability to respond to mechanical challenge. In this review, we will focus on how MSC function is regulated by mechanical challenges both in vitro and in vivo within the context of bone function specifically focusing on integrin, β-catenin and YAP/TAZ signaling. The importance of the nuclear envelope will be explored within the context of musculoskeletal diseases related to nuclear envelope protein mutations and nuclear envelope regulation of signaling pathways relevant to bone mechanobiology in vitro and in vivo.
Keywords: Nucleoskeleton, Nuclear envelope, Bone, Mechanical signals, Mesenchymal stem cells, Mechanobiology, LINC
1. Introduction
Bone protects and mechanically supports the physiologic functions of the body. Osteoblast and osteoclast activity constantly remodels bone to maintain its structure. While many factors such as age, diet, and genetics are important in regulating resident bone cell functions, mechanical signals remain the most important factor for enhancing bone structure [1]. The response of bone to mechanical force depends on both mature bone cell populations–osteoblasts, osteocytes, and osteoclasts– as well as of bone marrow mesenchymal stem cells (MSCs). The proliferation and osteoblastic differentiation of MCSs in response to mechanical stimulation is required for the maintenance and repair of bone. When physical loading is reduced, such as in astronauts, injured service personnel with long periods of bedrest, and physically inactive aged individuals, MSCs tend to enter into adipogenic lineage [2], resulting in decreased bone and increased fat content [3].
Characterization of bone microenvironments enabled by recent technologies such as CLARITY [4], single cell sequencing [5] and tracing studies [6–10] suggests that multipotent stromal cells populations with varying osteogenic and or adipogenic capacity exist both within and outside of bone marrow, including peri-arteriolar, abluminal multipotent stromal cells that significantly contribute to maintenance and mechanical regulation of bone. The emerging organizational and functional complexity of bone marrow microenvironments suggest that site specific mechanical information contributes to the functioning and structural organization of these niches. However, how mechanical information may interact with these different cell populations within bone marrow is outside of the scope of this review and thus the mechanical models we present takes a simplified view of the complex heterogeneous nature of bone marrow biology.
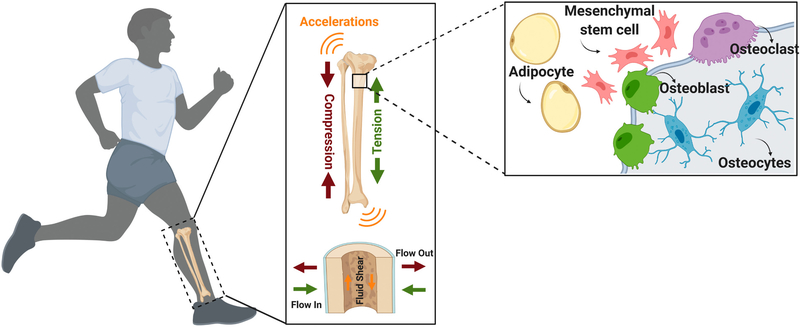
Inside the bone marrow, bone surfaces where bone cells reside are exposed to matrix deformations [11–15], accelerations [16–21], fluid flow [22–26] and changes in intramedullary pressure [27–29], each of which are inseparable [30]. During high physical activity, such as running, the bone is subjected to deformations with a magnitude of 2000–3500 microstrains (με) [12]. These local strain and pressure gradients induce local fluid flow within, as well as in and out, of the bone matrix. In vivo strain magnitudes around 400 με produce fluid velocities up to 100 μm/s within the lacunar–canalicular network generating fluid shear stress values as high as 5 Pa around osteocytes [31]. MSCs within bone marrow and osteoblastic cells that reside on or in proximity to bone surfaces also experience fluid flow associated with motions of the bone marrow relative to bone surfaces. During moderate running, tibial accelerations approach 2 g (1 g corresponds to 9.8 m/s2 - Earth’s gravitational pull) [32]. In addition to relatively high magnitude strains arising from physical activity, bone experiences a barrage of small strains. For example, measuring strain history of long bones within a 24 h period showed that large strains (>1000 με) occur relatively few times a day while very small strains (<10 με) occur thousands of times daily, suggesting that small magnitude events must also be physiologically relevant [33]. Not surprisingly, external application of high frequency (0–100 Hz), low magnitude (0.1–1 g) vibrations have been shown to increase bone and muscle indices in clinical studies [34,35]. While the predicted motions at the marrow-bone interface in response to strains and accelerations are smaller compared to canaliculi, the high viscosity of red marrow (400 cP) [36] results in appreciable fluid shear stress on cells residing in close proximity to these surfaces. In silico studies that model fluid shear stresses at bone-marrow interfaces reveal that sinusoidal vibrations within 0.1–2.0 g acceleration magnitude generate fluid shear stresses up to 2 Pa [37–40]. These finding clearly show that bone is a dynamic environment and simultaneously subjected to a multitude of mechanical signal intensities at both high and low frequencies (Fig. 1). Ultimately, the tissue level forces are transduced to bone cells and drive cellular function.
Fig. 1.
Physical activity exerts forces on bone at multiple length scales. Bone matrix is subjected simultaneously to numerous mechanical forces during physical activity including strains, accelerations, and fluid shear stress. These tissue level forces are transduced to various cell populations in bone, including osteoblasts, osteocytes, osteoclasts, adipocytes and mesenchymal stem cells, and provide a basis of mechano-regulation of bone at the cellular level.
Numerous studies have revealed how mechanical force regulates cell function through integrin-mediated signaling cascades and cytoskeletal structure; readers are encouraged to read excellent reviews on these topics for an in depth discussion on integrin and cytoskeleton related cell signaling [41–43]. In order to alter cell function, mechanical signals or mechanically-activated signaling molecules need to reach to cell nucleus. The nucleus, central to all cellular activity, relies on both direct mechanical input as well as its molecular transducers to sense external stimuli and respond by regulating intra-nuclear chromatin organization which ultimately determines cell function and fate. The nucleus was historically viewed as a passive organelle, however studies in the last decade have demonstrated that it is an active participant in mechanosensing and mechanosignaling.
The outermost layer of the nucleus is the nuclear envelope (NE), comprised of proteins occupying the inner and outer nuclear membranes, that acts as a unit to maintain a dynamic connectivity between the cytoskeleton and chromatin [44–46]. In addition to well-characterized proteins, such as nuclear pore complexes (NPC) and nuclear lamina proteins (Lamin A, Lamin B and Lamin C), the nuclear envelope has been shown to have >200 unique transmembrane proteins in the liver [47], leukocytes [48], skeletal muscle [49] and mesenchymal stem cells [50]. While the function of many of these proteins remains to be determined, the most notable include emerin, a small 35 kD protein that plays a role in cytoskeletal organization, signal molecule transduction and nuclear structuring [51,52], and a family of proteins that harbor KASH (Klarsicht, ANC-1, Syne Homology) and SUN (Sad1p, UNC-84) domains. These KASH domain harboring proteins (nesprins 1, 2, 3, and 4) and Sun proteins bind together in the nuclear envelope to form the LINC complex (Linker of Nucleoskeleton and Cytoskeleton) [53]. Our group and others have shown that LINC complexes not only play a critical role in providing physical connectivity between cytoplasmic and nuclear compartments, but also in maintaining cell mechanosensitivity [54,55], chromatin organization [56–58], and DNA repair mechanisms [59,60]. LINC complexes have also been shown to regulate the nuclear access of molecular transducers YAP/TAZ [61] and βcatenin [62], both of which are critical for bone function. Despite the close association between nuclear envelope mediated cell functions and bone health, as well as the clinically relevant musculoskeletal diseases that arise from mutations of nuclear envelope proteins, the role the nuclear envelope plays in the mechanical regulation of bone remains underexplored.
In this review, we will first consider how different mechanical signals may regulate bone structure and discuss the elements of the nuclear envelope that transmit these external forces into the cell nucleus via cytoskeletal connections. Owing to the lack of studies that focus on nuclear envelope function in osteoblasts, osteoclasts and osteocytes, we will focus on the influence of mechanical forces on MSCs as it relates to the osteogenic and adipogenic lineages. We will also explore the possible signaling mechanisms that regulate MSC lineage commitment in response to mechanical force. Using in vivo and in vitro evidence, we will explore how disrupting mechanocoupling at the nuclear envelope can lead to pathology and altered mechanobiology, as well as the consequences of mutations and loss of function of nuclear envelope elements on bone function.
2. Regulation of bone by mechanical signals
Exercise is one of the most commonly prescribed activities to combat the effects of bone loss in astronauts under microgravity [63] as well as aging populations on earth. Physically active adults are at reduced risk of hip and vertebral fracture and show increased skeletal muscle mass, strength, power, and intrinsic neuromuscular activation [64]. Early preclinical studies focused on loading regimes suggested that periosteal bone formation in response to exercise is associated with strain gradients rather than strain magnitude [63]. Later studies conformed that bone formation more strongly correlates with sites of high strain gradients, as opposed to strain magnitude, using finite element modeling in conjunction with bone histomorphometry in sheep models [65] and axial forearm loading in humans [66]. These findings suggest a role for strain rate in regulating bone modeling during exercise. In addition to matrix strains exercise results in periodic fluid motions within bone that produce fluid shear stress proportional to the change in fluid velocity between the fluid and bone surface. A recent tibia axial loading model in C57BL/6 mice correlated the bone modeling response with both experimentally measured strain magnitudes and computationally derived fluid flow velocity using a poroelastic finite element model with a constant shear rate (i.e. laminar flow) [67]. Results indicated that fluid flow magnitudes as small as 0.1 μ/s, but not strain energy, predicts endosteal bone formation suggesting a role of fluid shear stress in regulating bone during bone deformations.
Fluid shear stress also influences bone modeling in the absence of strain-induced fluid shear stress. Application of sinusoidal vibrations to bone explants at 0.3 g acceleration magnitude and 30 Hz frequency, a frequency/acceleration combination that does not generate appreciable strain on bones (<10 με) [19], results in a 5% increase of trabecular volume fraction within the bone marrow cavity [68]. Dynamic finite element simulations based on solid-fluid interaction formulas predict that this vibration regimen will produce an average fluid shear stress magnitude of 0.6 Pa on trabecular and endocortical bone surfaces. These regions of shear stress correlate with mineral deposition and resorption sites. Importantly, in all samples tested, fluid flow treatment resulted in less bone deposition when compared to vibration treated groups suggesting fluid shear stress alone cannot explain bone formation rates observed in vibrated groups. In agreement with vibration-induced fluid shear stress predictions in explant models [37,69] in vitro studies using osteoblasts [70], osteocytes [71] and MSCs [72] found no correlation between fluid shear stress and cell response during low intensity vibrations.. This suggests that bone can directly respond to high magnitude accelerations at the cellular level with no influence from vibration-generated fluid shear.
The natural frequency of dynamic muscle oscillation without electrical stimulation can be as high as 50 Hz [73] and up to 400 Hz when under external force [74]. This represents the physiological range by which bone may vibrate during daily activities. Importantly, these low-magnitude mechanical events generated by muscles decrease with age-related muscle weakness or disuse [75] providing a correlation between muscle deterioration and bone loss. In clinical studies treatment with low intensity vibrations (LIV),usually applied between 30 and 100 Hz, has been shown to promote bone quantity and quality in women with osteoporosis [34,76], and children with disabling conditions including cerebral palsy [35], and augment bone indices in child cancer survivors [77]. Animal studies demonstrate that external LIV application is sufficient to increase trabecular bone density and volume [16], enhance bone stiffness and strength [78], and slow bone loss caused by disuse [79]. Further, LIV enhanced muscle contractility [80], strength [81], and cross-sectional area [82], indicates that LIV signals are anabolic to skeletal muscle.
These studies across different loading regimes collectively show that bones are responsive to mechanical force and perhaps suggest that the type of stimuli is less important when compared to the magnitude of cellular responses they elicit. For example, our group compared the efficacy of different mechanical regimes in activating the FAK (focal adhesion kinase) phosphorylation at tyrosine 397, which is required for integrin engagement and subsequent activation of RhoA (Ras homolog family member A) mediated increase in cytoskeletal contractility [43]. Sequential or repeated applications of substrate strain, LIV, and lysophosphatidic acid (LPA), a biochemical RhoA-activator, all additively increased FAK phosphorylation. This uniformity of a cell’s ability to process different mechanical input types suggests that how these different signals are transmitted within the skeletal tissues, not the signal type, may lead to specific cell responses.
While the bone mechanobiology field has produced detailed models on how bone tissue strains, fluid flow, and even vibrations may result in loading events on osteocytes and osteoblasts that reside on or within bone surfaces [83–86], the mechanical environment of mesenchymal stem cells within bone marrow is underexplored due to the complexity of characterizing and studying this bone compartment [69,87]. Mesenchymal stem cells robustly respond to mechanical force, yet there is a lack of experimental and computational models to study cell-specific mechanical information in the bone marrow. This dearth of information precludes the determination of how mechanical factors affect MSCs inside the bone marrow. For example, using a 5-week treadmill intervention period on four-week-old C57BL/6 mice demonstrated increased proliferation of MSCs in exercised animals versus controls along with increased osteogenic differentiation indicated by higher levels of alkaline phosphatase activity, osteopontin and osteocalcin, as well as reduced bone marrow cavity fat [88]. Supporting these findings, a recent randomized clinical trial on pre-osteoporotic postmenopausal women demonstrated that LIV is protective against loss of mechanical strength in bones, and that LIV intervention minimizes the shift from the osteoblastic to the adipocytic lineage of MSCs [89]. One source of mesenchymal stem cells in limbs and calvaria are Prrx1 positive progenitors [90]. A recent study that explored the load-induced proliferation of Sca-1+Prrx1+and Sca-1− Prrx1+ cells of endosteal and periosteal surfaces in C57BL/6 mice found that both Sca-1+Prrx1+and Sca-1− Prrx1+ cells respond to load by increasing proliferation on periosteal bone surfaces while only Sca-1− Prrx1+ cells were responsive to load at endosteal surfaces [91]. Critically, increased proliferation was absent in aged animals. Evidence suggests that, in addition to possible age-related deficiencies in MSCs [88,92], physical changes in the niches of aging bone marrow may attenuate the mechanical signals that stimulate MSCs [93] reducing the efficacy of physical activity. Therefore, development of computational models - in combination with in vivo, ex vivo or tissue- engineering models - that can capture the mechanical and geometrical complexity of the bone marrow environments of MSCs may be critical in mechanistic approaches to understanding mechanical factors that drive MSC mechanoresponse at the cellular level.
3. Mechanical signal transduction in cells
Mechanical stimulation of bone travels within skeletal tissues and is ultimately transduced into the cell through cytoskeletal networks composed of actin, microtubules, and intermediate filaments. Mirroring the tissue level adaptations, these cytoskeletal networks respond to external mechanical forces by adapting cytoskeletal and nuclear mechanics as well as initiating signaling pathways to regulate cell function.
Cell structure is under a constant force-balance where compressive forces on microtubules balance the contractile pulling forces generated by F-actin stress fibers. This ever-maintained state is termed as tensegrity and allows instantaneous balancing of forces via deformations propagated within the cell by cytoskeletal networks, including into the nucleus [94] where intermediate filaments, actin, and DNA provide additional structural scaffolding [95]. Tensegrity based computational models predict equi-directional deformations of adherent cells under laminar fluid flow [96] as well as non-linear cell stiffening in response to external loads applied to cell membrane via magnetic beads [97,98]. Further, the transmittance of this fast stress propagation appears to be dependent on the baseline stress the cytoskeleton is under such that increasing the cellular tension via collagen-I coating directly increases the strain levels measured inside the cell nucleus upon external deformations [99]. In this way, extracellular deformations applied by magnetic beads on the cell surface have been shown to propagate via the cytoskeleton to deform chromatin [100]. In this study, inserting small bacterial transgene constructs engineered to express mouse DHFR mRNA upon stretching showed instantaneous DHFR mRNA expression proportional to the magnitude of chromatin stretching visualized with optical tracking. Importantly, chromatin stretching and DHFR mRNA expression were absent in cells treated with siRNA against the Sun-1 and Sun-2 elements of the LINC complex suggesting instantaneous mechanical nuclear stress propagation in intact cells. While these studies open up interesting areas of research in understanding how cytoskeletal forces may correlate with genomic function, measuring the cytoskeletal forces generated on the cell nucleus is a challenging task. Traditional approaches, like traction microscopy, can only estimate forces at the focal adhesions [101]. At the nuclear surface, one successfully utilized approach is the usage of FRET based sensors in conjunction with fluorophore-tagged nuclear envelope proteins such as Nesprin-2, and possibly others, to quantify deformations due to F-actin tension on the nuclear envelope [102,103]. One drawback of these approaches is that the measurements are relative and rely on overexpression of truncated or mutated versions of endogenous proteins that may affect cell physiology. In addition, experimental studies that utilized tracking of nuclear motion following laser-guided dissection of stress fibers have estimated, using standard linear solid model, that a single apical actin stress fiber can generate forces up to 65nN on the nucleus [104]. While these measurements are valuable in understanding the native forces on the nucleus, the estimations rely on certain assumptions and simplifications. Therefore, there is a need to estimate native intra-cellular cytoskeletal forces. One possible option to generalize and standardize these measurements across studies could be to incorporate of multiple inputs from cell level mechanical and imaging-based studies in finite element models to predict cell level forces in vivo.
Numerous proteins maintain the structure and contractility of the F-actin cytoskeleton. Polymerization of new actin filaments, as well as branch formations, is largely modulated by actin related protein (Arp) 2/3 complexes [105] while formin homology 1 (FH1) and 2 (FH2) domain containing proteins regulate the end-to-end actin formation [106]. Contractility and tension on actin fibers are largely regulated by small Rho GTPases, such as RhoA, Ras and CDC42A [107]. RhoA for example, recruits myosin light chain kinase to F-actin fibers through its effector protein ROCK, which in turn activates the dimerized motor protein myosin II to generate tension by pulling F-actin bundles together [108]. While force generation of F-actin on the cytoskeleton can act as a signal initiator for further cytoskeletal restructuring [109], such as recruitment of zyxin [110] to repair nano-cracks generated during F-actin contractions, the majority of cytoskeletal remodeling in response to external mechanical force is initiated at focal adhesions. Focal adhesions are <200 nm protein plaques comprised of integrins, focal adhesion kinase (FAK), talin, paxilin, vinculin, and zyxin, that enable direct connections between the extracellular matrix (ECM) and the cell [111].
Application of substrate strain in vitro recruits signaling complexes to focal adhesions, essentially turning them into intracellular signaling relays for extracellular mechanical information [112]. Upon mechanical challenge, structural elements, such as vinculin, paxilin and talin, as well as signaling molecules, including FAK, Src, and Akt, are recruited into focal adhesions [113–117]. Mechanically-driven changes in RhoA–Rock activity has been implicated in the osteogenic commitment of MSCs as they increase the activity of two early-stage osteogenic markers, osterix (Osx) and Runt-related transcription factor 2 (Runx2). We have recently reinforced the role of RhoA in MSC osteogenesis by demonstrating that regulation of RhoA activity through leukaemia-associated Rho guanine nucleotide exchange factor (LARG) and Rho GTPase-activating protein 18 (ARHGAP18) regulates osteogenic commitment in MSCs where activation of RhoA increased the osteogenic commitment of stem cells [118]. Similarly, directing RhoA activation through increased extracellular matrix stiffness results in increased osteogenesis and decreased adipogenesis and vice versa [119,120]. Instead of providing an indirect control of RhoA via substrate mechanics, expressing a dominant negative form of RhoA has been shown to induce adipogenesis while the constitutively active RhoA expression favors osteogenic commitment in human sourced MSCs [121]. In addition to static matrix properties, dynamic changes in cell environment including fluid flow, matrix strain and low intensity vibration have been shown to drive osteogenesis in MSCs. For example, application of cyclic strain in the absence of any soluble osteogenic factors is sufficient to upregulate osteogenic and suppress adipogenic mRNA markers [122]. Fluid flow, when applied in either oscillatory or in laminar form, can increase osteogenic commitment in MSCs [123,124]. When applied at high frequencies low intensity vibrations also increases osteogenic and suppresses adipogenic phenotypes of stem cells in both 2D and 3D culture systems [72,125]. These findings indicate that the RhoA-mediated cytoskeletal restructuring caused by static or dynamic mechanical cues, in addition to soluble factors, are powerful regulators of osteogenesis in vitro. Therefore approaches that leverage bioreactor systems and force-adaptive progenitor cells to generate bone-like constructs for bone tissue engineering and regeneration may present unique opportunities to not only drive osteogenic phenotypes, but also direct the structural qualities of these engineered scaffolds for improved functionality under physiologic demand [126].
Cytoskeletal restructuring events in cells result in activation of a number of signaling molecules including MAP kinases, β-catenin, and YAP/TAZ. Perhaps the most studied signaling proteins in bone derived stem cells are β-catenin and YAP/TAZ. Following a mechanical challenge, FAK activation initiates the Akt mediated inhibition of GSK3β function leading to increased levels of β-catenin and its nuclear accumulation [127,128]. Similarly, YAP and TAZ nuclear entry are triggered by soluble or mechanical factors that increase F-actin contractility such as LPA [129,130], increased substrate stiffness [131], and substrate stretch ranging from 3% to 15% [132,133]. We have recently reported that low intensity vibrations also result in increased nuclear YAP [134]. Not surprisingly, both βcatenin and YAP/TAZ have been shown to be highly-interdependent in their roles in regulating cell function [135]. Some examples include the requirement of both βcatenin and YAP for strain-mediated increase in cell proliferation [133] and the necessity of YAP-triggered βcatenin signaling during epithelial regeneration [136]. This suggests that the inter-dependent activity and compartmentalization of βcatenin and YAP/TAZ are critical for an effective cellular response. In bone, coordination of βcatenin, YAP, and TAZ are integral in skeletogenesis and bone regeneration. Not only does deletion of both βcatenin and YAP/TAZ result in skeletal deficits [137,138], they also modulate the function and expression of the master osteogenic transcription factor Runx2 in stem cells. For example, through canonical Wnt signaling, βcatenin/TCF1 binds and activates Runx2 expression in MSCs [139], TAZ forms complexes with Runx2 to increase its function [140], and TAZ nuclear presence positively drives MSC osteogenesis [140]. YAP on the other hand, maintains stem cell multipotentiality through repressing Runx2 function [141] and promoting the expression of Wnt inhibitory molecule Dkk-1 [142]. Further, we have shown that the absence of nuclear YAP amplifies osteogenesis in a Runx2 dependent manner [143]. Despite their overlapping and competing functionalities, both YAP/TAZ [144] and βcatenin [145,146] activities are indispensable to osteogenesis. Collectively, these studies show that mechanical information has to transmit through the nuclear envelope and into the nucleus to direct cell function and fate. This may occur through direct chromatin deformation, regulation of cytoskeletal contractility, or activation of mechanotransducers such as β-catenin and YAP/TAZ.
4. Role of nuclear envelope on cell mechanosignaling
Emerging evidence suggests that the nuclear envelope houses several mechanoregulatory proteins and has an active role in both cytoskeletal dynamics and nuclear access to molecular transducers of mechanical information. Mechanically, the cytoskeleton couples to the nucleus through the LINC complex proteins [147]. F-actin binds to a nesprin protein (Nesprin-1 or Nesprin-2), which are spectrin repeat proteins that pierce the nuclear envelope, connecting via its KASH domain to intra-membrane leaflet SUN proteins (Sun-1 and Sun-2) [147]. N-termini of giant nesprins share a calponin homology (CH) domain that binds to actin with high affinity. The CH domains found in Nesprin-1 and Nesprin-2 giant isoforms are identical to that found in α-actinin [148,149]. Nesprin CH domains promote actin polymerization in-vitro [149] and interact with microtubules through intermediate proteins such as dynein and kinesin [150,151]. Earlier studies showed that the mechanical connectivity provided by the LINC complex was critical in transmitting force to nuclei [152] and later studies utilized FRET based sensors to show nesprins are under constant force via the cytoskeleton [103]. This connectivity between F-actin and the nuclear envelope is co-regulated by other adaptor proteins such as FH1/FH2 domain-containing protein 1 (FHOD1) that binds to the spectrin repeat domain of giant nesprin-2 and increases the coupling strength between LINC and F-actin [153]. Recent studies further demonstrate that LINC complexes co-localize Rac1 selective GEF protein Sif and TIAM1-like Exchange Factor STEF to regulate the activity of non-muscle myosin IIB via Rac1 at the nuclear envelope [154]. Apical cytoskeletal F-actin also associates with LINC complex [155] and connects it to a subset of focal adhesions at the cell periphery [156,157]. In this way, depletion of Nesprin-1 [158] or Sun-1 [159] directly alters focal adhesion dynamics and upregulates cytoskeletal contractility and focal adhesion maturation while Sun-2 depletion results in reduced size and number of focal adhesions [160]. Nucleo-cytoskeletal connectivity has further implications on how focal adhesions respond to mechanical force and how nuclear mechanics adapt to external mechanical stimuli. Our group has reported in Sun-1 & 2 co-depleted or dominant negative KASH overexpressing MSCs that disconnecting Sun-Nesprin binding results in muted FAK phosphorylation at Tyr397 and impaired anti-adipogenic effect in response to LIV [127]. We have also recently reported that application of a single LIV regimen results in nuclear stiffening that can be measured from isolated cell nuclei via atomic force microscopy but not when LINC complex function was disabled [161]. This suggests that changes in cytoskeletal F-actin contractility is, in part, retained by the nucleus via increased nuclear stiffness and changes in heterochromatin structure in a LINC complex dependant manner. These findings open up an interesting possibility that regulation of LINC complex, whether through mechanical signals or other means, may result in altered mechanosensitivity of cells and skeletal tissues. Indeed, our group has reported that subjecting cells to simulated microgravity for 72 h results in decreased levels of Nesprin-2 and Sun-2 which were recovered by daily application of LIV [162]. Similarly, in healthy human MSCs application of LIV result in increased expression of LINC elements [163].
βcatenin signaling is recognized as critical for slowing down adipogenic differentiation [164,165] and maintenance of the proliferative, multipotential state of MCSs [166], as well as being an integral component of osteocyte-mediated bone mechanoresponse [138]. LINC complexes participate in βcatenin nuclear trafficking as evidenced by giant nesprins associating with αcatenin and βcatenin at the nuclear envelope [167,168]. Depletion of Nesprin 1 also reduces nuclear βcatenin levels [167]. βcatenin does not possess a classic nuclear localization signal; instead, it transits through the nuclear leaflets via direct contact with the nuclear pore complex (NPC) [169,170]. βcatenin is localized on the LINC element Nesprin-2 that appears to provide a ‘launching-pad’ for subsequent nuclear entry [167]. Our findings show that untethering Nesprin-2 from the nuclear envelope via co-depletion of Sun 1 and 2 proteins displaces βcatenin from the nuclear envelope, reduces its nuclear levels, and impedes its nuclear entry rate such that neither mechanical force nor pharmacological stabilization of βcatenin are recovered in LINC deficient MSCs [62]. YAP and its paralog TAZ are the other mechanosensitive molecular transducer proteins that in part rely on LINC function. YAP and TAZ alter their nuclear localization [131] in response to mechanical cues in order to direct MSC fate selection [171]. Loss of YAP/TAZ not only retards osteogenesis and promotes an adipogenic phenotype but also inhibits mechanical control of MSC differentiation [131]. Nuclear translocation of YAP is responsive to cytoskeletal contractility as evidenced by the application of substrate strain [132], Atomic Force Microscope-induced nuclear indentations [61], LIV [134], and pharmacologic RhoA-activators such as LPA [172] increasing YAP nuclear entry. Conversely, loss of cytoskeletal connec-tivity by disabling LINC function, either via depletion of Nesprin-1 [132] or overexpression of the Nesprin KASH domain [134], impedes me-chanical induction of YAP nuclear translocation.
Nucleoskeletal Lamin A/C lines the inner nuclear membrane providing mechanical resilience to the nucleus [173] and is implicated in modulating MSC differentiation as well as skeletal phenotypes. MSCs and osteoblastic cells possess robust Lamin A/C networks [174] and Lamin A/C levels increase when MSCs enter the osteogenic lineage [175]. This increase in Lamin A/C levels may also explain the increased cellular stiffness of osteoblasts [176]. Overexpression of Lamin A/C results in osteogenic differentiation of MSCs [177] while Lamin A/C decreases during adipogenic differentiation [178]. Although depleting Lamin A/C was associated with increased adipogenesis in MSCs [174], lipodystrophy-associated mutations of Lamin A/C were shown to slow adipogenic differentiation in cells [179]. Lmna knockout (Lmna − /−) mice have a significant reduction in bone mass and microarchitecture compared to WT mice as reflected by reduced osteoblast, osteocyte, and osteoclast numbers [180]. While these findings suggest a role for Lamin A/C in regulating the differentiated state of MSCs and osteoblasts, the role Lamin A/C plays in mechanical regulation of MSC and skeleton differentiation remains insufficiently explored.
Another important nuclear envelope associated regulator of mechanosensing is Torsin A. Torsin A is a nuclear envelope protein that belongs to AAA+ family (ATPases associated with various cellular activities) that utilizes ATP to unfold other proteins [181,182]. Torsin A interacts with Sun-1 [183], Nesprin 3α [184], lamina associated polypeptide 1 (LAP-1) [185], and emerin [186]. While the exact function of Torsin A in nuclear mechanotransduction is unclear, its functional role in the formation of perinuclear actin cables during rearward nuclear movement [187] suggests that it is important in regulating the cytoskeletal dynamics at the nuclear envelope.
An important protein that plays a prominent role in nuclear envelope mechanotransduction is emerin [188], which acts as a capping protein in vitro [189]. The application of substrate strain on epidermal stem cells results in emerin enrichment at the outer nuclear envelope where it recruits non-muscle myosin IIA to promote local actin polymerization at the perinuclear region [190]. Emerin is further shown to regulate nuclear export of βcatenin by depletion of emerin levels reducing nuclear βcatenin accumulation [191–193]. Furthermore, when magnetic beads were used to apply force to isolated nuclei, emerin was shown to undergo a Src dependent phosphorylation that eventually led to increased nuclear stiffness in a lamin A/C dependent manner [52].
While not directly perceived as a nuclear protein, actin has been emerging as a critical regulator of nuclear structure and thus skeletal health. We recently showed that cytochalasin D, a potent actin depolymerization agent, induces rapid influx of G-actin into the nucleus causing YAP exportation from the nucleus. YAP depletion results in derepression of Runx2 activity and increased osteogenic differentiation in MSCs as well as a strong increase in bone volume in mice [143]. Importantly, using actin branching inhibitor CK666 following cytochalasin D treatment mutes the osteogenic effect of nuclear actin suggesting that actin structure is important in regulating osteogenesis [143,194]. In agreement with these findings, knockdown of nuclear formin mDia2 results in increased osteogenesis and alters Lamin A/C levels [195]. Interestingly, nuclear F-actin polymerization was shown to be Lamin A/C and LINC dependent [196].
As summarized in Table 1, the nuclear envelope and its related proteins may plan an important role in skeletal mechanobiology.
Table 1.
Mechanotransduction elements involved in osteoblastic differentiation of MSCs.
| Protein | Outcome | References |
|---|---|---|
| Focal adhesions | - Act as signaling relays for mechanotransduction by recruiting vinculin, paxilin and talin as well as signaling molecules including FAK, Src, and Akt. - Subset of focal adhesions directly connect to nuclear envelope - Depletion of Sun and Nesprin proteins of LINC complex change focal adhesion dynamics and size- Provide a physical coupling between extracellular matrix and cytoskeleton. |
[113–117,156–159] |
| RhoA/Rock | - Regulates the actin cytoskeleton contractility. - LINC-cytoskeleton coupling regulates RhoA-GTPase levels - Inside the nucleus increases SP7 and Runx2 expression - Overexpression increases osteoblastogenesis- Deactivation leads to reduced osteogenesis |
[118–121,154,160] |
| FAK | - FAK activation initiates the Akt mediated inhibition of GSK3β function leading to increased levels of β-catenin and its nuclear accumulation | [127,128] |
| YAP/TAZ | - YAP and TAZ nuclear entry are triggered by soluble or mechanical factors that increase F-actin contractility, proliferation and differentiation into osteoblastic lineage. - YAP/TAZ nuclear entry is in-part depends on LINC complex function. - Depletion of YAP/TAZ decreases bone quality. |
[61,129–137,140,141] |
| βcatenin | - βcatenin nuclear envelope association and nuclear entry are in-part dependent on LINC complex function. - Wnt activated βcatenin/TCF1 complex binds and activates Runx2 expression in MSCs. - Increases cell proliferation and preserves multipotentiality by suppressing osteogenic gene loci through regulation of enhancer of zeste homolog 2, a key component of the polycomb repressive complex 2 in MSCs - Single allele deletion in osteocytes mutes bone anabolic response to loading- Promotes bone formation in osteoblasts |
[62,138,139,166,145,146] |
| Lamin A/C | - Lamin A/C levels increase during osteogenesis - Correlates with tissue stiffness and depletion leads to decreased osteoblastogenesis- Overexpression accelerates osteoblastogenesis. - Lmna − /− mice have significantly reduced bone mass and deteriorated microarchitecture. |
[174–180] |
| Torsin A | - Functional role in the formation of perinuclear actin cables during rearward nuclear movement suggests that it is important in regulating the cytoskeletal dynamics at the nuclear envelope | [183–187] |
| Nuclear F- actin | Nuclear F-actin increases osteogenic differentiation, this effect is blocked by depleting nuclear formin mDia2 or by CK666, an actin branching inhibitor. | [143,194,195] |
| Emerin | Recruits non-muscle myosin IIA to promote local actin polymerization and regulates nuclear export of βcatenin. | [188–193] |
5. Nuclear envelope related diseases of bone
The importance of nuclear envelope integrity on cell function has been thoroughly explored, but how we can use that information to improve skeletal health is less well studied. Evidence of the interdependence of nuclear envelope integrity on skeletal health manifests in clinically relevant skeletal diseases related to disruptions of the nuclear envelope architecture. Exploring the consequences and mechanisms of nuclear envelope dysfunction may lead to improved therapies and interventions for orthopedic pathologies. A brief summary is also presented in Table 2.
Table 2.
Disease impacts of the nuclear-mechanotransductive pathways.
| Disease | Gene | Outcome | References |
|---|---|---|---|
| HGPS, APSs, MADA | LMNA | Accumulation of progerin disrupts nuclear envelope integrity | [168,180,198,199] |
| MADB and RD | ZMPSTE24 | Accumulation of prelamin A which may lead to the abnormal skeletal phenotypes | [204–206] |
| NGPS | BANF1 | BAF protein which plays a role in nuclear assembly, chromatin organization. Decreased bone density and osteolysis, but no cardiovascular effects | [207] |
| Greenberg dysplasia | LBR | Interferes with cholesterol biosynthesis suggesting a primarily metabolic disease mechanism | [208–211] |
| Sclerosing bone dysplasias | LEMD3 | Involved in canonical TGF-β signaling which is crucial for in utero skeletal development and post-natal bone maintenance by promoting bone progenitor enrichment. Loss of lamin A/C in vivo causes an increase in MAN1 expression while decreasing MAN1/Runx2 colocalization, thus affecting osteogenesis of MSCs. | [212,213,180] |
5.1. Progeroid syndromes
Progeroid syndromes are clinically characterized by premature or accelerated aging, and therefore, result in age-related bone loss and skeletal abnormalities. Hutchinson-Gilford progeria syndrome (HGPS), atypical progeria syndromes (APSs), mandibuloacral dysplasias type A and B (MADA/MADB), restrictive dermopathy (RD), and Nestor-Guillermo progeria syndrome (NGPS) all present with similar bone pathologies and are secondary to nuclear envelope protein gene mutations in either LMNA, ZMPSTE24, or BANF1 genes [197].
HGPS is the most common and widely studied progeroid syndrome that occurs due to a de novo silent mutation in the LMNA gene. This mutation interferes with the maturation of lamin A from prelamin A resulting in a truncated version of lamin called progerin which disrupts nuclear envelope integrity due to abnormal accumulation. Transgenic mice with alterations in Lamin A have progeria with cardiomyopathy and sarcopenia, exhibit low bone mass, and increased marrow adiposity [180]. Mutated Lamin A/C forms may be detrimental to formation and maintenance of LINC/nucleoskeleton connections [198] and may contribute to age-related cell senescence [199]. Interestingly, similar to declining βcatenin signaling in LINC deficient cells [168], progeric mutations in mouse lines carrying a mutated LMNAL530P/L530P gene also diminish canonical Wnt signaling due to reduced nuclear localization and transcriptional activity of Lef1, leading to altered ECM synthesis [200]. Other HGPS studies similarly reported decreased nuclear βcatenin levels and diminished mineralization capacity both in vitro and in vivo [201]. These studies point out that the etiology of bone related problems in HGPS patients may be due to diminished nuclear connec-tivity between the cytoskeleton and βcatenin signaling. Interestingly, siRNA mediated depletion of Lamin A/C does not affect βcatenin nuclear access [62], suggesting a functional difference between the absence of Lamin A/C and progerin accumulation at the nuclear envelope. It has been recently reported that progerin leads to defects in nuclear F-actin dynamics as progerin lacks the actin-binding site of Lamin A where dysregulation of F-actin functionality resulted in altered nuclear morphology and jasplakinolide-induced Wnt/β-catenin signaling [202]. APSs are also caused by mutations in the LMNA gene and present with similar clinical characteristics to HGPS, but affected cells do not accu-mulate progerin in the same manner [203], suggesting that progeria symptoms can manifest in a progerin independent manner representing a multimodal breakdown of normal functionality.
MADA, MADB, and RD are caused by a mutation in the ZMPSTE24 gene which codes for the enzyme responsible for the final cleavage step of prelamin A to lamin A [204,205]. Similar to progerin accumulation in HGPS, this creates an accumulation of prelamin A which may lead to the abnormal skeletal phenotypes seen in patients with a ZMPSTE24 mutation [205]. NGPS is caused by a mutation in the BANF1 gene which codes for the BAF protein which is involved in nuclear assembly, chromatin organization, and regulating gene expression. This disease process presents with similar skeletal features as HGPS including decreased bone density and osteolysis, but lacks the cardiovascular effects [207]. Physical rehabilitation could improve skeletal health in patients with NGPS as indicated by bone growth of the proximal humerus and styloid process due to structural stress from muscle contraction and resorption phenomena in unloaded bone noted by Cabanillas et al. [207].
5.2. Greenberg skeletal dysplasia
Greenberg skeletal dysplasia (GSD), also known as hydrops-ectopic calcification-“moth eaten” (HEM) skeletal dysplasia, is a lethal process that presents with fetal hydrops, short-limbed dwarfism, and abnormal chondro-osseous calcification [208–210]. The latter resulting in a “moth eaten” appearance of long bones and the pelvis visualized on ultrasound radiography. GSD results from a mutation in the Lamin B receptor (LBR) protein, located on the inner nuclear envelope, which interferes with cholesterol biosynthesis suggesting a primarily metabolic disease mechanism [211].
5.3. Sclerosing bone dysplasias
Increased bone density and bony lesions characterize sclerosing bone dysplasias including osteopoikilosis, melorheostosis, and Buschke-Ollendorff Syndrome (BOS). These dysplasias are caused by a dysfunction in the MAN1 protein secondary to a mutation in the LEMD3 gene. MAN1 is involved in canonical TGF-β signaling which is fundamental during in utero skeletal development and post-natal bone maintenance by promoting bone progenitor enrichment [212,213]. Additionally, MAN1 is physically coupled to, and thus closely regulated by, lamin A/C and colocalizes with Runx2, both of which are important nuclear envelope proteins for bone health as previously described. Loss of lamin A/C in vivo causes an increase in MAN1 expression while decreasing MAN1/Runx2 colocalization, thus affecting osteogenesis of MSCs [180]. Therefore, this loss of signaling due to MAN1 dysfunction likely contributes to the skeletal abnormalities seen in sclerotic bone disease.
5.4. Other musculoskeletal conditions
Other conditions, including muscular dystrophy, joint disorders, normal aging, and cancer, also have deleterious effects on skeletal health. Mutations in nuclear envelope proteins emerin, nesrpin-1,and lamin A/C lead to various muscular dystrophies, such as Emery-Dreifuss Muscular Dystrophy, and joint disorders, such as arthrogryposis, that may have secondary effects on skeletal health due to reduced skeletal muscle movement from progressive muscle weakness and joint contractures [214,215], but are not directly related to bone pathologies.
Aging is a complex condition regulated by many factors including dysregulation of Lamin A/C and LINC. During the aging process, the Lamin A/C network decreases [175] and mutated forms of Lamin A/C have been identified in aged nuclei [216]. This suggests that bones may have altered mechanoresponses due to nuclear envelope changes in aged individuals. Mechanosensitivity studies conducted by comparing young and aged human primary bone cell response to fluid shear [217] and work from our laboratory comparing early and late passage MSC response to substrate strain [218] show that acute signaling response does not diminish during either chronological or in vitro aging. Being active, however, is not sufficient to stop age-related attenuation of exercise efficacy in older individuals [219]. While it is not clear why response to mechanical challenge diminishes with age, it is known that aging MSCs lose proliferative and differentiative capacity [220–223].
In contrast to the recommended high frequency of exercise for healthy aging and stronger musculoskeletal form, there are only a handful of studies that have investigated the role of exercise on MSC function as the majority of MSCs are generally derived from already young or aged sources. One study examined the effects of a 3-month-long daily 15 m/min treadmill activity on MSCs from adult 6-month-old female rats. Compared to neonatal MSCs (day 2), decreased osteogenic capacity in the sedentary adult group was partially recovered following exercise [224]. Another study investigated the effect of a 4-month-long daily ladder-climbing regimen. This study started at 17-months of age and compared aged and exercised cells to adult MSCs derived from 5-month-old rats. MSCs from aged cells showed decreased osteogenesis, which was partially restored by the 4-month-long exercise intervention [225]. While these studies highlight the benefits of exercise for MSCs, dynamic mechanical signals generated during exercise are accompanied by a systemic increase of heart rate, blood circulation, respiration, and caloric expenditure [226,227]. Therefore, it is hard to pinpoint if these observed effects are due to mechanical challenge alone or other physiological parameters including exercised-induced cardiovascular changes. In order to study the isolated effects of mechanical signals on aging MSC function, we applied LIV as an in vitro exercise mimetic to primary MSCs in a replicative senescence model where MSCs underwent 60 serial passages either with or without daily application of LIV. Our results indicated that mechanical signals protected declining proliferation, as well as adipogenic and osteogenic differentiation capacity in MSCs [218]. Simulated microgravity, another analog for unloading, also supports these findings. We have reported that consistent LIV delivery can normalize decreased cell proliferation and YAP protein levels under simulated microgravity [134,162]. These results suggest that the active mechanical environment may improve bone response to mechanical challenge long term by directly affecting MSCs proliferation, differentiation, and YAP signaling.
While not directly related to bone cells, breast cancer metastasis into bone impacts bone health through the activation of osteoclast function which results in increased bone loss [228]. Interestingly, breast cancer cells also display reduced levels of Lamin A/C and LINC complex elements [229], and application of LIV has been shown to alleviate cancer related bone loss in mice [230]. We recently examined the mechanisms through which cancer cells sense and respond to LIV. LIV decreased matrix invasion and impaired the secretion of osteolytic factors PTHLH, IL-11, and RANKL from cancer cells. Furthermore, transferring conditioned media from mechanically stimulated cancer cells reduced osteoclast differentiation and resorptive capacity. Disrupting the LINC complex by knockdown of Sun-1 & 2 impaired LIV-mediated suppression of metastatic cell invasion and osteolytic factor secretion, suggesting that LIV reduces the metastatic potential of human breast cancer cells through mechanosensing mediated by LINC complexes [231].
6. Conclusions
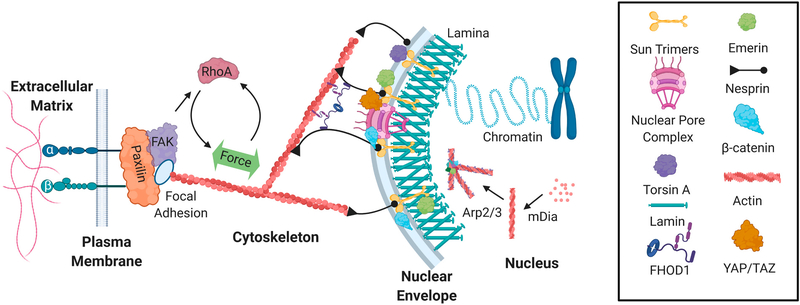
Bone is a mechanically rich ecosystem. The body of work discussed here shows that cells respond to wide variety mechanical signals irrespective of how they are applied and these signals eventually activate common signaling cascades and adaptation mechanisms. Thus, possible future studies investigating how bone is regulated by mechanical signals should focus on how mechanical signals are manifested at the cellular level to inform their function. In the last decade, considerable progress has been made in identifying the mechanisms by which cells sense and adapt to dynamic mechanical forces in their immediate environment. Mechanically derived adaptations in cytoskeletal and nuclear structure not only modulate the force transmission within cells, but lead to repositioning of signaling events and gene expression. At this juncture it appears that the nuclear envelope presents a critical barrier to the propagation of extracellular mechanical information into the nucleus through regulation of nuclear trafficking of transcriptional factors as well as the structural organization of chromatin through its connections with the cytoskeletal networks. To this end, future studies focusing on nuclear envelope mechanobiology as an integral part of the cellular mechanosensory mechanism would be valuable (Fig. 2).
Fig. 2.
The nucleoskeleton structurally and functionally couples the genome to extracellular dynamics. Extracellular forces activate integrin and focal adhesion signaling to increase F-actin contractility, which leads to increased polymerization of the actin cytoskeleton. LINC complexes composed of Sun trimers and Giant Nesprin mechanically couple the actin cytoskeleton and regulate the access of important mechanical transducers such as βcatenin and YAP/TAZ. Mechanical coupling of actin and LINC involves a cytoplasmic formin FHOD1 that attaches nesprin and actin at multiple points for a more robust association. Torsin A may also facilitate the LINC assembly at the nuclear envelope. Emerin associates with both sides of the nuclear envelope to regulate extra and intranuclear actin dynamics. Inside the nucleus, G-actin is assembled into linear and branched networks to regulate chromatin dynamics and gene access.
Acknowledgements
This study was supported by NIH awards AG059923, P20GM109095, P20GM103408, and NSF 1929188 and 2025505. The author(s) declare no competing interests financial or otherwise. We gratefully acknowledge expert help from Julianna Goelzer in generating figures and Anamaria Zavala writing support and helpful discussions.
References
- [1].Wolff J, Law of Bone Remodeling, SpringerVerlag, Berlin, 1986. [Google Scholar]
- [2].Meyers VE, et al. , RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity, J. Bone Miner. Res. 20 (10) (2005) 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ozcivici E, et al. , Mechanical signals as anabolic agents in bone, Nat. Rev. Rheumatol. 6 (1) (2010) 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Greenbaum A, et al. , Bone CLARITY: clearing, imaging, and computational analysis of osteoprogenitors within intact bone marrow, Sci. Transl. Med. (2017) 9(387). [DOI] [PubMed] [Google Scholar]
- [5].Tikhonova AN, et al. , The bone marrow microenvironment at single-cell resolution, Nature 569 (7755) (2019) 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Oguro H, Ding L, Morrison SJ, SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors, Cell Stem Cell 13 (1) (2013) 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chan CKF, et al. , Identification of the human skeletal stem cell, Cell 175 (1) (2018) (p. 43–56.e21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Acar M, et al. , Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal, Nature 526 (7571) (2015) 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shen B, et al. , A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis, Nature 591 (7850) (2021) 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Méndez-Ferrer S, et al. , Mesenchymal and haematopoietic stem cells form a unique bone marrow niche, Nature 466 (7308) (2010) 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rubin CT, Lanyon LE, Regulation of bone mass by mechanical strain magnitude, Calcif. Tissue Int. 37 (4) (1985) 411–417. [DOI] [PubMed] [Google Scholar]
- [12].Rubin CT, Lanyon LE, Regulation of bone formation by applied dynamic loads, J. Bone Joint Surg. Am. 66 (3) (1984) 397–402. [PubMed] [Google Scholar]
- [13].O’Connor JA, Lanyon LE, MacFie H, The influence of strain rate on adaptive bone remodelling, J. Biomech. 15 (10) (1982) 767–781. [DOI] [PubMed] [Google Scholar]
- [14].Rath B, et al. , Compressive forces induce osteogenic gene expression in calvarial osteoblasts, J. Biomech. 41 (5) (2008) 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Poliachik SL, et al. , 32 wk old C3H/HeJ mice actively respond to mechanical loading, Bone 42 (4) (2008) 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rubin C, et al. , Anabolism. Low mechanical signals strengthen long bones, Nature 412 (6847) (2001) 603–604. [DOI] [PubMed] [Google Scholar]
- [17].Oxlund BS, et al. , Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats, Bone 32 (1) (2003) 69–77. [DOI] [PubMed] [Google Scholar]
- [18].Tanaka SM, et al. , Effects of broad frequency vibration on cultured osteoblasts, J. Biomech. 36 (1) (2003) 73–80. [DOI] [PubMed] [Google Scholar]
- [19].Garman R, et al. , Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation, J. Orthop. Res. 25 (6) (2007) 732–740. [DOI] [PubMed] [Google Scholar]
- [20].Wren TA, et al. , Effect of high-frequency, low-magnitude vibration on bone and muscle in children with cerebral palsy, J. Pediatr. Orthop. 30 (7) (2010) 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pre D, et al. , The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment, Bone 49 (2) (2011) 295–303. [DOI] [PubMed] [Google Scholar]
- [22].McGarry JG, et al. , A comparison of strain and fluid shear stress in stimulating bone cell responses–a computational and experimental study, FASEB J. 19 (3) (2005) 482–484. [DOI] [PubMed] [Google Scholar]
- [23].Sikavitsas VI, et al. , Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces, Proc. Natl. Acad. Sci. U. S. A. 100 (25) (2003) 14683–14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bancroft GN, et al. , Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner, Proc. Natl. Acad. Sci. U. S. A. 99 (20) (2002) 12600–12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Weinbaum S, Cowin SC, Zeng Y, A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses, J. Biomech. 27 (3) (1994) 339–360. [DOI] [PubMed] [Google Scholar]
- [26].Reich KM, Gay CV, Frangos JA, Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production, J. Cell. Physiol. 143 (1) (1990) 100–104. [DOI] [PubMed] [Google Scholar]
- [27].Qin YX, Hu M, Intramedullary pressure induced by dynamic hydraulic pressure stimulation and its potential in treatment of osteopenia, Bone 48 (2011) S186. [Google Scholar]
- [28].Qin YX, Lam HY, Intramedullary pressure and matrix strain induced by oscillatory skeletal muscle stimulation and its potential in adaptation, J. Biomech. 42 (2) (2009) 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang P, et al. , Knee loading dynamically alters intramedullary pressure in mouse femora, Bone 40 (2) (2007) 538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chan ME, Uzer G, Rubin CT, The potential benefits and inherent risks of vibration as a non-drug therapy for the prevention and treatment of osteoporosis, Curr. Osteoporos. Rep. 11 (1) (2013) 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Price C, et al. , Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow, J. Bone Miner. Res. 26 (2) (2011) 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vainionpaa A, et al. , Intensity of exercise is associated with bone density change in premenopausal women, Osteoporos. Int. 17 (3) (2006) 455–463. [DOI] [PubMed] [Google Scholar]
- [33].Fritton SP, McLeod KJ, Rubin CT, Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains, J. Biomech. 33 (3) (2000) 317–325. [DOI] [PubMed] [Google Scholar]
- [34].Marin-Cascales E, et al. , Whole-body vibration training and bone health in postmenopausal women: a systematic review and meta-analysis, Medicine (Baltimore) 97 (34) (2018), e11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ritzmann R, Stark C, Krause A, Vibration therapy in patients with cerebral palsy: a systematic review, Neuropsychiatr. Dis. Treat. 14 (2018) 1607–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bryant JD, et al. , Rheology of bovine bone marrow, Proc. Inst. Mech. Eng. H 203 (2) (1989) 71–75. [DOI] [PubMed] [Google Scholar]
- [37].Coughlin TR, Niebur GL, Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration, J. Biomech. 45 (13) (2012) 2222–2229. [DOI] [PubMed] [Google Scholar]
- [38].Dickerson DA, Sander EA, Nauman EA, Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects, Biomech. Model. Mechanobiol. 7 (3) (2008) 191–202. [DOI] [PubMed] [Google Scholar]
- [39].Cabrita GJM, et al. , Hematopoietic stem cells: from the bone to the bioreactor, Trends Biotechnol. 21 (5) (2003) 233–240. [DOI] [PubMed] [Google Scholar]
- [40].Gordon M, Stem cells handbook, Bone Marrow Transplant. 33 (11) (2004) 1165. [Google Scholar]
- [41].Roca-Cusachs P, Iskratsch T, Sheetz MP, Finding the weakest link – exploring integrin-mediated mechanical molecular pathways, J. Cell Sci. 125 (13) (2012) 3025–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Scott A, et al. , Mechanotransduction in human bone in vitro cellular physiology that underpins bone changes with exercise, Sports Med. 38 (2) (2008) 139–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Thompson WR, Rubin CT, Rubin J, Mechanical regulation of signaling pathways in bone, Gene 503 (2) (2012) 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maurer M, Lammerding J, The driving force: nuclear mechanotransduction in cellular function, fate, and disease, Annu. Rev. Biomed. Eng. 21 (2019) 443–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].De Magistris P, Antonin W, The dynamic nature of the nuclear envelope, Curr. Biol. 28 (8) (2018) R487–r497. [DOI] [PubMed] [Google Scholar]
- [46].Graham DM, Burridge K, Mechanotransduction and nuclear function, Curr. Opin. Cell Biol. 40 (2016) 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schirmer EC, et al. , Nuclear membrane proteins with potential disease links found by subtractive proteomics, Science 301 (5638) (2003) 1380–1382. [DOI] [PubMed] [Google Scholar]
- [48].Korfali N, et al. , The leukocyte nuclear envelope proteome varies with cell activation and contains novel transmembrane proteins that affect genome architecture, Mol. Cell. Proteomics 9 (12) (2010) 2571–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wilkie GS, et al. , Several novel nuclear envelope transmembrane proteins identified in skeletal muscle have cytoskeletal associations, Mol. Cell. Proteomics 10 (1) (2011) (p. M110.003129). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cheng L-C, et al. , Identification of new transmembrane proteins concentrated at the nuclear envelope using organellar proteomics of mesenchymal cells, Nucleus (Austin, Tex.) 10 (1) (2019) 126–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Berk JM, Tifft KE, Wilson KL, The nuclear envelope LEM-domain protein emerin, Nucleus 4 (4) (2013) 298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Guilluy C, et al. , Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus, Nat. Cell Biol. 16 (4) (2014) 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Crisp M, et al. , Coupling of the nucleus and cytoplasm: role of the LINC complex, J. Cell Biol. 172 (1) (2006) 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Alam SG, et al. , The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity, Sci. Rep. 6 (2016) 38063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Uzer G, et al. , Cell mechanosensitivity to extremely low-magnitude signals is enabled by a LINCed nucleus, Stem Cells 33 (6) (2015) 2063–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ghosh S, et al. , Deformation microscopy for dynamic intracellular and intranuclear mapping of mechanics with high spatiotemporal resolution, Cell Rep. 27 (5) (2019) (p. 1607–1620.e4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Spichal M, et al. , Evidence for a dual role of actin in regulating chromosome organization and dynamics in yeast, J. Cell Sci. 129 (4) (2016) 681–692. [DOI] [PubMed] [Google Scholar]
- [58].Link J, Jahn D, Alsheimer M, Structural and functional adaptations of the mammalian nuclear envelope to meet the meiotic requirements, Nucleus 6 (2) (2015) 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lottersberger F, et al. , 53BP1 and the LINC complex promote microtubule-dependent DSB mobility and DNA repair, Cell 163 (4) (2015) 880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Swartz RK, Rodriguez EC, King MC, A role for nuclear envelope-bridging complexes in homology-directed repair, Mol. Biol. Cell 25 (16) (2014) 2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shiu J-Y, et al. , Nanopillar force measurements reveal actin-cap-mediated YAP mechanotransduction, Nat. Cell Biol. 20 (3) (2018) 262–271. [DOI] [PubMed] [Google Scholar]
- [62].Uzer G, et al. , Sun-mediated mechanical LINC between nucleus and cytoskeleton regulates betacatenin nuclear access, J. Biomech. 74 (2018) 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Greenleaf JE, et al. , Exercise-training protocols for astronauts in microgravity, J. Appl. Physiol. (1985) 67 (6) (1989) 2191–2204. [DOI] [PubMed] [Google Scholar]
- [64].Vuori I, Exercise and physical health: musculoskeletal health and functional capabilities, Res. Q. Exerc. Sport 66 (4) (1995) 276–285. [DOI] [PubMed] [Google Scholar]
- [65].Wallace IJ, et al. , Exercise-induced bone formation is poorly linked to local strain magnitude in the sheep tibia, PLoS One 9 (6) (2014), e99108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mancuso ME, Troy KL, Relating bone strain to local changes in radius microstructure following 12 months of axial forearm loading in women, J. Biomech. Eng. (2020) 142(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Carrieroa A, et al. , Spatial relationship between bone formation and mechanical stimulus within cortical bone: combining 3D fluorochrome mapping and poroelastic finite element modelling, Bone Rep. 8 (2018) 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Birmingham E, et al. , Mechanical stimulation of bone marrow in situ induces bone formation in trabecular explants, Ann. Biomed. Eng. 43 (4) (2015) 1036–1050. [DOI] [PubMed] [Google Scholar]
- [69].Metzger TA, et al. , The in situ mechanics of trabecular bone marrow: the potential for mechanobiological response, J. Biomech. Eng. (2015) 137(1). [DOI] [PubMed] [Google Scholar]
- [70].Uzer G, et al. , Separating fluid shear stress from acceleration during vibrations in vitro: identification of mechanical signals modulating the cellular response, Cell. Mol. Bioeng. 5 (3) (2012) 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Uzer G, et al. , Gap junctional communication in osteocytes is amplified by low intensity vibrations in vitro, PLoS One 9 (3) (2014), e90840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Uzer G, et al. , Vibration induced osteogenic commitment of mesenchymal stem cells is enhanced by cytoskeletal remodeling but not fluid shear, J. Biomech. 46 (13) (2013) 2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wakeling JM, Nigg BM, Modification of soft tissue vibrations in the leg by muscular activity, J. Appl. Physiol. (1985) 90 (2) (2001) 412–420. [DOI] [PubMed] [Google Scholar]
- [74].de Haan A, The influence of stimulation frequency on force-velocity characteristics of in situ rat medial gastrocnemius muscle, Exp. Physiol. 83 (1) (1998) 77–84. [DOI] [PubMed] [Google Scholar]
- [75].Huang RP, Rubin CT, McLeod KJ, Changes in postural muscle dynamics as a function of age, J. Gerontol. A Biol. Sci. Med. Sci. 54 (8) (1999) B352–B357. [DOI] [PubMed] [Google Scholar]
- [76].Gilsanz V, et al. , Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD, J. Bone Miner. Res. 21 (9) (2006) 1464–1474. [DOI] [PubMed] [Google Scholar]
- [77].Mogil RJ, et al. , Effect of low-magnitude, high-frequency mechanical stimulation on BMD among young childhood cancer survivors: a randomized clinical trial, JAMA Oncol. 2 (7) (2016) 908–914, 10.1001/jamaoncol.2015.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rubin C, et al. , Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention, J. Bone Miner. Res. 17 (2) (2002) 349–357. [DOI] [PubMed] [Google Scholar]
- [79].Rubin C, Xu G, Judex S, The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli, FASEB J. 15 (12) (2001) 2225–2229. [DOI] [PubMed] [Google Scholar]
- [80].McKeehen JN, et al. , Adaptations of mouse skeletal muscle to low-intensity vibration training, Med. Sci. Sports Exerc. 45 (6) (2013) 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mettlach G, et al. , Enhancement of neuromuscular dynamics and strength behavior using extremely low magnitude mechanical signals in mice, J. Biomech. 47 (1) (2014) 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Xie L, Rubin C, Judex S, Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations, J. Appl. Physiol. (1985) 104 (4) (2008) 1056–1062. [DOI] [PubMed] [Google Scholar]
- [83].Milovanovic P, et al. , Osteocytic canalicular networks: morphological implications for altered mechanosensitivity, ACS Nano 7 (9) (2013) 7542–7551. [DOI] [PubMed] [Google Scholar]
- [84].Verbruggen SW, Vaughan TJ, McNamara LM, Strain amplification in bone mechanobiology: a computational investigation of the in vivo mechanics of osteocytes, J. R. Soc. Interface 9 (75) (2012) 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bonewald LF, The amazing osteocyte, J. Bone Miner. Res. 26 (2) (2011) 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Han Y, et al. , Mechanotransduction and strain amplification in osteocyte cell processes, Proc. Natl. Acad. Sci. U. S. A. 101 (47) (2004) 16689–16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jansen LE, et al. , Mechanics of intact bone marrow, J. Mech. Behav. Biomed. Mater. 50 (2015) 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Maredziak M, et al. , The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells, Stem Cells Int. 2016 (2016) 2152435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rajapakse CS, et al. , Effect of low intensity vibration on bone strength, microstructure, and adiposity in pre-osteoporotic postmenopausal women: A randomized placebo-controlled trial, J. Bone Miner. Res. 36 (4) (2021) 673–684, 10.1002/jbmr.4229. Epub 2020 Dec 23. [DOI] [PubMed] [Google Scholar]
- [90].Ouyang Z, et al. , Prx1 and 3.2kb Col1a1 promoters target distinct bone cell populations in transgenic mice, Bone 58 (2014) 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cabahug-Zuckerman P, et al. , Site-specific load-induced expansion of Sca-1+ Prrx1+ and Sca-1−Prrx1+ cells in adult mouse long bone is attenuated with age, JBMR Plus 3 (9) (2019), e10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Choudhery MS, et al. , Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation, J. Transl. Med. 12 (2014) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Javaheri B, Pitsillides AA, Aging and mechanoadaptive responsiveness of bone, Curr. Osteoporos. Rep. 17 (6) (2019) 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ingber DE, Wang N, Stamenovic D, Tensegrity, cellular biophysics, and the mechanics of living systems, Rep. Prog. Phys. 77 (4) (2014), 046603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Capco DG, Wan KM, Penman S, The nuclear matrix: three-dimensional architecture and protein composition, Cell 29 (3) (1982) 847–858. [DOI] [PubMed] [Google Scholar]
- [96].Cooper LF, et al. , Incipient analysis of mesenchymal stem-cell-derived osteogenesis, J. Dent. Res. 80 (1) (2001) 314–320. [DOI] [PubMed] [Google Scholar]
- [97].Wang N, Butler JP, Ingber DE, Mechanotransduction across the cell-surface and through the cytoskeleton, Science 260 (5111) (1993) 1124–1127. [DOI] [PubMed] [Google Scholar]
- [98].McGarry JG, Prendergast PJ, A three-dimensional finite element model of an adherent eukaryotic cell, Eur. Cell. Mater. 7 (2004) 27–33 (discussion 33–4). [DOI] [PubMed] [Google Scholar]
- [99].Hu SH, et al. , Prestress mediates force propagation into the nucleus, Biochem. Biophys. Res. Commun. 329 (2) (2005) 423–428. [DOI] [PubMed] [Google Scholar]
- [100].Tajik A, et al. , Transcription upregulation via force-induced direct stretching of chromatin, Nat. Mater. 15 (12) (2016) 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Graham DM, et al. , Enucleated cells reveal differential roles of the nucleus in cell migration, polarity, and mechanotransduction, J. Cell Biol. 217 (3) (2018) 895–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhang Q, et al. , Mechanical stabilization of the glandular acinus by linker of nucleoskeleton and cytoskeleton complex, Curr. Biol. 29 (17) (2019) (p. 2826–2839.e4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Arsenovic PT, et al. , Nesprin-2G, a component of the nuclear LINC complex, is subject to myosin-dependent tension, Biophys. J. 110 (1) (2016) 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nagayama K, et al. , Estimation of the mechanical connection between apical stress fibers and the nucleus in vascular smooth muscle cells cultured on a substrate, J. Biomech. 0 (2014). [DOI] [PubMed] [Google Scholar]
- [105].Machesky LM, Insall RH, Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex, Curr. Biol. 8 (25) (1998) 1347–1356. [DOI] [PubMed] [Google Scholar]
- [106].Blanchoin L, et al. , Actin dynamics, architecture, and mechanics in cell motility, Physiol. Rev. 94 (1) (2014) 235–263. [DOI] [PubMed] [Google Scholar]
- [107].Jaffe AB, Hall A, Rho GTPases: biochemistry and biology, Annu. Rev. Cell Dev. Biol. 21 (1) (2005) 247–269. [DOI] [PubMed] [Google Scholar]
- [108].Riddick N, Ohtani K, Surks HK, Targeting by myosin phosphatase-RhoA interacting protein mediates RhoA/ROCK regulation of myosin phosphatase, J. Cell. Biochem. 103 (4) (2008) 1158–1170. [DOI] [PubMed] [Google Scholar]
- [109].Burridge K, Wittchen ES, The tension mounts: stress fibers as force-generating mechanotransducers, J. Cell Biol. 200 (1) (2013) 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Smith MA, et al. , A zyxin-mediated mechanism for actin stress fiber maintenance and repair, Dev. Cell 19 (3) (2010) 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kanchanawong P, et al. , Nanoscale architecture of integrin-based cell adhesions, Nature 468 (7323) (2010) 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Burridge K, et al. , Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton, Annu. Rev. Cell Biol. 4 (1988) 487–525. [DOI] [PubMed] [Google Scholar]
- [113].Grashoff C, et al. , Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics, Nature 466 (7303) (2010) 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Turner CE, Glenney JR Jr., Burridge K, Paxillin: a new vinculin-binding protein present in focal adhesions, J. Cell Biol. 111 (3) (1990) 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Pasapera AM, et al. , Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation, J. Cell Biol. 188 (6) (2010) 877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Sen B, et al. , Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells, Stem Cells 29 (11) (2011) 1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Sen B, et al. , mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells, J. Bone Miner. Res. 29 (1) (2014) 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Thompson WR, et al. , LARG GEF and ARHGAP18 orchestrate RhoA activity to control mesenchymal stem cell lineage, Bone 107 (2018) 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Engler AJ, et al. , Matrix elasticity directs stem cell lineage specification, Cell 126 (4) (2006) 677–689, 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [120].Khatiwala CB, et al. , ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK, J. Bone Miner. Res. 24 (5) (2009) 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].McBeath R, et al. , Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment, Dev. Cell 6 (4) (2004) 483–495. [DOI] [PubMed] [Google Scholar]
- [122].Carroll SF, Buckley CT, Kelly DJ, Cyclic tensile strain can play a role in directing both intramembranous and endochondral ossification of mesenchymal stem cells, Front. Bioeng. Biotechnol. 5 (2017) 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Case N, et al. , Steady and oscillatory fluid flows produce a similar osteogenic phenotype, Calcif. Tissue Int. 88 (3) (2011) 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Arnsdorf EJ, et al. , Mechanically induced osteogenic differentiation - the role of RhoA, ROCKII and cytoskeletal dynamics, J. Cell Sci. 122 (4) (2009) 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Karadas O, Mese G, Ozcivici E, Low magnitude high frequency vibrations expedite the osteogenesis of bone marrow stem cells on paper based 3D scaffolds, Biomed. Eng. Lett. 10 (3) (2020) 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rando TA, Ambrosio F, Regenerative rehabilitation: applied biophysics meets stem cell therapeutics, Cell Stem Cell 22 (3) (2018) 306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Case N, et al. , Mechanical regulation of glycogen synthase kinase 3beta (GSK3beta) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein, J. Biol. Chem. 286 (45) (2011) 39450–39456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sen B, et al. , Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node, J. Biol. Chem. 284 (50) (2009) 34607–34617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ho LTY, et al. , Lysophosphatidic acid induces ECM production via activation of the mechanosensitive YAP/TAZ transcriptional pathway in trabecular meshwork cells, Invest. Ophthalmol. Vis. Sci. 59 (5) (2018) 1969–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cai H, Xu Y, The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells, Cell Commun. Signal. 11 (1) (2013) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Dupont S, et al. , Role of YAP/TAZ in mechanotransduction, Nature 474 (7350) (2011) 179–183. [DOI] [PubMed] [Google Scholar]
- [132].Driscoll Tristan P., et al. , Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells, Biophys. J. 108 (12) (2015) 2783–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Benham-Pyle BW, Pruitt BL, Nelson WJ, Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry, Science 348 (6238) (2015) 1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Thompson M, et al. , Low-intensity vibration restores nuclear YAP levels and acute YAP nuclear shuttling in mesenchymal stem cells subjected to simulated microgravity, NPJ Microgravity 6 (1) (2020) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Azzolin L, et al. , YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response, Cell 158 (1) (2014) 157–170. [DOI] [PubMed] [Google Scholar]
- [136].Deng F, et al. , YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury, Cell Death Dis. 9 (2) (2018) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kegelman CD, et al. , Skeletal cell YAP and TAZ redundantly promote bone development by regulation of collagen I expression and organization, FASEB J. 32 (5) (2018. May) 2706–2721, 10.1096/fj.201700872R. Epub 2018 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Javaheri B, et al. , Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading, J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 29 (3) (2014) 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Gaur T, et al. , Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression, J. Biol. Chem. 280 (39) (2005) 33132–33140. [DOI] [PubMed] [Google Scholar]
- [140].Matsumoto Y, et al. , Reciprocal stabilization of ABL and TAZ regulates osteoblastogenesis through transcription factor RUNX2, J. Clin. Invest. 126 (12) (2016) 4482–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zaidi SK, et al. , Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription, EMBO J. 23 (4) (2004) 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Seo E, et al. , SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage, Cell Rep. 3 (6) (2013) 2075–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sen B, et al. , Intranuclear actin regulates osteogenesis, Stem Cells (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Tang Y, et al. , Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation, Nat. Cell Biol. 18 (2016) 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chen J, Long F, beta-catenin promotes bone formation and suppresses bone resorption in postnatal growing mice, J. Bone Miner. Res. 28 (5) (2013) 1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Day TF, et al. , Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis, Dev. Cell 8 (5) (2005) 739–750. [DOI] [PubMed] [Google Scholar]
- [147].Stewart-Hutchinson PJ, et al. , Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness, Exp. Cell Res. 314 (8) (2008) 1892–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Padmakumar VC, et al. , Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton, Exp. Cell Res. 295 (2) (2004) 330–339. [DOI] [PubMed] [Google Scholar]
- [149].Zhen Y-Y, et al. , NUANCE, a giant protein connecting the nucleus and actin cytoskeleton, J. Cell Sci. 115 (15) (2002) 3207–3222. [DOI] [PubMed] [Google Scholar]
- [150].Wilson MH, Holzbaur EL, Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells, Development 142 (1) (2015) 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Roux KJ, et al. , Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization, Proc. Natl. Acad. Sci. 106 (7) (2009) 2194–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lombardi ML, et al. , The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton, J. Biol. Chem. 286 (30) (2011) 26743–26753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kutscheidt S, et al. , FHOD1 interaction with nesprin-2G mediates TAN line formation and nuclear movement, Nat. Cell Biol. 16 (7) (2014) 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Woroniuk A, et al. , STEF/TIAM2-mediated Rac1 activity at the nuclear envelope regulates the perinuclear actin cap, Nat. Commun. 9 (1) (2018) 2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Versaevel M, et al. , Super-resolution microscopy reveals LINC complex recruitment at nuclear indentation sites, Sci. Rep. 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Chambliss AB, et al. , The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction, Sci. Rep. 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kim DH, et al. , Actin cap associated focal adhesions and their distinct role in cellular mechanosensing, Sci. Rep. 2 (2012) 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Chancellor TJ, et al. , Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation, Biophys. J. 99 (1) (2010) 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chen CY, et al. , Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies, Cell 149 (3) (2012) 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Thakar K, et al. , Opposing roles for distinct LINC complexes in regulation of the small GTPase RhoA, Mol. Biol. Cell 28 (1) (2017) 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Newberg J, et al. , Isolated nuclei stiffen in response to low intensity vibration, J. Biomech. (2020) 110012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Touchstone H, et al. , Recovery of stem cell proliferation by low intensity vibration under simulated microgravity requires intact LINC complex, npj Microgravity (2019) 5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Pongkitwitoon S, et al. , Cytoskeletal configuration modulates mechanically induced changes in mesenchymal stem cell osteogenesis, morphology, and stiffness, Sci. Rep. 6 (2016) 34791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Sen B, et al. , Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal, Endocrinology 149 (12) (2008) 6065–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Case N, et al. , β-Catenin levels influence rapid mechanical responses in osteoblasts, J. Biol. Chem. 283 (43) (2008) 29196–29205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Sen B, et al. , β-Catenin preserves the stem state of murine bone marrow stromal cells through activation of EZH2, J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. (2020), 10.1002/jbmr.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Neumann S, et al. , Nesprin-2 interacts with α-catenin and regulates Wnt signaling at the nuclear envelope, J. Biol. Chem. 285 (45) (2010) 34932–34938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Déjardin T, et al. , Nesprins are mechanotransducers that discriminate epithelial- mesenchymal transition programs, J. Cell Biol. 219 (10) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Koike M, et al. , β-Catenin shows an overlapping sequence requirement but distinct molecular interactions for its bidirectional passage through nuclear pores, J. Biol. Chem. 279 (32) (2004) 34038–34047. [DOI] [PubMed] [Google Scholar]
- [170].Tolwinski NS, Wieschaus E, A nuclear function for armadillo/beta-catenin, PLoS Biol. 2 (4) (2004), E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Hong J-H, et al. , TAZ, a transcriptional modulator of mesenchymal stem cell differentiation, Science 309 (5737) (2005) 1074–1078. [DOI] [PubMed] [Google Scholar]
- [172].Yasuda D, et al. , Lysophosphatidic acid–induced YAP/TAZ activation promotes developmental angiogenesis by repressing Notch ligand Dll4, J. Clin. Invest. 129 (10) (2019) 4332–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Lammerding J, et al. , Lamins A and C but not Lamin B1 regulate nuclear mechanics, J. Biol. Chem. 281 (35) (2006) 25768–25780. [DOI] [PubMed] [Google Scholar]
- [174].Swift J, et al. , Nuclear lamin-A scales with tissue stiffness and enhances matrix- directed differentiation, Science (2013) 341(6149). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Vidal C, et al. , Role of the nuclear envelope in the pathogenesis of age-related bone loss and osteoporosis, Bonekey Rep. 1 (2012) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Swift J, et al. , Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation, Science 341 (6149) (2013) 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bermeo S, et al. , Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/beta-catenin pathway, J. Cell. Biochem. 116 (10) (2015) 2344–2353. [DOI] [PubMed] [Google Scholar]
- [178].Constantinescu D, et al. , Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation, Stem Cells 24 (1) (2006) 177–185. [DOI] [PubMed] [Google Scholar]
- [179].Oldenburg A, et al. , A lipodystrophy-causing lamin A mutant alters conformation and epigenetic regulation of the anti-adipogenic MIR335 locus, J. Cell Biol. 216 (9) (2017) 2731–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Li W, et al. , Decreased bone formation and osteopenia in lamin a/c-deficient mice, PLoS One 6 (4) (2011), e19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Padmakumar VC, et al. , The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope, J. Cell Sci. 118 (15) (2005) 3419–3430. [DOI] [PubMed] [Google Scholar]
- [182].Tanabe LM, et al. , Primary dystonia: molecules and mechanisms, Nat. Rev. Neurol. 5 (11) (2009) 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Jungwirth M, et al. , The nuclear envelope localization of DYT1 dystonia torsinA-DeltaE requires the SUN1 LINC complex component, BMC Cell Biol. 12 (1) (2011) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Nery FC, et al. , TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton, J. Cell Sci. 121 (20) (2008) 3476–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Sosa BA, et al. , How lamina-associated polypeptide 1 (LAP1) activates Torsin, Elife 3 (2014), e03239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Shin JY, et al. , Lamina-associated polypeptide-1 interacts with the muscular dystrophy protein emerin and is essential for skeletal muscle maintenance, Dev. Cell 26 (6) (2013) 591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Saunders CA, et al. , TorsinA controls TAN line assembly and the retrograde flow of dorsal perinuclear actin cables during rearward nuclear movement, J. Cell Biol. 216 (3) (2017) 657–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Manilal S, et al. , The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein, Hum. Mol. Genet. 5 (6) (1996) 801–808. [DOI] [PubMed] [Google Scholar]
- [189].Holaska JM, Kowalski AK, Wilson KL, Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane, PLoS Biol. 2 (9) (2004), E231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Le HQ, et al. , Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment, Nat. Cell Biol. 18 (8) (2016) 864–875. [DOI] [PubMed] [Google Scholar]
- [191].Stubenvoll A, et al. , Attenuation of Wnt/beta-catenin activity reverses enhanced generation of cardiomyocytes and cardiac defects caused by the loss of emerin, Hum. Mol. Genet. 24 (3) (2015) 802–813. [DOI] [PubMed] [Google Scholar]
- [192].Tilgner K, et al. , Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of β-catenin, J. Cell Sci. 122 (3) (2009) 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Markiewicz E, et al. , The inner nuclear membrane protein Emerin regulates [beta]-catenin activity by restricting its accumulation in the nucleus, EMBO J. 25 (14) (2006) 3275–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Sen B, et al. , Intranuclear actin structure modulates mesenchymal stem cell differentiation, Stem Cells 35 (6) (2017) 1624–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Sankaran JS, et al. , Knockdown of formin mDia2 alters lamin B1 levels and increases osteogenesis in stem cells, Stem Cells 38 (1) (2020) 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Plessner M, et al. , Nuclear F-actin formation and reorganization upon cell spreading, J. Biol. Chem. 290 (18) (2015) 11209–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Carrero D, Soria-Valles C, López-Otín C, Hallmarks of progeroid syndromes: lessons from mice and reprogrammed cells, Dis. Model. Mech. 9 (7) (2016) 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Chen Z-J, et al. , Dysregulated interactions between lamin A and SUN1 induce abnormalities in the nuclear envelope and endoplasmic reticulum in progeric laminopathies, J. Cell Sci. 127 (8) (2014) 1792–1804. [DOI] [PubMed] [Google Scholar]
- [199].McClintock D, et al. , The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin, PLoS One 2 (12) (2007), e1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Hernandez L, et al. , Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria, Dev. Cell 19 (3) (2010) 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Choi JY, et al. , Diminished canonical β-catenin signaling during osteoblast differentiation contributes to osteopenia in progeria, J. Bone Miner. Res. 33 (11) (2018) 2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Takahashi Y, et al. , Impairment of nuclear F-actin formation and its relevance to cellular phenotypes in Hutchinson-Gilford progeria syndrome, Nucleus (Austin, Tex.) 11 (1) (2020) 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Barthélémy F, et al. , Truncated prelamin A expression in HGPS-like patients: a transcriptional study, Eur. J. Hum. Genet. 23 (8) (2015) 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Navarro CL, et al. , Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors, Hum. Mol. Genet. 14 (11) (2005) 1503–1513. [DOI] [PubMed] [Google Scholar]
- [205].Novelli G, et al. , Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C, Am. J. Hum. Genet. 71 (2) (2002) 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Agarwal AK, et al. , Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia, Hum. Mol. Genet. 12 (16) (2003) 1995–2001. [DOI] [PubMed] [Google Scholar]
- [207].Cabanillas R, et al. , Néstor–Guillermo progeria syndrome: a novel premature aging condition with early onset and chronic development caused by BANF1 mutations, Am. J. Med. Genet. A 155 (11) (2011) 2617–2625. [DOI] [PubMed] [Google Scholar]
- [208].Chitayat D, et al. , Hydrops-ectopic calcification-moth-eaten skeletal dysplasia (Greenberg dysplasia): prenatal diagnosis and further delineation of a rare genetic disorder, Am. J. Med. Genet. 47 (2) (1993) 272–277. [DOI] [PubMed] [Google Scholar]
- [209].Greenberg CR, et al. , A new autosomal recessive lethal chondrodystrophy with congenital hydrops, Am. J. Med. Genet. 29 (3) (1988) 623–632. [DOI] [PubMed] [Google Scholar]
- [210].Horn LC, et al. , Greenberg dysplasia: first reported case with additional non-skeletal malformations and without consanguinity, Prenat. Diagn. 20 (12) (2000) 1008–1011. [DOI] [PubMed] [Google Scholar]
- [211].Waterham HR, et al. , Autosomal recessive HEM/Greenberg skeletal dysplasia is caused by 3 beta-hydroxysterol delta 14-reductase deficiency due to mutations in the lamin B receptor gene, Am. J. Hum. Genet. 72 (4) (2003) 1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Wu M, Chen G, Li Y-P, TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease, Bone Res. 4 (1) (2016) 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Korman B, et al. , Mutation in LEMD3 (Man1) associated with osteopoikilosis and late-onset generalized morphea: a new Buschke-Ollendorf syndrome variant, Case Rep. Dermatol. Med. 2016 (2016) 2483041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Rac MWF, McKinney J, Gandhi M, Arthrogryposis, Am. J. Obstet. Gynecol. 221 (6) (2019) B7–B9. [DOI] [PubMed] [Google Scholar]
- [215].Wood CL, Straub V, Bones and muscular dystrophies: what do we know? Curr. Opin. Neurol. 31 (5) (2018). [DOI] [PubMed] [Google Scholar]
- [216].Scaffidi P, Misteli T, Lamin A-dependent nuclear defects in human aging, Science 312 (5776) (2006) 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Klein-Nulend J, et al. , Donor age and mechanosensitivity of human bone cells, Osteoporos. Int. 13 (2) (2002) 137–146. [DOI] [PubMed] [Google Scholar]
- [218].Bas G, et al. , Low intensity vibrations augment mesenchymal stem cell proliferation and differentiation capacity during in vitro expansion, Sci. Rep. 10 (1) (2020) 9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Razi H, et al. , Aging leads to a dysregulation in mechanically driven bone formation and resorption, J. Bone Miner. Res. 30 (10) (2015) 1864–1873. [DOI] [PubMed] [Google Scholar]
- [220].Puts R, et al. , Influence of donor age and stimulation intensity on osteogenic differentiation of rat mesenchymal stromal cells in response to focused low- intensity pulsed ultrasound, Ultrasound Med. Biol. 42 (12) (2016) 2965–2974. [DOI] [PubMed] [Google Scholar]
- [221].Meyer MB, et al. , Epigenetic plasticity drives adipogenic and osteogenic differentiation of marrow-derived mesenchymal stem cells, J. Biol. Chem. 291 (34) (2016) 17829–17847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Joiner DM, et al. , Bone marrow stromal cells from aged male rats have delayed mineralization and reduced response to mechanical stimulation through nitric oxide and ERK1/2 signaling during osteogenic differentiation, Biogerontology 13 (5) (2012) 467–478. [DOI] [PubMed] [Google Scholar]
- [223].Geißler S, et al. , Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells, PLoS One 7 (12) (2012), e52700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Hell RCR, et al. , Physical activity improves age-related decline in the osteogenic potential of rats’ bone marrow-derived mesenchymal stem cells, Acta Physiol. 205 (2) (2012) 292–301. [DOI] [PubMed] [Google Scholar]
- [225].Singulani MP, et al. , Effects of strength training on osteogenic differentiation and bone strength in aging female Wistar rats, Sci. Rep. 7 (2017) 42878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Speakman JR, Selman C, Physical activity and resting metabolic rate, Proc. Nutr. Soc. 62 (3) (2003) 621–634. [DOI] [PubMed] [Google Scholar]
- [227].Jackson AS, et al. , Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness, Arch. Intern. Med. 169 (19) (2009) 1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Le Pape F, Vargas G, Clézardin P, The role of osteoclasts in breast cancer bone metastasis, J. Bone Oncol. 5 (3) (2016) 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Matsumoto A, et al. , Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer, Bone 90 (2016. September) 69–79, 10.1016/j.bone.2016.05.014. Epub 2016 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Pagnotti GM, et al. , Low intensity vibration mitigates tumor progression and protect bone quantity and quality in a murine model of myeloma, Bone 90 (2016) 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Yi X, et al. , Mechanical suppression of breast cancer cell invasion and paracrine signaling to osteoclasts requires nucleo-cytoskeletal connectivity, Bone Res. 8 (1) (2020) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]