Key Points
Question
Does lithium augmentation of usual care reduce the rate of repeated suicide-related events in participants with bipolar disorder or depression who have survived a recent event?
Findings
This randomized clinical trial was stopped for futility after 519 veterans had been enrolled. No overall differences between lithium and placebo treatments were found.
Meaning
The findings of this study suggest that in patients who are actively being treated for mood disorders and substantial comorbidities, simply adding lithium to existing medication regimens is unlikely to be effective for preventing a broad range of suicide-related events.
Abstract
Importance
Suicide and suicide attempts are persistent and increasing public health problems. Observational studies and meta-analyses of randomized clinical trials have suggested that lithium may prevent suicide in patients with bipolar disorder or depression.
Objective
To assess whether lithium augmentation of usual care reduces the rate of repeated episodes of suicide-related events (repeated suicide attempts, interrupted attempts, hospitalizations to prevent suicide, and deaths from suicide) in participants with bipolar disorder or depression who have survived a recent event.
Design, Setting, and Participants
This double-blind, placebo-controlled randomized clinical trial assessed lithium vs placebo augmentation of usual care in veterans with bipolar disorder or depression who had survived a recent suicide-related event. Veterans at 29 VA medical centers who had an episode of suicidal behavior or an inpatient admission to prevent suicide within 6 months were screened between July 1, 2015, and March 31, 2019.
Interventions
Participants were randomized to receive extended-release lithium carbonate beginning at 600 mg/d or placebo.
Main Outcomes and Measures
Time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
Results
The trial was stopped for futility after 519 veterans (mean [SD] age, 42.8 [12.4] years; 437 [84.2%] male) were randomized: 255 to lithium and 264 to placebo. Mean lithium concentrations at 3 months were 0.54 mEq/L for patients with bipolar disorder and 0.46 mEq/L for patients with major depressive disorder. No overall difference in repeated suicide-related events between treatments was found (hazard ratio, 1.10; 95% CI, 0.77-1.55). No unanticipated safety concerns were observed. A total of 127 participants (24.5%) had suicide-related outcomes: 65 in the lithium group and 62 in the placebo group. One death occurred in the lithium group and 3 in the placebo group.
Conclusions and Relevance
In this randomized clinical trial, the addition of lithium to usual Veterans Affairs mental health care did not reduce the incidence of suicide-related events in veterans with major depression or bipolar disorders who experienced a recent suicide event. Therefore, simply adding lithium to existing medication regimens is unlikely to be effective for preventing a broad range of suicide-related events in patients who are actively being treated for mood disorders and substantial comorbidities.
Trial Registration
ClinicalTrials.gov Identifier: NCT01928446
This randomized clinical trial assesses whether the addition of lithium to existing medication regimens would prevent or delay repeated suicide-related events and whether it could be used safely.
Introduction
Suicide is a devastating clinical and public health problem. In 2017, suicide was the nation’s 10th leading cause of death.1 Up to 90% of suicides are attributable to mental illness2,3,4 and more than 20% to diagnosed affective disorders.5 Veterans accounted for 13% of all US deaths from suicide in 2017, with an age- and sex-adjusted rate for all veterans 1.5 times greater than other Americans.6 This increased risk led the US Department of Veterans Affairs (VA) to establish comprehensive programs, clinical services, and research to prevent suicide.6,7
Several treatments are available to reduce the risk of suicidal behavior.8 Effective psychotherapies are cognitive-behavioral, dialectical-behavioral, and problem-solving therapies.9,10,11 Clozapine is approved by the US Food and Drug Administration for decreasing suicidal behavior in patients with schizophrenia and schizoaffective disorder.12 There is ongoing research on ketamine,13 and although the Food and Drug Administration recently approved esketamine for major depressive disorder and acute suicidal ideation or behavior, whether this agent is effective for preventing suicide or reducing suicidal thoughts or actions is not known.14 Antidepressants may be associated with reduced suicide-related outcomes in older patients but increased suicide-related outcomes in younger patients.15,16,17
Numerous observational studies18,19 suggest that lithium may prevent suicide and suicide attempts in patients with bipolar disorder or depression, with some studies20,21 suggesting that this may be somewhat independent of lithium’s effects on mood. However, these observations could reflect practitioners’ propensity for prescribing lithium to patients less prone to suicide attempts. A cohort study22 of veterans using propensity score matching found no difference in suicide rates for patients with bipolar disorder taking lithium vs valproate. Randomized clinical trials23,24,25 to test whether lithium can prevent suicidal behavior in patients with bipolar disorder or depression have been underpowered.
In 2013, when this trial was planned, meta-analyses of trials of lithium vs placebo or active comparators that included patients with bipolar disorder or depression,26,27 most conducted to evaluate other outcomes, found that suicide was less common in patients receiving lithium than comparators. The most recent meta-analysis27 available at that time did not find differences for nonfatal deliberate self-harm. More recent meta-analyses,28,29 limited to studies of patients with major depression, raised questions about the association between lithium use and deaths from suicide. Nevertheless, the 2019 VA/US Department of Defense Clinical Practice Guideline for Assessment and Management of Patients at Risk for Suicide subsequently stated, “We suggest offering lithium alone (among patients with bipolar disorder) or in combination with another psychotropic agent (among patients with unipolar depression or bipolar disorder) to decrease the risk of death by suicide in patients with mood disorders.”10(p 27)
Questions remain about whether lithium, approved for bipolar disorders and used for adjunctive treatment for major depression, can prevent suicide-related behaviors in patients with these disorders. We conducted this trial to assess whether lithium would prevent or delay repeated suicide-related events and whether it could be used safely.
Methods
Patients
Veterans at 29 VA medical centers who had an episode of suicidal behavior or an inpatient admission to prevent suicide within 6 months were screened between July 1, 2015, and March 31, 2019. Veterans were enrolled after they provided written informed consent and their clinical practitioners concurred. Eligibility criteria included meeting Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision)30 criteria for bipolar I or II disorder or major depression, consenting to provide an emergency contact, and being cognitively intact31 and able to appreciate risks and benefits of participation.32 Exclusion criteria were schizophrenia; 6 or more previous lifetime suicide attempts; use of lithium within the past 6 months; history of significant adverse effects of lithium; unstable substance use or medical conditions; pregnancy, lactation, or not using birth control; participating in another randomized intervention trial; and current use of clozapine, haloperidol, or diuretics except amiloride. The study was sponsored by the VA Cooperative Studies Program, conducted in accordance with Good Clinical Practice Guidelines, and approved by the Human Rights Committee at the Boston VA Cooperative Studies Program Coordinating Center, the VA Central Institutional Review Board, and local VA research oversight committees at each site. Data were not submitted to the oversight committees. Only the data monitoring committee received deidentified data. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol can be found in Supplement 1.
Oversight
Data were collected by investigators at each site and analyzed at the coordinating center. The futility analysis was conducted by an independent statistician working with the Data Monitoring Committee and the coordinating center but not directly involved in the trial’s design or conduct. The Food and Drug Administration did not require an Investigational New Drug application.
Trial Design
The study was a double-blind, placebo-controlled, 52-week randomized clinical trial of extended-release lithium carbonate, in addition to usual VA management, to prevent repeated suicide-related behaviors or hospitalizations to prevent suicide (suicide-related events). Patients were recruited in person by research coordinators and then randomized within permuted blocks of 4 within each site and within 4 strata: bipolar disorder with prior suicide attempt, bipolar disorder without prior suicide attempt, depression with prior suicide attempt, and depression without suicide prior attempt.
Participants were randomized to receive lithium beginning at a dose of 600 mg/d (300 mg/d if there were contraindications to this dose) and titrated upward or placebo (98% microcrystalline cellulose). Lithium (or placebo) serum concentrations were determined by a central laboratory after each dose adjustment until steady state with a lithium concentration between 0.6 and 0.8 mEq/L (to convert to millimoles per liter, multiply by 1). If participants could not tolerate the dose needed to achieve the target concentration, they were given the maximum tolerated dose, at least 300 mg/d. Real or simulated lithium concentrations, creatinine concentration, estimated glomerular filtration rate, and review of symptoms were used to guide dosing by study physicians at each site. Medications were dispensed in blister cards that contained 1- or 2-week supplies. After steady state was achieved, lithium concentrations were determined monthly for 6 months and then quarterly. Lithium concentrations were measured more frequently if there were interacting medications or side effect concerns.
Baseline characteristics, including race, ethnicity, sex, and psychiatric and medical comorbidities, all known to be associated with suicidal behavior, were collected by participant self-report. Response options for each were defined by the study investigators and complied with sponsor policies that encouraged the collection of these data.
Mental health symptoms were measured by standardized instruments,33,34,35,36,37,38 including the Columbia–Suicide Severity Rating Scale33 and the Patient Health Questionnaire 9,34 the activation subscale of the Internal State Scale,35 the Barratt Impulsiveness Scale,36 and Buss-Perry Aggression Questionnaire.37
Study medications were added to usual VA mental health care, which included medications and psychosocial treatment for mental health conditions and a range of rehabilitation- and recovery-oriented services.
Outcomes
The primary study outcome was time to the first episode of any 1 of a set of suicide-related events (patient outcomes). These included nonfatal suicide attempts, interrupted attempts, deaths by suicide, and hospitalizations to prevent suicide over a 1-year follow-up period. These events were classified using the Self-directed Violence Classification System.39 In this classification, suicide attempts were equivalent to suicidal (or undetermined) self-directed violence, nonfatal and interrupted attempts were equivalent to suicidal self-directed violence, interrupted. All outcomes were adjudicated by an end points committee blinded to study treatment but with access to reports from site investigators and documents from the VA (and, when relevant and available, other) facilities.12 Adjudicators were asked to first determine whether an event should be considered a primary outcome and then to classify the event. Two members of the committee evaluated each end point independently. A third review was performed if there was disagreement.
Statistical Analysis
A modified intention-to-treat analysis of all randomized patients receiving at least 1 dose of their study medication was conducted. Univariate and multivariate time-to-event analyses for primary outcome were conducted with Cox proportional hazards regression models and adjusted for covariates and randomization strata. Per-protocol analyses compared participants in the lithium group with participants in the placebo group who had taken at least 80% of their medications based on pill counts at the time of their first event or study completion.
The primary hypothesis was powered to test whether lithium compared with placebo resulted in a reduction in the repeat event rate by 37.0%, estimated from literature values,23,24,25 and estimates of nonadherence. Specifically, the hypothesis was designed to test for a reduction from 15.0% to 9.45% for participants receiving lithium while those receiving placebo remained at 15.0%. On the basis of a 2-sided log-rank test (α = .05) the study had greater than 80% statistical power to detect hazards ratios (HRs) greater than 1.64 or less than 0.61 based on a target recruited sample of 1862 and a final evaluable sample of 1490, assuming a 20% attrition rate. A 2-sided P < .05 was considered to be statistically significant.
Total follow-up ended at 13 months to detect events reported in the 1 month after completion that might be affected by treatment. Follow-up included 2 components: active follow-up, while patients were actively participating in study assessments, and passive follow-up, when patients discontinued active participation but when events could be identified through surveillance of VA electronic medical records.
The complete protocol, plan of analysis, and futility analysis are available in Supplement 1.
Results
Patient Characteristics
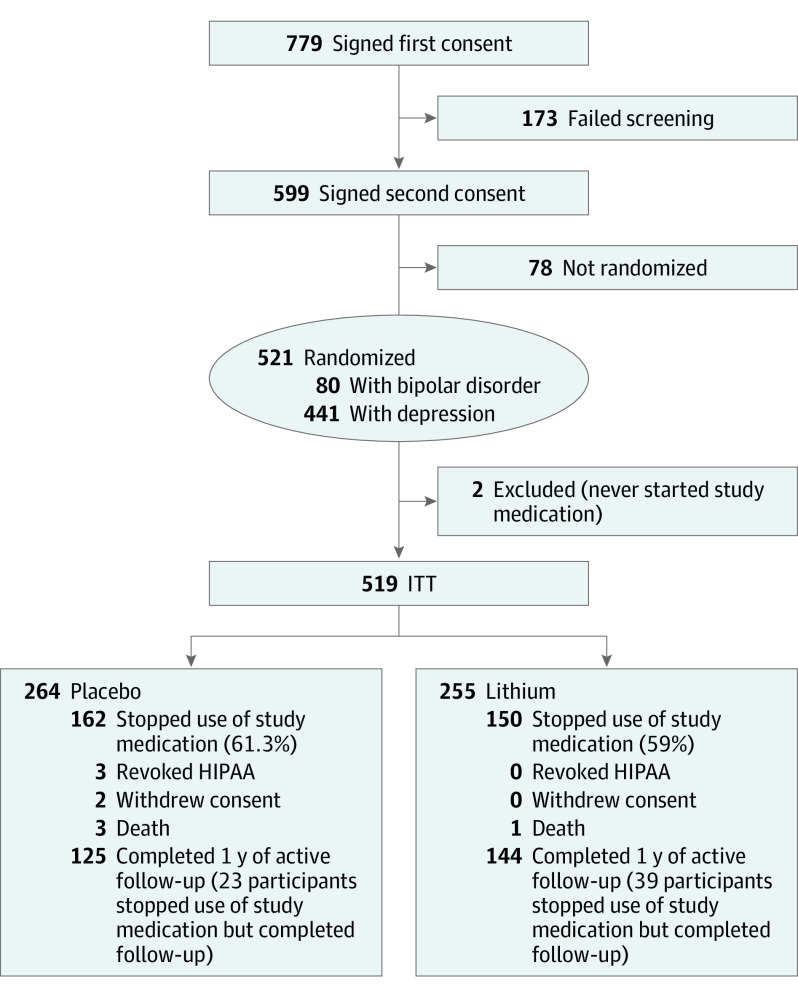
A total of 21 887 veterans with recent suicidal behavior or hospitalization were identified from 29 VA medical centers through electronic medical record data. Of these, 779 were eligible for and consented to a second screening, and 521 (66.9%) consented and were randomized (Figure 1; eFigure in Supplement 2).
Figure 1. CONSORT Flow Diagram.
HIPAA indicates Health Insurance Portability and Accountability Act; ITT, intention to treat.
Randomized participants were similar across a number of demographic characteristics, comorbid illnesses, mental health conditions, and rating scale values (Table 1). A total of 439 (84.6%) had major depression and 80 (15.4%) had bipolar disorder, but the treatment groups were balanced. Mean (SD) treatment exposure was 6.7 (4.5) months for participants with major depression and 5.6 (4.6) for participants with bipolar disorder. Overall mean (SD) lithium levels, including titration, were mean 0.42 (0.29) mEq/L, with means (SDs) at 3 months of 0.54 (0.25) mEq/L for patients with bipolar disorder and 0.46 (0.30) mEq/L for patients with major depressive disorder (n = 255; P = .11).
Table 1. Study Population and Baseline Measurementsa.
| Characteristic | Total (N = 519) | Lithium (n = 255) | Placebo (n = 264) |
|---|---|---|---|
| Age, mean (SD) [range], y | 42.8 (12.4) [20-72] | 43.2 (12.4) [21-72] | 42.4 (12.4) [20-71] |
| Age group, y | |||
| <36 | 181 (34.9) | 81 (31.8) | 100 (37.9) |
| 36-64 | 314 (60.5) | 163 (63.9) | 151 (57.2) |
| >64 | 24 (4.6) | 11 (4.3) | 13 (4.9) |
| Sex | |||
| Male | 437 (84.2) | 212 (83.1) | 225 (85.2) |
| Female | 82 (15.8) | 43 (16.9) | 39 (14.8) |
| Race | |||
| American Indian | 9 (1.7) | 5 (2.0) | 4 (1.5) |
| Asian | 5 (1.0) | 4 (1.6) | 1 (0.4) |
| Black or African American | 83 (16.0) | 39 (15.3) | 44 (16.7) |
| Native Hawaiian, Pacific Islander, or Maori | 7 (1.3) | 4 (1.6) | 3 (1.1) |
| White | 377 (72.6) | 185 (72.5) | 192 (72.7) |
| Multiple selected | 18 (3.5) | 10 (3.9) | 8 (3.0) |
| Unknown or not stated | 20 (3.9) | 8 (3.1) | 12 (4.5) |
| Ethnicity | |||
| Hispanic or Latino | 77 (14.8) | 35 (13.7) | 42 (15.9) |
| Not Hispanic or Latino | 437 (84.2) | 220 (86.3) | 217 (82.2) |
| Unknown or not stated | 5 (1.0) | 0 | 5 (1.9) |
| Educational level | |||
| Less than high school diploma | 3 (0.6) | 2 (0.8) | 1 (0.4) |
| High school diploma or GED | 106 (20.4) | 54 (21.2) | 52 (19.7) |
| Some college credit but no degree | 235 (45.3) | 105 (41.2) | 130 (49.2) |
| Associate’s degree | 79 (15.2) | 43 (16.9) | 36 (13.6) |
| Bachelor’s degree | 58 (11.2) | 30 (11.8) | 28 (10.6) |
| Master’s degree | 35 (6.7) | 18 (7.1) | 17 (6.4) |
| PhD or professional degree | 3 (0.6) | 3 (1.2) | 0 |
| Marital status | |||
| Married | 165 (31.8) | 88 (34.5) | 77 (29.2) |
| Divorced | 216 (41.6) | 99 (38.8) | 117 (44.3) |
| Never married | 106 (20.4) | 52 (20.4) | 54 (20.5) |
| Cohabitating | 17 (3.3) | 10 (3.9) | 7 (2.7) |
| Unknown or not stated | 3 (0.6) | 2 (0.8) | 1 (0.4) |
| Sexual orientation | |||
| Heterosexual | 450 (86.7) | 224 (87.8) | 226 (85.6) |
| Homosexual | 38 (7.3) | 19 (7.5) | 19 (7.2) |
| Bisexual | 18 (3.5) | 5 (2.0) | 13 (4.9) |
| Unknown or not stated | 13 (2.5) | 7 (2.7) | 6 (2.3) |
| Physical examination findings | 511 (98.5) | 252 (98.8) | 259 (98.1) |
| Tremor | 58 (11.4) | 22 (8.7) | 36 (13.9) |
| Thyroid enlargement | 5 (1.0) | 3 (1.2) | 2 (0.8) |
| Vital signs collected | 514 (99.0) | 253 (99.2) | 261 (98.9) |
| BMI, mean (SD) [range] | 30.1 (6.6) [17.3-73.1] | 30.3 (6.6) [17.7-58.3] | 30 (6.6) [17.3-73.1] |
| Mental health history | |||
| Bipolar disorder | 80 (15.4) | 37 (14.5) | 43 (16.3) |
| Major depressive disorder | 439 (84.6) | 218 (85.5) | 221 (83.7) |
| Posttraumatic stress disorder | 310 (59.7) | 143 (56.1) | 167 (63.3) |
| Panic disorder | 46 (8.9) | 26 (10.2) | 20 (7.6) |
| Generalized anxiety disorder | 141 (27.2) | 59 (23.1) | 82 (31.1) |
| Alcohol abuse or dependence | 251 (48.4) | 119 (46.7) | 132 (50.0) |
| Other substance abuse or dependence | 189 (36.4) | 88 (34.5) | 101 (38.3) |
| Personality disorder | 67 (12.9) | 32 (12.5) | 35 (13.3) |
| Other mental disorders | 126 (24.3) | 76 (29.8) | 50 (18.9) |
| Other depressive diagnoses | 113 (21.8) | 57 (22.4) | 56 (21.2) |
| No. of lifetime attempts, median (IQR) | 2 (1-3) | 2(1-3) | 2 (1-3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GED, General Educational Development.
Data are presented as number (percentage) of participants unless otherwise indicated.
Primary Study Outcome
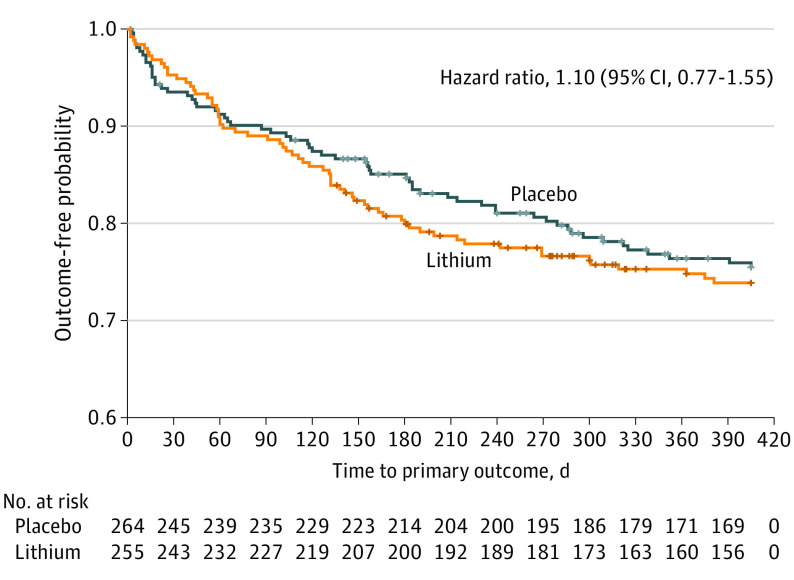
The trial was stopped for futility after 519 participants were randomized: 255 to lithium and 264 to placebo. Demographic characteristics of the participants included mean (SD) age, 42.8 (12.4) years; 437 (84.2%) male; 9 (1.7%) American Indian; 5 (1.0%) Asian; 83 (16.0%) Black or African American; 7 (1.3%) Native Hawaiian, Pacific Islander, or Maori; 377 (72.6%) White; 18 (3.5%) with multiple race selected; 20 (3.9%) of race unknown or not stated; 77 (14.8%) Hispanic or Latino; 437 (84.2%) not Hispanic or Latino; and 5 (1.0%) of ethnicity unknown or not stated. The mean (SD) total follow-up was 313 (134) days for the lithium group and 320 (133) days for the placebo group, and mean (SD) active follow-up was 272 (150) days for the lithium group and 266 (152) days for the placebo group. Of 429 candidate events considered by the end points committee, 197 events in 127 participants were adjudicated to be outcomes (suicide-related events). The first events in these 127 participants were primary outcomes (Table 2): 21 suicide attempts (suicidal self-directed violence), 28 interrupted suicide attempts (interrupted suicidal self-directed violence), 73 hospitalizations to prevent suicide, and 4 others (3 for which the end points committee determined that there was ambiguous evidence of intent and that the events should be considered undetermined self-directed violence and 1 for which the committee agreed that the event should be considered an outcome but for which there was disagreement about the classification). Of 255 participants randomized to receive lithium, 65 (25.5%) had primary outcome events; among 264 receiving placebo, 62 (23.5%) had primary outcome events. Overall, no treatment difference was found between lithium and placebo for the primary outcome (Figure 2) (log-rank test: HR, 1.10; 95% CI, 0.77-1.55; P = .61).
Table 2. Patient Outcomes by Treatment and Psychiatric Diagnosis: 127 Primary Outcomes and 70 Subsequent Events Among the Same Participants.
| Characteristic | Total No. | No. (%) | |
|---|---|---|---|
| Lithium (n = 255) | Placebo (n = 264) | ||
| Primary outcomes: first and subsequent events | 197 | 96 (48.7) | 101 (51.3) |
| Bipolar disorder | 30 | 10 | 20 |
| Major depressive disorder | 167 | 86 | 81 |
| Primary outcomes | 127 | 65 (25.5) | 62 (23.5) |
| Bipolar disorder | 16 | 7 | 9 |
| Major depressive disorder | 111 | 53 | 58 |
| Classes of suicidal behavior outcome events | |||
| Suicidal self-directed violence | 21 | 11 (4.3) | 10 (3.8) |
| Bipolar disorder | 2 | 1 | 1 |
| Major depressive disorder | 19 | 10 | 9 |
| Interrupted suicidal self-directed violence | 28 | 17 (6.7) | 11 (4.2) |
| Bipolar disorder | 4 | 2 | 2 |
| Major depressive disorder | 24 | 15 | 9 |
| Hospitalization to prevent suicide | 73 | 34 (13.3) | 39 (14.8) |
| Bipolar disorder | 10 | 4 | 6 |
| Major depressive disorder | 63 | 30 | 33 |
| Death from suicidea | 1 | 1 (0.4) | 0 |
| Bipolar disorder | 0 | 0 | 0 |
| Major depressive disorder | 1 | 1 | 0 |
| Otherb | 4 | 2 (0.8) | 2 (0.8) |
| Bipolar disorder | 0 | 0 | 0 |
| Major depressive disorder | 4 | 2 | 2 |
This table excludes 2 deaths by suicide in the placebo group that are discussed in the text. One participant had already experienced a primary outcome event. The other was identified through a search of data derived from the National Death Index after study data collection was closed.
There was consensus among the adjudicators that all the above events should be considered outcomes. For 3 participants, there was ambiguous evidence about the participants’ intent to die, and the consensus classification of the primary event was undetermined self-directed violence. For a fourth participant, there was ambiguous evidence about the degree of risk associated with the participant’s behavior that would allow the distinction between a suicide attempt and an interrupted attempt, and the consensus classification of the primary outcome was none of the above.
Figure 2. Time to Primary Outcome in the Lithium and Placebo Groups.
Cessation of Study Medication Use
Participants taking lithium or placebo who stopped treatment during the study had an increased rate of suicide-related events (HR, 2.86; 95% CI, 1.48-5.53; P = .007) without any difference between the 2 groups (HR, 1.11; 95% CI, 0.78-1.57; P = .55) (Table 3).
Table 3. Hazard Ratios for Tests Comparing Treatment (Lithium to Placebo) and Other Covariates.
| Outcome | Outcome events within covariate by treatment | Covariates | Hazard ratio (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|
| Lithium | Placebo | ||||||
| No./total No. | Event rate, % | No./total No. | Event rate, % | ||||
| Model 1: treatment only: unadjusted for other covariates | |||||||
| Primary outcome | 65/255 | 25 | 62/264 | 23 | Treatment | 1.10 (0.77-1.55) | .61 |
| Model 2: treatment only: unadjusted with site as a random effect | |||||||
| Primary outcome | 65/255 | 25 | 62/264 | 23 | Treatment | 1.08 (0.76-1.53) | .67 |
| Model 3: treatment and adjustment for stopping use of study medication while in the studya | |||||||
| Primary outcome | |||||||
| Stop | 59 | 31 | 40 | 35 | Stop | 2.86 (1.48-5.53) | .007 |
| No stop | 196 | 24 | 224 | 21 | Treatment | 1.11 (0.78-1.57) | .55 |
| Model 4: treatment and adjustment for stopping use of study medication while in the study a | |||||||
| Ceased attending study visits (dropout) | |||||||
| Stop | 59 | 21 | 40 | 27 | Stop | 1.62 (0.94-2.79) | .09 |
| No stop | 196 | 22 | 224 | 20 | Treatment | 1.24 (0.87-1.77) | .24 |
| Models 5 to 8: unadjusted, classes of the primary outcomes | |||||||
| Self-directed violence | 11/255 | 4 | 10/264 | 4 | Treatment | 1.14 (0.48-2.69) | .77 |
| Interrupted self-directed violence | 17/255 | 7 | 11/264 | 4 | Treatment | 1.61 (0.75-3.43) | .22 |
| Hospitalization to prevent suicide | 34/255 | 13 | 39/264 | 15 | Treatment | 0.92 (0.58-1.45) | .71 |
| Death from suicide | Too few deaths | ||||||
| Model 9: subgroup with ≥80% adherence | |||||||
| Primary outcome | 12/46 | 26 | 8/42 | 19 | Treatment | 1.49 (0.61-3.64) | .38 |
Models 3 and 4 have 2 covariates: treatment and medication use stop. The separate columns for lithium and placebo display the outcome event rates broken down by the categories stop and no stop.
Mental Health Symptoms
No difference in mental health symptoms was found in baseline scores on the standardized instruments33,34,35,36,37,38 between the treatment groups. Baseline scores for the Columbia–Suicide Severity Rating Scale33 and the Patient Health Questionnaire 934 but not the activation subscale of the Internal State Scale,35 Barratt Impulsiveness Scale,36 or Buss-Perry Aggression Questionnaire37 significantly estimated the primary outcome. Repeated-measures analyses for the Columbia–Suicide Severity Rating Scale, the Patient Health Questionnaire 9, and the activation subscale of the Internal State Scale identified no lithium-placebo differences over time.
Usual VA Mental Health Care
Study medications were added to usual VA mental health care, including medications and psychosocial treatment for mental health conditions and a range of rehabilitation- and recovery-oriented services. Participants in both study assignment groups had a mean (SD) of 1.15 (0.23) mental health service visits per month, without differences in treatment group, and 10 to 12 study visits during the year.
Per-Protocol Analyses
Only 1074 of 2154 lithium concentrations (49.9%) were 0.5 mEq/L or greater. Only 88 of 519 participants (17.0%) took 80% or more of their study medication (46 in the lithium group and 42 in the placebo group) and were considered substantially adherent. Twenty of these participants had primary outcomes (8 in the placebo group and 12 in the lithium group), a finding that was not significant and did not favor lithium treatment (HR, 1.49; 95% CI, 0.61-3.64) (Table 3).
In addition to assessing adherence, we gauged the success of double-blind procedures by asking participants and the practitioners who prescribed study medication to guess their treatment assignment at the end of the study. There was a higher tendency for participants taking lithium to be willing to guess (141 of 246 [57.3%] vs 116 of 256 [45.3%]) and to correctly guess their assignment. Among 151 participants taking lithium who made a guess, 96 (68.1%) were correct, whereas among 118 participants taking placebo who made a guess, only 57 (49.1%) were correct. Among 109 practitioners who made a guess for a participant taking lithium, 75 (68.8%) were correct, whereas among 89 practitioners who made a guess for a participant taking placebo, only 34 (38.2%) were correct.
Futility Analysis
As designed, the protocol called for an interim analysis when half of the planned sample was entered. Before that time, however, prompted by concerns about the rate of enrollment, the Data Monitoring Committee requested and reviewed a futility analysis (Supplement 1). At that time, after 43 months, there were 79 adjudicated primary outcomes. The futility analysis demonstrated that if study enrollment continued assuming existing recruitment rates for 2 more years, under the expected conditions of 37% fewer events in those treated with lithium, the probability of rejecting the null hypothesis would have been less than 10%. The Data Monitoring Committee recommended that the trial be stopped because of futility.
Safety
The incidence of serious adverse events was evenly distributed between the 2 groups. The most frequent serious adverse event was hospitalization to prevent suicide. Only 7 participants discontinued participation in the study because of serious adverse events. One developed lithium toxic effects. No serious cardiac arrhythmias or irreversible renal or thyroid abnormalities occurred. Nonserious adverse events were consistent with lithium’s well-known adverse event profile. Safety outcomes are reported in eTables 1 and 2 in Supplement 2.
Deaths
Four deaths occurred during the study, 3 in the first month of participation. One death occurred in the lithium group from a self-inflicted gunshot. Three deaths occurred in the placebo group. One, a suicide by self-inflicted gunshot, occurred after the participant had already experienced a primary outcome. Another was from an opioid overdose. The third occurred during the 13th month of study participation, but the cause could not be determined until 17 months after data collection was closed. The VA records and the National Death Index indicated that the cause of death was suicide by hanging, strangulation, or suffocation.
Discussion
To our knowledge, this is the largest randomized clinical trial of lithium to date that examines suicide-related behaviors as the primary outcome. Lauterbach et al23 and Girlanda et al25 studied patients with depression and found no significant effect of lithium. Oquendo et al24 studied patients with bipolar disorder and found no benefit of lithium over divalproex. None of these studies had adequate statistical power. The present double-blind, placebo-controlled study found no benefit of lithium over placebo for preventing or delaying suicide-related events (suicide attempts, interrupted attempts, hospitalizations to prevent attempts, or deaths from suicide) when it was added to usual VA mental health management.
Some issues require discussion. The study did not reach its original recruitment goal. One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/US Department of Defense Clinical Practice Guideline.10 Given that suicidal behavior occurs across diagnoses, the inclusion of patients with both major depression and bipolar disorder, including those with comorbidities, is a strength. However, the outcomes may have been sensitive to the distribution of demographic characteristics and psychiatric diagnoses in the study population. For example, the participants had a predominance of depression rather than bipolar disorder, the most common indication for lithium use, and most participants had substance use disorders, posttraumatic stress disorder, or both as comorbidities, possibly influencing outcomes. The study did not have enough participants to evaluate outcomes for patients with bipolar disorder, to test whether outcomes differed among patients with bipolar disorder and depression, or to assess whether comorbidities attenuated the effects of lithium. Data on responses to prior treatments to identify participants who were treatment resistant were insufficient.
The protocol increased participants’ contacts with the VA. Given that caring contacts, brief visits, educational sessions, postcards, letters, and telephone calls can prevent reattempts,38 the nonspecific elements of study participation may have affected outcomes. However, the estimate for rates of fatal and nonfatal suicide attempts and interrupted attempts based on historic VA electronic medical record data for the sample size calculations (15%) was substantially lower than the observed rate for primary outcomes, but it was higher than the observed rate of attempts and interrupted attempts (9.8%). In addition, the most frequent observed outcome was hospitalization to prevent suicidal behavior. All these findings are consistent with increased surveillance. Our finding that discontinuing use of study medication is associated with outcomes independent of treatment assignment may provide further evidence of the importance of nonspecific effects.
Limitations
This study has limitations, including high rates of attrition and low rates of substantial adherence with study medication, resulting in only approximately half (48.1%) of the serum lithium concentrations being 0.5 mEq/L or greater. Our findings are consistent with the 2013 meta-analysis27 that found no effect of lithium on nonfatal, deliberate self-harm, but we cannot address questions about whether lithium can prevent death by suicide.
Our findings are not necessarily generalizable to other health care settings or to other patient populations with differing proportions of individuals with bipolar disorder, lower rates of comorbidities, or higher treatment adherence. Our findings are particularly relevant to questions about outcomes for real-life patients and perhaps to speculations based on an ecologic study of lithium in drinking water40 that found that very low doses of lithium could be effective. Most important, our study suggests that in a population of patients with substantial comorbidities who are actively being treated for mood disorders and coexisting mental health or substance use disorders, simply adding lithium to existing medication regimens is unlikely to be effective for preventing an outcome that draws from a broad range of suicide-related events. However, lithium still has a role in the management of mood disorders, especially bipolar disorder.
Conclusions
This large randomized clinical trial of lithium treatment for suicidality did not find that lithium prevented suicide-related events when added to usual VA mental health care of veterans with major depressive disorder or bipolar disorder. Therefore, simply adding lithium to existing medication regimens is unlikely to be effective for preventing a broad range of suicide-related events in patients who are actively being treated for mood disorders and substantial comorbidities.
Trial Protocol and Statistical Analysis Plan
eTable 1. Nonserious Adverse Events by Treatment
eTable 2. Serious Adverse Events by Treatment
eFigure. CONSORT Diagram of Identification of Potentially Eligible Subjects
Nonauthor Collaborators. The Li+ plus Investigators
Data Sharing Statement
References
- 1.Hedegaard H, Curtin SC, Warner M. Suicide Mortality in the United States, 1999–2017. National Center for Health Statistics Data Brief 330. National Center for Health Statistics; 2018. [Google Scholar]
- 2.Bertolote JM, Fleischmann A. Suicide and psychiatric diagnosis: a worldwide perspective. World Psychiatry. 2002;1(3):181-185. [PMC free article] [PubMed] [Google Scholar]
- 3.Arsenault-Lapierre G, Kim C, Turecki G. Psychiatric diagnoses in 3275 suicides: a meta-analysis. BMC Psychiatry. 2004;4:37. doi: 10.1186/1471-244X-4-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilgen MA, Bohnert AS, Ignacio RV, et al. Psychiatric diagnoses and risk of suicide in veterans. Arch Gen Psychiatry. 2010;67(11):1152-1158. doi: 10.1001/archgenpsychiatry.2010.129 [DOI] [PubMed] [Google Scholar]
- 5.Too LS, Spittal MJ, Bugeja L, Reifels L, Butterworth P, Pirkis J. The association between mental disorders and suicide: a systematic review and meta-analysis of record linkage studies. J Affect Disord. 2019;259:302-313. doi: 10.1016/j.jad.2019.08.054 [DOI] [PubMed] [Google Scholar]
- 6.US Department of Veterans Affairs. National Veteran Suicide Prevention Annual Report. US Dept of Veterans Affairs; 2019. Accessed on May 27, 2020. https://www.mentalhealth.va.gov/suicide_prevention/data.asp
- 7.Veterans Affairs Office of Research and Development . VA research on suicide prevention. 2018. Accessed on July 13, 2020. https://www.research.va.gov/topics/suicide.cfm
- 8.Hawton K, Witt KG, Salisbury TL, et al. Psychosocial interventions for self-harm in adults. Cochrane Database Syst Rev. 2016;(5):CD012189. doi: 10.1002/14651858.CD012189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeCou CR, Comtois KA, Landes SJ. Dialectical behavior therapy is effective for the treatment of suicidal behavior: a meta-analysis. Behav Ther. 2019;50(1):60-72. doi: 10.1016/j.beth.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 10.US Department of Veterans Affairs and US Department of Defense. VA/DoD clinical practice guidelines assessment and management of patients at risk for suicide. 2019. Accessed May 27 2020. https://www.healthquality.va.gov/guidelines/MH/srb
- 11.D’Anci KE, Uhl S, Giradi G, Martin C. Treatments for the prevention and management of suicide: a systematic review. Ann Intern Med. 2019;171(5):334-342. doi: 10.7326/M19-0869 [DOI] [PubMed] [Google Scholar]
- 12.Meltzer HY, Alphs L, Green AI, et al. ; International Suicide Prevention Trial Study Group . Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91. doi: 10.1001/archpsyc.60.1.82 [DOI] [PubMed] [Google Scholar]
- 13.Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335. doi: 10.1176/appi.ajp.2017.17060647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson and Johnson . Janssen announces U.S. FDA Approval of SPRAVATO® (esketamine) CIII nasal spray to treat depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Accessed on August 28, 2020. https://www.jnj.com/janssen-announces-u-s-fda-approval-of-spravato-esketamine-ciii-nasal-spray-to-treat-depressive-symptoms-in-adults-with-major-depressive-disorder-with-acute-suicidal-ideation-or-behavior
- 15.Gibbons R, Hur K, Lavigne J, et al. Medications and suicide: High Dimensional Empirical Bayes Screening (iDEAS). Harv Data Sci Rev. 2019;1:2. doi: 10.1162/99608f92.6fdaa9de [DOI] [Google Scholar]
- 16.Gibbons RD, Brown CH, Hur K, Davis J, Mann JJ. Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):580-587. doi: 10.1001/archgenpsychiatry.2011.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone M, Laughren T, Jones ML, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ. 2009;339:b2880. doi: 10.1136/bmj.b2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand. 2001;104(3):163-172. doi: 10.1034/j.1600-0447.2001.00464.x [DOI] [PubMed] [Google Scholar]
- 19.Lewitzka U, Severus E, Bauer R, Ritter P, Müller-Oerlinghausen B, Bauer M. The suicide prevention effect of lithium: more than 20 years of evidence-a narrative review. Int J Bipolar Disord. 2015;3(1):32. doi: 10.1186/s40345-015-0032-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term medication. J Affect Disord. 1992;25(4):261-269. doi: 10.1016/0165-0327(92)90084-J [DOI] [PubMed] [Google Scholar]
- 21.Bocchetta A, Ardau R, Burrai C, Chillotti C, Quesada G, Del Zompo M. Suicidal behavior on and off lithium prophylaxis in a group of patients with prior suicide attempts. J Clin Psychopharmacol. 1998;18(5):384-389. doi: 10.1097/00004714-199810000-00006 [DOI] [PubMed] [Google Scholar]
- 22.Smith EG, Austin KL, Kim HM, et al. Suicide risk in Veterans Health Administration patients with mental health diagnoses initiating lithium or valproate: a historical prospective cohort study. BMC Psychiatry. 2014;14:357. doi: 10.1186/s12888-014-0357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauterbach E, Felber W, Müller-Oerlinghausen B, et al. Adjunctive lithium treatment in the prevention of suicidal behaviour in depressive disorders: a randomised, placebo-controlled, 1-year trial. Acta Psychiatr Scand. 2008;118(6):469-479. doi: 10.1111/j.1600-0447.2008.01266.x [DOI] [PubMed] [Google Scholar]
- 24.Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056. doi: 10.1176/appi.ajp.2011.11010163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girlanda F, Cipriani A, Agrimi E, et al. Effectiveness of lithium in subjects with treatment-resistant depression and suicide risk: results and lessons of an underpowered randomised clinical trial. BMC Res Notes. 2014;7:731. doi: 10.1186/1756-0500-7-731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819. doi: 10.1176/appi.ajp.162.10.1805 [DOI] [PubMed] [Google Scholar]
- 27.Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646 [DOI] [PubMed] [Google Scholar]
- 28.Riblet NBV, Shiner B, Young-Xu Y, Watts BV. Strategies to prevent death by suicide: meta-analysis of randomised controlled trials. Br J Psychiatry. 2017;210(6):396-402. doi: 10.1192/bjp.bp.116.187799 [DOI] [PubMed] [Google Scholar]
- 29.Roberts E, Cipriani A, Geddes JR, Nierenberg AA, Young AH. The evidence for lithium in suicide prevention. Br J Psychiatry. 2017;211(6):396. doi: 10.1192/bjp.211.6.396 [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. American Psychiatric Association; 2000. [Google Scholar]
- 31.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(6):734-739. doi: 10.1176/ajp.140.6.734 [DOI] [PubMed] [Google Scholar]
- 32.Jeste DV, Palmer BW, Appelbaum PS, et al. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry. 2007;64(8):966-974. doi: 10.1001/archpsyc.64.8.966 [DOI] [PubMed] [Google Scholar]
- 33.Posner K, Brown GK, Stanley B, et al. The Columbia–Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauer MS, Crits-Christoph P, Ball WA, et al. Independent assessment of manic and depressive symptoms by self-rating. Scale characteristics and implications for the study of mania. Arch Gen Psychiatry. 1991;48(9):807-812. doi: 10.1001/archpsyc.1991.01810330031005 [DOI] [PubMed] [Google Scholar]
- 36.Stanford MS, Mathias CW, Dougherty DM, et al. Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers Individ Dif. 2009;47:385–395. doi: 10.1016/j.paid.2009.04.008 [DOI] [Google Scholar]
- 37.Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63(3):452-459. doi: 10.1037/0022-3514.63.3.452 [DOI] [PubMed] [Google Scholar]
- 38.Milner AJ, Carter G, Pirkis J, Robinson J, Spittal MJ. Letters, green cards, telephone calls and postcards: systematic and meta-analytic review of brief contact interventions for reducing self-harm, suicide attempts and suicide. Br J Psychiatry. 2015;206(3):184-190. doi: 10.1192/bjp.bp.114.147819 [DOI] [PubMed] [Google Scholar]
- 39.Crosby AE, Ortega L, Melanson C. Self-directed Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2011. [Google Scholar]
- 40.Memon A, Rogers I, Fitzsimmons SMDD, et al. Association between naturally occurring lithium in drinking water and suicide rates: systematic review and meta-analysis of ecological studies. Br J Psychiatry. 2020;217(6):667-678. doi: 10.1192/bjp.2020.128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Nonserious Adverse Events by Treatment
eTable 2. Serious Adverse Events by Treatment
eFigure. CONSORT Diagram of Identification of Potentially Eligible Subjects
Nonauthor Collaborators. The Li+ plus Investigators
Data Sharing Statement