Abstract
cagA+ Helicobacter pylori strains have been linked to more severe gastric inflammation, peptic ulcer disease, and gastric cancer in adults, but there have been few studies of cagA in children. We examined the relationship between H. pylori cagA status and clinical status in Japanese children. Forty H. pylori-positive children were studied: 15 with nodular gastritis, 5 with gastric ulcers, and 20 with duodenal ulcers. H. pylori status was confirmed by biopsy-based tests and serum anti-H. pylori immunoglobulin G (IgG) antibody. As controls, 77 asymptomatic children with sera positive for anti-H. pylori IgG were enrolled. Levels of IgG antibodies to CagA in serum were measured by an antigen-specific enzyme-linked immunosorbent assay. In 16 patients with successful H. pylori eradication, posttreatment levels of CagA and H. pylori IgG antibodies also were studied. The CagA antibody seropositivities of asymptomatic controls (81.8%) and patients with nodular gastritis, gastric ulcers, and duodenal ulcers (80.0 to 95.0%) were not significantly different. Compared with pretreatment levels of CagA antibodies, posttreatment levels decreased progressively and significantly. We conclude that, as in Japanese adults, a high prevalence of cagA+ H. pylori strains was found in Japanese children, and that there was no association with nodular gastritis or peptic ulcer disease. In the assessment of eradicative therapies, monitoring of serum anti-CagA antibodies does not appear to offer any direct benefit over monitoring of anti-H. pylori antibodies.
It is widely recognized that colonization with Helicobacter pylori induces a persistent gastric tissue response and is an important risk factor for peptic ulcer disease and gastric cancer (4). However, the majority of H. pylori-positive persons are asymptomatic throughout their lifetime, and it is not known why only a subset of positive patients develop ulcer disease and cancer. Variation in clinical outcomes has been attributed to differences in bacterial strains, hosts, and environmental factors.
H. pylori strains are genetically diverse (13, 33). Although of unknown function, the cytotoxin-associated gene A (cagA) has been identified as a possible marker of virulence of H. pylori (5). Since the cytotoxin-associated gene product (CagA, 120 to 140 kDa) encoded by cagA is immunodominant (10, 34), a specific immune response to the CagA protein is induced as long as H. pylori colonization persists (6). Therefore, serum immunoglobulin G (IgG) antibodies to the CagA antigen may be a reliable marker of carriage of a cagA+ H. pylori strain (10, 12) which includes the cag pathogenicity island (9, 35). In Western populations, cagA+ H. pylori strains induce more severe gastric mucosal inflammation than cagA gene-negative strains (10, 15, 20) and are associated with higher risks of peptic ulcer disease (11, 12, 15) and gastric cancer (6, 16). However, there is wide geographical variation in the prevalence of cagA+ strains (1, 29, 37) and in their genotype (28), and it is unknown whether cagA+ strains represent a universal marker for these H. pylori-associated diseases. Among adults in Japan, there is no clear relationship between cagA+ H. pylori strains and enhanced risk of disease (21).
Childhood is the critical period for acquisition of H. pylori (2, 27). As in adults, H. pylori appears to be associated with both a tissue response (gastritis) and duodenal ulcer in children (32). However, there have been few studies of CagA seroprevalence in children (7, 20), and its role in peptic ulcer disease has not been studied. In this study, we examined whether H. pylori CagA status was associated in Japanese children with nodular gastritis, which is a unique endoscopic characteristic in childhood (18, 24), and with peptic ulcer disease.
MATERIALS AND METHODS
Patients.
A total of 40 H. pylori-positive dyspeptic patients were enrolled in this study: 20 patients with duodenal ulcer, 5 with gastric ulcer, and 15 with nodular gastritis alone (Table 1). Diagnoses were determined on the basis of findings by upper gastrointestinal endoscopy. Nodular gastritis, defined as endoscopically proven multiple nodularity in the antrum with lymphoid follicles and inflammatory cell infiltration in the lamina propria, is believed to be the major form of H. pylori gastritis in childhood (18, 24). The patients selected had no underlying diseases and were not taking medications, including nonsteroidal anti-inflammatory drugs. H. pylori status was assessed by biopsy-based tests (rapid biopsy urease test, histology, and culture) and testing for the presence of serum anti-H. pylori IgG antibody with a commercial enzyme-linked immunosorbent assay (ELISA) kit (HM-CAP; Enteric Products, Inc., Westbury, N.Y.). In adults, because H. pylori is often difficult to isolate in culture, nonculture techniques (histology, rapid biopsy urease test, serology, or urea breath test) are performed for diagnosing H. pylori infection (17). Our previous studies have demonstrated that compared with biopsy tests, the sensitivity of anti-H. pylori IgG and IgA antibodies were 88.2 and 91.2%, respectively (22). Even when H. pylori has not been cultured, the presence of the organism can be confirmed by a combination of these techniques. As controls, 77 asymptomatic children with positive anti-H. pylori IgG tests, who did not undergo endoscopy, were enrolled into this study. All sera were stored at −20°C until assay. Sixteen patients who received eradication therapy (proton pump inhibitor-based dual or triple regimens) and had successful eradication of H. pylori (23, 24) were studied at serial intervals. In these patients, pretreatment and posttreatment levels of H. pylori IgG antibodies were measured by using HM-CAP. Serum samples were taken pretreatment and at 3, 6, and 12 months after completion of eradication therapy. Informed consent was obtained from patients or their parents in all cases.
TABLE 1.
Characteristics of 117 study patients
| Clinical status | No. of patients
|
Mean age (yr) ± SD (range) | ||
|---|---|---|---|---|
| Male | Female | Total | ||
| Gastric ulcer | 2 | 3 | 5 | 12.2 ± 4.5 (4–16) |
| Duodenal ulcer | 16 | 4 | 20 | 12.3 ± 2.6 (5–16) |
| Nodular gastritis | 5 | 10 | 15 | 14.1 ± 1.4 (11–16) |
| Asymptomatic control | 33 | 44 | 77 | 12.0 ± 3.5 (3–16) |
| Total | 56 | 61 | 117 | 12.4 ± 3.3 (3–16) |
CagA antibodies.
Serum anti-CagA IgG antibody levels were assayed as previously described (6). Briefly, a recombinant protein fragment of CagA (ORV220; OraVax, Inc., Cambridge, Mass.) that was purified from Escherichia coli cell lysates was used as an antigen and was fixed to a 96-well plate in carbonate-bicarbonate buffer. After incubation of treated wells with serum diluted 1:100, alkaline phosphatase-conjugated goat anti-human IgG (1:1,000 dilution) was added. After addition of the phosphatase substrate, absorbance was read at 405 nm. Based on results from H. pylori-negative controls in adults, a value of ≥0.2 ELISA unit (EU) of CagA IgG antibodies was considered to be positive (21).
Statistical methods.
The difference in CagA seropositivity between asymptomatic controls and patients with each of the three clinical diagnoses was analysed by Fisher's exact test. Differences between pretreatment and posttreatment levels of CagA and differences between posttreatment levels of CagA and H. pylori IgG antibodies were analyzed by the paired t test. A value of P < 0.05 was regarded as statistically significant. Values were presented as means ± standard deviations.
RESULTS
CagA seropositivity.
Among the H. pylori-positive children in this study, a high percentage in each group were seropositive for anti-CagA IgG antibodies (Table 2). There were no significant differences in seropositivity rates and levels of CagA antibodies between asymptomatic controls and patients with ulcer disease or nodular gastritis.
TABLE 2.
Prevalence and levels of serum anti-CagA antibody in H. pylori-positive controls and patients
| Clinical status | No. of patients | % CagA antibody positive (95% CI)a | Pb | CagA level (EU)b | Pc |
|---|---|---|---|---|---|
| Gastric ulcer | 5 | 80.0 (44.9–100) | 1.00 | 1.2 ± 0.7 | 0.90 |
| Duodenal ulcer | 20 | 95.0 (85.5–100) | 0.18 | 1.2 ± 0.9 | 0.87 |
| Nodular gastritis | 15 | 93.3 (80.7–100) | 0.45 | 0.9 ± 0.5 | 0.10 |
| Asymptomatic control | 77 | 81.8 (73.2–90.4) | 1.3 ± 0.8 |
CI, confidence interval.
Compared with asymptomatic controls.
Among children who were CagA antibody positive.
Effect of treatment on CagA antibody levels.
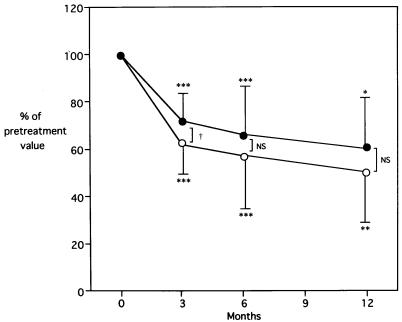
All 16 patients who had successful eradication were seropositive for CagA antibodies before therapy. In these patients, the mean pretreatment level of anti-CagA antibodies was 1.0 ± 0.6 EU. Posttreatment levels were significantly decreased at 3 months (0.7 ± 0.5 EU; P < 0.001), at 6 months (0.6 ± 0.4 EU; P < 0.001), and at 12 months (0.5 ± 0.3 EU; P < 0.01), compared with the pretreatment levels (Fig. 1). Nevertheless, by using the threshold of 0.2 EU as the indicator of seropositivity, 8 (64%) of 11 patients studied remained seropositive even at 12 months after therapy. At 3 months posttreatment, the percent decrease in CagA antibody levels was greater than that in H. pylori IgG levels (P < 0.05).
FIG. 1.
Levels of serum anti-CagA (open circles) and anti-H. pylori IgG antibodies (solid circles) after eradication therapy in 16 patients. Mean posttreatment levels at specified follow-up times are expressed as percentages of pretreatment levels. Error bars, standard deviations. ∗, P < 0.05 versus pretreatment level; ∗∗, P < 0.01; ∗∗∗, P < 0.001. NS, not significant. †, P < 0.05, comparing anti-H. pylori and anti-CagA antibodies.
DISCUSSION
In Western countries, patients with duodenal ulcer are more likely to be colonized by cagA+ H. pylori strains than patients with gastritis alone (10, 11, 15). In the West, H. pylori strains that are cagA+ also are associated with more-substantial gastric tissue involvement with increased neutrophil infiltration, in adults (10, 15) and in children (20). Similarly, in gastric mucosa from persons colonized with cagA+ strains, increased gastric epithelial cell interleukin-8 (IL-8) mRNA (38) and IL-8 secretion (14, 31) have been observed. However, similarly close correlations have not been universally observed (19). In addition, among Swedish children and adolescents, there was no significant correlation between degree of gastric inflammation and H. pylori cytotoxin production (8).
In Western patients, CagA seropositivity has been shown to be higher in those who have atrophic gastritis, which is a precursor to gastric cancer (3, 26). Although cagA+ strains may play a role in the development of gastric cancer, they are neither necessary nor sufficient for this process (6). In Japan, high CagA seropositivity rates have been observed in asymptomatic adults, reinforcing the insufficiency of carriage of a cagA+ strain for gastric cancer development (21). CagA seroprevalence varies geographically (1, 29, 37). The frequency of cagA+ strains observed in asymptomatic children has ranged from 76% in Mexico (7) to 40% in France (20). In Japan, cagA+ H. pylori strains are common in persons of all ages. Thus, available evidence from this and previous studies (1, 28, 29, 37) suggests that cagA+ strains are not significant as disease-specific pathogenetic markers.
Allelic differences within cagA that distinguish Western and East Asian cagA+ H. pylori strains have been reported (28, 36). These differences may reflect variation in other parts of the cag pathogenicity island, which has been considered to encode potential virulence-related genes. It is possible that Asian cagA+ H. pylori strains do not induce the accentuated tissue damage caused by Western cagA+ strains. In any event, both host and bacterial factors should be considered in order to understand the pathogenesis of H. pylori-associated diseases.
As a noninvasive assay, determination of serum CagA IgG antibody levels may be useful in the assessment of eradicative therapy, as is evaluation of H. pylori IgG antibody levels (25, 30). In this study, levels of serum CagA IgG antibodies decreased significantly after eradication of H. pylori using antimicrobial therapy. However, most successfully treated patients remained CagA seropositive for months during the follow-up period. Similarly, seroconversion to negativity for H. pylori IgG and IgA antibodies requires about 12 months after successful eradication (22). Thus, the humoral immune responses, not only to H. pylori group antigens but also to the CagA protein, do not quickly disappear after the organism is eliminated. Assessing anti-CagA antibodies as a marker for eradication does not appear to offer any direct benefit over use of anti-H. pylori antibodies alone.
ACKNOWLEDGMENTS
We thank Takuji Fujisawa (Kurume, Japan), Hitoshi Tajiri (Osaka, Japan), Mutsuko Konno (Sapporo, Japan), and Shun-ichi Maisawa (Morioka, Japan) for providing the sera of patients and controls.
This work was supported in part by the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Atherton J C. Genotypic analysis of vacA and cagA in Helicobacter pylori isolates from the U.S., Thailand, Peru, and China. Gut. 1995;37(Suppl. 1):A69. [Google Scholar]
- 2.Banatvala N, Mayo K, Megraud F, Jennings R, Deeks J J, Feldman R A. The cohort effect and Helicobacter pylori. J Infect Dis. 1993;168:219–221. doi: 10.1093/infdis/168.1.219. [DOI] [PubMed] [Google Scholar]
- 3.Beales I L P, Crabtree J E, Scunes D, Covacci A, Calam J. Antibodies to CagA protein are associated with gastric atrophy in Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1996;8:645–649. [PubMed] [Google Scholar]
- 4.Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 5.Blaser M J. Role of vacA and the cagA locus of Helicobacter pylori in human disease. Aliment Pharmacol Ther. 1996;10(Suppl. 1):73–77. doi: 10.1046/j.1365-2036.1996.22164008.x. [DOI] [PubMed] [Google Scholar]
- 6.Blaser M J, Perez-Perez G I, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stemmermann G N, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 7.Camorlinga-Ponce M, Torres J, Perez-Perez G, Leal-Herrera B, Gonzalez-Ortiz B, Madrazo de la Garza A, Gomez A, Munoz O. Validation of a serologic test for the diagnosis of Helicobacter pylori infection and the immune response to urease and CagA in children. Am J Gastroenterol. 1998;93:1264–1270. doi: 10.1111/j.1572-0241.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- 8.Celik J, Su B, Tiren U, Finkel Y, Thoresson A C, Engstrand L, Sandstedt B, Bernander S, Normark S. Virulence and colonization-associated properties of Helicobacter pylori isolated from children and adolescents. J Infect Dis. 1998;177:247–252. doi: 10.1086/517365. [DOI] [PubMed] [Google Scholar]
- 9.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiarn P, Borodovsky M, Rappouli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5793. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cover T L, Dooley C P, Blaser M J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990;58:603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cover T L, Glupczynski Y, Lage A P, Burette A, Tummuru M R, Perez-Perez G I, Blaser M J. Serologic detection of infection with cagA+Helicobacter pylori strains. J Clin Microbiol. 1995;33:1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cover T L, Tummuru M K R, Cao P, Thompson S A, Blaser M J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 14.Crabtree J E, Covacci A, Farmery S M, Xiang Z, Tompkins D S, Perry S, Lindley I J D, Rappouli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabtree J E, Taylor J D, Wyatt J I, Heatley R V, Shallcross T M, Tompkins D S, Rathbone B J. Mucosal IgA recognition of Helicobacter pylori 120-kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 16.Crabtree J E, Wyatt J I, Sobala G M, Miller G, Tompkins D S, Primrose J N, Morgan A G. Systemic and mucosal humoral responses to Helicobacter pylori in gastric cancer. Gut. 1993;34:1339–1343. doi: 10.1136/gut.34.10.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cutler A F, Havstad S, Ma C K, Blaser M J, Perez-Perez G I, Schubert T T. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995;109:136–141. doi: 10.1016/0016-5085(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 18.Czinn S, Dahms B, Jacobs H H, Kaplan B, Rothstein F C. Campylobacter-like organisms in association with symptomatic gastritis in children. J Pediatr. 1986;109:80–83. doi: 10.1016/s0022-3476(86)80579-0. [DOI] [PubMed] [Google Scholar]
- 19.Graham D Y, Genta R M, Graham D P, Crabtree J E. Serum CagA antibodies in asymptomatic subjects and patients with peptic ulcer: a lack of correlation of IgG antibody in patients with peptic ulcer or asymptomatic Helicobacter pylori gastritis. J Clin Pathol. 1996;49:829–832. doi: 10.1136/jcp.49.10.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husson M O, Gottrand F, Vachee A, Dhaenens L, Martin de la Salle E, Turck D, Houcke R, Leclerc H. Importance in diagnosis of gastritis of detection by PCR of the cagA gene in Helicobacter pylori strains isolated from children. J Clin Microbiol. 1995;33:3300–3303. doi: 10.1128/jcm.33.12.3300-3303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katagiri M, Takeda H, Kudo M, Kato M, Kamishima Y, Kagaya H, Nishikawa K, Sukegawa M, Ohtaki T, Kleanthous H, Blaser M J, Asaka M. Infection with cagA+ positive H. pylori strains is not associated with gastric cancer in Japan. Gastroenterology. 1997;112:A589. [Google Scholar]
- 22.Kato S, Furuyama N, Ozawa K, Ohnuma K, Iinuma K. Long-term follow-up study of serum IgG and IgA antibodies after Helicobacter pylori eradication. Pediatrics. 1999;104:E221–E225. doi: 10.1542/peds.104.2.e22. [DOI] [PubMed] [Google Scholar]
- 23.Kato S, Ritsuno H, Ohnuma K, Iinuma K, Sugiyama T, Asaka M. Safety and efficacy of one-week triple therapy for eradicating Helicobacter pylori in children. Helicobacter. 1998;3:278–282. doi: 10.1046/j.1523-5378.1998.08030.x-i1. [DOI] [PubMed] [Google Scholar]
- 24.Kato S, Takeyama J, Ebina K, Naganuma H. Omeprazole-based dual and triple regimens for Helicobacter pylori eradication in children. Pediatrics. 1997;100:E31–E35. doi: 10.1542/peds.100.1.e3. [DOI] [PubMed] [Google Scholar]
- 25.Kosunen T U, Seppala K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titers after eradication of Helicobacter pylori. Lancet. 1992;339:893–895. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 26.Kuipers E J, Perez-Perez G I, Meuwissen S G M, Blaser M J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 27.Mendall M A, Goggin P M, Molineaux N, Levy J, Toosy T, Strachan D, Camm A J, Northfield T C. Childhood living conditions and Helicobacter pylori seropositivity in adult life. Lancet. 1992;339:896–897. doi: 10.1016/0140-6736(92)90931-r. [DOI] [PubMed] [Google Scholar]
- 28.Miehlke S, Kibler K, Kim J G, Figura N, Small S M, Graham D Y, Go M F. Allelic variation in the cagA gene of Helicobacter pylori obtained from Korea compared to the United States. Am J Gastroenterol. 1996;91:1322–1325. [PubMed] [Google Scholar]
- 29.Perez-Perez G I, Bhat N, Gaensbauer J, Alan F, Taylor D N, Kuipers E J, Zhang L, You W C, Blaser M J. Country-specific constancy by age in cagA+ proportion of Helicobacter pylori infection. Int J Cancer. 1997;72:453–456. doi: 10.1002/(sici)1097-0215(19970729)72:3<453::aid-ijc13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Perez G I, Cutler A F, Blaser M J. Value of serology as a noninvasive method for evaluating the efficacy of treatment of Helicobacter pylori infection. Clin Infect Dis. 1997;25:1038–1043. doi: 10.1086/516089. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S A, Tummuru M K R, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman P M. Peptic ulcer disease in children. Diagnosis, treatment, and the implication of Helicobacter pylori. Gastroenterol Clin North Am. 1994;23:707–725. [PubMed] [Google Scholar]
- 33.Taylor D E, Eaton M, Chang N, Salama S M. Construction of a Helicobacter pylori genome map and demonstration of diversity at the genome level. J Bacteriol. 1992;174:6800–6806. doi: 10.1128/jb.174.21.6800-6806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tummuru M K R, Cover T L, Blaser M J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tummuru M K R, Sharma S A, Blaser M J. Helicobacter pylori PicB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 36.van Doorn L J, Figueiredo C, Sanna R, Blaser M J, Quint W G V. Distinct variants of Helicobacter pylori cagA are associated with vacA subtypes. J Clin Microbiol. 1999;37:2306–2311. doi: 10.1128/jcm.37.7.2306-2311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb P M, Crabtree J E, Forman D the Eurogast Study Group. Gastric cancer, cytotoxin-associated gene A-positive Helicobacter pylori, and serum pepsinogens: an international study. Gastroenterology. 1999;116:269–276. doi: 10.1016/s0016-5085(99)70122-8. [DOI] [PubMed] [Google Scholar]
- 38.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]