Abstract
Purpose
Lead Poisoning is a major health problem in Iran. We aimed to compare efficacy of a standard regimen (Succimer) with that of a low-priced combination of D-penicillamine and Garlic in outpatients with lead poisoning.
Methods
In this retrospective cross-sectional study, year-long clinical files of outpatients with lead poisoning in two referral toxicology clinics in Mashhad, Iran were reviewed. A total of 79 patients (all men), received either Succimer or a combination of D-penicillamen plus garlic (DPN + Gar), for 19 and 30 days, respectively. Clinical and laboratory data, including blood lead level (BLL), were analyzed and treatment expanses were compared between the two regimens.
Results
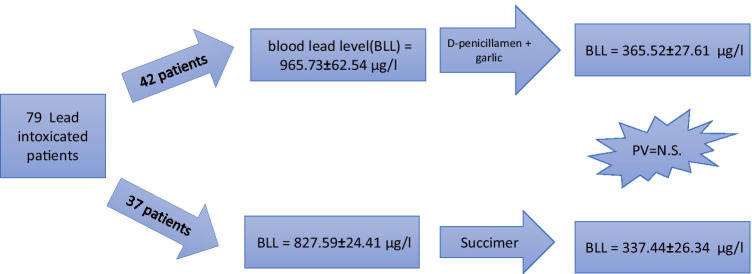
Of 79 male patients, 42 were treated by DPN + Gar and 37 received Succimer. Mean BLL of DPN + Gar group before treatment (965.73 ± 62.54 µg/L) was higher than that of the Succimer group (827.59 ± 24.41) (p < 0.001). After treatment, BLL in both groups significantly reduced to 365.52 ± 27.61 µg/L and 337.44 ± 26.34 µg/L, respectively (p < 0.001). The price of a 19-day treatment with Succimer was approximately 28.6 times higher than a one-month course of treatment with garlic plus DPN. None of the treatments caused serious side effects in the patients.
Conclusion
Combination therapy with DPN + Gar is as effective as Succimer in Pb poisoning, while treatment with Succimer is significantly more expensive.
Graphical abstract
Keywords: Lead, Succimer, D-penicillamine, Garlic, Addiction, Poisoning
Introduction
Lead (Pb), as a toxic heavy metal, is a common environmental and occupational pollutant which can cause serious health problems in most body organs, such as the gastrointestinal tract, liver, kidneys, hematopoietic, endocrine, cardiovascular, reproductive and neuropsychological systems, and impair the development of nervous and skeletal systems [1, 2]. Among all clinical manifestations, abdominal pain is a common symptom which brings the patients into medical care [3]. The sources of lead exposure vary among countries. In Iran, for instance, drug dealers and smugglers illegally add Pb to opium for the purpose of increasing opium weight to gain more financial profit [4]. Over the past decade, poisoning with lead-contaminated opium has become a major health problem and several cases of poisoning due to adulterated opium have been reported [3, 5–8].
Although acute lead poisoning is rare, particularly in adults, sub-acute and chronic poisonings are commonly seen in clinical practice [6]. Because of the serious and sometimes permanent complications of lead poisoning, cessation of lead exposure with or without chelator therapy is the cornerstone of treatment in symptomatic patients [3, 9]. Globally available chelating agents are utilized in two main forms: parenteral and oral. Treatment indications and type of chelator are determined based on several factors including the patient's age, body weight, clinical manifestations, and Blood Lead Level (BLL) [3, 10]. Although chelators have been generally shown to reduce BLLs, their safety, efficacy, availability, and prices remain a challenge in some countries [9–11]. One major problem particularly in low-income countries and among underprivileged people is medication affordability, which extensively affects clinical success and patient adherence to treatment plan.
Succimer (2,3-meso-dimercaptosucccinic acid or DMSA) is a universally-recognized chelating agent and a water soluble derivative of Dimercaprol (British Anti Lewisite = BAL), which is the only recommended oral chelator for effective and safe treatment of lead poisoning[12]. It is also the chelator of choice that has been solely approved for pediatrics use by the US FDA under the commercial name CHEMET [13].
Another chelator is D-Penicillamine (3-mercapto-D-valine or 3-Sulfanyl-D-valine or b,b-dimethylcysteine) or DPN, which has been used as an oral chelating agent for several decades. DPN is a third-line oral chelator for lead toxicity that can reduce BLL and reverse hematologic toxicity when used for 4 to 12 weeks [14, 15]. Recently, combination therapy with DPN and garlic in Pb poisoning has become a subject of interest and such treatment has been reported to be efficacious [10, 11, 16].
In Iran, Succimer is very costly and frequently unavailable in the market, whereas DPN and garlic are low-cost and conveniently accessible. Moreover, majority of patients with addiction-related lead poisoning, who are in need of chelator therapy, come from low socioeconomic status and could not afford Succimer. Hence, we aimed to compare the efficacy and expenses of a standard regimen (Succimer alone) with those of a low-priced combination therapy with D-penicillamine plus garlic.
Methods and participants
In this retrospective cross-sectional study, clinical files of all opium addicted patients above 18 years of age, whose lead poisoning had been confirmed clinically and laboratory, were reviewed. We included individuals whose poisonings were due to regular intake of lead-adulterated opium, and who had received outpatient treatment during 2018 in two referral and private toxicology clinics in Mashhad, Iran.
The symptomatic patients with BLLs above 700 µg/L had been treated by either the standard chelator, Succimer, for 19 days or a combination therapy of D-penicillamine and garlic tablets (DPN and Gar) for 30 days. The standard dosing for Succimer capsules (Succimer, 200 mg Iran, and Succicaptal® 100 or 200 mg, Serb pharmaceutical company, France) was 10 mg/kg/dose three times a day for 5 days, and then 10 mg/kg/dose two times a day for 14 days. The patients who had been treated with DPN, had received 250 mg capsules (Artamin® 250 mg, by Sandoz pharmaceutical company, Austria) advising to take one capsule two hours after each daily meal (750 mg/day) for three days, and if tolerated without any side effects, increase it to two capsules per day (1500 mg/day) for 30 days. They had been also directed to take 500 mg garlic tablets (Garcin®, Goldaru pharmaceutical company, Esfahan, Iran) after each meal (1500 mg/day) for three days, and if tolerated increase to 3000 mg daily for 30 days. The above mentioned doses were selected based on previous studies [10]. Patients with severe poisoning (symptomatic with complications, or BLL above 1000 µg/L) who had been candidates for parenteral chelator therapy, but either could not afford or refused hospitalization, had also been treated by one of the above oral regimens.
After detailed explanation of both regimens regarding possible side effects and costs, each patient had signed an informed consent and treatment had been started with either Succimer (Succimer group) or a combination of garlic tablets plus D-penicillamine capsules (DPN + Gar group). We designed the groups based on patients' clinical files. In both groups, blood lead levels had been measured by an approved toxicology laboratory using heated graphite technique of atomic absorption spectrometry method (Perkin Elmer, Model 3030, Chicago, USA) once before starting treatments and then one month after treatments completed. Other laboratory tests and any further assessments had been performed as clinically indicated. All demographic data, clinical and laboratory findings, were filed confidentially in the clinic database. A total of 79 patients (all men), who completed the course of treatment, were included in the study.
This study was approved by the ethics committee of Mashhad University of Medical Sciences (Ethical code: IR.MUMS.fm.REC.1397.388). We estimated treatment expenses and calculated the reduction percentage of BLL for each patient by the following equation:
Data were collected and analyzed by SPSS (ver. 11) using nonparametric statistical tests. Data are presented as mean ± SE and percent. P-values less than 0.05 were considered statistically significant.
Results
A total of 79 patients (all men), whose files showed a completed course of treatment, were entered the study. Of them, 42 individuals were treated by DPN + Gar, and 37 patients received Succimer. Before treatments, the mean BLLs in the DPN + Gar group (965.73 ± 62.54 µg/L) was higher than that in the Succimer group (827.59 ± 24.41) (p < 0.001). As expected, BLLs of patients receiving Succimer decreased significantly from 827.59 ± 24.41 to 337.44 ± 26.34 µg/L (p < 0.001). Similarly in the DPN + Gar group, BLLs also declined significantly from 965.73 ± 62.54 to 365.52 ± 27.61 µg/L (p < 0.001).
The patients who received DPN + Gar, had a significantly higher BLLs than that in the Succimer group, both before (p < 0.001) and after (p < 0.007) treatment. But, reduction percentages were similar (p = 0.196) as described in Table 1.
Table 1.
Comparison of blood lead level (BLL) in patients with D-penicillamine and garlic table (DPN + Gar) and Succimer at the start and at the end of the treatment, and BLL reduction by treatments. BLL is presented as means ± SE (unpaired T-test)
| Blood lead level µg/ml | Drug | Number | Mean ± SE (µg/L) | Minimum (µg/L) | Maximum (µg/L) | P-value |
|---|---|---|---|---|---|---|
| At the start of treatment | DPN + Gar | 42 | 965.73 ± 62.54 | 202 | 2438 | 0.001 |
| Succimer | 37 | 827.59 ± 24.41 | 405 | 1235 | ||
| At the end of treatment | DPN + Gar | 42 | 365.52 ± 27.61 | 38.9 | 824 | 0.007 |
| Succimer | 37 | 337.44 ± 26.34 | 60.19 | 984 | ||
| Reduction by treatment | DPN + Gar | 42 | 596.55 ± 54.82 | 102 | 1898.5 | 0.007 |
| Succimer | 37 | 488.20 ± 22.70 | 116 | 740 |
However, the mean of the absolute amount of reduction of BLL in the DPN + Gar group was higher than that in the Succimer group (p = 0.007) (Table 1). Both treatment regimens showed similar percent reduction of BLL (Table 2). The pretreatment BLL in both groups could not predict the post-treatment BLL (R2 = 0.6, P = 0.11 in DPN + Gar group; R2 = 0.57, P = 0.15 in Succimer group). Fortunately, no serious side effects were observed throughout the treatment with neither Succimer nor the DPN + Gar combination.
Table 2.
The means percentage of Blood lead level (BLL) reduction during treatment by DPN + Gar and Succimer. Percentage reduction of BLL is reported in means ± SE (unpaired T test)
| Group | Number | Mean ± SE (%) | Median % | Minimum% | Maximum % | P-value |
|---|---|---|---|---|---|---|
| DPN + Gar | 42 | 60.21 ± 2.65 | 64.18 | 14.60 | 88.24 | 0.175 |
| Succimer | 37 | 59.95 ± 2.43 | 59.27 | 11.60 | 92.27 |
In Iran, price of each 200 mg capsule of Succimer is nearly three USD, and given the standard dosing (10 mg/kg/day in divided doses every 8 h for 5 days, then every 12 h for 14 days), a complete 19-day course of treatment for an average adult (body weight = 60 kg) would cost approximately 180 USD. Each 250-mg capsule of DPN roughly costs 0.1 USD (10 Cents), and each 400-mg tablet approximately costs three Cents (prices in February 2021) (Table 3).
Table 3.
Comparison of dose, adverse effects, and price between Succimer, DPN, and garlic; GI: gastrointestinal
| Chelator | Suggested Dose in Pb poisoning | Major Adverse Effects | Price per Capsule/tablet |
|---|---|---|---|
| Succimer | 10 mg/kg/day in divided doses (every 8 h for 5 days, then every 12 h for 14 days, for a total of 19 days) | GI Discomfort, Rash, Mucocutaneous Reactions, Neutropenia, Liver Enzymes Elevation | 3 USD |
| DPN | 1 g/day or 10–15 mg/kg/day in 3–4 divided doses for at least 4 weeks | GI disturbances (anorexia, nausea, vomiting), changes/loss of taste, hair loss, hypersensitivity reactions, aplastic anemia, nephrotoxic effects, autoimmune reactions, headache | 0.1 USD |
| Garlic | 400 mg (1200 μg allicin or 2 g fresh garlic) trice a day for 4 weeks | In high doses: hypersensitivity, GI complications, headache, somnolence, euphoria, polyuria, and muscarinic manifestations | 0.03 USD |
Patients in both groups had declared their general satisfaction upon the end of the treatments duration considering normal lab results and resolution of clinical sign and symptoms. Over the course of treatment and later in post-treatment follow-ups, no significant complications were reported to physicians.
Discussion
In this study, we described the adequate efficacy and reasonable price of treatment with a combination of DPN and garlic in comparison with Succimer alone.
Succimer is the chelator of choice for treatment of lead poisoning and has significant benefits; for instance, it allows no Pb redistribution into the brain and is more effective than CaNa2EDTA in decreasing Pb levels in the brain and circulation [10, 13]. It has minimal adverse effects such as gastrointestinal discomfort, rash, mild to severe mucocutaneous reactions, mild neutropenia, mild elevation of liver enzymes etc. [10, 13, 17, 18]. As mentioned before, the suggested dose of Succimer in lead poisoning is 10 mg/kg/day in divided doses (every 8 h for 5 days, then every 12 h for 14 days, for a total of 19 days) for adults and children older than 12 months [13]. On the other hand, DPN can lead to several adverse effects including gastrointestinal disturbances (anorexia, nausea, vomiting), changes/loss of taste, hair loss, hypersensitivity reactions, aplastic anemia, nephrotoxic effects, and headache to name but a few. DPN has also the potential for eliminating essential nutrients such as zinc and iron and its absorption is affected by diet [10, 14, 15]. DPN is easily available in the Iranian market and its suggested dose for Lead poisoning is 1 g/day (250 mg every 6 h) or 10–15 mg/kg/day titrated in 3–4 divided doses per day for at least 4 weeks [15, 19–22]. A quick comparison between Succimer and DPA in terms of adverse effects, doses, and price is depicted as Table 3.
Recent studies have described garlic (Allium sativum) as an herbal medicine with very low price, which could successfully chelate Pb in animals and humans [10, 23–25]. In a controlled double-blind clinical trial among 117 workers of a car battery company, garlic could effectively reduce BLLs [11]. Garlic contains sulfur compounds such as S-allyl cystine, S-allyl mercaptocystein and allicin, which may have chemo-prophylactic and chelating effects in lead toxicity by enhancing urinary excretion and preventing gastrointestinal absorption of Pb [16, 25, 26]. A study by El-Khishin et al. also showed that treatment with garlic extract could decrease lead levels as efficacious as Succimer in a rodent model of lead toxicity [27]. Similarly, it has been indicated that garlic can improve Pb toxic effects by scavenging it from tissues and antioxidant activity, which result in decreased tissue exposure to Pb [28].
Adverse effects and possible toxicity of garlic are mostly observed in high doses and include hypersensitivity, gastrointestinal complications, headache, somnolence, euphoria, polyuria, and muscarinic manifestations [28, 29]. Extracted garlic tablets are easily available in Iran. The recommended dose of garlic in Lead poisoning is 400 mg (equivalent to 1200 μg allicin or 2 g fresh garlic) three times a day for at least 4 weeks [10, 11, 26]. The cost of a one-month course of treatment with garlic plus DPN would be less than three USD (price in February 2021).
As known, Succimer is the safest and most efficacious chelating agent (compared to other chelators) presently available [10, 13, 14, 17, 20]. However, its serious side effects, unavailability, and high price are some drawbacks for its use in Iran, which oblige the physicians to seek more affordable yet effective alternatives for treatment of lead poisoning. Some studies have shown that combination therapy with two chelators could be more promising and have less complications than single therapy [17, 22, 30, 31].
In this study, we observed no significant complications in neither of the groups. However, three patients in the Succimer group and four in the DPN + Gar group had stopped their medications due to mild dermal or gastrointestinal disorders. In general, clinical and para-clinical status of patients were improved after both treatments.
Limitations
Due to exceptional circumstances in our country regarding shortage of some expensive antidotes such as Succimer, and based on the clinical and research experience of the authors, we were urged to use a combination therapy with DPN + Gar. In all patients with high BLL, who could not afford either Succimer or hospitalization, mutual decision between the clinician and the patient had been made to begin the recommended combination regimen. Another limitation of this study was gender bias. A major reason was the fact that there were far fewer women with toxic lead levels who required rapid therapeutic action outside of standard protocols.
Conclusion
Combination therapy with DPN + Gar was as efficacious as Succimer. The price of a 19-day treatment with Succimer was 28.6 times higher than with a one-month course of treatment with garlic plus DPN. Therefore, it is imperative to consider alternative treatments with garlic and DPN in lead-poisoned patients, who require prompt chelating therapy while there is either shortage of Succimer or financial burdens. Additional prospective studies and randomized trials are required to address the limitations of this study.
Acknowledgements
The authors would like to express their gratitude to Ms. Adeleh Mahdizadeh and Ms. Parisa Rajaee for their kind assistance in this study.
Funding
This study was financially supported by Mashhad University of Medical Sciences (code number: 951453).
Declarations
Conflict of interest
None to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maryam Vahabzadeh, Email: vahabzadehm@mums.ac.ir.
Mahdi Balali-mood, Email: balalimoodM@mums.ac.ir.
Ali Banagozar Mohammadi, Email: alibanagozar@tbzmed.ac.ir.
Mohammad Moshiri, Email: moshirim@mums.ac.ir, Email: Moshirimo@gmail.com.
References
- 1.Ahamed M, Siddiqui MKJ. Environmental lead toxicity and nutritional factors. Clin Nutr. 2007;26(4):400–408. doi: 10.1016/j.clnu.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor D, Hou D, Ok YS, Lanphear BP. The effects of iniquitous lead exposure on health. Nat Sustain. 2020;3(2):77–79. doi: 10.1038/s41893-020-0475-z. [DOI] [Google Scholar]
- 3.Vahabzadeh M, Mégarbane B. Abdominal pain related to adulterated opium: An emerging issue in drug addicts. World J Psychiatry. 2020;10(5):95–100. doi: 10.5498/wjp.v10.i5.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soltaninejad K, Flückiger A, Shadnia S. Opium addiction and lead poisoning. J Substance Use. 2011;16(3):208–212. doi: 10.3109/14659891.2010.545860. [DOI] [Google Scholar]
- 5.Dadpour B, Afshari R, Mousavi SR, Kianoush S, Keramati MR, Moradi VA, et al. Clinical and Laboratory Findings of Lead Hepatotoxicity in the Workers of a Car Battery Manufacturing Factory. Iran J Psychiatry. 2016;10(2):1–6. [Google Scholar]
- 6.Ghane T, Zamani N, Hassanian-Moghaddam H, Beyrami A, Noroozi A. Lead poisoning outbreak among opium users in the Islamic Republic of Iran, 2016–2017. Bull World Health Organ. 2018;96(3):165. doi: 10.2471/BLT.17.196287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radfar SR, Nematollahi P, Farhoudian A, Noroozi A. Lead Poisoning among Opium Users in Iran, a Possible New Emerging Epidemic in the Region. Iran J Public Health. 2017;46(8):1152–1153. [PMC free article] [PubMed] [Google Scholar]
- 8.Zamani N, Mehrpour O, Hassanian-Moghaddam H, Jalali M, Amirabadizadeh A, Samie S, et al. A preliminary report on the largest ongoing outbreak of lead toxicity in Iran. Sci Rep. 2020;10(1):1–7. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farzaneh E, Habibzadeh A, Mehrpour O. Lead Toxicity among Oral Opium Addicts with Abdominal Pain: A Case Series of 17 Cases. Indian J Forensic Med Toxicol. 2017;11(2).
- 10.Kianoush S, Sadeghi M, Balali-Mood M. Recent Advances in the Clinical Management of Lead Poisoning. Acta Med Iran. 2015:327–36. [PubMed]
- 11.Kianoush S, Balali-Mood M, Mousavi SR, Moradi V, Sadeghi M, Dadpour B, et al. Comparison of therapeutic effects of garlic and d-Penicillamine in patients with chronic occupational lead poisoning. Basic Clin Pharmacol Toxicol. 2012;110(5):476–481. doi: 10.1111/j.1742-7843.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 12.Alinejad S, Aaseth J, Abdollahi M, Hassanian-Moghaddam H, Mehrpour O. Clinical Aspects of Opium Adulterated with Lead in Iran: A Review. Basic Clin Pharmacol Toxicol. 2018;122(1):56–64. doi: 10.1111/bcpt.12855. [DOI] [PubMed] [Google Scholar]
- 13.Kosnett MJ, et al. Succimer. In: Brent J, Burkhart K, Dargan P, Hatten B, Megarbane B, Palmer R, et al., editors. Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient. Cham: Springer International Publishing; 2017. pp. 2987–2991. [Google Scholar]
- 14.Aaseth J, Skaug MA, Cao Y, Andersen O. Chelation in metal intoxication—principles and paradigms. J Trace Elem Med Biol. 2015;31:260–266. doi: 10.1016/j.jtemb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Paeezi M, Zamani N, Hassanian-Moghaddam H, Shadnia S, Zamani N, Chaleshi V, et al. Treatment of adult lead poisoning with D-penicillamine. Drug Metab Pers Ther. 2019;34(2). [DOI] [PubMed]
- 16.Mumtaz S, Ali S, Khan R, Shakir HA, Tahir HM, Mumtaz S, et al. Therapeutic role of garlic and vitamins C and E against toxicity induced by lead on various organs. Environ Sci Pollut Res Int. 2020;27(9):8953–8964. doi: 10.1007/s11356-020-07654-2. [DOI] [PubMed] [Google Scholar]
- 17.Kim H-C, Jang T-W, Chae H-J, Choi W-J, Ha M-N, Ye B-J, et al. Evaluation and management of lead exposure. Ann Occup Environ Med. 2015;27(1):1–9. doi: 10.1186/s40557-014-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varma A, Bradberry SM, Vale JA. Rash and pyrexia after succimer (dimercaptosuccinic acid; DMSA) Clin Toxicol (Phila) 2017;55(7):680. doi: 10.1080/15563650.2017.1304553. [DOI] [PubMed] [Google Scholar]
- 19.Amiri M, Amini R. A comparison of blood-lead level (BLL) in opium-dependant addicts with healthy control group using the graphite furnace/atomic absorption spectroscopy (GF-AAS) followed by chemometric analysis. Iran Red Crescent Med J. 2012;14(8):488–491. [PMC free article] [PubMed] [Google Scholar]
- 20.Hauptman M, Bruccoleri R, Woolf AD. An update on childhood lead poisoning. Clin Pediatr Emerg Med. 2017;18(3):181–192. doi: 10.1016/j.cpem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitman SK, Huynh T, Bjarnason TA, An J, Malkhasyan KA. A case report and focused literature review of d-penicillamine and severe neutropenia: A serious toxicity from a seldom-used drug. Clin Case Rep. 2019;7(5):990. doi: 10.1002/ccr3.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shojaeepour S, Fazeli M, Mandegary A, Sayed-Mirzaei S-M, Ahmadi N, Saeedi A, et al. Evaluation of oxidative stress in combination therapy with d-penicillamine and n-acetylcysteine (NAC) in lead poisoning in opium addicts. Asia Pac J Med Toxicol. 2017;6(4):123–128. [Google Scholar]
- 23.Ghasemi S, Hosseini M, Feizpour A, Alipour F, Sadeghi A, Vafaee F, et al. Beneficial effects of garlic on learning and memory deficits and brain tissue damages induced by lead exposure during juvenile rat growth is comparable to the effect of ascorbic acid. Drug Chem Toxicol. 2017;40(2):206–214. doi: 10.1080/01480545.2016.1197238. [DOI] [PubMed] [Google Scholar]
- 24.Hamza G, Ibegbu A, Buraimoh A. Evaluation of the Effects of Aqueous Garlic Extract on Lead-Induced Changes on Cerebellum of Wistar rats. Afr J Cell Path. 2017;8(2):9–14. doi: 10.5897/AJCPATH17.002. [DOI] [Google Scholar]
- 25.Sadeghi A, Soleimani H, Nasseri-Moghadam S, Radmard AR. Lead contaminated opium as unusual cause of abdominal pain-case series. Iran J Radiol. 2017(5).
- 26.Parsi A, Ghorbani A, Hesam S, Hosseini M. Comparison of Garlic therapeutic effects and standard therapy with De Penicillamine in patients with Lead poisoning. J Adv Pharm Educ Res. 2020;10(S2):85. [Google Scholar]
- 27.El-Khishin IA, El-Fakharany YMM, Hamid OIA. Role of garlic extract and silymarin compared to dimercaptosuccinic acid (DMSA) in treatment of lead induced nephropathy in adult male albino rats. Toxicol Rep. 2015;2:824–832. doi: 10.1016/j.toxrep.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorrigiv M, Zareiyan A, Hosseinzadeh H. Garlic (Allium sativum) as an antidote or a protective agent against natural or chemical toxicities: A comprehensive update review. Phytother Res. 2020;34(8):1770–1797. doi: 10.1002/ptr.6645. [DOI] [PubMed] [Google Scholar]
- 29.Alare K, Alare T. Review of Toxicity of Allicin From Garlic. Open Access J Toxicol. 2020.
- 30.Jalali SM, Najafzadeh H, Bahmei S. Protective role of silymarin and D-penicillamine against lead-induced liver toxicity and oxidative stress. Toxicol Ind Health. 2017;33(6):512–518. doi: 10.1177/0748233716685660. [DOI] [PubMed] [Google Scholar]
- 31.Jalali SM, Najafzadeh H, Mousavi SM. Comparative effect of silymarin and d-penicillamine on lead induced hemotoxicity and oxidative stress in Rat. Iran J Toxicol. 2017;11(3):11–18. doi: 10.29252/arakmu.11.3.11. [DOI] [Google Scholar]