Abstract
Importance:
The practice of oncology will involve care of a growing population of individuals with mid- and late-life cancers. Managing these individuals is complex based on differences in biological age at diagnosis. Biological age is a measure of accumulated life course damage to biological systems, loss of reserve and vulnerability to functional deterioration and death. Biological age is important because it affects the ability to manage the rigors of cancer therapy, survivors’ function, and cancer progression. However, biological age is not always clinically apparent. We present a conceptual framework of life course biological aging, summarize candidate measures and describe a research agenda to facilitate clinical translation to oncology practice.
Observations:
Mid- and late-life cancers are chronic diseases arising from cumulative patterns of biological aging occurring over the life course. Before diagnosis each new patient was on a distinct course of biological aging related to past exposures, life experiences, genetics and non-cancer chronic disease. Cancer and its treatments can also affect biological aging. Several measures of biological age have been used in oncology research including p16INK4a, epigenetic age, telomere length, and inflammatory and body composition markers. One or more of these measures could be useful in cancer care, either alone or in combination with clinical history and geriatric assessments. However, further research will be needed before biological age assessment can be recommended in routine practice, including determination of situations where knowledge about biological age would change treatment, ascertaining whether treatment effects on biological aging are short-lived or persistent, and testing interventions to modify biological age, decrease treatment toxicities and maintain functional abilities.
Conclusions and Relevance:
Understanding differences in biological aging could ultimately allow clinicians to better personalize treatment and supportive care, develop tailored survivorship care plans, and prescribe preventive or ameliorative therapies and behaviors informed by aging mechanisms.
Keywords: Cancer, biological aging, geroscience, gero-oncology, older patients, survivorship, treatment, health outcomes
“Research on the basic mechanisms of aging and cancer will enhance our understanding of both subjects and will enable the development of new strategies for cancer prevention and treatment, as well as the optimal delivery of cancer interventions based on a person’s age.” 1
Several converging forces are driving unprecedented changes in the practice of adult oncology. Discovery and dissemination of new therapeutic paradigms are providing expanded treatment choices and improved survival.2 At the same time, demographic trends are driving increases in population life expectancy and cancer rates increase dramatically with advancing age. These clinical, demographic and epidemiological trends are resulting in large increases in the numbers of individuals being diagnosed with and surviving mid-(age 45-64) and late-life (age 65+) cancers.3 These factors promoted the American Society of Clinical Oncology to include optimization of care for older adults as a priority in its 2020 report on clinical cancer advances.2
Optimizing care for the growing number of older patients and survivors is complex based on differences in biological age at the time of diagnosis. Biological aging is generally defined as accumulated damage to biological systems over the life course, leading to loss of reserve and capacity to respond to challenges, vulnerability to chronic diseases including cancer, diabetes and cardiovascular disease, and deterioration in function and death.4
Biological age is not always clinically apparent in oncology settings, but it may be indirectly reflected in comorbidities, polypharmacy, and functional limitations.5–7 Together with other factors like social support, biological age effects the ability to manage the rigors of new cancer therapies and survivorship outcomes. Biological age is also important because it is a potentially modifiable driver of survivors’ physical, cognitive, emotional and physiological function and the course of chronic disease,8 including cancer and cancer progression.9
Cancer is unique among chronic diseases because treatments for most chronic diseases (e.g., diabetes, high blood pressure) serve to stabilize the system (control blood sugar, lower blood pressure), while most cancer treatments destabilize the system and cause additional damage accumulation. For this reason, cancer and its treatments can increase biological age and are considered disease-drivers of aging.10 Most literature in this area has focused on shared processes of biological aging and carcinogenesis in late-life 10–13 or aging-related treatment consequences in childhood and young adult survivors.14 The purpose of this narrative review is three-fold: 1) present a conceptual framework of life course biological aging, 3) summarize candidate measures of biological aging and 3) describe a research agenda to facilitate translation of life course biological aging into clinical practice.
Conceptual Framework
The interrelationships between aging and cancer are multidirectional (Figure 1). Aging increases the risk of developing cancer through accumulated damage and mutations.12 As noted above, aging also increases the risk of other diseases, and many of these, like diabetes further increase the risk of cancer if death from other causes does not occur earlier.12,15 Cancer and other chronic diseases can each promote additional aging10 and contribute to survivorship outcomes.
Figure 1. Interrelationships Between Aging And Cancer.
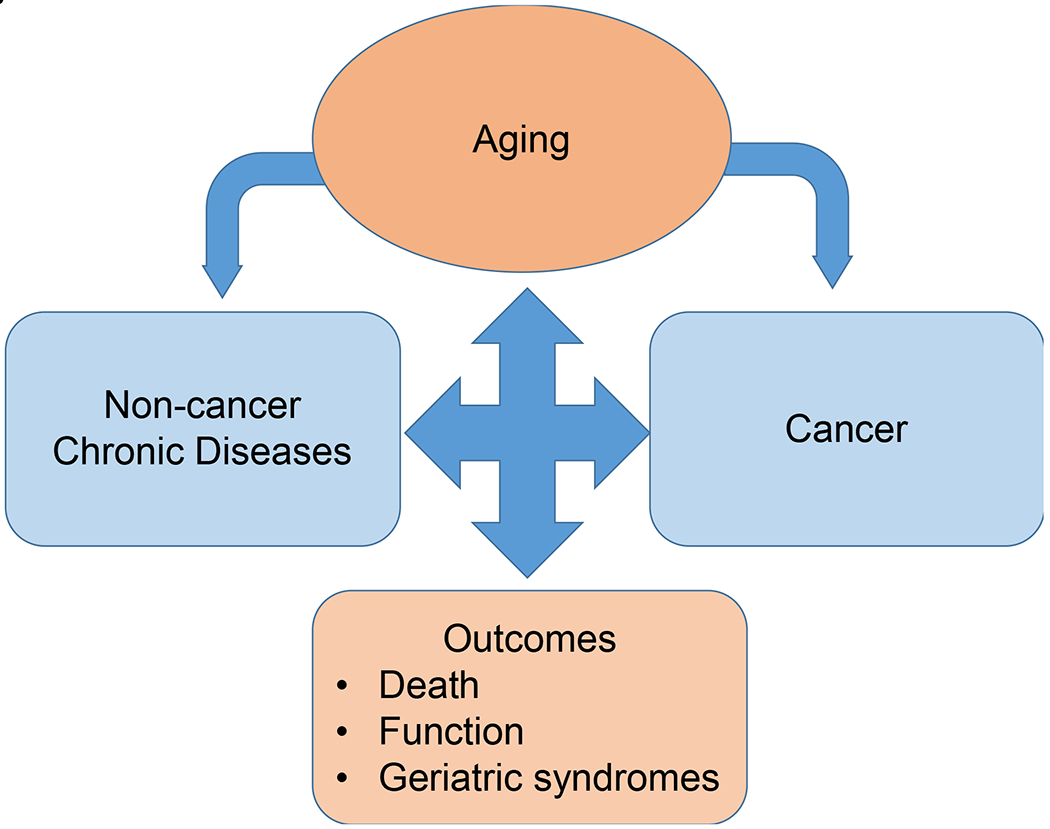
The relationships between aging and cancer are multidirectional. Aging increases the risk of developing cancer and non-cancer chronic diseases, and many of these diseases further increase the risk of cancer. Cancer and other chronic diseases can also promote additional aging each contributing to survivorship outcomes including functional ability, geriatric syndromes (e.g., falls, frailty) and death.
There is considerable heterogeneity in aging and the rate of aging over the life course (Figure 2). We use a longitudinal framework that considers cancer as a chronic disease arising from cumulative patterns of biological aging occurring over the life course. Most individuals age gradually (normative aging),4 while some age more slowly than the norm based on genetic, lifestyle, or yet undiscovered factors.16 These slow agers have a longer lifespan (e.g., centenarians) and exhibit a younger biological age than their chronological age. Slow agers may not develop cancer or non-cancer chronic disease, or if they do, they have very late disease onset.16 Others show accelerated aging, aging more rapidly than the norm. Accelerated aging can begin in early or later life. For instance, adverse childhood events can alter stress responses leading to increased inflammation and shift individuals to accelerated aging.17–19
Figure 2. Life Course Framework of Biological Aging in Cancer Patients and Survivors.
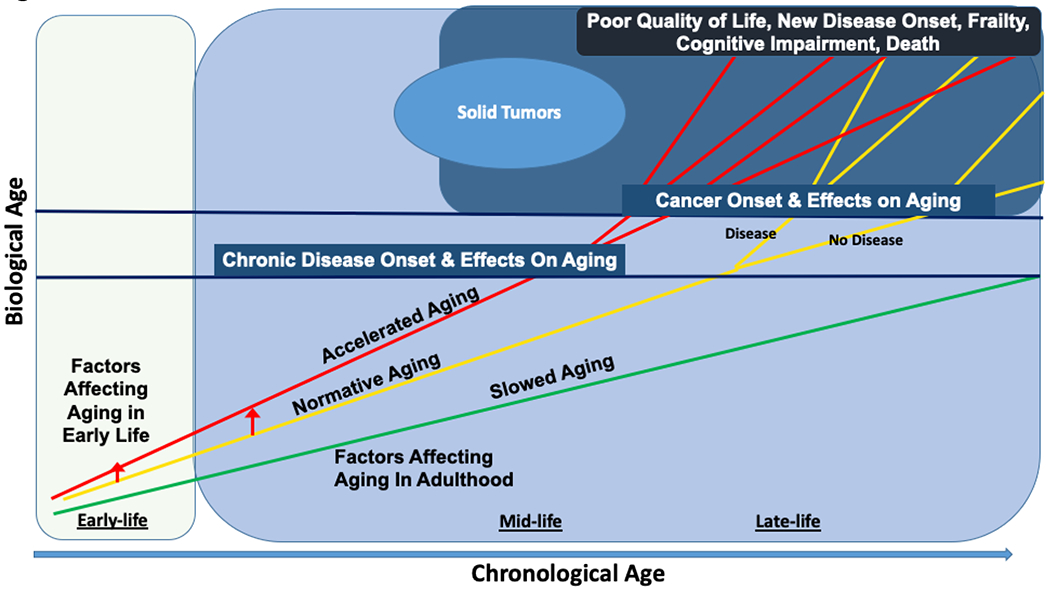
This figure depicts the relationships between biological aging and cancer over the life course. Biological aging begins at birth. Most individuals age gradually (“normative aging,” yellow line), some age slowly (green line), and others show “accelerated aging” (red line). Early life events can increase the risk of a change in slope of aging and result in a shift from normative to accelerated aging (red arrow). In adulthood, individuals may be exposed to factors that can shift their biological age from normative aging to accelerated aging, with a steeper slope of increase over time (red arrow). Non-cancer chronic diseases (first blue horizontal line) can occur or not at different chronological ages based on biological aging. The branching lines for each of the three aging trajectories show that those that develop non-cancer chronic disease proceed with a higher slope of biological aging, and those that do not develop chronic disease proceed along their original biological aging trajectory. The net effect of biological aging and non-cancer chronic disease can be to increase the risk of cancer if death does not occur earlier from other causes. After cancer and its therapies, biological age trajectories can branch again, where some individuals maintain their pre-cancer aging trajectory while others experience an increase in their slope of aging (i.e., have an increase in biological aging).
In adulthood, individuals may shift the slope of their biological aging trajectory from normative to accelerated aging due to environmental toxins, chronic life stress, unhealthy lifestyles, or genetic risks.14,16,20,21 Progeroid syndromes are examples of extreme accelerations of aging from birth and are not considered in our model.22
Normative or accelerated agers have greater potential to develop chronic diseases, including cancer than slow agers. Disease onset will generally be earlier for those experiencing accelerated vs. normative aging or slow aging.23 If non-cancer chronic disease occurs, the disease process itself can shift the existing biological age trajectory. For example, the onset of insulin resistance with type II diabetes is thought to exacerbate metabolomic dysfunction and inflammation, creating a feedback loop to promote further aging. Thus, the population surviving to develop mid- and late-life cancers includes a heterogenous group of individuals, each already on a distinct course of biological aging within and across chronological ages.
Cancer and its therapies (e.g. radiotherapy, chemotherapy) can further effect aging through increased DNA damage, inflammation and mitochondrial dysfunction.10,11,13 Inflammation via receptors of advanced glycated end-products (RAGE) signaling24 or senescence-associated pathways also increase risk of distant cancer progression. Additionally, the blood-brain interface integrity can be impaired by chemotherapy, creating a route for inflammatory cell migration and brain aging.25 Some patients will experience these cancer-related aging effects and have an increase in the slope of their biological aging, accelerating their pre-cancer trajectory. Others will experience some cancer-related aging effects but maintain the slope of their prior biological age trajectory (phase shift), while others do not have appreciable effects of their cancer on aging, and continue their pre-cancer aging trajectory.15,26
Implications for Clinical Research
The concepts embodied in our framework are well-established in non-cancer populations. Translation of this framework to oncology practice will require: 1) data on useful candidate measures specific to the cancer setting and 2) evidence that use of data about biological age provides better outcomes than currently recommended approaches. In this section we summarize recommendations for filling these translational research gaps.
Candidate Measures of Biological Aging
There are several requirements for life course biological aging biomarkers that are specific to the oncology setting, including: 1) capacity to reflect processes underlying aging beyond the effects of cancer and its treatments, 2) sensitivity to change, 3) ability to predict cancer outcomes better than chronological age, and 4) practicality in terms of costs and availability for use in care settings.8 Measures with current evidence of potential validity and utility in oncology settings are summarized here, on Table 1 and in several key studies and reviews.8,11,23,27,28
Table 1.
Biomarkers of Biological Age for Potential Use in Oncology Settings
| Measures | Process of Aging Measured | Sensitive to change? | Predicts outcomes? | Practicality | Citations |
|---|---|---|---|---|---|
| Biomarkers | |||||
| p16INK4 | Cellular senescence | Yes | Unknown | New approaches in development | 65,73,29,30 |
| Epigenetic clocks | DNA methylation/damage | Yes | Yes | High cost; common lab protocols | 4,31,32 |
| Telomere length | Cell specific aging | Yes | Yes; inconsistent findings | Low cost; strict technical protocols needed | 27,37–39 |
| IL-6, CRP, TNFRII | Inflammation | Yes | Yes | Low cost; common protocols | 8,40 |
| Clinical Indices 1 | |||||
| Pace of aging | Physiological system dysfunction 2 | Yes | Yes | Can be derived from medical record | 4,41 |
| Deficit accumulation indices | Loss of function 2 | Yes | Yes | Easy to estimate with patient-reported data and medical record | 6 |
| Frailty indices | Global measure2 | Yes | Yes | Requires personnel to administer | 44,74 |
| Geriatric assessments | Global measure 2 | unknown | Yes | Requires personnel to administer; takes about 15–20 minutes | 6,23,27,42–44,74,75 |
| Sarcopenia | Muscle atrophy | Yes | Yes | Requires CAT scan | 43 |
| Allostatic load | Physiological System Dysfunction | Yes | Yes | Can be derived from medical record | 27,33,50,51 |
Clinical indices use combinations of measures included on Table 1.
Global measures capture some combination of chronic diseases (comorbidity), organ system function and physiological reserve (e.g., glycated hemoglobin, forced expiratory volume in one second (FEV1), systolic blood pressure, total cholesterol, creatinine, blood urea nitrogen, hematocrit, etc.), function domains such as physical function (e.g., get up and go, grip strength, gait speed, walk stairs, etc.), cognitive function, and ability to perform activities of daily living.
Increased expression of p16INK4a, which reflects specific processes underlying aging (i.e., cellular senescence) has been reported after breast cancer chemotherapy and radiotherapy. Mid- and late-life breast cancer survivors have been observed to experience a change in p16INK4a expression comparable to 14 to 17 years of biological aging in the first 12-months after chemotherapy.29,30 Interestingly, a recent study of p16INK4a expression found that there were differences by chemotherapy regimen, with significantly greater aging after anthracycline-based vs. non-anthracycline regimens (23 to 26 years vs. 9 to 11 years). Additionally, women with lower pre-treatment p16INK4 (i.e., younger biological age) were observed to have greater increases in p16INK4a expression after chemotherapy than those starting with higher levels, suggesting possible non-linear or threshold effects.30 Although a number of laboratories are capable of measuring mRNA expression of the p16INK4a gene in whole blood and T-cell subsets, to date clinical labs do not assess this marker. At the point where measurement can be done in clinical labs, p16INK4a expression could be a useful marker of how treatment effects biological age and could identify survivors in need of surveillance and/or intervention.
DNA methylation changes, commonly referred to as “epigenetic clocks,” have also been studied in cancer patients. Epigenetic clock measures have been proposed as global markers of life course biological aging4,31,32 since they are highly correlated with chronological age33 and are predictive of earlier mortality declines in function, frailty and brain aging.32 In a study of adults with seven cancer types, epigenetic age of three or more years beyond chronological age was associated with cancer mortality independently of other risk factors.34 In studies of mid- and late-life breast cancer survivors, DNA methylation profiles have showed three years of biological aging in the one year post-treatment35 and greater aging than seen among non-cancer controls.36 While still relatively expensive, this group of tests has good predictive and concurrent validity and could be used in treatment decision making. Changes in epigenetic age from pre-to post treatment could also potentially guide survivorship care.
Telomere length may also have some utility as a marker of life course biological aging in the oncology setting since it serves a biological role in aging and shortens after exposure to cancer therpay.12 Telomere length also track with chronological age (although effects are modest), can be modified by interventions, and predicts overall mortality.37,38 although results have been inconsistent for other survivorship outcomes. 27 One study with childhood survivors found strong associations of telomere length and chronic disease development and function.39 Technical aspects of telomere length measurement are evolving and future advances could make this a useful marker done in clinical labs.
Inflammation is a central pillar of aging, so it is a logical measure of biological aging.8,12,40 Inflammation could be useful in oncology practice since: 1) higher levels of inflammatory markers are associated with frailty, functional and cognitive decline, and death in cancer patients, 2) inflammation can be modified by interventions like physical activity and 3) inflammatory markers are relatively easy to measure. Indeed, clinical labs frequently offer measures of inflammation such as C-reactive protein. Despite these advantages, it is difficult to separate the inflammatory effects of cancer and its therapies from biological aging. It is possible that persistent longitudinal elevations in inflammation beyond acute diagnosis and treatment could be useful to identify survivors with acceleration of biological aging. Thus inflammation may serve as a useful marker of biological aging.
Several composite indices like the Pace of Aging, which captures organ function and reserve have been evaluated in general populations and might be useful in oncology settings.4,41 While not a measure of life course biological aging, geriatric assessments which cover multiple domains of aging are feasible, require less than 30 minutes to administer, and are recommended to support treatment decisions for patients ages 65 and older.23,27,42 These assessments typically use clinical data to capture aging-related processes and include items like loss of muscle mass,43 difficulty getting out of a chair, accumulation of chronic diseases and impaired activities of daily living.6,44
Scores on pre-systematic treatment geriatric assessments have been associated with chemotherapy toxicity,45 hospitalizations45 and/or higher all-cause mortality in survivors of breast, colorectal, prostate, and other cancers.46 Unfortunately, geriatric assessments are still not widely used, require personnel to complete, are not generally assessed longitudinally, and are only recommended for patients ages 65 and older.
Measures of allostatic load have also been included in geriatric assessments or as independent makers of biological age. Allostatic load is defined as accumulated damage resulting from life course exposures and responses to the social environment.47,48 A high allostatic load manifests as deterioration of function across multiple physiological regulatory systems including cardiovascular, metabolic, immune, and nervous systems and is more common in Blacks than whites.49 Allostatic load can be determined through a combination of established clinical measures like blood pressure and pulse rate. This measure is prognostic of frailty, comorbidities and mortality and can be modified by intervention, making this a potentially useful measure of biological aging. 27,33,50,51
Another promising clinical assessment that likely reflects biological age is sarcopenia. Sarcopenia, the severe loss of muscle mass, has been shown to predict a variety of oncologic outcomes, including physical function, chemotherapy toxicity and mortality.52 It will be useful to evaluate how these clinical measures correlate with biological age tests and test the variation in outcomes explained by each.
Clinical Research Needs
As evidence about and access to testing for biomarkers of biological aging evolves, additional studies will be needed to inform recommendations about use in routine oncology practice. Biological aging is heterogenous and both mid-life and late-life cancer patients can have important variability in biological age without clinically apparent deficits. 53 However, attention to biological aging in cancer care is currently focused on patients ages 65 and older. Thus, an overarching research need is to develop and test sensitive methods to screen for risk of having biological age in excess of chronological age in a broader age range of patients (Table 2). Patients with a risk of advanced biological age could then undergo biomarker testing.
Table 2.
Clinical Measures of Life Course Biological Aging for Potential Use in Clinical Practice and Research Studies 1
| Domain | Exemplar Components | Measurement | |
|---|---|---|---|
| Clinical Encounters | Research | ||
| Childhood adversity | Parents divorced, abuse, homelessness | History | NIH toolkits |
| Chronic life stress | Family, job, life stress (divorce, death of family member) | History | NIH toolkits |
| Environment Toxins Systemic racism Access to healthcare |
Air pollution Life opportunities Ability to get care when needed |
History | NIH toolkits |
| Perceived discrimination | Experience of age, race, gender, or sexual identify discrimination in jobs, health care, housing | History | NIH toolkits |
| Chronic diseases | Diabetes, cardiovascular disease, others | Diseases and medications, age of onset | |
| Reserve | |||
| Cognitive | Education, cognition | MMSE, Blessed, Mini-cog | Fact-cog, PROMIS, EORTC |
| Physiological | HbA1C, renal function, anemia, lipids | Routine lab | |
| Psychological resilience | Optimism, coping strategies | ||
| Geriatric syndromes | Incontinence, Malnutrition Falls, Gait disorders, Hearing/vision deficits, Fatigue Frailty | History of each; history of unintentional weight loss, exhaustion, weakness, decrease in physical activity | IADLS ADLS FACT-fatigue |
| Functional ability | Physical function Emotional well-being Social and role function |
Ability to rise from chair unassisted, 6-minute walk test. FEV1 G8, Vulnerable Elders Survey-13 (VES-13) |
Frailty or deficits accumulation indices; FACT, PROMIS, EORTC, performance status |
| Body composition | Sarcopenia, visceral fat | CAT scan | |
| Depression, anxiety | Feeling sad, anxious | History | CES-D, STAI, Geriatric depression scale, NIH tool kit |
| Social support | Family or friends to buffer stress, provide tangible support | History | MOS Social support |
| Lifestyle | Smoking, activity, sleep quality and duration | History | Actigraphy, IPAQ |
| Life expectancy | Non-cancer competing mortality | ePrognosis | |
Adapted from 75
Next, since oncologists will not have biological age measures from prior to diagnosis, it will be useful to determine medical history factors associated with the risk of different life course aging trajectories. In non-cancer populations multi-level factors effect life course biological age and aging trajectory, including genotype, poor sleep, chronic life stress, having multiple chronic conditions, harmful environmental exposures, and limits in community resources, socioeconomic opportunity and healthcare access.14,16,20,21 Large longitudinal population datasets could identify factors associated with pre-cancer life course aging trajectories.
Ensuring that biological age research protocols include diverse participants will be important54 since lower socioeconomic position, chronic stress related to adverse childhood events, poverty, depression, and experiencing racism have been linked to increased inflammation, early development of chronic conditions and accelerated life course aging.17–19,54 Most of these factors are not considered in current clinical oncology assessments, but it could be useful to query early-life circumstances and social position over the life course to identify patients that may be at risk for being biologically older than their chronological age at diagnosis, especially those presenting with mid-life cancers.54
Another research need is to determine situations where knowledge about biological age would change treatment decisions. For instance, are dose reductions needed for patients based on biological age like chemotherapy dose adjustments for reduced renal function? Does biological age at the time of diagnosis predict risk of treatment toxicity independently of geriatric assessment-based algorithms?45 Are there differences in risk of toxicity with specific regimens based on the etiology of biological aging (e.g., accumulated DNA repair failures vs. chronically high levels of stress hormones)? These questions could be addressed in cancer clinical trial correlative studies by including a history of life course factors known to associated with biological age trajectories together with pre-post treatment measures of biological age.
For survivorship care, it would be useful to determine variables that predict which cancer survivors will shift towards a greater biological age after treatment since only some survivors experience increases in physical and brain aging, fatigue and cognitive problems, and declines in function.15,26,40,52,55 Treatment trials or large cohort studies with detailed history, pre- and post-treatment biospecimens and functional status measures post-treatment follow-up of functioning could inform risk of change in biological age after therapy. Longitudinal studies could also be useful to determine if treatment-related effects on biological aging persist, are maintained, or get worse over time. Additionally, studies will be needed to evaluate the impact of targeting survivors for added surveillance and early intervention based on biological age or changes in biological age.
Moving forward, it will also be important to test combinations of functional and biological age indicators in the oncology setting.56 Research to move the field forward could evaluate the utility of computer simulation and artificial intelligence methods to combine data,57 and test associations between longitudinal changes in biological age,27 geriatric assessment, and decline in function in cancer survivors compared to contemporaries without cancer. Longitudinal measures will be critical to refine our conceptual framework to determine whether biological age trajectories are linear with shifts after specific “hits” due to life events or chronic diseases like cancer, or have more complex patterns.
Lastly, research is also needed to test interventions to modify biological age and decrease cancer treatment toxicities and maximize healthy survivorship. Links between markers and their specific underlying aging-related pathways could be useful to identify and test the ability of mechanistically-based interventions targeting modifiers of aging. Senolytic drugs are one class of pharmacotherapeutics shown to minimize several diseases of aging, and it is possible that these drugs may reduce cancer risk or protect against acceleration of biological aging after cancer therpay.58 Discovering optimal targets and timing for these pharmacotherapeutic interventions will be another critical future research direction.
Several social and behavioral modifiers of biological aging could also be tested in treatment and survivorship interventions to improve outcomes, including obesity reduction, increased physical activity, better sleep health, and reducing socioenvironmental adversities (e.g., systemic racism, economic stress, interpersonal stress, social isolation).15,17,49,59–62 Stopping smoking and increasing physical activity are two examples of interventions with evidence for improving survivors’ health that may also directly deaccelerate biological aging (i.e., are gero-protective).16,63,64 Calorie restriction, intermittent fasting, foods rich in anti-oxidants and drugs like metformin and senolytic agents are being tested in gerontology research and may decrease biological aging, and some may lower risk of cancer progression.8,65–68 As knowledge advances, it will be important to translate these results to gero-oncology trials with biological aging endpoints.
Conclusion
Applying a life course approach to aging among cancer patients and survivors can provide a nuanced understanding of how aging impacts cancer, and cancer and its treatments impact aging. This approach could guide cancer and aging research,28 provide evidence for personalizing cancer care to biological age, support development of clinically useful measures of change in biological age and testing of supportive care interventions informed by aging mechanisms. Overall, this approach could ultimately provide evidence to guide care and policy to equitably improve the health of all adult cancer survivors by maximizing treatment benefits and healthy survivorship while minimizing costs and harms.69–72
Acknowledgements
Dr. Mandelblatt had full access to all the data reviewed for this narrative and takes responsibility for the integrity of the summary and accuracy of the data included in the manuscript.
Funding Source
This research was supported by the National Cancer Institute at the National Institutes of Health grants R01CA129769 and R35CA197289 and the National Institute of Aging at the National Institutes of Health grant R01AG068193 to JM. As a member of the Cancer and Aging Research Group (CARG), JM’s work on this project was also supported in part by grant R33AG059206 (MPI: W Dale, S Mohile, H Klepin) from the National Institute of Aging at the National Institutes of Health. The work of AJS was supported in part by the National Institute of Aging at the National Institutes of Health grants P30AG10133, R01AG19771 and R01LM01136. TAA was supported in part by National Cancer Institute at the National Institutes of Health grants R01CA172119, U54 CA137788, and P30CA008748. The work of JC was supported in part by the American Cancer Society Research Scholars grant 128660-RSG-15-187-01-PCSM and the National Cancer Institute at the National Institutes of Health grant R01CA237535. HJC was supported in part by the National Institute of Aging at the National Institutes of Health grant P30AG028716 for the Duke Pepper Center.
Role of Funder/Sponsor Statement
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Jeanne Mandelblatt, Judith Carroll, and Harvey J. Cohen were the writing committee for this manuscript.
References
- 1.National Cancer Institute. NCI Annual Plan and Budget Proposal for FY 2020. https://www.cancer.gov/about-nci/budget/plan/2020-annual-plan-budget-proposal.pdf. Published 2020. Accessed September 18, 2020.
- 2.Markham MJ, Wachter K, Agarwal N, et al. Clinical cancer advances 2020: Annual report on progress against cancer from the American Society of Clinical Oncology. Journal of Clinical Oncology. 2020;38(10):1081. [DOI] [PubMed] [Google Scholar]
- 3.Bluethmann S, Mariotto A, Rowland J. Anticipating the “Silver Tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belsky DW, Caspi A, Arseneault L, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;9:e54870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockwood K, Howlett SE. Fifteen years of progress in understanding frailty and health in aging. BMC Med. 2018;16(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkhus L, Šaltytė Benth J, Rostoft S, et al. Geriatric assessment is superior to oncologists’ clinical judgement in identifying frailty. Br J Cancer. 2017;117(4):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice JN, Ferrucci L, Newman AB, et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. Geroscience. 2018;40(5-6):419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nature Reviews Cancer. 2020;20(2):89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodes RJ, Sierra F, Austad SN, et al. Disease drivers of aging. Ann N Y Acad Sci. 2016;1386(1):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci L, Gonzalez-Freire M, Fabbri E, et al. Measuring biological aging in humans: A quest. Aging Cell. 2020;19(2):e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muhandiramge J, Orchard S, Haydon A, Zalcberg J. The acceleration of ageing in older patients with cancer. J Geriatr Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 14.Ness KK, Kirkland JL, Gramatges MM, et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J Clin Oncol. 2018;36(21):2206–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cespedes Feliciano EM, Hohensee C, Rosko AE, et al. Association of Prediagnostic Frailty, Change in Frailty Status, and Mortality After Cancer Diagnosis in the Women’s Health Initiative. JAMA Netw Open. 2020;3(9):e2016747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahadi S, Zhou W, Schüssler-Fiorenza Rose SM, et al. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nature Medicine. 2020;26(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rentscher KE, Carroll JE, Mitchell C. Psychosocial Stressors and Telomere Length: A Current Review of the Science. Annu Rev Public Health. 2020;41:223–245. [DOI] [PubMed] [Google Scholar]
- 18.van der Linden B, Cheval B, Sieber S, Kliegel M, Cullati S. Adverse childhood experiences are associated with frailty in old age. Innovation in Aging. 2018;2(suppl_1):892–892. [Google Scholar]
- 19.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood—brain barrier dysfunction predicting cognitive decline. Nature. 2020;581(7806):71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward-Caviness CK, Nwanaji-Enwerem JC, Wolf K, et al. Long-term exposure to air pollution is associated with biological aging. Oncotarget. 2016;7(46):74510–74525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramírez CL, Cadiñanos J, Varela I, Freije JM, López-Otín C. Human progeroid syndromes, aging and cancer: new genetic and epigenetic insights into old questions. Cell Mol Life Sci. 2007;64(2):155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jylhävä J, Pedersen NL, Hägg S. Biological Age Predictors. EBioMedicine. 2017;21:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson BI, Lippman ME. Targeting RAGE Signaling in Inflammatory Disease. Annu Rev Med. 2018;69:349–364. [DOI] [PubMed] [Google Scholar]
- 25.Wardill HR, Mander KA, Van Sebille YZ, et al. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int J Cancer. 2016;139(12):2635–2645. [DOI] [PubMed] [Google Scholar]
- 26.Soria-Valles C, López-Soto A, Osorio FG, López-Otín C. Immune and inflammatory responses to DNA damage in cancer and aging. Mech Ageing Dev. 2017;165(Pt A):10–16. [DOI] [PubMed] [Google Scholar]
- 27.Belsky DW, Moffitt TE, Cohen AA, et al. Eleven Telomere, Epigenetic Clock, and Biomarker-Composite Quantifications of Biological Aging: Do They Measure the Same Thing? Am J Epidemiol. 2018;187(6):1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubbard JM, Cohen HJ, Muss HB. Incorporating biomarkers into cancer and aging research. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(24):2611–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shachar SS, Deal AM, Reeder-Hayes KE, et al. Effects of Breast Cancer Adjuvant Chemotherapy Regimens on Expression of the Aging Biomarker, p16INK4a. JNCI Cancer Spectrum. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371–384. [DOI] [PubMed] [Google Scholar]
- 32.Marioni RE, Shah S, McRae AF, et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015;44(4):1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castagné R, Garès V, Karimi M, et al. Allostatic load and subsequent all-cause mortality: which biological markers drive the relationship? Findings from a UK birth cohort. Eur J Epidemiol. 2018;33(5):441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dugué P-A, Bassett JK, Joo JE, et al. DNA methylation-based biological aging and cancer risk and survival: Pooled analysis of seven prospective studies. International Journal of Cancer. 2018;142(8):1611–1619. [DOI] [PubMed] [Google Scholar]
- 35.Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao S, Hu Q, Kerns S, et al. Impact of chemotherapy for breast cancer on leukocyte DNA methylation landscape and cognitive function: a prospective study. Clinical Epigenetics. 2019;11(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjærg-Hansen A, Bojesen SE. Short Telomere Length, Cancer Survival, and Cancer Risk in 47102 Individuals. JNCI: Journal of the National Cancer Institute. 2013;105(7):459–468. [DOI] [PubMed] [Google Scholar]
- 38.Duggan C, Risques R, Alfano C, et al. Change in peripheral blood leukocyte telomere length and mortality in breast cancer survivors. Journal of the National Cancer Institute. 2014;106(4):dju035–dju035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song N, Li Z, Qin N, et al. Shortened Leukocyte Telomere Length Associates with an Increased Prevalence of Chronic Health Conditions among Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort. Clinical Cancer Research. 2020;26(10):2362–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alfano CM, Peng J, Andridge RR, et al. Inflammatory Cytokines and Comorbidity Development in Breast Cancer Survivors Versus Noncancer Controls: Evidence for Accelerated Aging? J Clin Oncol. 2017;35(2):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guida JL, Ahles TA, Belsky D, et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J Natl Cancer Inst. 2019;111(12):1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noam V, Reshma J, Efrat D, et al. NCCN Guidelines Insights: Older Adult Oncology, Version 2.2016. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw. 2016;14(11):1357–1370. [DOI] [PubMed] [Google Scholar]
- 43.Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncology. 2017;3(12):e172319–e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockwood K, Howlett SE. Age-related deficit accumulation and the diseases of ageing. Mech Ageing Dev. 2019;180:107–116. [DOI] [PubMed] [Google Scholar]
- 45.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol. 2016;34(20):2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerard EJ, Deal AM, Chang Y, et al. Frailty Index Developed From a Cancer-Specific Geriatric Assessment and the Association With Mortality Among Older Adults With Cancer. J Natl Compr Canc Netw. 2017;15(7):894–902. [DOI] [PubMed] [Google Scholar]
- 47.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157(19):2259–2268. [PubMed] [Google Scholar]
- 48.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. [DOI] [PubMed] [Google Scholar]
- 49.Van Dyke ME, Baumhofer NKi, Slopen N, et al. Pervasive Discrimination and Allostatic Load in African American and White Adults. Psychosomatic Medicine. 2020;82(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic Load and Frailty in Older Adults. Journal of the American Geriatrics Society. 2009;57(9):1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seeman T, Singer B, Ryff C, Dienberg-Love G, Levy-Storms L. Social relationships, gender and allostatic load across two age cohorts. Psychosomatic Medicine. 2002;64:395–406. [DOI] [PubMed] [Google Scholar]
- 52.Williams GR, Chen Y, Kenzik KM, et al. Assessment of Sarcopenia Measures, Survival, and Disability in Older Adults Before and After Diagnosis With Cancer. JAMA Network Open. 2020;3(5):e204783–e204783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dale W WG, MacKenzie A, Soto-Perez-de-Celis E, Maggiore R, Merrill JK, Kata S, Smith KT, Klepin HD. How Is geriatric assessment used in clinical practice for older adults with cancer? A survey of cancer providers by the American Society of Clinical Oncology. Journal of oncology practice. 2020. 10.1200/OP.20.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vineis P, Avendano-Pabon M, Barros H, et al. Special Report: The Biology of Inequalities in Health: The Lifepath Consortium. Front Public Health. 2020;8:118–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mandelblatt JS ZX, Small BJ, Ahn J, Zhai W, Ahles T, Extermann M, Graham D, Jacobsen PB, Jim H, McDonald BC, Patel S, Root JC, Saykin AJ, Cohen HJ, Carroll JE Deficit Accumulation Frailty Trajectories of Older Breast Cancer Survivors and Non-Cancer Controls: The Thinking and Living with Cancer Study. J Nat’l Cancer Inst. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bell CG, Lowe R, Adams PD, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019;20(1):249–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kendiukhov I AI-based investigation of molecular biomarkers of longevity. Biogerontology. 2020; 10.1007/s10522-020-09890-y. [DOI] [PubMed] [Google Scholar]
- 58.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The Clinical Potential of Senolytic Drugs. Journal of the American Geriatrics Society. 2017;65(10):2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forrester S, Jacobs D, Zmora R, Schreiner P, Roger V, Kiefe CI. Racial differences in weathering and its associations with psychosocial stress: The CARDIA study. SSM - Population Health. 2019;7:100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, et al. Exercise Attenuates the Major Hallmarks of Aging. Rejuvenation Research. 2014;18(1):57–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moskalev A, Chernyagina E, Tsvetkov V, et al. Developing criteria for evaluation of geroprotectors as a key stage toward translation to the clinic. Aging cell. 2016;15(3):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guida JL, Agurs-Collins T, Ahles TA, et al. Strategies to Prevent or Remediate Cancer and Treatment-Related Aging. J Natl Cancer Inst. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crescioli C Targeting Age-Dependent Functional and Metabolic Decline of Human Skeletal Muscle: The Geroprotective Role of Exercise, Myokine IL-6, and Vitamin D. Int J Mol Sci. 2020;21(3):1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belsky DW, Caspi A, Cohen HJ, et al. Impact of early personal-history characteristics on the Pace of Aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging cell. 2017;16(4):644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moskalev A, Chernyagina E, Kudryavtseva A, Shaposhnikov M. Geroprotectors: A Unified Concept and Screening Approaches. Aging Dis. 2017;8(3):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anisimov VN. Metformin for cancer and aging prevention: is it a time to make the long story short? Oncotarget. 2015;6(37):39398–39407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gill TM. Translational Geroscience: Challenges and Opportunities for Geriatric Medicine. J Am Geriatr Soc. 2019;67(9):1779–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guy GP, Yabroff KR, Ekwueme DU, Rim SH, Li R, Richardson LC. Economic Burden of Chronic Conditions Among Survivors of Cancer in the United States. Journal of Clinical Oncology. 2017;35(18):2053–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown GC. Living too long: the current focus of medical research on increasing the quantity, rather than the quality, of life is damaging our health and harming the economy. EMBO Rep. 2015;16(2):137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marsman D, Belsky DW, Gregori D, et al. Healthy ageing: the natural consequences of good nutrition-a conference report. European journal of nutrition. 2018;57(Suppl 2):15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demaria M, O’Leary MN, Chang J, et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017;7(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 75.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36(22):2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]


