Abstract
A comparison of antibody titers to JC virus (JCV) or BK virus (BKV) was made by hemagglutination inhibition (HI) and enzyme immunoassay (EIA) with 114 human plasma samples. Antibody titers to JCV or BKV determined by HI were lower than those determined by EIA. Nevertheless, as HI titers increased so did EIA titers. When antibody data were compared by the Spearman rank correlation test, highly significant correlations were found between HI and EIA titers. Results obtained by plotting EIA antibody titers for JCV against those for BKV generally showed a reciprocal relationship, i.e., samples with high antibody titers to JCV had lower antibody titers to BKV and vice versa. Some samples, however, had antibody titers to both viruses. Of the samples tested, 25.4% (25 of 114) had HI and EIA antibody titers to JCV and BKV which were identical or closely related. This is not the scenario one would expect for cross-reactive epitopes shared by the two viruses, but one suggesting that these samples were from individuals who had experienced infections by both viruses. Adsorption with concentrated JCV or BKV antigen of sera with high antibody titers to both JCV and BKV and testing by JCV and BKV EIA gave results which support this conclusion. Although 52.6% (51 of 97) of the samples from the Japanese population tested had very high antibody titers (≥40,960) to either JCV or BKV, none of the samples were found by a dot blot immunoassay to have antibodies which cross-reacted with simian virus 40. The results from this study, in agreement with those of others, suggest that humans infected by JCV or BKV produce antibodies to species-specific epitopes on their VP1 capsid protein, which is associated with hemagglutination and cellular binding.
Until recently, antibody titers to JC virus (JCV) were measured by inhibition of JCV hemagglutination (HA) activity. HA inhibition (HI) assays were used for this purpose because of the ease and rapidity with which they could be performed. Many contemporary assays for measuring antibodies to viral and other antigens employ enzyme immunoassay (EIA) techniques because of their greater sensitivity and precision relative to HI. Detection of JCV and BK virus (BKV) in urine by antigen capture EIA was reported over a decade ago (2). However, EIA for antigen capture or antibody detection did not become widely used because of the restricted range of cell types infectable by JCV, its lengthy growth cycle, and its poor replication capacity, thus making antigen preparation for use in EIAs labor intensive, time-consuming, and costly for testing large numbers of samples.
EIA development for JCV antibody detection was made easier by the creation of a permanent cell line, SVG, which supports the growth of JCV (19). Recently, Frye et al. utilized the SVG cell line to propagate Mad-4 strain 586 JCV for use in detecting antibodies to JCV by EIA (9). More recently, the VP1 structural protein of Mad-4 JC was cloned into pBlueBacIII and expressed in Spodoptera frugiperda clone 158 cells. Purified VP1 from this clone was then used to develop an EIA to study the systemic and intrathecal antibody responses to JCV infection of patients with progressive multifocal leukoencephalopathy (35). VP1 forms the outer coat of the JCV virion and makes up 75% the total virion protein, and epitopes of VP1 are responsible for its agglutination of erythrocytes (8, 22).
We have utilized another approach to facilitate JCV antigen production for use in EIA. Vacante and coworkers constructed a hybrid JCV-simian virus 40 (SV40) strain in which they ligated a regulatory sequence from SV40 into the regulatory region of JCV (33). Relative to unmodified natural strains of JCV, the hybrid construct replicates faster, produces higher titers of virus, and has an expanded range of infectable cell types of primate origin. Coupled with these improved characteristics, this hybrid, called JCV-SVE(Δ), still produces virions which retain the total antigenic capacity of JCV because the regulatory sequences of JCV, in their native or this hybrid form, do not code for the three structural proteins, VP1, VP2, and VP3, which make up its virion (33).
In this report, we describe development of EIAs by using hybrid JCV-SVE(Δ) or BKV (Gardner strain) as the antigen and study the relationship between HA and EIA antibody titers for these viruses. The Japanese population samples studied were also tested for antibodies to SV40.
MATERIALS AND METHODS
Source of plasma samples.
The plasma samples used in this study were from two groups. The first group consisted of 97 samples from a large collection made in Hiroshima, Japan, between 1967 and 1984 by personnel from the Radiation Effects Research Laboratory. These samples were collected to determine whether transmitted cytogenetic damage occurred among children born to parents exposed to radiation from the atomic bombing of Hiroshima, Japan. Many of the samples from this group were previously reported to have very high HI antibody titers to JCV (22). JCV and BKV have been reported to share extensive base sequence and amino acid homology (8). Probably only a limited portion of the total epitopes present in the JCV and BKV capsid proteins are involved in HI. Therefore, the very high HI antibody titers found in this Japanese sample group made it an excellent resource to study whether EIA detects antibodies to epitopes of a more global nature than HI. Because of the uniqueness of this Japanese group, 17 samples from a multiethnic group of normal laboratory personnel were studied as a control. All plasma samples were stored frozen at −70°C or below after collection.
Virus growth.
JCV was grown in SVG cells, a cell line established by immortalization of human fetal brain cells with an origin-defective mutant of SV40 (19). The hybrid Mad-1–SVEΔ strain of JCV, constructed by insertion of a regulatory region sequence from SV-40 into the regulatory region of the Mad-1–SVE strain of JCV, was used in these studies because of its greater replication capacity relative to unmodified JCV strains. Harvests of culture medium (Eagle minimum essential medium with 10% fetal bovine serum and 50 μg of gentamicin per ml) from JCV-infected cultures grown in 75-cm2 flasks (Costar Corp., Cambridge, Mass.) were begun about 2 weeks after infection when pronounced cytopathology was observed microscopically. To sustain production of high titers of JCV, about 5 × 106 uninfected SVG cells and fresh medium (25 ml) were added to infected cultures exhibiting pronounced cytopathology, and virus harvests were made at weekly intervals thereafter.
BKV (Gardner strain) was grown in low-passage human embryonic kidney (HEK) cells as described previously (10).
Virus purification.
Cellular debris was removed from harvests of both JCV and BKV by low speed centrifugation (1,500 × g for 15 min). The supernatant medium was passed through a 0.2-μm-pore-size Nalgene filter (Nalge Co., Rochester, N.Y.), and the virus in the filtered supernatant medium was pelleted by ultracentrifugation (105,000 × g for 90 min). JCV or BKV pellets were resuspended and layered onto continuous 25 to 60% Optiprep (Life Technologies, Gaithersburg, Md.) gradients and banded by density gradient ultracentrifugation (160,000 × g for 16 h). Gradients were fractionated, and fractions were monitored for levels of JCV or BKV by HA of human type O erythrocytes. Fractions containing peak JCV or BKV HA titers were pooled and used as antigen for enzyme immunoassays.
HI.
The HI assays were performed as described in detail previously (22, 24).
EIA.
The optimum concentrations of JCV or BKV antigen and the respective reagents used to perform EIA were determined by block titration. EIA was performed in 96-well, flat-bottom, Immulon-4 microtiter plates (Dynatech Laboratories, Chantilly, Va.). Coating solution, washing solution, blocking buffer, sample diluent solution, and other reagents specified were purchased from Kirkegaard & Perry Laboratories, Gaithersburg, Md., and used in accordance with the manufacturer's instructions.
Approximately 2,500 HA units of purified JCV or BKV were added to each plate well in 100 μl of coating solution, and the plates were incubated overnight (ca. 18 h) at 4°C to adsorb virus to surfaces of plate wells. After plate wells were washed to remove unadsorbed virus, blocking buffer (200 μl) was added to each plate well, and each plate was incubated for 1 h at room temperature (23 to 25°C) to minimize nonspecific binding of serum immunoglobulin and test reagents to the solid phase. Serum samples, starting at a 1:40 dilution (100 μl/plate well), were diluted in plate wells in serial fourfold increments, incubated at 37°C for 30 min, and washed. The following reagents (100 μl/plate well) were then added sequentially with plate washing between each reagent addition as follows: biotin-labeled goat antihuman immunoglobulin G (H&L), for 1 h at 37°C; peroxidase-labeled streptavidin, for 30 min at 37°C; and 3,3′,5,5′-tetramethylbenzidene substrate, for 30 min at 23 to 25°C. The substrate reaction was stopped by the addition of stop solution (100 μl/plate well), and sample absorbance was measured at 450 nm with a SpectraMAX Plus spectrophotometer (Molecular Devices Corp., Sunnyvale, Calif.). Serum samples with an absorbance of 0.05 greater than that of appropriate serum controls were considered positive.
Serum adsorption.
Cloned JCV or BKV VP1 (106 HA units/50 μl) was used to adsorb antibody from three undiluted sera (0.2 ml each) found upon HI and EIA testing to have antibodies to both JCV and BKV. The cloned VP1 strains, grown in baculovirus, were a generous gift from Stephan Frye and Peter Jensen (unpublished data), Laboratory of Molecular Medicine and Neuroscience, National Institute of Neurological Disorders and Stroke, Bethesda, Md. Adsorption with intermittent shaking was done for 2 h at room temperature (23 to 25°C) and overnight at 4°C. After adsorption was completed, adsorbing JCV or BKV antigen was removed by centrifugation at 4°C for 90 min at 100,000 × g.
Dot blot assay.
All plasma samples from the Japanese population utilized were tested for antibodies to SV40 by a dot blot immunobinding assay on nitrocellulose (11).
Statistical analysis of data.
Data were analyzed by the Spearman rank correlation test, a test which compares rank order number, rather than actual values, of two sets of variables to calculate a correlation coefficient. If a monotonic relationship exists between the two variables, one variable is invariably associated with an increase or decrease in the other (36).
RESULTS
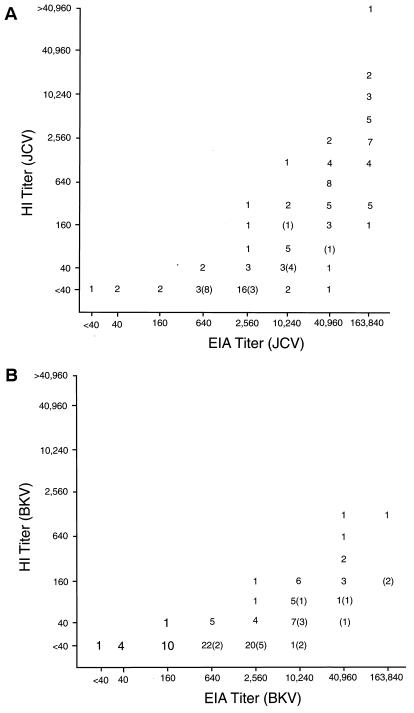
To clarify the relationship between antibody test results for BKV and JCV obtained by HI and EIA, we used both tests to determine antibody titers for both viruses on 97 plasma samples from the unique Japanese population described and on control samples from 17 laboratory personnel. Although a higher proportion of the Japanese population samples studied had higher JCV HI and EIA antibody titers than samples from the laboratory personnel studied, samples from both groups with similar JCV HI titers had similar EIA titers (Fig. 1A). The BKV HI and EIA antibody titers found in samples of both study groups were more similar in titer than those for JCV (Fig. 1B).
FIG. 1.
Relationship between HI and EIA antibody titers to JCV (A) or BKV (B) in samples from 97 children born to survivors of the atomic bombing of Hiroshima, Japan. The data for the 17 control laboratory personnel are in parentheses. The Spearman rank correlations for data from the Japanese and laboratory personnel groups were, respectively, 0.814 and 0.885 for the JCV antibody data in panel A and 0.696 and 0.844 for the BKV antibody data in panel B.
A highly significant relationship was found between HI and EIA antibody titers to JCV and BKV when data were analyzed by the Spearman rank correlation test (Fig. 1). Our results show that antibody titers to both JCV and BKV determined by HI are much lower than those determined by EIA. Nevertheless, results from both tests correlated well because, for most samples, as HI titers increased exponentially so did EIA titers. The reciprocal relationship existing between JCV and BKV HI and EIA antibody titers found in the majority of the samples studied provided evidence that the epitopes in the respective viruses to which antibodies were synthesized were predominantly species specific. Generally, samples found to have high BKV antibody titers by EIA were found to have lower EIA antibody titers to JCV. Conversely samples with high EIA antibody titers to JCV had lower antibody titers to BKV. Low or moderate EIA antibody titers to JCV and BKV were also found in a high percentage of samples (Fig. 1 and see also Table 2).
TABLE 2.
Relationship between HI and EIA titers for JCV and BKV
| Virus | HI-EIA titer category | EIA titer | No. of samples with EIA titer | HI titer | No. of samples with HI titer | % EIA test results in category (no. positive/total no.) |
|---|---|---|---|---|---|---|
| JCV | Low | ≤2,560 | 35 | <40 | 38 | 92.1 (35/37) |
| JCV | Moderately low | ≥2,560 | 11 | 40 | 13 | 84.6 (11/13) |
| JCV | Moderately high | ≥10,240 | 23 | 80 to 320 | 26 | 88.5 (23/26) |
| JCV | High | ≥40,960 | 36 | ≥640 | 37 | 97.3 (36/37) |
| BKV | Low | ≤2,560 | 64 | <40 | 67 | 95.5 (64/67) |
| BKV | Moderately low | ≥2,560 | 15 | 40 | 21 | 71.4 (15/21) |
| BKV | Moderately high | ≥10,240 | 21 | 80 to 320 | 23 | 91.3 (21/23) |
| BKV | High | ≥40,960 | 3 | ≥640 | 3 | 100 (3/3) |
Some samples studied, however, had relatively high antibody titers to both viruses. Antibody adsorption studies were performed to determine whether these high antibody titers to both viruses resulted from (i) the high degree of amino acid relatedness between JCV and BKV and, thus, to antibody responses to concomitant shared epitopes, or (ii) to infection of individuals by both viruses and the antibody responses to unique epitopes of each virus. Table 1 shows the results of selective adsorption of three sera found to have high antibody titers to JCV and BKV. Sera adsorbed with JCV-specific antigen and tested in a JCV EIA had essentially no antibody to JCV, whereas sera adsorbed with BKV specific antigen and tested in a BKV EIA had essentially no antibody to BKV. Conversely, sera adsorbed in the reciprocal relationship, i.e., BKV adsorbed and tested in a JCV EIA and JCV adsorbed and tested in a BKV EIA, had essentially the same antibody titer for JCV or BKV as that of their respective unadsorbed matched serum controls. These results strongly suggest that the individuals from which these sera were obtained had undergone both JCV and BKV infection at some time in their lives.
TABLE 1.
Adsorption with BKV and JCV antigens of sera with high antibody titers to both viruses
| Serum no. | EIA antibody titers with:
|
|||||
|---|---|---|---|---|---|---|
| JCV antigen
|
BKV antigen
|
|||||
| Unadsorbed serum control | JCV adsorbed serum | BKV adsorbed serum | Unadsorbed serum control | BKV adsorbed serum | JCV adsorbed serum | |
| 1 | 40,960 | 160 | 10,240 | 10,240 | 640 | 10,240 |
| 2 | 40,960 | <40 | 40,960 | 40,960 | 640 | 40,960 |
| 3 | 40,960 | <40 | 10,240 | 10,240 | 40 | 10,240 |
EIA has greater sensitivity and has the potential to detect a wider range of epitopes than HI. As shown by our data, threshold and endpoint antibody titers for JCV or BKV obtained by HI and EIA generally differ widely. As a rough guide to permit extrapolation of antibody titers to JCV or BKV from either HI or EIA data, we have subdivided our results into the four titer categories shown in Table 2: low, HI titer of <40 and EIA titer of ≤2,560; moderately low, HI titer of 40 and EIA titer of ≥2,560; moderately high, HI titer of 80 to 320 and EIA titer of ≥10,240; and high, HI titer of ≥640 and EIA titer of ≥40,960. Most sample results fell within these categories; however, some results were discordant. The majority of these discordant results came from samples with low to moderately low HI titers (≤40) but moderately high EIA titers (≥10,240). Of the samples tested, 9.6% (11 of 114) showed this relationship for antibodies to JCV and 12.2% (14 of 114) to BKV (Fig. 1).
None of the 97 samples from the Japanese population studied was found by dot blot immunoassay to have antibodies to SV40 (20). All of these samples were tested under code, and all three rhesus monkey control sera included with these samples were correctly identified: two SV40 antibody-positive samples and one SV40 antibody-negative sample.
DISCUSSION
Antibodies abrogate HA by JCV or BKV by combining with specific epitopes on their VP1 capsid proteins (8). Thus, relative HI antibody titers for these viruses should reflect the degree to which they share common epitopes on their VP1 capsid proteins. The amino acids coded by the JCV and BKV genomes have been reported to have 79% homology. Therefore, a high degree of cross-reactivity might be expected among antibodies produced in response to infection by either of these viruses. Our results, however, do not reflect this scenario. They show that individual sera have widely different antibody titers to JCV and BKV. In fact, 25 of 28 samples with high JCV HI titers ranging from 320 to >40,960 and a JCV EIA titer of 163,840, our highest EIA titer detected, had HI BKV titers of ≤40 and EIA BKV titers of ≤2560. Similarly, 10 of 11 samples with BKV HI titers ranging from 80 to 1,280 and an EIA titer of ≥40,960 had JCV HI titers of ≤40 and an EIA titer of ≤2,560. These results suggest that the major epitopes to which antibodies are directed are those which define JCV or BKV species specificity. This result may not be too surprising because hyperimmunization of animals was required before genus-specific antigens or minor cross-reacting capsid surface antigens could be demonstrated for JCV, BKV, and SV40 (34).
Infections by viruses which share epitopes with a close relative have been shown to elicit cross-reactive antibodies. However, antibody titers to the virus causing an infection usually are much higher than to its relative. Many of our test samples had antibodies to both JCV and BKV in HI and EIA tests. Of the two samples groups tested, 25.4% (29 of 114) showed dual reactivity. Many of these samples had antibody titers which were closely related or identical for both JCV and BKV, which is not the result one would expect for cross-reactive antibodies to related, unidentical epitopes. A plausible explanation of these results is that samples with similar antibody titers to JCV and BKV were from individuals who had experienced infections by both viruses. Our antibody adsorption studies support this conclusion. PCR analysis of urine samples by Shah et al. (27) also provide evidence for this conclusion. Their results show it to be fairly common for individuals to experience infection by both JCV and BKV. Of the immunosuppressed patients in that study, a low percentage also were found to have undergone simultaneous infection by both viruses.
As mentioned above, a low percentage of repeatly tested samples had low HI antibody titers and high EIA antibody titers to JCV or BKV. The reasons for these disparate results are unknown. Possible explanations are antibody responses to epitopes other than those involved with HI or to strain differences between the virus initiating an infection and that used in antibody assays.
All 97 of the plasma samples from Japanese patients tested for antibody to JCV or BKV by HI or EIA were also tested for antibody to SV40 by a dot blot assay (11, 20). None of the samples were found to have antibodies to SV40, albeit 52.6% (51 of 97) of the samples tested for JCV antibodies and 9.3% of the samples tested for BKV antibodies had titers of ≥40,960. The failure of high-titer antibodies to JCV or BKV to cross-react with SV40, even though about 70% of its amino acids are homologous to those of JCV and BKV, may be related to its inability to hemagglutinate erythrocytes. Our results show that infection of humans by JCV or BKV elicits antibodies primarily to species-specific epitopes strongly associated with HA.
As recently discussed by Liu et al. (17, 18), sialic acid has been shown to be a major receptor component for all polyomaviruses, except SV40 (12–14, 21, 25, 28). By comparing the crystal structure of SV40 and mouse polyomavirus, it was shown that the inability of SV40 to bind to sialic acids resided in the truncation of an 8-amino-acid segment from its VP1 protein (16, 30). In mouse polyomavirus, the 8-amino-acid segment missing in SV40 was shown to form an integral part of an external loop, located on the surface of its capsid, which directly interacts with sialic acids. JCV also differs from SV40 in that it does not use major histocompatibility complex class 1 proteins as a receptor to initiate infection of glial cells (17). Enzymatic removal of α(2-3) and α(2-6) sialic acid from erythrocytes and SVG cells resulted in loss of JCV's ability to agglutinate erythrocytes and to bind to SVG cells. However, removal of only α(2-3)-linked sialic acids from erythrocytes and SVG cells neither abrogated JCV HA nor its ability to bind to or to infect cells, suggesting that these JCV cellular interactions are critically linked to α(2-6) sialic acids (18). The ability of JCV to infect only a limited number of cell types has been shown to result from intracellular mechanisms involving viral gene transcription and viral DNA replication (1, 31, 32). Therefore, for antibody to be effective in neutralizing JCV and probably BKV, it likely must combine with species-specific epitopes on their VP1 capsid protein involved in cellular binding, thus preventing virus attachment and subverting infection. Indeed, previous data have shown that HI and neutralization of JCV correlate well (24). The correlation of our HI and EIA data further suggests that in JCV or BKV infections of humans, a major portion of antibodies produced are to epitopes on their VP1 capsid proteins involved with binding to cell membranes.
SV40 was shown to be a contaminant of early vaccines, and reports have linked it to infection of humans and cancer (3–5, 15). Other reports have failed to substantiate these claims (27, 29). The development of sensitive and specific tests for JCV, BKV, and SV40 may provide tools to resolve these issues.
ACKNOWLEDGMENTS
We thank James V. Neel, Department of Human Genetics, University of Michigan, Ann Arbor, Mich., for furnishing the Japanese population samples used in this study and James Dambrosia, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Md., for performing the statistical analysis of study data.
REFERENCES
- 1.Amemiya K, Traub R, Durham L, Major E O. Interaction of nuclear factor-1-like protein with the regulatory region of the human polyomavirus JC virus. J Biol Chem. 1989;264:7025–7032. [PubMed] [Google Scholar]
- 2.Arthur R R, Shah K V, Yolken R H, Charachi P. Detection of human papovaviruses BKV and JCV in urines by ELISA. In: Sever J L, Madden D L, editors. Polyomaviruses and human neurological disease. New York, N.Y: Liss; 1983. pp. 169–176. [PubMed] [Google Scholar]
- 3.Bergsagel D J, Finegold M J, Butel J S, Kupsky W J, Garcea R L. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med. 1992;326:988–993. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- 4.Carbone M, Pass H I, Rizzo P, Marinetti M, Di Muzio M, Mew D J Y, Levine A S, Procopio A. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9:1781–1790. [PubMed] [Google Scholar]
- 5.Carbone M, Rizzo P, Procopio A, Giuliano M, Pass H I, Gebhardt M C, Mangham C, Hansen M, Malkin D F, Bushart G, Pompetti F, Picci P, Levine A S, Bergsagel J D, Garcea R L. SV40-like sequences in human bone marrow tumors. Oncogene. 1996;13:527–535. [PubMed] [Google Scholar]
- 6.Chen M H, Benjamin T. Roles of N-glycans with alpha 2,6 as well as alpha 2,3 linked sialic acid in infection by polyoma virus. Virology. 1997;233:440–442. doi: 10.1006/viro.1997.8596. [DOI] [PubMed] [Google Scholar]
- 7.Flaegstad T, Traavik T. Detection of BK virus IgM antibodies by two enzyme-linked immunosorbent assays (ELISA) and a hemagglutination inhibition method. J Med Virol. 1985;17:195–204. doi: 10.1002/jmv.1890170212. [DOI] [PubMed] [Google Scholar]
- 8.Frisque R J, Bream G L, Cannella M T. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frye S, Trebst C, Dittmer U, Petry H, Bodemer M, Hunsmann G, Weber T, Luke W. Efficient production of JC virus in SVG cells and the use of purified viral antigens for analysis of specific humoral and cellular immune response. J Virol Methods. 1997;63:81–92. doi: 10.1016/s0166-0934(96)02117-9. [DOI] [PubMed] [Google Scholar]
- 10.Gardner S D, Field A M, Coleman D V, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;i:1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 11.Heberling R L, Kalter S S. Rapid dot-immunobinding assay on nitrocellulose for viral antibodies. J Clin Microbiol. 1986;23:109–113. doi: 10.1128/jcm.23.1.109-113.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann M, von der Leith C W, Stehling P, Reutter W, Pawlita M. Consequences of subtle sialic acid modification on the murine polyomavirus receptor. J Virol. 1997;71:5922–5931. doi: 10.1128/jvi.71.8.5922-5931.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keppler O T, Herrmann M, Oppenlander M, Meschede W, Pawlita M. Regulation of susceptibility and cell surface receptor for the B-lymphotropic papovavirus by N glycosylation. J Virol. 1994;68:6933–6939. doi: 10.1128/jvi.68.11.6933-6939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keppler O T, Stehling P, Herrmann M, Kayser H, Grunow D, Reutter W, Pawlita M. Biosynthetic modulation of silaic acid-dependent virus-receptor interactions of two primate polyoma viruses. J Biol Chem. 1995;270:1308–1314. doi: 10.1074/jbc.270.3.1308. [DOI] [PubMed] [Google Scholar]
- 15.Lednicky J A, Garcea R L, Bergsagel D J, Butel J S. Natural simian virus 40 strains are present in human choroid plexus and ependymoma tumors. Virology. 1995;212:710–717. doi: 10.1006/viro.1995.1529. [DOI] [PubMed] [Google Scholar]
- 16.Liddington R, Yan Y, Moulai J, Sahli R, Benjamin T, Harrison S. Structure of simian virus 40 at 3.8 Å resolution. Nature. 1991;354:278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 17.Liu C K, Hope A P, Atwood W J. The human polyoma virus, JCV, does not share receptor specificity with SV40 on human glial cells. J Neurovirol. 1998;4:49–58. doi: 10.3109/13550289809113481. [DOI] [PubMed] [Google Scholar]
- 18.Liu C K, Wei G, Atwood W J. Infection of glial cells by the human polyomavirus JCV is mediated by an N-linked glycoprotein containing α (2-6) linked sialic acids. J Virol. 1998;72:4643–4649. doi: 10.1128/jvi.72.6.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Major E O, Miller A E, Mourrain P, Traub R G, De Widt E. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci USA. 1985;82:1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Major E O, Neel J V. The JC and BK human polyoma viruses appear to be recent introductions to some South American Indian tribes: there is no serological evidence of cross-reactivity with the simian polyoma virus SV40. Proc Natl Acad Sci USA. 1998;95:15525–15530. doi: 10.1073/pnas.95.26.15525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantyjarvi R, Arstila P, Meurman O. Hemagglutination by BK virus, a tentative new member of the papovavirus family. Infect Immun. 1972;6:824–828. doi: 10.1128/iai.6.5.824-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neel J V, Major E O, Awa A A, Glover T, Burgess A, Traub R, Curfman B, Satoh C. Hypothesis: “rogue cell”-type chromosomal damage in lymphocytes is associated with infection with the JC human polyoma virus and has implications for oncogenesis. Proc Natl Acad Sci USA. 1996;93:2690–2695. doi: 10.1073/pnas.93.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padgett B L, Walker D L. Prevalence of antibodies in human sera against JV virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973;127:467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- 24.Padgett B L, Walker D L. New human papovaviruses. Prog Med Virol. 1976;22:1–35. [PubMed] [Google Scholar]
- 25.Seganti L, Mastromarino P, Superti F, Sinibaldi L, Orsi N. Receptors for BK virus on human erythrocytes. Acta Virol. 1981;25:177–181. [PubMed] [Google Scholar]
- 26.Shah K V, Daniel R W, Kelly T J., Jr Immunological relatedness of papovaviruses of the simian virus 40-polyoma subgroup. Infect Immun. 1977;18:558–560. doi: 10.1128/iai.18.2.558-560.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah K V, Daniel R W, Strickler H D, Goedert J J. Investigation human urine for genomic sequences of the primate polyoma viruses simian virus 40, BK virus and JC virus. J Infect Dis. 1997;176:1618–1621. doi: 10.1086/517340. [DOI] [PubMed] [Google Scholar]
- 28.Sinibaldi L, Goldoni P, Pietropaolo V, Cattani L, Peluso C, Di Taranto C. Role of phospholipids in BK virus infection and haemagglutination. Microbiologica. 1992;15:337–344. [PubMed] [Google Scholar]
- 29.Strickler H D, Goedert J J, Fleming M, Travis W D, Williams A E, Rabkin C S, Daniel R W, Shah K V. Simian virus 40 and pleural mesothelioma in humans. Cancer Epidemiol Biomarkers Prev. 1996;5:473–475. [PubMed] [Google Scholar]
- 30.Stehle T, Yan Y, Benjamin L, Harrison S C. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature. 1994;369:160–163. doi: 10.1038/369160a0. [DOI] [PubMed] [Google Scholar]
- 31.Sumner C, Shinohara T, Durham L, Traub R, Major E O, Amemiya K. Expression of multiple classes of the nuclear factor-1 family in the developing human brain: differential expression of two classes of NF-1 genes. J Neurovirol. 1996;2:87–100. doi: 10.3109/13550289609146542. [DOI] [PubMed] [Google Scholar]
- 32.Tamura T A, Inoue T, Nagata K, Mikoshiba K. Enhancer of human polyoma JC virus contains nuclear factor 1-binding sequences; analysis using mouse brain nuclear extracts. Biochem Biophys Res Commun. 1988;157:419–425. doi: 10.1016/s0006-291x(88)80265-1. [DOI] [PubMed] [Google Scholar]
- 33.Vacante D A, Traub R, Major E O. Extension of JC host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology. 1989;170:353–361. doi: 10.1016/0042-6822(89)90425-x. [DOI] [PubMed] [Google Scholar]
- 34.Walker D L, Frisque R J. The biology and molecular biology of JC virus. In: Salzman N P, editor. The Papovaviridae. Vol. 1. New York, N.Y: Plenum Press; 1986. pp. 327–377. [Google Scholar]
- 35.Weber T, Trebst C, Frye S, Cinque P, Vago L, Sindic C, Schulz-Schaeffer W J, Kretzschmar H A, Enzensberger W, Hunsmann G, Luke W. Analysis of the systemic and intrathecal humoral response in progressive multifocal leukoencephalopathy. J Infect Dis. 1997;176:250–254. doi: 10.1086/514032. [DOI] [PubMed] [Google Scholar]
- 36.Woolfon R F. Statistical methods for the analysis of biomedical data. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 268–270. [Google Scholar]