Key Points
Question
Does a combination of oral nicotinamide and pyruvate help with short-term improvement in visual function in individuals with glaucoma?
Findings
In this phase 2, randomized clinical trial, a combination of nicotinamide and pyruvate was safe and the number of improving visual field test locations was higher in the treatment group vs the placebo group over a median of 2.2 months.
Meaning
Oral nicotinamide and pyruvate can result in short-term improvement in visual function in patients with treated, manifest glaucoma.
This phase 2 randomized clinical trial tests the hypothesis that a combination of nicotinamide and pyruvate can improve retinal ganglion cell function in individuals with glaucoma as measured with standard automated perimetry.
Abstract
Importance
Open-angle glaucoma may continue to progress despite significant lowering of intraocular pressure (IOP). Preclinical research has suggested that enhancing mitochondrial function and energy production may enhance retinal ganglion cell survival in animal models of glaucoma, but there is scant information on its effectiveness in a clinical setting.
Objective
To test the hypothesis that a combination of nicotinamide and pyruvate can improve retinal ganglion cell function in human glaucoma as measured with standard automated perimetry.
Design, Setting, and Participants
In this phase 2, randomized, double-blind, placebo-controlled clinical trial at a single academic institution, 197 patients were assessed for eligibility. Of these, 42 patients with treated open-angle glaucoma and moderate visual field loss in at least 1 eye were selected for inclusion and randomized. A total of 32 completed the study and were included in the final analysis. The mean (SD) age was 64.6 (9.8) years. Twenty-one participants (66%) were female. Participant race and ethnicity data were collected via self-report to ensure the distribution reflected that observed in clinical practice in the US but are not reported here to protect patient privacy. Recruitment took place in April 2019 and patients were monitored through December 2020. Data were analyzed from January to May 2021.
Interventions
Ascending oral doses of nicotinamide (1000 to 3000 mg) and pyruvate (1500 to 3000 mg) vs placebo (2:1 randomization).
Main Outcomes and Measures
Number of visual field test locations improving beyond normal variability in the study eye. Secondary end points were the rates of change of visual field global indices (mean deviation [MD], pattern standard deviation [PSD], and visual field index [VFI]).
Results
Twenty-two of 29 participants (76%) randomized to the intervention group and 12 of 13 participants (92%) randomized to placebo received their allocation, and 32 participants (32 eyes; ratio 21:11) completed the study (21 from the intervention group and 11 from the placebo group). Median (IQR) follow-up time was 2.2 (2.0-2.4) months. No serious adverse events were reported during the study. The number of improving test locations was significantly higher in the treatment group than in the placebo group (median [IQR], 15 [6-25] vs 7 [6-11]; P = .005). Rates of change of PSD suggested improvement with treatment compared with placebo (median, −0.06 vs 0.02 dB per week; 95% CI, 0.02 to 0.24; P = .02) but not MD (0.04 vs −0.002 dB per week; 95% CI, −0.27 to 0.09; P = .35) or VFI (0.09 vs −0.02% per week; 95% CI, −0.53 to 0.36; P = .71).
Conclusions and Relevance
A combination of nicotinamide and pyruvate yielded significant short-term improvement in visual function, supporting prior experimental research suggesting a role for these agents in neuroprotection for individuals with glaucoma and confirming the need for long-term studies to establish their usefulness in slowing progression.
Trial Registration
ClinicalTrials.gov Identifier: NCT03797469
Introduction
Randomized clinical trials in glaucoma have demonstrated that lowering intraocular pressure (IOP) is associated with reduced risk of glaucoma onset and progression across the disease spectrum, regardless of severity.1,2,3,4,5,6 However, glaucoma continued to progress in many individuals despite significant IOP reduction, a finding also commonly seen in clinical practice. The consensus opinion of the authors is that this is owing to either insufficient lowering of IOP, IOP-independent risk factors, or a combination of the two.3,7,8 In an effort to further protect retinal ganglion cells (RGCs) and their axons, recent studies have investigated the association between nutritional biomarkers and the risk of glaucoma9,10 as well as the potential benefit of nutritional supplementation on measures of visual function.10,11,12,13
Mitochondrial abnormalities may be an early driver of neuronal dysfunction in glaucoma.14 In the DBA/2J mouse model (studying inherited, chronic, elevated IOP and glaucoma in mice) and in an inducible model of ocular hypertension in rats, these mitochondrial and metabolic changes occurred even before detectable neurodegeneration.15,16 In particular, investigators have reported that oral administration of nicotinamide (the amide of vitamin B3 and a precursor for nicotinamide adenine dinucleotide [NAD], a key molecule in energy and redox metabolism) was neuroprotective in the DBA/2J model. At the highest dose tested, 93% of eyes in DBA/2J did not develop glaucoma, as determined by the absence of RGC somal and axonal loss.15 Given the decrease in NAD with aging,15,17 which may render retinal neurons more vulnerable to disease-related insults, the investigators hypothesized that increasing NAD may support the mitochondrial activity of RGCs and decrease their susceptibility to glaucoma.18,19,20,21 In a rat model of ocular hypertension, retinal and optic nerve NAD declined as a function of IOP, while nicotinamide was neuroprotective at a range of doses.16 In addition, nicotinamide has been shown to be low in the sera of patients with primary open-angle glaucoma.10 These data further support a role for NAD in glaucoma. Furthermore, Harder et al22 reported that an IOP-mediated decrease in retinal pyruvate levels was associated with dysregulated glucose metabolism prior to detectable optic nerve degeneration in metabolic studies of DBA/2J mice. They also found that oral supplementation with pyruvate protected both rat and mouse models of glaucoma from neurodegeneration, with a combination of nicotinamide and pyruvate being the most protective in the chronic mouse model.
There is currently an unmet need for neuroprotective agents that are able to help slow rates of visual field progression in glaucoma beyond the known benefits of lowering IOP.23,24,25 Nonetheless, glaucoma is often a slowly progressive disease, which demands a relatively large sample size and long-term follow-up for clinical trials to provide sufficient statistical power to detect protective effects, particularly among patients already treated with IOP-lowering medications. Given the significant neuroprotective effects observed in recent studies,15,22 the similarities in cellular biology of NAD+ and pyruvate pathways between mice and humans, as well as the safety profile of these supplements, we investigated the effect of nicotinamide and pyruvate on the visual function of patients with treated manifest glaucoma.
Methods
The institutional review board of Columbia University Irving Medical Center approved this study, which adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all participants. Participants received compensation for participation in this trial. This study was registered in the National Library of Medicine and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol can be found in Supplement 1.
Patients
Patients were recruited from the Columbia University Irving Medical Center. Patients aged 40 to 80 years with best-corrected visual acuity equal to or better than 20/40 with a diagnosis of open-angle glaucoma and 24-2 standard automated perimetry (SAP) with mean deviation (MD) between −3.0 and −12.0 dB in both eyes were included in the study. The definition of glaucoma was based on the presence of glaucomatous optic neuropathy on optic disc photographs and confirmed with optical coherence tomography (OCT). A glaucomatous visual field was defined based on the presence of a glaucoma hemifield test result outside normal limits and/or a pattern standard deviation (PSD) less than 5% on at least 2 consecutive examinations performed prior to enrollment. To minimize learning effects, all patients were required to have undergone at least 3 visual field examinations in the 3 years prior to enrollment. All patients were taking IOP-lowering medications selected at the discretion of the treating physicians. Participant race and ethnicity data were collected via self-report to ensure the distribution reflected that observed in clinical practice in the US but are not reported here to protect patient privacy.
Patients with significant cataract or media opacity that could interfere with perimetry as well as those with ocular or systemic conditions known to affect the results were excluded from the study. Patients who underwent incisional glaucoma surgery or laser procedures within 6 months of screening were not included. No changes in therapy were performed during the duration of the study. Patients were required to have stopped taking any nutritional supplements for at least 1 week prior to enrollment. Patients with previous intolerance to nicotinamide or pyruvate and those unable to take multiple pills were also excluded.
Testing
After participants met the screening inclusion and exclusion criteria and provided written informed consent, they were scheduled to undergo visual field testing, optic disc photography, OCT, and standard ophthalmologic examinations per protocol. Participants underwent visual field testing with the SAP 24-2 Swedish interactive thresholding algorithm (SITA) standard program of the Humphrey field analyzer (Carl Zeiss Meditec).
Crabb and Garway-Heath26 demonstrated through computer simulation that the identification of visual field progression could be improved by clustering tests at the beginning and end of follow-up periods in the so-called wait-and-see approach. This approach appears to increase statistical power by minimizing the negative effects of variability on estimation of the MD slopes. This effect was later confirmed in a real-world sample of patients in the United Kingdom Glaucoma Treatment Study (UKGTS),5 in which a statistically significant difference between treatment and placebo was detected as early as the 12-month visit.
In parallel to this approach, participants in the current study underwent a cluster of 4 visual field examinations within 2 weeks prior to randomization, followed by an interim examination at week 6 and an additional cluster of 4 examinations within a 2-week period at the end of the study. For safety assessment, an additional visual field test was administered 1 week after the last dose. Reliability indices were required to be less than 15% for false positive responses and less than 20% for fixation losses. Although studies suggest that fixation losses may not be crucial parameters to assess test reliability in the clinical setting,27,28 in a clustered test paradigm in which subjects may be more likely to perform unreliable tests, we opted to set this criterion so as to not negatively impact the study power to detect small pointwise sensitivity changes. If a participant was unable to achieve reliable test results within a cluster of examinations, the examinations were repeated until the best results were obtained after being reviewed by a masked expert grader (C.G.D.M.).
Optic disc photography was performed within 6 months prior to the first study visit and at the end of study visit. Images were reviewed for safety assessment, to confirm diagnoses and to rule out retinal pathologies. All images were reviewed by a masked expert grader (C.G.D.M.).
Participants underwent OCT testing using Cirrus 5000 (Carl Zeiss Meditec) at baseline and end of study visits. OCT results were not used for primary end point determination but rather for diagnosis confirmation, safety analysis, and exclusion of other conditions that could influence the results. The optic nerve head and retinal nerve fiber layer (RNFL) scans included 200 × 200 cube scans. Good quality scans required signal strength greater than 6.
The Montréal Cognitive Assessment (MoCA) is a cognitive screening test designed to assist in the detection of cognitive impairment. MoCA scores range from 0 to 30. A score of 26 or higher is considered normal. Although we excluded participants based on self-reported causes of cognitive impairment, we used MoCA as a quantitative assessment of cognitive function that could potentially improve as a result of nicotinamide and pyruvate supplementation and that could interfere in the interpretation of our results. The questionnaire was administered at baseline, week 4, week 8, and end of study. Standard ophthalmic examinations, including IOP measurements with Goldmann applanation tonometry, medication review, medical and ocular history, safety monitoring, and adverse events were performed at every visit.
Agents
Nicotinamide and pyruvate doses were set at a high level determined to be safe in humans using body surface area calculations that reflect metabolic rate differences across species to calculate human doses equivalent to those effective against glaucoma in mice.15,22,29 A review of the safety of nicotinamide for different indications in humans revealed an excellent profile in doses higher than the one tested in this trial.30
Nicotinamide and pyruvate were combined, as the former increases NAD levels15 while the latter can both overcome deficits in glycolysis and act as an antioxidant.22 Recent studies demonstrated alterations in both glucose and pyruvate metabolism in a mouse model of glaucoma and support a deficit in glycolysis.22,31 The combination of both molecules at equivalent doses was more effective at preventing glaucoma in a murine model than either molecule alone.22 Placebo pills were chosen so they could best match shape and color of the active agents as well as the number of pills taken at each visit.
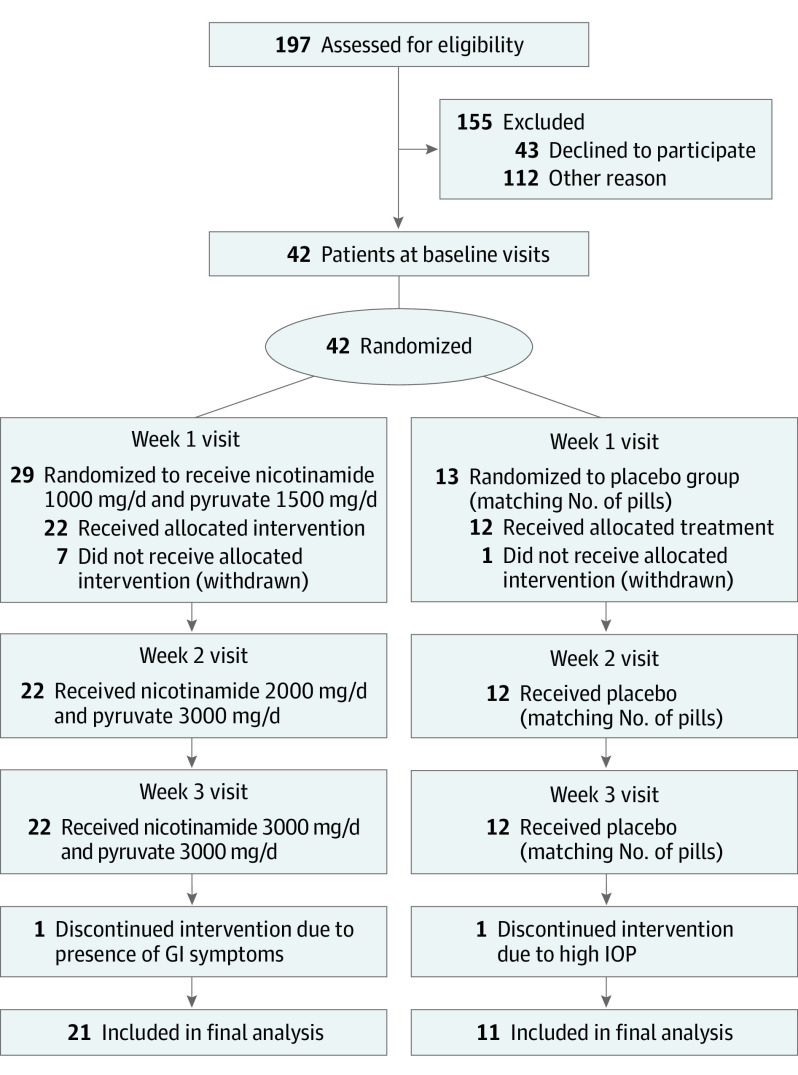
The schedule of visits and doses are depicted in the Figure. Before dosing, participants underwent the first cluster of 4 visual field tests that served as their baseline. Once these tests were completed, they returned for randomization. For both agents, we started with a low dose (1000 mg of nicotinamide and 1500 mg of pyruvate) for 1 week to assess tolerability, followed by an intermediate dose (2000 mg of nicotinamide and 3000 mg of pyruvate) for another week. At that point, another visual field test was performed to assess safety. Finally, the doses were raised to the final high dose (3000 mg of nicotinamide and 3000 mg of pyruvate) for 1 more week, at which point the second cluster of 4 visual field tests was administered. Once all tests were completed with participants taking the high dose, both agents were discontinued, and a final visual field test was performed at the end of study visit. At the end of each dose escalation, participants returned for ophthalmological examination, medication review, medical and ocular history, safety monitoring, and adverse events.
Figure. CONSORT Diagram.
GI indicates gastrointestinal; IOP, intraocular pressure.
Randomization (2:1) was performed with Stata version 14.2 (StataCorp). After obtaining consent, the research coordinator assessed the randomization log in a consecutive fashion and assigned treatment vs placebo accordingly. Placebo pills were selected to best mimic the active agents and the same number of pills was given at each escalation period so as to prevent participants from inferring their randomization status. Aside from the research coordinator in charge of assessing the randomization log and dispensing drugs, investigators, technicians, and participants were masked from randomization assignment at each visit and throughout the study. For each visual field test performed, an expert reviewed the test for quality assurance and when needed a repeat test was performed while maintaining masking from group randomization.
Statistical Analyses
Only 1 eye per participant was included in the analyses. If both eyes met the inclusion and exclusion criteria, the eye with better quality of visual field test results prior to enrollment was included. This study was primarily powered to detect differences in the number of improving test locations in the study eye based on the parameter estimates described32 for short-term enhancement of visual field sensitivity following surgical reduction of IOP. As a primary end point, we compared the number of test locations experiencing rates of change (slopes) of age-corrected sensitivities (total deviation) exceeding the upper 25th percentile of the limits of variability of the entire data set. For 80% statistical power (1-β; type II error) and α level (type I error) of 5%, SDs of 3, and a 2:1 sample allocation, a minimum of 36 patients (36 eyes) would be needed to detect a mean difference of 3.0 points between groups using an independent-samples t test. Assuming a dropout rate of 10%, a minimum of 40 participants needed to be enrolled.
Owing to the 2020 coronavirus pandemic and the temporary freeze in clinical studies at our institution, in addition to participants’ concerns regarding attending office visits for extended periods, we stopped enrollment after 32 participants had completed all visits. The data presented here correspond to that final sample and a post hoc power calculation was performed.
Continuous variables are described as means with SDs or medians with IQRs, whereas categorical variables are reported as proportions with percentages. The primary outcome measure was the difference in the number of improving test locations between groups. The preplanned secondary outcomes were differences in rates of change (slopes) of the MD, PSD, and visual field index (VFI). Preplanned exploratory outcomes were differences in rates of change in OCT circumpapillary RNFL (cpRNFL) and MoCA scores. Differences in improvement beyond the 25th percentile of the distribution of rates of change (MD, PSD, and VFI) were treated as post hoc outcomes.
Independent samples t test was used to test the primary end point. The rate of change in pointwise sensitivities (pointwise linear regression) was calculated with least squares linear regression using the total deviation values of the 24-2 pattern after excluding the points above and below the blind spot. Logistic mixed-effects regression was used to test for the association between the likelihood of significant pointwise improvement after adjusting for baseline sensitivity. In addition, ancillary end points were explored, such as differences in rates of change of MD, VFI, and PSD using linear mixed-effects models. The same model was used to assess differences in OCT metrics and MoCA scores. For OCT, changes in global cpRNFL between the 2 time points were analyzed as a secondary outcome measure. Statistical significance was defined at P < .05 (2-tailed) for the primary outcome. No multiple comparisons adjustment was performed for other analyses. Computerized statistical analyses were performed with Stata version 14.2 (StataCorp).
Results
A total of 32 participants (21 randomized to treatment and 11 to placebo) completed all planned visits and had complete data for analyses. Of these, 21 participants (66%) were female. Table 1 summarizes their clinical characteristics. Nineteen of 32 participants (59.38%) had moderate visual field loss (mean [SD] severity, −7.0 [3.0] dB). There were no differences between groups in any of the tested parameters, including medical or surgical therapy. There were also no significant differences between groups regarding visual field reliability indices. The Figure depicts the CONSORT diagram describing enrollment, allocation to treatment, disposition status, and how participants were analyzed in the trial.
Table 1. Clinical Characteristics of the Study Sample by Randomization Group.
| Characteristic | Mean (SD) | |
|---|---|---|
| Treatment group (21 patients [21 eyes]) | Placebo group (11 patients [11 eyes]) | |
| Age, y | 64.3 (10.5) | 65.2 (8.6) |
| Female, No. (%) | 13 (62) | 8 (73) |
| Male, No. (%) | 8 (38) | 3 (27) |
| Baseline | ||
| Mean deviation, dB | −6.8 (3.0) | −7.9 (2.7) |
| PSD, dB | 7.3 (3.1) | 9.5 (3.5) |
| VFI, % | 82.5 (9.8) | 77.7 (10.9) |
| Baseline global RNFL, µm | 64.2 (10.1) | 63.2 (10.7) |
| Study duration, median, IQR, mo | 2.2 (2.0-2.4) | 2.8 (1.0) |
| No. of antiglaucoma medications | 1.8 (1.0) | 2.4 (1.4) |
| Type of medication (prostaglandins), No. (%) | 15 (71.4) | 9 (81.8) |
| Surgery, No. (%) | ||
| Laser | 7 (33.3) | 7 (63.6) |
| Incisional | 9 (42.8) | 3 (27.2) |
Abbreviations: PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; VFI, visual field index.
There were no reported serious adverse events during the study. The most commonly reported adverse event was mild gastrointestinal discomfort reported in 10 of 32 participants (31%) (7 of 21 in the treatment group [33%] vs 3 of 11 [27%] in the placebo group; P > .99), none of which required any intervention for treatment and which improved with continued dosing. One participant in the nicotinamide and pyruvate group discontinued treatment (despite mild gastrointestinal symptoms) at the discretion of the study team. No clinically significant detrimental changes in visual function (eg, perimetry or visual acuity) or ocular anatomy (eg, slitlamp examination, disc photographs, or OCT) were noted during the treatment period or at the end of study visit.
Regarding the primary end point, the number of improving test locations was significantly higher in the treatment group vs the placebo group (median [IQR], 15 [6-25] vs 7 [6-11]; P = .005). The post hoc power calculation using the observed data revealed a statistical power of 84% (type II error of 16%). Logistic mixed-effects regression revealed that eyes treated with nicotinamide and pyruvate were more likely to experience improving test locations (odds ratio [OR], 3.20; 95% CI, 1.25-8.16; P = .01), which was independent from the baseline sensitivity of test locations (for each dB more positive, the odds of pointwise improvement were 9% lower; P < .001) (Table 2). These results suggest that treatment with nicotinamide and pyruvate tripled the likelihood of improvement of test locations relative to placebo. To assess whether these findings were not the result of chance alone, we compared the number of progressing (worsening) test locations based on the same criteria as for improvement. There was no difference between treatment and placebo groups (median [IQR], 12 [6-21] vs 11 [5-20]; P = .66).
Table 2. Bivariate Logistic Mixed-Effects Regression Testing the Association Between Randomization, Baseline Severity, and Pointwise Improvement.
| Parameter | OR (95% CI) | P value |
|---|---|---|
| Baseline sensitivity (per dB higher) | 0.91 (0.89-0.92) | <.001 |
| Randomization (treatment vs placebo) | 3.20 (1.25-8.16) | .02 |
Abbreviation: OR, odds ratio.
The median (IQR) of the baseline total deviation values of improving test locations (positive slopes) was −5 (−2 to −10) dB whereas the median (IQR) for those that deteriorated (negative slopes) was −3 (−1 to −7) dB (P < .001). Mixed-effects models revealed no significant difference between groups regarding baseline total deviation sensitivities within the range more likely to improve (ie, between −2 and −10 dB; mean difference, 0.42 dB; 95% CI, −0.40 to 1.25; P = .31).
The rates of visual field PSD improvement were higher in the treatment group (median [IQR], −0.06 [−0.30 to 0.06] dB per week vs 0.02 [−0.07 to 0.07] dB per week; 95% CI, 0.02 to 0.24; mixed-effects model P = .02) (Table 3). There was no difference between groups regarding rates of change of the visual field MD (median [IQR], 0.04 [−0.10 to 0.28] dB per week vs −0.002 [−0.10 to 0.05] dB per week; 95% CI, −0.27 to 0.09; P = .35). Yet, when looking at the number of eyes experiencing an MD slope more positive than the upper 25th percentile of the distribution of the entire sample (more than 0.13 dB per week), more eyes experienced improvement in the treatment group than in the placebo group (8 of 21 vs 0 of 11; Fisher exact test P = .02). Similarly, although there was no difference in rates of change of the VFI (median [IQR], 0.09% [−0.4 to 0.6] per week vs −0.02 [−0.2 to 0.1] per week; 95% CI, −0.53 to 0.36; P = .71), more eyes in the treatment group experienced improvement beyond the 25th percentile (more than 0.45% per week; 8 of 21 vs 0 of 11; P = .02).
Table 3. Univariable Linear Mixed-Effects Models Testing Differences in Rates of Change of Visual Field Global Indices Between Treatment Groups.
| Parameter | β Coefficient (95% CI)a | P value |
|---|---|---|
| Mean deviation, dB/wk | −0.08 (−0.27 to 0.09) | .36 |
| Pattern standard deviation, dB/wk | 0.12 (0.01 to 0.23) | .02 |
| Visual field index, %/wk | −0.08 (−0.53 to 0.36) | .71 |
The coefficient β corresponds to the difference in slopes between groups.
We found no effect of randomization on rates of change of OCT global retinal nerve fiber layer thickness (difference, 0.18; 95% CI, −0.06 to 0.43; P = .13). No differences in MoCA scores were noted between groups (difference, 0.06 units; 95% CI, −0.08 to 0.20; P = .42).
Discussion
In this randomized, double-blind, placebo-controlled clinical trial, patients with manifest open-angle glaucoma treated with a combination of nutritional supplements (nicotinamide and pyruvate) experienced a statistically significant improvement in visual function based on the number of improving test locations on SAP compared with the placebo group. In addition, the rate of change of the visual field PSD was significantly different and suggestive of improvement of global perimetric sensitivity. Although the rate of change of other global visual field indices and OCT global RNFL did not differ statistically between groups, all tested parameters changed in the same direction, suggesting improvement of structure and function. Of note, the results were not associated with any serious adverse reactions to the agents investigated.
The rationale for this clinical trial derived from neuroprotective effects seen in murine models.15,18,19,20,21,22 Williams et al15 have used RNA sequencing to analyze the RGCs of DBA/2J mice at different ages to elucidate the earliest molecular changes that occur in glaucoma. Notably, they identified mitochondrial dysfunction as one of the first changes within RGCs in that model. These results provided the basis for metabolic profiling studies that identified an age-dependent depletion of NAD as a primary driver of RGC vulnerability in glaucoma. By repleting NAD levels with a diet supplemented with nicotinamide, they reported significant neuroprotection from glaucoma in mice, as measured by synapse loss, RGC loss (assessed by soma counts), optic nerve degeneration, and pattern electroretinography. Additionally, the investigators have found that mice with genetically modified or gene-therapy increased NAD levels experienced neuroprotection, including protection against decreased dendritic field area, decreased branching complexity of RGCs, and synaptic loss.15,18,19,20 Further mouse-based studies uncovered abnormalities in glucose and pyruvate metabolism in glaucoma with combined nicotinamide and pyruvate treatment being the most effective.22 Despite this evidence favoring the use of nicotinamide for glaucoma neuroprotection, to our knowledge, no prior studies had been conducted in humans to support the clinical use of nicotinamide either alone or with pyruvate. The results of our trial support potential benefit in humans as well, at least for a short duration, which may serve as basis for larger, longer clinical trials that could provide more evidence for its use in the clinical setting.
Parallel to the present study, a recent report12 demonstrated neuroprotective effects of nicotinamide in humans in a randomized placebo-controlled clinical trial. The investigators gave 57 patients with treated glaucoma 1500 mg of nicotinamide per day for 6 weeks then 3000 mg per day for 6 weeks followed by crossover without washout. They observed a significant improvement in electroretinography parameters (photopic negative response) during that period as well as an improvement in the visual field MD. The present study showed a statistically significant effect on standard automated perimetry over a very short time period, which should be underscored as this is currently the reference standard to measure visual function and to assess patient-important outcomes in clinical and research settings.
The strongest signal identified in our study was in changes in pointwise visual field sensitivity. The number of improving test locations had an odds 3 times greater than in placebo-treated eyes. Most of these improving locations corresponded to areas of mild to moderate functional loss. This observation sheds light on the possible mechanism of action of the tested agents. Although some authors disagree that visual function can improve in glaucoma, many studies have confirmed that significant, sustained sensitivity improvement can occur following substantial IOP reduction.33,34,35 For instance, Caprioli et al34 reported that 57% of eyes had at least 10 improving visual field locations after trabeculectomy. One hypothesis is that, in addition to dead and healthy RGCs in patents with glaucoma, some cells may still be functioning at variable firing rates.36,37 Nicotinamide and pyruvate supplementation and their effects on the mitochondria and energetic status of these cells may rescue these cells to a state with better function, which is translated into improved perimetric and electroretinographic measures. In the present study, improving test locations tended to be those with mild to moderate sensitivity loss, likely because severely damaged or dead cells (or those with normal function) are less likely to respond to supplementation. In addition, these locations tended to be seen at the edge of scotomata, which are more likely to correspond to the location of RGCs with impaired (but not absent) electrical activity.36,37 Among the global indices tested, the strongest signal was seen in the PSD, which is a parameter often considered to be more specific for glaucoma, as it reflects asymmetry (or variability) in the island of vision typically seen in mild to moderate glaucoma. Although a weaker signal was seen in the MD and VFI, a subanalysis looking at thresholds for improvement analogous to the one conducted by Hui et al12 confirmed a similar and statistically significant result. Although OCT changes were not significant in the current study, this imaging modality was performed only twice (at baseline and end of study) for safety assessment and was therefore underpowered. Nonetheless, the nonsignificant OCT trend was also in the direction of improvement (ie, thicker RNFL). Although axons of RGCs in glaucoma may not regrow, they could increase in thickness due to improved mitochondrial activity and axonal transport.38,39 Our rationale for presenting these data was to show that at least no significant progression (or worsening) on OCT was observed, which could go against our functional finding.
Besides the investigation of a potential neuroprotective treatment for glaucoma, the present study used a novel design aimed at improving the statistical power to detect visual field changes using clusters of tests, which is called the wait-and-see approach.26 To date, studies suggesting the usefulness of this approach, which recommends intense testing at the beginning and end of follow-up, have been limited to computerized simulations or longer periods of follow-up than the present investigation.40,41 In the UKGTS,5 clustering was performed at baseline, 18 months, and 24 months. The investigators were able to detect statistically significant changes even at 12 months of follow-up. This is remarkable, as clinical trials in glaucoma to date have required large sample sizes and long follow-up to be able to detect statistically significant changes between groups. To our knowledge, the present study is the first to demonstrate the power of intensive clustering (median 2.2 months) to detect group changes over a highly compressed time frame in humans in a real-world setting. This has important implications for clinical trial design, suggesting that this approach is feasible and may indeed be able to reduce costs of future trials in glaucoma.
Limitations
This study has limitations. Our findings are applicable for short-term changes in visual function. We cannot yet extrapolate that supplementation with nicotinamide and pyruvate at the present dose will result in slower rates of visual field progression or sustained functional improvement over extended periods. As a lifelong and chronic disease, glaucoma demands treatment interventions that are able to slow or halt progression, such as decay in visual function, to the extent that vision-related quality of life is not significantly affected during the patient’s life span. Nevertheless, our findings show that this combination of agents could be used in larger clinical trials with a good safety profile and an underlying mechanism of action consistent with the protection of RGCs. Another limitation of the present study is that 76% of participants randomized to the intervention group and 100% of those randomized to placebo received their allocation, with only 1 participant in each group discontinuing intervention. These observations may limit the likelihood that the groups remained balanced by the randomization as well as the robustness of the intent-to-treat analysis. Although the reason was the same for all individuals (research restrictions owing to the COVID-19 pandemic in New York), this could potentially have led to differences in measured and unmeasured variables at baseline and hence have influenced the main outcomes. Additionally, although we did not find a significant effect of the active agent on cognition, one should consider that MoCA is a crude test for that purpose and a more extensive cognitive battery would ideally be used to test for this outcome. Moreover, as an ancillary end point, the sample size was not calculated for that purpose and hence the study may have been underpowered to test for changes in cognition.
Conclusions
In summary, nutritional supplementation with high doses of nicotinamide and pyruvate can yield short-term improvement in visual field sensitivity in treated glaucoma patients with moderate functional loss. The selection of agents targeting NAD and bioenergetic capacity to support cellular resilience may enable the development of new neuroprotective therapies for glaucoma patients. The use of a clustered visual field testing paradigm will likely prove useful for the testing of future neuroprotective agents.
Trial protocol
Data sharing statement
References
- 1.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-720. doi: 10.1001/archopht.120.6.714 [DOI] [PubMed] [Google Scholar]
- 2.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E; Early Manifest Glaucoma Trial Group . Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48-56. doi: 10.1001/archopht.121.1.48 [DOI] [PubMed] [Google Scholar]
- 3.The AGIS Investigators . The Advanced Glaucoma Intervention Study (AGIS): 7. the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429-440. doi: 10.1016/S0002-9394(00)00538-9 [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Normal-Tension Glaucoma Study Group . The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498-505. doi: 10.1016/S0002-9394(98)00272-4 [DOI] [PubMed] [Google Scholar]
- 5.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295-1304. doi: 10.1016/S0140-6736(14)62111-5 [DOI] [PubMed] [Google Scholar]
- 6.Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S; Low-Pressure Glaucoma Study Group . A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151(4):671-681. doi: 10.1016/j.ajo.2010.09.026 [DOI] [PubMed] [Google Scholar]
- 7.Susanna R Jr, De Moraes CG, Cioffi GA, Ritch R. Why do people (still) go blind from glaucoma? Transl Vis Sci Technol. 2015;4(2):1. doi: 10.1167/tvst.4.2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furlanetto RL, De Moraes CG, Teng CC, et al. ; Low-Pressure Glaucoma Treatment Study Group . Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am J Ophthalmol. 2014;157(5):945-952. doi: 10.1016/j.ajo.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 9.Huynh B, Shah P, Sii F, Hunter D, Carnt N, White A. Low systemic vitamin D as a potential risk factor in primary open-angle glaucoma: a review of current evidence. Br J Ophthalmol. 2021;105(5):595-601. doi: 10.1136/bjophthalmol-2020-316331 [DOI] [PubMed] [Google Scholar]
- 10.Kouassi Nzoughet J, Chao de la Barca JM, Guehlouz K, et al. Nicotinamide deficiency in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2019;60(7):2509-2514. doi: 10.1167/iovs.19-27099 [DOI] [PubMed] [Google Scholar]
- 11.Marino PF, Rossi GCM, Campagna G, Capobianco D, Costagliola C; Qualicos Study Group . Effects of citicoline, homotaurine, and vitamin E on contrast sensitivity and visual-related quality of life in patients with primary open-angle glaucoma: a preliminary study. Molecules. 2020;25(23):E5614. doi: 10.3390/molecules25235614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui F, Tang J, Williams PA, et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: a crossover randomized clinical trial. Clin Exp Ophthalmol. 2020;48(7):903-914. doi: 10.1111/ceo.13818 [DOI] [PubMed] [Google Scholar]
- 13.Rolle T, Dallorto L, Rossatto S, Curto D, Nuzzi R. Assessing the performance of daily intake of a homotaurine, carnosine, forskolin, vitamin B2, vitamin B6, and magnesium based food supplement for the maintenance of visual function in patients with primary open angle glaucoma. J Ophthalmol. 2020;2020:7879436. doi: 10.1155/2020/7879436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tribble JR, Vasalauskaite A, Redmond T, et al. Midget retinal ganglion cell dendritic and mitochondrial degeneration is an early feature of human glaucoma. Brain Commun. 2019;1(1):fcz035. doi: 10.1093/braincomms/fcz035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PA, Harder JM, Foxworth NE, et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355(6326):756-760. doi: 10.1126/science.aal0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tribble JR, Otmani A, Sun S, et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol. 2021;43:101988. doi: 10.1016/j.redox.2021.101988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes AP, Price NL, Ling AJ, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155(7):1624-1638. doi: 10.1016/j.cell.2013.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams PA, Harder JM, Foxworth NE, Cardozo BH, Cochran KE, John SWM. Nicotinamide and WLDS act together to prevent neurodegeneration in glaucoma. Front Neurosci. 2017;11:232. doi: 10.3389/fnins.2017.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams PA, Harder JM, Cardozo BH, Foxworth NE, John SWM. Nicotinamide treatment robustly protects from inherited mouse glaucoma. Commun Integr Biol. 2018;11(1):e1356956. doi: 10.1080/19420889.2017.1356956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harder JM, Braine CE, Williams PA, et al. Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc Natl Acad Sci U S A. 2017;114(19):E3839-E3848. doi: 10.1073/pnas.1608769114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams PA, Harder JM, John SWM. Glaucoma as a metabolic optic neuropathy: making the case for nicotinamide treatment in glaucoma. J Glaucoma. 2017;26(12):1161-1168. doi: 10.1097/IJG.0000000000000767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harder JM, Guymer C, Wood JPM, et al. Disturbed glucose and pyruvate metabolism in glaucoma with neuroprotection by pyruvate or rapamycin. Proc Natl Acad Sci U S A. 2020;117(52):33619-33627. doi: 10.1073/pnas.2014213117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Moraes CG, Liebmann JM, Levin LA. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog Retin Eye Res. 2017;56:107-147. doi: 10.1016/j.preteyeres.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordeiro MF, Levin LA. Clinical evidence for neuroprotection in glaucoma. Am J Ophthalmol. 2011;152(5):715-716. doi: 10.1016/j.ajo.2011.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawlor M, Danesh-Meyer H, Levin LA, Davagnanam I, De Vita E, Plant GT. Glaucoma and the brain: trans-synaptic degeneration, structural change, and implications for neuroprotection. Surv Ophthalmol. 2018;63(3):296-306. doi: 10.1016/j.survophthal.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 26.Crabb DP, Garway-Heath DF. Intervals between visual field tests when monitoring the glaucomatous patient: wait-and-see approach. Invest Ophthalmol Vis Sci. 2012;53(6):2770-2776. doi: 10.1167/iovs.12-9476 [DOI] [PubMed] [Google Scholar]
- 27.Bickler-Bluth M, Trick GL, Kolker AE, Cooper DG. Assessing the utility of reliability indices for automated visual fields. testing ocular hypertensives. Ophthalmology. 1989;96(5):616-619. doi: 10.1016/S0161-6420(89)32840-5 [DOI] [PubMed] [Google Scholar]
- 28.Yohannan J, Wang J, Brown J, et al. Evidence-based criteria for assessment of visual field reliability. Ophthalmology. 2017;124(11):1612-1620. doi: 10.1016/j.ophtha.2017.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27-31. doi: 10.4103/0976-0105.177703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knip M, Douek IF, Moore WP, et al. ; European Nicotinamide Diabetes Intervention Trial Group . Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43(11):1337-1345. doi: 10.1007/s001250051536 [DOI] [PubMed] [Google Scholar]
- 31.Jassim AH, Coughlin L, Harun-Or-Rashid M, Kang PT, Chen YR, Inman DM. Higher reliance on glycolysis limits glycolytic responsiveness in degenerating glaucomatous optic nerve. Mol Neurobiol. 2019;56(10):7097-7112. doi: 10.1007/s12035-019-1576-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright TM, Goharian I, Gardiner SK, Sehi M, Greenfield DS. Short-term enhancement of visual field sensitivity in glaucomatous eyes following surgical intraocular pressure reduction. Am J Ophthalmol. 2015;159(2):378-85.e1. doi: 10.1016/j.ajo.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 33.Musch DC, Gillespie BW, Palmberg PF, Spaeth G, Niziol LM, Lichter PR. Visual field improvement in the collaborative initial glaucoma treatment study. Am J Ophthalmol. 2014;158(1):96-104.e2. doi: 10.1016/j.ajo.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caprioli J, de Leon JM, Azarbod P, et al. Trabeculectomy can improve long-term visual function in glaucoma. Ophthalmology. 2016;123(1):117-128. doi: 10.1016/j.ophtha.2015.09.027 [DOI] [PubMed] [Google Scholar]
- 35.Ahmed OM, Waisbourd M, Spaeth GL, Katz LJ. Improvement in structure and visual function in patients with glaucoma: the possible key to better treatment? Surv Ophthalmol. 2021;66(4):644-652. doi: 10.1016/j.survophthal.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 36.Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005;46(9):3197-3207. doi: 10.1167/iovs.04-0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortune B, Burgoyne CF, Cull GA, Reynaud J, Wang L. Structural and functional abnormalities of retinal ganglion cells measured in vivo at the onset of optic nerve head surface change in experimental glaucoma. Invest Ophthalmol Vis Sci. 2012;53(7):3939-3950. doi: 10.1167/iovs.12-9979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota S, Takihara Y, Arimura S, et al. Altered transport velocity of axonal mitochondria in retinal ganglion cells after laser-induced axonal injury in vitro. Invest Ophthalmol Vis Sci. 2015;56(13):8019-8025. doi: 10.1167/iovs.15-17876 [DOI] [PubMed] [Google Scholar]
- 39.Takihara Y, Inatani M, Eto K, et al. In vivo imaging of axonal transport of mitochondria in the diseased and aged mammalian CNS. Proc Natl Acad Sci U S A. 2015;112(33):10515-10520. doi: 10.1073/pnas.1509879112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Z, Medeiros FA. Impact of different visual field testing paradigms on sample size requirements for glaucoma clinical trials. Sci Rep. 2018;8(1):4889. doi: 10.1038/s41598-018-23220-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Z, Medeiros FA. Sample size requirements of glaucoma clinical trials when using combined optical coherence tomography and visual field endpoints. Sci Rep. 2019;9(1):18886. doi: 10.1038/s41598-019-55345-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Data sharing statement